
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 08 May 2024
Sec. Aquatic Physiology
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1395339
 Cheng He1,2,3
Cheng He1,2,3 Ting Ye1,2,3
Ting Ye1,2,3 Junxi Zeng1,2,3
Junxi Zeng1,2,3 Hongkuan Zhang1,2,3
Hongkuan Zhang1,2,3 Yizhou Sun1,2,3
Yizhou Sun1,2,3 Chuanxu Zhang1,2,3
Chuanxu Zhang1,2,3 Xianqing He1,2,3
Xianqing He1,2,3 Jiale Qiu1,2,3
Jiale Qiu1,2,3 Huaiping Zheng1,2,3*
Huaiping Zheng1,2,3*Introduction: In aquatic animals, colors not only reflect their sensory qualities but also affect their nutritional components and market price. Noble scallop Chlamys nobilis is an importantly economical bivalve cultured in the south sea of China and renowned for its various shell colors.
Methods: In this study, the nutrient compositions were compared among golden, yellow, and brown shell colour noble scallops originating from the same population for breeding new varieties.
Results: Among three colour scallops, there is no significant difference in the content of moisture, ash, and total protein content (TPC) but significant differences in total carotenoid content (TCC), total lipid content (TLC), and amino acids (AAs) (P < 0.05), with the TCC of the brown scallop being about 2 times lower. The yellow scallop contained the same nutrients as TCC, TLC, ∑PUFAs (including EPA and DHA), and n-3/n-6 as the golden scallop, and both were significantly higher than the brown scallop (P < 0.05), but they had significantly lower ∑SFAs than the latter (P < 0.05). The correlation between the nutrient indices and the shell color of scallops was 67.5%. There is a significantly positive correlation between TCC and ∑PUFAs (P < 0.05), while a significantly negative correlation exists between TCC and ∑SFAs (P < 0.05). More importantly, the yellow scallop had significantly higher EAAs and FAAs than the golden scallop (P < 0.05).
Discussion: This study showed that the yellow noble scallop not only enriched in carotenoids and PUFAs but also enriched in amino acids. Therefore, the yellow scallop is more benefit to human health, which can provide high-quality food for customers.
The surface colour of organisms is the most visual feature, and the difference in color often represents important differences in material composition and information (Bolechová et al., 2019). As the most important high-quality protein source for human beings, the quality and nutritional values of aquatic products is significantly linked to the color, based on experience, tradition and customs (Cai and Leung, 2022; He et al., 2023; Wang et al., 2024), and the color of aquatic products significantly affects their purchasing decisions (Steine et al., 2007). Therefore, the body or muscle of most farmed aquatic animals are indirect or direct involving in coloration (Sun et al., 2023; Ding et al., 2024).
The mollusk is the second Phylum in the animal Kingdom, which can not only provide crucial ecological services (Pruden et al., 2021), significant economic benefits and huge amounts of high-quality protein to humans, but also provide great values of viewing and collecting for their pleasantly regular geometric shapes, striking colors and colour patterns (Evo-devo of shell colour in gastropods and bivalves). Therefore, studies on genetics in shell colors have been attended in many species (Williams, 2017), especially in bivalve, such as oysters (Wang et al., 2018; Han et al., 2021), scallops (Zheng et al., 2013; Liu et al., 2015), mussels (Newkirk, 1980; Sun et al., 2021), etc. With increasing attention of high quality of aquatic products, over 20 new varieties marked by colors have been bred in China since 2000. Subsequently, studies on nutrient comparison among different shell colors have also increased (Zheng et al., 2010; Zhu et al., 2018; Tan et al., 2021).
The noble scallop Chlamys nobilis is an important marine bivalve, which is famous for its brilliant colors including orange/golden, yellow, orange-purple, purple, or brown, etc (Tan et al., 2020). More interestingly, muscle colour in adductor and mantle of this scallop has a significantly positive correlation with the total carotenoids content (TCC), resulting in the golden and white muscle (Zheng et al., 2010), as well as the shell and muscle colors can be inherited stably (Zheng et al., 2013). Based on these traits, a new variety named the noble scallop “Nan’ao Golden Scallop” was bred by artificial selection in 2015 (Zheng et al., 2015), and rapidly cultured in the southern sea of China for its unique features (Tan et al., 2020). In recent years, a new strain with yellow shell and the same color of muscles as “Nan’ao Golden Scallop” was bred by three-generations artificial selection, however, the nutritional compositions of noble scallop with different shell colors (such as golden, yellow, and brown) are not yet compared. Therefore, to investigate the difference of nutrient compositions among noble scallops with golden, yellow, and brown shell color, the content of moisture, ash, total protein, total lipid, amino acid, fatty acid, and total carotenoids were compared. The results will provide useful information for customers to obtain high-quality food.
The golden, yellow, and brown noble scallops C. nobilis (Figure 1) originated from the same population, which were collected from different stocks established in 2022. These scallops had been cultured in the same environments at the field of the Marine Biology Experimental Station of Nan’ao, Shantou University (N:23°28’38.78”, E:117°06’53.69”), China, and sea water temperature fluctuates in the range of 15-28°C with the season (Sun et al., 2024). Ten adult individuals with twelve months were randomly picked from each stock in Spring 2023, respectively. The muscle tissues from adductor and mantle were sampled, mixed, weighed, and frozen in liquid nitrogen. The studies involving animals were reviewed and approved by Shantou University of ethics committee.

