- 1College of Ecology and Environment, Hainan University, Haikou, China
- 2State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan University, Haikou, China
- 3School of Marine Biology and Fisheries, Hainan University, Haikou, China
- 4China Ocean Engineering Construction General Bureau, Haikou, China
Introduction: In 2023, a comprehensive survey was conducted at Qiziwan National Marine Park (QZW) to assess marine environmental variables, coral communities, and ecosystem parameters. This study aimed to evaluate the current state of coral ecosystems across different regions within the park.
Methods: The survey covered 19 sampling stations distributed among four regions: Xiaojiao Park, Zhongjiao Park, Dajiao Park, and Shayutang Village. Various marine environmental parameters were measured, and coral species were identified and recorded.
Results: A total of 67 species of reef-building corals belonging to 25 genera were identified. Dominant species included Porites lutea, Goniopora columna, Platygyra sinensis, Favites flexuosa, Galaxea fascicularis, and Acropora millepora. Coral coverage was found to be low at Xiaojiao Park, with high levels of turbidity and dissolved inorganic nitrogen, likely due to the nearby Changhua Port. Dajiao Park's sandy substrate made it unsuitable for coral survival. In contrast, Zhongjiao Park and Shayutang Village exhibited the best coral ecosystems. Redundancy analysis indicated that turbidity and substrate types were significant factors influencing coral distribution.
Discussion: The live coral coverage at Xiaojiao Park, parts of Zhongjiao Park, and Dajiao Park declined from 14.03% in 2017 to 11.73% in 2023. This decline is potentially attributed to frequent marine construction and land use changes. Anthropogenic disturbances appear to have worsened environmental conditions, undermining coral reef survival at QZW.
Introduction
Coral reefs are vital components of marine ecosystems and play a crucial role in maintaining ocean health (González-Barrios et al., 2021). Despite covering less than 1% of the ocean floor, coral reefs support approximately 25% of global marine biodiversity (Hughes et al., 2023; Voolstra et al., 2023). In coral reef ecosystems, factors such as water quality, coral distribution, coral recruitment, fish, and the diversity and quantity of large benthic organisms are closely interrelated, forming an interdependent ecological network (Hempson et al., 2018). Water quality, including temperature, salinity, and nutrient levels, is a significant factor affecting coral health and distribution (Woods et al., 2016). Good water quality is essential for the successful settlement and growth of juvenile corals (Cruz and Harrison, 2017). Coral cover and recruitment rates are vital indicators of coral reef health (Harrison et al., 2021). Successful settlement and growth of juvenile corals are fundamental to maintaining and renewing coral reef communities (Monroe et al., 2018). Investigating the distribution and recruitment of corals in different regions provides valuable insights into the reproductive capacity and recovery potential of coral reefs, offering important references for the protection and restoration of coral reef ecosystems. The abundance and diversity of fish, along with the types and quantities of macrobenthic organisms, are important indicators of coral reef health (Massei et al., 2023). They contribute to the biodiversity of coral reefs and play a crucial role in maintaining the balance and functionality of coral reef ecosystems (Horoszowski-Fridman et al., 2024). In summary, comprehending the intricate interactions among corals, coral reef biodiversity, and environmental shaping factors is crucial for effectively protecting and managing coral reef ecosystems (Massei et al., 2023).
Despite the significant ecological and economic value of coral reef ecosystems, they face multiple threats from climate change, overfishing, pollution, and habitat destruction (Ruppert et al., 2018). Rising sea temperatures resulting from global climate change lead to coral bleaching, severely impacting coral survival and reproductive capacity (Figueiredo et al., 2022; van Woesik et al., 2022; Nama et al., 2023). On a local scale, human activities such as overfishing and pollution have immediate impacts on coral health (Cramer et al., 2020). Overfishing can disrupt the delicate balance in coral reef ecosystems by letting certain species become overly abundant (Bilan et al., 2023). Pollution (e.g., nutrient runoff, oil spills, and plastic waste) introduces toxic substances into the marine environment and sets off a chain of detrimental events through the entire coral ecosystem (Blanckaert et al., 2023). In addition, tourism and marine recreation may also harm coral reefs. Snorkelers, divers, and sightseers can unintentionally injure corals from physical contact, and they also damage the water quality from sunscreen usage, discharging wastewater, using boat anchors, etc (Worachananant et al., 2008; Tashiro and Kameda, 2013). Therefore, by understanding coral communities and the environmental pressures they face in typical marginal coral reefs, we can not only gain a deeper understanding of the resilience of these ecosystems, but also develop targeted strategies to mitigate threats.
Whereas for the Hainan Island in general, the rich fringing reefs near the coastline have decreased by at least 80% over the past 30 years (Huang et al., 2020). The Qiziwan National Marine Park (QZW), a tourist attraction on the west coast of Hainan Island in China, is renowned for its calm bay waters and abundant coral reef resources. It has a tropical monsoon climate, with low rainfall and minimal impact from extreme weather conditions such as typhoons and thunderstorms. There are minimal seasonal variations in the wind direction, and the prevailing winds are easterly and southerly throughout the year. However, it is still at risk due to the nearby ports and their dredging activities, as coral reef ecosystems become vulnerable in coastal desertification landscapes.
In this work, we report a comprehensive assessment of the coral reefs at QZW in 2023. We also surveyed the composition of benthic organisms and fish in the coral reefs, and comparisons were made against previous surveys carried out in 2015 and 2017. By analyzing the water quality and the local human activities, we examined various factors affecting the spatial distribution of coral reef organisms at QZW to find out the primary cause of localized coral reef degradation.
Materials and methods
Study area
QZW is off the west coast of Hainan Island. Figure 1 shows that the sampling stations of QZW belonged to four regions, i.e., Xiaojiao Park (Station 1), Zhongjiao Park (Stations 2–9), Dajiao Park (Stations 10–16), and Shayutang Village (Stations 17–19). Xiaojiao Park is near Changhua Port and is an area with significant human activities. Zhongjiao Park is on the west side of QZW and has received protection in recent years. Dajiao Park is on the north side of QZW. It is a tourist destination, and popular activities include swimming and fishing. Shayutang Village is on the east side of QZW. It has fewer visitors than Dajiao Park, and the scenic areas are mostly the sandy beaches along the shore.
To overcome logistical challenges and ensure the thoroughness and accuracy of data collection despite adverse weather conditions and limited manpower, the present survey was conducted in two phases. In the first phase (August 2023), the water quality was monitored at all 19 stations, and the coral cover was surveyed at Stations 1–9 and 12–16. In the second phase (September 2023), the survey was carried out at Stations 3-6, 10-11, and 17–19, examining not only the coral cover but also the juvenile corals, fish communities, and macrobenthic organisms.
Environmental variables
Seawater temperature, turbidity, salinity, and pH were measured in-situ using an OS310 CTD profiler, an AQUAlogger 310TY turbidity logger, and a YSI water quality analyzer. The monthly average sea surface temperature (SST) was obtained from MODIS 178 Aqua level-3 products (https://oceancolor.gsfc.nasa.gov/) for January 2014 to October 2023.
