
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 18 June 2024
Sec. Marine Conservation and Sustainability
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1391752
 Barak Azrieli1†
Barak Azrieli1† Eynav Cohen2*†
Eynav Cohen2*† Leigh Livne2†
Leigh Livne2† Debra Ramon2
Debra Ramon2 Anat Tsemel2
Anat Tsemel2 Eyal Bigal2
Eyal Bigal2 Eli Shemesh2
Eli Shemesh2 Ziv Zemah-Shamir2
Ziv Zemah-Shamir2 Adi Barash1
Adi Barash1 Dan Tchernov2
Dan Tchernov2 Aviad Scheinin2
Aviad Scheinin2The blackchin guitarfish Glaucostegus cemiculus has suffered severe declines and regional extirpation throughout its known distributions. While this species and its relative, the common guitarfish Rhinobatos rhinobatos, have been described in the Mediterranean Sea with co-occurring habitat ranges, no research has recorded the existence or extent of these two separate populations along the Israeli coastal waters. Along a particular coast in Israel, Ma’agan Michael, fishermen have reported annual observations of juvenile guitarfish between June to November for the last forty years. Based on these citizen-based observations the main research objective is to establish whether Ma’agan Michael fulfils all three criteria from the literature by Dr Michelle Heupel, allowing it to be acknowledged as a nursery ground for G. cemiculus. The methodology built for this objective integrates biological characteristics data with the identification of a recurrent seasonal distribution. Visual surveys exhibited a significantly higher abundance in Ma’agan Michael when compared to an adjacent area (Caesarea), with 2,096 recorded observations overall. Additionally, using a species-specific modified Catch and Release protocol, a total of 492 juveniles were captured with a beach seine net. During these capturing events, individuals were morphometrically measured and sampled for future genetic analyses. Out of these, 327 specimens were also fitted for PIT tags to track recaptures in subsequent captures. The highest abundance of neonates was caught from August to September each year (2017–2019), and all individuals captured during this study were identified in the field as G. cemiculus, ranging from 20–35 cm in length (85% of captures). Many specimens had an umbilical cord scar (n = 88), with a large percentage possessing visual remains of the yolk sac. For the first time, this study provides an inter-year description of the species Glaucostegus cemiculus present along the Israeli shoreline.
Guitarfishes (superorder Batoidea) comprise two major families (Glaucostegidae and Rhinobatidae), in which Glaucostegus cemiculus (Glaucostegidae) are bottom dwellers. Guitarfish have a unique morphology composed of a flat ventral side with an elongated wedge-shaped snout (Naylor et al., 2012a). They are widely distributed from northern Portugal to Angola in the Atlantic and most Mediterranean countries.
G. cemiculus was historically common throughout the northern MS but has undergone an > 80% population reduction over the last three generations (45 years) and is therefore assessed as Critically Endangered (Kyne and Jabado, 2019). Their K-selected status mainly, as well as foraging strategies and habitat preference, have led to their population decline. The high monetary value of their meat and fins makes it an attractive catch, whether caught directly or as a bycatch (McClenachan et al., 2012; Dulvy et al., 2016; Moore, 2017; Jabado et al., 2018). Since coastal areas are an important habitat for these species, anthropogenic activities along the coastline (such as infrastructure expansion, pollution, tourism, and aquatic activities) expose them to greater risks (Bradai et al., 2012; Wosnick et al., 2018). Like many species worldwide, the species are also affected by a swift change to their habitat due to climate change, and an increase in ocean temperature and acidification may affect their survival (Pörtner and Peck, 2010; Di Santo, 2016).
In recent years, with the ongoing threat to guitarfish populations in the Mediterranean (Chaikin et al., 2020), resulting in population reduction, there is a greater need to identify nursery areas for endangered and non-endangered species to protect them throughout crucial life stages better, inform conservation managers and prevent non-endangered species from reaching endangered status. Much effort has been made to identify such locations to understand better how such crucial habitats affect the population. However, difficulties arise when defining these areas due to a lack of uniform standards of what characterises nursery grounds. Furthermore, juveniles at a site do not automatically equate to a nursery ground. To create a unified set of standards, Heupel et al. (2007) postulated three criteria to delegate a site as a nursery area for sharks, which was later adapted to batoids (Martins et al., 2018):
i. Neonates/juveniles are more prevalent in a particular area compared to others.
ii. Neonates/juveniles continue to use the area for extended time periods (weeks or months).
iii. Neonates/juveniles are prevalent in the same area over successive years.
Each of these three criteria was addressed quantitatively using a non-distractive, non-invasive method of visual abundance surveys. For each criterion, demanding a different spatiotemporal resolution, a fitted experimental design was constructed and will be discussed here. As of the time of writing, no nursery grounds have been identified or delegated for G. cemiculus according to these criteria. The only information on guitarfish populations in the eastern Mediterranean has come from the local fishery and fishery surveys (Kyne et al., 2020). These surveys contained only general, and sometimes incomplete, information on the guitarfish landings; observers did not specify the species and listed the catch as “guitarfish” in reports (Sherman et al., 2023).
While two species of guitarfish have been described in the Mediterranean Sea with co-occurring habitat ranges, no research has recorded the existence or extent of these two separate populations along the Israeli coastal waters. From previous fisher observations and discussions with local community sea users, R. rhinobatos was considered the dominant species observed, with G. cemiculus having a smaller presence if any at all. With this substantial knowledge gap in the basic biological and ecological data, further conservation efforts cannot be made to protect these species. Currently, there is no species-specific plan for their conservation in Israel, and thus, a baseline for this corner of the Mediterranean is required (Bangley et al., 2018; Martins et al., 2018).
Along a particular coast in Israel, near Kibbutz Ma’agan Michael, fishermen have reported annual observations of juvenile guitarfish at certain sites between June and November for the last forty years (Figure 1). We hypothesise that the presence of young guitarfish, recorded in high abundance and over long periods across all years observed, suggests that this area is vital at early life stages (Heupel et al., 2007; 2018). The main research objective is to establish if Ma’agan Michael is a nursery ground for G. cemiculus and if it satisfies all three criteria. To meet this objective, the southern adjacent site of Caesarea was added for comparison, as both sites share similar topography and habitat characteristics.
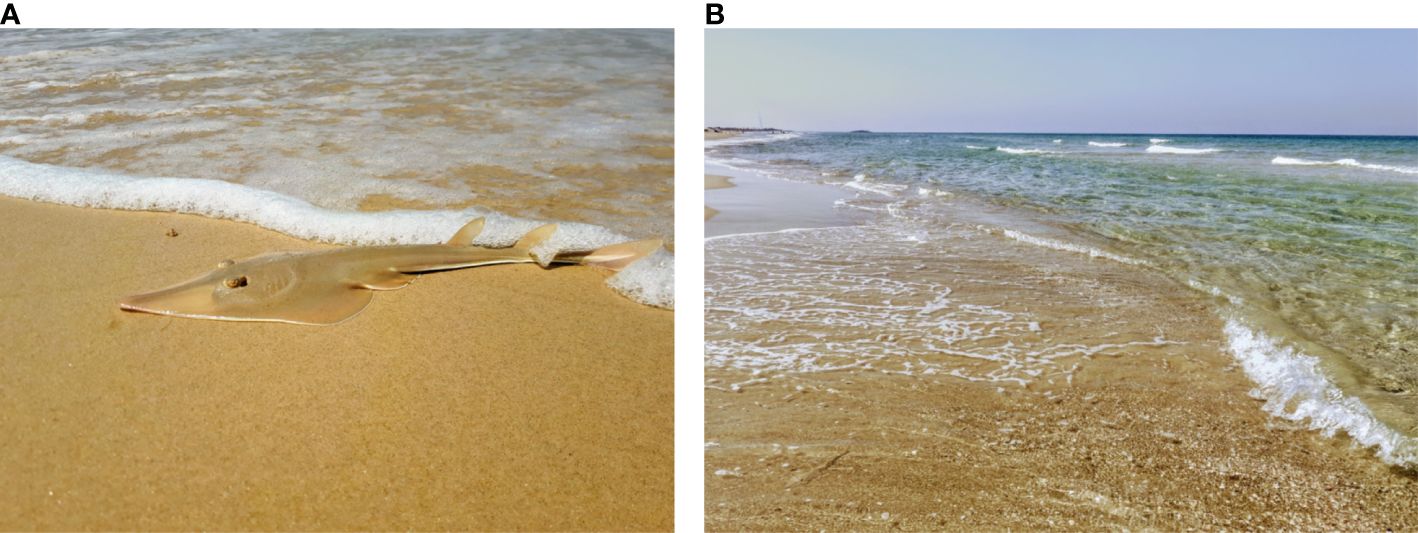
Figure 1 Glaucostegus cemiculus neonate ‘stranding’ (A) as seen in the intertidal zone during visual abundance surveys from afar (B).
