
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
DATA REPORT article
Front. Mar. Sci., 15 April 2024
Sec. Discoveries
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1305487
This article is part of the Research TopicNew Observations on the Behavior, Ecology, and Biology of Sharks and RaysView all 18 articles
 Austin J. Gallagher1*
Austin J. Gallagher1* Christine de Silva1,2
Christine de Silva1,2 Denley Delaney3
Denley Delaney3 S. David Harris1
S. David Harris1 Brennan T. Phillips2,4
Brennan T. Phillips2,4 Oliver N. Shipley1
Oliver N. Shipley1 James A. Sulikowski5
James A. Sulikowski5 Carlos M. Duarte6
Carlos M. Duarte6 Jonatha Giddens3
Jonatha Giddens3There are significant calls to scale efforts to advance our understanding of fundamental biological, ecological, and taxonomic information in the deep-sea to inform conservation and decision-making (Howell et al., 2020). There is growing interest into ocean depths between 200-1,000 m, building on recent research which suggested this depth band may contain up to 10 times the global fish biomass in all other marine habitats combined (Irigoien et al., 2014). A rich diversity of large marine predators is commonly found throughout this portion of the vertical water column, inspiring questions about the functional and ecological significance of this habitat (Braun et al., 2022).
The bluntnose sixgill shark (Hexchanchus griseus) is one of the largest species of deep-sea elasmobranchs (Barnett et al., 2012), reaching a confirmed total length of 482 cm (Bolivar, 1907), but with an unconfirmed report of 550 cm total length (Ebert and Compagno, 2012). The species is found circumglobally within tropical, temperate, and boreal latitudes (Finucci et al., 2020). While the species is known to have extremely large litters (47-108 pups, Ebert and Compagno, 2012), research also suggests a late age-at-maturity (females = 26.5 years; COSEWIC, 2007) and size at maturity (females at or near 400 cm TL; Ebert, 2002). The combination of these life history traits limits their population rebound potential, thus reducing their ability to sustain high levels of exploitation (Finucci et al., 2020). Due to its size and catchability, H. griseus is one of the better-studied deep-sea elasmobranchs, with a growing body of knowledge into its habitat use (Brooks et al., 2015; Comfort and Weng, 2015; Coffey et al., 2020) and trophic ecology (Reum et al., 2020). However, many aspects of H. griseus biology remain cryptic. For example, there is limited information on mating events, and thus the potential locations of reproductive grounds remain mostly speculative across its range (e.g., Ebert, 2002; Amor et al., 2017). The recent emergence of compact, innovative deep-sea observation platforms, such as video-equipped landers, is now rapidly improving our ability to detect rare deep-sea shark species’ biodiversity and new record behaviors performed in the deep ocean (e.g., Phillips et al., 2019; Gallagher et al., 2023).
Here we present rare observations of H. griseus from The Bahamas, including reports of body scarring, which provides some of the first clues to the spatial and temporal basis for mating behavior in a deep-sea elasmobranch. Given the challenges of determining where potential reproductive behaviors take place for deep-sea vertebrates, these observations also highlight how non-invasive remote lander video systems can provide timely information on critical habitat for poorly known species of deep-sea elasmobranchs.
The care and use of experimental animals complied with the Bahamas animal welfare laws, guidelines and policies as approved by the local government agencies. The authors declare no conflicts of interest. Research was conducted under permits from the Dept. Environment Protection and Planning and Dept. Marine Fisheries, Bahamas Government. This study used non-invasive visual observations of H. griseus, therefore animals were not experimentally manipulated in any way.
From 4 – 6 November 2021, two different types of custom, free-falling, deep baited remote underwater video systems (hereafter dBRUVs) were deployed 5 - 20 km northeast of Highbourne Cay, northwest Exuma Sound, The Bahamas (24.73405, -76.81480; Figure 1). The Exuma Sound is a deep-sea inlet of the Atlantic Ocean, where highly productive, neritic systems rapidly transition into deeper slopes that extend to ~2,000 m in the central region of the Sound (Buchan, 2000).
