- 1Coastal Oregon Marine Experiment Station, Oregon State University, Newport, OR, United States
- 2Fisheries, Wildlife and Conservation Sciences, Oregon State University, Corvallis, OR, United States
- 3Department of Commerce, National Marine Fisheries Service, National Oceanic and Atmospheric Association, Lacey, WA, United States
- 4Washington Department of Fish and Wildlife, Olympia, WA, United States
The broadnose sevengill shark (Notorynchus cepedianus) is a large, apex predator found in temperate waters around the world. Yet data on their distribution are limited, with reports of species occurrence typically restricted to specific bays or estuaries where they have been historically observed in high seasonal abundances. The Puget Sound is located in the southern portion of the Salish Sea, a large estuary spanning the border between northwestern Washington state, USA and southwestern British Columbia, Canada, and serves as an economic, cultural, and ecological hub. Until recently, there was only one verified record of broadnose sevengill sharks in the Salish Sea and none in the Puget Sound. However, our recent multi-agency collaborative effort revealed the presence of adult and sub-adult broadnose sevengill sharks in South Puget Sound, extending their previously known range hundreds of kilometers and into a new ecosystem. This work represents the first evidence of a significant presence of these apex sharks within the Salish Sea.
1 Introduction
Documenting spatial patterns of species occurrences is essential in explaining ecosystem dynamics and the processes that underlie them (Rangel et al., 2007; Cagnacci et al., 2010; Sims, 2010). As such, movement and distribution data have broad implications in research, conservation, and management, offering numerous insights to not only the species directly but also broader ecosystems and populations (Renshaw et al., 2023). This is particularly true for apex predators, as changes in their abundances can have significant impacts on ecosystem structure and function (e.g., Elton and Nicholson, 1942; Paine, 1969; Beschta and Ripple, 2009). Thus, improving our understanding of where predators are found, and how they are affected by human activity and environmental change, is critical for managing species and maintaining ecosystem health.
In marine ecosystems, sharks often occupy key ecological positions as higher-order predators, contributing to ecosystem structure and stability (Heithaus et al., 2008; Ferretti et al., 2010; Bizzarro et al., 2017). As such, there have been efforts to determine the spatial patterns and occurrences of many shark species through various means, such as the use of fisheries-dependent data (Holts et al., 1998; Punt and Walker, 1998; Kai et al., 2017), genetics (Van Houtan et al., 2020), and electronic tagging technologies (Block et al., 2011; Queiroz et al., 2016). However, given the cost, technological complexity, and spatial extent of such studies, the movements of many shark species remain unknown or are oversimplified from lack of data (Van Houtan et al., 2020). Specific nuances such as shark habitat type use, pupping grounds, migrations, and broader spatial and temporal patterns of distribution are often generalized or inferred (Renshaw et al., 2023).
Broadnose sevengill sharks (Notorynchus cepedianus; BSS) are large (up to ~3 m), generalist predators that consume a wide variety of prey items, including crustaceans, teleosts, chondrichthyans, and marine mammals (Ebert, 2002; Williams et al., 2012; Funes et al., 2024). They have been reported in temperate coastal oceans around the world (Ebert, 1996), playing significant roles in maintaining marine ecosystems by virtue of their highly diverse diets (Last and Stevens, 2009). However, information on their occurrence and spatial extent, and therefore inference about their ecosystem impact, is limited to a subset of specific regions or bays within their presumed distributions – notably South Africa (Ebert, 1996), Argentina (Lucifora et al., 2005), and within the western United States in San Francisco Bay (Ebert, 1989) and Willapa Bay (Williams et al., 2012).
