- Department of Gametes and Embryo Biology, Institute of Animal Reproduction and Food Research, Polish Academy of Sciences, Olsztyn, Poland
The natural resources of a country, including ichthyofauna, constitute a vital aspect of its national heritage. Fish populations are threatened with loss of biodiversity as a result of human activity (anthropopressure), resulting in water pollution, habitat destruction and overfishing. Additionally, the escalating threat is exacerbated by climate change, primarily manifested in periodic reservoir and watercourse desiccation. Genetic variability of captive is also threated as fish raised in hatcheries are susceptible to bacterial and viral diseases. Therefore, methodologies for fish sperm cryopreservation aimed at safeguarding the gene pool of both natural and captive fish populations assume paramount importance for their conservation and mitigation of irreversible losses, particularly crucial in light of increasing ecological disasters. This paper offers an overview of cryopreservation research in Poland, tracing back to early initiatives in the 1970s concerning carp (Cyprinus carpio) semen and culminating in recent advancements, where standardized cryopreservation methodologies were developed. We delve into the freezing results of semen of various fish species, encompassing both wild specimens like whitefish (Coregonus lavaretus) and lake minnows (Eupallasella percnurus), and farmed species such as sturgeons, carp, and numerous salmonid species. Additionally, we delineate projects that support such endeavors. Recent milestones in the establishment of fish sperm cryobanks in Poland catering to both wild and farmed species, including carp and rainbow trout (Oncorhynchus mykiss) – the most economically significant fish in Poland were presented. We also expound on the implementation of cryopreserved semen from sex-reversed rainbow trout in hatchery practices. Furthermore, we discuss significant challenges pertaining to sperm banking, particularly concerning funding and the practical utilization of cryostored semen samples for egg fertilization under hatchery conditions.
1 Introduction
The cryopreservation of fish semen offers a versatile technique for enhancing reproduction, breeding, and conservation efforts. It ensures the year-round availability of gametes, facilitates their transportation between farms, and supports artificial fertilization procedures (Cabrita et al., 2010; Martínez-Páramo et al., 2017). Cryopreserved sperm has proven instrumental in advancing genetic improvement programs, particularly in livestock like cattle, and holds promising potential for enhancing fish breeding initiatives (Torres et al., 2016). Furthermore, semen cryopreservation provides a secure means to safeguard genetic diversity in sperm banks, enabling the preservation and eventual reconstruction of original strains, populations, or genetic diversity. However, it is crucial to note that cryopreservation protocols must be meticulously tailored to each fish species. Despite ongoing research efforts aimed at establishing genome banks for sperm, the practical implementation of cryopreserved sperm in hatchery operations is still in its nascent stages for commercial applications (Tiersch, 2008; Cabrita et al., 2010). This review will focus on highlighting recent breakthroughs in fish sperm cryopreservation, which have paved the way for the establishment of sperm cryobanks in Poland.
2 Challenges of cryopreservation
The cryopreservation of sperm represents a multifaceted technique offering a plethora of advantages in fisheries management. These include the conservation of genetic diversity within fish populations, the preservation of gametes sourced from elite breeding individuals, facilitation of genetic material transport, and the provision of a reliable sperm reservoir independent of broodstock maturation synchronization (Judycka et al., 2019a; Bøe et al., 2021). However, despite considerable strides in standardizing cryopreservation protocols for fish semen, the practical application of cryopreserved sperm faces a myriad of challenges outweighing opportunities. A comprehensive analysis of these opportunities and challenges was previously presented in our review titled “Opportunities and challenges related to the implementation of sperm cryopreservation into breeding of salmonid fishes” (Judycka et al., 2019a). Within this context, a primary hurdle lies in upscaling cryopreservation methodologies to meet the demands of commercial fish breeders, particularly concerning the large-scale production of fertilized eggs and fry. Potential factors contributing to fish farmers’ hesitancy in adopting sperm cryopreservation techniques include perceived complexities and associated costs. Moreover, meticulous monitoring of cryopreserved semen containers is imperative to ensure samples are adequately submerged in liquid nitrogen. Additionally, given the species-specific variations in sperm quality and freezability, precise control and optimization of cryopreservation conditions are indispensable for each target species.
3 Advancements in fish sperm cryopreservation in Poland
3.1 History of cryopreservation and research groups in Poland
The first attempts at fish sperm cryopreservation in Southern Poland (Institute of Zootechnics, Kraków; Inland Research Institute, Zator) were initiated by Dr. Marek Moczarski for carp (Cyprinus carpio; Moczarski, 1977) and tench (Tinca tinca; Moczarski and Kołdras, 1982). Subsequent efforts were resumed in the 1990s by a group based in Northern Poland at the Warmia and Mazury University and the Institute of Animal Reproduction and Food Research, Olsztyn, led by Prof. Jan Glogowski and later continued by Dr. Radosław K. Kowalski’s group. Semen from several fish species were cryopreserved, including Northern pike (Esox lucius; Babiak et al., 1995), carp (Babiak et al., 1997), asp (Aspius aspius; Babiak et al., 1998a), rainbow trout (Oncorhynchus mykiss; Babiak et al., 1998b), and bream (Abramis brama; Glogowski et al., 1999). At the beginning of the 21st century, Prof. Andrzej Ciereszko joined the team, and cryopreservation studies progressed (Figure 1). New methods were developed for Siberian sturgeon (Acipenser baerii; Glogowski et al., 2002), research continued for whitefish (Coregonus lavaretus; Ciereszko et al., 2008), sex-reversed rainbow trout (Dietrich et al., 2014), and sex-reversed brook trout (Judycka et al., 2019b); brown trout (Salmo trutta; Nynca et al., 2014), huchen (Hucho hucho) and grayling (Thymallus thymallus; Nynca et al., 2015a), Siberian sturgeon (Judycka et al., 2015), lake minnow (Eupallasella percnurus; Dietrich et al., 2015), Northern pike (Dietrich et al., 2016), brook trout (Salvelinus fontinalis; Nynca et al., 2015b), salmon (Salmo salar; Nynca et al., 2016a), carp (Dietrich et al., 2017), sea trout (Salmo trutta m. trutta; Judycka et al., 2018a), European perch (Perca fluviatilis; Judycka et al., 2019c), Arctic charr (Salvelinus alpinus; Judycka et al., 2019d), pikeperch (Sander lucioperca; Judycka et al., 2021), as well as Atlantic sturgeon (Acipenser oxyrinchus; Judycka et al., 2024). Moreover, the aforementioned Team participated in 2016–2017 in establishing a cryopreserved semen bank for endangered fish in Poland, such as lake minnow, in collaboration with National Inland Fisheries Institute in Olsztyn storing a total of 400 straws (from 50 individuals of 8 populations). It has to be mentioned, that cryopreservation studies were complemented by research conducted by Dr. Katarzyna Dziewulska’s group from the University of Szczecin, Western Poland, focusing on the freezing of salmon and sea trout semen (Dziewulska et al., 2011; Dziewulska and Domagała, 2013).
