- 1Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao, China
- 2Laboratory for Marine Ecology and Environmental Science, Qingdao Marine Science and Technology Center, Qingdao, China
- 3College of Fisheries and Life Science, Shanghai Ocean University, Shanghai, China
In this study, we examined the short-term variation in the abundance of copepod Acartia specimens, identified previously as Acartia pacifica, in Laizhou Bay, an estuarine bay in the southern Bohai Sea in northern China. Monthly samples were collected from May 2011 to April 2012, excluding December 2011 and January to February 2012 due to ice. Based on its morphological characteristics, Acartia ohtsukai was distinguishable from A. pacifica for the first time in Laizhou Bay. A. ohtsukai was sporadically present from summer to autumn, with the highest abundance and occurrence in September. It appeared in June when the water temperature was above 20°C, and disappeared in November when the water temperature decreased to less than 10°C. During the surveyed months, A. ohtsukai was more commonly found inshore with salinity less than 28 than offshore in the bay. Correlation analysis revealed that temperature and chlorophyll a concentration significantly influenced the monthly variations in A. ohtsukai abundance. We also compared the occurrence of A. ohtsukai with that of three species from the genus Tortanus (Tortanus derjugini, Tortanus forcipatus, and Tortanus spinicaudatus) in Laizhou Bay. The coexistence of A. ohtsukai and T. derjugini in the bay suggests that their ecological habitats are similar to those of brackish-water species.
1 Introduction
The genus Acartia Dana, 1846 (Copepoda, Calanoida, Acartiidae) is widely distributed in coastal areas and estuaries globally. They can be indicators of hydrological conditions and respond to climate warming (Rice et al., 2015; Rice and Stewart, 2016; Borkman et al., 2018) in terms of their phenology, abundance, body size, and distribution pattern. Furthermore, many Acartia species are dominant in estuarine zooplankton communities (Wang et al., 2002; Borkman et al., 2018; Zuraire et al., 2018), and they are crucial as secondary producers, transferring energy through the food web to the fish stock, especially small pelagic fish such as anchovies and sprats, in the coastal ecosystems. The species Acartia (Odontacartia) ohtsukai is present in the estuarine and coastal waters of Korea, China, Vietnam, and Japan (Razouls et al., 2005–2023). It seasonally dominates the zooplankton community (Youn and Choi, 2008; Choi et al., 2021a) and is a good potential live prey for fish larvae through mass culture (Choi et al., 2021b). Acartia ohtsukai was originally identified as a separate species from its sibling species Acartia pacifica based on morphological and genetic differences (Ueda and Bucklin, 2006). These two Acartia species also have different habitat preferences. A. ohtsukai is a brackish species found along the coasts of the Northwest Pacific (Ueda and Bucklin, 2006; Moon et al., 2008; Sakaguchi et al., 2011), while A. pacifica is a coastal euryhaline species broadly distributed in offshore waters of the Indo-West Pacific (Ueda and Bucklin, 2006; Moon et al., 2008).
The population of A. ohtsukai in Ariake Bay, Japan, is considered a continental relict (Ueda and Bucklin, 2006) and is geographically segregated from the A. pacifica population in Gwangyang Bay, Korea (Moon et al., 2008). While A. ohtsukai has been frequently reported in bays and estuaries in Japan and Korea (Ueda and Bucklin, 2006; Moon et al., 2008; Youn and Choi, 2008, 2012; Kang, 2011; Park et al., 2013; Lee et al., 2017, 2020; Choi et al., 2019, 2021a, 2021b; Seo et al., 2021), and in Iraqi water (Jebir et al., 2021), its occurrence in the Chinese offshore waters is limited compared to that in the neighboring regions. On the other hand, A. pacifica, which is often confused with A. ohtsukai, is recognized as a common species on Chinese coasts, ranging from the Bohai Sea in the north to the South China Sea in the south (Chen and Zhang, 1965; Liu, 2008). It has also been reported in river mouths or estuaries with lower salinities, such as the Yellow River estuary and the turbid zone of the Yangtze River estuary (Liu et al., 2012; Feng and Liu, 2019).
The low salinity tolerance of A. pacifica observed in Chinese waters is inconsistent with its typical marine habitats in the NW Pacific (Steuer, 1915; Ueda and Bucklin, 2006). To address this inconsistency, researchers have investigated specimens previously identified as A. pacifica from various regions in China. Liu (2007) reported high genetic similarity and morphological resemblance between samples identified as A. pacifica in Xiamen Harbor and A. ohtsukai specimens in Ariake Bay, Japan (Ueda and Bucklin, 2006). Wang et al. (2011) suggested that the A. pacifica previously recorded in Jiaozhou Bay of the South Yellow Sea should be A. ohtsukai based on molecular sequencing. Moreover, Shih et al. (2022a) revised the species identification of A. pacific described by Chen and Zhang (1965) to A. ohtsukai on the China Acartia species list.
Laizhou Bay, one of the three largest bays in the Bohai Sea in North China, is a typical estuarine bay that receives runoff from more than ten rivers, including the Yellow River. The bay has a water salinity usually below 30–32, due to the influx of freshwater (Xia et al., 1991; Wang and Wu, 2018). Researchers have shown A. pacifica to be the dominant planktonic copepod species in Laizhou Bay during summer and autumn (Bi et al., 2001; Liu et al., 2012; Wang and Wu, 2018), while A. ohtsukai has not been reported in the region thus far. Correct identification of various Acartia species is essential for comprehending their geographic distribution and responses to changes in marine ecosystems. Given the low-salt conditions in the bay, which may not be suitable for the oceanic species A. pacifica, we re-examined samples collected in Laizhou Bay from 2011 to 2012. We focused on specimens initially identified as A. pacifica. We also investigated the influence of environmental factors on the distributions of these species in the bay. We aimed to clarify the classification of Acartia species and their habitat characteristics related to water conditions in Laizhou Bay, contributing to a better understanding of the Acartia species diversity and zoogeographic distribution in the region.
2 Materials and methods
2.1 Study area
Laizhou Bay is a semiclosed bay in the southern Bohai Sea, China. It has a bowl-shaped structure and an average depth of less than 10 m. The bay covers an area of approximately 7000 km2 and has a coastline of 320 km. The mouth of the bay is 96 km long, stretching from the Yellow River estuary (37°39′N, 119°16.6′E) in the west to Gaojiao of Kaimu Island (37°41′N, 120°13′E) in the east (Figure 1). Laizhou Bay receives water from more than ten rivers, including the Yellow River, the largest river in northern China. The bay has shallow topography and receives great input from river water, resulting in low salinity and high nutrient levels. A northwesterly coastal current flows into the bay, creating a counterclockwise circulation pattern that facilitates water exchange, along with the tidal influences (Jiang et al., 2018). During summer, the increased discharge from the Yellow River can create an eastward plume at the river mouth, affecting the adjacent sea. During the rainy season, the average salinity in the bay can decrease to less than 26. In winter, the Laizhou Bay zone experiences sea ice due to the combined effects of cold air, shallow water, and low salinity (Zhang et al., 2013).