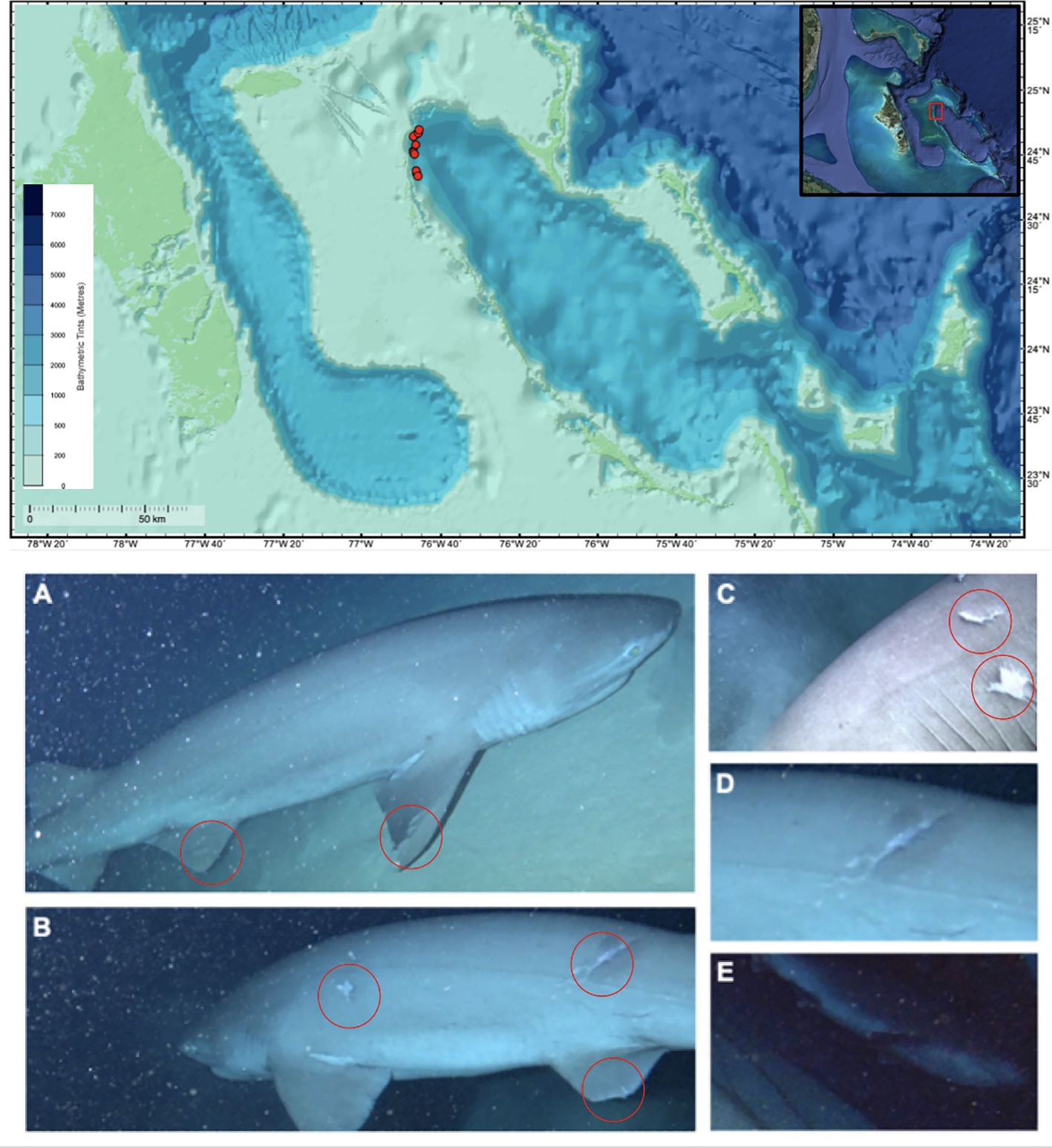
Figure 1 Top panel: Map of dBRUV deployments (red circles) in the Exuma Sound, The Bahamas, from the present study (with The Bahamas inset). Bottom panels: Hexanchus griseus recordings obtained from dBRUVs in the present study: (A, B) Large female sixgill shark bearing evidence of potential mating attempts on pectoral and pelvic fins (red circles), as well as on the body. (C) Tooth puncture marks from the same individual, indicative of distinctive H. griseus dentition (Corn et al., 2016), with (D) deep lacerations and bruising around the puncture site suggestive of recent (i.e., weeks) biting activity. (E) Unique individual male six gill shark. All individuals were visually estimated to be between 3.0 and 4.0 meters in total length sensu Gallagher et al. (2023).
