
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 07 June 2023
Sec. Coral Reef Research
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.928538
This article is part of the Research Topic Ornamental Fishing Industry View all 8 articles
Ornamental corals have become the main attention objects for many hobbyists and have already become a source for foreign exchange in developing countries. Through ornamental coral propagation, the continuity of ornamental coral trade can be guaranteed and the level of damage is very limited. The propagation technique has been developed by ornamental coral exporters in several locations in Indonesia. This paper has two main objectives: a) to determine the site suitability of ornamental coral propagation locations and b) to provide a detailed description of the development and progress of ornamental coral propagation in Indonesia with first-hand information from 14 companies. The present study showed that the highest growth rate was found on branched specimens of E. glabrescens (2.50 cm) after 5 months, and the lowest growth rate was found on branched specimens of E. paraancora (0.42 cm). Corals with larger branches or with two or three branches of E. glabrescens and E. paraancora have a low mortality and increased the number of branches to three to four branches after 5 months. Small colonies also survive with a mortality of 20%. The cultured corals can grow optimally and have exotic colors when placed at different depths. Best coral growth was obtained from fast-growing coral species at a shallow depth (<10 m). While medium depth (10–15 m) was suitable for corals with moderate growth rates, the deeper depths (> 15 m) were characterized by bright-colored corals with slow-growing rates. Color quality and growth of corals are closely related to some supporting factors such as the suitability of locations that have different physical, biological, and chemical factors in different depths and thus follow different requirements among coral species. Location choices determine the success of coral cultivation both for ornamental and restoration purposes. These results are valuable background information for traded ornamental corals that can be utilized by propagators.
Marine ornamental wildlife is traded globally and estimated to be worth at least half a billion dollars annually (Best, 2002; Carvalho et al., 2022). The estimate could be based on a conservative value due to data missing and unavailability or simply referenced to outdated data (Biondo and Calado, 2021). In the Indo-Pacific region, the industry has seen a continuously increasing trend in terms of volume and monetary value of the trading activities (Dee et al., 2014), of which Southeast Asia is the primary source of the organisms (Biondo and Calado, 2021). Ornamental corals are highly sought-after marine ornamental wildlife by marine aquarium enthusiasts, hobbyists, and even researchers for various reasons. For example, most ornamental corals are used by hobbyists for aquarium decoration because of their attractive shape, colorful polyps, and associated benefits to other aquarium organisms (Rinkevich and Shafir, 2000; Dee et al., 2014). There are at least 140 coral species traded annually in the world in the form of 12 million pieces or colonies (Wabnitz et al., 2003). From the number, Indonesia supplies the trading industry with as many as 71 species of ornamental corals produced from propagation. A few highly favored and traded species, such as Cynarina lacrymalis, Trachyphyllia geoffroyi, and Wellsophyllia radiata, remain supplied from the wild due to the developmental lag in their propagation techniques (UNEP-WCMC, 2015; Johan et al., 2018).
Furthermore, coral trade remains a hotly debated issue in policy and research discourses considering the continuing trend of coral reef ecosystem degradation. Moratoriums, tiered quota systems, and trade activities, particularly for corals of natural origin, are among of the discussed management strategies (Dee et al., 2014; Cannas et al., 2019; Schaar and Cox, 2021). A more balanced management strategy has been put in place by the Indonesian government that requires coral exporters and businesses to carry out coral transplantation trials as one of the obligations in running their business (Timotius and Syahrir, 2009; Williams et al., 2014). This measure dictates that an exporter or business is required to have trial transplantation activities and then shifts to fully use the transplanted ornamental coral to meet the market demands.
The commercial scale of coral transplantation has been carried out for more than two decades in several countries. For example, Northwestern Dominica transplanted and exported 35 coral species to fulfill overseas market demand using coral broodstocks from Indonesia (Bruckner and Borneman, 2010). In 1985, marine ornamental industries in Australia used 4% transplanted life coral, 86% wild coral, and 10% dead coral for various aquarium purposes. However, in 1999, the total use of ornamental corals was 22,827 pieces in Queensland (Harriott, 2001). On the basis of the coral propagation activities report, Australia has succeeded in that action so natural extraction could be reduced (Rinkevich and Shafir, 2000).
