- 1Key Laboratory of Tropical Marine Bio-resources and Ecology, Guangdong Provincial Key Laboratory of Applied Marine Biology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China
- 2Sanya National Marine Ecosystem Research Station; Tropical Marine Biological Research Station in Hainan, Chinese Academy of Sciences, Sanya, China
- 3CAS-HKUST Sanya Joint Laboratory of Marine Science Research and Key Laboratory of Tropical Marine Biotechnology of Hainan Province, Tropical Marine Biological Research Station in Hainan, Chinese Academy of Sciences, Sanya, China
- 4College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing, China
- 5Hong Kong Branch of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), The Hong Kong University of Science and Technology, Kowloon, Hong Kong SAR, China
- 6Institute of Space and Earth Information Science, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China
Observation of coral spawning times is valuable to detect spawning patterns and identify the potential mechanisms behind coral reproduction. Although large amount of data on global coral spawning records have become available over the past decades, information on coral spawning in the northern South China Sea remains scarce. In this study, we investigated the spawning patterns of scleractinian corals in Luhuitou fringing reef, Hainan Island, China, from 2009 to 2021 in relation to lunar cycles (month and day). The spawning times of 22 coral species from five genera (Acropora, Montipora, Platygyra, Dipsastraea, and Galaxea) within three families (Acroporidae, Merulinidae, and Euphylliidae) were recorded, with spawning occurring from lunar February to May 2009–2021. Recorded spawning events started at a period of increasing seawater temperature. Acropora, the most documented genus, spawned between lunar February and April, but primarily in lunar March. Importantly, the spawning time of Acropora was delayed for one lunar month in 2016 most likely due to a rapid decrease in monthly mean seawater temperature in lunar February. Spawning lunar days before, on or after full moon of corals in the Luhuitou Reef, including those of the Acropora species, were highly variable between years even for the same species. No predictable pattern of spawning times can thus be detected. Nonetheless, our results fill up an information gap on coral spawning patterns in the northern South China Sea that may be useful for further understanding of the reproductive biology of scleractinian corals throughout the Indo–West Pacific.
Introduction
Coral sexual reproduction is critical for the maintenance of coral populations (Baird et al., 2009b; Harrison, 2011). Two basic patterns of coral propagule release are evident: brooding and broadcast spawning, with the latter being more common (Baird et al., 2009b; Harrison, 2011). Broadcast spawning in scleractinian corals is typically highly synchronized. This facilitates the production of high concentrations of male and female gametes thereby augmenting the reproductive success of these coral species (Baird et al., 2009b; Harrison, 2011).
Sexual reproduction of scleractinian corals has been widely investigated in the Indo-Pacific and the Caribbean (Baird et al., 2021b), particularly in terms of the timing of its occurrence. The timing and extent of spawning synchronicity vary among coral taxa and locations (Table 1). Although spawning patterns have been identified for many reefs, little is known of these patterns in areas north of the Coral Triangle (Baird et al., 2021b). Luhuitou in southern Hainan Island, Southern China, supports one of the most extensive reef systems in northern South China Sea. We have been documenting coral spawning in Luhuitou over the last 15 years. The main objective of this study was therefore to synthesize the data collected over these years in order to understand coral reproductive patterns in Luhuitou and to address the existing knowledge gaps on questions related to which coral species spawn at what time for this locality. We hypothesized that coral spawning patterns in Luhuitou fringing reef are likely to reflect its geographical location, being located midway between the subtropical and tropical Indo-West Pacific, and share similarities between corals in these two general biogeographical realms.
Materials and methods
Coral spawning monitoring
Spawning activity was monitored on the Luhuitou fringing reef (18°12′ N, 109°28′ E) on southern Hainan Island in the northern region of the South China Sea. The reef is located within a protected outer shelf and has a hard coral cover of 21.58% (Sun et al., 2018) consisting of 69 species belonging to 24 genera and 13 families (Zhao et al., 2008). On the reef slope, Acropora is the dominant genus, followed by Porites as the second most dominant genus (Zhao et al., 2008). There are also other uncommon species, including Turbinaria peltata, Pavona decussata, and Astreopora myriophthalma. Generally, most corals are found between the depths of 1 m to 10 m below chart datum. The reef is periodically exposed to typhoons originated from the South China Sea or from the western Pacific. As coral spawning events are closely associated with phases of the moon, we monitored the timing of gametes released by corals at 2–5 m depth from 2009 to 2021 following the lunar cycles. We used the Gregorian names of each month to represent the equivalence of lunar month, e.g., lunar January to represent the first lunar month after the lunar new year, so forth and so on. The monitored records were collected every lunar month starting from 8–11 days before and up to15 days after each full moon in the period from lunar months off February to June over 2009–2021 (corresponding to around March to July in the Gregorian calendar), with the exception of 2012 and 2015. These periods were chosen because in 2009, Acropora robusta and A. millepora, were observed in a preliminary monitoring to carry immature and mature oocytes in lunar February and March respectively, suggesting that Acropora spawned during these lunar months (Huang, 2011; Huang et al., 2011). Thereafter, direct observations by SCUBA divers were conducted during the identified spawning periods from 20:00 to 22:00 hr. Two SCUBA divers would swim along a course routinely taken within the reef, carefully checking for signs of spawning and recording the spawning behaviors, times, and coral species using a digital camera (Tough TG-6, Olympus, Tokyo, Japan) with an underwater light (X8, Micolite, Yangjiang, China). Observations were not carried out under poor weather conditions.
Species identification
The spawning species were identified in situ using characteristics such as the colony growth form, polyp arrangement, and morphology. When necessary, coral fragments were sampled from uncommon or taxonomically challenging species. Coral species were identified based on Wallace (1999) and Veron (2000). Taxonomic names were checked against the World Register of Marine Species (WoRMS) and Huang et al. (2020) for recent taxonomic revisions of corals in China.
Environmental factors
From 2011 to 2020, seawater temperature was recorded at 30 min intervals using a HOBO temperature data logger (Onset Computer Corp., Bourne, MA, USA) deployed at 3 m depth in the monitoring area. Data on moon phases at the study location (Sanya City) were compiled from a time and date website (https://www.timeanddate.com).
