
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 07 February 2024
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1267381
 Giulio Tarantino1,2*†
Giulio Tarantino1,2*† Gregorio Motta1†
Gregorio Motta1† Paolo D’Ambrosio3,4
Paolo D’Ambrosio3,4 Serena Felline5
Serena Felline5 Valerio Sbragaglia6
Valerio Sbragaglia6 Stanislao Bevilacqua7
Stanislao Bevilacqua7 Perla Tedesco8
Perla Tedesco8 Giuseppe Scordella9
Giuseppe Scordella9 Antonio Terlizzi1,4,7
Antonio Terlizzi1,4,7Small-scale fishing plays a major role in regional economies worldwide and, with a large number of small vessels involved, it provides employment and livelihood to coastal communities. Generally recognized as more selective than other fishing practices, small-scale fishery can nevertheless be subjected to high rates of discards of both non-target species and small-sized individuals, which in turn could lead to both decreased incomes for fishers and increased depletion of fish stocks. However, if the relationship between fish size and price has long been assessed, the effect of enhanced size-selectivity of fishing gears and consequent economic gains has been little investigated. This study, set in the Porto Cesareo Marine Protected Area (Italy, Ionian Sea), aimed at testing effective strategies to improve trammel net selectivity, reducing discards and maximizing the income for fishers. Different mesh sizes (20, 22 and 24 mm) trammel nets were employed. The study consisted in 72 fishing days from July 2012 to September 2013 and each day involved experimental fishing with the three mesh sizes. A total of 16008 specimens (103 species) were collected but the analysis focused on the 18 most common species in the area for a total of 12782 individuals. Mesh size trammel nets of 20 mm and 22 mm yielded most of the biomass, 324.8 and 321.5 kg respectively, while the 24 mm mesh yielded 280.7 kg. The 24 mm mesh, even if accounted for lower income compared to the 22 mm mesh (2383.9 € vs 2590.5 €, respectively), provided significant 50% reduction of discards compared to the 20 and 22 mm mesh. The use of 24 mm mesh size was found to be an effective strategy to reduce the number of discarded organisms and, consequently, the pressure exerted on local fish stocks with associated higher revenue for fishers. The results of this study demonstrated that trammel net selectivity can improve and support conservation measures and concurrently increase profitability of local fishery.
Small-scale fishery (SSF), or artisanal fishery is an ancient fishing practice widespread throughout the world, involving different techniques and gears and targeting a wide variety of fish, crustacean, and mollusk species. SSF accounts for 15% of total catches worldwide (FAO, 2022) and contributes significantly to ensure food and livelihoods to coastal communities (Kurien, 2015). However, though SSF are commonly considered as a low-impact activity if compared to large-scale, industrial fisheries, it can result in detrimental effects to both commercial and non-commercial species and vulnerable marine habitats (Exton et al., 2019; Gutierrez et al., 2023).
In this context, management actions commonly aim to protect the health of marine ecosystems and reduce the impact of fishing activities and, in regions where fishing is tightly controlled, stock abundance has been shown to increase and remain within desired levels (Hilborn et al., 2020). Generally, guidelines from policy makers focused on reducing fishing pressure to marine ecosystems establishing several measures such as landing size limits (Halliday and Pinhorn, 2002; Hall and Mainprize, 2005), improving gears selectivity (Kennelly and Broadhurst, 2002) and reducing spatio-temporal fishing efforts through the restriction of fishing activity in priority areas (e.g., nursery areas) and/or in sensitive (e.g., reproductive) periods (Dunn et al., 2011).
The use of appropriate fishing gears for selective catching of individuals of optimal size, to attain a legal exploitation and to optimize the economic value of fish stocks, is also crucial to implementing sustainable fisheries (Sathianandan, 2017). The size of fish is related to their growth, maturation, and reproductive capacity (Uusi-Heikkilä et al., 2015; Birkeland and Dayton, 2005), with larger and older individuals showing higher reproductive potential, due to their higher fecundity and greater investment in offspring with respect to smaller ones (Barneche et al., 2018). Furthermore, larger and older individuals of predator fish play a key role in the ecosystem by exerting a regulative control through the trophic webs (Frank et al., 2005; Ruttenberg et al., 2011), and their reduction can trigger cascading effects with unexplored consequences (Friedlander and DeMartini, 2002; Frank et al., 2005; Scheffer et al., 2005; Myers et al., 2007; Bianchi et al., 2021; Cavan and Hill, 2022). From a commercial perspective, larger specimens command higher prices than smaller ones (Gates, 1974), influencing economic dynamics and management strategies (Reddy et al., 2013; Zimmermann and Jorgensen, 2015). At the same time, market demand can influence catch size, affecting population abundance, fishers’ income and even gear choice. However, the potential effect of the use of more selective gears on fishers’ income has not been adequately studied so far.
In the Mediterranean and Black Sea, SSF accounts for over 82% (68 800 vessels) of the fishing fleet and employs around 59% of people working in the fishery sector (FAO, 2022). In these basins, SSF mainly uses passive gears such as trammel nets and gillnets (Stergiou et al., 2006; FAO, 2022). Trammel nets are fixed nets kept vertical in the water column by floats and sinkers used to intercept fish, crustaceans, and cephalopods as they swim. They usually range in length from 1,000 m to 6,000 m, depending on the size of the vessel and the available manpower. Trammel nets consist of three panels of net, an inner panel that can be made of nylon, twisted or monofilament, and two outer panels with larger meshes, usually made of twisted nylon monofilament. Since static gears are generally considered more selective and less harmful to stocks and habitats than other gear types, their selectivity has been less studied. Their fishing effort is strongly related to net length and height, while selectivity depends mainly on mesh size, material, vertical slack, visibility of the net in the water and suspension ratio (Lucchetti et al., 2020).
The aim of this study was to test good practices that can be implemented within a Mediterranean Marine Protected Area (MPA) to improve gear selectivity, reduce discards, and maximize the economic income of local fishers facilitating potential changes in their fishing habits (Reddy et al., 2013). MPAs, due to their more streamlined and rapid administration, are a useful testing ground for conservation measures which, if successful, can then be extended to other areas. Here, we (1) assessed the selectivity of trammel nets with different mesh sizes (i.e., 20 mm, 22 mm, 24 mm) through the analysis of species composition, abundance, size and biomass of catches and discards, and (2) quantified the ensuing differences in fishers’ incomes associated to the use of these different mesh sizes.
The study was conducted within the Porto Cesareo MPA (Apulia, northern Ionian Sea). Porto Cesareo is the third MPA in Italy in terms of surface, with a surface of 16,554 hectares. The MPA extends over 32 km of coastline, covering a bathymetric range of 5-40 m and including 12 different habitats classified according to the nomenclature of the Regional Activity Centre for Specially Protected Areas (RAC/SPA). The MPA is divided into three different zones (Figure 1) characterized by different protection regimes. Two fully protected areas (Zones ‘A’) defined as “no-take, no-access”, two areas of general protection (Zones ‘B’) where human activities are restricted and fishing is allowed only for authorized small scale fishers with specific gears, and a buffer area (Zone ‘C’) of partial protection where human activities are regulated, commercial and recreational fishing activities are allowed with gears authorized by the MPA managing body. Trawling, purse seine fishing and underwater fishing are not permitted within the MPA. For fixed nets, the minimum mesh size allowed during the sampling period was 20 mm. Fishing for octopus (Octopus spp.) weighing less than 200 g is prohibited. The managing body holds the right to regulate the way in which fish resources are harvested in the interest of environmental requirements.