Ornamental coral production both sexually and asexually for large polyp coral must pay attention to several factors, where these methods vary and are well established. In general, coral growth is influenced by several primary limiting factors such as temperature, sunlight intensity, depth, and currents (Nybakken, 1992; Carpenter et al., 2008). Transplanted ornamental corals, however, require that corals show their best quality such as exotic growth forms and attractive colors, which can increase consumer interest. Important parameters are light intensity and color brightness associated with other color formation factors, so the ornamental corals develop attractive colors. According to Johan et al. (2018), environmental conditions related to unsuitable coral placement during cultivation can cause color degradation and unbright coral. On the basis of field experience, coral colors in the dry season are brighter compared with those in the rainy season. Thus, coral propagation in the dry season is highly recommended.
Location and water depth of installed propagation racks are arguably vital to ensure that the transplanted ornamental corals grow better and have attractive colors. However, the intricacies and relationships between these factors for different species of transplanted ornamental corals have rarely been studied, so it is not well described. Mostly, the ornamental coral cultivators only carry out trials without recording and describing scientifically, it only seems like being done by researchers.
Therefore, this study aims to determine the site suitability of ornamental coral propagation locations based on physical and chemical parameters as the supporting factors in coral propagation to obtain a high survival rate and a high quality of exotic ornamental coral in which consumers are interested. In addition, we provide a detailed description of the development and progress of ornamental coral propagation and the benefit of coral propagation for the surrounding environment, including ex situ coral propagation in Indonesia.
This research was conducted at ornamental coral propagation locations in Banyuwangi (East Java) in 2016, 2019, and 2021. This activity was carried out with two main activities.
This study was carried out in CV. Sri Kandi Akuarium’s facility by using the six selected coral species, known for their attractive colors and large polyp size: Cynarina lacrymalis, Nemenzophyllia turbida, Euphyllia paraancora, E. glabrescens, E. yaeyamaensis, and E. divisa. These corals are in very high demand in trade, whereas its availability in nature is limited. Even some of these species are still or have been banned from entering European countries.
Each species of corals was fragmented with different fragment size, namely, with one branch, two branches, and three branches with five replicates each in 17 m depth, so the total samples were 30 fragments. Mother colonies were kept in the rack for 8-12 months for the Euphyllia spp. and more than 24 months for the Cynarina lacrymalis. Each piece was attached to a prepared artificial substrate. Binding of the substrate with the coral colonies already attached by using a rubber car tire that has been cut to a size of 3 cm along the side of a table shelf with the same length and width of 1 m.
Growth data were collected twice from all six species by identifying the types of compromised health that attached to the substrate and transplant rack, such as algae, sponges, acidians, and sedimentation.
Growth data were collected using a ruler directly and using underwater photos with a standard size scale from a ruler that was in the same frame. The photos were analyzed using ImageJ computer software to obtain the size of the coral colony and the diameter. The number of additional branches was counted at the end of the study after 7 months of the study.
Scientific observations from 14 companies in Banyuwangi waters were carried out during the audit of the ornamental coral propagation program, where every coral culturist is required to audit their ornamental coral propagation business for obtaining the activity additional permit.
Types of equipment for this research were diving equipment, an underwater camera (Canon G16, USA) for documentation, underwater writing instruments, and a Hobo (Onset, USA) data logger for recording parameters for 2 weeks. Temperature and light intensity were recorded every 1 h.
Water samples were collected three times every 2 months and analyzed in the environmental laboratory of the Research Institute for Ornamental Fish Culture (Cibinong, Indonesia). Those water quality parameters were dissolved oxygen (DO), pH, turbidity, salinity, nitrate, nitrite, and phosphate concentrations. Water quality data for light intensity and temperature were statistically tested using one-way ANOVA and further tests using Tukey’s honestly significant difference to obtain differences between depths.