In August 2021, unverified anecdotal reports indicated that multiple (>10) BSS had been caught outside of their known distribution – in Hammersley Inlet, a small inlet located in the southern Puget Sound (Washington state, USA). The Puget Sound (6,200 km2), part of the larger Salish Sea that spans the Canadian-U.S. border, hosts over 7,000 species (Center for Biological Diversity 2005) and lucrative commercial and recreational fisheries (e.g., salmon, Dungeness crab, oysters; shrimp; McLeod et al., 2009; Wargo et al., 2013; Chasco et al., 2017; Morzaria-Luna, 2022), yet BSS are not known to be part of its marine ecosystems. Prior to 2021, the only verified record of BSS in the Salish Sea was located at Point Roberts, WA, near the Canadian border (Pietsch and Orr, 2019) (Figure 1). Unverified records include a single acoustic detection in the south Puget Sound of a tag attached to a BSS in Willapa Bay, WA (Williams et al., 2012).
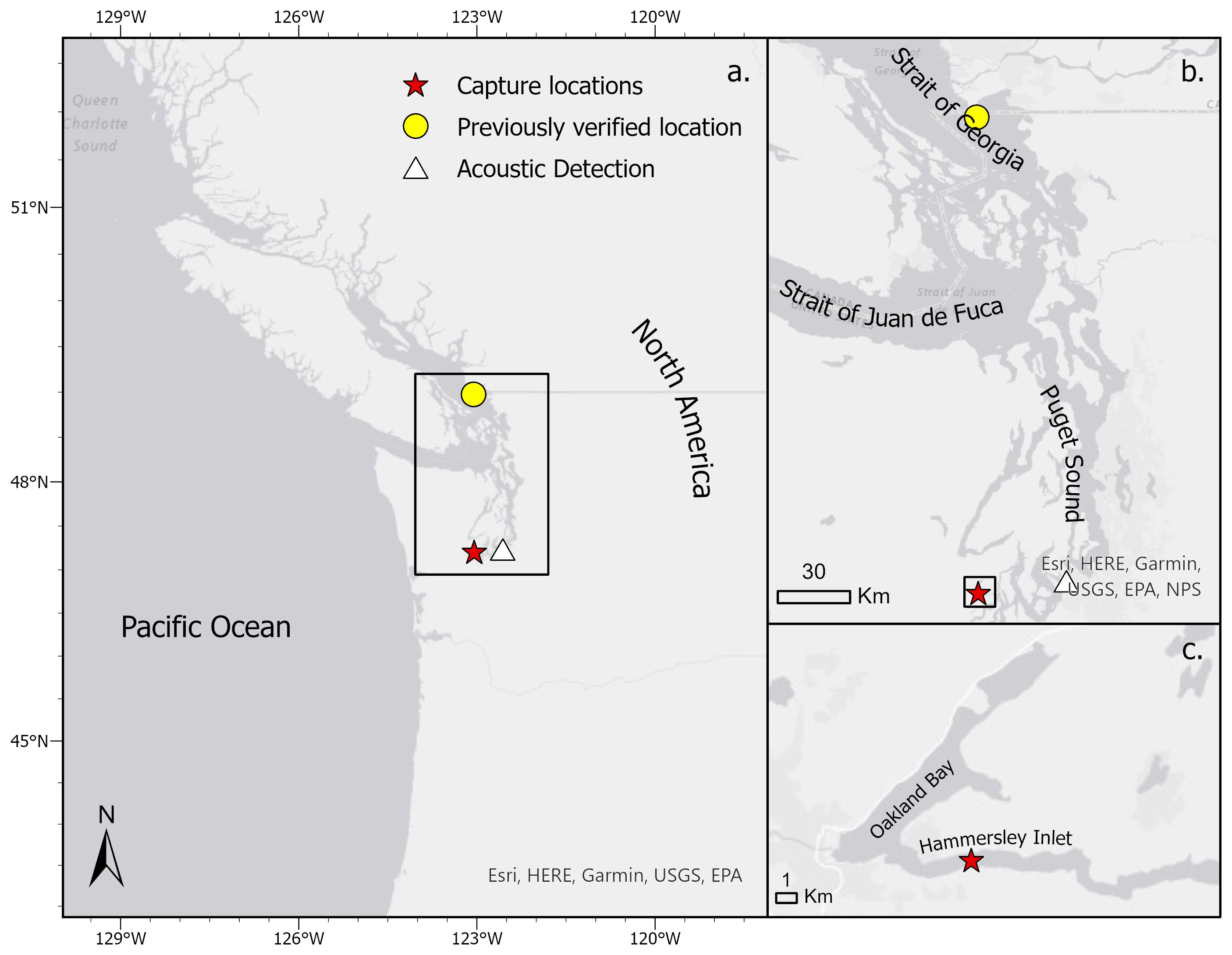
Figure 1 Map and locations of historical and recently identified broadnose sevengill shark locations in the (A) the Salish Sea between the northwest region of Washington state, USA and southern Canada, (B). a zoomed view of the previously reported locations of BSS in the Salish Sea and (C) current evidence in Hammersley Inlet and Oakland Bay. The yellow circle indicates the only verified record in the region prior to this study, located at Point Roberts, WA. The white triangle represents an acoustic detection of a tag placed on a BSS in Willapa Bay, WA (Williams et al., 2012). The red star shows new capture locations documented within this study. The black boxes indicate the area in the subsequent tile.
