
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 20 February 2024
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1339471
 June Kim1
June Kim1 Sung Hwoan Cho2*
Sung Hwoan Cho2*Incorporating feed stimulants and attractants in low fish meal (FM) diets is a very effective way to improve palatability and increase feed intake and fish growth. This research aims to elucidate the substitution impact of different levels of FM with various plant proteins in diets with jack mackerel meal (JMM) inclusion as feed stimulants on the growth and feed utilization of rockfish (Sebastes schlegeli). A two-way (2 substitution levels [25% and 50%] × 3 substitution sources [corn gluten meal (CGM), soybean protein concentrate (SPC), and corn protein concentrate (CPC)]) ANOVA experimental design was adopted. In total, 525 fish averaging 8.3 g were assigned to 21 tanks (25 juvenile/tank). Seven isoprotetic and isolipidic feeds were formulated. The control (Con) diet contained 55% FM. CGM, SPC, and CPC were replaced for 25% and 50% FM in the Con diet, and then 22% JMM as feed stimulants was included at the cost of FM, named as the CGM25, CGM50, SPC25, SPC50, CPC25, and CPC50 diets, respectively. All diets were assigned to triplicate groups of rockfish. Rockfish were hand-fed to apparent satiation twice a day for 8 weeks. Rockfish fed the 25% FM substitution diets achieved superior (p < 0.0001 for both) weight gain and specific growth rate (SGR) compared to rockfish fed the 50% FM substitution diets, while rockfish fed the CGM-replaced diets achieved superior (p < 0.0001 for both) weight gain and SGR compared to rockfish fed the SPC- and CPC-replaced diets. The weight gain and SGR of rockfish fed the Con and CGM25 diets were superior to rockfish fed all other diets. Rockfish fed the 25% FM substitution diets achieved superior (p < 0.0001 for both) feed efficiency (FE) and protein efficiency ratio (PER) compared to rockfish fed the 50% FM substitution diets, while rockfish fed the CGM-replaced diets achieved superior FE and PER compared to rockfish fed the CPC-replaced diets. Rockfish fed the 25% FM substitution diets led to superior (p < 0.0001) protein retention (PR) compared to rockfish fed the 50% FM substitution diets. The biological indices, proximate composition, and amino acid profiles of the whole body, as well as the plasma and serum parameters of rockfish, were not changed by either substitution level or substitution source. In conclusion, the replacement of CGM for 25% FM in the rockfish diet with JMM inclusion can be made without deteriorating growth performance, feed consumption and utilization, biochemical composition, and plasma and serum parameters.
The cost of aquaculture production has continued to increase due to the unstable price of fish meal (FM) in the international market (FAO, 2020). Furthermore, as FM supply has continued to dwindle due to overfishing of wild stock, and to ensure the sustainability of aquaculture by reducing the cost of production and the fish in/fish out ratio, there is a high need to expand the use of substitutable protein sources. In the last few decades, plant proteins have been increasingly used in aquafeeds due to the rise in aquaculture production and the need to develop cost-effective feed (Hardy, 2010). Despite their suitability, the use of plant proteins faces nutritional limitations, such as the presence of antinutritional factors (ANFs) and nonsoluble carbohydrates and the limited availability of essential amino acids (EAA) in plants (Hardy, 2010; Luthada-Raswiswi et al., 2021). ANFs in plant proteins can cause intestinal enteritis in fish, reduce protein uptake and digestion, deteriorate growth, and affect the immune response of farmed fish (Hardy, 2010).
Efforts to enhance the nutritional quality of plants in terms of balancing EAA and eliminating ANFs are underway. Production of plant concentrates and supplementation of EAA and minerals are some of the methods explored. However, these efforts are not enough to overcome some of these deficiencies (Hardy, 2010; Gatlin et al., 2007). For instance, despite being digestible, corn gluten meal (CGM) is deficient in EAA, such as arginine and lysine, and contains nonsoluble carbohydrates that are not nutritional to fish (Hardy, 2010; Gatlin et al., 2007). Nevertheless, CGM is commonly used in salmon and marine fish feeds (Gatlin et al., 2007). Furthermore, 40% and 60% FM could be successfully replaced by CGM with supplementation of EAA in juvenile olive flounder (Paralichthys olivaceus) (Kikuchi, 1999), and Japanese seabass (Lateolabrax japonicus) (Men et al., 2014) feeds, respectively. Lee et al. (1996) also unveiled that FM up to 18% could be replaceable with CGM without reducing weight gain and feed utilization of rockfish (Sebastes schlegeli) when juvenile rockfish were fed with a 55% FM-basal diet or one of the diets substituting 9%, 18%, and 27% FM with CGM in the 45-day feeding trial.
Corn protein concentrate (CPC), a product of the enzymatic removal of nonprotein components in corn with high protein but less than 1% starch, is being used to improve the nutritional defects of corn gluten (Khalifa et al., 2018). Partial replacement of FM by CPC in diets has been assessed in some fish species. For instance, 50% and 53.4% FM could be replaced with CPC in the feeds of red hybrid tilapia (Oreochromis sp.) (Ng et al., 2019) and Nile tilapia (Oreochromis niloticus) (Khalifa et al., 2018), respectively.
Soybean meal protein has found considerable usage in aquafeeds as well. However, it also contains ANFs that are harmful and may not be palatable to carnivorous fish (Kissil et al., 2000; National Research Council (NRC), 2011). Soy protein concentrate (SPC) is made by extracting defatted soy flakes in aqueous ethanol to remove ANFs (Hien et al., 2017). Although SPC is still lacking in methionine-contained carbohydrates that may deteriorate digestibility, it could replace the whole FM in the tambaqui (Colossoma macropomum) diet containing 45% FM without retardation in growth performance (Martins et al., 2020). Furthermore, FM up to 82.5% could be replaced with SPC in the black sea bream (Acanthopagrus schlegelii) diet without causing unfavorable impacts on growth and feed efficiency (Kalhoro et al., 2018).
Although reduced feed intake of fish resulted from poor palatability and limited substitutability of the alternative plant protein source for FM in fish feeds, these plant proteins (CGM, SPC, and CPC) could have high potential as a substitute for FM in the rockfish diet as well. The application of feed attractants and stimulants has been suggested to enhance the acceptability (palatability) of the low FM diet of hybrid striped bass (Morone chrysops × Morone saxatilis) (Blaufuss and Trushenski, 2012). Feed attractants can improve the feed intake of fish by stimulating olfactory and gustatory cues that elicit acceptance of feed (Zou et al., 2017). Attractants, including betaine, nucleosides, AA, and nucleotides, are available naturally in prey or as synthetic chemicals (Kasumyan and Døving, 2003; Yacoob and Browman, 2007). Furthermore, attractants, such as nucleotides and inosine monophosphate can also be found in the extracts of jack mackerel (Trachurus japonicus) (Hidaka et al., 2000; Takakuwa et al., 2019). In addition, incorporated jack mackerel meal (JMM), which exhibits the highest feed-attracting responses to rockfish (Kim et al., 2020) and olive flounder (Jeong et al., 2020) among various crude ingredients, including several FM sources commonly used as the protein sources in diets, has successfully improved feed intake and growth performance (Jeong et al., 2020; Baek et al., 2021; Kim and Cho, 2019).
In the Republic of Korea (hereafter, Korea), rockfish is one of the main farmed marine fish species, with a production of 21,571 metric tons in 2020, valued at $145.6 million (FAO, 2022). Like many carnivorous species, commercial rockfish feed contains a high percentage (50%–60%) of FM as the protein source. To ensure sustainable and cost-effective production of this species, searching for a suitable alternative for FM in rockfish feed receives great interest. To date, a study has explored the use of a blend of animal and plant protein sources, tuna by-product meal and soybean meal in rockfish feed (Jeon et al., 2014). To our knowledge, there is no study to evaluate the substitutability of plant protein sources for FM in rockfish diets, in which feed stimulants were added.
The present study, thus, aims to elucidate the replacement impacts of various plant proteins for different levels of FM in diets with JMM inclusion on the growth, feed consumption and utilization, and biochemical composition of rockfish.
Juvenile rockfish were purchased from a private hatchery (Buan-gun, Jeollabuk-do, Korea; 35°35′08.2″N, 126°31′52.6″E). Rockfish were acclimated to the experimental conditions and provided with a commercial extruded pellet containing 50% crude protein and 8% crude lipid (Suhyup Feed, Gyeongsangnam-do, Korea) for a fortnight. For the feeding experiment, 525 juvenile fish averaging 8.3 g were randomly assigned to 21, 50 L of flow-through tanks for each of the seven experimental diets (25 fish per tank) with triplicate groups. The assigned fish were carefully hand-fed twice daily (08:30 and 17:30) at an apparent satiation level for 8 weeks. The water supply into each tank was a mixture of sand-filtered seawater and underground seawater at a ratio of 1:1. The water flow rate of each tank was 4.5 L/min, and proper aeration was provided to each tank. Water temperature fluctuated from 20.7°C to 24.1°C (22.3°C ± 0.68°C; mean ± SD) and salinity fluctuated from 31.2 g/L to 33.6 g/L (32.5 ± 0.58 g/L), while pH fluctuated from pH 7.1 to 7.5 (7.3 ± 0.07) and dissolved oxygen fluctuated from 7.2 mg/L to 7.5 mg/L (7.4 mg/L ± 0.09 mg/L) measured by using a digital multimeter (AZ-8603, AZ Instrument, Taiwan) throughout the feeding experiment. To prevent water quality deterioration, the tanks were siphon-cleaned daily, and dead fish were eliminated immediately when observed.