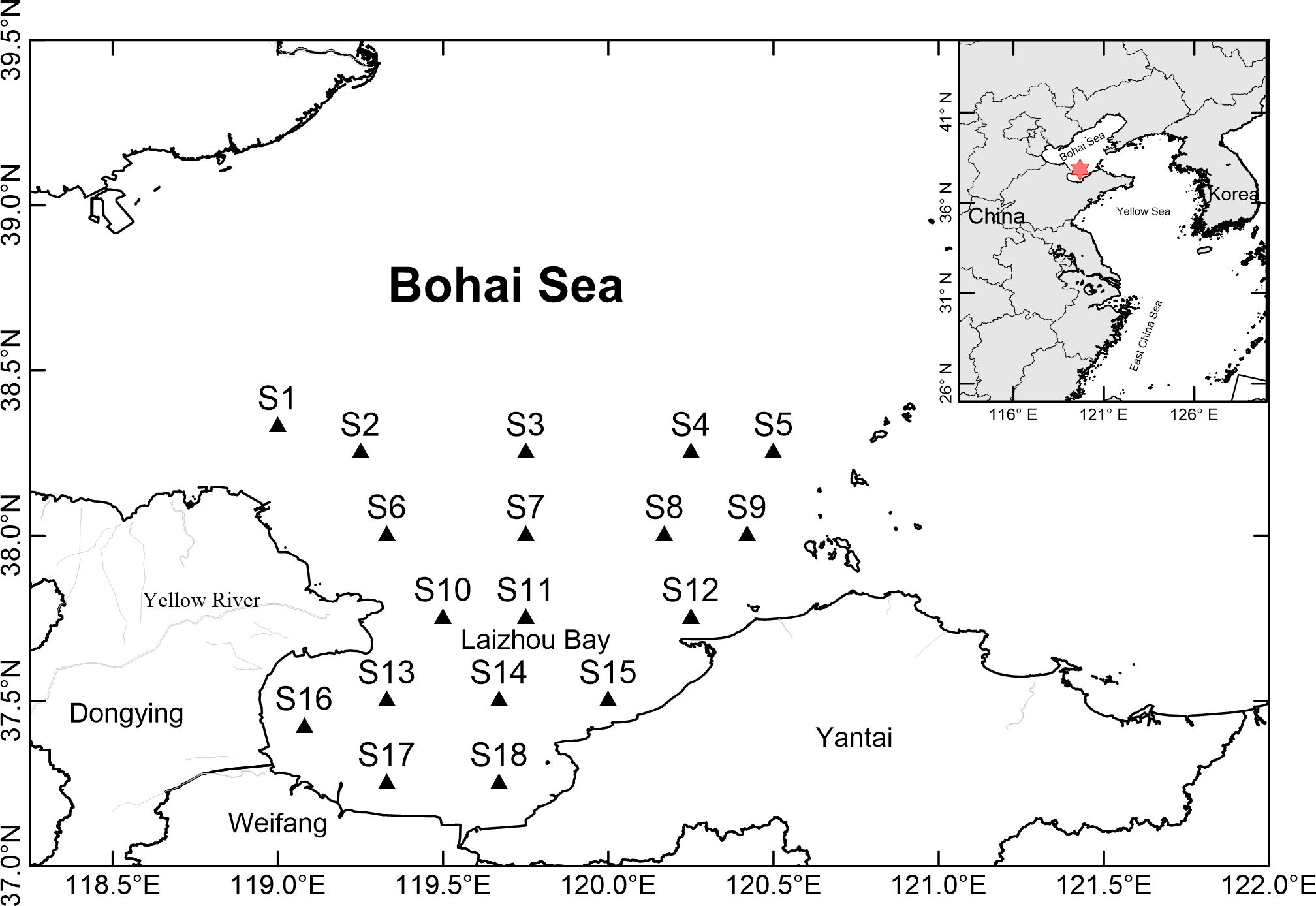
Figure 1 Map of the investigation region and location of sampling stations in Laizhou Bay, Bohai Sea, China.
2.2 Sample collection
From May 2011 to April 2012, monthly sampling was conducted in the bay area between 37.25°N and 38.75°N, 119°E and 120.75°E. Samples from December 2011 to February 2012 were excluded due to the presence of ice (Zhang et al., 2013). A total of eighteen stations were set (see Figure 1). At each station, vertical tows were made using a conical plankton net with a mesh size of 160 μm and a mouth diameter of 32 cm from the bottom to the surface layer. The volume of filtered water was estimated using a flow meter (Hydrobios, Germany) attached to the net mouth. Copepod samples were immediately fixed and stored in a buffered formaldehyde seawater solution at a final concentration of approximately 5%. Surface water temperature and salinity data were obtained from the Oceanographic Data Center, Chinese Academy of Sciences (CASODC) (http://msdc.qdio.ac.cn, accessed on 19th January 2024). The dissolved oxygen (DO) concentration and pH were measured at each station using YSI ProPlus (USA). Chlorophyll a (chl. a) concentration data were obtained from the OCEAN PRODUCTIVITY website (http://www.science.oregonstate.edu/ocean.productivity/index.php, Oregon State University, accessed on 25th September 2018).
Specimens of Acartia, previously identified as A. pacifica, were sorted from the samples and dissected under a stereomicroscope (Olympus SZX16, Japan). The developmental stages of the Acartia species were classified as copepodites (copepodids I-V) and adults following the criteria outlined by Li and Fang (1990). Prosome length (PL) was determined to the nearest 0.01 mm using a measuring reticule calibrated with a stage graticule on a dissecting microscope. Genetic analyses were not performed on the specimens due to the extended storage period of over a decade. Additionally, copepod specimens of the genus Tortanus were also sorted for further analysis, considering their possible coexistence with A. ohtsukai in estuarine waters, as suggested by Ueda and Bucklin (2006) and Moon et al. (2008).
2.3 Statistical analysis
The abundance of Acartia was measured as individuals per cubic meter (ind. m-3) at each station. Correlation analyses were conducted to investigate the impact of environmental factors (water temperature, salinity, DO, pH, and chl. a concentration) on the occurrence of Acartia. A statistical significance level of 0.05 was used. To account for autocorrelation among environmental factors, partial correlation analysis was performed with temperature as the control variable. The original abundance of Acartia was log-transformed. Principal component analysis (PCA) was used to determine the relationships between the factors and log-transformed Acartia abundance. The ordination component scores and the variable loading coefficients were plotted based on Pearson’s correlation matrix using STATISTICA 12.0 (StatSoft, Inc., Tulsa, OK, USA). Distribution contour and post maps were generated using Surfer 10.0 (Golden Software, Inc, USA) to visually represent the temporal and spatial distributions of Acartia.
3 Results
3.1 Morphological description
The adult female Acartia specimens had prosome lengths (PLs) ranging from 850 μm to 1100 μm, with a mean of 957 ± 301 μm. As Shown in Figure 2, the fifth pediger of the female had acute, slightly curved lateral projections and posterodorsal spines on each side. The genital double somite is as long as its width and has two dorsal spines. The cauda rami were relatively long, with a length/width ratio of approximately three or more. On the first antennule, the second segment had a short spine on the distal half of the dorsal surface, while the fourth and fifth segments had subterminal spines dorsally. The length of the fifth leg was approximately 1.5 times greater than the width of the fifth leg.
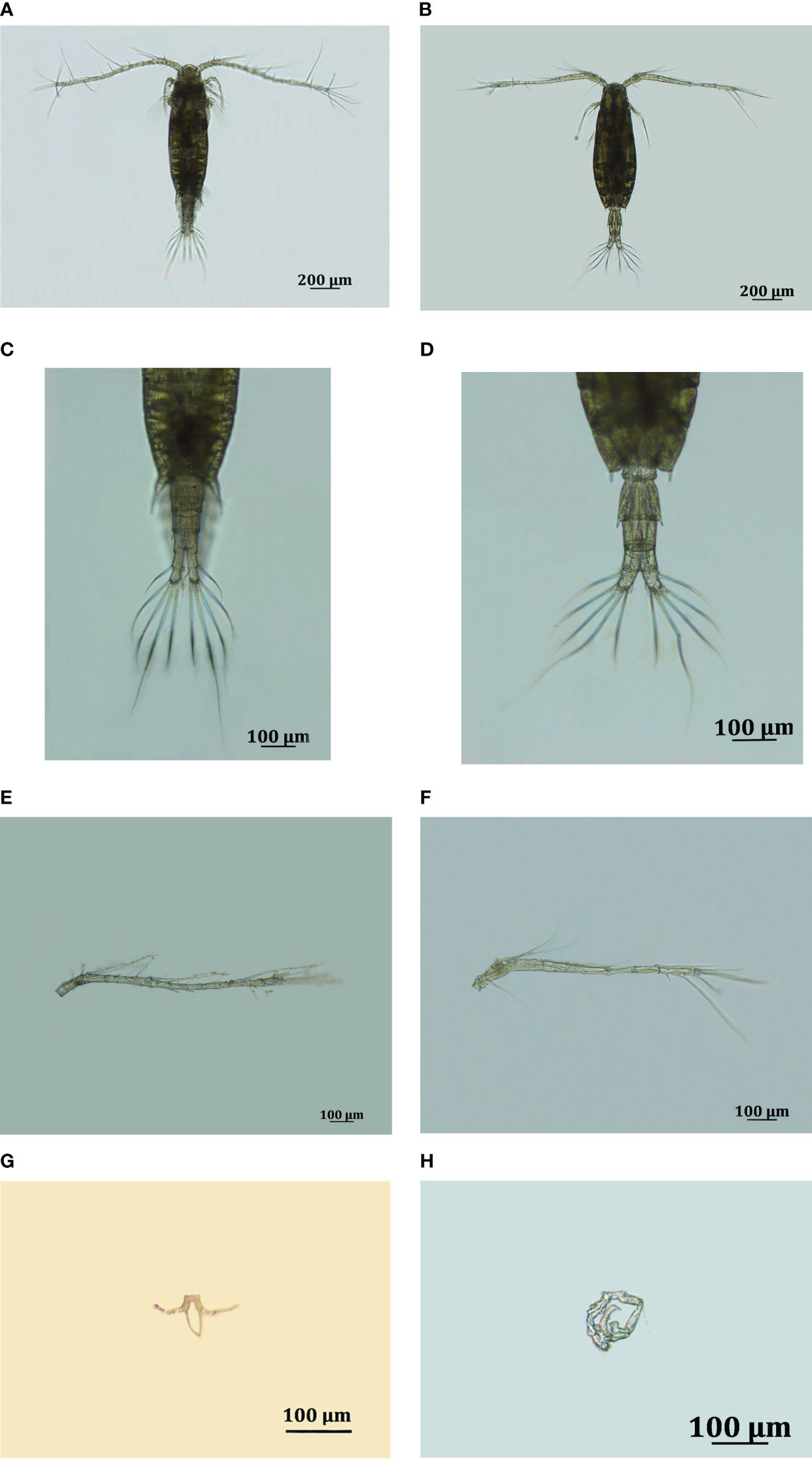
Figure 2 Photographs of Acartia ohtsukai sampled in Laizhou Bay, Bohai Sea, showing the (A) habitus (dorsal view), (B) urosome (dorsal view) and (C) antennule (right), (D) fifth leg of female and (E) habitus (dorsal view), (F) urosome (dorsal view) and (G) antennule (right), (H) fifth leg of male, respectively.
