- 1Atmosphere and Ocean Research Institute, University of Tokyo, Kashiwa, Chiba, Japan
- 2Institute of Marine Affairs and Resource Management, National Taiwan Ocean University, Keelung, Taiwan
- 3Center of Excellence for the Oceans, National Taiwan Ocean University, Keelung, Taiwan
Uroteuthis edulis (Hoyle, 1885) is an Indo-Pacific squid species widely distributing in the western Pacific, and commercially important especially in Japan and Taiwan. It has been suggested that some individuals are possibly transported from the spawning ground in north Taiwan to the coasts of Japan, however, the strength of population connectivity between those areas and its influence on U. edulis population dynamics were unveiled. To understand the U. edulis population connectivity in this area, the correlations between statolith trace elements and abiotic/biotic factors were examined first, and then squid experienced environments were postulated throughout their entire life cycle. Sr/Ca ratio showed a strongly negative correlation with ambient water temperature but no correlation with individual growth rate, suggesting that Sr/Ca ratio can be used to reflect squid experienced temperatures. Most squid caught in the Sea of Japan hatched in the areas having similar water temperature with where Taiwanese squid hatched, that would be off the north Taiwan or even warmer area. Statolith trace elements successfully distinguished the catch locations but not the hatching grounds, implying that hatching grounds of Japan and Taiwan squid were largely overlapped. Thus, we suggest that there is strong population connectivity of U. edulis population between southern Japan and northern Taiwan. As there was no clear evidence for existence of local population hatched in the Sea of Japan in this study, U. edulis population might display a source-sink population dynamics, that is, population in Taiwanese waters and/or further south as the source, and the one in the Sea of Japan as a sink population. As U. edulis should be considered as a metapopulation, collaboration among countries in the northwestern Pacific is required for sustainable fishery management of this species.
1 Introduction
Population connectivity, which is defined as the exchange of individuals among geographically separated subpopulations, has been regarded as one of the crucial processes for species evolution, population dynamics, and community persistence to climate change (Lowe and Allendorf, 2010; Kool et al., 2013). Population connectivity over large geographical areas has been commonly found in many squid species (Loligo reynaudii and Doryteuthis pealeii, Shaw et al., 2010; Sepioteuthis australis, Smith et al., 2015), that local populations are connected by not only the highly mobile adult stage but also long-distance transport of planktonic paralarval stage (Boyle and Rodhouse, 2005). For instance, adult individuals of large-size squid species migrate long distance between feeding and spawning grounds to expand their distributions (Illex argentines, Arkhipkin, 1993; Dosidicus gigas, Nigmatullin et al., 2001; Todarodes pacificus, Sakurai et al., 2002). On the other hand, currents dispersal during squid early life stages and the survival of the transported individuals is strongly associated with squid distribution (Illex illecebrosus, Dawe et al., 2007; Ommastrephes bartramii, Chen et al., 2012). Previous squid population studies mostly focus on genetic connectivity, indicating a high level of gene flow commonly found among squid subpopulations over large geographical areas (Shaw et al., 2010; Smith et al., 2015; McKeown et al., 2019). Many squid populations should be considered as metapopulations (Lipiński et al., 2016), however, demographic connectivity and its impacts on population dynamics have rarely been addressed due to the time-, labor- and cost-intensive nature of collecting demographic data at spatial and temporal scale (Drake et al., 2022). As ignoring demographic connectivity among different regions could easily lead to biases of impacts of connectivity on population dynamics and overexploitation (Fogarty and Botsford, 2007; Ying et al., 2011; Kerr et al., 2017), it is parament to incorporate demographic data (e.g. occupancy, abundance) into connectivity analysis to better understand the spatiotemporal dynamics of spatially structured populations for effective squid fishery management (Carson et al., 2011; Drake et al., 2022).
Squid statoliths are composed of aragonitic calcium carbonate similar to that in corals, shells of gastropod and bivalve, and fish otoliths (Smith et al., 1979; Campana and Neilson, 1985; Zacherl et al., 2003; calcium carbonate matrix of statolith: Arkhipkin, 2005). Trace elements in the statolith have been widely used as a proxy to distinguish different geographical groups and to identify natal origins (Loligo gahi, Arkhipkin et al., 2004; Dosidicus gigas, Liu et al., 2015; Sepioteuthis lessoniana, Ching et al., 2019; Illex argentinus, Avigliano et al., 2020a). For example, elemental signatures in the statolith of Loligo gahi varied significantly among geographical and seasonal cohorts (Arkhipkin et al., 2004). Even though no genetic difference was detected among the cohorts (Shaw et al., 2004), these cohorts were assumed as distinct subpopulations with sufficient gene flow (Arkhipkin et al., 2004). Warner et al. (2009) showed that concentrations of trace elements in statolith cores of the adult California market squid Doryteuthis opalescens closely resembled the elemental signatures in statolith of paralarvae caught on the spawning ground, which opened the possibility to identify the source populations for geographical and seasonal stocks. Thus, statolith trace elements can be used as natural markers for connectivity studies. Specifically, the Sr/Ca ratio showed potential as an indicator of ambient water temperature according to the negative correlation between water temperature at capture and the Sr/Ca ratio at the edge of statoliths in Doryteuthis gahi from the Southwest Atlantic (Arkhipkin et al., 2004). Ultimately, statolith trace elements and their relationships with environmental conditions enable to distinguish cohorts that hatched in different spawning grounds and further reconstruct squid experienced environments (Ikeda et al., 2003; Jones et al., 2018).
The swordtip squid Uroteuthis edulis (Hoyle, 1885) is a medium- to large-sized neritic squid (maximum mantle length ~ 410 mm) distributed widely from southern Japan, across the South China Sea to the Philippine Islands and perhaps even to the continental shelf of northern Australia (Jereb and Roper, 2010). It is a commercially important fishery species of the Northwest Pacific area (FAO, 2016), particularly in coastal waters off southern Japan and northern Taiwan (Kawano, 2006; Wang et al., 2008). Life-history traits of U. edulis show great geographical and seasonal variations (Pang et al., 2020, Pang et al., 2022). The main spawning season is summer for U. edulis population in Japan, and winter spawning squids of U. edulis in southern Japan showed higher growth rate than spring and summer spawning squids (Pang et al., 2020). U. edulis in northern Taiwan spawn all year around with two spawning peaks in spring and autumn (Wang et al., 2008), with spring-spawning squid mature at a younger age than autumn-spawning ones (Pang et al., 2020). The hatching ground of U. edulis in Taiwan was suggested to be in and around the coastal water of northern coast of Taiwan (Wang et al., 2008), yet the hatching ground of U. edulis caught in Japan was still unclear. Furthermore, population fluctuations of U. edulis in the localities around Japan and Taiwan display contrasting patterns: the catch in Taiwanese waters has greatly increased since 1990, while that in Japanese waters has decreased steadily since 1988. An analysis of the relationship between squid catch and environmental parameters revealed that high water temperature was correlated with both the positive effect on the Taiwanese catch and the negative effect on the Japanese catch (Pang et al., 2018). Yet, the underlying mechanisms of local U. edulis population fluctuations remained elusive.
Previous studies focused on the U. edulis migratory routes in northwest Pacific, which is strongly driven by complex current systems (Figure 1A). Previous studies suggested that U. edulis hatched in the southern East China Sea and migrates to Japan (Yamaguchi et al., 2015, Yamaguchi et al., 2018, Yamaguchi et al., 2020), specifically, individuals collected in Tsushima Straits in June and August might be transported from the south with Kuroshio Currents (Yamaguchi et al., 2015), while those collected in October with Taiwan Warm Current (Yamaguchi et al., 2018). However, the information from those studies were limited with discontinuous sampling months and a small number of individuals. Our previous work on U. edulis life cycle also implied that Taiwan population might recruit to the Japan summer spawning group as there were few mature individuals found in Japan during its hatching season (Pang et al., 2020). For better understanding of the U. edulis population dynamics, not only the migratory routes but also the strength of its population connectivity across spatiotemporal scales would be necessary.
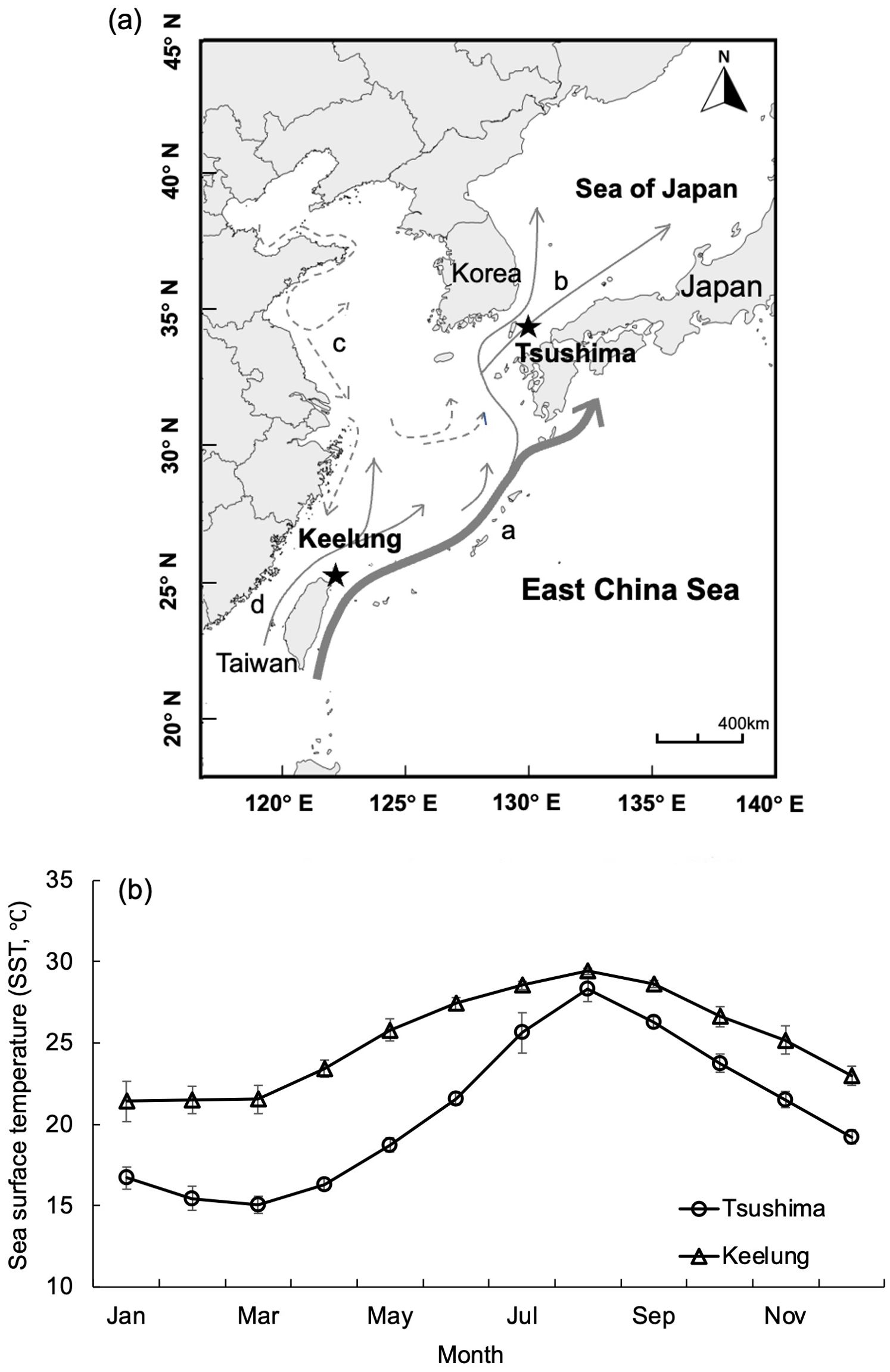
Figure 1. (A) Sampling localities (stars) off the Tsushima Islands (Japan) and off Keelung (Taiwan) and major current systems around the sampling locations. a, Kuroshio current; b, Tsushima warm current; c, East China Sea mainland coastal current; d, Taiwan Strait warm current. (B) Mean monthly sea surface temperature of waters off the Tsushima Islands and off Keelung during 2017-2020. The bars represent standard deviations.
Therefore, in this study, statolith elemental analysis was conducted on the U. edulis squid population collected in Japan and Taiwan simultaneously for continuous three years. Firstly, variation in statolith trace elements were examined considering biotic and abiotic factors. Secondly, life trajectories of Sr/Ca ratio were applied to postulate the experienced environment. Finally, we discuss the population connectivity between southern Japan and northern Taiwan and its potential impacts on squid survival and maturation, and the underlying mechanism of U. edulis populations fluctuation to provide useful guidance as to appropriate fisheries management of this species.
