- 1Safety and Emergency Department, Hong Kong–Zhuhai–Macao Bridge Authority, Zhuhai, China
- 2South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou, China
The location of offshore and coastal marine engineering projects often shows considerable overlap with the foraging and breeding grounds of marine mammals. Lingding Bay, located in the Chinese Pearl River Delta, is home to the world’s largest known population of Indo-Pacific humpback dolphins (Sousa chinensis). The bay is also the site of the Hong Kong–Zhuhai–Macao Bridge mega-engineering project. This study assessed the responses of the dolphins to the bridge construction. Data were collected on dolphin sightings by survey vessels following standard line-transect tracks, with surveys conducted during the pre-construction (2005–2006), construction (2015–2016), and post-construction (2020–2021) phases. The dolphin distribution patterns, density, group sizes, and presence of calves were compared across these three periods. Additionally, the range patterns of the dolphins were analyzed following the identification of individuals during the post-construction phase. The average distance at which humpback dolphins were sighted from the bridge was significantly shorter after the completion of the bridge than during the pre-construction and construction phases. Furthermore, the density of humpback dolphins in the southern region of the bay—where the bridge is located—was significantly higher post-construction compared with that recorded during the pre-construction and construction phases. A noticeable increase in dolphin group sizes post-construction may indicate a shift in foraging strategy. The post-construction phase coincided with the COVID-19 pandemic lockdown period, and the resultant reduced human activity in Lingding Bay may have influenced the distribution of dolphins and other animals to some extent. Individual identification results demonstrated that the waters near the bridge remained an integral habitat for the dolphins post-construction, as they freely traversed underneath the bridge. The results of this study hold considerable importance within the realm of marine engineering, offering valuable guidance and references for informed decision-making and operational practices in associated domains.
1 Introduction
With the rapid development and transformation of coastal areas, over 40% of the world’s population currently resides in these regions, and an increasing number of people are expected to migrate there in the future (Small and Nicholls, 2003; Lotze et al., 2006). This spatial pattern of human settlement, coupled with an increasing demand for resources and trade, has exerted tremendous pressure on coastal ecosystems and biota (Lotze et al., 2006). Coastal development involves the construction of bridges, waterways, docks, wind farms, and other structures, and the locations of such projects often overlap with the distribution ranges of marine mammals. However, research on the responses of dolphins to large-scale ocean engineering projects is limited, which hinders the development of effective protective environmental policies (Wright et al., 2011; Dähne et al., 2013; Pirotta et al., 2013).
Early research on the responses of marine mammals to construction primarily focused on their short-term behavioral adjustments and shifts in distribution range. A comprehensive examination of the historical distribution patterns of bowhead whales (Balaena mysticetus) revealed a notable shift in their habitat usage following exposure to offshore construction, reflecting the avoidance of areas characterized by heavy industrial activity (Richardson et al., 1995; Schick and Urban, 2000). In a study on bottlenose dolphins (Tursiops truncatus) and harbor porpoises (Phocoena phocoena) in northeast Scotland, the bottlenose dolphins were shown to spend less time in the vicinity of construction works that involved either impact or vibration piling (Graham et al., 2017). Furthermore, the abundance of female Australian humpback dolphins (Sousa sahulensis) in the southern regions of the Great Barrier Reef decreased significantly in response to port construction activities and a major concurrent flood; however, at the completion of this development project, the abundance of females returned to reflect original numbers (Cagnazzi et al., 2020). Additionally, notable changes in the swimming speed of Indo-Pacific humpback dolphins (Sousa chinensis) have been observed during piling operations and in the presence of marine vessels in the waters off Hong Kong (Würsig et al., 2000; Piwetz et al., 2012). In the early to mid-1990s, the waters of northwest Hong Kong were key areas frequently visited by humpback dolphins (Jefferson, 2000); however, since then, dolphin sightings have significantly declined (Jefferson et al., 2023). A previous study on humpback dolphins in Lingding Bay (LDB), an estuary of the Pearl River Delta in southern China, also suggested that the dolphins may have altered their habitat selection in the last 20 years, adapting to coastline disturbances caused by human activities (Wang et al., 2022).
The Hong Kong–Zhuhai–Macao Bridge (HZMB) is a bridge–tunnel system connecting Hong Kong with the cities of Zhuhai and Macao on the Chinese mainland. Located in LDB, the HZMB is the longest bridge across the sea in the world. It has been hailed as a mega-engineering project and represents a world-class cross-sea passage. The eastern end of the bridge is located on an artificial island near Hong Kong International Airport, from where the bridge spans the LDB westward to an artificial island near the port of Zhuhai–Macao (Figure 1). Construction of the bridge began in December 2009, and the HZMB was officially opened for trial operations in October 2018. Of note, LDB is a key habitat of the Indo-Pacific humpback dolphin (Sousa chinensis), also known as the Chinese white dolphin and hereafter referred to as the humpback dolphin, an animal under state protection in China. Humpback dolphins’ health status, population dynamics, trophic relationships, and levels of pollutants within their bodies comprehensively reflect the ecological health and environmental changes of estuaries and their surrounding marine areas. As top predators highly sensitive to changes in water quality, food resources, and habitat conditions, monitoring these factors provides critical insights into ecosystem health, the presence of pollutants, the condition of the food web, and the impacts of human activities on the environment.
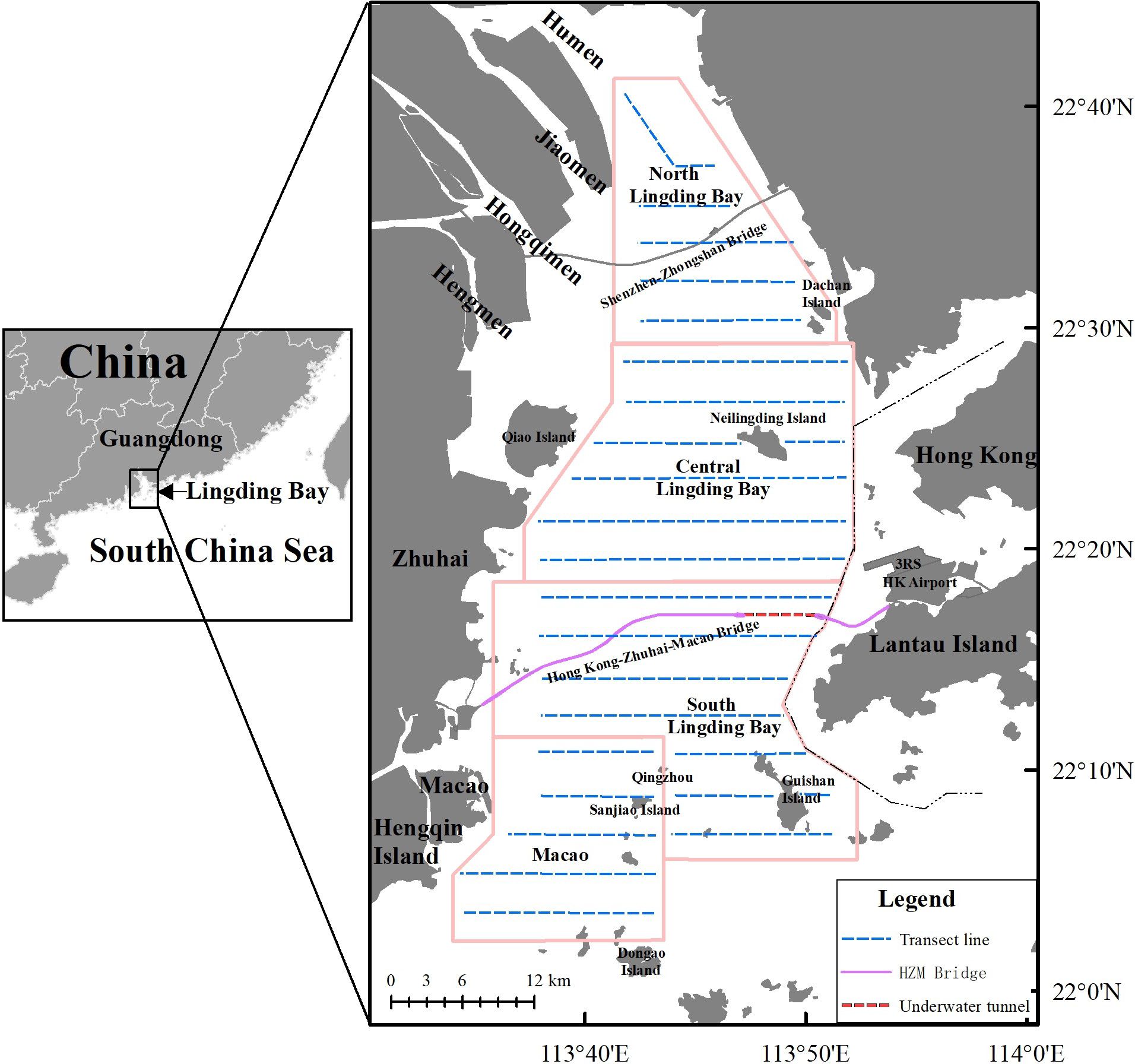
Figure 1. Map of the Lingding Bay study area in China, showing the locations of four line-transect survey regions—northern Lingding Bay, central Lingding Bay, southern Lingding Bay, and Macao. The pink-shaded area represents the boundary lines of the four survey regions.