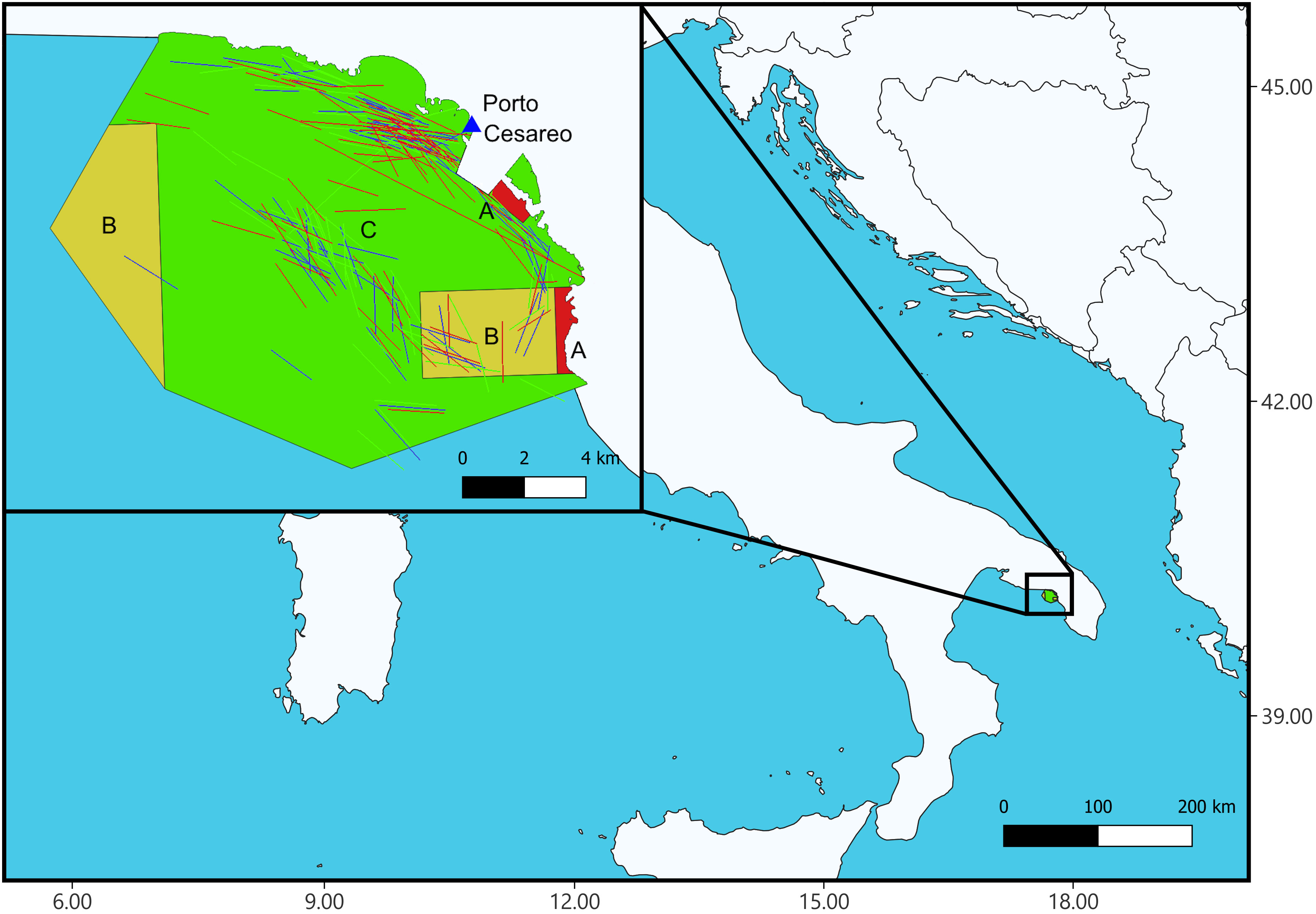
Figure 1 Map of the Porto Cesareo Marine Protected Area. In red the no-take, no-access areas (A zones), in yellow the areas of general protection (B zones), in green the partially protected area (C Zone). Colored tracks in the map represent samples (i.e., trammel net positions): 20 mm (red), 22 mm (green), 24 mm (blue). See text for further details.
The local fleet consists of 250 fishers and 130 registered small fishing boats. Most of the vessels operating in the area mainly use fixed gear as trammel nets and gill nets throughout the year. This fishery is multi-specific, with the most valuable species being cuttlefish (Sepia officinalis), octopus (Octopus vulgaris), mullet (Mullus surmuletus, Mullus barbatus) and the common pandora (Pagellus erythrinus).
All boats involved in this study are joined to the fishing cooperative operating within the Porto Cesareo MPA. The majority (70%) of fishing boats fell within the range of 6 to 10 m of total length. Approximately 21% of the boats exceeded 10 m, while 9% were smaller than 6 m. In terms of engine power, 70% of the boats had 80 HP power or lower, while the remaining 30% had engine power >80 HP. During the study, each boat took part in fishing activities for a maximum of 5 times.
Sampling was conducted from July 2012 to September 2013 for a total of 72 fishing days, with a single interruption in October 2012 due to the seasonal fishing closure. Sampling activities mostly occurred within the partially protected zone of the MPA (Figure 1), on sea bottoms characterized by the habitats ‘Biocenosis of coarse sands and fine gravels under the influence of bottom currents’ (2/3 of samples), ‘Posidonia oceanica meadows’ and ‘Coralligenous platform’ (1/3 of samples collectively).
On each fishing day, three vessels drawn at random from the fleet were involved, each equipped with a trammel net with different internal mesh sizes, 20 mm, 22 mm or 24 mm respectively (measured from knot to knot). The mesh size of the outer net panels, on the other hand, was 17 cm regardless of the size of the inner mesh. The nets, regardless of mesh size, were 2000 m long and 4 m high. The soak time of fishing nets was about 5 hours in all cases, although slightly varied depending on season, specific locations and prevailing weather and sea conditions, based on the usual fishing strategy of local fishermen. At least one member of the scientific staff was always onboard to supervise the execution of the sampling procedure.
Captured specimens were refrigerated and brought to the laboratory as soon as possible for taxonomic identification. Size (total length, i.e., the distance between the front end of the snout and the end of the longest lobe of the caudal fin, and standard length, i.e., the distance from the tip of the snout to the end of the caudal peduncle) and weight of each specimen were recorded. The size measurement was carried out in accordance with Annex IV of Regulation CR 1967/2006 “Measurement of the size of a marine organism” [(CE) n. 1626/94. 2006].
Commercial size for all landed species was determined based on fish length, while for invertebrates the commercial size was determined based on their weight. To assess the economic value of individual species, we conducted a comprehensive study tracking the average selling prices within the local market over a year. To improve the accuracy of our economic evaluations, particularly for rare species or remarkably large specimens, we integrated the assessment with expert judgement of by specialists from the local fishing cooperative, who provided valuable historical perspectives on species prices.
In addition, for the 10 species reported in Supplementary Table S1, we observed a correlation between fish size and price/kg, with larger specimens sold at higher prices. Based on market values, for each species, we determined a size threshold at which price/kg increased. Subsequently, we computed the number of specimens captured using each different mesh size, that exceeded these thresholds (Supplementary Table S1).
For the analysis, the full dataset was filtered by removing all species with very few observations and selecting the most abundant species (18 species) accounting for more than 70% of the total abundance, which were also the most important species on the local market. We estimated the percentage distribution of total landings, the ratio of non-commercial to commercial size individuals, the total biomass landed (expressed in grams), and the legal (i.e., considering only individuals of legal size) economic income (expressed in €) of the catch.
Discard in terms of both total abundance and biomass for Invertebrates, fish and the total of the two taxa were calculated for each net mesh according to the formula:
The catch per unit effort (CPUE) was calculated for each net mesh according to the following formula. Each net was 2 km long, was set for two hours for a total of 72 sampling days.
To test for temporal patterns in catches in relation to mesh size, a permutational multivariate analysis of variance (PERMANOVA; Anderson, 2014) based on Bray-Curtis similarity was performed on species abundance data (Supplementary Table S2). The design for the analysis consisted of four factors: net mesh (three levels, fixed), year (two levels, fixed and crossed to net mesh), season (four levels, fixed and nested in year), month (four levels, random, nested in season). The analysis was based on untransformed data and 9999 permutations. The same analysis was also performed on biomass data (Supplementary Table S3). Patterns of variation were depicted through MDS ordination of Season × Mesh centroids based on the Bray-Curtis similarity matrix for both abundance and biomass (Supplementary Figures S1, 2).
We used General Linear Models (GLM) to investigate statistical differences in catch among different mesh size. We implemented three GLMs (formulas, details and outputs of the models are reported in Supplementary Materials) for each species with weight, standard length and discards number as dependent variables and mesh size as fixed effect (three levels: 20 mm, 22 mm, 24 mm). The statistical models were developed under the R statistical environment v.3.6.2 (R Core Team, 2021) using package stats (see all details in Supplementary Tables S4–S6).
To test for statistical differences in economic revenue, a one-way PERMANOVA on the economic revenue with mesh size (three levels) as fixed factor were performed for each species. The test was performed using 999 permutations. When significant, post-hoc pair-wise comparisons were performed via PERMANOVA t statistic with 999 permutations (Supplementary Table S7). PERMANOVAs were performed using Primer 7 PERMANOVA+ (Anderson, 2014; Clarke and Gorley, 2015), R package Vegan (Jari Oksanen et al., 2018) and pair-wise PERMANOVA package RVAideMemoire (Hervé, 2018).
The 216 experimental fishing events (72 with each mesh size) collected a total of 16008 specimens and 103 species (76 were fishes, 18 molluscs, 7 crustaceans and 2 echinoderms). Raw data about captures are reported in Supplementary Table S8. The five most fished species, accounting for 56% of total catches, were Scorpaena porcus (17%), Mullus surmuletus (13%) Diplodus annularis (13%), Sepia officinalis (7%) and Pagellus erithrinus (6%). The remaining 44% of the total catch included the other 98 species.
Table 1 summarized the catch data across all species separately for each trammel net mesh size (see Supplementary Table S9 for details on single species). Filtered dataset consisted of 18 species and 12782 individuals, of which 43% (5475 individuals) were caught using 20 mm mesh, 37% (4748 individuals) were caught using 22 mm mesh, and 20% (2559 individuals) were caught using 24 mm mesh (Figure 2A). Considering the corresponding biomass, the 20 mm mesh size amounted to 324.8 kg, the 22 mm mesh size to 321.5 kg, and the 24 mm mesh size to 280.7 kg. The associated total income was 2222.9 € using the 20 mm mesh size, 2590.5 € using the 22 mm mesh size and 2383.9 € using the 24 mm mesh size (Figure 2B). Among the three mesh sizes, the 24 mm mesh recorded the highest values in terms of average standard length, weight of organisms caught and in terms of income for fishers (Table 1).