For adult males, the prosome length ranged from 835 μm to 1100 μm, with a mean of 835 ± 216 μm. The caudal ramus was approximately 1.5 times longer than its width. The length of the first exopod segment of the left fifth leg was approximately equal to that of the second exopod segment of the left fifth leg and shorter than the length of the first exopod segment of the right fifth leg. The second exopod segment of the right fifth leg was as long as its width and had a square medial projection.
Based on the above observations, the morphological characteristics of the specimens were consistent with the descriptions of Acartia ohtsukai by Ueda and Bucklin (2006).
3.2 Abundance and developmental compositions
During the nine-month surveys conducted in Laizhou Bay, the presence of A. ohtsukai was first observed sporadically in June (Figure 3). Its abundance gradually increased throughout July and August, peaking in September at a mean abundance of 557 ± 1423 ind m-3. However, there was a noticeable decline in October, with the abundance decreasing to less than 3 ind m-3. By November, A. ohtsukai had almost disappeared from the surveyed area.
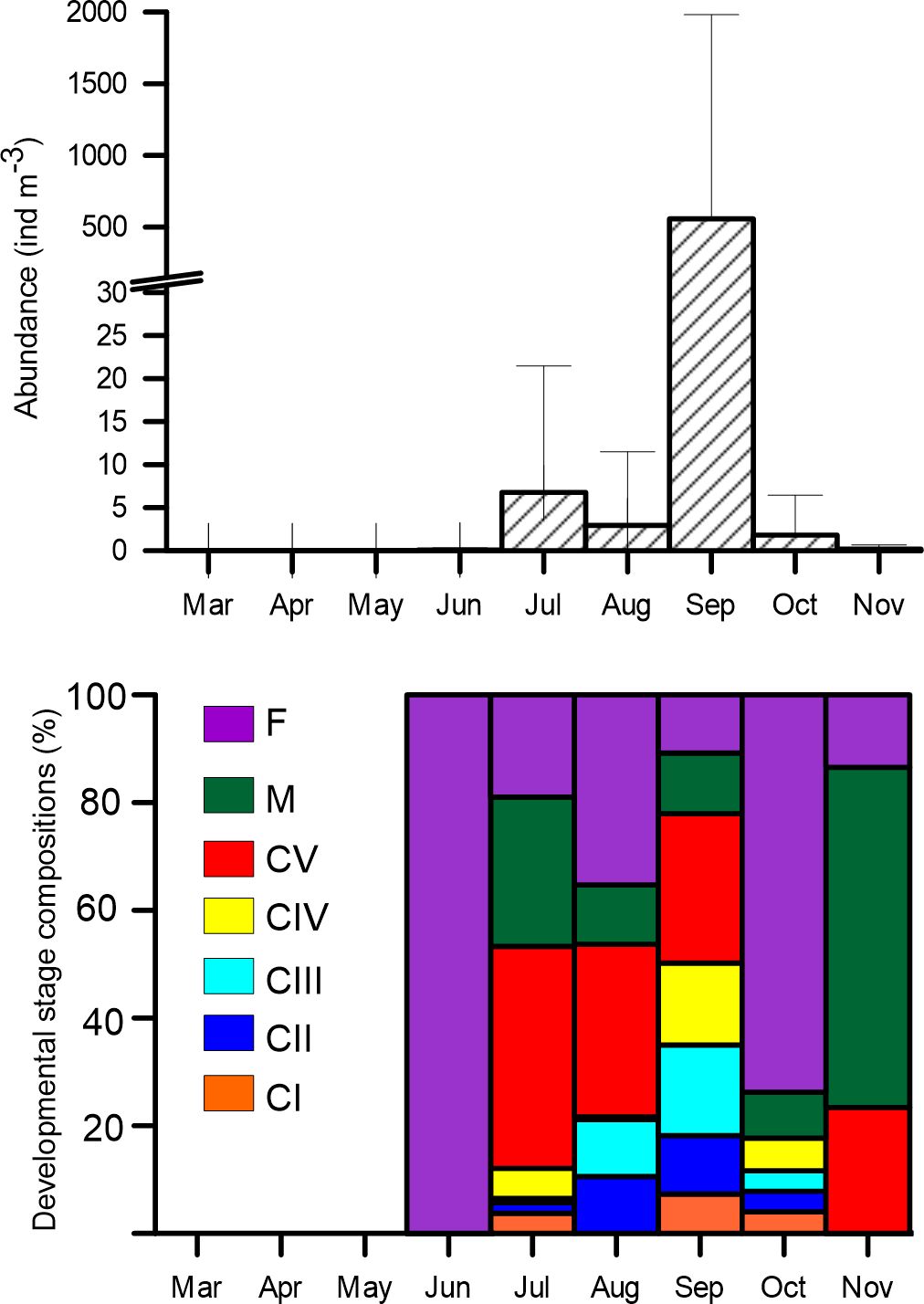
Figure 3 Monthly mean abundance and relative composition of Acartia ohtsukai developmental stages in Laizhou Bay, Bohai Sea.
From July to November, both female and male adults of A. ohtsukai coexisted. The female-to-male (F/M) ratio was approximately equal in July (1.35) and September (0.97). However, there was a noticeable female bias in adults during August and October, with F/M ratios of 3.90 and 4.52, respectively. Copepodite individuals appeared later than adults and dominated the population accouting for more than 50% of the total population from July to September.
3.3 Spatial distribution
Figure 4 illustrates the distribution pattern of A. ohtsukai in the bay. The species is mainly concentrated at the base of the bay and is rarely found in the central part of the bay. In June, A. ohtsukai was found only found at station S16 near the bay base. In July and August, the species began spreading from the bottom to the center and mouth of the bay. It was present in approximately 33% of the surveyed stations. In September, A. ohtsukai had the highest occurrence rate (55%). The distribution of A. ohtsukai was remarkably heterogeneous, with higher densities observed at the bottom of the bay than at the central part. The highest abundance of A. ohtsukai, with more than 5000 ind m-3 was observed at Station No.17. The neighboring stations No.16 and No.13 also had relatively higher abundances, with 3022 and 907 ind m-3, respectively. However, at other stations, the abundance of A. ohtsukai was less than 5 ind m-3. In October, the abundance of A. ohtsukai was less than 5 ind m-3 at all stations, although the occurrence rate (50%) was similar to that in September. In November, the species gradually disappeared from the bay and was sporadically found at only two stations.