The first dBRUV system (System A) (Giddens et al., 2021) consisted of a single Ultra-High Definition (HD) camera (Sony Handycam FDR-AX33) housed in a borosilicate glass pressure sphere (Vitrovex, NautilusMarine, GmbH). The sphere was situated above a stainless-steel detachable bottom shaft (41 cm long) that held the bait canister, and burnwire release mechanism attached to a temporary anchor. The pressure housing (33 cm diameter, 1.2 cm thick) housed the Ultra-HD (3840 x 2160) 4K video camera with 20.6 megapixel still image capability, fixed at a 45 declination from the horizontal plane. External high efficiency LED lighting (Cree XLamp CMT1930 LED), with two lights each placed 318 mm from the center of the sphere and angled downward at 45 degrees from the horizontal plane, provided an illumination of 1530 lm and a color temperature of 4000 K. A bank of 14.4 V, 2.6 Ah lithium-ion batteries provided 337 Wh for the camera and lighting system. A single waterproof bulkhead (BH10FTI, SubConn), externally located on top of the glass sphere, provided an electrical connection for battery charging and data retrieval. Depth was monitored using a boat-based echosounder.
The second dBRUV system (System B) consisted of a vertically oriented, carbon-fiber frame with pressure-tolerant flotation and an acoustic weight-release system (Phillips et al., 2019; Gallagher et al., 2023). A single GoPro Hero6 camera (GoPro), set to record 1080p video at 60 frames per second, was secured within a deep sea housing (GoBenthic, GroupB Incorporated, USA) and attached to the frame ~1.5 m above the bottom of the unit. Two potted LED lights were used to illuminate the seafloor (SiteLite, Juice Robotics, USA), and the cameras and lights were powered with a custom lithium-ion battery pack. Temperature and depth were monitored using a calibrated Starmon TD stand-alone logger (Star Oddi, Iceland).
A small amount of bait (bonito, Sarda spp.; 500 g) was used in both dBRUV systems to attract large marine predators and other marine life: in system B the bait was attached to a pole put in clear view of the camera; in System A the bait was placed into a perforated, 10 cm PVC tube that was out of the view of the camera. Each day, up to four dBRUVs (a maximum of two units per system) were deployed simultaneously every 1.5-2.0 km at depths between 700 – 1,100 meters, using an expendable drop weight of 20 kg. In System A, a burn wire was triggered to release the expendable weight at a pre-programmed time (5 hours). In System B, an acoustic release system (PORT LF-SD, EdgeTech, USA) was used to release the drop weight, allowing for the entire system to return to the surface upon command, resulting in deployments of 6-7 hours (as they were deployed and retrieved sequentially after System A). All dBRUVs were then located and retrieved at the surface using boat-based GPS unit and a VFH radio receiver unit (R410, 150-160 MHz; Advanced Telemetry Systems, USA). Upon retrieval, media cards from the cameras were downloaded and the footage was scanned. The presence of any large sharks was noted, as was their total time on camera, the number of passes they performed (sensu Shea et al., 2020), while noting biological and morphological details.
To further contextualize these findings, we undertook an extensive review of the published peer-reviewed scientific literature to quantify the relative number of studies that have used remote video camera systems to study H. griseus biology. Studies published prior to February 14th 2022 were included, and categorized as either behavioral, demographic, methodological, or locality record.
Video data were collected from 11 deployments along the continental shelf break of the northwest Exuma Sound (Table 1), at depths up to 1110m. This resulted in a total of 3151.1 minutes (52.5 hours) of footage (286.5 ± 16.5 min, mean ± SD). dBRUVs recorded footage from 09:14 AM to 12:09 PM, at depths ranging from 876 – 1017 m (981.6 ± 61.0 m, mean ± SD). Of the 11 total dBRUV deployments, three individual H. griseus (2 females, 1 male) were observed independently on three deployments (27% of deployments). The size of the sharks was estimated to be between 3.0 and 4.0 m in total length (Figure 1), using the dBRUV as a reference as done in Gallagher et al. (2023). Hexanchus griseus were recorded for a total of 10.84 minutes, making a total of 30 unique passes at the camera, ranging between 1 – 26 passes per individual.
