
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 12 February 2024
Sec. Marine Pollution
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1270026
The essential target of academics and the industrial sector is the innovation of an industrial ecology approach. Worldwide, cigarette butts (CBs) comprise the most predominant form of litter that spreads into the ecosystem and inland. In the meantime, oil is spilled into marine life from various activities and transportation. The result is a complex oil–water composition in a high concentration that causes severe hazards to the environment and to aquatic life. In this regard, the current investigation focuses on obtaining hydrophobic cellulose acetate from CBs for use as a filter media. The filter is applied in marine oil spill separation as a win–win industrial ecology technique. Initially, the separated CB residuals were prepared by successive washing. Subsequently, the obtained cellulose acetate fibers were characterized by Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM). Furthermore, the new CB-based filters were used in the separation/adsorption of marine oil wastewater spill. Subsequently, a group of experiments were conducted. The results showed that the cleanup efficiency could be up to 99% in terms of chemical oxygen demand (COD). Moreover, the products were recovered by washing with hot water for further use, which showed high removal activity that reached 95% after successive uses. In comparison to commercial oil sorption materials, the results were promising as the RP-C18 cartridge revealed a maximum oil removal of 90%. Such preliminary data on a cellulose acetate filter scheme are a good indicator in the development of an oil spill facility, being a suitable candidate for controlling oil wastewater effluent streams.
Modern society is suffering from a severe global challenge causing environmental water deterioration due to modern aquaculture activities (Thabet et al., 2022a). Crude oil and its derivatives could travel thousands of miles and over dozens of hands prior to their final use (Tehrim et al., 2021; Thabet et al., 2022b). Under industrial assessments, over 80% of this oil is shipped to transfer the crude oil for use; the result is an accidental oil spill into the aquatic environment. Oil is spilled onto water bodies through human activities such as leaking marine engines (Lessard and Demarco, 2000; Hussein et al., 2008). It is not only man-made human activities that cause the seeping of the oil spill into the environment, natural disasters also cause oil leaks into aquatic sources. Oil spilled into marine life causes severe damage and distress to the ecosystem. In addition, numerous activities, including household, recreational, and industrial applications, result in a growing demand for storage and oil transportation, increasing the chances of accidental offshore oil spills into water bodies (Li et al., 2006; Thabet et al., 2022c). Thus, oil spills threaten both the aquatic environment and human health. In comparison to the various water contamination sources, oil spill contamination is signified by its quick spreading on the surface of the water and covering large areas, forming air insulation that threatens aquatic life. Thus, oil spills should be cleaned up promptly (Lessard and Demarco, 2000; Zhang et al., 2020).
With this concept, research studies have emerged focusing on investigating appropriate oil–water separation technologies for both merits of saving the aquatic environment (Xu et al., 2019) and recovering the spilled oil for further use (Doyan et al., 2021). Conventionally, various oil–water separation techniques have been introduced, including physical separation such as gravity sedimentation, centrifugation, and electrolytic separation, as well as chemical/physical separation strategies such as adsorption processes or biodegradation techniques (Zhang et al., 2021; Zhang et al., 2021). Such techniques are based on the characteristics of oil–water, such as the oil phase and water phase densities and conductivities. However, these methodologies are expensive and not economically reliable, as well as inefficient since they do not prevent oil diffusion (Li et al., 2020). Moreover, all of the introduced techniques have been shown to be incapable and less effective in removing smaller oil droplets and oil emulsions (Abu-Danso et al., 2019).
Conventionally, numerous approaches have been applied for oil spill management (Al Salehin et al., 2019; Zhang et al., 2020). Such explorations include natural, chemical, biochemical, and mechanical techniques (Zhao et al., 2010; Moerman and Potts, 2011; Novotny et al., 2011; Soltani et al., 2014; Kazeem et al., 2018). However, some treatments are still not efficient due to biochemical processes requiring a long time, in addition to the requirement of specific temperatures to occur and the presence of organics and oxygen (Lee et al., 2014; Zang et al., 2015; Li et al., 2016; Ifelebuegu et al., 2018). Among other alternative techniques, oil/water separation through an oil absorbent technique has been shown to be an effective approach (Li et al., 2006; Nerendra and Yiqi, 2006). This is due to its easy operation and efficient removal (Lin and Liu, 2000). However, for low operational costs, the selection of an absorbent material is still a topic of research (Williams and Nur, 2018). To date, the utilized oil-absorbent materials are categorized into natural and synthetic products (Choi and Cloud, 1992; Wardley-Smith, 1993), which may be organic or inorganic, such as wooden materials, polyacrylate, polystyrene, polypropylene, and zeolite (El-Sayed and El-Samni, 2006; Tayeb et al., 2019). However, these traditional materials have shortcomings, including a low absorption capacity with minimum oil saving rates in addition to their high costs (Zang et al., 2015). Thus, a low-cost material with good absorbent capacity is recommended to accommodate an economical approach.
Disposed of smoked cigarettes, so-called cigarette butts (CBs), constitute the biggest solid waste causing significant environmental contamination and are the most collected form of litter in beach areas (Ou et al., 2016; Doyan et al., 2021). Smoked CBs comprise more than 4,000 individual chemicals that are toxic or that may be carcinogenic (Zhao et al., 2010). These chemicals are leached into the environment from the smoked filter butt and smoked tobacco (Zhang et al., 2021). These contaminants include heavy metals such as aluminum, iron, copper, lead, zinc, and chromium, as well as ethyl phenol and pesticide residues (Zhao et al., 2010; Moerman and Potts, 2011; Novotny et al., 2011). In recent years, researchers have paid more attention to the environment-friendly recycling of toxic non-biodegradable solid wastes into value-added materials (Li et al., 2020). Based on this concept, recycling the waste residuals of CBs allows the safe disposal of toxic chemicals while minimizing the disposal costs in order to diminish their hazardous effects on nature (Moerman and Potts, 2011). Thus, CBs have gained significant value as a hydrophobic functionalized waste to counter their deleterious environmental impact (Ou et al., 2016). A few published studies have focused on the hydrophobic functionalization nature of using CBs to eliminate pollutants through an adsorption technique (Ifelebuegu et al., 2018; Kazeem et al., 2018). However, the use of CB waste for the adsorptive/filtration treatment of oil/water separation has been rarely investigated.
Worldwide, the recent common trend is to valorize solid wastes into value-added materials. Based on this, and to manage the environmental impacts of CBs, they are valorized through turning the residuals into value-added materials. For instance, a previous study showed residual CBs being applied as an anti-corrosive material and used as an inhibitor in corrosive media (Zhao et al., 2010). In addition, a different study converted CBs into a carbon-based material, which showed promise as a suitable candidate for meeting energy demands (Lee et al., 2014). Furthermore, a study (Lessard and Demarco, 2000) applied this waste as an oil spill skimmer from water (Lessard and Demarco, 2000).
Egypt is well endowed with great marine life and is one of the most sensitive environmental habitats and tourist attractions. Hurghada City is a tourist destination on the Red Sea coast, particularly the Ras Ghareb shore that is bordered on the north by the Suez Governorate and Egypt at the Red Sea coast. However, oil spills due to human activities are considered an issue in the area. Hence, cleaning up oil spills in this marine environment is considered a special concern. The present investigation deals with the treatment of the oil spill in the marine area. The study is based on a win–win technology, using CB waste residuals and transforming them into a filter media for oil-contaminated water removal. The material is initially subjected to chemical treatment and then introduced as a hydrophobic filter for the separation of oil from water. System performance is investigated and the operational parameters are studied. Subsequently, the experimental data are compared with those of the available techniques in the literature.