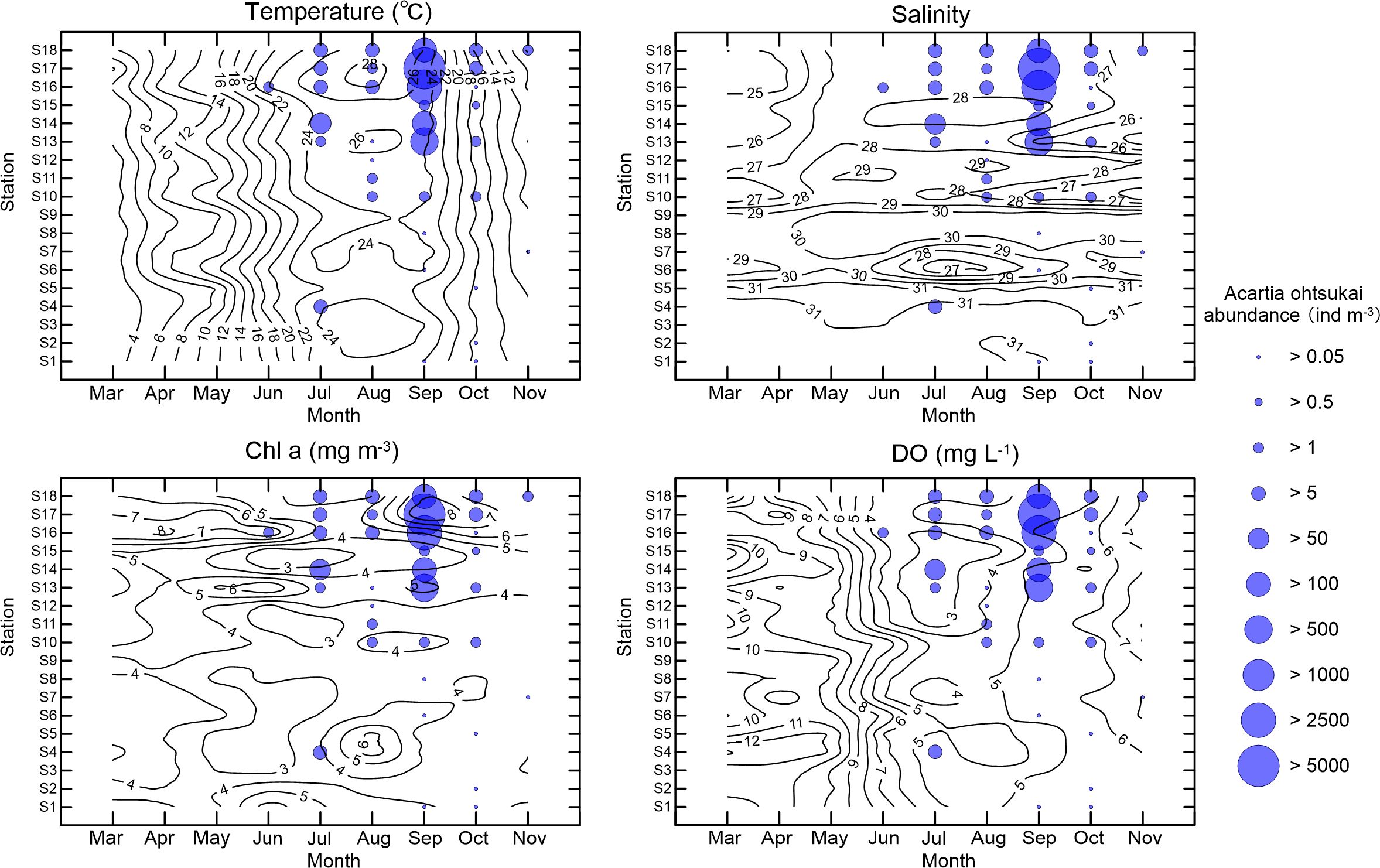
Figure 4 Spatial abundance (ind m-3) distributions of Acartia ohtsukai with contours of environmental factors in Laizhou Bay, Bohai Sea. Contour line: Environmental factors; Circle: Abundance of A. ohtsukai.
3.4 Relations with environmental effects
ANOVA showed significant differences in water temperature, salinity, chlorophyll, dissolved oxygen levels, and pH among the months and stations. Table 1 presents the correlation analysis results, indicating that water temperature, salinity, and chl. a concentration significantly impacted the abundance of A. ohtsukai. Importantly, there were seasonal variations in the correlation coefficients. A positive correlation between water temperature and A. ohtsukai abundance was observed in June and August, while a negative correlation with salinity was found in September and October. In June, there was a highly significant positive correlation between the chl. a concentration and A. ohtsukai abundance, which decreased from September to October.
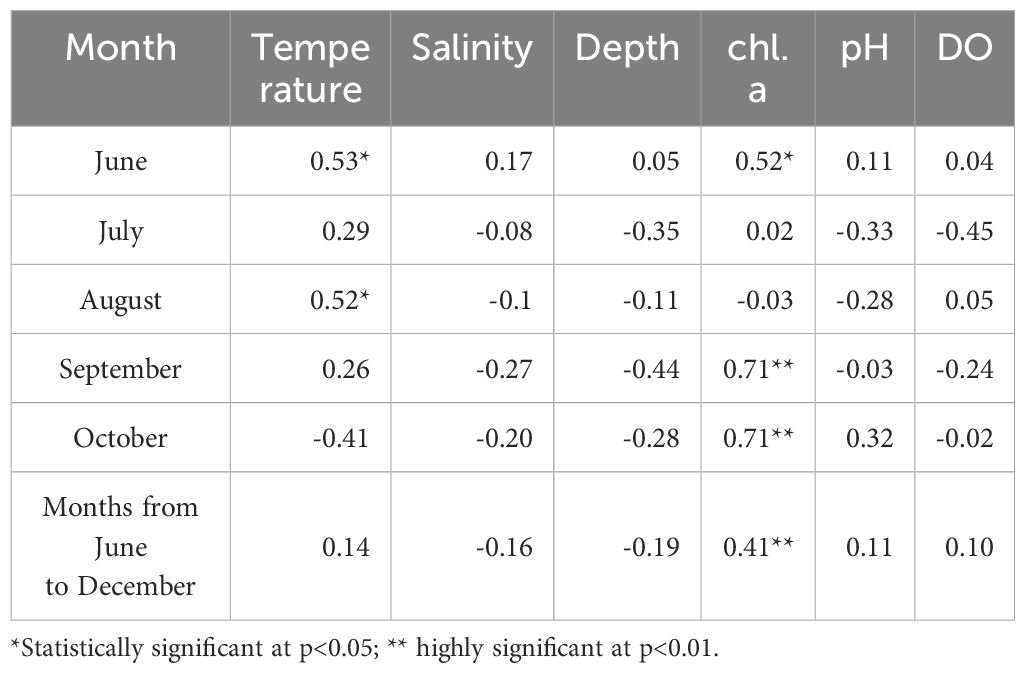
Table 1 Correlation coefficients between log-transformed Acartia ohtsukai abundance and environmental factors.
Figure 4 illustrates the relative distributions of the environmental factors and A. ohtsukai. A. ohtsukai was observed at stations with temperatures above 20°C in June and disappeared when the temperature decreased below 10°C in November. On average, A. ohtsukai occurred in waters with an average temperature of 24.9°C, a salinity of 27.4, and an average chl. a concentration of 7.33 mg m-3. Stations with A. ohtsukai abundances exceeding 500 ind m-3 were characterized by temperatures above 23°C, salinities below 28, and chl. a concentrations above 8 mg m-3.
We identified three Tortanus species, namely, T. derjugini, T. forcipatus, and T. spinicaudatus. No copepods of the genus Sinocalanus were observed in the samples. Figure 5 illustrates that Tortanus sp. was present from May to October, with the highest abundance occurring at station S17 in September. Over 46% of the Tortanus occurrences coincided with those of A. ohtsukai. The highest abundance of T. derjugini occurred in September at station S17, which was the same as that of A. ohtsukai. T. spinicaudatus had the greatest number of occurrences (18), occurring from May to September. T. derjugini rarely occurred at only two stations in August and September, but it had the highest total abundance. T. forcipatus appeared in September and disappeared in October, making it the latest occurring species. Due to the infrequent occurrence and small sample sizes, data from the three Tortanus spp. were combined for correlation analysis. Correlation analysis revealed a strong correlation between the total abundance of Tortanus sp. and that of A. ohtsukai (Spearman rank R = 0.25, p = 0.01<0.05).