A two-way [2 (substitution levels; 25 and 50%) × 3 (substitution sources; CGM, SPC, and CPC)] ANOVA experimental design was applied. The seven experimental diets were formulated to be isoproteic at 51.0% and isolipidic at 14.0% (Table 1). In the control (Con) diet, 55% FM and 12% fermented soybean meal were included as protein sources. In addition, 21.5% of wheat flour and 4.5% of each fish and soybean oils were contained as carbohydrate and lipid sources in the Con diet, respectively. In the Con diet, 25% and 50% FM were substituted with CGM, SPC, and CPC, respectively, and then 22% JMM, achieving the best growth and highest feed consumption of rockfish (Kim and Cho, 2023), was added at the sacrifice of FM in all plant-substituted diets, named as the CGM25, CGM50, SPC25, SPC50, CPC25, and CPC50 diets, respectively. All experimental feeds met the dietary protein and lipid requirements of rockfish (Cho et al., 2015). The ingredients of the experimental feeds were well blended thoroughly and made into pellets in two sizes (4 mm and 6 mm in die) using a laboratory pellet extruder (Dongsung Mechanics, Busan City, Korea). As the rockfish grew, the proper size of the experimental feed was supplied. All experimental feeds were dried at 40°C in an electronic dry machine (UDS-4522F, Kyung Dong Navien, Pyeongtaek-si, Gyeonggi-do, Korea) for 24 h, and then stored in a freezer (−20°C) until use.
At the termination of the feeding experiment, all live fish were unfed for 24 h and then sacrificed (anesthetic overdose MS-222 at 100 mg/L) for sampling collection and measurements. For the calculation of the biological indices of rockfish, 10 fish were randomly selected from each tank. Parameters were calculated as follows: specific growth rate (SGR, %/day) = (Ln final weight of fish − Ln initial weight of fish) × 100/days of feeding (56 days), feed efficiency (FE) = weight gain of fish/feed supply, protein efficiency ratio (PER) = weight gain of fish/protein supply, protein retention (PR) = protein gain of fish × 100/protein supply, condition factor (CF, g/cm3) = weight of fish (g) × 100/total length of fish (cm)3, viscerosomatic index (VSI, %) = viscera weight of fish × 100/weight of fish, and hepatosomatic index (HSI, %) = liver weight of fish × 100/weight of fish.
Blood from the caudal vein was collected from three fish from each tank, using a heparinized syringe. The blood was centrifuged at 2,700×g at 4°C for 10 min to obtain the plasma, which was later stored at −70°C. The plasma obtained was used for analyzing the aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (TB), total cholesterol (T-CHO), triglyceride (TG), total protein (TP), and albumin (ALB) using an automatic chemistry system (Fuji Dri-Chem NX500i, Fujifilm, Tokyo, Japan).
The centrifuged (2,700×g at 4°C for 10 min) samples from three fish stored at −70°C were used to analyze lysozyme activity and superoxide dismutase (SOD). The turbidimetric assay for lysozyme activity was carried out according to the procedure described by Lange et al. (2001). In the procedure, 100 µL of the serum was added to a 1.9-mL suspension of Micrococcus lysodeikticus (0.2 mg/mL; Sigma, St. Louis, MO, USA) in a sodium phosphate buffer (0.05 M, pH 6.2). The reaction was carried out at room temperature (25°C), and absorbance at 530 nm was read before and after the 30-min reaction period with a microplate reader. Lysozyme activity was described as the amount of enzyme required to lyse the gram-positive bacterium M. lysodeikticus. SOD was evaluated using a SOD ELISA Kit (MBS705758; MyBiosource Inc., San Diego, CA, USA), according to the manufacturer’s protocol. This assay employs the competitive inhibition enzyme immunoassay technique. The kit has been precoated with an antibody specific to SOD. After adding the substrate solution, the intensity of the color developed was inversely proportional to the concentration of SOD in the sample. The absorbance was measured at 450 nm using a microplate reader (Infinite® 200 PRO; TECAN, Männedorf, canton of Zürich, Switzerland), and the concentration was calculated by drawing a standard curve.
Ten fish at the beginning and all remaining fish from each tank at the completion of the 8-week experiment were sacrificed for the chemical analysis of the whole-body rockfish based on the AOAC standard procedures (AOAC, 1990). Moisture and ash content were determined by oven drying the samples for 24 h at 105°C, and the sample was incinerated at 550°C for 4 h using a muffle furnace, respectively. Kjeldahl method (Kjeltec 2100 Distillation Unit, Foss Tecator, Hoganas, Sweden) was used to determine the crude protein, while the lipid content was determined using an ether-extraction method (Soxtec TM 2043 Fat Extraction System, Foss Tecator, Hoganas, Sweden).
For the analysis of the AA, 18 components were analyzed by the ninhydrin postcolumn reaction method using ion-exchange chromatography. All AA in the experimental feeds and whole-body rockfish were analyzed by using an AA analyzer (L-8900 Auto-analyzer; Hitachi, Tokyo, Japan). In the case of 16 constituent AA (except for methionine and cysteine), 0.2 g of the sample was placed in a decomposition tube, 10 mL of 6 N HCl was added, nitrogen gas was injected, and the sample was hydrolyzed at 110°C for 24 h. After concentrating the filtrate with a reduced pressure concentrator, it was adjusted to 50 mL with 0.2 M sodium citrate buffer, and the filtrate filtered with a 0.20-μm cellulose acetate syringe filter was used as an analysis sample. On the other hand, samples were oxidized for the analysis of methionine and cysteine with performic acid for 24 h at under 5°C to obtain methionine sulfone and cysteic acid. They were then freeze-dried twice with deionized water, hydrolyzed, and analyzed according to the standard procedure used for the other AAs. On the other hand, the tryptophan content of samples was evaluated with high-performance liquid chromatography (S1125 HPLC pump system, Sykam, Germany).
Data were analyzed using a two-way ANOVA and Tukey’s HSD test to evaluate the effects of dietary treatments on fish. The difference in dietary treatments was considered significant at p < 0.05. The statistical analysis was carried out in SPSS version 24.0 (SPSS Inc., Chicago, IL, USA). Percentage data were arcsine-transformed prior to statistical analysis.
Among EAA, leucine and phenylalanine, and arginine and tryptophan content in CGM and CPC, and SPC were relatively high over those in FM (Table 2). Increased FM substitution levels with CGM and CPC, and SPC in diets led to increased leucine and phenylalanine, and arginine and tryptophan content, respectively. Glutamic acid was the richest among non-EAA in the experimental diets.
Survival exceeded 97%, but it was not (p > 0.7) significantly altered by dietary treatments (Table 3). The weight gain and SGR of rockfish fed the 25% FM replacement feeds were remarkably (p < 0.0001 for both) greater than those of rockfish fed the 50% FM replacement feeds. The weight gain and SGR of rockfish fed the CGM-replaced diets were also remarkably greater (p < 0.002 for both) than those of rockfish fed the SPC- and CPC-replaced diets. In addition, their remarkable (p < 0.004 for both) interactions on weight gain and SGR of rockfish were observed. The weight gain and SGR of rockfish fed the Con and CGM25 diets were remarkably (p < 0.0001) greater than those of rockfish fed all other diets. Weight gain and SGR of rockfish fed the SPC25 diet were also remarkably (p < 0.05) greater than those of rockfish fed the CGM50, SPC50, and CPC50 diets, but not remarkably (p > 0.05) different from those of rockfish fed the CPC25 diet.
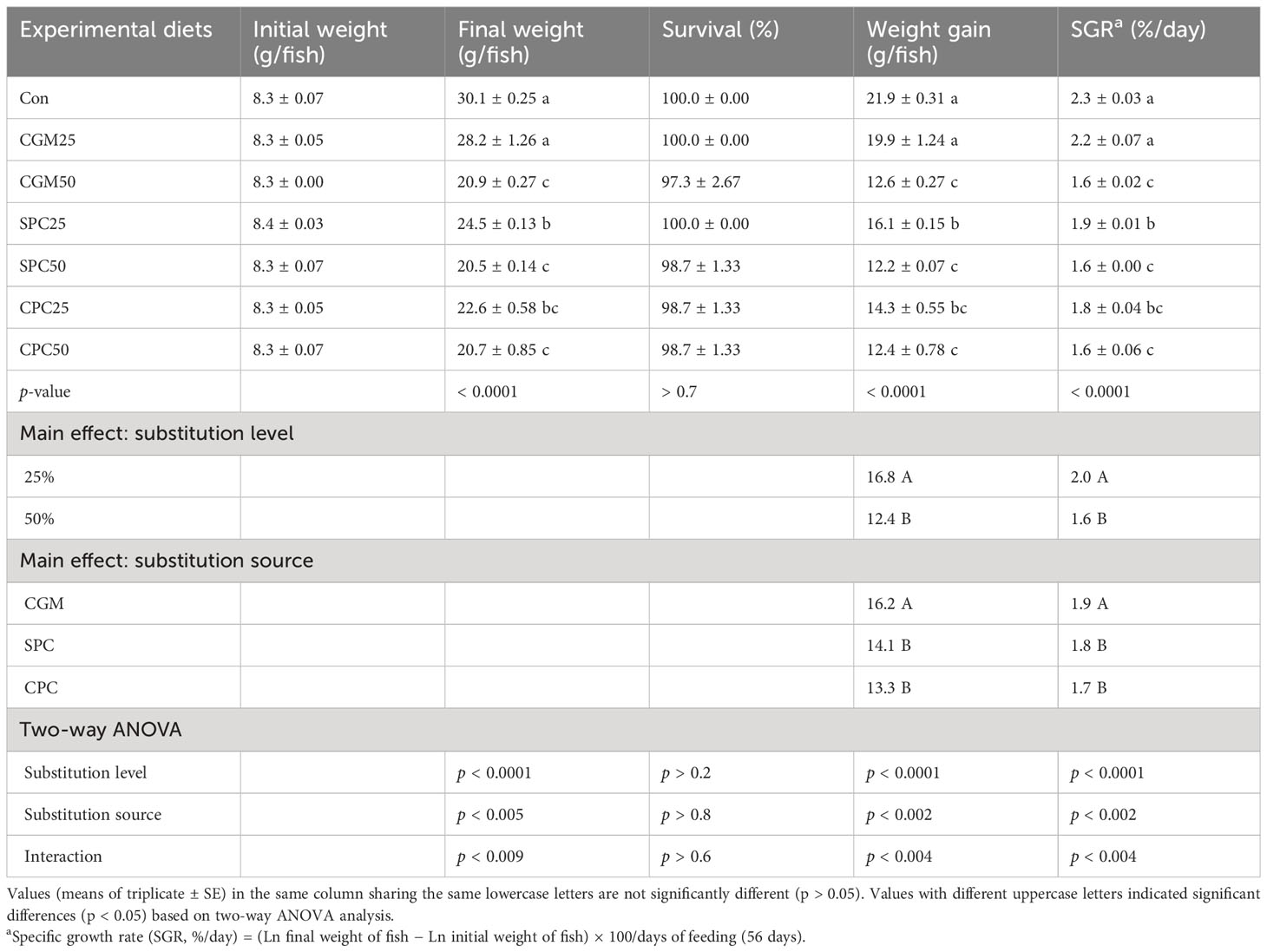
Table 3 Survival (%), weight gain (g/fish), and specific growth rate (SGR) of rockfish fed the experimental diets substituting 25% and 50% FM with various plant protein sources for 8 weeks.
