- 1Shandong Provincial Key Laboratory of Marine Ecology and Environment & Disaster Prevention and Mitigation, Qingdao, China
- 2Key Laboratory of Ecological Prewarning, Protection and Restoration of Bohai Sea, Ministry of Natural Resources, Qingdao, China
- 3Yantai Institute of Coastal Zone Research, Chinese Academy of Science, Yantai, China
Introduction: The macrobenthos plays a vital role within the ecosystem of seagrass beds, with its characteristics and spatial distribution serving as indicators of the well-being of the seagrass beds.
Methods: In August 2018, three seagrass beds located in the Yellow River Estuary of Dongying, the west coast of Yantai, and Swan Lake of Weihai, were investigated to compare the ecological influences of seagrass habitat on the benthic environment and macrobenthic community. Within each seagrass bed, porewater, sediment, and macrobenthos were sampled from three separate stations (center of seagrass bed, edge of seagrass bed and bare area).
Results and discussion: One-way ANOVA showed significant differences (p < 0.05) in environmental factors and macrobenthos species, abundance, biomass and diversity indices among the three seagrass beds. The present data did not show significant impacts on habitat and macrobenthos in the different coverage areas of seagrass beds at the investigated spatial scales, though crustacea and some carnivores were relatively more inclined to inhabit areas with higher seagrass densities. Aquaculture and eutrophication may trigger the loss of seagrass bed habitats, that affects macrobenthic biodiversity, and conservation measures are needed to protect seagrass bed habitats.
Introduction
Seagrass beds, as a crucial component of marine ecosystems, play a vital role in estuarine and coastal environments due to their rich biodiversity and productivity (Phillips and McCroy, 1980; Stoner, 1980; Costanza et al., 1997). Extensive research conducted over several decades attests to the importance of seagrass beds in delivering essential ecosystem services, including nutrient and carbon cycling and storage, coastal stabilization, and providing habitats and protection for marine organisms (Gartner et al., 2013). They can also alter water flow patterns and sedimentation rates, thereby impacting food supplies for macrobenthos (Jankowska et al., 2019). Furthermore, the ability of seagrass roots to absorb nutrients from sediments that are inaccessible to other primary producers allows the seagrass bed to create a substantial carbon reservoir (Huang et al., 2006).
Over the past few years, seagrass beds have experienced varying degrees of damage and are currently facing numerous artificial threats, resulting in a significant decrease in area, particularly in regions where humans have developed (Norris et al., 1997). The deterioration of seagrass ecosystems has now become a critical coastal protection issue that governments and scientists worldwide are anxiously trying to address. Habitat destruction of seagrass beds severely jeopardizes the existence of macrobenthic communities (Li et al., 2007). Synthesizing previous international findings, the most common response variables of macrobenthic populations and community structure to changes in seagrass bed habitats are density and number of species. Two-thirds of studies of individual or species functional processes showed significant correlations between seagrass bed size and benthic density, growth and mortality, significant effects on benthic habitat density were found in 80% of edge effect studies, and seagrass bed fragmentation was a significant predictor of benthic density in 75% of studies. However, different studies presented different results for the same reflective variables due to the compounding effects of study location, season, and target taxa (Boström et al., 2006). The macrobenthic community is a vital element of seagrass bed ecosystems, and its characteristics and distribution are closely connected to the area and density of seagrass beds (Blanchet et al., 2004), making it useful in assessing the health of these beds (Smith, 1981). Additionally, macrobenthic communities are crucial to seagrass bed ecosystem services as they impact biogenic structure, nutrient fluxes, and indirect effects like predation, food webs, and detritus cycling (Lundquist et al., 2018).
Prior studies have predominantly concentrated on seagrass beds in southern China, where a larger variety of species and denser plants are present, such as Guangxi (Li et al., 2007) and Hainan (Tu et al., 2016). However, few reports have focused on the effect of human activities on seagrass habitats in intertidal zones, which significantly impact macrobenthic assemblages. In Dongying Yellow River Estuary, Yantai west coast and Weihai Swan Lake, the growth and distribution of seagrass beds varies in different regions as a result of human activities such as coastal tourism and aquaculture. Therefore, it is essential to study macrobenthic communities in various areas of seagrass beds to evaluate the ecological service status of degraded seagrass beds in coastal intertidal ecosystems. Our study aims are to 1) explore the differences in macrobenthic community structure in seagrass beds at different sampling areas (center, edge and bare areas) and 2) reveal the relationship between macrobenthic communities and the habitat environment in seagrass beds at the intertidal zone of three different regions. This study hopes to provide essential information and data support for the protection and restoration of seagrass beds.
Materials and methods
Study area
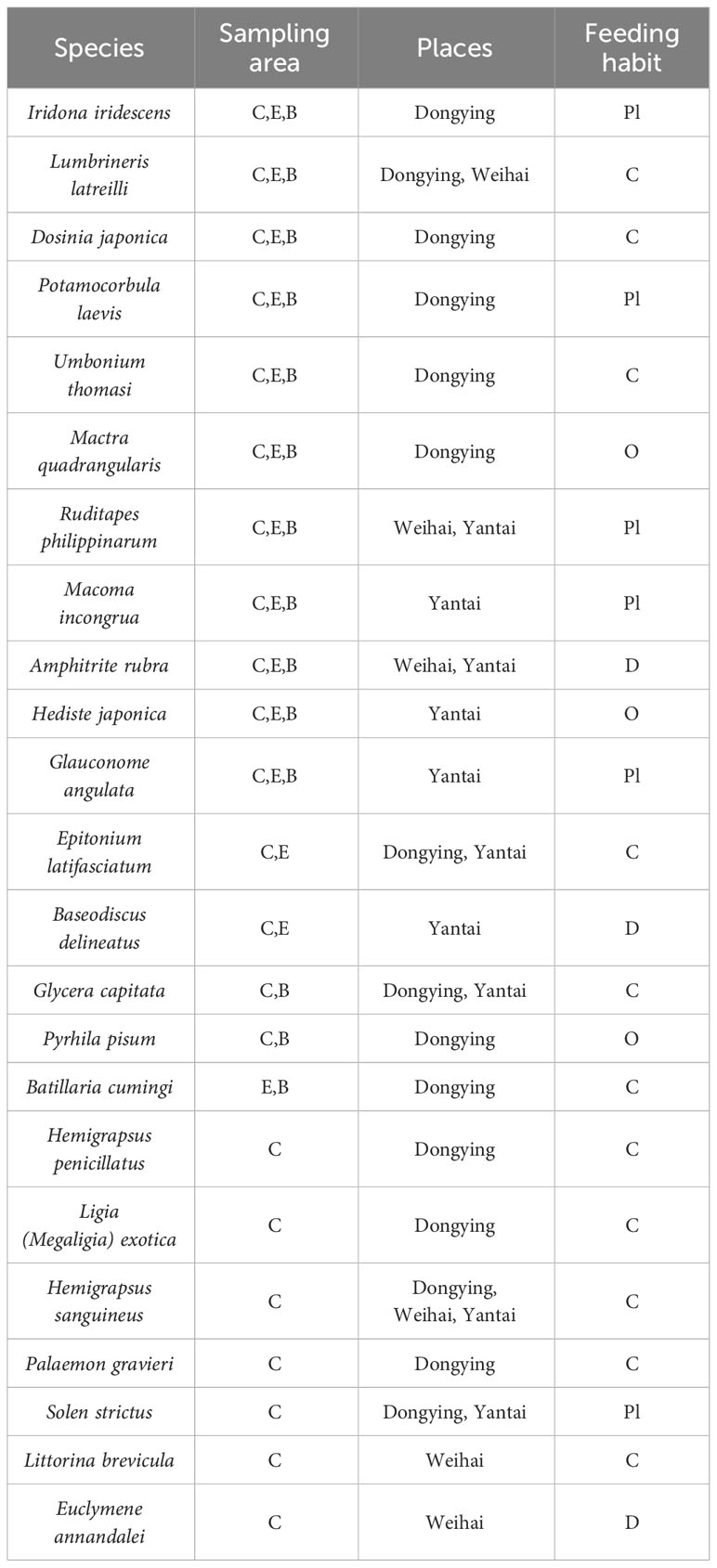
Macrobenthos samples were collected from three intertidal seagrass beds in Shandong Province, China (37°50’N-37°58’N, 119°15’E-122°55’E). The sampling locations and areas of the three seagrass beds are shown in Figure 1 and Table 1. The sampling stations of seagrass beds were determined according to the distribution and biomass of seagrass, the center area with flourishing seagrass (labeled C), the edge area with sparse seagrass (E) and the bare area with no seagrass (B). For example, DC means the center area of the seagrass bed in the intertidal zone of Dongying.
