
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci., 12 February 2024
Sec. Ocean Solutions
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1359614
Macroalgae cultivation is receiving growing attention as a potential carbon dioxide removal (CDR) strategy. Macroalgae biomass harvesting and/or intentional sinking have been the main focus of research efforts. A significant amount of biomass is naturally lost through erosion and breakage of cultivated or naturally growing seaweed, but the contribution of the resulting particulates to carbon sequestration is relatively unexplored. Here, we use a fully coupled kelp-biogeochemistry model forced by idealized parameters in a closed system to estimate the potential of macroalgal-derived particulate organic carbon (POC) sinking as a CDR pathway. Our model indicates that at a kelp density of 1.1 fronds m−3, macroalgal POC sinking can export 7.4 times more carbon to the deep sea (depths > 500m) and remove 5.2 times more carbon from the atmosphere (equivalent to an additional 336.0 gC m−2 yr−1) compared to the natural biological pump without kelp in our idealized closed system. The results suggest that CDR associated with POC sinking should be explored as a possible benefit of seaweed farming and point to the need for further study on organic carbon partitioning and its bioavailability to quantify the effectiveness and impacts of macroalgal cultivation as a CDR strategy.
Macroalgae (seaweed) cultivation is receiving growing attention as a potential carbon dioxide removal (CDR) strategy to mitigate climate change (Duarte et al., 2017; Gattuso et al., 2021; National Academies of Sciences, E. and Medicine, 2022; de Ramon N’Yeurt et al., 2012). The motivation behind this strategy stems from the high rate of primary production and biomass accumulation of seaweed (Duarte and Cebrián, 1996; Schiel and Foster, 2015; Duarte et al., 2017; Smale et al., 2020), the immense size of the ocean, the buffering capacity of seawater, and the durability of the ocean as carbon storage (Gruber et al., 2019; Siegel et al., 2021). However, important questions remain surrounding the effectiveness and impacts of this strategy (Boyd et al., 2022; Gallagher et al., 2022; Stafford, 2022).
Two recent studies have examined macroalgae cultivation and intentional harvesting or intentional sinking of the biomass as a CDR method (Wu et al., 2022; Arzeno-Soltero et al., 2023). The concept behind the intentional sinking strategy is that cultivated macroalgae are harvested and sunk to the deep ocean where the carbon will be potentially sequestered for a climate-relevant time scale (GESAMP, 2019; Gattuso et al., 2021; National Academies of Sciences, E. and Medicine, 2022). The results of Wu et al. (2022) suggested that macroalgae cultivation and sinking have considerable CDR potential which can be further boosted by artificial upwelling to alleviate nutrient limitation and that large-scale deployment of the strategy would have significant side effects, including the reduction of phytoplankton net primary production and the creation of new oxygen minimum zones on the seafloor. Arzeno-Soltero et al. (2023) simulated the potential of seaweed farming (including four seaweed types) to produce Gt-scale biomass carbon under two nitrate scenarios without explicitly accounting for feedback to nitrate cycling or competition with phytoplankton. The results indicated that 1 GtC yr−1 biomass carbon could be harvested by farming seaweed in the most productive 0.8% of exclusive economic zones worldwide (>1 million km2).
Macroalgal habitats are characterized by rapid biomass turnover, which results in large amounts of carbon entering the marine environment as detritus (particulate organic carbon or POC) through incremental blade erosion and dislodgement (Mann, 1973; Krause-Jensen and Duarte, 2016). It has been estimated that 82% of the global average productivity of kelp (large brown seaweeds that make up the order Laminariales) beds or forests (864 gC m−2 yr−1) are channeled to the detrital pool (Krumhansl and Scheibling, 2012). About 32% of the global carbon sequestration by macroalgae (173 TgC yr−1) has been estimated to occur through POC sedimentation and deep sea export (Krause-Jensen and Duarte, 2016). Similarly, for cultivated macroalgae, an estimated 8-49.4% of the annual gross production of Saccharina latissima was lost to the environment in Norway (Fieler et al., 2021). Carbon loss from a Saccharina japonica farm in China was estimated at about 61% of gross production (Zhang et al., 2012). POC thus has the potential to serve as one of the essential pathways of carbon sequestration and CDR (National Academies of Sciences, E. and Medicine, 2022).
POC production and export from cultivated macroalage have not been fully explored as a CDR pathway. In the model of Wu et al. (2022), the eroded macroalgal biomass was directly converted back to nutrients and dissolved inorganic carbon (DIC), and POC export was not directly included. Arzeno-Soltero et al. (2023) predicted potential seaweed biomass production for CDR, but didn’t report carbon export. The lack of quantitative data characterizing the POC sinking from cultivated macroalgae is a major gap yet to be filled in order to fully assess different CDR pathways and to develop a feasible implementation strategy.
Along with uncertainties surrounding POC export, two additional limitations of existing studies motivate us to develop a new model to study the macroalgae cultivation for ocean-based CDR. First, the CDR potential and the magnitude of possible ecological side effects of macroalage cultivation will depend on the macroalgal cultivation density (Boyd et al., 2022). Existing studies have not explicitly varied the macroalgal cultivation density, which limits our understanding of the impacts and sequestration potential of large-scale CDR deployments. Second, there has been a debate about the imported organic subsidies in an open ecosystem and their impact on the global seaweed net carbon balance (Gallagher et al., 2022; Stafford, 2022), which motivates us to consider a closed system where we can readily track all forms of carbon, nutrients, and other materials.
Here, we use OceanBioME (Strong-Wright et al., 2023a), an environment for modeling the kelp-biogeochemical interactions in an idealized closed 1D column configuration to study macroalgae cultivation as a CDR strategy. This fully coupled kelp-biogeochemical model enables us to predict macroalgae growth based on ambient open ocean conditions and to quantify the impact of kelp cultivation on the biogeochemistry and carbon fluxes. We focus on the vertical carbon flux due to sinking POC, referred to as the gravitational pump (Resplandy et al., 2019) and the air-sea CO2 flux, as a function of time and kelp cultivation density. The aims of the study are to evaluate whether POC sinking alone can be an effective pathway for carbon sequestration, to identify the relationship between carbon fluxes and kelp cultivation density, and to deepen our understanding of the potential impacts of large-scale macroalgae cultivation on the marine ecosystem.
Large-scale macroalgae cultivation in coastal waters may be limited by the relatively small coastal areas available for farming, or conflict with other blue economic activities such as fisheries, tourism, and marine energy (Azevedo et al., 2019; Wu et al., 2022). Here, we focus on macroalgae cultivation in the open ocean, building on the previous work of (Strong-Wright and Taylor, 2022) which modeled the growth potential of sugar kelp in the North Atlantic. Whereas Strong-Wright and Taylor (2022) did not consider the impacts of macroalgae cultivation on nutrients or phytoplankton, our fully coupled model enables us to analyze the two-way coupling between the seaweed and the ambient environment. Using an idealized 1D column model in a closed system, we are able to reproduce natural seasonal cycles and better understand the sensitivity to key parameters.
