
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 01 May 2024
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1363703
This article is part of the Research TopicTowards an Expansion of Sustainable Global Marine AquacultureView all 13 articles
The traditional method of sexual reproduction in Sargassum fusiforme can lead to difficulties in maintaining the stable inheritance of superior traits. However, technology for asexual proliferation of seedlings in seaweed tissue culture is not well-developed. Therefore, we established a tissue culture method to study, the effects of different parts of S. fusiforme, uniconazole (UIZ) concentrations, and culture methods on the regeneration of tissue-derived juveniles of S. fusiforme. The results showed that the optimal culture conditions were solid medium with modified Provassoli’s enriched seawater containing 3 μM UIZ for at least 17 days followed by transfer to liquid medium to induce rapid cell proliferation. These optimal conditions resulted in a callus-like/adventitious bud induction rate of 100%, callus-like/adventitious bud number per explant of 27.43 ± 4.57, and relative growth rate of 3.05 ± 0.27. The best plant parts for tissue culture were the filamentous holdfasts followed by the stem tip. In addition, UIZ treatment increased photosynthesis, resulting in soluble sugar and soluble protein contents of 30.47 mg·g-1 and 1.39 mg·g-1 of in the regenerated juveniles. Based on our results, S. fusiforme can be cultured using a tissue culture technique in which UIZ is added to a solid medium, followed by culture in liquid medium for proliferation. Sargassum fusiforme juveniles obtained using this technique can be cultured continuously until the next culture season and grow normally, providing a technical reference for indoor preservation and expansion of algal species.
Sargassum fusiforme (Harvey) Okamura (Sargassaceae, Phaeophyta) is a brown macroalga with high economic and nutritional value that grows in warm temperate and subtropical regions (Mantri et al., 2022; Pang et al., 2023). Sargassum fusiforme and other seaweeds are widely distributed in eastern China, Korea, and Japan, among other locations, and provide egg-laying and seedling resource sites for various marine organisms (Uji et al., 2015; Hwang et al., 2020; White and White, 2020). This algae is rich in polyunsaturated fatty acids, fucoidans, polysaccharides, and polyphenols, and exerts anti-obesity, anti-inflammatory, antioxidant, anti-tumor, and antiviral properties, making it applicable in the fields of nutraceuticals, pharmaceuticals and cosmetics (Akremi et al., 2017; Garcia-Poza et al., 2020; Kelly et al., 2020; Ali et al., 2021). The global annual production of S. fusiforme has increased from 109,597t in 2003 to 303,797 t in 2020; therefore, the seaweed aquaculture industry shows high development prospects (Tian et al., 2023).
Sargassum fusiforme has been traditionally cultivated in two ways; sexual propagation by fertilizing the male and female receptacles to form a zygote, which regenerates the holdfasts, and asexual propagation by artificially retaining the holdfasts of S. fusiforme during the harvest of the mature algal mass, followed by culturing the holdfasts in the following year (Xu et al., 2022b). However, the traditional method of seedling cultivation leads to the growth of weed algae, which compete with seedlings for nutrients and space, and it is challenging to control the temperature, pH, and light. Even during sexual reproduction, it is difficult to stabilize the inheritance of some heat- and cold-tolerant strains because of crossbreeding (Chen and Zou, 2014; Chen et al., 2019; Luo et al., 2023). Traditional seedling culture conditions are complicated and time-consuming, and the maintenance and replacement of cultivation equipment during cultivation can cause a serious economic burden (Largo et al., 2020; Collins et al., 2022; Arbaiza et al., 2023). Additionally, cultivation of seaweed in marine areas can alter the ecological structure, abundance, and diversity of phytoplankton, thereby adversely affecting ecosystems, organisms and the surrounding environment (Kelly et al., 2020; Eggertsen and Halling, 2021; Tian et al., 2023). Therefore, improved cultivation methods for S. fusiforme must be explored.
Tissue culture is the most common method of cultivation and broadly refers to use of the plant itself, such as isolated organs, tissues, cells, protoplasts, and mutants. This approach has the advantages of simple operation, easy cultivation in large quantities, short cultivation time, easy control of cultivation conditions, and a low mutation rate of cells (Custodio et al., 2022; Yan et al., 2022). Nutritional propagation is an effective means of tissue culture. According to the morphological changes that occur during tissue culture, propagation can be divided into direct regeneration and indirect regeneration (including callus culture and protoplast culture) to generate virus-free and fast-growing resistant algal strains (Avila-Peltroche et al., 2022; Jiksing et al., 2022). However, there are some limitations to tissue culture, such as the lack of development of tissue culture methods for different seaweeds, susceptibility to bacterial contamination during the culture process, uncertainty in the optimal composition of the culture medium, and difficulty in controlling the culture results (Baweja et al., 2009; Yong et al., 2014; Muhamad et al., 2018).
Tissue culture techniques can be used to induce the production of calluses, budding, and plant development by adding plant growth regulators, which are synthetic (or extracted from microorganisms), along with an organic compound similar to natural plant hormones that regulate the growth and development of biological organisms (Rademacher, 2015; Luo et al., 2023). Uniconazole (UIZ), known as a triazole-type cytochrome P450 enzyme inhibitor, is a plant growth regulator. Most studies of UIZ application were conducted in soybean, corn, and wheat to evaluate resistance to high salt, drought and low temperatures, and to induce germination and emergence, and its effects on the photosynthetic characteristics, antioxidant activity, and morphological parameters of plants. However, the application of UIZ to economic seaweeds has not been widely examined (Keshavarz and Khodabin, 2019; de Araújo Amatuzzi et al., 2020; Aghaei et al., 2021; Zhou et al., 2021; Hu et al., 2022).
Tissue culture technology has the advantages of simple operation, easy control of growth conditions, short cultivation time, high yield, and low cost. In this study, we utilized tissue culture technology for seedling propagation of S. fusiforme to investigate the effects of different parts, UIZ concentrations and culture methods on juvenile regeneration, growth, development, and nutrient accumulation. We developed a method for juvenile regeneration of S. fusiforme tissue, and provide technical support for seedling propagation and expansion of this algae.
Sargassum fusiforme was collected from Dongtou, Zhejiang province, during the harvesting season and brought to the laboratory within 2 h in a 4°C cryopreservation box. The surface of S. fusiforme was cleaned with brushes and tweezers to remove attached stray algae and plankton, rinsed with filtered and sterilized natural seawater. Healthy and uniformly sized algal organisms were selected for temporary incubation in a light-illuminated incubator (light incubator, Yanghui, Ningbo, China) for 7 days. The experimental conditions were as follows: temperature was 19°C, light intensity was 90 µmol photons·m-2 ·s-1, light:dark photoperiod (L:D) was 12 h:12 h, salinity of the culture solution was 26‰, and the culture medium was supplemented with 200 µmol·L-1 NaNO3 and 20 µmol·L-1 NaH2PO4. The culture solution was aerated continuously using an air pump (ACO-9730, Haili, Guangzhou, China).
After 7 days of domestication, healthy stem tips (2–4 cm in length), the lower end of the stem base (4–5 cm from the base), and filamentous holdfasts of S. fusiforme were selected and rinsed three times in sterile seawater. The seaweed was treated with a solution of 0.38% NaClO and 0.5% KI in a 1:1 volume ratio for 2 min, sterilized with sterile water, and rinsed. The samples were then cut into approximately 1.5 cm segments using a scalpel and cultured on modified Provassoli’s enriched seawater (PES) solid medium with different concentrations of UIZ (Macklin, Guangzhou, China). The tissue was cultivated at a temperature of 19°C, with a light intensity of 60 μmol photons·m-2 ·s-1 and L:D = 12 h:12 h. The solid medium was changed every month to prevent browning of the tissue (Kerrison et al., 2015; Uji et al., 2015; Muhamad et al., 2018; Xu et al., 2022a).
The experiment comprised of two phases. During the first phase, S. fusiforme explants were cultured in solid medium containing UIZ. A cross-design was used to divide the culture samples into 12 groups based on the source site of the explants (proximal stem tip, proximal stem base, and filamentous holdfasts) and concentration of UIZ (0, 3, 5, and 7 μM). The culture samples were exposed to a light intensity of 90 μmol photons ·m-2 ·s-1. During the second stage of culture, the S. fusiforme segments were transferred into modified PES liquid medium on days 14 (first batch), 17 (second batch), 20 (third batch), and 52 (fourth batch) after the first stage of culture. The callus-like/adventitious bud induction rate, callus-like/adventitious bud numbers per explant, relative growth rate (RGR), photosynthetic activity and morphology of S. fusiforme tissue culture were recorded. The liquid PES medium was replaced every three days during incubation.
Morphological changes in S. fusiforme explants were documented using a Sony camera (SONY a6000, Tokyo, Japan) after each medium change. The numbers of callus-like or adventitious buds in each explant segment were recorded. The morphology and cellular structure of the characteristic explants were observed at every 3 days using a stereomicroscope (Leica M80, Wetzlar, Germany) and an orthostatic microscope (Leica DM2700 P, Wetzlar, Germany).
The callus-like/adventitious bud induction rate and callus-like/adventitious bud numbers per explant of S. fusiforme were counted every 3 days during the incubation period. The specific formulas were as follows:
The RGR was calculated by determining the weight of the explants and regenerated juveniles at the time of medium change, using the following formula:
where W0 is the initial weight of the explant (g), Wt is the weight of the explant and regenerated juveniles on day t (g), and t is the number of culture (days).
The physiological state of regenerating S. fusiforme juveniles was determined by measuring their maximum quantum yield (Fv/Fm) and maximum relative electron transfer rate (rETRm) using Junior-Pam (Walz, Effeltrich, Germany). S. fusiforme explants together with regenerated juveniles were dark-adapted for 30 min, and the Fv/Fm and rETRm in the range of 0 – 820 μmol photons m-2 ·s-1 were determined, where Fv = Fm-F0, Fv is the variable fluorescence, Fm is the maximum fluorescence after dark-adaptation, and F0 is the minimum fluorescence after dark-adaptation. The rapid light curve was fitted according to the formula described by Platt et al. (1980):
The soluble carbohydrate (SC) content was determined using the anthrone method developed by Ershadi et al. (2015). Approximately 0.1 g of tissue culture from S. fusiforme juveniles was crushed in liquid nitrogen; 1 mL of distilled water was added during the crushing process, and then 2 mL of MgCO3 suspension was added. The samples were fixed to 10 mL with distilled water and centrifuged in a freezing centrifuge at 10277×g for 10 min. The supernatant (1 mL) was transferred into a test tube and mixed with 2 mL of distilled water, followed by addition of 10 mL of anthrone reagent. The mixture was shaken continuously until gas escaped and then heated in a boiling water bath at 100°C for 5 min. After the mixture cooled, the absorbance was measured at 620 nm using a UV spectrophotometer with distilled water as a reference. The SC content was calculated from the standard curve of absorbance values(mg·g-1).