At each station, seawater samples were collected from the bottom layer near the coral reef substrate using a water sampler at both 3 m and 8 m depth (1 L each), then stored in polypropylene sampling bottles at -20°C. The dissolved inorganic nutrient salts, including dissolved inorganic nitrogen (DIN, as the sum of NH4+, NO3−, and NO2−), inorganic silicates, and phosphates, were analyzed using an AA3 HR Nutrient Autoanalyzer (SEAL, Germany). The total alkalinity of the sampled seawater was measured using a Hanon T960 Basic Automatic Titrator, and the average value from multiple measurements was taken.
Survey of coral, benthic organisms, and fish communities
The survey was conducted according to the method of Zhu et al. (2022) with the following adaptations. At each station, the Linear Point Intercept Method was used to assess the coverage of macroalgae, colonial anemones, rocks (>15 cm), rubbles (0.5–15 cm), sand (<0.5 cm), and other substrates along a 60 m belt. Within 2.5 m on both sides of the belt, the composition and distribution density were surveyed for both fish species and large invertebrate species in the coral reef. The density of juvenile corals (0.4–5 cm in diameter) was carefully counted by visual census in at least 40 random quadrates (0.5 m × 0.5 m) along each 60 m transect (Edmunds et al., 1998). Juvenile corals were identified to genus level. Stony corals, soft corals, and gorgonians were identified to species level according to Veron (2000); Dai (2009), and Zou (2001) by He Zhao, and then confirmed by Yuxiao Ren. The coverage of dead coral on the transect was determined by analyzing the camera images.
Statistical analysis
All data were analyzed and visualized using Microsoft Excel 2023, SPSS 26.0, Origin 2023b, or R. The monitored water quality indicators and identified biological data were recorded using Microsoft Excel 2023. The dissolved inorganic carbon (DIC) was calculated using the CO2SYS software (Pierrot et al., 2006), and graphs were generated using Origin 2023b. The multivariate relationships between coral cover and water quality parameters were analyzed using the Correlation Plot APP in Origin 2023b, and the similarity between stations were compared through systematic clustering analysis in Origin 2023b. The niche width of corals was calculated using the SPecies Association Analysis package in R. The vegan package in R was used to calculate the Shannon diversity index (H’), the Margalef richness index (d), and the Pielou’s evenness index (J).
Results
Satellite remote sensing of SST
Although the water temperature shown in the graph fluctuates significantly, there is no significant long-term upward or downward trend in the seawater temperature of QZW (Supplementary Figure S1). This indicates that while the seawater temperature in the region has remained generally stable over the past 10 years, there are still significant seasonal changes and extreme high temperature events. The water temperature of QZW has approached or exceeded 30°C multiple times, particularly in the years 2014, 2017, 2019, 2020, and 2022. The high temperatures during these years may be caused by global warming or local climate anomalies. In 2023, there were also multiple instances where the water temperature exceeded 30°C.
Environmental variables
Significant spatial variations were observed in key environmental parameters, including seawater temperature (WT), turbidity, dissolved oxygen (DO), salinity (SAL), pH, dissolved inorganic carbon (DIC), dissolved inorganic nitrogen (DIN), phosphate (PO43−), and silicate (SiO32−), across the 19 stations at QZW (Supplementary Figures S2–S7).Overall, WT at QZW ranged from 28.70 to 30.27°C (mean 29.44°C) at 3 m depth and from 28.61 to 29.81°C (mean 29.29°C) at 8 m depth. Notably, Dajiao Park exhibited the highest mean temperature (29.64°C at 3 m, 29.55°C at 8 m), suggesting a localized thermal anomaly. Turbidity levels varied significantly, with the highest turbidity recorded at Dajiao Park at 3 m depth (20.79 FTU) and at Xiaojiao Park at 8 m depth (30.29 FTU), indicating differing levels of sediment disturbance across sites. Dissolved oxygen levels were highest at Dajiao Park, with averages of 6.46 mg·L−1 at 3 m and 6.54 mg·L−1 at 8 m. This elevated DO concentration may enhance aerobic processes and support diverse marine life in this area. Salinity showed minimal spatial variation, although Shayutang Village had the highest values (33.21‰ at 3 m, 33.29‰ at 8 m), possibly due to localized evaporation effects or unique water exchange dynamics. The pH levels remained consistently alkaline across QZW, with minor variation (8.134 at 3 m and 8.133 at 8 m), indicating a stable carbonate buffering system conducive to coral growth. The highest DIC concentrations were recorded at Shayutang Village (1979.13 μmol·L−1 at 3 m, 1972.57 μmol·L−1 at 8 m). This stability in DIC levels suggests efficient mixing and carbon cycling within the water column, minimizing vertical stratification.
Spatial heterogeneity was also evident in the distribution of nutrients across the 19 stations at QZW (Supplementary Table S1). The DIN reached its highest levels at Zhongjiao Park at 3 m depth (8.96 μmol·L−1) and at Shayutang Village at 8 m depth (9.96 μmol·L−1). This variation in DIN concentrations likely reflects differences in nutrient input and local biogeochemical processes. The PO43− concentrations were found to be highest at Shayutang Village at both 3 m (0.111 μmol·L−1) and 8 m (0.205 μmol·L−1). These elevated phosphate levels suggest localized sources of phosphorus, potentially from anthropogenic activities. The SiO32− concentrations were also highest at Zhongjiao Park, with values of 6.82 μmol·L−1 at 3 m depth and 7.10 μmol·L−1 at 8 m depth. The elevated silicate levels at this site may be attributed to terrestrial runoff or benthic fluxes, influencing the availability of this essential nutrient for diatom growth.
These findings underscore the spatial heterogeneity of environmental conditions at QZW, highlighting the need for site-specific management strategies to address ecological challenges and support the conservation of coral reef ecosystems.
Spatial distribution of benthic composition and coral communities
The distribution of substrate types across the surveyed stations is depicted in Figure 2A. Coral coverage varied significantly, ranging from 0.00% to 17.83% (mean 6.40%) at 3 m depth and from 0.00% to 24.83% (mean 10.77%) at 8 m depth. Notably, at Xiaojiao Park (Station 1), coral coverage at 3 m depth was 9.67%, whereas the substrate at 8 m depth was entirely sand with no coral presence. This suggests significant spatial variability in substrate composition. At 3 m depth, distinct clusters of substrate types were observed among Stations 12, 13, 14, and 15, and another cluster among Stations 1, 2, 5, and 6 (Figure 2B). Similarly, at 8 m depth, Stations 12, 13, 14, and 15 formed one cluster, while Stations 16 and 17 formed another. These clusters indicate spatial heterogeneity in substrate types across different depths.

Figure 2 Analysis of benthic composition and coral communities. (A) Substrate Type: Distribution of different substrate types (e.g., sand, rubble, rock) across the sampling stations. (B) Cluster Analysis: Grouping of sampling stations based on similarities in benthic composition. (C) Coral Species Composition: Relative abundance of different coral species at each station. (D) Coral Diversity (Shannon diversity index, Species richness, Species evenness index) of coral communities.