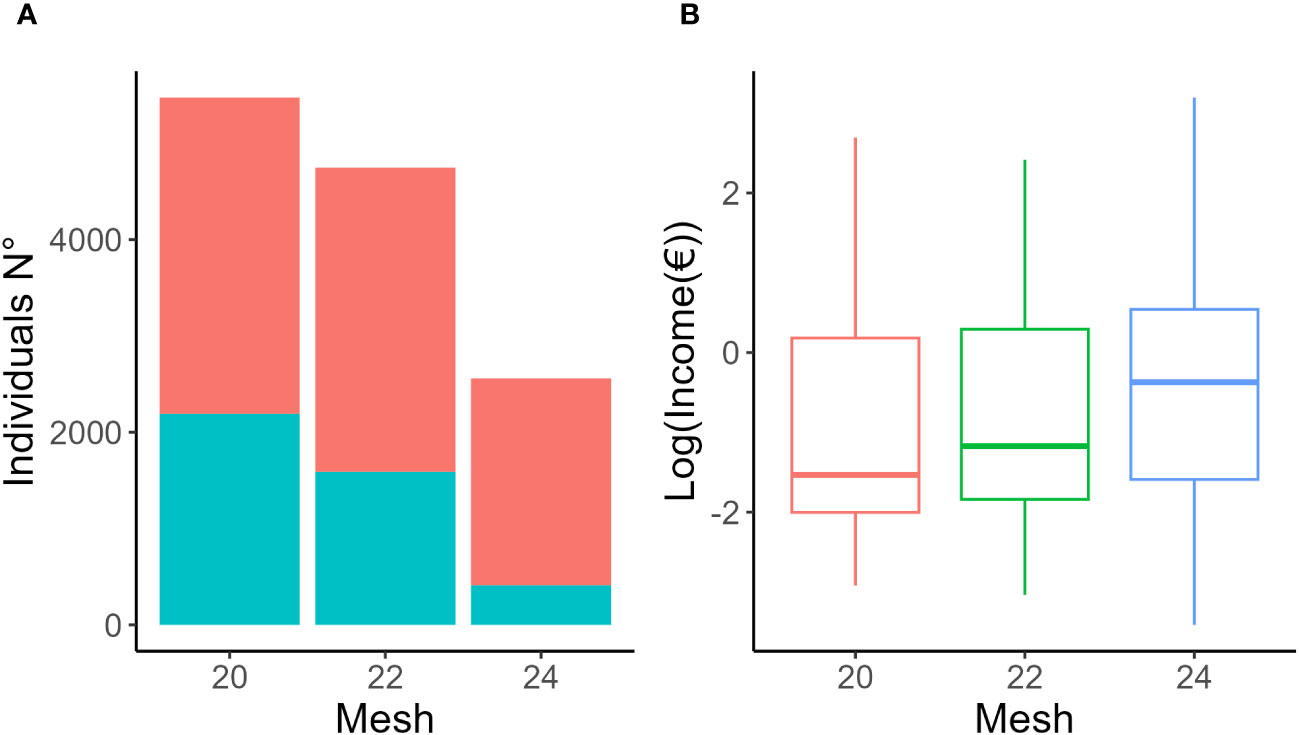
Figure 2 (A) Number of individuals over (red) and under (blue) the legal sale size as established by Regulation EC 1967/2006. (B) Mean income in € (log transformed) for each mesh size. 20 mm (red), 22 mm (green), 24 mm (blue).
Focusing on discards, a ratio for discards in terms of total abundance and total biomass for each net mesh has been measured for both invertebrates, fish and the cumulative value (Tables 2, 3). A similar trend was observed with the 24 mm mesh net accounting for the lower discards compared to the other nets.
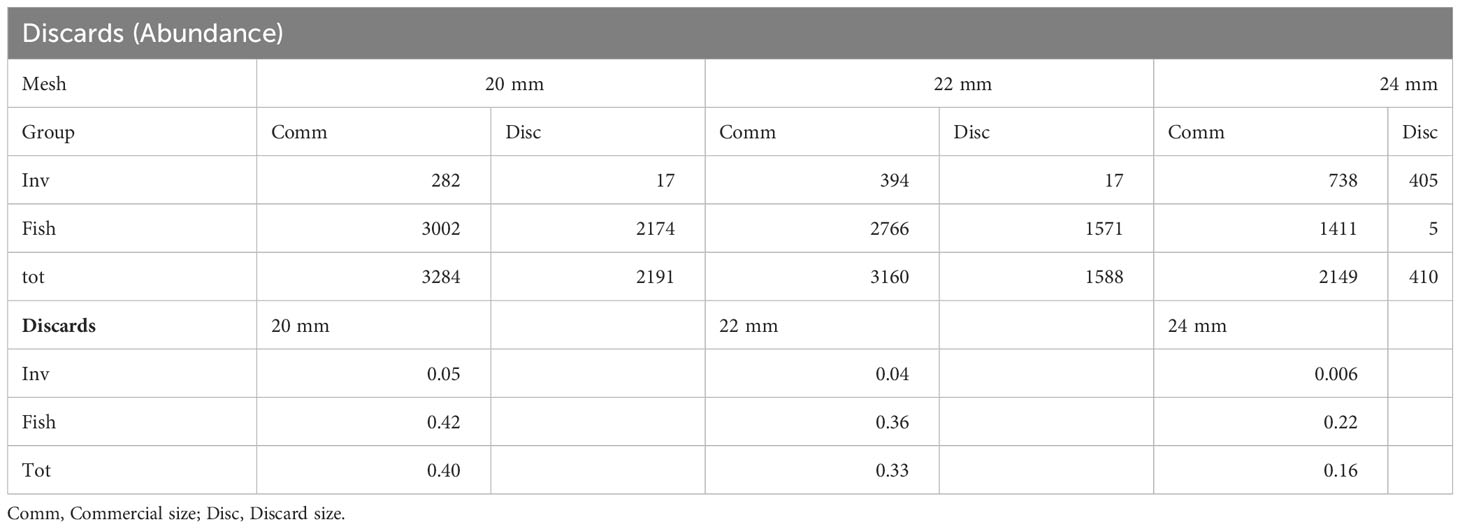
Table 2 Summary of discards for both invertebrates, fish and total by net mesh expressed for total abundance (number of individuals).
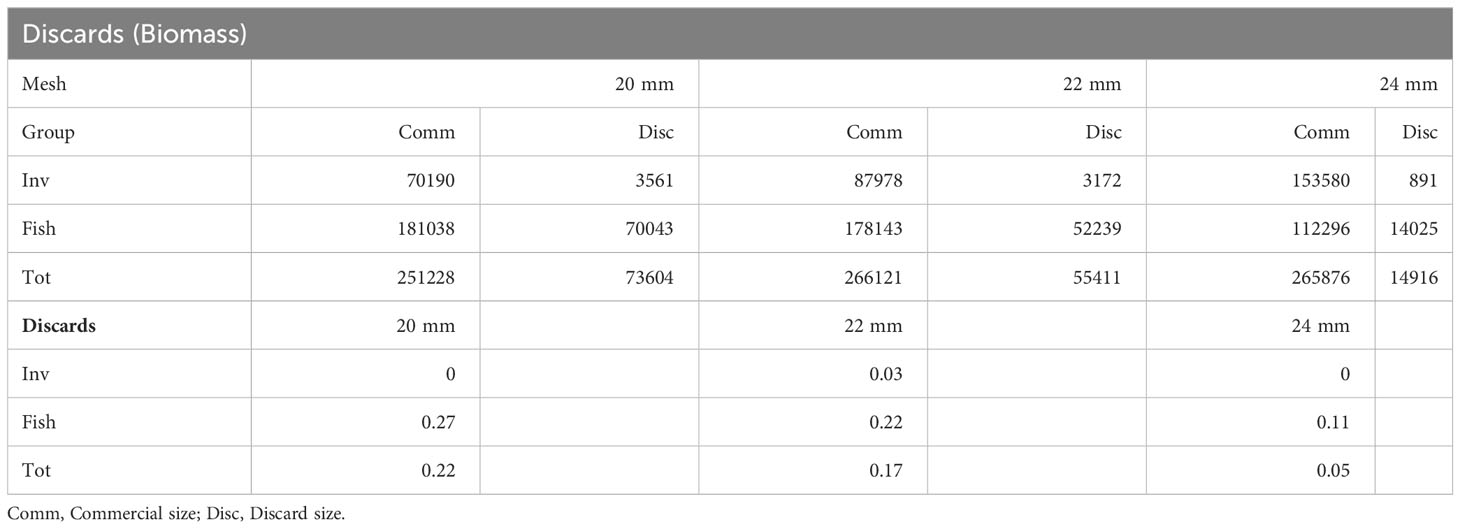
Table 3 Summary of discards for both invertebrates, fish and total by net mesh expressed for total biomass (g).