2 Methods
2.1 Sampling
Uroteuthis edulis Hoyle were collected in Japanese and Taiwanese waters (Figure 1A). Sea surface temperature (SST) in the waters off northern Taiwan was warmer and less variable throughout the year (19.7°C – 29.7°C, mean 25.2°C) compared to that in the waters off southern Japan, which was cooler and more variable among months (14.4°C – 29.2°C, mean 20.7°C; Japan Meteorological Agency, 2022, Figure 1B). Information of the samples were summarized in Table 1. Samples from Japanese waters were collected each month from May 2017 to August 2020, using inshore set-nets located off northern Tsushima Island (depth 30–36 m, 34.33–34.39°N, 129.17–129.30°E, Figure 1A). Fishermen separated the squid into six size classes, with about 4 kg of squid sampled from each size class (about 15 individuals for the largest class and 120 for the smallest). Measurement data were scaled by the total catch weight of each size class on any given sampling date to avoid size selection biases. Squid from Taiwanese waters were obtained by bottom-trawl during two main spawning seasons in May-July and September-November from 2017 to 2019, at 110 m depth in the southern East China Sea (25.50–26.00°N, 121.50–122.50°E, Figure 1A). Both samples from Japan and Taiwan were collected between sunset and sunrise. As the catches in Taiwan were small and unsorted, all collected squid were taken to the lab as samples for further analysis. Due to the long-distance transportation, all the samples delivered to the lab were frozen, and thawed on the day of dissection. For each squid, mantle length (ML) was measured to 1mm, and the stage of sexual maturity was determined based on stages reported for Doryteuthis plei (Perez et al., 2002; Pang et al., 2020, Pang et al., 2022). In this study, for each sampling month, the proportion of mature individual (the sum of mature females and males to all individuals) was calculated. Catch information of the squid samples is summarized in Table 1.

Table 1. Summary of catch data for Uroteuthis edulis sampled from the waters off (A) Tsushima and (B) Keelung.
Due to the large monthly variation in catches and the proportion of mature individuals in the Japanese population every year (Table 1), samples from the continuous three years were used to eliminate the sampling bias and understand the population structure accurately. For Japanese squid, we categorize catch into the four boreal seasons of spring (March-May), summer (June-August), autumn (October-December) and winter (December-February). We attribute mature individuals to spawning seasons based on the month of capture, for example, mature individuals caught during March-May in waters off southern Japan are considered to be spring spawning group (J spring). Taiwan population was divided into two major spawning groups: spring spawning group (T spring, May-July) and autumn spawning group (T autumn, September-November).
2.2 Age determination
Statoliths is formed in the statocysts, cavities in the cranial cartilage, before hatching and continue growing after hatching by the deposition of an organic-rich (translucent) zone and an organic-poor (opaque) zone every day (Barker et al., 1997). This deposition process provides an accurate daily increment, which is commonly accepted for age determination in many loliginid squid species (Jackson, 2004). Thus, growth increments on the statoliths of U. edulis are assumed to be deposited daily (Natsukari et al., 1988), as in other loliginid squids (Patterson, 1988; Villanueva, 2000; Arkhipkin, 2005). Representative sub-samples were selected from both female and male squids across the size distribution of squids for statolith microstructure analysis and age determination. The left statolith was used for age determination, and the right one was kept for further trace elemental analysis. Statoliths were attached to a microscope slide using Crystalbond 509, ground on both sides along a transverse plane using fine waterproof sandpapers (1000–4000 grit), and polished with cloth before observation. Statolith microstructure was examined by light microscope under transmitted light at ×400 magnification. The hatching ring was defined as the first thick black ring around the nucleus (Arkhipkin, 2005). Growth increments of each statolith were manually read from the hatching ring to the edge of the rostrum using a light microscope connected to a video monitor and an otolith reading system (Otolith 8 software, Ratoc System Engineering Co., Ltd.). The statolith growth increments were counted twice, with an interval of at least 3 months between readings. Only when the counts differed by less than 10%, the mean of the two counts was used as the estimated squid age in days. When increment definition was poor, or the statolith was over-ground, the sample was discarded. Age of 1268 Japanese, and 371 Taiwanese squids were successfully determined.
2.3 Trace element analysis
From these individuals whose age were successfully read, representative sub-samples were selected for statolith microstructure analysis. The right statoliths were cleaned using distilled water and fixed in the resin with the anterior side on the surface for grounding. Statoliths were grounded using fine waterproof sandpapers (2000-4000 grit) until the nucleus and statolith increments could be clearly observed. Each resin sample with grounded statolith was cut into 5mm cylinder cube and contaminants were removed from the ground surfaces using ethanol prior to analysis. The elemental analysis was performed successfully for, using sector field inductively coupled plasma mass spectrometry (ICP-MS, Element XR, Thermo Fischer Scientific), coupled to the 193 nm ArF excimer laser ablation system (RESOlution M-50, Resonetics) installed at the Atmosphere and Ocean Research Institute (Amekawa et al., 2016). Total 426 individuals, including female and male squids across a wide size distribution from both Taiwan and Japan, were successfully analyzed. The following trace elements were quantified at each ablation spot: 88Sr, 24Mg, 37Ba, 11B, 131P, 55Mn, 238U, with 42Ca used as an internal standard to account for variation in ablation yield. The concentrations of elements Mn and U were occasionally below zero, therefore those were removed from further statistics analysis.
For each statolith, ablation spots were made along the axis of growth from the nucleus to the marginal edge of the rostrum at even intervals (Figure 2). The diameter of each ablation spot was 60 μm, which contains approximately 15-20 days of statolith increments. The interval between two ablation spots was 120 μm. The number of ablation spots ranged from 8 to 12 per statolith, depending on the total length of statolith. To minimize the risk of cross-calibration and establish appropriate and reliable calibration standards, two ablation spots of a glass standard (NIST-SRM614) and two ablation spots of a pressed powder carbonate standard material (JCp-1) with the same size (60 μm) were added to both before and after analysis of each statolith. Each ablation spot was measured with an energy density of ~7 J/cm2, a pulse rate of 5 Hz, a signal integration time of 60 s and a continuous He gas stream to the ICP-MS (Supplementary Table S1).
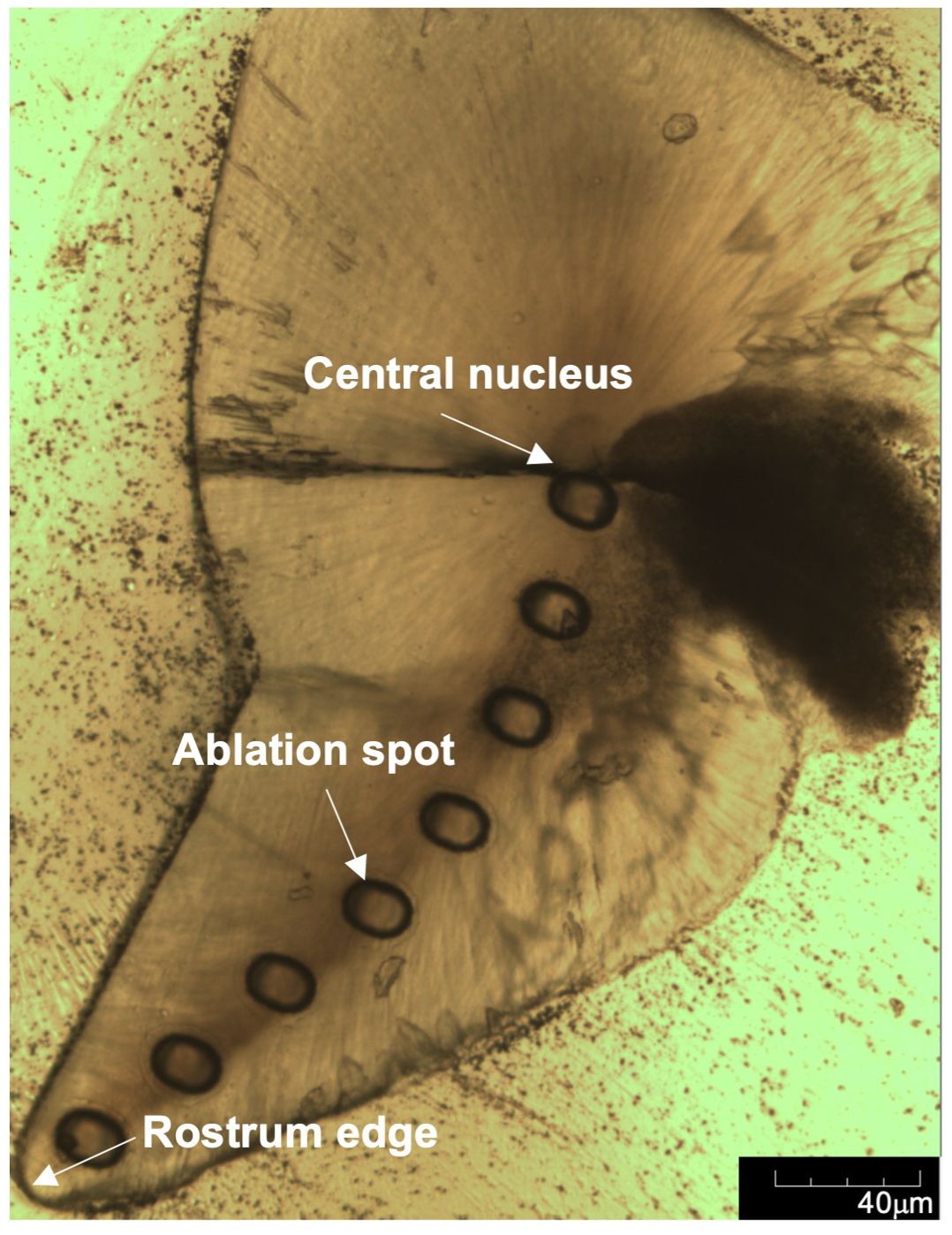
Figure 2. Image of ground statolith with ablation spots from the central nucleus to the rostrum edge.
2.4 Statistics analysis
Elemental ratios were calculated in comparison to certified values of JCp-1 (Okai et al., 2002; Amekawa et al., 2016). Each element was standardized to calcium as trace element/Ca ratios. As the squid conduct diel vertical migration at a water layer of 50-75m, monthly data on 50m depth water temperatures (50m WT) and SST at both sampling locations were collected from the database of Japan Meteorological Agency.
The correlation between all the statolith trace element concentrations at the ablation spot on rostrum edge, which is the part precipitated the most recently, and 50 m WT or SST at capture were examined using Pearson correlation analysis (Berman, 2016). To examine if individual instantaneous growth rate would affect elemental uptake rate, the correlations between average statolith increment width (SIW) and statolith trace element concentrations at the corresponding ablation spot were examined. Seven-days moving average of SIW was calculated for each individual. Two ablation spots were selected: the one located at the highest average SIW, and the another at the edge of rostrum. Then the average SIW (around 20 increments per spot) at these two spots were calculated for each individual to conduct further correlation analysis with statolith trace element concentrations.
Since Sr/Ca ratio showed the strongest correlation with ambient water temperature (Table 2), Sr/Ca ratio at the statolith nucleus/rostrum edge from the same hatch/catch month were averaged and compared between Japan-caught and Taiwan-caught individuals using one-way ANOVA and pairwise post hoc test (Tukey method). As the assumption of ANOVA test is the normal distribution of the data, Levene’s test was applied to check the homogeneity of variances across groups. If the assumption was not met, the alternative Kruskal-Walls rank sum test was applied.
