Abstract
Introduction:
The Aotearoa New Zealand ghost shrimp of the infraorders Axiidea and Gebiidea have never been comprehensively reviewed, with recent work uncovering a diverse regional fauna representing eight of the 14 known families.
Methods:
Using standard morphological and DNA sequencing tools, the family Callianopsidae is, for the first time, recorded off New Zealand, represented by a new species of Vulcanocalliax.
Results:
The new species was found near hydrocarbon seeps on the Hikurangi Margin, on the eastern New Zealand continental slope, and is only the second species now known in this genus. The single congener, V. arutyunovi, is only known from a mud volcano in the Gulf of Cádiz, off the Iberian Peninsula. Vulcanocalliax sp. nov. was formerly reported as an unnamed host of a new endemic New Zealand rhizocephalan barnacle Parthenopea australis and is here formally described as Vulcanocalliax beervana sp. nov.
Discussion:
The new species differs, e.g., in the shape of the anterior carapace margin (convexly rounded and without elevated postantennal shoulder present in V. arutyunovi), the ocular peduncle having a convex anterolateral margin (compared to a straight margin), and the uropodal exopod has a dorsomedian ridge, lacking the elevated anterior portion that is distinct in V. arutyunovi. This brings the number of described New Zealand ghost shrimp species to 18. A key to all known Callianopsidae is provided.
ZOOBANK:
LSID urn:lsid:zoobank.org:pub:4EDE6CE1-B644-4FFB-94CA-C8725A7549DD.
Introduction
The small group of ghost shrimp in the family Callianopsidae Manning and Felder, 1991 currently recognizes six recent species (DecaNet eds., 2024, accessed 1 August 2024). Originally described as a subfamily Callianopsinae in a new family Ctenochelidae by Manning and Felder (1991), it was designated to accommodate a single species Callianopsis goniophthalma (Rathbun, 1902); Sakai (2011) subsequently places it in the new family Callianopsidae.
As the other members in the broader group commonly referred to as ghost shrimp that, for example, include the abundant and diverse coastal callianassids, they are pale in life color and inhabit burrows that the animals build into soft sediments [see references in the work of Poore and Ahyong (2023)]. The ghost shrimp taxonomy and systematics have recently undergone substantial reviews with many new genera derived from a combined molecular-morphology phylogeny (Poore et al., 2019; Robles et al., 2020), and there was convincing support for the validity of the family Callianopsidae as a basal group of the “callianassoid” family tree. The term “Callianassoidea” commonly refers to a group of families of ghost shrimp of “Callianassidae and related families”; however, Poore et al. (2019) questioned this superfamily as some of their proposed diagnostic morphological characters overlap with other axiidean families that are not included in this term. Nevertheless, Robles et al. (2020), who include Gary Poore as an author, continued to use the term and pointed out that it formed a monophyletic and well-supported clade within the axiidean phylogeny. The study by Robles et al. (2020) supported close evolutionary relationships between the families Callianopsidae and the Eucalliacidae (Manning and Felder, 1991) at the base of this clade. These two taxa share the broadly expanded maxilliped 3 dactylus that bears a dense field of setae on a truncate margin (typically for the other ghost shrimp, the dactylus is narrow and digitate) and between the Callianopsidae and the Ctenochelidae that share a simple uropodal exopod, i.e., without an elevated dorsal plate (compared to the dorsal plate presenting as a triangular thickening with a distal row of stiff setae that is typical for other ghost shrimp like the Callianassidae or Callichiridae).
Three genera are recognized in the Calllianopsidae: Callianopsisde Saint Laurent, 1973 with four species being the most speciose, with a fragmented distribution in all major oceans and reported from shelf to slope depths (~300–700 m); BathycalliaxSakai and Türkay, 1999 containing the single species B. geomar Sakai and Türkay, 1999 known only from the northeastern Pacific at around 630-m depth and representing the first record of a ghost shrimp potentially associated with a deep-water cold seep; and VulcanocalliaxDworschak and Cunha, 2007 for a single species V. arutyunovi Dworschak and Cunha, 2007, the second record of a ghost shrimp from chemoautotrophic communities, described from a mud volcano in the Gulf of Cádiz off Spain, and the deepest record for this family with just over 1,300-m depth. Lörz et al. (2008) reported an undescribed species of Vulcanocalliax as the host of an undescribed species of kentrogonid rhizocephalan, subsequently described by Lützen et al. (2009) as Parthenopea australis from a single specimen collected during the 2006 RENEWZ voyage sampling cold seep communities along the east coast of Aotearoa New Zealand (Baco et al., 2010). Here, the new species of Vulcanocalliax is formally described; its distribution remains restricted to two samples collected during the RENEWZ voyage between 650- and 808-m depth (Figure 1). Samples have only been acquired through sediment samplers (grab and multi corer), indicating that more typical gear used to collect epibenthic fauna is insufficient to sample burrowing benthic infauna. This new species represents the first and only callianopsid known from the New Zealand region and is named with our gratitude to Ryan McArthur, the manager of the biggest New Zealand beer festival Beervana, who has contributed to the success of the 10th International Crustacean Congress held in Wellington in 2023.
Figure 1

Map of the Aotearoa New Zealand region (inset) and the Hikurangi Margin off the east coast of the North Island. The black stars indicate the sampling locations of the specimens of Vulcanocalliax beervana sp. nov., in the vicinity of the two cold seep sites known as “Builder’s Pencil” and “Rock Garden” sampled during the 2006 RENEWZ voyage (Baco et al., 2010).
Material and methods
Sample collections
Specimens were collected from two locations during the 2006 RENEWZ voyage conducted by the R/V Tangaroa (Stations TAN0616/13 and TAN0616/35) and using the van Veen grab and multicorer (Figure 1). Specimens were extracted from the sediment on board and preserved in 80% ethanol.
Morphological examination
Specimens were examined using a Leica MZ 9.5 stereomicroscope. Size is expressed as carapace length (cl.), including rostrum, in mm. The description covers the holotype female of the new species, with comments on variation on the males and juvenile covered in the Remarks section below. Material examined is deposited in the National Institute of Water and Atmospheric Research Invertebrate Collection, Wellington (NIWA).