BSS have been shown to be ecologically important in the habitats where they live (Lucifora et al., 2005; Funes et al., 2024). However, because they are not thought to reside in Puget Sound waters, BSS are conspicuously absent from ecological models there. Updated information on their range could be relevant to both BSS species management as well as the ongoing management of other species in the Salish Sea (McLeod et al., 2009; Chasco et al., 2017; Morzaria-Luna, 2022). Therefore, in this study, we aimed to collect such data by verifying the presence of BSS in the Puget Sound.
2 Methods
Field work took place in Hammersley Inlet (Figure 1) in spring and summer of 2022 and summer through winter of 2023. Hammersley Inlet is a narrow, shallow (<15 m) body of water which connects Oakland Bay to the rest of South Puget Sound. Sampling periods were chosen opportunistically around slack tides, given that Hammersley Inlet has fast currents that can prevent fishing lines from staying in place. Fishing took place by research vessel or from shore. Fishing was done using heavy tackle rods or with droplines, which consisted of a floating surface buoy attached to a vertical nylon line (test strength 1600 psi), lightly weighted at the bottom to allow free swimming of sharks if/when hooked. Each dropline was fitted with 2-3 wire leaders with 14/0 or 16/0 baited circle hooks along the vertical line, arranged so at least one hook was close to the bottom and others were suspended in the water column. Bait consisted of farmed Atlantic salmon (Salmo salar), Pacific herring (Clupea pallasii), or opportunistically collected salmon (Oncorhynchus spp.) carcasses. Methods were approved under Oregon State University Institutional Animal Care and Use Committee protocol number 2020-0092 and Washington Department of Fisheries and Wildlife permit number 21-273.
Baited droplines were checked every 30 minutes for presence of sharks (either directly or for bite marks on bait) and/or to replace bait. GPS locations were noted using the boat’s on-board electronics system (Garmin) when fishing from boat. When caught from the research vessel, sharks were restrained alongside the boat, securing the animal for measurements while allowing it to remain in the water and obtain aeration over the gills (Figure 2A). When captured from shore, animals were brought into the shallows and secured on top of a custom-made cradle, allowing head and gills to remain submerged while morphometric measurements were taken (Figure 2B). For each shark, we measured lengths in centimeters (cm; pre-caudal, fork, and total length in natural position), sex, as well as clasper length and rigidity (i.e., degree of calcification) in males to determine sexual maturity. Sharks were externally marked with individually numbered conventional tags (Floy Tag & Manufacturing, Inc., Seattle, WA) to identify individuals if recaptured. Hooks were removed before all sharks were released. Photos were taken (Figures 2A, B) for verification purposes by the University of Washington’s Ichthyology Collection at the Burke Museum (Seattle, WA).

Figure 2 Broadnose sevengill sharks caught in Oakland Bay from boat (A) and from shore (B) during field efforts. Species identification was confirmed using the number of gills and the spotting pattern, and verified by the University of Washington Ichthyology Collection at the Burke Museum (catalog number UW202515).
