
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 29 February 2024
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1340522
To investigate the spatial–temporal distribution characteristics of Harpadon nehereus in the Yangtze River Estuary (YRE) and its relationship with environmental factors, this study used the data from resource and environmental surveys conducted in the YRE and adjacent waters during August (summer) and November (autumn), 2017–2022. Generalized additive models (GAM) were employed to analyze the relationships between the relative resources of H. nehereus and environmental factors and to predict the spatial–temporal distribution of H. nehereus resources in 2022. Our results revealed that the best model deviance explained in summer and autumn was 64.89% and 49.90%, with average effect sizes of 0.75 and 0.70, respectively, for cross-validated regression slopes. Water temperature and salinity were identified as the key environmental factors influencing the relative resources of H. nehereus in the YRE. Overall, there were notable seasonal differences in the relationship between the relative resources of H. nehereus and environmental factors. The relative resources of H. nehereus in the YRE were higher in the summer than in autumn. In summer, both water temperature and salinity exhibited multi-wave nonlinear relationships with the relative resources of H. nehereus, while in autumn, the relative resources of H. nehereus showed a positive linear relationship with water temperature and a non-linear relationship with salinity. Additionally, the predicted and observed values of the relative resources of H. nehereus in 2022 showed similar spatial distribution patterns. The relative resources of H. nehereus was higher in the northern branch than in the southern branch and the offshore regions compared to the near-estuary regions. Altogether, our study provides a scientific basis for conservation management and sustainable utilization of H. nehereus resources in the YRE, thereby contributing to the restoration and management of fishery resources in the region.
The Yangtze River Estuary (YRE) is located in the subtropical monsoon climate zone of eastern Eurasian and has a unique geographical location and environmental conditions (Kindong et al., 2020). Owing to the runoff of the Yangtze River, the YRE provides a rich bait base for the growth of a variety of economic fish and their juveniles. Moreover, YRE is the largest migratory corridor for saltwater and freshwater fish as well as a breeding and hatchery site in China (Chen et al., 2021). However, anthropogenic factors, such as overfishing, water pollution, and wetland reclamation, has altered the structure of aquatic communities in the YRE (Yang et al., 2020), reduced the stability of the ecosystem, and decreased the resources of some large fishes with long lifespans, and increased the resources of small fishes, such as Coilya nasus and Harpadon nehereus (Yang et al., 2022). H. nehereus has become a dominant species in the YRE (Sun et al., 2015).
Being a carnivorous fish, H. nehereus occupies a high trophic level and an important niche in the ecosystem (Pan and Cheng, 2011). H. nehereus has experienced rapid population growth along the coast of China in recent decades, compressing the ecological niches of other marine species and disrupting existing food webs (Wang et al., 2021; Jiang et al., 2022). Wang et al. (2021) pointed to a northward shift in overall suitable habitat for H. nehereus as the climate changes, which may further affect the stability of the ecological community. Therefore, understanding the variability of the spatial–temporal distribution of H. nehereus can provide theoretical references and a scientific basis for the effective management and conservation of fishery resources (Zhao et al., 2014; Huang et al., 2015). Variations in the distribution of fish stocks are closely associated with environmental factors (Ma et al., 2020). Thus far, several studies have been conducted on the relationship between spatial–temporal distribution and environmental factors. For instance, Li et al. (2021) found that changes in water temperature affect the relative abundance of Larimichthys polyactis, and Ma et al. (2022a) demonstrated that temperature and salinity have a significant impact on the relative abundance of Engraulis japonicus.
Being a short-distance migratory fish, the population of H. nehereus has obvious dynamic-change characteristics. Additionally, its spatial–temporal distribution varies with environmental conditions, and there is a non-linear relationship with the environmental factors (Du, 2018). Among the common species distribution models, the generalized additive model (GAM) is better able to analyze the complex connections between the data and is commonly used to construct relationships between a response variable and multiple explanatory variables, which can reflect the effect of each environmental factor on the abundance of fish stocks. Therefore, GAM has been widely used in analyzing the spatial–temporal distribution of fishery resources and their relationship with environmental factors (Wu et al., 2019).
Thus far, studies on H. nehereus have primarily focused on its biological characteristics (Luo, 2012), morphological features (Wang et al., 2020), feeding habits (Liu et al., 2021), and aquatic product processing (Cao et al., 2020). Among these, studies on H. nehereus in the YRE are limited, with most of them focusing on trophic ecology (Pan and Cheng, 2011). Moreover, there are no reports on the spatial–temporal distribution of H. nehereus and its relationship with environmental factors. Therefore, this study utilized fish resource survey data collected from the YRE and its adjacent waters during the period from 2017 to 2022. The data was analyzed using the GAM to investigate the relationship between the resource distribution characteristics of the YRE and environmental factors. The results of our study provide a scientific basis for the sustainable use and management of the aquatic biological resources of the YRE.
Since H. nehereus rarely occur in the waters of the YRE in May (spring) and February (winter), the stock and environmental data for H. nehereus in this paper were obtained from the fishery resource surveys conducted in August (summer) and November (autumn) from 2017 to 2022 in the YRE and adjacent waters (Table 1). The survey area consisted of the southern and northern branches of the YRE and adjacent waters. The sampling is fixed-point sampling. The netting gear used is a truss rod trawl, with an opening length of 6 m, an opening width of 1.8 m, a body length of 11m, a body mesh of 0.02 m, a number of 2 bladder nets, and a bladder mesh of 0.01 m. The survey vessel was operated at a speed of 2 knots for 0.5 h. A total of 18 survey stations were set up per season using the fixed-point sampling method. However, some of the survey stations were adjusted in 2021 (Figure 1). The samples were taken to the laboratory for the measurement of biological data, such as body length, weight, and gonadal maturity. Environmental data were measured simultaneously at each station during the survey. Water samples were taken from each station at high and low tides, and the sampling method was determined according to the requirements of Part III of the Specification for Marine Monitoring (GB 17378.3-2007). Surface water temperature (°C), salinity (S, ‰), pH, and dissolved oxygen (DO, mg/L) were measured using a WTW Multi 3430 water quality meter.