Initially, the location for the collection of samples was chosen based on the oil spill in the area. Hence, 150 km to the north of Hurghada shore on the Red Sea coast at Ras Ghareb, which is bordered on the north by the Suez Governorate in Egypt, was chosen as the sample collection location. CBs were collected and thereafter taken to the laboratory for preparation. The CB filters used in the current study varied in their dimensions and the squashing degree and humidity. The collected butts generally ranged in length from 12 to 26 mm, while the diameter commonly ranged from 6 to 8 mm. However, to attain a structure like the commercial acoustic products, the collected butts were treated prior to use. Firstly, after removal of the remaining tobacco from the butts, the CBs were subjected to water swelling in order to remove the warped papers around them. Subsequently, the washed collected material was squeezed and exposed to successive (five times) soaking in sulfuric acid (0.02%, w/v) according to a process described elsewhere (Soltani et al., 2014). Afterward, it was also successively (four times) subjected to NaOH aqueous solution (5%, w/v), followed by successive (six times) washing with ethanol prior to final rinsing with water through stirring for 1 h in each cycle. Finally, to obtain the filter cartridge, the cleaned filters were dried in an electric oven (80°C for 48 h) to prepare them for use. However, after the filter was subjected to treatment, it was regenerated for cyclic use by cleaning with hot water (100°C). The preparation steps for converting the used filter butts into a filter cartridge are displayed in Figure 1. This study introduces a win–win technology through the conversion of cigarette filter waste into a value-added material for the elimination of oil from oil spill wastewater.

Figure 1 Preparation steps for cigarette butt filter sheet cartridge. (A) Unwrapped filter butts. (B) Filter butts after acid and alkali washing. (C) Cleaned filter butts after ethanol and water washing. (D) Warped sheet in the filter cartridge.
The study area selected was Hurghada City, with longitude and latitude of 33°48′ E and 27°15′ N, respectively. The seawater temperature in this location is approximately 20°C. The oil spilled onto the location due to the marine life. The oil discharged from ships and the collected samples were medium oil mixed with water. The samples were collected in the period from February to July 2023. Oily water was collected near the seashore, and the samples were then taken to the laboratory for analysis and use. The collected oil/water samples were retained at 4°C until use following standard methods (APHA, 2005).
The collected oil/water effluent was poured into a tank while stirring continuously. Afterward, the solution was pumped into the prepared filter media for treatment. The solution in the tank consisted of a mixture of oil and water with an oil content of 5,000 mg/L. The solution after oil separation was collected in another tank and the reclaimed water was taken to the laboratory and subjected to analysis. A Hach spectrophotometer (model DR2000) was used for total suspended solids (TSS) and chemical oxygen demand (COD). Furthermore, the oil concentration was determined according to a standard technique following the oil-in-water methodology of the DR2000 spectrophotometer that was properly calibrated by adequate manufacturing standards. Initially, the wavelength was adjusted to 450 nm, and each sample was dissolved in 1,1,1-titrachlomethane, which was used as the reagent. The oil content after treatment was then determined and compared with the initial oil content in the sample. Thereby, the percentage of oil removal after treatment was calculated according to Equation 1:
where Ct is the oil concentration at time t and C0 is the initial oil concentration. A digital Adwa pH meter (model AD1030) was used for pH measurement. Furthermore, for comparison, commercial castor oil was used as a model pollutant for synthetic wastewater preparation. This oil is a pale yellowish color. Castor oil was dispersed in water via mechanical stirring prior to treatment. The schematic representation of the experimental setup is shown in Figure 2.
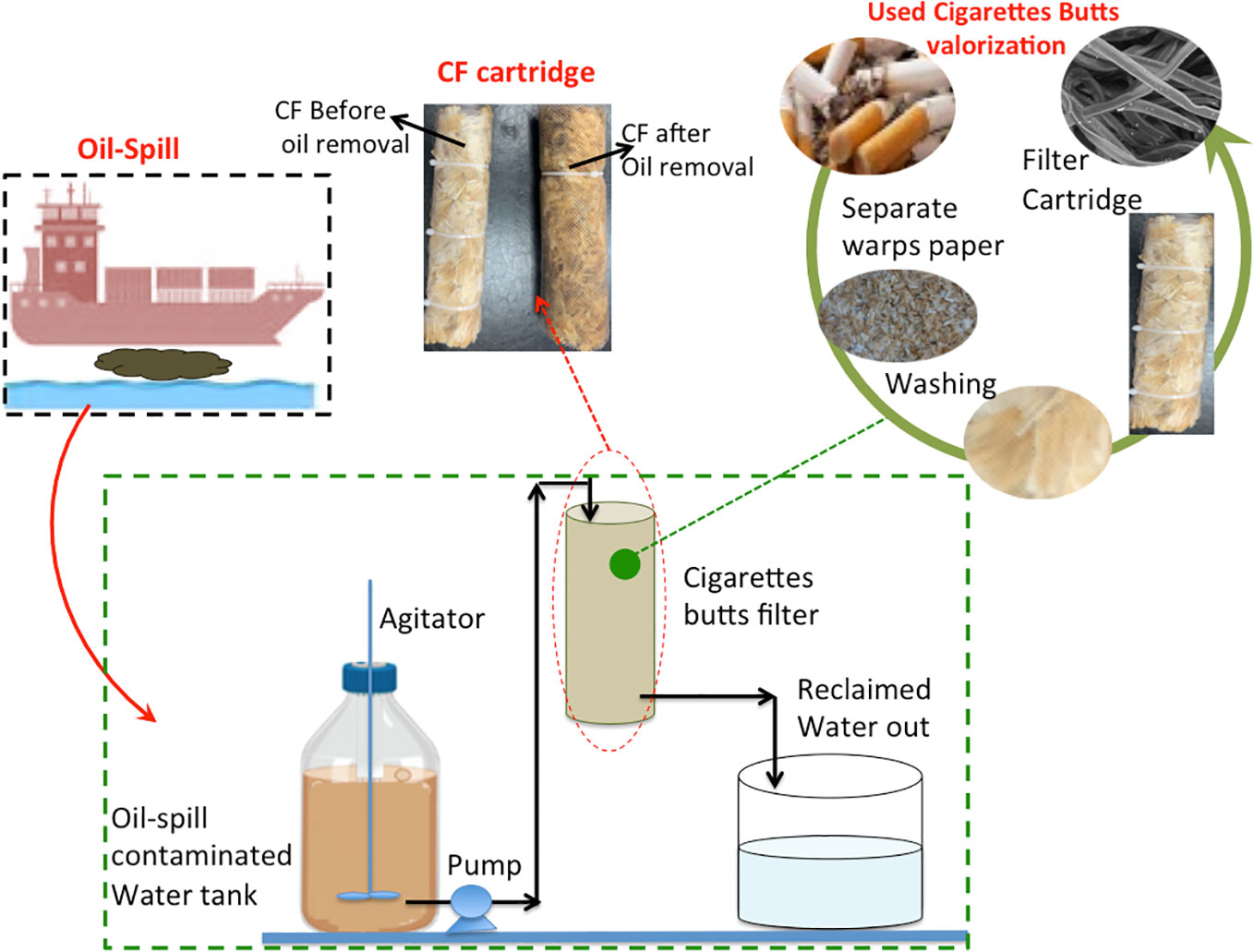
Figure 2 Graphical illustration of the oil spill wastewater treatment using cellulose acetate filter from cigarette butts.