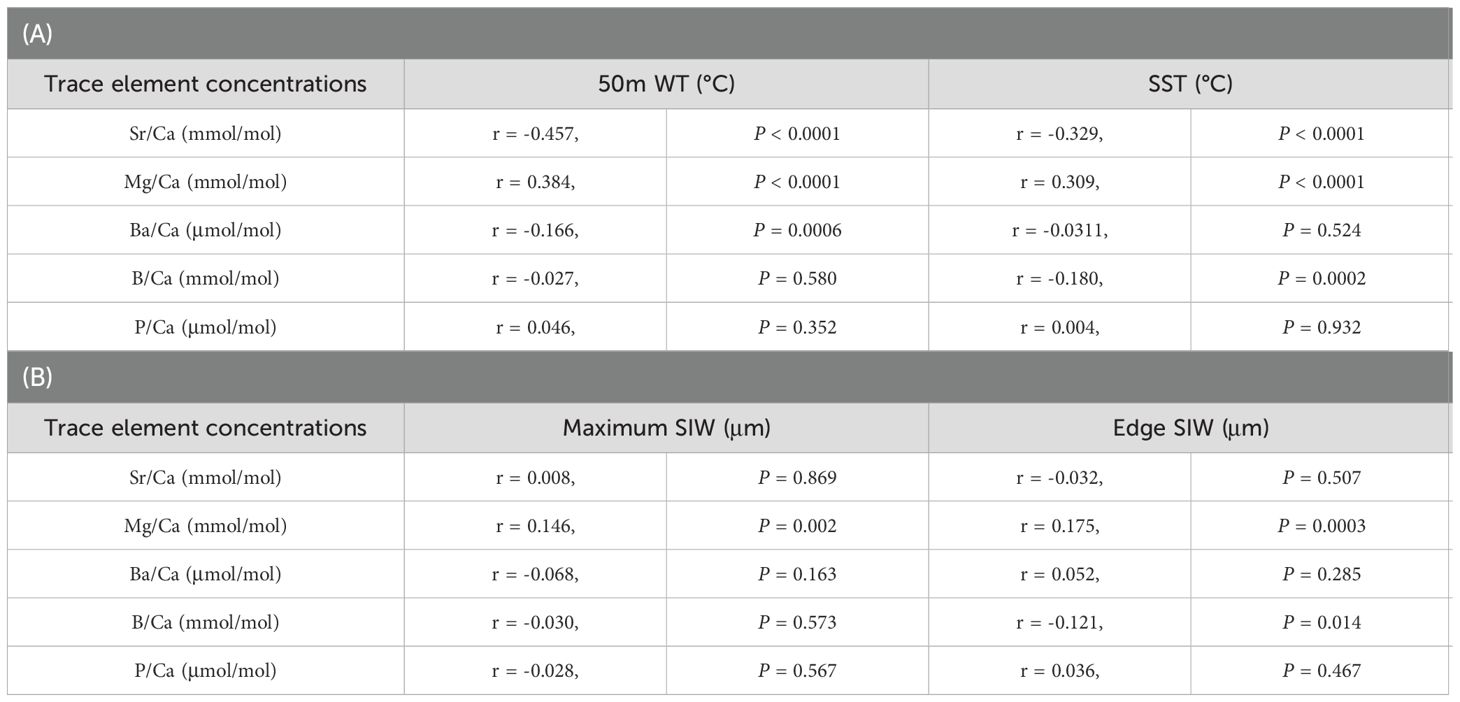
Table 2. Correlations between trace element concentrations of the ablation spot at the statolith rostrum edge and (A) 50-m water temperature (WT) or SST at capture (n = 419); (B) maximum statolith increment width (SIW) or SIW at the rostrum edge (n = 417).
Furthermore, data of Sr/Ca ratios from the nucleus to the rostrum edge were applied to reflect the experienced environment of each mature individual throughout all life stages. The sample size, hatching period, age range, and ML range of mature individuals applied in establishing Sr/Ca ratio life trajectory for each spawning group were listed in the Table 3. Since age determination and trace elemental analysis were conducted on each of the two statoliths for each individual, the age interval of each individual was calculated by dividing the age of the individual by the number of its statolith ablation spots minus one. For each spawning group, a life trajectory of Sr/Ca ratios was established: estimated age at each ablation spot of mature individuals was used as independent variable, and the corresponding Sr/Ca ratio in the transect from nucleus to the rostrum edge as dependent variable. Polynomial models with different degrees from two to six were tested and compared using AIC (Akaike information criterion) to find the fit model for Sr/Ca ratio trajectories. A biquadratic polynomial function with a degree of four was selected because the AIC values polynomial models with a degree between four and five showed small differences, and we found it sufficient to describe the relationship between Sr/Ca ratio and its corresponding age while not overfitting.
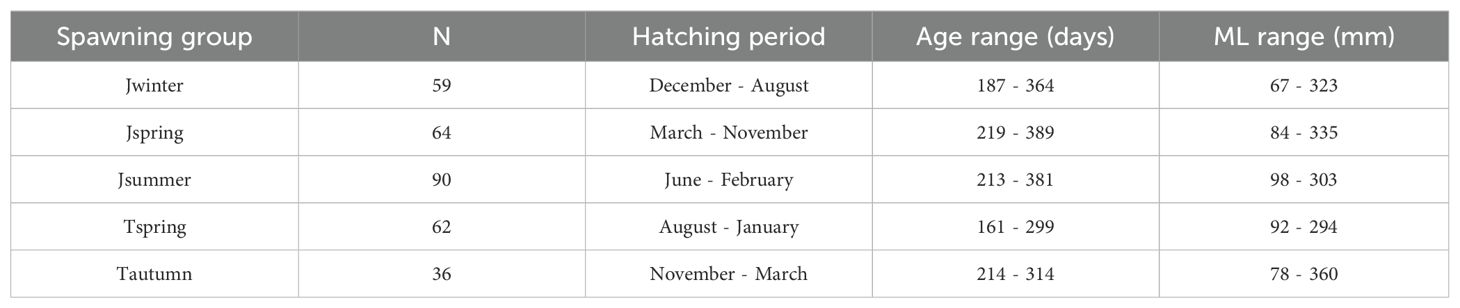
Table 3. Sample size (N), hatching period, age range, and ML range of mature individuals applied in Sr/Ca ratio life trajectories for each spawning group.
Principal component analysis (PCA) is a classical linear classification analysis, which has been widely applied for high dimensional data to facilitate visualization and interpretation (Chen et al., 2018; Feng et al., 2019). To better identify statolith trace elements for distinguishing geographical groups, a PCA was conducted to distinguish the difference between catch locations (Japan or Taiwan) using the concentration ratios of five trace elements in the ablation point at the rostrum edge. Similarly, the concentrations of five trace elements at the statolith nucleus were applied in the PCA to examine the difference of hatching grounds between Japan- and Taiwan-caught squid. All the statistical analysis were conducted using R-4.1.2.
3 Results
3.1 Correlations of statolith trace elements with water temperature and instantaneous growth rate
Both 50 m WT and SST showed significant negative correlations with Sr/Ca and positive correlations with Mg/Ca ratios (Table 2A; Figure 3). These correlations were stronger for 50 m WT than SST. Negative correlations were also found with the Ba/Ca ratio and 50 m WT as well as the B/Ca ratio and SST. The negative correlation between Sr/Ca ratio and 50m WT was the strongest among all trace elements. No significant correlations were observed between P/Ca ratio and water temperature.
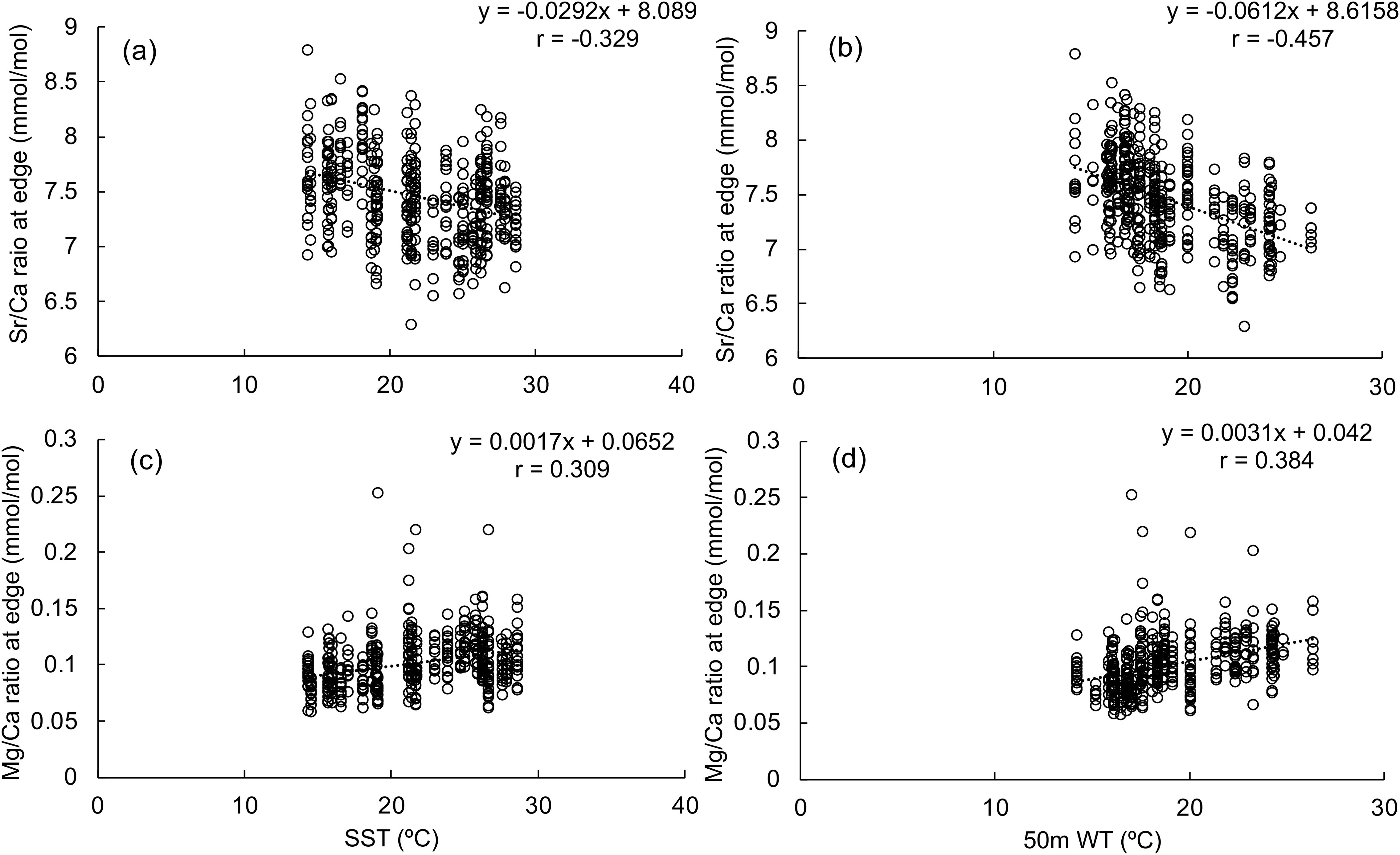
Figure 3. Correlations between (A) SST and Sr/Ca ratio at the rostrum edge; (B) 50-meter water temperature and Sr/Ca ratio at the rostrum edge; (C) SST and Mg/Ca ratio at the rostrum edge; (D) 50-meter water temperature and Sr/Ca ratio at the rostrum edge.
Correlations between statolith trace elements with individual instantaneous growth rate were examined further (Table 2B). Only Mg/Ca ratio had a strong positive correlation with instantaneous growth rate represented by either maximum SIW or SIW at the rostrum edge. Therefore, excluding from the influence of individual growth, the Sr/Ca ratio was considered to be the most suitable elemental signature to represent the water temperature experienced by each individual.
3.2 Comparison of Sr/Ca ratio at the statolith nucleus and rostrum edge among the months of catch and hatching
Since the monthly difference in 50 m WT was negligible throughout the year (approximately 0.5°CC difference for each month among years), means were calculated for the entire three years of data of 50 m WT and Sr/Ca ratios at the corresponding ablation spots for each catch and hatching month. Sr/Ca ratios at the statolith rostrum edge caught in each month are compared for Japanese and Taiwanese individuals in Figure 4A. Sr/Ca ratios at the rostrum edge were higher in Japanese individuals than those in Taiwanese individuals from the same catch month (May: F = 52.96, P < 0.001; June: F = 11.015, P = 0.0016; July: F = 10.638, P = 0.0016; November: F = 8.517, P = 0.010) except October (F = 1.432, P = 0.247).