The number of coral genera at each station ranged from 0 to 9 (mean 5.8) at 3 m depth and from 0 to 10 (mean 7.8) at 8 m depth (Figure 2C). The coral communities were predominantly composed of Porites, Goniopora, Galaxea, and Acropora. Zhongjiao Park (Stations 2-9) exhibited the highest coral presence, followed by Shayutang Village (Stations 17-19). Coral diversity was notably higher at 8 m depth compared to 3 m depth (Figure 2D). These indices underscore the variability in coral diversity and evenness among different stations and depths.
These results highlight the significant spatial heterogeneity in substrate types and coral communities across the study area. The observed patterns in coral coverage, genera distribution, and diversity indices suggest that local environmental conditions and habitat characteristics play crucial roles in shaping coral reef ecosystems.
Coral communities, fish communities, and macrobenthic organisms
Analysis of the recorded videos captured on-site facilitated the identification of a diverse array of reef-building corals, comprising 67 species from 25 genera (Supplementary Table S2). Notably, among the identified species, Porites lutea, Goniopora columna, Platygyra sinensis, Favites flexuosa, Galaxea fascicularis, and Acropora millepora emerged as dominant (Figure 3A). These dominant species collectively demonstrate a broad ecological niche, as evidenced by their widespread distribution and abundance across the surveyed habitat (Figure 3B).
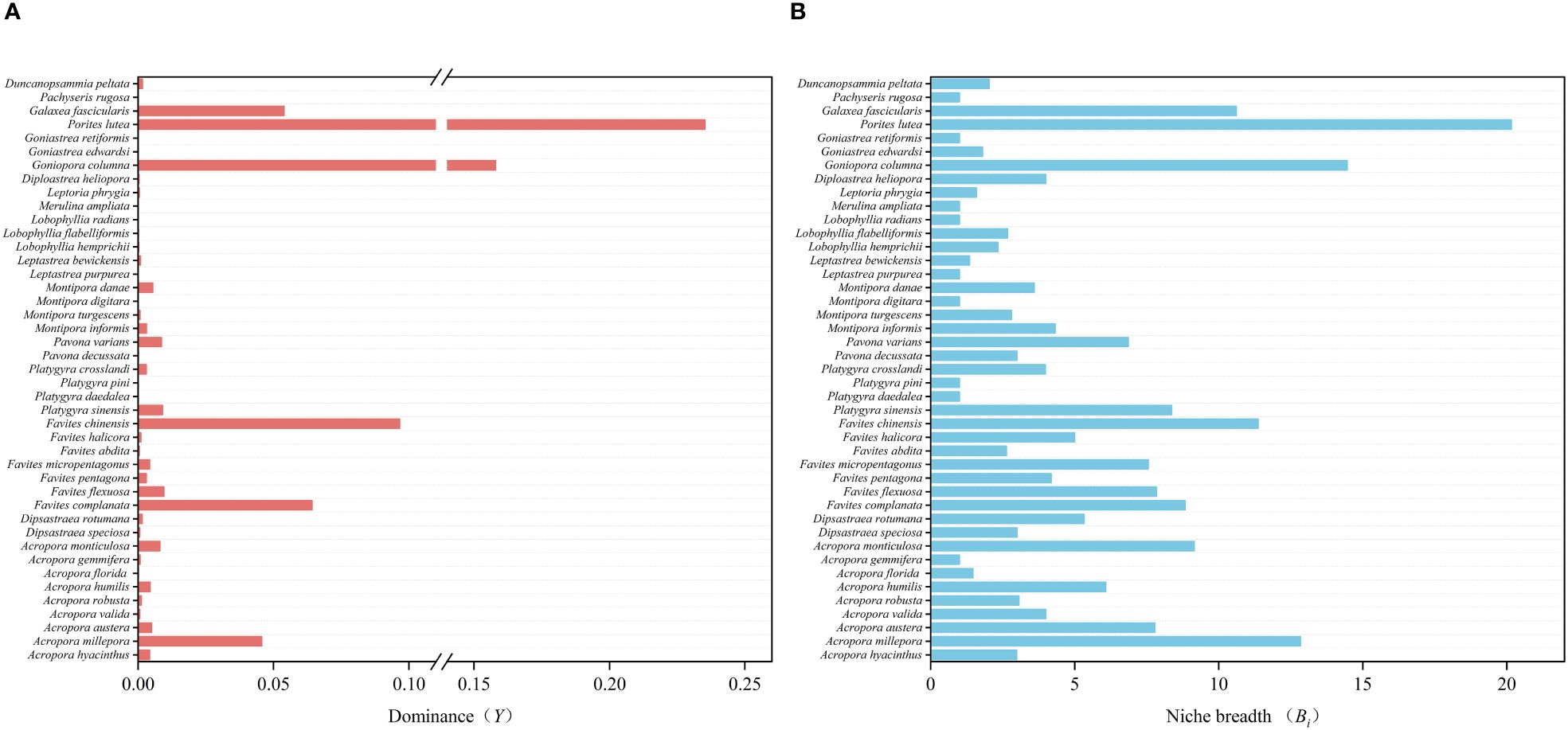
Figure 3 Analysis of coral communities. (A) Dominance: Dominance index of coral species, indicating the most prevalent species in the community. (B) Ecological Niche: Analysis of the ecological niches occupied by different coral species, illustrating their functional roles within the ecosystem.
Due to the constraints outlined previously, the examination of juvenile corals, fish communities, and benthic organisms was confined to stations characterized by higher coral coverage (Stations 3-6, 10, 11, 17-19). Analysis of the findings reveals notable disparities across these stations and depths. In Figure 4A, coral recruitment displayed considerable variability across stations and depths. Station 6 exhibited the highest recruitment, with 3.2 ind.·m−2 at 3 m depth and 2.8 ind.·m−2 at 8 m depth. In contrast, Station 10 recorded the lowest recruitment at 3 m depth (0.4 ind.·m−2), while Station 11 had the lowest recruitment at 8 m depth (0.4 ind.·m−2). This indicates spatial variability in coral recruitment, possibly influenced by local environmental conditions. Primary coral recruitment was attributed to Porites and Goniopora, with supplementary recruitment from Acropora. This underscores the importance of these coral genera in facilitating reef recovery and resilience. Figure 4B highlights variations in fish density across stations and depths. At 3 m depth, the highest fish density was observed at Station 6 (3.33 ind.·100m−2), while Station 11 recorded no fish. Similarly, at 8 m depth, Station 19 exhibited the highest density (12.78 ind.·100m−2), whereas both Stations 10 and 11 had the lowest density (0.40 ind.·100m−2). These findings suggest a heterogeneous distribution of fish populations, likely influenced by factors such as habitat complexity and resource availability. Figure 4C depicts the abundance of macrobenthic organisms, with Station 18 recording the highest count (6 species) at 3 m depth and Station 10 recording the highest count (5 species) at 8 m depth. This indicates variability in benthic community composition.