The model used in this study provided by OceanBioME is based on a modified version of the Lodyc Ocean Biogeochemical Simulation Tools for Ecosystem and Resources (LOBSTER) model (Lévy et al., 2005), coupled with a carbonate chemistry model (Resplandy et al., 2009) and a kelp growth model (Broch and Slagstad, 2012; Broch et al., 2013; Fossberg et al., 2018; Broch et al., 2019). Details of the model formulation and implementation are available in the archive Strong-Wright et al. (2023b). Saccharina latissima (sugar kelp) was chosen because it is well-studied and widely used in aquaculture and has been proposed as a candidate for offshore macroalgae farms (Broch et al., 2019; RunningTide, 2021; Strong-Wright and Taylor, 2022). To simulate kelp growth we use a model for individual fronds which integrate three coupled equations for the frond area, the carbon and the nitrogen contents of the kelp. Specifically, the kelp uptakes nitrate, ammonium, and dissolved inorganic carbon from the surrounding water and releases dissolved and particulate organic matter. It is assumed that nitrogen is the limiting nutrient in the kelp growth model because either its demand/supply ratio is higher in the ocean compared with other nutrients [e.g. phosphorus (Atkinson and Smith, 1983; Martiny et al., 2014)], or the potential constraints of other micronutrients [e.g. iron (Paine et al., 2023)] can be overcome by designing a cultivation platform equipped with a nutrient supply. Similarly, in a global model of kelp growth Arzeno-Soltero et al. (2023) assumed that nitrogen is the limiting nutrient, while Wu et al. (2022) considered nitrogen and phosphorous but neglected the limitation by micronutrients. If additional nutrients limit kelp growth in the open ocean, this would likely reduce the kelp primary production and CDR potential, while potentially increasing competition between kelp and phytoplankton. OceanBioME uses the fluid dynamics package Oceananigans (Ramadhan et al., 2020) to integrate the tracer conservation equation and track the properties of biological particles. A schematic of the model is presented in Figure 1 and the details of the model are provided in the Supplementary Material.
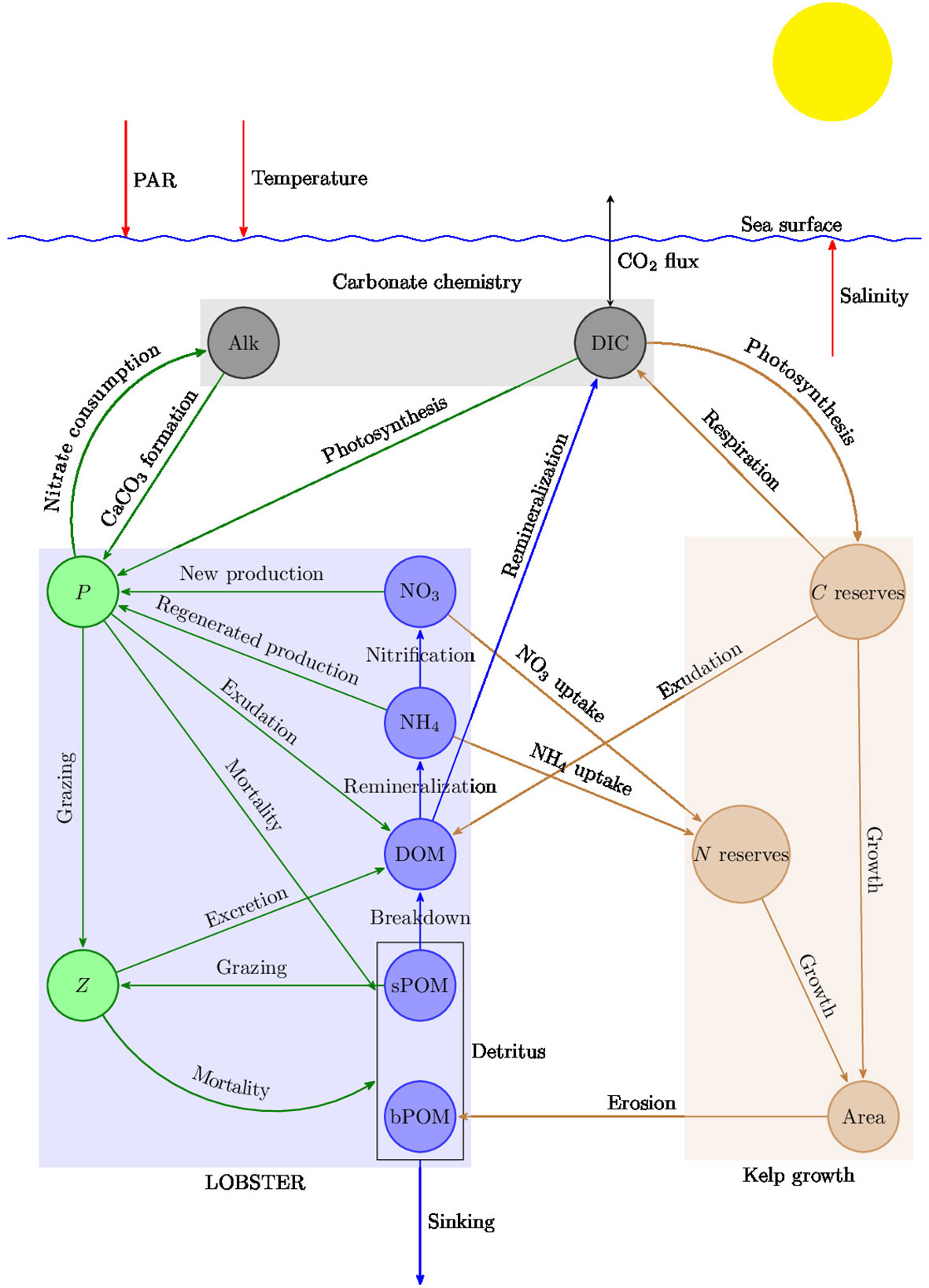
Figure 1 Schematic of OceanBioME. The state variables include phytoplankton (P), zooplankton (Z), nitrate (NO3), ammonium (NH4), semi-labile dissolved organic matter (DOM), small particulate organic matter (sPOM), and big particulate organic matter (bPOM), expressed in terms of their nitrogen content (mmolN m−3), dissolved inorganic carbon (DIC) in mmolC m−3, alkalinity (Alk) in meq m−3, frond area in dm2, nitrogen reserves in gram N per gram structural mass (g N (g sw)−1), and carbon reserves in g C (g sw)−1.