The coastline of Ma’agan Michael (32° N, 34° E) stretches approximately eight kilometres and is characterised by sandy beaches (Figure 2). The southern point of the study area is bordered by the Taninim River estuary, which originates inland at Ramot Menashe and yields into the Mediterranean Sea at the coastal village of Jisr Al Zarqa. This river carries large quantities of organic matter, which flows directly into the sea basin. Other habitat features on the southern border of the estuary include Tel Taninim (a kurkar ridge) and submerged rocky sandstone (kurkar reef patches), which are common to this region. These submerged rocky reef patches also occur at the northern border of this site (Tel Dor) and provide a natural sheltering effect to the exposed coastline. Inland and along the coast, there are multiple freshwater fishponds used for cultivating fish for human consumption, and this effluent contains higher levels of organic matter that is directly discharged into the sea via six canals along the coastline. For the purposes of the research objective, a comparison of this main site to an adjacent site was necessary, so Caesarea (Figure 2) was selected for this purpose.
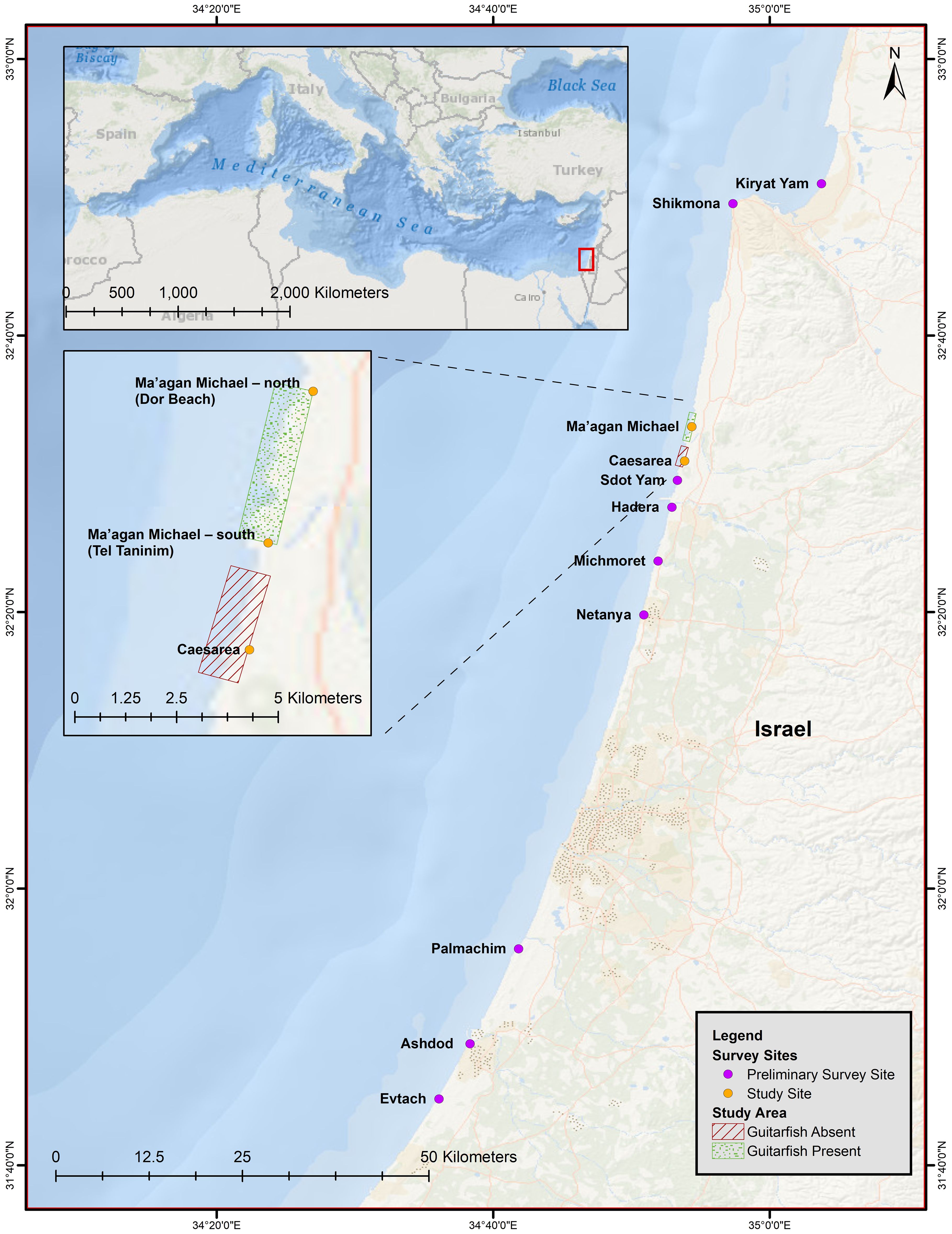
Figure 2 Overview of sites surveyed during the study along the Israeli coastline (orange and purple circles). A map of the Mediterranean Sea, with Israel indicated by the red rectangle. The study sites of Ma’agan Michael and Caesarea (orange circles). Surveys in Ma’agan Michael stretched from the Tel Taninim estuary to Dor Beach.
A total of 487 specimens were examined morphologically for species identification. Rhinobatos rhinobatos have a wider separated rostral ridge and separated nasal lobes, and their snout and whole disc shapes are wider. This is compared to G. cemiculus, whose rostral ridges are narrowly separated, nasal lobes are elongated, and its snout is more pointed. Differences in rostral ridge width can be observed both dorsally and ventrally (Supplementary Figure 4).
A total of 40 samples from 12 different sampling days were chosen randomly (two to three samples for each sampling day) from Ma’agan Michael. The sex ratio was equally maintained. An additional sample was taken from a neonate guitarfish at the Gottesman Family Israel Aquarium after a pregnant female guitarfish was caught at sea and brought into the aquarium. The neonate was identified as R. rhinobatos. Following all the lab procedures and sequencing steps, the analysis involved 38 nucleotide sequences for NADH and 37 nucleotides for CO1.
Two phylogenetic trees were inferred based on the two markers: CO1 and NADH (Supplementary Figure 3). The evolutionary history was described using the Maximum Likelihood method and General Time Reversible model for CO1 and the Tamura-Nei model (Tamura & Nei, 1993) for NADH. The sequences have been uploaded to the NCBI database as of the publication of this manuscript.
Caesarea was chosen to determine a significantly higher abundance of neonates in Ma’agan Michael compared to an adjacent coastal site (surveyed in the preliminary surveys) named Caesarea. Six temporally paired surveys were conducted in each site throughout the aggregation season, from September to November.
Possessing geographical and habitat characteristics similar to Ma’agan Michael, Caesarea is typical of most of the Israeli shoreline, with sandy, shallow-sloping beaches and kurkar ridges. It is also a unique amalgamation of archaeological ruins, showcasing remnants of ancient harbours, docks, breakwaters, and architectural structures.
To prove that G. cemiculus neonates continue to use the area for an extended period of time, routine visual abundance surveys were conducted from July 2017 to December 2019. To address the second criterion, the time of the neonate’s appearance and disappearance in Ma’agan Michael was highlighted. Regarding the third criterion, the research continued its monitoring efforts for an extent of three years (2017–2019).