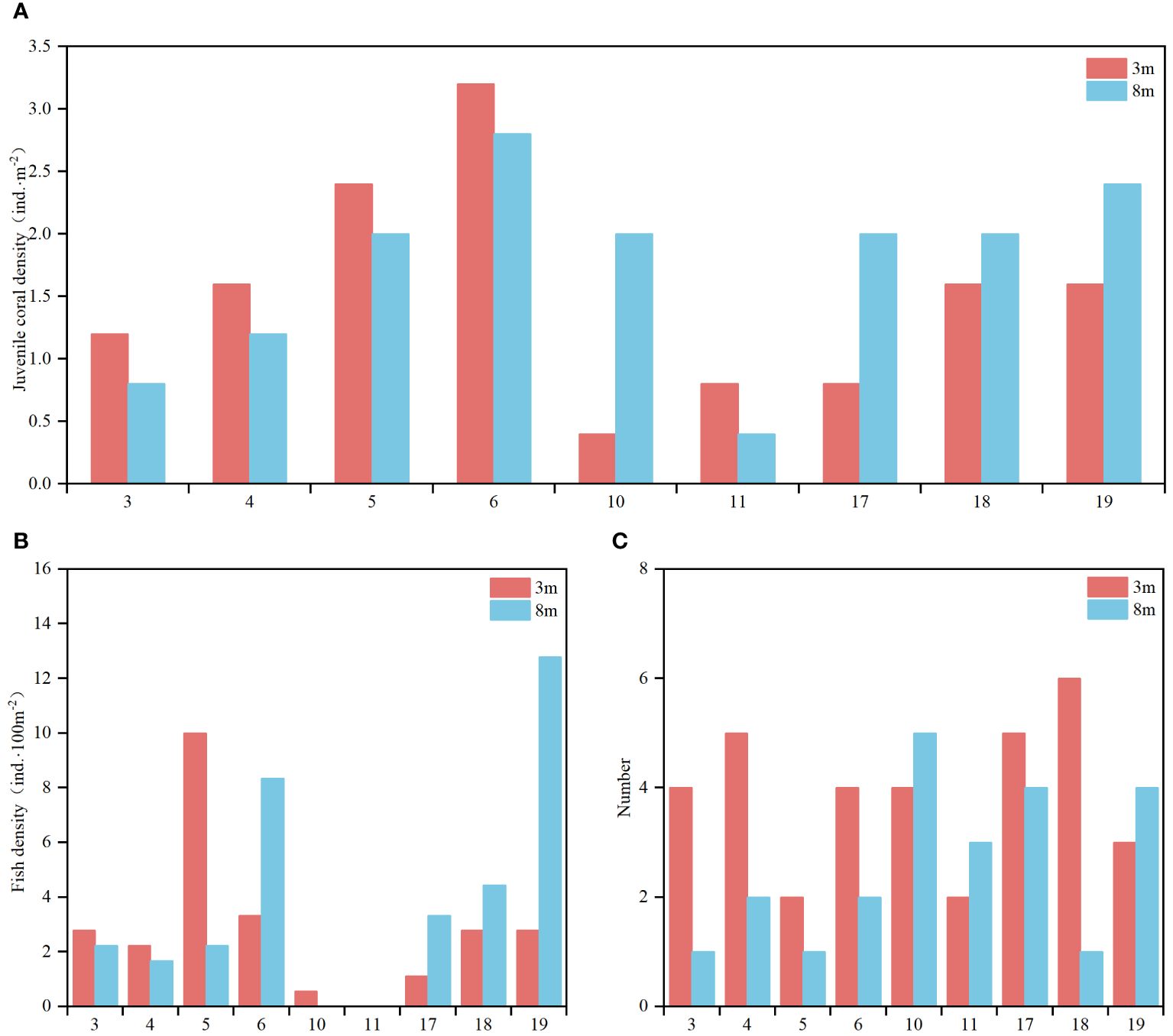
Figure 4 Analysis of juvenile corals, fish communities, and macrobenthic organisms. (A) Density of juvenile coral: Number of coral larvae per unit area at each sampling station. (B) Fish Density: Number of fish per unit area, providing insight into the health and biodiversity of fish communities. (C) Number of Macrobenthic Organism Species: Species count of macrobenthic organisms at each station.
The relationship between environmental parameters and reef condition
Environmental variables significantly influenced the spatial variations in reef benthic composition and coral communities. As shown in Figure 5A, Redundancy Analysis (RDA) revealed that the first and second axes explained 94.91% and 2.51% of the variance in reef benthic substrata, respectively. Key environmental factors, including turbidity (P = 0.001), sand (P = 0.001), and rubble (P = 0.001), significantly impacted reef benthic substrata. These findings indicate that water turbidity was crucial factors affecting the structure of reef ecosystems, while the proportions of sand and rubble could influence habitat suitability and stability for benthic organisms.
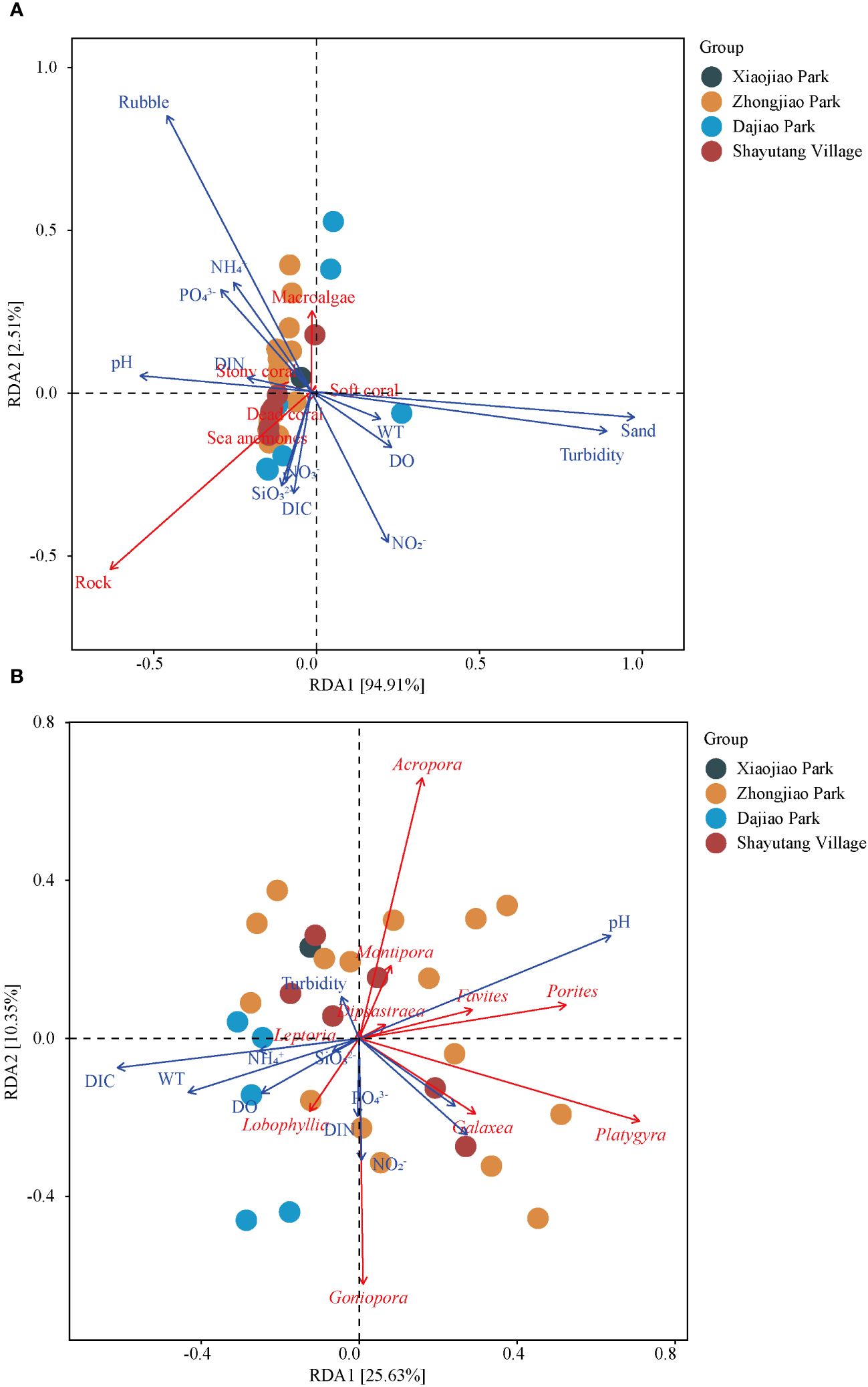
Figure 5 Redundancy analysis between environmental variables and ecological parameters. (A) Reef Benthic Substrata: Correlation between environmental variables (e.g., temperature, salinity) and the composition of benthic substrata. (B) Coral Communities: Correlation between environmental variables and the structure of coral communities, identifying key factors influencing coral health.