Feed consumption of rockfish fed the 50% FM substitution feeds was remarkably (p < 0.03) higher than that of rockfish fed the 25% FM substitution feeds (Table 4). However, feed consumption of rockfish was not (p > 0.2) different among dietary treatments.
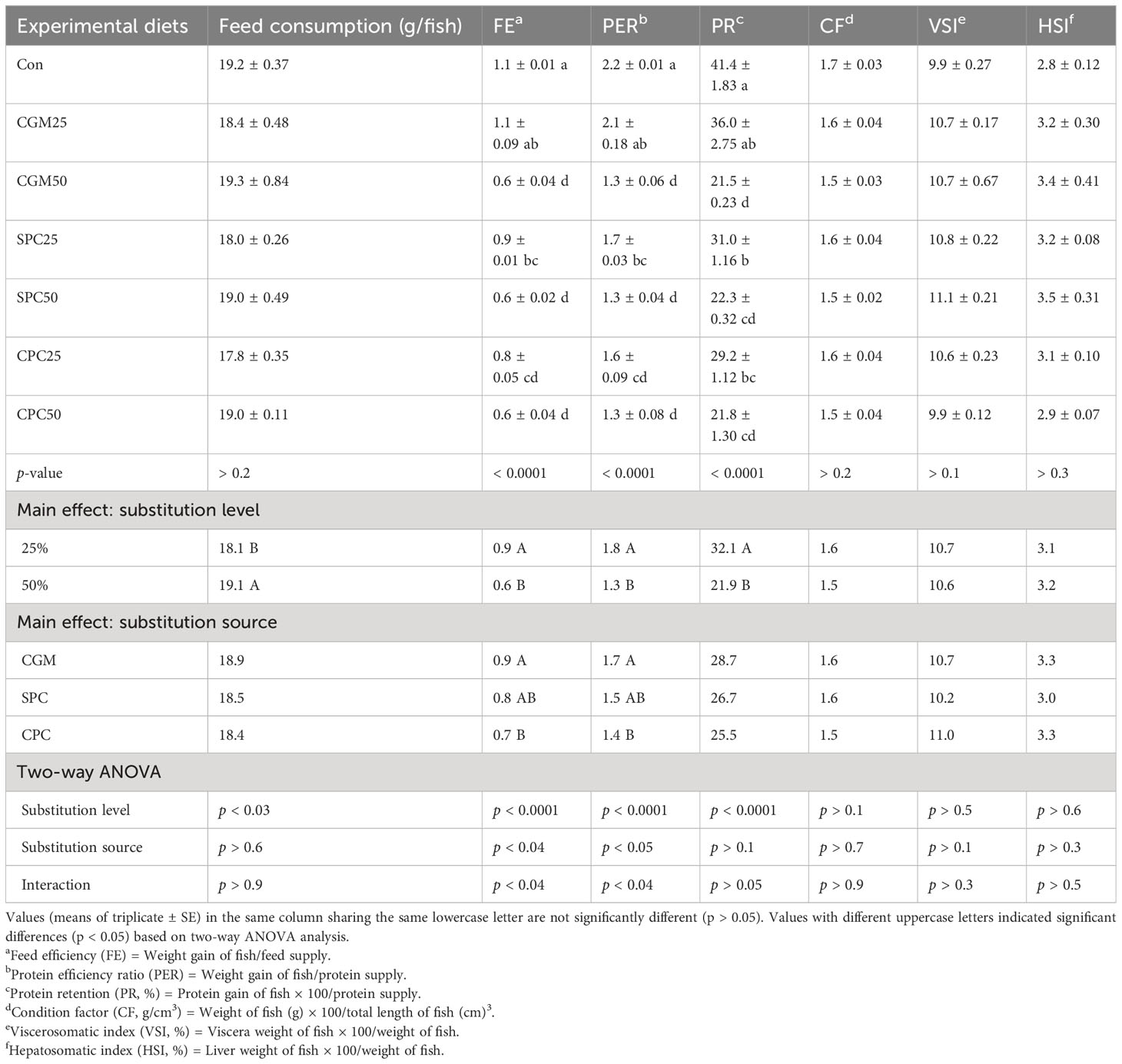
Table 4 Feed consumption (g/fish), feed efficiency (FE), protein efficiency ratio (PER), protein retention (PR), condition factor (CF), viscerosomatic index (VSI), and hepatosomatic index (HSI) of rockfish fed the experimental diets substituting 25% and 50% FM with various plant protein source for 8 weeks.
FE and FER of rockfish fed the 25% FM substitution feeds were remarkably (p < 0.0001 for both) higher than those of rockfish fed the 50% FM substitution feeds. FE and FER of rockfish fed the CGM-substituted feeds were remarkably (p < 0.04 and p < 0.05, respectively) higher than those of rockfish fed the CPC-substituted feeds, but not remarkably (p > 0.05) different from those of rockfish fed the SPC-substituted feeds. Furthermore, their significant (p < 0.04 for both) interactions on FE and PER of rockfish were observed. FE and PER of rockfish fed the Con diet were remarkably (p < 0.0001 for both) higher than those of rockfish fed the CGM50, SPC25, SPC50, CPC25, and CPC50 diets, but not remarkably (p > 0.05) different from those of rockfish fed the CGM25 diet. PR of rockfish fed the 25% FM replacement diets was remarkably (p < 0.0001) higher than that of rockfish fed the 50% FM replacement diets. PR of rockfish fed the Con diet was remarkably (p < 0.0001) higher than that of rockfish fed the CGM50, SPC25, SPC50, CPC25, and CPC50 diets, but not remarkably (p > 0.05) different from that of rockfish fed the CGM25 diet.
However, none of the CF, VSI, and HSI of rockfish were remarkably (p > 0.05) changed by either substitution level or substitution source.
The moisture content of the whole-body rockfish ranged from 69.9% to 71.4%, and the crude protein content ranged from 16.7% to 18.0% (Table 5). The crude lipid content of the whole-body rockfish ranged from 7.4% to 7.6%, and the ash content ranged from 4.1% to 4.4%. These parameters were not remarkably (p > 0.05) affected by either substitution level or substitution source. None of these parameters were remarkably (p > 0.7, p > 0.1, p > 0.8, and p > 0.9, respectively) different among dietary treatments.