The Indo-Pacific humpback dolphin—listed as Vulnerable in the IUCN Red List— is a small, toothed cetacean residing in coastal and estuarine habitats and is predominantly found in the coastal waters of the Eastern Indian Ocean and Western Pacific Ocean (Jefferson et al., 2017). Its distribution extends from the northern coast of China (with the northernmost sighting record located in the Yellow Sea) southward throughout Southeast Asia and westward to the border between Bangladesh and Myanmar (Jefferson and Rosenbaum, 2014; Jefferson and Smith, 2016; Jefferson et al., 2017; Lin et al., 2022). In China, humpback dolphins primarily inhabit the coastal regions of Xiamen, the western coasts and estuaries of Taiwan, the waters around Nanao Island in Shantou, the coast of the Pearl River Estuary up to the Moyang River, Leizhou Bay in Zhanjiang, the Beibu Gulf in Guangxi, and the waters around Sanya in Hainan (Chan, 2019; Chen et al., 2008; Chen et al., 2010; Chen et al., 2016; Jefferson, 2000; Li et al., 2019; Li et al., 2016; Peng et al., 2020; Wang et al., 2016a; Wang et al., 2016b; Xu et al., 2015). Among these regions, the largest known population is found between the Pearl River Estuary and the Moyang River (PRE-MR), boasting an estimated 2,600 individuals, which significantly surpasses the populations of the other regions (Jefferson, 2018; Chan, 2019; Li et al., 2019). Therefore, the population of this flagship species in PRE-MR serve as an ecological indicator for the Guangdong–Hong Kong–Macao Greater Bay Area, thereby playing a pivotal role in biodiversity conservation and ecosystem health assessments.
The construction of the HZMB has provided an opportunity to investigate the potential impact of marine construction on humpback dolphins in LDB. Due to the lack of research addressing the effect of bridge construction on humpback dolphins, wildlife conservation authorities still require insight into the potential adverse effects of such a mega-marine project on these animals. This study, inspired by the “opportunistic experiment” of Richardson et al., investigated the distribution of humpback dolphins before, during, and after construction of the HZMB (Richardson et al., 1985). Our research objectives encompassed assessments of the following three aspects: (1) the distribution and density of humpback dolphins in areas associated with the HZMB project, comparing these values with historical data; (2) group structure changes, such as calf presence or aggregations into large groups; and (3) whether humpback dolphins traversed underneath the completed bridge.
2 Methods
2.1 Study area
The study area covered most of the humpback dolphin distribution in the LDB and was bordered by Humen to the north, Dong’ao Island to the south, the boundary of Guangdong–Hong Kong waters to the east, and the coastlines of Zhuhai, Macao, and Hengqin Islands to the west. Previous surveys have shown that humpback dolphins are unevenly distributed in the LDB (Chen et al., 2010). Dividing the study area into four regions allows for a more accurate reflection of the humpback dolphins’ density and distribution characteristics in each region, thereby improving the precision of the survey results. Additionally, subdividing the area helps balance the sample size across regions, reducing sampling errors. Consistent survey efforts were maintained across these regions to ensure unbiased data collection and analysis. Our study area was subdivided into four regions: northern Lingding Bay (NLDB), central Lingding Bay (CLDB), southern Lingding Bay (SLDB), and Macao (MA). The exact geographic locations of all monitoring zones and observation transects are illustrated in Figure 1.
2.2 Vessel survey
A survey team used standard line-transect methods to conduct regular vessel surveys (Buckland et al., 2001). To ensure the continuity and comparability of observational data, these surveys comprised the same visual observation and operational protocols as previous dolphin surveys in the Pearl River Estuary (Jefferson, 2000; Chen et al., 2010).
The observation vessel was a shrimp trawler with an observation platform fitted to its foredeck, approximately 4-5 m above the waterline. Under suitable observational conditions (0-5 on the Beaufort scale, no heavy rain, and visibility ≥1200 m), the survey vessel traveled along predetermined transects at 7-8 knots. Data were recorded simultaneously by a team of two observers, consisting of a primary observer using marine compass binoculars (7 × 50; Bushnell, Overland Park, KS, USA) and a secondary observer conducting naked-eye observations. To alleviate fatigue, the primary and secondary observers exchanged roles every 30 min. Both observers underwent training in observation and recording methods, visual distance estimation (corrected using a laser rangefinder), and dolphin species identification, and both had experience in offshore cetacean surveys.
For each line-transect, the following data were recorded: the times at which transect observations began and ended; the geographic location of the transect; boat speed; boat heading; sea state; visibility; and total distance covered. The survey vessel approached dolphin groups closely to allow an estimation of the number of individuals, observation of group composition and behavior, and photography of individuals from different angles for identification purposes (using a high-speed SLR camera and telephoto lens). For each dolphin sighting, the following data were collected: initial sighting time, geographic location, sighting angle, distance of dolphins from the vessel (estimated visually by the observers), number of dolphins, group composition, and behavior. A handheld GPS (eXplorist; magellan, San Dimas, CA, USA) was used to determine geographic locations, survey vessel speed, and distance covered, whereas angles were measured using compass binoculars. Group composition was determined according to age classes, comprising unspotted calves (UC), unspotted juveniles (UJ), mottled (SJ), speckled (SS), spotted adults (SA), and unspotted adults (UA) (Jefferson, 2000; Jefferson et al., 2012).
2.3 Photo-identification
Humpback dolphins bear distinctive markings on their bodies, such as notches on the dorsal fin or scar tissue (Chan and Karczmarski, 2024). Occasionally, body coloration patterns can also serve as references that allow researchers to distinguish between individuals. When humpback dolphins were sighted during our line-transect surveys, the survey was paused, and the vessel slowly approached the dolphins from the side and rear to allow accurate photography. Since body coloration and markings are not necessarily symmetrical, efforts were made to capture images of both sides of each dolphin. After the photos had been sorted into a reference library enabling the identification of individuals, they were subsequently used to analyze the movement patterns of individual dolphins.
2.4 Data analysis
The survey data were categorized into three phases relating to the construction of the HZMB: (1) pre-construction (February 2005 to January 2006), (2) construction (August 2015 to August 2016), and (3) post-construction (June 2020 to April 2021). Although the dataset provides important information regarding dolphin activity during the different phases of the HZMB construction, the use of a one-year dataset for each phase presents limitations.
2.4.1 Distribution and population density
Geographic information system (GIS) analysis was employed to determine changes in dolphin distribution in the study area during the three phases of HZMB construction. The data were projected onto the UTM49N coordinate system to minimize distortions in distance and area measurements. A cost/distance grid was created to measure the distance between each grid (400 m2) and the bridge, allowing a calculation of the distance from each dolphin sighting location to the bridge (Buckstaff et al., 2013). The non-parametric Kruskal–Wallis test was used to test our null hypothesis that the distances from dolphin sighting locations to the HZMB did not differ significantly across the three construction phases.
To assess changes in the density of humpback dolphins in the study area across our three time periods, a distance sampling method was used to evaluate dolphin density in various regions (Buckland et al., 2001). For subsequent data analysis, we used DISTANCE software (Version 6.0, Release 2) to estimate dolphin density and related statistical parameters (Thomas et al., 2009). The formulae used for calculating density and the coefficient of variation were as follows:
where D is the density of the dolphins, n is the number of on-effort sightings (sample size), f(0) is the trackline probability density at zero distance, E(s) is an unbiased estimate of average group size, L is the length of the transect line, g(0) is the trackline detection probability, CV is the coefficient of variation, and var is variance.
2.4.2 large group dolphin distribution and calf occurrence analyses
A frequency distribution of different activities and behaviors can identify whether any LDB regions are associated with distinct behaviors in the humpback dolphins. In terms of group size, larger dolphin groups are closely linked to the availability of food resources and level of human interference: locations containing larger groups are typically indicative of richer food resources and lower levels of human activity (Hung, 2008; Liu et al., 2021). Additionally, the presence and well-being of calves are crucial, affecting the overall health and survival of the population. Calves are generally more sensitive to construction activities, and monitoring changes in their distribution provides a vital indicator of the impact of projects on the dolphin population. Therefore, we overlaid the locations of large dolphin groups (≥10 individuals) and calf sightings with the cost/distance grid, extracted the distances from sighting locations to the bridge for the three construction phases, and assessed changes in dolphin distribution over time.
To analyze the variation in the frequency of dolphin occurrences within specific distances from the bridge, the study separately calculated the distribution of Indo-Pacific humpback dolphins, the presence of calves, and the formation of large aggregations within 0–0.99 km, 1–4.99 km, and 5–9.99 km of the Hong Kong–Zhuhai–Macao Bridge. The differences among these groups were also analyzed at various stages of the bridge’s construction.
3 Results
3.1 Dolphin distribution and density
Humpback dolphins were located at significantly different distances from the HZMB during the three phases of its construction (Kruskal–Wallis, H (2, 567) = 14.45, p = 0.0007). The average distances from sighting locations to the bridge during the pre-construction, construction, and post-construction phases were 11.56 km (SD = 8.16, n = 182), 11.32 km (SD = 6.48, n = 175), and 8.93 km (SD = 5.57, n = 210), respectively (Figure 2). Density overlay maps of dolphin-sighting locations relative to the bridge are presented for these time periods in Figure 3.