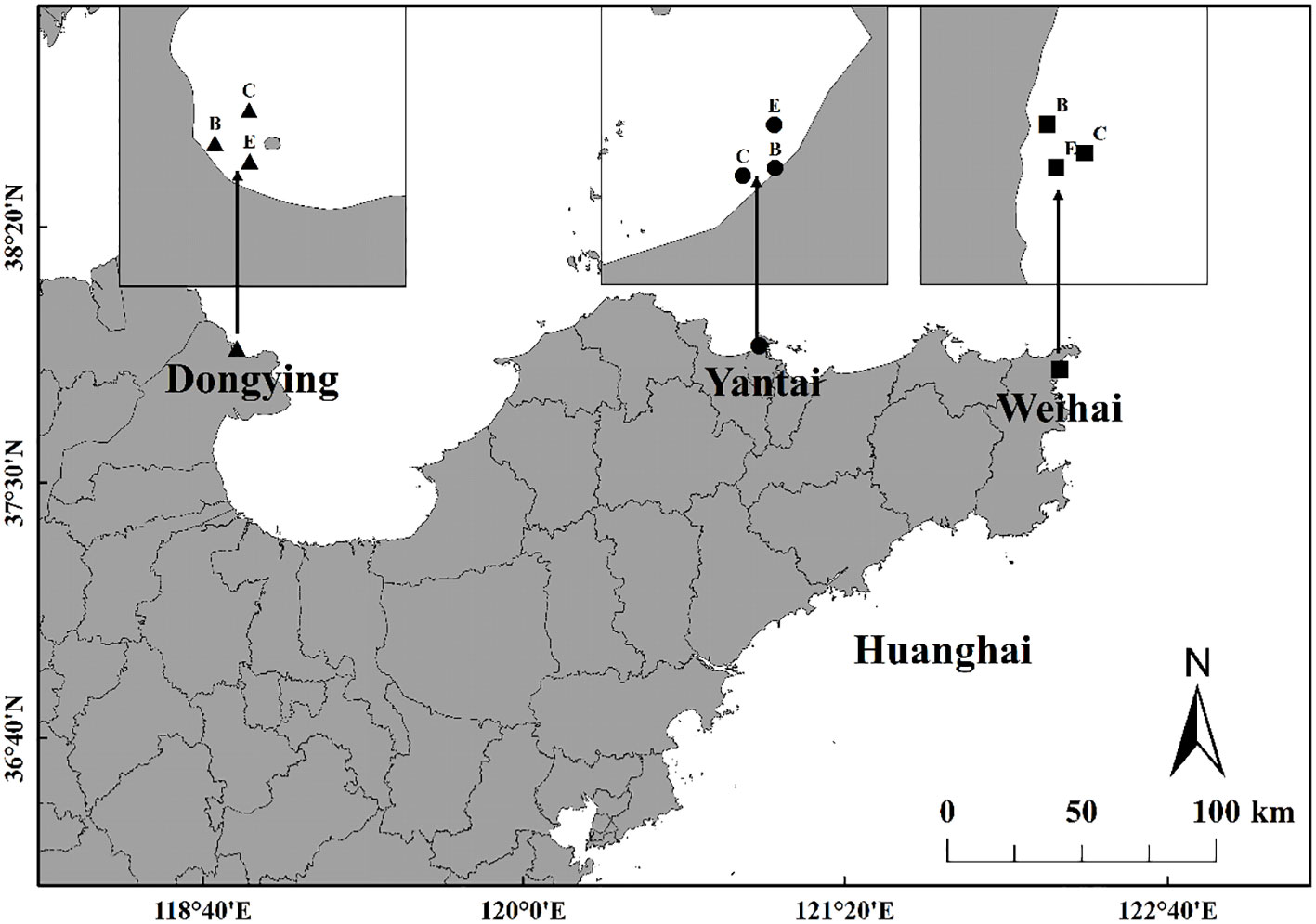
Figure 1 Locations of three sampling stations in Shandong Province, China (C means center of seagrass beds, E means edge of seagrass beds, B means bare area).
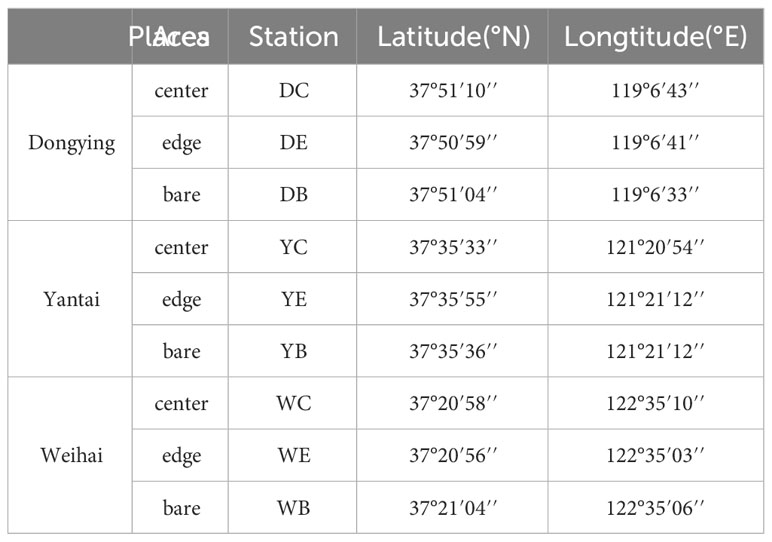
Table 1 Coordinates of the 9 sampling stations in Shandong Province, China (DC means the center area of seagrass bed in intertidal zone of Dongying, and so on).
Sampling methods and procedure
In August 2018, seagrass growth was prolific, and comprehensive surveys were conducted in three intertidal seagrass beds to collect water, sediment and macrobenthos samples. Additionally, the YSI Pro multimeter was utilized to measure the oxygen concentration, salinity and temperature at each station in situ.
Three replicates of porewater samples were collected by using capillaries (Agro Business Park, Netherland) and 20 ml syringes at each station, then stored in 50 ml bottles. Porewater refers to the free water in the sediment voids, based on which the relevant hydrochemical parameters were determined. Samples were transported into iceboxes before being frozen at the laboratory. The NO3−, NO2−, NH4+ and SiO32− were measured using a nutrient autoanalyzer utilizing gas-segmented continuous flow analysis (AutoAnalyzer 3, Branluebbe, Germany).
Three parallel samples of seagrass and macrobenthos samples were collected using a 33× 33× 30 cm stainless steel sample box at each station. During the process of sampling, large macrobenthic individuals were picked out first and then washed through a 0.5 mm mesh sieve, after which the remaining macrobenthos were separated from seagrass plants. The macrobenthos were preserved in 75% alcohol with a tag, and the seagrass plants were kept in an ice box for further identification and measurement in the laboratory. The macrobenthos samples were identified to the lowest possible taxon (usually species level), counted and weighed. Bivalve biomass was calculated as the whole animal including shell. The seagrass components (roots, stems, and leaves) were carefully separated using forceps and a scalpel. Each component was then weighed separately, and the recorded weight was an average of parallel samples.
Sediment samples were collected by inserting a clean and single use plastic 20 ml syringe into the sediment to collect the top 5 cm of sediment. Three parallel samples were taken from each station, once sampled, sediment was placed into individual plastic bottles and conserved in iceboxes for transportation to the laboratory, where it was stored in a freezer before analysis. Sediment grain size measurements were performed on freeze-dried samples using a Mastersizer 2000 Laser particle Sizer (Malvern Instruments Limited, UK). TN and TOC in sediment were analyzed by a CNS Analyzer (Vario MACRO CN) on dry and ground samples.
Data analysis
Three biodiversity indices, diversity index H’, species richness index d and evenness index J’, were calculated to analyze the dynamics of macrobenthic community structure, species composition and abundance. The following three formulas were adopted (Shannon and Weaver, 1949; Margalef, 1968; Pielou, 1975):
where s is the species number at each station; N is the total abundance at each station; Pi is the percentage of the abundance of the i-th species to the total abundance (Bi et al., 2023).
Multivariate analysis was conducted using Primer 7.0 software (Plymouth Routines in Multivariate Ecological Research) to analyze the community structure and its relationship with environmental factors in the BIOENV and BVSTEP analyses. Furthermore, cluster analysis and multidimensional scaling (MDS) were conducted by using Bray–Curtis similarity data after square root transformation of the species abundance data. A distance-based linear model (DistLM) was used to analyze the relationship between benthic traits and environmental factors among different sampling places, distance-based redundancy analysis (dbRDA) was used to visualize the fitted model, and stepwise regression (Stepwise) was used to select a subset of optimal environmental factors.
Results
Environmental variables analysis
The NO3−, PO43− of porewater as well as sediment grain size and TOC among the three seagrass beds showed significant variations (p< 0.05) (Tables 2, 3). Of the total, porewater PO43− and sediment MGS (median grain size) in Dongying seagrass bed were both highly significantly lower than those in Yantai and Weihai (p< 0.01), and sediment TOC content was significantly higher than that in Weihai (p< 0.05), while porewater NO3− in Weihai was highly significantly higher than those in Dongying and Yantai (p< 0.01). However, the results of investigations at different stations in the same seagrass bed showed that no significant differences were exhibited in the environmental factors, except that porewater PO43− in the edge area of the seagrass bed in Yantai was significantly lower than that in the center and bare area (p< 0.05), and sediment MGS in the edge area of the seagrass bed in Dongying was significantly higher than that in the center area (p< 0.05). It is noteworthy that porewater DO (dissolved oxygen) and salinity as well as sediment MGS and TOC in the three seagrass beds showed a slight decreasing trend from the center, edge to bare areas, although the differences were not significant.