Based on the preliminary surveys, a protocol was designed for the visual abundance surveys in this study and was implemented for the comparative as well as the routine main study site visual abundance surveys as follows; walking along the beach at the tideline at a consistent, steady pace and counting individual guitarfish observed from the tideline up to two meters into the sea. Surveys were conducted only when sea conditions were considered suitable (defined as a wave height of 0 - 0.5 meters). Surveys that were conducted under unsuitable sea conditions, without the application, or did not follow protocol were excluded from the results. All surveys took place between 0700 and 1000 hours in the morning, at a walking pace of 5 to 6 km per hour. Suitable conditions were pre-determined by a field experiment before this method was implemented. The method considered different hours of the day, pace, and sea conditions, including wave height, wind speed, and direction. Easterly winds resulted in a lower observation rate and were preferably avoided. After determining the most suitable conditions for the surveys, most (if not all) surveys were conducted according to this protocol, with surveys at different conditions excluded from the results. The same individual performed over 95% of visual surveys to avoid sample and observation bias.
A reliable platform was required to achieve maximum accuracy and consistency during the survey periods. The “Guitarfish” app was created voluntarily to assist animal conservation. The app was designed as a mobile application specifically for this project and has been utilised for all surveys since July 2017. The application shows the exact GPS location of the survey while tracking the movement and documenting the surveyor’s route. Every guitarfish sighting was documented in the application to an accuracy of 20 m. All guitarfish that overlapped within a 20 m2 area were combined and reported as a total number of observed individuals. The application also documents the distance, time, and pace to exclude any bias due to large shifts in the survey.
In addition to the environmental data, anthropogenic data, such as the presence and number of humans utilising the beach, was documented in the application. The number of humans was categorised into four sub-categories: 1: no audience, 2: small audience, 3: crowded, and 4: very crowded. The number of long-term camping tents on the beach was also recorded for future research on the combined effects of noise, light pollution, and swimmer presence on the presence or absence of guitarfish. Drainage points from the fishing ponds to the sea were also marked to assess whether these points may impact observations. The application also documents pictures and videos of guitarfish, as well as field notes and observed phenomena.
For the analysis and determination of whether each one of the three criteria was met, a transformation from the number of observations per survey to density per survey was calculated as follows:
As criterion one requires, to elucidate that the main study site does occupy a significantly higher abundance of guitarfish than its adjacent, a two-sample paired t-test was done for the 6 paired surveys from each site (n=12) based on the calculated density using PAST 3.04 statistics software (Hammer et al., 2001).
Between July 2017 and December 2018, 30 field days were conducted at Ma’agan Michael Beach, excluding January to March 2018, due to unsuitable sea conditions, with 13 field days in 2017 and 17 field days in 2018. During those days, 186 nets were deployed (a mean of six nets per day). Nets were not deployed between January to March 2018 due to unsuitable sea conditions.
Net deployment was conducted with a permit from the Fishery and Aquaculture Department (permit number: 10017474 for 2017–2019) and a permit from Israel’s Nature and Parks Authority (permit numbers: 41738, 42036, and 42286 for 2017–2019, respectively). Nets were deployed at selected locations along the studied area based on observations made during the visual surveys. With the same protocol, they were set from 10–30 m out to a maximum depth of 1.3 m (example provided in Supplementary Figures 1A-C). The number of deployments on each sampling day depended on sea conditions and the number of guitarfish caught in each net. As mentioned above, suitable conditions for net deployments were pre-determined for the visual abundance surveys. On most field days, an average of six nets were deployed in six different locations, with a minimum of three nets per survey day. Net deployments were standardised to mean captured individuals per net.
Specimens caught in the net were carefully removed by hand and immediately placed within a temporary container pre-filled with 140 L of seawater and some sand. Each specimen was removed from the holding container, placed on a flat tray, and measured for the following using a measuring tape to the nearest mm: total length (TL), disc length (DL), and disc width (DW; Supplementary Figure 2). Weight was measured using a lab scale (VETEX model: super ss) to the nearest gram. Additionally, sex was documented and determined based on the presence or absence of claspers. Dorsal and ventral photos of each individual were taken for documentation purposes. To approximate size at birth, the presence of yolk sac remains or scarring on the neonate’s ventral side was documented throughout the study (Duncan & Holland, 2006; Chin et al., 2015). Total length and weight (g) of specimens with either one of these features were used to calculate the average size at birth. Any abnormal observations, such as the presence of a black spot on the chin, injury-inflicted dimorphism, markings, or abnormalities, were also documented. Specimens with missing data were removed, and data from 2019 from the Ma’agan Michael study area was removed due to low data resolution. Duplicates were removed from the recaptured specimens, and only the first capture was considered. A total of 548 individuals were captured in this study.
Neonates captured in Ma’agan Michael were tagged with Biomark MiniHPT8 passive integrated transponder (PIT) tags from a pre-loaded needle to maximise sterilization (Das Mahapatra et al., 2001; Farrugia et al., 2011). A small incision (with a sterile scalpel) was made just under the dorsal fin, and with a dedicated syringe, the tag was inserted (Supplementary Figure 2C). The incision was then sterilised using a specific solution (Chlorhexidine Gluconate 1.5% W/V, Cetrimide 15% W/V dilution 1:100). Following the first tagging event, all individuals were scanned for the presence or absence of a PIT tag, allowing for confirmation of recapture (Supplementary Figure 2D).
Additionally, tissue samples were taken from the tip of the first dorsal fin (fin clip) using sterile scissors (Supplementary Figure 2B). This part of the protocol is also necessary to inform the surveyor if the individual is a recapture. The maximum handling time was set to five minutes to reduce stress on the specimen. After collecting all measurements and data, the individual was confirmed to be in good condition by a standardised observation period before being released back to the sea (Farrugia et al., 2011; Supplementary Figure 2E). Net bycatch (i.e., crustaceans, fish, etc.) was also documented for further research. All guitarfish caught and handled were returned to the sea without injury within 10 minutes of capture, with no mortality recorded because of the operation.
All morphometric data were analysed using SPSS software version 23. For any data that was not normally distributed, a non-parametric Spearman’s Rank test was used to test the significance. The length-weight relationship (LWR) was calculated based on 420 neonates measured during three seasons by using a power-type equation as follows:
where W= total weight (grams), L= total length (cm), a = constant (intercept), b = allometric growth coefficient (slope of regression line).
All 500 individuals captured were morphologically identified in the field as G. cemiculus. Dead specimens collected either in the field from fishermen or from the Israeli Oceanography and Limnological Research (IOLR) fishing surveys resulted in 16 guitarfish identified as R. rhinobatos and eight identified as G. cemiculus (Supplementary Figure 5).
The phylogenetic tree for both COI and NADH segments showed maximum similarity between all 37 and 38 (respectively) field specimens to G. cemiculus reference sequences, confirming the sampled neonates from Ma’agan Michael belong to this species, while the comparative sample from the Gottesman Family Israel Aquarium, named “Guitarfish B/C (Aquarium)” clustered with R. rhinobatos.
The authenticity of the undocumented neonate aggregation phenomenon was confirmed in eight out of the 11 locations surveyed in the preliminary surveys. Ma’agan Michael, one of the eight locations where guitarfish neonates were observed, was selected as the main study site after analysis of the comparative surveys exhibited a significantly higher abundance of neonates (n=6 per site) in Ma’agan Michael than its southern neighbour Caesarea (t test=8.6183, p-value = 0.03).
A total of 47 surveys were conducted in the routine main study site survey series between June 2017 and November 2019, with 2,096 neonates observed and recorded. In 2018, there were no surveys between January and March and January 2019 due to unsuitable sea conditions. The results exhibited a clear trend of high abundance during mid-August and September, with abundance declining until November (Figure 3). In December, sightings were recorded much less frequently. In April and May of both years, there was another peak of sightings, but of lower abundance (up to 1.3 individuals per km in 2018 and 0.66 individuals per km in 2019). The individual density (expressed as an average) from July to November was higher in 2017 (avg. 11.84 ind./km) compared with 2018 and 2019 (avg. 1.98 ind./km and 3.24 ind./km, respectively). During 2017, the distribution patterns exhibited a higher individual abundance in the middle of the study area between Ma’agan Michael Beach and Ma’ayan Tzvi Beach compared with the outskirts.