DNA sequencing for molecular taxonomy
Total genomic DNA was extracted from a single pleopod from the last three pairs of the specimen using a QIAamp DNA Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The extracted DNA was eluted in 100 μL of sterile distilled H2O (RNase (Ribonuclease) free), quantified by 1.5% agarose gel electrophoresis and using N60 NanoPhotometer (Implen, Munich, Germany). Then, the total genomic DNA solution was sent to Berry Genomics Co., Ltd (Beijing, China) for library preparation and whole-genome sequencing. Paired-end libraries were constructed with an insert size of 300 base pairs (bp) and sequenced (2 × 150 bp) on the Illumina NovaSeq 6000 platform, and approximately 6 Gb of raw data were produced. Trimmomatic v 0.39 (Bolger et al., 2014) was used to remove adaptors and low-quality reads. Spades v 3.15.4 (Bankevich et al., 2012) was applied with multiple k-mer strategies to assemble the reads into contigs. Contigs containing mitochondrial ribosomal RNA sequences were identified using BLAST+ v 2.12.0 (Camacho et al., 2009) and checked by mapping to corresponding reference sequences using Unipro UGENE v. 42.0 (Rose et al., 2019). The 16S rRNA sequence was extracted for the purposes of this study and was aligned with reference sequences available on GenBank using Geneious (v 2021.1.1) (http://www.geneious.com; Kearse et al., 2012). The default Clustal Omega alignment function parameters were applied to calculate the percentage identity and patristic distance matrix (Tamura-Nei distance mode, neighbor-joining tree build method, outgroup not specified). The sequences for the paratype of the new species (NIWA 69439) are deposited on GenBank under accession numbers: PQ137440.
Results
Family Callianopsidae Manning and Felder, 1991
Genus VulcanocalliaxDworschak and Cunha, 2007
Vulcanocalliax Dworschak and Cunha, 2007: 37—Sakai (2011): 350–351—Poore et al. (2019): 102—Robles et al. (2020): phylogeny.
Diagnosis: Carapace, longitudinal carina absent; cardiac sulci absent. Rostrum obsolete or obtusely triangular, flat, not reaching cornea. Pleonite 1 with anterolateral lobe, not overlapping with posterolateral lobe of carapace; pleonite 6 without prominent lateral projections. Maxilliped 2 with arthrobranch. Maxilliped 3 exopod absent or rudimentary. Epipods present on maxilliped 3 through P4. Minor cheliped merus with one to two small proximal teeth on lower margin. Male pleopod 2 with appendix interna, appendix masculina absent [adjusted from the work ofPoore and Ahyong (2023)].
Type species: Vulcanocalliax arutyunovi (Dworschak and Cunha, 2007).
Remarks: The family Callianopsidae contains two hitherto monotypic genera BathycalliaxSakai and Türkay, 1999 and VulcanocalliaxDworschak and Cunha, 2007, in addition to Callianopsis de Saint Laurent, 1973 that currently contains four recent and nine fossil species (DecaNet eds., 2024, accessed 1 August 2024). Callianopsis is easily distinguished from the others by the presence of a dorsal carina on the rostrum (versus carina absent in the other two genera) and pleomere 6 with prominent lateral projections (versus absent in the other two genera). Vulcanocalliax differs from Bathycalliax in the absence of cardiac sulci and the male pleopod is reduced, not flagellate as in Bathycalliax. The presence (Bathycalliax geomar) or absence (Vulcanocalliax arutyunovi) of an exopod of maxilliped 3 cannot be considered diagnostic at a generic level with the finding of a rudimentary exopod in the new species of Vulcanocalliax (see below). The presence of an anterolateral lobe of the first abdominal somite is present in both species of Vulcanocalliax and appears present in Bathycalliax geomar (Sakai and Türkay, 1999: fig. 1), but it remains to be confirmed across other callianopsids. Dworschak and Cunha (2007) proposed this character as unique to Vulcanocalliax and argued that it is not homologous to the anterolateral projection in other axiidean families.
Table 1 provides the comparative branchial formula for callianopsids. Shared diagnostic characters are provided by the epipods present on the maxillipeds and the first three pereiopods in Bathycalliax and Vulcanocalliax; epipods are only present on maxilliped 1 and rudimentary on maxilliped 2 in Callianopsis anovalisLin et al., 2007. The two Vulcanocalliax species, furthermore, share an arthrobranch on maxilliped 2, which is absent in both Bathycalliax and Callianopsis.
Table 1
| Maxillipeds | Pereopods | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 4 | 5 | |
| Callianopsis anovalis Lin et al., 2007 | ||||||||
| Arthrobranch | — | — | 2 | 2 | 2 | 2 | 2 | — |
| Podobranch | — | r | — | — | — | — | — | — |
| Epipods | 1 | r | — | — | — | — | — | — |
| Exopods | 1 | 1 | — | — | — | — | — | — |
| Bathycalliax geomar Sakai and Türkay, 1999 | ||||||||
| Arthrobranch | — | — | 2 | 2 | 2 | 2 | 2 | — |
| Podobranch | — | 1 | — | — | — | — | — | — |
| Epipods | 1 | 1 | 1 | 1 | 1 | 1 | r | — |
| Exopods | 1 | 1 | r | — | — | — | — | — |
| Vulcanocalliax arutyonovi Dworschak and Cunha, 2007 | ||||||||
| Arthrobranch | — | 1 | 2 | 2 | 2 | 2 | 2 | — |
| Podobranch | — | — | — | — | — | — | — | — |
| Epipods | 1 | 1 | 1 | 1 | 1 | 1 | r | — |
| Exopods | 1 | 1 | — | — | — | — | — | — |
| Vulcanocalliax beervana sp. nov. | ||||||||
| Arthrobranch | — | 1 | 2 | 2 | 2 | 2 | 2 | — |
| Podobranch | — | 1 | — | — | — | — | — | — |
| Epipods | 1 | 1 | 1 | 1 | 1 | 1 | r | — |
| Exopods | 1 | 1 | r | — | — | — | — | — |
Comparative branchial formula for select Callianopsidae: Callianopsis anovalisLin et al., 2007, Bathycalliax geomarSakai and Türkay, 1999, Vulcanocalliax arutyunovi Dworschak and Cunha, 2007, and Vulcanocalliax beervana sp. nov. (r, rudimentary).
Vulcanocalliax beervana sp. nov.