3.2 Methods
3.2.1 Cryopreservation techniques
The initial attempts at cryopreserving sperm involved the use of vials, with freezing performed in nitrogen vapor (Moczarski and Kołdras, 1982). For most of cryopreservation trials in the 90-ties, sperm was cryopreserved mainly by pelleting on dry ice (-79°C). In the first decade of the 21st century, the method of pelleting on dry ice was gradually abandoned and 0.25 ml and 0.5 ml plastic straws (IMV Technologies, L’Agile, France) were introduced (Glogowski et al., 2002). These straws were frozen 3 cm above liquid nitrogen (in the vapor of liquid nitrogen) for 5 min in a Styrofoam box with an isolating Neopor block (Minitübe GmbH, Tiefenbach, Germany). In 2014, an efficient glucose-methanol extender was developed (Ciereszko et al., 2014) which was subsequently refined to optimize the cryopreservation of fish semen (primarily salmonids). This optimization involved controlling the final concentration of spermatozoa and cryoprotectants (Nynca et al., 2017; Judycka et al., 2018a; Judycka et al., 2019b, c, d, 2020, 2021).
3.2.2 Measurements of sperm motility
During the first decades, sperm motility of fresh and cryopreserved semen was subjectively evaluated. However, in the first decade of the 21st century, Computer-Assisted Sperm Analysis (CASA) was introduced for objective and advanced sperm motility analysis. The first system introduced was the Hobson Sperm Cell Tracker, developed by Hobson Vision Ltd., Bakewell, UK (Ciereszko et al., 2008). Subsequently, other systems followed, such as the Sperm Class Analyzer v. 4.0.0 developed by Microptic S.L, Barcelona, Spain (Dziewulska et al., 2011), the CRISMAS computer-assisted sperm analysis software (Image House Company, Copenhagen, Denmark; Judycka et al., 2015; Sarosiek et al., 2016a); and the CEROS II System (Hamilton Thorne, Beverely MA, USA; Judycka et al., 2019c).
3.2.3 Measurements of sperm concentration
Fish spermatozoa are small, therefore counting under the microscope is time-consuming. Fast methods for sperm concentration measurements are prerequisite, especially for the quick and successful execution of standardized cryopreservation procedures, which rely on controlling the number of spermatozoa in straws. Three methods are commonly used for this purpose: i) spectrophotometric method based on estimation of sperm concentration (Ciereszko and Dabrowski, 1993), ii) computer-aided fluorescence microscopy using NucleoCounter SP-100 (Chemometec, Allerød, Denmark; Nynca and Ciereszko, 2009), and iii) flow cytometry employing a portable cytometer, Muse Count and Viability Assay Reagent (Millipore, Burlington, MA, USA) (Nynca et al., 2016b). The latter two methods can also assess sperm viability.
3.2.4 Evaluation of fertilizing ability of cryopreserved semen
The fertilizing ability represents the most crucial and ultimate parameter of cryopreserved semen and has been measured in several studies, both at the stage of eyed eggs and hatched larvae, preferably employing a controlled sperm-to-egg ratio (for example, (Babiak et al., 1998b, Babiak et al., 1997; Glogowski et al., 2002; Ciereszko et al., 2008; Dziewulska et al., 2011; Dziewulska and Domagała, 2013; Ciereszko et al., 2014; Dietrich et al., 2014; Nynca et al., 2014; Judycka et al., 2015; Nynca et al., 2015b; Dietrich et al., 2016; Sarosiek et al., 2016b; Judycka et al., 2018b; Judycka et al., 2019c; Judycka et al., 2019d).
3.3 Existing sperm banks
3.3.1 Common carp
Semen cryopreservation has been utilized to establish a semen bank housing valuable carp genetic lines at the Institute of Ichthyobiology and Aquaculture of the Polish Academy of Sciences in Gołysz. A leader in carp breeding since the 1950s, the Institute has curated a unique collection of 17 breeding lines, forming a “living gene bank”. This living gene bank plays a pivotal role in national and ex situ programs for the protection and reproduction of aquatic animals, aligning with Poland’s commitment to the Convention on Biological Diversity. Semen cryopreservation activities for the carp breeding lines maintained in Gołysz were conducted in accordance with “National programs for maintaining the genetic reserve of farmed fish and the conservation of genetic resources of farm animals” (2003 to 2008) founded by Ministry of Agriculture and Rural Development, operational program “Sustainable development of the fisheries sector and coastal fishing areas”, 2.2. project title – “Protection of genetic resources of original pure lines of farmed carp” (2013–2016) and operational program “Fisheries and Sea” (PO RYBY 2014–2020), actions within Priority 2 - Supporting environmentally sustainable, resource-efficient, innovative, competitive, and knowledge-based aquaculture, the operation titled “Protection of the possessed genetic resources of fish implemented in accordance with national environmental protection programs and biodiversity restoration” (2017–2023). The primary goal of these programs is to preserve genetic variability and selection material for future breeding by safeguarding the gene pool of males across various breeding lines.