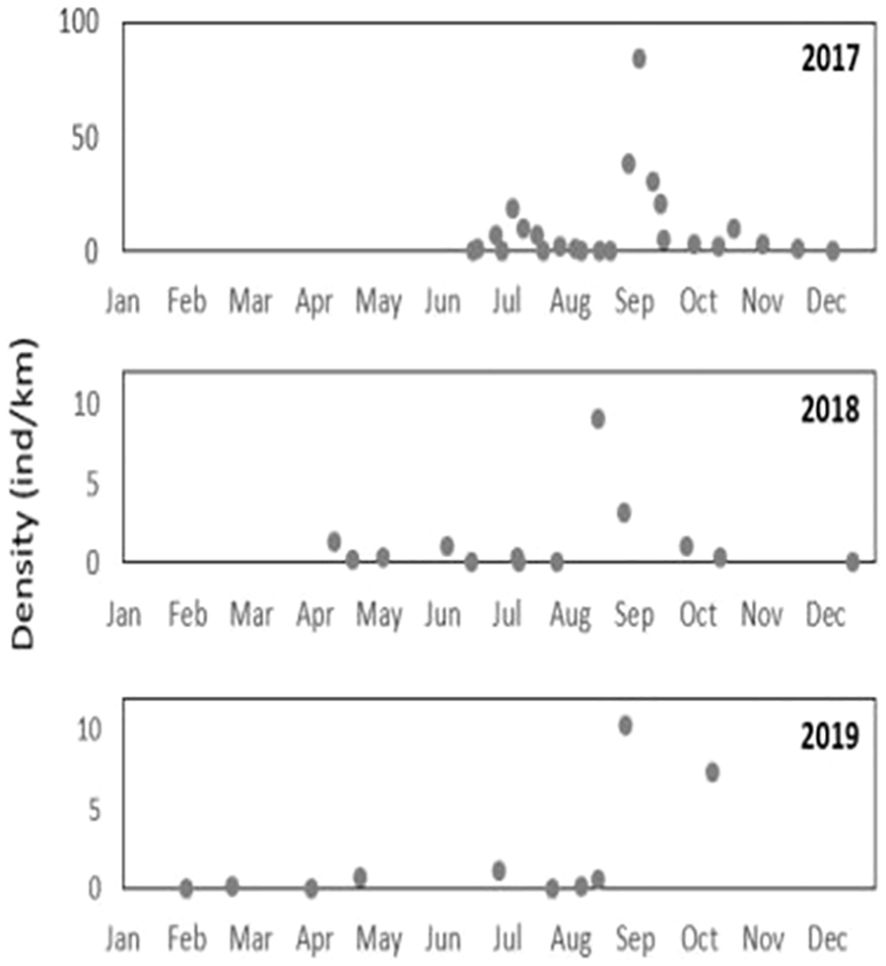
Figure 3 Visual abundance surveys conducted at Ma’agan Michael between June 2017 to October 2019. Each point on the plot marks a single survey. The density is calculated by the number of individuals sighted during a survey divided by distance (km).
During the field days, there were two peaks in the average number of guitarfish captured per net deployed in September 2017 and August 2018 (average of 7.8 and 10.29 individuals captured per net, respectively, Figure 4). In the following months, there was a decline in capture rates, with a minimum in December of 2017 and 2018 (average of 0.56 and 0.25 individuals captured per net, respectively). In April and May of 2018, there was a noticeable second peak in average capture rates (average of 2.5 and one individual captured per net, respectively), followed by a decline in capture rates until August.
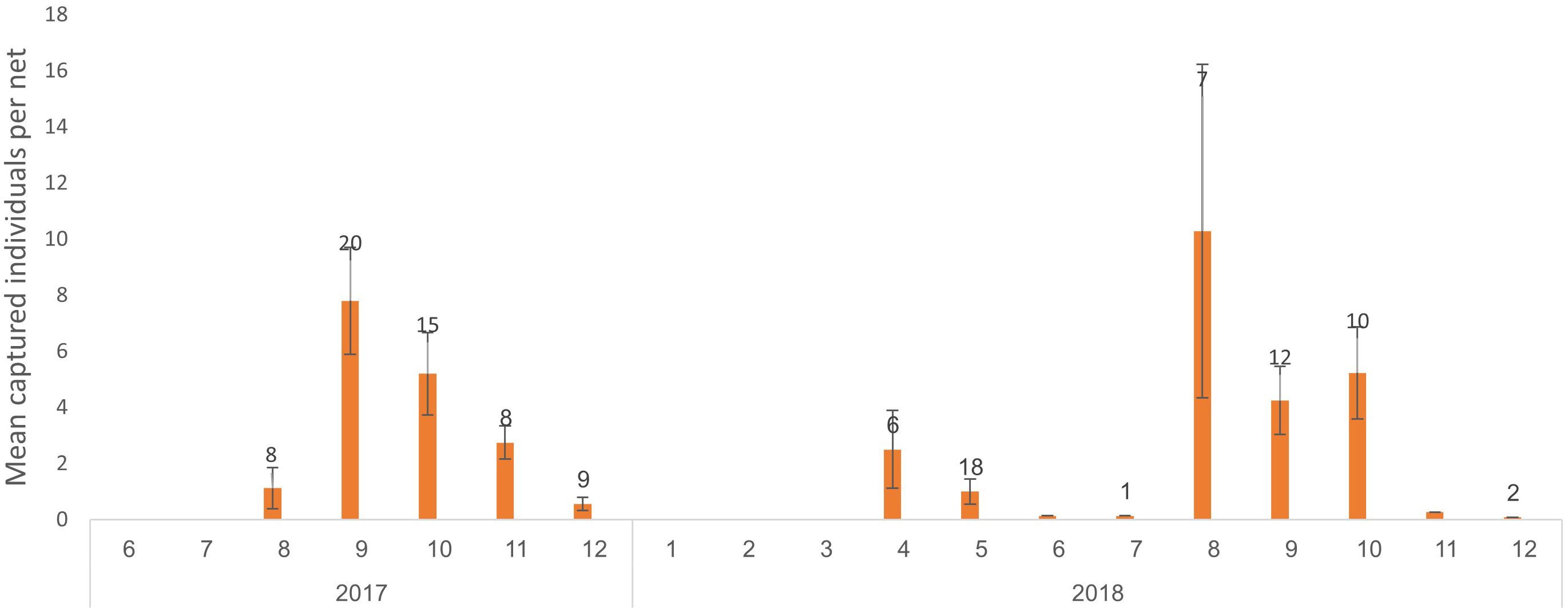
Figure 4 The mean number of captured individuals per net at the Ma’agan Michael site. Red bars represent fabric net deployment, and blue bars represent nylon net deployment. The numbers above the bar represent the raw number of nets deployed each month. Line bars represent SD of mean captured individuals per net.
Of those captured, 327 guitarfish were equipped with PIT tags (Biomark). Nine of the guitarfish equipped with tags were recaptured, and one (captured in October 2017) was recaptured twice (three in total; Supplementary Table 1). An additional 10 guitarfish were recaptured based on the fin clip scar, but since they were not equipped with PIT tags, it was hard to determine which individual we caught and if they were captured more than once. The tagged recapture rate is approximately 3% of the total capture. The average growth was 0.38 mm per day (median 0.3). One individual showed no growth between captures. One individual grew quickly (1.65 mm/d), faster than all captured guitarfish. The growth rate was, on average, approximately one cm per month.
The captured guitarfish ranged from 23 cm to 81.3 cm in total length (mean: 34.9 ± 6.3) and weighed 45 g to 1810 g (mean 144.9 ± 145.7). The ratio of males to females did not differ from 1:1 (218 males and 240 females; Chi-Square Test, significant to 0.304). However, the null hypothesis stating the size (Unequal Variance t-test (Welch’s t-test): 1.2373, p-value: 0.20346) and weight (Unequal Variance t-test: 1.0327, p-value: 0.30233) between males and females has no significant difference in the means of the two groups was rejected. 78% of the captured guitarfish belonged to the 30–35 cm bin. The 20–35 cm group bin represented 84.46% of all specimens captured.