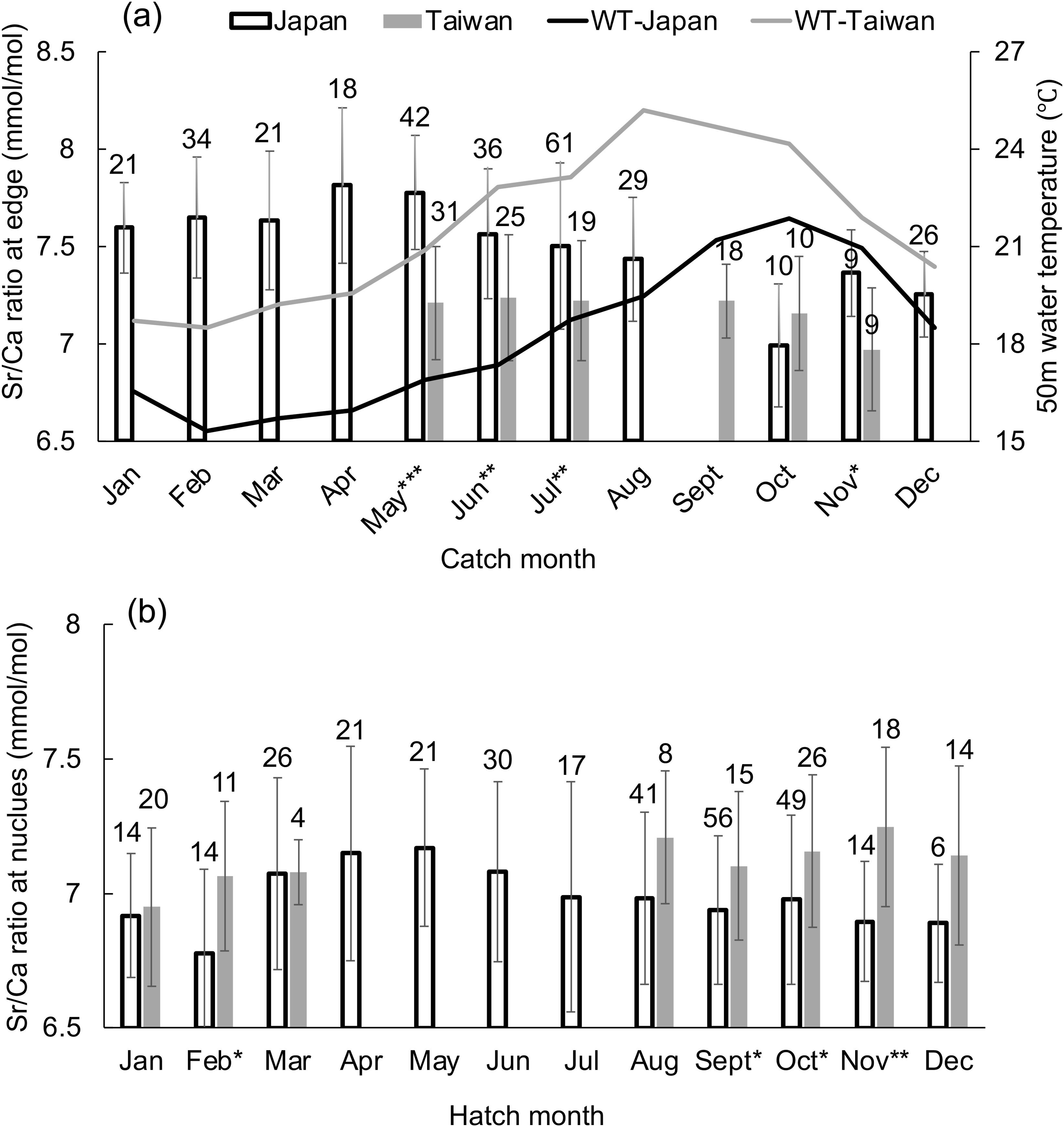
Figure 4. (A) Mean ± SD for Sr/Ca ratios at the ablation spot at the statolith rostrum edge for each month for squids sampled monthly from Japan (Tsushima) and Taiwan (Keelung). Superimposed is the mean monthly 50 m water temperature (WT) at these locations. (B) Mean Sr/Ca ratios at the ablation spot of the statolith nucleus for each month from Japan and Taiwan. The number of squid sampled each month is shown above each bar. Significant differences between Japan and Taiwan were present for the months indicated by asterisks: *P < 0.05, **P < 0.01, ***P < 0.001.
Similarly, mean Sr/Ca ratios at the nucleus (hatching) were calculated for each month and compared between Japanese and Taiwanese individuals (Figure 4B). The Sr/Ca ratios at the nucleus were higher in Taiwanese than in Japanese individuals in September (F = 4.126, P = 0.046), October (F = 5.969, P = 0.017), November (F = 12.267, P = 0.002) and February (F = 5.719, P = 0.025).
In addition, the Sr/Ca ratio at the nucleus of Japanese individuals was similar or even lower than that at the rostrum edge of individuals caught in Taiwan in the same month (Figure 5, September: F = 15.904, P < 0.001), suggesting that Japanese individuals hatched in the waters at a similar temperature with that off northern Taiwan, or an even warmer area.
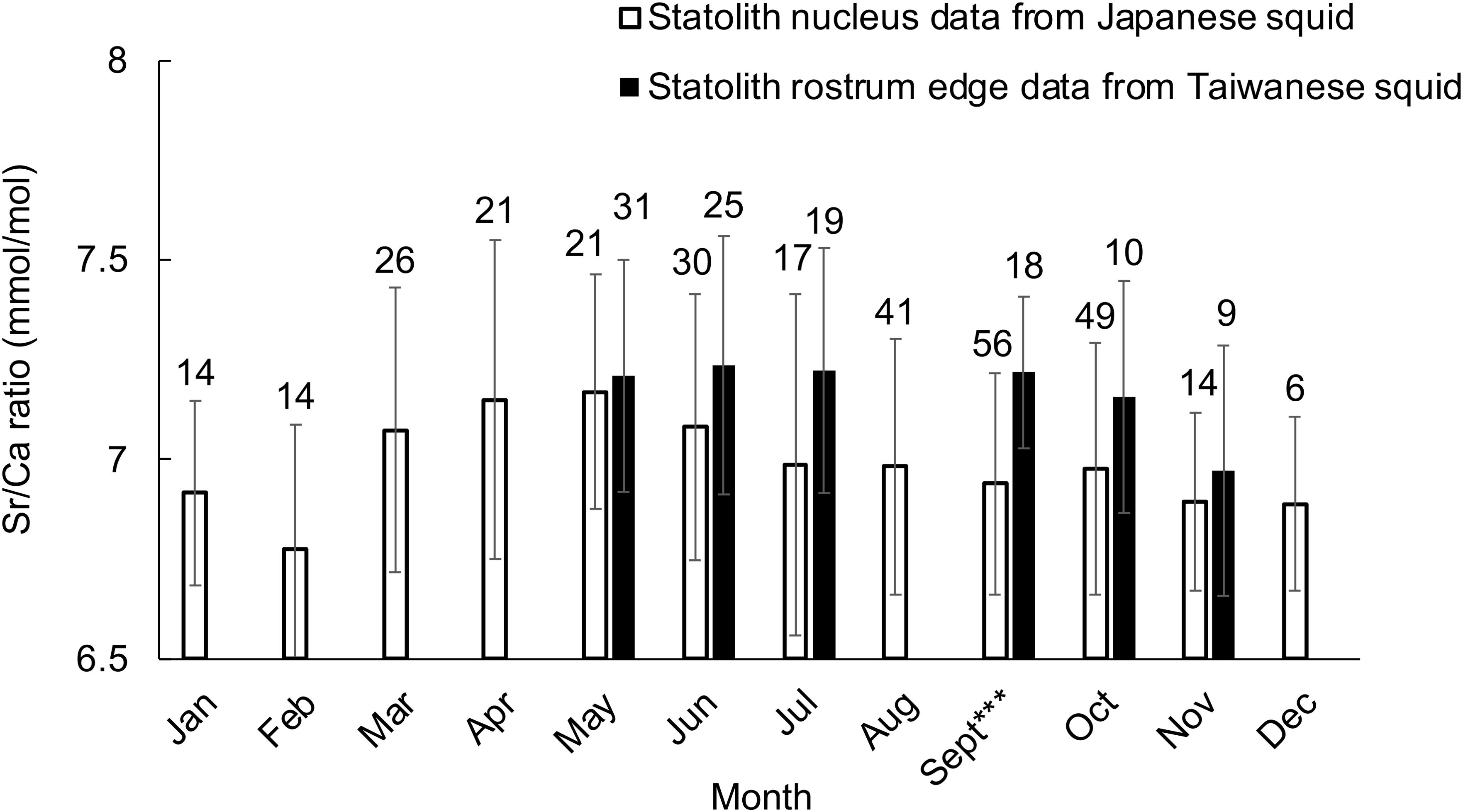
Figure 5. Mean Sr/Ca ratios at the ablation spot at the statolith nucleus for each month from Japanese squid, and that at the statolith rostrum edge for each month from Taiwanese squid. Sample numbers are indicated above the bar for each data set. Significant differences between Japan and Taiwan are indicated by asterisks: *P < 0.05, **P < 0.01, ***P < 0.001.
3.3 Polynomial model describing individual Sr/Ca ratio life trajectory
To illustrate the changes in water temperature experienced throughout the entire squid life cycle, a polynomial model was applied to each seasonal spawning group of Japan and Taiwan to reflect squid experienced temperatures change (Supplementary Table S2).
As for Sr/Ca ratio life trajectories of Japanese mature individuals, great seasonal variation was observed among the spawning groups (Figure 6). Even though Jwinter spawning group hatched in a warmer environment than Jspring spawning group, Jwinter and Jspring spawning groups experienced a similar stable temperature change during May-January, and then moved to a colder environment when approaching the spawning grounds off southern Japan from January to May (Figures 6A, B). On the opposite, the water temperature that Jsummer spawning group experienced slightly decreased during the transport in September-May, and increased during the spawning season (Figure 6C). Overall, Jsummer spawning group experienced relatively overall higher temperature profile throughout its life cycle.
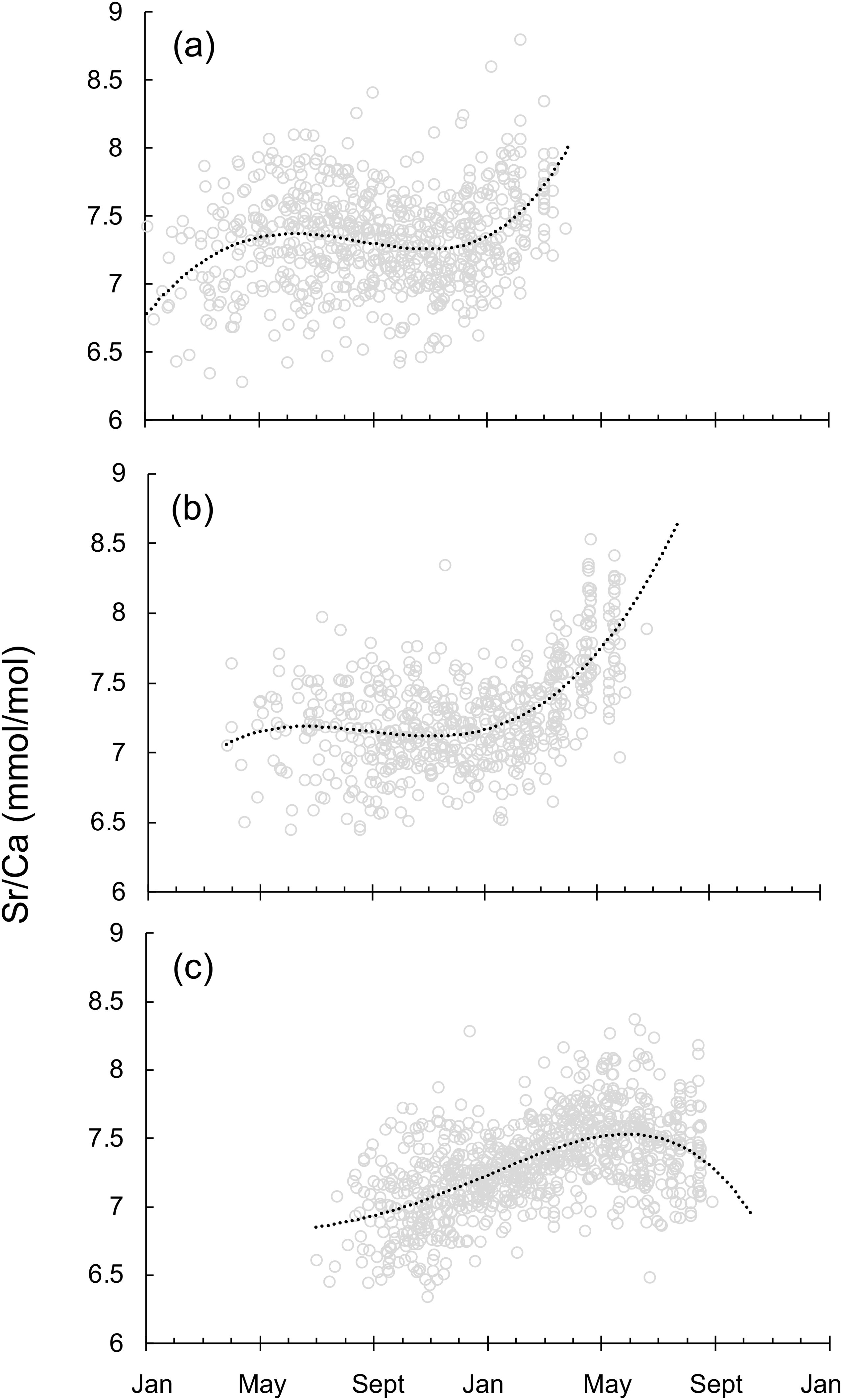
Figure 6. Sr/Ca ratio life trajectories on a real time scale for squid sampled off Japan. (A) the winter (December to February) spawning group (n=59), (B) the spring (March to May) spawning group (n=64), (C) the summer (June to August) spawning group (n=90) (see text for sampling details). Lines represent polynomial functions fitted to individuals that hatched at different times.
On the other hand, Tspring and Tautumn spawning groups also showed distinct Sr/Ca ratio life trajectories (Figure 7). Tspring spawning group lived in a relatively steady temperature throughout the life cycle (Figure 7A), while Tautumn spawning group hatched in a warmer environment, moving to a colder area in May and then came back to a warmer spawning group in autumn (Figure 7B).