Results
Spawning species
The number of spawned species recorded in each spawning day before, at or after full moon is provided in Table 2, and the general patterns summarized in Table 3. A total of 22 scleractinian coral species from five genera (Acropora, Montipora, Platygyra, Dipsastraea, and Galaxea) representing three families (Acroporidae, Merulinidae, and Euphylliidae) were observed to have spawned from 2009 to 2021. We recorded 14 species belonging to the genus Acropora, four belonging to Montipora (both Acroporidae), two belonging to Platygyra, one belonging to Dipsastraea (Merulinidae), and one belonging to Galaxea (Euphylliidae) (Table 3). Acroporidae was by far the largest recorded family, and Acropora, the most dominant genus with the highest number of species that were observed to spawn on the Luhuitou fringing reef. Spawning activities of six representative coral species are illustrated in Figure 1.
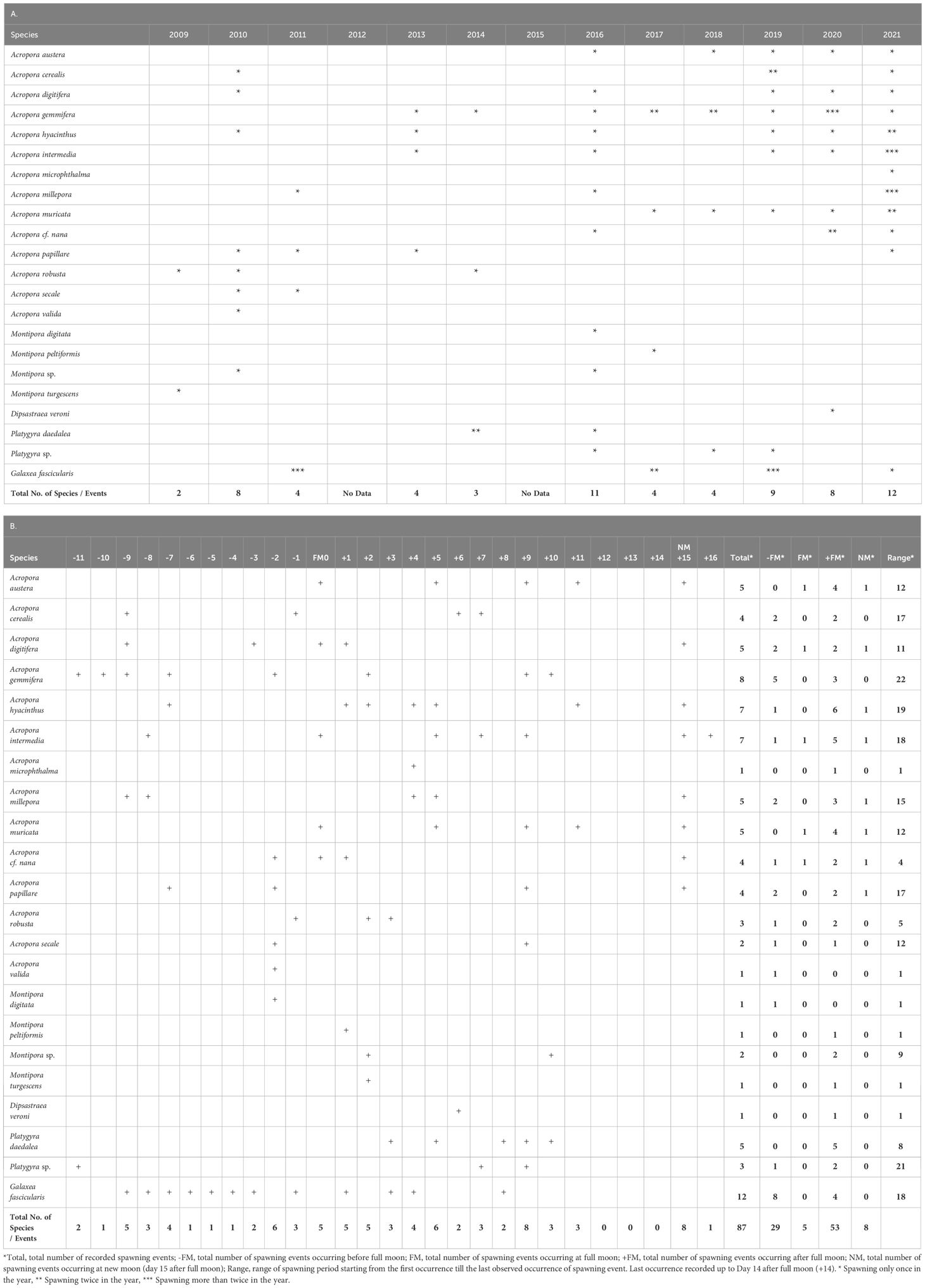
Table 2 (A) Record of spawning of the coral species in Luhuitou Fringing reef and (B) Summary of spawning records of coral species in Luhuito Fringing Reef in Hainan Island, northern South China Sea showing the spawning events recorded number of days before (-) and after (+) full moon.