Data were related to coral types and placement according to depth. All types of coral and depth positions were recorded related to its rearing place. Depth distinction was divided into three groups: shallow water (<5–9 m), medium depth (10–15 m), and deep depth (>15 m). Fragmented coral colonies were attached to an artificial substrate and tied to the placement racks.
Each of the 14 companies had a shelf plan according to depth locations, made by the company for observation purposes. The different companies had between 50 and 500 racks, depending on the company’s business scale. From these data, the number of broodstock and seedlings was obtained as coral transplantation data.
The growth rate of Euphyllia spp. shows (Figure 1) that not all coral colonies that initially had a large size (three-branched) had a rapid growth rate with a large colony size at the end of the research. Interestingly, small colonial corals (one-branched) can exceed the growth rate of two-branched corals of E. divisa, three-branched corals of E. paradivisa, and two- and three-branched corals of E. yaeyamaensis. The data also show that only three-branched corals have a larger size than two-branched corals and those occur in E. divisa and E. glabrescens corals after 5 months of study. Meanwhile, the bifurcated corals at the end of the study had the highest growth rate in coral E. paradivisa. The highest growth rate was found on three-branched specimens of E. glabrencens (2.50 cm) after 5 months, and the lowest growth rate was found on one-branched specimens of E. paraancora (Figure 1).
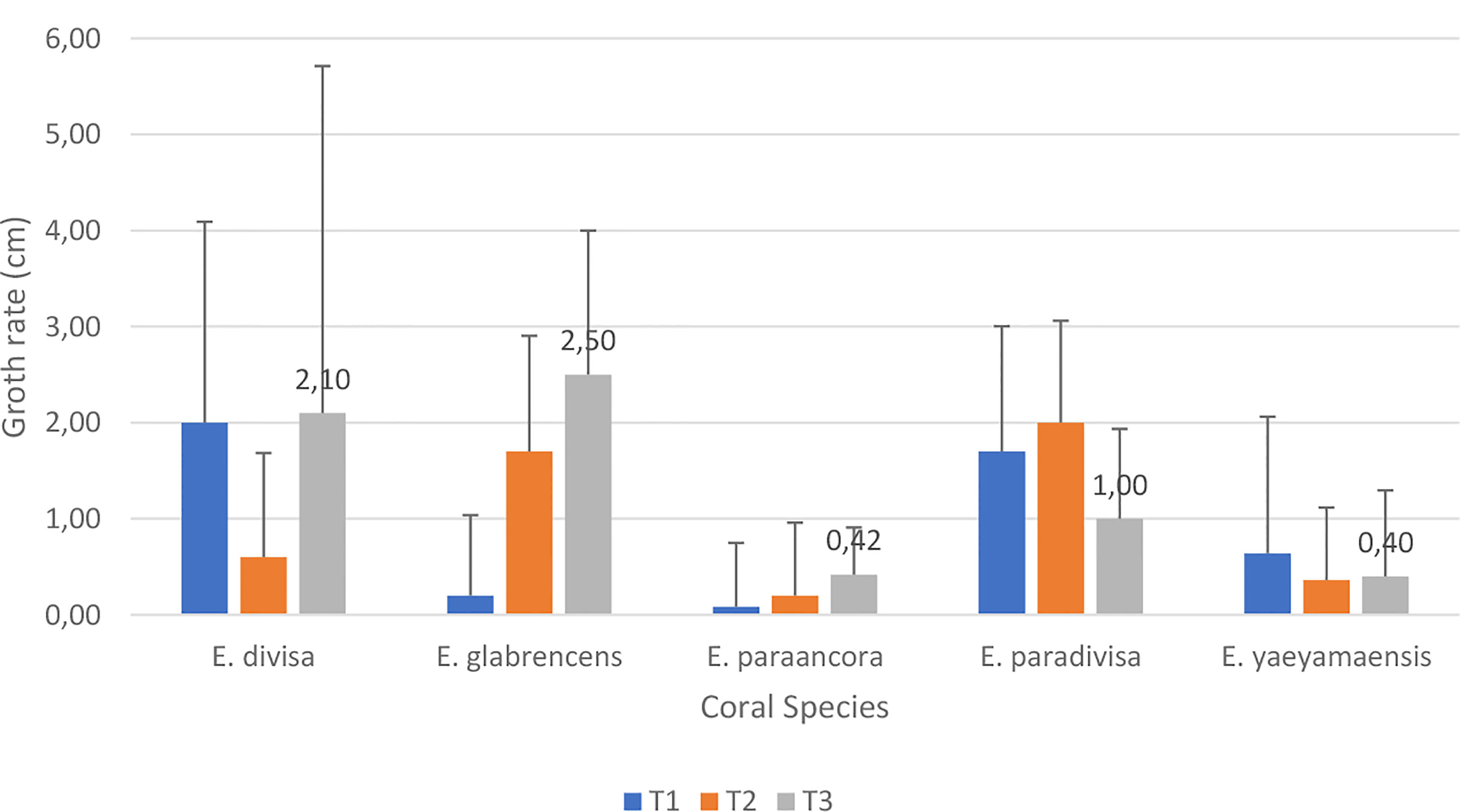
Figure 1 The coral growth rate of five Euphyllia coral species in 17 m depth after 5 months, shown as mean ± SD, n = 5.
The increase in the number of branches is not always based on the number of initial branches when treatments started. Corals with more branches, namely, two and three branches, experienced an increase in the number of branches, the most occurred in E. glabrescens and E. paraancora, which reached a mean of three branches after 5 months of observation. On the other hand, corals E. divisa and E. paradivisa (2.8 branches) grew faster than single-branched corals as shown in Figure 2.