Figure 2
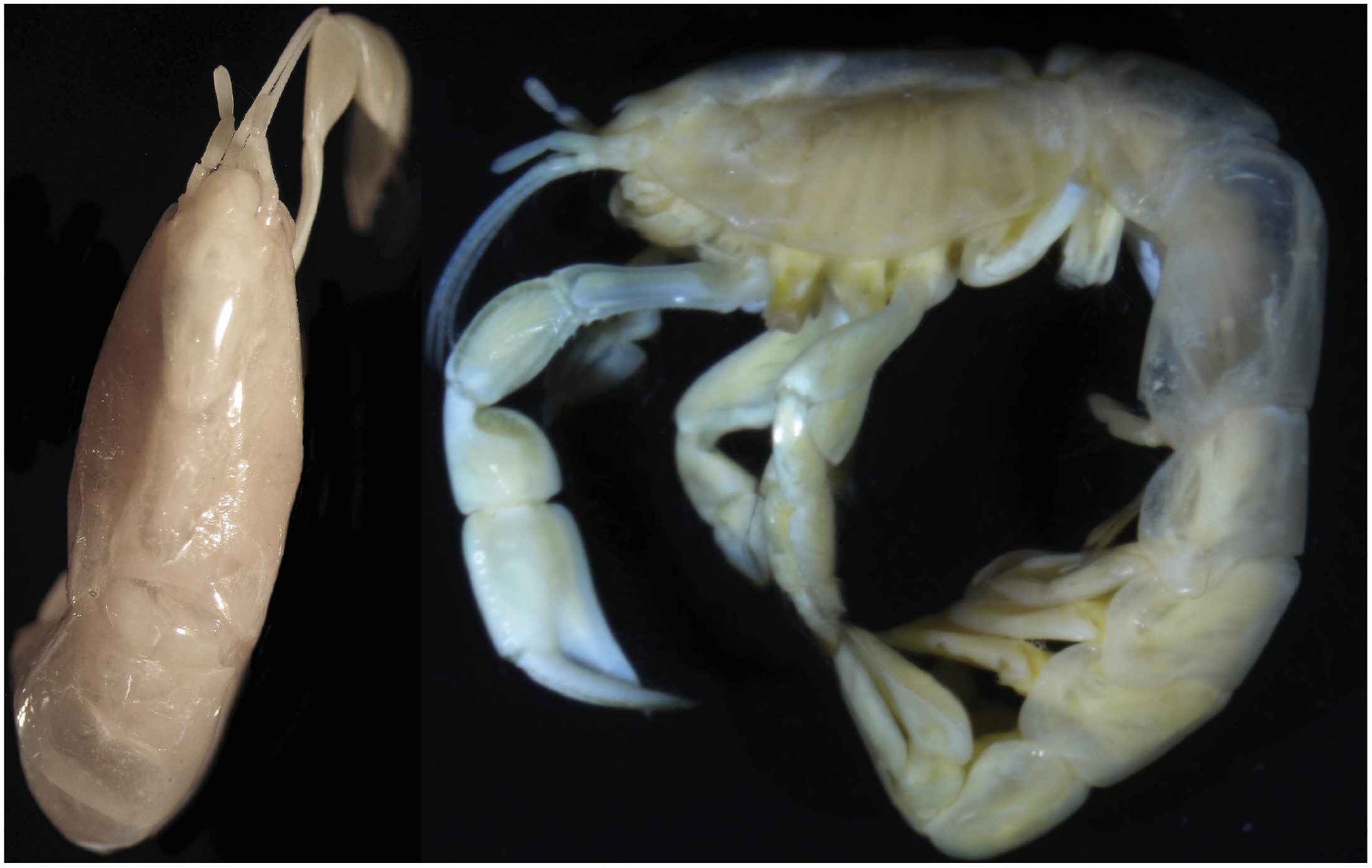
Vulcanocalliax beervana sp. nov.: Holotype female (NIWA 29413; cl., 9.5 mm); left, dorsal habitus; right, lateral habitus.
Figure 3

Vulcanocalliax beervana sp. nov.: Holotype female (NIWA 29413; cl., 9.5 mm); (A) carapace, abdominal somite 1, dorsal view with detail of rostrum and right eye; (B) abdominal somites 2–6, dorsal view; (C) telson, left urosome, dorsal view; (D) left uropodal endopod and exopod, lateral view; (E) anterior carapace, eyestalk, antennule, antenna, lateral view; (F) major (left) cheliped, lateral face; (G) major cheliped, mesial face; (H) minor (right) cheliped, lateral face; (I) pereopod 2; (J) pereopod 3; (K) pereopod 4; (L) pereopod 4 distal propodus and dactylus inside view; (M) pereopod 5; (N) pereopod 5 detail of distal propodus and dactylus, oblique inside view; (O) pleopod 1; (P) pleopod 2. Scale bars, 2 mm.
Figure 4

Vulcanocalliax beervana sp. nov.: Holotype female (NIWA 29413; cl., 9.5 mm); (B) first maxilla; (C) second maxilla; (E) second maxilliped with detail of proximal portion of endopod (en.) and exopod (ex.) and branchiae; (F) maxilliped 3. Paratype male (NIWA 69439; cl., 6. 0 mm); (A) mandible, excluding paragnaths; (D) first maxilliped; (G) first pleomere with paired reduced pleopods. Scale bars: (A, D) 2 mm and (B, C, E–G) 1 mm.
ZOOBANK LSID urn:lsid:zoobank.org:act:3F10DC90-F860-4BD9-A46B-B570735A2551
Type material: Holotype: female (9.5 mm), R/V Tangaroa Stn. TAN0616/13, Rock Garden, 40.0328 S, 178.1563 E, 650 m, van Veen Grab, 04 Nov 2006 (NIWA 29413).
Paratypes: 3 males (6.0, 5.8, and 5.3 mm), same as holotype (NIWA 69439).
1 juvenile (4.1 mm), R/V Tangaroa Stn. TAN0616/35, Builder’s Pencil, 39.5437 S, 178.3348 E, 808 m, Multicorer, 06 Nov 2006 (NIWA 29414).