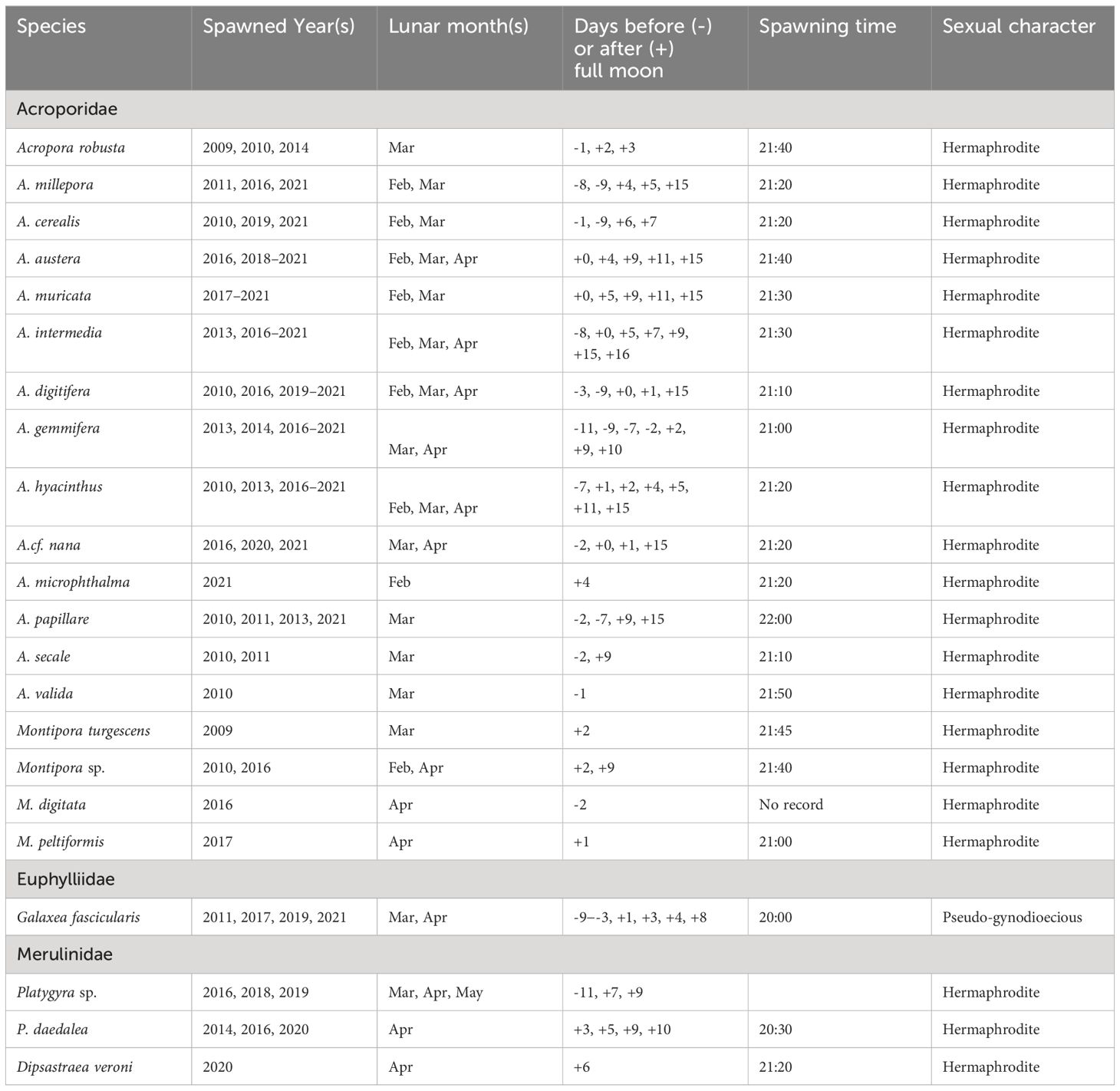
Table 3 Observed in situ spawning days and times of 22 scleractinian corals species before or after full moon on the Luhuitou fringing reef from 2009–2021.
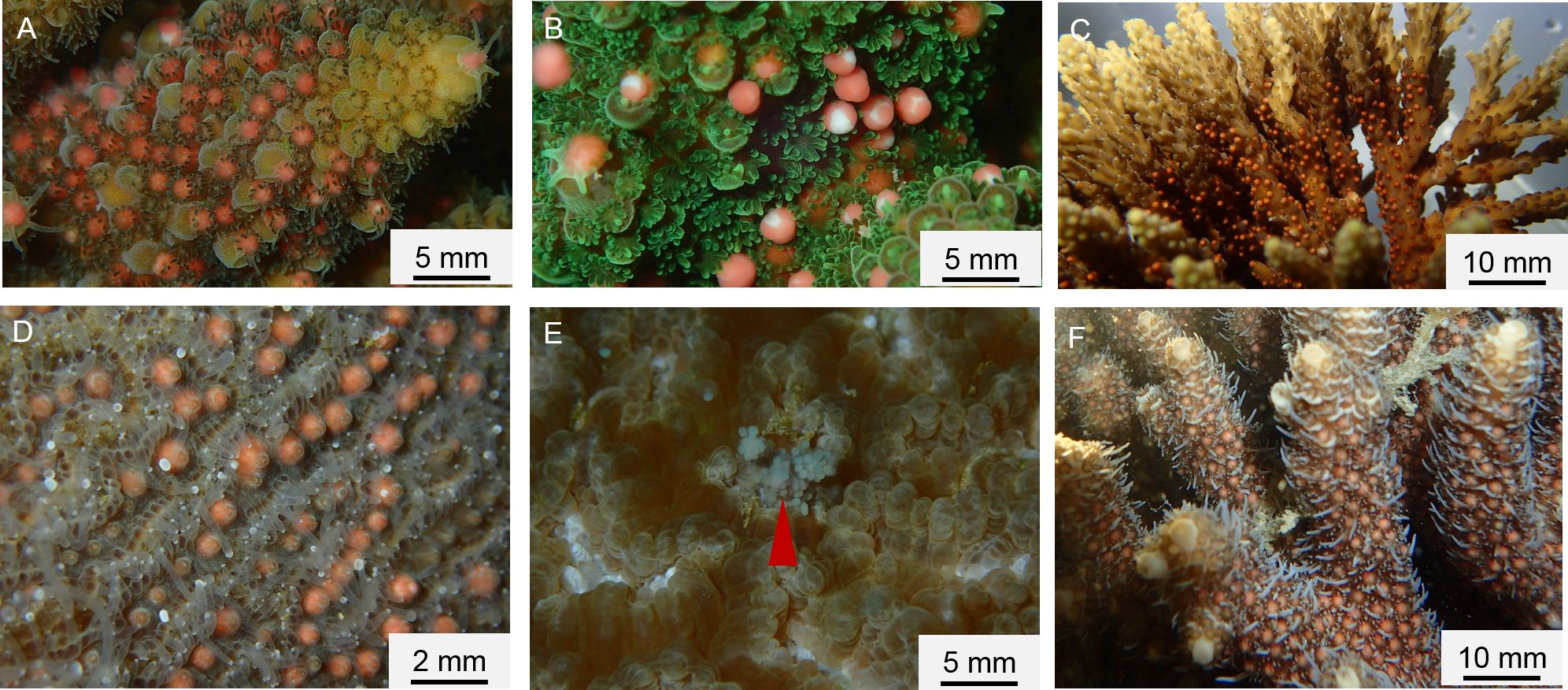
Figure 1 Polyps ready to release egg-sperm bundles, with bundles visible beneath the oral disc. The color of bundles is red in (A-D, F) and light cyan in (E). The red arrowhead points to bundles (light cyan color) of Dipsastraea veroni. (A) Acropora gemmifera; (B) Acropora austera; (C) Acropora cf.nana; (D) Platygyra daedalea; (E) Dipsastraea veroni; and (F) Acropora millepora.