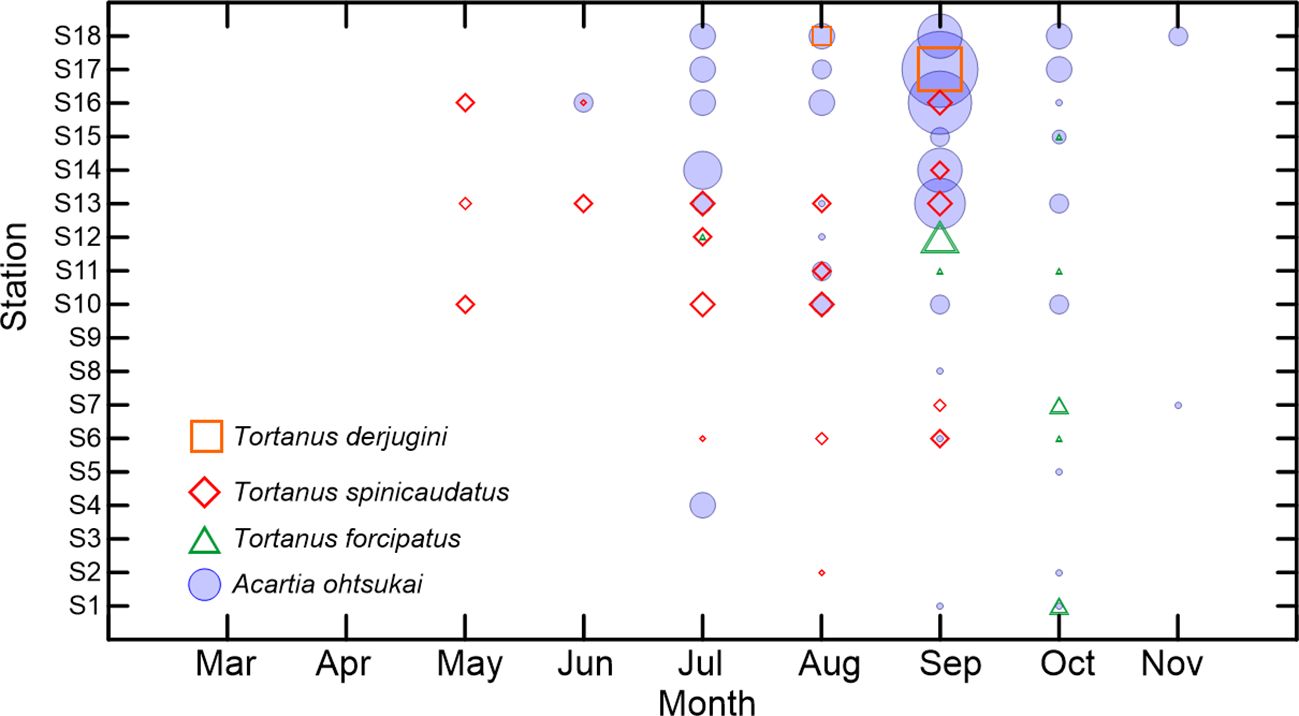
Figure 5 Occurrence distributions of Tortanus derjugini, T. forcipatus, and T. spinicaudatus among months and stations inLaizhou Bay, Bohai Sea. The scale legend of the symbols is similar to that in Figure 4.
Figure 6 presents a biplot illustrating the results of PCA for environmental factors during summer and autumn. The two-axis ordination diagram explains more than 65% of the variance, with the first two principal components explaining 36.63% and 28.68% of the variance, respectively. The biplot indicates that the scores on Axis I were mostly influenced by factors such as chl. a, salinity, and depth, which were closely related to Acartia abundance. The loadings of factors on Axis II indicate that the scores on the second component were mostly influenced by temperature, pH, and dissolved oxygen (DO). However, A. ohtsukai contributed only slightly (6%) to the variability of Axis II. Therefore, chlorophyll, salinity, and depth appeared to be more sensitive to A. ohtsukai than to the other three factors from June to November. Additionally, the principal discriminating endpoints between the abundances of Acartia and Tortanus were closely distributed, indicating a similar response to environmental factors.
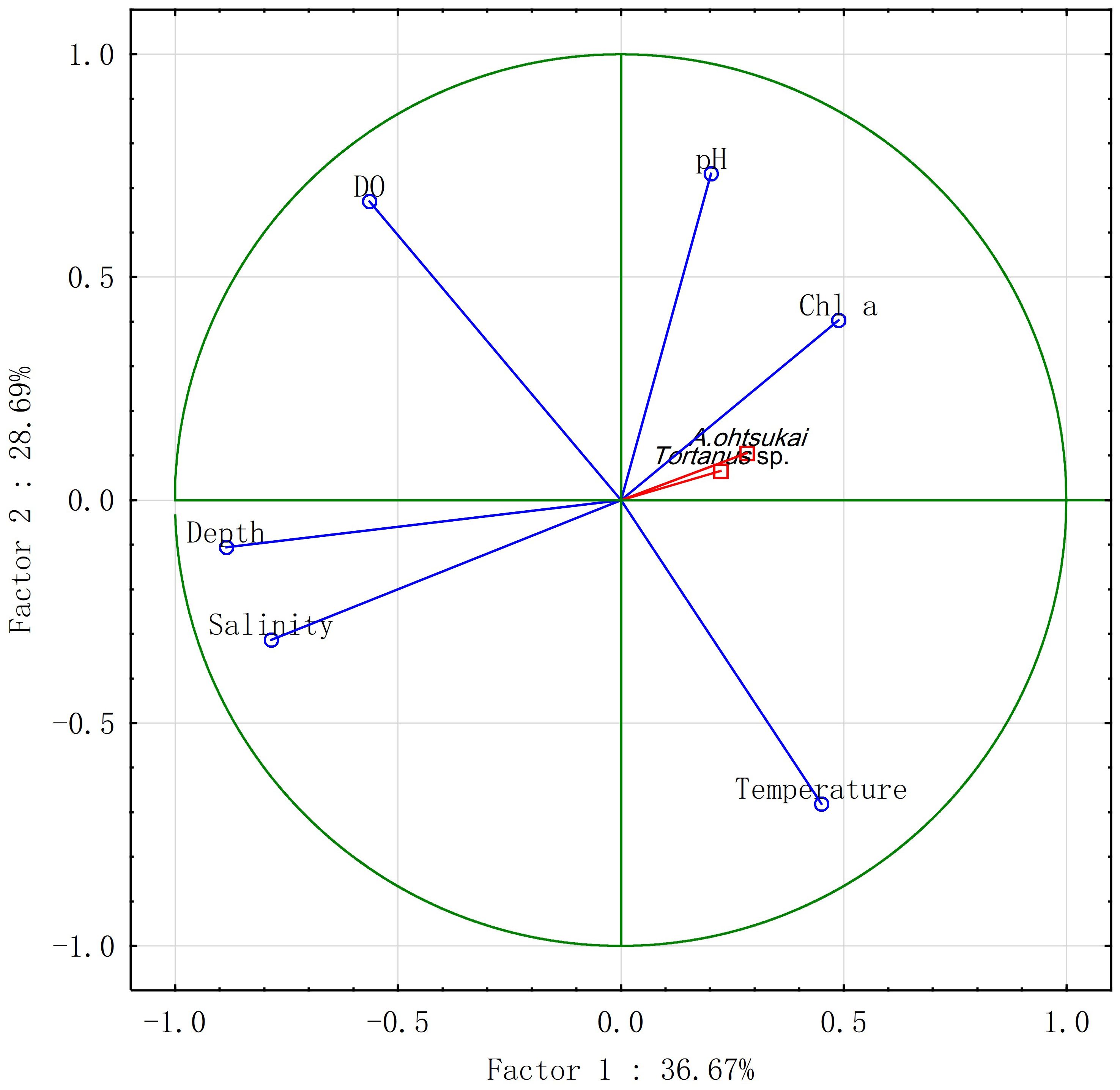
Figure 6 Principal component analysis (PCA) plot considering environmental factors and Acartia ohtsukai abundance. The first two axes are shown, and the percentages of variance explained are indicated.
4 Discussion
4.1 Species determination
A. ohtsukai is not commonly reported along the Chinese coasts, unlike its closely related species A. pacifica, which has been extensively reported in coastal or estuarine waters in China since the 1950s. However, after Ueda and Bucklin (2006) described A. ohtsukai as a distinct species, Chinese researchers re-examined Acartia specimens from estuaries and coasts. According to morphology and genetic sequences, specimens previously identified as A. pacifica in Jiaozhou Bay in the southern Yellow Sea (Wang et al., 2011) and in Xiamen Harbor in the East China Sea (Liu, 2007) should be reclassified as A. ohtsukai. There have been no records of A. ohtsukai in Laizhou Bay. Although we did not perform genetic analysis on the Acartia specimens due to their preservation in formaldehyde for more than a decade, the results indicated that Acartia specimens collected in 2011 from Laizhou Bay, previously identified as A. pacifica, should be considered A. ohtsukai based on their morphology, specifically the characteristics of the caudal rami and antennule in females and the urosomal somites and the fifth leg in males, as described by Ueda and Bucklin (2006).