3 Results
We caught 9 BSS over 10 days of field sampling (Table 1). Species were verified as BSS by the University of Washington Burke Museum Ichthyology Collection using data and photos obtained while in the field (catalog number UW202515; Figure 2). They were positively identified by their seven paired gill slits, broad head shape, and unique spotting pattern covering the dorsal surface of the body (Barnett et al., 2012). All were males except for one female caught on the first sampling day. Male total lengths ranged from 136 cm to 212 cm (n=8; mean=166; SD=28.09); the female caught was 138 cm TL. Based on Ebert (2003) and calcification of claspers, all individuals were considered adult or subadult; no neonates or young-of-the-year (<120 cm) were caught. All sharks were captured from May to August, while none were caught during sampling efforts between September and November 2023 (Table 1). All animals were released and swam without difficulty from the capture site.
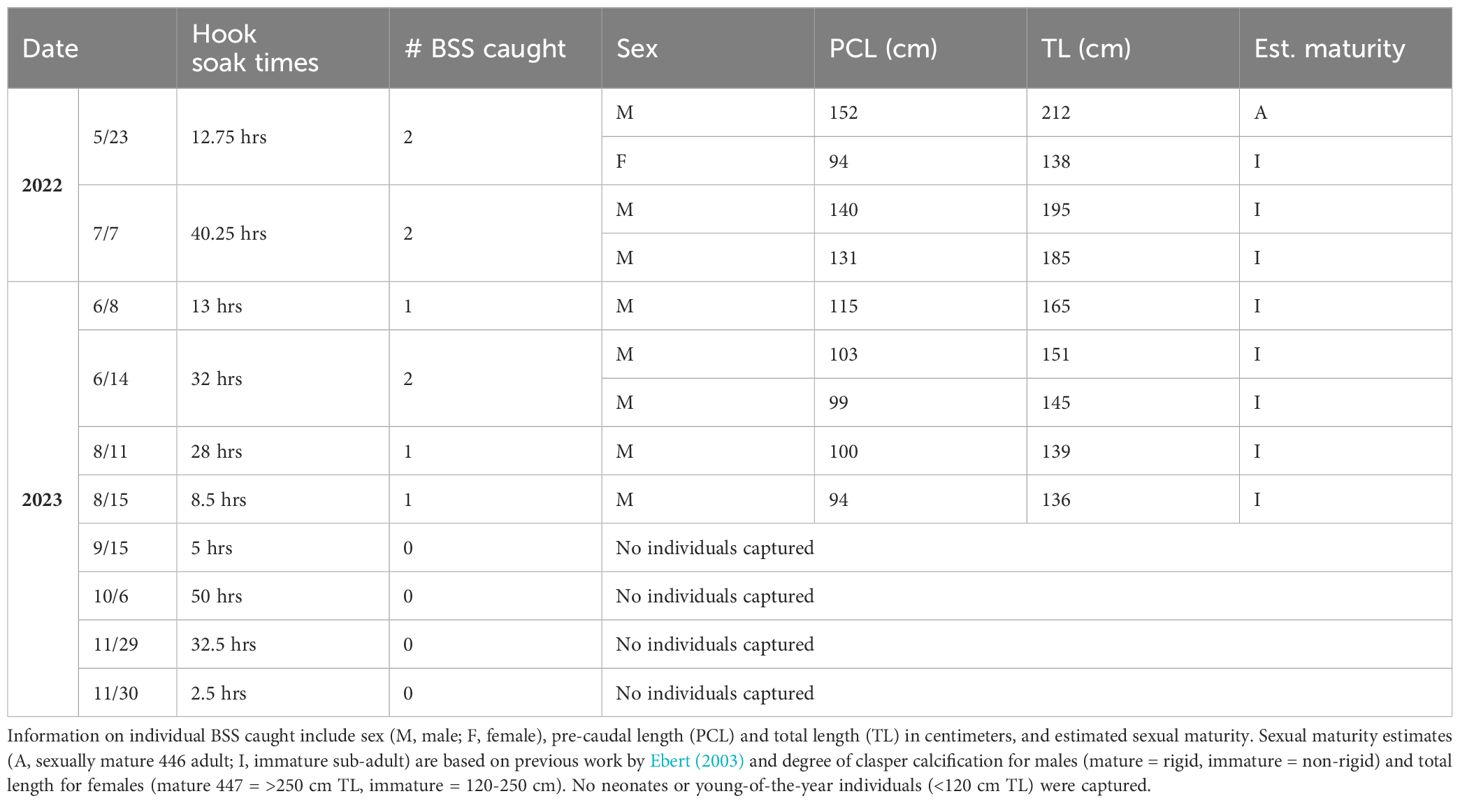
Table 1 Dates of targeted broadnose sevengill shark (BSS) fishing, hook soak times (field hours x # of hooks), and total number of BSS caught each day.
