
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 05 June 2024
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1395728
This article is part of the Research Topic Sustainable Aquaculture: Innovating Aquafeed With Solid-State Fermentation. View all 3 articles
Market instability, increased competition, escalating price and reduced availability of conventional ingredients warrants the researchers to rely on alternative feed ingredients. This approach may help in producing aqua feeds in a sustainable and cost-effective way to accomplish the global food and nutritional securities. Mahua oil cake (Bassia latifolia) is an underutilized non-conventional ingredient that holds promise for incorporation into aqua feed following nutrient enhancement by solid-state fermentation. A five-month pond feeding trial was carried out to investigate the effects of Sachharomyces cerevisiae and Bacillus subtilis fermented mahua oil cake (MOC) on the production performance, nutrient utilization, digestive capacity, and innate immunological responses of Labeo rohita fingerlings. For this, two iso-nitrogenous feed were formulated and prepared incorporating fermented MOC at different levels i.e. 0 and 40 percentage replacing soybean meal and other feed ingredients and fed to rohu fingerlings of two treatment groups in pond culture for 5 months duration. Improved growth performance, feed conversion ratio, feed intake, protein efficiency ratio and digestive capacity were observed in fish fed diets with 40 percent of fermented MOC compared to control. Innate immune responses parameters (respiratory burst activity, myeloperoxidase, lysozyme and hemaglutination activities) were significantly higher (P < 0.05) in fishes fed with fermented MOC. Therefore, we conclude incorporation of solid state fermented mahua oil cake up to 40% level in diet of L. rohita fingerlings in pond culture without any adverse effects on growth, nutrient utilization and innate immune response.
Fish is a highly nutritious and health-promoting component of the human diet and has great potential to achieve the United Nations Sustainable Development Goals (FAO, 2020), especially food and nutritional security. The only way to increase fish production and feed an ever-growing population (9.7 billion people by 2050, according to the UN, 2019) is through aquaculture, as captured fish production has reached a plateau. By 2030, an additional 121.6 metric tons (MT) of fish from global aquaculture will be required to meet the expected demand of 183 MT (Brugère and Ridler, 2004). It specifies the huge requirement for feed and feedstuffs to achieve mammoth-targeted fish production. Historically, fish meal has been the main source of dietary protein in aqua feed due to its superior nutritional profile. However, due to the relatively low level of marine fish production and the high market price for fish meal, several studies have proposed replacing fish meal with other plant protein sources, either in part or in its entirety, by feedstuffs from plant origin (Fournier et al., 2004; Kim and Cho, 2024). Most commercially produced carp do not need marine-derived proteins in their diet (FAO, 2016; Daniel, 2017). Soybean meal (SBM) has been the most preferred plant protein source in carp feed due to its excellent nutritional profile (Yue and Zhou, 2008). However, in the recent past, the escalating price due to the widening demand and supply gap and competition from other food-producing sectors has put economic pressure on the carp farming industry. The price of soybean meal is expected to rise further owing to insufficient supply and high demand, as well as restrictions on horizontal expansion due to environmental concerns associated with soybean cultivation. Hence, it is paramount to search for other alternative ingredients that are sustainably sourced.
Mahua oil cake (MOC, Bassia latifolia) is a by-product of oil recovery from mahua seeds and is currently being used as fertilizer, bio-pesticide, and a component of livestock feed (Gupta et al., 2012; Ramadan et al., 2016. The estimated annual production of mahua oil cake (MOC) in India is 140 million metric tons (Mani et al., 2020). The MOC contains 18-20% crude protein, 7-8% ether extract, 7-8% crude fiber. In addition, it is reported that MOC contains anti-nutritional factors (ANFs) such as saponin (8-9%) and tannin (6-7%), which limit its usage as a fish feed component (Das et al., 2022a). Saponins, found in various plant-derived feed ingredients for fish, can have detrimental effects on fish growth by damaging the respiratory epithelium of the gills, increasing the permeability of small intestinal mucosal cells, inhibiting active nutrient transport and by reducing the protein digestibility of the ingredient by forming sparingly digestible saponin–protein complexes (Francis et al., 2001). Tannin inhibits digestive processes by binding enzymes, proteins, vitamin B, and minerals, causing growth depression in fish (Liener, 1989).
Therefore, optimization of processing parameters to partially or completely remove the ANFs that are present in MOC is the avenue for effective utilization of MOC as a fish feed component. In this regard, solid-state fermentation (SSF) is a cost-effective technique in which microorganisms grow on solid substrates in the absence of free liquid (Srivastava et al., 2019) and it is developing as a viable alternative to submerged/liquid fermentation (Nigam and Pandey, 2009). SSF improves nutritional quality of plant-based ingredients by increasing protein content by microbial hydrolysis. The microorganisms in the SSF process consume soluble sugars and organic acids to synthesize amino acids, fatty acids, and vitamins, hence increasing the nutritious content of the substrate ingredient (Ramachandran et al., 2005; Ghosh & Mandal, 2015). This method improves protein digestibility, reduces larger polypeptides, produces novel bioactive peptides, modulates amino acid profiles, and partially or fully removes anti-nutritional substances like saponin, trypsin inhibitors, and tannins (Feng et al., 2023). It might be due to the action of microorganisms, which metabolize anti-nutrients or toxicants into less toxic compounds (Shamna et al., 2015).
The utilization of fermented feedstuff in aqua feed as feed component to partially replace fishmeal or soybean meal is a trending research area. Some of the studies concluded that the fermented soy pulp (FSP) increased the growth and health status of Clarias gariepinus (Kari et al., 2022); fermented soybean meal can replace portion of fish meal without negative effect on growth in largemouth bass (He et al., 2020) and coho salmon (Zhang et al., 2023); 10% FM protein can be replaced with fermented rice protein in hybrid grouper (He et al., 2021); fermented poultry by-product meal showed better growth performance in tilapia (Dawood et al., 2020); fermented soybean meal enhanced the growth, antioxidant status and reduced inflammatory response of turbot juveniles (Dan et al., 2022); dietary fermented wheat bran improved the growth and feed efficiency in nile tilapia (Mohammady et al., 2023).
Although there is some information on the effects of fermented mahua oil cake (MOC) on growth performance of rohu carp, it is only available in small-scale controlled laboratory studies (Das et al., 2022b). Therefore, the purpose of this study was to investigate the effect of dietary fermented MOC in terms of production performance in Labeo rohita in large-scale commercial pond trials.
The pure strain of yeast (Saccharomyces cerevisiae) employed for the solid-state fermentation of mahua oil cake was procured from ICAR-National Dairy Research Institute (ICAR-NDRI), Karnal, India. The culture was retrieved using yeast extract-peptone-dextrose (YPD) media. The YPD medium, comprising yeast extract (1.0g), peptone (2.0g), dextrose (2.0g), and distilled water (100 ml), was prepared. Subsequently, S. cerevisiae was added to the mix and incubated at 37°C for 48 hours. The culture was kept in YPD medium and stored at 4°C until use. Previously isolated strain of Bacillus subtilis was transferred to universal bacterial medium after a 24-hour incubation period at 37°C until use.
