
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 26 January 2024
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1334896
In May 2020, a bottom-trawl survey in the southern Bohai Sea collected the portunid crab Charybdis bimaculata, a species formerly found in the northern Yellow Sea. In subsequent surveys, C. bimaculata was found to be abundant and likely to occupy habitats and niches of native species. To study the suitability of habitat in the southern Bohai Sea for this crab, nine trawl surveys were conducted between 2020 and 2022 to monitor its dispersal. Using Biomod2 software and combining species occurrence and environmental data, a distribution model for C. bimaculata in the southern Bohai Sea is developed. We analyze relationships between this and other crustacean species by comparing niche widths and their overlap. A random forest model outperforms eight others, and has the highest evaluation indices among single algorithm species-distribution models. The evaluation index of an ensemble model is significantly higher than those of single algorithm models, indicating its greater accuracy and robustness. We report suitable habitat for C. bimaculata to occur mainly in central and northeastern Laizhou Bay, and for this habitat suitability to shift over years from the middle to northeastern waters. Niche width showed a negative trend from 2020 to 2022, and is greater in May than August for each year. Niche overlaps between C. bimaculata and other major crustaceans in the southern Bohai Sea exist. We consider that increased sea surface temperature caused by climate change enabled invasion of C. bimaculata from northern Yellow Sea waters into the southern Bohai Sea, where it can overwinter and complete its life cycle. These results provide a scientific basis upon which monitoring of C. bimaculata in the Bohai Sea can be strengthened to better cope with its invasion and any negative impact on local biodiversity.
Climate change affects the distributions of marine species by changing their environment (e.g., water temperature, ocean currents) (Hazen et al., 2013; Becker et al., 2019). These changes can reduce appropriate habitat for entire marine communities, cause local species extinctions, create conditions suitable for non-native species to exploit (Stachowicz et al., 2002; Bellard et al., 2013; Sorte et al., 2013; Hulme, 2017), and enables invasive species establishment and distribution expansion (Perry et al., 2005; Knutsen et al., 2013; Xu et al., 2022; Neumann et al., 2013).
The Bohai Sea, a semi-enclosed shallow (average depth 18 m) water body in northeastern China, is heavily impacted by climate change (Chen et al., 2021), and its waters are warming rapidly (Belkin, 2009). Because the southern Bohai Sea has many oil-drilling platforms, and the Yellow River, China’s second longest, discharges into it, contributing more than 75% of the total freshwater input into the Bohai Sea (Ren et al., 2002), its ecology is also vulnerable to the impacts of human activity, terrestrial runoff, and river input (Wang et al., 2022). Changes in these large volumes of freshwater, and nutrients within, dramatically affect salinity and dissolved oxygen, primary productivity, phytoplankton biomass, and chlorophyll-A concentrations. This can result in rapid environmental changes. Climate change and runoff have changed environmental conditions in the southern Bohai Sea, changed the suitability of habitat for native species, and provided suitable habitat for invasive species.
The crab Charybdis bimaculata (Portunidae) is small and widely distributed in Japanese waters, and coastal waters of the Yellow and East China seas. Throughout its range it can be abundant, and in terms of biomass, a dominant species in the crustacean community (Teruyoshi, 2008; Luan et al., 2018). In the Bohai Sea area of China, C. bimaculata was first reported from a 1982 bottom-trawl survey in Laizhou Bay (Wang and Wu, 2018), but in 11 consecutive years (2009–2019) of spring and summer trawl surveys in these waters, the Shandong Institute of Marine Resources and Environment has not reported it once, nor has it been reported in any other study from this area. However, in six trawl surveys between May 2020 and August 2022 in the Bohai Sea, many C. bimaculata were found. Because this species, which lives for about one year, has been found many times over in three consecutive years, it appears to have become established in these waters.
While C. bimaculata is not a target species in any current trawl fishery, it represents a potentially important prey species for many other fishes (Zhang et al., 2011; Wang et al., 2012), and may now occupy an important ecological position in the Bohai Sea food web. Accordingly, for C. bimaculata we report its: 1) distribution and that of suitable habitat in the southern Bohai Sea; 2) niche width and ecological overlap with other native crustacean species in the southern Bohai Sea; (3) likely mechanism of invasion into these waters; and (4) possible impact on other species of crustacean, and in niche.