4 Discussion
We present verified and repeated evidence of BSS in South Puget Sound, the southernmost portion of the Salish Sea, more than 320 km beyond their previously documented range along the outer Washington coast. Despite the heavy anthropogenic activity in the Puget Sound, prior to our work, there was only one verified report of BSS in the entirety of the Salish Sea (over 200 km away), which occurred over two decades ago (Figure 1). Thus, BSS were not thought to occur in the Salish Sea with regularity nor are they currently considered in management efforts there (Harvey et al., 2012; Preikshot and Bobbi Cheney, 2015). However, our data suggest a seasonally consistent presence far into South Puget Sound, which has only one outlet to the north Pacific Ocean, meaning the species must occupy waters of the central Salish Sea on at least a transitory basis. With their high trophic status and ecosystem impact in other locations, these findings may suggest a need to revise our understanding of ecological dynamics in the Salish Sea. Moreover, our work has broader implications for our understanding of BSS movements globally, highlighting the value of further exploration into the range of this species.
The lack of observations of BSS in the Salish Sea prior to this study makes it difficult to determine whether BSS presence is a new development, or if they have been simply missed from surveys and fishing efforts in this area. Previous efforts to observe sharks in the Puget Sound noted other elasmobranch species, but not BSS. For instance, thousands of trawl surveys spanning decades, completed by the Washington Department of Fisheries and Wildlife, found other shark species but never a single BSS (Quinnell, 1991; Palsson et al., 2002, Palsson et al., 2003; Blaine et al., 2020). Additionally, the nearby Seattle Aquarium aimed to specifically assess shark presence in the area using baited remote underwater video cameras (BRUV), deployed at night every other month for over 12 years (2002-2005, 2008-2015) from under the aquarium in downtown Seattle (Griffing et al., 2014). This work successfully recorded >250 observations of bluntnose sixgill sharks (Hexanchus griseus), but no BSS. Both bluntnose sixgill sharks and BSS scavenge for carrion (Ebert, 1991, Ebert, 1994) and can co-occur at the depth range of these surveys since both species move into shallower waters at night (Andrews et al., 2009; Barnett et al., 2010); therefore, it is reasonable that extensive nighttime BRUV efforts capturing bluntnose sixgill sharks would have also observed BSS had they been in the area. No bluntnose sixgill sharks were taken as bycatch during BSS target fishing for this study, likely due to their deeper depth preferences during the day compared with broadnose sevengill sharks (Andrews et al., 2009; Barnett et al., 2010). However, annual presence of pinnipeds, a key prey species for BSS (Lucifora et al., 2005; Funes et al., 2024), has steadily increased in this region since the 1990s. Carretta et al. (2016) hypothesized that natural pinniped predators, such as orcas, may increase their presence in this region with this increasing prey abundance. It is therefore possible that BSS presence in this region may be the result of a recent distributional shift in response to changes in local prey resources.
