- 1North Carolina Aquariums, NCSU CMAST, Morehead, NC, United States
- 2Coastal Carolina University, Department of Marine Science, Douglas Hall, Conway, SC, United States
- 3Blue Elements Imaging & Exploration, Fredericksburg, VA, United States
- 4Wild Me, Portland, OR, United States
Sand tigers are frequently observed at shipwrecks and other artificial reef habitats off North Carolina (USA), but data about occupancy, movement ecology, and site fidelity are lacking. Spot A Shark USA researchers used Wildbook© photoidentification software to spot map sand tigers in images provided by recreational SCUBA divers, or collected from remotely operated vehicles, and an offshore live-streaming camera. We uniquely identified 1837 sand tigers, 101 of which were resighted on more than one date between 2005–2021. Sand tigers of both sexes and various ages were found year-round along the northern, central and southern coast. We identified shipwrecks or artificial reef sites with consistently high numbers of shark encounters reported, sometimes with seasonal occupancy patterns. Resighted sharks were often encountered at the same or nearby locations, confirming high levels of residency and site fidelity to specific locations. Together, the mating scars seen on 121 female sand tigers and 202 females documented with rotund abdomens consistent with pregnancy highlight the importance of NC waters for reproduction. We also quantified other characteristics of the sand tigers visible in the photographs including wounds, parasitic copepods, and attached fishing gear. Our results reflect the importance of habitats off the NC coast to the movement and reproductive ecology of sand tigers at multiple life history stages. Sand tiger populations have declined in the Northwest Atlantic, so information about residence at specific locations, seasonal patterns of occupancy, and sex-dependent behaviors associated with migration and reproduction are important to future management and conservation of the species.
1 Introduction
The sand tiger Carcharias taurus Rafinesque, 1810, is a large shark found in the neritic zone in the Northwest Atlantic (NWA) from the Gulf of Maine to Florida and the northern Gulf of Mexico (Campagno, 1984; Gilmore, 1993). Though previously described in the United States (US) by the National Oceanic and Atmospheric Administration, National Marine Fisheries Service (NOAA, NMFS; Carlson et al., 2009) as a species of concern, this designation is not a legal classification under the Endangered Species Act and is no longer in use. However, since 2000 NMFS has prohibited the take of sand tiger sharks (NOAA and NMFS, 2000). Life history characteristics, including slow growth, delayed maturity, and low productivity, make sand tigers susceptible to population decline (Goldman et al., 2006; Ha, 2006; Rigby et al., 2021) and ongoing mortality as bycatch and in targeted catch-and-release fisheries in the US (Kneebone et al., 2013; Kilfoil et al., 2017) and elsewhere within the global range (Dicken et al., 2006; Lucifora et al., 2009) contributes to their continued vulnerability (Carlson et al., 2009). Globally, the species is designated as Critically Endangered on the IUCN Red List (Rigby et al., 2021). In parts of its range, including the southwest Atlantic and Mediterranean, populations decreased by over 80% in the last century and few or no individuals are now encountered there. In east Australia and South Africa, populations with similar declines may be recovering following management measures to reduce fishing mortality. The NWA population is estimated to have declined 30–49% (Musick et al., 1993; Ha, 2006; Rigby et al., 2021). Management strategies in the US to reverse this decline include prohibition on harvest and designation of Essential Fish Habitat (EFH) and Habitat Areas of Particular Concern (HAPC) in portions of its range (NMFS, 2009, 2017).
Sand tigers make long seasonal migrations correlated with their reproductive cycles (Gilmore, 1993; Lucifora et al., 2002; Dicken et al., 2007; Teter et al., 2015; Haulsee et al., 2018; Dwyer et al., 2023) and often display high site fidelity (Olbers and Smith, 2019; Paxton et al., 2019; Marens, 2021; Hoschke et al., 2023). Along the US Atlantic coast, their generalized movement pattern includes annual north-south migrations. They move out of northern waters (i.e., coastal waters off New England) southward at the end of summer and early fall towards mating grounds off the southeastern United States (SEUS) coast, including North Carolina (NC; Gilmore, 1993; Teter et al., 2015). After mating in late winter and early spring (Feb–May), continued migrations as far south as Florida occur before northward movements in summer (Gilmore, 1993; Teter et al., 2015; Kohler and Turner, 2019; Marens, 2021). These NWA migratory patterns vary based on sex, age, and reproductive status, making it difficult to assign a single consistent migratory behavior. Some sand tigers, including females pregnant after mating in spring, may remain in NC waters through winter gestation and only migrate out of NC post-partum in spring or remain resident year-round (Marens, 2021). In contrast, sand tigers migrating through Gray’s Reef National Marine Sanctuary (<200 nm south of the NC border) suggested short residencies at that location since most of the sharks passed through the area within 1–4 days (Williams et al., 2019). Teter et al. (2015) found seven tagged male sand tigers (168–232 cm total length) followed expected migration trends and moved out of Delaware Bay and southward along the continental shelf margin between August and October, often pausing at “rest-stops” before reaching the Cape Hatteras area by December. Once in NC waters, all males continued to occupy neritic habitats between Cape Hatteras and Cape Fear until the last tags detached in February. In contrast, three females (197–228 cm total length) leaving Delaware Bay moved in a different direction than the males, swimming east to the edge of the continental slope off New Jersey where the tags popped off after 76–151 days. Tracks for all three females were spatially and temporally similar, but such eastward movement by females had not been documented previously. The authors suggested these females were recently matured and perhaps had not yet mated. They hypothesized the offshore movements could be to avoid or delay reproduction or to take advantage of high productivity in warmer offshore waters.
Several areas on the US east coast are consistently important for sand tiger life history stages, with some individuals showing strong site fidelity to these sites. Kneebone et al. (2012) identified summer nursery grounds for juvenile sand tigers in Plymouth, Kingston, Duxbury Bay, Massachusetts. In a 2008–2011 telemetry study, juveniles were caught there as late in the year as October (Kneebone et al., 2013, 2014). Kneebone et al. (2014) reported detections for tagged sharks from the Gulf of Maine to Cape Canaveral, Florida, during all months. While summer (May–Oct) residency in New England was ubiquitous among the tagged sharks, juveniles could be found off both NC and Florida during winter (Nov–Apr). Some juvenile and adult sand tigers of both sexes consistently returned to Delaware Bay (Teter et al., 2015; Haulsee et al., 2016; Kilfoil et al., 2017), with some forming aggregations biased toward association with conspecifics of similar maturity and showing evidence of habitat preference within the bay and adjacent coastal ocean (Haulsee et al., 2018; Roose et al., 2022).
Sand tigers are found in many coastal benthic and pelagic habitats in the NWA — estuaries, nearshore (<5 miles from shore) environments, natural hard bottoms, artificial reefs including shipwrecks, and offshore to the continental shelf. There appear to be differential dependencies on specific habitats based on life or reproductive stage, sex, and time of year. Such intraspecific variability in habitat usage and movement ecology, makes it imperative to understand the role each habitat plays. Little is known about fine-scale habitat selection, occupancy, and site fidelity off the SEUS.
The NC coast has long been reported to be important habitat for this shark (Smith, 1907; Radcliffe, 1916). Gilmore et al. (1983) and NOAA NMFS (2017) suggested sand tigers give birth along the SEUS coast and recent research suggests an important role for continental shelf waters of NC for mating, gestation and possibly pupping (Marens, 2021; Wyffels et al., 2022). Sand tigers are reported year-round in North and South Carolina coastal waters, often associated with artificial reefs and shipwrecks (Farmer, 2004; Schwartz et al., 2013; Paxton et al., 2019, 2020a, Marens, 2021). Since the 1960s (Claud Hull, personal communication; Supplementary Figure 1), SCUBA divers have documented sand tigers at shipwrecks in the “Graveyard of the Atlantic,” as the NC coast is known, due to the over 5000 wrecks in this area (Figure 1; Babits, 2002; Hoyt et al., 2014). SCUBA charter operators offer highly popular trips for divers to observe individual and aggregating sand tigers at shipwrecks off the coast and popular dive magazines feature stories about diving with sand tigers (e.g., https://www.scubadiving.com/drive-and-dive-sand-tiger-sharks-galore-in-morehead-city-nc; visited 26 Feb 2024). Sand tigers tend to be highly tolerant of diver presence (Campagno, 1984; Pollard et al., 1996; Bennett and Bansemer, 2004; Barker et al., 2011), making it both feasible and safe to photograph them at close range.
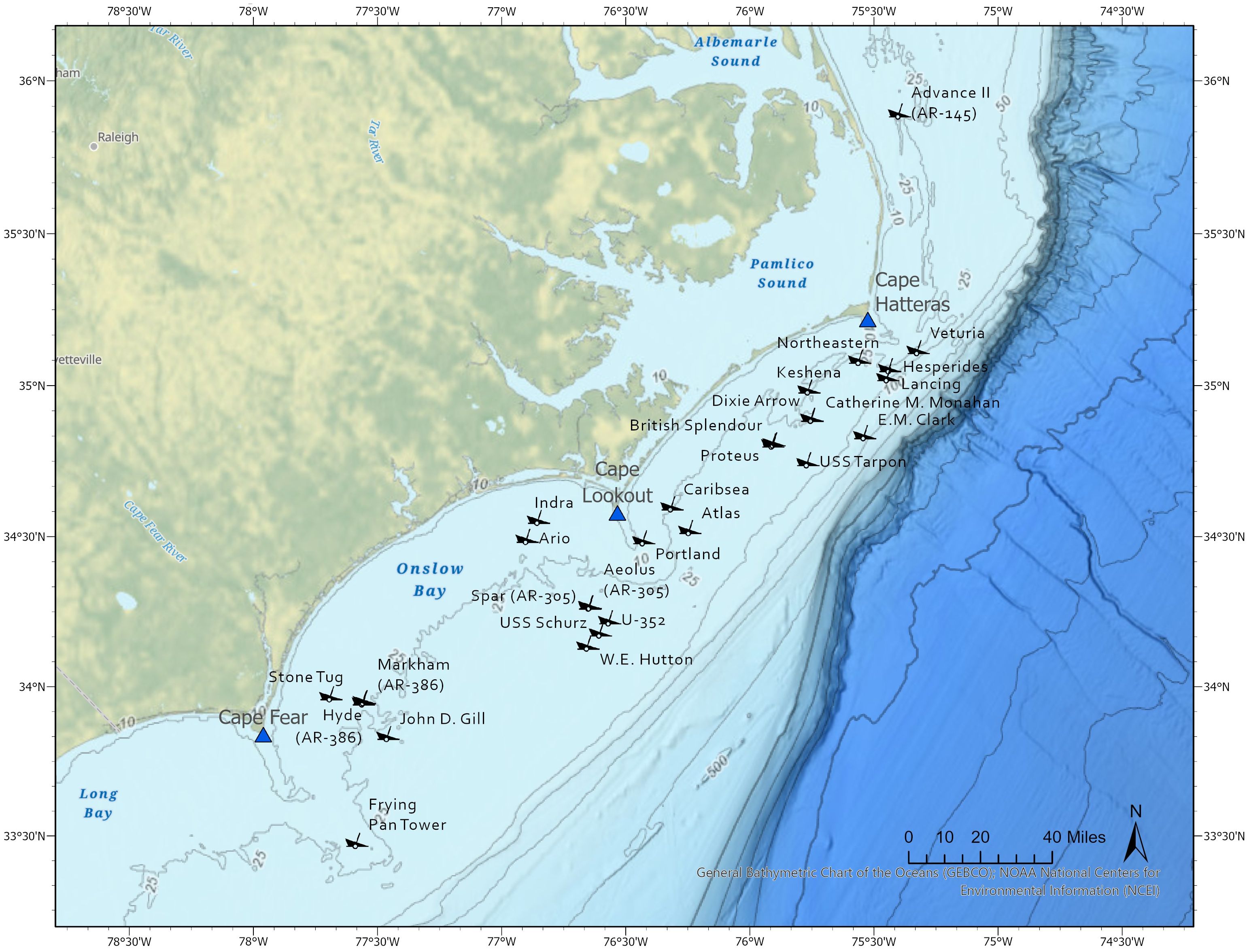
Figure 1 Locations of artificial reefs and shipwrecks off North Carolina, USA, where sand tiger sharks were encountered by community divers contributing to Spot A Shark USA.
To take advantage of the serendipitous co-occurrences of individually-identifiable sand tigers, shipwrecks, and SCUBA divers in coastal NC, Spot A Shark USA (SAS; www.spotashark.com) provides a public online platform where recreational divers can upload images of sand tigers they encounter. SAS uses Wildbook© software (Arzoumanian et al., 2005; Berger-Wolf et al., 2017) to map a spot pattern (spot map; Figure 2) for each shark and stores it in a photo library database. Individual sharks are identifiable because of reddish or brownish spots (Campagno, 1984) irregularly scattered laterally along their bodies. Spots are both persistent and individually unique (Van Tienhoven et al., 2007; Bansemer and Bennett, 2008).