Trawl surveys were conducted by the Shandong Institute of Marine Resources and Environment in the southern Bohai Sea (Figure 1), covering important ecological areas such as Laizhou Bay and the Yellow River Estuary (37–38.5°N, 117.7–121°E). In total, 394 stations were sampled over nine surveys in May, August, and October, from 2020–2022 (Table 1). Limitations in investigation time and marine conditions resulted in some station locations differing between surveys.
Scientific-survey fisheries data were collected by bottom trawl each year using the same 260 kW power survey vessel “LU CHANG YU 60003,” and a single bottom trawl of mouth area 30.6 m2, bag mesh 20 mm, and diameter ~8 m when towing. Stations were trawled for 1 h at ~2 kn. Survey data included counts of all crustaceans. Count data for each station were standardized and converted into numbers of crustacean individuals (ind) h−1; these data are used to calculate niche width and overlap. Population occurrence data (presence or absence) for C. bimaculata were transformed on a spatial resolution grid of 0.1° latitude × 0.1° longitude to facilitate input data for species distribution models.
Bottom temperature (BT), bottom salinity (BS), bottom dissolved oxygen (BO), chlorophyll-a concentration (Chla), primary productivity (PP), and phytoplankton (Phy) data were obtained from the Copernicus Marine Service (http://marine.copernicus). Water depth (Depth, D) data were obtained from the global marine environmental data set (GMED, https://gmed.auckland.ac.nz/index.html). All environmental data were downloaded at a 0.1° spatial resolution, and monthly temporal resolution.
The species distribution model (SDM) is based on ecological niche theory and analyzes habitat suitability by establishing a correlation between a species’ distribution and environmental factors, spatial characteristics, and biological factors. We used the Biomod2 software package in R (V4.0.2) (Thuiller et al., 2016) to investigate habitat suitability for C. bimaculata in the southern Bohai Sea. Nine models [generalized linear model (GLM), multiple adaptive regression splines (MARS), generalized boosting model (GBM), classification tree analysis (CTA), artificial neural network (ANN), surface range envelope (SRE), flexible discriminant analysis (FDA), random forest (RF), and maximum entropy (MaxEnt)] were examined.
The Biomod2 software package requires known presence and absence points, but information regarding absence points is seldom available. We follow Thuiller et al. (2016) in generating pseudoabsence points using known presence points. Using C. bimaculata presence/absence data, we randomly generated three groups with 50 pseudoabsence records in each (Shi et al., 2023). To evaluate model accuracy, we randomly selected 70% of occurrence data for use as a training data set and the remainding 30% as a validation data set; each model was run 100 times for cross-validation. The area under the curve (AUC) of the receiver operating characteristic (ROC) (Hanley and McNeil, 1982), the true skill statistic (TSS) (Allouche et al., 2006), and Cohen’s kappa (Kappa) (Cohen, 1960), were used as indices to evaluate model performance. The closer the measured values of TSS, Kappa, and AUC are to 1, the more reliable the prediction results are (Pearce and Ferrier, 2000). The relative importance of environmental variables to the distribution of C. bimaculata was also calculated to better understand which ones best described this species’ distribution. The relative importance of environmental variables is calculated using methods in the Biomod2 software package. First, the reference values are calculated using the model constructed with all the variables; the predicted values are then obtained using a new model constructed by randomizing the individual variables; Spearman coefficients of reference and predicted values are then estimated; and finally, the Spearman coefficient is subtracted from 1 to obtain the importance value.
To reduce uncertainty in the single algorithm model and data generation process (mainly pseudo-deletion sites), we developed an ensemble species distribution model to predict the spatial distribution of suitable habitat for C. bimaculata. Single models with AUC ≥ 0.7, TSS ≥ 0.5, and Kappa ≥ 0.4, were retained (Chen et al., 2021) to construct the distribution of C. bimaculata within the study area. Six survey ensemble models were constructed, and the distribution results of potentially suitable habitat for C. bimaculata based on each ensemble model were normalized. The normalization process was realized by Fuzzy membership in ArcGIS 10.3. A habitat distribution map for C. bimaculata was prepared using ArcGIS 10.3. The grid value in the figure represents the habitat suitability index (HSI)—the probability of a habitat being suitable for a species. The HSI ranges 0 to 1; the closer the grid value is to 1 the higher the probability of species occurrence. We define that area with HSI ≥ 0.6 to be suitable habitat for C. bimaculata following Yu et al. (2020).