The current work utilized two different types of microorganisms, namely B. subtilis and S. cerevisiae, as the inoculum for the solid-state fermentation of mahua oil cake. S. cerevisiae was added to consume the oxygen in the fermenting flask, allowing Bacillus subtilis to thrive as anaerobic bacteria (Hu et al., 2008). Mahua oil-cake that had been dried and finely powdered was subjected to SSF in a circular drum. To get the fermentation mix’s final moisture level to 20%, sterile water was added. S. cerevisiae (4.0 log colony forming unit/ml) was added to the wet fermentation mix in a 4:1 ratio and incubated for 24 hours at 37°C. Following the first fermentation stage, B. subtilis (4.5 log cfu/ml) was added to the cultured mixture in the same ratio. After that, it was cultured in an anaerobic setting for 24 hours at 37°C. After 48 hours of anaerobic fermentation, wet samples were collected and autoclaved at 105°C for 30 minutes to end the continuous fermentation process. Following a 24-hour drying process at 60°C in a hot air oven, the fermented autoclaved samples were cooled, ground, packed, and stored at -20°C until required.
Following the guidelines of Bureau of Indian Standards (BIS), IS number: IS 16150 (Part 1), two iso-proteic diets (28%) were prepared for rohu (Labeo rohita) fingerlings (Table 1). Thirty percent of the rohu fingerlings’ control diet consisted of soybean meal. Forty percent fermented MOC was used to replace soybean meal and other ingredients in the test diet formulation. In brief, all dried feed ingredients were weighed, pulverized through an 80 mesh screen of 100 µ, completely mixed, and floating feed of 2 mm size was prepared using a twin-screw extruder (screw speed 27 rpm; barrel temperature 120°C, Jinan Saibainuo Machinery Co. Ltd., China), The pellets were stored until needed after being dried at the ICAR-CIFA feed mill in Bhubaneswar.
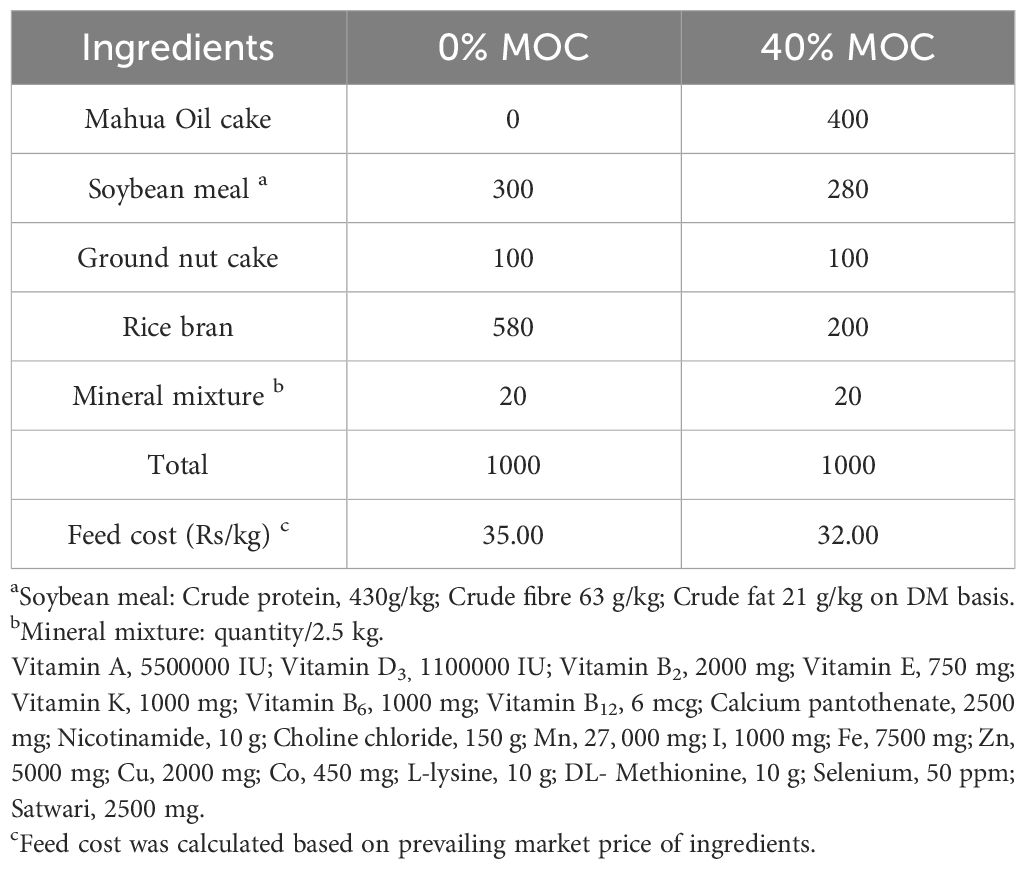
Table 1 Formulation of experimental diets (g/kg) for Labeo rohita with two levels of fermented mahua oil cake.
Healthy rohu fingerlings (2400 nos; avg. wt. 13.5 g) were stocked in the experimental pond facility of ICAR-CIFA, Bhubaneswar. During the three-week acclimatization period, fishes were fed control feed. Three ponds, each measuring 0.06 acres (20m X10m X 1.5m) were assigned for each treatment. They were stocked with 350 fish in total, resulting in a stocking density of 14,414 fish per hectare (Ayyappan and Jena, 2003). The ponds were fertilized by following the standard protocol (Jena and Das, 2006). The fishes were fed ad libitum with roughly 3% of their weight twice a day for five months. The water quality parameters, such as temperature, pH, dissolved oxygen (DO), total hardness, ammonia-N, and total alkalinity, were estimated fortnightly by following standard method of APHA (2005) and recorded as follows: temperature (26.05 ± 0.48 0C), pH (7.4 ± 0.12), dissolved oxygen (5.48 ± 0.14 mg l-1), total hardness (66 ± 5 mg CaCO3 l-1), ammonia-N (0.68 ± 0.23 mg l-1), and total alkalinity (7.7 ± 5 mg CaCO3 -1). Plankton samples were collected from each pond and then preserved in 4% formaldehyde for subsequent quantitative analysis. Following preservation, the samples underwent analysis using the direct census method (Jhingran et al., 1969). The total plankton counts varied within a range of 12360 to 14520 nos l-1.