The soluble protein (SP) content was determined using Coomassie Brilliant Blue G-250 dye as described by Bradford (1976). Approximately 0.1 g of S. fusiforme juveniles was ground in liquid nitrogen in a mortar, suspended in 10 mL 0.1 mol/L phosphate buffer (pH = 6.8) and centrifuged at 10277×g for 10 min for 10 min at 4°C in an ultra-high-speed freezer centrifuge. The supernatant (1 mL) was collected and mixed with 4 mL of Coomassie Brilliant blue G-250 staining solution. The mixture was allowed stand for 3 min at 25°C and the absorbance measured at 595 nm. For the control group, 1 mL of PBS was mixed with 4 mL of Coomassie Brilliant Blue G-250 staining solution. The mixture was incubated at 25°C for 3 min before absorbance was recorded using a UV spectrophotometer. The SP content was calculated using a bovine serum protein standard curve (mg·g-1).
The mannitol content was determined as described by Chen et al. (2020). A dried juvenile S. fusiforme sample (0.1 g) was collected from a tissue culture source. The sample was milled using liquid nitrogen, and 30mL of HCl with a mass fraction of 1% was added. After the mixture was stirred for 4 min in a 100°C water bath, the supernatant was collected and diluted 100 times. The diluted solution (1 mL) was added to a centrifuge tube. Sodium periodate (1mL) of was then added, mixed well, then it was placed at 25°C for 15 min. 2mL of 0.1% rhamnose was added, mixed thoroughly, and left to wait for 1 min. Nash reagent (4.0 mL) was added and mixed thoroughly. The mixture was heated in a constant temperature water bath at 53°C for 15 min and cooled to room temperature after the mixture changed color. Finally, the absorbance of mannitol was measured at 413 nm using distilled water as a blank. The standard curve of mannitol was drawn using the absorbance a value as the vertical coordinate and the concentration as the horizontal coordinate(mg·g-1).
All data were preliminarily processed using Excel (Microsoft, Redmond, WA, USA) and subjected to analysis of variance chi-square (F-test), one-way analysis of variance, and two-way analysis of variance using SPSS 25.0 software (SPSS, Inc., Chicago, IL, USA), with the significance level set at P<0.05. Graphs were drawn using origin 2023 (OriginLab, Northampton, MA, USA), and experimental results are expressed as the mean ± standard deviation.
Figure 1 shows the morphological changes that occurred during the regeneration of different parts of S. fusiforme on days 34 and 62 under various batch and UIZ concentration treatments. When using a shorter time to induce callus-like tissues/adventitious buds on solid medium, and higher concentrations of UIZ, S. fusiforme was less likely to germinate and sprout compared with low concentration group. In the first bath culture, regenerating buds were induced from S. fusiforme filamentous holdfasts, and a few stem tips were produced; production occurred only under conditions of low UIZ content in the medium (0, 3, and 5 UIZ). As the induction time in solid medium was increased, S. fusiforme filamentous holdfasts, stem tips and stem bases were induced with different degrees of callus-like tissues or adventitious buds. The most pronounced morphological changes in S. fusiforme explants were observed in the 3 μM UIZ-cultured filamentous holdfasts, in which a large number of leaves, cylindrical protuberances and a few holdfasts were induced, whereas only cylindrical protuberances were induced at the stem tip and stem base. Most filamentous holdfasts and a few stem tips appeared as callus-like tissues/adventitious buds in the first stage of culture for approximately 9 days, whereas the stem base appeared after at least 30 days of cultivation in solid medium. Furthermore, in this method, adventitious buds continued to regenerate into gas vesicles or leaves after isolation (Figure 1). After initial morphological optimization screening, we cultured the tissue-derived 3 μM UIZ-activated S. fusiforme germlines for a longer period and continued this culture until the next breeding season (Figure 2).

Figure 1 Morphological changes in regeneration of various parts of S. fusiforme at 34 and 62 days under different batch and UIZ concentration treatments.
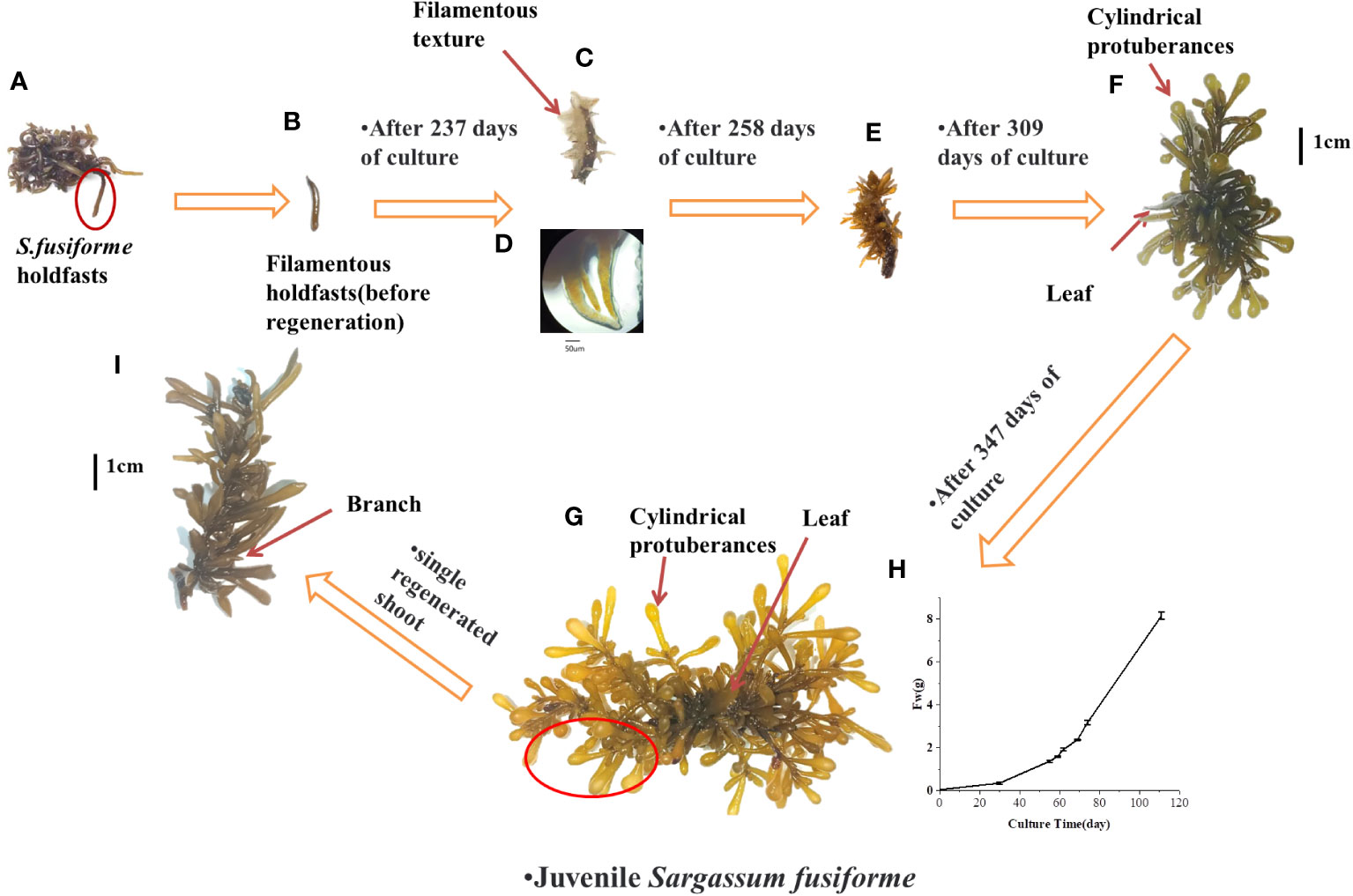
Figure 2 Induction of S. fusiforme into seedling by prolonged cultivation (A) complete holdfast (B) filaments holdfasts of S. fusiforme before regeneration (C) formation of yellowish/transparent filamentous textures; (D) morphology of c under the microscope (E) filamentous textures begin to diverge for colouring (F) formation of cylindrical protuberances and leaf (G) formation of S. fusiforme juveniles (H) change in the fresh weight of S. fusiforme seedling in f over time (I) growth of single S. fusiforme branch into an adult juveniles.
Changes in the explant cells were periodically observed during the culture period using a stereomicroscope and a Leica microscope. Explants treated with UIZ exhibited four distinct morphological structures, whereas regenerating buds formed directly from explants that were not treated with UIZ. The four types involves irregularly structured fragile mulberry embryo-like tissue (Figures 3A–C), hard spherical protuberances (Figures 3D–F), a colorless or light-yellow filamentous texture (Figures 3G–I), and direct regeneration of adventitious buds through the tips or cuts of the S. fusiforme filamentous holdfasts (Figures 3J–L). Depending on the site of explant germination and its structure, callus-like tissue can form a hyaline or yellowish bulge or callus through the medullary region at the center of the S. fusiforme holdfast (Figure 3C), or regenerative tissue can form through epidermal cells (Figures 3E, F).