When looking at the temporal size distribution of guitarfish caught in Ma’agan Michael from August to November during two successive years, 2017–2018, the smallest mean and median for guitarfish total length were found in August (31.58 cm and 31.6 cm, respectively), and the highest mean and median were found in November (36.23 cm and 34.2 cm, respectively). (Figure 5). A significant correlation was found between the total length measured and the date (Spearman’s Rank Test, p< 0.0001, Adjusted R-squared: 0.42). The linear model resulted in TL (cm) = 0.006*Days + 30.3, setting the general growth rate at 0.006 cm per day, equivalent to 1.98 cm per month (30 days).
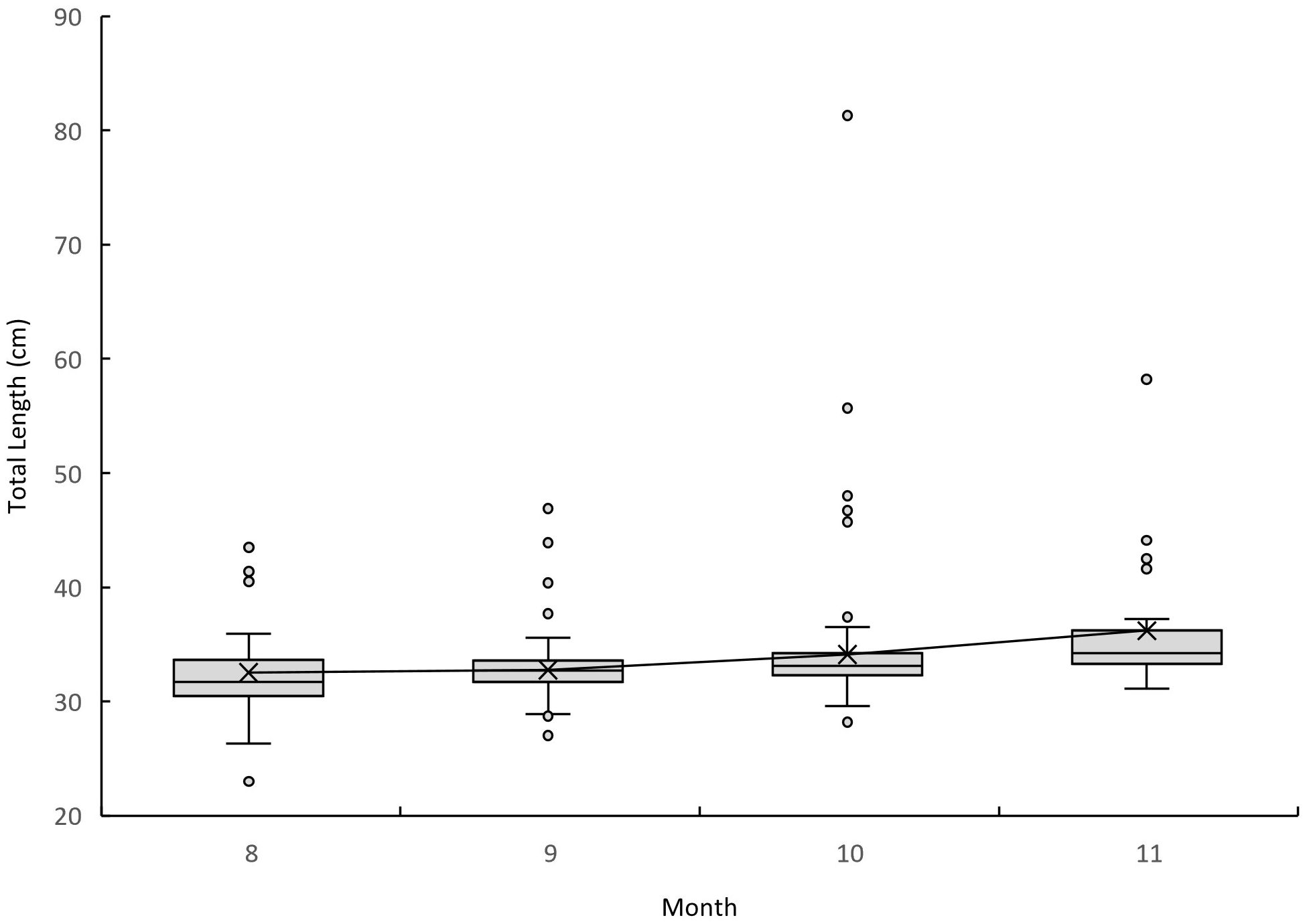
Figure 5 Monthly temporal size distribution of all guitarfish captured in the Ma’agan Michael site for 2017 and 2018 combined. The number of nets deployed each month is presented in the brackets below.
According to length-weight relationship equations, the ratio between the total length and weight of the guitarfish sampled in Ma’agan Michael is presented in Figure 6. The values of exponent b in the equations were approximately 3, indicating an isometric growth with a high correlation coefficient (r2). The value of LLR (r2 > 0.9) indicated that they are highly significant and highly correlated. A significant strong correlation exists between weight and total length (Spearman Rank Correlation, p-value<0.01).
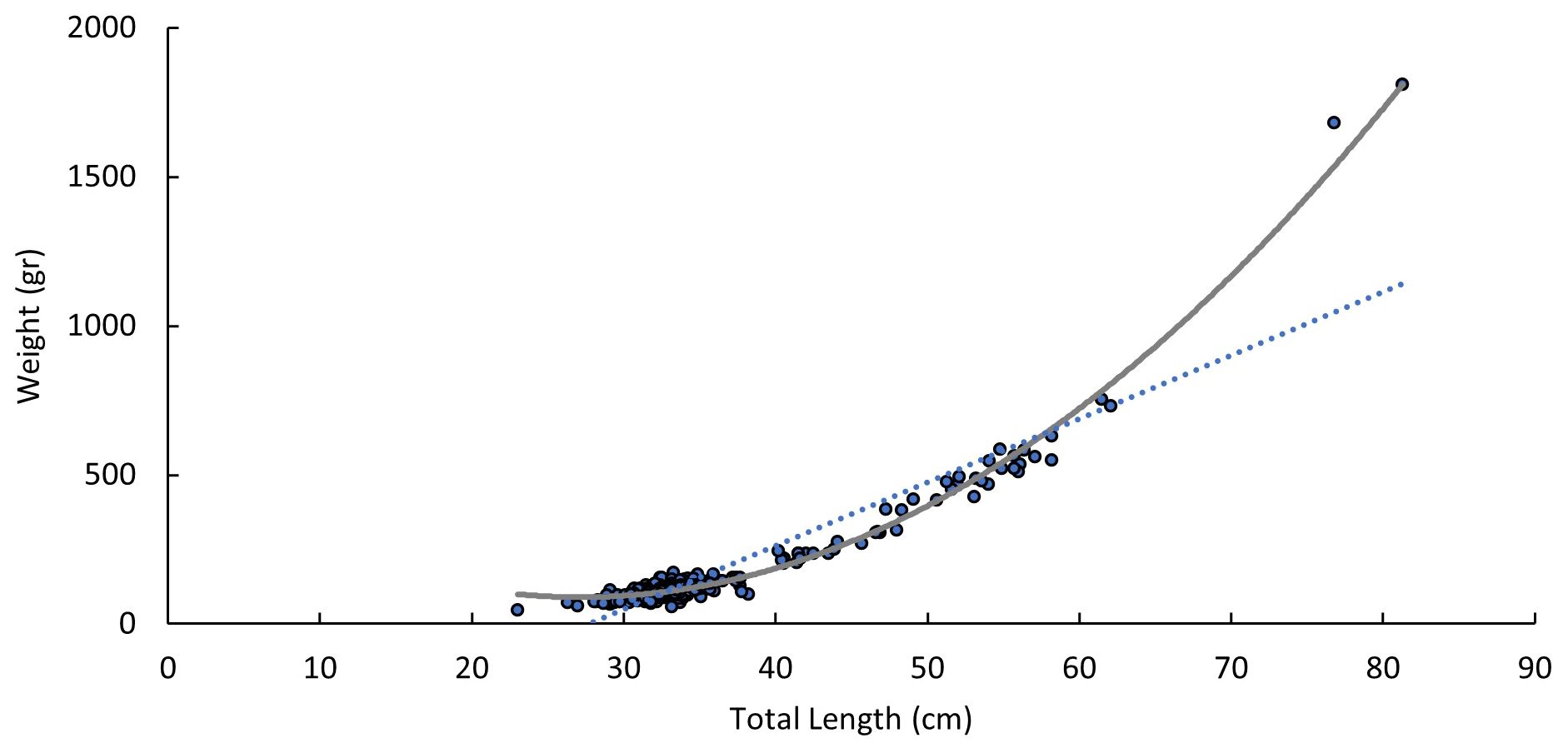
Figure 6 Length-Weight Relationship of guitarfish caught in Ma’agan Michael (n=469). Specimens that did not undergo a full workup of measurements were omitted from statistical analysis.