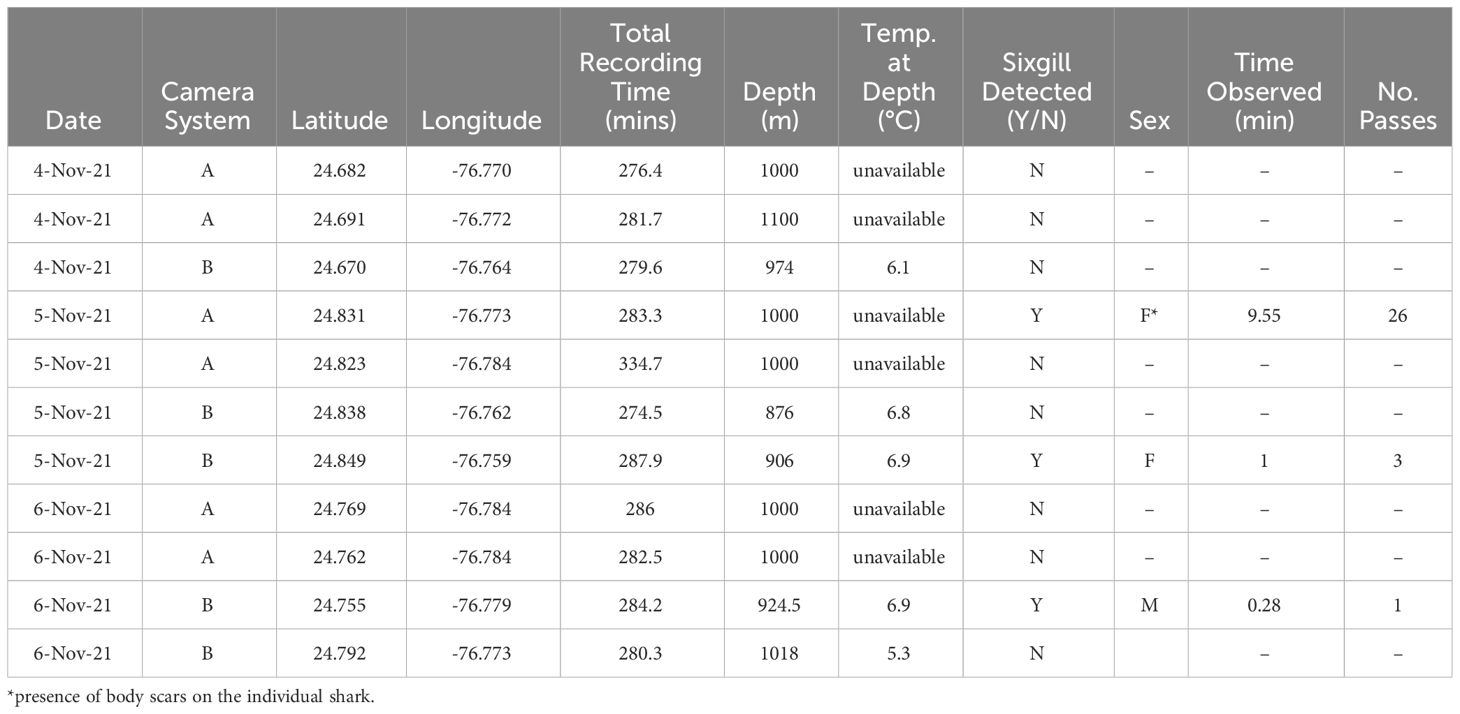
Table 1 Deep baited remote underwater video (dBRUV) deployments during the study period, with information on location, time of day, duration, shark occurrence, behavior, and notes.