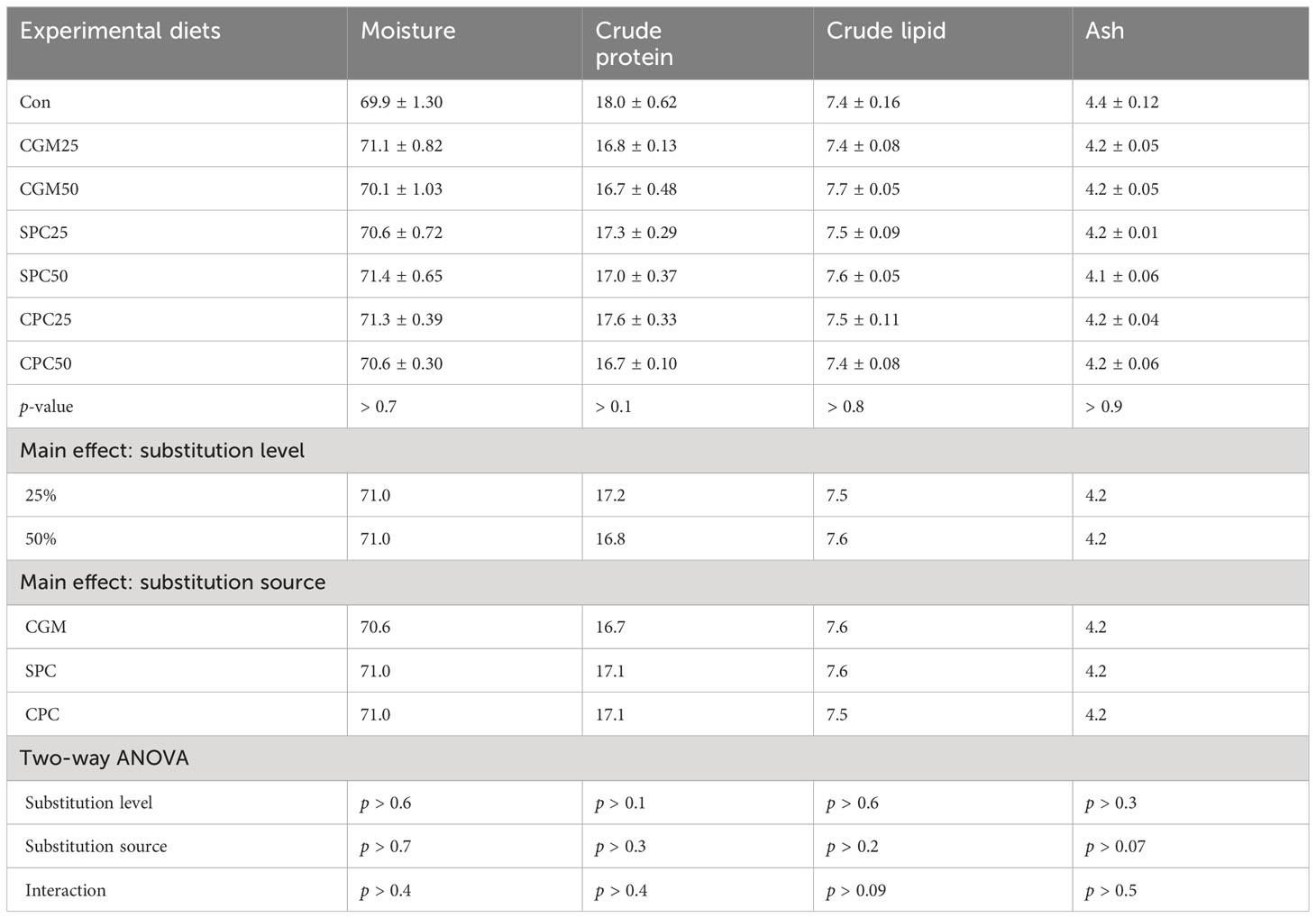
Table 5 Proximate composition (%) of the whole body of rockfish fed experimental diets substituting 25% and 50% FM with various plant protein sources for 8 weeks.
The AA profiles of the whole-body rockfish were not remarkably (p > 0.05) affected by either substitution level or substitution source (Table 6).
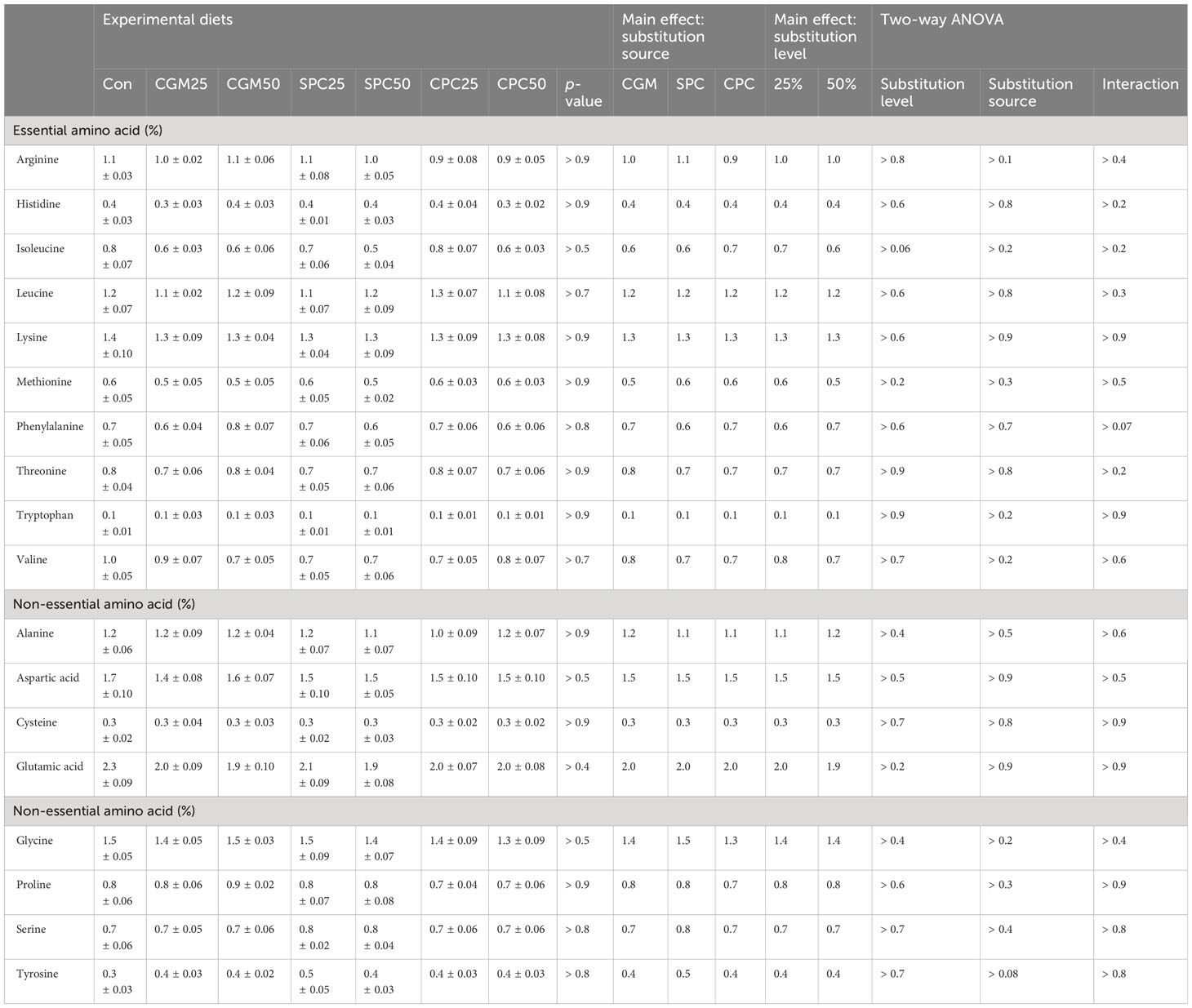
Table 6 Amino acid profiles (% of wet weight) of the whole body of rockfish fed the experimental diets substituting 25% and 50% FM with various plant protein sources for 8 weeks.
None of the plasma parameters (AST, ALT, ALP, TB, T-CHO, TG, TP, and ALB) of rockfish were remarkably (p > 0.05) changed by either substitution level or substitution source (Table 7). Plasma AST, ALT, ALP, TB, T-CHO, TG, TP, and ALB were not remarkably (p > 0.9, p > 0.2, p > 0.6, p > 0.8, p > 0.2, p > 0.2, p > 0.8, and p > 0.8, respectively) different among dietary treatments.
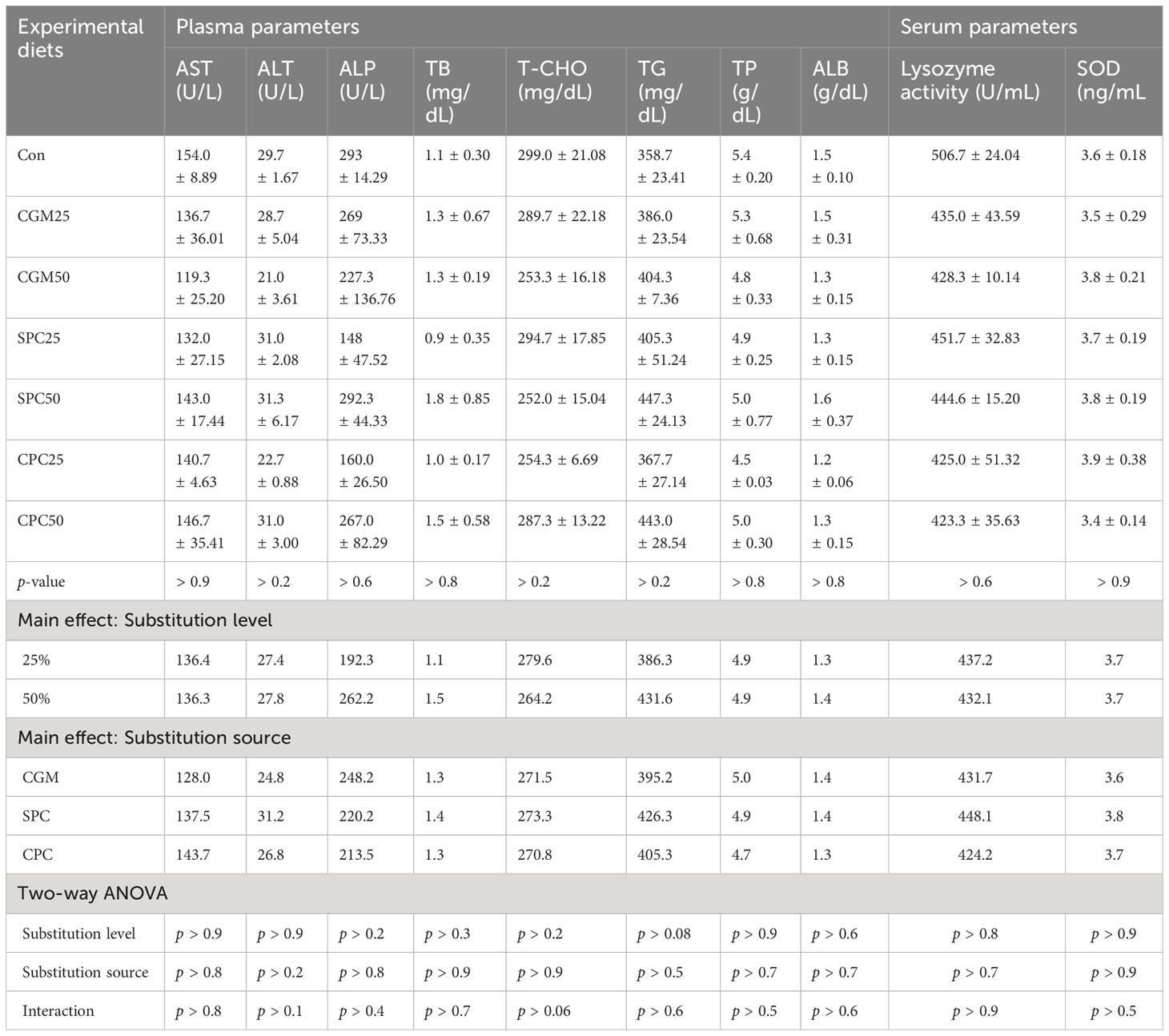
Table 7 Plasma and serum parameters of rockfish fed the experimental diets substituting 25% and 50% FM with various plant protein sources for 8 weeks.