Many specimens were caught and reported to have remains of their yolk sac intact (Figure 7). The time period for this observation is from the beginning of October to the end of October. The average TL at birth was calculated to be 31.9 cm (SE 2 cm, n=88), Sizes ranged between 23–35.9 cm (mean 31.87 cm, SD 2.016), and weight ranged between 45–165 g (mean 110.95 g, SD 22.68, n=88). The size and weight of male and female neonates did not differ significantly (Unequal Variance t-test (Welch’s t-test), all p values > 0.05).
Our findings from the aggregation of blackchin guitarfish in Ma’agan Michael aligned with the basic description of a nursery ground proposed by Heupel et al. (2007) and later adopted for batoids (Martins et al., 2018). The quantitative definition translates to three criteria, as mentioned before, which were addressed through a series of visual abundance surveys and the neonates’ biological characteristics over successive years.
Before addressing the nursey definition, it is important to clarify which species were predominantly caught and analysed in this paper. There exists a known overlap in the distributions of R. rhinobatos and G. cemiculus, reported in Tunisia, Egypt, and Turkey (Abdel-Aziz et al., 1993a; Çek et al., 2009; Echwikhi et al., 2013; Filiz et al., 2016). Based on this study’s morphometric and genetic species identification results, it appears that R. rhinobatos does not occupy the nearshore areas of G. cemiculus. The main method for modern species identification was defined by Ward et al. (2005), who suggested CO1 (cytochrome oxidase subunit 1) as a region in the mitochondrial DNA that is the best preserved for differentiating between species. CO1 has ~ 630 bp and is used as the global barcoding method for taxonomically separating species.
In elasmobranchs, it is also prevalent to use another marker, the nicotinamide adenine dinucleotide hydrogen subunit 2 (NADH 2) gene that has ~1044 bp and was found to be successful in separating closely related elasmobranch species by Naylor et al. (2012b). Since there are very few sequences of the two species available on the international online databases, in this study, both markers were chosen to increase the success and accuracy of the examination. Both markers are part of the control region of the mitochondrial DNA.
In addition, R. rhinobatos juveniles are reportedly caught by fishers in the deeper waters of the Ma’agan Michael site at 20–40 m depths, directly in front of the postulated G. cemiculus nursery. With no R. rhinobatos being caught close to shore in this study, it is a possible indicator of niche partitioning between the two species and that the deeper waters are an important site for R. rhinobatos. Partitioning of resources, especially that of prey, can allow the two sympatric species to reduce competition and enable co-existence, thus increasing survival rates during crucial life stages for both (McPeek, 2014; Martins et al., 2018). Therefore, the two species can utilise the same area but at different depths. Additional studies investigating deeper waters within the area are required to confirm this hypothesis, as it is still unclear how R. rhinobatos utilise this area. Parturition seasons have been documented in the Mediterranean Sea for R. rhinobatos alone, with Tunisia reporting parturition during June/July (Enajjar et al., 2008) and Egypt between July and September (Abdel-Aziz et al., 1993b) and at the time of submission, there is still a knowledge gap concerning when and where mating occurs.
Prior to the methodology implemented here, most of the guitarfish data in the Mediterranean Sea had been based on commercial fishery landings, which limits the accuracy and applicability of collected data (Newell, 2016). This study’s experimental design aimed to research guitarfish in their natural environment with minimally invasive methodologies without sacrificing guitarfish or fatal injury. These two methods of visual and net surveys are highly complementary to one another. They can be easily adapted to similar sites in different countries, especially those with limited funding. However, these methods are highly susceptible to environmental conditions and require a certain level of expertise to reduce sampling bias.
G. cemiculus population is projected to decline by 50% over the next three generation periods (Notarbartolo di Sciara et al., 2016); there are not many sites with high concentrations of guitarfish, and to date, no nursery ground has been declared at the time of publication. There is a large gap in our knowledge of these species in the eastern Mediterranean, with some studies conducted in neighbouring countries and some records of juvenile presence. However, no nursery grounds of spatiotemporal distribution study have been attempted before. To answer the first criterion, stating that neonates/juveniles are more prevalent in a particular area than in others, it has been shown utilising temporally paired comparative visual abundance surveys.
Ma’agan Michal hosts a significantly higher density of guitarfish per km compared to an adjacent location- additionally, the unquantified preliminary surveys, other than justifying the study site selection, indicate the spatial extent of this phenomenon. It is important to note that there were reports from seven additional locations along the Israeli shore that were outside the scope of the present study; we recommend re-visiting and surveying these locations to examine their potential as a nursery ground for G. cemiculus.
The second criterion states that neonates/juveniles continue to use the area for extended time periods (weeks or months). The site-specific seasonal scope of habitat usage in the Ma’agan Michael intertidal zone was delineated both by the routine main study site visual abundance surveys and net deployments for at least three months (mid-August to mid-November). Furthermore, the increase in neonates TL during each season, as it has been shown in the temporal size distribution analysis, indicates that it is the same population occupying Ma’agan Michael for that specific season. From 2017 to 2019, between January and March, there was no information on guitarfish presence due to unsuitable sea conditions for net deployment and visual abundance surveys. It is possible that the guitarfish were using this area throughout those years. In addition, recaptured individuals from the same location, as indicated by their PIT tags, showed that some individuals are being recaptured up to 10 months from the initial capture.
The third criterion states that neonates/juveniles are prevalent in the same area over successive years; this was shown in Ma’an Michael, the main study site, from September 2016 to 2019 from both capture by the seine nets and during visual abundance surveys. It is important to note that during the year 2023, monitoring efforts for G. cemiculus neonates in Ma’agan Michael were reinitiated, but the research team confirmed the ongoing presence of this species in this proposed to be nursery ground.
Other than addressing the three criteria, a potential benefit of Ma’agan Michael’s intertidal zone as a nursery ground is that it can protect it from both predators and harsh sea conditions. Neonate guitarfish use shallow waters to avoid predation or simply by happenstance of residing in shallow intertidal waters at this life stage (Newell, 2016; Moore, 2017; Martins et al., 2018). In general, the shallow areas of the study site may limit the entrance of large predators by creating a depth barrier (Carrier and Pratt, 1998; Bethea et al., 2014). However, it has been shown that nursery areas are not necessarily free of predators (Martins et al., 2018). A second possible advantage relates to neonates exhibiting novel stranding behaviour for short periods (up to one minute) throughout the day. This may demonstrate a strategy that is possible only in the intertidal zone to escape predation in shallow waters, although the motivation for this behaviour remains a mystery and is not fully understood. Lastly, the geographic structure of the area, with the submerged rocky reef and small islands, provides protection against mechanical wave action, which may serve as a third advantage of this area as a nursery ground.
Length-weight ratio data (LWR) are useful and standard practice for biological and oceanographic modelling studies and crucial for species conservation and management plans. Determining length-weight relationships in fish provides information about the growth pattern, general health, and habitat conditions relevant to nursery grounds. Growth behaviour will be isometric when the allometric growth coefficient b=3, and negative allometric growth is determined when b< 3. In terms of the ecology of the neonates and the environmental conditions in the locations in which they were sampled, results can indicate if the habitat provides suitable conditions for the species to grow and thrive. For example, the value of the allometric growth coefficient may be influenced by the availability of food resources. If neonates have access to abundant and nutritious food, an increase in TL and weight will be exhibited, resulting in b= ~3. Conversely, a significantly lower value of the allometric growth coefficient, which was not found in our results, might indicate environmental stressors, such as pollution, habitat degradation, or resource limitations, which can impact growth. Since nursery grounds are defined as essential habitats for population persistence and provide important services to the neonates, benefiting their survival and fitness, the allometric growth coefficient found here serves as evidence for detecting a nursery ground for G. cemiculus, in the main study site, Ma’agan Michael. It is important to note that the appropriate approach for using the LWR equation for the estimation of the LWR parameters requires a large sample size and a minimum of one-year time scale of data collection to capture all the size ranges and to truly reflect the ontogenetic growth performance of a specific aquatic species in a specific location. Both requirements were met in this research and allowed the utilisation of this equation.