Two female H. griseus were observed on 5th November 2021, one on each camera system. These two individuals appeared to be in different life history stages and exhibited divergent behaviors. Mating scars were observed prominently on one female shark (estimated to be ~3.5 m in total length). Scars were attributed to relatively recent (i.e., within months of observation), coercive mating (and/or attempts) based on previous criteria outlined by Ritter and Amin (2019). These included subcutaneous bite marks around the gills (Figure 1C) and pectoral and pelvic fin areas (Figures 1A, B, D), which are unlikely to be reflective of competitive and predator bouts (Ritter and Amin, 2019). The mating scars on this individual bore a large bite radius, bruising around the bite area, and clear indentations suggestive of H. griseus teeth (Corn et al., 2016, Figures 1C, D). This individual female shark was observed for the longest period of time (9.55 minutes), and made contact with the lander system 30 times, biting the apparatus and moving the entire unit with the drop weight >5 meters. Another individual female H. griseus, uniquely identified by pectoral fin markings, was observed for one minute, but did not make any contact with the dBRUV system. This individual was larger, and exhibited lateral bloating, bulging near its pelvic fins, and inability to turn when swimming (this individual instead used its fins to reposition itself). A sole male H. griseus was seen on 6th November 2021 in the same area (on camera system B, Figure 1E), although it was only seen once for less than one minute.
A total of 18 published studies were identified that used remote deep sea camera systems and reported various aspects of H. griseus biology. Most studies were undertaken in the Pacific Ocean and reported aspects of shark behavior (i.e., feeding behaviors, 38%) and demographic information (i.e., population trends, 38%). Notably, no published studies reported observations related to the observation of mating scars or notable reproductive behaviors (Supplementary Table S1).
Knowledge of the habitats associated with reproductive behaviors of sharks remains both a challenge and priority for their conservation, and this is especially the case for deep-sea sharks which remain challenging to access and survey. In the present study, we describe several novel, twilight zone observations of live bluntnose sixgill sharks - the largest predatory shark living in the deep-sea in global oceans. Unique to our study is the documentation of large individuals and the presence of teeth marks on at least one individual, suggestive of potential mating events.
While we believe the observed scarring is indeed a result of mating behavior, we cannot validate this claim, and there are several other potential explanations for scarring. For example, sixgill sharks are known to aggregate at depth to scavenge on carcasses, and competition for access to scavenging resources could result in agonistic bites between individuals. Furthermore, predatory behavior between sympatric sixgill sharks and other large species (i.e., tiger sharks, Galeocerdo cuvier) cannot be ruled out. However, group mating events have been suggested for the species, supported by observations of extreme polyandry (Larson et al., 2011). Indeed, a single female bluntnose sixgill shark contained 71 pups sired by nine fathers (Larson et al., 2011). This aspect of their biology provides support for the increased prevalence of mating scars when individuals of reproductive size overlap in space and time, as reported and corroborated in our study. If the observed scars were indeed related to mating, it is plausible that the mating attempts could have occurred in months prior to the observation. Evidence from white sharks (Carcharodon carcharias) observed at Guadalupe Island, Mexico, suggest that scars and body lacerations may be visible for up to several months (Domeier and Nasby-Lucas, 2007).
The proposed reproductive grounds of H. griseus were previously inferred from the physical capture of multiple life-history stages. For example, Ebert (2002) reported a potential pupping ground of H. griseus in South African waters owing to the capture of both mature adults and small pups, while King and Surry (2017) reported the capture of pregnant females in the strait of Georgia. Though evidence for geographically distinct maternal lineages supports the existence of regional reproductive grounds (Vella and Vella, 2017), key information on the habitat-use of individuals prior to, and during gestation are yet to be detailed. Our systematic review of the scientific literature highlighted that previous video-based research on H. griseus has been historically restricted to the northeast Pacific Ocean. Many of these studies have reported demographic information, such as temporal changes in population structure through time (e.g., Dunbrack and Zielinski, 2003; 2005 Dunbrack, 2008;). It was notable, however that there was an absence of studies reporting the location of habitats associated with potential reproductive events, presenting a key conservation challenge for H. griseus populations, and deep-sea sharks more broadly.