Fourier transform infrared spectroscopy (FTIR) (model Jasco, FT/IR-4100, type A) was performed to investigate the functional groups responsible for oil sorption. Furthermore, scanning electron microscopy (SEM) (Quanta FEG 250) was used to assess the microstructure morphology of the prepared CBs and the loaded CBs with oil after filtration, imaged using a field-emission scanning electron microscope. This instrument was supplemented with energy-dispersive X-ray (EDX) spectroscopy. EDX analysis was conducted to investigate the principal elemental contents in the CBs, which were obtained by energy-dispersive spectra.
FTIR transmittance spectrum analysis is useful for identifying the elemental composition of the CBs. The FTIR analysis comparing the clean and unused CBs and the used, but washed and cleaned, CBs is displayed in Figures 3A, B, respectively). Figure 3B shows the removal of nicotine contaminants in the waste CBs. For this process, acid, alkali, ethanol, and water were used as the cleaning agents. Thus, cleaning the CBs with water and treatment with acid, alkali, and ethanol enhanced the surface modification, which reflected the presence of the available COOH groups, which also enhanced the adsorption uptake.
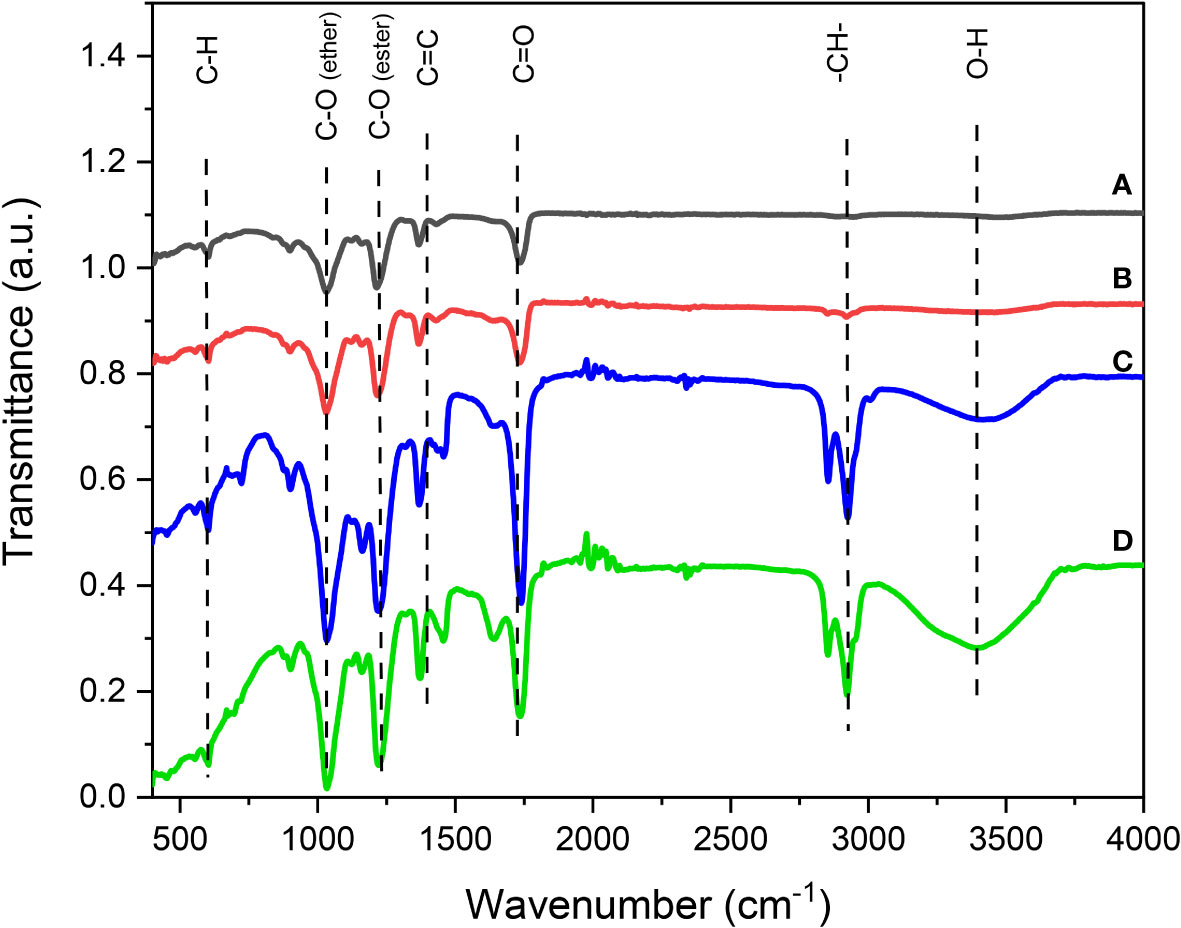
Figure 3 Fourier transform infrared (FTIR) spectra of the cigarette butts: clean cigarette butts (A) washed cigarette butts (B) cigarette butts saturated with castor oil (C) and cigarette butts saturated with spilled oil (D).
It is worth mentioning that, although the presence of pores and the increase in surface area also improve the adsorption capacity for more binding sites and enhance the adsorption process, hydrophobicity is the main driving force of adsorption (Poorsargol et al., 2020). However, commonly, the COOH groups have been found to have an effective adsorption capacity; adsorption is thus significant even in the hydrophilic surface. The COOH groups in the sample improved the hydrophobicity of the cellulose acetate fiber substance, thus promoting the interaction of oil in water with the substance. Water is readily adsorbed onto the hydrophilic surface by forming hydrogen bonds with the COOH groups of the substrate. Furthermore, hydrogen bonding with the pre-adsorbed water molecules provides the water uptake mechanism. The surface coverage of the adsorbed water film grows continuously beyond a monolayer formation. The hydrogen bonding and the electrostatic interaction of the oil with the COOH groups on the cellulose acetate fiber could be the force driving oil adsorption on the cellulose acetate fiber (Nam et al., 2014; Poorsargol et al., 2020; Zhao et al., 2023). Thus, a higher COOH content could be the reason for the better performance of the cellulose acetate fiber in removing oil from water.