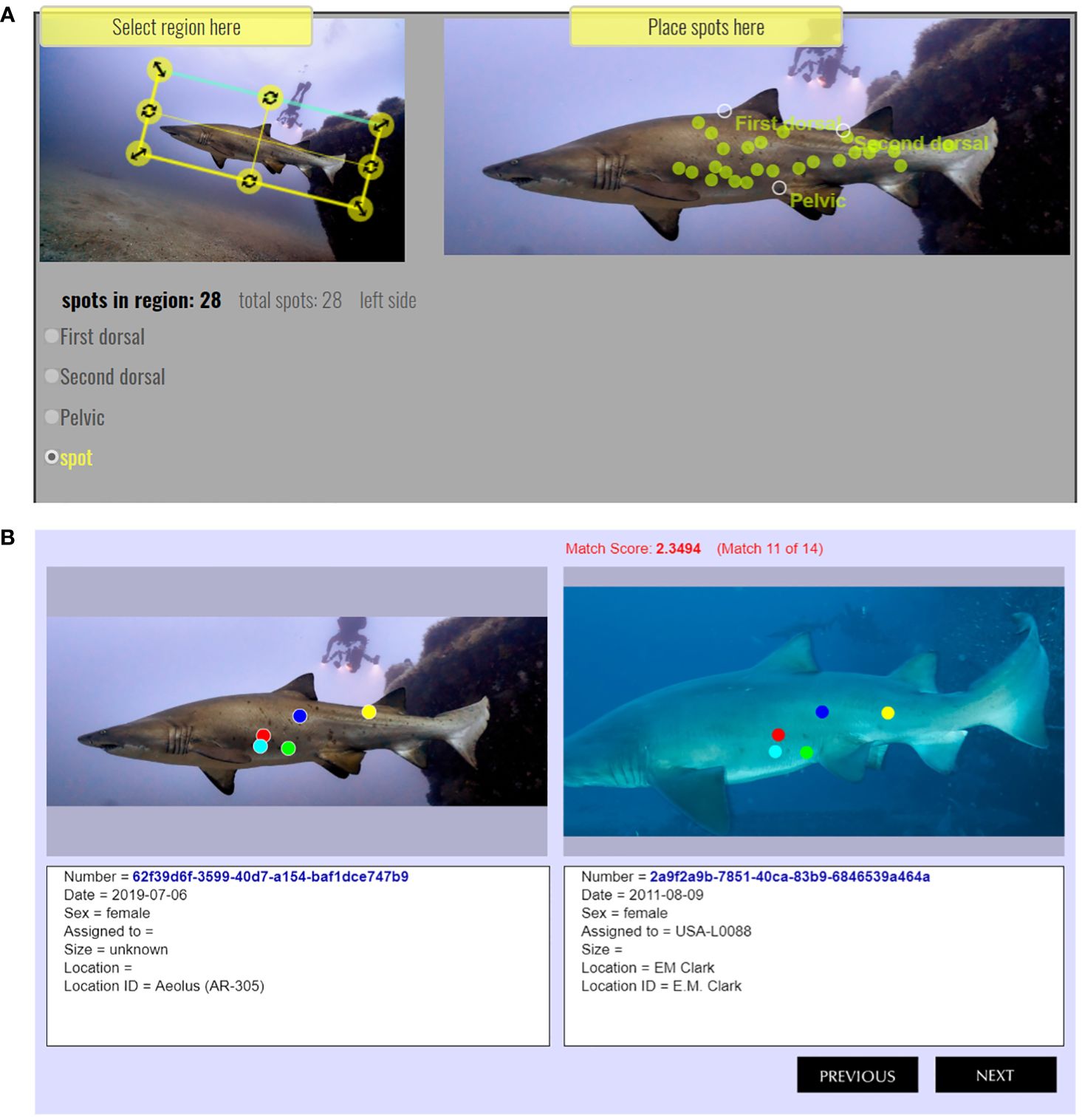
Figure 2 Examples from a left-sided sand tiger shark showing: (A) spot mapping results with location of dorsal and pectoral fins as anatomical landmarks and dots placed over spots and, (B) a possible match (right) for a spot mapped shark left, which is not a match.
Wildbook© is an autonomous computational system that applies deep convolutional neural networks to identify individuals of species that are striped, spotted, wrinkled, or notched (Berger-Wolf et al., 2017). Wildbook’s machine learning processes apply both modified Groth (Groth, 1986) and Interactive Individual Identification System (I3S) algorithms (Van Tienhoven et al., 2007) to find possible matches for novel individuals to existing records within the SAS photographic dataset based upon spot maps. Additional biological, ecological, spatial, and temporal data may be added to individual records within SAS, providing a non-invasive method for studying sand tigers (Bennett and Bansemer, 2004; Bansemer and Bennett, 2008).
Based upon earlier records of sand tigers submitted to SAS, Paxton et al. (2019) reported site fidelity in six females to three shipwreck sites off NC. They were observed at the shipwrecks Aeolus, Atlas, and Spar, with encounters separated by 1–72 months and 0–46 km. Expanded participation in SAS allows for broader analysis of how sand tigers are using artificial reef habitats, most notably shipwrecks. Specifically, we used SAS data to address the following: 1) are sand tigers consistently found at NC artificial reefs in enough numbers that photographic data can be used to inform movement and reproductive ecology, 2) are there artificial reef sites that are especially important for sand tigers, 3) are there temporal patterns in habitat use or site fidelity, 4) are there difference in site use between males and females, and 5) is NC important for reproductive ecology of sand tigers?
2 Materials and methods
In 2017, the North Carolina Aquariums and WildMe© (www.wildme.org) developed SAS by building upon an existing Wildbook© research platform used to photo-document and track individual sand tigers in Australia (Barker and Williamson, 2010). SAS was made available to the US public in summer 2018 and has been in operation since. We do not include any Australian records in our SAS dataset or the results reported here.
2.1 Image collection
Sand tiger images were collected from multiple sources. SCUBA divers uploaded photos directly through the SAS website. The NCA routinely conducted outreach campaigns to local dive clubs, shops, and guides to raise awareness about this community science project. In peak visitor seasons (1–3 days per week depending on weather between Jun-Aug beginning in 2021), we engaged directly with divers to collect photos as they got off dive charter boats at two SCUBA diving charter operators near Morehead City, NC. Divers interacted with SAS technicians to download their photos. Technicians collected and uploaded all pertinent data and images to the SAS platform. Some divers also submitted images from their previous years of diving prior to SAS being launched. In addition to still photos, technicians downloaded video data with permission, typically from GoPro® action cameras (GoPro, Inc., San Mateo, CA) from which still sand tiger images were extracted.
Sand tiger images for SAS were also extracted from video obtained from a remotely operated vehicle (ROV; Teledyne Benthos Stingray) footage collected from researchers conducting observations at shipwrecks Caribsea (16 Jul 2018), U-352 (07 Aug 2018), and W.E. Hutton (07 Aug 2018).
Finally, SAS images were retrieved from video recordings and still images taken at Frying Pan Tower (Figure 1) as part of SharkCam, a live-streaming, publicly-viewable camera hosted and maintained by Explore.org (https://explore.org/livecams/frying-pan/shark-cam; visited 26 Feb 2024). The Frying Pan Tower structure (33°29′ N, 77°35′ W) is a privately-owned (https://fptower.org; visited 26 Feb 2024), decommissioned US Coast Guard Light Station located 51 km off Cape Fear, NC. Frying Pan Tower is situated above natural hard bottom habitat (Figure 1; NC Division of Marine Fisheries, 2022) in approximately 17 m of water at the seaward edge of Frying Pan Shoals. Additional details about the camera and setting are found in Burge and Harris (2021) and Coleman and Burge (2021). Still images from SharkCam were collected from a gallery of snapshots submitted by viewers, by extracting them from recorded video files, and as screenshots taken in real-time while technicians watched the live feed.
2.2 Image processing
All images were uploaded with metadata that included the submitter or SAS technician, image date of picture(s), and the location (typically at a known shipwreck). Each record of an individual shark on a given day at a specific location was considered an encounter. Each encounter submission received a unique alphanumeric identifier automatically generated by Wildbook©, which remains with that encounter for its lifetime. Ancillary information about sex, relative size, environmental conditions, shark behavior, observed injuries, or other observations could also be entered for each encounter. Multiple images of a shark are included in a single encounter when images were taken from the same date and location. If multiple sharks were visible in a submitted photo, technicians copied the photo(s) and uploaded them as separate encounter(s), with notes added to each encounter to direct data managers to which shark is associated with each encounter.
2.3 Spot mapping
Wildbook© software was used by trained technicians to spot map the shark in each encounter. This software is used for photoidentification of terrestrial (Parham et al., 2018; Verschueren et al., 2023) and marine species (Blount et al., 2022, Weideman et al., 2020), including other elasmobranchs (Rohner et al., 2013; Norman et al., 2017). To spot map a shark’s photograph, the technician first rotated the photo to align the image horizontally (Figure 2A). Next, reference marks were placed on the image at the anterior insertion of the first and second dorsal fins, and the pelvic fin, as anatomical landmarks. Finally, between 3–30 additional marks were placed over discernable spots on the left or right flank of the shark. Images which did not contain the full body of the shark were usable if the three anatomical landmarks were visible. Once all spot marks were placed, the spot map was uploaded to Wildbook© for comparison to all other spot maps in the SAS database. The spot map comparisons yielded a list of possible matches (typically between 5–50) from the existing database of all SAS encounters for the newly uploaded spot map (Figure 2B). Technicians then compared the new encounter to matching encounters to verify matches or establish a lack of match, in which case the encounter represented a new shark.
New encounters received a unique SAS alphanumeric identification sequence (SAS ID) following the convention of USA, plus L or R to designate side of the shark that was mapped, plus a sequential number; for example, USA-L0003 and USA-R0458. For encounters that returned matches with an existing shark in the database, a second technician confirmed that it was a match. Once confirmed, the new encounter was assigned the same SAS ID. Thus, a unique SAS ID may include multiple encounters with the same shark on different dates and possibly at different locations. These records are referred to as resighted sharks.
Some submitted images were not suitable for spot mapping due to a blurry or dark photo, spots being obscured by other fish, the shark having no discernable spots, or missing reference landmarks. For unusable images, the encounter was labeled as UNID and given a sequential numerical identifier (e.g., UNID-16). Data from these encounters were included in our analysis of temporal occupancy but were not useable to track movement of resighted individuals over time.
2.4 Keywords
As each new encounter image was processed, technicians collected additional data and assigned a sex to the animal. Adult males were readily identifiable by the presence of fully calcified claspers extending from between the pelvic fins (Lucifora et al., 2002; Smale, 2002). Subadult males have smaller claspers (Supplementary Figure 2) that do not project at all or only slightly beyond the pelvic fin, but, if visible, were also used for sex determination. A clear view of the cloacal area made assignment of female straightforward. If not, other features of the shark were used to assign a sex of female, including the absence of claspers on a shark that appeared to be above sub-adult size in length and girth. Younger, smaller sharks tend to be longer relative to body height (slimmer), while adults are stouter. In some photos, relative size could be estimated by comparison to other sharks or fishes present, human divers in the photo or shipwreck features. Using these clues about size, large sharks with no claspers visible were assigned as females. Additionally, presence of scars at the base of the pectoral fins consistent with wounds acquired during mating helped with assigning sharks as females (Supplementary Figure 3). Finally, because females give live birth to young around 1 m in length, they become very rotund in late fall and winter months in later stages of gestation (Gilmore, 1993; Bansemer and Bennett, 2008; Wyffels et al., 2020). Sharks with prominently rounded abdomens consistent with pregnancy (Supplementary Figure 4) were assigned as females. If there were no clear sex markers, then the sex of the shark was assigned as unknown.
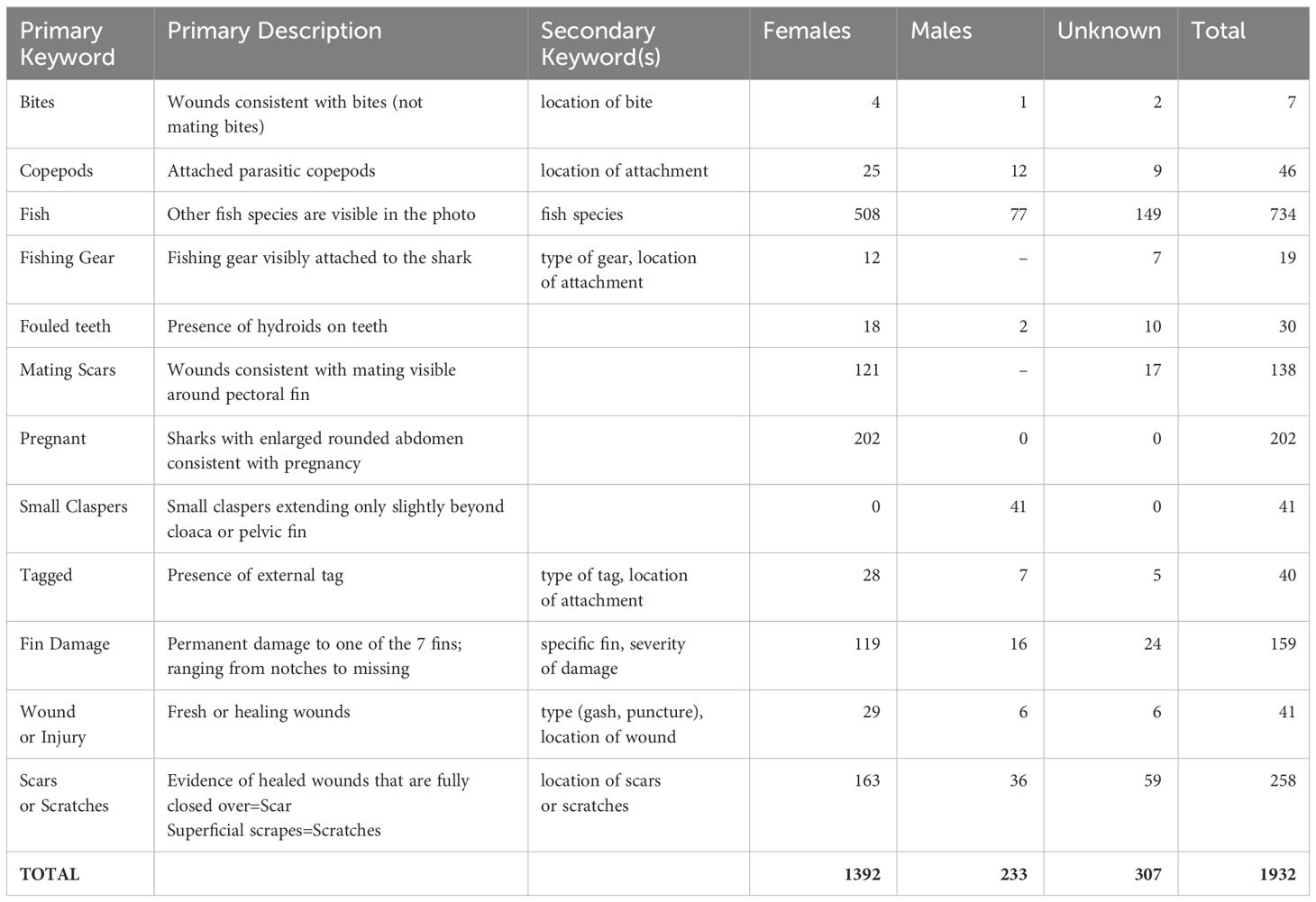
Technicians also added informational keywords to encounters (Table 1). An encounter may have no or several keywords. Primary keywords were used to maintain consistency in assignment, while secondary keywords further described what was observed. For example, a shark with a part of its caudal fin missing was assigned keywords of “fin damage” plus “caudal.” More detailed information about an encounter was sometimes recorded in a comment area by either the submitter or the technician. These comments were reviewed for keyword attribution as well.
2.5 Statistical analyses
The dataset was downloaded from the SAS website on 22 Jan 2022. We recognize the limitations of the Spot A Shark dataset with regards to statistical hypothesis testing because 1) as a community science project sampling was not standardized, 2) data were collected in multiple formats, and 3) sampling effort was not balanced spatially or temporally. Therefore, we limited our statistical analyses to descriptive metrics using SAS 9.4 or Microsoft Excel.