Regarding the catch per unit effort (CPUE) (Table 4; Supplementary Tables S10), in terms on total biomass the highest CPUE is observed for the 20 and 22 mesh. When looking at the legal size biomass, the 24 mesh net records the highest CPUE in terms of commercial size biomass together with the 22 mm mesh net and the lowest discards CPUE compared to the two other nets.
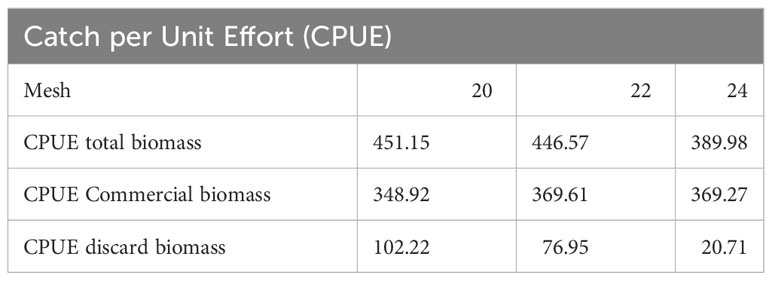
Table 4 Summary of catch per unit effort (CPUE) for total, commercial and discards biomass for each net mesh.
PERMANOVAs on fish abundance and biomass detected in both cases a significant interaction between factors ‘net mesh’ and ‘season’, indicating significant changes in the structure of catches among seasons that varied with net mesh (Supplementary Tables S2, 3), with a lower seasonal changes observed for the 24 mm mesh if compared to those observed for the other two mesh sizes, which instead both showed larger and similar seasonal patterns of changes (Supplementary Figures S1, 2).
Results of GLMs for each species (weight, standard length, under/over legal-size specimens) and PERMANOVA (for income) are reported in Figures 3–6 and Supplementary Tables S4–S6. In particular, focusing on the five most frequently harvested species (S. porcus, M. surmuletus, D. annularis, S. officinalis, P. erithrinus), we found that the use of trammel nets with the 24 mm mesh size resulted in a significant increase (p-value<0.001 for all significant tests, Supplementary Tables S3–S7) in the selectivity of the organisms caught if compared to the other two nets. We observed an increase in terms of both average weight (Figure 3) and standard length (Figure 4), a decrease in the number of discards (Figure 5), and an increase in fishers’ income (Figure 6) for most of the species, except for the undercut/oversized ratio in M. surmuletus and for S.officinalis, where no differences were found for all variables.
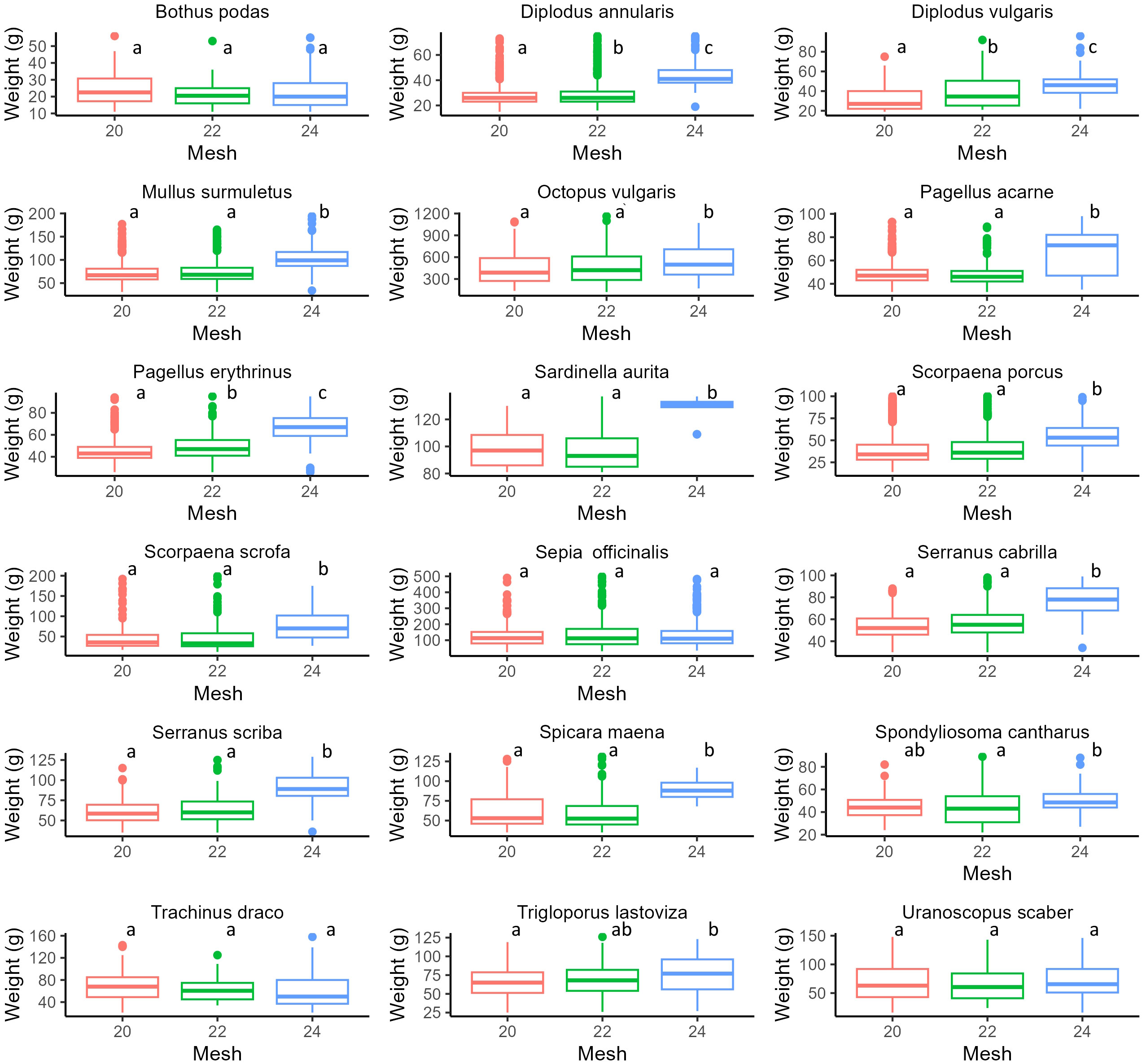
Figure 3 Boxplot of individual weight for each species (filtered dataset) each mesh size. 20 mm (Red), 22 mm (Green), 24 mm (Blue). For each plot, the thick horizontal line represents the median of the distribution, the box includes 50% of the data (25th-75th percentiles), and the whiskers reach the highest and lowest value. Open circles represent outliers. Statistical differences are reported as letters, p-values for each pair-wise contrast are reported in Supplementary Table S4.
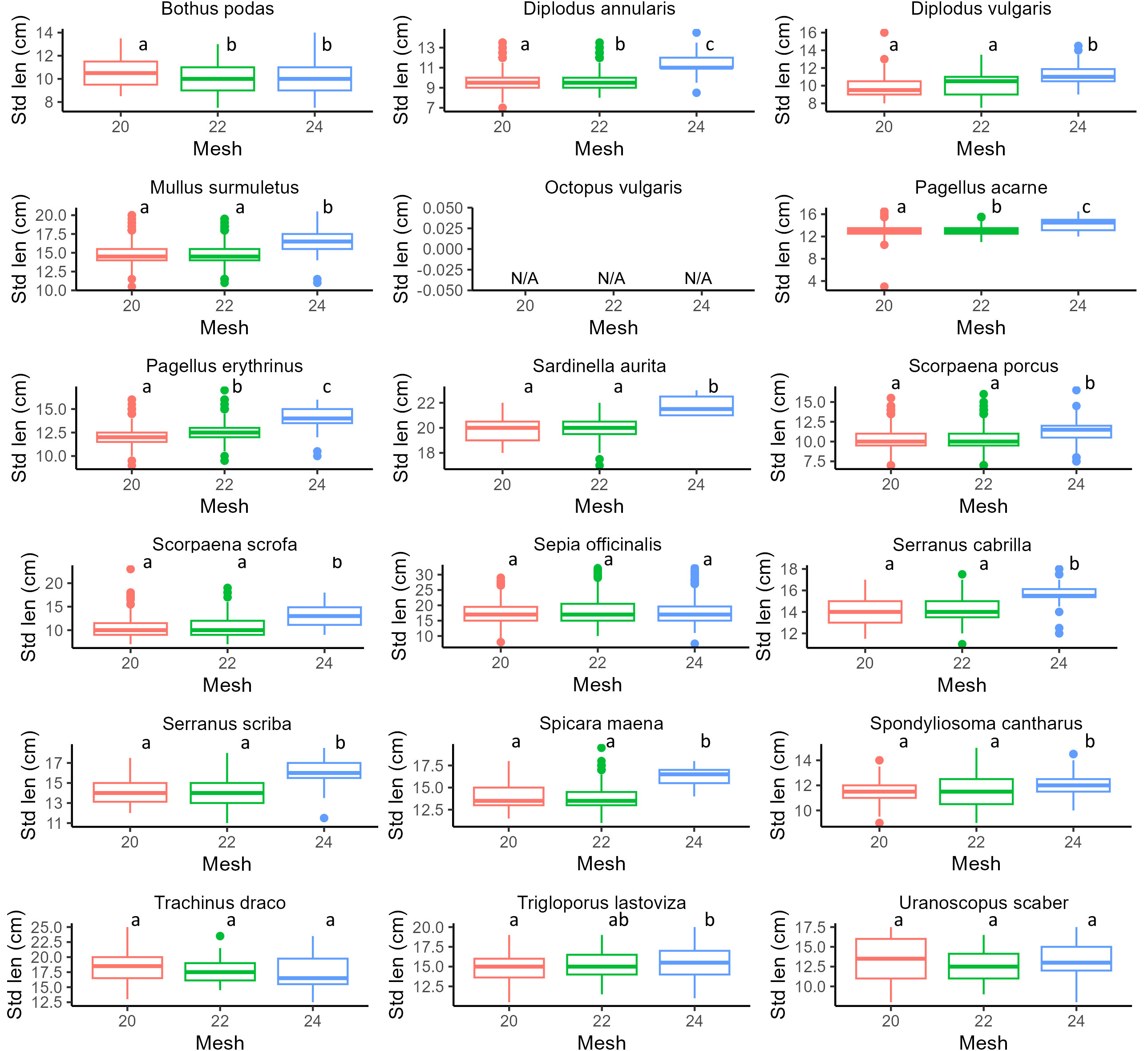
Figure 4 Boxplot of individual standard length for each mesh size. 20 mm (Red), 22 mm (Green), 24 mm (Blue). For each plot, the thick horizontal line represents the median of the distribution, the box includes 50% of the data (25th-75th percentiles), and the whiskers reach the highest and lowest value. Open circles represent outliers. Statistical differences are reported as letters, p-values for each pair-wise contrast are reported in Supplementary Table S5. N/A, Not Assigned.