Figure 1 Shell and soft tissues of golden, yellow, and brown noble scallops C. nobilis. He et al., 2024.
The frozen sample was first dried in a vacuum freeze-dryer (Alpha 1-2 LDplus, Germany). Then, the moisture content is determined by the formula
Next, the dried sample was placed into powder to be grinded, taking 0.1 g to place in a muffle furnace, and calcining at 800°C for 8 hours. Finally, ash was weighed and determined by the formula
The 0.5 g dried sample was accurately weighed and placed in a glass tube, and the protein digestive fluid was added to react until the liquid in the tube was green and transparent. After the liquid has cooled and been transferred to a 50-mL measuring flask, adjust the volume to 50 mL by deionized water. According to the National Food Safety Standard of China (GB/T5009.5-2016), the total protein content was determined and calculated using an automatic Kjeldahl nitrogen analyzer (FOSS kjeltec-8400, Denmark).
Determination of amino acids (AAs) was according to Tan et al. (2019). The amino acid content in the sample was analyzed according to the National Food Safety Standard of China (GB/T5009.124-2016) using a high-performance liquid chromatography and mass spectrometry (TSQ-Endura system, Thermo Fisher, USA).
Determination of total lipid content and fatty acids content was according to Zhu et al. (2022) using a high-performance gas chromatography (Shimadzu, Japan) and a GC workstation (Shimadzu CLASS-GC10, Japan).
Determination of total carotenoid content was according to the method used by Zheng et al. (2010) using a vacuum freeze-drying (Alpha 1-2 LDplus, Germany) and an UV-Vis recording spectrophotometer (UV-2501PC, Shimadzu, Japan).
All data in this study were expressed as the mean ± standard deviation (SD), and the data follows a normal distribution. ANOVA and multiple comparisons were completed, and Pearson correlation and PCA analysis were performed on all nutritional indicators. The PCA was analyzed using the Omicshare data processing platform developed by GENE DENOVO (https://www.omicshare.com), with default parameters. All statistical analyses were performed on the Statistical Package for the Social Sciences (SPSS, vision 26.0) and significance for all analyses was set to P < 0.05 unless noted otherwise.
Proximate compositions including moisture, ash, TPC, and TLC of golden, yellow, and brown scallops are listed in Table 1. No significant difference in moisture, ash, and TPC existed among three scallops, but both golden and yellow scallops had significantly higher TLC than brown one (P < 0.05).

Table 1 Comparison of proximate compositions among golden, yellow, and brown noble scallops C. nobilis.
The golden and yellow scallops were both had significantly higher TCC than the brown scallop (P < 0.05). But the golden and yellow scallops had no significant differences (Figure 2).
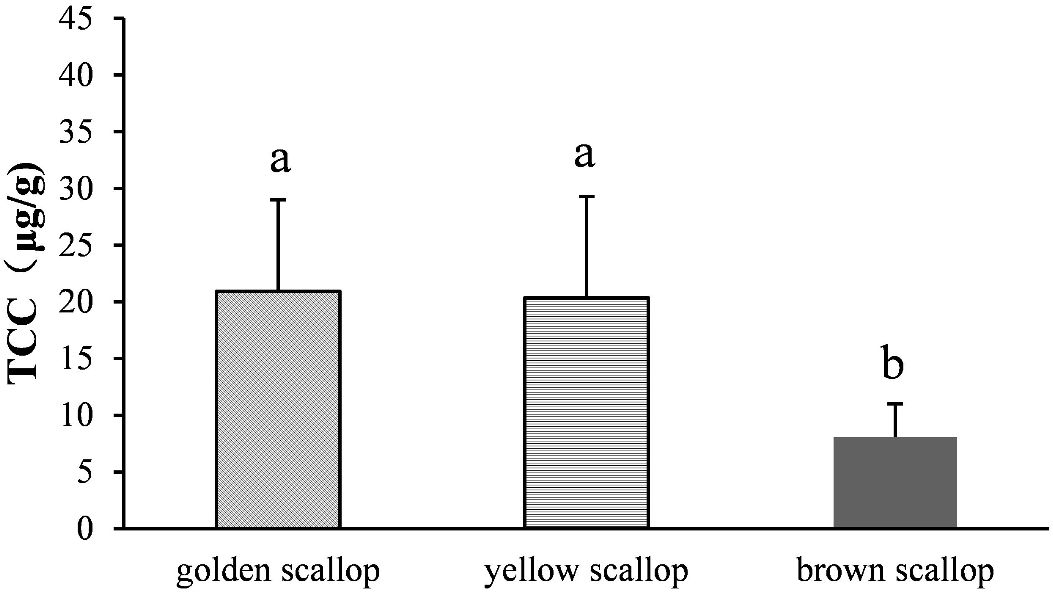
Figure 2 Comparison of total carotenoid content (TCC) in muscle among golden, yellow, and brown noble scallops C. nobilis. The different letters indicate a significant difference (P < 0.05). He et al., 2024.