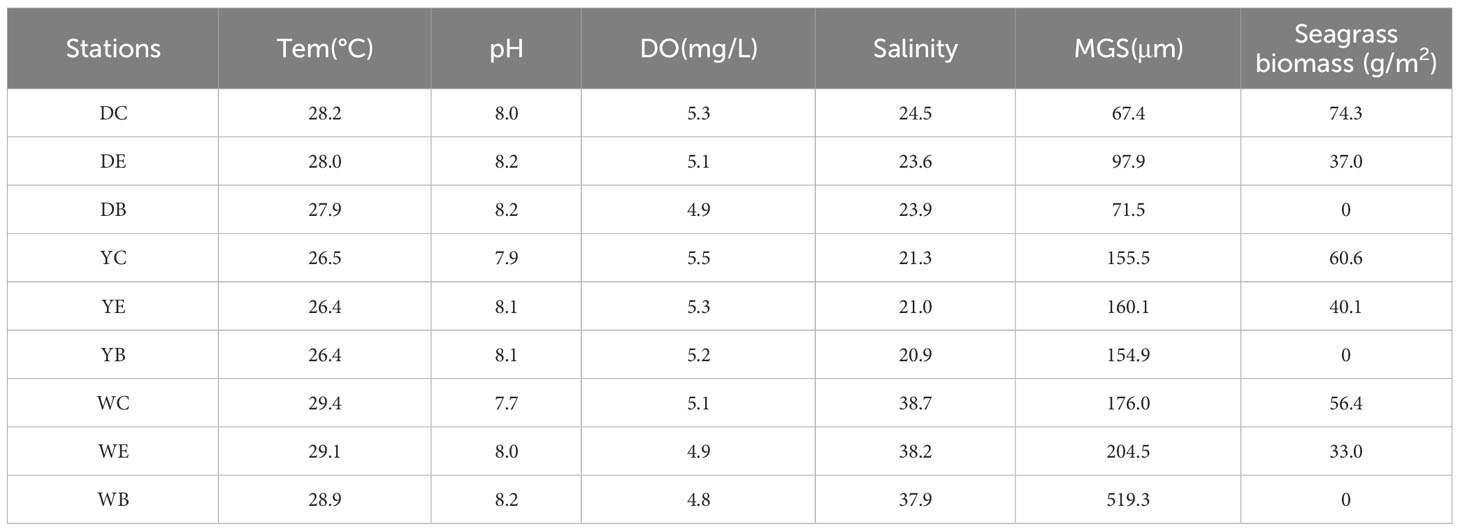
Table 2 Results of water temperature, pH, DO, salinity, sediment median grain size (MGS) and seagrass biomass at 9 sampling sites.
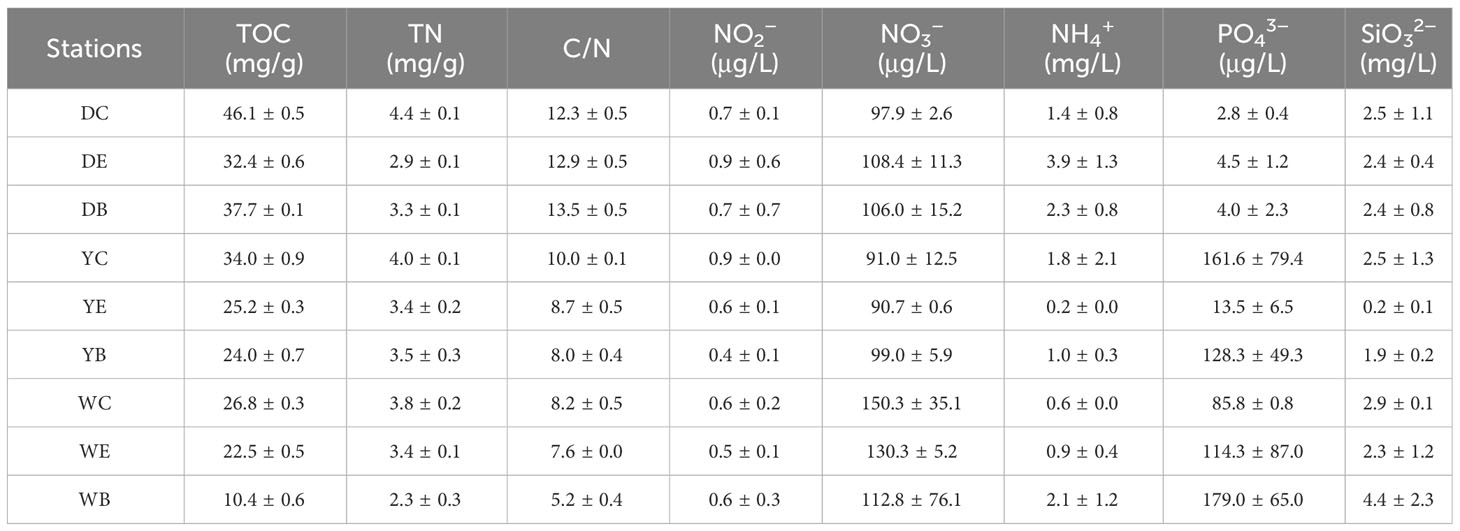
Table 3 Results of sediment total organic carbon (TOC), total nitrogen (TN) and water nutrients at 9 sampling sites.
C/N is a vital indicator for identifying the origin of organic matter in sediments, making it a common method for discerning the source of organic matter. It is widely employed to determine the endogenous and exogenous sources of vegetation in sediment organic matter (Talbot, 1990). The C/N in the surface sediments of the three intertidal seagrass beds ranged from 5.2 to 13.5, with Dongying having significantly higher values compared to the other two regions (p< 0.01). In Dongying, the C/N of the seagrass beds showed that the bare area had higher values than the edge and center areas (p< 0.05), all exceeding 12. In Weihai, the C/N exhibited a decreasing trend from the center area to the bare area (p< 0.01). However, the difference between sampling sites of the seagrass beds in Yantai was not significant (p > 0.05).
Species composition
A total of 23 different species were identified, of which 12 species belonged to the mollusca, 5 to polychaeta, 5 to crustacea, and 1 to another category. The number of species in the three seagrass beds had a common pattern, i.e., the number of species decreased from the center area to the bare area, but only the number of species in the center area of Weihai seagrass bed was significantly higher than that in the bare area (p< 0.05) (Figure 2). In addition, ANOVA analysis showed that the number of macrobenthos species in the three seagrass beds differed significantly (p< 0.05). Mollusca species were found to be the dominant taxa in the three intertidal seagrass beds, encompassing over 50% of the total species number in both Dongying and Yantai. Specifically, Umbonium thomasi was prevalent across all sampling stations in Dongying, Amphitrite rubra was the dominant species in Yantai, whereas Ruditapes philippinarum was found at every sampling station in Weihai.
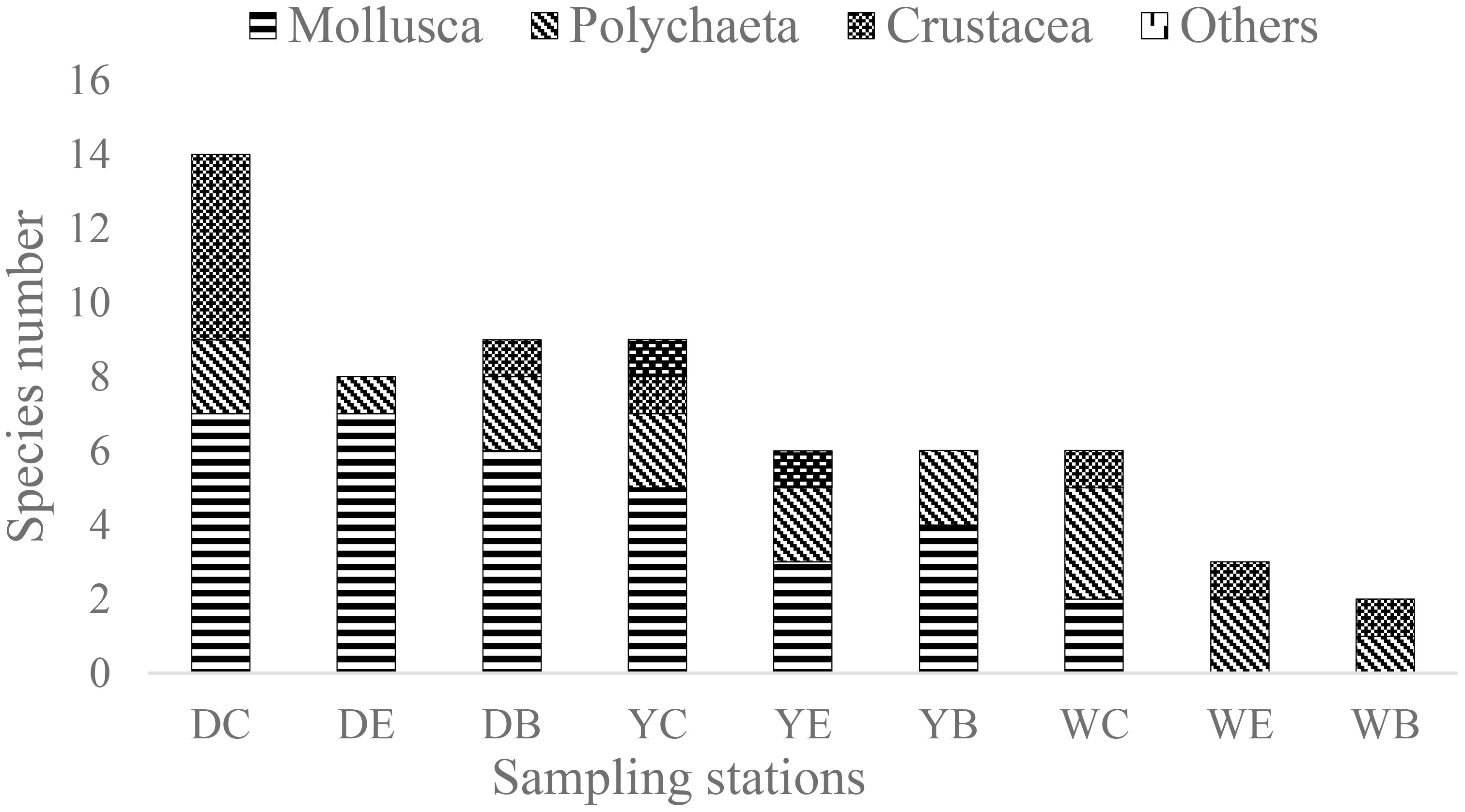
Figure 2 Species number and composition of macrobenthos in seagrass beds at the 9 sampling stations.
In terms of species composition, crustacea were most common in the center area, accounting for 35.7% of the species composition, and were not found or occurred less frequently in the edge and bare areas. Certain larger polychaete and crustacea species, such as Pyrhila pisum and Hemigrapsus penicillatus, were only found in the center area, and Hemigrapsus sanguineus was identified only in areas with high seagrass biomass.