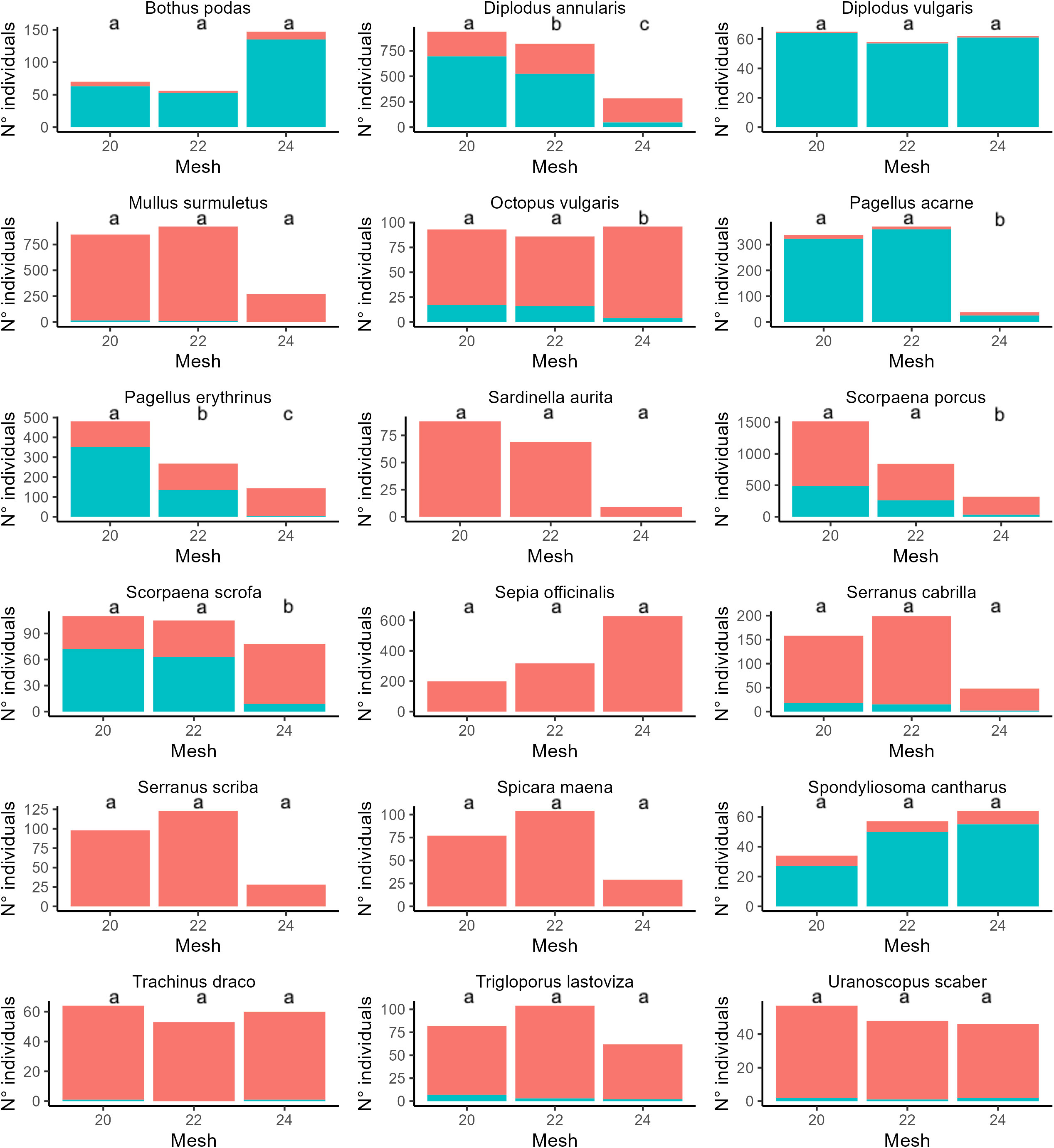
Figure 5 Number of individuals over (Red) and under (Blue) the legal sales size as established by Regulation EC 1967/2006. Statistical differences are reported as letters, p-values for each pair-wise contrast are reported in Supplementary Table S6.
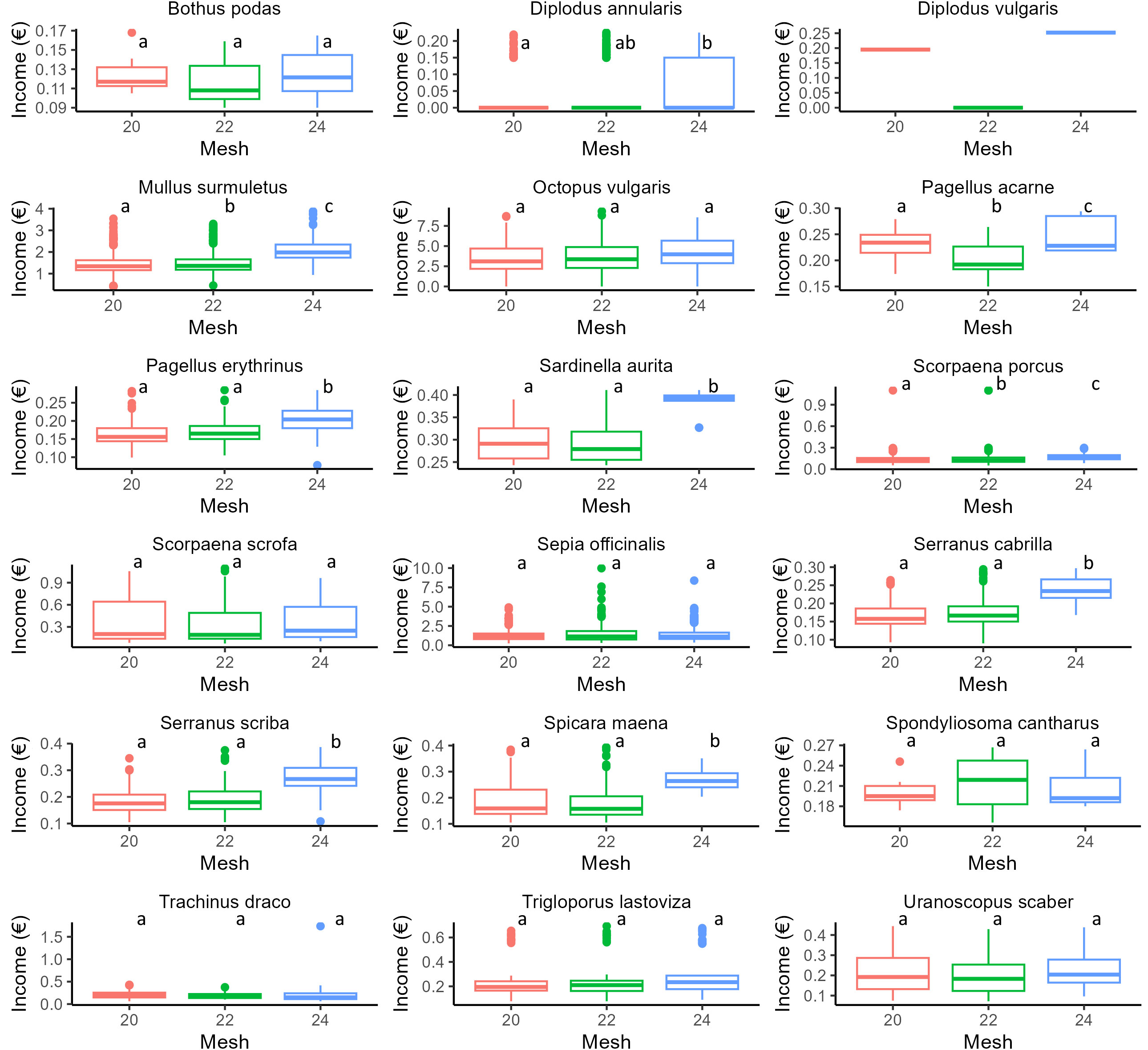
Figure 6 Boxplot for income (expressed in € per individual) for each mesh size. 20 mm (Red), 22 mm (Green), 24 mm (Blue). For each plot, the thick horizontal line represents the median of the distribution, the box includes 50% of the data (25th-75th percentiles), and the whiskers reach the highest and lowest value. Open circles represent outliers. Statistical differences are reported as letters, p-values for each pair-wise contrast are reported in Supplementary Table S7.