Spawning lunar months
Observed coral species released gametes between lunar February and May (spring to early summer) over the 13-year observation period. All Acropora spawned within a three-month window with 8, 13, and 5 spawning species observed, respectively, in lunar February, March and April (Table 3). An exception was observed in 2016 wherein all of the seven Acropora species (A. austera, A. digtifera, A. gemmifera, A. hyacinthus, A. intermedia, A millepora, and A. nana) spawned in lunar April and not in lunar March. Altogether, lunar March and April appeared to have the largest scale of multi-specific spawning events with 16 and 14 respectively of the 22 studied coral species recorded to have spawned (Table 3).
For some of the coral species, spawning was only recorded occasionally. More extensive spawning data were recorded for Acropora species, among these A. austera, A. digtifera, A. gemmifera, A. hyacinthus, A. intermedia, and A. muricata were recorded to spawn in consecutive years especially from 2016/2017 to 2021 (Table 2A). Except for A. gemmifera, these species were also recorded to spawn both during full moon and new moon (i.e., 15 days after full moon, which is already the new moon of the next lunar month) (Table 2B). These events, however, did not occur in the same year. Spawning of A. austera, A. digtifera, A. hyacinthus, A. intermedia, and A. muricata occurred during new moon of lunar April (2016), whereas A.austera, A. digtifera, A. muricata, and A. intermedia were recorded to spawn at the full moon of lunar March (2020) (Table 2B). At least 11 species Acropora, including A. digtifera, A. gemmifera, and A. intermedia also occasionally spawned before full moon (Table 2B); and except A. valida, all the other 13 Acropora species spawned after full moon.
Multispecies spawning in Luhuitou Reef occurred in 2016, 2018, 2019, and 2020, with at least three Acropora species spawning in the same night. More often, however, split spawning between species or between colonies of the same species occurred. For instance, A. gemmifera spawned at least twice in 2017, 2018 and 2020; same for A. intermedia, A. hyacinthus, and A. muricata in 2021. For non-Acropora species, split spawning events have also been recorded in 2014 and 2020, for example, in Platygyra daedalea.
Spawning lunar days
In contrast to what is conventionally assumed, the studied coral species released gametes over a wide range of period around full moon during the 13-year observations (Table 2). Different species of Acropora spawned 9–11, 7, and 1–3 days before full moon and 0–7, 9–11, and 15–16 days after full moon, with a maximum range covering 19 lunar days for A. hyacinthus and 20 lunar days for A. gemmifera. Interestingly, some gaps were noted with no Acropora spawning 4–6 days before full moon or 8 and 12–14 days after full moon during these 13 years of observations. Except for A. valida, most other Acropora species typically spawned more frequently after full moon (Table 2). Several Acropora species spawned more than once a year, suggesting that split spawning involving different coral colonies of the same species occurred. For example, A. gemmifera spawned 11 and 2 days before full moon in 2017; 7 days before full moon and 9 days after full moon in 2018; 7, 9, and 10 days before full moon but none after full moon in 2020. Acropora millepora spawned 9 and 8 days before full moon and 5 days after full moon in 2021; A. intermedia spawned only 5, 15, and 16 days after full moon but none before full moon in 2021.
Similar patterns were observed for the other four genera monitored. Montipora, Platygyra, Dipsastraea, and Galaxea spawned both before and after full moon. Montipora spawned 2 days before full moon and 1–2 and 10 days after full moon. Platygyra spawned 11 days before full moon and 3, 5, 7–10 days after full moon. In particular for Platygyra, spawning occurred in the last quarter moon. In D. veroni, spawning occurred 6 days after full moon. Galaxea fascicularis spawned successively over several nights around full moon, ranging between 3–9 days before full moon and 1, 3, 4, and 8 days after full moon. Other than Acropora spp., G. fascicularis is one other species that has a maximum range of spawning covering 18 days (Table 2A). Overall, although variability in the spawning time of the 22 coral species was high, there is a higher chance (61%) of spawning occurring after full moon. Considering that 15 days after full moon is actually new moon day, there were five cases of spawning occurring exactly on the full moon day, and eight cases of spawning during new moon.
Spawning hours
Despite high variability in the days of spawning, the spawning hours of Acropora, Montipora, and D. veroni were more consistent and predictable during the monitoring period (Table 3), beginning after 21:00 hr and continuing for approximately 1 hr. The spawning times of Platygyra species and G. fascicularis started at approximately 20:00 and 20:30 hr, respectively.
Temperature
The mean annual seawater temperature at 2–3 m depth in the Luhuitou fringing reef from 2011–2020 is approximately 27°C. The annual temperature data for 2009, 2010, and 2021 were incomplete and thus are not presented. Most of the 22 coral species spawned from lunar February to April with monthly mean (± SD) seawater temperature of 24.31 ± 1.39, 27.01 ± 1.44, and 29.05 ± 1.00°C respectively from 2011–2020 (Figure 2).