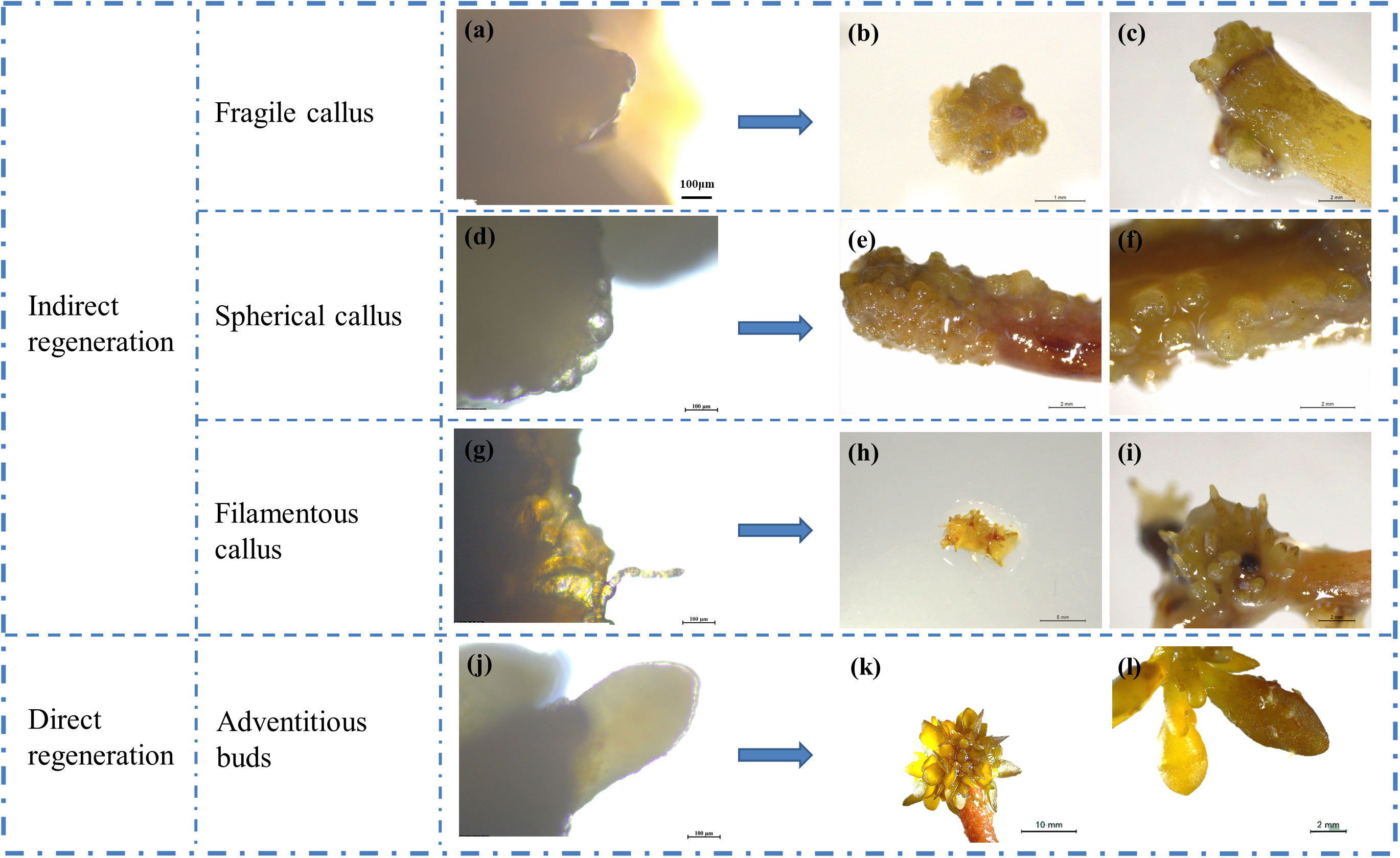
Figure 3 Types of regeneration in S. fusiforme tissue culture. (A–C) friable mulberry-like tissue cells; (D–F) hard spherical or cylindrical protuberances; (G-I) colorless or transparent filamentous texture; (J–L) directly regenerated into leaves.
The cross-sectional structure of S. fusiforme holdfast cells was arranged from outside to inside as follows: epidermis, subepidermis, exodermis, endodermis, and medulla in the middle. The epidermal cells were small, whereas the cortical cells increased in size from the outermost to the innermost layer. Medullary cells were the smallest, and the intercellular boundaries were well-defined (Figure 4). The outer epidermal cells of S. fusiforme holdfasts showed a polygonal outline and were darkest in color, as they are rich in pigments and starch grains. The pith, which has denser cells and fewer starch grains than other regions of S. fusiforme, exhibited an intermediate morphology. In contrast, the medullary zone of S. fusiforme adventitious buds were not necessarily located at the mid-end of the morphology. Morphogenesis was induced in the epidermis and subepidermis of earlier tissues (Figures 4B, D, F). The diameter of the nascent buds cells was smaller than that of the filamentous holdfasts of mature S. fusiforme (Figure 4).
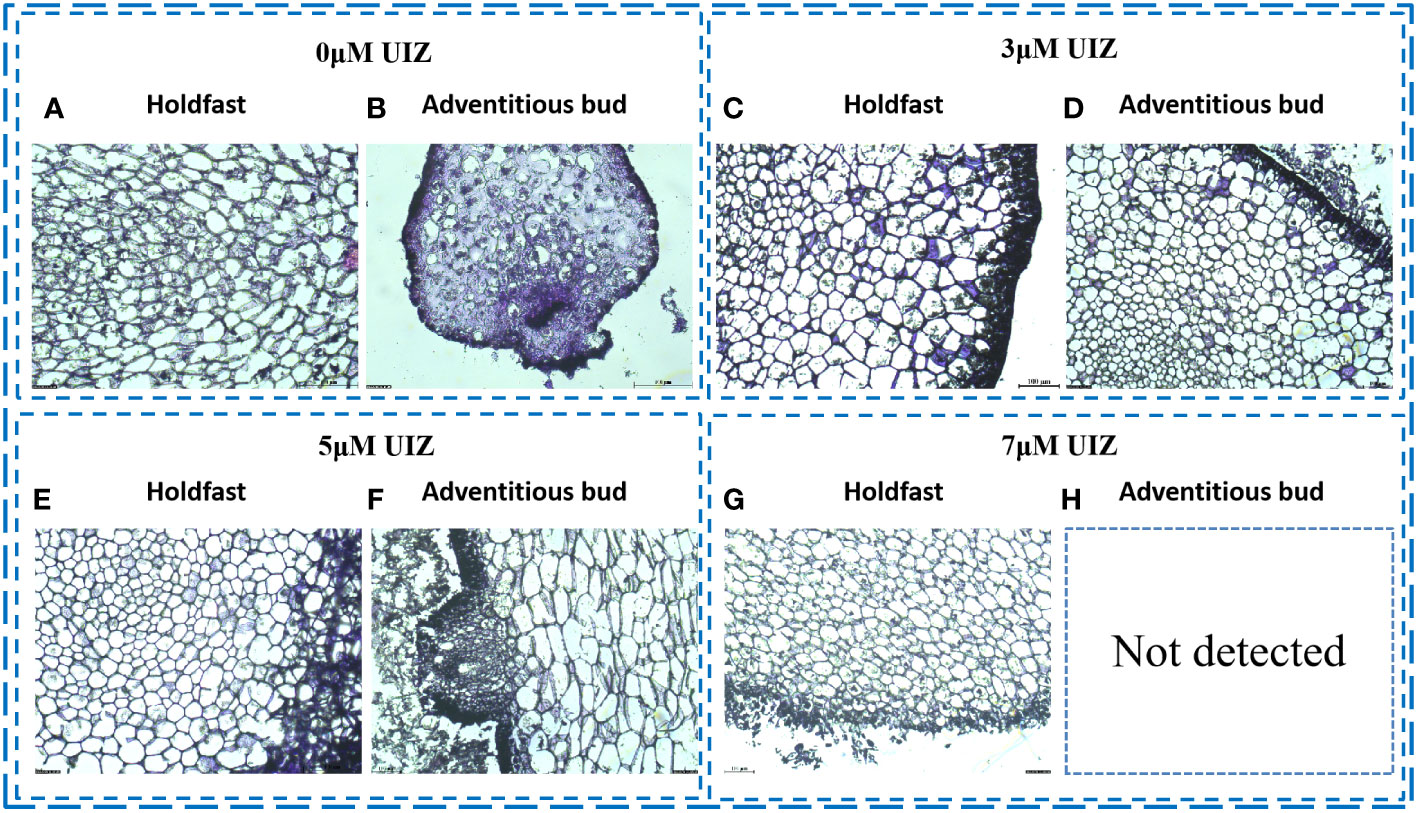
Figure 4 Frozen sections of S. fusiforme cells at different UIZ concentrations (A) cross-sectional view of 0 μM UIZ-treated filamentous holdfasts; (B) cross-sectional view of 0 μM UIZ-treated adventitious buds; (C) cross-sectional view of 3 μM UIZ-treated filamentous holdfasts; (D) cross-sectional view of 3 μM UIZ-treated adventitious buds; (E) cross-sectional view of 5 μM UIZ-treated filamentous holdsfasts; (F) cross-sectional view of 5 μM UIZ-treated adventitious buds; (G) cross-sectional view of 7 μM UIZ-treated filamentous holdfasts. (H) not detected.
In both stages of culture, the callus-like/adventitious bud induction rate and callus-like/adventitious buds numbers per explant were significantly affected by the UIZ concentration at the S. fusiforme explant site (p< 0.05, Figure 5). Addition of low concentrations of UIZ significantly increased the callus-like/adventitious bud numbers per explant and induction rate of S. fusiforme near the stem tips. The induction rate of filamentous holdfasts treated with 3 μM UIZ was 100%. Additionally, 27.43 ± 4.58 callus-like/adventitious buds were produced per explant segment (p< 0.05, Figure 5). The number of callus-like or adventitious buds per explant was higher in all parts of the explant under the 3 μM UIZ treatment condition compared to that observed under other UIZ concentrations (Figure 6B). Furthermore, there was a significant difference in the number of callus-like/adventitious bud per explant between the first batch of the earliest liquid medium transfer and the second, third, and fourth batches of the culture(p< 0.05, Figure 6D).This result suggests that a minimum of 17 days of cultivation in solid medium is necessary. Batch 4 produced 7.19 ± 3.13 adventitious shoots/callus-like tissues per branch segment (p< 0.05, Figure 6D). Batch 4 as the group treated with 3 μM UIZ and with the longest solid induction time showed the highest germination rate, indicating that solid medium was beneficial for inducing adventitious buds/callus-like tissues (Figure 6).