Considering the cold-water temperatures, slow growth, and likely reduced metabolism of a large-bodied deep sea chondrichthyan, we also cannot fully discount potential mating behaviors occurring away from the study location. However, the unique oceanographic properties of the Exuma Sound would certainly explain why this region may be of notable biological significance for H. griseus – for which multiple life-history stages have been observed (Brooks et al., 2015). Northern regions of the Sound are known to support high predator diversity (Brooks et al., 2015), which may be driven by high allochthonous neritic energetic inputs from adjacent coastal ecosystems. For example, Shipley et al. (2017a) predicted that neritic subsidies from extensive coastal habitats flanking the Exuma Sound may be modulated by strong onshore-offshore physical transport in addition to the movement and trophic interactions of deep-diving predators, such as Caribbean reef sharks (Carcharhinus perezi, Shipley et al., 2017b). Recent work also suggests that the bluntnose sixgill shark is likely an important vector of blue carbon (Shipley et al., 2023), which corroborates the notion that large sharks may form ecological associations with carbon sinks in the deep ocean (Dixon and Gallagher, 2023). These observations suggest this region could be an ecological hotspot that could support many important components of organismal life-histories, including that of H. griseus. Further observations of multiple females bearing similar scarring would further support this region as a potential mating ground for this species, although information on shark movement would be needed.
The conservation potential of deep-sea ecosystems is intrinsically tied to knowledge of key habitats supporting critical stages of organismal life-histories, such as reproduction. Our study provides new clues into the highly cryptic reproductive behavior of a large deep-sea shark, detailing the potential location of potential reproductive grounds in the elusive H. griseus. Such information is critical for implementing contemporary management frameworks, such as marine protected areas, to conserve biodiversity and functionality in the deep-sea. The use of innovative technologies to survey the deep-sea, as done and reported here, hold great promise for identifying future priority habitats for the conservation of deep-sea sharks.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AG: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing, Data curation, Supervision, Visualization. CDS: Investigation, Writing – review & editing. DD: Funding acquisition, Investigation, Resources, Writing – review & editing. SDH: Investigation, Resources, Writing – review & editing. BP: Resources, Supervision, Validation, Writing – review & editing. OS: Conceptualization, Methodology, Writing – review & editing. JS: Methodology, Validation, Writing – review & editing. CMD: Supervision, Validation, Writing – review & editing. JG: Investigation, Resources, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research performed in this article. Funding was provided from the National Geographic Society via an Explorer Grant to AG, as well as from a grant from the National Geographic Exploration Technology Lab to AG.
We thank our Bahamian partners and stakeholders who have enabled and supported this work: L. Gittens and the Dept. Marine Fisheries, as well as the staff from the Dept. Environment Protection and Planning. We thank crew of the R/V Tigress for field assistance, particularly S. Gray. We also thank the staff from the University of Rhode Island Lab and The National Geographic Society Exploration Technology Lab.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AG declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1305487/full#supplementary-material
Amor K. O. B., Amor M. M. B., Zaouali J., Capapé C. (2017). On the capture of a pregnant bluntnose sixgill shark Hexanchus griseus (Chondrichthyes: Hexanchidae) from the Gulf of Tunis (Central Mediterranean Sea). J. Black Sea / Mediterr. Environ. 23, 177–182.
Barnett A., Braccini J. M., Awruch C. A., Ebert D. A. (2012). An overview on the role of Hexanchiformes in marine ecosystems: biology, ecology and conservation status of a primitive order of modern sharks. J. Fish Biol. 80, 966–990. doi: 10.1111/j.1095-8649.2012.03242.x
Bolivar I. (1907). Indicacion de algunas peces notable de la Corufia. Boletin la Real Sociedad Espanola Hist. Natural 7, 206–209.