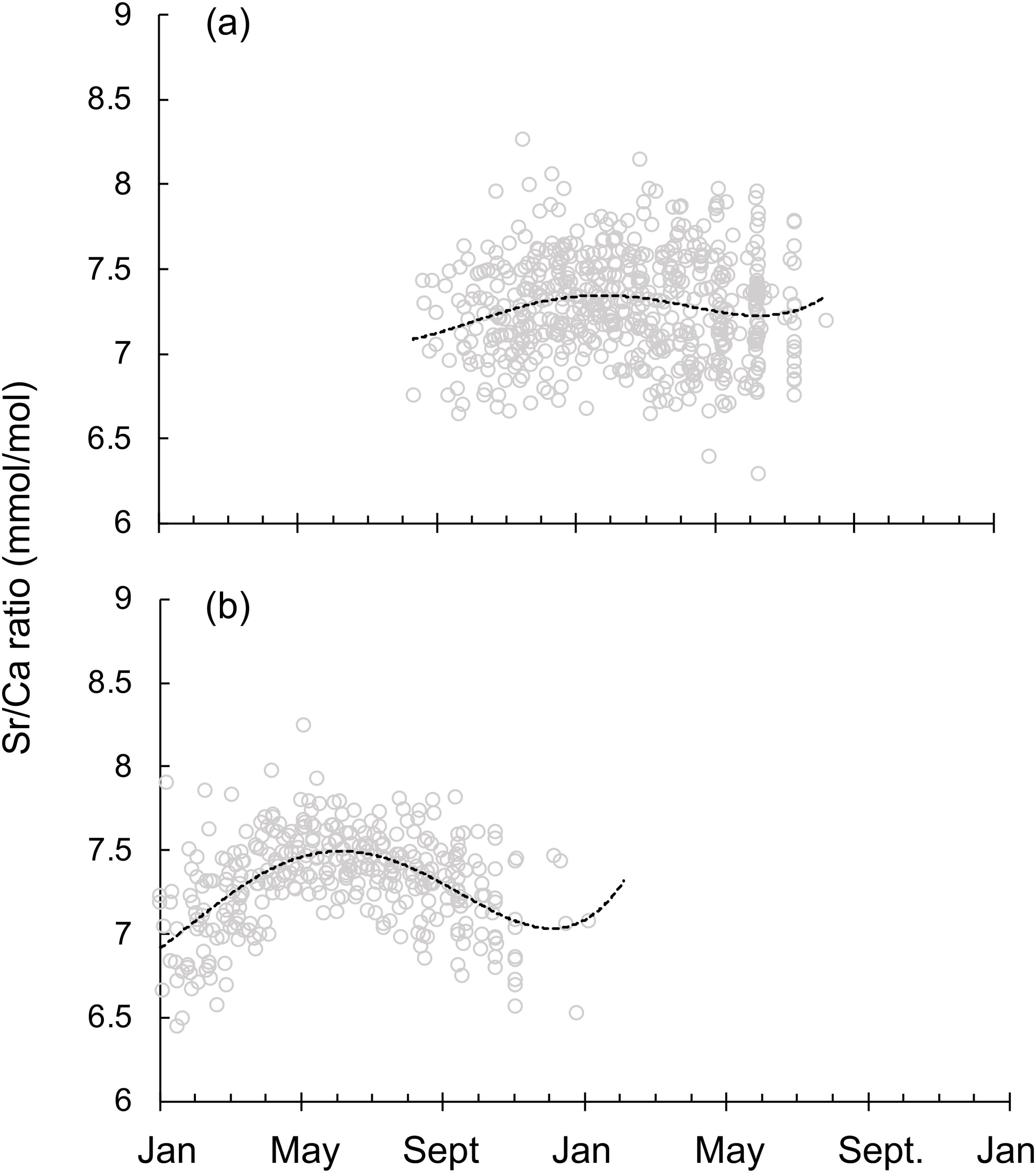
Figure 7. Sr/Ca ratio life trajectories for squid captured off Taiwan on a real time scale for (A) the spring spawning group (n=62), and (B) the autumn spawning group (n=36) (see text for sampling details). Lines represent fitted polynomial functions.
3.4 Distinguishing populations using trace elements at the statolith rostrum edge and nucleus
To examine the capacity of statolith trace elements to distinguish populations, the variation in elemental concentrations in the ablation spot at the rostrum edge were analyzed using PCA. PC1 explained 29.49% and PC2 22.65% of the total variance (Figure 8A). All trace elements were negatively correlated with PC1, except Mg/Ca ratio. Sr/Ca ratio contributed the most to the PC1. The score plot shows that most of the individuals caught in Taiwan showed higher values of PC1 than many individuals caught in Japan.
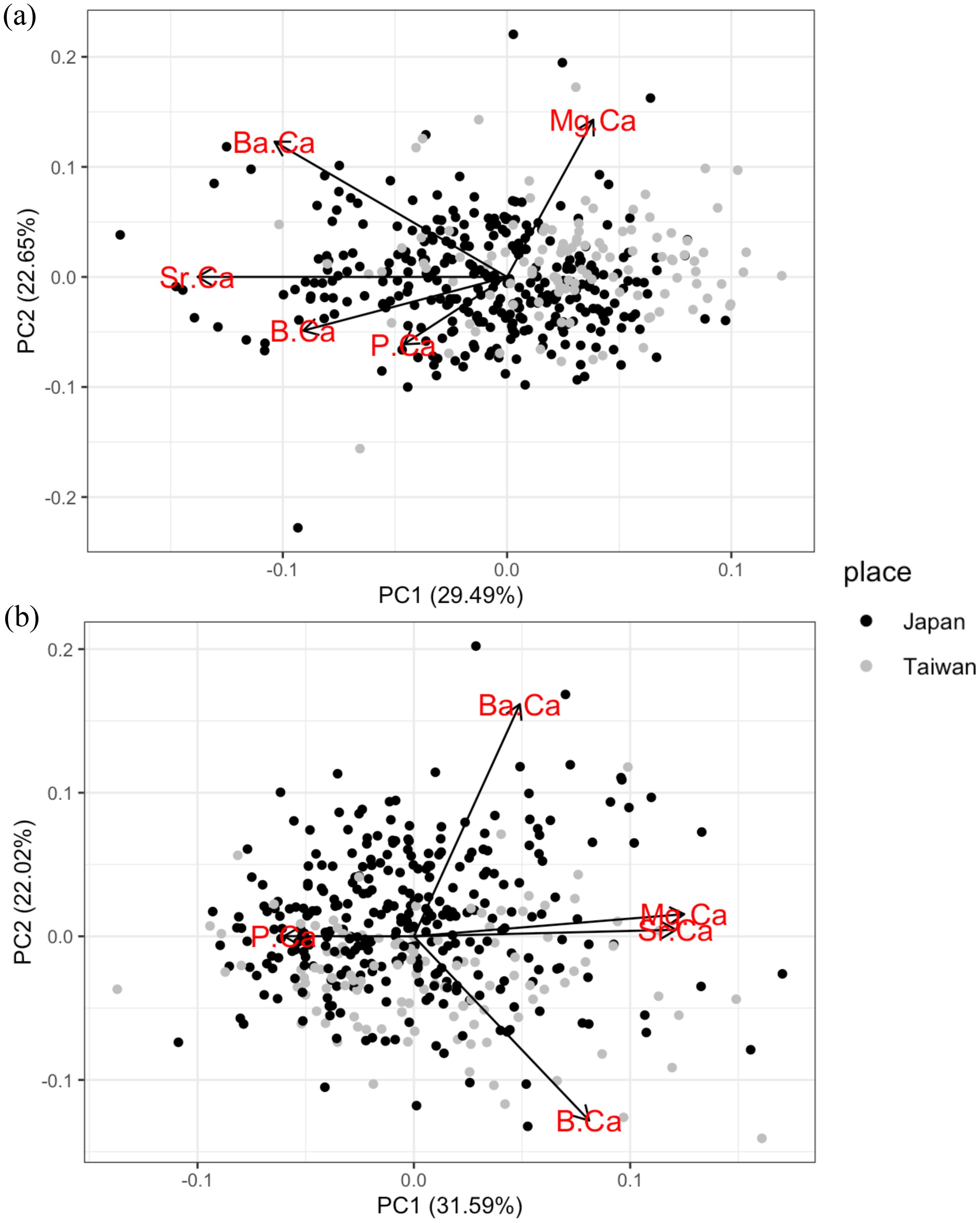
Figure 8. Principal component analysis biplot of five trace elements (Sr/Ca, Mg/Ca, Ba/Ca, P/Ca and B/Ca) in the ablation spot at the statolith rostrum edge (A) and statolith nucleus (B). The score plot points indicate the value for each individual squid at the location of capture (black symbols: Japan; grey symbols: Taiwan).
To distinguish the hatching environment between individuals caught in Japan and Taiwan, another PCA was applied to the variation of elemental concentrations in the ablation spot at the statolith nucleus. The first two factors, PC1 and PC2, explained 31.59% and 22.02% of the total variance, respectively (Figure 8B). Both the Mg/Ca and Sr/Ca ratios were positively correlated with PC1. The P/Ca ratio was negatively correlated with PC1. However, for both PC1 and PC2, the score plots of elemental concentrations in the ablation spot at the statolith nucleus overlapped broadly between squid individuals from Japan and Taiwan. This implies that the hatching environments of Japan individuals were also highly overlapped with Taiwan individuals, which could be in and around northern coast of Taiwan.
4 Discussion
4.1 Variation in statolith trace elements of U. edulis and its associated factors
Various abiotic and biotic factors influence Sr/Ca ratio in the otoliths/statoliths, such as water temperature, ambient water elemental concentration, diet and metabolic effects (Walther and Thorrold, 2006; Doubleday et al., 2013). Strontium has been found to be significantly correlated with water temperature (Smith et al., 1979; Rodhouse et al., 1994; Ikeda et al., 2003). Previous studies have used the mean trace element concentration of the whole statolith and the mean temperature experienced throughout the individual life cycle to examine the correlations between trace elements and ambient water temperature, which cannot be precise (Arkhipkin et al., 2004; Liu et al., 2015). In the present study, multiple data of the Sr/Ca ratio at the rostrum edge were obtained to examine its relationship with ambient water temperature at capture, and provided more convincing evidence of the influence of water temperature on the individual uptake rate of strontium. Our result suggested that Sr/Ca ratio in the ablation spot at the statolith rostrum edge was negatively correlated with 50 m WT in U. edulis, which is in consistent with negative correlation between Sr/Ca ratio and water temperature obtained from experiments on captive U. edulis by Yamaguchi et al. (2015). However, as observed in this study, variance was large. Therefore, a direct estimation of the water temperature experienced by squid from the correlation with Sr/Ca ratio would not be robust. Hence, this study was not aimed to estimate the absolute value of water temperature through Sr/Ca ratio, but to contribute to a better understanding of water temperature conditions throughout squid life cycle.
Strontium concentration in the oceanic water can be affected by inshore factors and show great geographical difference (Lebrato et al., 2020). However, in this study, the hatching/fishing grounds of squid are far enough from inshore, which can be considered the similar environment as open ocean. In addition, winter Sr/Ca ratio of coral skeleton around Kyushu area, southern Japan is 9.8 mmol/mol (Hirabayashi et al., 2013), which is about 9.2 mmol/mol at Ishigaki Island near northern Taiwan (Mishima et al., 2010). Hence, though the ocean environments are different between southern Japan and northern Taiwan, strontium concentration reflected from coral skeletons suggested that the difference of Sr/Ca ratio in squid statolith owing to geographical differences appears to be not great in this area.
Although elemental composition was assumed to be primarily influenced by the physiochemical properties of the water, biological process such as diet, metabolism, ontogeny and diel vertical migration, would also influence the elemental composition in the statoliths. For example, some laboratory rearing experiments with the cuttlefish Sepia officinalis found a lack of temperature effects on its statolith Sr/Ca ratio, but did show the effect of diet on it (Zumholz et al., 2006, Zumholz et al., 2007). Most of the U. edulis specimens examined in the present study had empty stomachs. Even if a few individuals had something remaining in their stomach, it would provide only a snapshot of the diet and cannot provide a meaningful measure of the influence of diet on statolith elemental signatures. Therefore, dietary impact was excluded in the present study.