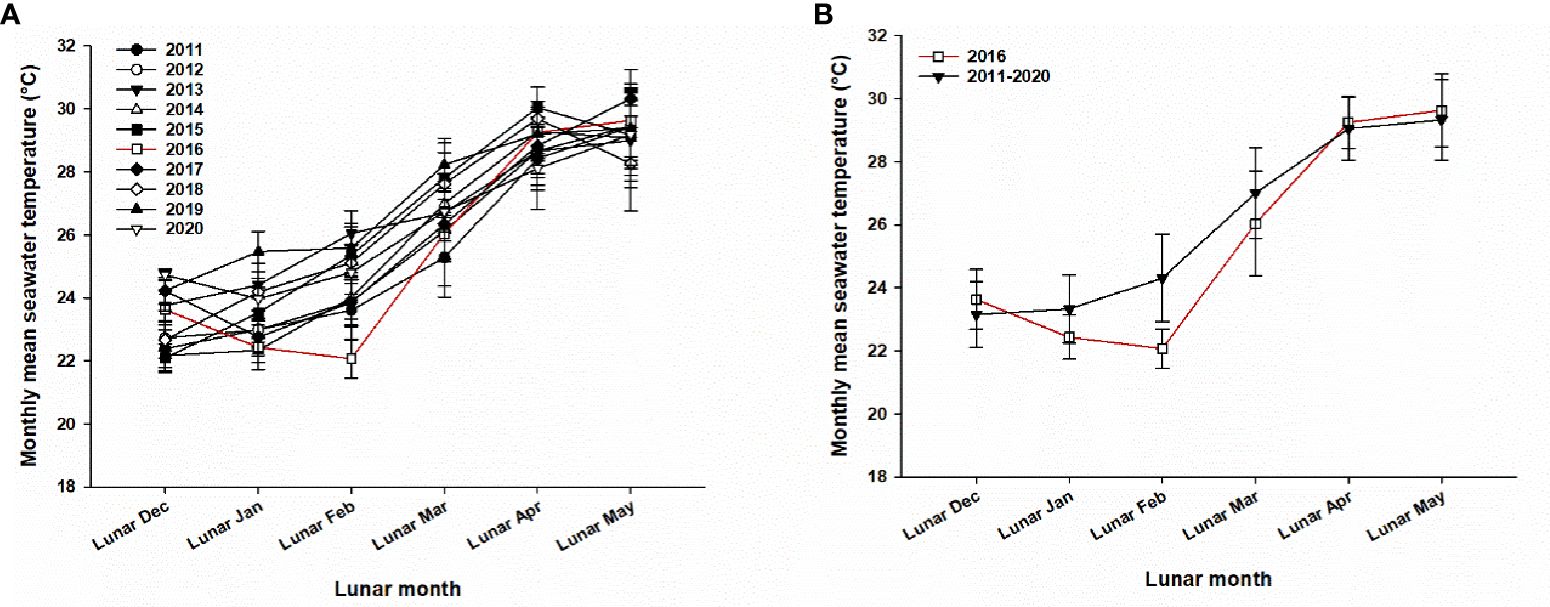
Figure 2 Variation in (A) monthly mean (SD) seawater temperatures of each year from 2011–2020 recorded at 3 m depth in the Luhuitou fringing reef, and (B). mean (SD) monthly seawater temperature in the Luhuitou fringing reef for 2011–2020, and that for 2016 alone showing the exceptionally low temperature recorded in 2016.
The monthly mean seawater temperatures from lunar January to March 2016, especially that in lunar February 2016, were lower than those recorded in the same period in the other years of the study (Figure 2). The monthly mean (± SD) seawater temperatures at the same site in 2016 were 22.43 ± 0.70, 22.07 ± 0.61, and 26.04 ± 1.66°C in lunar January, February, and March, respectively, indicating that the mean seawater temperature decreased by 0.9°C, 2.24°C, and 0.97°C respectively in these months, compared with those in 2011–2020.
Discussion
Compared to other reef sites in the Indo–West Pacific, very little detailed research has been conducted on spawning patterns of scleractinian corals in the northern South China Sea. This study is the first systematic investigation of in situ scleractinian coral spawning patterns in the Luhuitou fringing reef at Hainan Island. Twenty-two species from five genera and three families of scleractinian corals spawned from lunar February to May over the 13 years of the study. One important observation, however, should be noted. Although monitoring for coral spawning throughout these 13 years was carried out within the same general area of the reef following an established route, the data cannot be completely exhaustive. It is often difficult to ascertain whether lack of a spawning record is result of lack of spawning or lack of observation. There is always a possibility that the absence of a spawning record for any particular species may simply be a result of lack of observation. In particular, for example, Porites spp. are second in dominance next only to Acropora spp. in Luhuitou Reef but no record of their spawning was made. It being dioecious, observation of its spawning is difficult. Furthermore, although only two species were observed to have spawned in 2009, eight were observed to spawn in the subsequent year of 2010. Thereafter, the number of species that spawned varied widely, with as few as three species in 2014 and up to 11 and 12 species in 2016 and 2021 respectively (Table 2A). This suggestions that variability in the sampling efforts and there was no clear pattern of increasing number of spawning species from 2009 to 2021.
More importantly, clear spawning patterns of these coral species were not evident, especially for Acropora species. This indicates that while a general spawning season is predictable, the exact spawning day remains elusive and highly unpredictable. Many studies (Harrison and Wallace, 1990; Mangubhai and Harrison, 2008; Lin and Nozawa, 2017) have been conducted to find the proximate clue that drives coral spawning pattern. To date, detection of the proximate cues remains difficult because of the many major uncertainties involved (Harrison and Wallace, 1990; Mangubhai and Harrison, 2008). Given the high irregularity in the spawning patterns observed in our present study, it is unlikely that a single cue is involved especially at the daily temporal scale. Nonetheless, a few general patterns may still be observed from our long-term spawning data.