Feeding habit
Table 4 outlines the feeding habits of macrobenthos present within three distinct seagrass beds. The table indicates that 11 of the 23 species are categorized as carnivorous (C). Additionally, 6 species fall under the planktophagous habit (Pl), while 3 species are classified as detritivorous (D) and omnivorous (O). Notably, we did not observe any herbivorous crab species within the seagrass beds. Spatially, we identified a greater presence of carnivorous species in central areas of the seagrass beds. Conversely, planktophagous and omnivorous species appeared to be concentrated within the edge and bare regions of the beds.
Distinct variations were observed in the feeding habits present in the three seagrass beds. Specifically, in Dongying, three feeding habits (Pl, O, and C) were detected, with the carnivorous group holding the largest representation of 66.7% of the total species. Weihai, on the other hand, revealed four feeding habits (Pl, O, C, and D), with the carnivorous group being prominent, accounting for 50% of the total species. Yantai also presented four habits (Pl, O, C, and D), but no dominant habit was identified.
Abundance and biomass
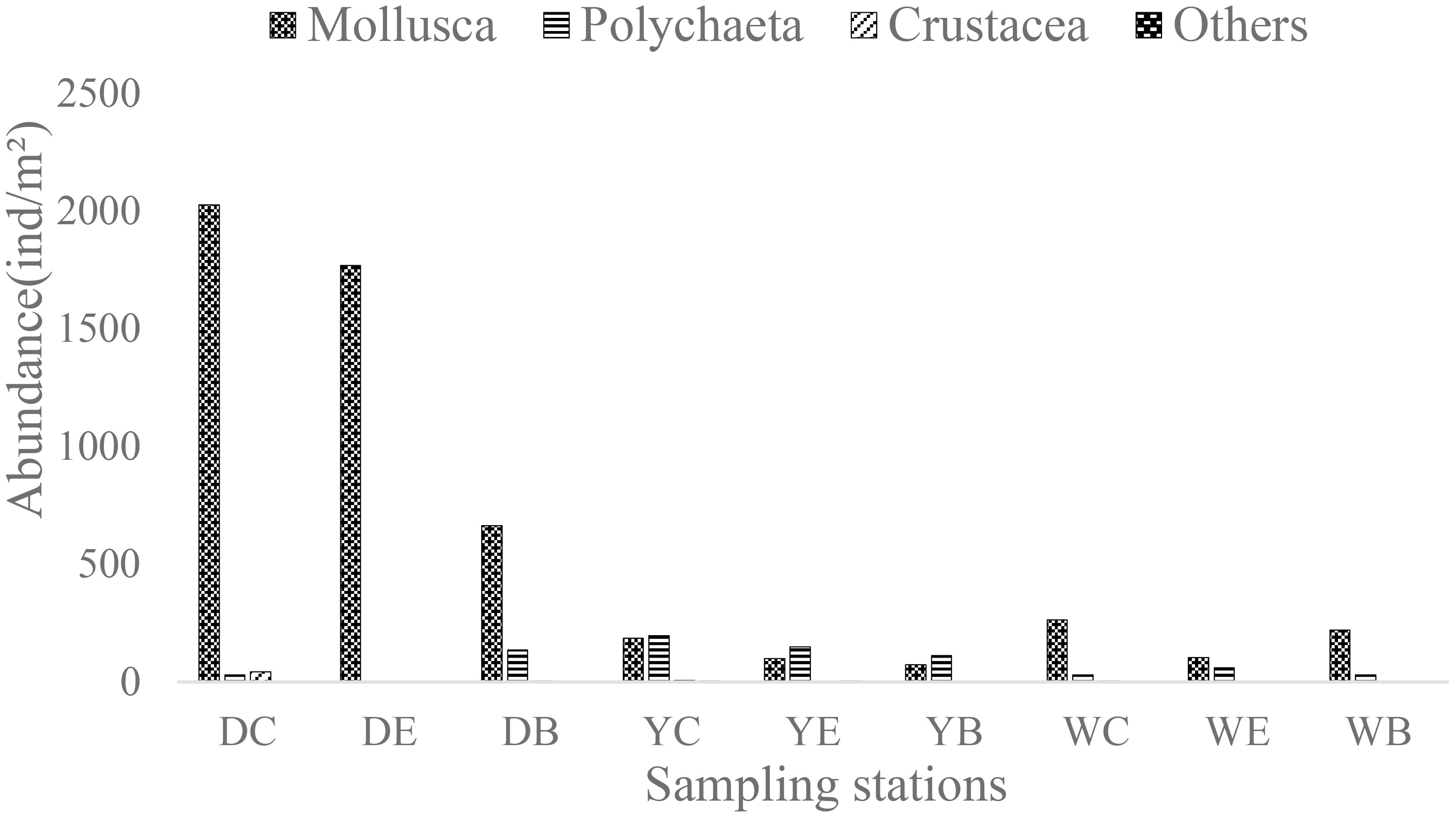
The results of one-way ANOVA showed that the differences in macrobenthic abundance in the center, edge and bare areas of the seagrass beds were not statistically significant (p > 0.05), while the differences in macrobenthic abundance among the three seagrass beds were highly significant (p< 0.01), as shown by a highly significant higher abundance in Dongying than in Yantai and Weihai (p< 0.01) (Figure 3). The average abundance in Dongying area was 1558.0 ± 550.3 ind/m2, among which the single mollusca Potamocorbula laevis had an average abundance value as high as 963.3 ± 486.3 ind/m2. In addition, the mollusca abundance from the center area to the bare area showed a decreasing trend. The average abundance of Yantai was 277.8 ± 86.1 ind/m2, and the abundance of polychaeta was slightly higher than that of mollusca. The dominant species Amphitrite rubra was mainly distributed in the center and edge areas. Finally, in Weihai, the average abundance of macrobenthos was 236.7 ± 55.2 ind/m2, with mollusca showing a dominant abundance of 195.6 ± 67.6 ind/m2. Ruditapes philippinarum was the most important contributing species.
Similar to the abundance results, although there were differences in biomass among the three sampling stations (center, edge, and bare areas), the differences were not statistically significant (p > 0.05), whereas the differences in macrobenthic biomass among the three seagrass beds were significant (p< 0.05) (Figure 4). In Dongying, the mean biomass was 214.4 ± 40.6 g/m2, with mollusca having the largest biomass of 202.1 ± 48.4 g/m2. In Yantai, the mean biomass was 140.9 ± 16.7 g/m2, and the dominant taxon was mollusca, with a mean value of 113.9 ± 11.6 g/m2. In Weihai, the mean biomass was 364.5 ± 76.8 g/m2, which was significantly higher than that in Dongying (p< 0.05) and highly significant than that in Yantai (p< 0.01). The contribution of mollusca was more than 99.0% in all three sampling stations, with the dominant species Ruditapes philippinarum having a high biomass of 358.0 ± 72.7 g/m2.
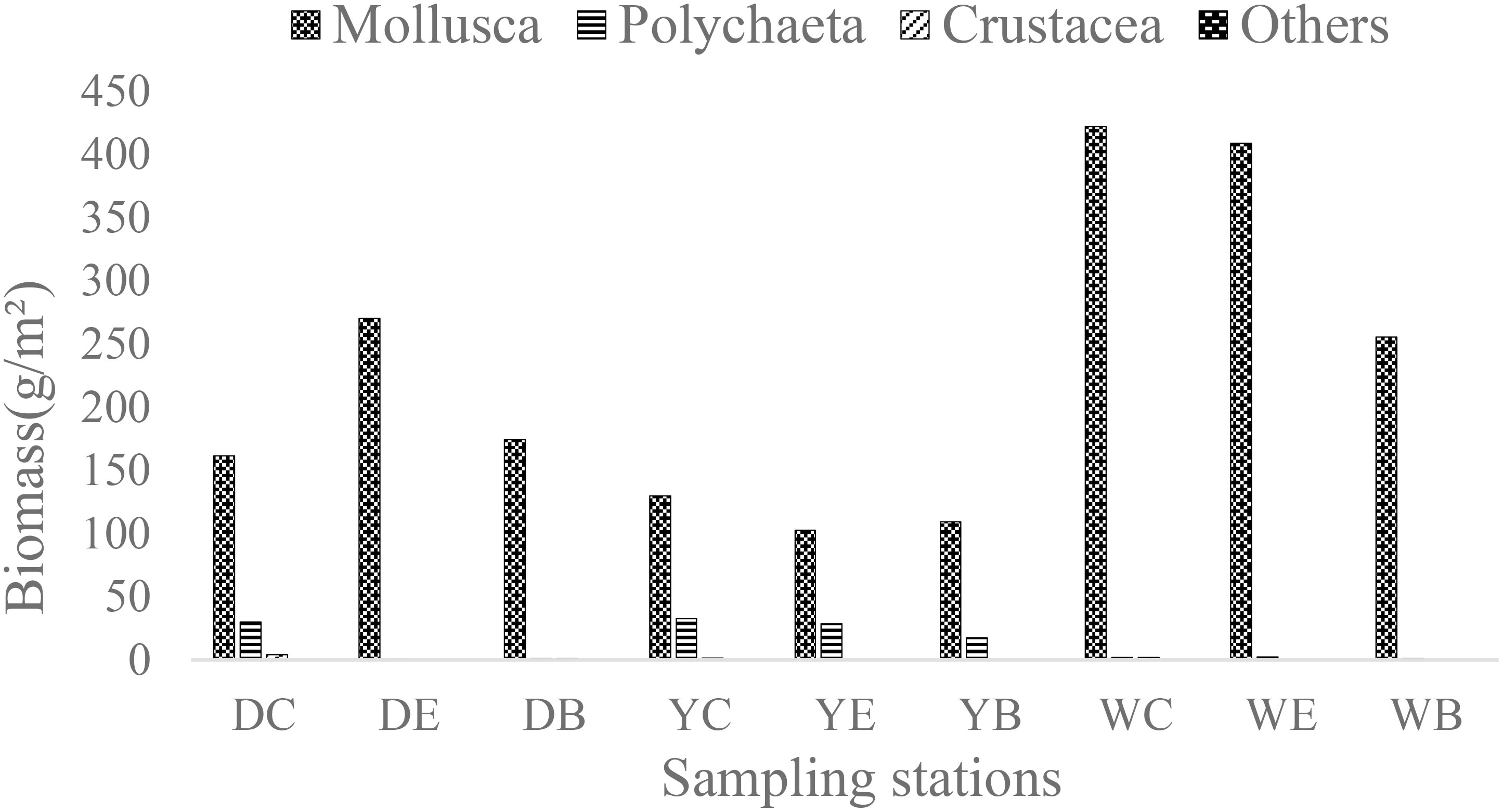
Figure 4 Biomass of macrobenthos at the 9 sampling stations in the three seagrass bed biodiversity indices.