The spectra of the unused CBs and the cleaned recycled CBs contained the same functional groups. However, as shown in Figures 3C, D, the CBs were loaded with different types of oil removed from water, i.e., castor oil and spilled oil in seawater. In these figures, new groups were represented, or what could be more intensive peaks that were not shown in the curves in Figures 3A, B displaying unsaturated fiber with oil. These groups include the spectrum at 3,493 cm−1 demonstrating the increase of the hydroxyls (OH) in the CBs saturated with oil spill rather than those loaded with castor oil. However, this OH group did not appear in the unsaturated CB with oil (Figures 3A, B). Moreover, at around 2,878 cm−1, the peak of the aliphatic C–H stretching appeared in the saturated oil CB samples that were disappearing in the unused filters. However, the characteristic peaks at 778, 1,030, 1,104, and 1,721 cm−1 appeared in the four cellulose acetate fibers that were saturated or unsaturated with oil. Peak 1,104 cm−1 presented the carbonyl group (C–O). Moreover, 1,030 cm−1 was attributed to the ether group (O=C), at around 1,721 cm−1, the peak of the aliphatic C–H stretching. The band of the 778-cm−1 spectrum attributable to the out-of-plane bending of CH was also present in the spectra of all the samples (Nascimento et al., 2016; Poorsargol et al., 2020).
The morphology of the used CBs as the starting material was investigated prior to removal of the residual oil from wastewater contaminated with oil spill. The results are displayed in Figure 4. Firstly, the clean CBs were evaluated for comparison. The data displayed in Figures 4A–D show the carbon precursor consisting of numerous bundles of Y-shaped head cellulosic fibers in a completely random fashion.

Figure 4 Scanning electron microscopy (SEM) images of the cigarette butts: (A) Clean cigarette butts; (B) Washed cigarette butts; (C) Cigarette butts saturated with castor oil; (D) Cigarette butts saturated with oil spill.
The SEM morphology images of the clean cigarette filter that was unused or cleaned and recycled are shown in Figures 4A, B, respectively. Unattached fibers with the oil molecules are obvious from the images. It was found that the volume of the constituent specimen fibers of the cellulose acetate increased when saturated with oil (Figures 4C, D). Notably, after oil separation, the oil was loaded into the fibers, which were firmly attached to the surface of the cellulose acetate fibers of CBs. However, it is worth mentioning that the type of oil separated by cellulose acetate fibers affects the shape and volume of the fiber. This might be associated with the natural adhesive property of such oils on cellulose acetate fibers, as shown in Figure 4C. The volume of the fibers after castor oil removal was higher than that of the oil spill separated oil, as displayed in Figures 4C, D, respectively. Furthermore, it was verified from the SEM images that the fibrous structure of the CBs remained undisturbed after oil sorption.
The surface elements of the cellulose acetate fibers were investigated to determine the percentages of the elements on the hydrophobic surface, which reflects the adsorption tendency of the surface. To investigate the regional elemental composition of the CB material, EDX analysis was conducted. Table 1 presents the EDX spectra of the used cigarette filter butts and the compositional analysis of the used cigarette filters. The residual smoke constituents of the smoked cigarette material were trapped throughout the filter after smoking, as well as the cellulosic material. Notably, carbon is the main chemical constituent used in cigarette filter butts, which represents 58% of the total raw material. Furthermore, it is worth mentioning that the metals detected in cigarette filters might be from the additives usually added to the tobacco in cigarettes. For instance, potassium is an auxiliary material added to tobacco as potassium sorbate. Moreover, titanium in tobacco in the form of titanium dioxide is another tobacco additive (Soltani et al., 2014).
It is also worth mentioning that the presence of carbon, titanium, and aluminum might improve the adsorption tendency since these elements have previously shown potential in adsorption performance. This is associated with the electrostatic attraction and the π–π electron interaction between the adsorbent and the adsorbate. Hence, the presence of multiple elements in such materials induces interactions between the CB adsorbent and the dye molecules (Kazeem et al., 2018).
In order to determine the sorption time for maximum oil uptake, it is essential to investigate the optimal oil uptake time. In this regard, oil removal was studied as a function of contact time. The results are presented in Figure 5. The results revealed that oil separation increased with the increase in contact time between the filter medium and the oil molecules. It is also observed from the data in Figure 5 that the time increase resulted in better oil removal efficiency. It is indicated that the oil removed reached 99% when the contact time reached 5 min. However, it was only 88% when the contact time was 1 min. Interestingly, the pore structure of the filter butts helped with oil adhesion on the surface of the filter material (Tayeb et al., 2019; Cai et al., 2021).
The concentration of oil in the feed before and after the filtration tests and the other water characteristics were identified, and the results are shown in Table 2. As seen from the data in the table, the COD was reduced from 6,650 to 836 mg-COD/L. In addition, the oil content decreased from 137 to 8 mg/L. This verified the importance of the CB filter in the removal of oil. The work demonstrated high removal of oil molecules from oil spill wastewater, therefore adding value to waste CBs as an effective filter cartridge material in an attempt to produce an effective and economic filter for the removal of oil from oil spilled to seawater streams. These results are in accordance with the compared data in previous works by Zang et al. (2015), Rohrbach et al (Lee et al., 2014), and Li et al. (2016), who removed 98% of oil using various fiberglass cloth filters. It is worth mentioning that the current investigation showed the same high separation efficiency using discarded waste material in comparison to the available synthetic materials. Notably, an industrial ecology approach is suggested in the current study.
The prepared CB filter was further examined for use in the separation of different oil types. The data are presented in Figure 6. The studied oil types included castor oil as a synthetic wastewater model and oil spill as a real polluted wastewater sample. It is obvious from the results that the initial removal was 88% for castor oil compared to 88.4% for oil spill (Poorsargol et al., 2020). However, the final removal reached 99% and 99.8% for castor oil and oil spill, respectively. It is suggested that CBs possess a number of functional groups that might support the adsorption process. Furthermore, the spongy structure of CBs might have helped the absorption process to occur. According to the FTIR results displayed in Figure 3, the presence of the COOH groups in CBs not only provides a strong affinity to the CB substrate but also delivers more active sites for oil capture. Since the cellulose acetate in CBs hydrolyzed in the aqueous phase, the CBs were converted into cellulose and acetic acid. Thus, the OH group appeared in the sample. Therefore, the OH group was augmented with the C=O group and COOH was obtained, which helped improve the adsorption tendency. The COOH groups formed in the medium helped improve the hydrophobicity of the cellulose acetate fiber substance, thus promoting the interaction of oil in water with the substance. In addition, hydrogen bonding and the electrostatic interaction of oil with the COOH groups drive the adsorption of oil (Poorsargol et al., 2020).
It is evident from Figure 6 that the electrostatic attraction between the OH surface groups on the CB adsorbent and the cationic groups in oil played important roles in the oil adsorption mechanism. In general, for hydroxyl-rich adsorbent materials, the hydroxyl groups interact electrostatically with the cationic pollutants and, hence, undergo strong hydrogen bonding. However, it has been reported that peroxy groups could undergo non-covalent surface–π attraction interactions with organics containing aromatic groups, including oil. Therefore, the introduction of cellulose acetate enriched with the hydroxyl groups enhances the adsorption affinity of the material (Nam et al., 2014; Zhao et al., 2023). Thus, the hydrogen bonding and electrostatic attraction between the filter surface and oil molecules is responsible for oil removal. Moreover, it was notable that the type and the structure of the oil did not have a significant role in the oil removal efficiency on the cellulose acetate fibers. Thus, the results exhibited the superior performance of the CB filters regardless of the type of oil used for separation. This is not in accordance with the results of Ifelebuegu et al. (2018), who reported on various oil types including vegetable oil, diesel oil, and motor oil with modified cigarette filters with the highest removal efficiency of 75%. This might be attributed to the nature of the oil removed from wastewater playing a significant role in the removal efficiency.