Additionally, as shown in Figure 5B, the first and second RDA axes explained 25.63% and 10.35% of the variance in coral community composition, respectively. Significant influences on coral communities were attributed to pH (P = 0.010) and DIC (P = 0.029). This suggests that variations in seawater pH and the carbonate system play important roles in the health and distribution of coral communities.
Temporal changes of coral communities
A comparative analysis of coral community data from Qi (2017) and the present study reveals temporal changes in coral coverage at QZW. As shown in Figure 6A, the average coral coverage decreased from 14.03% in 2017 to 11.73% in 2023. Further comparison with the findings of Zhou et al. (2017), who surveyed Shayutang Village and other coastal areas of Hainan from 2014 to 2015, provides additional context. In 2015, the live coral coverage at Shayutang Village was approximately 21.9%, with an H’ of 3.48. In the current survey, Shayutang Village exhibited a mean coral coverage of 5.72% at 3 m depth and 17.56% at 8 m depth, with an H’ of 3.229 (Figures 6B, C). This decline in coral coverage and diversity indicates a shift in the coral community structure over time. These findings highlight the dynamic nature of coral ecosystems at QZW, underscoring the importance of continuous monitoring and adaptive management strategies.

Figure 6 Ecological changes in Qiziwan National Marine Park. (A) Changes in Coral Coverage at Stations 1-10 from 2017 to 2023: Temporal trends in coral coverage, highlighting areas of growth or decline. (B) Change in Coral Coverage at Shayutang Village from 2015 to 2023: Long-term changes in coral coverage at a specific village site. (C) H’ of Coral Species at Shayutang Village: Variation in species diversity over time, indicating ecological stability or shifts.
Discussion
Degradation of coral ecosystems
Due to global warming and local climate anomalies, the frequency of extreme high-temperature events in the QZW area has increased over the past decade, potentially causing serious impacts on the local ecosystem. Environmental changes, along with human activities such as overfishing, pollution, and tourism, have put pressure on the local coral reefs in QZW (Cabral-Tena et al., 2020; Vaher et al., 2022). Compared to 2017, the average coral coverage at Stations 1-10 decreased from 14.03% to 11.73% (Qi, 2017). Similarly, compared to 2015, the mean coral coverage in Shayutang Village at 3 m water depth dropped from 21.9% to 5.72% (Zhou et al., 2017). The notable decrease in coral coverage at QZW suggests significant degradation of the coral ecosystems. QZW has a lower amount of coral juvenile supplementation, indicating a decline in the resilience of coral reefs (Harrison et al., 2021). The abundance of fish and benthic species in Qiziwan National Marine Park is relatively low, which may negatively affect the health, stability, and sustainability of the local coral reef ecosystem (Massei et al., 2023). According to local fishermen, QZW has been affected by overfishing, physical damage, and other human disturbances in the past. The deteriorating coral and reduced biodiversity indicate that the coral reef ecosystem of QZW is deteriorating, posing a threat to its health, stability, and sustainability.
Influencing factors on coral ecosystems
The type of substrate significantly impacts coral coverage (Otaño-Cruz et al., 2019). No live corals or coral recruitment were observed at Station 1 (8 m depth) or at Stations 12, 13, 14, and 15 (Dajiao Park) at both 3 m and 8 m depths. These sites featured purely sandy substrates, which are unfit for coral survival. It has been suggested that hard and stable substrates, such as rocks or existing coral skeletons, provide a better environment for coral attachment (Hoffmann et al., 2022). Stable attachment is challenging for juvenile corals on sandy substrates due to the high mobility of sand particles. Corals often die when transported or buried by sand (Sherman et al., 2016; dela Cruz and Harrison, 2020). At Station 16, the substrate was a mix of sand and reef, providing a less detrimental environment for coral attachment. Consequently, coral coverage was no longer zero.
Seawater turbidity, typically caused by suspended solid particles, algae, and other organic matter, significantly affects coral distribution by impacting light penetration and sedimentation (Bessell-Browne et al., 2017). High turbidity harms coral growth and health by reducing light penetration, resulting in inadequate energy levels, weaker growth, and reduced reproduction (Carlson et al., 2022; Travaglione et al., 2023). Stephen and Ruth (2004) summarized threshold values for Red Sea coral reef systems (<100 m depth), indicating that minimally impaired corals require turbidity levels less than 2.0 NTU. However, at QZW, the average turbidity was 8.57 FTU at 3 m depth and 11.04 FTU at 8 m depth. This indicates that higher turbidity is a key factor affecting coral distribution (Morgan et al., 2016). Elevated turbidity at Station 1 is partially attributed to its proximity to Changhua Harbor, where fishing activities and harbor construction significantly influence turbidity. Additionally, the sandy substrate at 8 m depth at Station 1 leads to an accumulation of excessive suspended solids. Due to the unique topography of QZW, geographically similar and closely positioned stations, such as Station 9 and Station 10, exhibit significant differences in turbidity. Noticeable differences in coral cover and coral species exist between these two locations. Station 10 exhibited higher coral cover and the presence of anemones and soft corals. This variation may result from the specific topography and substrate types surrounding the sites, emphasizing the interplay of these factors. Turbidity significantly influences the distribution of coral species. Corals thriving in turbid regions are predominantly robust, colony-forming species with greater tolerance. Acropora are mainly found in areas with low turbidity due to their low resilience and inability to adapt to high turbidity environments.