The number of H. nehereus caught per unit of time per net (g/h-net) was used as the response variable of the GAM to reflect the relative resources of H. nehereus in the region. During processing, a constant (1) was added to the response variables to avoid the conversion error of the zero value (Tian et al., 2009) and to enable the application of GAM with identity as the link function. Considering the short-distance migration of H. nehereus (Sun, 2022), longitude and latitude were selected as the influencing factors of the spatial distribution of H. nehereus. Water temperature influences the rate of gonadal development in H. nehereus (Sun, 2022), salinity affects fish growth and development (Ran et al., 2020), water depth influences fish distribution (Wang et al., 2020), and DO and pH drive the spatial–temporal variability of the fish community across seasons (Zhang, 2012); therefore, longitude, latitude, water temperature, salinity, water depth, pH, and DO were chosen as the explanatory variables of the model. The full factorial expression of GAM was as follows:
where y is relative resource, s is spline smoothing function, Lat is latitude, Lon is longitude, T is water temperature, S is salinity, Dep is water depth, DO is dissolved oxygen, Ɛ is relative error, and family is the distribution mode set to Gaussian distribution.
The variance inflation factor (VIF) was used to test for collinearity of explanatory variables by season (Hou et al., 2021). In this study, the threshold value of the VIF test was set to 5, and the collinearity was considered to exist when the VIF threshold value was > 5 (Ma et al., 2022a). When the VIF of only one factor was > 5, the factor was removed and the collinearity test was conducted for the other factors with VIF< 5; however, when the VIF of multiple environmental factors was > 5, the influence factor with the largest variance expansion coefficient was removed and the collinearity test was conducted again.
Akaike information criterion (AIC) can be used to measure the goodness-of-fit of multi-group models, and it is generally believed that the lower the AIC value, the better the model fit (Planque et al., 2007). After screening, all the explanatory variables were added stepwise to the GAM for both seasons, and the model with the smallest AIC value was selected as the best-fitting model for each season. The AIC was calculated as follows:
where k is the number of parameters and L is the likelihood function.
The predictive abilities of the selected models were cross-validated with a 5-fold cross-validation method described by Rodríguez et al. (2010) and by constructing a linear relationship between the predicted values (lnY) and the observed values (lny), using the following equation:
This process is repeated 100 times and the mean values of a and b reflect the prediction bias. For instance, a = 0 and b = 1 indicate the best model prediction performance, with no deviation between the predicted and observed values (Li et al., 2015).
Based on the environmental survey data of each site in each season of 2022, the survey area was divided into 0.025° × 0.025° grids, and the center point of each grid was determined. Thereafter, the inverse distance weighting method was applied to obtain the environmental data of the center point of each grid (Philip and Watson, 1982). The optimal model for each season was applied to predict the distribution of H. nehereus for the corresponding season in 2022, which was then compared with the measured resource distribution.
Model construction, cross-validation, and prediction were performed in the R software V4.1.2, and the model was implemented through the “mgcv” package (Wood et al., 2016). The survey stations and resource distribution maps were drawn in Arcmap 10.8.
The results revealed that in summer, the VIF values of all the environmental factors were< 5, while in autumn, the VIF values of water temperature and DO were > 5; however, after removing the VIF value of DO (highest), the VIF values of all the influencing factors were< 5 (Table 2).
Based on the minimum AIC principle, the variable combination of the best model in summer was T + S + Lon + Lat (Table 3), with a deviance explained of 64.89%. Among these, salinity had the largest contribution (34.12%), followed by longitude (22.74%), latitude (6.99%), and water temperature (1.04%). Salinity and longitude had an extremely significant effect on the abundance (P< 0.001, Table 4), while water temperature had a significant effect on the abundance of H. nehereus (P< 0.01, Table 4). The variable combination of the optimal model for autumn was consistent with that of the summer (Table 3), and the model deviance explained was 49.90%. Among the variables, the contribution of water temperature and salinity was very significant, accounting for 21.31% and 10.86%, respectively. The water temperature had an extremely significant effect on the relative resources of H. nehereus in the YRE in autumn (P< 0.001, Table 4).
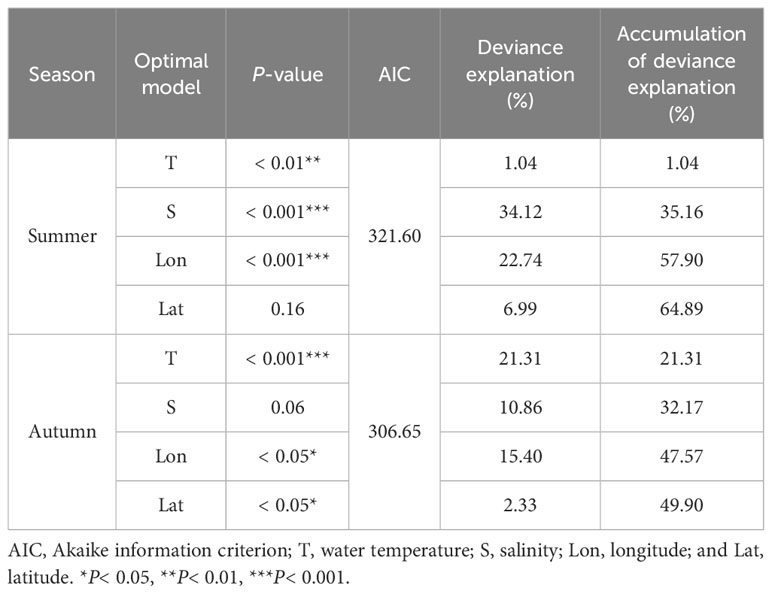
Table 4 Parameters associated with the best-fit model for the relative resources of Harpadon nehereus in the Yangtze River Estuary in summer and autumn (2017–2022).
In summer, the relative resources of H. nehereus in the YRE showed a non-linear relationship with water temperature, salinity, and longitude (Figures 2A–C). It showed a wavy non-linear relationship with water temperature and fluctuated greatly with an increase in water temperature, reaching the maximum value at approximately 30.4 °C (Figure 2A). Meanwhile, it showed a more complicated multi-wave non-linear relationship with salinity in the range of 0–30‰, reaching the maximum value at approximately 26 (Figure 2B). Additionally, it showed a non-linear relationship with longitude, which increased and then decreased with an increase in longitude in the range of 121.2–122.2°E (Figure 2C). Lastly, it showed a negative linear relationship with latitude, which decreased with an increase in latitude (Figure 2D).