The Shannon–Wiener index was used to calculate niche width (Dice, 1945).
Pianka’s index was used to calculate niche overlap (Shannon and Weiner, 1963)
where Pij = nij/Nij represents the proportion of the number of individuals of species i in the j resource state to the number of all individuals of that kind, where j represents a certain survey station. Qij is the overlap index, and its value is between 0 and 1; the larger the value, the higher the overlap. When Qij > 0.75, overlap is considered to be significant (Pianka, 1973).
Based on cross-validation evaluation, the AUC (Figure 2A), TSS (Figure 2B), and Kappa (Figure 2C), and values of 2700 single algorithm models were calculated. The RF model performs best, for which the three evaluation indices are the highest. GBM and ANN models follow.
The average relative importance of environmental variables in the RF model is shown in Figure 3. The main factors influencing the distribution of C. bimaculata vary with time: in May 2020 they are BS, BO, and BT; August 2020, BO, D, and BT; May 2021, D, Phy, BT, and Chla; August 2021, BO, Phy, and Pro; May 2022, BO, Pro, and BT; and August 2022, Pro, BS, and BT. Of these, BT (in 5 surveys) and BO (in 4 surveys) most-greatly affect the distribution of C. bimaculata.
Suitable habitat for C. bimaculata (HSI ≥ 0.6) occurs mainly in the central (37°30–37°50’N, 119°20–119°50’E) and northeastern (37°50–38°10’N, 119°50–120°30’E) waters of Laizhou Bay, and gradually shifts over time from the former to the latter. The area of suitable habitat in May 2020 is significantly larger than in August of 2020 and 2022, but smaller than it is in August of 2021 (Figure 4). The proportion of pixels where the corresponding probability of HSI is ≥ 0.6 for surveys in May 2020 is 16.4%, and for August 2020 (12.6%), May 2021 (16.4%), August 2021 (17.9%), May 2022 (19.8%), and August 2022 (13.0%).
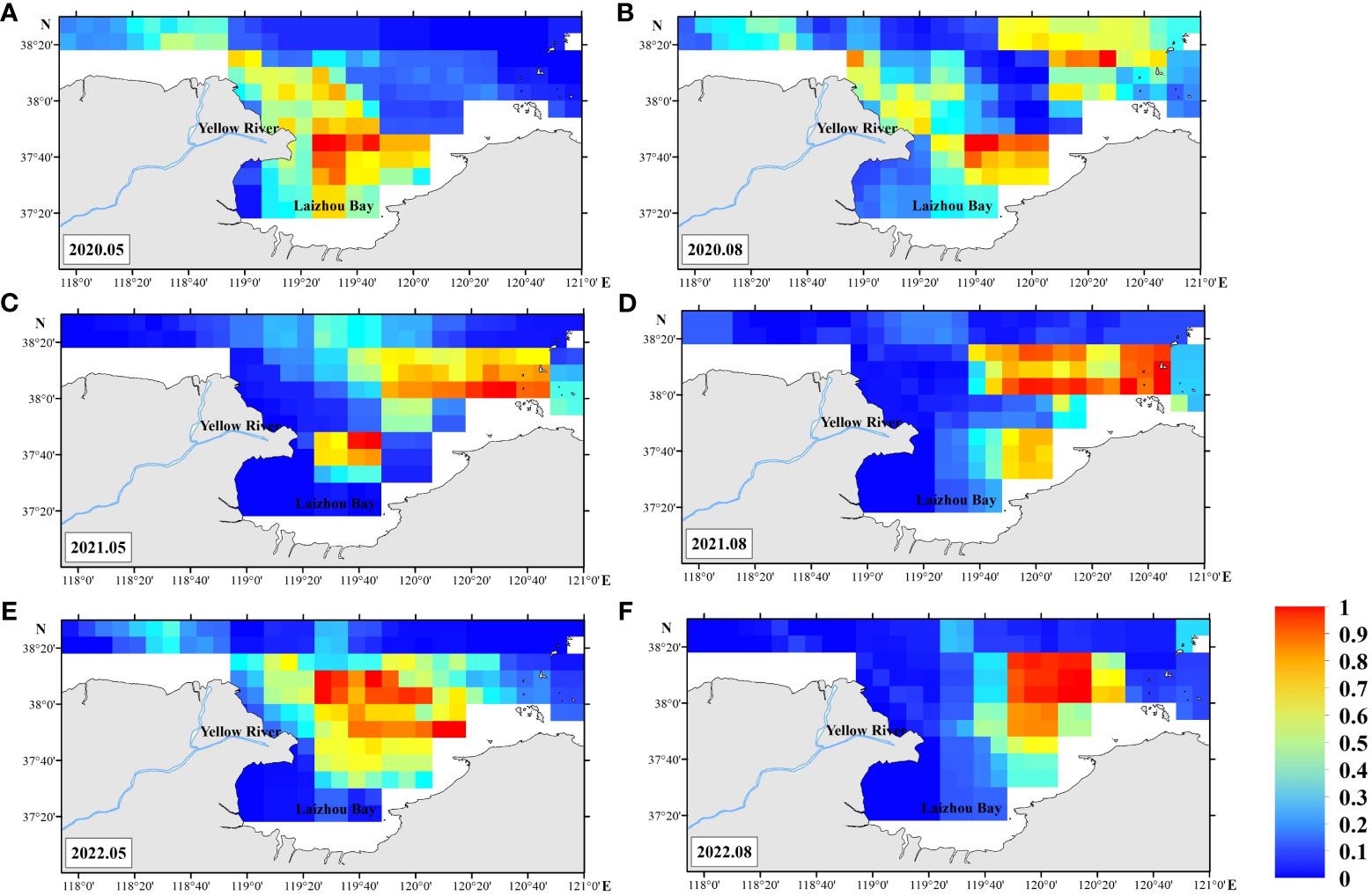