Here, we run OceanBioME in a 1D column configuration. This removes the direct influence of advection and allows us to consider the carbon fluxes in a closed system. Although the 1D model does not calculate horizontal gradients, we still need to specify a nominal horizontal domain size to couple the kelp growth model with the ecosystem and carbonate chemistry models. In all cases, we use a nominal domain size of 20m×20m×600m (depth), and the vertical grid spacing is 6m. We repeated the calculations with higher vertical resolution and obtained very similar results. We integrate one kelp growth model per vertical meter within the upper 100m of the water column, mimicking kelp grown from a vertical rope. One way to interpret this configuration is a very large kelp farm where properties are homogeneous in the horizontal direction. Although we always use one individual kelp model per vertical meter, we vary the kelp density from 0.025 to 250 fronds m−3 scaling the interactions between the kelp and the ecosystem and carbonate chemistry models. The range of kelp densities that we selected was chosen to cover a large range of densities in the literature (Broch et al., 2013; Forbord et al., 2020; Wu et al., 2022).
The model is forced by an idealized annual cycle of surface PAR data derived from observations (NASA Goddard Space Flight Center, Ocean Ecology Laboratory, Ocean Biology Processing Group, 2021) and temperature, salinity, and mixed layer depth (MLD) data from a reanalysis product (E.U. Copernicus Marine Service Information, 2023a) averaged over the region between 20° and 25° W and 55° and 60° N in the North Atlantic (see the Supplementary Material). Piecewise linear functions were used to idealize the forcing parameters and the annual cycle of the mixed layer depth is made up of distinct regimes due to the combination of mechanical and surface density forcings such as winds, tides, solar radiation, heat and freshwater exchange, etc. (Williams and Follows, 2011). Details of the idealization of the forcing parameters are shown in the Supplementary Material. Vertical mixing is parameterized by prescribing a vertical diffusivity as a quadratic function of depth in the mixed layer with a maximum diffusivity of 0.08 m2 s−1and a constant diffusivity of 0.0001 m2 s−1below the mixed layer (see the Supplementary Material for details). The characteristic values of turbulent diffusivity were chosen to better match the results of the Mercator model (see Section 2.3). The models were run for a period of 2 years starting from 1 January with a time step of 3.5 minutes. The initial conditions for the kelp state variables are A0 = 0.1 dm−2, N0 = 0.01 g N (g sw)−1, and C0 = 0.1 g C (g sw)−1. The initial area A0 is much smaller than the area when the kelp is fully grown and hence the model results are not very sensitive to the initial carbon, C0, and nitrogen, N0, reserves (Strong-Wright and Taylor, 2022).
We compared our model without kelp to a reanalysis product (E.U. Copernicus Marine Service Information, 2023b, henceforth referred to as the Mercator model). This allows us to ensure that our baseline case without kelp (or the counterfactual to a kelp farm) captures the key features of the biogeochemical state in the North Atlantic ocean. Our baseline column model reproduces the key features of the Mercator state estimate. For example, the timing and the amplitude of phytoplankton blooms (Figure 2A) and the seasonality of the partial pressure of CO2 (pCO2) in surface seawater (Figure 2B) agree well between our column model and the Mercator model. In particular, both models show distinct regimes: a decreasing pCO2 in the spring as primary production removed inorganic carbon from the water, an increasing pCO2 in late spring and summer due to reduced solubility of CO2 associated with seasonal warming, a decreasing pCO2 in the autumn due to seasonal cooling, a relatively constant pCO2 in the winter and early spring due to the balance between cooling, exchange of carbon-rich deep waters, and low primary production. The small difference in the pCO2 between the OceanBioME and Mercator models might be due to the idealization of the annual cycle used here in OceanBioME. The simulation without kelp is referred to as the baseline case in the following analysis.
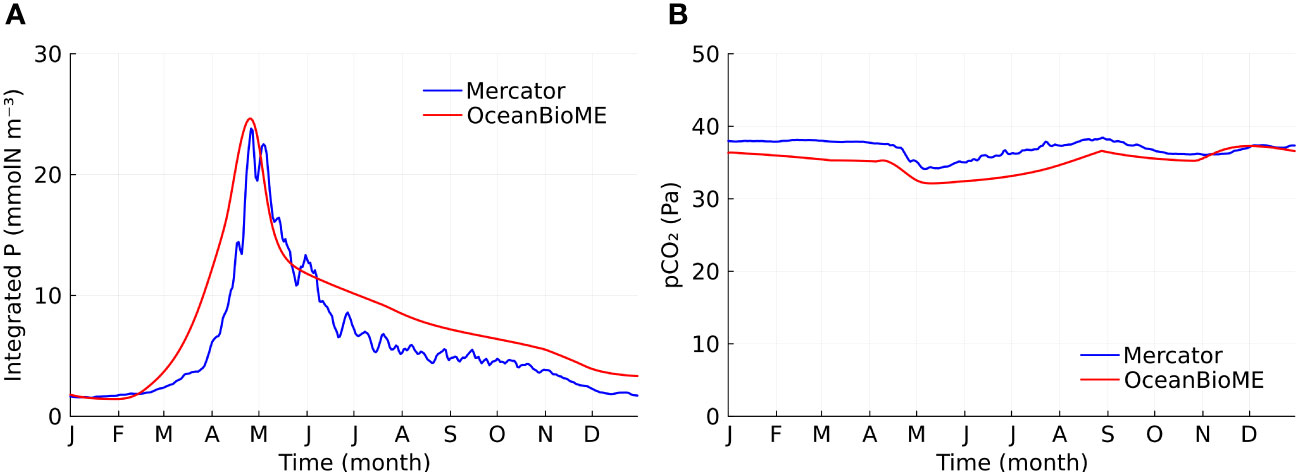
Figure 2 Model validation. (A) Time series of vertically integrated phytoplankton (P) concentration for the two models. (B) Time series of partial pressure of CO2 in surface seawater for the two models.
Sugar kelp can perform ‘luxury uptake’, whereby they uptake nutrients when the concentrations in the surrounding water are high and use the nutrients later in the year. Sugar kelp growth also exhibits distinct seasonal patterns due to varying light, temperature, nutrient conditions, and innate seasonality (Broch and Slagstad, 2012). In our model, the rate of change in the frond area is negative in summer (due to the relative rate of frond erosion being greater than the specific growth rate) and remains low with the kelp increasing carbohydrate reserves through photosynthesis until mid-autumn. Figure 3A illustrates this by showing a time series of kelp frond area and nitrogen and carbon reserves at a depth of 3m for a kelp density of 0.25 fronds m−3. An increase in frond area resumes from mid-autumn until winter, and during this period stored carbohydrates are utilized for growth, as can be seen by the corresponding reduction in carbon reserves.