The standard method of AOAC (2012) was used to analyze the proximate composition of mahua oil cake (before and after fermentation) and experimental diets. To summarize, crude percent protein was calculated by estimating nitrogen content by micro-Kjeldahl method (Kelplus, PELICAN, India) and multiplying with a factor of 6.25. Ether extract was measured by solvent extraction with petroleum ether, boiling point 40-60 0C (Soxtec system, Pelican equipment, Chennai, India) where as crude fibre was determined by acid digestion (1.25%) followed by alkali digestion (1.25%) with Fibra Plus equipment (Pelican, India).
The tannin content of MOC was measured using Folin-Ciocalteu reagents, as described by Makkar et al. (2007). With slight modifications, vanillin-H2SO4 method of Hiai et al. (1976) was used to calculate the cake’s saponin content.
Sampling of the fish was carried out every month in each pond to estimate the average weight and accordingly, biomass was calculated to adjust the daily feed ration. The various growth indices were calculated as follows.
For the assay of digestive enzymes, three fish were randomly selected from each pond. Following anaesthetizing the fishes with a buffered solution containing 120 mg l−1 tricaine methane sulphonate (MS-222; Sigma) (Sethi et al., 2022; Kumari et al., 2024), the intestinal tissue was carefully removed, pooled, and kept at -80°C until the enzyme activity was assayed. Using a mortar and pestle, frozen pooled intestinal samples were hygienically ground in liquid nitrogen. A 5% crude enzyme extract was prepared using chilled 0.25 M sucrose (w/v). After centrifugation of homogenized tissues at 10,000 g for 15 minutes at 4°C, the supernatant was collected and stored at -20°C until use. Bradford (1976) method was used to quantify the total protein concentration in tissue homogenates. Soluble starch (1% w/v) was used as a substrate to estimate intestinal amylase activity (Rick and Stegbauer, 1974). Using the casein digestion method, the protease activity was measured (Liu et al., 1991).
Five fish from each pond were randomly selected at the conclusion of the experimental trial and anesthetized with 120 mg l-1 of tricaine methane sulphonate (MS-222; Sigma). Blood was drawn from the ventrolateral caudal area, near the spinal cord, with a disposable hypodermic needle (2.0 ml). Individual fish blood sample was immediately transferred into two tubes: one was a 1.5 ml micro-centrifuge tube (used for collecting serum), and the other was an EDTA tube with a thin coating of the anticoagulant ethylene diamine tetra acetic acid (EDTA). To stop the collected blood from clotting and hemolysis, the EDTA tube was gently shaken. To collect serum, the blood sample without anticoagulant was left undisturbed in a slanting position at room temperature for two hours to facilitate clot formation. The sample was then centrifuged at 4000 g for 10 minutes in a refrigerated centrifuge. Serum samples were collected and stored at -20°C until use.
Respiratory burst activity of blood was measured by the reduction of nitro-blue tetrazolium (NBT) according to the technique described by Secombes (1990) and later modified by Stasiak and Baumann (1996). The serum myeloperoxidase activity (MPO) was determined using the procedures outlined by Quade and Roth (1997). In brief, 15 μl of fish serum was diluted in 135 μl of Hank’s balanced salt solution (free of Ca2+ and Mg2+). Subsequently, 50 μl of 20 mM 3, 3′, 5, 5′-tetramethylbenzidine and 5 mM hydrogen peroxide were added to the same well. The mixture was then incubated for two minutes at room temperature. The final reaction was stopped by adding 4 M sulfuric acid, and the optical density (OD) was measured at 450 nm with a UV-VIS Spectrophotometer (Thermo Spectronic, UK).
Lysozyme assay was conducted following the procedure outlined in Ellis (1990). A freshly prepared solution of 130 μl lyophilized Micrococcus lysodeikticus (Sigma, USA), at a concentration of 0.6 mg/ml (in 0.02 M sodium citrate buffer), was added to a mixture comprising 10 μl of fish serum samples and 10 μl of 0.02 M sodium citrate buffer. The initial OD was measured at 450 nm immediately after adding the bacterial solution. After incubating the samples at 24°C for 1 hour, the OD of the samples was measured again at 450 nm. A standard curve was generated using a mixture of 20 μl working standard and 130 μl of M. lysodeikticus solution. Lysozyme activity was quantified in units/ml, where one unit is defined as a decrease in absorbance of 0.001 per minute.
The hemagglutination activity was quantified using Blazer and Wolke (1984) methodology. To sum up, equal amounts of NSS and 25 μl of fish serum sample that had been inactivated for 30 minutes at 45°C were mixed. A freshly prepared 1% New Zealand white rabbit red blood cell (RBC) suspension (25 μl) was added to the wells and incubated at room temperature for two hours. By measuring the reciprocal of the maximum blood dilution at which every RBC had fully agglutinated, the activity was determined.
The experiment’s results were statistically analyzed using Prism software (version 4.0, Graph Pad Software, San Diego, CA, USA). Results were presented as mean ± SEM, with P values < 0.05 indicating significance.
Proximate composition of feed and effect of solid -state fermentation (SSF) on nutritional composition and anti-nutritional factor (total saponin and total tannin) of mahua oil cake (MOC) was presented in Tables 2, 3, respectively. The fermentation of MOC with S. cerevisiae and B. subtilis resulted in significant (p < 0.05) increase in the protein content (16.3%). A reduction of 37.6% and 24.9% in crude fiber and ether extract, respectively, was recorded following SSF of MOC. The fermentation of MOC with S. cerevisiae and B. subtilis resulted in significant decrease (p < 0.05) in the total saponin and total tannin content. Fermentation resulted in a decrease in the total saponin and total tannin contents of MOC by 62.72 and 75.78%, respectively.
Enhanced growth performance and nutrient utilization were observed in the treatment group. The weight gain %, SGR, FCR, and PER were higher in the rohu fingerlings fed with 40% fermented mahua oil cake incorporated feed compared to control feed without fermented mahua oil cake (Table 4) during five months of feeding trial. There was no incidence of disease in fishes of both the groups.