Copepods belonging to the genus Acartia are prevalent and widely distributed in estuarine and coastal waters worldwide. However, each species has specific temperature and salinity requirements, limiting its spatial-temporal distribution (Kasahara et al., 1975; Bradford, 1976; Kang, 2011; Peck et al., 2015). Therefore, ecological habits, along with morphological characteristics and genetic sequences, are crucial for determining Acartia species. A. ohtsukai predominantly resides in river mouths, estuaries, and harbors temperate waters during the hot season, particularly in areas with abundant freshwater inputs (Ueda and Bucklin, 2006; Youn and Choi, 2008; Park et al., 2013; Jebir et al., 2021). This species exhibits limited tolerance to low temperatures, requiring a minimum water temperature of 16.5°C (Youn and Choi, 2008) and tolerating a maximum of 29°C (Ueda and Bucklin, 2006). A. ohtsukai also exhibits favorable tolerance to low salinity and is found in waters with a minimum salinity of 0.8 (Sakaguchi et al., 2011) and a maximum salinity of 32 (Choi et al., 2021a). The optimal temperature and salinity for its population density are suggested to be approximately 25~26°C (Youn and Choi, 2008; Kang, 2011; Choi et al., 2019) and below 30~32 (Ueda and Bucklin, 2006; Moon et al., 2008; Kang, 2011), respectively. On the other hand, A. pacifica is mostly found on temperate and tropical coasts during warm seasons (summer and autumn). It can tolerate a minimum water temperature of 12°C (Moon et al., 2008) and a maximum exceeding 29°C (Zuraire et al., 2018). A. pacifica has a high salinity tolerance, with a minimum of 21 (Kang, 2011) and a maximum of 36.5 (Greenwood, 1981) in its habitat. Typically, the A. pacifica population occurs in waters with temperatures above 22°C (Greenwood, 1981; Kang, 2011; Zuraire et al., 2018) and salinities greater than 30~32 (Greenwood, 1981; Ueda and Bucklin, 2006; Moon et al., 2008). A study conducted in Gwangyang Bay revealed a distinct separation of A. ohtsukai and A. pacifica in response to changes in salinity (Moon et al., 2008). A. ohtsukai is primarily a brackish species in estuarine waters with salinities less than 30~32 during the summer, while A. pacifica is relatively hypersaline and is found in offshore waters with salinities greater than 30~32 during the warm season. As a result, the two congener copepods are suggested to be distinguished by their salinity preferences (Kang, 2011). Laizhou Bay, characterized by its shallow topography and abundant river input, has low salinity levels. The salinity during the study period was lower than 31.5 (Figure 4), which may not be optimal for the survival of typical A. pacifica. Moreover, there was a negative correlation between the abundance of A. ohtsukai and salinity during the abundant months of September and October (Table 1). There was also a preference for relatively low salinity (< 28) (Figure 4), which is consistent with the temperature and salinity characteristics of the ecological habitats of A. ohtsukai in Korean and Japanese waters (Ueda and Bucklin, 2006; Moon et al., 2008; Soh, 2010; Kang, 2011).
4.2 Effects of environmental factors
In this study, we found that A. ohtsukai in Laizhou Bay appeared sporadically and seasonally during the summer and autumn, similar to bays and estuaries in Korea (Kang, 2011; Choi et al., 2021a) and Jiaozhou Bay in the South Yellow Sea (Zhong and Xiao, 1992a, 1992b; Sun et al., 2012). The seasonality of A. ohtsukai emergence and population increase is likely linked to the production and hatching of resting eggs. These eggs remain on the surface substrate during winter and hatch when temperatures become favorable. Research by Zhong and Xiao (1992a) supports this hypothesis. The initial surge in Acartia sp. larvae was not a result of normal reproduction but the hatching of dormant eggs (Zhong and Xiao, 1992a; Wang et al., 2005). The hatching of dormant eggs is strongly influenced by water temperature (Uye, 1985). Incubation experiments demonstrated that A. ohtsukai had high spawning rates and hatching success at temperatures of 25°C and 30°C and salinities of 27 (Choi et al., 2021b). In Jiaozhou Bay, southern Yellow Sea, the species exhibited a high rate of dormant egg hatching at 15°C (Zhong and Xiao, 1992a) and continued spawning when the water temperature ranged between 8°C and 24°C. The highest egg production rate occurred at a temperature of 23°C (Sun et al., 2011). Moreover, A. ohtsukai mortality was relatively low at 20–30°C across a salinity range of 20–33 (Choi et al., 2021b). Therefore, water temperature is likely to be the crucial factor triggering the population increase of this species. Our data, presented in Table 1, show significant positive correlations between species abundance and temperature during the early seasons of occurrence. Additionally, as presented in Figure 4, A. ohtsukai first emerged in June when the water temperature was above 20°C, then decreased as the water temperature decreased below 20°C and disappeared when the temperature decreased below 10°C. This may be due to the high mortality rate of A. ohtsukai females at the same temperature, as reported by Choi et al. (2021b).
In addition, the concentration of chl. a was also significantly correlated with the distribution of A. ohtsukai in Laizhou Bay (Table 1). The density of A. ohtsukai was highest inshore where the chl. a concentrations were relatively high (>8 mg m-3) (Figure 4). Similarly, the chl. a concentration was suggested to influence the occurrence of Acartia erythraea in Incheon and Ulsan (Kang, 2011). A high chl. a concentration of (>10 mg L-1) was recommended for maintaining relatively high species richness and diversity of Acartia sp. during summer (Choi et al., 2021a). The low concentration of suitable food in seawater compared to that in estuaries may explain the failure of A. tonsa to develop in coastal waters with lower chl. a concentrations <1 ug L-1 ppm (Paffenhöfer and Stearns, 1988). The chl. a concentration also affects the daily production of Acartia omorii in Ilkang Bay, Korea (Kang et al., 2007), and the spawning rate of the copepod Acartia hongi in Gyeonggi, Korea (Youn and Choi, 2007), which in turn affects population size.
Exposure to hypoxic conditions(< 2.0 mg L-1 dissolved oxygen) negatively affects egg production, developmental rate, and size at maturity in Acartia tonsa, especially during the summer when the copepod population is most abundant and growing rapidly (Richmond et al., 2006). A. ohtsukai has been observed to avoid hypoxic waters with naturally depleted DO (Kang, 2011). However, in this study, the distribution of A. ohtsukai in the bay cannot be explained by DO as a limiting factor, as the bay has a relatively high DO concentration (>3.0 mg L-1). Although A. ohtsukai did not occur in the waters with the highest DO concentration, its density was found in waters with a DO concentration above 4 mg L-1, similar to that of A. ohtsukai in ports of Korea, which occurred within a relatively narrow range of DO concentrations from 4.30 to 9.32 mg L-1 (Kang, 2011).
4.3 Coexistence with Tortanus sp.
Ueda and Bucklin (2006) reported that A. ohtsukai, a continental relict species, coexisted with S. sinensis and T. derjugini/T. dextrilobatus in turbid estuaries within Ariake Bay, Japan. Similar patterns of coexistences were observed in Korean estuaries (Moon et al., 2008). In this study, we identified three species of the genus Tortanus in Laizhou Bay: T. forcipatus, T. spinicaudatus, and T. derjugini. These species are common in East Asian continental waters during the warm season, with T. forcipatus and T. spinicaudatus preferring coastal waters and T. derjugini being restricted to estuaries (Chen and Zhang, 1965; Ohtsuka et al., 1995; Soh, 2010; Shih et al., 2022b). S. sinensis was not found in our samples, possibly because this species mainly occurs in the innermost part of the oligohaline water where the salinity is less than 15 (Youn and Choi, 2008; Suzuki et al., 2013; Seo et al., 2021), which is outside the scope of our survey area. Our results indicated that T. forcipatus, T. derjugini, and T. spinicaudatus overlap temporally and geographically with A. ohtsukai, particularly T. derjugini, which was observed at the same station as A. ohtsukai in September (Figure 5). This suggests that they have similar ecological habits to warm-season and estuarine species (Soh, 2010). The coexistence of T. derjugini and A. ohtsukai may be due to their shared optimum water temperature of approximately 15°C for dormant egg hatching in their growth cycles (Kasahara and Uye, 1979; Zhong and Xiao, 1992a), while T. forcipatus emerges later than A. ohtsukai, probably due to its higher optimum water temperature (>15°C) for dormant egg hatching at 25°C (Kasahara et al., 1975; Kasahara and Uye, 1979). Nevertheless, our findings showed that T. derjugini occurs rarely and does not completely overlap in spatial and temporal distribution with A. ohtsukai in Laizhou Bay. Seo et al. (2021) also reported that A. ohtsukai and T. dextrilobatus appeared in the same seasons from spring to autumn in the Tamjin River estuary, but T. derjugini appeared only in the spring. Sakaguchi et al. (2011) observed the co-occurrence of three continental relict species, A. ohtsukai, S. sinensis, and T. derjugini/T. dextrilobatus, in estuaries in southern and western Korea but not in estuaries in western Japan. Suzuki et al. (2013) suggested that these semi-endemic species may have different preferences for estuarine habitat factors, such as turbidity and food concentration, in addition to temperature and salinity, leading to different distribution patterns over time and space on the southeastern coasts of the Eurasian continent and in neighboring waters.