Compositions and content of FAs are listed in Table 2. Content of 11 FAs including 3 saturated fatty acids (SFAs), 4 monounsaturated fatty acids (MUFAs), and 4 polyunsaturated fatty acids (PUFAs) were determined. There was no significant difference in ∑MUFAs among three colour scallops. However, the golden and yellow scallops had significantly lower ∑SFAs and higher ∑PUFAs than the brown one (P < 0.05). Furthermore, the golden and yellow scallops contained significantly higher EPA and DHA, and n-3/n-6 than the brown one (P < 0.05).
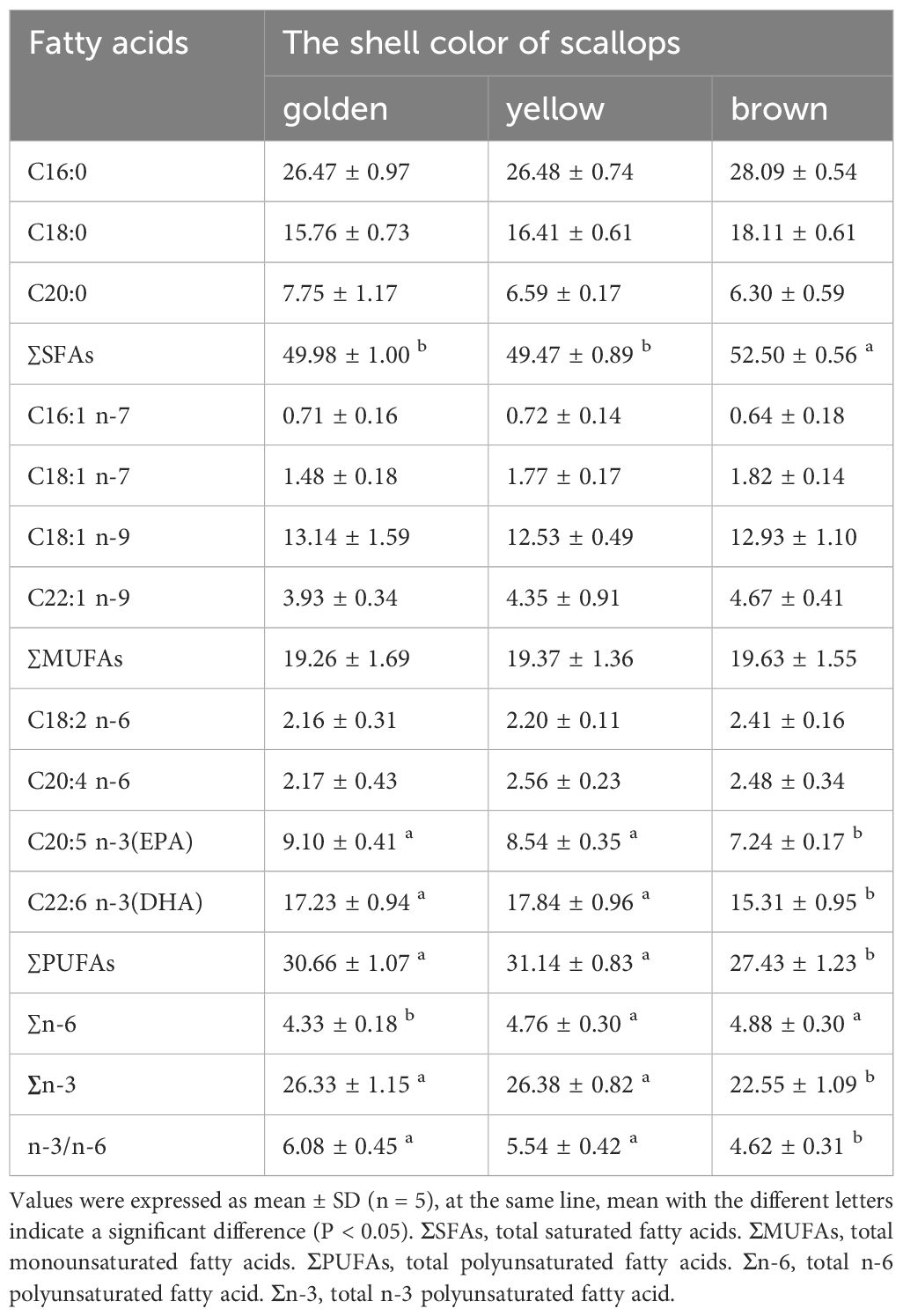
Table 2 Comparison of fatty acid contents (%) among golden, yellow, and brown noble scallops C. nobilis.
Totally, 17 amino acids were detected in all scallops, including seven essential amino acids (EAAs) and six flavor amino acids (FAAs) (Table 3). The content of EAAs (Lys, Thr, Met, Ile, Leu, and Phe) and FAAs (Phe, Asp, Glu, Gly, and Tyr) had significant differences among three color scallops (P < 0.05), Among them, the content of AAs in the brown scallops is the highest, followed by the yellow scallops, and the golden scallops are the lowest.