Serum lysozyme activity of rockfish changed from 423.3 U/mL to 506.7 U/mL, and SOD changed from 3.4 ng/mL to 3.9 ng/mL. Both substitution level and source had no remarkable (p > 0.05) impacts on lysozyme activity and SOD. Neither lysozyme activity nor SOD were remarkably (p > 0.6 and p > 0.9, respectively) different among dietary treatments.
The alternative use of plant proteins for FM in fish farming can make aquaculture more sustainable (Gatlin et al., 2007). This study evaluated the substitutability of CGM, SPC, and CPC for 25% and 50% of FM in rockfish diets with 22% JMM inclusion, as reported in our previous studies (Baek et al., 2021; Kim and Cho, 2019) showing the desirable inclusion effect of JMM in feeds on improvement in feed consumption and growth of rockfish. Increased FM substitution levels in the diets of rockfish lowered weight gain and SGR in this study, which is consistent with other studies showing that increased FM replacement levels in diets deteriorated the growth performance of fish (Kissil et al., 2000; Hien et al., 2017; Blaufuss and Trushenski, 2012; Baek et al., 2023). Furthermore, dietary FM replacement with CGM produced superior weight and SGR in rockfish compared to dietary FM replacement with SPC or CPC in this experiment. CGM has been considered a practical FM substitute because it has few ANFs, and a low level of fiber content, and a relatively balanced AA profile (Pereira and Oliva-Teles, 2003). Dietary FM replacement with CGM was successfully conducted in various fish species, such as olive flounder (Kikuchi, 1999), sunshine sea bass (Lewis and Kohler, 2008), and Asian seabass (Lates calcarifer) (Nandakumar et al., 2017), without producing an unfavorable impact on growth.
No discernible differences in weight gain, SGR, feed intake, and feed utilization were found in rockfish fed the Con and CGM25 diets in the present experiment, implying that 25% FM replacement with CGM in the diet with JMM inclusion as feed stimulants did not cause any undesirable impacts on growth, feed consumption, and feed utilization of rockfish. This was consistent with the upper inclusion limit of CGM (20%–25% of diets for salmon and marine fish, but 10%–15% range in general) suggested by Gatlin III et al (Gatlin et al., 2007). However, Lee et al. (1996) revealed that substitution of CGM for FM up to 18% could be successfully made in the 55% FM-basal diet of juvenile rockfish without causing a reduction in weight gain in the 45-day feeding trial. Improved substitutability of FM with CGM from 18% in the study of Lee et al. (1996) to 25% (in this study) in the 55% FM-based diets of rockfish could be partially explained by the desirable effect of JMM as feed stimulants in the CGM25 diet on rockfish in the present study. Incorporating JMM instead of FM in the diet, substituting 25% FM with CGM (CGM25 diet), improved feed acceptability by rockfish and eventually brought comparable growth performance, feed consumption, and feed utilization to rockfish fed the 55% FM-basal diet (Con diet). Similarly, incorporating JMM at the sacrifice of FM in the 55% and 60% FM basal diets effectively elevated the growth performance of rockfish (Kim and Cho, 2019) and olive flounder (Jeong et al., 2020), respectively, directly responding to increased feed intake.
Recently, however, Baek et al. (2023) unveiled that CGM, SPC, and CPC could replace 25% FM in the feeds of olive flounder without producing undesirable impacts on growth and feed utilization when juvenile olive flounder were provided with a 60% FM-basal feed or one of the feeds replacing 25% and 50% of FM by CGM, SPC, and CPC with 12% JMM inclusion as feed stimulants for 56 days. Jeong and Cho (2023) also proved that diets substituting 25 and 50% FM with chicken by-product meal (CBM) and tuna by-product meal (TBM), respectively, achieved superior growth and feed consumption of olive flounder compared to fish fed a 60% FM-based diet when fish were fed with a 60% FM-based diet or one of the diets replacing 25% and 50% FM with TBM, CBM, and meat meal with 12% JMM inclusion as feed stimulants. However, substitutability of CGM for FM seems to be very limited in the rockfish feed compared to fish feeds in other studies, in which dietary replacement of FM up to 40% and 60% with CGM achieved comparable growth performance to catfish (Pseudobagrus ussuriensis) (Bu et al., 2018), olive flounder (Kikuchi, 1999), and seabass (Men et al., 2014) fed the 50%, 75%, and 52% FM-basal feeds, respectively. These disparities could also result from with or without EAA supplementation in the specific replacer (CGM) for FM in fish feeds.
Comparable growth performance and feed utilization of rockfish fed the CGM25 diet to rockfish fed the Con diet in the present experiment might have resulted from improved protein digestibility in CGM, supported by the fact that the apparent protein digestibility of CGM in rockfish was 79.1%, but 92.6%, 87.5%, and 76.2% for rockfish muscle, white FM, and soybean meal, respectively (Bai et al., 2001), and CGM was an efficient protein source in rockfish feeds (Lee, 2002). Nevertheless, inferior growth and feed utilization (FE, PER, and PR) of rockfish fed the 50% FM substitution feeds with various plant proteins compared to rockfish fed the 25% FM substitution feeds in this study could have been caused by the limited availability of EAA (Gatlin et al., 2007; Men et al., 2014). Lysine and methionine appeared to be the limiting AA in all plant proteins in the current study. Lysine content decreased from 2.81% in the CGM25 diet to 2.18% in the CGM50 diet, and this was also the same for methionine, which decreased from 1.15% to 1.04%. The decrease in these two limiting AAs might have also caused the growth reduction of rockfish fed the CGM50 diet due to AA imbalance, as reported in juvenile catfish (Bu et al., 2018) and gilthead sea bream (Sparus aurata) (Pereira and Oliva-Teles, 2003). These trends toward further reduction in weight gain and SGR of rockfish receiving the 50% FM substitution diets were also found in other plant proteins, SPC and CPC, in the present experiment. Interestingly, Kikuchi (1999) and Men et al. (2014) suggested that CGM could substitute FM up to 40% and 60% in diets supplemented with limiting AA without deteriorating growth of olive flounder and Japanese seabass, respectively, while CGM could substitute 40% FM in the feed of catfish (Bu et al., 2018), respectively.
Digestibility of soybean meal, SPC, and soy protein isolate was limited in carnivorous fish, including hybrid striped bass (Blaufuss and Trushenski, 2012). This might explain why rockfish fed the SPC25 and SPC50 diets produced inferior growth and feed utilization compared to rockfish fed the Con diet in this study. Similarly, there was an inverse relationship between dietary replacement levels of SPC for FM and the growth of gilthead seabream (Kissil et al., 2000). Unlike this study, however, 66.9% FM could be replaced with SPC in hybrid grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) feeds without reducing growth performance when fish were fed with a 67.6% FM-basal diet or diets replacing 28.4%, 41.1%, 54.0%, 66.9%, and 76.8% FM with SPC supplemented with taurine for 6 weeks (Faudzi et al., 2017). Furthermore, incorporating EAA in low FM diets replacing 33% and 57% FM with SPC achieved comparable growth of mutton snapper (Lutjanus analis) to fish fed a 36% FM-basal diet, but inferior growth for fish fed a diet replacing 77% FM with SPC when juvenile mutton snapper were fed with a 36% FM-basal diet or diets replacing 33%, 57%, and 77% with SPC with AA (lysine and methionine) supplementation for 55 days (Freitas et al., 2011).
CPC is a novel ingredient that can be combined with other protein ingredients to improve their nutrients and AA profiles (Novriadi et al., 2019; Rossi et al., 2013; Rossi et al., 2015). There is a paucity of studies on the use of CPC in fish feeds. Replacement of the whole poultry by-product meal by 15% CPC with lysine supplementation in the Florida pompano (Trachinotus carolinus) diet achieved comparable growth to fish fed a 15% poultry by-product meal-basal diet but led to a morphological change in the intestinal tract, thus affecting protein digestion (Novriadi et al., 2019). Nevertheless, CPC with limiting AA supplementation could be used as a substitute for 75% FM in diets without deteriorating the growth performance of red drum (Sciaenops ocellatus) and shortfin corvine (Cynoscion parvipinnis) (Minjarez-Osorio et al., 2016), but in a similar experiment with red drum, fish fed a diet replacing 50% FM by CPC with AA supplementation led to poorer weight gain than fish fed a 50% FM-basal diet (Rossi et al., 2013). The reason for this is unknown, but we can speculate that it might be because of CPC processing methods or biological differences among fish species. Unlike this study, Ng et al. (2019) proved that CPC was also effective in replacing FM up to 50% in red hybrid tilapia feeds (Ng et al., 2019). The combined CPC and SPC have been shown to be a successful replacer for FM in the feeds of red drum (Rossi et al., 2015) and Pacific white shrimp (Litopenaeus vannamei) (Guo et al., 2020), and this may be replicated in rockfish feeds to lower FM protein and production cost in future studies.