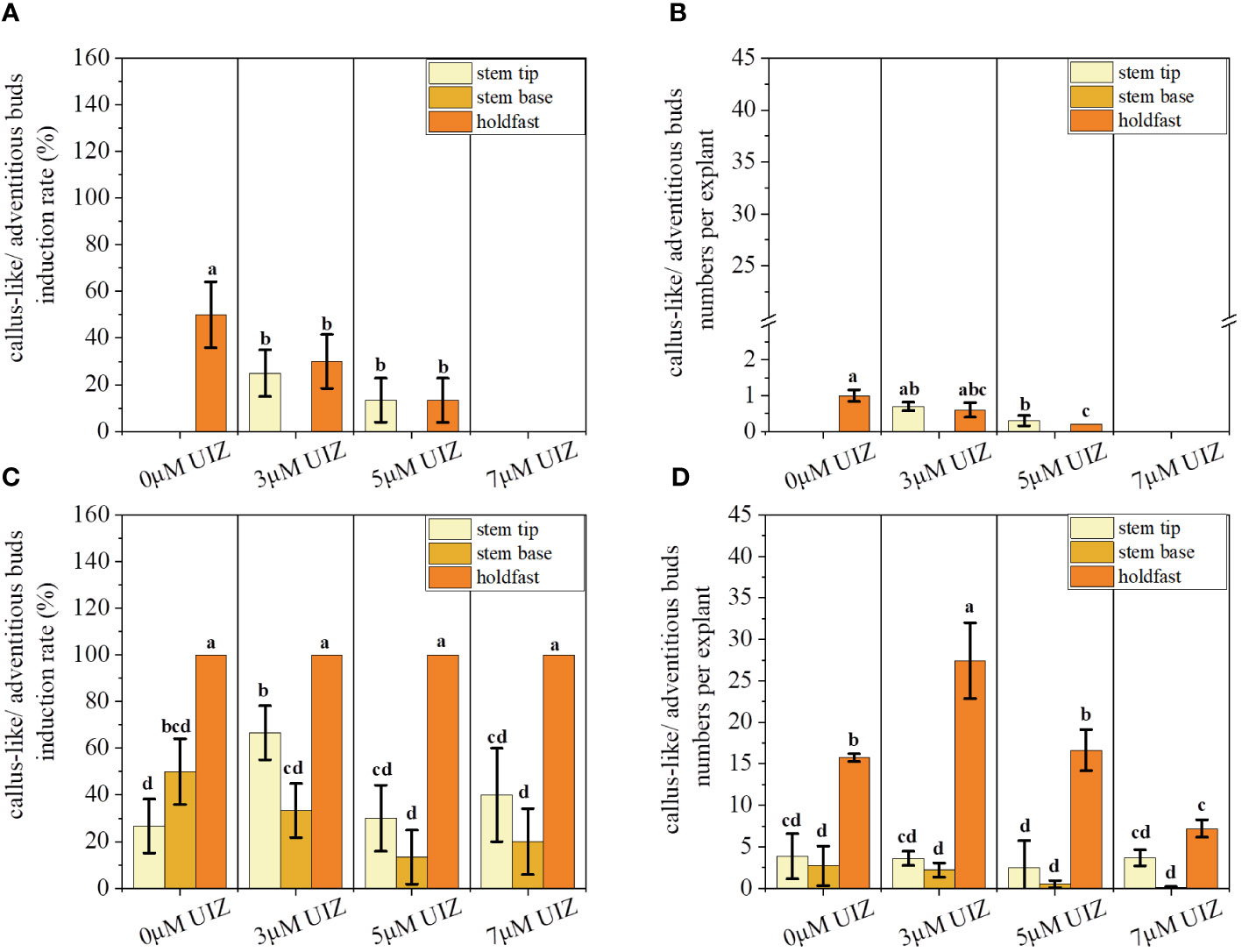
Figure 5 Effects of callus-like/adventitious buds induction rate and callus-like/adventitious buds numbers per explant at different culture stages. (A) callus-like/adventitious buds induction rate at solid culture stage; (B) callus-like/adventitious buds numbers per explant at solid culture stage; (C) callus-like/adventitious buds induction rate at batch culture stage; (D) callus-like/adventitious buds numbers per explant at batch culture stage. Values are expressed as mean ± standard deviation (n = 3). Different letters indicate significant differences between culture conditions (p< 0.05).
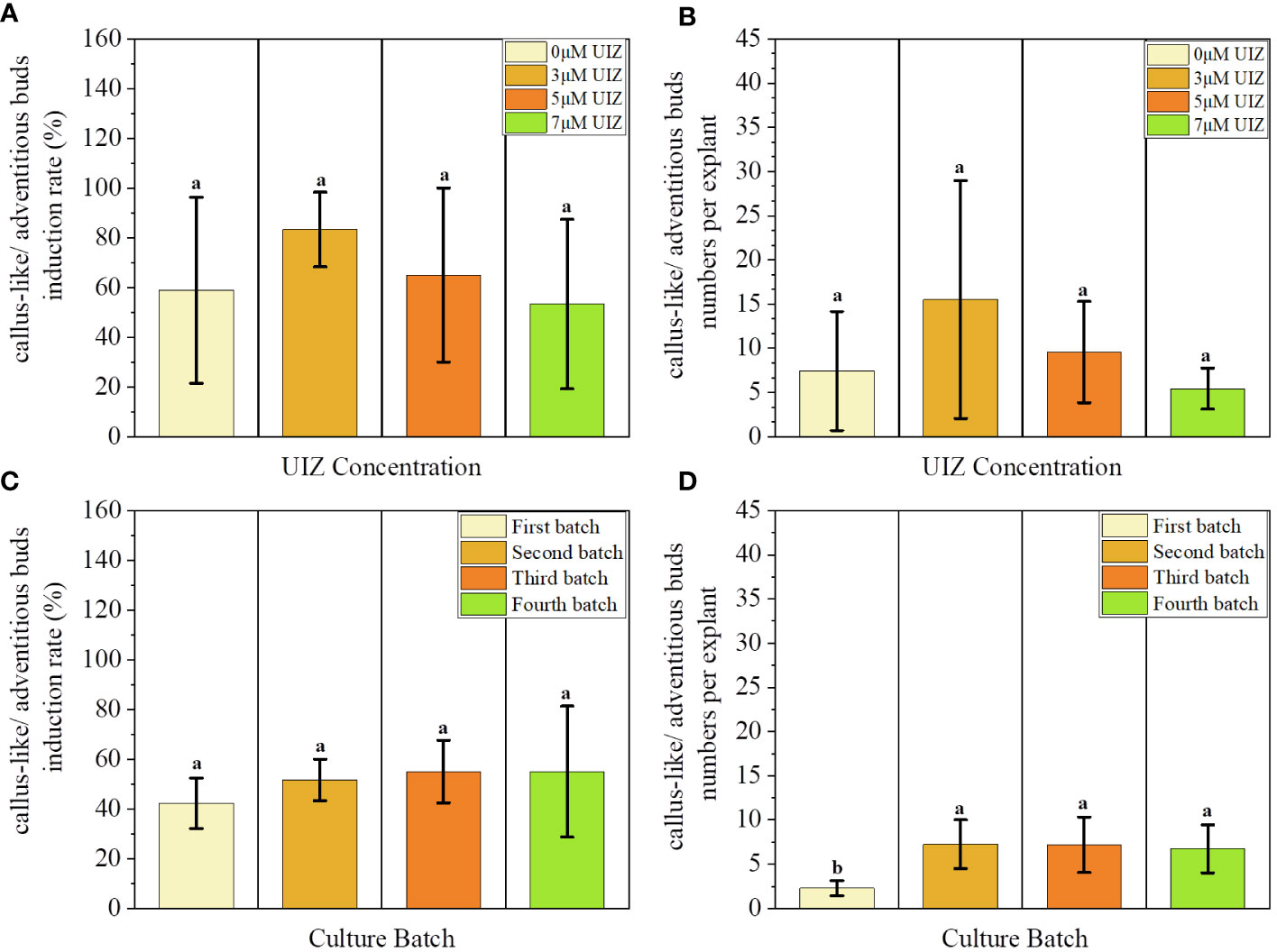
Figure 6 Effects of UIZ concentration and culture batch on callus-like/adventitious buds induction rate and callus-like/adventitious buds numbers per explant of S. fusiforme. (A) callus-like/adventitious buds induction rate of different UIZ concentration treatments; (B) callus-like/adventitious buds numbers per explant of different UIZ concentration treatments; (C) callus-like/adventitious buds induction rate of different batches of culture; (D) callus-like/adventitious buds numbers per explant of different batches of culture. Values are expressed as mean ± standard deviation (n = 3). Different letters indicate significant differences between different culture conditions (p< 0.05).
Changes in the length of S. fusiforme were significantly affected by different concentrations of UIZ (Figure 7). The greatest effect was observed at 3 μM UIZ (p< 0.001, Figure 7B). The length of regenerated juveniles varied among the different explant sites of S. fusiforme. Additionally, changes in S. fusiforme’s filamentous holdfasts were greater than those in the stem tips (p< 0.05). The 3 μM UIZ treated filamentous holdfasts provided lengths of up to 3.43 ± 0.39 cm (p< 0.05).
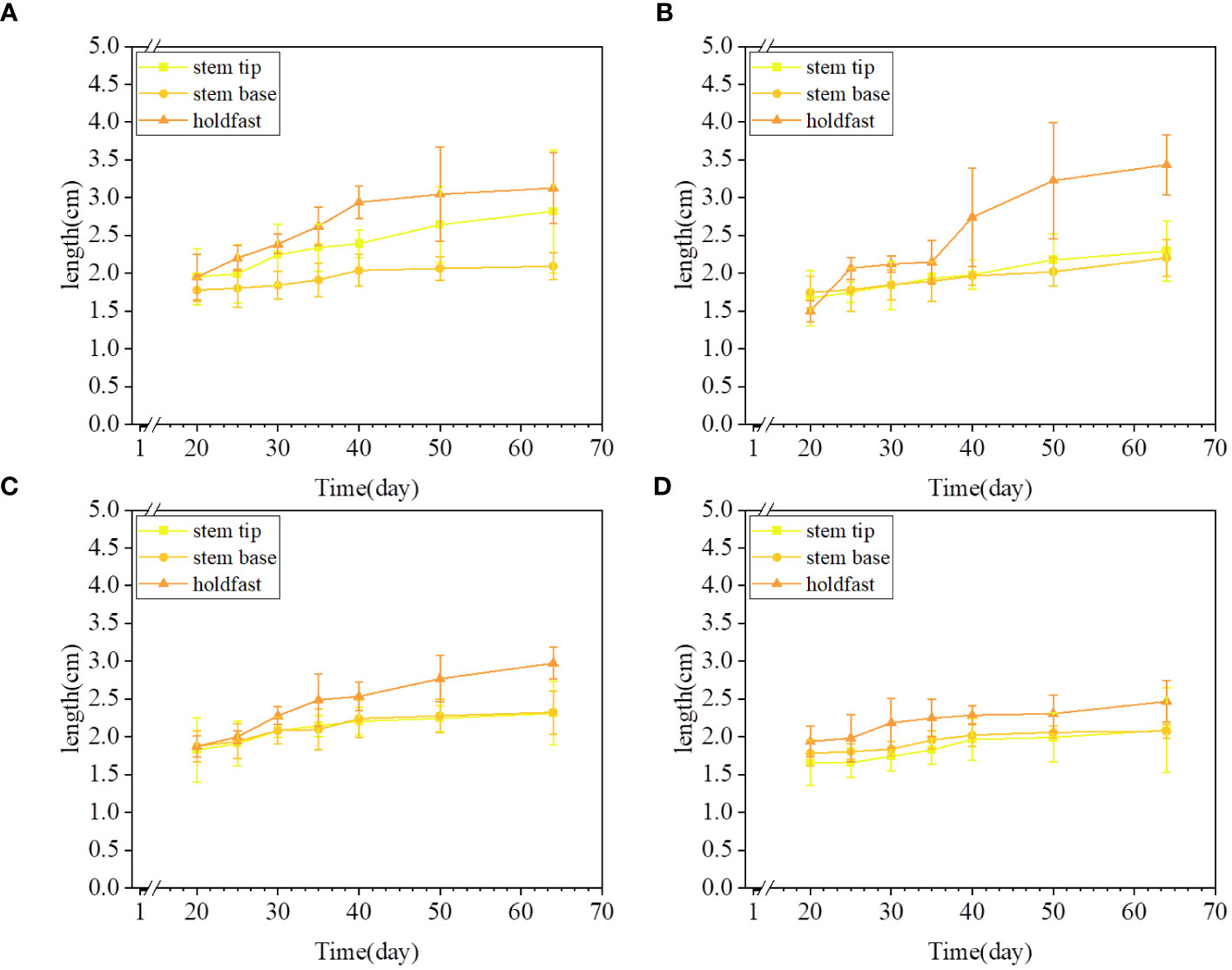
Figure 7 Variation in culture length of S. fusiforme treated with different concentrations of UIZ. (A) length change for 0 μM UIZ treatment; (B) length change for 3 μM UIZ treatment; (C) length change for 5 μM UIZ treatment; (D) length change for 7 μM UIZ treatment. Values are expressed as mean ± standard deviation (n = 3). Different letters indicate significant differences between different culture conditions (p< 0.05).