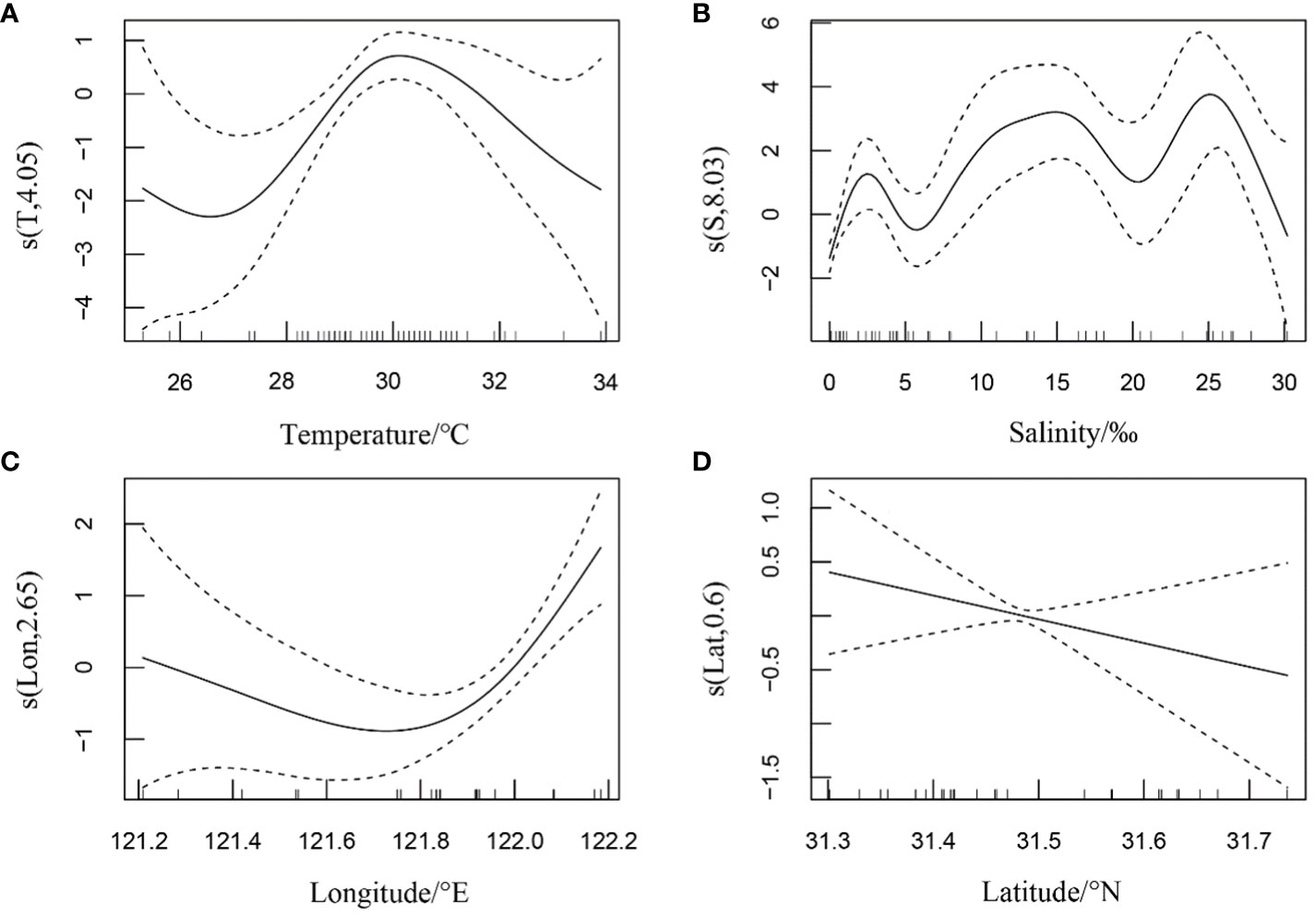
Figure 2 Relationship between the relative resources of Harpadon nehereus in the Yangtze River Estuary and the environmental factors in summer. (A: Temperature. B: Salinity. C: Longitude. D: Latitude.)
In autumn, the relative resources of H. nehereus in the YRE demonstrated a positive linear relationship with water temperature. Additionally, non-linear relationships were observed between the relative resources of H. nehereus and factors such as salinity, longitude, and latitude (Figure 3). It increased with an increase in water temperature in the range of 10–20°C (Figure 3A). Additionally, it increased with an increase in salinity at< 20, but gradually decreased with an increase in salinity at > 20 (Figure 3B). Furthermore, it showed an overall positive correlation with longitude in the range of 121.2–122.2°E (Figure 3C) and a multi-wave non-linear relationship with latitude in the range of 31.3–31.7°N, attaining a maximum value at 31.6°N (Figure 3D).