Figure 4 Random forest (RF) model prediction of Charybdis bimaculata habitat in the southern Bohai Sea. May 2020 (A), August 2020 (B), May 2021 (C), August 2021 (D), May 2022 (E), August 2022 (F).
AUC, TSS, and Kappa values of an ensemble model are significantly higher than those of the single-algorithm model (Table 2, Figure 5), indicating that the integrated model is more accurate and robust.
The importance of environmental variables for different survey ensemble models is similar to RF model results. However, the integrated model emphasizes the importance of fewer factors (one environmental factor may be much more important than others). In this way, the ensemble model improves model accuracy.
The distributions of suitable habitat in the ensemble and RF models are similar, but the area of suitable habitat is significantly higher. The proportion of pixels for which the HSI probability is ≥ 0.6 for each survey differs over time: May 2020 (30.9%), August 2020 (13.0%), May 2021 (22.7%), August 2021 (22.2%), May 2022 (26.6%), and August 2022 (15.9%) (Figure 6).

Figure 6 Ensemble model prediction of Charybdis bimaculata habitat in the southern Bohai Sea. May 2020 (A), August 2020 (B), May 2021 (C), August 2021 (D), May 2022 (E), August 2022 (F).
The highest niche width (3.56) occurred in May 2020, and the lowest (1.49) in August 2021. Niche width trended down from 2020 to 2022 and is higher in May than in August of all years (Figure 7). High numbers indicate overlap in niches between C. bimaculata and other main crustaceans in southern Bohai Sea waters; niche overlap is higher in May 2020 and August 2021, and lower in all months of 2022. There are significant overlaps (> 0.75) with Oratosquilla oratoria, Metapenaeopsis dalei, Crangon hakodatei, and Palaemon gravieri in May 2020, M. dalei in May 2021, and Alpheus distinguendus, O. oratoria, Latreutes planirostris, Trachysalambria curvirostris, and Alpheus japonicus in August 2021 (Figure 8).