Counts and frequency tables were constructed for shark occurrence by site, region, month, year, and season and combinations thereof. Regional categories for sites and seasonal categories for dates were designated consistently with those reported by Marens (2021; Table 2; Supplementary Table 1). Seasons were set as Winter (Dec, Jan, Feb), Spring (Mar, Apr, May), Summer (Jun, Jul, Aug) and Fall (Sep, Oct, Nov), corresponding to the meteorological seasons for NC. We calculated counts and frequencies for the keywords listed in Table 1.
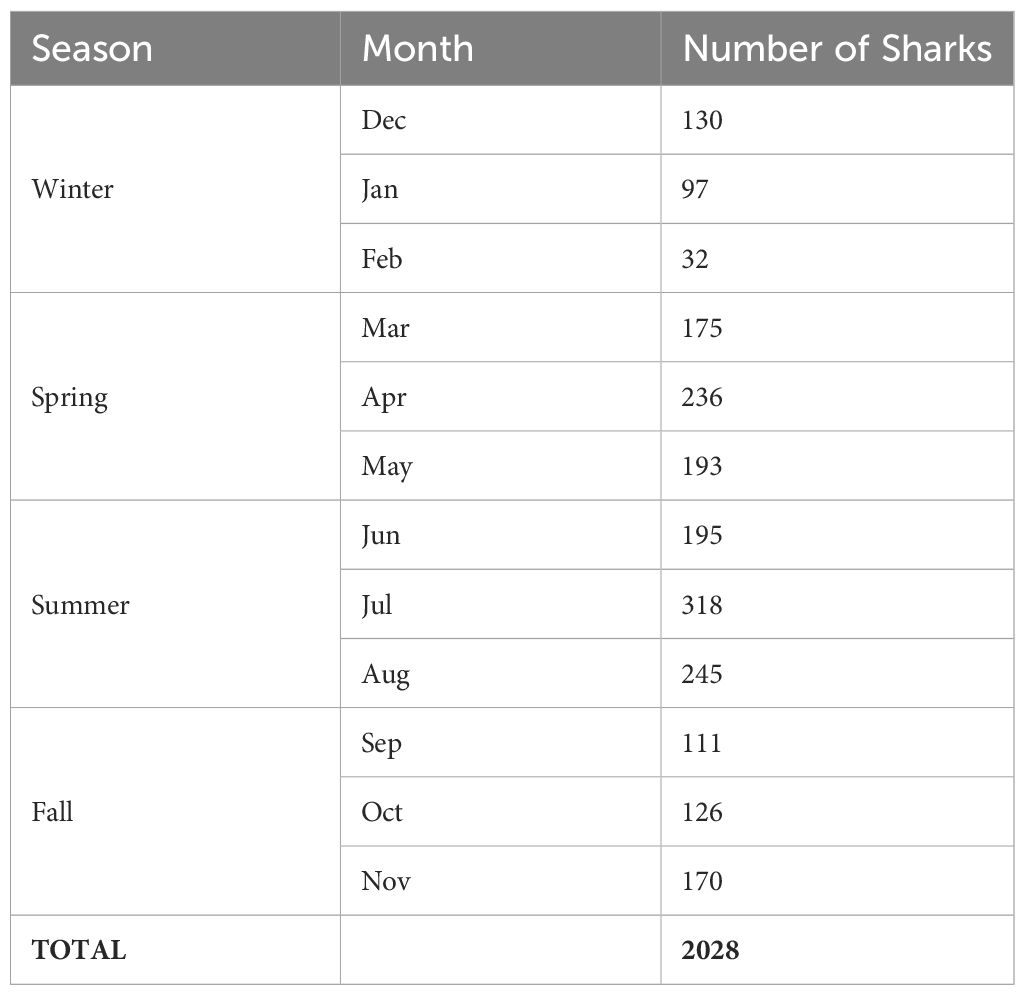
Table 2 Number of sand tiger encounters in the Spot A Shark USA database compiled across months and seasons for all years (2005–2021).
For resighted sharks, we calculated the days at liberty (DAL) between all sightings. To calculate distances travelled between sites by resighted sharks, we compiled a matrix of coordinates for all sites (Comer and Love-Adrick, 2016; NOAA, 2022) and computed the distances in nautical miles (nm) between all possible combinations of sites using the formula:
where: Lat_place_1 = latitude of location 1, Lat_place_2 = Latitude of location 2, Lon_place_1 = longitude of location 1, Lon_place_2 = longitude of location 2 and PI() = pi
Distance calculations were converted to km using the formula:
3 Results
In total, 2028 unique sand tiger encounters representing 1837 individual sharks with encounter dates ranging between 2005–2021 were included in this dataset. Of these, 824 were spot mapped on the right side, 924 on the left and 89 were UNID and did not get spot mapped. We note many of our results, especially those related to temporal and spatial patterns, reflect total available images (diver effort, SharkCam/Explore.org footage availability). To emphasize this, we reference our findings as “sand tiger encounters” throughout this section to acknowledge the data summarize diver interactions with sharks and availability of footage from Explore.org/SharkCam. We recognize these images are not representative of the sand tiger population as a whole.
Females made up 56.8% of the sharks, males 13.5%, and unknown sex 29.7%. All three categories were observed in all months, with females outnumbering males in all months (Table 3; Supplementary Figure 5). We received images from 30 sites, with Frying Pan Tower and Artificial Reef 255 (AR-255) Bridge Frame being the only sites that are not shipwrecks (see Supplementary Table 1 for additional details on all sites including links to archaeological and historical details). The sites are in water 6–73 m deep and lie 0.2–51 km offshore. One encounter submitted from the South Carolina shipwreck Tauracavor, and 16 submitted from unknown locations on the central NC coast, were included in the SAS dataset, but were eliminated from some analyses. Fifty-one community divers submitted photographs.
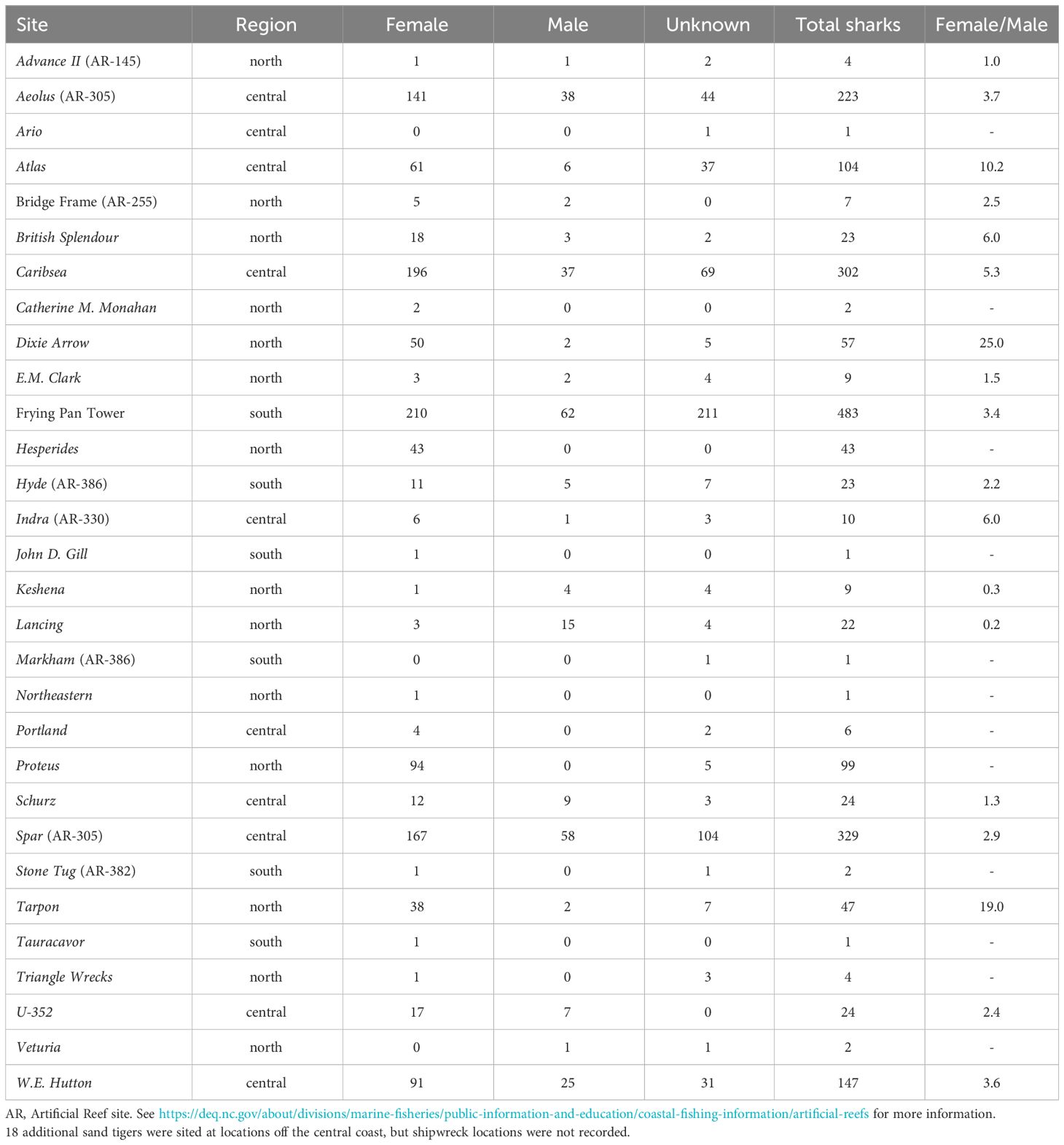
Table 3 Sites of sand tiger Carcharias taurus images cataloged in the Spot A Shark USA database (www.spotashark.com) by sex and female:male ratio.
3.1 Spatial and temporal occupancy
Though sharks were photographed at 30 sites, 90% of images came from just 10 sites, with Frying Pan Tower accounting for 24% of all submissions (Figures 3, 4). The other most frequent locations were Spar (16%), Caribsea (15%), Aeolus (11%), W.E. Hutton (7%), and Atlas, Proteus, Dixie Arrow, Tarpon, and Hesperides (each 2–5%). The remainder of the sites had fewer than 25 shark encounters (range=1–24). For the shipwrecks where sand tigers were photographed, 14 were designated as northern, 10 central and 6 southern (Table 3; Supplementary Table 1). Nearly 60% of encounters were from shipwrecks off the central coast, with 23% from southern and 17% from northern locations (Figure 3).
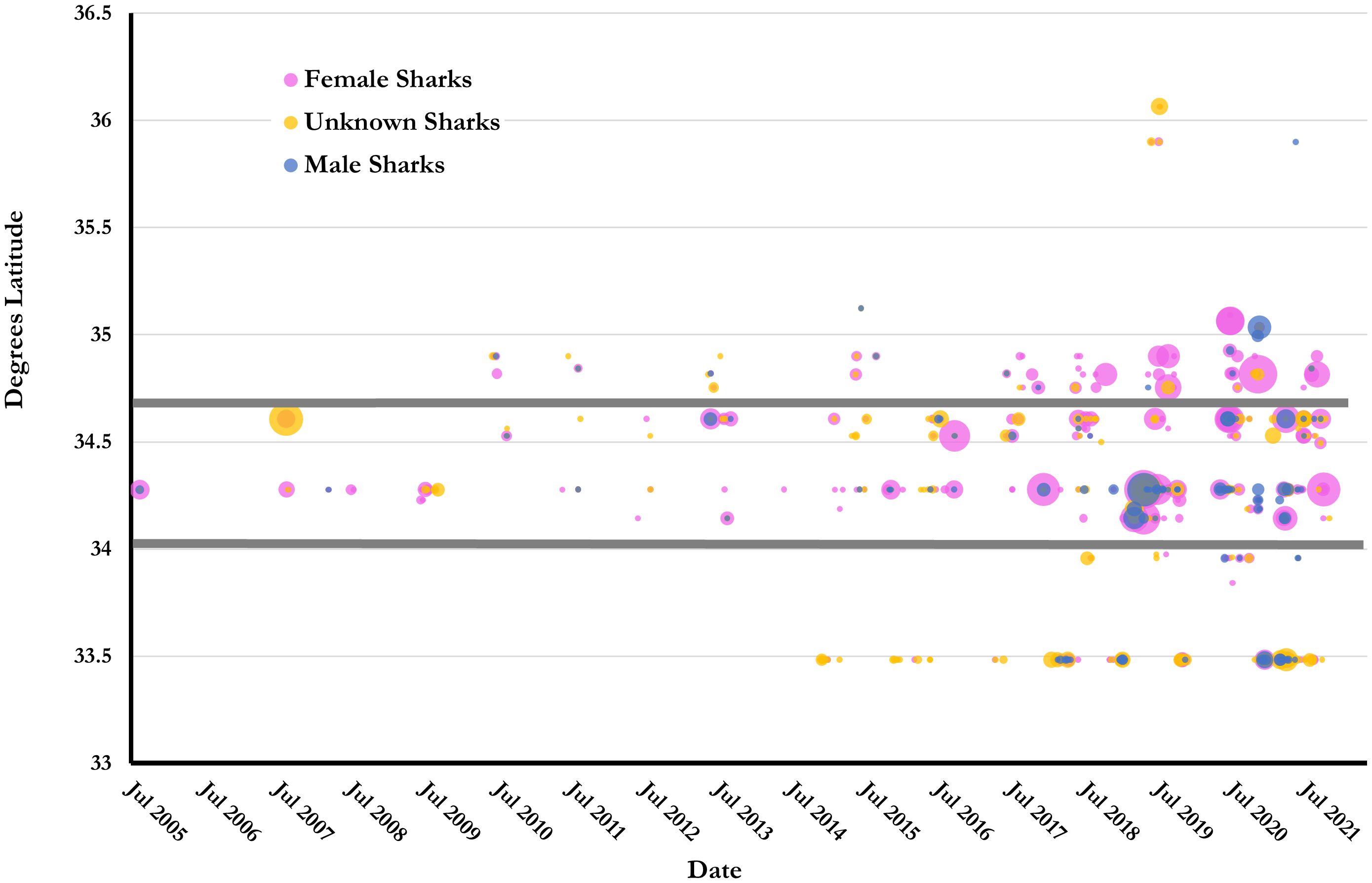
Figure 3 Latitudinal distribution of Spot A Shark sand tiger shark encounters in North Carolina, USA, from 2004-2021. Each dot represents a single encounter. Large dots are scaled to represent multiple encounters at the same location. Grey horizontal lines demark boundaries between the northern, central and southern regions.