A. ohtsukai and Tortanus sp. were present in Laizhou Bay during the warm season, and their populations were concentrated at the base of the bay. They first appeared at the western base of the bay near the river mouth in early summer and then spread from west to east and from the inner to the middle bay from July to September. Finally, they retreated to the base of the bay in late autumn and winter. This indicates that the inner bay serves as the source of replenishment for their populations. The seasonal occurrence patterns of the populations are consistent with the dynamics of low-salt runoff water in Laizhou Bay. The hydrology in Laizhou Bay is mostly influenced by the Yellow River and coastal river runoff, Bohai Sea waters, and monsoons (Jiang et al., 2018). The water salinity on the west side at the base of the bay remains low due to runoff from the Yellow River and land runoff along the coast throughout the year, which provides a continuous brackish habitat for A. ohtsukai and Tortanus sp. In late spring and early summer, when the water temperature rises, the resting eggs of A. ohtsukai and Tortanus sp. on the west base of the bay hatch and form pelagic populations. During the rainy seasons of summer and early autumn, the low-salt water expands from the base of the bay to the middle and outer bay due to the increase runoff from the Yellow River, land runoff, and the southeastern monsoon. Consequently, the A. ohtsukai and Tortanus sp. populations also extend to the middle bay with runoff. During the dry seasons of autumn and winter, the low-salinity water retreats southward to the bay bottom, and the relatively high-salinity water of the Bohai Sea enters the bay from the eastern part of the mouth of the bay in a compensatory way. As a result, the A. ohtsukai and Tortanus sp. also retreat toward the bay base and disappear in the middle and outer bay.
5 Conclusion
Laizhou Bay, which has relatively low salinity, provides a suitable habitats for brackish-water organisms. The Acartia specimen previously identified as Acartia pacifica in Laizhou Bay is Acartia ohtsukai, based on its habitat and morphological characteristics. Monthly surveys indicate that Acartia species are present in Laizhou Bay during the warm seasons of summer and autumn. They are found near the river mouth where the chl. a concentration is high, the water temperature exceeds 20°C, and the salinity is below 28. These findings align with the habitat preference of A. ohtsukai as a brackish-water species, as described by Ueda and Bucklin (2006). Water temperature may also function in the occurrence of A. ohtsukai, while the chl. a concentration influences its distribution. The co-occurrence of A. ohtsukai with three Tortanus species in Laizhou Bay was observed, although there were spatial and seasonal variations among the Tortanus species.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
TZ: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. DW: Formal analysis, Visualization, Writing – review & editing. YL: Data curation, Writing – review & editing. MN: Formal analysis, Writing – review & editing. ZC: Formal analysis, Writing – review & editing. JW: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Project of Yellow River Fisheries Resources and Environment Investigation (grant no.HHDC-2022) and Fisheries Development Grant “Habitat Survey of Valuable and Endangered Aquatic Wildlife” from the MARA, P. R. China, the Shandong Provincial Natural Science Foundation (grant no. 2009ZRB02317), and the China National Offshore Oil Corporation Foundation (grant no. CF-MEEC/TR/2023–16).
Acknowledgments
We are grateful to Mr. CHEN Junfeng, Ms. WANG Xiuxia, and Mr. WU Qiang, Mr. SUN Jianqiang, Mr. CHEN Ruisheng for their help in field work at sea and laboratory sample processing. Thanks for the data service provided by the Oceanographic Data Center, Chinese Academy of Sciences (CASODC) (http://msdc.qdio.ac.cn). Thank Mr. ZHANG Haiyan for his monthly dataset of mean temperature, salinity, and current of the Surface and Bottom Layer of Bohai, Yellow Sea and East China Sea between 1997 and 2016 on CASODC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bi H. S., Sun S., Gao S. W., Zhang G. T. (2001). The ecological characteristics of zooplankton community in the Bohai Sea II. The distribution of copepoda abundance and seasonal dynamics. Acta Ecol. Sin. 21, 177–185. doi: 10.3321/j.issn:1000-0933.2001.02.001
Borkman D. G., Fofonoff P., Smayda T. J., Turner J. T. (2018). Changing Acartia spp. phenology and abundance during a warming period in Narragansett Bay, Rhode Island, USA: 1972–1990. J. Plankton Res. 40, 580–594. doi: 10.1093/plankt/fby029
Bradford J. M. (1976). Partial revision of the Acartia subgenus Acartiura (Copepoda: Calanoida: Acartiidae). New Zeal. J. Mar. Fresh. 10, 159–202. doi: 10.1080/00288330.1976.9515606
Chen Q. C., Zhang S. Z. (1965). The planktonic copepods of the Yellow Sea and the East China Sea, I. Calanoida. Studia Marina Sin. 7, 20–131.
Choi S. Y., Lee E. H., Soh H. Y., Jang M.-C. (2021b). Effects of temperature and salinity on egg production, hatching, and mortality rates in Acartia ohtsukai (Copepoda, Calanoida). Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.704479
Choi S. Y., Seo M. H., Shin K., Jang M. C., Soh H. Y. (2019). Spatial distribution of Acartia (Copepoda, Calanoida) species in the southern coastal waters of Korea during summer. Korean J. Environ. Biolog. 37, 299–308. doi: 10.11626/KJEB.2019.37.3.299
Choi S. Y., Seo M. H., Soh H. Y. (2021a). Short-term variation in abundance of four Acartia species (Copepoda, Calanoida) in a eutrophic bay. PeerJ 9, e10835. doi: 10.7717/peerj.10835
Feng Y., Liu Z. (2019). Environmental determinants of zooplankton assemblages during summer hypoxia in the Changjiang Estuary and its adjacent waters, China. Reg. Stud. Mar. Sci. 32, 100838. doi: 10.1016/j.rsma.2019.100838
Greenwood J. G. (1981). Occurrence of congeneric pairs of Acartia and Pseudodiaptomus species (Copepoda, Calanoida) in Moreton Bay, Queensland. Estuar. Coast. Shelf Sci. 13, 591–596. doi: 10.1016/S0302-3524(81)80060-6
Jebir A. M., Ajeel Shaker G., Khalaf Talib A. (2021). Ecological study of zooplankton in South Shatt Al-Arab River, Southern Iraq. J. Biol. Stud. 4, 1–23. doi: 10.62400/jbs.v4i1.5609
Jiang H., Liu D., Song X., Ma Y., Wang Y., Liu A., et al. (2018). Response of phytoplankton assemblages to nitrogen reduction in the Laizhou Bay, China. Mar. pollut. Bull. 136, 524–532. doi: 10.1016/j.marpolbul.2018.09.042
Kang H. K., Kang Y. J., Park C. (2007). Production of Acartia omorii (Copepoda: Calanoida) in Ilkwang Bay, southeastern coast of Korea. J. Mar. Syst. 67, 236–244. doi: 10.1016/j.jmarsys.2006.05.014
Kang J. (2011). The occurrence of Acartia species and their environmental characteristics at three ports in Korea. Ocean Sci. J. 46, 219–237. doi: 10.1007/s12601-011-0018-7
Kasahara S., Onbe T., Kamigaki M. (1975). Calanoid Copepod eggs in sea-bottom muds. III. Effects of temperature, salinity and other factors on the hatching of resting eggs of Tortanus forcipatus. Mar. Biol. 31, 31–35. doi: 10.1007/BF00390645
Kasahara S., Uye S. (1979). Calanoid copepod eggs in sea-bottom muds. V. Seasonal changes in hatching of subitaneous and diapause eggs of Tortanus forcipatus. Mar. Biol. 55, 63–68. doi: 10.1007/BF00391718
Lee E. H., Choi S. Y., Seo M. H., Lee S. J., Soh H. Y. (2020). Effects of temperature and pH on the egg production and hatching success of a common Korean copepod. Diversity 12, 372. doi: 10.3390/d12100372
Lee E. H., Seo M. H., Yoon Y. H., Choi S. D., Soh H. Y. (2017). Environmental factors affecting zooplankton community in Gwangyang Bay. Korean J. Environ. Biolog. 35, 631–639. doi: 10.11626/KJEB.2017.35.4.631
Li S., Fang J. C. (1990). Marine planktonic copepod larvae in China Seas (Bejing: China Ocean Press), 115–118.
Liu C. C. (2007). Molecular phylogeny of two Acartia species in China Southeast Coastal Seas (Xiamen, China: Xiamen University).
Liu A. Y., Song X. K., Liu L. J., Ren L. H., Jin Y., Liu Y. H., et al. (2012). Community structure of zooplankton in Laizhou bay in summer. Mar. Sci. 36 (10), 61–67.