The SDM model is widely used in studies on marine organisms, including to determine habitat distribution (Rubec et al., 2016; Shi et al., 2023), impacts of climate change on marine habitats (Chen et al., 2021; Martina et al., 2022), and biological invasions by non-native species (Neumann et al., 2013). We use Biomod2 software to reveal the distribution of suitable habitat for C. bimaculata from the Yellow to southern Bohai seas. By comparing evaluation indices of single and integrated models, we determine that RF models outperform single ensemble models. As a machine-learning model (Han et al., 2021), the RF model performs better in an environment where data are few (Hernandez et al., 2006; Mi et al., 2017). The GBM and MAXENT models are both machine-learning models, and they also perform well. The ensemble model that integrates multiple models that perform well at predicting C. bimaculata habitat is generally better than a single model for evaluating indicators, and is becoming increasingly widely applied in fisheries modeling (Zhang et al., 2019; Hao et al., 2020; Chen et al., 2023; Ye et al., 2021; Liu et al., 2022). Our application of the SDMs model confirms that it is appropriate for studying habitat distribution during the early stages of species invasion. Accurate prediction of the potential distribution of C. bimaculata can aid the management of this species, which is important for conservation of biodiversity in the Bohai Sea. Accurate prediction can also identify the potential risk posed by species invasion in these waters.
The life cycle of C. bimaculata involves a planktonic larval stage and largely benthic post-settlement stage; adults have poor swimming ability and cannot migrate long distances (Pan et al., 2012). Population genetic structure suggests that larval C. bimaculata disperse from spawning grounds, and are transported in currents to new places. Their high larval dispersal ability leads to homogenization of genetic variation in the Yellow and East China seas (Han et al., 2015). It follows that C. bimaculata likely spread as larvae from the northern Yellow Sea to the southern Bohai Sea.
Spawning of C. bimaculata occurs throughout the year, except during winter, and is greater in summer when water temperatures are higher (Doi et al., 2008). The super typhoon “Lekima” (August 4–13, 2019) occurred when C. bimaculata was spawning, and caused major storm surges in the Bohai Sea. After entering Shandong Peninsula, the typhoon’s path moved west and lingered in Laizhou Bay for 30 h, producing sustained southeast winds in Bohai Strait and northeasterly winds above force 8 in Bohai Bay, preventing the Yellow Sea storm surge into the Bohai Sea from returning (Fu et al., 2021). It is probable that plankton containing the larvae of C. bimaculata carried by this storm surge settled in the southern Bohai Sea, and the following year many C. bimaculata became apparent.
An extreme and accidental climate event such as super typhoon likely provided an opportunity for C. bimaculata to spread, but climate change explains how the species can persist in the southern Bohai Sea. The semi-enclosed Bohai Sea is severely affected by climate change, with sea surface temperatures within it increasing five times faster than the global average (Belkin, 2009). In recent decades, bottom temperatures in the southern Bohai Sea have also increased on average by 0.013°C annually (Ning et al., 2010). Sea bottom salinity, which was relatively stable at 28.7 from the 1950s to 1980s has risen to 30 at the beginning of 21st century, and increases annually by 0.105 (Ning et al., 2010; Zhou et al., 2012). Increased sea temperatures enable C. bimaculata to successfully overwinter, reproduce, and complete its life history, resulting in the expansion in distribution of this species.
Larvae require a suitable habitat in which to settle (Peterson, 2003). We predict suitable habitat using the SDMs, report this to occur mainly in central and northeastern Laizhou Bay, and for this area to generally shift from the middle to the northeast of this bay over time. This suggests that invasive species adapt to the new environment, leading to instability in the species distribution model (Elith et al., 2010). Charybdis bimaculata continues to adapt to habitat in the southern Bohai Sea, and will survive better in more optimal habitat. The distribution of C. bimaculata is affected by different environmental factors, and the effect of the same environmental factor differs over time. Although BO and BT are the main variables affecting the distribution of this crab in the southern Bohai Sea, other environmental variables also affect its distribution over time. After invading the southern Bohai Sea, its niche width and niche overlap with other crustacean species decreased significantly, suggesting that competition between it and other crustaceans was reducing. Most Bohai Sea crustaceans occur throughout the Yellow and East China seas with C. bimaculata. This indicates that these species can coexist in the Bohai Sea. Charybdis bimaculata is often an abundant and, in terms of biomass, dominant species. As a small crab, it represents a food source for fish, crustaceans and cephalopods, so its spread to the southern Bohai Sea may contribute to increased biodiversity.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The manuscript presents research on animals that do not require ethical approval for their study.