Specimens caught in Ma’agan Michael were observed with scars or remains of the yolk sac (Figure 7). We believe this to be a strong indicator of recent birthing, as bottom dwellers create constant friction with the bottom substrate, thus removing any remaining tissues. This indicator may remain on the guitarfish for hours to days. However, no investigations on this subject have been conducted. One specimen was captured with the remaining tissues of its yolk sac, indicating that it was born within a few hours of capture. The scar can remain longer, as previous studies have highlighted (up to six weeks, Simpfendorfer, 2000; Chin et al., 2015). Thus, the yolk sac and remains or scars act as a timeline indicating recent birthing; this allowed us to estimate the size of birth at 31.9 SE ± 2 cm for G. cemiculus in local waters. There are, however, specimens caught that deviate from the average birth size, which can be explained by the mother’s size. Larger or older mothers are logically more experienced and use their surrounding resources more efficiently, which gives the pups a size advantage (Hussey et al., 2010).
As G. cemiculus juveniles reach approximately 35–36 cm, which equates to a three- to four-month residency time, they are less frequently caught and observed. One explanation for this is nursery abandonment, where the juvenile no longer requires the safety of the sheltered area (Duncan & Holland, 2006; Bethea et al., 2014; Farrugia et al., 2011; Heupel et al., 2018), or a shift in the use of the site, such as migrating to deeper habitats. Another reason for this decreased presence may be predation, which we believe to be occurring based on observed injuries. However, guitarfish were observed to heal rapidly from these injuries, as observed from this study and described in previous studies with sharks (Simpfendorfer, 2000; Bird, 2012; Chin et al., 2015). Alternatively, it may be that not all individuals abandon the site at a certain size and instead continue to use it over a prolonged period.
During this study, G. cemiculus was reassessed by the IUCN Red List and, due to its severe population decline, has been redefined from Endangered to Critically Endangered (Kyne & Jabado, 2019). The status change emphasises the immediate need for establishing proper species-specific management and identification of important habitats throughout their entire distribution. G. cemiculus has especially suffered in European countries bordering the Mediterranean Sea, with overfished populations and no important habitat sites identified for the species and allocated for their conservation (Moore, 2017).
Based on the results of this research, we believe that Ma’agan Michael should be designated as a nursery ground for G. cemiculus, and we urge the local scientific community and stakeholders to pursue this designation in future. Temporally, the neonates use this habitat as a nursery from late July to early December. While neonates were observed and caught all year around (excluding January to March, when sea conditions prevented fieldwork), a relatively low density of neonates was recorded from April to July. Since guitarfish are vulnerable to all types of fishing methods, from pole and line, beach, and seine nets to artisanal fishing vessels in shallow waters, it is recommended to prohibit all activities at this location during peak seasons. However, as year-round protection is difficult to implement, we urge a seasonal, species-specific management plan to be developed for the nearshore Ma’agan Michael area from late July to early December. Further research will be required to understand the depth distribution of the species and movement patterns within the nursery. Following the legislative measures already in place protecting elasmobranchs in Israel, allocating species critical habitats for the critically endangered G. cemiculus should be the next step taken for their conservation. Nursery grounds are essential habitats for population persistence and provide important services to juveniles, benefiting their survival and fitness (Martins et al., 2018; Heupel et al., 2018). G. cemiculus conservation can benefit the entire marine food web through top-down control (Jackson et al., 2001; Ritchie and Johnson, 2009; Heupel et al., 2014).
The data presented in the study are deposited in the NCBI repository, accession numbers PP851828 and PP856405.
The animal study was approved by Israel Nature and Parks Authority. The study was conducted in accordance with the local legislation and institutional requirements.
BA: Visualization, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization, Writing – original draft, Supervision, Project administration, Funding acquisition. EC: Writing – review & editing, Visualization, Validation, Resources, Project administration, Methodology, Investigation, Formal analysis. LL: Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition. DR: Writing – original draft. ZZ: Writing – review & editing, Methodology. AB: Writing – review & editing, Conceptualization. AT: Writing – review & editing. EB: Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. ES: Writing – review & editing. DT: Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization, Writing – review & editing. AS: Writing – review & editing, Supervision, Resources, Project administration, Investigation, Funding acquisition, Data curation, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We thank The Kahn Foundation and Blue Marine Foundation for funding this project.
We thank all the members of the Deep Med Lab and Morris Kahn Marine Research Station for supporting this project, all the volunteers from the station’s network who assisted us during the field collection days, and the staff at the Israeli Nature and Parks Authority for their continued support, which makes this project feasible year after year.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1391752/full#supplementary-material
Abdel-Aziz S. H., Khalil A. N., Abdel-Maguid S. A. (1993a). Food and feeding habits of the common guitarfish, Rhinobatos rhinobatos in the Egyptian Mediterranean waters. Indian J. Mar. Sci. 22, 287–290.
Abdel-Aziz S. H., Khalil A. N., Abdel-Maguid S. A. (1993b). Reproductive cycle of the common guitarfish, Rhinobatos rhinobatos (Linnaeus 1758), in Alexandria waters, Mediterranean Sea. Mar. Freshw. Res. 44, 507–517. doi: 10.1071/MF9930507
Bangley C. W., Paramore L., Dedman S., Rulifson R. A. (2018). Delineation and mapping of coastal shark habitat within a shallow lagoonal estuary. PloS One 13. doi: 10.1371/journal.pone.0195221
Bethea D. M., Smith K. L., Casselberry G. A., Carlson J. K., Hendon J., Grubbs R. D., et al. (2014). Shark Nursery Grounds and Essential Fish Habitat Studies: GULFSPAN Survey – 2014 Gulf of Mexico Shark Pupping and Nursery Survey. Report to NOAA Fisheries, Highly Migratory Species Division. National Marine Fisheries Service Panama City Laboratory Contribution 15-01. 46 pp.
Bird P. M. (2012). Tissue regeneration in three carcharhinid sharks encircled by embedded straps. Copeia 1978, 2, 345–349. doi: 10.2307/1443580
Bradai M. N., Saidi B., Enajjar S. (2012). Elasmobranchs of the Mediterranean and Black Sea: status, ecology and biology. Bibliographic Analysis. Available at: https://www.fao.org/4/i3097e/i3097e.pdf. Accessed on: January 20, 2018.
Carrier J. C., Pratt H. L. (1998). Habitat management and closure of a nurse shark breeding and nursery ground. Fish. Res. 39, 209–213. doi: 10.1016/S0165-7836(98)00184-2
Çek Ş., Başusta N., Demirhan S. A., Karalar M. (2009). Biological observations on the common guitarfish Rhinobatos rhinobatos from İskenderun Bay (Turkey, Eastern Mediterranean). Anim. Biol. 59, 211–230. doi: 10.1163/157075609X437727
Chaikin S., Belmaker J., Barash A. (2020). Coastal breeding aggregations of threatened stingrays and guitarfish in the Levant. Aquat. Conserv.: Mar. Freshw. Ecosyst. 30, 1160–1171. doi: 10.1002/aqc.3305
Chin A., Mourier J., Rummer J. L. (2015). Blacktip reef sharks (Carcharhinus melanopterus) show a high capacity for wound healing and recovery following injury. Conserv. Physiol. 3, 1–9. doi: 10.1093/conphys/cov062
Das Mahapatra K., Gjerde B., Reddy P. V. G. K., Sahoo M., Jana R. K., Saha J. N., et al. (2001). Tagging: On the use of passive integrated transponder (PIT) tags for the dentification of fish. Aquac. Res. 32, 47–50. doi: 10.1046/j.1365-2109.2001.00526.x
Di Santo V. (2016). Intraspecific variation in physiological performance of a benthic elasmobranch challenged by ocean acidification and warming. J. Exp. Biol. 219, 1725–1733. doi: 10.1242/jeb.139204
Dulvy N. K., Allen D. J., Ralph G. M., Walls R. H. (2016). The conservation status of sharks, rays, and chimaeras in the Mediterranean Sea [Brochure]. IUCN, Malaga, Spain.