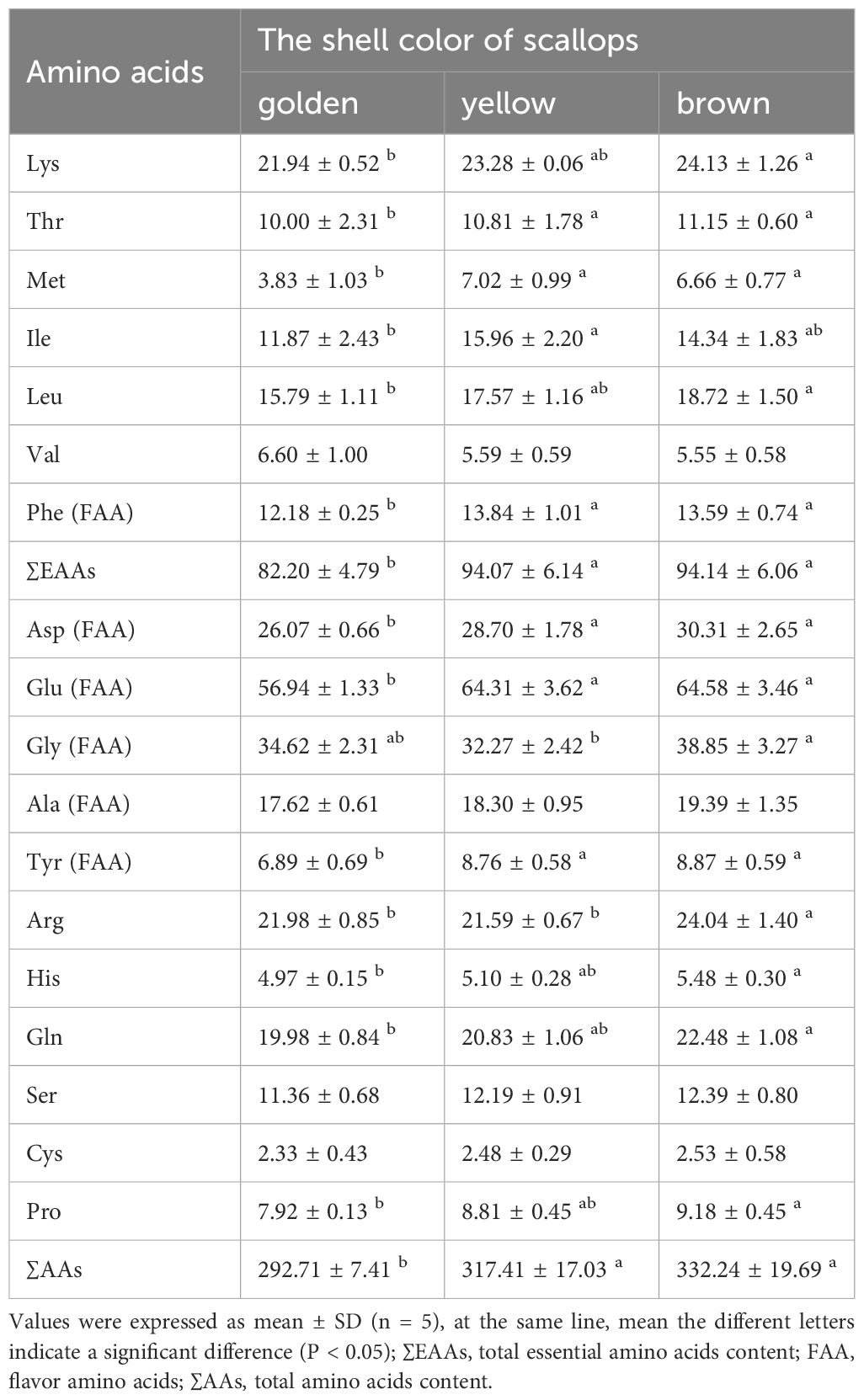
Table 3 Comparison of amino acid content (mg/g) among golden, yellow, and brown noble scallops C. nobilis.
The results of PCA showed that the correlation between nutrient indices and shell color was 67.5% (PCA1 was 47.2%, PCA2 was 20.3%), and the nutrient indices of golden and yellow scallops were similar (Figure 3). Among eight nutritional indexes (Table 4), significant positive correlations were found between TPC and TLC, as well as between AAs and EAAs (P < 0.01). Additionally, there was a significant positive correlation between TCC and PUFAs, TPC and PUFAs, SFAs and AAs (P < 0.05). However, there were significant negative correlations between SFAs and TCC (P < 0.05), SFAs and TLC (P < 0.05), SFAs and PUFAs (P < 0.01).