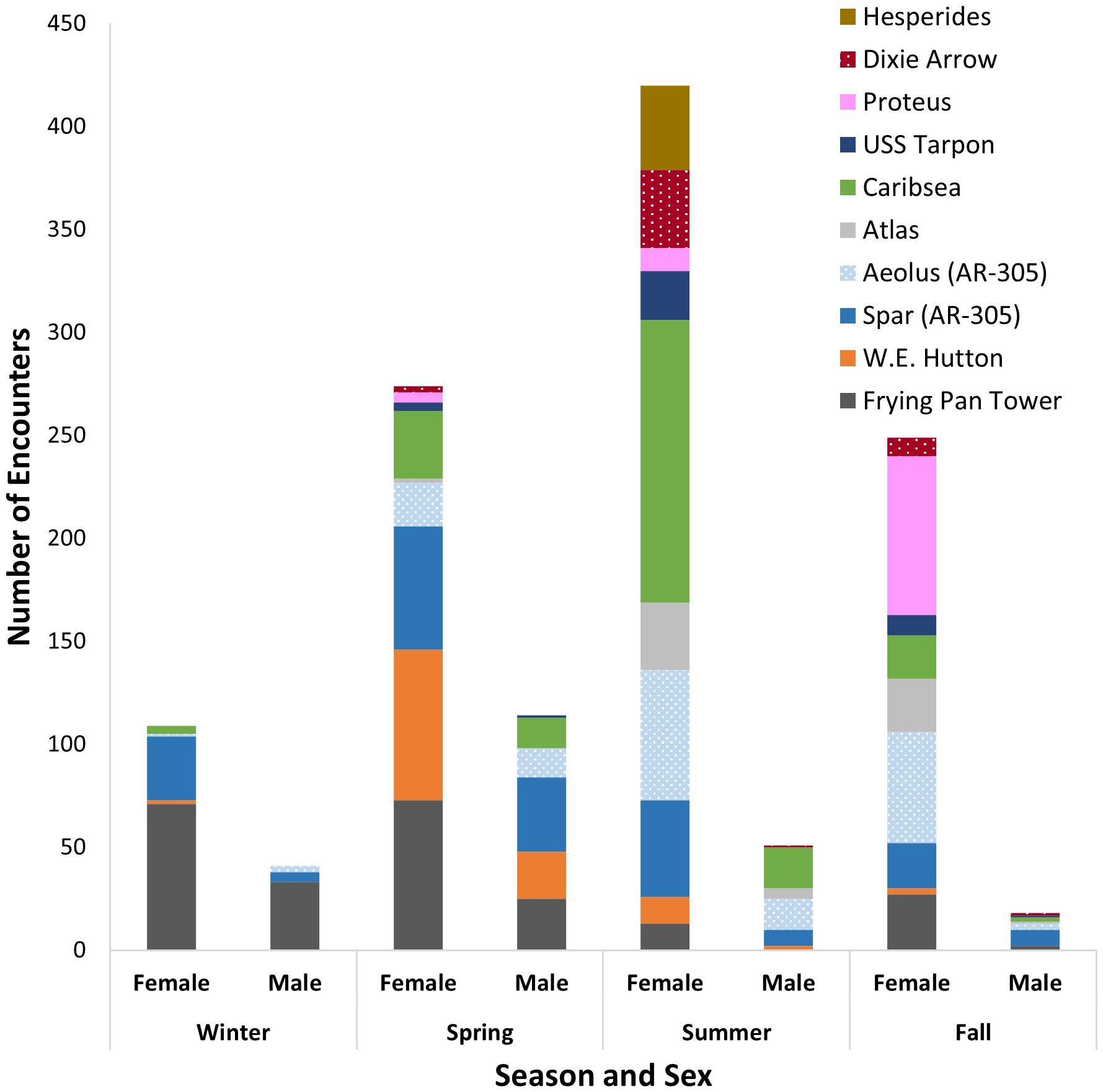
Figure 4 The number of sand tiger shark encounters in the Spot A Shark database by sex and season at the 10 most-frequented locations. Only locations which had at least 40 sand tiger shark encounters recorded are included. The locations are oriented from north (Hesperides) to south (Frying Pan Tower) on each bar.
3.1.1 Site by sex
At all sites with at least 10 submitted encounters, a mixture of female, male and sex unknown sharks were photographed, with two exceptions. Only female or unknown sex sharks were reported at the Proteus (n=99) and Hesperides (n=44) wrecks. Some locations had proportionally high numbers of females present across all years. For example, Dixie Arrow had 25 times more females (n=50) than males (n=2) reported. The Tarpon had 19 times as many females (n=38) as males (n=2) reported. Atlas had 10 times as many females (n=61) reported as males (n=6). Only at the Lancing did males exceed females with 15 males reported compared to only 3 females.
3.1.2 Temporal patterns
We examined the frequency of occurrence by month and season (Table 2). Sharks were photographed in all months of the year, with 59% encountered between Apr and Aug, corresponding to the months with highest recreational diver activity (Supplementary Figure 5). In northern sites, data gaps exist in late fall and winter months. None of the sites in the central region have encounters from all months. However, a few sites have 9–11 months coverage: Aeolus (Mar–Dec), Caribsea (Feb–Oct), Spar (no Sept records). At FPT, where the live camera was deployed and diver comfort is removed as a bias to image gathering, shark encounters were most frequent in late fall through spring, but there were encounters for all months. When grouped by season, 20% of all encounters are from fall, 30% from spring, 37% from summer and 13% from winter.
To examine differences in seasonal occupancy by sex, we tabulated encounters of 1052 female and 224 male sharks at ten sites along the north-south latitudinal gradient. Included sites had 40 or greater total encounters (Table 3; Figure 4) and represented 63% of our total 2028 encounters. In the northern region, Hesperides, the northern-most shipwreck had only females (n=41) reported on only two dates (04 and 05 Jul 2020). Dixie Arrow had 50 female sharks reported in spring, summer and fall, but only 1 male in spring and 1 male in summer, with 76% of those females in the summer months. Proteus only had female sharks (n=93) in spring, summer and fall, with 83% of encounters reported in fall. Tarpon had 38 females in spring, summer and fall, with 63% from summer. There were only 2 males reported, one in spring and one in fall. Caribsea had a total of 195 female sharks with highest numbers in summer (70%). Males (n=35) were reported mostly in spring and summer with only 2 reported in fall. Atlas had females in spring and summer (n=59) and fall (n=2), and males (n=6) in summer and fall. Aeolus had females (n=139) and males (n=35). Highest numbers of females were reported in summer and fall (84%), with most males reported in spring and summer (83%). Spar had females (n=160) and males (n=57). Highest numbers of females were reported in spring (38%) and summer (29%), with most males reported in spring (63%). W.E. Hutton had females (n=91) and males (n=25) in spring and summer. About 80% of females and 92% of males were reported in spring. In the southern region, Frying Pan Tower had females (n=184) year-round and males (n=60) in fall, winter and summer. Both females and males were reported in highest numbers in winter (39% females, 55% males) and spring (40% females, 42% males).
3.2 Photo-recaptured sharks
Most sharks were encountered only once, but 101 sharks (5.5% resighted) were observed on more than one date. Of these, 74 were seen twice, 13 were seen 3 times, 8 were seen 4 times, 3 were seen 5 times, 1 was seen 8 times, and 2 were seen 14 times. DAL between sightings ranged from 1–2176 days (mean=171.5 ± 356.4 days; median=16 days). Typically, sharks were observed at the same location during a relatively short window for all encounters, but 28 were resighted at a different location from the previous encounter. The distance between sightings ranged from 0 (i.e., seen at the same location on different dates) to 213.2 km. For sharks observed at a different location in the subsequent encounter, the distance between sightings averaged 21.1 ± 48.2 km. Below, we detail resightings of selected sharks arranged by location where they were first encountered.
3.2.1 Frying Pan Tower
Sharks first seen at Frying Pan Tower accounted for over a third of resighted sharks (n=37). All sharks first encountered at Frying Pan Tower were only resighted at the same location. The most observed shark was female USA-R0272, seen on 11 days between 28 Oct–12 Nov 2019 and next seen over a year later on 21 Dec 2020. The last dates she was seen were 12 and 15 Mar 2021. These 14 sightings spanned 504 DAL. In all photos, this shark is missing a large portion of the upper lobe of her caudal fin. In the Oct–Nov 2019 images she displayed a large abdomen and appeared pregnant (Figures 5A, B). In photos from the following winter of 2020–2021, she did not appear pregnant (Figure 5C).

Figure 5 Female Spot A Shark sand tiger shark USA-R0272 encountered at Frying Pan Tower: (A) first observed, rotund abdomen consistent with pregnancy, portion of upper lobe of caudal fin missing, 28 Oct 2019; (B) rotund abdomen consistent with pregnancy, 12 Nov 2019; (C) abdomen no longer rotund the following winter, 21 Dec 2020. Photo credit: All extracted from explore.org video files.
Male shark USA-R0536 was seen 8 times at Frying Pan Tower. He was first encountered on 23 Nov 2019 with a nick on the lower lobe of his caudal fin. The following winter he was seen on 7 dates between 27 Nov 2020 and 18 Mar 2021, this time with additional injuries including a nick on the trailing edge of the left pectoral fin, and numerous scrapes on his snout, head and body. There were three sharksuckers Echeneis sp. Linnaeus, 1758 attached on his left and right flanks. On 28 Nov 2019, a size comparison with other sharks was possible, indicating that this shark was relatively smaller than several large females also visible on the live camera that day. Most of the scrapes were no longer visible on the last day the shark was photographed (18 Mar 2021).
The remainder of the resighted FPT sharks were encountered on 2—5 separate dates. The average time between sightings was 105 ± 248 days (median=6 days), ranging from 1 to 1033 DAL. Of these, 5 sharks had greater than one year between encounters. Though resightings were reported at FPT in all seasons, most occurred fall through winter. Most sharks with multiple encounters were female (61.1%), with 19.4% males and 19.4% unknown sex.
3.2.2 Aeolus
A small male, USA-R0128 (Figure 6) was photographed at the wrecks of Aeolus and nearby Spar (0.26 km apart) on 14 days between 14 May 2019 and 26 June 2021. His estimated length at the first encounter at Aeolus was approximately 1 m (T. Houppermans, personal observation), so it is possible this was a young-of-year shark. This small shark was photographed 7 more times on Aeolus that year on 19 May, 27 June, 01, 05 and 07 Aug, 15 and 19 Oct. In 2020, he was encountered at Spar on 08 and 09 June and at Aeolus on 30 Jun. In 2021 he was seen twice at Aeolus on 07 and 25 Jun. This shark was typically found in and around the stern section of Aeolus, in sheltered areas (around 27 m depth). When first encountered, the shark had a deep, wide gash on his right flank extending from the base of the second dorsal to the lateral line. The lower posterior edge of the second dorsal had been severed. Photos showed healing through summer and fall, with fully closed scars present by Oct 2019. He was last observed on 23 June 2022, swimming just above the wreck.
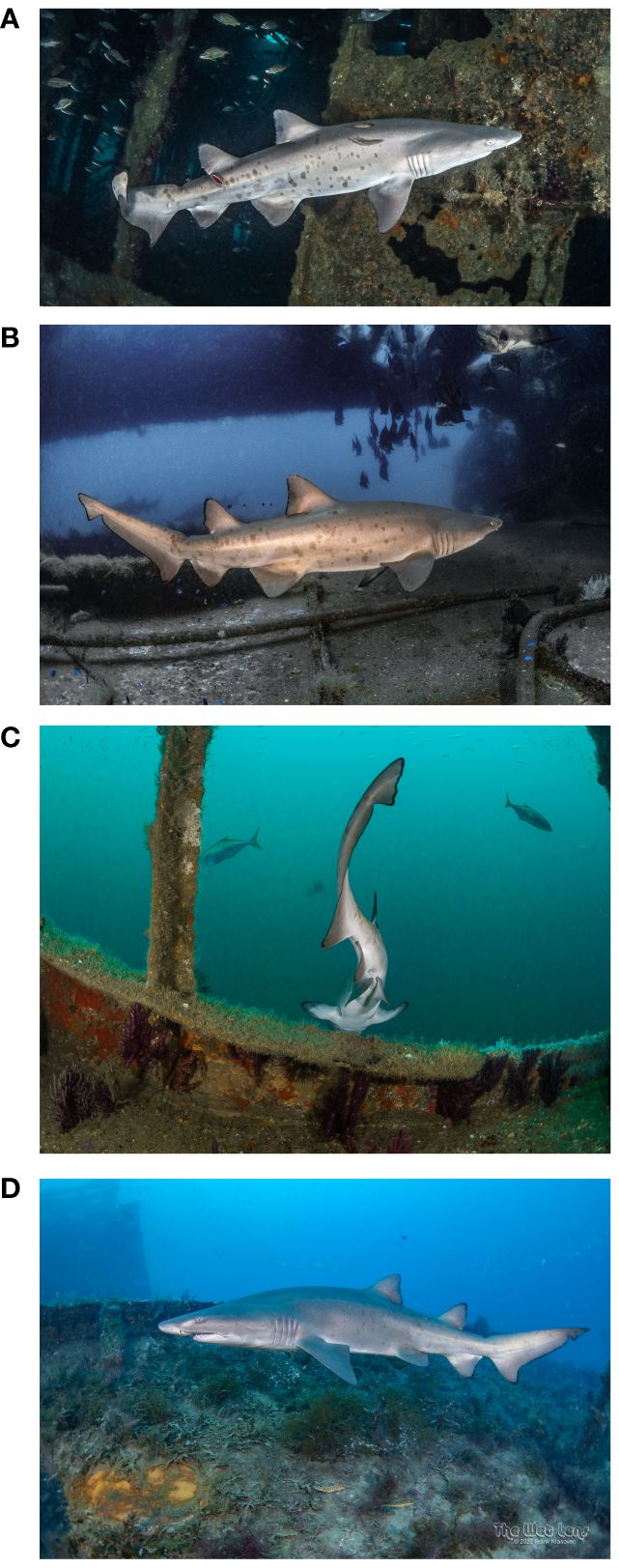
Figure 6 Small Spot A Shark male sand tiger shark USA-R0128 encountered: (A) first siting, bottom of second dorsal fin missing and fresh wound on side below second dorsal, length estimate <1m by diver, Aeolus, 14 May 2019; (B) wound appears healed, Aeolus, 07 Aug 2019; (C) subadult claspers visible, Spar, 07 Jun 2021; (D) last siting, Aeolus,23 Jun 2022. Photo credits: 3. (B) Tanya Houppermans; (D) Frank Krasovec.