We observed significant differences in the RGR of S. fusiforme based on variations in the culture site, UIZ concentration, and transfer medium (p< 0.05, Figure 8). A longer induction time in solid culture resulted in a higher RGR. However, it is necessary to culture the sample in liquid medium for 17days to achieve proliferative culture (Figure 8). Significant variations in the RGR were observed among different parts of S. fusiforme explants. Filamentous holdfasts exhibited the highest RGR of approximately 3.05% when cultured in 3μM UIZ (p< 0.05). The RGR trend in batch 4 of cultured S. fusiforme explants differed from that of the first three batches because the last batch had a long induction time in solid medium and insufficient time for the proliferation of regenerating buds in liquid medium (Figure 9).
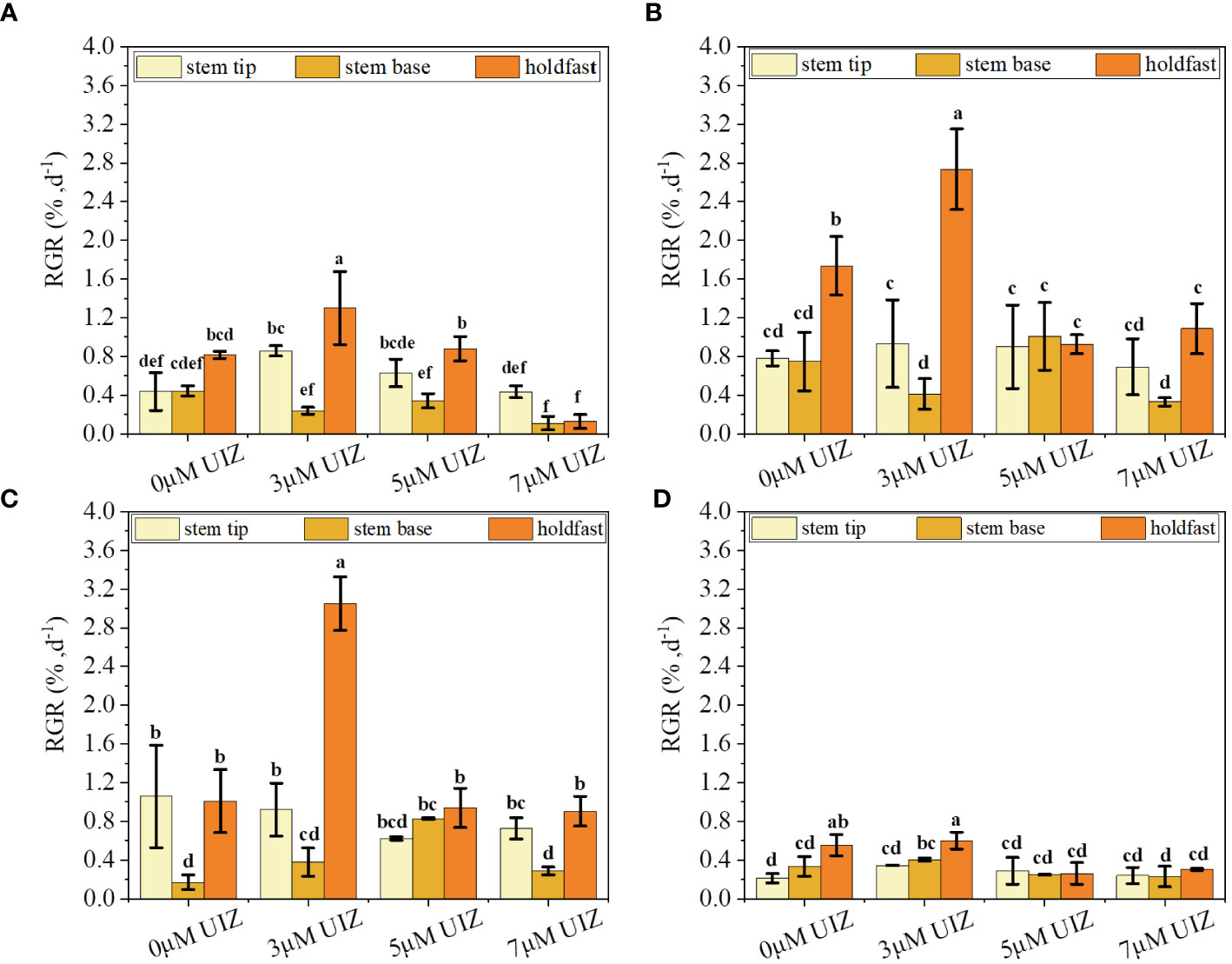
Figure 8 Effect of UIZ concentration and S. fusiforme parts on RGR of regenerated juveniles under batch culture stage. (A) RGR of the first batch of culture; (B) RGR of the second batch of culture; (C) RGR of the third batch of culture; (D) RGR of the fourth batch of culture. values are expressed as mean ± standard deviation (n = 3). Different letters indicate significant differences between different culture conditions (p< 0.05).
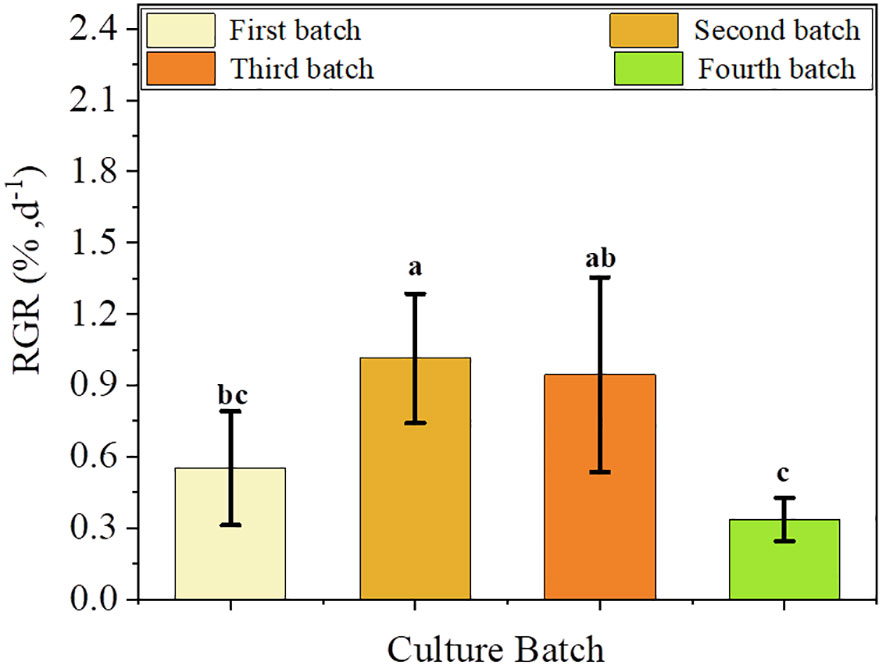
Figure 9 Effect of batch culture on changes in RGR of regenerated juveniles of S. fusiforme. Values are expressed as mean ± standard deviation (n = 3). Different letters indicate significant differences between different culture conditions (p< 0.05).
Different batches, explant locations, and UIZ concentrations played influenced the Fv/Fm, rETRm and fast light curves of S. fusiforme juveniles. The effect of the S. fusiforme explant site and UIZ concentration in culture batches 1 and 2 did not significantly affect Fv/Fm (P > 0.05, Figure 10A), whereas the effect on rETRm was significant up to a maximum of 39.82 ± 3.86 (P< 0.05, Figures 10D, E). The Fv/Fm, rETRm and rapid light curve disparity of S. fusiforme varied significantly in batch 4 according to the explant location and UIZ concentration, with the lowest Fv/Fm was 0.356 ± 0.04 and lowest rETRm was 8.98 ± 0.79 at the stem tip of S. fusiforme in the presence of UIZ compared to in the absence of UIZ (P< 0.05, Figure 10).