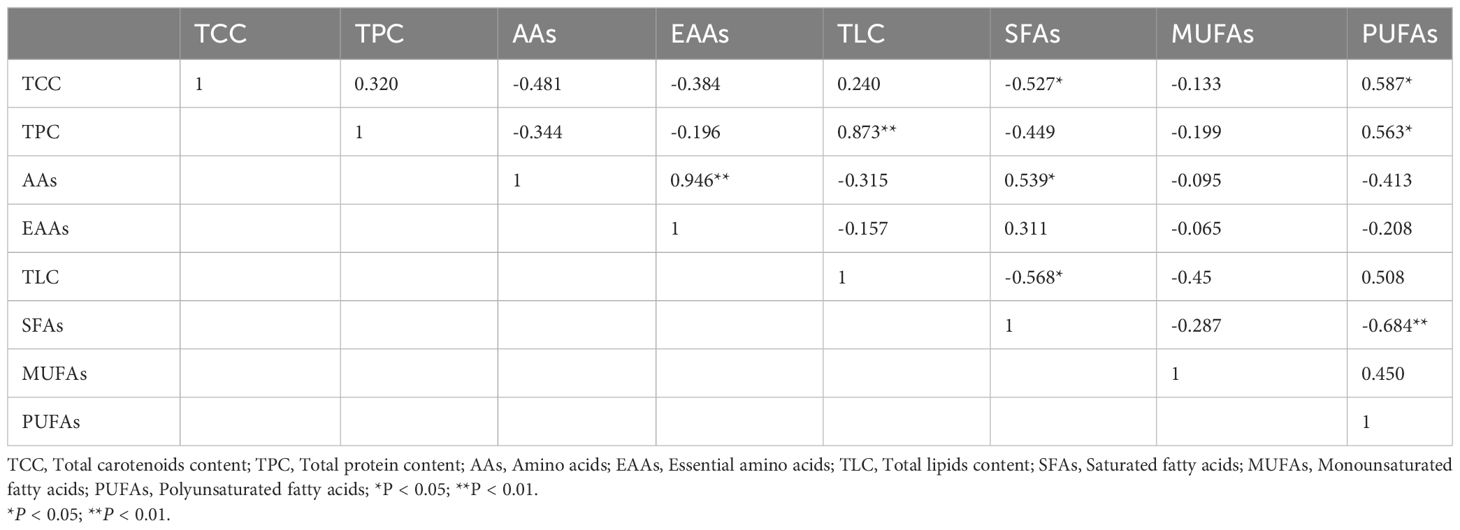
Table 4 Correlation coefficients (r) among different nutritional components in noble scallops C. nobilis.
For aquatic products, colour is the most striking feature and serves as an important reference index for people to evaluate its quality (Zhang et al., 2024). For some important aquatic products, bright colors not only have unique ornamental value (Jiang et al., 2019), but also have higher nutritional value (Li et al., 2020). They often contain special pigments (such as carotenoids) or essential nutrients. In this study, the three different colors of scallops have the same environment and natural food supply, so it is presumed that the differences in their nutritional composition are genetically or inherited determined rather than due to environmental factors.
It is well known that carotenoids are high-potency antioxidants, crucial for human and animal health. In the present study, TCC in the golden scallop was significantly higher than the brown one, which is consistent with our previous report on the species (Zheng et al., 2010). Interestingly, the yellow scallop had significantly higher TCC than the brown scallop as well. Both golden and yellow scallops enriched carotenoids in their muscles, which is the reason for their golden muscles. Moreover, TCC and muscle colors is steadily heritable in the golden and yellow noble scallops (unpublished data). Previously, a new variety of noble scallop “Nan’ao Golden Scallop” was enriched in carotenoids be bred by color (Zheng et al., 2015) and widely farmed on a large scale in China (Tan et al., 2020). Therefore, the yellow noble scallop is expected to breed another high-quality new variety, and worthy of further study.
Nowadays, people are paying more attention to a healthy diet. The composition and proportion of lipids in organisms are often used to assess their health status as food, particularly n-3 polyunsaturated fatty acids (n-3PUFAs), such as EPA and DHA, have received more attention (Nettleton, 1991; Xiao and Ou, 2005; Zhang et al., 2019; Djuricic and Calder, 2021). Moreover, excessive SFAs have been regarded as harmful to human health (Little et al., 2012; Karupaiah and Sundram, 2013). Therefore, new varieties with lower SFAs but higher PUFAs in aquatic animals are bred increasingly (He et al., 2020). In this study, the golden and yellow scallops contained significantly lower ∑SFAs and higher ∑PUFAs (especially EPA and DHA) and n-3/n-6 than the brown scallops, which may be more in line with the nutritional needs of humans.
In the present study, there is a significant positive correlation between TCC and ∑PUFAs, and a significant negative correlation between TCC and ∑SFAs, which is consistent with our previous result in this species (Tan et al., 2021). Studies have shown that carotenoids (such as lutein and lycopene) can regulate the lipid metabolism pathway, improve the efficiency of cell mitochondria, and accelerate fat decomposition, and regulate fat structure (Wang et al., 2019; Tian et al., 2020; Wang et al., 2020; Wu et al., 2020), which may be the reason why the golden and yellow scallops are rich in both carotenoids and PUFAs. Actually, the new variety of noble scallop “Nanao Golden Scallop” has widely farmed in southern China and mainly occupied the market since 2015 due to its enrich in carotenoids and PUFAs. Therefore, the yellow scallops, which are also rich in TCC and PUFAs, may have the same market appeal as the “Nan’ao Golden Scallop”.
More importantly and interestingly, the yellow scallop had significantly higher EAAs and FAAs than the golden scallop. More and more studies have shown that the EAAs rich in aquatic animal food are of great benefit to human health (Tacon and Metian, 2013; Norman et al., 2019), and the same is true for bivalve shellfish food (Idayachandiran et al., 2014; Wright et al., 2018; Thilagavathi and Christy, 2019). Rich in FAAs are also an important characteristics of aquatic animal food (Guo et al., 2019), especially the high content of Glu and Asp will directly improve the fresh and sweet taste (Babarro et al., 2011; Karnjanapratum et al., 2013). In addition, carotenoids can play a role in regulating the taste of food (Francis, 2000; Murillo et al., 2022). Therefore, the yellow scallop is more benefit to human health than the golden scallop.