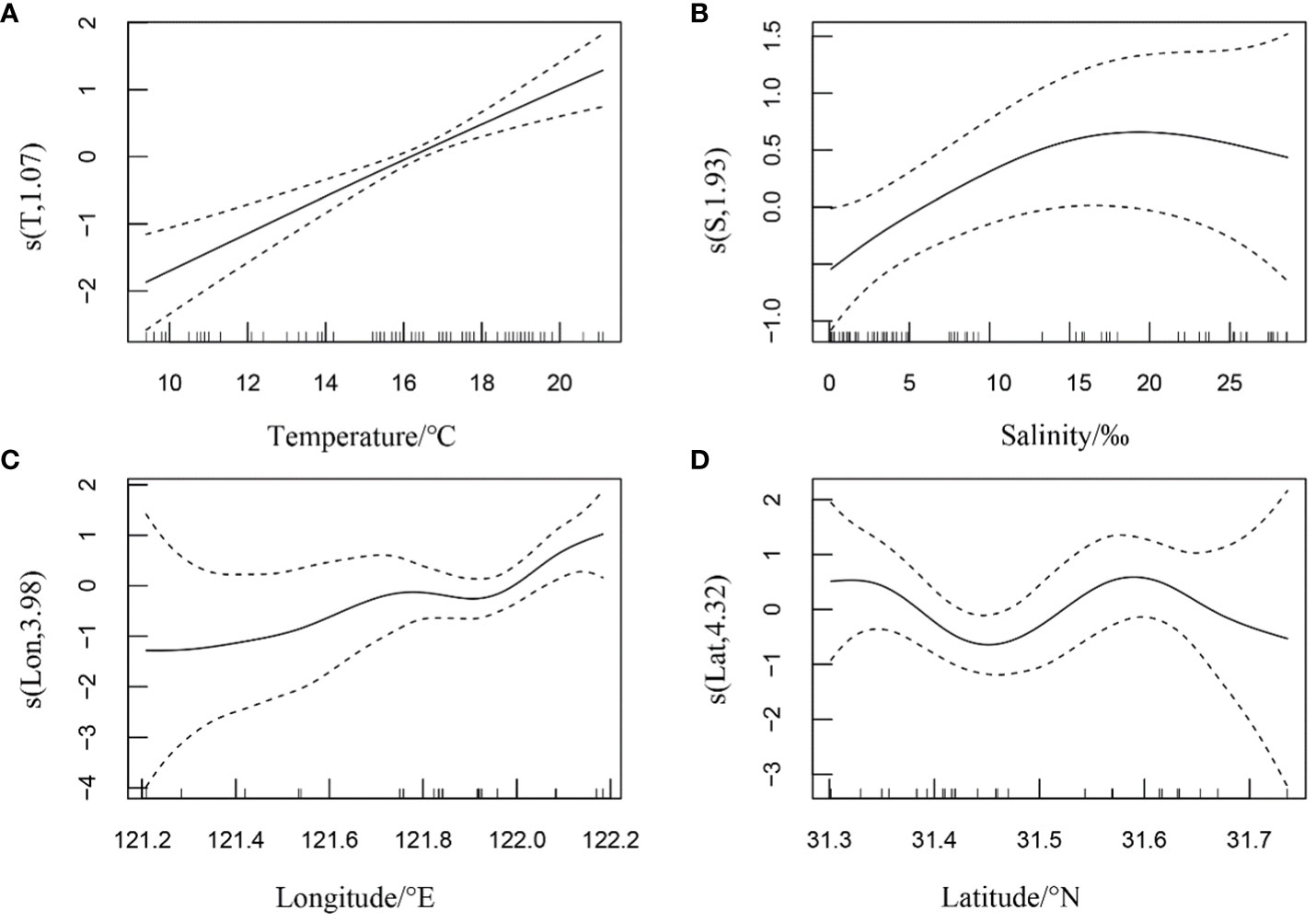
Figure 3 Relationship between the relative resources of Harpadon nehereus in the Yangtze River Estuary and the environmental factors in autumn. (A:Temperature. B: Salinity. C: Longitude. D: Latitude.)
The results of the cross-validation of the best GAM showed (Figure 4) that the mean cross-validated linear regression slope, mean intercept, and mean coefficient of determination (r2) were 0.75, 1.79, and 0.29 for summer and 0.70, 1.81, and 0.27 for autumn, respectively.
Figure 4 Linear regression of cross-validation test for the best generalized additive models for summer and autumn.
In terms of temporal distribution, there were inter-annual and seasonal variations in the relative resource of H. nehereus in the YRE. The inter-annual variations ranged between 625.95–3,780.80 g/h-net during 2017–2022 and showed an increasing trend from 2017 to 2021, with a significant increase in 2021. However, the relative resource decreased in 2022, although it was relatively higher than that during 2017–2020. Seasonally, the relative resource of H. nehereus was significantly greater in the summer than in the autumn (Figure 5).
In terms of spatial distribution, the H. nehereus showed an overall trend of higher relative resources in the northern branch than in the southern branch, and higher relative resources in the offshore than in the near-estuary, a feature that was even more pronounced in the autumn. The distribution of predicted values of relative resources of H. nehereus in the YRE in 2022 showed the similar distribution of true values, and the range of sizes of predicted values was similar to that of true values (Figure 6).
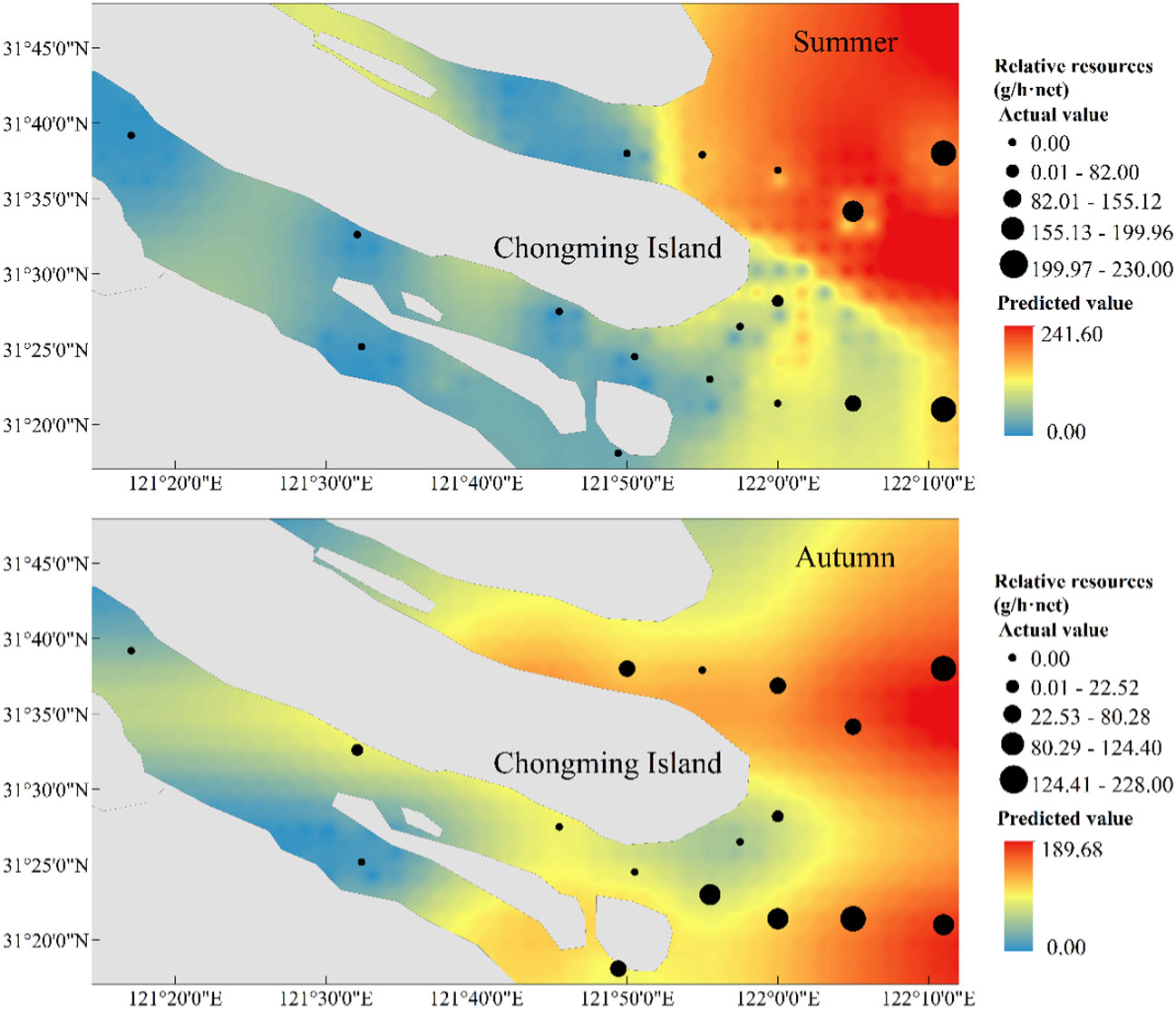
Figure 6 Predicted and observed spatial distribution of Harpadon nehereus in the Yangtze River Estuary in 2022.