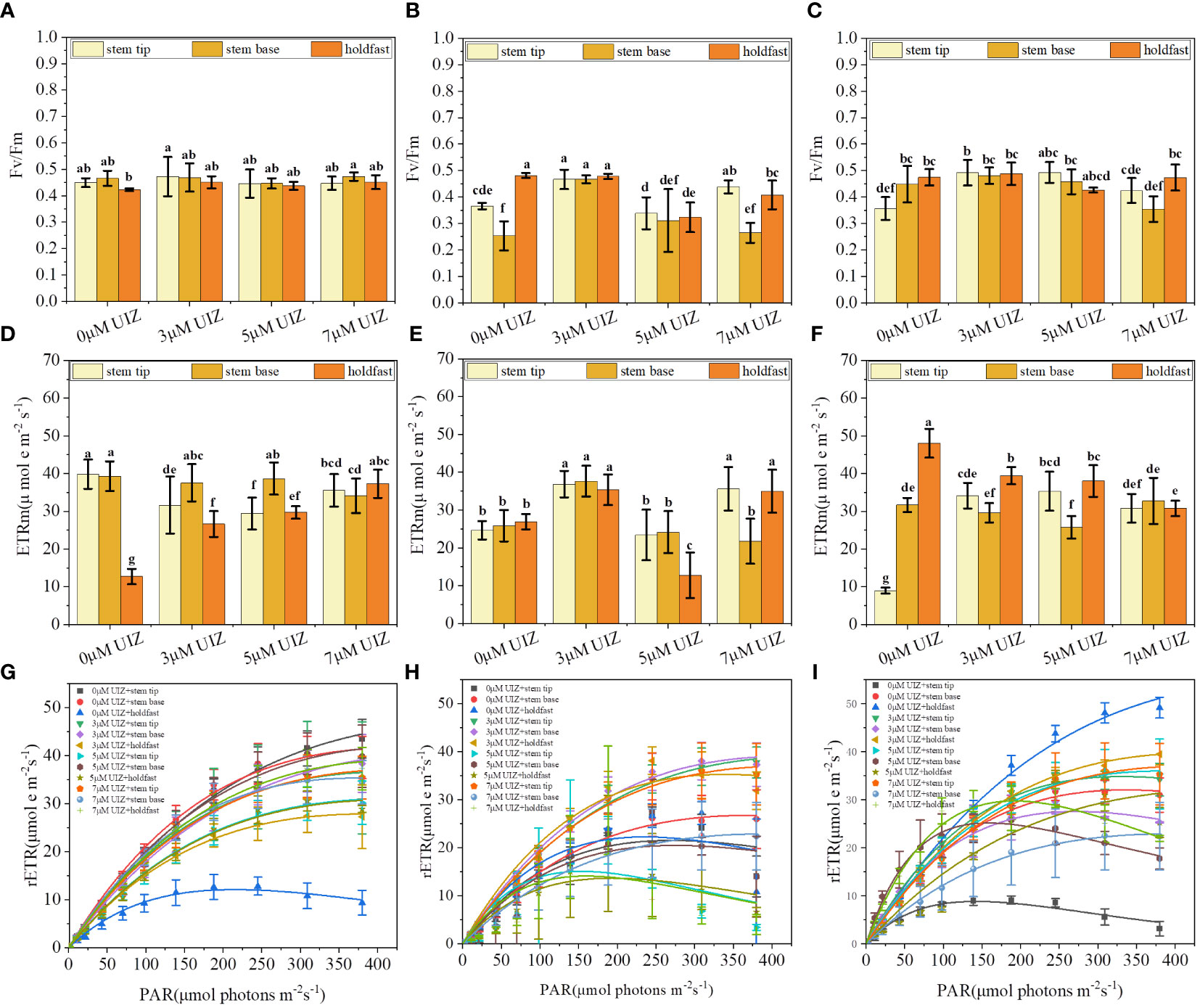
Figure 10 Effects of S. fusiforme explant parts and UIZ concentration on maximum quantum yield (Fv/Fm) and maximum electron transfer rate (rETRm) of PSII in batch culture. (A–C) Fv/Fm of batch 1, 2, and 4 cultures in batch culture; (D–F) rETRm of batch 1, 2, and 4 cultures in batch culture; (G–I) RLC of batch 1, 2, and 4 cultures in batch culture. Values are expressed as mean ± standard deviation (n = 3). Different letters indicate significant differences between different culture conditions (p< 0.05).
During the culture process, the concentration of UIZ significantly affected the SC and SP, which increased with increasing UIZ concentrations, The SC contents in the 3, 5 and 7 μM UIZ treatment groups were 105%, 115% and 163% of the control, respectively, with the highest SC content observed in culture containing 7 μM UIZ (P< 0.05, Figure 11A). The SP contents in the 3, 5, and 7 μM UIZ groups were 124%, 179%, and 276.2% of the control, respectively. The SP content was significantly affected by 5 and 7 μM UIZ treatment (P< 0.05, Figure 11B). As the concentration of UIZ was increased, the mannitol content was 59%, 65%, and 70% of the control. Exogenously added UIZ significantly reduced the mannitol content compared to that in the control group. However, there were no significant differences among the samples treated with different concentrations of UIZ. The most significant change in the mannitol content of S. fusiforme tissue culture-derived juveniles was observed under the treatment with 3 μM UIZ (P< 0.05, Figure 11C).
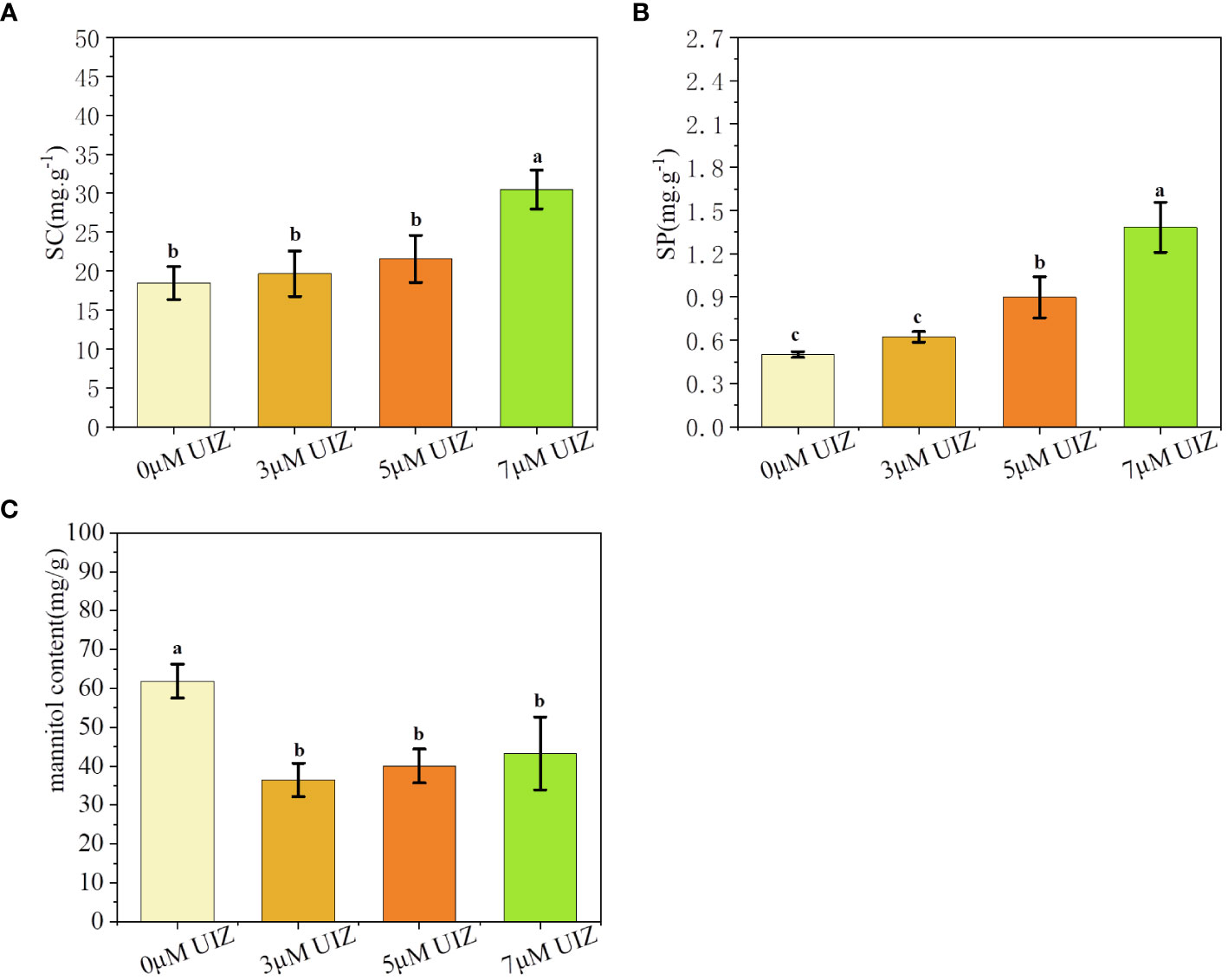
Figure 11 Effects of UIZ concentration on the content of soluble carbohydrates, soluble proteins, and mannitol in regenerated juveniles of S. fusiforme. (A) soluble carbohydrate content; (B) soluble protein content; (C) mannitol content. Values are expressed as mean ± standard deviation (n = 4). Different letters indicate significant differences between different culture conditions (p< 0.05).
We observed that the callus-like/adventitious bud induction rate and number of bud per explant varied widely among explants from different parts of S. fusiforme after tissue culture. The holdfasts and stems of S. fusiforme can serve as primary materials for tissue culture (Luo et al., 2023). Different parts of the stems were cultivated in groups, with adventitious buds more easily induced near the upper stem tip than near the stem base, possibly because S. fusiforme is polarized, similar to other macroalgae. Moreover, the holdfast structures of S. fusiforme contain a higher proportion of fibrous components in their cell walls, making them more resilient to high temperatures and nutrient-poor conditions (Endo et al., 2021). Finally, the regenerative capacity of the tissues may be related to their differentiation capacity. Tissues with low differentiation capacity often exhibit a higher regenerative capacity. Recovery from plant trauma may be influenced by key signaling factors involved in this process (Kavale et al., 2021; Liang et al., 2023). Thus, we showed that S. fusiforme filamentous holdfasts are the optimal material for asexual propagation and that stem tips have a greater regenerative capacity than do previously grown stem bases.
The regenerating tissues of S. fusiforme can form bumps in the epidermal, cortical, and medullary tissue zones. The regeneration process occurs mainly in the outer epidermal pigmentation zone, leading to the growth of adventitious buds and callus-like tissues. Most S. fusiforme cell appeared as round or oval in the transverse view, and most cells of the nascent buds were smaller than those of the mature filamentous holdfasts; the medullary tissue area of mature tissues was located in the center, whereas the medullary tissue area of nascent buds regenerated and grew from the side. This may be because of the greater totipotency of plant cells that are more pigmented compared with that of cells that are less pigmented (Kumar et al., 2006; Lin et al., 2021). In addition, after trauma caused by segmentation, trauma-induced wound regeneration may occur when reactive oxygen species induce cellular reconstruction by triggering transduction of wound signals and regulating cell cycle proteins and related genes, leading to the spreading and division of cells inward from the edge of the wound, and finally inducing tissue regeneration (Guan et al., 2022).
We utilized staged culture to induce changes in temperature and culture condition in accordance with different time periods during algae growth state. For instance, S. fusiforme was first cultured in nutrient-rich solid medium at low temperature and light intensity to facilitate better germination and generation of adventitious buds. The explants were then transferred to liquid medium to provide sufficient light, space, and nutrient salts for rapid proliferation. During algal growth, the density of the culture and its light intensity influence subsequent growth, photosynthesis, and substance accumulation (Chen et al., 2019; Cao et al., 2022). Kavale et al. (2023) induced bud germination using staged temperature and light controls. Most studies have been limited to laboratory culture conditions or single investigations of large-scale valve cultures, without combining the two factors (Obando et al., 2022; Tirtawijaya et al., 2022). Culturing pre- and post-seedlings at different stages may enhance the efficiency and effectiveness of continuous algal culture.