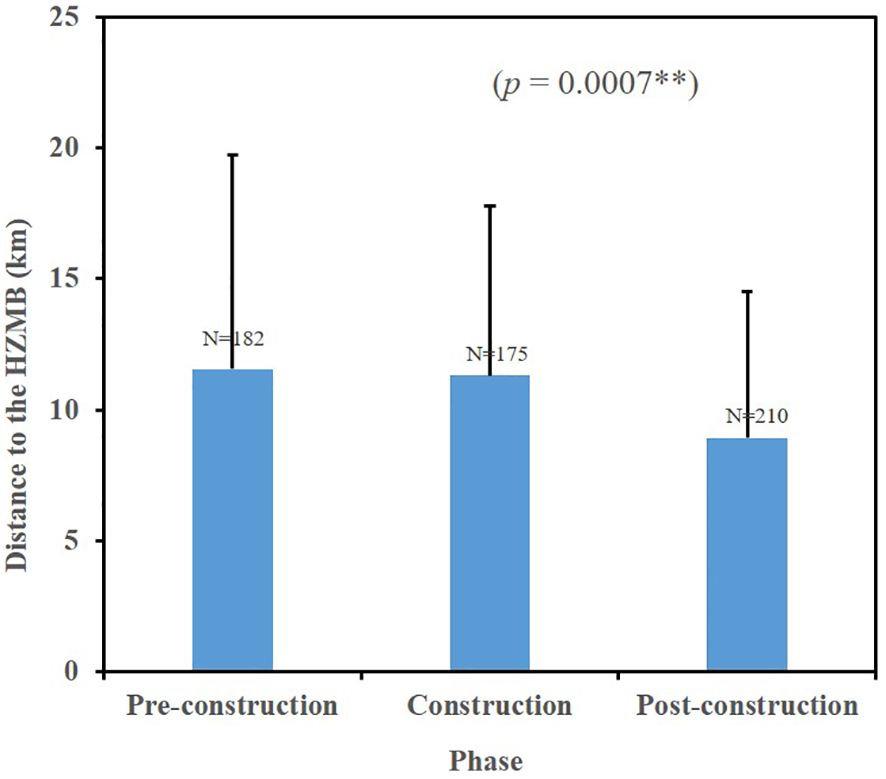
Figure 2. The average distance of humpback dolphins from the Hong Kong–Zhuhai–Macao Bridge (HZMB) during different phases of its construction. Error bars indicate SD, ** denotes significant differences.
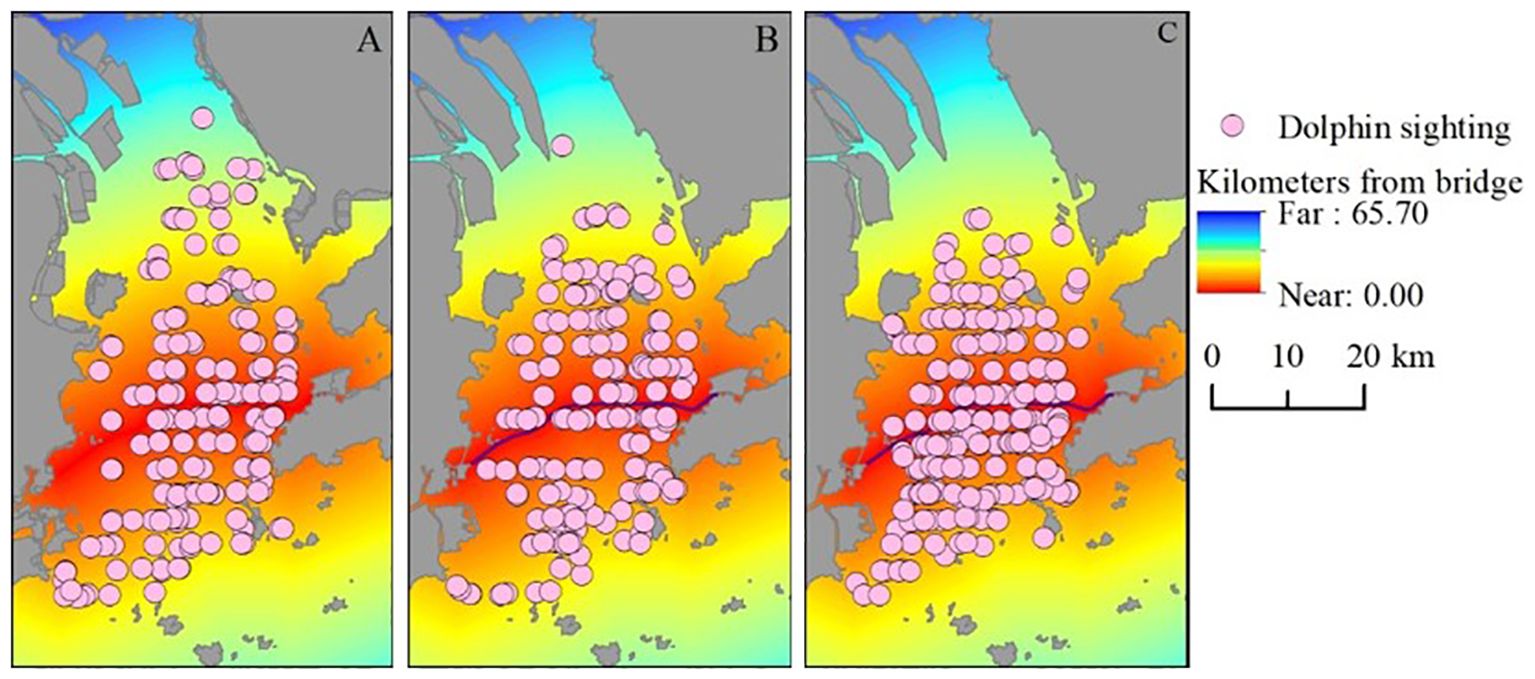
Figure 3. Dolphin group sightings during three different phases of the Hong Kong–Zhuhai–Macao Bridge (red line) construction: (A) pre-construction, (B) construction, and (C) post-construction. Sighting locations are overlaid with a cost/distance grid to extract the distances of the sighting locations from the bridge.
The CLDB and SLDB are located in proximity to the HZMB, and the abundance of dolphins in these two areas can be expected to reflect the response of the dolphins to bridge construction most accurately. The population density of dolphins in the CLDB showed little variation over the three investigational phases, remaining at 50-60 individuals/100km2. In contrast, the SLDB exhibited a significantly higher abundance of dolphins in the post-construction phase, whereas the population density remained relatively stable in the pre-construction and construction phases (Figure 4).
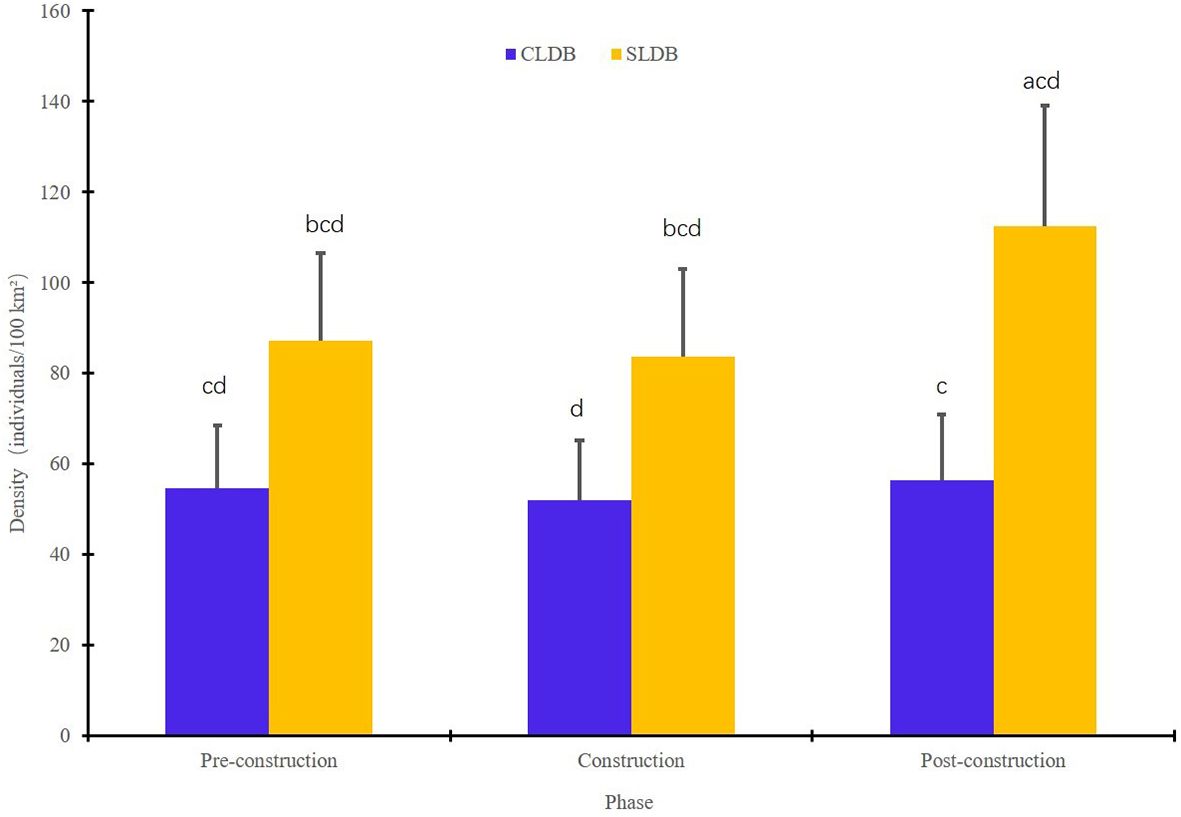
Figure 4. Changes in humpback dolphin density in the central Lingding Bay (CLDB) and south Lingding Bay (SLDB) regions during different construction phases of the Hong Kong–Zhuhai–Macao Bridge. Letters above the bars indicate the significance of differences between groups, where the same letter denotes no significant difference (α = 0.05).