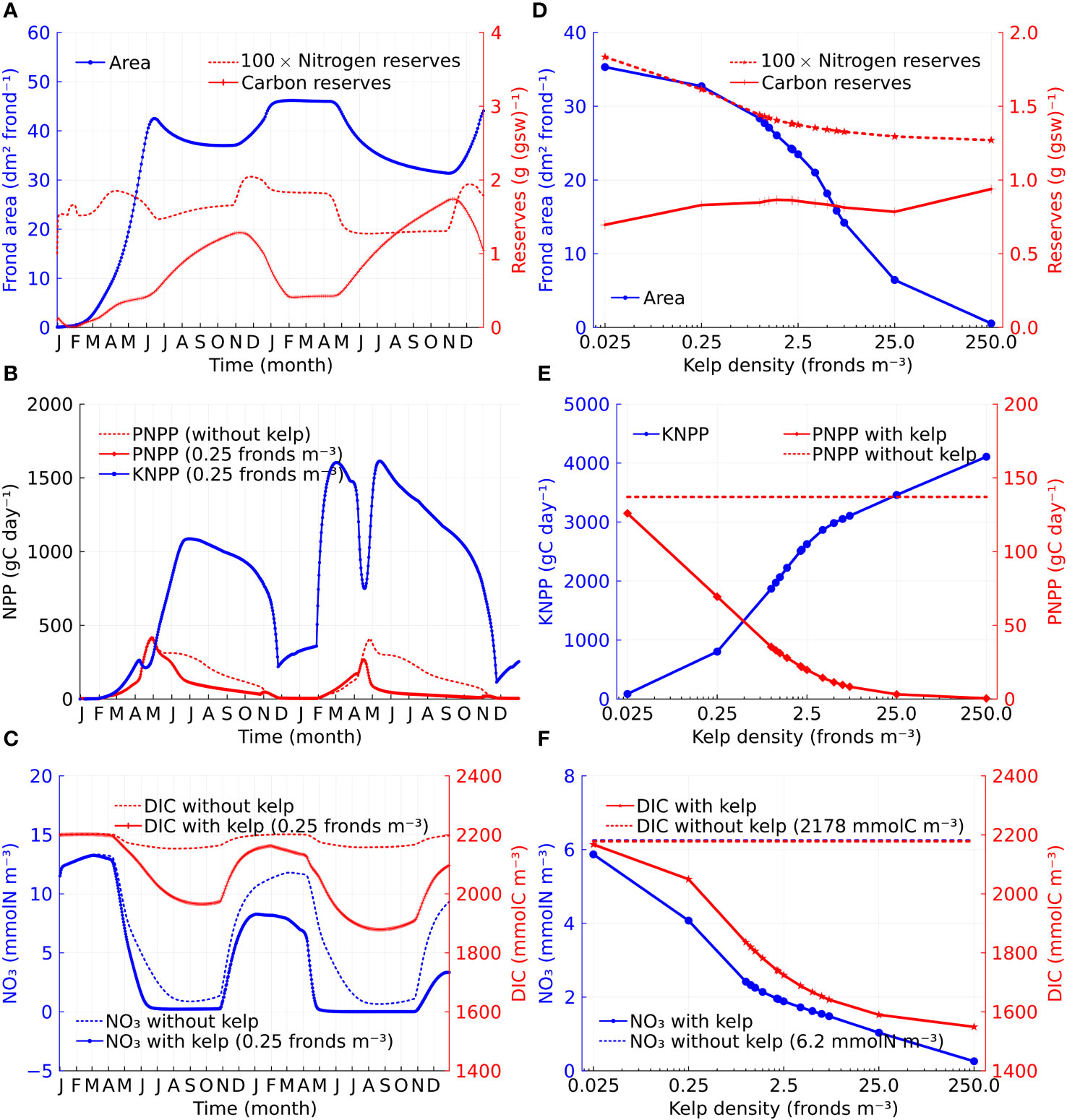
Figure 3 Time series and density dependence of kelp growth, volume-integrated NPP, and nitrate and DIC concentrations. (A) Time series of individual kelp properties at 3 m depth (in the case of kelp cultivation density at 0.25 fronds m−3): frond area, carbon reserves (gC (gsw)−1), and nitrogen reserves (gN (gsw)−1) which are shown as 100-fold of its value for presentation purposes. (B) Comparison between volume-integrated KNPP and PNPP. PNPP without kelp is given for reference. (C) Nitrate and DIC concentration comparison between cases with and without kelp at 3 m depth. (D) Density dependence of individual kelp properties at 3 m depth: frond area, carbon reserves, and 100-fold nitrogen reserves. (E) Density dependence of volume-integrated KNPP and PNPP. The dashed line represents the total time-averaged PNPP without kelp which is 137.1 gC day−1. (F) Density dependence of nitrate and DIC concentrations at 3 m depth. The dashed lines represent the time-averaged nitrate (blue) and DIC (red) concentrations at 3 m depth without kelp respectively.
Kelp interacts with phytoplankton by competing for nutrients. Adding kelp reduces phytoplankton net primary production-the total rate of organic carbon production by phytoplankton minus the rate of respiration (Sigman and Hain, 2012), although this reduction is highly seasonal and is largely confined to the period from April to November (Figure 3B). Note, however, that when kelp are included, the sum of kelp net primary production (KNPP) and phytoplankton net primary production (PNPP) is significantly larger than the PNPP in the case without kelp. The reduction in PNPP with the inclusion of kelp appears to occur due to ‘nutrient reallocation’ (Bach et al., 2021) which is evidenced by the reduction in the seawater nitrate concentrations when kelp is included (Figure 3C).
The seasonal pattern of nitrate concentration is controlled by the uptake by phytoplankton and kelp and the entrainment of nutrient-rich waters during periods of mixed layer deepening. During late autumn and early winter, the influence of kelp on PNPP is minimal. The kelp net primary production (KNPP) also exhibits distinct seasonal patterns due to varying light that influences kelp photosynthesis and temperature that influences both photosynthesis and respiration of kelp.
In our model, we do not include the effect of shading from kelp. This assumption is motivated by the relatively large effective horizontal spacing (20m) between vertical lines of kelp in our model. However, we do include the effect of shading from phytoplankton. The effect of shading from phytoplankton is more significant during the spring bloom than at other times of the year, which leads to a sharp decrease in KNPP in April (Figure 3B).
Figure 3D shows the two-year average of frond area and nitrogen and carbon reserves for kelp at a depth of 3m as a function of kelp density. The mean kelp area decreases rapidly for densities above about 2.5 fronds m−3. Commensurate with this decrease in kelp area, the time-averaged nitrate concentration in the seawater at 3m depth (Figure 3F) decreases with increasing kelp density. In a model of sugar kelp growth in the North Atlantic, Strong-Wright and Taylor (2022) found that kelp did not grow well when the mean nitrate concentration was below 0.5 mmol m−3. Note from Figure 3F that the mean nitrate concentration only falls to this level for the highest kelp densities, although this level occurs seasonally even at relatively low kelp densities (Figure 3C). The KNPP increases monotonically with kelp density, although the increase is sublinear. The sublinear increase in KNPP can be explained by the combination of the decrease in kelp frond area and the increase in kelp cultivation density (Figure 3D). In contrast, the PNPP decreases monotonically with kelp density due to nutrient reallocation (Figure 3E).