USA-L0199 (Supplementary Figure 2) was first seen at Aeolus on 01 Jun 2016 with many scrapes on his head and left flank, then again on 27 Sep 2016 with wound healing evident. On these first two dates, he was assigned as sex unknown. On 28 Jun 2019, small claspers were visible. In the last photograph taken 17 Jul 2020 at British Splendor (90.7 km northeast of Aeolus), claspers appeared fully developed. The tip of his caudal fin was missing, a healing cut (estimated 10–15 cm long) was noted anterior of the left eye, and parasitic copepods were attached on the upper left jaw.
Twelve additional sharks first observed at Aeolus were later resighted. One female with most of the first dorsal fin severed, but fully healed, was first seen on 06 Jul 2019 and then 4 more times Apr–Jul 2021 on either Aeolus or Spar (730 DAL) with scrapes on her head that healed over those months. Another three sharks, two females and one male, were resighted four times at either Aeolus only (n=2) or Spar (=1). There were eight sharks (n=6 females) sighted on two dates, at the Aeolus or Spar both times. The other two were seen at W.E. Hutton (female; 14.8 km south; 1506 DAL) and Advance (male; 213 km northeast; 2176 DAL).
3.2.3 Spar
Of the 23 sharks first observed at Spar, most were female (n=19) and seen only twice (n=18). Additionally, 1 male and 3 unknown sex sharks were resighted. DAL ranged from 8-2059. Resighted sharks were most frequently seen again at either Spar (n=12) or Aeolus (n=8). Total DAL ranged from 8–2059 (mean=438.2 ± 470.5 SD; median). Female USA-R0184 was first seen on Spar on 16 Nov 2015 and appeared pregnant. Another 24 sand tigers are visible (but too indistinct for spot mapping) in this submitted photograph including several more with large abdomens. She was next encountered over 5.5 years later on 06 July 2021 at Atlas (46.3 km northeast), representing the longest DAL in our dataset. Female USA-R0222 was seen twice on Spar in Aug 2019 and noted to have notches on the trailing edge of first dorsal fin (Supplementary Figure 6). She was subsequently encountered at Aeolus on 16 May 2020. A portion of her right pectoral fin was missing, and the wound appeared sealed and edged in white scar tissue. She was seen on 14 Jul 2020 and again on 12 Aug 2020 with the wound appearing completely healed over.
Other sharks with notable DAL intervals include female USA-L0275 observed above the sea floor adjacent to Spar on 05 July 2019. She was next observed at Aeolus on 12 and 14 Oct 2021 (832 DAL). Female USA-L0195 was seen at Spar on 01 May and 26 Jun 2019. When resighted on 06 Jun 2021 at Spar (777 DAL) she exhibited large, red scrapes on and below her second dorsal fin and extending over half of her caudal fin, as well as less significant scrapes dorsally above the gills. USA-L0120 is an unknown sex shark seen first at Spar on 09 Sept as a juvenile based on morphology and size relative to other fishes in the image. The shark was resighted on 09 Aug 2011 at Aeolus (699 DAL). Female USA-L0109 was at Spar on 15 Aug 2007 amid a large school of tomtate Haemulon aurolineatum Cuvier in Cuvier & Valenciennes, 1830 and then encountered again on Aeolus on 08 Jul 2009 (693 DAL). Female USA-R0181 was observed at Spar 01 May 2019 and then at Frying Pan Tower (124 km southeast) after 681 DAL on 12 Mar 2021. Adult male USA-L0378 was resighted at the U-352 (9.3 km southeast of Spar) after 569 DAL, with two sharksuckers attached ventrally.
3.2.4 Caribsea
Of 10 resighted sharks first encountered at Caribsea, only two were seen more than twice and only one was resighted at another location. This was USA-R0294, an unknown sex shark first seen on 10 Jun 2016 with several small scrapes on its head and snout. He was photographed again on 03 Aug 2020 (1515 DAL) at Atlas (11.1 km southeast) also with scrapes near the right eye. Sex could be determined in this second encounter. Of the remaining 9 sharks, five were females and four were males. Female shark USA-R0154 was seen at Caribsea on 01 Sept 2018 with healing mating scars on the left pectoral fin. She was seen again on 22 and 25 June 2020 (663 DAL) also with mating scars on right pectoral fin and additional wounds along the right flank. Another female, USA-L0522, was also reported at Caribsea with mating scars on her left pectoral fin and other wounds on her flank on 25 Jun and 05 Jul 2020. Two sub-adult males were resighted at Caribsea. First, USA-L0514 was observed on 25 Jun and 03 Aug 2020 with visible small claspers, the second dorsal fin folded over and parasitic copepods attached at the upper front jaw and tip of the snout. USA-L0819 was photographed at Caribsea on 06 Jul, 28 Aug, and 29 Sep 2021, with small claspers visible each date.
3.2.5 W.E. Hutton
Female USA-R0219 was seen at W.E. Hutton on 27 Jun 2019 and described by the submitting diver as a small sub-adult. She was resighted twice on Aeolus (14.8 km south) on 06 Jul and 03 Aug 2019 (37 DAL). A female appearing pregnant, USA-L0161, was encountered at W.E. Hutton on 30 April 2019 (Supplementary Figure 7). She was seen again at the same location on 27 June (58 DAL), but no longer displayed a large abdomen.
3.2.6 Hesperides
On 04 Jul 2020, a diver photographed 20 female sand tigers at Hesperides, 9 of which exhibited mating scars. The following day the same diver encountered 21 females, five of which were resightings.
3.3 Keywords
Over 1900 keywords were assigned to sharks during image processing (Table 1). We observed 19 sharks (12 female, 7 unknown sex) with attached fishing gear. Of these, six were at Aeolus, three at Frying Pan Tower, two at Hyde and the rest distributed among eight other sites. Sharks with attached fishing gear were mostly photographed in summer and early fall months. For 17 of these sharks, fishing hooks and sometimes trailing line were visible. Hooks were in the jaw for all but one shark, which had the hook embedded in its head. One shark had a section of gillnet tangled in its teeth and one had a length of rope (estimated 1.5 m long) around its caudal peduncle.
External tags were observed on 28 females, seven males, and five unknown sex sharks. Most were photographed at Frying Pan Tower, Aeolus, Caribsea and W.E. Hutton. Most tag numbers could not be discerned because the tags were fouled, numbers were not visible, or the resolution was too poor. However, we were able to confirm USA-R0756 carried a tag numbered #399299 when encountered on 30 March 2021 at Aeolus. The tag was reported to the NOAA Cooperative Tagging Project, which confirmed this male shark measuring 155 cm fork length had been tagged in Delaware Bay on 12 August 2019. It was visually estimated by the SAS contributing diver to be 170 cm long.
Parasitic copepods (Supplementary Figure 8) were observed attached to 25 female, 12 male and 9 unknown sex sharks, with most of those encounters submitted in Jun and Jul. Copepods were generally attached on the sharks’ heads, often around the nostrils and mouth. Most sharks with copepods attached were photographed at Caribsea (n=25), Spar (n=8) and Aeolus (n=4). It was not possible to determine the species of copepod from any images. We observed 30 sharks with hydroids on their teeth (Supplementary Figure 9), of which 18 were female, 2 male and 10 unknown sex. Most of these were photographed at Caribsea (n=9), Spar (n=8) and Aeolus (n=6) in summer months.
Small claspers were noted for 41 male sharks. These were observed in encounters from all months except Feb, with the most reported in May (n=6) and Jun (n=8). Most of these sub-adult males were photographed at Frying Pan Tower (n=12), Spar (n=9) and Caribsea (n=9) with the remainder at the wrecks of Aeolus, Hyde, Atlas, W.E. Hutton and British Splendor.
Wounds or scars consistent with mating injuries (Supplementary Figure 3) were observed on 121 female and 17 unknown sex sharks. Of these, 41% were photographed on Caribsea, 14% on Hesperides, and 9% on British Splendor. These wounds were most often reported in Jun (30%) and Jul (44%), though scars were visible on sharks in various stages of healing throughout the year.
We found 202 female sharks at 18 sites displaying morphology consistent with pregnancy (Figure 7; Supplementary Figure 4). While 87% of these encounters were submissions from Jun–Dec, this keyword was assigned to sharks throughout the year. The two sites with the most females appearing pregnant are Frying Pan Tower and Spar, each with 41 encounters and collectively accounting for 41% of these observations. Other sites with possible pregnant females reported include Caribsea and Dixie Arrow, each with 20; Proteus and Aeolus, each with 18; Tarpon with 11; and the remaining sites, including all those from the northern region, which each had 1–6 reported.
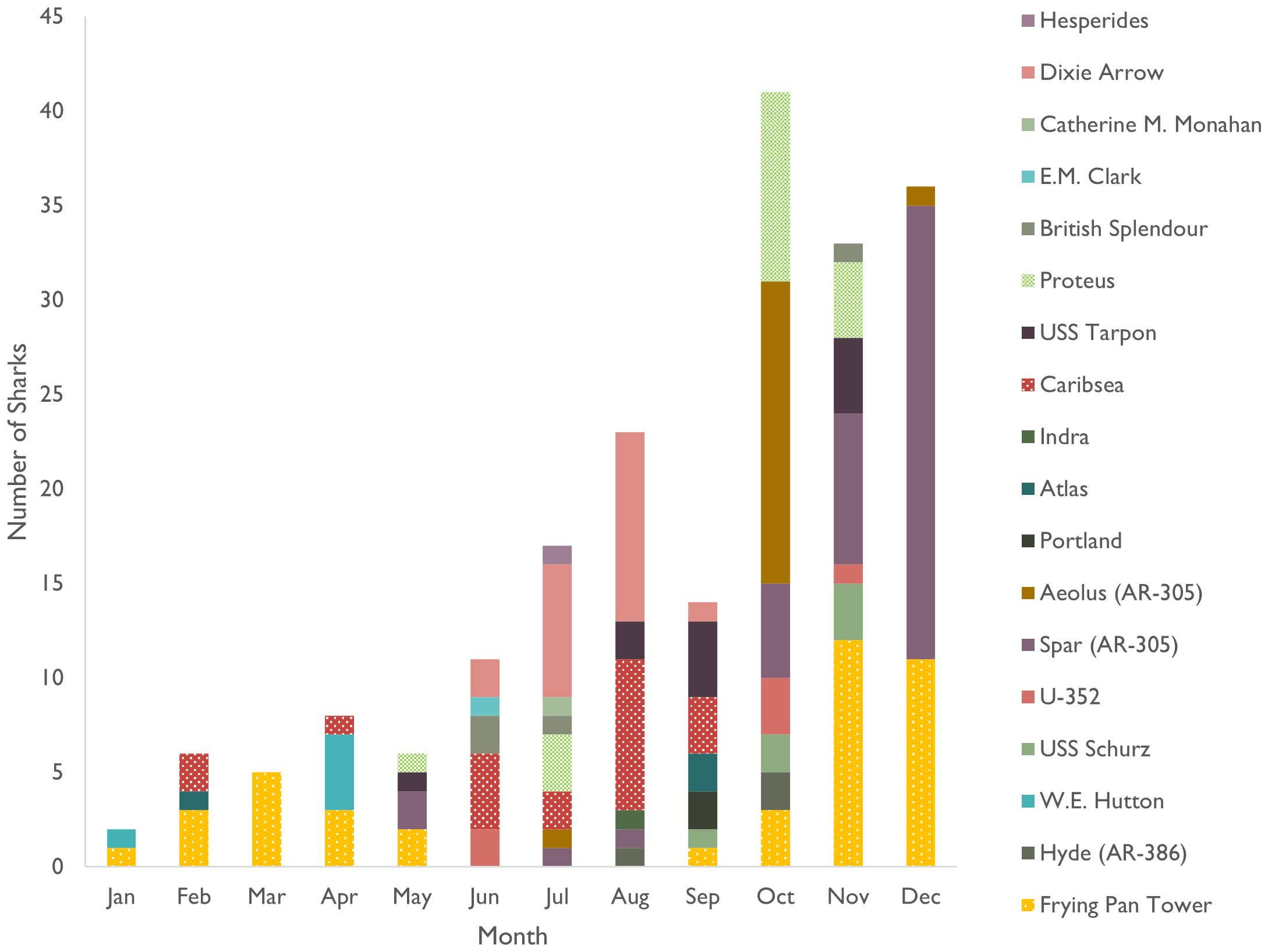
Figure 7 Number of female sand tiger sharks in the Spot A Shark database assigned the keyword "pregnant" in all years combined by month and location. Locations are ordered from north (Hesperides) to south (Frying Pan Tower) in all bars.
Fin damage was observed on 119 females, 16 males and 24 unknown sharks (Supplementary Figure 10). Of these, 25 were missing all or a portion of the fin. Most often damage was to pectoral (n=55), caudal (n=52) and dorsal (n=46) fins, with less damage to pelvic (n=16) or anal (n=2) fins. It was not possible to discern the etiologies of these injuries. More severe injuries were observed ranging from fresh wounds to healing injuries. Injuries included puncture wounds, and deeper cuts or gashes with incomplete healing or scarring (Supplementary Figure 11). Such wounds were observed on 29 females (not assigned as mating injuries), 6 males and 6 unknown sex sharks. For these wounds, the location(s) of the injuries were recorded. Three of these injuries appeared to be consistent with rope damage. We found an additional 7 sharks with significant bite wounds (Supplementary Figure 12). Two of these were females that also had some mating wounds at the base of the pectoral fin. Because scars and scratches could not always be differentiated, they were grouped. We observed 258 sharks (n=163 female, 36 male and 59 unknown sex) with scars or scratches visible. For these, the body location was assigned as head, body, caudal or any combination of these three areas (Supplementary Figure 13). Many sharks (n=81) had scars or scratches only on their bodies, often on dorsal or lateral areas. Scratches on the head (n=45) were typically on the snout and top of the head. The most common combination of scratch or scar locations was the head and body (n=62), and 20 sharks had scars or sctatches present on all three body locations.
4 Discussion
This study adds to our knowledge of sand tiger ecology and provides further evidence for the importance of the NC coast, and specifically shipwreck and artificial reef habitats, throughout their lives. Community science projects, including SAS, can have limitations (Berger-Wolf et al., 2017; Earp and Liconti, 2020) but are a proven research tools for elasmobranchs (Holmberg et al., 2008; Barker and Williamson, 2010; Norman et al., 2017; Giovos et al., 2019; Pottie et al., 2021), and other marine (Dudgeon et al., 2019; Dunbar et al., 2021; Blount et al., 2022) and terrestrial species (Bradsworth et al., 2017; Parham et al., 2018; Mason and Arathi, 2019; Gould et al., 2021). Globally, sand tigers exhibit variable patterns of migration based upon reproductive status, sex and age, and our data supports the notion that the NWA population does as well.