Exploring marker traits with economic potential has been thought to be an important step in shellfish genetic breeding. In this study, yellow shell color, as an obviously mark in appearance, can be applied in breeding the new variety of the noble scallop. More importantly, the yellow scallop is not only richer in carotenoids and PUFAs than the brown scallop, but also richer in EAAs and FAAs than the golden scallop, which is the first time report in molluscs. We believe that the yellow scallop is more benefit to human health. It is expected that the new strain of the yellow noble scallop “Shanda Golden Scallop” will be able to be bred a new variety and provide high-quality food for customers.
Nutrient compositions were compared among golden, yellow, and brown shell color noble scallops originating from the same population. The correlation between the nutrient indices and the shell color of scallops was 67.5%. The golden and yellow scallops have more TCC, TLC, and PUFAs, and lower SFAs than the brown scallop. Moreover, the yellow scallop contained more AAs (including EAAs and FAAs) than golden one. Therefore, the yellow scallop is more benefit to human health, which is expected to have potential market.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal studies were approved by Shantou University of ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
CH: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Investigation, Resources, Visualization. TY: Data curation, Formal analysis, Writing – original draft. JZ: Data curation, Investigation, Writing – original draft. HoZ: Funding acquisition, Writing – review & editing. YS: Data curation, Formal analysis, Writing – review & editing. CZ: Data curation, Formal analysis, Writing – original draft. XH: Data curation, Investigation, Writing – original draft. JQ: Data curation, Formal analysis, Writing – original draft. HuZ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the Department of Science and Technology of Guangdong Province, China (2021B0202020003), National Natural Science Foundation of China (32273119, 42206102), and China Agriculture Research System of MOF and MARA (CARS-49).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Babarro J. M. F., Fernández reiriz M. J., Labarta U., Garrido J. L. (2011). Variability of the total free amino acid (TFAA) pool in Mytilus galloprovincialis cultured on a raft system. Effect body size. Aquac Nutri 17, e448–e458. doi: 10.1111/j.1365-2095.2010.00781.x
Bolechová P., Chaloupková H., Hradec M., Jánová E., Doležalová J. (2019). Fur color change and hormonal development in captive females of northern white-cheeked (Nomascus leucogenys) and buff-cheeked (Nomascus gabriellae) gibbons. Gen. Comp. Endoc 282, 113210. doi: 10.1016/j.ygcen.2019.113210
Cai J. N., Leung P. S. (2022). Unlocking the potential of aquatic foods in global food security and nutrition: A missing piece under the lens of seafood liking index. Global Food Secur 33, 100641. doi: 10.1016/j.gfs.2022.100641
Ding P., Yu Y. S., Zhao Z. H., Li X., Wang X. J., Wang H. Y., et al. (2024). Behavior, intestinal health, and growth of small sea cucumbers Apostichopus japonicus in different color morphs. Mar. Env. Res. 193, 106300. doi: 10.1016/j.marenvres.2023.106300
Djuricic I., Calder P. C. (2021). Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2021. Nutrients 13, 2421. doi: 10.3390/nu13072421
Guo Q., Kong X. F., Hu C. J., Zhou B., Wang C. T., Shen Q. W. (2019). Fatty acid content, flavor compounds, and sensory quality of pork loin as affected by dietary supplementation with L-arginine and glutamic acid. J. Food Sci. 84, 3445–3453. doi: 10.1111/1750-3841.14959
Han Z. Q., Li Q., Xu C. X., Liu S. K., Yu H., Kong L. F. (2021). QTL mapping for orange shell color and sex in the Pacific oyster (Crassostrea gigas). Aquaculture 530, 735781. doi: 10.1016/j.aquaculture.2020.735781
He X. M., Wen Y. L., Li Z. L., Zhou Y., Hu W. J., Sun J. M., et al. (2023). Body color selection of domesticated carp (Cyprinus carpio) in traditional agricultural systems: Insight provided by growth performance, nutritional quality, and genetic diversity. Aquaculture 572, 739528. doi: 10.1016/j.aquaculture.2023.739528
He Z. J., Xie D. Z., Nie G. X. (2020). Comprehensive strategies for increasing n-3 highly unsaturated fatty acids content in farmed fish. Chin. J. Ani Nutri 32, 5089–5104. doi: 10.3969/j.issn.1006-267x.2020.11.015
Idayachandiran G., Muthukumar A., Kumaresan S., Balasubramanian T. (2014). Nutritional value of marine bivalve, Donax cuneatus (Linnaeus 1758) from Cuddalore coastal waters, southeast coast of India. Inventi Impact: Life Style 1, 15–19.