Diagnosis: Carapace anterior margin convexly rounded on either side of rostrum, not elevated behind second antenna. Ocular peduncle distolateral margin convex. Uropodal exopod with dorsomedian ridge, anterior portion of exopod smooth, not elevated. Maxilliped 2 with podobranch. Maxilliped 3 with rudimentary exopod.
Etymology: Named after New Zealand’s annual beer festival Beervana, with our gratitude for the organizer Ryan McArthur who supported the 10th International Crustacean Congress in Wellington 2023. Used as noun in apposition.
Description of female holotype (additional specimens noted where aspects of holotype are missing): Dorsally, carapace slightly longer than abdominal somites 1 and 2 combined. Frontal margin of carapace with narrow triangular acute rostrum, postorbital margin smoothly descending (prominences absent); rostrum extending to 0.3 times the visible length of eyestalks in dorsal view, ventrally setose. Lateral projections of carapace setose dorsally. Carapace lacking distinct dorsal oval and dorsal carina. A single median pit dorsally halfway between rostrum and distinct cervical groove, one pair of smaller pits anterior to cervical groove. Indistinct cardiac prominence bearing median pit in posterior quarter of carapace. Transverse sutures absent. Linea thalassinica well-defined, parallel to midline of carapace. Lateral surface of carapace finely tuberculate, ventral margin with short setae.
Eyestalks dorsally flattened and depressed, slightly convex ventrally, keeled laterally, in dorsal view reaching beyond basal antennal article; mesial surfaces flattened so eyestalks abut closely at midline over entire length; distolateral margin convexly rounded, not pigmented.
Antennular peduncle shorter than antennal peduncle, barely reaching base of antennal article 4; basal article with long setae dorsally near distal end; second article 0.65 times length of basal article with few dorsal setae near distal end; third article about 1.9 times length of second, with ventrolateral row of long, ventrally directed setae continued onto ventral ramus of flagellum; rami of flagellum about equal length; dorsal ramus with few short setae, subterminal articles of dorsal ramus thicker than those of ventral rami, distal 4 rami bearing dense line of ventral aesthetascs.
Antennal peduncle 1.8 times length of antennular peduncle; basal article with ventrolaterally produced excretory pore; second article with deep, diagonal ventrolateral furrow, distally with field of long setae below ventrolateral suture; small, rounded, articulated dorsal scale at joint proximal to third article; third article elongate, same length as fourth or combined length of first two; fourth article narrower than third; flagellum missing (nearly twice length of cl. in paratypes NIWA 69439).
Mandible with large, terminally setose, 3-segmented palp, third article of palp terminally rounded; incisor process thin, regular row of small teeth on cutting margin, mesial surface with lip giving rise to molar process proximal to incisor process.
First maxilla with narrow endopodal palp, terminal article deflected proximally at articulation; proximal endite setose on slightly concave margin, terminally with field of thick simple setae; distal endite elongate, terminally truncate and armed with stiff simple setae.
Second maxilla with endopod narrowed distally, basal and coxal endites each longitudinally subdivided; exopod forming large, broad scaphognathite, lacking very long setae on posterior lobe.
First maxilliped with endopod reduced; proximal endite triangular; distal endite elongate, lateral surface and all margins heavily setose, mesial surface concave; exopod without transverse suture; distal part broadened, with long marginal setation at its mesial end, proximal part with field of mesially directed setae near mesial end; epipod large, broad, anterior end tapered, angular.
Second maxilliped with long endopod; merus straight, mesial surface concave, slightly thicker in proximal half than in distal, flexor margin with dense fringe of long, close-set setae; carpus short; propodus weakly arcuate, length 1.6 times width, about one-third length of merus; dactylus short, less than half-length of propodus, densely setose; exopod as long as endopodal merus and carpus combined, fringed marginally by long setae; epipod small, leaf-shaped; podobranch long, lancet-shaped; single arthrobranch small.
Third maxilliped with rudimentary exopod; endopod robust, with long dense setation on mesial margin; endopodal ischium subrectangular, 1.5 times as long as broad, proximomesial lip rounded, mesial surface with medial longitudinally oriented elevation bearing well-defined curved row of 18 sharp teeth; merus oval, slightly shorter than wide, mesial surface with setose elevation proximally and distally; carpus subtriangular, with setose lobe on flexor margin; propodus large, subrectangular, about as long as broad; dactylus 1.3 times as long as broad, ovate distally, fringed with very dense field of close-set, stiff, serrated setae.
Branchial formula (Table 1) includes exopods, epipods, and a podobranch as described for the first, second, and third maxillipeds above; branchiae limited to single arthrobranch on second maxilliped, pair of arthrobranchs on third maxilliped, and pair of arthrobranchs on each of the first through fourth pereopods. Epipods are present on all maxillipeds and pereopods 1–3.
Chelipeds strongly calcified, unequal. Left (major) cheliped larger; coxa with strong, posteriorly curved spine posteriomesially, simple epipod laterally; ischium slender, nearly as long as merus, extensor margin proximally concave, flexor margin with row of small spines, distalmost largest, length about 2.5 times distal breadth; merus stout, length about 1.6 times breadth at midlength, extensor margin curved, flexor margin with row of spines along proximal three-fourth; carpus broad, broadest distally, flexor margin arcuate and keeled, ventrodistally round, with small granule at corner, dorsal margin straight; propodus heavy, length (including fixed finger) about 1.6 times width, mesial surface of palm smooth; dorsal and ventral propodal margins slightly curved, tufts of setae on inner face above ventral margin, absent below dorsal margin; fixed finger thick, prehensile margin armed with low tooth at about mid-length, proximally with denticles, terminating in rounded tip; excavated below the prehensile margin on mesial face; dactylus heavy, curved, line of several setose punctae on mesial side of dorsal margin, cutting edge sinusoidal, smooth, tip strongly curved.
Minor cheliped (right); coxa with strong, posteriorly curved spine posteriomesially, simple epipod laterally; ischium slender, extensor margin slightly concave, flexor margin with spines increasing in size distally, length about 2.5 times distal breadth; merus stout, length about 1.8 times breadth at midlength, extensor and flexor margins evenly convex, flexor margin with two spines proximally; carpus broad, broadest distally, flexor margin arcuate and keeled, distoventrally rounded, extensor margin straight; propodus less heavy than major cheliped, length (including fixed finger) about 1.9 times width, mesial surface of palm smooth bar a few setose punctae; dorsal propodal margin straight, ventral margin slightly curved, tufts of setae on outer face below dorsal margin and above ventral margin; fixed finger narrows to slender tip, directed straight forward, prehensile margin with few tiny corneous teeth proximally, gently concave; dactylus narrow, longer than fixed finger and distinctly crossing fixed finger, nearly straight, line of several setose punctae on dorsal margin, cutting edge with few tiny corneous teeth.