The high number of encounters in the SAS database (n=2028) allows us to examine broad patterns in STS habitat use and opens pathways of investigation into other aspects of their ecology. Additional data gathered from photographs offers insight into the prevalence of injuries, such as those from fishing activities. Fishing related injuries in our study were less than what was observed in Australia by Bansemer and Bennett (2010) who found up to 29% of females and 52% of males with attached gear or injuries attributed to fishing. Many SAS sharks were observed with wounds and injuries of varying severity, but aside from mating scars it is rarely possible to determine the cause. Healing was observed in several resighted sharks, and recovery and functionality was further evidenced by sharks with significant wounds being resighted and even pregnant. Hydroids have been observed in the teeth of sand tigers elsewhere in their range and may indicate cessation of feeding (Pollard et al., 1996). We also collected information about associations with parasites, commensals and other fish species. Data about sand tigers and intraspecific fish associations are currently being assessed (A. McClanahan, unpublished) and will add to work on sand tiger, round scad, and mesopredator associations reported by Coleman & Burge (2021).
Below we evaluate our findings relative to the five questions posed for this project by combining temporal and spatial data from encounters, including from resighted sharks, and keyword data.
4.1 Are sand tigers consistently found around NC artificial structures, including shipwrecks, in enough numbers that photographic data can be used to inform movement and reproductive ecology?
The SAS database of 1837 sand tigers reflects what has previously been reported for sand tiger distribution in NC waters. Since STS are federally protected from harvest (NMFS, 2009, 2017) anecdotal reports of captures in recreational and commercial fisheries are rare (Mcclellan Press et al., 2016; Kilfoil et al., 2017). A 27-year systematic longline survey in Virginia suggest sand tigers are a minor constituent of the total abundance of sharks with only 135 sand tigers caught among 4830 total individuals during 577 longline sets (Latour and Gartland, 2020). Similarly, sand tigers were rarely reported in a decades-old scientific survey of South Carolina waters with 2 of 297 sharks captured on bottom longlines during 74 sets (Low and Ulrich, 1984). The most comprehensive and recent information available on sand tiger populations in the NWA (Carlson et al., 2009) synthesized scientific catch data from 1974–2004, concluding that trends in abundance of sand tigers showed only modest declines between 0.2% and 6.2% and that there was not growth overfishing as would be expected for such a long-lived and very low productivity species. Despite these encouraging signs for abundance and demographics in this species the authors note that “exceptionally low productivity of sand tigers and the relatively low sample sizes on which we based our trend analyses” argued for a continued listing by NMFS as a species of special concern (Carlson et al., 2009).
We received submissions from locations coastwide in NC and throughout the year. Some sites are more heavily represented, such as the complex of wrecks in the central region that includes Aeolus, Spar, Caribsea and Atlas (n=958 of 2028 encounters), as these are frequently visited SCUBA destinations (Gerken, 2013). Because of the live camera at Frying Pan Tower, we have a high proportion of observations from that location as well. Collectively, our observations are consistent with Paxton et al. (2020a) who found up to four times as many sand tigers at artificial habitats compared to natural hard bottom or natural ledge habitats. Others also document sand tigers at artificial reef habitats in the northern and central NC coasts (Whitfield et al., 2011; Brown et al., 2020). Using acoustic telemetry, Marens (2021) reported extended sand tiger residency at Frying Pan Tower; multiple shipwreck sites including Caribsea, Proteus, Tarpon, Atlas, Papoose, Schurz; and multiple artificial reef sites (e.g., AR-285, 275, 255) that are not popular dive sites (Dottie Benjamin, pers. comm.).
Valuable information about the occurrence of sand tigers comes from anecdotal and photographic evidence from the dive community about the sites frequented by SCUBA divers where sand tigers are most consistently observed. Conducting field research on sharks often requires labor-intensive and expensive methods to deploy divers, angle specimens and launch telemetry equipment, resulting in relatively fewer locations surveyed, fewer sampling days and lower numbers of sharks documented overall compared to the nearly 1900 sharks in SAS. Thus, because of its crowd-sourcing approach, SAS provided a cost-effective and non-invasive research tool. Resighting sharks within and between years will continue to illuminate finer-scale patterns of behavior.
4.2 Are there artificial reef sites that are especially important for sand tigers?
Despite the non-random sampling inherent to SAS, we can draw conclusions about which sites seem to be especially important. In the southern region, Frying Pan Tower has sand tigers year-round (Figure 4), and it is the potential role it may be playing for females during winter that is of particular interest as detailed below. The complex of shipwrecks in the central region is occupied by sand tigers year-round. Likely due to accessibility for dive operators and consistently good visibility, shipwrecks in this region are highly represented in the SAS database. Unbalanced sampling makes it difficult to draw rigorous conclusions about which sites may be most important for sand tigers, but the resighting data from 101 sharks indicate sand tigers display site fidelity within and between years and residency to shipwrecks in this region. Most sharks were resighted at the same or a nearby location, typically within a relatively short time span, indicating some sand tigers may be residential at certain sites for days to weeks in a given year. However, a few sand tigers were observed at the same location with months or even years between sightings. Given the high proportion of sand tigers in the SAS database recorded at Frying Pan Tower (24%), it is noteworthy that, of the 37 resighted sharks first recorded there, none were ever photographed at any other NC locations. In contrast, 28 of the resighted sharks were observed at more than one location, averaging more than 20 km away and up to 2176 DAL, indicating that individuals move between sites within and across years.
We have the least data from the northern shipwrecks (n=329 of 2028), perhaps due to the many days when marine conditions preclude diving. While this makes it difficult to ascribe more importance to any one site in this region, Dixie Arrow, Tarpon and Proteus, are noteworthy as possibly being important seasonally as aggregation sites for females. Marens (2021) found overall lower average residency times for sand tigers in the northern region (2 days) compared to southern (4 days) and central (6 days) sites. Though no explanation for this is evident, it is possible that environmental, physiological, or social drivers of occupancy and movement patterns may differ between these coastal regions in NC.
Our data showed most resightings occurred at the same or nearby locations, confirming previous findings that sand tiger sharks show high levels of residency and site fidelity to specific NC artificial reef sites (Paxton et al., 2019; Marens, 2021). This was especially evident at Frying Pan Tower where all the sharks first encountered there were resighted only there, including female USA-0272 who was seen there on 14 dates in 3 years. At several other locations, sharks were often resighted at the same site on sequential days or weeks and were also shown to return to the same site after presumably migrating out of NC waters for months or even years. Given the proximity of Aeolus and Spar and the high number of sharks resighted between those two sites, this area could be functionally perceived by the sharks as a single site as illustrated by many resighted sharks moving frequently between these two locations.
Our findings are consistent with telemetry data from Marens (2021) showing Atlas, Caribsea, Aeolus, and Papoose in the central region had the longest residency of male as well as immature and pregnant female sand tiger sharks (ranging from hours to 75+ days) compared to the other two regions. Coleman and Burge (2021) quantified frequency of sand tiger shark occurrence at Frying Pan Tower in more than 1000 video clips from Nov 2014–Jan 2019, reliably finding them every year fall through winter.
4.3 Are there temporal patterns in habitat use or site fidelity?
SAS data suggest sand tiger occupancy patterns show differential use of sites based upon season. This was true of Dixie Arrow and Tarpon where female occupancy was highest in summer, while at Proteus it was highest in fall. Interestingly, the occupancy patterns of sharks at Frying Pan Tower appear to be shifting. Prior to 2020, high numbers of sand tigers appeared in Oct, remained abundant through the winter, and left in April, with summer months having few or no records. In the last three years, live camera data suggest sand tigers are increasingly abundant year-round, though winter residency remains the time of peak abundance (E. Burge and A. McClanahan, unpublished data).
SAS has the most shark encounters in summer months. Consistent with generalized migratory patterns for this species driven by reproductive cycles, we begin to see higher numbers of shark encounters in spring and through summer. This is also the time of year when mixed groups of sand tigers are known to be migrating to and inhabiting summer grounds further north (Haulsee et al., 2018). Marens (2021) noted variability in movement of females in summer with some going north, while 7 were never observed outside of NC during her 3-year study. It is unknown if the sharks observed in NC in summer months are migratory, residential or a combination of these, and more research into their movement ecology during this period is needed. Because SCUBA divers who are SAS submitters are more active in summer months, our data cannot be relied upon to provide unbiased detection, and a more rigorous sampling protocol to investigate this is required.
4.4 Are there differences in site use between male and females?
Overall, we see about four times as many females as males in the SAS dataset, with nearly a third of the sharks’ sex not assignable. There is not a clear explanation for the unbalanced sex ratio, as we can find no published accounts that the population as a whole demonstrates a skewed sex ratio. Except for the two locations noted previously, sharks of both sexes were encountered at all locations. However, there is some indication that males and females may be utilizing habitats differently and at times may be sexually segregated. For example, at Atlas, Proteus, Tarpon and Dixie Arrow, females far outnumbered males. In contrast, other sites had male to female ratios more consistent with that of the SAS database, including Spar, Aeolus and W.E. Hutton. Studies in other populations have also found skewed sex ratios for this species during portions of the year, attributing this to sexual differences in movement patterns and differences in habitat requirements between juveniles and adults (Parker and Bucher, 2000; Lucifora et al., 2002; Smale, 2002; Lynch et al., 2013; Klein et al., 2019). Frying Pan Tower was one site were a higher proportion of males, including sub-adult males, were present contemporaneously with females, including in winter months. This was a different dynamic from any other location both in terms of timing of peak abundances in winter and sex ratios closer to parity.
Differences in female and male SAS encounter numbers could be attributed to males, especially adults, being more transient once mating season has passed. This is supported by most male encounters being in spring and summer months. If this is the case, then males may be more likely to be moving between shipwrecks and artificial reef sites and may display shorter residency times, thus having overall lower probability of being encountered by divers. Similar disparity in male versus female residency times, though not occupancy, which was similar, was found by Marens (2021) particularly in the central region. Those data also demonstrated that males that had migrated to NC waters after being fitted with acoustic telemetry tags in Delaware were absent in summer, unlike females that showed greater variability in summer residency patterns (Marens, 2021).
4.5 Is NC important for reproductive ecology of sand tigers?
The number of female sharks exhibiting mating scars indicate mating takes place in NC after males and females arrived following winter migrations. Given that females with healed mating scars continued to be observed through summer and fall, it appears at least a portion of pregnant females are remaining in NC waters for all or part of gestation. Some female sharks overwintered at Frying Pan Tower and many females observed there and elsewhere in winter appeared to be pregnant. Two videos from Frying Pan Tower (Erin Burge/Explore.org; 9 Sep 2021 https://www.youtube.com/watch?v=sZr3Jk45hF0, 11 Mar 2021 https://www.youtube.com/watch?v=tg6i79iZVgI) and one from Aeolus (12 Oct 2021; Ethan Simmons; https://youtu.be/E2_KmZb256I) show seemingly pregnant females with distinctive movement inside their abdomens likely to be live gestating pups. While this cannot be confirmed with photographic data alone, it is consistent with ultrasonography that confirmed 21 ovulatory and gravid females in NC between Jun-Jan (Marens, 2021; Wyffels et al., 2022; James Sulikowski, pers. comm.). Two of these sharks were part of Marens (2021) telemetry study and displayed extended winter residency at Frying Pan Tower before moving north in spring, presumably after parturition. SAS data suggest other locations that possibly serve as gestation aggregation sites in winter months included Spar, Aeolus, Schurz, and Tarpon, in the central region, and Proteus in the northern region. Marens (2021) similarly found pregnant tagged females off all regions of NC during winter months. This reproductive philopatry and aggregatory behavior of pregnant females is consistent with observations for this species in Australia (Bansemer and Bennett, 2009; Barker and Williamson, 2010; Lynch et al., 2013), South Africa (Smale, 2002; Dicken et al., 2007) and South America (Lucifora et al., 2002, 2009).
Mating and birth have not been observed in situ for this species in NC, and exact timing and location of these events remains unknown. SAS encounters of females both with mating scars and appearing pregnant are reported over spans of several months. Marens (2021) reported fresh mating scars were evident on females as late as early Aug. An extended mating timespan from Mar-Jun was described (Gilmore et al., 1983; Gilmore, 1993) as it may be occurring over a wide geographic range from NC to Florida. Although sand tigers are not thought to store sperm (Gilmore et al., 1983; Gilmore, 1993; Wyffels et al., 2022), one study did find they exhibit both behavioral and genetic polyandry in South Africa (Chapman et al., 2013). Females are investing significant energetic resources to produce only one or two offspring each reproductive cycle. If females in NWA are copulating with multiple males to enhance offspring fitness, they may be extending individual mating windows to increase the likelihood of encountering fit males. Gestation lasts between 9-12 months and may be determined by water temperature for this species (Bennett and Bansemer, 2004; Tokunaga et al., 2022). Dispersal of pregnant females along the NC coast in varying depths likely means they may be experiencing a range of winter temperature regimes. Thus, mating, gestation and parturition may be occurring over protracted and overlapping periods of time, rather than in tight synchrony. While these reproductive life history events may not all be taking place in NC for all sand tigers in the NWA population, SAS data reflects the importance of this area of the SEUS for this shark, particularly for mature females.
Based upon their size at encounter, USA-0128 and USA-L0817 were likely young of year (YOY) sharks, suggesting parturition may be occurring in NC waters. We have additional photographs not useable for SAS and anecdotes from divers of very small sand tigers, less than 1.2 m in length (the maximum size at birth for sand tigers (Gilmore, 1993; Gilmore et al., 2005) that support this possibility (C. Price, unpublished data). The exact locations of NWA pupping grounds have not been established for sand tigers. Our data contain no records of possible YOY sand tigers at Frying Pan Tower (E. Burge, unpublished data), nor have we observed small sharks there to suggest pupping is occurring at or near this location, despite its importance for overwintering pregnant females. One possibility is that females give birth closer inshore at natural hard bottom sites. This habitat type is widespread, but highly patchy, in NC (NCDEQ, 2016; Steward et al., 2022), not often frequented by dive charter operators, and often exhibits highly turbid conditions not conducive to underwater photography. In Australia, females do not give birth in estuaries, but are believed to prefer rocky reef habitats on the coast (Bennett and Bansemer, 2004). In South Africa, pupping grounds are also thought to be nearshore (Smale, 2002; Dicken et al., 2007).