Regardless of the persistence or novelty of BSS in South Puget Sound, our findings emerge at a timely junction in the management of the Puget Sound’s resources. Given the importance of apex predators in ecosystem regulation, studies of ecosystem and resource management should expand to include sharks such as BSS in the Puget Sound. To support this management, future research efforts should focus on better defining the ecological role of BSS here specifically. By assessing BSS population abundance, identifying their fine- and large-scale movements, exploring patterns of seasonal presence, connectivity with other populations, and examining BSS foraging ecology within Puget Sound and the Salish Sea at large, we can better quantify the impact they are likely having in this productive region.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee (IACUC). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JS: Conceptualization, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. EP: Conceptualization, Investigation, Methodology, Writing – review & editing, Visualization. DL: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. LH: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – review & editing. AM: Writing – review & editing. TC: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding was provided by support from the Washington Department of Fish and Wildlife.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andrews K. S., Williams G. D., Farrer D., Tolimieri N., Harvey C. J., Bargmann G., et al. (2009). Diel activity patterns of sixgill sharks, Hexanchus griseus: the ups and downs of an apex predator. Anim. Behav. 78, 525–536. doi: 10.1016/j.anbehav.2009.05.027
Barnett A., Abrantes K. G., Stevens J. D., Bruce B. D., Semmens J. M. (2010). Fine-scale movements of the broadnose sevengill shark and its main prey, the gummy shark. PLoS One 5, e15464. doi: 10.1371/journal.pone.0015464
Barnett A., Braccini J. M., Awruch C. A., Ebert D. (2012). An overview on the role of Hexanchiformes in marine ecosystems: biology, ecology and conservation status of a primitive order of modern sharks. J. Fish Biol. 80, 966–990. doi: 10.1111/j.1095-8649.2012.03242.x
Beschta R. L., Ripple W. J. (2009). Large predators and trophic cascades in terrestrial ecosystems of the western United States. Biol. Conserv. 142, 2401–2414. doi: 10.1016/j.biocon.2009.06.015
Bizzarro J. J., Carlisle A. B., Smith W. D., Cortés E. (2017). Diet composition and trophic ecology of northeast pacific ocean sharks. Adv. Mar. Biol. 77, 111–148. doi: 10.1016/bs.amb.2017.06.001
Blaine J., Lowry D., Pacunski R. (2020). 2002-2007 WDFW scientific bottom trawl surveys in the southern Salish Sea: species distributions, abundance, and population trends. Fish Program Technical Report FPT 20-01. Olympia, WA: Washington Department of Fish and Wildlife.
Block B. A., Jonsen I. D., Jorgensen S. J., Winship A. J., Shaffer S. A., Bograd S. J., et al. (2011). Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90. doi: 10.1038/nature10082
Cagnacci F., Boitani L., Powell R. A., Boyce M. S. (2010). Animal ecology meets GPS-based radiotelemetry: a perfect storm of opportunities and challenges. Philos. Trans. R. Soc B Biol. Sci. 365, 2157–2162. doi: 10.1098/rstb.2010.0107
Carretta J. V., Marie O., Erin M., Baker J. D., Weller D. W., Lang A. R., et al. (2016). U.S. Pacific marine mammal stock assessments, 2015. doi: 10.7289/V5/TM-SWFSC-561
Center for Biological Diversity. (2005). The Puget Sound Basin—A biodiversity assessment. Available at: http://sanjuans.org/wp-content/uploads/2016/11/PugetSoundBasinBiodiversityAssessment.pdf.
Chasco B. E., Kaplan I. C., Thomas A., Acevedo-Gutiérrez A., Noren D., Ford M. J., et al. (2017). Estimates of Chinook salmon consumption in Washington State inland waters by four marine mammal predators from 1970 to 2015. Can. J. Fish. Aquat. Sci. 74 (8), 1173–1194. doi: 10.1139/cjfas-2016-0203
Ebert D. (1989). Life history of the sevengill shark, Notorynchus cepedianus Peron, in two northern California bays. Calif. Fish Game 75, 102–112.
Ebert D. (1991). Diet of the seven gill shark Notorynchus cepedianus in the temperate coastal waters of southern Africa. South Afr. J. Mar. Sci. 11, 565–572. doi: 10.2989/025776191784287547
Ebert D. A. (1994). Diet of the sixgill shark Hexanchus griseus off southern Africa. South Afr. J. Mar. Sci. 14, 213–218. doi: 10.2989/025776194784287030
Ebert D. (1996). Biology of the sevengill shark Notorynchus cepedianus (Peron 1807) in the temperate coastal waters of southern Africa. South Afr. J. Mar. Sci. 17, 93–103. doi: 10.2989/025776196784158545
Ebert D. (2002). Ontogenetic changes in the diet of the sevengill shark (Notorynchus cepedianus). Mar. Freshw. Res. 53, 517. doi: 10.1071/MF01143
Ebert D. (2003). Sharks, Rays, and Chimaeras of California. Berkeley, CA: University of California Press, 284 pp.