We compared the average humpback dolphin densities between the experimental subregions during the three construction periods. These average densities were significantly different between the CLDB and SLDB areas during the same periods (p < 0.05). When considering the CLDB in isolation, humpback dolphin abundance did not differ significantly between the pre-construction and construction phases or between the pre-construction and post-construction phases (p > 0.05); however, the dolphin density was significantly different between the construction and post-construction phases (p < 0.05). Additionally, when viewing only the SLDB, dolphin density did not differ significantly between the pre-construction and construction phases (p > 0.05); however, significant differences were observed between the pre-construction and post-construction phases, as well as between the construction and post-construction phases (p < 0.05).
3.2 Group size and calf occurrence
The mean sizes of dolphin groups during the pre-construction, construction, and post-construction phases were 5.03 (SD = 4.43, n = 182), 4.70 (SD = 6.48, n = 175), and 6.18 individuals (SD = 5.15, n = 210), respectively.
Group sizes were initially compared between the pre-construction, construction, and post-construction datasets via analysis of variance (ANOVA) followed by Tukey’s honest significant difference (HSD) post-hoc test, with the initial assessments including Levene’s test for homogeneity of variances. The results from Levene’s test indicated that an assumption of equal variances across the three datasets could not be rejected at a significance level (α) of 0.05 (F = 2.7043, p = 0.0682). Subsequent ANOVAs (focusing on the equality of means) similarly failed to reject the null hypothesis at the 0.05 significance level (F = 2.8154, p = 0.0612). However, Tukey’s HSD post-hoc test identified significant mean differences between the group sizes observed during the construction and post-construction phases (mean difference = 1.4793, p = 0.0476, reject = true). In other words, although overall mean differences did not differ significantly, specific pairwise comparisons revealed significant differences (particularly between the construction and post-construction datasets).
Recent studies highlight that environmental pressures, including food scarcity and anthropogenic factors, play a significant role in cetacean group formation. Research suggests that larger dolphin groups are often formed to improve foraging efficiency when food resources are scarce, as highlighted by Bearzi et al. (1999), while environmental factors such as prey distribution can also significantly influence group dynamics, as noted by Gowans et al. (2007).The distribution of humpback dolphin sightings comprising large groups (10 or more individuals) is illustrated for the different monitoring periods in Figure 5. Large groups were observed on both sides of the HZMB during the three construction phases. The distances of these groups from the HZMB did not differ significantly between the three periods (Kruskal-Wallis H (2,85) = 2.85, p = 0.2402). The average distances of large groups from the bridge during pre-construction, construction, and post construction were 11.13 km (SD = 6.35, n = 25), 9.75 km (SD = 5.12, n = 21), and 8.59 km (SD = 5.71, n = 38), respectively. Density overlay maps of large group sightings in relation to the bridge during these three construction phases are shown in Figure 5.
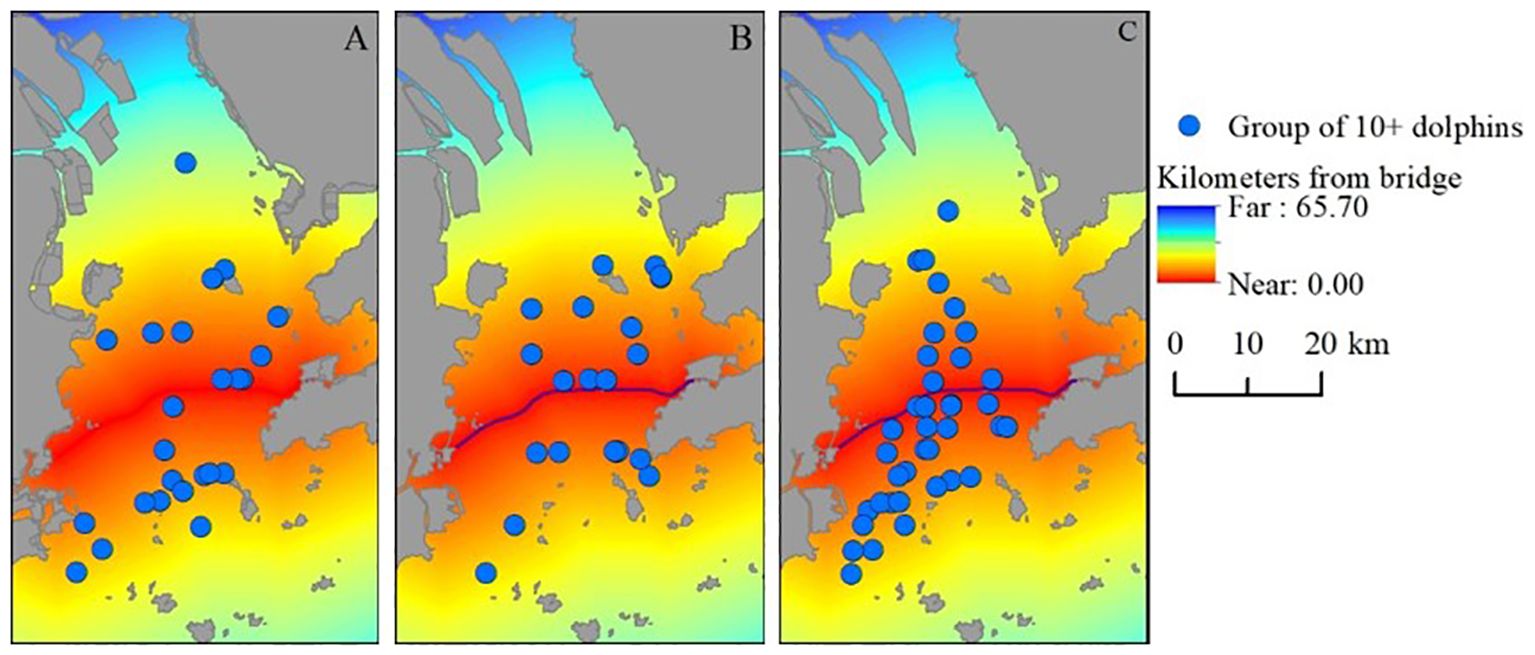
Figure 5. Sightings of large humpback dolphin groups (≥10 individuals) during the (A) pre-construction, (B) construction, and (C) post-construction phases of the Hong Kong–Zhuhai–Macao Bridge (red line). Sighting locations are overlaid with a cost/distance grid to extract the distances from sighting points to the bridge.
In this study, “calves” refers collectively to the age groups designated UC and UJ. Figure 6 displays the proportion of calves among the different age groups as recorded during the three different phases of bridge construction. Notably, during the construction period, the proportion of calves was the lowest, contrasting with its peak during the pre-construction period.
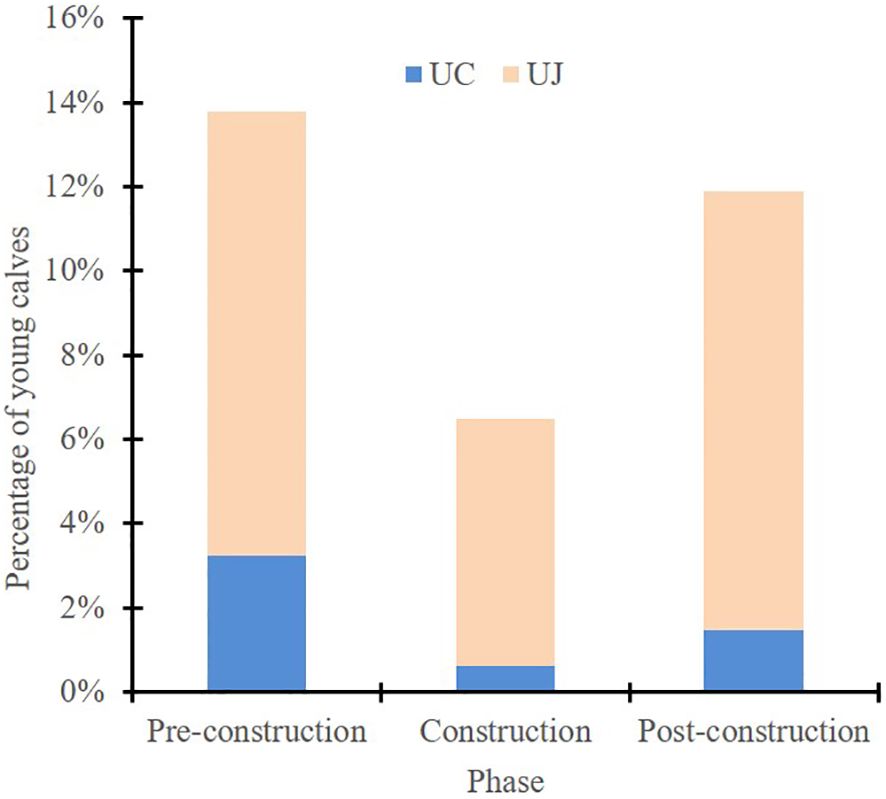
Figure 6. Percentages of young calves [i.e., unspotted calves (UC) and unspotted juveniles (UJ)] observed among all dolphin age groups during the three different phases of construction of the Hong Kong–Zhuhai–Macao Bridge.
The distribution of calf sightings as recorded in each construction phase is shown in Figure 7. During the pre-construction phase, calves were observed throughout the LDB. However, during the construction phase, the number of calf sightings significantly decreased, with fewer sightings on both sides of the HZMB and almost none in the NLDB. During the post-construction period, calves were again observed in all regions, with a higher density of sightings near the bridge. Notably, the distances from calf sightings to the bridge did not differ significantly between the three periods (Kruskal-Wallis, H (2, 172) = 5.98, p = 0.0504). During the pre-construction, construction, and post-construction phases, these average distances comprised 11.89 km (SD = 7.29, n = 66), 9.48 km (SD = 5.15, n = 33), and 9.06 km (SD = 5.62, n = 73), respectively. Figure 7 displays the density overlay maps of calf sightings in relation to the HZMB during the three construction periods.