5 Conclusions
SAS data indicated coastal NC may serve as a connective hotspot (Lowerre-Barbieri et al., 2021) linking sharks throughout their life stages at large and small spatial resolutions. Adult males and females, and sub-adults, are consistently present, even in months when conspecifics are residing further north or south. More than 25 SAS sharks appeared to be juveniles based upon slimmer body shape and size relative to other fishes or wreck features of known size. The presence of males with small claspers, suggested that young sharks may leave as-yet-undiscovered pupping areas to inhabit artificial reef sites which may be areas of high prey fish abundance (Rosemond et al., 2018). It is unknown how many of these juveniles may be out-migrating seasonally to established summer nursery areas further north (Kneebone et al., 2012, 2014) or using artificial reefs in NC as alternative nursery areas. Many shipwrecks off NC exhibit high rugosity relative to their surroundings (Paxton et al., 2017), and such three-dimensional structure offers considerable physical and ecological complexity in otherwise homogenous seascapes, simultaneously providing access to prey and protection from predators (Paxton et al., 2020a, b; Gámez and Harris, 2022). These data will aid future evaluation by NMFS of sand tiger management status, stock assessments, consideration of additional Habitat Areas of Particular Concern (HAPC) designations, and conservation efforts. New EFH in coastal Massachusetts for YOY and juveniles and HAPC in Delaware Bay for all life stages were only recently designated following research identifying the importance of these areas (NMFS, 2017). Locating sand tiger pupping grounds and nursery areas (Heupel et al., 2007) in NC are a high priority for a more complete understanding of the role NC waters play for sand tigers throughout their lives.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by NC Aquariums internal research review board. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AMc: Conceptualization, Data curation, Investigation, Visualization, Writing – review & editing. EB: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing. TH: Conceptualization, Investigation, Writing – review & editing. JH: Software, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The NC Aquarium Society provided financial support for Spot A Shark technicians.
Acknowledgments
This work would not be possible without the many generous divers who shared their photographs with us, especially Shawn Harper, Frank Krasovec, and Devin Daniels. Over 100 undergraduates at Coastal Carolina University harvested snapshots of sand tigers from Explore.org’s SharkCam. We appreciate the ongoing collaboration with Explore.org and Richard Neal at Frying Pan Tower. We thank the NC Aquarium Society, and the South-east Alliance for Reproduction and Conservation for funding to develop and operate Spot A Shark USA. We are grateful to local dive operators, particularly Bobby Purifoy and Dottie Benjamin, who spread the word about Spot a Shark and advocate for the conservation of sharks and all marine life. Finally, we owe a big thank you to the dedicated technicians that spent countless hours creating spot maps - Michael Mann, Erica Blair, Kirsten Boleyn, Julie Thomas, Mikayla Beeson, Ben Rinehart, Ben Regester, and Anna Gibbons.
Conflict of interest
Author TH was employed by company Blue Elements Imaging & Exploration.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1362703/full#supplementary-material
References
Arzoumanian Z., Holmberg J., Norman B. (2005). An astronomical pattern-matching algorithm for computer-aided identification of whale sharks Rhincodon typus. J. Appl. Ecol. 42, 999–1011. doi: 10.1111/j.1365-2664.2005.01117.x
Babits L. E. (2002). “Maritime archaeology in North Carolina,” in International handbook of underwater archaeology. The springer series in underwater archaeology. Eds. Ruppé C. V., Barstad J. F. (Springer, Boston, MA), 119–126. doi: 10.1007/978-1-4615-0535-8_9
Bansemer C. S., Bennett M. B. (2008). Multi-year validation of photographic identification of grey nurse sharks, Carcharias taurus, and applications for non-invasive conservation research. Mar. Freshw. Res. 59, 322–331. doi: 10.1071/MF07184
Bansemer C. S., Bennett M. B. (2009). Reproductive periodicity, localised movements and behavioural segregation of pregnant Carcharias taurus at Wolf Rock, southeast Queensland, Australia. Mar. Ecol. Prog. Ser. 374, 215–227. doi: 10.3354/meps07741
Bansemer C. S., Bennett M. B. (2010). Retained fishing gear and associated injuries in the east Australian grey nurse sharks (Carcharias taurus): implications for population recovery. Mar. Freshw. Res. 61, 97–103. doi: 10.1071/MF08362
Barker S. M., Peddemors V. M., Williamson J. E. (2011). A video and photographic study of aggregation, swimming and respiratory behaviour changes in the Grey Nurse Shark (Carcharias taurus) in response to the presence of SCUBA divers. Mar. Freshw. Behav. Physiol. 44, 75–92. doi: 10.1080/10236244.2011.569991
Barker S. M., Williamson J. E. (2010). Collaborative photo-identification and monitoring of grey nurse sharks (Carcharias taurus) at key aggregation sites along the eastern coast of Australia. Mar. Freshw. Res. 61, 971–979. doi: 10.1071/MF09215
Bennett M., Bansemer C. (2004). Investigations of Grey Nurse Shark in Queensland to fulfil actions under the Recovery Plan for Grey Nurse Shark (Carcharias taurus) in Australia regarding impact of divers, and establishment of a photographic database to improve knowledge of migratory movements, localised site movements and estimation of bycatch. Australian Natural Heritage Trust (Queensland, Australia: Commonwealth of Australia), 53. Available at: https://espace.library.uq.edu.au/view/UQ:84407.
Berger-Wolf T. Y., Rubenstein D. I., Stewart C. V., Holmberg J. A., Parham J., Menon S., et al. (2017). Wildbook: Crowdsourcing, computer vision, and data science for conservation. arXiv preprint arXiv:1710.08880. Bloomberg Data for Good Exchange Conference. 24 Sept 2017, Chicago, IL. doi: 10.48550/arXiv.1710.08880
Blount D., Gero S., Van Oast J., Parham J., Kingen C., Scheiner B., et al. (2022). Flukebook: an open-source AI platform for cetacean photo identification. Mamm. Biol. 102, 1005–1023. doi: 10.1007/s42991-021-00221-3
Bradsworth N., White J. G., Isaac B., Cooke R. (2017). Species distribution models derived from citizen science data predict the fine scale movements of owls in an urbanizing landscape. Biol. Conserv. 213, 27–35. doi: 10.1016/j.biocon.2017.06.039
Brown C. M., Paxton A. B., Taylor J. C., Van Hoeck R. V., Fatzinger M. H., Silliman B. R. (2020). Short-term changes in reef fish community metrics correlate with variability in large shark occurrence. Food Webs 24, e00147. doi: 10.1016/j.fooweb.2020.e00147
Burge E. J., Harris E. S. (2021). SharkCam fishes: A guide to nekton at Frying Pan Tower. Los Angeles: Explore.org Ocean Frontiers, 228 pp. doi: 10.5281/zenodo.5842340
Campagno L. J. V. (1984). FAO Species Catalogue. Vol. 4. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part 2 - Carcharhiniformes Vol. 125 (Rome: FAO Fish. Synop. FAO Fish. Synop. FAO), 251–655.
Carlson J. K., McCandless C. T., Cortés E., Grubbs R. D., Andrews K. I., Musick J. A. (2009). An update on the status of the sand tiger shark, Carcharias taurus, in the northwest Atlantic Ocean. NOAA Technical Memorandum NMFS-SEFSC-585: U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Southeast Fisheries Science Center, Panama City Laboratory, Panama City, FL.
Chapman D. D., Wintner S. P., Abercrombie D. L., Ashe J., Bernard A. M., Shivji M. S., et al. (2013). The behavioural and genetic mating system of the sand tiger shark, Carcharias taurus, an intrauterine cannibal. Biol. Lett. 9, 20130003. doi: 10.1098/rsbl.2013.0003
Coleman N. C., Burge E. J. (2021). Association behavior between sand tiger sharks and round scad is driven by mesopredators. PeerJ 9, e11164. doi: 10.7717/peerj.11164
Comer A. M., Love-Adrick R. (2016). North Carolina division of marine fisheries artificial reef guide (Morehead City, NC: North Carolina Department of Environmental Quality), 131.
Dicken M. L., Booth A. J., Smale M. J., Cliff G. (2007). Spatial and seasonal distribution patterns of juvenile and adult raggedtooth sharks (Carcharias taurus) tagged off the east coast of South Africa. Mar. Freshw. Res. 58, 127–134. doi: 10.1071/MF06018
Dicken M. L., Smale M. J., Booth A. J. (2006). Shark fishing effort and catch of the ragged-tooth shark Carcharias taurus in the South African competitive shore-angling fishery. Afr. J. Mar. Sci. 28, 589–601. doi: 10.2989/18142320609504209
Dudgeon C. L., Kilpatrick C., Armstrong A., Armstrong A., Bennett M. B., Bowden D., et al. (2019). “Citizen Science photographic identification of marine megafauna populations in the Moreton Bay Marine Park,” in Moreton Bay Quandamooka & Catchment: Past, present, and future. Eds. Tibbetts I. R., Rothlisberg P. C., Neil D. T., Homburg T. A., Brewer D. T., Arthington A. H. (Brisbane, Australia: The Moreton Bay Foundation), 447–490. Available at: https://moretonbayfoundation.org/.
Dunbar S. G., Anger E. C., Parham J. R., Kingen C., Wright M. K., Hayes C. T., et al. (2021). HotSpotter: Using a computer-driven photo-id application to identify sea turtles. J. Exp. Mar. Biol. Ecol. 535, 151490. doi: 10.1016/j.jembe.2020.151490
Dwyer R. G., Rathbone M., Foote D. L., Bennett M., Butcher P. A., Otway N. M., et al. (2023). Marine reserve use by a migratory coastal shark, Carcharias taurus. Biol. Conserv. 283, 110099. doi: 10.1016/j.biocon.2023.110099
Earp H. S., Liconti A. (2020). “Science for the Future: The use of citizen science in marine research and conservation,” in YOUMARES 9 - The Oceans: Our Research, Our Future: Proceedings of the 2018 conference for YOUng MArine RESearcher in Oldenburg, Germany. Eds. Jungblut S., Liebich V., Bode-Dalby M. (Cham: Springer International Publishing), 1–19. doi: 10.1007/978-3-030-20389-4_1
Farmer C. H. (2004). Sharks of South Carolina (Charleston, SC: South Carolina Department of Natural Resources Marine Resources Division).
Gámez S., Harris N. C. (2022). Conceptualizing the 3D niche and vertical space use. Trends Ecol. Evol. 37, 953–962. doi: 10.1016/j.tree.2022.06.012
Gerken M. (2013). Top 10 wreck dives of North Carolina (Scuba Diving). Available online at: https://www.scubadiving.com/photos/top-10-wreck-dives-north-carolina (Accessed on 28 May 2023).
Gilmore R. G. (1993). Reproductive biology of lamnoid sharks. Environ. Biol. Fishes 38, 95–114. doi: 10.1007/BF00842907
Gilmore R., Dodrill J., Linley P. (1983). Reproduction and embryonic development of the sand tiger shark, Odontaspis taurus (Rafinesque). Fishery Bull. 81, 201–225.
Gilmore R. G., Outz O., Dodrill J. W. (2005). “Oophagy, intrauterine cannibalism and reproductive strategy in lamnoid sharks,” in Reproductive biology and phylogeny of Chondrichthyes. Ed. Hamlett W. M. (Science Publishers, Inc., Enfield, NH), 435–463.
Giovos I., Stoilas V., Al-mabruk S. A., Doumpas N., Marakis P., Maximiadi M., et al. (2019). Integrating local ecological knowledge, citizen science and long-term historical data for endangered species conservation: Additional records of angel sharks (Chondrichthyes: Squatinidae) in the Mediterranean Sea. Aquat. Conservation: Mar. Freshw. Ecosyst. 29, 881–890. doi: 10.1002/aqc.3089
Goldman K. J., Branstetter S., Musick J. A. (2006). A re-examination of the age and growth of sand tiger sharks, Carcharias taurus, in the western North Atlantic: the importance of ageing protocols and use of multiple back-calculation techniques. Environ. Biol. Fishes 77, 241–252. doi: 10.1007/s10641-006-9128-y
Gould J., Clulow J., Clulow S. (2021). Using citizen science in the photo-identification of adult individuals of an amphibian based on two facial skin features. PeerJ 9, e11190. doi: 10.7717/peerj.11190http://doi.org/10.7717/peerj.11190
Groth E. J. (1986). A pattern-matching algorithm for two-dimensional coordinate lists. Astronomical J. 91, 1244–1248. doi: 10.1086/114099
Ha D. S. (2006). Ecology and conservation of Virginia shark species: Analysis of thirty years of Virginia long-line shark census data, 1974–2004. [Dissertation]. [Williamsburg (VA)]: College of William and Mary. doi: 10.25773/v5-masw-m267
Haulsee D. E., Breece M. W., Brown L. M., Wetherbee B. M., Fox D. A., Oliver M. J. (2018). Spatial ecology of Carcharias taurus in the northwestern Mid-Atlantic coastal ocean. Mar. Ecol. Prog. Ser. 597, 191–206. doi: 10.3354/meps12592
Haulsee D. E., Fox D. A., Breece M. W., Brown L. M., Kneebone J., Skomal G. B., et al. (2016). Social network analysis reveals potential fission-fusion behavior in a shark. Sci. Rep. 6, 34087. doi: 10.1038/srep34087
Heupel M. R., Carlson J. K., Simpfendorfer C. A. (2007). Shark nursery areas. Mar. Ecol. Prog. Ser. 337, 287–297. doi: 10.3354/meps337287
Holmberg J., Norman B., Arzoumanian Z. (2008). Robust, comparable population metrics through collaborative photo-monitoring of whale sharks Rhincodon typus. Ecol. Appl. 18, 222–233. doi: 10.1890/07-0315.1
Hoschke A. M., Whisson G. J., Haulsee D. (2023). Population distribution, aggregation sites and seasonal occurrence of Australia’s western population of the grey nurse shark Carcharias taurus. Endangered Species Res. 50, 107–123. doi: 10.3354/esr01225
Hoyt J., Delgado J. P., Barr B., Terrell B., Grussing V. (2014). Graveyard of the Atlantic: An overview of North Carolina’s maritime cultural landscape. Maritime Heritage Program Series: Number 4 NOAA Office of National Marine Sanctuaries (Silver Spring, MD), 58 pp.