Sr/Ca ratio showed no correlation with the instantaneous growth rate as estimated from either maximum SIW or SIW at the rostrum edge in this study. No difference of Sr/Ca ratio in the statoliths of jumbo flying squid Dosidicus gigas was detected between those sampled in El Niño years and normal years, though there was a 2.5°CC difference in water temperature between these two years (Ikeda et al., 2002a). Such results are possibly due to the occurrence of vertical migration (Yatsu et al., 1998; Ikeda et al., 2002b) and daily periodicity in the Sr/Ca ratio (Ikeda et al., 2002b), which mask its relationship with ambient water temperature. On the other hand, experiments on captured squid maintained at a constant temperature showed great individual variability of statolith Sr/Ca ratio in Heterololigo bleekeri (Hosono et al., 2022). Therefore, the observed strong variability in the relationship between the Sr/Ca ratio and water temperature would be an intrinsic individual difference in the squid species.
Magnesium is another element of interest in the statolith chemistry (Arkhipkin et al., 2004; Warner et al., 2009; Liu et al., 2011). Although magnesium is distributed uniformly in the ocean, the precipitation of magnesium has been found to be physiologically regulated in cephalopods (Kristensen, 1980; Arkhipkin and Bjørke, 2000). The Mg/Ca ratio in the statolith of U. edulis was positively correlated with ambient water temperature, consistent with the relationship observed in coral skeletons (Mitsuguchi et al., 1996). Similar to coral skeletons (Smith et al., 1979), the statolith Mg/Ca ratio was positively correlated with growth rate in the embryos of cuttlefish Sepia apama, adults of Doryteuthis gahi and Dosidicus gigas (Arkhipkin et al., 2004; Gillanders et al., 2013; Liu et al., 2011). The present study found a similar positive relationship between the Mg/Ca ratio and the instantaneous growth rate as estimated from either maximum SIW or SIW at the rostrum edge, which suggested that Mg/Ca ratio is influenced by squid metabolism and not suitable to indicate the environmental conditions.
Barium enters coastal seas from river water, runoff and upwelling from deep water (Chan et al., 1977). The concentration of barium increases with depth, and has a nutrient-type distribution in the ocean (Lea et al., 1989). Ba/Ca ratio is negative to temperature and showed no relation with salinity in the statolith of the S. officinalis (Zumholz et al., 2007). In this study, barium showed a relatively significant negative correlation with 50 m WT, though the correlation was not that strong that with strontium.
Boron is another key element to discriminate the difference between Japanese and Taiwanese squids, however, very little is known about boron in marine biogenic carbonate and the factors leading to observed differences of boron concentrations in squid statoliths. Recent studies suggest that boron has little information content both in fish otoliths and cephalopod statoliths (Andrus and Crowe, 2002; Lu et al., 2014, Lu et al., 2019), except for one study on octopus statoliths which found that boron can be used to discriminate among different geographical groups (Daryanani et al., 2021). However, the mechanism of boron uptake in squid and octopus remains elusive.
As phosphorus incorporation is poorly understood for fish otoliths or cephalopod statoliths, it is largely thought to be physiologically-regulated, related to metabolic pathways (Hüssy et al., 2021; Martino et al., 2021). In the present study, no contribution of phosphorus was found to distinguish U. edulis from Japan and Taiwan.
4.2 Experienced environments estimated from Sr/Ca life trajectory and its potential impacts on squid survival and maturity
Sr/Ca ratio has been successfully applied to reconstruct the life-long experienced environment of many fish species based on its strong correlation with ambient water temperature (Chang et al., 2004; Avigliano et al., 2017, Avigliano et al., 2020b). Previous studies using Sr/Ca ratio to reconstruct experienced environment is limited in squid species due to the considerable individual variation (Ikeda et al., 2003; Jones et al., 2018), which was also reflected in this study. Since remarkable squid sample size was applied to each seasonal/geographical spawning group, we believe that Sr/Ca ratio was robust enough to display the tendency of changes in the experienced water temperature of each group.
As most Japanese squid were considered to hatch around the waters off north Taiwan or further south, they would be transported to Japanese waters by currents. Passive tracer experiments showed that half of tracers in the Tsushima Strait originated in the Taiwan Strait while the other half came from the Kuroshio in summer, and the contribution from Taiwan Strait decreased to 20% while those from the Kuroshio increased up to 80% in winter (Guo et al., 2006). Moreover, the survival rate would differ dramatically due to the difference of environmental conditions that individuals experienced through transportation (Hirano and Fujimoto, 1970; Sassa et al., 2007; Kasai et al., 2008; Chen et al., 2012). For example, eggs and larvae of jack mackerel Trachurus japonicus spawned off the northern Taiwan are transported more to the Pacific Ocean than northern East China Sea, but more survive in the northern East China Sea than in the Pacific Ocean (Kasai et al., 2008). In this study, high catch along with high proportion of mature individuals were found in Jsummer spawning group (Table 1A). In accordance with the experienced temperature reflected by Sr/Ca ratio life trajectory, Jsummer spawning group might be transported by the warm and slow-drifting water in Taiwan-Tsushima Warm Current (Park et al., 2013). In addition to the nutrients-rich coastal area in the East China Sea (Gong et al., 2003; Wang et al., 2003; Huang et al., 2020), the transport of Taiwan-Tsushima Warm Current would be greatly associated with high survivorship and recruitment in Japanese waters. On the other side, low catch, short fishing season and low proportion of mature individuals occurred in winter and spring season (Table 1A). As suggested by the Sr/Ca ratio life trajectories, Jwinter and Jspring spawning groups experienced a steady temperature during the current transport, these individuals would be rapidly transported northeastward through the Kuroshio Current and restricted from the coastal areas of China (Li et al., 2014). Therefore, the low catch and short fishing season of U. edulis during winter season in Sea of Japan might be attributed to the low survival rate of eggs and larvae under the relatively unfavorable water condition in Kuroshio Current. As maturation of U. edulis was suggested to be associated with empirical water temperature and duration of the transport (Yamaguchi et al., 2019, Yamaguchi et al., 2022), low proportion of mature individuals in winter might be caused by limited growth resulted from the fast transport by Kuroshio Current, while warm and slow-drifting Taiwan-Tsushima Warm Current explained high maturation rate in summer spawning group.
Distribution of fish larva and plankton assemblages in the Taiwan Strait are influenced by seasonal monsoons (Hsieh et al., 2005, Hsieh et al., 2010, Hsieh et al., 2011, Hsieh et al., 2013). In summer (May-August), the Kuroshio Current remains offshore in east Taiwan, and the north of Taiwan is characterized by waters from Taiwan Strait driven by southwestern monsoons (Jan et al., 2002, Jan et al., 2006). In winter (October-March), strong northeastern monsoons oppose the Kuroshio Current, and a portion of it then flows onto the shelf, overlying a productive cold dome off northeastern Taiwan (Jan et al., 2010). As shown by the Sr/Ca ratio life trajectories in this study, Tspring spawning group experienced a stable temperature change through squid life cycle. Thus, Tspring spawning group hatched around autumn, might be contained in the cold productive dome off northeastern Taiwan to grow and mature, until spawning in the spring season (Cheng et al., 2022). On the other hand, Tautumn spawning group hatched around winter, might migrate to the East China Sea by Taiwan Warm Current driven by southwestern monsoons, and move back to north Taiwan when the strong northeastern monsoons begin (Wang et al., 2008). This is in accordance with our results of Sr/Ca ratio trajectory that Tautumn spawning group moving to a colder area and then coming back to a warmer spawning ground. A rather stable and nutritious environment experienced by Tspring spawning group might also explain its early maturation than Tautumn spawning group.
Even though the present study applied a great number of statolith samples to measure changes in trace element concentrations from statolith nucleus to rostrum, future research could improve the accuracy through performing age determination after trace element analysis using the same statolith, and increase the resolution by applying smaller ablation spots representing shorter time period.
4.3 Population connectivity and its association with U. edulis local population dynamics
Present results support studies suggesting that loliginid squids typically display connected populations over large geographical areas unless specific oceanographic features restrict gene flow (Shaw et al., 2010; McKeown et al., 2019). Our study not only agreed with previous findings that squid caught in Tsushima were most likely transported from areas waters off northern Taiwan (Yamaguchi et al., 2019, Yamaguchi et al., 2020), but also indicated the potential hatching grounds further south, where could be northern South China Sea or the Philippine Islands (Jin et al., 2018). Even though the exact hatching ground of U. edulis sampled in this study was not clearly identified, the population should be considered as a metapopulation across northwest Pacific, where several U. edulis subpopulations (Taiwan, Japan and etc.) are spatially structured and heavily mixed (Kritzer and Sale, 2004). In addition to the population genetic study using mitochondrial DNA analysis among several regions from Japan to Taiwan suggested U. edulis are genetically connected in this area (Tomano et al., in prep), this study also showed that the sub-population size in Tsushima, southern Japan is largely determined by strength of transport from sub-population in northern Taiwan and larval survivorship during the transport.
Egg mass of U. edulis was recorded on the sandy bottom of Tsushima Strait (Natsukari, 1976), however, there was no strong evidence on the existence of local U. edulis population in Japan in this study. If local U. edulis population in Japan was small, the next generation of squid transported to Japan remains elusive. Natsukari et al (1988) found rather high natural mortality rate and low larvae survival rate in the northwestern Kyushu area, and fishing has probably little effects on the stocks. Thus, one plausible explanation is that squid would be constantly transported from Taiwanese waters or further south to spawn, however, great larvae loss might occur due to the unfavorable environmental conditions in the spawning ground off the coast of southern Japan, which leads to the low recruitment in Japanese waters. As limited sampling site in the Sea of Japan was included in this study, investigation in the northern area than Tsushima Strait and the Pacific coast of Japan is required to further examine the existence of local population in Japan.
Uroteuthis edulis local population fluctuations might be driven by different mechanisms. Life-history traits in warmer environments, such as fast growth and early maturation, high fecundity, and high frequency of sneaker occurrence as a flexible male reproductive tactic, might contribute greatly to maintaining the Taiwanese population at high abundance and therefore a relatively stable resource (Pang et al., 2020, Pang et al., 2022). In contrast, U. edulis individuals are constantly transported from Taiwanese waters (or further south) to Japanese waters. However, high paralarval mortality is likely to occur due to unfavorable environmental conditions with elevated temperatures, which may lead to a small local population in Japanese waters. Thus, the pattern of population connectivity of U. edulis in the northwestern Pacific results in the source-sink dynamics of a metapopulation, where the southern population is the source and the Japanese population is a sink population, with the Japanese population size presumably driven by current transport.
Uroteuthis edulis became the dominant cephalopod species fished in the East China Sea after the population of Sepiella japonica collapsed in the 1980s (Pang et al., 2018). The catch and CPUE of U. edulis have been very high off Mainland China and the East China Sea since the 1990s (Li et al., 2020). Thus, the intensive catch of U. edulis in the south might be the main factor driving the catch of Japanese squid to decrease since the 1990s, especially in the East China Sea. Since the U. edulis population in the Northwest Pacific Ocean should be considered as a metapopulation with multiple straddling stocks that are simultaneously exploited in different regions (Arkhipkin et al., 2023), all the countries involved in exploitation should collaborate to ensure that it is managed sustainably.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
YP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. YY: Methodology, Resources, Supervision, Validation, Writing – review & editing. TA: Data curation, Software, Supervision, Validation, Writing – review & editing. TI: Methodology, Software, Supervision, Validation, Writing – review & editing. CC: Data curation, Methodology, Supervision, Validation, Writing – review & editing. TK: Supervision, Validation, Writing – review & editing. YI: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported financially by Japan Society for the Promotion of Science Kakenhi Grants 17K15309 and 19H03029 to Y.I., 20JI4792 to Y.P., and Japan Science and Technology Agency CREST Grant JPMJCR23J6 to Y.Y.