Cryopreservation activities were carried out in two phases, from 2003 to 2008 and 2013 to 2023, involving the freezing of semen from carp breeding lines: Polish lines (Landek line (2), Ochaby line (3), Gołysz line (6) and Knyszyn line (K)) and foreign lines such as Hungarian (8, 7, 0, W, T), Lithuanian (B, BVP), Ukrainian (U, Up), French (F), Israeli (D), German (N), Yugoslavian (J) as detailed in Table 1. The lines designated for freezing were selected by the staff of Institute in Gołysz. The selection process focused on endangered lines, particularly those whose breeding stock had diminished in recent years. The lines with the fewest breeders were prioritized for preservation, as were lines experiencing significant declines. This allowed us to protect the lines in the weakest state and those facing the greatest threats. Semen was collected from hormonally stimulated carp and samples showed a minimum of 70% motility were selected for cryopreservation. From 2003 to 2005, an effective pellet freezing method was used, involving the dilution of semen in Kurokura’s extender (with 10% DMSO and 10% egg yolk, Kurokura et al., 1984) before being dripped into solidified carbon dioxide (dry ice, -79°C) wells. The frozen semen took the form of pellets, which were then transferred into labeled plastic boxes and stored in liquid nitrogen (-196°C). This method ensured a high fertilization and hatching rate, typically ranging from 80% to 90%. Subsequently, in 2006, to align with modern cryopreservation standards, the method transitioned to plastic straw (volume 0.5 mL) freezing. The great advantage of straws was possibility to print the information concerning individual fish directly on straws with the use of straw printer. The developed method involved diluting semen in the Kopeika, Lahnsteiner or “Pike” extender (at 1:6 or 1:9 ratio) (Kopeika, 1986; Lahnsteiner et al., 2000; Bernáth et al., 2017). Semen was immediately packed into 0.5-mL straws and held in liquid nitrogen vapor 3 cm over the surface of the liquid nitrogen for 5 min. Afterward, the straws were placed into liquid nitrogen for storage. From 2013, each male’s semen was evaluated by thawing one straw in a 40°C water bath for 13 s, and post-thaw sperm motility showed a range of 42 to 66%. The cryopreserved semen, stored in both pellets and straws in Cryobank at the Cryogene company in Poland, is maintained under the supervision of the Institute in Gołysz. Detailed records of cryopreservation protocols, including extender compositions, dilution factors, and post-thaw motility, are documented, contributing significantly to the field of aquaculture and genetic preservation research.
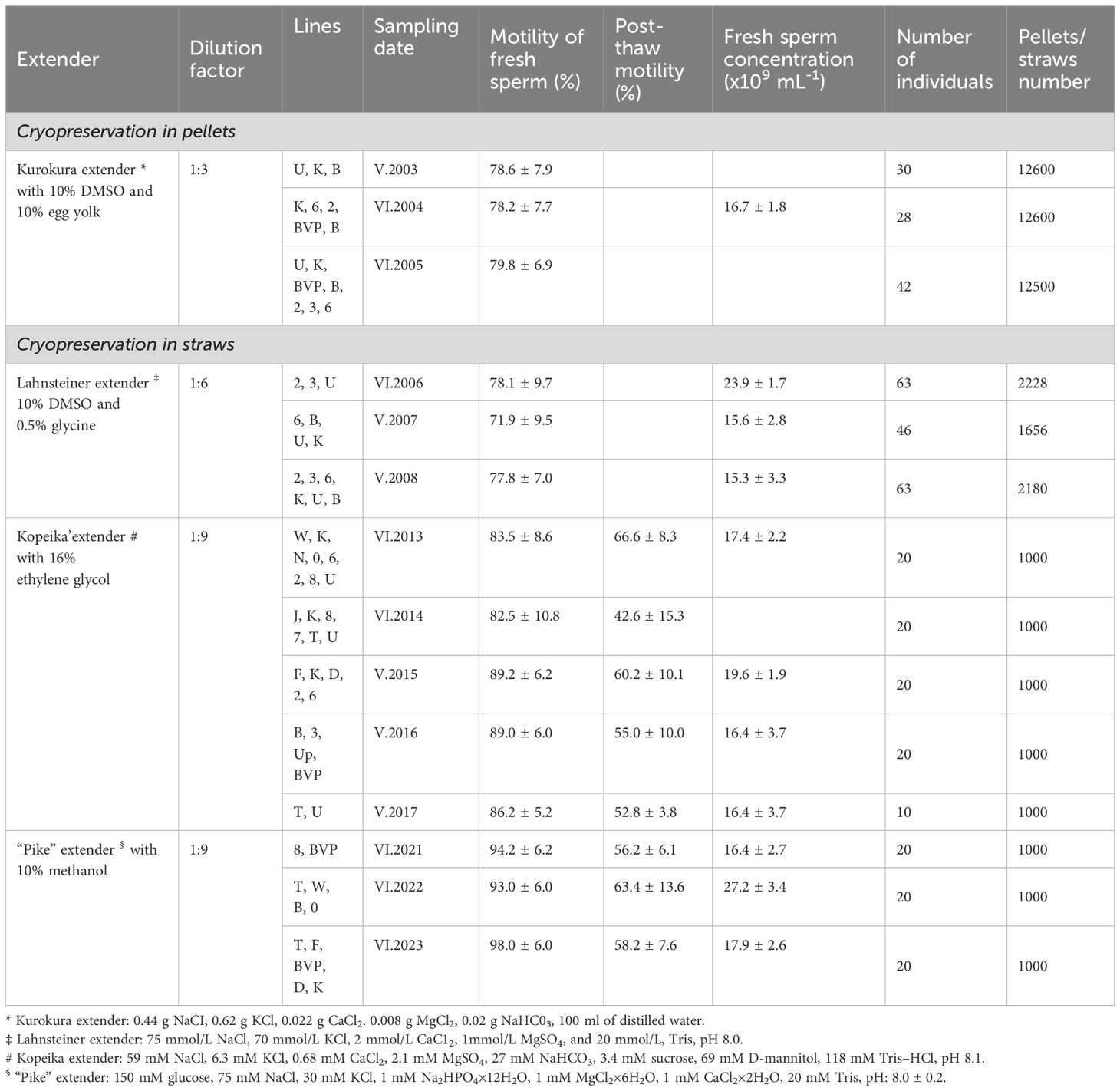
Table 1 Summary of semen cryopreservation protocols for common carp breeding lines maintained at the Institute of Ichthyobiology and Aquaculture, Gołysz.
In 2023, as part of the project “Protection of genetic resources of farmed and environmentally valuable freshwater and dual-environment fish”, we performed cryopreservation of semen from four breeding lines of carp (Izraelian: DOR-70, Bubianski: B, Zatorski: Z and Starzawski: SK) maintained at the Experimental Fish Farm in Zator, National Inland Fisheries Research Institute. Semen was collected from 60 non-stimulated male carps, and concurrently, a fin sample was taken from each individual for genetic analysis to facilitate future crossbreeding. The cryopreservation process involved diluting the semen at a 1:9 ratio with a ‘Pike’ extender, resulting in a post-cryopreservation motility of 43–51%, varying by lines (Table 2). Semen from 60 males were cryopreserved and is being stored in Fish Sperm Bank in Olsztyn. It has been emphasized, that these works started in 2022 when dr Kowalski’s team cryopreserved semen in 180 straws from 45 individuals.