The timing of coral spawning has often been examined with respect to the three principal determinants of reproduction time known for marine invertebrates: lunar cycles, the diurnal light cycle, and annual temperature variation (Korringa, 1947). More recently, the timing of coral spawning has mainly been assessed in relation to two features: the lunar cycles (Lin et al., 2021; Baird et al., 2021a) and seawater temperature (Nozawa, 2012; Keith et al., 2016; Lin and Nozawa, 2017). Previous studies have demonstrated that coral spawning timing was correlated with lunar cycles (Willis et al., 1985) so that phases of the moon, or days after full moon were used to predict the broadcast spawning night with some accuracy. However, reference to lunar month has not been widely applied in many coral spawning studies. In the present study, we found that lunar month instead of Gregorian (or common calendar) month was more suitable and precise in documenting coral spawning at the monthly level. For example, A. gemmifera spawned twice in lunar March (2017) and March (2018). If we use Gregorian month to describe this pattern, A. gemmifera would have been recorded to spawn in two consecutive calendar months of April and May in both 2017 and 2018. Furthermore, transcriptome analysis of A. gemmifera over an entire lunar month showed that genes associated with biological clocks and circadian processes (i.e. cry1, bzip1, and clock) change their diurnal cycles, indicating that lunar cycle controls transcriptional processes in corals (Oldach et al., 2017). Thus, corals are likely to respond to lunar signals directly and regulate their biological rhythms (Oldach et al., 2017). This finding provides the physiological basis to explain why many in situ corals usually spawn after full moon.
In the Great Barrier Reef, the mass spawning of corals took place between the full and last quarter moon in late spring (Babcock et al., 1986). Acropora and Montipora spawning occurred 1–11 days after full moon between 2010–2016 in Lyudao, Taiwan (Lin and Nozawa, 2017). Dipsastraea speciosa usually spawned 5 days after full moon, demonstrating that the period of darkness between sunset and moonrise triggered mass spawning in this species (Lin et al., 2021). However, the highly variable coral spawning patterns on the Luhuitou Reef showed that many corals also spawned before full moon, and the timing differed highly between years even for the same species. In Kochi, southern Japan, Acropora species spawned throughout most lunar phases, i.e. 0 - 3 days before full moon and 0–7, 13 days after full moon from 1989–1991 (Hayashibara et al., 1993), and 9–14 d and 0–2 days before the full moon and 1–15 days after full moon from 2002 to 2006 (Mezaki et al., 2007). The general asynchrony has also been documented in the Red Sea (Shlesinger and Loya, 2019). Although Luhuitou Reef in Hainan Island is located in the tropics in the northern South China Sea, it would appear that the coral spawning pattern in this reef is more similar to that of the subtropical sites like Kochi, than to other tropical sites like Lyudao and the Great Barrier Reef.
Seawater temperature has long been considered as a primary cue influencing coral spawning pattern because temperature affects the rate of gametogenesis, which in turn affects the timing of coral spawning (Harrison and Wallace, 1990; Keith et al., 2016). The timing of coral spawning events varies geographically, with spawning events reported in every month of the year at different locations around the world. Although spawning events at most geographical sites occur mainly during warmer seasons (Keith et al., 2016), higher or lower monthly mean seawater temperature during gametogenesis could lead to earlier or later spawning times respectively. As observed in the present study, Acropora species spawned in lunar April 2016 instead of lunar February and March as in other years. This delay in spawning corresponded to the lowest seawater temperatures recorded in lunar February and March in 2016 (Figure 2). This is consistent with previous findings in which a rapid decrease in seawater temperature during the late period of gametogenesis provided a dominant proximate cue for delayed spawning. In Lyudao, the spawning time of acroporids and merulinids in 2015 were delayed to May and June due to the very low temperatures in January and February (Lin and Nozawa, 2017). On Solitary Islands, Western Australia, the delay in the timing of coral spawning coincided with a delayed increase in sea temperature in the subtropics (Wilson and Harrison, 2003). Overall, these findings contribute to the growing concern that lower monthly mean seawater temperatures during gametogenesis before spawning could lead to earlier or delayed spawning and the implication of this on coral recruitment success.
Other than temperature, data on other physical parameters like rainfall, salinity change, tidal fluctuation, nutrients and other water quality indicators are not available to allow a more extensive investigation into the potential proximate clues affecting coral spawning patterns in Luhuitou Reef. Nonetheless, these 13 years of spawning observations fill a critical information gap on coral spawning in the northern South China Sea that may be useful for further understanding of coral spawning patterns and mechanisms in the Indo-West Pacific. Further work is needed to establish the generality of these spawning patterns by investigating other coral species, such as Porties and Montipora, and to identify plausible mechanism to explain these patterns in relation to the presence of environmental cues. Focus may also be extended to other months of the year as variant morphotypes of Acropora divaricate is Sesoko, Okinawa were found to spawn in August-September, in contrast to other colonies of the same species that normally spawn in May-June (Furukawa et al., 2020, 2024). Similar variants or cryptic species could potentially be present in Luhuitou Reef. Ability to more precisely predict the spawning pattern of coral species is critical if further works on understanding coral reproduction are to be undertaken. Moreover, reef restoration works using sexual recruits will ultimately have to depend on the ability to obtain sources of egg bundles during coral spawning for larval culture. All these will continue to present challenges to our understanding of coral reproductive patterns and the potential changes in these patterns due to environmental variability in the foreseeable future especially under the pressures of global climate change.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
YS: Funding acquisition, Investigation, Resources, Validation, Writing – original draft, Writing – review & editing. YZ: Investigation, Writing – review & editing. LJ: Investigation, Writing – review & editing. XY: Investigation, Writing – review & editing. LH: Investigation, Software, Validation, Writing – review & editing. TY: Investigation, Writing – review & editing. JY: Investigation, Writing – review & editing. JL: Investigation, Writing – review & editing. CL: Investigation, Writing – review & editing. PA: Writing – review & editing. HH: Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Key R&D Program of China (2021YFC3100500), Science and Technology Project of Guangdong Provincial Department of Natural Resources (GDNRC[2022]40), National Natural Science Foundation of China (42206153), Natural Science Foundation of Guangdong Province, China (2023A1515010810), Science and Technology Projects in Guangzhou (2023A04J0200), and Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (SMSEGL20SC01).