Elton C., Nicholson M. (1942). The ten-year cycle in numbers of the lynx in Canada. J. Anim. Ecol. 11, 215–244. doi: 10.2307/1358
Ferretti F., Worm B., Britten G. L., Heithaus M. R., Lotze H. K. (2010). Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 13, 1055–1071. doi: 10.1111/j.1461-0248.2010.01489.x
Funes M., Wysiecki A. M. D., Bovcon N. D., Jaureguizar A. J., Irigoyen A. J. (2024). Understanding the feeding ecology of the broadnose sevengill shark (Notorynchus cepedianus) in Patagonia, Argentina. Food Webs 38, e00339. doi: 10.1016/j.fooweb.2024.e00339
Griffing D., Larson S., Hollander J., Carpenter T., Christiansen J., Doss C. (2014). Observations on abundance of bluntnose sixgill sharks, hexanchus griseus, in an urban waterway in puget sound 2003-2005. PLoS One 9, e87081. doi: 10.1371/journal.pone.0087081
Harvey C. J., Williams G. D., Levin P. S. (2012). Food web structure and trophic control in central puget sound. Estuaries Coasts 35, 821–838. doi: 10.1007/s12237-012-9483-1
Heithaus M., Frid A., Wirsing A. J., Worm B. (2008). Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202–210. doi: 10.1016/j.tree.2008.01.003
Holts D. B., Julian A., Sosa-Nishizaki O., Bartoo N. W. (1998). Pelagic shark fisheries along the west coast of the United States and Baja California, Mexico. Fish. Res. 39, 115–125. doi: 10.1016/S0165-7836(98)00178-7
Kai M., Thorson J. T., Piner K. R., Maunder M. N. (2017). Predicting the spatio-temporal distributions of pelagic sharks in the western and central North Pacific. Fish. Oceanogr. 26, 569–582. doi: 10.1111/fog.12217
Last P. R., Stevens J. D. (2009). Sharks and rays of Australia. 2nd ed (Cambridge, Mass: Harvard University Press).
Lucifora L. O., Menni R. C., Escalante A. H. (2005). Reproduction, abundance and feeding habits of the broadnose sevengill shark Notorynchus cepedianus in north Patagonia, Argentina. Mar. Ecol. Prog. Ser. 289, 237–244. doi: 10.3354/meps289237
McLeod K., Crowder L. B., Leslie H., Aburto M., Alessa L., de los Angeles Carvajal M., et al. (2009) Ecosystem-Based Management for the Oceans (Washington, D. C., UNITED STATES: Island Press). Available online at: http://ebookcentral.proquest.com/lib/osu/detail.action?docID=3317513 (Accessed January 16, 2024).
Morzaria-Luna H. N., Kaplan I. C., Harvey C. J., Girardin R., Fulton E. A., MacCready P., et al. (2022). Design and Parameterization of a Spatially Explicit Atlantis Ecosystem Model for Puget Sound. NOAA technical memorandum NMFS-NWFSC-177. Northwest Fisheries Science Center, 247 pp. doi: 10.25923/tnp6-mf67
Paine R. T. (1969). A note on trophic complexity and community stability. Am. Nat. 103, 91–93. doi: 10.1086/282586
Palsson W. A., Clarke P., Hoffmann S., Beam J. (2002). Results From The 2000 Transboundary Trawl Survey of the Eastern Strait of Juan De Fuca and Discovery Bay. Mill Creek, WA: Washington Department of Fish and Wildlife, 76 pp.
Palsson W. A., Hoffmann S., Clarke P., Beam J. (2003). Results from the 2001 transboundary trawl survey of the southern Strait of Georgia, San Juan Archipelago and adjacent waters. Washington Department of Fish and Wildlife Technical Report FPT 03-09. Olympia, WA: Washington Department of Fish and Wildlife, 109 pp.