Most species show a variation in market price as a function of weight (Supplementary Table S1). The total catches of species characterized by higher price/kg at higher sizes (compared to baseline price at minimum commercial size) yielded by the 20 mm, 22 mm, and 24 mm mesh fell over the size at which the price/kg increase for 24%, 34% and 28% respectively (Table 5).
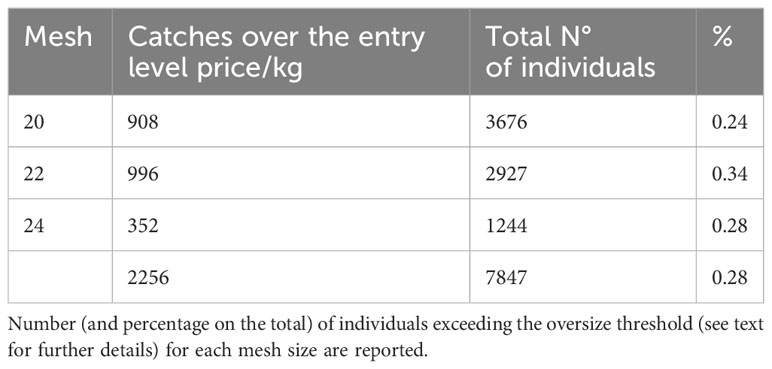
Table 5 Total number of caught individuals belonging to species whose price/kg varies at different sizes.
The effectiveness of current management strategies and conservation measures to reduce fishing pressure on wild populations of target fishes is often difficult to assess and the effects on stock health are still uncertain (Guillen et al., 2018; Maynou et al., 2018; Lucchetti et al., 2020; Borges, 2021);. For example, selective harvesting of larger individuals, usually considered a “good practice”, can be equally detrimental than less selective fishing practices. As size is closely linked to growth, maturation, reproductive output and survival (Birkeland and Dayton, 2005; Zimmermann and Jorgensen, 2015), size-selective harvesting can have both short and long-term effects on fish populations by reducing their reproductive potential and genetic variability (Bruggemann et al., 1996; Friedlander and DeMartini, 2002; Birkeland and Dayton, 2005; Uusi-Heikkilä et al., 2015; Zimmermann and Jorgensen, 2015; Gandra et al., 2021). On the other hand, size-unselective fishing tools remove individuals across all size classes, thus mining the persistence of fish populations in two ways by combining potentially negative effects of removing larger and older specimens to those deriving from killing individuals before they achieve sexual maturity and reproduce.
Indeed, the use of size-selective tools could alleviate fishing pressure on target populations and increase socio-ecological benefits of fishing. This is particularly true for small-scale fisheries due to their commonly scarce economic income (Gomez et al., 2006; Vasilakopoulos et al., 2014; FAO, 2022) and the potential threats of these activities on fish stocks (Exton et al., 2019; Gutierrez et al., 2023). Here, we found that the use of the 24 mm mesh trammel net, compared to 20 mm and 22 mm mesh, increased catch size-selectivity, reduced discards, and resulted in larger fish size.
Focusing on gears selectivity, several studies on trap and gill net mesh sizes pointed out that the mesh size of different gears is directly related to body size (weight and standard length) of fish caught (Petrakis and Stergiou, 1995; Petrakis and Stergiou, 1996; Mahon and Hunte, 2001). However, other factors such as the different body shapes of the various species and their capability to pass through the mesh, greatly influence the selectivity of fishing gears and the whole picture is still poorly understood and difficult to predict (Mahon and Hunte, 2001; Fabi et al., 2002). The significant differences we found for the majority of commercially valuable species, both in terms of weight and standard length, suggested the greater effectiveness of the 24 mm mesh in catching larger specimens respect to the 20 mm and the 22 mm mesh. Such findings agreed with other studies carried out in the Mediterranean Sea aimed at assessing the effect of varying mesh size on catches. Fabi et al. (2002) found that the use of trammel nets and gillnets with larger mesh sizes (respectively of 70 mm and 90 mm) allowed the capture of larger specimens compared to 45 mm mesh net. Petrakis and Stergiou (1995; 1996) stated that, on average, larger meshes lead to catch larger specimens for some target species of high commercial value, such as Mullus surmuletus, Pagellus acarne and P. erythrinus.
Catching larger specimens often turns into higher incomes for fishers’ due to their higher selling price (€/kg) compared to the small and medium-sized counterparts of the same species (Colloca et al., 2013; Tsikliras and Polymeros, 2014). The price of fish is a factor that greatly influences the behavior of fishers, who tend to select valuable species and plan fishing strategies in order to maximize the potential income (Battaglia et al., 2010; Tsikliras and Polymeros, 2014). Our study indicated that the use of 24 mm mesh nets resulted in up to double mean income per individual for fishers with respect to the 20 mm and 22 mm mesh. As far as the total income, catches from 20 mm net mesh led to a lower gain with respect the larger mesh (2222.9 €, 2590.5 €, 2383.9 € using the 20, 22, 24 mm mesh respectively). Indeed, the 22 mm mesh net seemed to perform even better than the 24 mm mesh net. However, it is worth noting that due to the high rates of discards (i.e., two times the discards recorded for the 24 mm mesh net), the use of the 22 mm net may probably lead to higher costs for manpower (e.g., landing, specimens size selection activities, net cleaning, discard removal, etc.) which may, in turn, reduce or even counterbalance its higher income. Such findings may act as an incentive to increase the compliance towards the management measures aimed at increasing the selectivity of fishing tools and the sustainability of local fishery in the study area. Convincing fishermen that management decisions at European scale works also in terms of their payback is a key step towards better results. For instance, closed areas and technical measures such as legal minimum sizes, gear sizes and fishing restrictions (European Regulation (EU) 1967/2006; European Regulation (EU) 2019/1241, 2019), if supported by local fishermen, may lead to both economic and ecological benefits.
Another major global issue for fisheries management concerns discards (FAO, 2022), defined by the FAO as ‘The portion of the total organic material of animal origin in the catch that is thrown away or discarded at sea for any reason. It does not include plant materials and post-harvest discards, such as offal. Discards may be alive or dead.’, which includes all those species or individuals discarded by fishers because of their size (below the legal limit), and low (or null) commercial value (Hall et al., 2000; FAO, 2022). Discards affect the survival of the stocks, removing juvenile and undersized individuals before they reach optimal size (Cowan Jr. et al., 2010), and can reduce the income of fishers (Catchpole et al., 2005). In the Mediterranean Sea, discard rate in small scale fisheries, estimated by the General Fisheries Commission for the Mediterranean (GFCM) to range between 3% and 15%, led to reduced valuable catches, increased depletion of fish stocks, and subsequent potential effects on the structure and functioning of marine ecosystems (FAO, 2022). However, these assessments have covered only a small portion of fisheries of the Mediterranean and Black Sea, and discard rates are often poorly estimated or completely unknown (FAO, 2022).
In the current depleted stock scenario, the reduction of discards is a key aspect within fisheries management and conservation strategies to manage fishery’s strains on ecosystems (Tzanatos et al., 2007; Fauconnet et al., 2019). With this in mind, since 2015 the EU’s Common Fisheries Policy (CFP) has introduced a discard ban for all commercial species subject to catch limits or minimum landing sizes (landing obligation; Article 15 of Regulation (EU) No 1380/2013), with the aim of reducing unwanted catches by incentivizing better selectivity. Beyond negative effects on the overall health of fish stocks (Diamond and Beukers-Stewart, 2011; Heath et al., 2014), harvesting individuals under the legal commercial size may also result in higher costs for fishers (Macher et al., 2008) due to lost revenues. Discard levels can be related to a multitude of factors including local species diversity, environmental contexts, constrains of fishing activity, as well as weather factors (Batista et al., 2009), making comparisons between different managements plans or gear choice difficult to be done. However, large mesh sizes are generally expected to catch fewer discards than smaller mesh. As our results showed, their abundance often declines exponentially with gear size both within and between species (Jennings et al., 2001; Stergiou et al., 2006). Batista et al. (2009), investigating the discard from trammel net fisheries in the south of Portugal, found a very high value for percentage of discards equal to 52.8% of total catch numbers, with large socio-economic implications due to the waste of fishing efforts and the ensuing loss of revenues. Other studies, carried out in the same area, detected lower level of discards ranging between 13% (Borges et al., 2001) and 49% (Gonçalves et al., 2007). In our study, the use of a larger mesh size significantly decreased the rejection rate for both fish and invertebrates, with the 24 mm mesh reducing discards to 16%, half the values (33% and 40%) recorded for the smaller mesh nets.