Jiang J. F., Nuez-Ortin W., Angell A., Zeng C. S., Nys R. D., Vucko M. J. (2019). Enhancing the coloration of the marine ornamental fish Pseudochromis fridmani using natural and synthetic sources of astaxanthin. Algal Res. 42, 101596. doi: 10.1016/j.algal.2019.101596
Karnjanapratum S., Benjakul S., Kishimura H., Tsai Y. H. (2013). Chemical compositions and nutritional value of Asian hard clam (Meretrix lusoria) from the coast of Andaman Sea. Food Chem. 141, 4138–4145. doi: 10.1016/j.foodchem.2013.07.001
Karupaiah T., Sundram K. (2013). Modulation of human postprandial lipemia by changing ratios of polyunsaturated to saturated (P/S) fatty acid content of blended dietary fats: a cross-over design with repeated measures. Nutri J. 12, 1–11. doi: 10.1186/1475-2891-12-122
Li Q. Q., Zu L., Cheng Y. X., Wade N. M., Liu J. G., Wu X. G. (2020). Carapace color affects carotenoid composition and nutritional quality of the Chinese mitten crab, Eriochier sinensis. LWT 126, 109286. doi: 10.1016/j.lwt.2020.109286
Little J. P., Madeira J. M., Klegeris A. (2012). The saturated fatty acid palmitate induces human monocytic cell toxicity toward neuronal cells: exploring a possible link between obesity-related metabolic impairments and neuroinflammation. J. Alzheimers Dis. 30, 179–183. doi: 10.3233/JAD-2011-111262
Liu H. L., Zheng H. P., Zhang H. K., Deng L. H., Liu W. H., Wang S. Q., et al. (2015). A de novo transcriptome of the noble scallop, Chlamys nobilis, focusing on mining transcripts for carotenoid-based coloration. BMC Genomics 16, 44. doi: 10.1186/s12864-015-1241-x
Murillo C. M. C., Rodrigues N., Dias M. I., Bermejo-Román R., Veloso A. C. A., Pereira J. A., et al. (2022). Monovarietal olive oils fortified with carotenoids: Physicochemical and sensory trends and taste sensor evaluation. J. Am. Oil Chem. Soc 99, 1113–1126. doi: 10.1002/aocs.12650
Nettleton J. (1991). Omega-3 fatty acids: Comparison of plant and seafood sources in human nutrition. J. Am. Diet Assoc. 91, 331–337. doi: 10.1016/S0002-8223(21)01118-4
Newkirk G. F. (1980). Genetics of shell color in Mytilus edulis L. and the association of growth rate with shell color. J. Exp. Mar. Biol. Ecol. 47, 89–94. doi: 10.1016/0022-0981(80)90140-9
Norman R., Crumlish M., Stetkiewicz S. (2019). The importance of fisheries and aquaculture production for nutrition and food security. Rev. Sci. Tech Oie 38, 395–407. doi: 10.20506/rst.issue.38.2.2990
Pruden M. J., Dietl G. P., Handley J. C., Smith J. A. (2021). Using molluscs to assess ecological quality status of soft-bottom habitats along the Atlantic coastline of the United States. Ecol. Indic 129, 107910. doi: 10.1016/j.ecolind.2021.107910
Steine G., Guttormsen A. G., Alfnes F., Kolstad K. (2007). Economic values for quality traits, the colour of salmon fillet. Aquaculture 272, S312–S313. doi: 10.1016/j.aquaculture.2007.07.190
Sun T. Y., He Z. R., Bai Z. Y., Zheng H. F., Li J. L. (2021). Estimates of genetic parameters and genotype-by-environment interaction for inner shell color and inner shell luster in the golden strain of the freshwater mussel Hyriopsis cumingii. Aquac Rep. 22, 100980. doi: 10.1016/j.aqrep.2021.100980
Sun W. K., Qiao L. K., Li X. T., Gu J. Y., Zhou Y. G., Wang P. (2023). The relationship between the surface color and astaxanthin concentration of different fillet parts of Atlantic salmon under mariculture and freshwater farming modes. J. Food Compos Anal. 123, 105506. doi: 10.1016/j.jfca.2023.105506
Sun Y. Z., Zhang C. X., Liu F. X., Zhang H. K., Du H., Zhang Y. H., et al. (2024). Effects of different aquaculture patterns on growth, survival and yield of diploid and triploid Portuguese oysters (Crassostrea angulata). Aquaculture 579, 740264. doi: 10.1016/j.aquaculture.2023.740264
Tacon A. J., Metian M. (2013). Fish matters: Importance of aquatic foods in Human nutrition and global food supply. Rev. Fish Sci. 21, 22–38. doi: 10.1080/10641262.2012.753405
Tan K. S., Leng X. M., Zhao Y., Liu H. X., Cheng D. W., Ma H. Y., et al. (2019). Amino acid variations in polymorphic noble scallops, Chlamys nobilis. J. Food Process Perserv 42, e14262. doi: 10.1111/jfpp.14262
Tan K. S., Liu H. L., Zhang H. K., Li S. K., Ma H. Y., Zheng H. P. (2021). Carotenoids content and lipid nutritional quality variation in tissues of male and female polymorphic (golden and brown) noble scallops Chlamys nobilis. Aquaculture 536, 736483. doi: 10.1016/j.aquaculture.2021.736483
Tan K. S., Zhang H. K., Lim L., Zheng H. P. (2020). Selection breeding program of Nan’ao Golden Scallop Chlamys nobilis with higher nutritional values and less susceptible to stress. Aquaculture 517, 734769. doi: 10.1016/j.aquaculture.2019.734769
Thilagavathi M., Christy P. D. A. (2019). Nutritional value of marine bivalve, Donax variabilis (Linnaeus 1758) from Porayar coastal area, Nagapattinam District Tamil Nadu India. Pramana Res. J. 9, 812–819.