Pietsch T. W., Orr J. W. (2019) Fishes of the Salish Sea (University of Washington Press). Available online at: https://uwapress.uw.edu/book/9780295743745/fishes-of-the-salish-sea (Accessed April 7, 2024).
Preikshot D., Bobbi Cheney D. (2015). Ecosystem models of species changes in South Puget Sound from 1970 to 2012 and simulations of species changes from 2012 to 2054 (Seattle, WA: Washington Sea Grant). Available at: https://repository.library.noaa.gov/view/noaa/44166.
Punt A. E., Walker T. I. (1998). Stock assessment and risk analysis for the school shark (Galeorhinus galeus) off southern Australia. Mar. Freshw. Res. 49, 719–731. doi: 10.1071/MF96101
Queiroz N., Humphries N. E., Mucientes G., Hammerschlag N., Lima F. P., Scales K. L., et al. (2016). Ocean-wide tracking of pelagic sharks reveals extent of overlap with longline fishing hotspots. Proc. Natl. Acad. Sci. 113, 1582–1587. doi: 10.1073/pnas.1510090113
Quinnell S. (1991). Abundance of Puget Sound demersal fishes: 1987 research trawl survey results (Olympia, WA: State of Washington, Department of Fisheries, Marine Fish/Shellfish Program).
Rangel T. F. L. V. B., Diniz-Filho J. A. F., Colwell R. K. (2007). Species richness and evolutionary niche dynamics: A spatial pattern–oriented simulation experiment. Am. Nat. 170, 602–616. doi: 10.1086/521315
Renshaw S., Hammerschlag N., Gallagher A. J., Lubitz N., Sims D. W. (2023). Global tracking of shark movements, behaviour and ecology: A review of the renaissance years of satellite tagging studies 2010–2020. J. Exp. Mar. Biol. Ecol. 560, 151841. doi: 10.1016/j.jembe.2022.151841
Sims D. (2010). “Tracking and analysis techniques for understanding free-ranging shark movements and behavior,” in Sharks and Their Relatives II, (CRC Press). 351–392. doi: 10.1201/9781420080483-c8
Van Houtan K. S., Gagné T. O., Reygondeau G., Tanaka K. R., Palumbi S. R., Jorgensen S. J. (2020). Coastal sharks supply the global shark fin trade. Biol. Lett. 16, 20200609. doi: 10.1098/rsbl.2020.0609
Wargo L., Ayres D., Cheng Y. W. (2013) Washington Coastal Spot Shrimp Fishery. Available online at: https://wdfw.wa.gov/sites/default/files/publications/01532/wdfw01532.pdf.
Keywords: elasmobranch, marine ecology, movement, occurrence, predator
Citation: Schulte JM, Personius EM, Lowry D, Hillier L, McInturf AG and Chapple TK (2024) Advancing the ecological narrative: documentation of broadnose sevengill sharks (Notorynchus cepedianus) in South Puget Sound, Washington, USA. Front. Mar. Sci. 11:1430962. doi: 10.3389/fmars.2024.1430962
Received: 10 May 2024; Accepted: 06 June 2024;
Published: 27 June 2024.
Edited by:
Emily Lester, Australian Institute of Marine Science (AIMS), AustraliaReviewed by:
Steven T. Kessel, Shedd Aquarium, United StatesThomas Marcellin Grothues, Rutgers, The State University of New Jersey, United States
Copyright © 2024 Schulte, Personius, Lowry, Hillier, McInturf and Chapple. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica M. Schulte, SmVzc2ljYS5zY2h1bHRlQG9yZWdvbnN0YXRlLmVkdQ==
 Jessica M. Schulte
Jessica M. Schulte Ethan M. Personius
Ethan M. Personius Dayv Lowry
Dayv Lowry Lisa Hillier
Lisa Hillier Alexandra G. McInturf
Alexandra G. McInturf Taylor K. Chapple
Taylor K. Chapple