Braun C. D., Arostegui M. C., Thorrold S. R., Papastamatiou Y. P., Gaube P., Fontes J., et al. (2022). The functional and ecological significance of deep diving by large marine predators. Annu. Rev. Mar. Sci. 14, 129–159. doi: 10.1146/annurev-marine-032521-103517
Brooks E. J., Brooks A. M., Williams S., Jordan L. K., Abercrombie D., Chapman D. D., et al. (2015). First description of deep-water elasmobranch assemblages in the Exuma Sound, The Bahamas. Deep Sea Res. Part II: Topical Stud. Oceanogr. 115, 81–91. doi: 10.1016/j.dsr2.2015.01.015
Coffey D. M., Royer M. A., Meyer C. G., Holland K. N. (2020). Diel patterns in swimming behavior of a vertically migrating deepwater shark, the bluntnose sixgill (Hexanchus griseus). PloS One 15, e0228253. doi: 10.1371/journal.pone.0228253
Comfort C. M., Weng K. C. (2015). Vertical habitat and behaviour of the bluntnose sixgill shark in Hawaii. Deep Sea Res. Part II: Topical Stud. Oceanogr. 115, 116–126. doi: 10.1016/j.dsr2.2014.04.005
Corn K. A., Farina S. C., Brash J., Summers A. P. (2016). Modelling tooth–prey interactions in sharks: the importance of dynamic testing. R. Soc. Open Sci. 3, 160141. doi: 10.1098/rsos.160141
COSEWIC (2007). “Assessment and status report on the bluntnose sixgill shark Hexanchus griseus in Canada,” in Committee on the Status of Endangered Wildlife in Canada (Ottawa: Environment and Climate Change Canada (ECCC), Government of Canada).
Dixon O. F. L., Gallagher A. J. (2023). Blue carbon ecosystems and sharks: connected networks, species interactions, and future research needs. Front. Mar. Sci. 10, 1202972. doi: 10.3389/fmars.2023.1202972
Domeier M. L., Nasby-Lucas N. (2007). Annual re-sightings of photographically identified white sharks (Carcharodon carcharias) at an eastern Pacific aggregation site (Guadalupe Island, Mexico). Mar. Biol. 150, 977–984. doi: 10.1007/s00227-006-0380-7
Dunbrack R. (2008). Abundance trends for Hexanchus griseus, Bluntnose Sixgill Shark, and Hydrolagus colliei, Spotted Ratfish, counted at an automated underwater observation station in the Strait of Georgia, British Columbia. Can. field-naturalist 122, 124–128. doi: 10.22621/cfn.v122i2.570
Dunbrack R., Zielinski R. (2003). Seasonal and diurnal activity of sixgill sharks (Hexanchus griseus) on a shallow water reef in the Strait of Georgia, British Columbia. Can. J. Zool. 81, 1107–1111. doi: 10.1139/z03-087
Dunbrack R., Zielinski R. (2005). Body size distribution and frequency of anthropogenic injuries of bluntnose sixgill sharks, Hexanchus griseus, at Flora Islets, British Columbia. Can. Field-Naturalist 119, 537–540. doi: 10.22621/cfn.v119i4.184
Ebert D. A. (2002). Some observations on the reproductive biology of the sixgill shark Hexanchus griseus (Bonnaterre 1788) from South African waters. Afr. J. Mar. Sci. 24, 359–363. doi: 10.2989/025776102784528439
Ebert D. A., Compagno L. J. V. (2012). “FAO species catalogue for fishery purposes,” in Sharks of the World. An Annotated and Illustrated Catalogue of Shark Species Known to Date, vol. 1. (FAO, Rome).