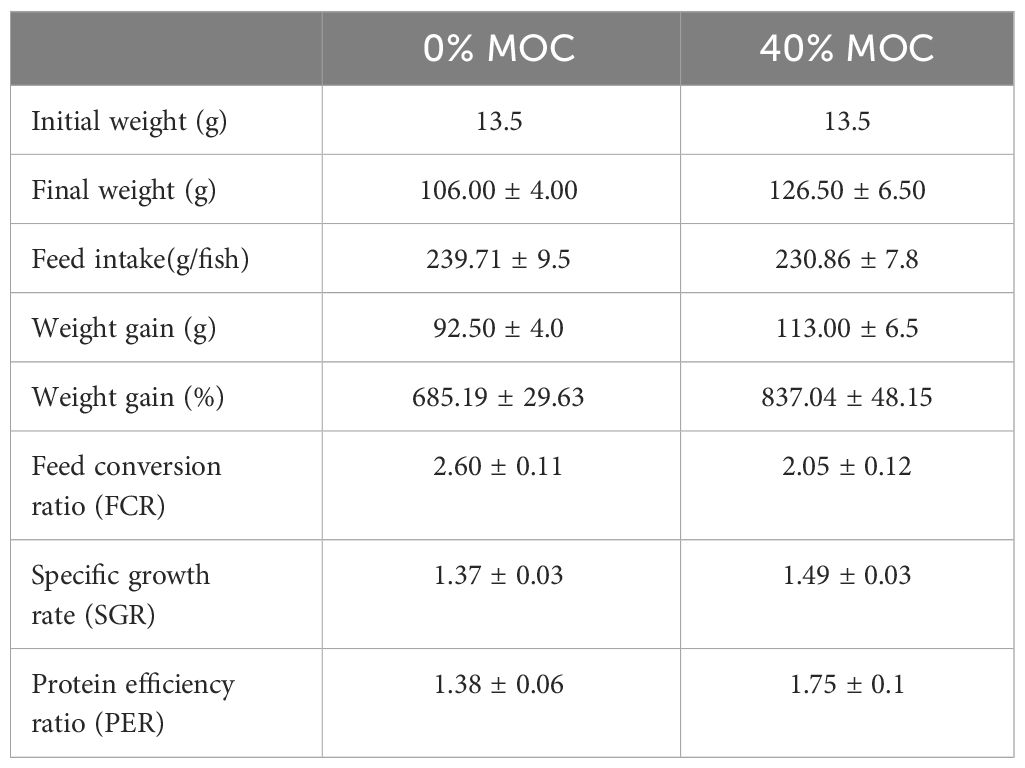
Table 4 Growth performance of rohu fed with two levels of fermented mahua oil cake in pond experiment.
Intestinal amylase and protease activities were higher in the fishes fed with 40% fermented mahua oil cake incorporated feed compared to control feed (Figure 1).
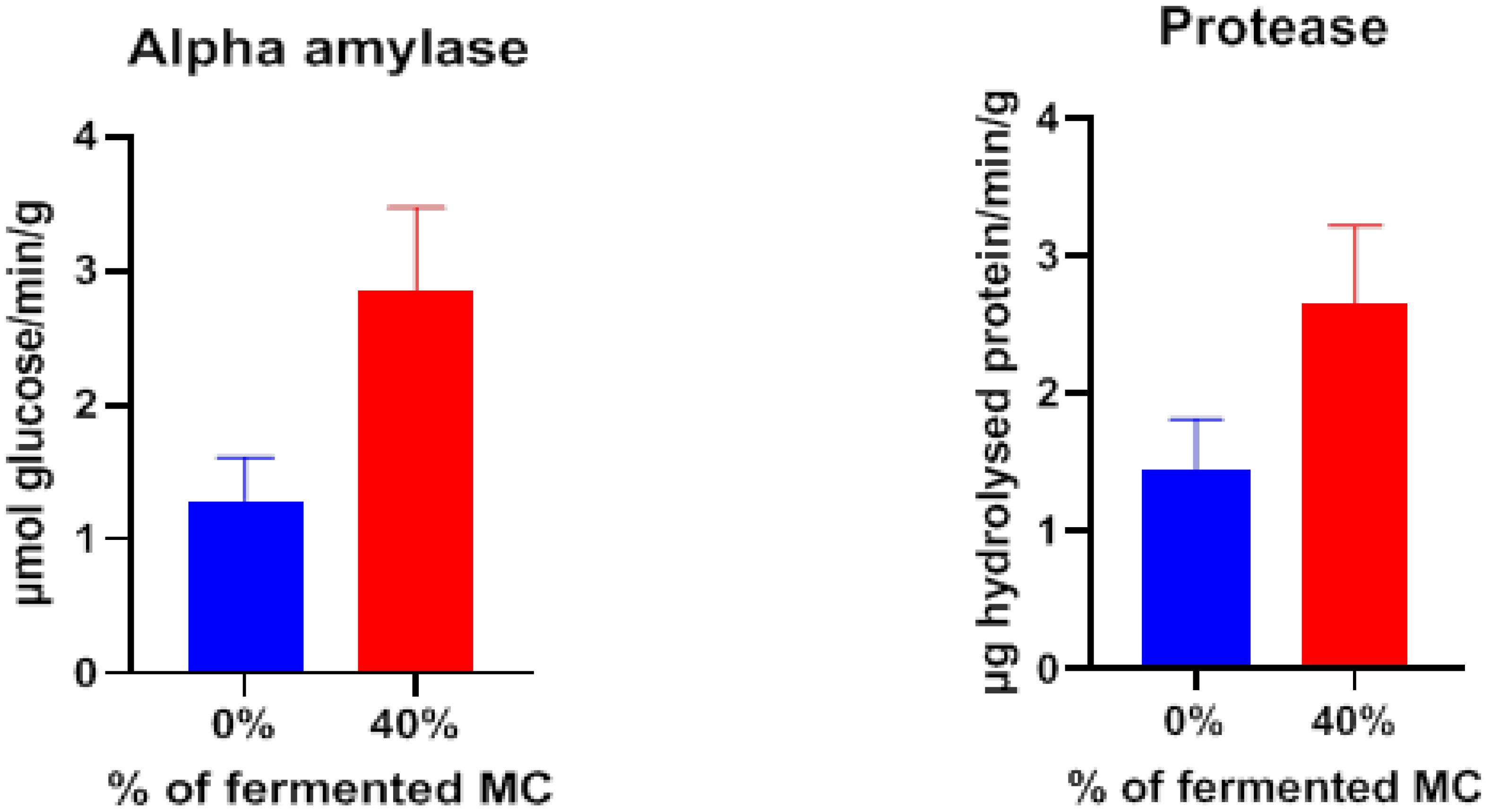
Figure 1 Gut enzyme activity of rohu fed with two levels of fermented mahua oil cake in pond experiment.
Dietary fermented mahua oil cake incorporated feed demonstrated a significant impact on nonspecific immune parameters of rohu fingerlings (Table 5). There was significant (p<0.05) increase in RBA, MPO, lysozyme and hemagglutination activity in 40% fermented MOC incorporated feed compared to control feed.