Acknowledgments
We thank Prof. Yoshiro Watanabe, Prof. Shin-ichi Ito and Prof. Hideaki Kidokoro for valuable discussions, Dr. Nobuhiro Ogawa for his help on experiment instruction, Dr. Kei Zenimoto for his help with sample collecting, Seiya Kudo for his help in the laboratory, Prof. Ian Gleadall for editing this manuscript, and two reviewers for their insightful comments on revising this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1424397/full#supplementary-material
References
Amekawa S., Kubota K., Miyairi Y., Seki A., Kawakubo Y., Sakai S., et al. (2016). Fossil otoliths, from the Gulf of Kutch, Western India, as a paleo-archive for the mid-to late-Holocene environment. Quat. Int. 397, 281–288. doi: 10.1016/j.quaint.2015.07.006
Andrus C. F. T., Crowe D. E. (2002). Alteration of otolith aragonite: effects of prehistoric cooking methods on otolith chemistry. J. Archaeol. Sci. 29, 291–299. doi: 10.1006/jasc.2001.0694
Arkhipkin A. (1993). Age, growth, stock structure and migratory rate of pre-spawning short-finned squid Illex argentinus based on statolith ageing investigations. Fish. Res. 16, 313–338. doi: 10.1016/0165-7836(93)90144-V
Arkhipkin A. I. (2005). Statoliths as ‘black boxes’(life recorders) in squid. Mar. Freshw. Res. 56, 573–583. doi: 10.1071/MF04158
Arkhipkin A. I., Campana S. E., FitzGerald J., Thorrold S. R. (2004). Spatial and temporal variation in elemental signatures of statoliths from the Patagonian longfin squid (Loligo gahi). Can. J. Fish. Aquat. Sci. 61, 1212–1224. doi: 10.1139/f04-075
Arkhipkin A. I., Nigmatullin C. M., Parkyn D. C., Winter A., Csirke J. (2023). High seas fisheries: the Achilles’ heel of major straddling squid resources. Rev. Fish Biol. Fish. 33, 453–474. doi: 10.1007/s11160-022-09733-8
Avigliano E., Ivanovic M., Prandoni N., Méndez A., Pisonero J., Volpedo A. V. (2020a). Statolith chemistry as a stock tag in the Argentine shortfin squid Illex argentinus. Reg. Stud. Mar. Sci. 38, 101355. doi: 10.1016/j.rsma.2020.101355
Avigliano E., Pisonero J., Dománico A., Sánchez S., Volpedo A. V. (2017). Migration and brackish environment use of Prochilodus lineatus (Characiformes: Prochilodontidae) inferred by Sr: Ca ratio transects of otolith. Neotrop. 15. doi: 10.1590/1982-0224-20170055
Avigliano E., Pouilly M., Bouchez J. (2020b). Strontium isotopes (87Sr/86Sr) reveal the life history of freshwater migratory fishes in the La Plata Basin. River Res. Appl. 36, 1985–2000. doi: 10.1002/rra.3727
Berman J. J. (2016). Data simplification: taming information with open source tools (Morgan Kaufmann).
Campana S. E., Neilson J. D. (1985). Microstructure of fish otoliths. Can. J. Fish. Aquat. Sci. 42, 1014–1032. doi: 10.1139/f85-127
Carson H. S., Cook G. S., López-Duarte P. C., Levin L. A. (2011). Evaluating the importance of demographic connectivity in a marine metapopulation. Ecology 92, 1972–1984. doi: 10.1890/11-0488.1
Chan L. H., Drummond D., Edmond J. M., Grant B. (1977). On the barium data from the Atlantic GEOSECS expedition. Deep-Sea Res. 24, 613–649. doi: 10.1016/0146-6291(77)90505-7
Chang C. W., Iizuka Y., Tzeng W. N. (2004). Migratory environmental history of the grey mullet Mugil cephalus as revealed by otolith Sr: Ca ratios. Mar. Ecol. Prog. Ser. 269, 277–288. doi: 10.3354/meps269277
Chen X., Cao J., Chen Y., Liu B., Tian S. (2012). Effect of the Kuroshio on the spatial distribution of the red flying squid. Ommastrephes bartramii Northwest Pacific Ocean. Bull. Mar. Sci. 88, 63–71. doi: 10.5343/bms.2010.1098
Chen F., Lin J., Qian B., Wu Z., Huang P., Chen K., et al. (2018). Geochemical assessment and spatial analysis of heavy metals in the surface sediments in the eastern Beibu Gulf: A reflection on the industrial development of the south China coast. Int. J. Env. Res. Pub He 15, 496. doi: 10.3390/ijerph15030496
Cheng Y. C., Jan S., Chen C. C. (2022). Kuroshio intrusion and its impact on swordtip squid (Uroteuthis edulis) abundance in the Southern East China sea. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.900299
Ching T. Y., Chen C. S., Wang C. H. (2019). Spatiotemporal variations in life-history traits and statolith trace elements of Sepioteuthis lessoniana populations around northern Taiwan. J. Mar. Biolog. Assoc. U. K. 99, 203–213. doi: 10.1017/S0025315417001801
Daryanani D. S., Martino J. C., Doubleday Z. A. (2021). Statolith chemistry: a new tool to understand the ecology and provenance of octopus. Rev. Fish Biol. 31, 923–934. doi: 10.1007/s11160-021-09671-x
Dawe E. G., Hendrickson L. C., Colbourne E. B., Drinkwater K. F., Showell M. A. (2007). Ocean climate effects on the relative abundance of short-finned (Illex illecebrosus) and long-finned (Loligo pealeii) squid in the northwest Atlantic Ocean. Fish. Oceanogr. 16, 303–316. doi: 10.1111/j.1365-2419.2007.00431.x
Doubleday Z. A., Izzo C., Woodcock S. H., Gillanders B. M. (2013). Relative contribution of water and diet to otolith chemistry in freshwater fish. Aquat Biol. 18, 271–280. doi: 10.3354/ab00511
Drake J., Lambin X., Sutherland C. (2022). The value of considering demographic contributions to connectivity: a review. Ecography 2022, e05552. doi: 10.1111/ecog.05552
FAO Fisheries and Aquaculture Department (2016). The state of world fisheries and aquaculture (Food and Agriculture Organization of the United Nations).
Feng B., Chen B., Zhuo W., Chen Q., Zhang Y., Zhang W. (2019). Seasonal and spatial distribution of atmospheric tritiated water vapor in mainland China. Environ. Sci. Technol. 53, 14175–14185. doi: 10.1021/acs.est.9b03855
Fogarty M. J., Botsford L. W. (2007). Population connectivity and spatial management of marine fisheries. Oceanography 20, 112–123. doi: 10.5670/oceanog
Gillanders B. M., Wilkinson L. M., Munro A. R., de Vries M. C. (2013). Statolith chemistry of two life history stages of cuttlefish: effects of temperature and seawater trace element concentration. Geochim. Cosmochim. Acta 101, 12–23. doi: 10.1016/j.gca.2012.10.005
Gong G. C., Wen Y. H., Wang B. W., Liu G. J. (2003). Seasonal variation of chlorophyll a concentration, primary production and environmental conditions in the subtropical East China Sea. Deep Sea Res. II: Top. Stud. Oceanogr. 50, 1219–1236. doi: 10.1016/S0967-0645(03)00019-5
Guo X., Miyazawa Y., Yamagata T. (2006). The Kuroshio onshore intrusion along the shelf break of the East China Sea: The origin of the Tsushima Warm Current. J. Phys. Oceanogr. 36, 2205–2231. doi: 10.1175/JPO2976.1
Hirabayashi S., Yokoyama Y., Suzuki A., Kawakubo Y., Miyairi Y., Okai T., et al. (2013). Coral growth-rate insensitive Sr/Ca as a robust temperature recorder at the extreme latitudinal limits of Porites. Geochem. J. 47, e1–e5. doi: 10.2343/geochemj.2.0259
Hirano T., Fujimoto M. (1970). Preliminary results of investigation of the Kuroshio functioning as a means of transportation and diffusion of fish eggs and larvae. The Kuroshio, 405–416. doi: 10.1515/9780824885830-041
Hosono S., Irie T., Yamamoto J., Nakaya M., Sakurai Y., Kawamura T., et al. (2022). Negative temperature dependence of statolith Sr/Ca and its intraspecific variability in experimentally maintained spear squid Heterololigo bleekeri. J. Mar. Biol. Assoc. U. K. 102 (5), 315–321. doi: 10.1017/S0025315422000546
Hsieh C. H., Chen C. S., Chiu T. S. (2005). Composition and abundance of copepods and ichthyoplankton in Taiwan Strait (western North Pacific) are influenced by seasonal monsoons. Mar. Freshw. Res. 56, 153–161. doi: 10.1071/MF04058
Hsieh H. Y., Lo W. T., Wu L. J., Liu D. C., Su W. C. (2011). Comparison of distribution patterns of larval fish assemblages in the Taiwan Strait between the northeasterly and southwesterly monsoons. Zool. Stud. 50, 491–505.
Hsieh H. H., Yen H. Y., Min-Hung S. (2010). Moho depth derived from gravity data in the Taiwan Strait area. Terr. Atmospheric Oceanic Sci. 21, 2. doi: 10.3319/TAO.2009.03.05.01(T
Hsieh H. Y., Yu S. F., Lo W. T. (2013). Influence of monsoon-driven hydrographic features on siphonophore assemblages in the Taiwan Strait, western North Pacific Ocean. Mar. Freshw. Res. 64, 348–358. doi: 10.1071/MF12151
Huang T. H., Chen C. T. A., Bai Y., He X. (2020). Elevated primary productivity triggered by mixing in the quasi-cul-de-sac Taiwan Strait during the NE monsoon. Sci. Rep. 10, 1–9. doi: 10.1038/s41598-020-64580-6
Hüssy K., Limburg K. E., de Pontual H., Thomas O. R. B., Cook P. K., Heimbrand Y., et al. (2021). Trace element patterns in otoliths: the role of biomineralization. Rev. Fish. Sci. Aquac. 29, 445–477. doi: 10.1080/23308249.2020.1760204
Ikeda Y., Arai N., Kidokoro H., Sakamoto W. (2003). Strontium: calcium ratios in statoliths of Japanese common squid Todarodes pacificus (Cephalopoda: Ommastrephidae) as indicators of migratory behavior. Mar. Ecol. Prog. Ser. 251, 169–179. doi: 10.3354/meps251169
Ikeda Y., Okazaki J., Sakurai Y., Sakamoto W. (2002b). Periodic variation in Sr/Ca ratios in statoliths of the Japanese Common Squid Todarodes pacificus Steenstru (Cephalopoda: Ommastrephidae) maintained under constant water temperature. J. Exp. Mar. Biol. Ecol. 273, 161–170. doi: 10.1016/S0022-0981(02)00143-0
Ikeda Y., Yatsu A., Arai N., Sakamoto W. (2002a). Concentration of statolith trace elements in the jumbo flying squid during El Niño and non-El Niño years in the eastern Pacific. J. Mar. Biolog. Assoc. U. K. 82, 863–866. doi: 10.1017/S0025315402006264
Jackson G. D. (2004). Advances in defining the life histories of myopsid squid. Mar. Freshw. Res. 55, 357–365. doi: 10.1071/MF03152
Jan S., Sheu D. D., Kuo H. M. (2006). Water mass and throughflow transport variability in the Taiwan Strait. . J. Geophys. Res. Oceans 111. doi: 10.1029/2006JC003656
Jan S., Tseng Y. H., Dietrich D. E. (2010). Sources of water in the Taiwan strait. J. Oceanogr. 66, 211–221. doi: 10.1007/s10872-010-0019-7
Jan S., Wang J., Chern C. S., Chao S. Y. (2002). Seasonal variation of the circulation in the Taiwan Strait. J. Mar. Syst. 35, 249–268. doi: 10.1016/S0924-7963(02)00130-6
Japan Meteorological Agency (2022). 10-day mean SSTs and 50 m temperatures. Available online at: https://www.data.jma.go.jp/gmd/kaiyou/data/db/kaikyo/jun/sst_HQ.html (Accessed 2022/01/05).
Jereb P., Roper C. F. (2010). “Cephalopods of the world-an annotated and illustrated catalogue of cephalopod species known to date. Vol 2,” in Myopsid and oegopsid squids (No. 2) (FAO).
Jin Y., Liu B., Chen X., Staples K. (2018). Morphological beak differences of loliginid squid, Uroteuthis chinensis and Uroteuthis edulis, in the northern South China Sea. J. Oceanol. Limnol. 36, 559–571. doi: 10.1007/s00343-017-6285-0
Jones J. B., Arkhipkin A. I., Marriott A. L., Pierce G. J. (2018). Using statolith elemental signatures to confirm ontogenetic migrations of the squid Doryteuthis gahi around the Falkland Islands (Southwest Atlantic). Chem. Geol. 481, 85–94. doi: 10.1016/j.chemgeo.2018.01.034
Kasai A., Komatsu K., Sassa C., Konishi Y. (2008). Transport and survival processes of eggs and larvae of jack mackerel Trachurus japonicus in the East China Sea. Fish. Sci. 74, 8–18. doi: 10.1111/j.1444-2906.2007.01491.x
Kawano M. (2006). An egg mass of Photololigo edulis found in coastal waters off Yamaguchi prefecture [Japan] (Southwestern Japan Sea: Bulletin of Yamaguchi Prefectural Fisheries Research Center (Japan).