Duncan K. M., Holland K. N. (2006). Habitat use, growth rates and dispersal patterns of juvenile scalloped hammerhead sharks Sphyrna lewini in a nursery habitat. Mar. Ecol. Prog. Ser. 312, 211–221. doi: 10.3354/meps312211
Echwikhi K., Saidi B., Bradai M. N., Bouain A. (2013). Preliminary data on elasmobranch gillnet fishery in the Gulf of Gabès, Tunisia. J. Appl. Ichthyol. 29, 1080–1085. doi: 10.1111/jai.12022
Enajjar S., Bradai M. N., Bouain A. (2008). New data on the reproductive biology of the common guitarfish of the Gulf of Gabès (southern Tunisia, central Mediterranean). J. Mar. Biol. Assoc. UK 88, 1063–1068. doi: 10.1017/S0025315408001550
Farrugia T. J., Espinoza M., Lowe C. G. (2011). Abundance, habitat use and movement patterns of the shovelnose guitarfish (Rhinobatos productus) in a restored southern California estuary. Mar. Freshw. Res. 62, 648–657. doi: 10.1071/MF10173
Filiz H., Bilge G., Giannetto D., Yapici S. (2016). New sighting of the endangered species blackchin guitarfish, Rhinobatos cemiculus, in the South Aegean Sea. Mar. Biodivers. Records 9, 6–9. doi: 10.1186/s41200-016-0009-6
Hammer Ø., Harper D. A. T., Ryan P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electronica 4, 1–9.
Heupel M. R., Carlson J. K., Simpendorfen C. A. (2007). Shark nursery areas: concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 337, 287–297. doi: 10.3354/meps337287
Heupel M. R., Kanno S., Martins A. P. B., Simpfendorfer C. A. (2018). Advances in understanding the roles and benefits of nursery areas for elasmobranch populations. Mar. Freshw. Res. 70, 897–907. doi: 10.1071/MF18081
Heupel M. R., Knip D. M., Simpfendorfer C. A., Dulvy N. K. (2014). Sizing up the ecological role of sharks as predators. Mar. Ecol. Prog. Ser. 495, 291–298. doi: 10.3354/meps10597
Hussey N. E., Wintner S. P., Dudley S. F. J., Cliff G., Cocks D. T., Aaron MacNeil M. (2010). Maternal investment and size-specific reproductive output in carcharhinid sharks. J. Anim. Ecol. 79, 184–193. doi: 10.1111/j.1365-2656.2009.01623.x
Jabado R. W., Kyne P. M., Pollom R. A., Ebert D. A., Simpfendorfer C. A., Ralph G. M., et al. (2018). Troubled waters: Threats and extinction risk of the sharks, rays and chimaeras of the Arabian Sea and adjacent waters. Fish Fish. 19, 1043–1062. doi: 10.1111/faf.12311
Jackson J. B., Kirby M. X., Berger W. H., Bjorndal K. A., Botsford L. W., Bourque B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. science 293, 629–637. doi: 10.1126/science.1059199
Kyne P., Jabado R. (2019). Glaucostegus cemiculus Vol. 8235 (The IUCN Red List of Threatened Species 2019), e.T104050689A104057239. doi: 10.2305/IUCN.UK.2019-2.RLTS.T104050689A104057239.en
Kyne P. M., Jabado R. W., Rigby C. L., Dharmadi, Gore M. A., Pollock C. M., et al. (2020). The thin edge of the wedge: Extremely high extinction risk in wedgefishes and giant guitarfishes. Aquat. Conserv.: Mar. Freshw. Ecosyst. 30, 1337–1361. doi: 10.1002/aqc.3331
Martins A. P. B., Heupel M. R., Chin A., Simpfendorfer C. A. (2018). Batoid nurseries: Definition, use, and importance. Mar. Ecol. Prog. Ser. 595, 253–267. doi: 10.3354/meps12545
McClenachan L., Cooper A. B., Carpenter K. E., Dulvy N. K. (2012). Extinction risk and bottlenecks in the conservation of charismatic marine species. Conserv. Lett. 5, 73–80. doi: 10.1111/j.1755-263X.2011.00206.x
McPeek M. A. (2014). Limiting factors, competitive exclusion, and a more expansive view of species coexistence. Am. Nat. 183, iii–iv. doi: 10.1086/675305
Moore A. B. (2017). Are guitarfishes the next sawfishes? Extinction risk and an urgent call for conservation action. Endangered Species Res. 34, 75–88. doi: 10.3354/esr00830
Naylor G. J. P., Caira J. N., Jensen K., Rosana K. A. M., Straube N., Lakner C. (2012a). “Elasmobranch phylogeny: A mitochondrial estimate based on 595 species,” in Biology of Sharks and Their Relatives, 2nd Edition. Eds. Carrier J. C., Musick J. A., Heithaus M. R. (CRC Press, Boca Raton, Florida), 31–56.
Naylor G. J., Caira J. N., Jensen K., Rosana K. A. M., White W. T., Last P. R. (2012b). A DNA sequence–based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bull. Am. Museum Natural History 2012, 1–262. doi: 10.1206/754.1
Newell B. (2017). Draft Status Review Report of Two Species of Guitarfish: Rhinobatos rhinobatos and Rhinobatos cemiculus. Natl. Mar. Fish. Service 62.
Notarbartolo di Sciara G., Bradai M. N., Morey G., Brahim K., Camara L., Litvinov F., et al. (2016). Glaucostegus cemiculus (The IUCN Red List of Threatened Species 2016), e.T63132A104009894.
Pörtner H. O., Peck M. A. (2010). Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 77, 1745–1779. doi: 10.1111/j.1095–8649.2010.02783.x
Ritchie E. G., Johnson C. N. (2009). Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998. doi: 10.1111/j.1461-0248.2009.01347.x
Sherman C. S., Simpfendorfer C. A., Haque A. B., Digel E. D., Zubick P., Eged J., et al. (2023). Guitarfishes are plucked: Undermanaged in global fisheries despite declining populations and high volume of unreported international trade. Mar. Policy 155. doi: 10.1101/2022.10.05.510982
Simpfendorfer C. A. (2000). Growth rates of juvenile dusky sharks, Carcharhinus obscurus (Lesueur 1818), from southwestern Australia estimated from tag-recapture data. Fishery Bull. 98, 811–81a.
Tamura K., Nei M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–26. doi: 10.1093/oxfordjournals.molbev.a040023
Ward R. D., Zemlak T. S., Innes B. H., Last P. R., Hebert P. D. (2005). DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B: Biol. Sci. 360, 1847–1857. doi: 10.1098/rstb.2005.1716
Keywords: abundance, conservation, Glaucostegus cemiculus, blackchin guitarfish, morphometrics, nursery ground, Eastern Mediterranean Sea
Citation: Azrieli B, Cohen E, Livne L, Ramon D, Tsemel A, Bigal E, Shemesh E, Zemah-Shamir Z, Barash A, Tchernov D and Scheinin A (2024) Characterising a potential nearshore nursery ground for the blackchin guitarfish (Glaucostegus cemiculus) in Ma’agan Michael, Israel. Front. Mar. Sci. 11:1391752. doi: 10.3389/fmars.2024.1391752
Received: 26 February 2024; Accepted: 16 May 2024;
Published: 18 June 2024.
Edited by:
Wen-Cheng Wang, National Taiwan Normal University, TaiwanReviewed by:
Alfonso Aguilar-Perera, Universidad Autónoma de Yucatán, MexicoCopyright © 2024 Azrieli, Cohen, Livne, Ramon, Tsemel, Bigal, Shemesh, Zemah-Shamir, Barash, Tchernov and Scheinin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eynav Cohen, bmF2aWMwMDdAZ21haWwuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.