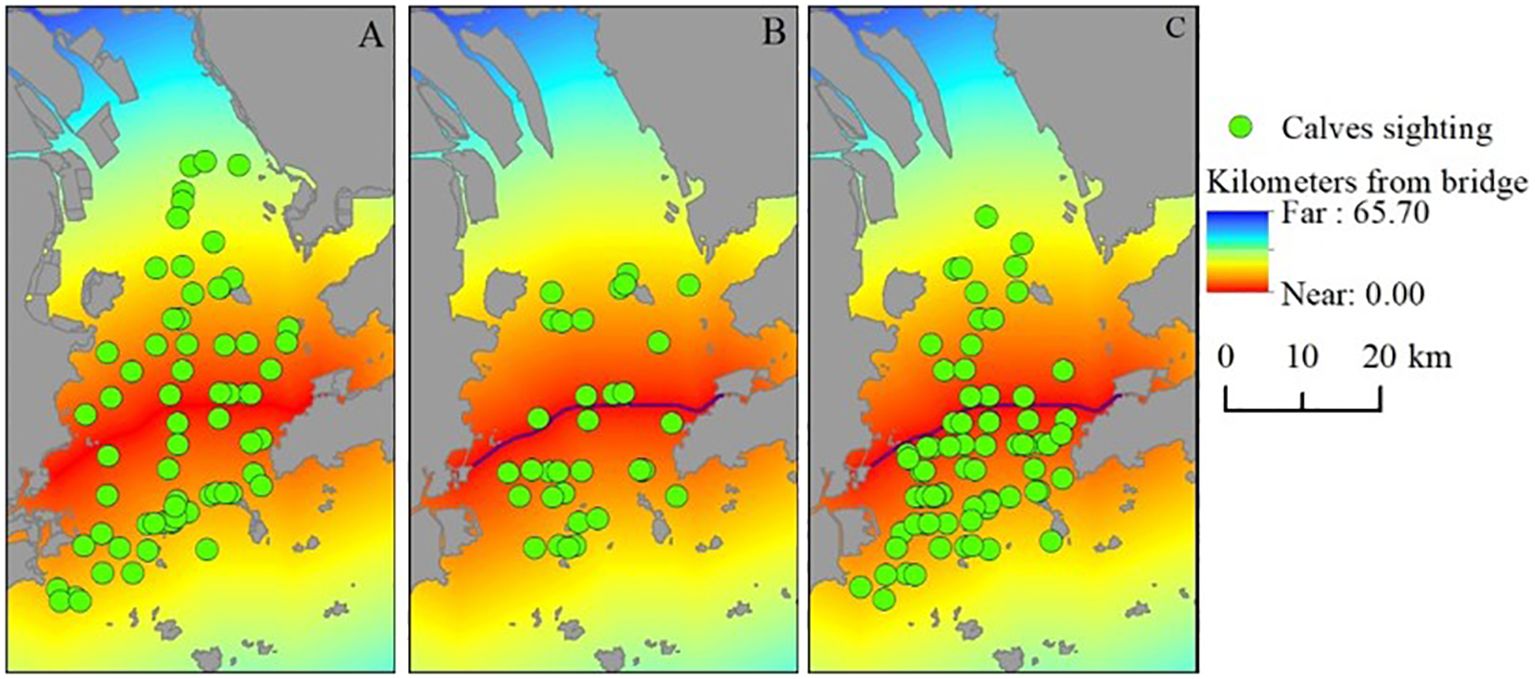
Figure 7. Locations of calf sightings during the (A) pre-construction, (B) construction, and (C) post-construction phases of the Hong Kong–Zhuhai–Macao Bridge (red line). These locations were overlaid with a cost/distance grid to extract their distances from the bridge.
Based on the statistical analysis of three distribution categories—dolphin sightings, large group sightings, and calf sightings—across different time periods (pre-construction, construction, and post-construction) in various distance intervals (0–1, 1–5, 5–10) (Table 1), the Fisher’s Exact test results indicate that the differences in the 0-1 interval across different years are not statistically significant (p = 1.0, which is greater than the 0.05 significance level). This suggests no significant changes in dolphin sightings within this interval over time. However, the differences in the 1–5 and 5–10 intervals are highly significant, with p-values of less than 0.05 (p < 0.05), specifically. This indicates that dolphin activity in these farther distance intervals has changed significantly over time.
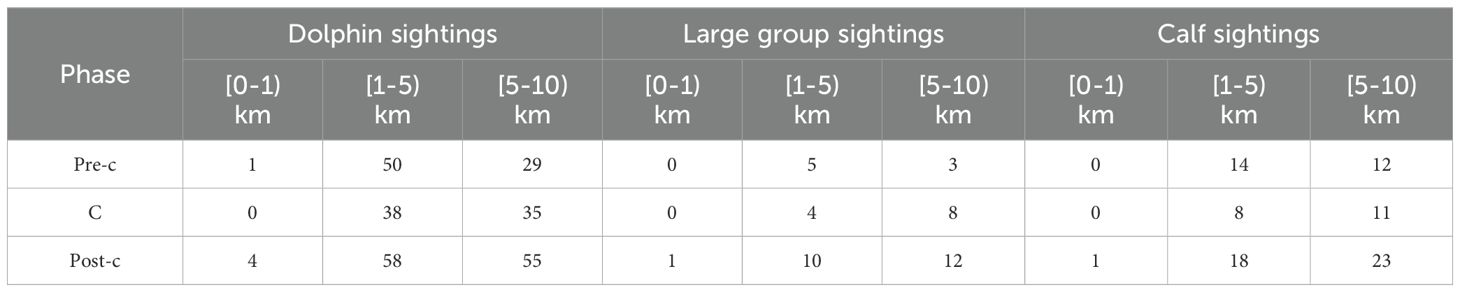
Table 1. Dolphin sightings, large group sightings, and calf sightings within specific ranges from the bridge during different phases: pre-construction (pre-c), construction (c), and post-construction (post-c).
3.3 Range use and movement patterns
During the post-construction survey, 449 humpback dolphin individuals were identified via photographs. Of these, approximately 174 individuals were sighted twice or more at different locations, accounting for 467 of the total number of sightings throughout the study area and period. The remaining 275 dolphins (61.2%) were photographed only once. Of the repeatedly identified individuals, 19.5% were observed exclusively in the waters to north of the bridge (Figure 8A), ~42.0% were found only in the waters to south of the bridge (Figure 8B), and ~38.5% exhibited a range use that spanned the HZMB (Figure 8C). Additionally, 46.6% of the dolphins displayed an activity range that was consistently close to the bridge, where they were repeatedly sighted (Figure 8D).
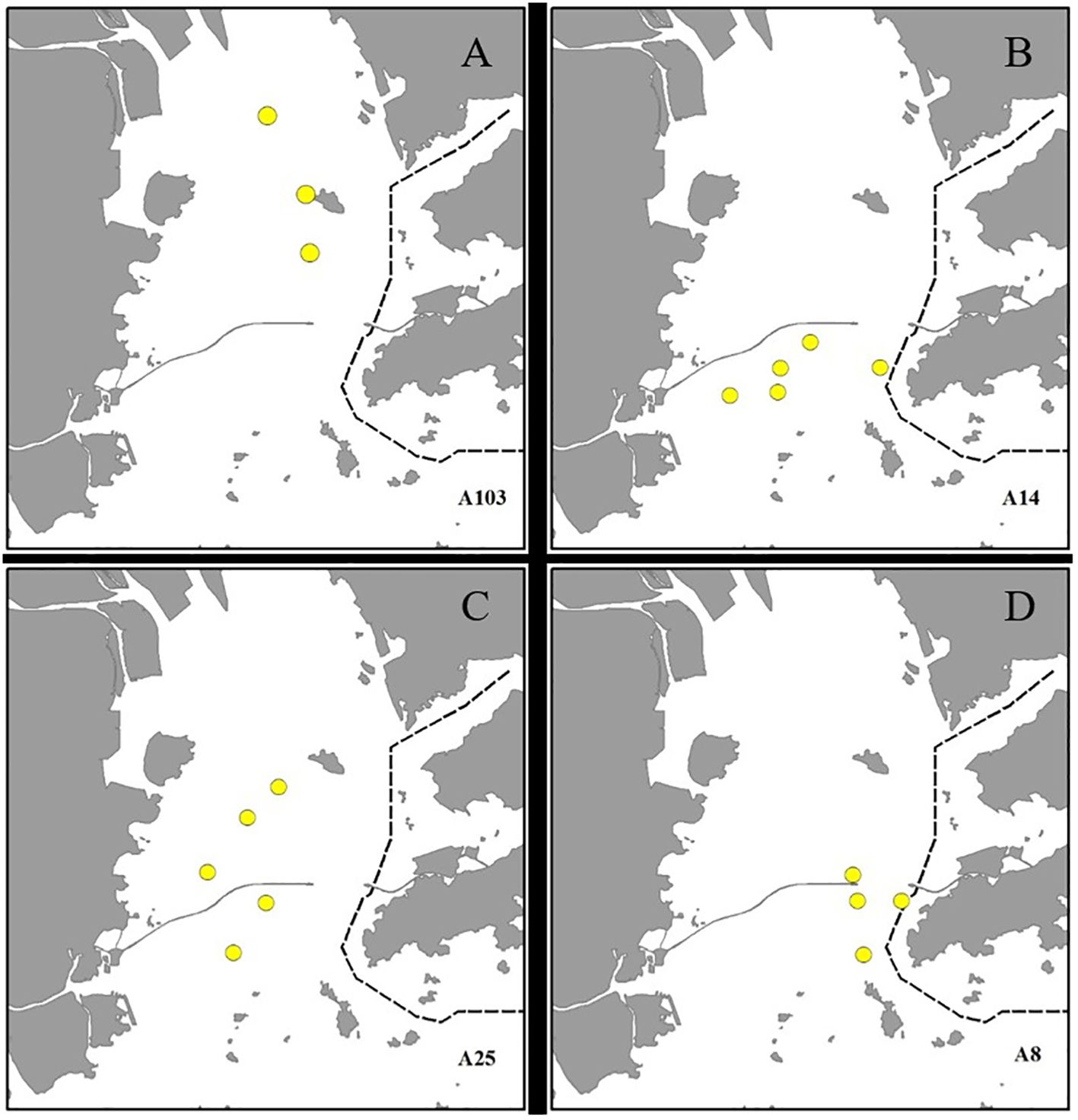
Figure 8. Patterns of humpback dolphin range use across the study area. (A) Individuals occurring exclusively in the waters to the north of the Hong Kong–Zhuhai–Macao Bridge (HZMB). (B) Individuals encountered exclusively in the waters to the south of the HZMB. (C) Individuals that were sighted on both sides of the HZMB. (D) Individuals that were frequently sighted in waters close to the HZMB.