Conventional top-down management actions are generally unenforceable in small scale fisheries due to poor tractability of these fisheries. Historically, the most successful form of governance involved local communities in a co-management with local organizations (Worm et al., 2009; Guidetti and Claudet, 2010; Di Franco et al., 2016; Giakoumi et al., 2018). Similarly, the collaboration between stakeholders and the management bodies is widely recognized as a determining factor for the success of conservation objectives of an MPA (Edgar et al., 2014; Watson et al., 2014; Di Franco et al., 2016; Di Lorenzo et al., 2016). As a consequence of the results of the present study, Porto Cesareo MPA, in joint agreement with fishers operating within its boundaries, set the minimum mesh of nets to 24 mm and banned 20 and 22 mm nets, with the aim of mitigating the impact on local fish stocks, favor their recover together with maintaining a substantial income for fishers. The experimentation of good practices for fishing activities within delimited and controlled areas such as MPAs has proven to be effective and useful for testing new conservation strategies and gear and, at the same time, to enhance the relationships between users of marine resources and conservation strategies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The manuscript presents research on animals that do not require ethical approval for their study.
GT: Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing, Supervision. GM: Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. PD’A: Investigation, Methodology, Supervision, Writing – review & editing. SF: Data curation, Investigation, Methodology, Writing – review & editing. VS: Conceptualization, Writing – review & editing. SB: Investigation, Methodology, Writing – review & editing. PT: Data curation, Investigation, Methodology, Writing – original draft. GS: Formal analysis, Investigation, Methodology, Writing – original draft. AT: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union – NextGenerationEU; Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP C63C22000520001 Project title “National Biodiversity Future Center - NBFC”.
This study was conducted thanks to the cooperation between the Porto Cesareo MPA authorities and fishers’ cooperative and thanks to the sampling support and logistical organization work done by Sergio Fai, Cristian Vaglio, Ilario Peluso, Graziano Maccagnano e Pierpaolo Patarnello. Trevor Willis kindly provided an extensive revision of the language.
Author GS is employed by the company Methodo Scientific Consulting, Monteroni di Lecce LE, Italy.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1267381/full#supplementary-material
Anderson M. J. (2014). Permutational multivariate analysis of variance (PERMANOVA) (John Wiley & Sons, NJ, USA: Wiley statsref), 1–15.
Barneche D. R., Robertson D.R., White C. R., Marshall D. J. (2018). Fish reproductive-energy output increases disproportionately with body size. Science 360, 642–645. doi: 10.1126/science.aao6868
Batista M. I., Teixeira C. M., Cabral H. N. (2009). Catches of target species and bycatches of an artisanal fishery: The case study of a trammel net fishery in the Portuguese coast. Fisheries Res. 100 (2), 167–177. doi: 10.1016/j.fishres.2009.07.007
Battaglia P., Romeo T., Consoli P., Scotti G., Andaloro F. (2010). Characterization of the artisanal fishery and its socio-economic aspects in the central Mediterranean Sea (Aeolian Islands, Italy). Fisheries Res. 102 (1-2), 87–97. doi: 10.1016/j.fishres.2009.10.013
Bianchi D., Carozza D. A., Galbraith E. D., Guiet J., DeVries T. (2021). Estimating global biomass and biogeochemical cycling of marine fish with and without fishing. Sci. Adv. 7 (41), eabd7554.
Birkeland C., Dayton P. K. (2005). The importance in fishery management of leaving the big ones. Trends Ecol. Evol. 20 (7), 356–358. doi: 10.1016/j.tree.2005.03.015
Borges L. (2021). The unintended impact of the European discard ban. ICES J. Mar. Sci. 78 (1), 134–141. doi: 10.1093/icesjms/fsaa200
Borges T. C., Erzini K., Bentes L., Costa M. E., Goncalves J. M., Lino P. G. (2001). By-catch and discarding practices in five Algarve (southern Portugal) métiers. J. Appl. Ichthyology 17 (3), 104–114. doi: 10.1046/j.1439-0426.2001.00283.x
Bruggemann J. H., Van Kessel A. M., Van Rooij J. M., Breeman A. M. (1996). Bioerosion and sediment ingestion by the Caribbean parrotfish Scarus vetula and Sparisoma viride: implications of fish size, feeding mode and habitat use. Mar. Ecol. Prog. Ser. 134, 59–71. doi: 10.3354/meps134059
Catchpole T. L., Frid C. L., Gray T. S. (2005). Discards in North Sea fisheries: causes, consequences and solutions. Mar. Policy 29 (5), 421–430. doi: 10.1016/j.marpol.2004.07.001
Cavan E. L., Hill S. L. (2022). Commercial fishery disturbance of the global ocean biological carbon sink. Global Change Biol. 28, 1212–1221. doi: 10.1111/gcb.16019
Clarke K. R., Gorley R. N. (2015). “Getting started with PRIMER v7,” in PRIMER-E: plymouth (Plymouth Marine Laboratory), 20 (1).
Colloca F., Cardinale M., Maynou F., Giannoulaki M., Scarcella G., Jenko K. (2013). Rebuilding Mediterranean fisheries: a new paradigm for ecological sustainability. Fish fisheries 14 (1), 89–109. doi: 10.1111/j.1467-2979.2011.00453.x
Cowan J. H. Jr (2011). Red snapper in the gulf of mexico and US south atlantic: data, doubt, and debate. Fisheries 36 (7), 319–331.
Diamond B., Beukers-Stewart B. D. (2011). Fisheries discards in the North Sea: waste of resources or a necessary evil. Rev. Fisheries Sci. 19 (3), 231–245. doi: 10.1080/10641262.2011.585432
Di Franco A., Thiriet P., Di Carlo G., Dimitriadis C., Francour P., Gutiérrez N. L., et al. (2016). Five key attributes can increase marine protected areas performance for small-scale fisheries management. Sci. Rep. 6 (1), 38135. doi: 10.1038/srep38135
Di Lorenzo M., Claudet J., Guidetti P. (2016). Spillover from marine protected areas to adjacent fisheries has an ecological and a fishery component. J. Nat. Conserv. 32, 62–66. doi: 10.1016/j.jnc.2016.04.004
Dunn D. C., Boustany A. M., Halpin P. N. (2011). Spatio-temporal management of fisheries to reduce by-catch and increase fishing selectivity. Fish Fisheries 12 (1), 110–119. doi: 10.1111/j.1467-2979.2010.00388.x
Edgar G. J., Stuart-Smith R. D., Willis T. J., Kininmonth S., Baker S. C., Banks S., et al. (2014). Global conservation outcomes depend on marine protected areas with five key features. Nature 506, 216–220. doi: 10.1038/nature13022
Exton D. A., Ahmadia G. N., Cullen-Unsworth L. C., Jompa J., May D., Rice J., et al. (2019). Artisanal fish fences pose broad and unexpected threats to the tropical coastal seascape. Nat. Commun. 10 (1), 2100.