Nitrogen has been identified as the primary factor causing water eutrophication, and the concentration of DIN is a critical environmental factor in coral reef areas. The DIN at QZW (8.5 μmol/L) was also well above the threshold (1.0 μmol/L) considered suitable for coral reefs (Stephen and Ruth, 2004). Both NO2− and NH4+ were identified by RDA as key factors negatively affecting coral distribution (Figure 5). A high level of NO2−, which is typically an intermediate product in nitrogen cycling, signals the accumulation of nitrogen waste. A high level of NH4+, which is a primary nitrogen source, may be linked to pollution and human activities. The ongoing expansion of Changhua Port may create nitrogen-rich wetlands and sediments in estuarine and coastal areas, which escalates the discharge of nitrogen into the seawater (Song et al., 2023). In addition, the fertilizers used in agriculture near Changhua Town contain nitrates and ammonium salts, which can enter rivers and estuaries through runoff and end up in the ocean, thus increasing nitrogen levels in the seawater (Maggi et al., 2023).
The H’ and CR indices of the coral reefs in Shayutang Village have significantly declined compared to previous survey results (Zhou et al., 2017). Such changes are typically the result of the interaction between multiple natural and human factors (Vaher et al., 2022). In 2022, a famous Chinese entertainment program was filmed in Shayutang Village, attracting a large number of tourists to the small village. The influx of tourists may have exceeded the environmental carrying capacity of the area, greatly impacting the corals. Furthermore, since the trial operation of the Changjiang Haini Nuclear Power Plant began in 2015, the role of hot water discharge in coral degradation still needs to be determined through water quality measurements.
Environmental factors such as substrate type, turbidity, and DIN are not isolated, and they collectively shape coral reef ecosystems. Future research needs to delve deeper into analyzing and understanding their intricate relationships, and the response of coral ecosystems to multiple factors needs to be examined to develop comprehensive management strategies (Dong et al., 2018; Tan et al., 2023).
The ecological functions of dominant coral species
Dominant species typically play a leading role in ecosystems due to their strong growth and reproductive capabilities (Xu et al., 2017). The dominant corals compete and coexist with other corals and organisms, providing survival opportunities for different species (Connell, 1961; van Woesik et al., 2020). The hard skeletal structures formed by reef-building corals facilitate sedimentation, stabilize sediments, reduce turbidity, improve water quality, and mitigate the impact of waves and storm surges, thereby protecting the coastline (Saha et al., 2016). The branching structures or tentacles of A. millepora and G. columna provide shelter for numerous marine organisms, including small fish, crustaceans, and other invertebrates (George et al., 2021), thus allowing them to reproduce in safe spaces. Compared to faster-growing branching corals, such as P. lutea, P. sinensis, F. flexuosa, and G. fascicularis, massive corals are better suited to environmental changes, such as rising water temperatures and nutrient enrichment (Schlöder and D’Croz, 2004). Their adaptation strategies and resource utilization capabilities help them survive despite climate change and other stressors. The dominant coral species at QZW mainly included P. lutea, G. columna, P. sinensis, F. flexuosa, G. fascicularis, and A. millepora. These six species contributed significantly to the stability and diversity of the ecosystem because of their crucial roles as the dominant species in coral reef ecosystems. Therefore, the predominant types of coral recruits at QZW belonged to the genera Porites, Goniopora, and Acropora.
The results of this study indicate that despite the ongoing degradation, there are signs of community turnover in the coral reefs at QZW, which help maintain the fragile ecological balance. For example, although the six dominant coral species have broad niche widths, there is relatively little overlap in their ecological niches with other corals. It can be inferred that through their ecological niches and resource utilization strategies, the dominant coral species prevent the coral reef ecosystem at QZW from falling into rapid decline (Conti-Jerpe et al., 2022).
Protection and management of coral ecosystems
The significant loss of coral reefs at QZW is comparable to the general situation at Hainan Island (Huang et al., 2020). Although large-scale construction and tourism development remain limited at QZW, the region is still impacted by frequent marine engineering projects. Currently, to manage resources and promote the recovery of degraded coral reefs, the local government has implemented measures such as prohibiting overfishing and removing predators. Nevertheless, abandoned fishing nets and arrows have caused irreparable damage to coral reefs, and these waste materials need to be carefully cleaned up in the future.
The coral reefs at Zhongjiao Park exhibited the highest health status based on established evaluation criteria, including live coral coverage and juvenile coral supplementation (Huang et al., 2012; Williams et al., 2013; Zhao et al., 2013). These criteria consider factors such as minimal human interference, good water quality, and rich biodiversity, which collectively contribute to the overall health of coral reefs. These thriving reefs effectively promote the recovery and development of nearby coral reef ecosystems. The protection of the Zhongjiao Park region is crucial as its reefs have high ecological value and are indispensable in maintaining the overall ecological balance at QZW. We recommend designating the Zhongjiao Park region as a protected core zone and intensifying the ecological conservation efforts therein. To avoid irreversible harm to coral reefs, activities that may disrupt the coral reef ecosystem within this core protected area should be minimized if not eliminated.
This study also reveals the significance of coral management and protection at QZW. The results clarified the impact of environmental factors on coral reef health, which could assist the development of more effective conservation measures. It is particularly important to mitigate the adverse impacts by controlling human activities, pollution, and excessive development. Additional research can further explore these issues to enhance our understanding of how to protect the valuable ecosystem. Based on our findings, we recommend closer collaboration with local governments, environmental organizations, and international partners to develop sustainable ecological conservation policies. Our study also offers prospects for advancing ecotourism to stimulate local economic development. We emphasize the importance of education and public awareness to ensure that more people understand the significance of coral reefs and conservation measures.