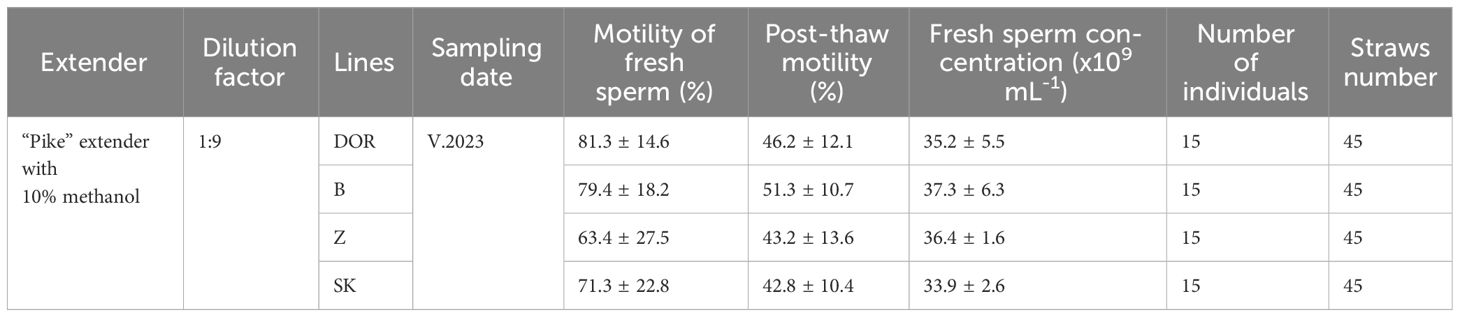
Table 2 Line-specific cryopreservation characteristics for selected carp breeding lines maintained at the Experimental Fish Farm in Zator, National Inland Fisheries Research Institute.
Summing up, currently, semen from carp lines maintained in two Polish breeding stations are under a cryopreservation program. This should secure the variability of carp in Poland, allowing the constant development of aquaculture for this species.
3.3.2 Autochthonous population of common whitefish in Łebsko Lake
The autochthonous population of common whitefish in Lake Łebsko, located within the Słowiński National Park, was first recognized in the 19th century and documented at the beginning of the 20th century (Ciereszko et al., 2008). This population is currently classified as endangered and is supported by stocking programs.
In 2006–2007, as part of the grant project “Protection of the autochthonous population of common whitefish in Lake Łebsko,” funded by EKOFUNDUSZ, Warsaw, we conducted initial research focusing on semen characteristics and the development of a semen cryopreservation procedure. Fish were collected by fishermen in 2006 (testicular and stripped, n = 17; Table 3) and 2007 (stripped, n = 19). Obtaining good-quality semen from captured fish posed a significant challenge, partially resolved through close cooperation with the fishermen. Semen characteristics, including volume, sperm concentration, motility parameters, and seminal plasma parameters, were established (Ciereszko et al., 2008). The efficacy of four cryoprotectants—methanol, glycerol, DMSO, and DMA—was evaluated. Methanol emerged as superior, ensuring both post-thaw sperm motility and fertilizing ability. Consequently, the cryopreservation procedure was refined, involving the use of an extender consisting of 0.3 M glucose and 10 or 15% methanol, with a semen-extender ratio of 1:3. Extended semen was then filled into 0.25 ml plastic straws and frozen in nitrogen vapor, positioned 3 cm above the surface of the liquid nitrogen. This procedure resulted in a decrease in sperm motility to half of the values observed in fresh semen, while maintaining similar fertilizing ability. Gonadal semen from six males (collection in 2006) was cryopreserved and currently stored in the Fish Sperm Bank in Olsztyn (Ciereszko et al., 2008).
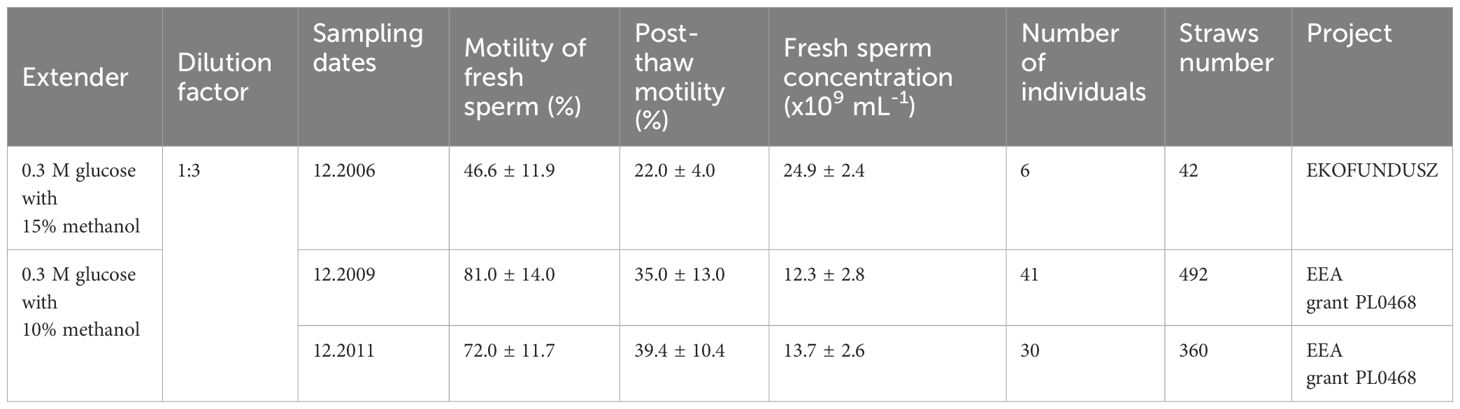
Table 3 Characteristics of fresh and cryopreserved sperm and number of straws of European whitefish maintained in the sperm bank of the Institute of Animal Reproduction and Food Research Polish Academy of Sciences in Olsztyn.
The cryopreservation efforts continued under the grant project “Ichthyological Biodiversity of Lakes – Elaborating a Model for Problem Solution: A Case Study of Natural Resources of Autochthonous Common Whitefish in Lake Łebsko (Łebsko Lake Whitefish),” funded by EEA grants. In 2009, semen from 41 males was collected using an improved procedure based on careful fish collection and limited semen specimen handling (5–6 per hour), characterized, and subsequently cryopreserved using the glucose (0.3 M)-methanol (10%) extender. Previously, methanol concentration at 10% resulted in obtaining similar fertilization ability to 15% methanol, therefore we decided to use in further experiments 10% methanol. Post-thaw sperm viability and motility were measured at 67% and 35%, respectively. In 2011, cryopreservation was performed for an additional 30 males, followed by cryopreservation of semen from 12 males in 2013. All these samples are currently stored in the Fish Sperm Bank in Olsztyn.