Fabi G., Sbrana M., Biagi F., Grati F., Leonori I., Sartor P. (2002). Trammel net and gill net selectivity for Lithognathus mormyrus (L), diplodus annularis (L) and mullus barbatus (L) in the adriatic and ligurian seas. Fisheries Res. 54 (3), 375–388. doi: 10.1016/S0165-7836(01)00270-3
FAO (2022). Report of the forty-fourth session of the General Fisheries Commission for the Mediterranean (GFCM) – Online, 2–6 November 2021. GFCM Report No. 44 (Rome). doi: 10.4060/cc0292en
Fauconnet L., Frangoudes K., Morato T., Afonso P., Pita C. (2019). Small-scale fishers’ perception of the implementation of the EU landing obligation regulation in the outermost region of the Azores. J. Environ. Manage. 249, 109335. doi: 10.1016/j.jenvman.2019.109335
Frank K. T., Petrie B., Choi J. S., Leggett W. C. (2005). Trophic cascades in a formerly cod-dominated ecosystem. Science 308 (5728), 1621–1623. doi: 10.1126/science.1113075
Friedlander A. M., DeMartini E. E. (2002). Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian Islands: the effects of fishing down apex predators. Mar. Ecol. Prog. Ser. 230, 253–264. doi: 10.3354/meps230253
Gandra M., Assis J., Martins M. R., Abecasis D. (2021). Reduced global genetic differentiation of exploited marine fish species. Mol. Biol. Evol. 38, 1402–1412. doi: 10.1093/molbev/msaa299
Gates J. M. (1974). Demand price, fish size and the price of fish. Can. J. Agric. Economics/Revue Can. d’agroeconomie 22 (3), 1–12. doi: 10.1111/j.1744-7976.1974.tb00931.x
Giakoumi S., McGowan J., Mills M., Beger M., Bustamante R. H., Charles A., et al. (2018). Revisiting “success” and “failure” of marine protected areas: a conservation scientist perspective. Front. Mar. Sci., 223. doi: 10.3389/fmars.2018.00223
Gomez S., Lloret J., Demestre M., Riera V. (2006). The decline of the artisanal fisheries in Mediterranean coastal areas: the case of Cap de Creus (Cape Creus). Coast. Manage. 34 (2), 217–232. doi: 10.1080/08920750500531389
Gonçalves J. M. S., Stergiou K. I., Hernando J. A., Puente E., Moutopoulos D. K., Arregi L., et al. (2007). Discards from experimental trammel nets in southern European small-scale fisheries. Fisheries Res. 88 (1-3), 5–14. doi: 10.1016/j.fishres.2007.06.017
Guidetti P., Claudet J. (2010). Comanagement practices enhance fisheries in marine protected areas. Conserv. Biol. 24 (1), 312–318. doi: 10.1111/j.1523-1739.2009.01358.x
Guillen J., Holmes S. J., Carvalho N., Casey J., Dörner H., Gibin M., et al. (2018). A review of the European Union landing obligation focusing on its implications for fisheries and the environment. Sustainability 10 (4), 900. doi: 10.3390/su10040900
Gutierrez N. L., Funge-Smith S., Gorelli G., Mancha-Cisneros M. M., Defeo O., Johnson A. F., et al. (2023). “Production and environmental interactions of small-scale fisheries,” in 2023. Illuminating Hidden Harvests: the contributions of small-scale fisheries to sustainable development (Rome, FAO;Durham, USA: Duke University; Penang, Malaysia, WorldFish).
Hall M. A., Alverson D. L., Metuzals K. I. (2000). By-catch: problems and solutions. Mar. Poll. Bull. 41, 204–219. doi: 10.1016/S0025-326X(00)00111-9
Hall S. J., Mainprize B. M. (2005). Managing by-catch and discards: how much progress are we making and how can we do better. Fish Fisheries 6 (2), 134–155. doi: 10.1111/j.1467-2979.2005.00183.x
Halliday R. G., Pinhorn A. T. (2002). A review of the scientific and technical bases for policies on the capture of small fish in North Atlantic groundfish fisheries. Fisheries Res. 57 (3), 211–222. doi: 10.1016/S0165-7836(02)00079-6
Heath M. R., Cook R. M., Cameron A. I., Morris D. J., Speirs D. C. (2014). Cascading ecological effects of eliminating fishery discards. Nat. Commun. 5 (1), 3893. doi: 10.1038/ncomms4893
Hervé M. (2018). RVAideMemoire: testing and plotting procedures for biostatistics. R Package version 0.9-69, 3.
Hilborn R., Amoroso R. O., Anderson C. M., Baum J. K., Branch T. A., Costello C., et al. (2020). Effective fisheries management instrumental in improving fish stock status. Proc. Natl. Acad. Sci. 117 (4), 2218–2224. doi: 10.1073/pnas.1909726116
Jari Oksanen F. G. B., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P. R., et al. (2018). Vegan: community ecology package. R Package version 2 (6).
Jennings S., Pinnegar J. K., Polunin N. V., Boon T. W. (2001). Weak cross-species relationships between body size and trophic level belie powerful size-based trophic structuring in fish communities. J. Anim. Ecol., 934–944. doi: 10.1046/j.0021-8790.2001.00552.x
Kennelly S. J., Broadhurst M. K. (2002). By-catch begone: changes in the philosophy of fishing technology. Fish Fisheries 3 (4), 340–355. doi: 10.1046/j.1467-2979.2002.00090.x
Kurien J. (2015). Voluntary guidelines for securing sustainable small-scale fisheries in the context of food security and poverty eradication: summary.
Lucchetti A., Virgili M., Petetta A., Sartor P. (2020). An overview of gill net and trammel net size selectivity in the Mediterranean Sea. Fisheries Res. 230, 105677. doi: 10.1016/j.fishres.2020.105677
Macher C., Guyader O., Talidec C., Bertignac M. (2008). A cost–benefit analysis of improving trawl selectivity in the case of discards: the Nephrops norvegicus fishery in the Bay of Biscay. Fisheries Res. 92 (1), 76–89. doi: 10.1016/j.fishres.2007.12.021
Mahon R., Hunte W. (2001). Trap mesh selectivity and the management of reef fishes. Fish Fisheries 2 (4), 356–375. doi: 10.1046/j.1467-2960.2001.00054.x
Maynou F., del Mar Gil M., Vitale S., Giusto G. B., Foutsi A., Rangel M., et al. (2018). Fishers’ perceptions of the European Union discards ban: perspective from south European fisheries. Mar. Policy 89, 147–153. doi: 10.1016/j.marpol.2017.12.019
Myers R. A., Baum J. K., Shepherd T. D., Powers S. P., Peterson C. H. (2007). Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315 (5820), 1846–1850. doi: 10.1126/science.1138657
Petrakis G., Stergiou K. I. (1995). Gill net selectivity for diplodus annularis and mullus surmuletus in greek waters. Fisheries Res. 21 (3-4), 455–464.
Petrakis G., Stergiou K. I. (1996). Gill net selectivity for four fish species (Mullus barbatus, pagellus erythrinus, pagellus acarne and spicara flexuosa) in greek waters. Fisheries Res. 27 (1-3), 17–27.
R Core Team (2021). R: A language and environment for statistical computing (Vienna: R Foundation for Statistical Computing).
Reddy S. M., Wentz A., Aburto-Oropeza O., Maxey M., Nagavarapu S., Leslie H. M. (2013). Evidence of market-driven size-selective fishing and the mediating effects of biological and institutional factors. Ecol. Appl. 23 (4), 726–741. doi: 10.1890/12-1196.1
Ruttenberg B. I., Hamilton S. L., Walsh S. M., Donovan M. K., Friedlander A., DeMartini E., et al. (2011). Predator-induced demographic shifts in coral reef fish assemblages. PloS One 6 (6), e21062. doi: 10.1371/journal.pone.0021062
Scheffer M., Carpenter S., de Young B. (2005). Cascading effects of overfishing marine systems. Trends Ecol. Evol. 20 (11), 579–581. doi: 10.1016/j.tree.2005.08.018
Stergiou K. I., Moutopoulos D. K., Soriguer M. C., Puente E., Lino P. G., Zabala C., et al. (2006). Trammel net catch species composition, catch rates and métiers in southern European waters: A multivariate approach. Fisheries Res. 79 (1-2), 170–182. doi: 10.1016/j.fishres.2006.03.003
Tsikliras A. C., Polymeros K. (2014). Fish market prices drive overfishing of the ‘big ones’. PeerJ 2, e638. doi: 10.7717/peerj.638
Tzanatos E., Somarakis S., Tserpes G., Koutsikopoulos C. (2007). Discarding practices in a Mediterranean small-scale fishing fleet (Patraikos Gulf, Greece). Fisheries Manage. Ecol. 14 (4), 277–285. doi: 10.1111/j.1365-2400.2007.00556.x
Uusi-Heikkilä S., Whiteley A. R., Kuparinen A., Matsumura S., Venturelli P. A., Wolter C., et al. (2015). The evolutionary legacy of size-selective harvesting extends from genes to populations. Evol. Appl. 8 (6), 597–620. doi: 10.1111/eva.12268
Vasilakopoulos P., Maravelias C. D., Tserpes G. (2014). The alarming decline of Mediterranean fish stocks. Curr. Biol. 24 (14), 1643–1648. doi: 10.1016/j.cub.2014.05.070
Watson J. E., Dudley N., Segan D. B., Hockings M. (2014). The performance and potential of protected areas. Nature 515, 67–73. doi: 10.1038/nature13947
Worm B., Hilborn R., Baum J. K., Branch T. A., Collie J. S., Costello C., et al. (2009). Rebuilding global fisheries. science 325, 578–585. doi: 10.1126/science.1173146
Keywords: small-scale fisheries, gear selectivity, trammel net, sustainability, selective fishing
Citation: Tarantino G, Motta G, D’Ambrosio P, Felline S, Sbragaglia V, Bevilacqua S, Tedesco P, Scordella G and Terlizzi A (2024) Increasing trammel mesh size reduces biomass removal, mitigates discards and increases economic revenue in artisanal fisheries. Front. Mar. Sci. 11:1267381. doi: 10.3389/fmars.2024.1267381
Received: 26 July 2023; Accepted: 24 January 2024;
Published: 07 February 2024.
Edited by:
Tomaso Fortibuoni, Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA), ItalyReviewed by:
Taner Yildiz, Istanbul University, TürkiyeCopyright © 2024 Tarantino, Motta, D’Ambrosio, Felline, Sbragaglia, Bevilacqua, Tedesco, Scordella and Terlizzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulio Tarantino, Z2l1bGlvLnRhcmFudGlub0Bzem4uaXQ=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.