The effect of the agitation speed of oily wastewater on the filtration efficiency of such oil was examined at 100–475 rpm at a sorption time of 1–5 min. In this regard, the data displayed in Figure 7 demonstrate that the increase in agitation speed enhanced the efficiency of oil removal from 80% in the first minute, reaching 93%. This verified that an increase in mixing speed enhanced the rate of mass transfer, leading to a lessening in surface film resistance, thus allowing residual oil to be easily spread on the surface of the CBs (Lin and Liu, 2000). Furthermore, it could be confirmed that the solution contaminated with oil became very turbulent and erratic in nature, resulting in the transfer of oil into the filter material.
This result is in accordance with that of a previous investigation by Williams and Nur (2018), who confirmed the spread of pollutant on the adsorbent material at low agitation and its accumulation on the filter bed at higher speed.
To further understand the filtration and oil sorption technique with the modified oil filter, the filter weight and thickness comparison data were assessed. This effect was investigated by taking three different weights at constant initial oil concentration and recording the separation time through the use of different filter doses. In Figure 8, it is clearly observed that the removal rate was enhanced with the increase in CB weight from 8.9, 3.25, and 18.43 g through the increase in the separation and adsorption time. This phenomenon was clear at the minimum time (1 min) of separation. In addition, by increasing the filter bed dose, the removal efficiency also increased, but the removal rate later almost reached a constant value with the increase in time. The filter media was saturated with the oil even at a higher weight, which might account for this (Tehrim et al., 2021). This might have been due to the fact that the porosity of the surface helps capture the oil where the pores increase the strength of the sorption mechanism through increasing the effective forces between the oil molecules on the surface of the CBs.
The enhancement and increment in removal efficacy were attributed to the increase in the available adsorption sites that are mainly responsible for oil separation. Hence, the optimum adsorbent dose was considered to be 8.9 g as, with the increase in time, an almost complete removal of approximately 99% was attained at 5 min of separation in all filter doses. The saturation of the sorbent pores with oil begins at a critical filter thickness. Beyond this thickness, the removal efficiency decreases (Li et al., 2022). Thus, this approach supports natural recovery and restricts artificial cleanup to industrial ecology levels using waste filter material in minimal doses.
The reusability of the filter medium from CBs was investigated for its suitability for multiple and successive uses. To perform the desorption experiments, hot water was used for filter washing in order to prepare the used filter saturated with oil for reuse. The sorption efficiency per cycle was referenced from the sorbent oil onto the filter bed. The reusability test results are displayed in Figure 9. After three sorption cycles of oil spill at 1, 2, 3, 4, and 5 min, the efficiency decreased from 100% to 98%. Although the results for fresh use were higher than those for multiple uses, the efficiency was still almost as high, especially at higher contact times. This might be due to the squeezing of the filter resulting in the deformation of the hollow lumen on the fiber, thereby decreasing the spaces available for oil uptake. Thus, the improved surface roughness delayed the oil from easily escaping the fiber after the third sorption cycle. These results were compared to those of raw CB filters with a smooth surface (Nascimento et al., 2016).
It is essential to investigate the significance of the current work commercially to further illustrate its merit. For comparison and to assess and validate the suggested cartridge using recycled cellulose acetate derived from CBs, a synthetic sportive material, i.e., RP-C18 delivered by OPEC (Oil Pollution Environmental Control Ltd.), was used as a filter cartridge media. The effect of the oil removal and sorption from the oil–water emulsion on the sorption treatment is shown in Figure 10. The data in the figure illustrated that increasing the contact time increases the fraction of oil removed.
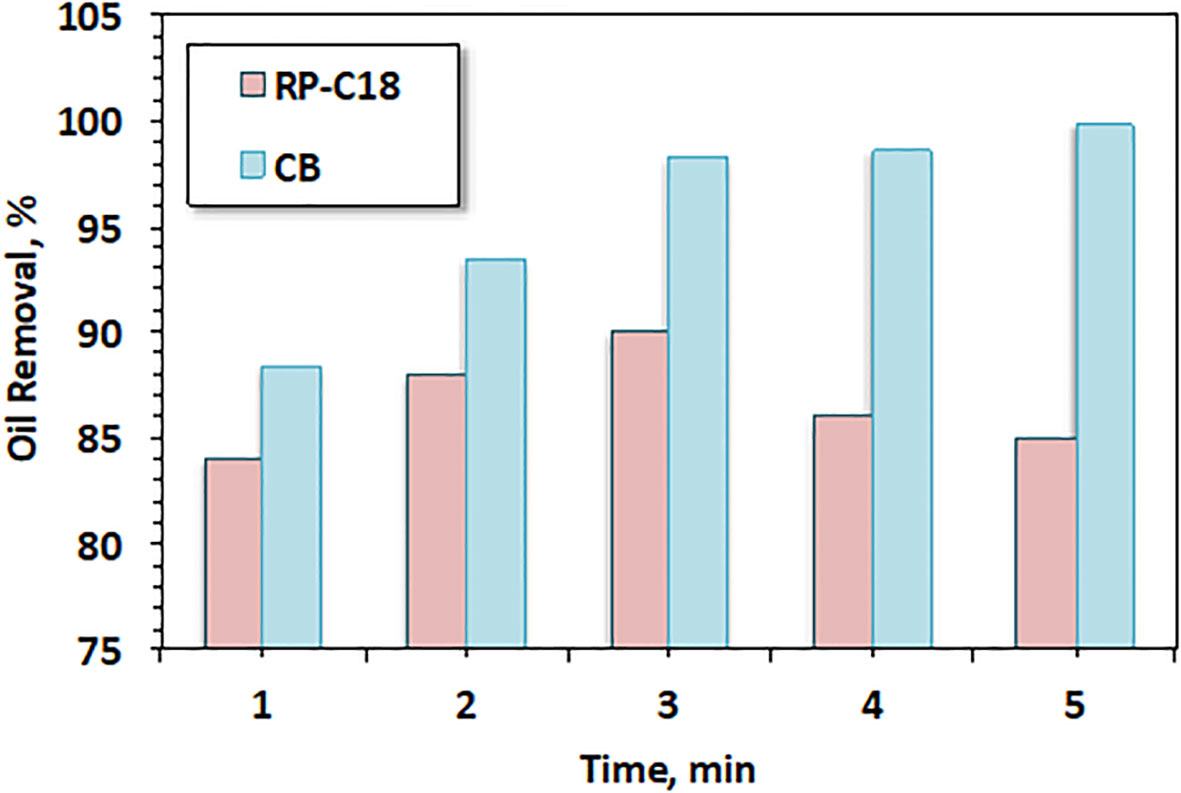
Figure 10 Effect of oil spill separation efficiency using RP-C18 as a function of filtration time compared with cigarette butts (CBs).