PES medium was used as the basal medium with regulated components throughout the cultivation process. Solid medium was used to induce the formation of callus-like tissue/adventitious buds, and liquid medium was used to ensure rapid proliferation. The optimal conditions for culture of S. fusiforme explants callus-like/adventitious buds led to an induction rate of up to 100%, callus-like/adventitious bud numbers per explant of 27.43 ± 4.58, and RGR of up to 3.05 ± 0.28. Thus, the concentration of the medium is critical. A nutrient concentration that is too high can lead to the formation of micro-organisms, weed algae, and other unwanted growth factors that compete with the desired algae. However, a nutrient concentration that is too low is not sufficient to support the daily growth and development of algae. Therefore, it is important to adjust the nutrient composition during the cultivation process to meet the needs of algae and promote optimal growth (Sampath-Wiley et al., 2008; Xu et al., 2022b). Previous studies showed that solid medium is more effective in inducing callus tissue formation, whereas liquid PES culture did not result in callus tissue formation (Li et al., 2015).
Endogenous plant hormones undergo dynamic regulation throughout the algal growth stages in response to various stimuli (Pang et al., 2023). External plant growth regulators are essential for achieving the desired effect; however, using regulator concentrations that are too high can be toxic to the plant and affect its future development, whereas concentrations that are too low will not produce the desired effect. UIZ is a plant growth retardant that is commonly used to preserve plant growth (Zhang et al., 2020; Lv et al., 2022). We demonstrated that appropriate concentrations of UIZ were beneficial for determining number of callus-like/adventitious buds per explant and induction rate of callus-like/adventitious buds. The highest induction rate was observed at lower concentrations in the short term. Low concentrations of UIZ had little retardation effect, allowing for rapid germination and sprouting. However, as the culture time increased, the plants adapted to a high concentration of UIZ, resulting in the sprouting and emergence of buds. High concentrations of foreign stimulants can delay or inhibit growth but may induce bud the formation (Carmo et al., 2020). The results demonstrate that explants treated with UIZ produced a significantly higher number of buds than did untreated seaweed. Uji et al. (2015) reported that UIZ treatment promoted germination, likely by regulating healing tissue proliferation and bud differentiation through modulation of endogenous auxin and abscisic acid levels. We found that the most suitable site for tissue culture was the filamentous holdfast, followed by the stem tip and stem base. The number of callus-like buds per explant greatly increased in the batch stage of culture, indicating that solid medium is favorable for inducing the germination of adventitious/adventitious buds and that liquid medium is favorable for the regeneration and proliferation of buds. Callus-like tissues can be induced when the stem tip is transferred to liquid culture conditions for a sufficient number of explants. Therefore, switching to liquid-culture conditions is recommended.
Furthermore, UIZ treatment impacted photosynthesis. Specifically, 3 μM UIZ had a significant effect on rETRm, and varying concentrations of UIZ resulted in an increase or decrease in its relative electron transfer rate. This may be because the UIZ regulates photosynthesis in S. fusiforme similarly to its effects in soybean and wheat. Excessive reactive oxygen species can be inhibited by controlling relevant photosynthetic genes, regulating photosynthetic antenna proteins, and enhancing the antioxidant capacity, ultimately leading to increased photosynthesis (Jiang et al., 2021; Hu et al., 2022; Yu et al., 2022).
Our results revealed that the concentration of UIZ was positively correlated with the SC and SP contents of S. fusiforme. These effects may be attributed to the ability of UIZ to enhance the antioxidant system and reduce mitochondrial damage, resulting in increased contents of SC and SP (Keshavarz and Khodabin, 2019; Zhou et al., 2021). The mannitol content in the UIZ-treated group was significantly lower than that in the control group. This effect decreased with increasing of UIZ concentrations, possibly because the low concentration of UIZ promoted the production of more adventitious buds, which consumed mannitol stored inside the alga. In contrast, high concentrations of UIZ had a growth slowing effect, which inhibited some regenerating buds. As a result, the mannitol content in the body was higher than that in the low concentration UIZ group.
Filamentous holdfasts of S. fusiforme can be used as primary sites for tissue culture. The stem tip, stem base and filamentous holdfasts of S. fusiforme were cultured in solid PES medium with 60 μmol photons·m-2 ·s-1 light at 19°C, salinity is 27–28‰, L:D = 12 h:12 h, and 3 μM UIZ added for at least 17 days of induction culture before culture in liquid PES medium with 90 μmol photons·m-2 ·s-1 light. This method can be used to regenerate S. fusiforme juveniles from tissue culture. Tissue cultures of the stem tip of S. fusiforme produce fine, dense air vesicles and secondary branches. Finally, UIZ significantly increased the number of callus-like/adventitious buds per explant of regenerated juveniles of S. fusiforme, and photosynthesis, SC, and SP. The results showed that this culture method was economical, easy to operate, and did not cause any damage to the ecosystem. Thus, the method can be used for tissue culture and germplasm expansion of S. fusiforme in industry.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
LG: Writing – original draft, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation. GP: Writing – original draft, Methodology, Investigation. LL: Writing – original draft, Methodology, Investigation. CG: Writing – review & editing, Methodology, Investigation. BC: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. ZM: Writing – review & editing, Supervision, Methodology.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Wenzhou Science and Technology Plan Project (No. N20220005), the National Key R&D Program of China (No. 2018YFD0901503), the Wenzhou Science and Technology Association project (No. kjfw0196), and the National Natural Science Foundation of China (No. 41706147 and 41876124).
The authors also would like to appreciate all staffs for their helpful assistance during the materials collection. In addition, we are very grateful to Prof. Mingjiang Wu of Wenzhou University, who had given us great help in the collection of materials, the provision of experimental projects and funding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aghaei F., Seyed Sharifi R., Narimani H. S. (2021). Effects of uniconazole and biofertilizers application on yield and some biochemical characteristics of wheat under soil salinity stress. Environ. Stresses. Crop Sci. 14, 487–499. doi: 10.22077/escs.2020.2810.1730
Akremi N., Cappoen D., Anthonissen R., Verschaeve L., Bouraoui A. (2017). Phytochemical and in vitro antimicrobial and genotoxic activity in the brown algae Dictyopteris membranacea. S. Afr. J. Bot. 108, 308–314. doi: 10.1016/j.sajb.2016.08.009
Ali O., Ramsubhag A., Jayaraman J. (2021). Biostimulant properties of seaweed extracts in plants: implications towards sustainable crop production. Plants (Basel) 10, 531. doi: 10.3390/plants10030531
Arbaiza S., Avila-Peltroche J., Castaneda-Franco M., Mires-Reyes A., Advincula O., Baltazar P. (2023). Vegetative Propagation of the Commercial Red Seaweed Chondracanthus chamissoi in Peru by Secondary Attachment Disc during Indoor Cultivation. Plants (Basel) 12, 1940. doi: 10.3390/plants12101940
Avila-Peltroche J., Won B. Y., Cho T. O. (2022). An improved protocol for protoplast production, culture, and whole plant regeneration of the commercial brown seaweed Undaria pinnatifida. Algal Res. 67, 102851. doi: 10.1016/j.algal.2022.102851
Baweja P., Sahoo D., Garco,/j.algal P., Robaina R. R. (2009). Review: Seaweed tissue culture as applied to biotechnology: Problems, achievements and prospects. Phycol Res. 57, 45–58. doi: 10.1111/j.1440-1835.2008.00520.x
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Cao C., Zhang T., Wu M., Chen B., Ma Z. (2022). Differential growth and physiological responses of Sargassum fusiforme and epiphytic Ulva lactuca to culture densities and interspecific competition. Reg. Stud. Mar. Sci. 56, 102671. doi: 10.1016/j.rsma.2022.102671
Carmo L. P., Moura C. W. N., Lima-Brito A. (2020). Red macroalgae extracts affect in vitro growth and bud formation in Comanthera mucugensis (Giul.) L. R. Parra & Giul., an endemic dry flower species from the Chapada Diamantina (Brazil). S. Afr. J. Bot. 135, 29–34. doi: 10.1016/j.sajb.2020.07.033
Chen B., Zou D. (2014). Growth and photosynthetic activity of Sargassum henslowianum(Fucales, Phaeophyta) seedlings in responses to different light intensities, temperatures and CO2 levels under laboratory conditions. Mar. Biol. Res. 10, 1019–1026. doi: 10.1080/17451000.2013.872798
Chen B., Zou D., Ma Z., Yu P., Wu M. (2019). Effects of light intensity on the photosynthetic responses of Sargassum fusiforme seedlings to future CO2 rising. Aquac Res. 50, 116–125. doi: 10.1111/are.13873
Chen M., Zhang W., Wu H., Guang C., Mu W. (2020). Mannitol: physiological functionalities, determination methods, biotechnological production, and applications. Appl. Microbiol. Biotechnol. 104, 6941–6951. doi: 10.1007/s00253-020-10757-y
Collins N., Kumar Mediboyina M., Cerca M., Vance C., Murphy F. (2022). Economic and environmental sustainability analysis of seaweed farming: Monetizing carbon offsets of a brown algae cultivation system in Ireland. Bioresour Technol. 346, 126637. doi: 10.1016/j.biortech.2021.126637