Tian H. C., Liu G. B., Guo Y. Q., Li Y. K., Deng M., Liu D. W., et al. (2020). Lycopene supplementation regulates the gene expression profile and fat metabolism of breeding hens. J. Anim. Physiol. Anim. Nutri 104, 936–945. doi: 10.1111/jpn.13344
Wang J., Geng T. H., Zou Q. H., Yang N. R., Zhao W. Y., Li Y. T., et al. (2020). Lycopene prevents lipid accumulation in hepatocytes by stimulating PPARα and improving mitochondrial function. J. Funct. Foods 67, 103857. doi: 10.1016/j.jff.2020.103857
Wang J. L., Li Q., Zhong X. X., Song J. L., Kong L. F., Yu H. (2018). An integrated genetic map based on EST-SNPs and QTL analysis of shell color traits in Pacific oyster Crassostrea gigas. Aquaculture 492, 226–236. doi: 10.1016/j.aquaculture.2018.04.018
Wang N., Wang D. X., Zhou J., Luo G. (2019). Lutein prevents the excessive fat deposition in liver and abdominal tissues by activating SIRT1 and up-regulating ATGL and HSL in high fat diet rats (FS06-01-19). Curr. Dev. Nutr. 3, nzz029.FS06-01-19. doi: 10.1093/cdn/nzz029.FS06-01-19
Wang L. M., Xiong J. R., Xu C. C., Qin C. B., Zhang Y. R., Yang L. P., et al. (2024). Comparison of muscle nutritional composition, texture quality, carotenoid metabolites and transcriptome to underling muscle quality difference between wild-caught and pond-cultured Yellow River carp (Cyprinus carpio haematopterus). Aquaculture 581, 740392. doi: 10.1016/j.aquaculture.2023.740392
Wright A. C., Fan Y., Baker G. (2018). Nutritional value and food safety of bivalve molluscan shellfish. J. Shellfish Res. 37, 695–708. doi: 10.2983/035.037.0403
Wu T., Gao Y. F., Hao J. Y., Geng J. T., Zhang J. J., Yin J. J., et al. (2020). Capsanthin extract prevents obesity, reduces serum tmao levels and modulates the gut microbiota composition in high-fat-diet induced obese C57BL/6J mice. Food Res. Int. 128, 108774. doi: 10.1016/j.foodres.2019.108774
Xiao M., Ou Z. Q. (2005). Research progress of the physiological function and mechanism of two kinds of fatty acid (EPA and DHA) in the fish oil of deep sea. Food Sci. 26, 522–526.
Zhang K. X., Li N., Wang Z. H., Feng D. D., Liu X. Y., Zhou D. Y., et al. (2024). Recent advances in the color of aquatic products: Evaluation methods, discoloration mechanism, and protection technologies. Food Chem. 434, 137495. doi: 10.1016/j.foodchem.2023.137495
Zhang T. T., Xu J., Wang Y. M., Xue C. H. (2019). Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 75, 100997. doi: 10.1016/j.plipres.2019.100997
Zheng H. P., Liu H. L., Chen X. Q., Zhang B., Zhang Q., Wang Y. J., et al. (2015). “A new variety of the noble scallop “Nan’ao Golden Scallop” Chlamys nobilis (Bivalve: Pectinidae) (Ed by the National Aquaculture Technique Extension Central Station of China),” in Guide for 2015 Extending Aquatic Varieties (Chin Agri Press, Beijing), 123–132.
Zheng H. P., Liu H. L., Zhang T., Wang S. Q., Sun Z. W., Liu W. H., et al. (2010). Total carotenoid differences in scallop tissues of Chlamys nobilis (Bivalve: Pectinidae) with regard to gender and shell colour. Food Chem. 122, 1164–1167. doi: 10.1016/j.foodchem.2010.03.109
Zheng H. P., Zhang T., Sun Z. W., Liu W. H., Liu H. L. (2013). Inheritance of shell colors in the noble scallop Chlamys nobilis (Bivalve: Pectinidae). Aquac Res. 44, 1229–1235. doi: 10.1111/are.2013.44.issue-8
Zhu Y. J., Li Q., Zhang J. X., Yu H., Kong L. F. (2018). Analysis and evaluation of nutrient composition in different tissues of Pacific oyster (Crassostrea gigas) with five shell colors. J. Fish Sci. China 25, 354–360. doi: 10.3724/SP.J.1118.2018.17198
Keywords: scallop, shell colour, nutrient, carotenoids, PUFA, high-quality food
Citation: He C, Ye T, Zeng J, Zhang H, Sun Y, Zhang C, He X, Qiu J and Zheng H (2024) Nutrient comparisons among the noble scallops Chlamys nobilis with three different shell colours to provide advices for consumers to choose high-quality food. Front. Mar. Sci. 11:1395339. doi: 10.3389/fmars.2024.1395339
Received: 03 March 2024; Accepted: 24 April 2024;
Published: 08 May 2024.
Edited by:
Samad Rahimnejad, University of Murcia, SpainReviewed by:
Xi Shi, Henan Normal University, ChinaCopyright © 2024 He, Ye, Zeng, Zhang, Sun, Zhang, He, Qiu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaiping Zheng, aHB6aGVuZ0BzdHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.