Rockfish fed the 25% FM replacement diets had inferior feed intake to rockfish fed the 50% FM replacement diets in this experiment. However, feed consumption was not influenced by dietary treatments. Unlike this study, dietary increased FM replacement levels with various alternative sources tended to bring about reduced feed intake of fish (Khalifa et al., 2018; Kissil et al., 2000; Hien et al., 2017; Baek et al., 2023). Significant or slight improvements in weight gain, SGR, FE, and PER of rockfish fed the CGM diets compared to rockfish fed the SPC and CPC diets implied that CGM seemed to be a more suitable replacer for FM than either SPC or CPC in the rockfish feeds in this experimental conditions. Increased FM replacement levels in diets lowered the FE, PER, and PR of rockfish, which directly responded to the reduced growth of rockfish in this experiment. Unlike this experiment, feed intake of olive flounder was reduced with dietary increased FM substitution levels, but no remarkable difference in feed utilization was found among dietary treatments (Baek et al., 2023). Feed consumption and growth performance of large yellow croaker (Larimichthys crocea) were not negatively affected by dietary FM replacements with SPC when fish were fed with a 40% FM-based diet or one of the diets replacing 25%, 50%, 75%, and 100% FM by SPC with limiting AA supplementation for 8 weeks (Wang et al., 2017). In snakeheads (Channa striata), diets replacing higher than 40% FM with SPC deteriorated growth, feed utilization, and digestibility of protein and lipid when juvenile snakeheads were fed with a 60.7% FM-basal diet or one of the diets replacing 40%, 60%, 80%, and 100% FM with SPC supplemented with lysine and methionine for 42 days (Hien et al., 2017). In addition, dietary replacement of SPC for FM up to 40% was recommended in the starry flounder (Platichthys stellatus) feeds when juvenile starry flounder were fed with a 68% FM-based diet or one of the diets replacing 20%, 40%, 60, 80%, and 100% FM by SPC with limiting AA supplementation for 10 weeks (Li et al., 2015). Incorporating AA in low FM feeds substituting FM with SPC seems to critically influence the substitutability of SPC for FM in fish feeds.
The whole FM could be replaced by the combined soybean and canola meals with AA supplementation in the diets of blunt snout bream (Megalobrama amblycephala) without producing any negative impacts on growth and feed utilization when blunt snout bream were provided with diets substituting 50% and 100% FM by the combined soybean and canola meals with or without limiting AA supplementation (Ahmed et al., 2019). Likewise, the whole (30.7%) FM protein could be substitutable with the mixture of plant proteins supplemented with limiting AA in the feeds of gibel carp (Carassius gibelio) without producing any undesirable effects on growth and feed utilization when gibel carp were provided with a 30.7% FM-based diet or diets replacing 20.7% and 30.7% FM protein by the mixture of plant proteins (soybean meal, rapeseed meal, cottonseed meal, distillers dried grains with soluble, wheat gluten, SPC, and corn gluten meal) with or without limiting AA (lysine, methionine, and threonine) supplementation; however, a diet replacing the whole FM with the mixture of plant proteins without AA supplementation led to poorer growth and feed utilization than those of gibel carp fed all other diets (Cai et al., 2022).
Nevertheless, the substitutability of FM to a limited amount of plant protein with AA supplementation is also reported in fish feeds. For example, dietary increased FM substitution with isolated soy protein lowered growth and feed utilization of silvery-black porgy (Sparidentex hasta) regardless of limiting AA supplementation when silvery-black porgy was provided with a 62% FM-based diet or one of the diets replacing 45%, 60%, and 75% FM by isolated soy protein with or without limiting AA (lysine and methionine) supplementation (Yaghoubi et al., 2020). They suggested that poor growth performance of silvery-black porgy fed the isolated soy protein-replaced diets would result from the ANFs in soybean protein products rather than lysine and methionine deficiency in low FM diets. In addition, the inclusion of hydroxyproline, which is considered conditionally free EAA in aquafeeds, in a high plant protein diet did not improve the growth performance of turbot (Scophthalmus maximus) (Li et al., 2009). Dietary FM replacement with plant proteins without AA supplementation frequently led to changes in intestinal morphology and function, and enteritis due to the ANFs, and nutrient imbalance associated with plant products (Zhang et al., 2013; Glencross et al., 2020).
The CF, VSI, and HSI of rockfish were not influenced by either substitution level or substitution source in this experiment. Similarly, no remarkable difference in the CF, HSI, and VSI were found in juvenile hybrid grouper (Epinephelus fuscoguttatus × E. lanceolatus) fed diets replacing various levels of FM with SPC (Faudzi et al., 2017). Unlike this study, CF and HSI were reduced at high replacement levels of SPC for FM in pearl gentian grouper (Epinephelus lanceolatus ♂ × E. fuscoguttatus ♀) diets (Chen et al., 2019). In addition, dietary FM substitution with SPC and CGM brought about significant differences in the CF, HSI, and VSI of the red drum (Rossi et al., 2013).
The proximate composition and AA profiles of the whole-body rockfish were not different among dietary treatments in this experiment. This is consistent with the previous findings showing no differences in the chemical composition of Nile tilapia (Oreochromis niloticus) (Lin and Luo, 2011) and hybrid grouper (Faudzi et al., 2017) fed diets substituting FM with soybean meal and SPC, respectively. Baek et al. (2023) and Jeong and Cho (2023) also demonstrated that the whole-body proximate composition, AA, and FA profiles of olive flounder were not influenced by dietary FM substitution source or substitution level. Similarly, the whole-body chemical composition was not altered by FM replacement with the combined SBM, CGM, and CPC in summer flounder (Paralichthys dentatus) (Enterria et al., 2011) and combined SBM, canola meal, and cotton seed meals in blunt snout bream (Ahmed et al., 2019) feeds, respectively. However, diets replacing FM with soybean meal, SPC, and CPC altered the chemical composition of shortfin corvina but not for red drum (Minjarez-Osorio et al., 2016). Chen et al. (2019) explained that dietary FM substitution with SPC altered the whole-body proximate composition and AA profiles of pearl gentian grouper.
Fish health can be assessed by evaluating the fish’s serum parameters (Hernández et al., 2019). Administration of dietary plant proteins can affect the plasma protein and cholesterol of gilthead sea bream (Sktjà-Bobadilla et al., 2005). However, plasma and serum measurements of rockfish were not different among dietary treatments in this study, agreeing with the previous study (Baek et al., 2023; Jeong and Cho, 2023; Seong et al., 2018) that dietary replacements of various animal and plant proteins for FM did not influence the serum measurements of olive flounder. Similarly, FM replacement with tuna by-product meal and soybean meal in diets did not affect the plasma chemistry parameters of rockfish (Jeon et al., 2014). Further research may be undertaken on changes in the serum parameters of fish to better understand when fish are treated with various alternative plant proteins for FM in feeds.
In fish species that are susceptible to plant feedstuffs, the nutritional imbalance caused by dietary partial FM replacement can adversely affect their immune system (Hernández et al., 2021). Lysozyme activity and SOD are frequently used as indicators of innate immunity (Saurabh and Sahoo, 2008) and as antioxidant defense enzymes that repel attacks from reactive oxygen species (Trenzado et al., 2006). Likewise, lysozyme activity decreased with dietary increased CGM levels in the juvenile Pseudobagrus ussuriensis (Bu et al., 2018). Furthermore, the lysozyme activity of shortfin corvina was changed by dietary substitution of soybean meal, SPC, and CPC for FM (Minjarez-Osorio et al., 2016). Dietary FM substitution with SPC influenced both lysozyme activity and SOD in starry flounder (Li et al., 2015). It has been suggested that there is species-specific tolerance for plant-based feedstuffs (Minjarez-Osorio et al., 2016) as well as a dose-dependent effect on fish immunity (Saurabh and Sahoo, 2008), which may explain the conflicting results. Our observation, however, indicated that replacing 50% FM with various plant proteins did not adversely deteriorate rockfish’s plasma and serum measurements.
In the rockfish diets with 22% JMM inclusion, 25% FM could be substituted with CGM at the expense of FM without causing any negative impacts on growth performance, feed consumption and utilization, biochemical composition, and plasma and serum measurements. The feasibility of the CGM25 diet should be tested prior to the practical feeding trial.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The feeding trial and subsequent handling and sampling of experimental fish were carried out as per the ethical guideline of the Korea Maritime and Ocean University (KMOU IACUC 2021-03). The study was conducted in accordance with the local legislation and institutional requirements.
JK: Data curation, Investigation, Writing – original draft. SC: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R12C1009903).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmed M., Liang H., Chisomo Kasiya H., Ji K., Ge X., Ren M., et al. (2019). Complete replacement of fish meal by plant protein ingredients with dietary essential amino acids supplementation for juvenile blunt snout bream (Megalobrama amblycephala). Aquac. Nutr. 25, 205–214. doi: 10.1111/anu.12844
AOAC (1990). Official methods of analysis. 15th ed (Arlington, VA, USA: Association of Official Analytical Chemists. AOAC International).