4 Discussion
We systematically analyzed the responses of humpback dolphins to various phases of a large-scale marine engineering project by using vessel-based line-transect data to assess changes in their distribution, density, group size, and group composition. Additionally, we examined the post-construction range use and movement patterns of the dolphins via the identification of individuals.
Dolphin abundance showed little change in the CLDB over periods before, during, and after the construction of the HZMB. In contrast, their abundance increased in the SLDB after the bridge construction. The increased abundance observed in the SLDB may have resulted from alternative causes, such as a reduction in human activities due to the COVID-19 pandemic that may have led to an increase in the number of dolphins entering the SLDB. However, our results suggest that the bridge construction had a minimal impact on the habitat use of humpback dolphins in the LDB, retaining it as a key habitat in their distribution range. During the COVID-19 lockdown, large marine mammals that had not been observed for generations reemerged near coastlines and in marine channels (Bates et al., 2020). This unexpected behavior has been linked to the reduced anthropogenic noise in the environment during human confinement (Rutz et al., 2020; Thomson and Barclay, 2020).
During the post-construction phase, an increased dolphin density near the HZMB could be attributable to two reasons: (1) an increase in food resources (reef effect) and/or (2) a reduction in disturbances (sheltering effect). The introduction of hard substrates on an originally uniform sandy seabed can alter the species composition of the habitat, subsequently increasing the abundance of marine organisms (Pedersen et al., 2006; Petersen and Malm, 2006). Furthermore, with reduced fishing activity near the bridge, the fish community is also likely to change. The waters around the HZMB in the Pearl River Estuary are under significant fishing pressure, especially from bottom and shrimp trawling operations (Liu et al., 2011; Zhou et al., 2019). Safety alert lines were established on either side of the HZMB in January 2020, thereby restricting vessel operations within 5 km of the bridge. In the Guangdong waters around the HZMB, all vessel traffic is prohibited in the bridge waters and a marginal 5 km buffer zone. With the exception of emergency disposal, official duties, and water activities permitted by maritime authorities, no vessels may enter these waters beyond the first-level alert line in bridge channels and tunnel areas. Such restrictions provide a sheltering effect, contributing to the observed increase in dolphin abundance in the SLDB, which is the region in which the HZMB is located. Dolphins dive for longer periods in areas with heavy vessel traffic or in the presence of an oncoming vessel (Ng and Leung, 2003). Therefore, the humpback dolphins may find the protected waters around the HZMB in the SLDB more attractive than other areas.
Additionally, the distance of humpback dolphin sightings from the bridge decreased significantly in the post-construction period. However, distances from the bridge did not differ significantly between large groups and calves during the different construction phases. Indeed, an analysis of the movement patterns of individual dolphins revealed that both sides of the bridge continued to be essential distribution areas for dolphins in the post-construction period. This finding underscores the importance of the marine area around the HZMB as a crucial corridor for humpback dolphins in the LDB, with the regions on both sides persisting as preferred habitats.
Group size plays a vital role in facilitating communication and maintaining social dynamics among marine mammals. In the LDB, the average group size of humpback dolphins was significantly larger during the post-construction period compared to the construction phase. This increase in group size likely reflects a behavioral adaptation to cope with the elevated disturbances caused by shipping traffic, noise pollution, and habitat modifications associated with construction activities. For instance, the heightened shipping activity may have disrupted the dolphins’ natural foraging areas, prompting them to form larger groups to improve hunting success and reduce individual energy expenditure (Connor, 2000; Silk, 2007). Furthermore, the alteration in prey availability and distribution due to construction-induced changes in the marine environment could have driven dolphins to form larger groups, as cooperative foraging becomes advantageous when prey is sparse or patchily distributed.
The variation in group sizes across geographical locations likely results from intraspecific trade-offs between the advantages of increased social cooperation—such as enhanced foraging efficiency—and the potential costs, such as increased competition for limited resources (Gygax, 2002; Gowans, 2019). These trade-offs may vary depending on local environmental conditions, leading to different social structures. Coastal dolphins exhibit dynamic social systems that can shift according to temporal changes in environmental pressures, such as seasonal shifts in prey abundance or increased human activities. Such flexibility allows dolphins to adapt their social behavior to fluctuating conditions within their habitats (Gómez de Segura et al., 2006; Sutaria et al., 2019).
In recent years, overfishing has significantly impacted fishery resources in LDB, leading to a 70% decrease in the biomass of coastal fish from 2004 to 2014 in the northern part of the bay (Yu et al., 2016). Additionally, the post-construction monitoring period of our study coincided with the COVID-19 pandemic. As a result, the number of fishing vessels, high-speed passenger ships, and other oceangoing boats was significantly reduced compared with the traffic present during the construction of the HZMB. This substantial decrease in the interference from various vessels benefits dolphin communication. These factors collectively suggest that alterations in dolphin foraging strategies, driven by the variable availability of prey and the reduction of interference from seafaring vessels, could be linked with fluctuations in group size.
In terms of age groups, the percentage of young calves observed during the post-construction phase was significantly higher than that recorded for the construction period, yet it remained slightly lower than that noted for the pre-construction period. An analysis of stranded dolphin mortalities suggested that pollutants such as polychlorinated biphenyls, Cu, and perfluorooctane sulfonate may contribute significantly to the low calf survival rate in this population (Sun et al., 2022). Calves are more susceptible to anthropogenic disturbances than other age classes. Given the escalating anthropogenic activities in their habitats (including offshore construction projects, vessel traffic, fishing activities, and pollutant discharge), a restoration of the calf sighting ratio to that of the pre-construction period poses serious challenges.
The distances of calf sightings from the bridge did not differ significantly between the three HZMB construction periods. In the NLDB, a higher number of calves were present during the pre-construction phase of the bridge; however, during the construction and post-construction phases, this number decreased noticeably, likely linked to the extensive and prolonged development in this area, such as more than a decade of sand mining. In the CLDB, calf sightings decreased during the construction phase of the bridge, suggesting a potential decline in habitat quality that may stem from diminished food resources or increased human activities. South of the HZMB, an increased presence of calves was recorded during the construction phase, which could be attributed to two factors. Firstly, a decline in NLDB habitat quality may have prompted calves to migrate southward. Secondly, during post-construction, the suspension of ferry services between Hong Kong and MA due to the COVID-19 pandemic resulted in reduced water traffic in the northern waters of MA, possibly leading to the eastward migration of dolphins from the western Pearl River Estuary. However, whether this phenomenon is related to the impact of the HZMB construction requires further investigations utilizing extended monitoring data.
Based on our monitoring results, the construction of the HZMB had a minimal impact on the north–south movement of humpback dolphins in the LDB. The annual average distribution density of dolphins remained at approximately 50-60 individuals per 100km2 in the CLDB throughout the pre-construction period (2005-2006), construction period (2015-2016), and post-construction period (2020-2021). Humpback dolphins have been shown to exhibit seasonal north–south migration patterns in LDB, moving toward the north during the dry season and toward the south during the wet season (Jia et al., 2000). Currently, the annual average population density of humpback dolphins remains stable in the CLDB. Further monitoring has shown significant seasonal fluctuations in dolphin sightings in the CLDB (unpublished report), again suggesting that the seasonal north–south migration of the dolphins may not have been affected by the bridge construction.
In terms of distribution range, some dolphins were seen both north and south of the bridge at different times. This suggests that the movement of individual dolphins is unlikely to be hindered by the HZMB. One previous study on the ranging patterns of humpback dolphins in LDB found that the estimated mean range size (± SD) of 40 humpback dolphins was 99.5 ± 61.04 km2 (range: 23.76-303.84 km2), with several individuals occurring exclusively in LDB (Hung and Jefferson, 2004). A similar study in the waters of Xiamen found that the individual ranges of humpback dolphins along this part of the coastline were similar to those of their conspecifics in the LDB (Chen et al., 2011). Our observation that some humpback dolphins did not traverse the HZMB could be an artefact of their habitual ranging patterns. The presence of the bridge may have also had an influence, leading to a change in distribution. Age class, associations with fishing boats, the distribution and availability of food resources, and human activities and disturbances have all been shown to influence the ranging patterns of humpback dolphins in the Pearl River Estuary (Hung and Jefferson, 2004). Future studies need to assess the significance of short-term behavioral changes relative to long-term survival and reproductive success, although long-term effects should not be assumed (Bejder et al., 2006). It should also be noted that the HZMB had just started operating during the post-construction survey period and was influenced by the COVID-19 pandemic, experiencing a low traffic volume. The habitat use of dolphins near the bridge is still subject to further assessments once the traffic volume increases.