GAM is one of the most common regression models (Yin et al., 2019) that is commonly used to study the spatial and temporal distribution of fish populations (Ji et al., 2020). In this study, we constructed two seasonal (summer and autumn) models for H. nehereus resources in the YRE during 2017–2022 based on seven environmental factors, namely water temperature, salinity, DO, pH, water depth, longitude, and latitude. Our results revealed that the optimal model combinations for both the summer and autumn seasons were T + S + Lon + Lat, indicating that water temperature, salinity, longitude, and latitude are the primary environmental factors influencing the relative resources of H. nehereus in the YRE. Cross-validation is commonly used to verify the predictive ability of a model (Jensen et al., 2005). The results of linear regression analysis of 100 cross-validations of both the best-fit models showed the mean effect of 100 linear regressions was systematically biased from the orthogonal line of the 1:1 regression line, which may be caused by insufficient data or the absence of important influencing variables (Zhu et al., 2012). However, there was a clear positive correlation between the predicted and observed values of the models, suggesting a relatively high accuracy of the models in predicting spatial–temporal distributions of H. nehereus in the YRE. In addition, the model deviance explained was lower in both summer and autumn, which may be due to the higher influence of other factors, such as bait, on resource distribution compared to environmental factors. Relevant studies have shown (Liu et al., 2021) that H. nehereus has a wide feeding range of over 40 species, primarily consisting of fish and shrimp, which also undergo seasonal variations and further affect the seasonal distribution and migration of H. nehereus. The combined effect of environmental factors may also affect the model deviation rate. For example, high water temperature and low DO synergistically affect the metabolic rate of fish, suggesting that DO may also influence H. nehereus distribution (Yu and Xian, 2009; Hajisamae and Yeesin, 2010).
The results of our study showed that environmental factors, such as water temperature and salinity, considerably influence the distribution of H. nehereus in the YRE. Water temperature is considered to be the most important influencing factor on the distribution of fish stocks (Zhao et al., 2020), which affects not only the feeding, growth, reproduction, and metabolism of fish (Liu et al., 2020) but also the abundance and distribution of their natural bait (Lewin et al., 2014), which further affect the migratory activities of the fish (Ma et al., 2020). Our results showed that the relative resources of H. nehereus in the YRE showed a wavy non-linear relationship with water temperature in summer and a positive linear relationship with the water temperature in autumn. Thus, the higher the water temperature the greater the resource of H. nehereus in this water temperature range (Sun, 2022). In addition, there were more pronounced seasonal variations in the relationship between H. nehereus distribution and water temperature, which may be associated with seasonal variations in the runoff from the YRE (Zhang, 2015). The Yangtze River runoff affects the distribution of temperature and salinity, location of spawning ground, and nutrient transport, which further affected the structure of the fish community (Rakocinski et al., 1996; Shan et al., 2004), resulting in a more pronounced relationship between fishery resources and water temperature.
Salinity influences all the life stages of fish (Ma et al., 2022b), and its tolerance varies among different developmental stages. H. nehereus adults migrate to high-salinity environments during the reproductive stage, while H. nehereus juveniles thrive in low-salinity environments due to their low tolerance to high-salinity (Luo et al., 2012). For instance, Sun (2022) found that in the coastal waters of Zhejiang province, H. nehereus adults tend to migrate to high-salinity environments (≥ 30) before the spawning and reproductive period, while H. nehereus juveniles are abundant in low-salinity environments (< 27). These findings are consistent with the influence of salinity on the distribution of H. nehereus observed in this study. The geographical location of the YRE is unique as it constitutes both freshwater and saltwater. Consequently, the salinity distribution in this study area spans a wide range and its effect on the relative resources of H. nehereus is more complex, with obvious seasonal variations between summer and autumn. The relative resources of H. nehereus in the YRE showed a multi-wave non-linear relationship with salinity in the summer and a non-linear relationship with salinity in autumn. This may be due to the spawning bloom of H. nehereus in summer (Zhuang et al., 2018), where fish at different developmental stages are present and have different salinities suitable for survival, while most of the H. nehereus in autumn are in the juvenile stage and prefer low-salinity environments for optimal growth and development, and so the relative resources of H. nehereus showed a decreasing trend as the salinity increased to a certain salinity level.