Baek S. I., Cho S. H., Kim H. S. (2021). Dietary inclusion effect of various levels of jack mackerel meal on the growth performance, feed efficiency and whole body composition of rockfish (Sebastes schlegeli). J. Fish. Aquat. Sci. 24, 311–317. doi: 10.47853/FAS.2021.e30
Baek S. I., Joeng H. S., Cho S. H. (2023). Replacement effect of fish meal by plant protein sources in olive flounder (Paralichthys olivaceus) feeds with an addition of jack mackerel meal on growth, feed availability, and biochemical composition. Aquac. Nutr., 7965258. doi: 10.1155/2023/7965258
Bai S. C., Choi S. M., Kim K. W., Wang X. J. (2001). Apparent protein and phosphorus digestibilities of five different dietary protein sources in Korean rockfish, Sebastes schlegeli (Hilgendorf). Aquac. Res. 32, 99–105. doi: 10.1046/j.1355-557x.2001.00009.x
Blaufuss P., Trushenski J. (2012). Exploring soy-derived alternatives to fish meal: Using soy protein concentrate and soy protein isolate in hybrid striped bass feeds. N. Am. J. Aquac. 74, 8–19. doi: 10.1080/15222055.2011.635782
Bu X., Lian X., Zhang Y., Chen F., Tang B., Ge X., et al. (2018). Effects of replacing fish meal with corn gluten meal on growth, feed utilization, nitrogen and phosphorus excretion and IGF-I gene expression of juvenile Pseudobagrus ussuriensis. Aquac. Res. 49, 977–987. doi: 10.1111/are.13545
Cai W., Liu H., Han D., Zhu X., Jin J., Yang Y., et al. (2022). Complete replacement of fishmeal with plant protein ingredients in gibel carp (Carassius auratus gibelio) diets by supplementation with essential amino acids without negative impact on growth performance and muscle growth-related biomarkers. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.759086
Chen Y., Ma J., Huang H., Zhong H. (2019). Effects of the replacement of fishmeal by soy protein concentrate on growth performance, apparent digestibility, and retention of protein and amino acid in juvenile pearl gentian grouper. PloS One 14, e0222780. doi: 10.1371/journal.pone.0222780
Cho S. H., Kim H. S., Myung S. H., Jung W., Choi J., Lee S. (2015). Optimum dietary protein and lipid levels for juvenile rockfish (Sebastes schlegeli, Hilgendorf 1880). Aquac. Res. 46, 2954–2961. doi: 10.1111/are.2015.46.issue-12
Enterria A., Slocum M., Bengtson D. A., Karayannakidis P. D., Lee C. M. (2011). Partial replacement of fish meal with plant protein sources singly and in combination in diets for summer flounder, Paralichthys dentatus. J. World Aquac. Soc 42, 753–765. doi: 10.1111/j.1749-7345.2011.00533.x
FAO (2020) The State of World Fisheries and Aquaculture (SOFIA): Sustainability in action (Rome: Food and Agriculture Organization). Available online at: https://www.fao.org (Accessed 2023-01-24).
FAO (2022)Fisheries and Aquaculture Information and Statistics. Available online at: https://www.fao.org (Accessed 2023-02-17).
Faudzi N. M., Yong A. S. K., Shapawi R., Senoo S., Biswas A., Takii K. (2017). Soy protein concentrate as an alternative in replacement of fish meal in the feeds of hybrid grouper, brown-marbled grouper (Epinephelus fuscoguttatus) × giant grouper (E. lanceolatus) juveniles. Aquac. Res. 49, 431–441. doi: 10.1111/are.13474
Freitas L. E. L., Nunes A. J. P., do Carmo Sá M. V. (2011). Growth and feeding responses of the mutton snapper, Lutjanus analis (Cuvier 1828), fed on diets with soy protein concentrate in replacement of anchovy fish meal. Aquac. Res. 42, 866–877. doi: 10.1111/are.2011.42.issue-6
Garling D. L., Wilson R. P. (1976). Optimum dietary protein to energy ratios for channel catfish fingerlings, Ictalurus punctatus. J. Nutr. 106, 1368–1375. doi: 10.1093/jn/106.9.1368
Gatlin D. M. III, Barrows F. T., Brown P., Dabrowski K., Gaylord T. G., Hardy R. W., et al. (2007). Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac. Res. 38, 551–579. doi: 10.1111/j.1365-2109.2007.01704.x
Glencross B. D., Baily J., Berntssen M. H. G., Hardy R., MacKenzie S., Tocher D. R. (2020). Risk assessment of the use of alternative animal and plant raw material resources in aquaculture feeds. Rev. Aquac. 12, 703–758. doi: 10.1111/raq.12347
Guo J., Huang Y., Salze G., Roy L. A., Davis D. A. (2020). Use of plant-based protein concentrates as replacement for fishmeal in practical diets for the Pacific white shrimp (Litopenaeus vannamei) reared under high stocking density and low salinity conditions. Aquac. Nutr. 26, 225–232. doi: 10.1111/anu.12982
Hardy R. W. (2010). Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquac. Res. 41, 770–776. doi: 10.1111/are.2010.41.issue-5
Hernández C., Hurtado-Oliva M. A., Peña E., Hernández C., Hurtado-Oliva M. A., Peña E. (2019). Effect of short-term starvation on hematological and blood biochemical parameters in juvenile spotted rose snapper Lutjanus guttatus (Steindachner, 1869). Lat. Am. J. Aquat. Res. 47, 9–17. doi: 10.3856/vol47-issue1-fulltext-2
Hernández C., Lizárraga-Velázquez C. E., Contreras-Rojas D., Sánchez-Gutiérrez E. Y., Martínez-Montaño E., Ibarra-Castro L., et al. (2021). Fish meal replacement by corn gluten in feeds for juvenile spotted rose snapper (Lutjanus guttatus): Effect on growth performance, feed efficiency, hematological parameters, protease activity, body composition, and nutrient digestibility. Aquaculture 531, 735896. doi: 10.1016/j.aquaculture.2020.735896
Hidaka I., Kohbara J., Araki T., Morishita T., Miyajima T., Shimizu S., et al. (2000). Identification of feeding stimulants from a jack mackerel muscle extract for young yellowtail Seriola quinqueradiata. Aquaculture 181, 115–126. doi: 10.1016/S0044-8486(99)00221-5
Hien T. T. T., Phu T. M., Tu T. L. C., Tien N. V., Duc P. M., Bengtson D. A. (2017). Effects of replacing fish meal with soya protein concentrate on growth, feed efficiency and digestibility in diets for snakehead Channa striata. Aquac. Res. 48, 3174–3181. doi: 10.1111/are.13147
Jeon G. H., Kim H. S., Myung S. H., Cho S. H. (2014). The effect of the dietary substitution of fishmeal with tuna by-product meal on growth, body composition, plasma chemistry and amino acid profiles of juvenile Korean rockfish (Sebastes schlegeli). Aquac. Nutr. 20, 753–761. doi: 10.1111/anu.2014.20.issue-6
Jeong H. S., Cho S. H. (2023). Inclusion effect of jack mackerel meal as feed stimulants in diets replacing different levels of fish meal with various animal protein sources on growth performance of olive flounder (Paralichthys olivaceus). Aquac. Rep. 28, 101450. doi: 10.1016/j.aqrep.2022.101450
Jeong H. S., Choi D. G., Lee K. W., Cho S. H., Lim S. G., Lee B. J., et al. (2020). Attractiveness of various crude feed ingredients to juvenile olive flounder (Paralichthys olivaceus, Temminck & Schlegel) and its application to aquaculture. Aquac. Res. 51, 4517–4532. doi: 10.1111/are.14797
Kalhoro H., Zhou J., Hua Y., Ng W. K., Ye L., Zhang J., et al. (2018). Soy protein concentrate as a substitute for fish meal in diets for juvenile Acanthopagrus schlegelii: effects on growth, phosphorus discharge and digestive enzyme activity. Aquac. Res. 49, 1896–1906. doi: 10.1111/are.13645
Kasumyan A. O., Døving K. B. (2003). Taste preferences in fishes. Fish Fish. 4, 289–347. doi: 10.1046/j.1467-2979.2003.00121.x
Khalifa N. S. A., Belal I. E. H., El-Tarabily K. A., Tariq S., Kassab A. A. (2018). Evaluation of replacing fish meal with corn protein concentrate in Nile tilapia Oreochromis niloticus fingerlings commercial diet. Aquac. Nutr. 24, 143–152. doi: 10.1111/anu.12542
Kikuchi K. (1999). Partial replacement of fish meal with corn gluten meal in diets for Japanese flounder Paralichthys olivaceus. J. World. Aquac. Soc 30, 357–363. doi: 10.1111/j.1749-7345.1999.tb00686.x
Kim H. S., Baek S. I., Lee K. W., Jeong H. S., Cho S. H. (2020). Attractiveness of various protein sources to juvenile rockfish (Sebastes schlegeli, Hilgendorf 1880). J. Appl. Aquac. 32, 205–220. doi: 10.1080/10454438.2019.1655516
Kim H. S., Cho S. H. (2019). Dietary inclusion effect of feed ingredients showing high feeding attractiveness to rockfish (Sebastes schlegeli Hilgendorf 1880) on the growth performance, feed utilization, condition factor and whole body composition of fish (II). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 231, 66–73. doi: 10.1016/j.cbpa.2019.01.011
Kim J., Cho S. H. (2023). Inclusion effect of jack mackerel, Trachurus japonicus, meal in the diet of rockfish, Sebastes schlegeli, on growth, feed utilization, biochemical composition, and innate immune responses. J. World Aquac. Soc 54, 1137–1161. doi: 10.1111/jwas.13003
Kissil G. W., Lupatsch I., Higgs D. A., Hardy R. W. (2000). Dietary substitution of soy and rapeseed protein concentrates for fish meal, and their effects on growth and nutrient utilization in gilthead seabream Sparus aurata L. Aquac. Res. 31, 595–601. doi: 10.1046/j.1365-2109.2000.00477.x
Lange S., Gudmundsdottir B. K., Magnadottir B. (2001). Humoral immune parameters of cultured Atlantic halibut (Hippoglossus hippoglossus L.). Fish Shellfish Immunol. 11, 523–535. doi: 10.1006/fsim.2000.0333
Lee S. (2002). Apparent digestibility coefficients of various feed ingredients for juvenile and grower rockfish (Sebastes schlegeli). Aquaculture 207, 79–95. doi: 10.1016/S0044-8486(01)00751-7
Lee S., Yoo J., Lee J. Y. (1996). The use of soybean meal, corn gluten meal, meat meal, meat and bone meal, or blood meal as a dietary protein source replacing fish meal in Korean rockfish (Sebastes schlegeli). Korean J. Anim. Nutr. Feedstuffs. 20, 21–30.