Acknowledgments
We thank Prof. Andrew Baird for valuable suggestions and comments on the manuscript. We also thank the staff of Tropical Marine Biological Research Station in Hainan for providing technical assistance and facilities for conducting this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Babcock R., Bull G., Harrison P. L., Heyward A., Oliver J., Wallace C., et al. (1986). Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90, 379–394. doi: 10.1007/BF00428562
Baird A. H., Birrel C. L., Hughes T. P., Mcdonald A., Nojima S., Page C. A., et al. (2009a). Latitudinal variation in reproductive synchrony in Acropora assemblages: Japan vs. Australia. Galaxea J. Coral Reef Stud. 11, 101–108. doi: 10.3755/galaxea.11.101
Baird A. H., Cumbo V. R., Gudge S., Keith S. A., Maynard J. A., Tan C.-H., et al. (2015). Coral reproduction on the world’s southernmost reef at Lord Howe Island, Australia. Aquat Biol. 23, 275–284. doi: 10.3354/ab00627
Baird A. H., Edwards A. J., Guest J. R., Harii S., Hatta M., Lachs L., et al. (2021a). A coral spawning calendar for Sesoko Station, Okinawa, Japan. Galaxea J. Coral Reef Stud. 24, 41–49. doi: 10.3755/galaxea.G2021_S10O
Baird A. H., Guest J. R., Edwards A. J., Bauman A. G., Bouwmeester J., Mera H., et al. (2021b). An Indo-Pacific coral spawning database. Sci. Data. 8, 35. doi: 10.1038/s41597-020-00793-8
Baird A. H., Guest J. R., Willis B. L. (2009b). Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol. Evol. Syst. 40, 551–571. doi: 10.1146/annurev.ecolsys.110308.120220
Baird A. H., Prior S., Bridge T., Cowman P. (2023). First records of coral spawning on Norfolk Island. Galaxea J. Coral Reef Stud. 25, 3–4. doi: 10.3755/galaxea.G25-2
Carroll A., Harrison P., Adjeroud M. (2006). Sexual reproduction of Acropora reef corals at Moorea, French Polynesia. Coral Reefs. 25, 93–97. doi: 10.1007/s00338-005-0057-6
Chamberland V. F., Snowden S., Marhaver K. L., Petersen D., Vermeij M. J. (2017). The reproductive biology and early life ecology of a common Caribbean brain coral, Diploria labyrinthiformis (Scleractinia: Faviinae). Coral Reefs. 36, 83–94. doi: 10.1007/s00338-016-1504-2
Chelliah A., Amar H. B., Hyde J., Yewdall K., Steinberg P. D., Guest J. R. (2015). First record of multi-species synchronous coral spawning from Malaysia. PeerJ 3, e777. doi: 10.7717/peerj.777
Furukawa M., Kitanobo S., Ohki S., Teramoto M. M., Hanahara N., Morita M. (2024). Integrative taxonomic analyses reveal that rapid genetic divergence drives Acropora speciation. Mol. Phylogenet. Evol. 195, 108063. doi: 10.1016/j.ympev.2024.108063
Furukawa M., Ohki S., Kitanobo S., Fukami H., Morita M. (2020). Differences in spawning time drive cryptic speciation in the coral Acropora divaricata. Mar. Biol. 167, 163. doi: 10.1007/s00227-020-03781-z
Gan S. H., Waheed Z., Chung F. C., Spiji D. A., Sikim L., Saleh E., et al. (2021). In situ observations of coral spawning and spawn slick at Lankayan Island, Sabah, Malaysia. Mar. Biodivers. 51, 1–7. doi: 10.1007/s12526-020-01158-5
Gouezo M., Doropoulos C., Fabricius K., Olsudong D., Nestor V., Kurihara H., et al. (2020). Multispecific coral spawning events and extended breeding periods on an equatorial reef. Coral Reefs. 39, 1107–1123. doi: 10.1007/s00338-020-01941-7
Harrison P. L. (2011). “Sexual Reproduction of Scleractinian Corals,” in Coral Reefs: An Ecosystem in Transition (Springer Netherlands, Dordrecht), 59–85.
Harrison P. L., Babcock R. C., Bull G. D., Oliver J. K., Wallace C. C., Willis B. L. (1984). Mass spawning in tropical reef corals. Science 223, 1186–1189. doi: 10.1126/science.223.4641.1186
Harrison P. L., Wallace C. C. (1990). “Reproduction, dispersal and recruitment of scleractinian corals,” in Coral reefs (Elsevier, Amsterrdam), 133–207.
Hayashibara T., Shimoike K., Kimura T., Hosaka S., Heyward A., Harrison P., et al. (1993). Patterns of coral spawning at Akajima Island, Okinawa, Japan. Mar. Ecol. Prog. Ser. 101, 253–262. doi: 10.3354/meps101253
Huang J. (2011). The sexual reproductive biology of hermatypic corals in Luhuitou, Sanya, China.PhD. Beijing, China: Graduate school of the Chinese Academy of Sciences.
Huang J., Huang H., Zhang Y., Yang J., You F. (2011). Embryonic and larval development of Montipora turgescens and Acropora robusta. J. Trop. Oceanogr. 30, 67–73.
Huang L., Huang H., Jiang L. (2020). A revised taxonomy for Chinese hermatypic corals. Biodivers. Sci. 28, 515–523. doi: 10.17520/biods.2019384
Jamodiong E. A., Maboloc E. A., Leriorato J. C., Tañedo M. C. S., Diaz L. A., Tabalanza T. D., et al. (2018). Coral spawning and spawn-slick observation in the Philippines. Mar. Biodivers. 48, 2187–2192. doi: 10.1007/s12526-017-0680-9
Jordan A. C. (2018). Patterns in Caribbean Coral Spawning. Master of Science. Davie, FL, USA: Nova Southeastern University.