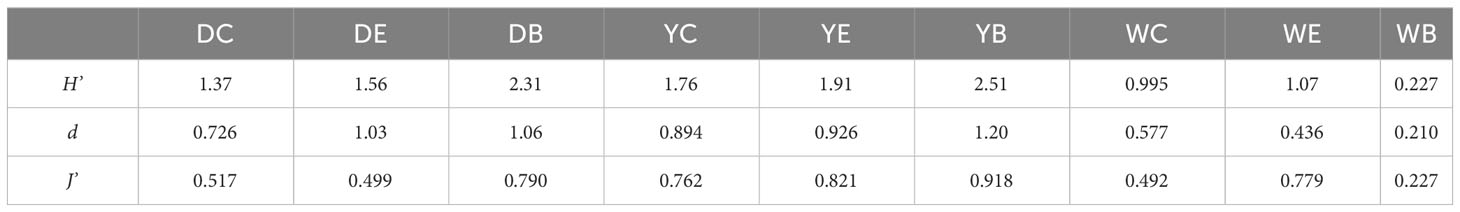
H’ (Shannon-Weiner diversity index) and d (Margalef richness index) of the three seagrass beds were significantly different (p< 0.05), and the lowest of both indices was in Weihai (Table 5). The H’ and J’ (Pielou evenness index) showed a tendency to increase from the center to the bare area in Dongying (p< 0.05). In Yantai, the H’ and d increased from the center to the bare area, of which the H’ differed highly significantly among the three sampling stations (p< 0.01).
Community structure
Cluster analysis based on Bray-Curtis similarity with abundance combined with Simprof analysis (Figures 5A–C) showed that macrobenthos of different sampling stations in seagrass beds were classified into the same group, and the center, edge, and bare areas did not show obvious regional divisions. In Dongying (Figure 5A) and Yantai (Figure 5B), stations in the center and edge areas of the seagrass beds were the first to be clustered together, while in Weihai (Figure 5C), there was a crossover in the cluster of sampling stations in different regions. In terms of spatial distribution, macrobenthic communities differed significantly (p< 0.05) among the three intertidal seagrass beds (as shown in Figure 5D), which was also confirmed by multidimensional scaling (MDS) analyses (Figure 6), with a stress value of 0.01 indicating a good sorting result.

Figure 5 Cluster analysis based on the Bray–Curtis similarity of the abundance of macrobenthos at 9 sampling stations (A-C) refer to the replicate samples for Dongying, Yantai, Weihai, respectively, and (D) refer to three locations together).
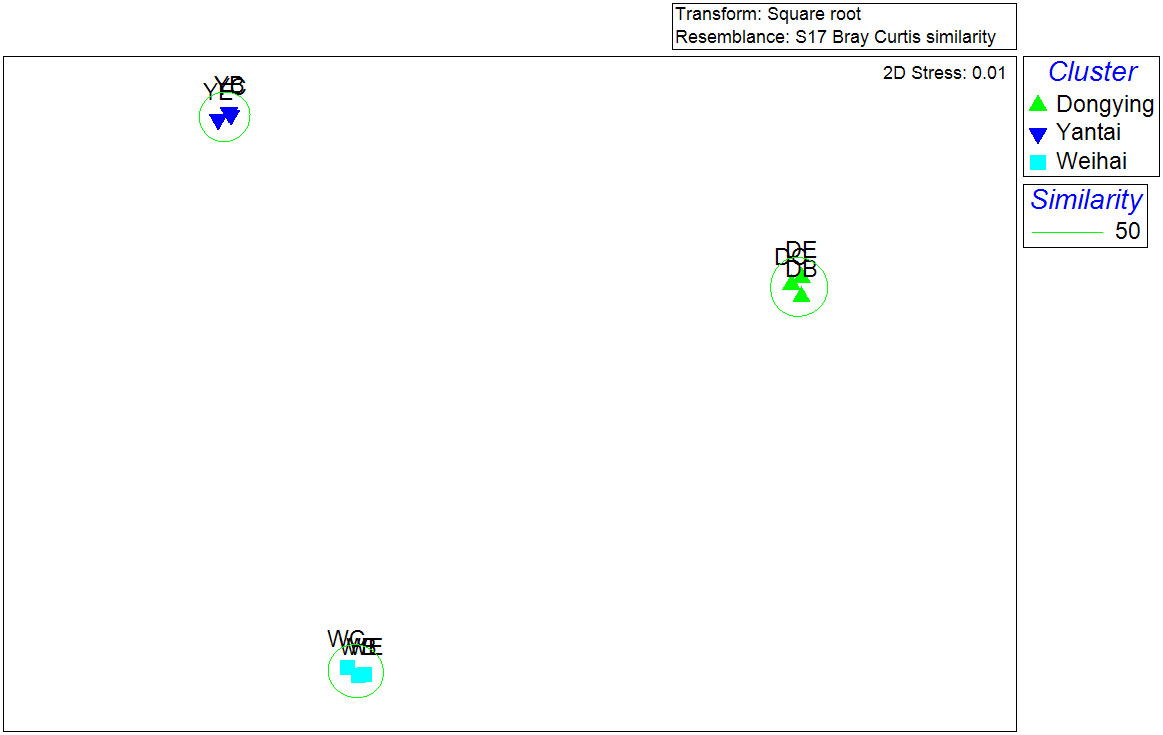
Figure 6 MDS analysis based on the Bray–Curtis similarity of the abundance at three sampling stations.
SIMPER analysis of macrobenthic communities in three seagrass beds revealed significant differences in dominant taxa or species between locations. Specifically, in the Dongying group, the three stations exhibited an average similarity of 69.5%, with Potamocorbula laevis and Umbonium thomasi as the dominant species contributing 32.7% and 27.1%, respectively. In the Yantai group, the average similarity was 72.8%, and Amphitrite rubra, Macoma incongrua, and Glauconome angulata were the dominant species, with contribution rates of 28.9%, 19.7%, and 19.4%, respectively. Last, in the Weihai group, the average similarity was 69.3%, with Ruditapes philippinarum and Lumbrineris latreilli as the dominant species contributing 72.9% and 20.0%, respectively.
Relationship between macrobenthos and environmental factors
The BIOENV and BVSTEP analyses of environmental factors and macrobenthic abundance data revealed significant correlations between nutrient concentration, sediment median grain size, and macrobenthic abundance. The combination of environmental factors with the highest contribution were sediment median grain size (MGS), total organic carbon (TOC), and porewater salinity.
The dbRDA analysis revealed that a set of environmental factors, including salinity, temperature, pH, MGS, TOC, NO2−, and TN, played a crucial role in determining macrobenthos distribution. The first two dbRDA axes captured a considerable portion of the variability in the data, with 91.2% of the variability accounted for in the fitted model and 90.9% represented in the data cloud (Figure 7). Specifically, the first dbRDA1 axis (that explained 58.1% of the total variation) was negatively associated with salinity, MGS, and TN and positively correlated with the remaining four environmental factors. On the other hand, the second dbRDA2 axis (32.8% of the total variation) was positively correlated with salinity, MGS, and temperature. The spatial arrangement of the sampling stations revealed that Weihai stations were located in the upper left region of the ordination diagram, indicating higher tolerance to higher salinity and larger substrate sizes. The Dongying stations were positively correlated with pH, NO2−, and TOC, while the Yantai stations were associated with TN only. Importantly, environmental elements demonstrated a salient relationship with the benthic community’s distribution, with temperature, pH, and salinity significantly impacting the distribution characteristics of benthic communities (F = 22.13, p< 0.01; F = 5.17, p< 0.05; F = 3.49, p< 0.05, respectively), as demonstrated by the results of the ordination diagrams.