Finucci B., Barnett A., Bineesh K. K., Cheok J., Cotton C. F., Dharmadi, et al. (2020). Hexanchus griseus. IUCN Red List Threatened Species 2020, e.T10030A495630. doi: 10.2305/IUCN.UK.2020-3.RLTS.T10030A495630.en
Gallagher A. J., Shipley O. N., De Silva C., Kohler J. K., Fernandes T. F., Austin T., et al. (2023). First records of the blurred lantern shark Etmopterus bigelowi from the Cayman Islands, Western Atlantic. Front. Mar. Sci. 10, 1165207. doi: 10.3389/fmars.2023.1165207
Giddens J., Turchik A., Goodell W., Rodriguez M., Delaney D. (2021). The national geographic society deep-sea camera system: A low-cost remote video survey instrument to advance biodiversity observation in the deep ocean. Front. Mar. Sci. 1157. doi: 10.3389/fmars.2020.601411
Howell K. L., Hilário A., Allcock A. L., Bailey D. M., Baker M., Clark M. R., et al. (2020). A blueprint for an inclusive, global deep-sea ocean decade field program. Front. Mar. Sci. 999. doi: 10.3389/fmars.2020.584861
Irigoien X., Klevjer T. A., Røstad A., Martinez U., Boyra G., Acuña J. L., et al. (2014). Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Commun. 5, 1–10. doi: 10.1038/ncomms4271
King J. R., Surry A. M. (2017). Seasonal and daily movements of the bluntnose sixgill shark (Hexanchus griseus) in the strait of Georgia from satellite tag data. Environ. Biol. Fishes 100, 1543–1559. doi: 10.1007/s10641-017-0664-4
Larson S., Christiansen J., Griffing D., Ashe J., Lowry D., Andrews K. (2011). Relatedness and polyandry of sixgill sharks, Hexanchus griseus, in an urban estuary. Conserv. Genet. 12, 679–690. doi: 10.1007/s10592-010-0174-9
Phillips B. T., Shipley O. N., Halvorson J., Sternlicht J. K., Gallagher A. J. (2019). First in situ observations of the sharpnose sevengill shark (Heptranchias perloff), from the Tongue of the Ocean, Bahamas. J. Ocean Sci. Foundation 32, 17–22. doi: 10.5281/zenodo.2539708
Reum J. C., Williams G. D., Harvey C. J., Andrews K. S., Levin P. S. (2020). Trophic ecology of a large-bodied marine predator, bluntnose sixgill shark Hexanchus griseus, inferred using stable isotope analysis. Environ. Biol. Fishes 103, 147–162. doi: 10.1007/s10641-019-00941-z
Ritter E. K., Amin R. W. (2019). Mating scars among sharks: evidence of coercive mating? Acta Ethologica 22, 9–16. doi: 10.1007/s10211-018-0301-z
Shea B. D., Benson C. W., de Silva C., Donovan D., Romeiro J., Bond M. E., et al. (2020). Effects of exposure to large sharks on the abundance and behavior of mobile prey fishes along a temperate coastal gradient. PloS One 15, e0230308. doi: 10.1371/journal.pone.0230308
Shipley O. N., Howey L. A., Tolentino E. R., Jordan L. K., Ruppert J. L., Brooks E. J. (2017b). Horizontal and vertical movements of Caribbean reef sharks (Carcharhinus perezi): conservation implications of limited migration in a marine sanctuary. R. Soc. Open Sci. 4, 160611. doi: 10.1098/rsos.160611
Shipley O. N., Matich P., Hussey N. E., Brooks A. M., Chapman D., Frisk M. G., et al. (2023). Energetic connectivity of diverse elasmobranch populations–implications for ecological resilience. Proc. R. Soc B 290, 20230262. doi: 10.1098/rspb.2023.0262
Shipley O. N., Polunin N. V., Newman S. P., Sweeting C. J., Barker S., Witt M. J., et al. (2017a). Stable isotopes reveal food web dynamics of a data-poor deep-sea island slope community. Food Webs 10, 22–25. doi: 10.1016/j.fooweb.2017.02.004
Keywords: chondrichthyes, baited remote underwater video, deep-ocean, reproduction, lander, shark, twilight zone
Citation: Gallagher AJ, de Silva C, Delaney D, Harris SD, Phillips BT, Shipley ON, Sulikowski JA, Duarte CM and Giddens J (2024) Novel behavioral observations and body scarring for the bluntnose sixgill shark (Hexanchus griseus) offer clues to reproductive patterns and potential mating events. Front. Mar. Sci. 11:1305487. doi: 10.3389/fmars.2024.1305487
Received: 01 October 2023; Accepted: 21 March 2024;
Published: 15 April 2024.
Edited by:
André Sucena Afonso, Center for Marine and Environmental Sciences (MARE), PortugalReviewed by:
Gonzalo Mucientes Sandoval, Spanish National Research Council (CSIC), SpainCopyright © 2024 Gallagher, de Silva, Delaney, Harris, Phillips, Shipley, Sulikowski, Duarte and Giddens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Austin J. Gallagher, YXVzdGluQGJlbmVhdGh0aGV3YXZlcy5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.