Custodio L., Charles G., Magne C., Barba-Espin G., Piqueras A., Hernandez J. A., et al. (2022). Application of in vitro plant tissue culture techniques to halophyte species: A review. Plants (Basel) 12, 126. doi: 10.3390/plants12010126
de Araújo Amatuzzi J. C., do Nascimento Vieira L., Sant’Anna-Santos B. F., Noseda M. D., Pacheco de Freitas Fraga H. (2020). Improved in vitro development of Epidendrum secundum (Orchidaceae) by using aqueous extract of the seaweed Kappaphycus alvarezii (Rhodophyta, Solieriaceae). Acta Physiol. Plant 42, 1–9. doi: 10.1007/s11738-020-03129-6
Eggertsen M., Halling C. (2021). Knowledge gaps and management recommendations for future paths of sustainable seaweed farming in the Western Indian Ocean. Ambio 50, 60–73. doi: 10.1007/s13280-020-01319-7
Endo H., Sugie T., Yonemori Y., Nishikido Y., Moriyama H., Ito R., et al. (2021). Vegetative reproduction is more advantageous than sexual reproduction in a canopy-forming clonal macroalga under ocean warming accompanied by oligotrophication and intensive herbivory. Plants (Basel) 10, 1522. doi: 10.3390/plants10081522
Ershadi A., Karimi R., Mahdei K. N. (2015). Freezing tolerance and its relationship with soluble carbohydrates, proline and water content in 12 grapevine cultivars. Acta Physiol. Plant 38, 1–10. doi: 10.1007/s11738-015-2021-6
Garcia-Poza S., Leandro A., Cotas C., Cotas J., Marques J. C., Pereira L., et al. (2020). The evolution road of seaweed aquaculture: cultivation technologies and the industry 4.0. Int. J. Environ. Res. Public Health 17, 6528. doi: 10.3390/ijerph17186528
Guan X., Mao Y., Stiller J. W., Shu S., Pang Y., Qu W., et al. (2022). Comparative gene expression and physiological analyses reveal molecular mechanisms in wound-induced spore formation in the edible seaweed nori. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.840439
Hu H., Feng N., Shen X., Zhao L., Zheng D. (2022). Transcriptomic analysis of Vigna radiata in response to chilling stress and uniconazole application. BMC Genomics 23, 205. doi: 10.1186/s12864-022-08443-6
Hwang E. K., Choi H. G., Kim J. K. J. B. M. (2020). Seaweed resources of korea. Bot. Mar. 63, 395–405. doi: 10.1515/bot-2020-0007/html
Jiang Y., Sun Y., Zheng D., Han C., Cao K., Xu L., et al. (2021). Physiological and transcriptome analyses for assessing the effects of exogenous uniconazole on drought tolerance in hemp (Cannabis sativa L.). Sci. Rep. 11, 14476. doi: 10.1038/s41598-021-93820-6
Jiksing C., Ongkudon M. M., Thien V. Y., Rodrigues K. F., Yong W. T. L. (2022). Recent advances in seaweed seedling production: a review of eucheumatoids and other valuable seaweeds. Algae 37, 105–121. doi: 10.4490/algae.2022.37.5.11
Kavale M. G., Alexander H. J., Malarvizhi J., Manivannan M., Ram S. (2021). Short note: Preliminary observations on the propagule production of Sargassum polycystum C. Agardh from stoloniferous branches. Aquaculture 534, 736322. doi: 10.1016/j.aquaculture.2020.736322
Kavale M. G., Largo D. B., de la Torre E. O., Baritugo A. T., Azcuna-Montaño M. (2023). Plantlets directly developed from secondary phyllodes of Sargassum siliquosum J. Agardh: Implication for seedling production during the off-reproductive season. Aquaculture 563, 738977. doi: 10.1016/j.aquaculture.2022.738977
Kelly E. L. A., Cannon A. L., Smith J. E. (2020). Environmental impacts and implications of tropical carrageenophyte seaweed farming. Conserv. Biol. 34, 326–337. doi: 10.1111/cobi.13462
Kerrison P. D., Le H. N., Hughes A. D. (2015). Hatchery decontamination of Sargassum muticum juveniles and adults using a combination of sodium hypochlorite and potassium iodide. J. Appl. Phycol 28, 1169–1180. doi: 10.1007/s10811-015-0672-8
Keshavarz H., Khodabin G. (2019). The role of uniconazole in improving physiological and biochemical attributes of bean (Phaseolus vulgaris L.) subjected to drought stress. J. Crop Sci. Biotechnol. 22, 161–168. doi: 10.1007/s12892-019-0050-0
Kumar G. R., Reddy C. R. K., Jha B. (2006). Callus induction and thallus regeneration from callus of phycocolloid yielding seaweeds from the Indian coast. J. Appl. Phycol 19, 15–25. doi: 10.1007/s10811-006-9104-0
Largo D. B., Rance G. M. S., Diola A. G., Aaron-Amper J. (2020). Method for the mass production of seedlings of the tropical brown seaweed Sargassum (Phaeophyceae, Ochrophyta). MethodsX 7, 100854. doi: 10.1016/j.mex.2020.100854
Li H., Liu J., pang T. (2015). Callus induction and morphogenesis of callus in Kappaphycus alvarezii (Rhodophyta, Solieriaceae). Haiyang Xuebao 37, 52–61. doi: 10.3969/j.issn.0253-4193.2015.04.005
Liang Y., Heyman J., Lu R., De Veylder L. (2023). Evolution of wound-activated regeneration pathways in the plant kingdom. Eur. J. Cell. Biol. 102, 151291. doi: 10.1016/j.ejcb.2023.151291
Lin L., Ma Z., Chen B., Wu M. (2021). Analysis of physiological and ecological functions to mature sporophyte of cultivation Sargassun fusiforme based on its organ morphilogical structure. Oceanol. Limnol. Sin. 52, 1047–1057. doi: 10.11693/hyhz20210100008
Luo L., Zuo X., Guo L., Pang G., Ma Z., Wu M., et al. (2023). Effects of exogenous hormones on the regeneration of juveniles from Sargassum fusiforme holdfasts. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.1072391
Lv R., Zhang W., Xie X., Wang Q., Gao K., Zeng Y., et al. (2022). Foliar application uniconazole enhanced lodging resistance of high-quality indica rice (Oryza sativa L.) by altering anatomical traits, cell structure and endogenous hormones. Field Crop Res. 277, 108425. doi: 10.1016/j.fcr.2021.108425
Mantri V. A., Veeragurunthan V., Sambhwani K., Anisoddin Kazi M. (2022). Concise review of industrially important red seaweed Gracilaria dura (C. Agardh) J. Agardh. J. Appl. Phycol 34, 1825–1841. doi: 10.1007/s10811-022-02755-6
Muhamad S. N. S., Ling A. P.-K., Wong C.-L. (2018). Effect of plant growth regulators on direct regeneration and callus induction from Sargassum polycystum C. Agardh. J. Appl. Phycol 30, 3299–3310. doi: 10.1007/s10811-018-1649-1
Obando J. M. C., dos Santos T. C., Martins R. C. C., Teixeira V. L., Barbarino E., Cavalcanti D. N. (2022). Current and promising applications of seaweed culture in laboratory conditions. Aquaculture 560, 738596. doi: 10.1016/j.aquaculture.2022.738596
Pang G., Luo L., Guo L., Gao C., Sheng X., Ma Z., et al. (2023). Temporal and spatial changes of major endogenous phytohormones during the regeneration of juveniles from Sargassum fusiforme holdfasts. J. Appl. Phycol. 35, 2995–3006. doi: 10.1007/s10811-023-03113-w
Platt T., Gallegos C., Harrison W. G. (1980). Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 38, 687–701. doi: 10.1093/pasj/57.2.341
Rademacher W. (2015). Plant growth regulators: backgrounds and uses in plant production. J. Plant Growth Regul. 34, 845–872. doi: 10.1007/s00344-015-9541-6
Sampath-Wiley P., Neefus C. D., Jahnke L. S. (2008). Seasonal effects of sun exposure and emersion on intertidal seaweed physiology: Fluctuations in antioxidant contents, photosynthetic pigments and photosynthetic efficiency in the red alga Porphyra umbilicalis Kützing (Rhodophyta, Bangiales). J. Exp. Mar. Biol. Ecol. 361, 83–91. doi: 10.1016/j.jembe.2008.05.001
Tian S., Chen B., Wu M., Cao C., Gu Z., Zheng T., et al. (2023). Are there environmental benefits derived from coastal aquaculture of Sargassum fusiforme? Aquaculture 563, 738909. doi: 10.1016/j.aquaculture.2022.738909
Tirtawijaya G., Negara B. F. S. P., Lee J.-H., Cho M.-G., Kim H. K., Choi Y.-S., et al. (2022). The influence of abiotic factors on the induction of seaweed callus. J. Mar. Sci. Eng. 10, 513. doi: 10.3390/jmse10040513
Uji T., Nanaumi D., Kawagoe C., Saga N., Miyashita K. (2015). Factors influencing the induction of adventitious bud and callus in the brown alga Sargassum horneri (Turner) C. Agardh. J. Appl. Phycol 28, 2435–2443. doi: 10.1007/s10811-015-0745-8
White L. N., White W. L. (2020). Seaweed utilisation in New Zealand. Bot. Mar. 63, 303–313. doi: 10.1515/bot-2019-0089
Xu L., Lin L., Luo L., Zuo X., Cao C., Jin X., et al. (2022a). Organic acid treatment for removal of epiphytic Ulva L. attached to Sargassum fusiforme seedlings. Aquaculture 547, 737533. doi: 10.1016/j.aquaculture.2021.737533
Xu L., Luo L., Zuo X., Cao C., Lin L., Zheng H., et al. (2022b). Effects of temperature and irradiance on the regeneration of juveniles from the holdfasts of Sargassum fusiforme, a commercial seaweed. Aquaculture 557, 738317. doi: 10.1016/j.aquaculture.2022.738317
Yan K., Du X., Mao B. (2022). Production of virus-free chrysanthemum (Chrysanthemum morifolium ramat) by tissue culture techniques. Methods Mol. Biol. 2400, 171–186. doi: 10.1007/978-1-0716-1835-6_17
Yong Y. S., Yong W. T. L., Thien V. Y., Ng S. E., Anton A., Yassir S. (2014). Acclimatization of micropropagated Kappaphycus alvarezii (Doty) Doty ex Silva (Rhodophyta, Solieriaceae) in outdoor nursery system. J. Appl. Phycol 27, 413–419. doi: 10.1007/s10811-014-0289-3
Yu M., Huang L., Feng N., Zheng D., Zhao J. (2022). Exogenous uniconazole enhances tolerance to chilling stress in mung beans (Vigna radiata L.) through cross talk among photosynthesis, antioxidant system, sucrose metabolism, and hormones. J. Plant Physiol. 276, 153772. doi: 10.1016/j.jplph.2022.153772
Zhang M., Yang J., Pan H., Pearson B. J. (2020). Dwarfing effects of chlormequat chloride and uniconazole on potted baby primrose. HortTechnology 30, 536–543. doi: 10.21273/HORTTECH04646-20
Keywords: Sargassum fusiforme, tissue culture, seaweed, hormone, seedling propagation
Citation: Guo L, Pang G, Luo L, Gao C, Chen B and Ma Z (2024) Asexual proliferative seedling technology for Sargassum fusiforme constructed using tissue culture method. Front. Mar. Sci. 11:1363703. doi: 10.3389/fmars.2024.1363703
Received: 31 December 2023; Accepted: 09 April 2024;
Published: 01 May 2024.
Edited by:
Yngvar Olsen, NTNU, NorwayReviewed by:
Leonel Pereira, University of Coimbra, PortugalCopyright © 2024 Guo, Pang, Luo, Gao, Chen and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binbin Chen, YmluYmNoQDE2My5jb20=; Zengling Ma, bWF6ZW5nbGluZ0B3enUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.