Lewis H. A., Kohler C. C. (2008). Corn gluten meal partially replaces dietary fish meal without compromising growth or fatty acid composition of sunshine bass. N. Am. J. Aquac. 70, 50–60. doi: 10.1577/A06-091.1
Li P., Mai K. S., Trushenski J., Wu G. Y. (2009). New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37, 43–53. doi: 10.1007/s00726-008-0171-1
Li P. Y., Wang J. Y., Song Z. D., Zhang L. M., Zhang H., Li X. X., et al. (2015). Evaluation of soy protein concentrate as a substitute for fishmeal in diets for juvenile starry flounder (Platichthys stellatus). Aquaculture 448, 578–585. doi: 10.1016/j.aquaculture.2015.05.049
Lin S., Luo L. (2011). Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia, Oreochromis niloticus × O. aureus. Anim. Feed Sci. Technol. 168, 80–87. doi: 10.1016/j.anifeedsci.2011.03.012
Luthada-Raswiswi R., Mukaratirwa S., O’Brien G. (2021). Animal protein sources as a substitute for fishmeal in aquaculture diets: A systematic review and meta-analysis. Appl. Sci. 11, 3854. doi: 10.3390/app11093854
Martins G. P., Mazini B. S. M., Campos M. A. F., Oliveira D. S., Guimarães I. G. (2020). Effect of replacing fish meal protein by crystalline amino acid and soy protein concentrate on growth, feed utilization, and metabolism of tambaqui Colossoma macropomum juveniles. J. World Aquac. Soc 51, 1250–1269. doi: 10.1111/jwas.12688
Men K., Ai Q., Mai K., Xu W., Zhang Y., Zhou H. (2014). Effects of dietary corn gluten meal on growth, digestion and protein metabolism in relation to IGF-I gene expression of Japanese seabass, Lateolabrax japonicus. Aquaculture 428–429, 303–309. doi: 10.1016/j.aquaculture.2014.03.028
Minjarez-Osorio C., Castillo-Alvarado S., Gatlin D. M., González-Félix M. L., Perez-Velazquez M., Rossi W. (2016). Plant protein sources in the diets of the sciaenids red drum (Sciaenops ocellatus) and shortfin corvina (Cynoscion parvipinnis): A comparative study. Aquaculture 453, 122–129. doi: 10.1016/j.aquaculture.2015.11.042
Nandakumar S., Ambasankar K., Ali S. S. R., Syamadayal J., Vasagam K. (2017). Replacement of fish meal with corn gluten meal in feeds for Asian seabass (Lates calcarifer). Aquacult. Int. 25, 1459–1505. doi: 10.1007/s10499-017-0133-2
National Research Council (NRC) (2011). Nutrient Requirements of Fish and Shrimp, Animal Nutrition Series (Washington DC. USA: The National Academies Press). Available at: https://www.nrc.re.kr.
Ng W. K., Leow T. C., Yossa R. (2019). Effect of substituting fishmeal with corn protein concentrate on growth performance, nutrient utilization and skin coloration in red hybrid tilapia, Oreochromis sp. Aquac. Nutr. 25, 1006–1016. doi: 10.1111/anu.12918
Novriadi R., Spangler E., Allen Davis D. (2019). Comparative effect of advanced soy products or corn protein concentrate with porcine meal on growth, body composition, and distal intestine histology of Florida pompano, Trachinotus carolinus. J. World Aquac. Soc 50, 433–447. doi: 10.1111/jwas.12547
Pereira T. G., Oliva-Teles A. (2003). Evaluation of corn gluten meal as a protein source in diets for gilthead sea bream (Sparus aurata L.) juveniles. Aquac. Res. 34, 1111–1117. doi: 10.1046/j.1365-2109.2003.00909.x
Rossi W., Moxely D., Buentello A., Pohlenz C., Gatlin D. M. III (2013). Replacement of fishmeal with novel plant feedstuffs in the diet of red drum Sciaenops ocellatus: an assessment of nutritional value. Aquac. Nutr. 19, 72–81. doi: 10.1111/anu.2013.19.issue-s1
Rossi W., Tomasso J. R., Gatlin D. M. (2015). Production performance and non-specific immunity of cage-raised red drum, Sciaenops ocellatus, fed soybean-based diets. Aquaculture 443, 84–89. doi: 10.1016/j.aquaculture.2015.03.012
Saurabh S., Sahoo P. K. (2008). Lysozyme: an important defence molecule of fish innate immune system. Aquac. Res. 39, 223–239. doi: 10.1111/j.1365-2109.2007.01883.x
Seong M., Lee S., Lee S., Song Y., Bae J., Chang K., et al. (2018). The effects of different levels of dietary fermented plant-based protein concentrate on growth, hematology and non-specific immune responses in juvenile olive flounder, Paralichthys olivaceus. Aquaculture 483, 196–202. doi: 10.1016/j.aquaculture.2017.10.023
Sktjà-Bobadilla A., Peña-Llopis S., Gómez-Requeni P., Médale F., Kaushik S., Pérez-Sánchez J. (2005). Effect of fish meal replacement by plant protein sources on non-specific defence mechanisms and oxidate stress in gilthead sea bream (Sparus aurata). Aquaculture 249, 387–400. doi: 10.1016/j.aquaculture.2005.03.031
Takakuwa F., Masumoto T., Fukada H. (2019). Identification of feeding stimulants for greater amberjack Seriola dumerili in muscle tissue of jack mackerel Trachurus japonicus. Fish. Sci. 85, 387–395. doi: 10.1007/s12562-018-01285-w
Trenzado C., Hidalgo M. C., García-Gallego M., Morales A. E., Furné M., Domezain A., et al. (2006). Antioxidant enzymes and lipid peroxidation in sturgeon Acipenser naccarii and trout Oncorhynchus mykiss. A comparative study. Aquaculture 254, 758–767. doi: 10.1016/j.aquaculture.2005.11.020
Wang P., Zhu J., Feng J., He J., Lou Y., Zhou Q. (2017). Effects of dietary soy protein concentrate meal on growth, immunity, enzyme activity and protein metabolism in relating to gene expression in large yellow croaker Larimichthys crocea. Aquaculture 477, 15–22. doi: 10.1016/j.aquaculture.2017.04.030
Yacoob S. Y., Browman H. I. (2007). Prey extracts evoke swimming behavior in juvenile Atlantic halibut (Hippoglossus hippoglossus). Aquaculture 270, 570–573. doi: 10.1016/j.aquaculture.2007.05.003
Yaghoubi M., Torfi mozanzadeh M., Ghafle Marammazi J., Safari O., Hekmatpour F., Gisbert E. (2020). Lysine and methionine supplementation in high soy protein content diets for silvery-black porgy (Sparidentex hasta) juveniles. Iran J. Fish. Sci. 19, 1329–1343. doi: 10.22092/ijfs.2019.119235
Yan Q., Xie S., Zhu X., Lei W., Yang Y. (2006). Dietary lysine requirement for Sebastes schlegeli. Acta Hydrobiol. Sin. 30, 459–465.
Yan Q., Xie S., Zhu X., Lei W., Yang Y. (2007). Dietary methionine requirement for juvenile. Aquac. Nutr. 13, 164–169. doi: 10.1111/j.1365-2095.2007.00461.x
Zhang K., Ai Q., Mai K., Xu W., Liufu Z., Zhang Y., et al. (2013). Effects of dietary hydroxyproline on growth performance, body composition, hydroxyproline and collagen concentrations in tissues in relation to prolyl 4-hydroxylase α(I) gene expression of juvenile turbot, Scophthalmus maximus L. fed high plant protein diets. Aquaculture 404–405, 77–84. doi: 10.1016/j.aquaculture.2013.04.025
Zou Q., Huang Y., Cao J., Zhao H., Wang G., Li Y., et al. (2017). Effects of four feeding stimulants in high plant-based diets on feed intake, growth performance, serum biochemical parameters, digestive enzyme activities and appetite-related genes expression of juvenile GIFT tilapia (Oreochromis sp.). Aquac. Nutr. 24, 1076–1085. doi: 10.1111/anu.2017.23.issue-5
Keywords: low fish meal feed, fish meal replacement, plant proteins, substitution level, feed availability
Citation: Kim J and Cho SH (2024) Substitution effect of fish meal with various plant protein sources on growth performance and feed utilization in rockfish (Sebastes schlegeli) diets including jack mackerel meal used as feed stimulants. Front. Mar. Sci. 11:1339471. doi: 10.3389/fmars.2024.1339471
Received: 16 November 2023; Accepted: 29 January 2024;
Published: 20 February 2024.
Edited by:
Seyyed Morteza Hoseini, Iranian Fisheries Science Research Institute (IFSRI), IranReviewed by:
Imtiaz Ahmed, University of Kashmir, IndiaCopyright © 2024 Kim and Cho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung Hwoan Cho, Y2hvc3VuaEBrbW91LmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.