The percentage of oil removal ranged from 84% to 90%, with an increase in contact time from 1 to 3 min. This removal efficiency was attributed to the sorption capacity of the synthetic sorbent. This was most likely due to the fact that, at minimum contact time, oil removal occurred only on the outer surface of the synthetic fiber. However, by increasing the contact time, the sorbent material became more saturated with oil and, therefore, sorption took place through the interior bulk of the material. The cartridge material was more fibrous and fluffy than the outer surface. Subsequently, there was more oil sorbed with the time of contact (Tayeb et al., 2019). Furthermore, for comparison, according to the results in Figure 10, the cellulose acetate from the CB filter showed a pronounced effect compared to the commercial RP-C18 material. The removal efficiency of oil spill when the CB filter was used could reach 99%, but was 90% when the commercial adsorbent (RP-C18) was used. These results confirm the significance of the developed filter from waste material.
On the other hand, with the increase in contact time beyond 3 min, the tendency for oil sorption declined. This could be attributed to the saturation of RP-C18 occurring at a higher contact time with oil. Notwithstanding, the saturation of the sorbent material per unit area should be constant for a specified material, notably for the synthetic sorbent material, which declined with time. This phenomenon might be associated with the fact that the sorption behavior depends on the number of available sites on the surface of the sorbent material (Cai et al., 2021).
Comparison of the CB removal and separation efficiencies between the experimental data obtained from the current investigation and those from the literature data was conducted. Although it was difficult to make an accurate comparison, it provided a general explanation of the unique and different findings of the current investigation compared to data from the existing literature. In addition, it showed the significance of the current work. The proposed approach was the use of washed CBs as an industrial ecology filter in the separation of oil from water. The approach of the current study and those reported in the literature for the treatment of various effluents using CBs are listed in Table 3. Encouragingly, only the washed CBs showed high removal rates, which reached 98% and 99%, with minimum reaction time, i.e., 5 min, in comparison to the thermally and chemically treated CBs, which recorded from 30 min to 12 h of contact time. However, a lower reaction time was needed in the current study compared to other systems. Notably, the chemical and ultrasonic treatment might have reduced the contact time to 4 min. However, this treatment strategy increased the management and treatment costs.
An efficient superior treatment is described in the current study since the activation technique is economical. It should be mentioned that other systems deal with less adaptable pollutants, such as heavy metals, in comparison to the oil spill used in the current study. Moreover, these studies exhibited a number of disadvantages, including the long reaction time and the quantities of chemical agents; in contrast, the current activation system only needed minimal use of chemicals and water. Thus, the waste CBs in the current investigation, as well as in the cited literature, introduced a cradle-to-cradle technology for treatment in a cost-efficient manner. Moreover, it is worth mentioning that the proposed system is the most economical approach compared to those in the literature since its activation tendency is costless. Therefore, this is endorsing such technology for oil spill removal.
Regarding the oil separation techniques, numerous treatment methods could be used for various types of oil–water emulsion separation. Table 3 lists various examples of oil separation techniques. Table 4 presents a comparison of the results from the literature with those obtained in the present study. Several separation techniques are also displayed in Table 4, including biochemistry using microorganisms (Li and Bai, 2006), photocatalytic oxidation (Hussain et al., 2020), physical separation using filtration (Tayeb et al., 2019), and combined systems such as filtration/coagulation (Yue et al., 2018) and biochemical/adsorption (Liu et al., 2015). The oil separation mechanism was based on an oil demulsification and separation mechanism. With the exception of (Li and Bai, 2006) (Luna et al., 2015), and (Wei et al., 2018), the removal efficiencies for oily wastewaters reported in the literature varied from 68% to 99%, while that of the present study was 99%. This range of removal efficiencies is attributable to the variety of hydrocarbon compounds and their nature in wastewaters. However, some techniques showed poor removal efficiencies (Li and Bai, 2006; Luna et al., 2015; Wei et al., 2018). The current study was based on the use of hydrophobic waste based on waste valorization.
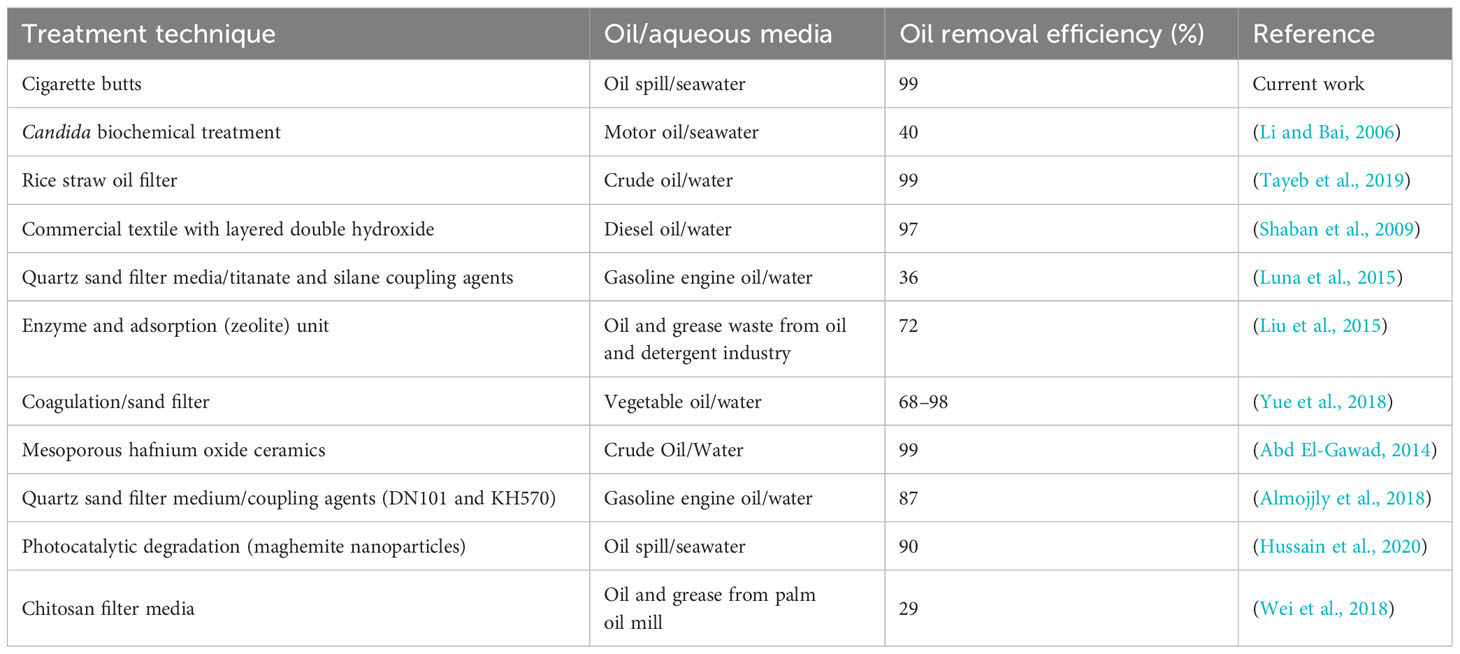
Table 4 Removal efficiency in oil separation using various oil separation systems for different oil types.
From an economic point of view, the cellulose acetate from CBs is considered a cost-efficient filter due to its high removal efficiency with its reuse option. Moreover, such a reuse technique helps in litter minimization. Thereby, cellulose acetate can be considered a viable hydrophobic alternative filter with almost complete oil separation. Furthermore, as abovementioned, although other separation systems such as biochemical treatment achieved high removal efficiency, the cost of biotreatment is considered high compared to the current costless material. Moreover, other treatments are combined systems, which increase the operational costs. Furthermore, the search for a solution to widen the application of oil/water separation is still an attractive research point. The current system could be more reliable when it is automated for marine site separation facilities.