XZ: Writing – original draft, Writing – review & editing, Data curation, Investigation. YS: Methodology, Software, Visualization, Writing – review & editing. SL: Data curation, Investigation, Writing – review & editing. YY: Data curation, Writing – review & editing. BX: Investigation, Writing – review & editing. XW: Data curation, Writing – review & editing. HS: Data curation, Writing – review & editing. FL: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Innovation Development Program of Yantai (contracts 2023YD081 and 2023JCYJ101), the Science and Technology Innovation Program of the Laoshan Laboratory (No. LSKJ202203803), the Natural Science Foundation of Shandong Province (No. ZR2020KE050), and the opening foundation of the Observation and Research Station of Bohai Strait Eco-Corridor, MNR, grant BH202304.
We thank all of our colleagues who contributed to this study. We thank Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Allouche O., Tsoar A., Kadmon R. (2006). Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 43, 1223–1232. doi: 10.1111/j.1365-2664.2006.01214.x
Becker E. A., Forney K. A., Redfern J. V., Barlow J., Jacox M. G., Roberts J. J., et al. (2019). Predicting cetacean abundance and distribution in a changing climate. Divers. Distrib. 25, 626–643. doi: 10.1111/ddi.12867
Belkin I. M. (2009). Rapid warming of large marine ecosystems. Prog. Oceanogr. 81, 207–213. doi: 10.1016/j.pocean.2009.04.011
Bellard C., Thuiller W., Leroy B., Genovesi P., Bakkenes M., Courchamp F. (2013). Will climate change promote future invasions? Global. Change. Biol. 19, 3740–3748. doi: 10.1111/gcb.12344
Chen Y. L., Shan X. J., Gorfine H., Dai F. Q., Wu Q., Yang T., et al. (2023). Ensemble projections of fish distribution in response to climate changes in the Yellow and Bohai Seas, China. Ecol. Indic .146, 109759. doi: 10.1016/j.ecolind.2022.109759
Chen Y. L., Shan X. J., Ovando D., Yang T., Dai F. Q., Jin X. S. (2021). Predicting current and future global distribution of black rockfish (Sebastes schlegelii) under changing climate. Ecol. Indic. 128, 107799. doi: 10.1016/j.ecolind.2021.107799
Cohen J. (1960). A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 20, 37–46. doi: 10.1177/001316446002000104
Dice L. R. (1945). Measures of the amount of ecologic association between species. Ecology 26, 297–302. doi: 10.2307/1932409
Doi W., Yokota M., Strüssmann C. A., Watanabe S. (2008). Growth and reproduction of the portunid crab Charybdis bimaculata (Decapoda:Brachyura) in Tokyo Bay. J.Crustacean.Biol 28, 641–651. doi: 10.1651/07-2964.1
Elith J., Kearney M., Phillips E. (2010). The art of modelling range-shifting species. Methods Ecol. .Evol .1, 330–342. doi: 10.1111/j.2041-210X.2010.00036.x
Fu C. F., Li T., Liu S. C., Gao Y., Dong J. X. (2021). Characteristics of the storm surge and UAV disaster investigation caused by the typhoon Lekima (No.1909) in the Bohai Bay. Mar. Forecasts. 38, 17–23. doi: 10.11737/j.issn.1003-0239.2021.05.003
Han Z. Q., Zheng W., Chen G. B., Shui B. N., Liu S. F., Zhuang Z. M. (2015). Population genetic structure and larval dispersal strategy of portunid crab Charybdis bimaculata in Yellow sea and East China sea. Mitochondr.DNA 26, 402–408. doi: 10.3109/19401736.2013.840592
Han X. R., Zhu G. P., Men Y. L., Yang Z. (2021). Ensemble predicting of Spodoptera frugiperda potential distribution. J. Biosafety. 30, 65–71. doi: 10.3969/j.issn.2095-1787.2021.01.011
Hanley J. A., McNeil B. J. (1982). The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143, 29–36. doi: 10.1148/radiology.143.1.7063747
Hao T., Elith J., Lahoz-Monfort J. J., Guillera-Arroita G. (2020). Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography .43, 549–558. doi: 10.1111/ecog.04890