Figure 2 Coral growth rate according to increase of branch number of five species of Euphyllia spp. after 5 months, data show mean ± SD, n = 5.
Statistical analysis revealed significant growth differences in the five species E. glabrencensvs. E. divisa, E. yaeyamaensis vs. E. divisa, and E. divisa vs. E. paraacncora as shown in Table 1. High differences occur between one branch and three branches between the species 2 (E. yaeyamaensis) and species 3 (E. divisa).
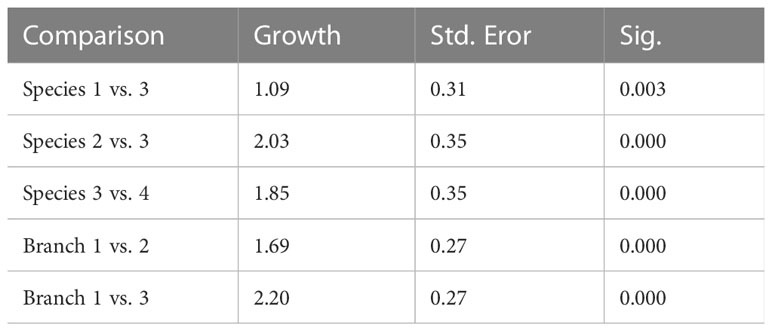
Table 1 Comparative analysis of growth average (cm) of five species of Euphyllia [E. glabrencens (1), E. yaeyamaensis (2), E. divisa (3), E. paraancora (4), and E. paradivisa (5)] based on direct counts and length measurements.
Corals that experienced the highest mortality were E. glabrescens corals with three branches, namely, three colonies (60%); this was because the coral was covered by sponges attached to all parts of the coral colony. Mortality also occurred in two colonies (40%) of three-branched corals of E. paradivisa. In single-branched E. paradivisa and E. glabrescens corals, only one colony (20%) died (Table 2). Coral mortality was generally caused by competition with other biotas, especially sponges.
Ornamental coral maintenance by companies was found in several locations such as in Binuangeun (Banten), Kepulauan Seribu (DKI Jakarta), and Sarangan (Bali). Ornamental corals that can grow well at a depth of 0–5 m generally are fast-growing corals characterized by forms of growth, including branching, encrusting, and folious. Those branching species belong to the following genera: Acropora spp., Montipora spp., Pocillopora spp., Hydnophora spp., Seriatopora spp., Echinophyllia spp., Echinopora spp., Hydnophora spp., and many other types as shown in Table 3.