Conclusions
This survey carried out in 2023 at QZW recorded 67 hard coral species from 25 genera. Compared with previous surveys, the decrease in live coral coverage indicated that local coral populations in QZW were gradually deteriorating. Eutrophication was evident in the water bodies at QZW primarily due to nitrogen, and substrate type and turbidity were key environmental factors affecting the corals. The species of fish and large invertebrates in QZW were relatively few, exhibiting low density and poor biodiversity. This indicated that the fishery resources of QZW have not been effectively protected. The current findings suggest that environmental factors and human activities play crucial roles in shaping the structure of coral communities. Restoring valuable coral reef ecosystems relies on improving land management and reducing human pressures.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HZ: Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. JZ: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. HY: Writing – review & editing, Writing – original draft, Visualization, Resources, Project administration, Funding acquisition. YPL: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. XLiu: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. YCL: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. WZ: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. AW: Writing – review & editing, Visualization, Validation, Resources, Project administration, Funding acquisition. XLi: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work were financially supported by the National Natural Science Foundation of China (42161144006 or 3511/21 and 42076108), the Innovative Talent Foundation of Hainan Province (KJRC2023C39) and Coral Reef Protection Project in Qiziwan National Marine Park (HD-KYH-2023192).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1407803/full#supplementary-material
References
Bessell-Browne P., Negri A. P., Fisher R., Clode P. L., Duckworth A., Jones R. (2017). Impacts of turbidity on corals: The relative importance of light limitation and suspended sediments. Mar. Pollut. Bull. 117, 161–170. doi: 10.1016/j.marpolbul.2017.01.050
Bilan M., Gori A., Grinyó J., Biel-Cabanelas M., Puigcerver-Segarra X., Santín A., et al. (2023). Vulnerability of six cold-water corals to sediment resuspension from bottom trawling fishing. Mar. Pollut. Bull. 196, 115423. doi: 10.1016/j.marpolbul.2023.115423
Blanckaert A. C. A., Grover R., Marcus M.-I., Ferrier-Pagès C. (2023). Nutrient starvation and nitrate pollution impairs the assimilation of dissolved organic phosphorus in coral-Symbiodiniaceae symbiosis. Sci. Total Environ. 858, 159944. doi: 10.1016/j.scitotenv.2022.159944
Cabral-Tena R. A., López-Pérez A., Alvarez-Filip L., González-Barrios F. J., Calderon-Aguilera L. E., Aparicio-Cid C. (2020). Functional potential of coral assemblages along a typical Eastern Tropical Pacific reef tract. Ecol. Indic. 119, 106795. doi: 10.1016/j.ecolind.2020.106795
Carlson R. R., Li J., Crowder L. B., Asner G. P. (2022). Large-scale effects of turbidity on coral bleaching in the Hawaiian islands. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.969472
Connell J. H. (1961). The influence of interspecific competition and other factors on the distribution of the barnacle chthamalus stellatus. Ecology 42, 710–723. doi: 10.2307/1933500
Conti-Jerpe I. E., Pawlik J. R., Finelli C. M. (2022). Evidence for trophic niche partitioning among three temperate gorgonian octocorals. Coral Reefs 41, 907–920. doi: 10.1007/s00338-022-02279-y
Cramer K. L., Jackson J. B. C., Donovan M. K., Greenstein B. J., Korpanty C. A., Cook G. M., et al. (2020). Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci. Adv. 6, eaax9395. doi: 10.1126/sciadv.aax9395
Cruz D., Harrison P. L. (2017). Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Sci. Rep. 7, 13985. doi: 10.1038/s41598-017-14546-y
Dai C. F. (2009). Scleractinia Fauna of Taiwan: Complex Group Vol. 1 (Taibei: National Taiwan University Press).
dela Cruz D. W., Harrison P. L. (2020). Enhancing coral recruitment through assisted mass settlement of cultured coral larvae. PloS One 15, e0242847. doi: 10.1371/journal.pone.0242847
Dong Z.-g., Chen Y.-h., Ge H.-x., Li X.-y., Wu H.-l., Wang C.-h., et al. (2018). Response of growth and development of the Pacific oyster (Crassostrea gigas) to thermal discharge from a nuclear power plant. BMC Ecol. 18, 31. doi: 10.1186/s12898-018-0191-y
Edmunds P. J., Aronson R. B., Swanson D. W., Levitan D. R., Precht W. F. (1998). Photographic versus visual census techniques for the quantification of juvenile corals. Bull. Mar. Sci. 62, 937–946.
Figueiredo J., Thomas C. J., Deleersnijder E., Lambrechts J., Baird A. H., Connolly S. R., et al. (2022). Global warming decreases connectivity among coral populations. Nat. Climate Change 12, 83–87. doi: 10.1038/s41558-021-01248-7
George E. E., Mullinix J. A., Meng F., Bailey B. A., Edwards C., Felts B., et al. (2021). Space-filling and benthic competition on coral reefs. PeerJ 9, e11213. doi: 10.7717/peerj.11213
González-Barrios F. J., Cabral-Tena R. A., Alvarez-Filip L. (2021). Recovery disparity between coral cover and the physical functionality of reefs with impaired coral assemblages. Global Change Biol. 27, 640–651. doi: 10.1111/gcb.15431
Harrison P. L., dela Cruz D. W., Cameron K. A., Cabaitan P. C. (2021). Increased coral larval supply enhances recruitment for coral and fish habitat restoration. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.750210
Hempson T. N., Graham N. A. J., MacNeil M. A., Hoey A. S., Wilson S. K. (2018). Ecosystem regime shifts disrupt trophic structure. J. Appl. Ecol. 28, 191–200. doi: 10.1002/eap.1639
Hoffmann J. J. L., Michaelis R., Mielck F., Bartholomä A., Sander L. (2022). Multiannual seafloor dynamics around a subtidal rocky reef habitat in the North sea. Remote Sens. 14, 2069. doi: 10.3390/rs14092069
Horoszowski-Fridman Y. B., Izhaki I., Katz S. M., Barkan R., Rinkevich B. (2024). Shifting reef restoration focus from coral survivorship to biodiversity using Reef Carpets. Commun. Biol. 7, 141. doi: 10.1038/s42003-024-05831-4
Huang J., Wang F., Zhao H., Xu H., Liu S., Xu Q., et al. (2020). Reef benthic composition and coral communities at the Wuzhizhou Island in the south China sea: The impacts of anthropogenic disturbance. Estuarine Coast. Shelf Sci. 243, 106863. doi: 10.1016/j.ecss.2020.106863
Huang H., Zhang C. L., Yang J. H., Feng Y., Lian J. S., Tan Y. H. (2012). Scleractinian coral community characteristics in Zhubi reef sea area of Nansha lslands. J. Appl. Oceanogr. 31, 79–84.
Hughes T. P., Baird A. H., Morrison T. H., Torda G. (2023). Principles for coral reef restoration in the anthropocene. One Earth 6, 656–665. doi: 10.1016/j.oneear.2023.04.008
Maggi F., Tang F. H. M., Tubiello F. N. (2023). Agricultural pesticide land budget and river discharge to oceans. Nature 620, 1013–1017. doi: 10.1038/s41586-023-06296-x
Massei K., Souza M. C. S., Silva R., Costa D., Vianna P. C. G., Crispim M. C., et al. (2023). Analysis of marine diversity and anthropogenic pressures on Seixas coral reef ecosystem (northeastern Brazil). Sci. Total Environ. 905, 166984. doi: 10.1016/j.scitotenv.2023.166984
Monroe A. A., Ziegler M., Roik A., Röthig T., Hardenstine R. S., Emms M. A., et al. (2018). In situ observations of coral bleaching in the central Saudi Arabian Red Sea during the 2015/2016 global coral bleaching event. PloS One 13, e0195814. doi: 10.1371/journal.pone.0195814
Morgan K. M., Perry C. T., Smithers S. G., Johnson J. A., Gulliver P. (2016). Transitions in coral reef accretion rates linked to intrinsic ecological shifts on turbid-zone nearshore reefs. Geology 44, 995–998. doi: 10.1130/G38610.1
Nama S., Shanmughan A., Nayak B. B., Bhushan S., Ramteke K. (2023). Impacts of marine debris on coral reef ecosystem: A review for conservation and ecological monitoring of the coral reef ecosystem. Mar. Pollut. Bull. 189, 114755. doi: 10.1016/j.marpolbul.2023.114755
Otaño-Cruz A., Montañez-Acuña A. A., García-Rodríguez N. M., Díaz-Morales D. M., Benson E., Cuevas E., et al. (2019). Caribbean near-shore coral reef benthic community response to changes on sedimentation dynamics and environmental conditions. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00551
Pierrot D., Lewis E., Wallace R., Wallace D., Wallace W., Wallace D. W. R. (2006). MS Excel Program Developed for CO2 System Calculations. ORNL/CDIAC-105a (Oak Ridge, Tennessee: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy).