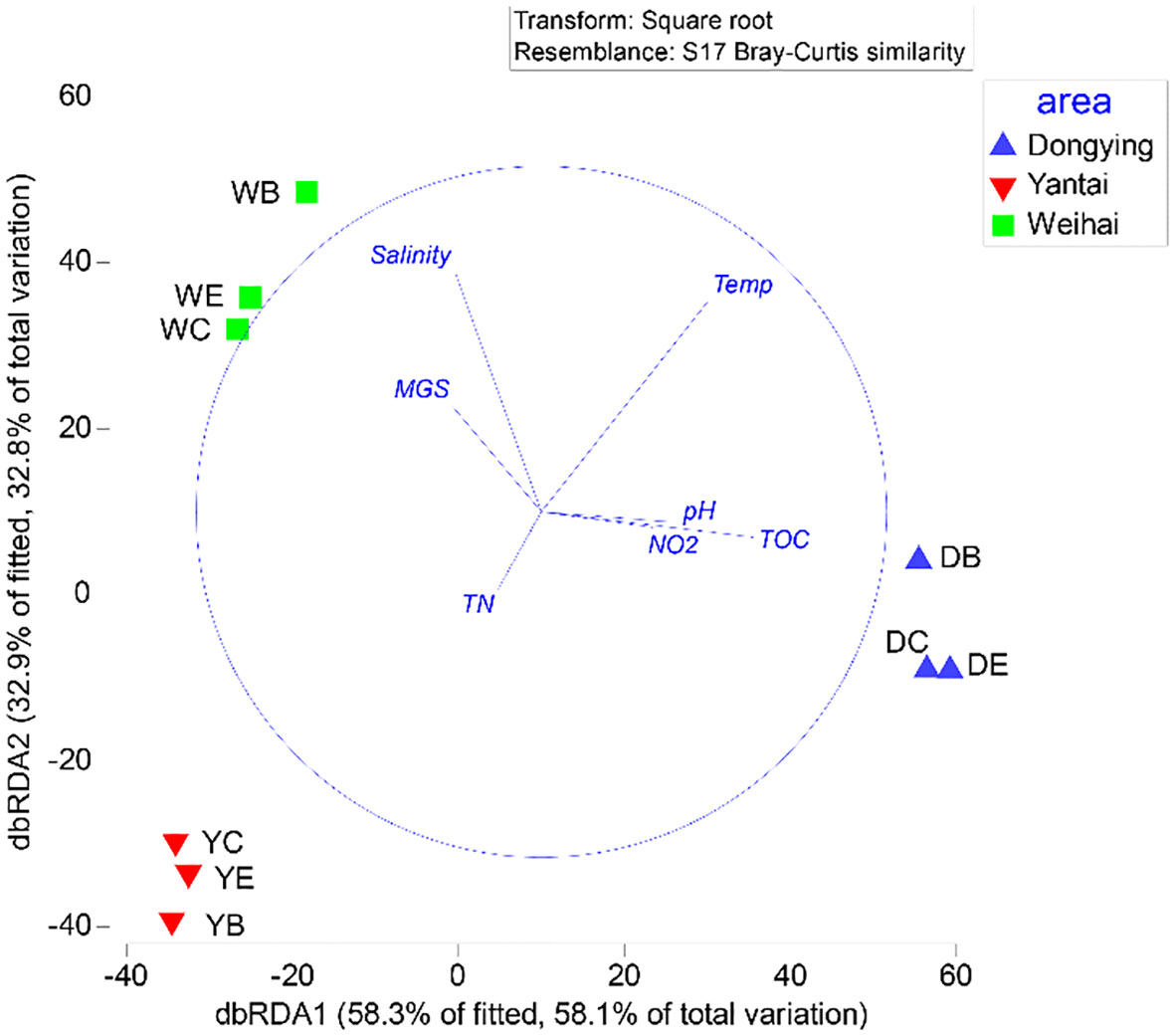
Figure 7 Two-dimensional distance-based redundancy analysis (dbRDA) ordination representing the model of spatial variation in macrobenthos community structure related to the environmental variables selected by the best linear models based on distance (DistLM).
Discussion
Differences in seagrass species and seagrass bed habitats among the three regions
Zostera japonica is the seagrass species prevalent in Dongying, while Zostera marina is the dominant seagrass species in Yantai and Weihai. Zostera japonica has a small body size, prefers substrates with high sand content and is able to live in shallow intertidal zones with large fluctuations in water temperature and light and relatively strong environmental disturbances (Zhang et al., 2013). Zostera marina, on the other hand, is the most widespread seagrass in the northern hemisphere, primarily found in shallow intertidal and subtidal waters with high water transparency and slow currents (Neckles et al., 2005). Unlike Zostera japonica, it is less tolerant to environmental changes. During the field survey, the seagrass beds in Dongying were located far from the towns and there was no aquaculture. However, there were numerous petroleum extraction facilities in close proximity. Although there was no direct river confluence in the seagrass bed area, the estuary of the Yellow River, Chinese second-largest river, was approximately 15km northeast of the region. The Yellow River is responsible for depositing approximately 1.1 × 109 t of sediment into the ocean annually, which constitutes around 5.5% of the global river sediments that reach the sea (Milliman and Syvitski, 1992). Additionally, the amount of particulate organic carbon that the river transports into the sea ranges from 1.76 × 106 to 8.14 × 106 t per year, accounting for 0.4% to 1.9% of the world’s total organic carbon from rivers that reaches the ocean (Schlünz and Schneider, 2000). The seagrass bed at Swan Lake in Weihai was located in an aquaculture plant and was strongly influenced by the aquaculture activities of Apostichopus japonicus and Ruditapes philippinarum, especially during the harvesting seasons of sea cucumbers and clams. Finally, human activities in the seagrass bed in Yantai, which located in the intertidal zone of bathing beach, were more frequent than the other two seagrass beds, and tourists and beach combers could be seen at the site traveling around the area of the seagrass beds, which caused somewhat negative impacts on the growth of the local seagrasses.
The results of this study showed that the sediment MGS and porewater PO43- concentration were the lowest in Dongying seagrass bed, while the sediment TOC content was the highest, and the porewater NO3- concentration was the highest in Weihai seagrass bed. The sediment MGS in Dongying and the porewater PO43- concentration in Yantai showed significant differences across the center, edge, and bare areas of the seagrass beds. Specifically, the sediment MGS was found to be the smallest in the center area of the seagrass bed in Dongying, while the porewater PO43- concentration was the lowest in the edge area of the seagrass bed in Yantai. It has been shown that coastal tourism and aquaculture may have noteworthy environmental consequences, including reduced seawater quality, red tide hazards, and increases in macroalgae and attached plants (Burdick and Short, 1999). The higher nutrient salt concentration of seagrass beds in Yantai and Weihai verified the above observation. The lowest MGS and highest TOC content in the sediments of the Dongying seagrass beds can be attributed to two factors. Firstly, the fine-grained sediments from the Yellow River settle in the peripheral waters of the Yellow River estuary due to tidal currents and near-shore circulation. These sediments undergo chemical and biological modifications before settling, resulting in higher TOC content in the adjacent sea area (Qiao et al., 2011). Secondly, the large surface area of the fine particles promotes microbial growth and organic film adsorption, leading to the enrichment of more nutrients (Hyland et al., 2005). In addition, seagrass beds can affect water flow, dissipate turbulence, and reduce wave action, which reduces sediment resuspension and increases sedimentation in seagrass beds (Lundquist et al., 2018), so the sediment MGS in the center of the different seagrass beds in this study were relatively smaller and the TOC content decreased slightly from the center to the bare area. There were no significant differences in environmental factors measured in the center, edge, and bare areas of the three seagrass beds surveyed. According to Edgar et al. (1994), differences between seagrass-covered and unvegetated areas in terms of key physicochemical parameters, such as temperature, salinity, light, and nutrients, may be small compared to the direct and indirect differences caused by the vegetative structure.
Distribution of macrobenthos in seagrass beds
Seagrasses exhibit a structural heterogeneity which serves as a valuable habitat for some macrobenthos species that would not be capable of surviving on exposed surfaces. Additionally, variations in substrate composition and water flow amongst different locations offer a diverse array of microhabitats for macrobenthos (Edgar et al., 1994). Consequently, Dense seagrass areas are generally considered to provide favorable environmental conditions for the growth of macrobenthos, ultimately leading to higher biodiversity indices. Conversely, areas with less seagrass and low primary productivity tend to result in poor habitat conditions, leading to slower growth of macrobenthos and lower biodiversity indices (Hillman et al., 1995). There were few notable distinctions in the diversity indices among the center, edge, and bare areas of the two seagrass beds, except for Weihai. Surprisingly, the bare area exhibited higher biodiversity. The seagrass beds in Weihai were positioned within the aquaculture zone, where Ruditapes philippinarum dominated, comprising over 80% of the biomass and abundance at each sampling site. This dominance contributed to a decline in macrobenthic biodiversity indices. Also, no significant differences in macrobenthos species, abundance, and biomass were found across the center, edge, and bare areas of the seagrass beds, with the exception of a significantly higher number of species in the center area of the seagrass bed in Weihai than in the bare area. The study found that the variations in benthic biodiversity across the sampling sites in the three seagrass beds were relatively small when compared to previous research. Bell et al. (2001) proposed that detecting the effects of seagrass bed habitat change on benthos might be challenging due to the limited spacing between seagrass bed patches, which allows for the active and passive dispersal of organisms in currents. Reed and Hovel (2006) supported this notion by demonstrating that small-scale seagrass habitat changes had minimal impact on the composition of benthic communities. However, when the area of existing seagrass beds in the region was significantly reduced beyond a threshold level (90%), there was a rapid decline in species richness and abundance of macrobenthos. A study in Barker Inlet found that the differences between unvegetated and seagrass-covered areas, as observed by human samplers, were primarily influenced by the above-ground portion of the seagrass. Interestingly, the edge of the seagrass beds may extend 2 m beyond the marked area due to the presence of substantial below-ground seagrass biomass in the sediments (Webster et al., 1998; Tanner, 2005). The absence of substantial variations in the macrobenthos composition of seagrass beds across different sampling sites may be attributed to several factors. Firstly, despite the varying sizes and levels of human-induced disruptions among the three intertidal seagrass beds, none of them reached a critical point of diminishing in size. Additionally, the bare areas were sampled not far from seagrass beds (~5 m), and seagrass root biomass may remain in the sediments.