Second pereopod chelate, most of flexor margins of ischium and merus lined with evenly spaced long setae, similar setae restricted primarily to distal patches on flexor margin in carpus, flexor margin of propodus densely setose, long proximally, progressively more reduced in length and stiffened distally; prehensile margins of both fingers corneous, finely microserrate along straight edge over most of length; dorsal margin of dactylus straight, with patches of stiff, arched setae becoming increasingly reduced in length.
Third pereopod ischium short, one-quarter length of merus; merus length about three times breadth, with several tufts of setae; carpus subtriangular, forming distal lobe on flexor margin, distal width about 0.7 times mid-length, flexor margin terminally with field of long arched setae; propodus extensor margin convex, flexor margin sub-rectangular in shape, 0.75 times as broad as long, lateral surfaces with fields of setae, long setae on margins; dactylus tear-shaped, length about 1.8 times width, terminating in narrow, straight tip, setose on margins, lateral face crossed by fields of short setae.
Fourth pereopod semichelate, ventrodistal corner of propodus expanded, with spiniform setae along tip of finger, single strong serrate spiniform seta at base; dense fields of setae on lateral surface of both propodus and drop-shaped dactylus proximally field of thick, serrate setae near articulation with dactylus.
Fifth pereopod chelate, opposable surfaces of propodus and minute dactylus excavate, terminally rounded with marginal spiniform setae; dactylus forming beak-like chela, with distodorsal spine, obscured by dense fields of simple and serrate setae on distal half of propodus and surface of dactylus.
Abdomen long, unarmed; dorsal length ratio (along midline) of first to sixth abdominal somites: 1.0: 1.29: 0.79: 0.84: 0.89: 0.76. First somite slightly narrowed anteriorly, pleuron not expanded laterally or ventrally, rounded. Second somite with straight anterior margin, posterior margin expanded posterolaterally, ventral and posterior margin bearing sparse setae. Third to fifth somites each distinctly shorter than second somite, posterior margins expanded posterolaterally; pleura each with pairs of crescent-shaped setal tufts midlaterally, and simple setae on posteroventral margin. Sixth somite trapezoid in dorsal view, narrowed posteriorly, ventral margin of pleonite with short setae, posterior margin with tuft of long setae on each side.
Telson about as long as broad, broadest proximally, narrowing distally, posterolateral margin rounded, with few short simple setae, dorsally smooth and convex, with few simple long setae.
Uropod with endopod oval, 1.4 times as long as broad, slightly overreaching telson, posterolateral and posterior margins with few simple setae, dorsal surface convex and smooth, without longitudinal carina; exopod oval, about 1.7 times as long as broad, anterior portion smooth, not elevated, longitudinal carina developed dorsally, lateral margin with fringe of short, spiniform setae and simple setae, posterodistal margin of exopod with few simple setae.
First female pleopod uniramous, composed of two articles, total length less than half (0.37) that of second pleopod; proximal article half as long as distal article, straight; terminal article straight.
Second female pleopod biramous, with appendix interna; dense setation largely restricted to distal lobe of basipod, lateral margin of exopod, and mesial margin of endopod; bearing small appendix interna with cincinnuli.
Third to fifth pleopod pairs forming large, posteriorly cupped fans when cross-linked by hooked setae of appendices internae on opposed margins of endopods; endopod of each triangular. Appendices internae stubby, movably articulated to mesial margin of endopod.
Remarks on male and juvenile form: The remaining material is comprised of three small males (NIWA 69439) that may not be fully developed yet. The largest specimen has gonopores on coxa of the fifth pereopod, indistinct in the smaller specimens, in all specimens the first pleopods are vestigial and simple (Figure 4G). The smallest specimen (NIWA 29414) is considered a juvenile as it does not have any discernible gonopores or pleopods 1 developed. The body and appendages of these specimens are generally similar to that of the female holotype with the minor exceptions as follows: antennal article three is slightly shorter than (not same length as) the fourth article (largest male; cl., 6.90 mm; NIWA 69439); there are both a right and a left detached major cheliped retained in the sample of three males (NIWA 69439); and cheliped merus is slightly longer at 1.70–1.75 length-width (compared to 1.6), as is the propodus (1.66–1.70 for males and 1.6 for holotype female). Proportions of abdominal segments differ slightly with ranges of segments 1 to 6 presented as 1: 1.26–1.54: 0.67–0.92: 0.59–0.75: 0.74–0.92: 0.8–1.0.
Color: Not documented but probably pale in life color as typical for the family.
Distribution: Known only from the Hikurangi Margin, east of the North Island of Aotearoa New Zealand, 650–808 m (Figure 1).
Remarks: Vulcanocalliax beervana sp. nov. is morphologically and genetically similar to V. arutyunovi, but some distinct morphological differences are apparent: the anterior carapace margin without the rostrum is distinctly convex in V. beervana, lacking the elevated postantennal shoulder present in V. arutyunovi; the anterolateral shape of the eye stalk is convexly rounded in V. beervana but acutely angled with a straight margin in V. arutyunovi; the second maxilliped has a long podobranch in V. beervana, which is absent in V. arutyunovi; the third maxilliped is furnished with a rudimentary exopod in V. beervana, which is absent in V. arutyunovi; the shape of the chelipeds differ, e.g., the flexor margin of the ischium is concave in V. beervana but straight in V. arutyunovi; the ventrodistal corner of the carpus is rounded in V. beervana but subacute in V. arutyunovi; the cutting edge of the fixed finger of both the major and minor chelipeds are lacking prominent triangular teeth in V. beervana, which are present in V. arutyunovi; and the dorsal surface of the uropodal exopod differ with only a simple longitudinal ridge near the midline in V. beervana, whereas V. arutyunovi has an exopod with a proximal elevation and no median ridge.