Keith S. A., Maynard J. A., Edwards A. J., Guest J. R., Bauman A. G., Van Hooidonk R., et al. (2016). Coral mass spawning predicted by rapid seasonal rise in ocean temperature. Proc. R. Soc B. 283, 20160011. doi: 10.1098/rspb.2016.0011
Korringa P. (1947). Relations between the moon and periodicity in the breeding of marine animals. Ecol. Monogr. 17, 347–381. doi: 10.2307/1948665
Lacks A. L. (2000). Reproductive ecology and distritution of the scleractinian coral Fungia scutaria in Kane’ohe Bay, O’ahu, Hawai’i. University of Hawai ‘i, Honolulu., HI, USA
Lin C.-H., Nozawa Y. (2017). Variability of spawning time (lunar day) in Acropora versus merulinid corals: a 7-yr record of in situ coral spawning in Taiwan. Coral Reefs. 36, 1269–1278. doi: 10.1007/s00338-017-1622-5
Lin C., Takahashi S., Mulla A. J., Nozawa Y. (2021). Moonrise timing is key for synchronized spawning in coral Dipsastraea speciosa. Proc. Natl. Acad. Sci. U.S.A. 118, e2101985118. doi: 10.1073/pnas.2101985118
Liu P., Fan T., Dai C. (2005). Timing of larval release by the blue coral, Heliopora coerulea, in southern Taiwan. Coral Reefs. 24, 30–30. doi: 10.1007/s00338-004-0455-1
Mangubhai S., Harrison P. L. (2008). Asynchronous coral spawning patterns on equatorial reefs in Kenya. Mar. Ecol. Prog. Ser. 360, 85–96. doi: 10.3354/meps07385
Mendes J., Woodley J. (2002). Timing of reproduction in Montastraea annularis: relationship to environmental variables. Mar. Ecol. Prog. Ser. 227, 241–251. doi: 10.3354/meps227241
Mezaki T., Hayashi T., Iwase F., Nakachi S., Nozawa Y., Miyamoto M., et al. (2007). Spawning patterns of high latitude scleractinian corals from 2002 to 2006 at Nishidomari, Otsuki, Kochi, Japan. Kuroshio Biosphere. 3, 33–47.
Nozawa Y. (2012). Annual variation in the timing of coral spawning in a high-latitude environment: influence of temperature. Biol. Bull. 222, 192–202. doi: 10.1086/BBLv222n3p192
Oldach M. J., Workentine M., Matz M. V., Fan T. Y., Vize P. D. (2017). Transcriptome dynamics over a lunar month in a broadcast spawning acroporid coral. Mol. Ecol. 26, 2514–2526. doi: 10.1111/mec.14043
Padilla-Gamiño J. L., Gates R. D. (2012). Spawning dynamics in the Hawaiian reef-building coral Montipora capitata. Mar. Ecol. Prog. Ser. 449, 145–160. doi: 10.3354/meps09530
Penland L., KlouleChad J., Idip D., Van Woesik R. (2004). Coral spawning in the western Pacific Ocean is related to solar insolation: evidence of multiple spawning events in Palau. Coral Reefs. 23, 133–140. doi: 10.1007/s00338-003-0362-x
Rosser N., Gilmour J. (2008). New insights into patterns of coral spawning on Western Australian reefs. Coral Reefs. 27, 345–349. doi: 10.1007/s00338-007-0335-6
Shlesinger T., Loya Y. (2019). Breakdown in spawning synchrony: A silent threat to coral persistence. Science 365, 1002–1007. doi: 10.1126/science.aax0110
Sun Y., Lei X., Lian J., Yang J., Wu Y., Huang H. (2018). Ecosystem status and health assessment of Sanya Coral Reef National Nature Reserve. Biodivers. Sci. 26, 258–265. doi: 10.17520/biods.2017312
Veron J. E. N. (2000). Corals of the World (Townsville, Queensland, Australia: Australian Institute of Marine Science).
Wallace C. (1999). Staghorn corals of the world: a revision of the genus Acropora (Melbourne: CSIRO Publishing). doi: 10.1071/9780643101388
Wijayanti D. P., Indrayanti E., Wirasatriya A., Haryanto A., Haryanti D., Sembiring A., et al. (2019). Reproductive seasonality of coral assemblages in the Karimunjawa Archipelago, Indonesia. Front. Mar. Sci. 6, 195. doi: 10.3389/fmars.2019.00195
Willis B., Babcock R., Harrison P. L., Oliver J., Wallace C. (1985). Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. Proceedings of International Coral Reef Congress 5, 343–348.
Wilson J., Harrison P. L. (2003). Spawning patterns of scleractinian corals at the Solitary Islands a high latitude coral community in eastern Australia. Mar. Ecol. Prog. Ser. 260, 115–123. doi: 10.3354/meps260115
Yu X., Sun Y., Zhang Y., Huang H. (2022). First record of coral split spawning in the genus Acropora at Luhuitou fringing reef, Sanya, China. B Mar. Sci. 99, 65–66. doi: 10.5343/bms.2022.0039
Zhao M., Yu K., Zhang Q., Shi Q. (2008). Spatial pattern of coral diversity in Luhuitou fringing reef, Sanya, China. Sheng Tai Xue Bao. 28, 1419–1428. doi: 10.1016/S1872-2032(08)60051-7
Keywords: coral spawning, Acropora, lunar month, lunar day, the South China Sea
Citation: Sun Y, Zhang Y, Jiang L, Yu X, Huang L, Yuan T, Yang J, Lian J, Liu C, Ang P and Huang H (2024) Coral spawning patterns on the Luhuitou fringing reef in Hainan Island of the northern South China Sea. Front. Mar. Sci. 11:1418942. doi: 10.3389/fmars.2024.1418942
Received: 17 April 2024; Accepted: 24 June 2024;
Published: 15 July 2024.
Edited by:
Yehuda Benayahu, Tel Aviv University, IsraelReviewed by:
Masaya Morita, University of the Ryukyus, JapanThamasak Yeemin, Ramkhamhaeng University, Thailand
Copyright © 2024 Sun, Zhang, Jiang, Yu, Huang, Yuan, Yang, Lian, Liu, Ang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Put Ang, YXB1dDk2MjI5QGdtYWlsLmNvbQ==; Yuyang Zhang, eXl6aGFuZ0BzY3Npby5hYy5jbg==; Hui Huang, aHVhbmdodWlAc2NzaW8uYWMuY24=
†These authors have contributed equally to this work
 Youfang Sun
Youfang Sun Yuyang Zhang
Yuyang Zhang Lei Jiang
Lei Jiang Xiaolei Yu1,4
Xiaolei Yu1,4 Lintao Huang
Lintao Huang Chengyue Liu
Chengyue Liu Put Ang
Put Ang Hui Huang
Hui Huang