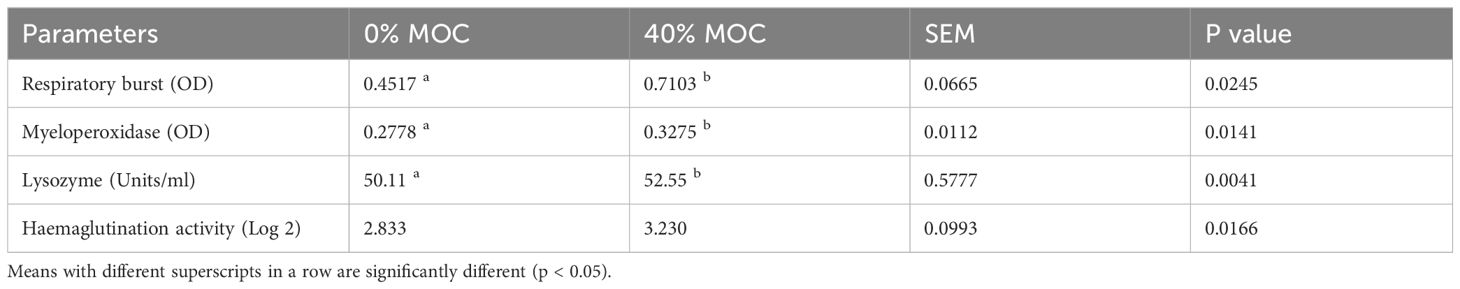
Table 5 Nonspecific immune parameters of rohu fed with two levels of fermented mahua oil cake in pond experiment.
Several researchers have explored the possibilities of using plant proteins as viable substitutes for fish meal in aqua feed. Despite having a high crude protein content, the use of plant-based ingredients in fish feed has limitations due to the presence of several anti-nutritional factors that cause poor nutrient availability and digestion (Kumari et al., 2013; Phulia et al., 2018; Siddaiah et al., 2023). Various detoxification approaches are being employed to eliminate or reduce anti-nutrients present in plant proteins, increasing the nutrient contents and bio-availability, and so providing value to the product for improved usage (Ranjan et al., 2019). In the current study, MOC was subjected to SSF with S. cerevisiae and B. subtilis, and the result showed a considerable reduction in total saponin and total tannin content. This is consistent with Anand et al. (2020), who observed a significant decrease in total saponin and total tannin content following SSF of Sesbania leaf meal. Similarly, fermented MOC showed enhanced protein and decreased crude fiber content, which was in congruence with the findings of Sun et al. (2012). The conditions utilized in SSF are conducive to the growth of microbes, as they closely resemble the natural environment (Zepf and Jin, 2013). These microbes produce a variety of enzymes that aid in the degradation of starch, non-starch polysaccharides, and other polymeric forms of molecules in the substrate into soluble monomers, resulting in a beneficial increase in protein content and a decrease in fiber content (Gao, 2011; Banerjee and Ghosh, 2016). The analyzed water quality parameters fell within the optimal range for rohu fingerlings, implying that the experimental fish were not stressed. Fish fed diets with 400 g kg−1 fermented MOC showed higher WG (%), SGR, and PER values, but lower FCR values compared to the control group during the five months pond trial. In contrary to this, when the experiment was conducted indoor in tank system, enhanced growth was reported only up to inclusion level of 20% fermented MOC (Das et al., 2022a). However, better growth performance of rohu was recorded in pond culture up to 40% inclusion level of solid state fermented MOC, which was likely caused by the presence of residual saponin and natural planktons.
Saponin supplemented diet (150-450 mg Kg-1) significantly enhanced body weight of common carp (Serrano, 2013) and Nile tilapia (Francis et al., 2001).
The significant decrease in anti-nutritional factors and increase in protein content of mahua oil cake following SSF might have contributed for growth-promoting effect in fish (Qazi et al., 2012). The improved growth performance observed in the group fed fermented MOC compared to the control implies that the former could be a suitable dietary protein source. It could replace traditional protein sources such as soybean meal in rohu diets without compromising growth performance or nutrient utilization. Shamna et al. (2015) reported the beneficial effect of feeding fermented jatropha protein concentrate to rohu fingerlings.
The fermented MOC-fed group also had a reduced FCR, which helped in economizing production. The improvement of an animal’s performance, specifically in relation to growth and nutrient utilization is often correlated with the positive impact of digestive enzymes on the process of digestion. During solid state fermentation, microbes secrete enzymes such as amylase, lipase, cellulase, protease, chitinase, and more, which remain in the fermented ingredients and later incorporated into diets (Vieira et al., 2023) which aid in digestion (Ofuya and Nwajiuba, 1990; Pandey et al., 1999; Iyayi and Losel, 2001, Prakasham et al., 2006). In this experiment, enhanced protease and amylase activity in fish fed with 40% of fermented mahua oil cake correlates with their enhanced growth performances. Exogenous enzyme supplementation has been shown to improve digestive enzyme activity. Consistent with our findings, Kumari et al. (2013) reported significantly enhanced activities of digestive enzymes in rohu fingerlings fed with a diet containing nano-encapsulated trypsin. Similarly, enhanced activities of lipase and amylase were reported in Jian carp (Jiang et al., 2014) when fed a xylanase supplemented diet. Similar to this, a diet supplemented with carbohydrase enhanced the activity of these enzymes in turbot (Diógenes et al., 2018) and White Sea bream (Magalhães et al., 2018).
Immunomodulation is a highly effective strategy for preventing frequent disease outbreak in fishes, which is a major concern in today’s intensive aquaculture system (Jahan et al., 2021). Non-specific immune parameters provide insight into a fish’s overall health and well-being and can be employed as bio-markers for assessing the health status of fish (Swain et al., 2019; Siddaiah et al., 2022). In the present investigation, the innate immune parameters of rohu fingerlings, such as respiratory burst activity, lysozyme activity, hemaglutination activity, and myeloperoxidase contents, showed a significant increase in the diets that contained 40% fermented MOC compared to the control. The ability of activated phagocytes to release superoxide anions within the host is indicated by the respiratory burst activity (RBA) assessed in terms of NBT reduction (Gokulakrishnan et al., 2022; Sethi et al., 2022). Significantly (p < 0.05) enhanced RBA activity clearly suggests that inclusion of 40% fermented MOC in diet was favorable for improving the non-special immunity of rohu fingerlings by increasing phagocytosis. Myeloperoxidase (MPO) is an antibacterial enzyme found in phagocytic cells, specifically neutrophil azurophilic granules (Das et al., 2022a) which play a major role in nonspecific cellular immunity. Increased NBT and MPO activity in our results imply higher phagocytosis activity, which demonstrates immunostimulatory action of fermented MOC. The results are concurrent with the previous findings of Bui et al. (2014) in red sea bream fingerlings who found that replacing fish meal with fish protein hydrolysate increased the amount of myeloperoxidase. Lysozyme, a leucocytic enzyme with mucolytic characteristics has been widely used as indices of the immunity of fish in numerous studies (Zhang et al., 2023). In this investigation, the significant increase in serum lysozyme activity justifies the benefits of incorporating fermented MOC into the diet, hence boosting L. rohita immunity. Fermented soybean meal has shown to be effective in boosting immune responses in juvenile olive flounder (Kim et al., 2010). Our results also corroborates with the findings of previous studies (Maeda et al., 2014; Ashouri et al., 2020), which suggest that lysozyme is elicited by different immunostimulating substances and acts as an integral component of aquatic animal antibacterial defense mechanisms. Increase in haemaglutination activity in rohu fingerlings fed with fermented MOC indicates protection against microbial invasion.