3.3.3 Sex-reversed females brook trout and rainbow trout
We have developed a cryopreservation procedure for Salmonid fish semen based on the glucose-methanol extender, whose effectiveness was further confirmed by high fertilization rates (Nynca et al., 2017; Judycka et al., 2018b; Judycka et al., 2019b; Judycka et al., 2019d). This formed the basis for establishing cooperation with the “Dąbie” Fish Hatchery and joint implementation of the NCRD project (Innovative Hatchery Project – Implementation of Semen Cryopreservation in the Development of Breeding Programs of Salmonid Fish, CRYOHATCH TANGO 1/266953/NCBR/2015), where a standardized cryopreservation procedure (in terms of sperm concentration and cryoprotectant concentrations) for sex-reversed female rainbow and brook trout semen was developed. The optimal conditions for cryopreservation of semen were 3.0×109 spermatozoa ml−1, 7.5% methanol and 0.15 M glucose for sex-reversed females rainbow trout, or 0.19 M glucose for sex-reversed females brook trout. In this elaborated procedure, semen mixed with cryoprotectants (glucose and methanol) was loaded into 0.5 ml plastic straws (IMV Technologies, L’Aigle, France), which were placed on a floating rack and equilibrated for 15 min on ice. After equilibration, the straws were frozen 3 cm above liquid nitrogen (in the vapor of liquid nitrogen) for 5 min in a Styrofoam box with an isolating Neopor block (Minitübe GmbH, Tiefenbach, Germany) and then placed in liquid nitrogen. The straws were then thawed by immersion in a water bath at 40°C for 10 s.
The cryopreservation of semen was performed in 2017–2018 resulting in the creation of a bank of cryopreserved semen from one line of sex-reversed female brook trout and nine lines of sex-reversed rainbow trout in cooperation with the Fish Hatchery “Dąbie”. A total of 19730 straws of cryopreserved semen were collected from 153 sex-reversed females (Table 4). This quantity ensured unlimited availability of semen for hatchery operations and enabled the execution of breeding crosses. Post-thaw sperm motility ranged from 4 to 77%, and it is noteworthy that different lines exhibited varying qualities of sperm motility and concentration in fresh semen, thus differing significantly in their suitability for cryopreservation. The lines designated for freezing were selected by the hatchery staff, and the cryopreserved semen is stored at the “Dąbie” Fish Hatchery.
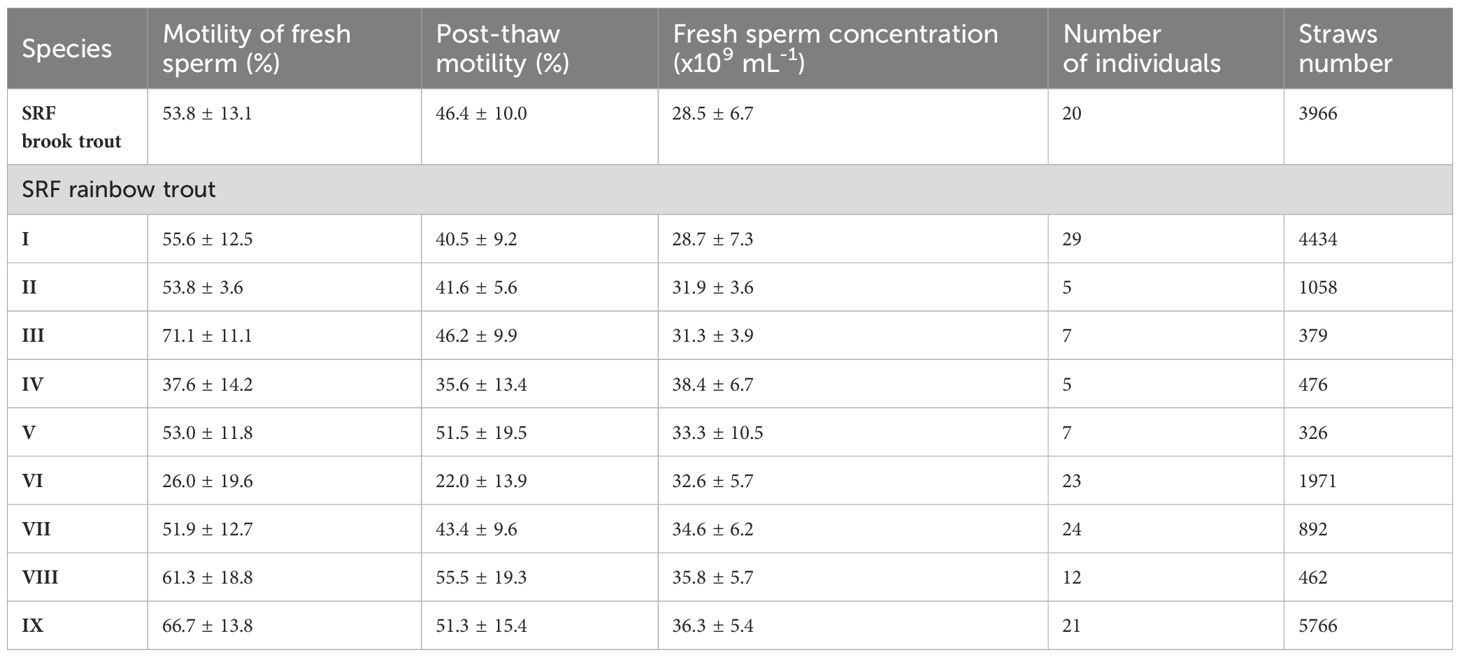
Table 4 Characteristics of fresh and cryopreserved sperm and number of straws of sex-reversed females brook trout and different lines of sex-reversed females rainbow trout maintained in the sperm bank of the Dabie hatchery.
3.3.4 Salmonid species and sturgeon
The experience gained during the development of cryopreservation procedures for different fish species led to the realization of the project titled “Protection of Genetic Resources of Farmed and Environmentally Valuable Freshwater and Dual-Environment Fish” in cooperation with the National Inland Fisheries Research Institute in Olsztyn. Within this project, a sperm cryobank is being established for rainbow trout (both spring and autumn lines), Atlantic salmon, whitefish, sea trout, grayling, and Atlantic sturgeon farmed in the Institute’s Stations (Table 5). The project commenced in 2022, and the cryopreservation of semen for each species will be conducted over the next few years. To date, approximately 1380 straws of cryopreserved semen from Salmonid fish and sturgeon are stored in this bank.