Kilfoil J. P., Wetherbee B. M., Carlson J. K., Fox D. A. (2017). Targeted catch-and-release of prohibited sharks: Sand tigers in coastal Delaware waters. Fisheries 42, 281–287. doi: 10.1080/03632415.2017.1306974
Klein J. D., Bester-van der Merwe A. E., Dicken M. L., Mmonwa K. L., Teske P. R. (2019). Reproductive philopatry in a coastal shark drives age-related population structure. Mar. Biol. 166, 1–12. doi: 10.1007/s00227-019-3467-7
Kneebone J., Chisholm J., Bernal D., Skomal G. (2013). The physiological effects of capture stress, recovery, and post-release survivorship of juvenile sand tigers (Carcharias taurus) caught on rod and reel. Fisheries Res. 147, 103–114. doi: 10.1016/j.fishres.2013.04.009
Kneebone J., Chisholm J., Skomal G. (2014). Movement patterns of juvenile sand tigers (Carcharias taurus) along the east coast of the USA. Mar. Biol. 161, 1149–1163. doi: 10.1007/s00227-014-2407-9
Kneebone J., Chisolm J., Skomal G. B. (2012). Seasonal residency, habitat use, and site fidelity of juvenile sand tiger sharks Carcharias taurus in a Massachusetts estuary. Mar. Ecol. Prog. Ser. 471, 165–181. doi: 10.3354/meps09989
Kohler N. E., Turner P. A. (2019). Distributions and movements of Atlantic shark species: A 52-year retrospective atlas of mark and recapture data. Mar. Fisheries Rev. 81, 1–93. doi: 10.7755/MFR.81.2.1
Latour R. J., Gartland J. (2020). Dynamics of the shark community in the Mid-Atlantic Bight. Mar. Biol. 167, 100. doi: 10.1007/s00227-020-03720-y
Low R. A., Ulrich G. F. (1984). Survey of the shark resource in shelf waters off South Carolina. Marine Resources Division, South Carolina Wildlife and Marine Resources Department, Charleston, South Carolina. Technical report Number 61. 31 pp. Available at: https://dc.statelibrary.sc.gov/bitstream/handle/10827/30367/DNR_Survey_of_the_Shark_1984-5.pdf?sequence=1.
Lowerre-Barbieri S. K., Friess C., Griffin L. P., Morley D., Skomal G. B., Bickford J. W., et al. (2021). Movescapes and eco-evolutionary movement strategies in marine fish: Assessing a connectivity hotspot. Fish Fisheries 22, 1321–1344. doi: 10.1111/faf.12589
Lucifora L. O., García V. B., Escalante A. H. (2009). How can the feeding habits of the sand tiger shark influence the success of conservation programs. Anim. Conserv. 12, 291–301. doi: 10.1111/j.1469-1795.2009.00247.x
Lucifora L. O., Menni R. C., Escalante A. H. (2002). Reproductive ecology and abundance of the sand tiger shark, Carcharias taurus, from the southwestern Atlantic. ICES J. Mar. Sci. 59, 553–561. doi: 10.1006/jmsc.2002.1183
Lynch T. P., Harcourt R., Edgar G., Barrett N. (2013). Conservation of the critically endangered eastern Australian population of the grey nurse shark (Carcharias taurus) through cross-jurisdictional management of a network of marine-protected areas. Environ. Manage. 52, 1341–1354. doi: 10.1007/s00267-013-0174-x
Marens M. (2021). Movement and habitat use of sand tiger sharks (Carcharias taurus) in North Carolina coastal waters. [Master’s Thesis]. [Wilmington (NC)]: University of North Carolina Wilmington.
Mason L., Arathi H. S. (2019). Assessing the efficacy of citizen scientists monitoring native bees in urban areas. Global Ecol. Conserv. 17, e00561. doi: 10.1016/j.gecco.2019.e00561
Mcclellan Press K., Mandelman J., Burgess E., Cooke S. J., Nguyen V. M., Danylchuk A. J. (2016). Catching sharks: recreational saltwater angler behaviours and attitudes regarding shark encounters and conservation. Aquat. Conserv.: Mar. Freshw. Ecosyst. 26, 689–702. doi: 10.1002/aqc.2581
Musick J. A., Branstetter S., Colvocoresses J. A. (1993). “Trends in shark abundance from 1974 to 1991 for the Chesapeake Bight region of the U.S. Mid-Atlantic Coast,” in Conservation biology of elasmobranchs. Ed. Branstetter S. (NOAA Technical Report NMFS 115), 1–18. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Springfield, VA. Available at: https://spo.nmfs.noaa.gov/Technical%20Report/tr115.pdf.
NCDEQ. (2016). “North Carolina coastal habitat protection plan source document,” in Anonymous. NC (Division of Marine Fisheries, Morehead City, NC).
NC Division of Marine Fisheries. (2022). Location of hard bottom habitat. https://deq.nc.gov/about/divisions/marine-fisheries/habitat-information/coastal-habitat-types/hard-bottom (Accessed 09 December 2022).
NMFS (National Marine Fisheries Service). (2009). FAtlantic Highly Migratory Species; Essential Fish Habitat 74 Fed. Reg. 28018–28025 (June 12, 2009) NDepartment of Commerce, National Oceanic and Atmospheric Administration. RIN 0648–AV00. 1600. Available at: https://www.govinfo.gov/content/pkg/FR-2009-06-12/pdf/E9-13866.pdf.
NMFS. (2017). Final amendment 10 to the 2006 consolidated Atlantic Highly Migratory Species Fishery Management Plan: Essential fish habitat (Office of Sustainable Fisheries Silver Spring, MD. 75 pp: National Marine Fisheries Service).
NOAA. (2022). Wrecks and obstructions database. Available at: https://wrecks.nauticalcharts.noaa.gov/viewer/.
NOAA, NMFS. (2000). Atlantic highly migratory species (HMS) fisheries; prohibited shark species; large coastal shark species; commercial fishery closure change. Federal register 65:120 (21 june 2000) (Accessed 25 January 2024).
Norman B. M., Holmberg J. A., Arzoumanian Z., Reynolds S. D., Wilson R. P., Rob D., et al. (2017). Undersea Constellations: The global biology of an endangered marine megavertebrate further Informed through citizen science. Bioscience 67, 1029–1043. doi: 10.1093/biosci/bix127
Olbers J., Smith G. (2019). Ragged-tooth shark (Carcharias taurus) abundance and behaviour during their annual gestation period on Quarter-mile reef, Sodwana Bay, iSimangaliso Wetland Park, South Africa. Season Report for 2018/2019. Ezemvelo KZN Wildlife Internal Report, Pietermaritzburg, South Africa. 11 pp. doi: 10.13140/RG.2.2.26675.09768
Parham J., Stewart C., Crall J., Rubenstein D., Holmberg J., Berger-Wolf T. (2018). “An animal detection pipeline for identification,” in 2018 IEEE Winter Conference on Applications of Computer Vision (WACV), 12–15 March 2018 (Lake Tahoe, NV), 1075–1083.
Parker P., Bucher D. J. (2000). Seasonal variation in abundance and sex ratio of grey nurse (sand tiger) sharks Carcharias taurus in northern New South Wales, Australia: A survey based on observations of recreational SCUBA divers. Pacific Conserv. Biol. 5, 336–346. doi: 10.1071/PC000336
Paxton A. B., Blair E., Blawas C., Fatzinger M. H., Marens M., Holmberg J., et al. (2019). Citizen science reveals female sand tiger sharks (Carcharias taurus) exhibit signs of site fidelity on shipwrecks. Ecology 100, e02687. doi: 10.1002/ecy.2687
Paxton A. B., Newton E. A., Adler A. M., Van Hoeck R. V., Iversen E. S., Taylor J. C., et al. (2020a). Artificial habitats host elevated densities of large reef-associated predators. PloS One 15, e0237374. doi: 10.1371/journal.pone.0237374
Paxton A. B., Pickering E. A., Adler A. M., Taylor J. C., Peterson C. H. (2017). Flat and complex temperate reefs provide similar support for fish: Evidence for a unimodal species-habitat relationship. PloS One 12. doi: 10.1371/journal.pone.0183906
Paxton A. B., Shertzer K. W., Bacheler N. M., Kellison G. T., Riley K. L., Taylor J. C. (2020b). Meta-analysis reveals artificial reefs can be effective tools for fish community enhancement but are not one-size-fits-all. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00282
Pollard D. A., Lincoln Smith M. P., Smith A. K. (1996). The biology and conservation status of the grey nurse shark (Carcharias taurus Rafinesque 1810) in New South Wales, Australia. Aquat. Conservation: Mar. Freshw. Ecosyst. 6, 1–20. doi: 10.1002/(SICI)1099-0755(199603)6:1<1::AID-AQC177>3.0.CO;2-%23
Pottie S., Flam A. L., Keeping J. A., Chivindze C., Bull J. C. (2021). Quantifying the distribution and site fidelity of a rare, non-commercial elasmobranch using local ecological knowledge. Ocean Coast. Manage. 212, 105796. doi: 10.1016/j.ocecoaman.2021.105796
Radcliffe L. (1916). The sharks and rays of Beaufort, North Carolina (Beaufort, NC: United States Fisheries Biological Station), 58.
Rigby C. L., Carlson J., Derrick D., Pacoureau N., Simpfendorfer C. (2021) Carcharias taurus. The IUCN red list of threatened species 2021 (IUCN) (Accessed 02 January 2023). e.T3854A2876505.
Rohner C. A., Pierce S. J., Marshall A. D., Weeks S. J., Bennett M. B., Richardson A. J. (2013). Trends in sightings and environmental influences on a coastal aggregation of manta rays and whale sharks. Mar. Ecol. Prog. Ser. 482, 153–168. doi: 10.3354/meps10290
Roose R., Oliver M., Haulsee D., Breece M., Carlisle A., Fox D. (2022). The sociality of Atlantic sturgeon and sand tiger sharks in an estuarine environment. Anim. Behav. 193, 181–191. doi: 10.1016/j.anbehav.2022.08.008
Rosemond R. C., Paxton A. B., Lemoine H. R., Fegley S. R., Peterson C. H. (2018). Fish use of reef structures and adjacent sand flats. Mar. Ecol. Prog. Ser. 587, 187–199. doi: 10.3354/meps12428
Schwartz F. J., Hoey J. J., McCandless C. T., Peterson C. H. (2013). Longline Shark Survey Data Report: 1972-2011. University of North Carolina at Chapel Hill, Institute of Marine Sciences UNC-IMS Data Report (Morehead City, NC). Available at: http://ims.unc.edu/files/2013/11/sharksurvey.pdf.
Smale M. J. (2002). Occurrence of Carcharias taurus in nursery areas of the Eastern and Western Cape, South Africa. Mar. Freshw. Res. 53, 551–556. doi: 10.1071/MF01129
Smith H. M. (1907). The fishes of North Carolina (Raleigh, NC: North Carolina Geological and Economic Survey), 453. doi: 10.5962/bhl.title.56126
Steward D. N., Paxton A. B., Bacheler N. M., Schobernd C. M., Mille K., Renchen J., et al. (2022). Quantifying spatial extents of artificial versus natural reefs in the seascape. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.980384
Teter S. M., Wetherbee B. M., Fox D. A., Lam C. H., Kiefer D. A., Shivji M. (2015). Migratory patterns and habitat use of the sand tiger shark (Carcharias taurus) in the western North Atlantic. Mar. Freshw. Res. 66, 158–169. doi: 10.1071/MF14129
Tokunaga S., Watanabe Y. Y., Kawano M., Kawabata Y. (2022). Factors affecting gestation periods in elasmobranch fishes. Biol. Open 11, bio059270. doi: 10.1242/bio.059270
Van Tienhoven A. M., Hartog J. E. D., Reijns R. A., Peddemors V. M. (2007). A computer-aided program for pattern-matching of natural marks on the spotted raggedtooth shark Carcharias taurus. J. Appl. Ecol. 44, 273–280. doi: 10.1111/j.1365-2664.2006.01273.x
Verschueren S., Fabiano E. C., Kakove M., Cristescu B., Marker L. (2023). Reducing identification errors of African carnivores from photographs through computer-assisted workflow. Mammal Res. 68, 121–125. doi: 10.1007/s13364-022-00657-z
Weideman H. J., Stewart C. V., Parham J. R., Holmberg J., Flynn K., Calambokidis J., et al. (2020). “Extracting identifying contours for african elephants and humpback whales using a learned appearance model,” in 2020 IEEE Winter Conference on Applications of Computer Vision (WACV). Presented at the 2020 IEEE Winter Conference on Applications of Computer Vision (WACV), pp. 1265–1274. doi: 10.1109/WACV45572.2020.9093266
Whitfield P. E., Muñoz R. C., Buckel C. A., Heeseman L. M. (2011). Fish Community and Habitat Assessments on North Carolina Shipwrecks: Monitor National Marine Sanctuary, Battle of the Atlantic, June 2010 Marine Sanctuaries Conservation Series ONMS-11-03. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Office of National Marine Sanctuaries, Silver Spring, MD. 39 pp.
Williams B. L., Roberson K., Young J., Kendall M. S. (2019). Using Acoustic Telemetry to Understand Connectivity of Gray’s Reef National Marine Sanctuary to the U.S. Atlantic Coastal Ocean. NOAA Technical Memorandum NOS NCCOS 259. National Oceanic and Atmospheric Administration. Silver Spring, MD. 82 pp. doi: 10.25923/r2ma-5m96
Wyffels J. T., Coco C., Schreiber C., Palmer D., Clauss T., Bulman F., et al. (2020). Natural environmental conditions and collaborative efforts provide the secret to success for sand tiger shark Carcharias taurus reproduction in aquaria. Zoo Biol. 39, 355–363. doi: 10.1002/zoo.21558
Keywords: movement ecology, site fidelity, shipwreck habitat, shark photo identification, reproductive ecology, Carcharias taurus
Citation: Price CS, McClanahan AL, Burge EJ, Houppermans T and Holmberg J (2024) Community science informs movement and reproductive ecology of sand tigers Carcharias taurus off North Carolina, United States of America. Front. Mar. Sci. 11:1362703. doi: 10.3389/fmars.2024.1362703
Received: 29 December 2023; Accepted: 07 March 2024;
Published: 03 May 2024.
Edited by:
Emily Lester, Australian Institute of Marine Science (AIMS), AustraliaReviewed by:
Peter J. Auster, Mystic Aquarium, United StatesJosé Pedro Andrade, University of Algarve, Portugal
Copyright © 2024 Price, McClanahan, Burge, Houppermans and Holmberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carol Seals Price, Y2Fyb2wucHJpY2VAbmNhcXVhcml1bXMuY29t
 Carol Seals Price
Carol Seals Price Ara Lupton McClanahan1,2
Ara Lupton McClanahan1,2 Erin J. Burge
Erin J. Burge