Analysis of macrobenthos taxa and feeding habits showed that crustacea were almost exclusively found in the center of seagrass beds compared to edge and bare areas, and that the feeding habits of macrofauna in the center areas were mainly omnivorous and carnivorous, a feature that might be related to seagrass density. In a field experiment, it was found that decapods were more common in dense seagrasses with or without predators, and that low decapod abundance in patches with low seagrass coverage density was not due to an increase in predation, but rather because decapods preferred high-density seagrass habitats, where more food was available (Connolly, 1997). Similarly, as the primary food for associated fish species in shallow waters, small fish spent more time in seagrass-covered areas where more food was available, i.e., fish preferred vegetated habitats or congregated where food utilization was higher (Connolly, 1994). Thus, the effects of seagrass bed patchiness may be magnified for larger organisms that are more dependent on the size of seagrass beds, such as fish (Frost et al., 1999). Based on the above studies, it can be hypothesized that although the center, edge, and bare areas of seagrass beds in the three regions investigated were not clearly distinguished in macrobenthic abundance, biomass, and clustering analyses, it is possible that high densities of seagrasses protect and provide food for organisms with a variety of feeding habits, including a number of high trophic level predators, which can influence the population structure and productivity of organisms within the seagrass beds.
Correlation between macrobenthic communities and environmental factors
Our study showed that the macrobenthic communities in seagrass beds in the three regions showed obvious differentiation, with the Shannon-Weiner diversity index and Margalef richness index in Dongying and Yantai being significantly higher than that in Weihai, and the combination of the results of BIOENV, BVSTEP and dbRDA analyses suggests that the main environmental factors affecting the distribution of macrobenthos are porewater salinity, sediment MGS and TOC content. Sediment TOC content in Dongying either exceeded or approached established upper TOC thresholds (about 3.5% TOC) considered to pose a high risk of benthic fauna impairment (Hyland et al., 2005), but the biodiversity and species richness remained high. By comparing the environmental data, no obvious exceedance or eutrophication was found in the seagrass bed in Dongying for all nutrient salt indicators except for the high sediment TOC content, and the DO concentration in water was all above 2 mg/L, which would not cause potential hazards to the benthic fauna. In transitional waters, the degradation of organic matter at the sediment surface can lead to oxygen depletion and the production of toxic by-products such as ammonia and sulphides. These processes can have a significant impact on the distribution of benthos. However, these negative effects can be mitigated by the oxygen produced through photosynthesis by autotrophs and the uptake of oxygen from the atmosphere. Additionally, the reuse of decomposition products by microbes and water exchange can lower the concentration of toxic metabolites in the sediment, reducing the stress on benthos (Tagliapietra et al., 2012). The high TOC content in Dongying’s surface sediments does not pose a significant issue due to the seagrass beds’ location in the intertidal zone. The exposed sediments and tidal action, along with the high concentration of DO and alkaline pH in the water, create oxidizing conditions that restrict the accumulation of biologically detrimental by-products.
Organic matter in marine sediments originates from marine and terrestrial sources. Marine input is derived from in situ primary production and decomposition processes, while terrestrial input comes from surface runoff, primarily from terrestrial plants and human activities (Yi et al., 2022). The composition of organic matter in sediments can be determined by the molar ratio of TOC to TN (TOC/TN). A higher TOC/TN suggests a greater presence of land-derived organic matter, whereas a lower TOC/TN indicates a dominance of marine-derived organic matter (Meyers, 1997). Specifically, when the TOC/TN ratio falls between 4 to 8, marine sources predominate (Bordovskiy, 1965; Prahl et al., 1980), whereas a ratio above 12 signifies a land-based origin (Thornton and Mcmanus, 1994; Ogrinc et al., 2004). Ratios between 8 to 12 indicate a mixture of both marine and land sources (Milliman et al., 1984). The C/N value of surface sediments in the Dongying seagrass beds exceeds 12, suggesting that land-based inputs dominate the organic matter source in this region. This is likely due to the presence of the Yellow River estuary within Dongying’s boundaries. Each year, a significant amount of sediment from the Yellow River accumulates near the estuary, resulting in land-based materials coated with Yellow River sand being the primary influence on the sediments in this area. Shellfish biodeposition, seaweed farming, and soil organic matter are potential sources of sediment organic matter in aquaculture areas (Xia et al., 2018; Sui et al., 2019). The seagrass beds in Weihai, located within the Ruditapes philippinarum aquaculture area, contain a significant amount of protein from shellfish and seagrass organisms. Despite the high nutrient concentration in the water column due to human activities, the organic matter content in the surface sediment is lower than that in the sea area adjacent to the Yellow River estuary in Dongying. This difference results in a relatively low C/N value, highlighting the influence of the Yellow River conveyance on the organic matter composition in the surrounding sea waters (Yu G. L. et al., 2019).
Conservation measures are needed to prevent ecological risks to the ecosystem caused by the degradation of seagrass beds. At present, the most common methods for restoring and conserving seagrass beds are habitat restoration, seeding, transplanting, and turfing. By boosting the number and density of seagrass plants in specific intertidal areas, new seagrass beds can be constructed to compensate for the reduction in existing beds (Yu S. et al., 2019). Furthermore, steps must be taken to protect existing seagrass beds from further loss and to restore their ecological functions and structures (Guo, 2019). One approach involves improving water quality within seagrass beds to increase water column transparency and encourage recovery (Short and Wyllie-Echeverria, 1996). This necessitates addressing the issue at the source by lowering land-based inputs, particularly industrial wastewater, domestic sewage, and other high-nutrient pollutant discharges. Along with close monitoring of seagrass beds, several additional measures merit attention, such as controlling anthropogenic factors, raising public awareness, conducting local seagrass studies and experiments, creating a knowledge base of seagrass characteristics in distinct locales, gathering data on seagrass bed habitats and biodiversity profiles, and adopting a site-specific approach to restore seagrass beds (Wu and Zhang, 2018).
Conclusion
A total of 23 macrobenthos species were collected and identified in the three intertidal seagrass beds. Through analysis, we can draw the following conclusions:
(1) There are significant differences in environmental factors and macrobenthos species composition, community structure and biodiversity in seagrass beds in Dongying, Yantai and Weihai.
(2) The study revealed limited variation in environmental factors and the number, abundance, and biomass of macrobenthos among the sampling sites in seagrass beds. The distribution of crustacea and species that feed carnivorously and omnivorously were higher in the center of the seagrass beds.
(3) Habitat loss and aquaculture may adversely affect the concentration of nutrients in the environment and the diversity of macrobenthos, and corresponding ecological protection measures are needed to ensure no further degradation of existing seagrass beds.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Yantai Institute of Coastal Zone Research, Chinese Academy of Science. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YJ: Writing – original draft. BS: Writing – original draft. JX: Funding acquisition, Project administration, Writing – review & editing. SJ: Writing – original draft. LC: Writing – original draft. BL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (42276142, 42176160), the Youth Science and Technology Talent Project supported by Ministry of Natural Resources (recipient: Jiangling Xu), the Key Laboratory of Ecological Prewarning, Protection and Restoration of Bohai Sea, Ministry of Natural Resources (2023110), the found of International Partnership Program of the Chinese Academy of Sciences (133137KYSB20200002), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA23050304; XDA23050202).
Acknowledgments
We would like to thank all staff of the Yellow River Delta National Nature Reserve for their great support during the sampling. We also appreciate reviewers for their suggestions and comments on the earlier version of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bell S. S., Brooks R. A., Robbins B. D., Fonseca M. S., Hall M. O. (2001). Faunal response to fragmentation in seagrass habitats: implication for seagrass conservation. Biol. Conserv. 100, 115–123. doi: 10.1016/S0006-3207(00)00212-3
Bi P. R., Chen G. C., Wang J. M., Hu H. Z., Jiang Z. J., An W. S., et al. (2023). Comparison of macrofaunal assemblages in temperate seagrass meadows and neighboring seabeds along the southeastern coast of Shandong Peninsula, China. Marine Pollution Bulletin 190, 1–10. doi: 10.1016/j.marpolbul.2023.114847
Blanchet H., de Motaudouin X., Lucas A., Chardy P. (2004). Heterogeneity of macrozoobenthic assemblages within a Zostera noltii seagrass bed: diversity, abundance, biomass and structuring factors. Estuar. Coast. Shelf Sci. 61, 111–123. doi: 10.1016/j.ecss.2004.04.008
Bordovskiy O. K. (1965). Transformation of organic matter in bottom sediments and its early diagenesis. Mar. Geol. 3, 83–114. doi: 10.1016/0025-3227(65)90005-8
Boström C., Jackson E. L., Simenstad C. A. (2006). Seagrass landscapes and their effects on associated fauna: A review. Estuar. Coast. Shelf Sci. 68, 383–403. doi: 10.1016/j.ecss.2006.01.026
Burdick D. M., Short F. T. (1999). The Effects of boat docks on eelgrass beds in coastal waters of Massachusetts. Environ. Manage. 23, 231–240. doi: 10.1007/s002679900182
Connolly R. M. (1994). Removal of seagrass canopy: effects on small fish and their prey. J. Exp. Mar. Biol. Ecol. 184, 99–110. doi: 10.1016/0022-0981(94)90168-6
Connolly R. M. (1997). Differences in composition of small, motile invertebrate assemblages from seagrass and unvegetated habitats in a southern Australian estuary. Hydrobiologia 346, 137–148. doi: 10.1023/A:1002970100662
Costanza R., d’Arge R., de Groot R., Grasso M., Hannon B., Paruelo J. (1997). The value of the world’s ecosystem services and natural capital. Nature 387, 253–260. doi: 10.1038/387253a0
Edgar G. J., Shaw C., Watson G. F., Hammond L. S. (1994). Comparisons of species richness, size-structure and production of benthos in vegetated and unvegetated habitats in Western Port, Victoria. J. Exp. Mar. Biol. Ecol. 176, 201–226. doi: 10.1016/0022-0981(94)90185-6
Frost M. T., Rowden A. A., Attrill M. J. (1999). Effect of habitat fragmentation on the macroinvertebrate infaunal communities associated with the seagrass Zostera marina L. Aquat. Conserv.: Mar. Freshw. Ecosyst. 9, 255–263. doi: 10.1002/(SICI)1099-0755(199905/06)9:3<255::AID-AQC346>3.0.CO;2-F
Gartner A., Tuya F., Lavery P. S., McMahon K. (2013). Habitat preferences of macroinvertebrate fauna among seagrasses with varying structural forms. J. Exp. Mar. Biol. Ecol. 439, 143–151. doi: 10.1016/j.jembe.2012.11.009
Guo Y. (2019). Eco-economic value evaluation of seagrass beds in Guangxi Beibu Gulf and protection countermeasures. Modern Agric. Sci. Technol. 2), 170–173. doi: 10.3969/j.issn.1007-5739.2019.02.107
Hillman K., McComb A. J., Walker D. I. (1995). The distribution, biomass and primary production of the seagrass Halophila ovalis in the Swan/Canning Estuary, Western Australia. Aquat. Bot. 51, 1–54. doi: 10.1016/0304-3770(95)00466-D
Huang X., Huang L., Li Y., Xu Z., Fong C. W., Huang D., et al. (2006). Main seagrass beds and threats to their habitats in the coastal sea of South China. Chin. Sci. Bull. 51, 136–142. doi: 10.1007/s11434-006-9136-5
Hyland J., Balthis L., Karakassis I., Magni P., Petrov P., Shine J., et al. (2005). Organic carbon content of sediments as an indicator of stress in the marine benthos. Mar. Ecol. Prog. Ser. 295, 91–103. doi: 10.3354/meps295091
Jankowska E., Michel L. N., Lepoint G., Włodarska-Kowalczuk M. (2019). Stabilizing effects of seagrass meadows on coastal water benthic food webs. J. Exp. Mar. Biol. Ecol. 510, 54–63. doi: 10.1016/j.jembe.2018.10.004
Li Y., Huang X., Xu Z., Huang L. (2007). Threat and protection measurement of Hepu seagrass beds in Guangxi. Mar. Environ. Sci. 26, 587–590.