Both the gravitational pump and air-sea CO2 flux show a strong dependence on kelp density and seasonal patterns that reflect the kelp and phytoplankton growth (Figure 4). The gravitational pump, or the carbon flux due to sinking particulates, is computed by multiplying the concentration of POC at 500 m depth with the sinking speed of POC. The gravitational pump (two-year average) increases with kelp density and peaks at a density of 1.1 fronds m−3 (Figure 4C). Further increasing densities, characterized by smaller frond areas, reduces the gravitational pump. It is likely that the exact value of this limit is a result of the model assumptions, but since the kelp growth is clearly suboptimal at these very high densities (with low frond area and severe nutrient limitation), we do not focus on the results at very high densities. Without kelp, the gravitational pump peaks during late spring, coinciding with the peak of the spring phytoplankton bloom (Figure 2A). When kelp dominates the net primary production, the gravitational pump peaks in the late winter or early spring and decreases during summer and autumn (Figure 4A), reflecting the period when the kelp frond area is maximum (Figure 3A).
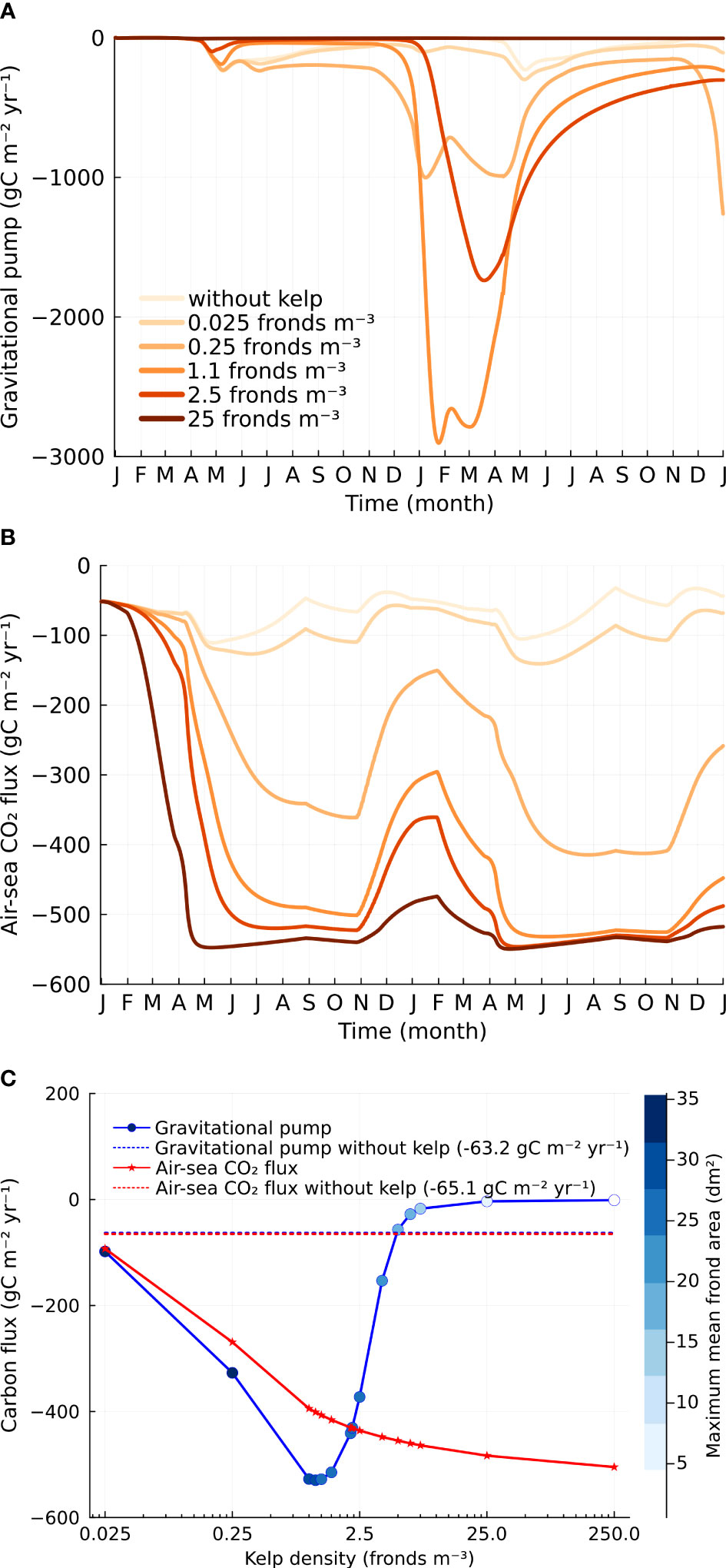
Figure 4 Carbon flux. (A) Time series of gravitational pump at varying kelp cultivation densities. (B) Time series of air-sea CO2 flux. Refer to the legends in (A). (C) Dependence of gravitational pump and air-sea CO2 flux on kelp density. Frond areas corresponding to different kelp cultivation densities are indicated on the color bar. The gravitational pump and air-sea CO2 flux in the baseline case are -63.2 (blue dashed line) and -65.1 (red dashed line) gC m−2yr−1 respectively. At a kelp density of 1.1 fronds m−3, macroalgae cultivation can export 7.4 times more carbon to the deep sea and remove 5.2 times more carbon from the atmosphere (equivalent to an additional 336.0 gC m−2yr−1) compared to the baseline case.
The air-sea CO2 flux also exhibits a strong dependence on kelp density and strong interseasonal fluctuations when kelp dominates the net primary production. The air-sea CO2 flux (two-year average) increases monotonically with kelp density and appears to saturate at about -520 gC m−2 yr−1 (Figure 4C). Remarkably, this is about 8 times larger than the air-sea CO2 flux without kelp. The seasonality of air-sea CO2 flux becomes less significant with increased kelp densities (Figure 4B). At high kelp densities, the enhancement in the air-sea CO2 flux occurs very soon after the start of the model when the kelp area is still small (Figure 3A).
It is important to note that we do not supply nutrients into our system and due to the sinking of POC through the gravitational pump, the system does not reach an equilibrium. The upper ocean carbon budget is also unsteady as can be seen by comparing the gravitational pump and the air-sea CO2 flux (Figure 4C). For the smaller kelp densities, the gravitational pump exceeds the air-sea CO2 flux while for large kelp densities, the gravitational pump is smaller than the air-sea CO2 flux. The reduction in the gravitational pump associated with the production of POC at high kelp density is accompanied by increased exudation of DOC. The observation may be explained by the overflow hypothesis, whereby DOC is exuded to reduce the build-up of photosynthetic products and maintain cellular homeostasis when low nutrient levels limit the synthesis of cellular structural components (Fogg, 1983; Paine et al., 2021). This is consistent with the findings of Abdullah and Fredriksen (2004) who found in their in situ incubation that more DOC was exuded from Laminaria hyperborea during non-growth phase and suggested that the decrease in growth is a consequence of the depletion of nutrients. The difference in carbon flux between the air-sea CO2 flux and the gravitational pump at high kelp cultivation density contributes to both kelp biomass carbon and exuded DOC.