5 Conclusion
The HZMB is a mega marine engineering project conducted within the habitat of the largest known population of humpback dolphins in the world. We evaluated the responses of the dolphins to construction activity via systematic line-transect sampling during various stages of the project. Results revealed a significant decrease in the average sighting distance of humpback dolphins from the bridge during the post-construction period compared to that in both the pre-construction and construction phases. Additionally, a notable increase in dolphin group sizes post-construction indicated a potential shift in foraging strategy. The proportion of calves recorded among the different age groups initially increased, followed by a subsequent decline and eventual recovery.
Monitoring these distribution changes of humpback dolphins over time offers valuable insights into the adaptive capacity of the species and facilitates the development of targeted conservation measures. Future endeavors should prioritize ongoing research and monitoring of humpback dolphins in the vicinity of the HZMB to monitor their long-term responses to the infrastructure and to form adaptive management approaches. Through the incorporation of scientific findings into conservation and management frameworks, stakeholders can collaboratively mitigate potential threats and ensure the enduring survival of this iconic dolphin in the region.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Academic Board of South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZH: Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft. LF: Formal Analysis, Investigation, Writing – review & editing. HW: Formal Analysis, Investigation, Writing – review & editing. KZ: Formal Analysis, Investigation, Writing – review & editing. XW: Conceptualization, Resources, Supervision, Writing – original draft, Writing – review & editing. TC: Conceptualization, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (42230413), Technical Services for the Environmental Monitoring of the Chinese white dolphin at the completion of the Hong Kong-Zhuhai-Macao Bridge (HZMBENSC20201700), and the Hainan Provincial Science and Technology Plan in the Science and Technology Innovation Joint Project of the Sanya Yazhou Bay Science and Technology City (2021CXLH0004).
Acknowledgments
The authors are grateful to Yujian Chen and Yuezhong Wang of the South China Sea Fisheries Research Institute for their diligent work in conducting the line-transect vessel surveys.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1407937/full#supplementary-material
References
Bates A. E., Primack R. B., Moraga P., Duarte C. M. (2020). COVID-19 pandemic and associated lockdown as a “Global Human Confinement Experiment” to investigate biodiversity conservation. Biol. Conserv. 248, 108665. doi: 10.1016/j.biocon.2020.108665
Bearzi G., Politi E., di Sciara G. N. (1999). Diurnal behavior of free-ranging bottlenose dolphins in the Kvarneric (northern Adriatic Sea). Mar. Mammal Sci. 15, 1065–1097. doi: 10.1111/j.1748-7692.1999.tb00878.x
Bejder L., Samuels A., Whitehead H., Gales N. (2006). Interpreting short-term behavioural responses to disturbance within a longitudinal perspective. Anim. Behav. 72, 1149–1158. doi: 10.1016/j.anbehav.2006.04.003
Buckland S. T., Anderson D. R., Burnham K. P., Laake J. L., Borchers D. L., Thomas L. (2001). Introduction to distance sampling: estimating abundance of biological populations (London: Oxford University Press).
Buckstaff K. C., Wells R. S., Gannon J. G., Nowacek D. P. (2013). Responses of bottlenose dolphins (Tursiops truncatus) to construction and demolition of coastal marine structures. Aquat. Mamm. 39, 174–186. doi: 10.1578/am.39.2.2013.174
Cagnazzi D., Parra G. J., Harrison P. L., Brooks L., Rankin R. (2020). Vulnerability of threatened Australian humpback dolphins to flooding and port development within the southern Great Barrier Reef coastal region. Global Ecol. Conserv. 24, e01203. doi: 10.1016/j.gecco.2020.e01203
Chan C. Y. (2019). Demography and socio-ecology of Indo-Pacific humpback dolphin (Sousa chinensis) metapopulation in the Pearl River estuary. Hong Kong: The University of Hong Kong.
Chan S. C. Y., Karczmarski L. (2024). Broad-scale impacts of coastal mega-infrastructure project on obligatory inshore delphinids: A cautionary tale from Hong Kong. Sci. Total Environ. 920, 169753. doi: 10.1016/j.scitotenv.2023.169753
Chen B., Zheng D., Ju J., Xu X., Zhou K., Yang G. (2011). Range ratterns of resident Indo-Pacific humpback dolphins (Sousa chinensis, Osbeck 1765) in Xiamen, China: implications for conservation and management. Zool. Stud. 50, 751–762.
Chen T., Hung S. K., Qiu Y. S., Jia X. P., Jefferson T. A. (2010). Distribution, abundance, and individual movements of Indo-Pacific humpback dolphins (Sousa chinensis) in the Pearl River Estuary, China. Mammalia 74, 117–125. doi: 10.1515/Mamm.2010.024
Chen B., Zheng D., Zhai F., Xu X., Sun P., Wang Q., et al. (2008). Abundance, distribution and conservation of Chinese White Dolphins (Sousa chinensis) in Xiamen, China. Mammalian Biol. 73 (2), 156–164. doi: 10.1016/j.mambio.2006.12.002
Chen B., Xu X., Jefferson T. A., Olson P. A., Qin Q., Zhang H., et al. (2016). Conservation status of the Indo-Pacific humpback dolphin (Sousa chinensis) in the northern Beibu Gulf, China. Adv. Mar. Biol. 73, 119–139. doi: 10.1016/bs.amb.2015.10.001
Connor R. C. (2000). Group living in whales and dolphins (Chicago: The University of Chicago Press).
Dähne M., Gilles A., Lucke K., Peschko V., Adler S., Krügel K., et al. (2013). Effects of pile-driving on harbour porpoises (Phocoena phocoena) at the first offshore wind farm in Germany. Environ. Res. Lett. 8, 25002. doi: 10.1088/1748-9326/8/2/025002
Gómez de Segura A., Crespo E. A., Pedraza S. N., Hammond P. S., Raga J. A. (2006). Abundance of small cetaceans in waters of the central Spanish Mediterranean. Mar. Biol. 150, 149–160. doi: 10.1007/s00227-006-0334-0
Gowans S. (2019). Grouping behaviors of dolphins and other toothed whales. Ethol. Behav. Ecol. Odontocetes, 3–24. doi: 10.1007/978-3-030-16663-2_1
Gowans S., Würsig B., Karczmarski L. (2007). “The social structure and strategies of delphinids: predictions based on an ecological framework,” in Advances in Marine Biology (San Diego, CA: Academic Press), 195–294.
Graham I. M., Pirotta E., Merchant N. D., Farcas A., Barton T. R., Cheney B., et al. (2017). Responses of bottlenose dolphins and harbor porpoises to impact and vibration piling noise during harbor construction. Ecosphere 8, e01793. doi: 10.1002/ecs2.1793
Gygax L. (2002). Evolution of group size in the superfamily Delphinoidea (Delphinidae, Phocoenidae and Monodontidae): a quantitative comparative analysis. Mammal Rev. 32, 295–314. doi: 10.1046/j.1365-2907.2002.00114.x
Hung S. K. (2008). Habitat use of Indo-pacific humpback dolphins (Sousa chinensis) in Hong Kong. Hong Kong: The University of Hong Kong.
Hung S. K., Jefferson T. A. (2004). Ranging patterns of Indo-Pacific humpback dolphins (Sousa chinensis) in the Pearl River Estuary, People’s Republic of China. Aquat. Mamm. 30, 159–174. doi: 10.1578/am.30.1.2004.159
Jefferson T. A. (2000). Population biology of the Indo-Pacific hump-backed dolphin in Hong Kong waters. Wildl. Monogr. 144, 1–65.
Jefferson T. A. (2018). Hong Kong’s Indo-Pacific humpback dolphins (Sousa chinensis): Assessing past and future anthropogenic impacts and working toward sustainability. Aquat. Mamm. 44, 711–728. doi: 10.1578/am.44.6.2018.711
Jefferson T. A., Becker E. A., Huang S. L. (2023). Influences of natural and anthropogenic habitat variables on Indo-Pacific humpback dolphins Sousa chinensis in Hong Kong. Endangered Species Res. 51, 143–160. doi: 10.3354/esr01249
Jefferson T. A., Hung S. K., Robertson K. M., Archer F. I. (2012). Life history of the Indo-Pacific humpback dolphin in the Pearl River Estuary, southern China. Mar. Mammal Sci. 28, 84–104. doi: 10.1111/j.1748-7692.2010.00462.x
Jefferson T. A., Rosenbaum H. C. (2014). Taxonomic revision of the humpback dolphins (Sousa spp.), and description of a new species from Australia. Mar. Mammal Sci. 30, 1494–1541. doi: 10.1111/mms.12152
Jefferson T. A., Smith B. D. (2016). Re-assessment of the conservation status of the Indo-Pacific humpback dolphin (Sousa chinensis) using the IUCN red list criteria. Adv. Mar. Biol. 73, 1–26. doi: 10.1016/bs.amb.2015.04.002
Jefferson T. A., Smith B. D., Braulik G. T., Perrin W. (2017). “Sousa chinensis (errata version published in 2018),” in The IUCN Red List of Threatened Species 2017. e.T82031425A123794774. Available online at: https://www.iucnredlist.org/species/82031425/123794774 (Accessed Nov 12, 2024).