Lundquist C. J., Jones T. C., Parkes S. M., Bulmer R. H. (2018). Changes in benthic community structure and sediment characteristics after natural recolonization of the seagrass Zostera muelleri. Sci. Rep. 8, 1–9. doi: 10.1038/s41598-018-31398-2
Margalef F. R. (1968). Perspective in Ecological Theory (Chicago: University of Chicago Press), 111.
Meyers P. A. (1997). Organic geochemical proxies of paleoceanographic, paleolimnologicm, and paleclimatic processes. Organic Geochemistry Proxies 27, 213–250. doi: 10.1016/S0146-6380(97)00049-1
Milliman J. D., Syvitski J. P. M. (1992). Geomorphic/tectonic control of sediment discharge to the ocean: the importance of small mountainous rivers. J. Geol. 100, 525–544. doi: 10.1086/629606
Milliman J. D., Xie Q. C., Yang Z. S. (1984). Transfer of particulate organic carbon and nitrogen from the Yangtze River to the ocean. Am. J. Sci. 284, 824–834. doi: 10.2475/ajs.284.7.824
Neckles H. A., Short F. T., Barker S., Kopp B. S. (2005). Disturbance of eelgrass Zostera marina by commercial mussel Mytilus edulis harvesting in Maine: dragging impacts and habitat recovery. Mar. Ecol. Prog. Ser. 285, 57–73. doi: 10.3354/meps285057
Norris J. G., Wyllie-Echeverria S., Mumford T., Bailey A., Turner T. (1997). Estimating basal area coverage of subtidal seagrass beds using underwater videography. Aquat. Bot. 58, 269–287. doi: 10.1016/S0304-3770(97)00040-5
Ogrinc N., Fontolan G., Faganeli J. (2004). Carbon and nitrogen isotope compositions of organic matter in coastal marine sediments (The Gulf of Trieste, N Adriatic Sea): indicator of sources and preservation. Mar. Chem. 95, 163–181. doi: 10.1016/j.marchem.2004.09.003
Phillips R. C., McCroy C. P. (1980). Handbook of Seagrass Biology: An Ecosystem Perspective (NewYork: Garland STPM Press).
Prahl F. G., Bennett J. T., Carpenter R. (1980). The early diagenesis of aliphatic hydrocarbons and organic matter in sedimentary particulates from Dabob Bay, Washington. Geochim. Cosmochim. Acta 44, 1967–1976. doi: 10.1016/0016-7037(80)90196-9
Qiao S. Q., Shi X. F., Bai Y. Z., Xiong L. F., Zhu A. M., Liu Y. G., et al. (2011). Distribution of organic carbon, nitrogen in suspended and surface sediments and their controlling factors off the Huanghe (Yellow River) mouth and the nearby Bohai sea. Acta Sedimentologica Sin. 29, 354–362. doi: 10.14027/j.cnki.cjxb.2011.02.011
Reed B. J., Hovel K. A. (2006). Seagrass habitat disturbance: how loss and fragmentation of eelgrass Zostera marina influences epifaunal abundance and diversity. Mar. Ecol. Prog. Ser. 326, 133–143. doi: 10.3354/meps326133
Schlünz B., Schneider R. R. (2000). Transport of terrestrial organic carbon to the oceans by rivers: re-estimating flux-and burial rates. Int. J. Earth Sci. 88, 599–606. doi: 10.1007/s005310050290
Shannon C. E., Weaver W. (1949). The mathematical theory of communication (Urbana: University of Illinois Press), 117.
Short F. T., Wyllie-Echeverria S. (1996). Natual and human-induced disturbance of seagrasses. Environ. Conserv. 23, 17–27. doi: 10.1017/S0376892900038212
Smith S. V. (1981). Marine macrophytes as a global carbon sink. Science 211, 838–840. doi: 10.1126/science.211.4484.838
Stoner A. W. (1980). The role of seagrass biomass in the organization of benthic macrofaunal assemblages. Bull. Mar. Sci. 30, 537–551.
Sui J., Zhang J., Ren S. J., Lin F. (2019). Organic carbon in the surface sediments from the intensive mariculture zone of Sanggou Bay: Distribution, seasonal variations and sources. J. Ocean Univ. China 18, 985–996. doi: 10.1007/s11802-019-3768-y
Tagliapietra D., Sigovini M., Magni P. (2012). Saprobity: a unified view of benthic succession models for coastal lagoons. Hydrobiologia 686, 15–28. doi: 10.1007/s10750-012-1001-8
Talbot M. R. (1990). A review of the palaeohydrological interpretation of carbon and oxygen isotopic ratios in primary lacustrine carbonates. Chem. Geol. 80, 261–279. doi: 10.1016/0168-9622(90)90009-2
Tanner J. E. (2005). Edge effects on fauna in fragmented seagrass meadows. Austral Ecol. 30, 210–218. doi: 10.1111/j.1442-9993.2005.01438.x
Thornton S. F., Mcmanus J. (1994). Application of organic carbon and nitrogen stable isotope and C/N ratios as source indicators of organic matter provenance in estuarine systems: evidence from the Tay Estuary, Scotland. Estuary Coast. Shelf 38, 219–233. doi: 10.1006/ecss.1994.1015
Tu Z. G., Han T. S., Chen X. H., Wu R., Wang D. R. (2016). Thecommunity structure and diversity of macrobenthos in Linshui Xincungang and Li’ angang seagrass special protected area, Hainan. Mar. Environ. Sci. 35, 41–48. doi: 10.13634/j.cnki.mes.2016.01.007
Webster P. J., Rowden A. A., Attrill M. J. (1998). Effect of shoot density on the infaunal macro-invertebrate community within a Zostera marina seagrass bed. Estuary Coast. Shelf 47, 351–357. doi: 10.1006/ecss.1998.0358
Wu R., Zhang H. (2018). Changes in seagrass beds and conservation measures in Hepu, Guangxi. China Sci. Technol. Inf. 22), 68–69.
Xia B., Han Q., Chen B. J., Sui Q., Jiang T., Sun X. M., et al. (2018). Influence of shellfish biodeposition on coastal sedimentary organic matter: A case study from Sanggou Bay, China. Continental Shelf Res. 172, 12–21. doi: 10.1016/j.csr.2018.11.002
Yi J. J., Wang W. H., Wang Y., Wan X. M., Wang H. B., Feng K. C. (2022). Distribution characteristics and pollution assessment of carbon, nitrogen and phosphorus in surface sediments of Nan’ao Island, eastern Guangdong Province. Guangxi Sci. 29, 871–880. doi: 10.13656/j.cnki.gxkx.20221116.007
Yu G. L., Li B., Li F., Qi Z. H., Zhang M. L. (2019). Carbon, nitrogen geochemical character and organic matter source study in the coastal sediment of Yellow River Estuary. Mar. Environ. Sci. 38, 862–867. doi: 10.12111/j.mes20190607
Yu S., Zhang J., Cui L., Jiang Z., Zhang L., Huang X. (2019). Preliminary study on seed-based restoration for Enhalus acoroides meadow. J. Trop. Oceanogr. 38, 49–54.
Keywords: seagrass bed, macrobenthos, community structure, biodiversity, environmental factor
Citation: Ji Y, Song B, Xu J, Jiang S, Chen L and Li B (2024) Distribution and influencing factors of macrobenthos on three seagrass beds in the intertidal zone of Shandong province, China. Front. Mar. Sci. 11:1349131. doi: 10.3389/fmars.2024.1349131
Received: 04 December 2023; Accepted: 15 February 2024;
Published: 29 February 2024.
Edited by:
Xiaoshou Liu, Ocean University of China, ChinaReviewed by:
Wenzhe Xu, Tianjin University of Science and Technology, ChinaPaolo Magni, National Research Council (CNR), Italy
Copyright © 2024 Ji, Song, Xu, Jiang, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baoquan Li, YnFsaUB5aWMuYWMuY24=
 Yinglu Ji
Yinglu Ji Bo Song3
Bo Song3 Shaoyu Jiang
Shaoyu Jiang Linlin Chen
Linlin Chen Baoquan Li
Baoquan Li