Our model results suggest that sinking particulate organic carbon resulting from natural erosion of kelp fronds (a byproduct of seaweed aquaculture) could provide a significant pathway for removing carbon from the surface ocean and enhancing the air-sea CO2 flux. Our findings further suggest that this pathway and the impact on phytoplankton net primary production through nutrient reallocation is highly dependent on the density at which the kelp is planted.
When kelp density increases, the area of fronds decreases due to nutrient limitation, leading to a decreased POC production for each kelp frond. The kelp growth model assumes that erosion increases with frond area and is negligible when the area is very small (Broch and Slagstad, 2012), which is based on the observation that longer fronds erode more easily than shorter ones (Sjøtun, 1993). The net effect of the decreased POC production per frond and increased kelp densities results in a maximum gravitational pump at a kelp density of about 1.1 fronds m−3. Since reduced POC production at high densities is indeed due to nutrient limitation, the geometry and environment external to the kelp farm will influence this result. Here, we model a closed system with no net inflow of nutrients. In an open system, ambient water flowing into the kelp farm would replenish the nutrients inside the farm to some extent, and alleviate severe nutrient stress.
The shift from POC to DOC at high kelp densities has implications for carbon export and storage. For example, previous model results indicate that the DOC/POC export flux ratio decreases with depth (Hansell et al., 2009). The shift from POC to DOC might change the relative contributions of both forms of organic carbon to the biological pump. The increased exudation of DOC by kelp at high cultivation densities could also enhance the microbial carbon pump (MCP), the successive transformation of labile DOC and semi-labile DOC through microbial activities to recalcitrant DOC which is resistant to rapid bacterial degradation. Jiao et al. (2010) proposed the MCP as a conceptual framework to address the role of microbial generation of recalcitrant dissolved organic matter. Further work is needed to assess the organic carbon partitioning among biomass carbon, POC, and DOC (and its bioavailability) in order to assess the role of large-scale macroalgae cultivation on the global carbon cycle and the effectiveness of different CDR pathways.
The air-sea CO2 flux is influenced by the ability of sugar kelp to deplete DIC (defined as the sum of the concentrations of the three carbonate species: dissolved CO2, and ) in the surface water (Maberly, 1990). Restricted to low dissolved CO2 concentration in seawater, a large number of marine macroalgae use for photosynthesis. However, the ability to deplete and, as a result, DIC varies among species (Sand-Jensen and Gordon, 1984). The DIC uptake rate of sugar kelp decreases when the DIC concentration is reduced, and the minimum DIC achievable is estimated to be around 1282 mmol m−3 (Maberly, 1990).
It has been hypothesized that the relatively long equilibration times associated with carbon uptake of low pCO2 water (Jones et al., 2014) can limit the effectiveness of ocean CDR (Bach et al., 2021). Since our one-dimensional model does not include the subduction of mixed layer water into the ocean interior, we are not able to test this hypothesis. If the low pCO2 water is subducted into the interior before equilibrating with the atmosphere, the air-sea CO2 flux could be reduced. Future studies considering physical transport would be necessary to provide a comprehensive quantification of CDR from the atmosphere, for example studying the residence-time-limited CDR potential.
The significant enhancement of the gravitational pump when the kelp density is lower than 2.2 fronds m−3 indicates that POC sinking alone without biomass harvesting and intentional sinking can be an effective pathway of exporting carbon to the deep ocean. Since total harvest costs on average represent about 19% of total seaweed farming costs (DeAngelo et al., 2023), POC sinking alone without biomass harvesting and intentional sinking may be a cost-effective CDR pathway at lower seaweed cultivation densities. A kelp density of 1.1 fronds m−3 is found to maximize carbon export through POC sinking in the current configuration. When the kelp density is higher than this density, although air-sea CO2 flux is still significant, more fixed carbon by kelp is channeled to biomass carbon or exuded DOC. In this case, intentional biomass harvesting or sinking might be a more effective option to sequester carbon.
As acknowledged in Broch and Slagstad (2012), the erosion rate and its dependence on parameters (hydrodynamic conditions, frond age, etc.) are highly uncertain and more research is needed to reduce this uncertainty. To test the influence of the erosion rate on the gravitational pump and air-sea CO2 flux, we varied the parameter controlling the dependence of the erosion rate on the kelp area by ±50% for the case with a kelp density of 1.1 fronds m−3 and found that the maximum amplitude of the gravitational pump and the air-sea CO2 flux changed by at most 13.7% and 2.4% respectively compared to the baseline value (see the Supplementary Material for details).
Here, we only consider the portion of the water column above 500m depth. POC that sinks below this depth will either get consumed in the water column (e.g. in the mesopelagic) or settle on the seafloor. Benthic biological and physical processes will determine the ultimate fate of the material that reaches the seafloor and ultimately the sequestration time. The accumulation of organic material on the seafloor could also have a major impact on the benthic community structure and oxygen levels (Bach et al., 2021).
Our model also indicates that kelp cultivation can have a significant impact on nutrient availability and phytoplankton net primary production. Nutrient limitations driven by macroalgae cultivation and lowered recycling rate of nutrients within the macroalgal biomass (Chapman and Craigie, 1977) will also alter phytoplankton community structure. The shift from small POC (dominated by microalgal detritus) to large POC (dominated by macroalgal detritus) and the shift in timing of maximum POC export have implications for the food web. There may also be significant ecological effects on deep-ocean communities associated with the enhanced particulate flux to the deep ocean (Boyd et al., 2022).
In summary, the results presented here reveal an important pathway for CDR and suggest that including POC export is important for assessing macroalgal cultivation as a CDR strategy. On the other hand, our model indicates that macroalgal cultivation can significantly influence phytoplankton net primary production, and the ecological impacts of this need to be fully assessed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
SC: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. JSW: Methodology, Software, Writing – original draft, Writing – review & editing. JRT: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is funded by grants from the Centre for Climate Repair and the Gordon and Betty Moore Foundation in collaboration with the Kelp Forest Foundation.