In the latter shape of the telson, uropods, and chelipeds and the presence of a rudimentary epipod on maxilliped 3, V. beervana is more similar to Bathycalliax geomar from the eastern Pacific, but the new species lacks cardiac sulci of the carapace (present in B. geomar) and has an arthrobranch on maxilliped 2 (absent in B. geomar) and the eyestalks are anterolaterally convex (subtriangular in B. geomar). The first male pleopod is uniquely flagellate in B. geomar, whereas, in both V. arutyunovi and V. beervana, the first pleopods are vestigial; however, only small males are available for both species of Vulcanocalliax and the adult form of the first male pleopod might yet be unknown.
Vulcanocalliax beervana sp. nov. was collected during a 2006 voyage that explored hydrocarbon seeps along the Hikurangi Margin off New Zealand’s east coast. Neither station where this new ghost shrimp was collected (TAN0616/13 and 35) were in the immediate vicinity of observed chemoautotrophic fauna or seepage flares detected in echograms (Greinert et al., 2010: Baco et al., 2010). Organisms collected in the same grab sample (TAN0616/13) and deposited in the NIWA Invertebrate Collection and database included non-chemoautotrophic fauna such as corals (stylasterid, primnoid, and a small solitary hard coral); therefore, V. beervana does not appear to be associated with the chemoherms and their local biological communities dominated by Calyptogena clams. Whereas diffuse seepage occurs along many areas along the Hikurangi Margin, neither of the grabs or multicorers nearer the known seeps sampled any further ghost shrimps. Considering that both relatives V. arutyunovi and Bathycalliax geomar were collected in the vicinity of seeps (Gulf of Cádiz at around 1330 m and off Oregon at 627 m, respectively), it appears that this group of species are at least tolerant of the environmental conditions present around areas of hydrocarbon seeps.
The single holotype of the kentrogonid rhizocephalan Parthenopea australis described by Lützen et al. (2009) remains the only specimen for this species. It was found as a parasite of one specimen of Vulcanocalliax sp. nov. collected at the same station (TAN0616/13) as the female holotype of V. beervana. Unfortunately, the host specimen cannot be located, but it almost certainly belongs to the same species described here.
Molecular taxonomy
The multi-gene analysis presented for 123 species of Callianassidae and related families by Robles et al. (2020) included sequences for the holotype of Vulcanocalliax arutyunovi (NHMW 21927) and resolved the two representatives of the family Callianopsidae (V. arutyunovi and Callianopsis goniophthalma) as a basal family of the remaining “callianassoid” families with high nodal support.
Only a 16S sequence could be generated for the new species V. beervana for comparative purposes (kindly provided by Qi Kou, Chinese Academy of Sciences, GenBank accession number PQ137440, with a partial 328-bp fragment generated for NIWA 69439 (paratype) aligning with GenBank sequence EU874919 for V. arutyunovi, with 80.55% sequence identity (Table 2). Out of 328 bp, 64 bp differ, including five insertions for V. beervana sp. nov. Sequences for the nearest proposed relative Bathycalliax geomar are so far not available; the only other callianopsid voucher available is provided by Callianopsis goniophthamla [MN237708, deposited by Robles et al. (2020)] that aligns with a sequence similarity of 76.596% and 75.445% for V. arutyunovi and V. beervana sp. nov., respectively. All other sequences for a range of Ctenochelidae, Eucalliacidae, and Callianassidae representatives included in the Robles et al. (2020) phylogeny resolve with higher sequence divergences (Table 2).
Table 2
| Family | Taxon | PQ137440 | EU874919 | MN237708 | MN237676 | MN237850 | MN237662 | MN237848 | MN237763 | EU882926 | EU874925 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Callianopsidae | Vulcanocalliax beervana sp. nov. (PQ137440) | — | |||||||||
| Callianopsidae | Vulcanocalliax arutyunovi (EU874919) | 80.547 | — | ||||||||
| Callianopsidae | Callianopsis goniophthalma (MN237708) | 75.455 | 76.596 | — | |||||||
| Ctenochelidae | Ctenocheloides almeidai (MN237676) | 70.000 | 71.818 | 71.733 | — | ||||||
| Ctenochelidae | Gourretia sp. ULLZ 9707 (MN237850) | 70.303 | 71.818 | 74.772 | 77.204 | — | |||||
| Ctenochelidae | Ctenocheles balssi (MN237662) | 74.503 | 75.497 | 76.744 | 76.412 | 85.284 | — | ||||
| Ctenochelidae | Dawsonius aff. latispina (MN237848) | 72.424 | 73.333 | 76.292 | 78.116 | 79.205 | 79.933 | — | |||
| Eucalliacidae | Calliaxina novaebritanniae (MN237763) | 72.205 | 73.414 | 68.182 | 74.164 | 72.340 | 73.667 | 73.556 | — | ||
| Eucalliacidae | Eucalliaxiopsis jonesi (EU882926) | 70.181 | 72.892 | 74.320 | 75.152 | 72.205 | 74.917 | 75.529 | 73.414 | — | |
| Callianassidae | Callianassa aqabaensis (EU874925) | 68.882 | 67.976 | 67.879 | 70.303 | 71.212 | 71.761 | 73.636 | 69.091 | 71.084 | — |
Pairwise percentage genetic distances for aligned partial 16S rRNA gene (328 bp) between Vulcanocalliax beervana sp. nov. and published GenBank sequences for a selection of families and species previously presented by Robles et al. (2009, 2020).
Discussion
Vulcanocalliax beervana sp. nov. is the first and only species of the family Callianopsidae recorded in New Zealand, the wider southwestern Pacific region and the southern hemisphere. Other callianopsid species are known from the northeastern Atlantic (V. arutyunovi and Callianopsis mauritana), the Bay of Bengal in the Indian Ocean (C. coecigena), the eastern Pacific from Alaska to Mexico (C. goniophthalma and Bathycalliax geomar), and Taiwan in the northwestern Pacific (C. anovalis) (Sakai, 2011). A key to the currently known callianopsid species is provided below.
Webber et al. (2010) provided a first summary of the New Zealand axiidean ghost and sponge shrimp fauna, which have since expanded to 14 species with descriptions of a callianassid by Schnabel et al. (2023b) and a challichirid by Poore et al. (2022). Current efforts continue to, for the first time, comprehensively inventory the Zealand Axiidea, with a number remaining to be formally described (Schnabel et al., 2023a).