Jia X., Chen T., Zhou J., Guo Z. (2000). Preliminary investigation on the Sousa chinensis in the Pearl River Estuary. China Environ. Sci. 20, 80–82.
Li M., Wang X., Hung S. K., Xu Y., Chen T. (2019). Indo-Pacific humpback dolphins (Sousa chinensis) in the Moyang River Estuary: The western part of the world’s largest population of humpback dolphins. Aquat. Conserv.: Mar. Freshw. Ecosyst. 29, 798–808. doi: 10.1002/aqc.3055
Li S., Lin M., Xu X., Xing L., Zhang P., Gozlan R. E., et al. (2016). First record of the Indo-Pacific humpback dolphins (Sousa chinensis) southwest of Hainan Island, China. Mar. Biodiver. Rec. 9(1), 1–6. doi: 10.1186/s41200-016-0005-x
Lin W., Wu L., Zeng Q., Leng X., Mo Y., Serres A., et al. (2022). First live sighting of an Indo-Pacific humpback dolphin (Sousa chinensis) in the Yellow Sea, the northern-most record of the species range. J. Mar. Biol. Assoc. United Kingdom 102, 333–337. doi: 10.1017/S0025315422000534
Liu M., Lin M., Lusseau D., Li S. (2021). The biogeography of group sizes in humpback dolphins (Sousa spp.). Integr. Zool. 16, 527–537. doi: 10.1111/1749-4877.12542
Liu W., Lin Z., Jiang Y., Huang Z. (2011). Spatial distribution of demersal fishery resources in the continental shelf of the northern South China Sea. J. Trop. Oceanogr. 30, 95–103. doi: 10.11978/j.issn.1009-5470.2011.05.095
Lotze H. K., Lenihan H. S., Bourque B. J., Bradbury R. H., Cooke R. G., Kay M. C., et al. (2006). Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312, 1806–1809. doi: 10.1126/science.1128035
Ng S. L., Leung S. (2003). Behavioral response of Indo-Pacific humpback dolphin (Sousa chinensis) to vessel traffic. Mar. Environ. Res. 56, 555–567. doi: 10.1016/s0141-1136(03)00041-2
Pedersen J., Leonhard S. B., Klaustrup M., Hvidt C. B. (2006). Benthic communities at Horns Rev before, during and after construction of Horns Rev offshore wind farm. Vattenfall, 1–134.
Peng C., Wu H., Wang X., Zhu Q., Jefferson T. A., Wang C. C., et al. (2020). Abundance and residency dynamics of the Indo‐Pacific humpback dolphin, Sousa chinensis, in the Dafengjiang River Estuary, China. Mar. Mamm. Sci. 36 (2), 623–637. doi: 10.1111/mms.12663
Petersen J. K., Malm T. (2006). Offshore windmill farms: threats to or possibilities for the marine environment. AMBIO: A J. Hum. Environ. 35, 75–80. doi: 10.1579/0044-7447(2006)35[75:OWFTTO]2.0.CO;2
Pirotta E., Laesser B. E., Hardaker A., Riddoch N., Marcoux M., Lusseau D. (2013). Dredging displaces bottlenose dolphins from an urbanised foraging patch. Mar. pollut. Bull. 74, 396–402. doi: 10.1016/j.marpolbul.2013.06.020
Piwetz S., Hung S., Wang J., Lundquist D., Wuersig B. (2012). Influence of vessel traffic on movements of Indo-Pacific humpback dolphins (Sousa chinensis) off Lantau Island, Hong Kong. Aquat. Mamm. 38, 325–331. doi: 10.1578/am.38.3.2012.325
Richardson W. J., Finley K. J., Miller G. W., Davis R. A., Koski W. R. (1995). Feeding, social and migration behavior of bowhead whales, Balaena mysticetus, in Baffin Bay vs. the Beaufort Sea—regions with different amounts of human activity. Mar. Mammal Sci. 11, 1–45. doi: 10.1111/j.1748-7692.1995.tb00272.x
Richardson W. J., Fraker M. A., Würsig B., Wells R. S. (1985). Behaviour of Bowhead Whales Balaena mysticetus summering in the Beaufort Sea: Reactions to industrial activities. Biol. Conserv. 32, 195–230. doi: 10.1016/0006-3207(85)90111-9
Rutz C., Loretto M.-C., Bates A. E., Davidson S. C., Duarte C. M., Jetz W., et al. (2020). COVID-19 lockdown allows researchers to quantify the effects of human activity on wildlife. Nat. Ecol. Evol. 4, 1156–1159. doi: 10.1038/s41559-020-1237-z
Schick R. S., Urban D. (2000). Spatial components of bowhead whale (Balaena mysticetus) distribution in the Alaskan Beaufort Sea. Can. J. Fish. Aquat. Sci. 57, 2193–2200. doi: 10.1139/f00-196
Silk J. B. (2007). The adaptive value of sociality in mammalian groups. Philos. Trans. R. Soc. B: Biol. Sci. 362, 539–559. doi: 10.1098/rstb.2006.1994
Small C., Nicholls R. J. (2003). A global analysis of human settlement in coastal zones. J. Coast. Res. 19, 584–599. doi: 10.2112/1551-5036(2003)19[584]2.0.CO;2
Sun X., Guo L., Luo D., Yu R.-Q., Yu X., Liang Y., et al. (2022). Long-term increase in mortality of Indo-Pacific humpback dolphins (Sousa chinensis) in the Pearl River Estuary following anthropic activities: Evidence from the stranded dolphin mortality analysis from 2003 to 2017. Environ. pollut. 307, 119526. doi: 10.1016/j.envpol.2022.119526
Sutaria D., Kelkar N., Araújo-Wang C., Santos M. (2019). Cetacean sociality in rivers, lagoons, and estuaries. Ethol. Behav. Ecol. Odontocetes, 413–434. doi: 10.1007/978-3-030-16663-2_19
Thomas L., Laake J., Strindberg S., Marques F., Buckland S., Borchers D., et al. (2009). Distance 6.0. Release 2. Research unit for wildlife population assessment, university of St. Andrews, UK. Lama guanicoe.
Thomson D. J., Barclay D. R. (2020). Real-time observations of the impact of COVID-19 on underwater noise. J. Acoust. Soc. America 147, 3390–3396. doi: 10.1121/10.0001271
Wang X., Chen T., Li M., Wang Y. (2022). Long-term changes in habitat use of Indo-Pacific humpback dolphins (Sousa chinensis) in response to anthropogenic coastline shift in Lingding Bay of Pearl River Estuary, China. Acta Ecol. Sin. 42, 2962–2973. doi: 10.5846/stxb202101070066
Wang X., Wu F., Chang W.-L., Hou W., Chou L.-S., Zhu Q. (2016a). Two separated populations of the Indo-Pacific humpback dolphin (Sousa chinensis) on opposite sides of the Taiwan Strait: Evidence from a larger-scale photo-identification comparison. Mar Mamm Sci 32 (1), 390–399. doi: 10.1111/mms.12257
Wang X., Wu F., Ding X., Zhu Q. (2016b). Record of an Indo-Pacific humpback dolphin (Sousa chinensis) without its upper rostrum in Xiamen Bay, Fujian Province, China. New Zealand J. Zool. 43(3), 299–306. doi: 10.1080/03014223.2016.1155997
Wright A. J., Deak T., Parsons E. (2011). Size matters: management of stress responses and chronic stress in beaked whales and other marine mammals may require larger exclusion zones. Mar. pollut. Bull. 63, 5–9. doi: 10.1016/j.marpolbul.2009.11.024
Würsig B., Greene C. Jr., Jefferson T. (2000). Development of an air bubble curtain to reduce underwater noise of percussive piling. Mar. Environ. Res. 49, 79–93. doi: 10.1016/S0141-1136(99)00050-1
Xu X., Song J., Zhang Z., Li P., Yang G., Zhou K. (2015). The world's second largest population of humpback dolphins in the waters of Zhanjiang deserves the highest conservation priority. Scientific Rep. 5, 8147. doi: 10.1038/srep08147
Yu J., Chen Z., Xu S. (2016). Land reclamation and its impact on fisheries resources in the Nansha wetland of Pearl River Estuary. J. Fish. Sci. China 23, 661–671. doi: 10.3724/SP.J.1118.2016.15352
Keywords: Indo-Pacific humpback dolphin, line-transect survey, distribution, behavior, marine engineering, Lingding Bay
Citation: Huang Z, Fang L, Wen H, Zhang K, Wang X and Chen T (2024) Responses of Indo-Pacific humpback dolphins (Sousa chinensis) to construction of the Hong Kong–Zhuhai–Macao Bridge. Front. Mar. Sci. 11:1407937. doi: 10.3389/fmars.2024.1407937
Received: 27 March 2024; Accepted: 01 November 2024;
Published: 21 November 2024.
Edited by:
Stelios Katsanevakis, University of the Aegean, GreeceReviewed by:
Thomas A. Jefferson, Clymene Enterprises, United StatesMingli Lin, Chinese Academy of Sciences (CAS), China
Kimberly Nielsen, Oceans Initiative, United States
Copyright © 2024 Huang, Fang, Wen, Zhang, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinxing Wang, wangxinxing@scsfri.ac.cn; Tao Chen, chent@scsfri.ac.cn
 Zhixiong Huang1
Zhixiong Huang1