The simple technique of solid state fermentation, which employs S. cerevisiae and B. subtilis, can significantly reduce anti-nutritional factors in mahua oil cake, and this fermented mahua oil cake can be included in aquafeed up to 40% level without compromising fish growth and well-being during pond culture. This study concludes that fermented MOC holds promise as an alternative ingredient in the diet of L. rohita.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
The animal study was approved by The Institutional Animal Ethics Committee (IAEC) of ICAR-CIFA, Bhubaneswar. The study was conducted in accordance with the local legislation and institutional requirements.
KD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. AM: Writing – review & editing. PS: Data curation, Methodology, Writing – review & editing. PR: Investigation, Methodology, Writing – review & editing. RK: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research received funding support by Indian council of agricultural research, New Delhi.
The authors are grateful to the Director, ICAR-CIFA, for provision of facilities to carry out the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
American Public Health Association (1926). Standard methods for the examination of water and wastewater Vol. 6 (New York, NY: American Public Health Association).
Anand G., Srivastava P. P., Varghese T., Sahu N. P., Harikriskna V., Xavier M., et al. (2020). Sesbania aculeata leaf meal as replacer of de-oiled rice bran in aquaculture feed: growth, IGF-1 expression, metabolic and biochemical responses in Cyprinus carpio (Linnaeus 1758). Aquac. Res. 51, 2483–2494. doi: 10.1111/are.14591
AOAC (2012). Official methods of analysis of AOAC International. 19th edn (Washington, DC, USA: AOAC International).
Ashouri G., Soofiani N. M., Hoseinifar S. H., Jalali S. A. H., Morshedi V., Valinassab T., et al. (2020). Influence of dietary sodium alginate and Pediococcus acidilactici on liver antioxidant status, intestinal lysozyme gene expression, histomorphology, microbiota, and digestive enzymes activity, in Asian sea bass (Lates calcarifer) juveniles. Aquaculture 518, 734638. doi: 10.1016/j.aquaculture.2019.734638
Ayyappan S., Jena J. K. (2003). Grow-out production of carps in India. J. Appl. Ichthyol. 13, 251–282. doi: 10.1300/J028v13n03_04
Banerjee S., Ghosh K. (2016). Bio-processing of linseed oil-cake through solid state fermentation by non-starch polysaccharide degrading fish gut bacteria. J. Ferment. Technol. 5, 1–10. doi: 10.4172/2167-7972.1000127
Blazer V. S., Wolke R. E. (1984). The effects of α-tocopherol on the immune response and non-specific resistance factors of rainbow trout (Salmo gairdneri Richardson). Aquaculture 37, 1–9. doi: 10.1016/0044-8486(84)90039-5
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Brugère C., Ridler N. (2004). Global aquaculture outlook in the next decades: an analysis of national aquaculture production forecasts to 2030. FAO Fisheries Circular 1001, 1–47.
Bui H. T. D., Khosravi S., Fournier V., Herault M., Lee K. J. (2014). Growth performance, feed utilization, innate immunity, digestibility and disease resistance of juvenile red seabream (Pagrus major) fed diets supplemented with protein hydrolysates. Aquaculture 418, 11–16. doi: 10.1016/j.aquaculture.2013.09.046
Dan Z., Zhang W., Zheng J., Gong Y., Cui K., Mai K., et al. (2022). Effects of fishmeal substitution by four fermented soybean meals on growth, antioxidant capacity and inflammation responses of turbot juveniles (Scophthalmus maximus L.). Aquaculture 560, 738414. doi: 10.1016/j.aquaculture.2022.738414
Daniel N. (2017). Status of aquaculture with respect to nutrition and feed. Int. J. fisheries Aquat. Stud. 5 (1), 333–345.
Das K. C., Mohanty S., Sahoo P. K., Sahoo S., Prakash B., Swain P. (2022a). Inclusion of different levels of solid-state fermented mahua oil cake on growth, digestibility and immunological parameters of rohu (Labeo rohita). Aquaculture 553, 738049. doi: 10.1016/j.aquaculture.2022.738049
Das P. C., Nayak A., Sarkar S., Choudhary P., Kumari R., Mohanty S. (2022b). Growth performance and immune responses of pengba (Osteobrama belangeri) during high-density fingerling rearing in biofloc system. Aquac. Res. 53, 6378–6388. doi: 10.1111/are.16111
Dawood M. A., Magouz F. I., Mansour M., Saleh A. A., Asely A. M. E., Fadl S. E., et al. (2020). Evaluation of yeast fermented poultry by-product meal in Nile tilapia (Oreochromis niloticus) feed: Effects on growth performance, digestive enzymes activity, innate immunity, and antioxidant capacity. Front. Vet. Sci. 6. doi: 10.3389/fvets.2019.00516
Diógenes A. F., Castro C., Carvalho M., Magalhães R., Estevão-Rodrigues T. T., Serra C. R., et al. (2018). Exogenous enzymes supplementation enhances diet digestibility and digestive function and affects intestinal microbiota of turbot (Scophthalmus maximus) juveniles fed distillers' dried grains with solubles (DDGS) based diets. Aquaculture 486, 42–50. doi: 10.1016/j.aquaculture.2017.12.013
Feng X., Ng K., Ajlouni S., Zhang P., Fang Z. (2023). Effect of solid-state fermentation on plant-sourced proteins: A review. Food Rev. Int. 1, 1–38. doi: 10.1080/87559129.2023.2274490
Fournier V., Huelvan C., Desbruyeres E. (2004). Incorporation of a mixture of plant feedstuffs as substitute for fish meal in diets of juvenile turbot (Psetta maxima). Aquaculture 236, 451–465. doi: 10.1016/j.aquaculture.2004.01.035
Francis G., Makkar H. P. S., Becker K. (2001). Effects of Quillaja saponins on growth, metabolism, egg production and muscle cholesterol in individually reared Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 129, 105–114. doi: 10.1016/S1532-0456(01)00189-2
Gao Y. (2011) Improved nutritional value of fish feed with plant protein ingredients by means of organic acid salts and solid state fermentation. Available at: http://hdl.handle.net/11250/2434102.