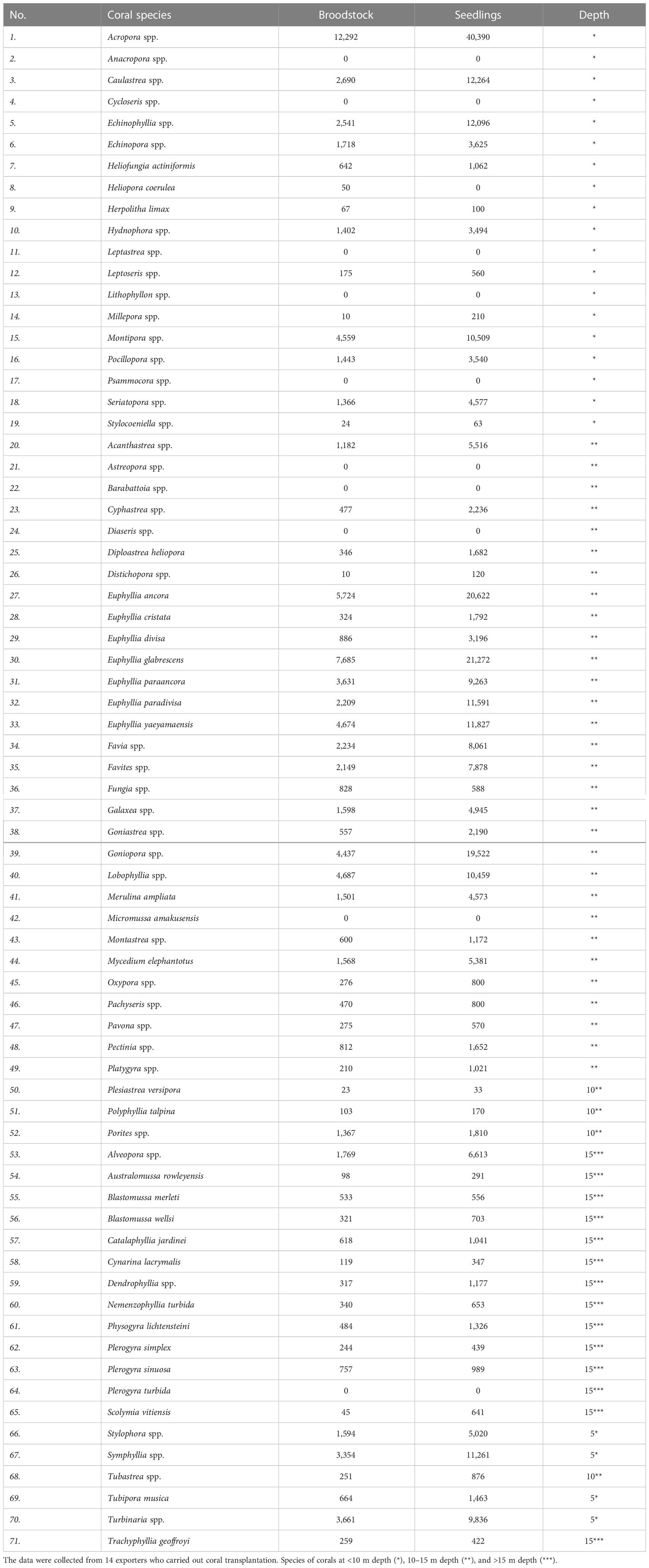
Table 3 Coral species and the number of available broodstock and fragmented corals, which were placed on the basis of the suitable depths for transplanting corals.