Hazen E. L., Jorgensen S., Rykaczewski R. R., Bograd S. J., Foley D. G., Jonsen I. D., et al. (2013). Predicted habitat shifts of Pacific top predators in a changing climate. Nat. Clim. Change. 3, 234–238. doi: 10.1038/nclimate1686
Hernandez P. A., Graham C. H., Master L. L., Albert D. L. (2006). The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29, 773–785. doi: 10.1111/j.0906-7590.2006.04700.x
Hulme P. E. (2017). Climate change and biological invasions: evidence, expectations, and response options. Biol. Rev. 92, 1297–1313. doi: 10.1111/brv.12282
Knutsen H., Jorde P. E., Gonzalez E. B., Robalo J., Albretsen J., Almada V. C. (2013). Climate change and genetic structure of leading edge and rear end populations in a northwards shifting marine fish species, the corkwing wrasse (Symphodus melops). PLoS. One 8, e67492. doi: 10.1371/journal.pone.0067492
Liu T., Liu H. Y., Tong J. B., Yang Y. X. (2022). Habitat suitability of neotenic net-wingedbeetles (Coleoptera: Lycidae) in China using combined ecological models, with implications for biological conservation. Divers. Distrib. 00, 1–18. doi: 10.1111/ddi.13545
Luan J., Zhang C. L., Xu B. D., Xue Y., Ren Y. P. (2018). Relationship between catch distribution of Portunid crab (Charybdis bimaculata) and environmental factors based on three species distribution models in Haizhou Bay. J. Fish. China. 42, 889–901. doi: 10.11964/jfc.20170610878
Martina I., Allan T. S., Ester D., Carlos A. (2022). Influence of climate change and extreme weather events on an estuarine fish community. Sci.Total.Environ 827, 154190. doi: 10.1016/j.scitotenv.2022.154190
Mi C., Huettmann F., Guo Y., Han X., Wen L. (2017). Why choose Random Forest to predict rare species distribution with few samples in large undersampled areas? Three Asian crane species models provide supporting evidence. PeerJ 5, e2849. doi: 10.7717/peerj.2849
Neumann H., Boois I. ,. D., Kröncke I., Reiss H. (2013). Climate change facilitated range expansion of the non-native angular crab Goneplax rhomboides into the North Sea. Mar. Ecolprog. Ser. 484, 143–153. doi: 10.3354/meps10299
Ning X. R., Lin C. L., Su J. L., Liu C. G., Hao Q., Le F. F., et al. (2010). Long-term environmental changes and the responses of the ecosystems in the Bohai Sea during 1960–1996. Deep-Sea. Res. Pt. II. 57, 1079–1091. doi: 10.1016/j.dsr2.2010.02.010
Pan G. L., Zhu Z. J., Zhang H. L., Zhou Y. D. (2012). Distribution of the biomass of Charybdis bimaculata and its relationships with the environmental factors in the coastal spawning ground of South Zhejiang during spring. J. Zhejiang Ocean Univ. (Natural Sci. Edition). 31, 482–486.
Pearce J., Ferrier S. (2000). Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Modell. 133, 225–245. doi: 10.1016/S0304-3800(00)00322-7
Perry A. L., Low P. J., Ellis J. R., Reynolds J. D. (2005). Climate change and distribution shifts in marine fishes. Science 308, 1912–1915. doi: 10.1126/science.1111322
Peterson A. T. (2003). Predicting the geography of species’invasions via ecological niche modeling. Q .Rev. Biol. 78, 419–433. doi: 10.1086/378926
Pianka E. R. (1973). The structure of lizard communities. Annu.Rev.Ecol.Evol.S 4, 53–74. doi: 10.1146/annurev.es.04.110173.000413
Ren L., Wang M., Li C., Zhang W. (2002). Impacts of human activity on river runoff in the northern area of China. J. Hydrol. 261, 204e217. doi: 10.1016/S0022-1694(02)00008-2
Rubec P. J., Kiltie R., Leone E., Flamm R. O., McEachron L., Santi C. (2016). Using delta-generalized additive models to predict spatial distributions and population abundance of juvenile pink shrimp in Tampa Bay, Florida. Mar.Coast.Fish 8, 232–243. doi: 10.1146/annurev.es.04.110173.000413
Shannon C. E., Weiner W. (1963). The Mathematical Theory of Communication. (Urbana-Champagne: University of Illinois Press).