The authors are grateful to the Centre for Climate Repair, the Kelp Forest Foundation, and Running Tide for stimulating conversations and encouraging this work. The authors also thank Samantha Deane, Max Chalfin, Dennis Hansell, Ole Jacob Broch, Silje Forbord, and Brian von Herzen for helpful discussions. This study has been conducted using data from E.U. Copernicus Marine Service Information and NASA’s Ocean Biology Processing Group.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1359614/full#supplementary-material
Abdullah M. I., Fredriksen S. (2004). Production, respiration and exudation of dissolved organic matter by the kelp Laminaria hyperborea along the west coast of Norway. J. Mar. Biol. Assoc. United Kingdom 84 (5), 887–894. doi: 10.1017/S002531540401015Xh
Arzeno-Soltero I. B., Saenz B. T., Frieder C. A., Long M. C., Deangelo J., Davis S. J., et al. (2023). Large global variations in the carbon dioxide removal potential of seaweed farming due to biophysical constraints. Commun. Earth Environ 185, 1–12. doi: 10.1038/s43247-023-00833-2
Atkinson M. J., Smith S. V. (1983). C:N:P ratios of benthic marine plants. Limnology Oceanography 28, 568–574. doi: 10.4319/LO.1983.28.3.0568
Azevedo I. C., Duarte P. M., Marinho G. S., Neumann F., Sousa-Pinto I. (2019). Growth of Saccharina latissima (Laminariales, Phaeophyceae) cultivated offshore under exposed conditions. Phycologia 58, 504–515. doi: 10.1080/00318884.2019.1625610
Bach L. T., Tamsitt V., Gower J., Hurd C. L., Raven J. A., Boyd P. W. (2021). Testing the climate intervention potential of ocean afforestation using the Great Atlantic Sargassum Belt. Nat. Commun. 12, 1–10. doi: 10.1038/s41467-021-22837-2
Boyd P. W., Bach L. T., Hurd C. L., Paine E., Raven J. A., Tamsitt V. (2022). Potential negative effects of ocean afforestation on offshore ecosystems. Nat. Ecol. Evol. 6, 675–683. doi: 10.1038/s41559-022-01722-1
Broch O. J., Alver M. O., Bekkby T., Gundersen H., Forbord S., Handå A., et al. (2019). The kelp cultivation potential in coastal and offshore regions of Norway. Front. Mar. Sci. 5. doi: 10.3389/FMARS.2018.00529
Broch O. J., Ellingsen I. H., Forbord S., Wang X., Volent Z., Alver M. O., et al. (2013). Modelling the cultivation and bioremediation potential of the kelp Saccharina latissima in close proximity to an exposed salmon farm in Norway. Aquaculture Environ. Interact. 4, 187–206. doi: 10.3354/AEI00080
Broch O. J., Slagstad D. (2012). Modelling seasonal growth and composition of the kelp Saccharina latissima. J. Appl. Phycology 24, 759–776. doi: 10.1007/s10811-011-9695-y
Chapman A. R., Craigie J. S. (1977). Seasonal growth in Laminaria longicruris: Relations with dissolved inorganic nutrients and internal reserves of nitrogen. Mar. Biol. 40, 197–205. doi: 10.1007/BF00390875/METRICS
DeAngelo J., Saenz B. T., Arzeno-Soltero I. B., Frieder C. A., Long M. C., Hamman J., et al. (2023). Economic and biophysical limits to seaweed farming for climate change mitigation. Nat. Plants 9, 45–57. doi: 10.1038/s41477-022-01305-9
de Ramon N’Yeurt A., Chynoweth D. P., Capron M. E., Stewart J. R., Hasan M. A. (2012). Negative carbon via Ocean Afforestation. Process Saf. Environ. Prot. 90, 467–474. doi: 10.1016/j.psep.2012.10.008
Duarte C. M., Cebrián J. (1996). The fate of marine autotrophic production. Limnology Oceanography 41, 1758–1766. doi: 10.4319/LO.1996.41.8.1758
Duarte C. M., Wu J., Xiao X., Bruhn A., Krause-Jensen D. (2017). Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00100
E.U. Copernicus Marine Service Information (2023a). Global ocean 1/12° physics analysis and forecast updated daily, Dataset. Mercator Ocean International Toulouse, France. doi: 10.48670/moi-00016
E.U. Copernicus Marine Service Information (2023b). Global ocean biogeochemistry analysis and forecast, Dataset Mercator Ocean International Toulouse, France. doi: 10.48670/moi-00015
Fieler R., Greenacre M., Matsson S., Neves L., Forbord S., Hancke K. (2021). Erosion dynamics of cultivated kelp, saccharina latissima, and implications for environmental management and carbon sequestration. Front. Mar. Sci. 8. doi: 10.3389/FMARS.2021.632725
Fogg G. E. (1983). The ecological significance of extracellular products of phytoplankton photosynthesis. Botanica Marina 26, 3–14. doi: 10.1515/botm.1983.26.1.3
Forbord S., Steinhovden K. B., Solvang T., Handå A., Skjermo J. (2020). Effect of seeding methods and hatchery periods on sea cultivation of Saccharina latissima (Phaeophyceae): a Norwegian case study. J. Appl. Phycology 32, 2201–2212. doi: 10.1016/j.algal.2020.102160
Fossberg J., Forbord S., Broch O. J., Malzahn A. M., Jansen H., Handå A., et al. (2018). The potential for upscaling kelp (Saccharina latissima) cultivation in salmon-driven integrated multi-trophic aquaculture (IMTA). Front. Mar. Sci. 9. doi: 10.3389/FMARS.2018.00418
Gallagher J. B., Shelamoff V., Layton C. (2022). Seaweed ecosystems may not mitigate CO2 emissions. ICES J. Mar. Sci. 79, 585–592. doi: 10.1093/icesjms/fsac011
Gattuso J.-P., Williamson P., Duarte C. M., Magnan A. K. (2021). The potential for oceanBased climate action: negative emissions technologies and beyond. Front. Climate 2. doi: 10.3389/fclim.2020.575716
GESAMP (2019). High level review of a wide range of proposed marine geoengineering techniques. GESAMP Rep. Stud 98, 144.