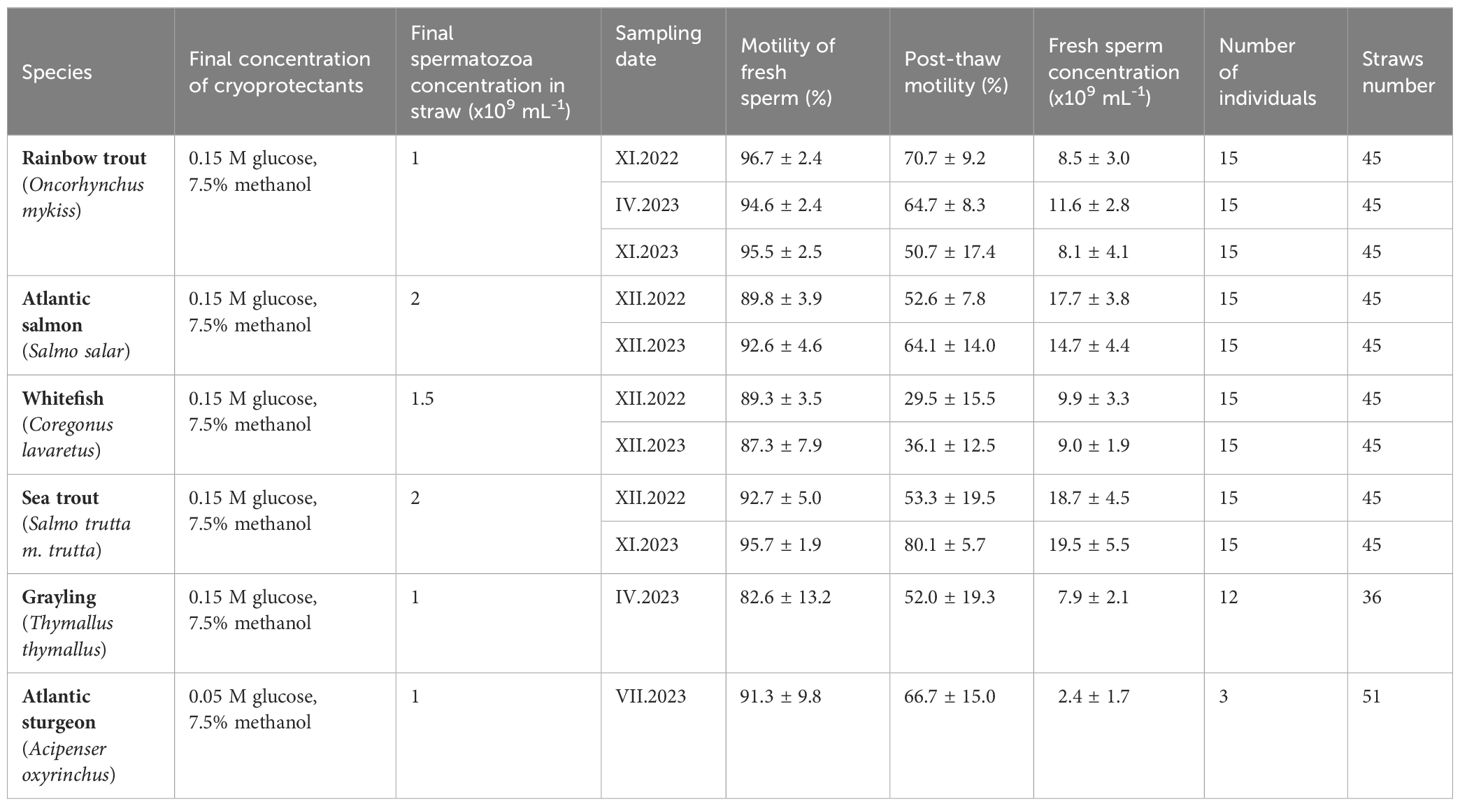
Table 5 Characteristics of fresh and cryopreserved sperm and number of straws of rainbow trout, Atlantic salmon, whitefish, sea trout grayling and Atlantic sturgeon cryopreserved with cooperation with National Inland Fisheries Research Institute.
Each cryopreservation trial started with the evaluation of fresh semen characteristics, such as sperm concentration and sperm motility parameters. Additionally, a fin clip was collected from each male to assess genetic variability, which will be utilized for more effective breeder selection in the future. The primary cryopreservation protocol involves freezing semen using a glucose-methanol extender and 0.5 ml plastic straws (as indicated above). However, it should be noted that species-specific differences in cryopreservation protocols were identified. For example, the optimal glucose concentration for almost all Salmonid fish species (except sex-reversed females brook trout) was found to be 0.15 M, whereas for Atlantic sturgeon, it appears to be 0.05 M. Moreover, also differences in the optimal values of final spermatozoa concentration were determined, ranging from 1 to 2×109 spermatozoa ml−1 (see Table 5). These differences can be attributed to variable values of sperm concentration in fresh semen for each species. Under optimal conditions, post-thaw sperm motility values ranged from approximately 30% for whitefish semen to 80%, which were recorded for sea trout.
4 Summary
In retrospect, research endeavors in fish sperm cryopreservation have spanned over the past five decades. Throughout this period, protocols for cryopreserving semen from a diverse array of fish species have been meticulously developed, alongside the establishment of comprehensive semen repositories catering to both wild and cultivated stocks. These repositories play a pivotal role in safeguarding genetic diversity, particularly in imperiled species, while concurrently serving as indispensable resources for advancing selective breeding programs. Furthermore, given the recent surge in ecological adversities, there arises an imperative to prioritize the cryopreservation of semen from wild fish species indigenous to their native habitats.