Key to Callianopsidae Manning and Felder, 1991
1. Pleomere 6 with prominent lateral projections; carapace with longitudinal carina running from rostrum; epipods absent on pereopods Callianopsis (four species) (2).
- Pleomere 6 without prominent lateral projections; rostrum dorsally smooth, without longitudinal carina; epipods present on maxilliped 3 to pereopod 4 (5).
2. Lateral spines or spine-like projections on abdominal pleura 2–6 Callianopsis caecigena (Alcock and Anderson, 1894).
- Abdominal pleura 2–4 laterally and ventrally unarmed, distoventral or lateral projections present on pleura 5 and/or 6 (3).
3. Abdominal pleuron 5 unarmed, ventral projection only on the sixth pleuron Callianopsis mauritana (Sakai et al., 2015).
- Abdominal pleura 5 and 6 with acute ventral projections (4).
4. Dorsal oval on carapace absent; abdominal tergite 1 distinctly notched anteriorly; maxilliped 2 exopod simple, not flagellate Callianopsis anovalisLin et al., 2007.
- Dorsal oval on carapace present; abdominal tergite 1 straight anteriorly; maxilliped 2 exopod distally flagellate Callianopsis goniophthalma (Rathbun, 1902).
5. Two cardiac sulci present; minor cheliped merus with more than two proximal spines on flexor margin; maxilliped 2 without arthrobranch; male pleopod 1 distally flagellate Bathycalliax geomar (monotypic genus).
- Cardiac sulci absent; minor cheliped merus with two proximal spines on flexor margin; maxilliped 2 with arthrobranch; male pleopod 1 vestigial Vulcanocalliax (two species) (6).
6. Carapace anterior margin concave lateral to rostrum, produced behind second antenna; ocular peduncle anterolateral margin subtriangular, straight; uropodal exopod thickened anterodorsally (strongly curved, descending to distal margin) Vulcanocalliax arutyunoviDworschak and Cunha, 2007.
- Carapace anterior margin convexly rounded on either side of rostrum, round behind second antenna; ocular peduncle anterolateral margin convex; uropodal exopod with dorsomedian ridge, anterior portion of exopod smooth, not elevated Vulcanocalliax beervava sp. nov.
Statements
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: NCBI GenBank, accession number PQ137440 (https://www.ncbi.nlm.nih.gov/nuccore/PQ137440.1/). Vulcanocalliax beervana Schnabel & Peart, 2024 is registered with ZooBank under (https://zoobank.org/NomenclaturalActs/3f10dc90-f860-4bd9-a46b-b570735a2551).
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because we used preserved animals deposited in a natural history collection.
Author contributions
KS: Writing – original draft. RP: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. KS and RP were funded by NIWA under Coasts and Oceans Research Programme 2 Marine Biological Resources: discovery and definition of the marine biota of New Zealand (2023–2024 SCI).
Acknowledgments
We are grateful to the staff of the NIWA Invertebrate Collection for their tireless work. We thank Qi Kou (Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China) for sequence data for the new species and Gary Poore (Museums Victoria, Melbourne, Australia) for guidance on comparative morphology of callianassoid shrimp. Specimens were collected by the voyage TAN0616 – RENEWZ I/NEW ZEEPS, the first component of the project ‘Exploration of Chemosynthetic Habitats of the New Zealand Region, funded by NOAA Ocean Exploration and NIWA, with co-funding from Woods Hole Oceanographic Institution, Scripps Oceanographic Institution, and the University of Hawai’i. We thank the reviewers for their time and helpful suggestions
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Alcock A. Anderson A. R. S. (1894). Natural history notes from H.M. Royal Indian Marine Survey Steamer “Investigator”, commander C.F. Oldham, R.N., commanding. - Series II, No. 14. An account of a recent collection of deep-sea Crustacea from the Bay of Bengal and Laccadive Sea. J. Asiatic Soc. Bengal (2) (Natural History)63, 141–185.
2
Baco A. R. Rowden A. A. Levin L. A. Smith C. R. Bowden D. A. (2010). Initial characterization of cold seep faunal communities on the New Zealand Hikurangi margin. Mar. Geol.272, 251–259. doi: 10.1016/j.margeo.2009.06.015
3
Bankevich A. Nurk S. Antipov D. Gurevich A. A. Dvorkin M. Kulikov A. S. et al . (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol.19, 455–477. doi: 10.1089/cmb.2012.0021
4
Bolger A. M. Lohse M. Usadel B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics30, 2114–2120. doi: 10.1093/bioinformatics/btu170
5
Camacho C. Coulouris G. Avagyan V. Ma N. Papadopoulos J. Bealer K. et al . (2009). BLAST+: architecture and applications. BMC Bioinf.10, 421. doi: 10.1186/1471-2105-10-421
6
DecaNet eds (2024). DecaNet. Accessed through: World Register of Marine Species. Available online at: https://www.decanet.info/aphia.php?p=taxdetails&id=1214590 (Accessed August 1, 2024).
7
de Saint Laurent M. (1973). Sur la systématique et la phylogénie des Thalassinidea: définition des familles des Callianassidae et des Upogebiidae et diagnose de cinq genres nouveaux. Comptes Rendus Hebdomadaires Séances l’Académie Des. Sci. Paris277, 513–516.
8
Dworschak P. C. Cunha M. R. (2007). A new subfamily, Vulcanocalliacinae n.subfam., for Vulcanocalliax arutyunovi n.gen,. n.sp. from a mud volcano in the Gulf of Cadiz (Crustacea, Decapoda, Callianassidae). Zootaxa1460, 35–46. doi: 10.11646/zootaxa.1460.1
9
Greinert J. Lewis K. B. Bialas J. Pecher I. A. Rowden A. Bowden D. A. et al . (2010). Methane seepage along the Hikurangi Margin, New Zealand: Overview of studies in 2006 and 2007 and new evidence from visual, bathymetric and hydroacoustic investigations. Mar. Geol.272, 6–25. doi: 10.1016/j.margeo.2010.01.017
10
Kearse M. Moir R. Wilson A. Stones-Havas S. Cheung M. Sturrock S. et al . (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics28, 1647–1649. doi: 10.1093/bioinformatics/bts199
11
Lin F. J. Komai T. Chan T. Y. (2007). First record of the thalassinidean genus Callianopsis de Saint Laurent 1973 (Decapoda, Ctenocheildae) in the West Pacific, with the description of a new species from Taiwan. Crustaceana80, 1193–1203. Available at: http://www.jstor.org/stable/20107910.