Gruber N., Clement D., Carter B. R., Feely R. A., van Heuven S., Hoppema M., et al. (2019). The oceanic sink for anthropogenic CO2 from 1994 to 2007. Science 363, 1193–1199. doi: 10.1126/science.1097403
Hansell D. A., Carlson C. A., Repeta D. J., Schlitzer R. (2009). Dissolved organic matter in the ocean a controversy stimulates new insights. Oceanography 22, 202–211. doi: 10.5670/OCEANOG.2009.109
Jiao N., Herndl G. J., Hansell D. A., Benner R., Kattner G., Wilhelm S. W., et al. (2010). Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nat. Rev. Microbiol. 8, 593–599. doi: 10.1038/nrmicro2386
Jones D. C., Ito T., Takano Y., Hsu W. C. (2014). Spatial and seasonal variability of the air-sea equilibration timescale of carbon dioxide. Global Biogeochemical Cycles 28, 1163–1178. doi: 10.1002/2014GB004813
Krause-Jensen D., Duarte C. M. (2016). Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci 9, 737–742. doi: 10.1038/ngeo2790
Krumhansl K. A., Scheibling R. E. (2012). Production and fate of kelp detritus. Mar. Ecol. Prog. Ser. 467, 281–302. doi: 10.3354/meps09940
Lévy M., Gavart M., Mémery L., Caniaux G., Paci A. (2005). A four-dimensional mesoscale map of the spring bloom in the northeast Atlantic (POMME experiment): Results of a prognostic model. J. Geophysical Research: Oceans 110, 1–23. doi: 10.1029/2004JC002588
Maberly S. C. (1990). Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. J. Phycology 26, 439–449. doi: 10.1111/J.0022-3646.1990.00439.X
Mann K. H. (1973). Seaweeds: Their productivity and strategy for growth. Science 182, 975–981. doi: 10.1126/SCIENCE.182.4116.975
Martiny A. C., Vrugt J. A., Lomas M. W., Marine (2014). Concentrations and ratios of particulate organic carbon, nitrogen, and phosphorus in the global ocean OPEN SUBJECT CATEGORIES. Sci. Data 1, 1–7. doi: 10.1038/sdata.2014.48
NASA Goddard Space Flight Center, Ocean Ecology Laboratory, Ocean Biology Processing Group (2021) Visible and infrared imager/radiometer suite (viirs) photosynthetically available radiation data (Accessed 22/07/2021). Dataset.
National Academies of Sciences, E. and Medicine (2022). A Research Strategy for Ocean-based Carbon Dioxide Removal and Sequestration (Washington, DC: The National Academies Press).
Paine E. R., Boyd P. W., Strzepek R. F., Ellwood M., Brewer E. A., Diaz-Pulido G., et al. (2023). Iron limitation of kelp growth may prevent ocean afforestation. Commun. Biol. 2023 6 (1), 1–9. doi: 10.1038/s42003-023-04962-4
Paine E. R., Schmid M., Boyd P. W., Diaz-Pulido G., Hurd C. L. (2021). Rate and fate of dissolved organic carbon release by seaweeds: A missing link in the coastal ocean carbon cycle. J. Phycology 57, 1375–1391. doi: 10.1111/JPY.13198
Ramadhan A., Wagner G. L., Hill C., Campin J.-M., Churavy V., Besard T., et al. (2020). Oceananigans.jl: Fast and friendly geophysical fluid dynamics on GPUs. J. Open Source Software 5, 2018. doi: 10.21105/joss.02018
Resplandy L., Lévy M., D’Ovidio F., Merlivat L. (2009). Impact of submesoscale variability in estimating the air-sea CO2 exchange: Results from a model study of the POMME experiment. Global Biogeochemical Cycles 23, 1–19. doi: 10.1029/2008GB003239
Resplandy L., Lévy M., McGillicuddy D. J. (2019). Effects of eddy-driven subduction on ocean biological carbon pump. Global Biogeochemical Cycles 33, 1071–1084. doi: 10.1029/2018GB006125
RunningTide (2021). Stripe Spring21 CDR Purchase Application, Dataset. Portland, ME (USA): Running Tide Technologies.
Sand-Jensen K., Gordon D. M. (1984). Differential ability of marine and freshwater macrophytes to utilize and CO2. Mar. Biol. 80, 247–253. doi: 10.1007/BF00392819/METRICS
Schiel D. R., Foster M. S. (2015). The Biology and Ecology of Giant Kelp Forests. 1st edn (155 Grand Avenue Suite 400 Oakland, CA 94612–3758: University of California Press).
Siegel D. A., DeVries T., Doney S. C., Bell T. (2021). Assessing the sequestration time scales of some ocean-based carbon dioxide reduction strategies. Environ. Res. Lett. 16, 104003. doi: 10.1088/1748-9326/ac0be0
Sigman D. M., Hain M. P. (2012). The biological productivity of the ocean. Nat. Educ. Knowledge 3, 1–16.
Sjøtun K. (1993). Seasonal Lamina Growth in two Age Groups of Laminaria saccharina (L.) Lamour. in Western Norway. Botanica Marina 36, 433–442. doi: 10.1515/botm.1993.36.5.433
Smale D. A., Pessarrodona A., King N., Burrows M. T., Yunnie A., Vance T., et al. (2020). Environmental factors influencing primary productivity of the forest-forming kelp Laminaria hyperborea in the northeast Atlantic. Sci. Rep. 10, 1–12. doi: 10.1038/s41598-020-69238-x
Stafford R. (2022). Comment on “Seaweed ecosystems may not mitigate CO2 emissions” by Gallagher et al (2022). ICES J. Mar. Sci. 79, 1701–1702. doi: 10.1093/icesjms/fsac087
Strong-Wright J., Chen S., Constantinou N. C., Silvestri S., Wagner G. L., Taylor J. R. (2023). OceanBioME.jl: A flexible environment for modelling the coupled interactions between ocean biogeochemistry and physics (v0.9.1). Zenodo. doi: 10.5281/zenodo.10136061
Strong-Wright J., Chen S., Constantinou N. C., Silvestri S., Wagner G. L., Taylor J. R. (2023b). OceanBioME.jl: A flexible environment for modelling the coupled interactions between ocean biogeochemistry and physics. doi: 10.5281/zenodo.10136061. Dataset.
Strong-Wright J., Taylor J. R. (2022). Modeling the growth potential of the kelp saccharina latissima in the North Atlantic. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.793977
Williams R. G., Follows M. J. (2011). Ocean Dynamics and the Carbon Cycle: Principles and Mechanisms (Shaftesbury Road Cambridge CB2 8EA: Cambridge University Press). doi: 10.1017/CBO9780511977817
Wu J., Keller D. P., Oschlies A. (2022). Carbon dioxide removal via macroalgae open-ocean mariculture and sinking: an Earth system modeling study. Earth System Dynamics Discussions 2022, 1–52. doi: 10.5194/esd-2021-104
Keywords: macroalgae cultivation, carbon dioxide removal, carbon sequestration, particulate organic carbon, kelp, climate change mitigation, ocean biogeochemical modeling, ocean-based solution
Citation: Chen S, Strong-Wright J and Taylor JR (2024) Modeling carbon dioxide removal via sinking of particulate organic carbon from macroalgae cultivation. Front. Mar. Sci. 11:1359614. doi: 10.3389/fmars.2024.1359614
Received: 21 December 2023; Accepted: 22 January 2024;
Published: 12 February 2024.
Edited by:
Wei-Bo Chen, National Science and Technology Center for Disaster Reduction (NCDR), TaiwanReviewed by:
Antoine De Ramon N’Yeurt, University of the South Pacific, FijiCopyright © 2024 Chen, Strong-Wright and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John R. Taylor, Si5SLlRheWxvckBkYW10cC5jYW0uYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.