Ghosh K., Mandal S. (2015). Nutritional evaluation of groundnut oil cake in formulated diets for rohu, Labeo rohita (Hamilton) fingerlings after solid state fermentation with a tannase producing yeast, Pichia kudriavzevii (GU939629) isolated from fish gut. Aquaculture Reports. 2, 82–90. doi: 10.1016/j.aqrep.2015.08.006
Gokulakrishnan M., Kumar R., Pillai B. R., Nanda S., Bhuyan S. K., Kumari R., et al. (2022). Dietary brewer’s spent yeast enhances growth, hematological parameters, and innate immune responses at reducing fishmeal concentration in the diet of climbing perch, Anabas testudineus fingerlings. Front. Nutr. 9. doi: 10.3389/fnut.2022.982572
Gupta A., Chaudhary R., Sharma S. (2012). Potential applications of mahua (Madhuca indica) biomass. Waste Biomass Valori. 3, 175–189. doi: 10.1007/s12649-012-9107-9
He Y., Guo X., Tan B., Dong X., Yang Q., Liu H., et al. (2021). Replacing fish meal with fermented rice protein in diets for hybrid groupers (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂): Effects on growth, digestive and absorption capacities, inflammatory-related gene expression, and intestinal microbiota. Aquacult. Rep. 19, 100603. doi: 10.1016/j.aqrep.2021.100603
He M., Li X., Poolsawat L., Guo Z., Yao W., Zhang C., et al. (2020). Effects of fish meal replaced by fermented soybean meal on growth performance, intestinal histology and microbiota of largemouth bass (Micropterus salmoides). Aquac. Nutr. 26, 1058–1071. doi: 10.1111/anu.13064
Hiai S., Oura H., Nakajima T. (1976). Color reaction of some sapogenins and saponins with vanillin and sulfur1c acid. Planta Med. 29, 116–122. doi: 10.1055/s-0028-1097639
Hu J., Lu W., Wang C., Zhu R., Qiao J. (2008). Characteristics of solid-state fermented feed and its effects on performance and nutrient digestibility in growing-finishing pigs. Asian-Aust. J. Anim. . Sci. 21, 1635–1641. doi: 10.5713/ajas.2008.80032
Iyayi E. A., Losel D. M. (2001). Changes in carbohydrate fractions of cassava peel following fungal solid state fermentation. J. Food Technol. Afr. 6, 101–103.
Jahan N., Islam S. M., Rohani M. F., Hossain M. T., Shahjahan M. (2021). Probiotic yeast enhances growth performance of rohu (Labeo rohita) through upgrading hematology, and intestinal microbiota and morphology. Aquaculture 545, 737243. doi: 10.1016/j.aquaculture.2021.737243
Jena J. K., Das P. C. (2006). “Carp culture,” in Handbook of fisheries and aquaculture (Indian Council of Agricultural Research, New Delhi, India), 265–282.
Jhingran V. G., Natarajan A. V., Banerjea S. M., David A. (1969). Methodology on reservoir fisheries investigations in India (Barrackpore, India: Central Inland Fisheries Research Institute), 109.
Jiang T. T., Feng L., Liu Y., Jiang W. D., Jiang J., Li S. H., et al. (2014). Effects of exogenous xylanase supplementation in plant protein-enriched diets on growth performance, intestinal enzyme activities and microflora of juvenile J ian carp (C yprinus carpio var. J ian). Aquac. Nutr. 20, 632–645. doi: 10.1111/anu.2014.20.issue-6
Kari Z. A., Kabir M. A., Dawood M. A., Razab M. K. A. A., Ariff N. S. N. A., Sarkar T., et al. (2022). Effect of fish meal substitution with fermented soy pulp on growth performance, digestive enzyme, amino acid profile, and immune-related gene expression of African catfish (Clarias gariepinus). Aquaculture 546, 737418. doi: 10.1016/j.aquaculture.2021.737418
Kim J., Cho S. H. (2024). Substitution effect of fish meal with various plant protein sources on growth performance and feed utilization in rockfish (Sebastes schlegeli) diets including jack mackerel meal used as feed stimulants. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1339471
Kim S. S., Pham M. A., Kim K. W., Son M. H., Lee K. J. (2010). Effects of microbial fermentation of soybean on growth performances, phosphorus availability, and antioxidant activity in diets for juvenile olive flounder (Paralichthys olivaceus). Food Sci. Biotechnol. 19, 1605–1610. doi: 10.1007/s10068-010-0227-3
Kumari R., Gupta S., Singh A. R., Ferosekhan S., Kothari D. C., Pal A. K., et al. (2013). Chitosan nanoencapsulated exogenous trypsin biomimics zymogen-like enzyme in fish gastrointestinal tract. PloS One 8, e74743. doi: 10.1371/journal.pone.0074743
Kumari R., Srivastava P. P., Mohanta K. N., Das P., Kumar R., Sahoo L., et al. (2024). Optimization of weaning age for striped murrel (Channa striata) based on expression and activity of proteases. Aquaculture 579, 740277. doi: 10.1016/j.aquaculture.2023.740277
Liener I. E. (1989). “Antinutritional factors in legume seeds: state of the art,” in Recent advances of research in antinutritional factors in legume seeds. Eds. Huisman J., van der Poel A. F. B., Liener I. E. (Wageningen: Pudoc), 6–14.
Liu Y. M., Zhu J. Z., Wu H. Y., Shi D. Z. (1991). Studies on digestive enzymes and amino acid of larval and post larval stages of prawn Penaeus chinensis. Oceanol. Lminol. Sin. 22, 571–575.
Maeda M., Shibata A., Biswas G., Korenaga H., Kono T., Itami T., et al. (2014). Isolation of lactic acid bacteria from kuruma shrimp (Marsupenaeus japonicus) intestine and assessment of immunomodulatory role of a selected strain as probiotic. Mar. Biotechnol. 16, 181–192. doi: 10.1007/s10126-013-9532-1
Magalhães R., Díaz-Rosales P., Diógenes A. F., Enes P., Oliva-Teles A., Peres H. (2018). Improved digestibility of plant ingredient-based diets for European seabass (Dicentrarchus labrax) with exogenous enzyme supplementation. Aquacu. Nutr. 24, 1287–1295. doi: 10.1111/anu.12666
Mani Y., Devaraj T., Devaraj K., AbdurRawoof S. A., Subramanian S. (2020). Experimental investigation of biodiesel production from Madhuca longifolia seed through in situ transesterification and its kinetics and thermodynamic studies. Environ. Sci. pollut. Res. 27, 36450–36462. doi: 10.1007/s11356-020-09626-y
Mohammady E. Y., Aboseif A. M., Soaudy M. R., Ramadan E. A., Hassaan M. S. (2023). Appraisal of fermented wheat bran by Saccharomyces cerevisiae on growth, feed utilization, blood indices, intestinal and liver histology of Nile tilapia. Oreochromis niloticus. Aquaculture 575, 739755. doi: 10.1016/j.aquaculture.2023.739755
Nigam P. S. N., Pandey A. (Eds.) (2009). Biotechnology for agro-industrial residues utilisation: utilisation of agro-residues (Dordrecht: Springer Science & Business Media).