Kerr L. A., Hintzen N. T., Cadrin S. X., Clausen L. W., Dickey-Collas M., Goethel D. R., et al. (2017). Lessons learned from practical approaches to reconcile mismatches between biological population structure and stock units of marine fish. ICES J. Mar. Sci. 74, 1708–1722. doi: 10.1093/icesjms/fsw188
Kool J. T., Moilanen A., Treml E. A. (2013). Population connectivity: recent advances and new perspectives. Landsc. Ecol. 28, 165–185. doi: 10.1007/s10980-012-9819-z
Kritzer J. P., Sale P. F. (2004). Metapopulation ecology in the sea: from Levins’ model to marine ecology and fisheries science. Fish Fish 5, 131–140. doi: 10.1111/j.1467-2979.2004.00131.x
Lea D. W., Shen G. T., Boyle E. A. (1989). Coralline barium records temporal variability in equatorial Pacific upwelling. Nature 340, 373–376. doi: 10.1038/340373a0
Li G., Chen X., Lei L., Guan W. (2014). Distribution of hotspots of chub mackerel based on remote-sensing data in coastal waters of China. Int. J. Remote Sens. 35, 4399–4421. doi: 10.1080/01431161.2014.916057
Li N., Zhou F., Chen X. J. (2020). Study on microstructure and growth characteristics of Uroteuthis edulis statolith in East China Sea. South China Fish. Sci. 16, 21–31. doi: 10.12131/20200078
Lipiński M. R., van der Vyver J. S. F., Shaw P., Sauer W. H. H. (2016). Life cycle of chokka-squid Loligo reynaudii in South African waters. Afr. J. Mar. Sci. 38, 589–593. doi: 10.2989/1814232X.2016.1230074
Liu B. L., Chen Y., Chen X. J. (2015). Spatial difference in elemental signatures within early ontogenetic statolith for identifying Jumbo flying squid natal origins. Fish. Oceanogr. 24, 335–346. doi: 10.1111/fog.12112
Liu B., Chen X., Chen Y., Lu H., Qian W. (2011). Trace elements in the statoliths of jumbo flying squid off the Exclusive Economic Zones of Chile and Peru. Mar. Ecol. Prog. Ser. 429, 93–101. doi: 10.3354/meps09106
Lowe W. H., Allendorf F. W. (2010). What can genetics tell us about population connectivity? Mol. Ecol. 19, 3038–3051. doi: 10.1111/j.1365-294X.2010.04688.x
Lu J., Gomes L. C., Nunes R. W., Castro Neto A. H., Loh K. P. (2014). Lattice relaxation at the interface of two-dimensional crystals: graphene and hexagonal boron-nitride. Nano Lett. 14, 5133–5139. doi: 10.1021/nl501900x
Lu W., Li Y., Kinjo R. (2019). Crystalline tetraatomic boron (0) species. J. Am. Chem. Soc 141, 5164–5168. doi: 10.1021/jacs.9b02173
Martino J. C., Doubleday Z. A., Fowler A. J., Gillanders B. M. (2021). Corrigendum to: Identifying physiological and environmental influences on otolith chemistry in a coastal fishery species. Mar. Freshw. Res. 72, 922–924. doi: 10.1071/MF20196_CO
McKeown N. J., Arkhipkin A. I., Shaw P. W. (2019). Genetic analysis reveals historical and contemporary population dynamics in the longfin squid Doryteuthis gahi: implications for cephalopod management and conservation. ICES J. Mar. Sci. 76, 1019–1027. doi: 10.1093/icesjms/fsz009
Mishima M., Suzuki A., Nagao M., Ishimura T., Inoue M., Kawahata H. (2010). Abrupt shift toward cooler condition in the earliest 20th century detected in a 165 year coral record from Ishigaki Island, southwestern Japan. Geophys. Res. Lett. 37. doi: 10.1029/2010GL043451
Mitsuguchi T., Matsumoto E., Abe O., Uchida T., Isdale P. J. (1996). Mg/Ca thermometry in coral skeletons. Science 274, 961–963. doi: 10.1126/science.274.5289.961
Natsukari Y. (1976). “Scuba diving observations on the spawning ground of the squid,” in Doryteuthis kensaki (Wakiya et Ishikaw) (Venus: Food and Aquaculture Organization).
Natsukari Y., Nakanose T., Oda K. (1988). Age and growth of loliginid squid Photololigo edulis (Hoyl ). J. Exp. Mar. Biol. Ecol. 116, 177–190. doi: 10.1016/0022-0981(88)90054-8
Nigmatullin C. M., Nesis K. N., Arkhipkin A. I. (2001). A review of the biology of the jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae). Fish. Res. 54, 9–19. doi: 10.1016/S0165-7836(01)00371-X
Okai T., Suzuki A., Kawahata H., Terashima S., Imai N. (2002). Preparation of a new geological survey of Japan geochemical reference material: Coral JCp-1. Geostand. Newsl. 26, 95–99. doi: 10.1111/j.1751-908X.2002.tb00627.x
Pang Y., Chen C. S., Iwata Y. (2020). Variations in female swordtip squid Uroteuthis edulis life history traits between southern Japan and northern Taiwan (Northwestern Pacific). Fish. Sci. 86, 1005–1017. doi: 10.1007/s12562-020-01465-7
Pang Y., Chen C. S., Kawamura T., Iwata Y. (2022). Environmental influence on life-history traits in male squid Uroteuthis edulis with alternative reproductive tactics. Mar. Biol. 169, 1–12. doi: 10.1007/s00227-022-04017-y
Pang Y., Tian Y., Fu C., Wang B., Li J., Ren Y., et al. (2018). Variability of coastal cephalopods in overexploited China Seas under climate change with implications on fisheries management. Fish. Res. 208, 22–33. doi: 10.1016/j.fishres.2018.07.004
Park Y. G., Yeh S. W., Hwang J. H., Kim T. (2013). Origin of the Tsushima Warm Current in a high resolution ocean circulation model. J. Coast. Res. 65, 2041–2046. doi: 10.2112/SI65-345.1
Patterson K. R. (1988). Life history of Patagonian squid Loligo gahi and growth parameter estimates using least-squares fits to linear and von Bertalanffy models. Mar. Ecol. Prog. Ser. 47, 65–74. doi: 10.3354/meps047065
Perez J. A. A., de Aguiar D. C., Oliveira U. C. (2002). Biology and population dynamics of the long-finned squid Loligo plei (Cephalopoda: Loliginidae) in southern Brazilian waters. Fish. Res. 58, 267–279. doi: 10.1016/S0165-7836(01)00397-6
Rodhouse P. G., Robinson K., Gajdatsy S. B., Daly H. I., Ashmore M. J. S. (1994). Growth, age structure and environmental history in the cephalopod Martialia hyadesi (Teuthoidea: Ommastrephidae) at the Antarctic Polar Frontal Zone and on the Patagonian Shelf Edge. Antarct. Sci. 6, 259–267. doi: 10.1017/S0954102094000398
Sakurai Y., Kiyofuji H., Saitoh S. I., Yamamoto J., Goto T., Mori K., et al. (2002). Stock fluctuations of the Japanese common squid, Todarodes pacificus, related to recent climate changes. Fish. Sci. 68, 226–229. doi: 10.2331/fishsci.68.sup1_226
Sassa C., Kawaguchi K., Taki K. (2007). Larval mesopelagic fish assemblages in the Kuroshio–Oyashio transition region of the western North Pacific. Mar. Bio. 150, 1403–1415. doi: 10.1007/s00227-006-0434-x
Shaw P. W., Arkhipkin A. I., Adcock G. J., Burnett W. J., Carvalho G. R., Scherbich J. N., et al. (2004). DNA markers indicate that distinct spawning cohorts and aggregations of Patagonian squid, Loligo gahi, do not represent genetically discrete subpopulations. Mar. Bio. 144, 961–970. doi: 10.1007/s00227-003-1260-z
Shaw P. W., Hendrickson L., McKeown N. J., Stonier T., Naud M. J., Sauer W. H. H. (2010). Discrete spawning aggregations of loliginid squid do not represent genetically distinct populations. Mar. Ecol. Prog. Ser. 408, 117–127. doi: 10.3354/meps08593
Smith S. V., Buddemeier R. W., Redalje R. C., Houck J. E. (1979). Strontium-calcium thermometry in coral skeletons. Science 204, 404–407. doi: 10.1126/science.204.4391.404
Smith T. M., Green C. P., Sherman C. D. (2015). Patterns of connectivity and population structure of the southern calamary Sepioteuthis australis in southern Australia. Mar. Freshw. Res. 66, 942–947. doi: 10.1071/MF14328
Villanueva R. (2000). Differential increment-deposition rate in embryonic statoliths of the loliginid squid Loligo vulgaris. Mar. Biol. 137, 161–168. doi: 10.1007/s002270000323
Walther B. D., Thorrold S. R. (2006). Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fish. Mar. Ecol. Prog. Ser. 311, 125–130. doi: 10.3354/meps311125
Wang K. Y., Liao C. H., Lee K. T. (2008). Population and maturation dynamics of the swordtip squid (Photololigo edulis) in the southern East China Sea. Fish. Res. 90, 178–186. doi: 10.1016/j.fishres.2007.10.015
Wang B. D., Wang X. L., Zhan R. (2003). Nutrient conditions in the yellow sea and the East China sea. Estuar. Coast. Shelf Sci. 58, 127–136. doi: 10.1016/S0272-7714(03)00067-2
Warner R. R., Hamilton S. L., Sheehy M. S., Zeidberg L. D., Brady B. C., Caselle J. E. (2009). Geographic variation in natal and early larval trace-elemental signatures in the statoliths of the market squid Doryteuthis (formerly Loligo) opalescens. Mar. Ecol. Prog. Ser. 379, 109–121. doi: 10.3354/meps07903
Yamaguchi T., Aketagawa T., Takayama K., Hirose N., Matsuyama M. (2019). Migratory routes of different sized swordtip squid (Uroteuthis edulis) caught in the Tsushima Strait. Fish. Res. 209, 24–31. doi: 10.1016/j.fishres.2018.08.008
Yamaguchi T., Kawakami Y., Matsuyama M. (2015). Migratory routes of the swordtip squid Uroteuthis edulis inferred from statolith analysis. Aquat. Biol. 24, 53–60. doi: 10.3354/ab00635
Yamaguchi T., Kawakami Y., Matsuyama M. (2018). Analysis of the hatching site and migratory behaviour of the swordtip squid (Uroteuthis edulis) caught in the Japan Sea and Tsushima Strait in autumn estimated by statolith analysis. Mar. Biol. Res. 14, 105–112. doi: 10.1080/17451000.2017.1351616
Yamaguchi T., Takayama K., Hirose N. (2022). Influence of migratory route on early maturation of swordtip squid, Uroteuthis edulis, caught off western Kyushu Island, Japan. Fish. Res. 249, 106233. doi: 10.1016/j.fishres.2022.106233
Yamaguchi T., Takayama K., Hirose N., Matsuyama M. (2020). The Sea of Amakusa playing the role of a distributor of swordtip squid (Uroteuthis edulis) migrating from the East China Sea to the east and west sides of Japan. Fish. Res. 225, 105475. doi: 10.1016/j.fishres.2019.105475
Yatsu A., Mochioka N., Morishita K., Toh H. (1998). Strontium/calcium ratios in statoliths of the neon flying squid, Ommastrephes bartrami (Cephalopoda), in the North Pacific Ocean. Mar. Biol. 131, 275–282. doi: 10.1007/s002270050320
Ying Y., Chen Y., Lin L., Gao T. (2011). Risks of ignoring fish population spatial structure in fisheries management. Can. J. Fish. Aquat. Sci. 68, 2101–2120. doi: 10.1139/f2011-116
Zacherl D. C., Manríquez P. H., Paradis G., Day R. W., Castilla J. C., Warner R. R., et al. (2003). Trace elemental fingerprinting of gastropod statoliths to study larval dispersal trajectories. Mar. Ecol. Prog. Ser. 248, 297–303. doi: 10.3354/meps248297
Zumholz K., Hansteen T. H., Klügel A., Piatkowski U. (2006). Food effects on statolith composition of the common cuttlefish (Sepia officinalis). Mar. Biol. 150, 237–244. doi: 10.1007/s00227-006-0342-0
Keywords: Sr/Ca ratio, life trajectory, experienced environments, source-sink dynamics, metapopulation
Citation: Pang Y, Yokoyama Y, Aze T, Irie T, Chen C-S, Kawamura T and Iwata Y (2024) Population connectivity of the swordtip squid Uroteuthis edulis between southern Japan and northern Taiwan using statolith trace elemental analysis. Front. Mar. Sci. 11:1424397. doi: 10.3389/fmars.2024.1424397
Received: 28 April 2024; Accepted: 03 September 2024;
Published: 03 October 2024.
Edited by:
Brett W. Molony, Oceans and Atmosphere (CSIRO), AustraliaReviewed by:
Levent Bat, Sinop University, TürkiyeSajikumar Kk, Central Marine Fisheries Research Institute (ICAR), India
Copyright © 2024 Pang, Yokoyama, Aze, Irie, Chen, Kawamura and Iwata. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumeng Pang, eXVtZW5nLnBhY2lmaWNhQGdtYWlsLmNvbQ==
†Present address: Yumeng Pang, Ocean Nexus Center, University of Washington, Seattle, WA, United States
 Yumeng Pang
Yumeng Pang Yusuke Yokoyama
Yusuke Yokoyama Takahiro Aze1
Takahiro Aze1 Takahiro Irie
Takahiro Irie Chih-Shin Chen
Chih-Shin Chen Yoko Iwata
Yoko Iwata