While the hazardous environmental effects of discarded cigarette filters on the biosphere have been reported, their recycling potential has been less explored. In the current study, cellulose acetate derived from used cigarette filters was successfully extracted and used as a filter bed for oil spill separation. The experimental results of the study revealed that the cellulose acetate-based CB filter media could successfully remove almost 99% of the oil spilled. Moreover, castor oil was also found to show high removal efficiency. The required filtration time was only 5 min. The oil could be removed at room temperature and using its original pH value (7.26). Furthermore, the cleanup efficiency for a staged oil spill was above 95% for the third cyclic use of CBs. Based on these results, i.e., in the cleanup of different oil types and the high cleanup efficiency and their reusability, CBs might represent a promising approach both to lessen their adverse effects and to promptly clean up oil spills.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
HN: Funding acquisition, Project administration, Software, Supervision, Visualization, Writing – review & editing. MA: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. MT: Conceptualization, Data curation, Formal analysis, Investigation, Writing – review & editing. NA: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors extend their appreciation to Prince Sattam bin Abdulaziz University for funding this research work through project number PSAU/2023/01/2194222.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abd El-Gawad H. S. (2014) Oil and grease removal from industrial wastewater using new utility approach. Adv. Environ. Chem. 2014, 916878. doi: 10.1155/2014/916878
Abu-Danso E., Bagheri A., Bhatnagar A. (2019). Facile functionalization of cellulose from discarded cigarette butts for the removal of diclofenac from water. Carbohydr. Polymers 219, 46–55. doi: 10.1016/j.carbpol.2019.04.090
Almojjly A., Johnson D., Oatley-Radcliffe D., Hilal N. (2018). Removal of oil from oil-water emulsion by hybrid coagulation/sand filter as pre-treatment. J. Water Process Eng. 26, 17–27. doi: 10.1016/j.jwpe.2018.09.004
Al Salehin P. Z., Moeinpour F., Mohseni Shahri F. S. (2019). Adsorption isotherm and thermodynamic studies of As(III) removal from aqueous solutions using used cigarette filter ash. Appl. Water Sci. 9, 172. doi: 10.1007/s13201-019-1059-9
APHA (2005). Standard methods for the examination of water & Wastewater (Port City Press; Baltimore, MD, USA: American Water Works Association, Water Environment Federation).
Cai L., Ying D., Liang X., Zhu M., Lin X., Xu Q, et al. (2021). A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem. Eng. J. 410, 128404. doi: 10.1016/j.cej.2021.128404
Choi H., Cloud R. M. (1992). Natural sorbents in oil spill cleanup. Environ. Sci. Technol. 26, 772–776. doi: 10.1021/es00028a016
Doyan A., Leong C. L., Bilad M. R., Kurnia K. A., Susilawati S., Prayogi S., et al. (2021). Cigarette butt waste as material for phase inverted membrane fabrication used for oil/water emulsion separation. Polymers 13, 1907. doi: 10.3390/polym13121907
El-Sayed M. A., El-Samni T. M. (2006). Physical and Chemical Properties of Rice Straw Ash and Its Effect on the Cement Paste produced from different cement types materials and methods. J. King Saud University: Eng. Sci. 19 (1), 21–30. doi: 10.1016/S1018-3639(18)30845-6
Hussain F. A., Zamora J., Ferrer I. M., Kinyuac M., Velázqueza Jesús M. (2020). Adsorption of crude oil from crude oil-water emulsion by mesoporous hafnium oxide ceramics. Environ. Science: Water Res. Technol. 6, 2035–2042. doi: 10.1039/x0xx00000x
Hussein M., Amer A., Sawsan I. (2008). Oil spill sorption using carbonized pith bagasse: trial for practical application. Int. J. Environ. Sci. Technol. 5 (2), 233–242. doi: 10.1007/BF03326017
Ifelebuegu A. O., Lale E. E., Mbanaso F. U., Theophilus S. C. (2018). Facile fabrication of recyclable, superhydrophobic, and oleophilic sorbent fromWaste cigarette filters for the sequestration of oil pollutants from an aqueous environment. Processes 6, 140. doi: 10.3390/pr6090140
Kazeem T. S., Lateef S. A., Ganiyu S. A., Qamaruddin M., Tanimu A., Sulaiman K. O., et al. (2018). Aluminium-modified activated carbon as efficient adsorbent for cleaning of cationic dye in wastewater. J. Cleaner Production 205, 303–312. doi: 10.1016/j.jclepro.2018.09.114
Lee M., Kim G., Song H. D., Park S., Yi J. (2014). Preparation of energy storage material derived from a used cigarette filter for a supercapacitor electrode. Nanotechnology 25, 345–601. doi: 10.1088/0957-4484/25/34/345601
Lessard R. R., Demarco G. (2000). The signifcance of oil spill dispersants. Spill Sci. Technol. Bull. 6 (1), 59–68. doi: 10.1016/S1353-2561(99)00061-4
Li N., Bai R. (2006). Development of chitosanbased granular adsorbents for enhanced and selective adsorption performance in heavy metal removal. Water Sci. Technol. 54, 103–113. doi: 10.2166/wst.2006.736
Li L., Jia C., Zhu X., Zhang S. (2020). Utilization of cigarette butt waste as functional carbon precursor for supercapacitors and adsorbents. J. Cleaner Production 256, 120326. doi: 10.1016/j.jclepro.2020.120326
Li Z., Li J., Tan J., Jiang M., Fu S., Zhang T., et al. (2022). In situ synthesis of novel peroxo-functionalized Ti3C2Tx adsorbent for aqueous pollutants removal: Role of oxygen-containing terminal groups. Chemosphere 286 (2), 131801. doi: 10.1016/j.chemosphere.2021.131801
Li J., Yan L., Tang X. H., Feng H., Hu D. C., Zha F. (2016). Robust superhydrophobic fabric bag filled with PU sponges used for vacuum-assisted continuous and ultrafast absorption and collection of oils from water. Adv. Mater. Interfaces 3, 1500770. doi: 10.1002/admi.201500770
Li Y. S., Yan L., Xiang C. B., Hong L. J. (2006). Treatment of oily wastewater by organic–inorganic composite tubular ultrafiltration (UF) membranes. Desalination 196, 76–83. doi: 10.1016/j.desal.2005.11.021
Lin C. C., Liu H. S. (2000). Adsorption in a centrifugal field: Basic dye adsorption by activated carbon. Ind. Eng. Chem. Resour. 39, 161–167. doi: 10.1021/ie9902333
Liu X., Ge L., Li W., Wang X., Li F. (2015). Layered double hydroxide functionalized textile for effective oil/water separation and selective oil adsorption. ACS Appl. Mater. Interfaces 7 (1), 791–800. doi: 10.1021/am507238y
Luna J. M., Rufino R. D., Jara A. M. A. T., Brasileiro P. P. F., Sarubbo L. A. (2015). Environmental applications of the biosurfactant produced by Candida sphaerica cultivated in low-cost substrates. Colloids Surf. A Physicochem. Eng. Asp. 480, 413–418. doi: 10.1016/j.colsurfa.2014.12.014
Moerman J., Potts G. (2011). Analysis of metals leached from smoked cigarette litter. Tobacco Control 20, i30–i35. doi: 10.1136/tc.2010.040196
Nam S. W., Choi D. J., Kim S. K., Her N., Zoh K. D. (2014). Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard Mater. 270, 144–152. doi: 10.1016/j.jhazmat.2014.01.037
Nascimento T. A., Dutra Fl´aviaV. A., Pires B. C., Tarley CésarR. T., Manoa V., Borges K. B. (2016). Preparation and characterization of a composite based on polyaniline, polypyrrole and cigarette filters: adsorption studies and kinetics of phenylbutazone in aqueous media. RSC Adv. 6, 64450. doi: 10.1039/C6RA14071H
Nerendra R., Yiqi Y. (2006). Properties of high –quality long Natural Cellulose Fibers from Rice Straw. J. Agric. Food Chem. 54 (21), 8077–8081. doi: 10.1021/jf0617723
Novotny E. T., Hardin S. N., Hovda L. R., Novotny D. J., McLean M. K., Shan S. (2011). Tobacco and cigarette butt consumption in humans and animals. Tobacco J. 20 (1), i17–i20. doi: 10.1136/tc.2011.043489
Ou J., Wan B., Wang F., Xue M., Wu H., Li W. (2016). Superhydrophobic fibers from cigarette filters for oil spill cleanup. RSC Adv. 6, 44469. doi: 10.1039/C6RA01303A
Poorsargol M., Razmara Z., Amiri M. M. (2020). The role of hydroxyl and carboxyl functional groups in adsorption of copper by carbon nanotube and hybrid graphene–carbon nanotube: insights from molecular dynamic simulation. Adsorption 26, 397–405. doi: 10.1007/s10450-020-00214-7
Shaban A. M., Kamal M. M., Kenawy N., El Manakhly H. (2009). Role of interior structure of agro and non-agro materials for industrial wastewater treatment. J. Appl. Sci. Res. 5 (8), 978–985.