Ofuya C. O., Nwajiuba C. J. (1990). Microbial degradation and utilization of cassava peel. World J. Microbiol. Biotechnol. 6, 144–148. doi: 10.1007/BF01200933
Pandey A., Selvakumar P., Soccol C. R., Nigam P. (1999). Solid state fermentation for the production of industrial enzymes. Curr. Sci., 149–162. https://www.jstor.org/stable/24102923
Phulia V., Sardar P., Sahu N. P., Fawole F. J., Shamna N., Gupta S. (2018). Substitution of soybean meal with fermented Jatropha kernel meal: effect on growth performance, body composition, and metabolic enzyme activity of Labeo rohita. Fish Physiol. Biochem. 44, 475–487. doi: 10.1007/s10695-017-0447-z
Prakasham R. S., Rao C. S., Sarma P. N. (2006). Green gram husk—an inexpensive substrate for alkaline protease production by Bacillus sp. solid-state fermentation. Bioresource Technol. 97 (13), 1449–1454.
Qazi J. I., Nadir S., Shakir H. A. (2012). Solid state fermentation of fish feed with amylase producing bacteria. Punjab Univ. J. Zool. 27, 1–7.
Quade M. J., Roth J. A. (1997). A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 58, 239–248. doi: 10.1016/S0165-2427(97)00048-2
Ramachandran S., Bairagi A., Ray A. K. (2005). Improvement of nutritive value of grass pea (Lathyrus sativus) seed meal in the formulated diets for rohu, Labeo rohita (Hamilton) fingerlings after fermentation with a fish gut bacterium. Bioresour. Technol. 96, 1465–1472. doi: 10.1016/j.biortech.2004.12.002
Ramadan M. F., Mohdaly A. A. A., Assiri A. M., Tadros M., Niemeyer B. (2016). Functional characteristics, nutritional value and industrial applications of Madhuca longifolia seeds: an overview. J. Food Sci. Technol. 53, 2149–2157. doi: 10.1007/s13197-015-2095-6
Ranjan A., Sahu N. P., Deo A. D., Kumar S. (2019). Solid state fermentation of de-oiled rice bran: Effect on in vitro protein digestibility, fatty acid profile and anti-nutritional factors. Food Res. Int. 119, 1–5. doi: 10.1016/j.foodres.2019.01.054
Rick W., Stegbauer H. (1974). “α-Amylase measurement of reducing groups,” in Methods of enzymatic analysis (New York, NY: Academic Press), 885–890. doi: 10.1016/B978-0-12-091302-2.50074-8
Secombes C. (1990). Isolation of salmonid macrophages and analysis of their killing activity. Techniques fish Immunol. 1, 137–163.
Serrano J. A.E. (2013). Effects of Q uillaja saponins on growth, feed efficiency, digestive enzyme activities and metabolism of common carp (Cyprinus carpio L). Aquac. Nutr. 19, 468–474. doi: 10.1111/anu.2013.19.issue-4
Sethi J. P., Choudhary P., Sarkar S., Kumari R., Mohanty S., Das P. C. (2022). Screening wheat flour and molasses as carbon sources in biofloc system for increasing growth and non-specific immune responses of pengba, Osteobrama belangeri (Valenciennes). J. Appl. Ichthyol. 38, 531–539. doi: 10.1111/jai.14343
Shamna N., Sardar P., Sahu N. P., Pal A. K., Jain K. K., Phulia V. (2015). Nutritional evaluation of fermented jatropha protein concentrate in Labeo rohita fingerlings. Aquac. Nutr. 21, 33–42. doi: 10.1111/anu.2015.21.issue-1
Siddaiah G. M., Kumar R., Kumari R., Chandan N. K., Debbarma J., Damle D. K., et al. (2023). Dietary fishmeal replacement with Hermetia illucens (Black soldier fly, BSF) larvae meal affected production performance, whole body composition, antioxidant status, and health of snakehead (Channa striata) juveniles. Anim. Feed Sci. Technol. 297, 115597. doi: 10.1016/j.anifeedsci.2023.115597
Siddaiah G. M., Kumar R., Kumari R., Damle D. K., Rasal K. D., Manohar V., et al. (2022). Dietary supplementation of fish protein hydrolysate improves growth, feed efficiency and immune response in freshwater carnivore fish, Channa striata fingerlings. Aquac. Res. 53, 3401–3415. doi: 10.1111/are.15848
Srivastava N., Srivastava M., Ramteke P. W., Mishra P. K. (2019). Solid-state fermentation strategy for microbial metabolites production: An overview. New Future developments Microbial Biotechnol. Bioengineering, 345–354. doi: 10.1016/B978-0-444-63504-4.00023-2
Stasiak S. A., Baumann P. C. (1996). Neutrophil activity as a potential bioindicator for contaminant analysis. Fish Shellfish Immunol. 6, 537–539. doi: 10.1006/fsim.1996.0050
Sun H., Tang J. W., Yao X. H., Wu Y. F., Wang X., Feng J. (2012). Improvement of the nutritional quality of cottonseed meal by bacillus subtilis and the addition of papain. Int. J. Agric.Biol. 14, 74.
Swain P., Das R., Das A., Padhi S. K., Das K. C., Mishra S. S. (2019). Effects of dietary zinc oxide and selenium nanoparticles on growth performance, immune responses and enzyme activity in rohu, Labeo rohita (Hamilton). Aquacu. Nutr. 25, 486–494. doi: 10.1111/anu.2019.25.issue-2
UN. (2019). World population prospects 2019 (New York: Department of Economic and Social Affairs. United Nations Organization). Available at: https://www.jstor.org/stable/resrep33108.20.
Vieira L., Filipe D., Amaral D., Magalhães R., Martins N., Ferreira M., et al. (2023). Solid-state fermentation as green technology to improve the use of plant feedstuffs as ingredients in diets for European sea bass (Dicentrarchus labrax) juveniles. Animals 13, 2692. doi: 10.3390/ani13172692
Yue Y. R., Zhou Q. C. (2008). Effect of replacing soybean meal with cottonseed meal on growth, feed utilization, and hematological indexes for juvenile hybrid tilapia, Oreochromis niloticus× O. aureus. Aquaculture 284 (1-4), 185–189.
Zepf F., Jin B. (2013). Bioconversion of grape marc into protein rich animal feed by microbial fungi. Chem. Eng. Process Tech. 1, 1011–1018.
Keywords: fermentation, mahua oil cake, growth performance, digestive capacity, immune response
Citation: Das KC, Mohanty A, Swain P, Routray P and Kumari R (2024) Fermented mahua oil cake in the diet of Labeo rohita: effects on growth performance, digestive enzyme activity and immune response. Front. Mar. Sci. 11:1395728. doi: 10.3389/fmars.2024.1395728
Received: 04 March 2024; Accepted: 08 May 2024;
Published: 05 June 2024.
Edited by:
Amit Ranjan, Tamil Nadu Fisheries University, IndiaReviewed by:
Prasanta Jana, Birsa Agricultural University, IndiaCopyright © 2024 Das, Mohanty, Swain, Routray and Kumari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rakhi Kumari, cmFraGlzLmNpZmFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.