Our study found that the relative resources of H. nehereus in the YRE showed a significant upward trend in both summer and autumn during 2017–2021. This may be due to the increase in seasonal or migratory fish in the YRE in recent years due to anthropogenic factors such as fishing (Yang et al., 2022). China has been protecting the key waters of the Yangtze River, including the YRE, since 2003 and implemented a ten-year fishing ban policy as of January 2021. H. nehereus grows rapidly and generally reaches sexual maturity at the age of one (Zhuang et al., 2018), which may explain the sharp increase in the resources of H. nehereus in the YRE in 2021. Moreover, relative to 2021, the resources of H. nehereus decreased in 2022, but was comparatively higher than during 2017–2020. This decrease in the relative resources of H. nehereus may be due to the invasion of salty tidal waves in the YRE in the summer of 2022 (Wang et al., 2023). The predicted and observed values of H. nehereus distribution in the YRE in 2022 were similar in range and presented similar spatial distribution characteristics. The relative resources of H. nehereus were higher in the northern branch compared to the southern branch and in the offshore regions than in the near-estuary regions, which indicates a good prediction and fitting effect of the selected models.
Seasonal variations in the relative resources of H. nehereus in the YRE were obvious, with the relative resources in the summer being significantly greater than that in the autumn. These results were consistent with a previous report by Guo et al. (2019) on the resources of H. nehereus in the Min River Estuary. The unique ecological environment of the YRE is affected by runoff and hydrodynamic factors. In summer, the YRE has abundant water and the hydrodynamics of the north and south branches are stronger, enabling better growth and survival of the marine migratory fishes, especially in the peak reproductive stage, and reducing intraspecies struggles and interspecies competitions (Shen et al., 2011). Therefore, the estuarine area can be used as a good baiting and fattening ground for marine migratory fishes (Shen et al., 2011). H. nehereus exhibits migratory activity from July to September, during which time they move towards the coast for spawning migration and bait solicitation (Sun and Chen, 1986; Luo et al., 2012). After October, when the water temperature decreases, H. nehereus gradually migrate offshore to deeper waters for overwintering (Guo et al., 2019), which explains the relatively high resources of H. nehereus in the YRE during summer and in the seaward side during autumn.
Salinity is an important factor influencing the spatial distribution of fish communities in the YRE, which constitutes both saltwater and freshwater (Ye et al., 2023). In terms of spatial distribution, the relative resources of H. nehereus in the YRE are higher in the northern branch compared to the southern branch and offshore compared to the near-estuary regions. This is possibly due to the significantly high salinity in the northern and offshore waters compared to the southern and near-estuary waters, respectively (Guo, 2022), which is suitable for H. nehereus as an oceanic migratory fish. Additionally, the southern branch has a navigation channel with frequent vessel traffic, which indirectly destroys the ecology of the watershed (Lewin et al., 2014). This may explain the low relative resources of H. nehereus in these waters.
In this study, the GAM model was used to analyze the resource distribution of H. nehereus on in the YRE and its relationship with environmental factors from 2017 to 2022. The study found several resource distribution characteristics of H. nehereus: The resource distribution of H. nehereus was higher in summer compared to autumn. The resource distribution of H. nehereus was higher in the waters of the northern branch of the YRE compared to the waters of the southern branch. Additionally, the waters of the far shore exhibited higher resource distribution than the waters of the near shore. The main environmental factors identified to affect the resource distribution characteristics of H. nehereus were water temperature and salinity. However, in addition to the environmental factors selected in this paper, other environmental factors and their combined effects also influence the resource distribution of estuarine fishes (Huang et al., 2013). Therefore, in future studies, more environmental factors will be selected, biological factors will be added as well as the joint effects among environmental factors will be considered. In this way, the resource distribution characteristics of H. nehereus in the YRE and their influencing factors will be more accurately investigated. These studies will be of great significance to the resource management, protection, and sustainable use of H. nehereus and the YRE resources.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
YL: Data curation, Investigation, Validation, Visualization, Writing – original draft. CG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Writing – review & editing. JC: Data curation, Funding acquisition, Investigation, Project administration, Writing – review & editing. QW: Conceptualization, Formal analysis, Funding acquisition, Writing – review & editing. JZ: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Shanghai Municipal Science and Technology Commission Local Capacity Building Program for Universities (23010502500; 20dz1204703).
The authors of this research would like to thank the teachers and students from the Research Laboratory of Quantitative Assessment and Management of Fisheries Resources and Ecosystems, Shanghai Ocean University and Shanghai Aquatic Wildlife Conservation Research Center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer, ZW, declared a shared parent affiliation with the authors, YL, CG, and JZ.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Cao R. L., Zhong Y. L., Lan H., Chen W. Y. C., Feng J. H., Chen X. E. (2020). Effects of different freezing methods on the quality of Harpadon nehereus. Food Ind. 41, 5–8.
Chen J. H., Wang X. F., Tian S., Wu J. H., Dai L. B., Gao C. X., et al. (2021). A review of the development of fishery resources monitoring in the yangtze river estuary and its adjacent waters. Resour. Environ. Yangtze. Basin. 30, 122–136. doi: 10.11870/cjlyzyyhj202101012
Du X. X. (2018). Biological characteristics and spatial distribution pattern of Harpadon nehereus in offshore water of southern zhejiang (Shanghai Ocean University).
Guo J. H., Li J., Shen C., Shi Y., Feng C., He X. B., et al. (2019). Estimation of biological parameters and stock of Harpadon nehereus in the min river estuary, east China sea. J. Guangdong. Ocean. Univ. 39, 56–64. doi: 10.3969/j.issn.1673-9159.2019.05.009
Guo L. X. (2022). Temporal and spatial distribution characteristics of dissolved organic matter in the north and south branches of the yangtze river estuary (Shanghai Ocean University).