Soltani S. M., Yazdi S. K., Hosseini S. (2014). Effects of pyrolysis conditions on the porous structure construction of mesoporous charred carbon from used cigarette filters. Appl. Nanosci 4, 551–569. doi: 10.1007/s13204-013-0230-0
Tayeb A. M., Farouq R., Mohamed O., Tony M. A. (2019). Oil spill clean-up using combined sorbents: a comparative investigation and design aspects. Int. J. Environ. Analytical Chem. doi: 10.1080/03067319.2019.1636976
Tehrim A., Dai M., Wu X., Umair M. M., Ali I., Amjed M. A., et al. (2021). Citric acid modified waste cigarette filters for adsorptive removal of methylene blue dye from aqueous solution. J. Appl. Polym Sci., e50655. doi: 10.1002/app.50655
Thabet R. H., Fouad M. K., El Sherbiny S. A., Tony M. A. (2022a). Solar assisted green photocatalysis for deducing carbamate insecticide from agriculture stream into water reclaiming opportunity. Int. J. Environ. Analytical Chem. doi: 10.1080/03067319.2022.2027930
Thabet R. H., Fouad M. K., El Sherbiny S. A., Tony M. A. (2022b). Zero-waste approach: Assessment of aluminum-based waste as a photocatalyst for industrial wastewater treatment ecology. Int. J. Environ. Res. 16, 36. doi: 10.1007/s41742-022-00414-9
Thabet R. H., Fouad M. K., El Sherbiny S. A., Tony M. A. (2022c). A circular economy use of waste drinking water treatment plant sludge for magnetic photocatalyst composite production into wastewater treatment. Int. J. Environ. Analytical Chem. doi: 10.1080/03067319.2022.2097009
Wardley-Smith J. (1993). The control of oil pollution (Gaithersburg, MD: Graham and Trotman, Inc.), 39–145.
Wei B., Liu J., Wang G., Chang Q. (2018). Filtration of oil from oily wastewater via hydrophobic modified quartz sand filter medium. J. Water Reuse Desalination. doi: 10.2166/wrd.2018.052
Williams N. E., Nur P. A. (2018). KOH modified Thevetia Peruviana shell activated carbon for sorption of dimethoate from aqueous solution. J. Environ. Sci. Health, 2–15.
Xu E. G., Richardot W. H., Li S., Buruaem L., Wei H.-H., Dodder N. G., et al. (2019). Assessing toxicity and in vitro bioactivity of smoked cigarette leachate using cell-based assays and chemical analysis. Chem. Res. Toxicol. 32, 1670–1679. doi: 10.1021/acs.chemrestox.9b00201
Yue C., Liu J., Zhang H., Dai L., Wei B., Chang Q. (2018). Increasing the hydrophobicity of filter medium particles for oily water treatment using coupling agents. Heliyon 4, e00809. doi: 10.1016/j.heliyon.2018.e00809
Zang D. L., Liu F., Zhang M., Niu X. G., Gao Z. G., Wang C. Y. (2015). Superhydrophobic coating on fiberglass cloth for selective removal of oil from water. Chem. Eng. J. 262, 210–216. doi: 10.1016/j.cej.2014.09.082
Zhang Q., Cheng Y., Fang C., Shi J., Chen J., Han H. (2021). Novel and multifunctional adsorbent fabricated by Zeolitic imidazolate framworks-8 and waste cigarette filters for wastewater treatment: Effective adsorption and photocatalysis. J. Solid State Chem. 299. doi: 10.1016/j.jssc.2021.122190
Zhang J., Xu H., Guo J., Chen T., Liu H. (2020). Superhydrophobic polypyrrole-coated cigarette filters for E_ective oil/water separation. Appl. Sci. 10, 1985. doi: 10.3390/app10061985
Zhang X., Yu M., Li Y., Cheng F., Liu Y., Gao M., et al. (2021). Effectiveness of discarded cigarette butts derived carbonaceous adsorbent for heavy metals removal from water. Microchemical J. 168, 106474. doi: 10.1016/j.microc.2021.106474
Zhao J., Zhang N. S., Qu C. T., Wu X. M., Zhang J. T., Zhang X. (2010). Cigarette butts and their application in corrosion inhibition for N80 steel at 90C in a hydrochloric acid solution, ind. Eng. Chem. Res. 49, 3986–3991. doi: 10.1021/ie100168s
Keywords: oil spill clean-up, filtration, cellulose acetate, cigarette butt valorization, adsorption
Citation: Nabwey HA, Abdelkreem M, Tony MA and Al Hoseny NF (2024) Smart win–win waste management: superhydrophobic filter using valorized cellulose acetate from discarded cigarette butts for cleaning up marine oil spill at Hurghada Red Sea shore in Egypt. Front. Mar. Sci. 11:1270026. doi: 10.3389/fmars.2024.1270026
Received: 11 August 2023; Accepted: 18 January 2024;
Published: 12 February 2024.
Edited by:
Bing Chen, Memorial University of Newfoundland, CanadaReviewed by:
Zhiwen Zhu, Memorial University of Newfoundland, CanadaCopyright © 2024 Nabwey, Abdelkreem, Tony and Al Hoseny. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hossam A. Nabwey, ZW5nX2hvc3NhbTIxQHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.