Shi Y. C., Kang B., Fan W., Xu L. L., Zhang S. M., Cui X. S., et al. (2023). Spatio-temporal variations in the potential habitat distribution of pacific sardine (Sardinops sagax) in the northwest Pacific Ocean. Fishes-Basel 8, 86. doi: 10.3390/fishes8020086
Sorte C. J. B., Ibez I., Blumenthal D. M., Molinari N. A., Miller L. P., Grosholz E. D., et al. (2013). Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecol. Lett. 16, 261–270. doi: 10.1111/ele.12017
Stachowicz J. J., Terwin J. R., Whitlatch R. B., Osman R. W. (2002). Linking climate change and biological invasions: ocean warming facilitates nonindigenous species invasions. Proc. Natl. Acad. Sci. 99, 15497–15500. doi: 10.1073/pnas.242437499
Teruyoshi N., Monthon G., Hideo S. (2008). Population dynamics of portunid crab Charybdis bimaculata in Ise Bay, central Japan. Fisheries .Sci. 74, 24–40. doi: 10.1111/j.1444-2906.2007.01494.x
Thuiller W., Georges D., Engler R., Breiner F. (2016) biomod2: Ensemble platform for species distribution modeling. Available at: https://cran.r-project.org/package=biomod2.
Wang J., Wu Q. (2018). Fishery resources and habitat in Laizhou Bay. (Bei jing: BJ:China Agriculture Press).
Wang X. J., Yang S. M., Zhang Q. (2022). Coupling effect of phytoplankton community structure and environmental factors in the Bohai Sea of China. Mar. pollut. Bull. 179, 113707. doi: 10.1016/j.marpolbul.2022.113707
Wang K., Zhang S. Y., Wang Z. H., Zhao J., Xu M. (2012). A preliminary study on fishery biology of Johnius belangerii off Ma’an Archipelago. J. Fish. China. 36, 228–237. doi: 10.3724/SP.J.1231.2012.27691
Xu S. C., Zhang Y., Zhou Y., Xu S., Yue S. D., Liu M. J., et al. (2022). Warming northward shifting southern limits of the iconic temperate seagrass (Zostera marina). iScience 25, 104755. doi: 10.1016/j.isci.2022.104755
Ye P. C., Zhang G. F., Zhao X., Chen H., Si Q., Wu J. Y. (2021). Potential geographical distribution and environmental explanations of rare and endangered plant species through combined modeling: A case study of Northwest Yunnan, China. Ecol.Evol 11, 13052–13067. doi: 10.1002/ece3.7999
Yu W., Wen J., Zhang Z., Chen X. J., Zhang Y. (2020). Spatio-temporal variations in the potential habitat of a pelagic commercial squid. J. Mar. Syst. 206, 103339. doi: 10.1016/j.jmarsys.2020.103339
Zhang B., Jin X. S., Dai F. Q. (2011). Feeding habits and their variation of seasnail (Liparis tanakae) in the central and southern Yellow Sea. J. Fish. China. 35, 1199–1207. doi: 10.3724/SP.J.1231.2011.17505
Zhang Z. X., Xu S. Y., Capinha C., Weterings R., Gao T. X. (2019). Using species distribution model to predict the impact of climate change on the potential distribution of Japanese whiting Sillago japonica. Ecol. Ind. 104, 333–340. doi: 10.1016/j.ecolind.2019.05.023
Keywords: Charybdis bimaculata, species invasion, Bohai Sea, species distribution model, suitable habitat, niche
Citation: Zhang X, Shi Y, Li S, Yang Y, Xu B, Wang X, Su H and Li F (2024) Climate change enables invasion of the portunid crab Charybdis bimaculata into the southern Bohai Sea. Front. Mar. Sci. 11:1334896. doi: 10.3389/fmars.2024.1334896
Received: 08 November 2023; Accepted: 09 January 2024;
Published: 26 January 2024.
Edited by:
Jinghui Fang, Chinese Academy of Fishery Sciences (CAFS), ChinaReviewed by:
Matteo Zucchetta, National Research Council (CNR), ItalyCopyright © 2024 Zhang, Shi, Li, Yang, Xu, Wang, Su and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Li, bGlmYW44MTEyMzBAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.