Hajisamae S., Yeesin P. (2010). Patterns in community structure of trawl catches along coastal area of the south China sea. Raffles. Bull. Zool. 58, 357–368. doi: 10.2108/zsj.27.771
Hou G., Feng Y. T., Chen Y. Y., Wang J. R., Wang S. J., Zhao H. (2021). Spatiotemporal distribution of threadfin porgy evynnis cardinalis in beibu gulf and its relationship with environmental factors. J. Guangdong. Ocean. Univ. 41, 8–16. doi: 10.3969/j.issn.1673-9159.2021.04.002
Huang X. Y., Li F., Chen J. K. (2015). Reserve network planning for fishes in the middle and lower yangtze river basin by systematic conservation approaches. Sci. Sin. Vitae. 45, 1244–1257. doi: 10.1007/s11427-015-4950-0
Huang L. M., Zhang H. J., Zhang Y. Z., Lin T., Li J., Wang J. Q., et al. (2013). Research and progress of relationship between fish and aquatic environmental factors in estuarine waters. Trans. Oceanol. Limnol. 1), 61–68. doi: 10.13984/j.cnki.cn37-1141.2013.01.009
Jensen O. P., Seppelt R., Miller T. J., Bauer L. J. (2005). Winter distribution of blue crab callinectes sapidus in chesapeake bay: Application and cross-validation of a two-stage generalized additive model. Mar. Ecol. Prog. Ser. 299, 239–255. doi: 10.3354/meps.299239
Ji Y. P., Li M. K., Han D. Y., Wang J., Zhang C. L., Ren Y. P. (2020). The relationship between spatiotemporal distribution of crangon affinis and environmental factors in the southern coastal waters of shandong. Period. Ocean. Univ. China 50, 56–62. doi: 10.16441/j.cnki.hdxb.20190011
Jiang R. J., Sun H. Q., Li X. F., Zhou Y. D., Chen F., Xu K. D., et al. (2022). Habitat suitability evaluation of Harpadon nehereus in nearshore of Zhejiang province, China. Front. Mar. Sci. 9. doi: 10.3389/FMARS.2022.961735
Kindong R., Wu J. H., Gao C. X., Dai L. B., Tian S. Q., Dai X. J., et al. (2020). Seasonal changes in fish diversity, density, biomass, and assemblage alongside environmental variables in the yangtze river estuary. Environ. Sci. pollut. Res. 27, 25461–25474. doi: 10.1007/s11356-020-08674-8
Lewin W.-C., Mehner T., Ritterbusch D., Brämick U. (2014). The influence of anthropogenic shoreline changes on the littoral abundance of fish species in german lowland lakes varying in depth as determined by boosted regression trees. Hydrobiologia 724, 293–306. doi: 10.1007/s10750-013-1746-8
Li B., Cao J., Chang J. H., Wilson C., Chen Y. (2015). Evaluation of effectiveness of fixed-station sampling for monitoring american lobster settlement. N. Am. J. Fish. Manage. 35, 942–957. doi: 10.1080/02755947.2015.1074961
Li Y. D., Zhang C. L., Ji Y. P., Xue Y., Xu B. D., Ren Y. P. (2021). Spatio-temporal distribution of larimichthys polyactis in southern waters off the shandong peninsula and its relationship with environmental factors. J. Fish. Sci. China 28, 442–450. doi: 10.12264/JFSC2020-0288
Liu Z. H., Han D. Y., Gao C. X., Ye S. (2021). Feeding habits of bombay ducks (Harpadon nehereus) in the offshore waters of southern zhejiang, based on predator cpue weighting. J. Fish. Sci. China 28, 482–492. doi: 10.12264/JFSC2020-0249
Liu M. Y., Lv Z. H., Tian S., Gao C. X., Dai L. B., Ye S. (2020). Spatial distribution of larimichthys polyactis in wen-tai fishing ground based on habitat suitability index. J. Shanghai. Ocean. Univ. 29, 92–101. doi: 10.12024/jsou.20190102518
Luo H. Z. (2012). Study of main biology character and analysis of resource status on the Harpodon nehereus (Zhejiang Ocean University).
Luo H. Z., Zhang H. D., Li P. F., Zhou Y. D. (2012). Analysis of the current situation of fishery biology of Harpodon nehereus in the east China sea. J. Zhejiang. Ocean. Univ. (Nat. Sci.) 31, 202–205, 233. doi: 10.3969/j.issn.1008-830X.2012.03.003
Ma W., Gao C. X., Tang W., Qin S., Ma J., Zhao J. (2022a). Relationship between engraulis japonicus resources and environmental factors based on multi-model comparison in offshore waters of southern zhejiang, China. J. Mar. Sci. Eng. 10, 657. doi: 10.3390/jmse10050657
Ma J., Huang J. L., Chen J. H., Li B., Zhao J., Gao C. X., et al. (2020). Analysis of spatiotemporal fish density distribution and its influential factors based on generalized additive model (gam) in the Yangtze river estuary. J. Fish. China 44, 936–946. doi: 10.11964/jfc.20190911940
Ma W., Qin S., Zhao J. (2022b). Distribution characteristics and influencing factors of fish resources in the offshore waters south of zhejiang. Prog. Fish. Sci. 43, 1–11. doi: 10.19663/j.issn2095-9869.20210610001
Pan X., Cheng J. (2011). Feeding ecology of Harpadon nehereus in areas adjacent to changjiang river estuary. J. Fish. Sci. China 18, 1132–1140. doi: 10.3724/SP.J.1118.2011.01132
Philip G. M., Watson D. F. (1982). A precise method for determining contoured surfaces. Aust. Pet. Explor. Assoc. J. 22, 205–212.
Planque B., Bellier E., Lazure P. (2007). Modelling potential spawning habitat of sardine (sardina pilchardus) and anchovy (engraulis encrasicolus) in the bay of biscay. Fish. Oceanogr. 16, 16–30. doi: 10.1111/j.1365-2419.2006.00411.x
Rakocinski C. F., Lyczkowski-Shultz J., Richardson S. L. (1996). Ichthyoplankton assemblage structure in mississippi sound as revealed by canonical correspondence analysis. Estuar. Coast. Shelf. S. 43, 237–257. doi: 10.1006/ecss.1996.0067
Ran F. X., Jin W. J., Huang S., Liu C. X., Li Z. X., Li C. Z. (2020). Research progress on the effects of salinity change on fish. J. Northwest. A&F. Univ. (Nat. Sci. Ed.) 48, 10–18. doi: 10.13207/j.cnki.jnwafu.2020.08.002
Rodríguez J. D., Pérez A., Lozano J. A. (2010). Sensitivity analysis of kappa-fold cross validation in prediction error estimation. Ieee. T. Pattern Anal. 32, 569–575. doi: 10.1109/TPAMI.2009.187
Shan X. J., Xian W., Wu Y. F. (2004). Progress of studies on ichthyoplankton ecology of changjiang river estuary. Trans. Oceanol. Limnol. 4), 87–93. doi: 10.3969/j.issn.1003-6482.2004.04.015
Shen X. Q., Shi Y. R., Chao M., Huang H. J., Tang F. H. (2011). Fish community structure of the yangtze river estuary in summer and autumn. J. Fish. China 35, 700–710. doi: 10.3724/SP.J.1231
Sun H. Q. (2022). A study on the biological characteristics, temporal and spatial distribution, and the relationship with environmental factors of Harpadon nehereus in zhejiang province coastal waters (Zhejiang Ocean University).