Author contributions
SJ: Writing – review & editing, Writing – original draft, Project administration, Funding acquisition, Conceptualization. MD: Writing – review & editing, Writing – original draft. JN: Writing – review & editing, Writing – original draft. AC: Writing – review & editing, Writing – original draft, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Research Projects No. 2018/29/N/NZ9/00761 and 2019/35/B/NZ9/03501 funded by the National Science Centre; and the project “Protection of Genetic Resources of Farmed and Environmentally Valuable Freshwater and Dual-Environment Fish”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Babiak I., Glogowski J., Brzuska E., Szumiec J., Adamek J. (1997). Cryopreservation of sperm of common carp, Cyprinus carpio L. Aquac. Res. 28, 567–571. doi: 10.1111/j.1365-2109.1997.tb01075.x
Babiak I., Glogowski J., Kujawa R., Kucharczyk D., Mamcarz A. (1998a). Cryopreservation of sperm from asp aspius aspius. Prog. Fish-Cult 60, 146–148. doi: 10.1577/1548-8640(1998)060<0146:COSFAA>2.0.CO;2
Babiak I., Glogowski J., Luczynski M. J., Goryczko K., Dobosz S., Kuzminski H. (1998b). The effect of individual male potency on fertilization ability of fresh and cryopreserved milt of rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 29, 337–340. doi: 10.1046/j.1365-2109.1998.00197.x
Babiak I., Glogowski J., Luczynski M. J., Kucharczyk D., Luczynski M. (1995). Cryopreservation of the milt of the northern pike. J. Fish. Biol. 46, 819–828. doi: 10.1111/j.1095-8649.1995.tb01604.x
Bernáth G., Ittzés I., Szabó Z., Horváth Á., Krejszeff S., Lujić J., et al. (2017). Chilled and post-thaw storage of sperm in different goldfish types. Reprod. Domest. Anim. 52, 680–686. doi: 10.1111/rda.12951
Bøe K., Bjøru B., Tangvold Bårdsen M., Nordtug Wist A., Wolla S., Sivertsen A. (2021). Opportunities and challenges related to sperm cryopreservation in Atlantic salmon gene banks. Conserv. Sci. Pract. 3. doi: 10.1111/csp2.552
Cabrita E., Sarasquete C., Martínez-Páramo S., Robles V., Beirão J., Pérez-Cerezales S., et al. (2010). Cryopreservation of fish sperm: applications and perspectives. J. Appl. Ichthyol. 26, 623–635. doi: 10.1111/jai.2010.26.issue-5
Ciereszko A., Dabrowski K. (1993). Estimation of sperm concentration of rainbow trout, whitefish and yellow perch using a spectrophotometric technique. Aquaculture 109, 367–373. doi: 10.1016/0044-8486(93)90175-X
Ciereszko A., Dietrich G. J., Nynca J., Dobosz S., Zalewski T. (2014). Cryopreservation of rainbow trout semen using a glucose-methanol extender. Aquaculture 420–421, 275–281. doi: 10.1016/j.aquaculture.2013.11.014
Ciereszko A., Dietrich G. J., Wojtczak M., Sobocki M., Hliwa P., Kuźmiński H., et al. (2008). Characterization and cryopreservation of whitefish (Coregonus lavaretus L.) semen from Lake Lebsko, Poland. Fundam. Appl. Limnol. 173, 59–65. doi: 10.1127/1863-9135/2008/0173-0059
Dietrich G. J., Nynca J., Dobosz S., Zalewski T., Ciereszko A. (2014). Application of glucose–methanol extender to cryopreservation of semen of sex-reversed females rainbow trout results in high post-thaw sperm motility and fertilizing ability. Aquaculture 434, 27–32. doi: 10.1016/j.aquaculture.2014.07.015
Dietrich G. J., Nynca J., Szczepkowski M., Dobosz S., Szczepkowska B., Ciereszko A. (2016). The effect of cryopreservation of semen from whitefish (Coregonus lavaretus) and northern pike (Esox lucius) using a glucose-methanol extender on sperm motility parameters and fertilizing ability. Aquaculture 464, 60–64. doi: 10.1016/j.aquaculture.2016.06.015
Dietrich G. J., Wolnicki J., Słowińska M., Sikorska J., Hliwa P., Kamiński R., et al. (2015). Short-term storage and cryopreservation of lake minnow (Eupallasella percnurus (Pallas 1814)) sperm. J. Appl. Ichthyol. 31, 75–78. doi: 10.1111/jai.12727
Dietrich M. A., Irnazarow I., Ciereszko A. (2017). Proteomic identification of seminal plasma proteins related to the freezability of carp semen. J. Proteomics 162, 52–61. doi: 10.1016/j.jprot.2017.04.015
Dziewulska K., Domagała J. (2013). Spermatozoa concentration influences cryopreservation success in sea trout (Salmo trutta m. trutta L.). Theriogenology 80, 659–664. doi: 10.1016/j.theriogenology.2013.06.013
Dziewulska K., Rzemieniecki A., Czerniawski R., Domagała J. (2011). Post-thawed motility and fertility from Atlantic salmon (Salmo salar L.) sperm frozen with four cryodiluents in straws or pellets. Theriogenology 76, 300–311. doi: 10.1016/j.theriogenology.2011.02.007
Glogowski J., Babiak I., Kucharczyk D., Luczynski M., Piros B. (1999). Some properties of bream Abramis brama L. sperm and its cryopreservation. Aquac. Res. 30, 765–772. doi: 10.1046/j.1365-2109.1999.00399.x
Glogowski J., Kolman R., Szczepkowski M., Horváth Á., Urbányi B., Sieczyński P., et al. (2002). Fertilization rate of Siberian sturgeon (Acipenser baeri, Brandt) milt cryopreserved with methanol. Aquaculture 211, 367–373. doi: 10.1016/S0044-8486(02)00003-0
Judycka S., Żarski D., Dietrich M. A., Palińska-Żarska K., Karol H., Ciereszko A. (2019c). Standardized cryopreservation protocol of European perch (Perca fluviatilis) semen allows to obtain high fertilization rates with the use of frozen/thawed semen. Aquaculture 498, 208–216. doi: 10.1016/j.aquaculture.2018.08.059
Judycka S., Dietrich M. A., Żarski D., Karol H., Palińska-Żarska K., Błażejewski M., et al. (2021). Towards standardization of the cryopreservation procedure of cultured pikeperch (Sander lucioperca) semen. Aquaculture 538, 736539. doi: 10.1016/j.aquaculture.2021.736539
Judycka S., Dietrich M. A., Szczepkowska B., Szczepkowski M., Liszewska E., Ciereszko A. (2024). Evaluation of the effects of glucose concentration in extender and sperm concentration in straw for cryopreservation of Atlantic sturgeon (Acipenser oxyrinchus) semen. Aquac. Rep. 35, 101995. doi: 10.1016/j.aqrep.2024.101995
Judycka S., Nynca J., Ciereszko A. (2019a). Opportunities and challenges related to the implementation of sperm cryopreservation into breeding of salmonid fishes. Theriogenology 132, 12–21. doi: 10.1016/j.theriogenology.2019.03.022
Judycka S., Nynca J., Dietrich M. A., Liszewska E., Ilgert J., Ciereszko A. (2019d). Development of an efficient and standardized method for the cryopreservation of Arctic charr milt and its use in the fertilization of brook trout eggs to produce ‘sparctic’ hybrids. Aquaculture 513, 734363. doi: 10.1016/j.aquaculture.2019.734363
Judycka S., Nynca J., Liszewska E., Dobosz S., Grudniewska J., Ciereszko A. (2018a). Optimal sperm concentration in straws and final glucose concentration in extender are crucial for improving the cryopreservation protocol of salmonid spermatozoa. Aquaculture 486, 90–97. doi: 10.1016/j.aquaculture.2017.12.019
Judycka S., Nynca J., Liszewska E., Dobosz S., Słowińska M., Różyński R., et al. (2018b). Cryopreserved rainbow trout semen can be used for the fertilization of up to 8000 eggs in a single application. Aquaculture 490. doi: 10.1016/j.aquaculture.2018.02.026
Judycka S., Nynca J., Liszewska E., Mostek A., Ciereszko A. (2019b). Comparative analysis of sperm freezability of sex-reversed female brook trout and sex-reversed female rainbow trout semen. Aquaculture 498. doi: 10.1016/j.aquaculture.2018.08.064
Judycka S., Słowińska M., Nynca J., Liszewska E., Dobosz S., Ciereszko A. (2020). Effects of glucose, methanol concentration, and time of equilibration on post-thaw sperm motility of rainbow trout semen. Aquaculture 520, 734996. doi: 10.1016/j.aquaculture.2020.734996
Judycka S., Szczepkowski M., Ciereszko A., Dietrich G. J. (2015). New extender for cryopreservation of Siberian sturgeon (Acipenser baerii) semen. Cryobiology 70, 184–189. doi: 10.1016/j.cryobiol.2015.02.005
Kopeika E. F. (1986). “Instruction on low temperature preservation of sperm carp,” in VNIRO on Fish Breeding (Publ. Co. Rybnoye).