Moon S. Y., Ohtsuka S., Ueda H., Soh H. Y. (2008). Acartia (Odontacartia) ohtsukai ueda and bucklin 2006 (Copepoda, Calanoida, Acartiidae): first record of its occurrence in Korean waters and habitat segregation from its sibling species A. pacifica steue. Zootaxa 1841, 61–64. doi: 10.11646/zootaxa.1841.1.5
Moon S. Y., Seo M. H., Shin Y., Soh H. Y. (2012). Seasonal variation of mesozooplankton communities in the semi-enclosed Muan Bay, Korea. Ocean Polar Res. 34, 1–18. doi: 10.4217/OPR.2012.34.1.001
Ohtsuka S., Ueda H., Lian G. (1995). Tortanus derjugini Smirnov (Copepoda: Calanoida) from the Ariake Sea, western Japan, with notes on the zoogeography of brackish-water calanoida copepods in East Sea. Bull. Plankton Soc. Japan 42, 147–162.
Paffenhöfer G. A., Stearns D. E. (1988). Why is Acartia tonsa (Copepoda, Calanoida) restricted to nearshore environments? Mar. Ecol. Prog. Ser. 42, 33–38. doi: 10.3354/meps042033
Park E. O., Rahman M. S., Seo M. H., Kim J. J., Soh H. Y. (2013). Distribution patterns of Calanoid Copepods along the Seomjin River Estuary in Southern Korea during summer. Korean J. Environ. Biolog 31, 165–171. doi: 10.11626/KJEB.2013.31.2.165
Peck N., Peters J., Diekmann R., Laakmann S., Renz J. (2015). Interactive effects of temperature and salinity on population dynamics of the calanoid copepod Acartia tonsa. J. Plankton Res. 37, 197–210. doi: 10.1093/plankt/fbu093
Razouls C., Desreumaux N., Kouwenberg J., de Bovée F. (2005–2023) ) (Sorbonne University, CNRS). Available online at: http://copepodes.obs-banyuls.fr/en (Accessed December 20, 2023).
Rice E., Dam H., Stewart G. (2015). Impact of climate change on estuarine zooplankton: surface water warming in Long Island Sound is associated with changes in copepod size and community Structure. Estuaries Coasts 38, 13–23. doi: 10.1007/s12237-014-9770-0
Rice E., Stewart G. (2016). Decadal changes in zooplankton abundance and phenology of Long Island Sound reflect interacting changes in temperature and community composition. Mar. Environ. Res. 120, 154–165. doi: 10.1016/j.marenvres.2016.08.003
Richmond C., Marcus N. H., Sedlacek C., Miller G. A., Oppert C. (2006). Hypoxia and seasonal temperature: Short-term effects and long-term implications for Acartia tonsa Dana. J. Exp. Mar. Biol. Ecol. 328, 177–196. doi: 10.1016/j.jembe.2005.07.004
Sakaguchi S. O., Ueda H., Ohtsuka S., Soh H. Y., Yoon Y. H. (2011). Zoogeography of planktonic brackish-water calanoid copepods in western Japan with comparison with neighboring Korean fauna. Plankton Benthos Res. 6, 18–25. doi: 10.3800/pbr.6.18
Seo M. H., Kim H. J., Lee S. J., Kim S. Y., Yoon Y. H., Han K. H., et al. (2021). Environmental factors affecting the spatiotemporal distribution of copepods in a small mesotidal inlet and estuary. Diversity 13, 389. doi: 10.3390/d13080389
Shih C., Chen Q. C., Lan Y. C., Hsiao S. H., Weng C. Y. (2022a). Key to the species of Acartiidae Acartia occurring in the China Seas. J. Mar. Sci. Tech. 30, 119–145. doi: 10.51400/2709-6998.2593
Shih C.-T., Chen Q.-C., Lan Y.-C., Hsiao S.-H., Weng C.-Y. (2022b). Key to the species of Tortanidae Tortanus occurring in the China Seas. J. Mar. Sci. Tech 30, 884–909. doi: 10.51400/2709-6998.2679
Soh H. Y. (2010). Invertebrate fauna of Korea. Volume 21, number 3. Arthropoda: Crustacea: Copepoda: Calanoida, Cyclopoida, Marine Planktonic Copepods (Incheon: National Institute of Biological Resources, Republic of Korea), 18–23.
Sun X., Sun S., Li C., Wang M. X. (2012). Seasonal change in body length of important small copepods and relationship with environmental factors in Jiaozhou Bay, China. Chin. J. Ocean. Limnol. 30, 404–409. doi: 10.1007/s00343-012-1140-9
Sun X. H., Sun S., Li C. L., Zhang G. T. (2011). Seasonal and spatial variability in egg production, abundance and production of small copepods in and near Jiaozhou Bay, China. J.Plankton R. 33, 741–750. doi: 10.1093/plankt/fbq135
Suzuki K. W., Nakayama K., Tanaka M. (2013). Distinctive copepod community of the estuarine turbidity maximum: comparative observations in three macrotidal estuaries (Chikugo, Midori, and Kuma Rivers), southwestern Japan. J. Oceanogr. 69, 15–33. doi: 10.1007/s10872-012-0151-7
Ueda H., Bucklin A. C. (2006). Acartia (Odontacartia) Ohtsukai, a new brackish-water Calanoid Copepod from Ariake Bay, Japan, with a redescription of the Closely Related A. pacifica from the Seto Inland Sea. Hydrobiologia 560, 77–91. doi: 10.1007/s10750-005-9513-0
Uye S. (1985). Resting egg production as a life history strategy of marine planktonic copepods. Bull. Mar. Sci. 37, 440–449. doi: 10.3354/meps08635
Wang M. X., Cheng F. P., Li C. L., Sun S. (2011). DNA barcoding of zooplankton in the Jiaozhou Bay for species identification. Haiyang Yu Huzhao 42, 702–710. doi: 10.1080/0144929X.2011.553739
Wang G., Jiang X., Li S., Wu L., Wu D. (2005). A potential source of recruitment of Acartia pacifica nauplii: viable benthic resting eggs. Acta Oceanol. Sin. 24, 151–158. doi: 10.1029/2003JC002236
Wang J., Wu Q. (2018). Fishery resource and habitat in Laizhou Bay (Beijing: Chinese Aquaculture Press), 11–54.
Wang R., Zhang H. Y., Wang K., Zuo T. (2002). Distribution and population dynamics of Paracalanus parvus, Paracalanus crassirostris, and Acartia bifilosa (Copepoda, Calanoida) in the Bohai Sea. Chin. J. Oceanol. Limn. 20, 348–357. doi: 10.1007/BF02847926
Xia D. X., Wang W. H., Liu C. X., Han L. Z., Zheng P. Y. (1991). China’s coastal embayment Vol. 3: Bays of Northern and Eastern Shandong Peninsula (Beijing: China Ocean Press), 9–26.
Youn S. H., Choi J. K. (2007). Egg production of the copepod Acartia hongi in Kyeonggi Bay, Korea. J. Mar. Syst. 64, 217–224. doi: 10.1016/j.jmarsys.2006.05.017
Youn S. H., Choi J. K. (2008). Distribution pattern of zooplankton in the Han River estuary with respect to tidal cycle. Ocean Sci. J. 43, 135–146. doi: 10.1007/BF03020694
Zhang X. L., Zhang Z. H., Xu Z. J., Li G., Sun Q., Hou X. J. (2013). Sea ice disasters and their impacts since 2000 in Laizhou Bay of Bohai Sea, China. Nat. Haz. 65, 27–40. doi: 10.1007/s11069-012-0340-0
Zhong X. F., Xiao Y. C. (1992a). Resting eggs of Acartia bifilosa Giesbrecht and A. pacifica Steuer in Jiaozhou Bay. Mar. Sci. 16 (5), 55–59.
Zhong X. F., Xiao Y. C. (1992b). Seasonal cycles of abundance of three copepod species population in Jiaozhou Bay. Mar. Sci. 16 (1), 44–48.
Keywords: Acartia pacifica, Acartia ohtsukai, copepod, Laizhou Bay, Bohai Sea
Citation: Zuo T, Wang D, Li Y, Niu M, Cheng Z and Wang J (2024) Occurrence of the calanoid copepod Acartia (Odontacartia) ohtsukai in Laizhou Bay, the Bohai Sea, China, and its relationship with environmental factors. Front. Mar. Sci. 11:1378085. doi: 10.3389/fmars.2024.1378085
Received: 29 January 2024; Accepted: 20 May 2024;
Published: 03 June 2024.
Edited by:
Sm Sharifuzzaman, University of Chittagong, BangladeshReviewed by:
Xiaoshou Liu, Ocean University of China, ChinaPaulinus Chigbu, University of Maryland Eastern Shore, United States
Copyright © 2024 Zuo, Wang, Li, Niu, Cheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Zuo, enVvdGFvQHlzZnJpLmFjLmNu
 Tao Zuo
Tao Zuo Di Wang1,3
Di Wang1,3 Yongtao Li
Yongtao Li