Sun P. F., Dai F. Q., Chen Y. L., Shan X. J., Jin X. S. (2015). Seasonal variations in structure of fishery resource in the yangtze river estuary and its adjacent waters. Prog. Fish. Sci. 36, 8–16. doi: 10.11758/yykxjz.20150602
Tian S. Q., Chen X. J., Chen Y., Xu L. X., Dai X. J. (2009). Standardizing cpue of ommastrephes bartramii for chinese squid-jigging fishery in northwest pacific ocean. Chin. J. Oceanol. Limnol. 27, 729–739. doi: 10.1007/s00343-009-9199-7
Wang Y. Q., Li C., Liu A. Q., Wu L. Y., Ge J. Z. (2023). Dynamic mechanism of saltwater intrusion at yangtze river estuary in summe. Yangtze. River. 54, 7–14. doi: 10.16232/j.cnki.1001-4179.2023.04.002
Wang Y. Y., Yang T. Y., Meng W., Si S. J., Chu M. J., Wang Z. (2020). Multivariate analysis of Harpadon nehereus populations from coastal areas of China based on morphological characters. J. Fish. Sci. China 27, 1234–1242. doi: 10.3724/SP.J.1118.2020.20062
Wang L. L., Zhang Z. X., Lin L. S., Peng X., Lin L., Kang B. (2021). Redistribution of the lizardfish Harpadon nehereus in coastal waters of China due to climate change. Hydrobiologia 848, 4919–4932. doi: 10.1007/s10750-021-04682-y
Wood S. N., Pya N., Säfken B. (2016). Smoothing parameter and model selection for general smooth models. J. Am. Stat. Assoc. 111, 1548–1563. doi: 10.1080/01621459.2016.1180986
Wu J. H., Dai L. B., Dai X., Tian S., Liu J., Chen J. H., et al. (2019). Comparison of generalized additive model and boosted regression tree in predicting fish community diversity in the yangtze river estuary, China. Chin. J. Appl. Ecol. 30, 644–652. doi: 10.13287/j.1001-9332.201902.037
Yang K. E., Chen J. H., Zhao J., Wang X. F., Wu J. H., Zhang S., et al. (2022). Size structure and community stability assessment of fish community in the yangtze river estuary. J. Fish. China, 1–11. doi: 10.11964/jfc.20210913057
Yang Y., Liu P. X., Zhou H. H., Xia L. H. (2020). Evaluation of the biodiversity variation and ecosystem health assessment in changjiang estuary during the past 15 years. Acta Ecol. Sin. 40, 8892–8904. doi: 10.5846/stxb201912272811
Ye S., Ma W., Chen W. F., Liu W. C. (2023). Distribution and influencing factorsof pacific chub mackerel scomber japonicus resources in southern offshore waters of zhejiang province in spring and summer. Fish. Sci. 42, 395–403. doi: 10.16378/j.cnki.1003-1111.21140
Yin J., Wang J., Zhang C. L., Xu B. D., Xue Y., Ren Y. P. (2019). Spatial and temporal distribution characteristics of larimichthys polyactis eggs in haizhou bay and adjacent regions based on two-stage gam. J. Fish. Sci. China 26, 1164–1174. doi: 10.3724/SP.J.1118.2019.19032
Yu H., Xian W. (2009). The environment effect on fish assemblage structure in waters adjacent to the changjiang (yangtze) river estuary, (1998–2001). Chin. J. Oceanol. Limnol. 27, 443–456. doi: 10.1007/s00343-009-9155-6
Zhang Y. Q. (2012). Environmental impact on the fish assemblage structure in adjacent sea area of the yangtze river estuary (Institute of Oceanography, Chinese Academy of Sciences).
Zhang M. (2015). Seasonal variation of river and tide energy in yangtze estuary and the influence on regime equilibrium (East China Normal University).
Zhao J., Liu X. X., Wu J. H., Han D. Y., Tian S., Ma J. (2020). Application of zero-inflated model in predicting the distribution of rare fish species: A case study of coilia nasus in yangtze estuary, China. Chin. J. Ecol. 39, 3155–3163. doi: 10.13292/j.1000.4890.202009.028
Zhao J., Zhang S. Y., Lin J., Zhou X. J. (2014). A comparative study of different sampling designs in fish community estimation. Chin. J. Appl. Ecol. 25, 1181–1187. doi: 10.13287/j.1001-9332.2014.0081
Zhu G. P., Zhu X. Y., Xu Y. Y., Xia H., Li Y. C., Xu P. X., et al. (2012). The spatiotemporal distribution of fishing grounds for antarctic krill (euphausia superba) around the south orkney islands in austral summer-autumn and its relation to environmental factors based on a generalized additive model. Chin. J. Polar. Res. 24, 266–273. doi: 10.3724/SP.J.084.2012.00266
Keywords: Harpadon nehereus, Yangtze River Estuary (YRE), spatial-temporal distribution, generalized additive models (GAM), environmental factors
Citation: Li Y, Gao C, Chen J, Wang Q and Zhao J (2024) Spatial–temporal distribution characteristics of Harpadon nehereus in the Yangtze River Estuary and its relationship with environmental factors. Front. Mar. Sci. 11:1340522. doi: 10.3389/fmars.2024.1340522
Received: 18 November 2023; Accepted: 05 February 2024;
Published: 29 February 2024.
Edited by:
Stephen J. Newman, Western Australian Fisheries and Marine Research Laboratories, AustraliaReviewed by:
Longshan Lin, State Oceanic Administration, ChinaCopyright © 2024 Li, Gao, Chen, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhao, anpoYW9Ac2hvdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.