Qi Z. J. (2017). Chess Bay Marine Tourism Complex Project Marine Environmental Impact Assessment Report (Ocean and Fisheries Bureau of Changjiang Li Autonomous County: Guangdong Sanhai Environmental Protection Technology Company Limited). e67–e79
Ruppert J. L. W., Vigliola L., Kulbicki M., Labrosse P., Fortin M.-J., Meekan M. G. (2018). Human activities as a driver of spatial variation in the trophic structure of fish communities on Pacific coral reefs. Global Change Biol. 24, e67–e79. doi: 10.1111/gcb.13882
Saha N., Webb G. E., Zhao J.-X. (2016). Coral skeletal geochemistry as a monitor of inshore water quality. Sci. Total Environ. 566-567, 652–684. doi: 10.1016/j.scitotenv.2016.05.066
Schlöder C., D’Croz L. (2004). Responses of massive and branching coral species to the combined effects of water temperature and nitrate enrichment. J. Exp. Mar. Biol. Ecol. 313, 255–268. doi: 10.1016/j.jembe.2004.08.012
Sherman C., Schmidt W., Appeldoorn R., Hutchinson Y., Ruiz H., Nemeth M., et al. (2016). Sediment dynamics and their potential influence on insular-slope mesophotic coral ecosystems. Continental Shelf Res. 129, 1–9. doi: 10.1016/j.csr.2016.09.012
Song Y., Tong M., Li M., Liu X., Yao H., Fang Y., et al. (2023). The biogenic elements retention in reservoirs of the Yangtze River basin and effect on the nutrient flux into the sea. Global Planet. Change 230, 104280. doi: 10.1016/j.gloplacha.2023.104280
Stephen C. J., Ruth A. K. (2004). A review of indicators of land-based pollution stress on coral reefs, national oceanic and atmospheric administration. Natl. Centers Coast. Ocean Sci., 1–55.
Tan Z., Wu F., Rao Y., Pan C., Hou G., Huang H. (2023). Spatial and temporal distribution of fish egg communities in the adjacent waters of Daya Bay nuclear power plant and their relationship with environmental factors. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1182213
Tashiro Y., Kameda Y. (2013). Concentration of organic sun-blocking agents in seawater of beaches and coral reefs of Okinawa Island, Japan. Mar. pollut. Bull. 77, 333–340. doi: 10.1016/j.marpolbul.2013.09.013
Travaglione N., Evans R., Moustaka M., Cuttler M., Thomson D. P., Tweedley J., et al. (2023). Scleractinian corals rely on heterotrophy in highly turbid environments. Coral Reefs 42, 997–1010. doi: 10.1007/s00338-023-02407-2
Vaher A., Kotta J., Szava-Kovats R., Kaasik A., Fetissov M., Aps R., et al. (2022). Assessing cumulative impacts of human-induced pressures on reef and sandbank habitats and associated biotopes in the northeastern Baltic Sea. Mar. pollut. Bull. 183, 114042. doi: 10.1016/j.marpolbul.2022.114042
van Woesik R., Roth L. M., Brown E. J., McCaffrey K. R., Roth J. R. (2020). Niche space of corals along the Florida reef tract. PloS One 15, e0231104. doi: 10.1371/journal.pone.0231104
van Woesik R., Shlesinger T., Grottoli A. G., Toonen R. J., Vega Thurber R., Warner M. E., et al. (2022). Coral-bleaching responses to climate change across biological scales. Global Change Biol. 28, 4229–4250. doi: 10.1111/gcb.16192
Voolstra C. R., Peixoto R. S., Ferrier-Pagès C. (2023). Mitigating the ecological collapse of coral reef ecosystems. EMBO Rep. 24, e56826. doi: 10.15252/embr.202356826
Williams J. E., Eric J. C., Jamison M. G., Enric Sala. and Stuart A. S. (2013). Benthic communities at two remote Pacific coral reefs: effects of reef habitat, depth, and wave energy gradients on spatial patterns. PeerJ 1, e81. doi: 10.7717/peerj.81
Woods R. M., Baird A. H., Mizerek T. L., Madin J. S. (2016). Environmental factors limiting fertilisation and larval success in corals. Coral Reefs 35, 1433–1440. doi: 10.1007/s00338-016-1494-0
Worachananant S., Carter R. W., Hockings M., Reopanichkul P. (2008). Managing the impacts of SCUBA divers on Thailand’s coral reefs. J. Sustain. Tour. 16, 645–663. doi: 10.1080/09669580802159677
Xu L., Yu K., Li S., Liu G., Tao S., Shi Q., et al. (2017). Interseasonal and interspecies diversities of Symbiodinium density and effective photochemical efficiency in five dominant reef coral species from Luhuitou fringing reef, northern South China Sea. Coral Reefs 36, 477–487. doi: 10.1007/s00338-016-1532-y
Zhao M. X., Yu K. F., Shi Q., Chen T. R., Zhang H. L., Chen T. G. (2013). Coral communities of the remote atoll reefs in the Nansha Islands, southern South China Sea. Environ. Monit. Assess. 185, 7381–7392. doi: 10.1007/s10661-013-3107-5
Zhou H. Y., Yao X. M., Li L., Geng T. N., Zhang Y. (2017). Scleractinian coral community structure and distribution in the coastal waters surrounding Hainan Island. Biodivers. Sci. 25, 1123–1130. doi: 10.17520/biods.2017079
Zhu W., Ren Y., Liu X., Huang D., Xia J., Zhu M., et al. (2022). The impact of coastal upwelling on coral reef ecosystem under anthropogenic influence: Coral reef community and its response to environmental factors. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.888888
Keywords: coral reefs, marine environmental variables, ecosystem parameters, Hainan Island, Qiziwan
Citation: Zhao H, Zhang J, Yang H, Li Y, Liu X, Liu Y, Zhu W, Wang A and Li X (2024) Coral reefs at Qiziwan National Marine Park in 2023: spatial variability and their relationship with environmental factors. Front. Mar. Sci. 11:1407803. doi: 10.3389/fmars.2024.1407803
Received: 27 March 2024; Accepted: 18 June 2024;
Published: 12 July 2024.
Edited by:
Aldo Cróquer, The Nature Conservancy, Dominican RepublicReviewed by:
Guowei Zhou, Chinese Academy of Sciences (CAS), ChinaHajime Kayanne, The University of Tokyo, Japan
Copyright © 2024 Zhao, Zhang, Yang, Li, Liu, Liu, Zhu, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiubao Li, eGl1YmFvbGlAaGFpbmFudS5lZHUuY24=; Haijun Yang, MzE1MDI2ODQ4QHFxLmNvbQ==
 He Zhao1,2
He Zhao1,2 Wentao Zhu
Wentao Zhu Aimin Wang
Aimin Wang Xiubao Li
Xiubao Li