12
Lörz A.-N. Glenner H. Lützen J. (2008). New records of Rhizocephala (Cirripedia) from New Zealand, including the first rhizocephalan records from hot vents and cold seeps. Crustaceana81, 1013–1019. doi: 10.1163/156854008X354911
13
Lützen J. Glenner H. A-Lörz N. (2009). Parasitic barnacles (Cirripedia: Rhizocephala) from New Zealand off-shore waters. New Z. J. Mar. Freshw. Res.43, 613–621. doi: 10.1080/00288330909510027
14
Manning R. B. Felder D. L. (1991). Revision of the American callianassidae (Crustacea: decapoda: thalassinidea. Proc. Biol. Soc. Washington104, 764–792.
15
Poore G. C. B. Ahyong S. T. (2023). Marine Decapod Crustacea - A Guide to Families and Genera of the World (Clayton South, Australia: CSIRO Publishing).
16
Poore G. C. Dworschak P. C. Robles R. Mantelatto F. L. Felder D. L. (2019). A new classification of Callianassidae and related families (Crustacea: Decapoda: Axiidea) derived from a molecular phylogeny with morphological support. Memoirs Museum Victoria78, 73–146. doi: 10.24199/j.mmv.2019.78.05
17
Poore G. C. B. Dworschak P. C. Schnabel K. (2022). Articullichirus, a new genus of ghost shrimp (Crustacea: Axiidea: Callichiridae) with two new species. Memoirs Museum Victoria81, 123–133. doi: 10.24199/j.mmv.2022.81.05
18
Rathbun M. J. (1902). Descriptions of new decapod crustaceans from the west coast of North America. Proc. United States Natl. Museum24, 885–905. doi: 10.5479/si.00963801.1272.885
19
Robles R. Dworschak P. C. Felder D. L. Poore G. C. B. Mantelatto F. L. (2020). A molecular phylogeny of Callianassidae and related families (Crustacea: Decapoda: Axiidea) with morphological support. Invertebrate Systematics34, 113–132. doi: 10.1071/IS19021
20
Robles R. Tudge C. C. Dworschak P. C. Poore G. Felder D. (2009). Molecular phylogeny of the Thalassinidea based on nuclear and mitochondrial genes. In: MartinJ. W.CrandallK. A.FelderD. L. (Eds). Crustacean IssuesDecapod crustacean phylogenetics. Boca Raton, FL, USA: CRC Press. 18, 309–326. doi: 10.1201/9781420092592-c15
21
Rose R. Golosova O. Sukhomlinov D. Tiunov A. Prosperi M. (2019). Flexible design of multiple metagenomics classification pipelines with UGENE. Bioinformatics35, 1963–1965. doi: 10.1093/bioinformatics/bty901
22
Sakai K. (2011). Axioidea of the world and a reconsideration of the Callianassoidea (Decapoda, Thalassinidea, Callianassida). Crustaceana Monogr.13, 1–616. doi: 10.1163/9789047424185
23
Sakai K. Türkay M. (1999). A new subfamily, Bathycalliacinae n. subfam., for Bathycalliax geomar n. gen., n. sp. from deep water cold seeps off Oregon, USA. Senckenbergiana biologica79, 203–209.
24
Sakai K. Türkay M. Beuck L. Freiwald A. (2015). A collection of the Infraorder Callianassidea (Decapoda, Pleocyemata) with one new genus and five new species from the Eastern Atlantic off Mauritania (R/V Maria S. Merian cruise MSM 16/3 “PHAETON”). Mar. Biodiversity45, 113–133. doi: 10.1007/s12526-014-0227-2
25
Schnabel K. E. Peart R. A. Bradford-Grieve J. Eagar S. Hosie A. Buckeridge J. (2023a). “Kingdom Animalia, Phylum Arthropoda, Subphylum Crustacea,” in The Marine Biota of New Zealand: Updating the New Zealand Inventory of Biodiversity to 2020. Eds. KellyM.MillsS.NelsonW.TerezowM. (NIWA Biodiversity Memoir, Wellington), 412–445.
26
Schnabel K. Rowden A. A. Poore G. C. B. (2023b). A new species of Arenallianassa (Decapoda: Axiidea: Callianassidae) from hydrothermal vents with notes on its ecology and a redescription of Arenallianassa arenosa (Poore 1975). Memoirs Museum Victoria82, 55–69. doi: 10.24199/j.mmv.2023.82.03
27
Webber W. R. Fenwick G. D. Bradford-Grieve J. M. Eagar S. H. Buckeridge J. S. Poore G. C. B. et al . (2010). “Subphylum Crustacea - shrimps, crabs, lobsters, barnacles, slaters, and kin,” in New Zealand Inventory of Biodiversity Volume 2. Chaetognatha, Ecdysozoa, Ichnofossils. Ed. GordonD. P. (Canterbury University Press, Christchurch), 98–232.
Summary
Keywords
ghost shrimp, integrative taxonomy, Crustacea, 16S rRNA, southwestern Pacific
Citation
Schnabel KE and Peart RA (2024) First record of the family Callianopsidae (Decapoda: Axiidea) and a new species of Vulcanocalliax from the Hikurangi Margin off Aotearoa New Zealand, with a key to species of Callianopsidae. Front. Mar. Sci. 11:1412024. doi: 10.3389/fmars.2024.1412024
Received
04 April 2024
Accepted
05 August 2024
Published
10 September 2024
Volume
11 - 2024
Edited by
Les Watling, University of Hawaii at Manoa, United States
Reviewed by
William Santana, Regional University of Cariri, Brazil
Ajit Kumar Mohanty, Indira Gandhi Centre for Atomic Research (IGCAR), India
Updates
Copyright
© 2024 Schnabel and Peart.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kareen E. Schnabel, Kareen.Schnabel@niwa.co.nz
†ORCID: Kareen E. Schnabel, orcid.org/0000-0002-9965-9010; Rachael A. Peart, orcid.org/0000-0001-5968-0811
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.