Kurokura H., Hirano R., Tomita M., Iwahashi M. (1984). Cryopreservation of carp sperm. Aquaculture 37, 267–273. doi: 10.1016/0044-8486(84)90159-5
Lahnsteiner F., Berger B., Horvath A., Urbanyi B., Weismann T. (2000). Cryopreservation of spermatozoa in cyprinid fishes. Theriogenology 54, 1477–1498. doi: 10.1016/S0093-691X(00)00469-6
Martínez-Páramo S., Horváth Á., Labbé C., Zhang T., Robles V., Herráez P., et al. (2017). Cryobanking of aquatic species. Aquaculture 472, 156–177. doi: 10.1016/j.aquaculture.2016.05.042
Moczarski M. (1977). Deep freezing of carp Cyprinus carpio L. sperm. Bull. Acad. Pol. Sci. Biol. 25, 187–190.
Moczarski M., Kołdras M. (1982). Properties of tench - Tinca tinca L. sperm and experiments with freezing it at -196°C. Acta Ichthyol. Piscat. 12, 41–49. doi: 10.3750/AIP
Nynca J., Ciereszko A. (2009). Measurement of concentration and viability of brook trout (Salvelinus fontinalis) spermatozoa using computer-aided fluorescent microscopy. Aquaculture 292, 256–258. doi: 10.1016/j.aquaculture.2009.04.020
Nynca J., Dietrich G. J., Dobosz S., Grudniewska J., Ciereszko A. (2014). Effect of cryopreservation on sperm motility parameters and fertilizing ability of brown trout semen. Aquaculture 433, 62–65. doi: 10.1016/j.aquaculture.2014.05.037
Nynca J., Dietrich G. J., Dobosz S., Zalewski T., Ciereszko A. (2015b). Effect of postthaw storage time and sperm-to-egg ratio on fertility of cryopreserved brook trout sperm. Theriogenology 83, 253–256. doi: 10.1016/j.theriogenology.2014.09.009
Nynca J., Dietrich G. J., Grudniewska J., Dobosz S., Liszewska E., Krzyś M., et al. (2015a). Efficient method for cryopreservation of European huchen (Hucho hucho L.) and grayling (Thymallus thymallus L.) semen. Aquaculture 435, 146–151. doi: 10.1016/j.aquaculture.2014.09.031
Nynca J., Dietrich G. J., Liszewska E., Judycka S., Karol H., Dobosz S., et al. (2016b). Usefulness of a portable flow cytometer for sperm concentration and viability measurements of rainbow trout spermatozoa. Aquaculture 451, 353–356. doi: 10.1016/j.aquaculture.2015.09.027
Nynca J., Judycka S., Liszewska E., Dobosz S., Ciereszko A. (2017). Standardization of spermatozoa concentration for cryopreservation of rainbow trout semen using a glucose-methanol extender. Aquaculture 477, 23–27. doi: 10.1016/j.aquaculture.2017.04.036
Nynca J., Judycka S., Liszewska E., Dobosz S., Grudniewska J., Arai K., et al. (2016a). Utility of different sugar extenders for cryopreservation and post-thaw storage of sperm from Salmonidae species. Aquaculture 464, 340–348. doi: 10.1016/j.aquaculture.2016.07.014
Sarosiek B., Dryl K., Judycka S., Dobosz S., Grudniewska J., Kowalski R. K. (2016b). Cryopreservation method for whitefish (Coregonus lavaretus) semen possible for use in large-scale fertilization. Aquac. Res. 47. doi: 10.1111/are.2016.47.issue-12
Sarosiek B., Dryl K., Krejszeff S., Żarski D. (2016a). Characterization of pikeperch (Sander lucioperca) milt collected with a syringe and a catheter. Aquaculture 450, 14–16. doi: 10.1016/j.aquaculture.2015.06.040
Tiersch T. R. (2008). Strategies for commercialization of cryopreserved fish semen. Rev. Bras. Zootecn. 37, 15–19. doi: 10.1590/S1516-35982008001300003
Keywords: sperm, cryopreservation, germplasm, cryobanking, biodiversity
Citation: Judycka S, Dietrich MA, Nynca J and Ciereszko A (2024) Preservation of fish male germplasm in Poland. Front. Mar. Sci. 11:1407895. doi: 10.3389/fmars.2024.1407895
Received: 27 March 2024; Accepted: 09 May 2024;
Published: 31 May 2024.
Edited by:
Yusuf Bozkurt, Iskenderun Technical University, TürkiyeReviewed by:
Viktoriya Dzyuba, University of South Bohemia in České Budějovice, CzechiaElias Figueroa, Universidad Católica de Temuco, Chile
Copyright © 2024 Judycka, Dietrich, Nynca and Ciereszko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sylwia Judycka, cy5qdWR5Y2thQHBhbi5vbHN6dHluLnBs
 Sylwia Judycka
Sylwia Judycka Mariola A. Dietrich
Mariola A. Dietrich Joanna Nynca
Joanna Nynca Andrzej Ciereszko
Andrzej Ciereszko