Coral species that can grow at a depth of 6–10 m came from moderate-growing corals such as Euphyllia spp., Galaxea astreata, G. fascicularis, Favia spp., Favites spp., Goniopora spp., Porites nigrescens, P. cylindrica, and other species as shown in Table 3. Some of these corals have long tentacles for catching food or prey so these corals can live even in turbid waters. Such observations have been found in Masaran, Jepara, where the location had a high sedimentation rate and the salinity fluctuated, because it was located in front of a river mouth. However, also, specimens from coral genera Goniopora spp. and Euphyllia spp. could survive and even dominate in one location under these conditions (Johan et al., 2007).
Corals that can grow at depths above 15 m are usually types of corals that have long tentacles, have attractive colors, and normally are preferred by consumers. In general, this type of coral species is rarely found, and distribution is very limited. This requires that the utilization of those species of corals is strictly regulated with a smaller quota limit compared with other species being collected.
In case the photosynthesis process is reduced because of limited light or high turbidity, these corals can generally take advantage of their tentacles to catch food. Usually, the prey is stung with stinging cells to immobilize prey. A certain water flow is needed so that prey can pass through coral colonies and be easily caught.
Fast-growing corals (*) were mostly found below 10 m depth, characterized by bright color, especially in the dry season. Whereas, the intermediate growth (**) was found in the range of 10–15 m, depending on the location’s brightness level. The species list of the rapid and intermediate growth rates is shown in Table 3.
Corals that can grow at a depth of 16–30 m usually have a slow growth rate (***). Apart from their unique and exotic characteristics, usually, their habitat substrates are dominated by sand and dead coral fragments, interspersed with large colonies of massive corals. This is the kind of place where valuable ornamental corals are found. These coral species include Trachyphyllia geoffroyi, Cynarina lacrymalis, Catalaphyllia jardinei, and Nemenzophyllia turbida, and some moderate-growing corals can be found in these locations (Table 3).
Fast-growing corals such as Acropora spp. can produce three to five colonies three times a year from each broodstock piece. In contrast, the medium-growth corals can only produce three colonies about two times per year. In addition, slow-growth corals can only be fragmented after more than 2 years. Mostly, the branching and foliose corals are easy to fragment and have a rapid growth rate.
On the basis of temperature and light intensity data (Table 4; Figure 3) at depths of 5, 10, and 15 m, it can be seen that kinds of corals at a depth of 15 m have a lower light intensity (statistically significant compared with those at 10 and 5 m depth). This is one of the reasons why you find different coral species at 15 m as compared with those at 5 m. Kind of species, growth, color and survival rate are related with the most suitable depth in culturing corals.
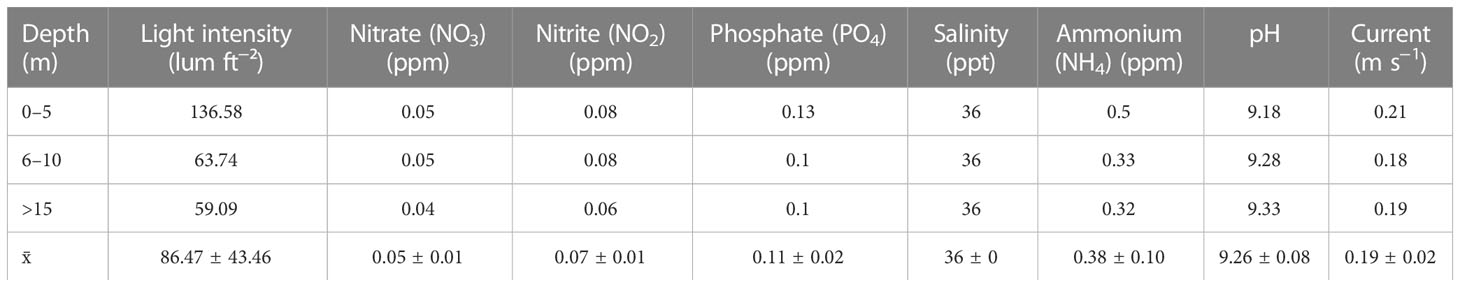
Table 4 Water quality, light intensity, and current at coral transplantation CV. Sri Kandi Akuarium’s site. Mean ± SD is presented in the last row.
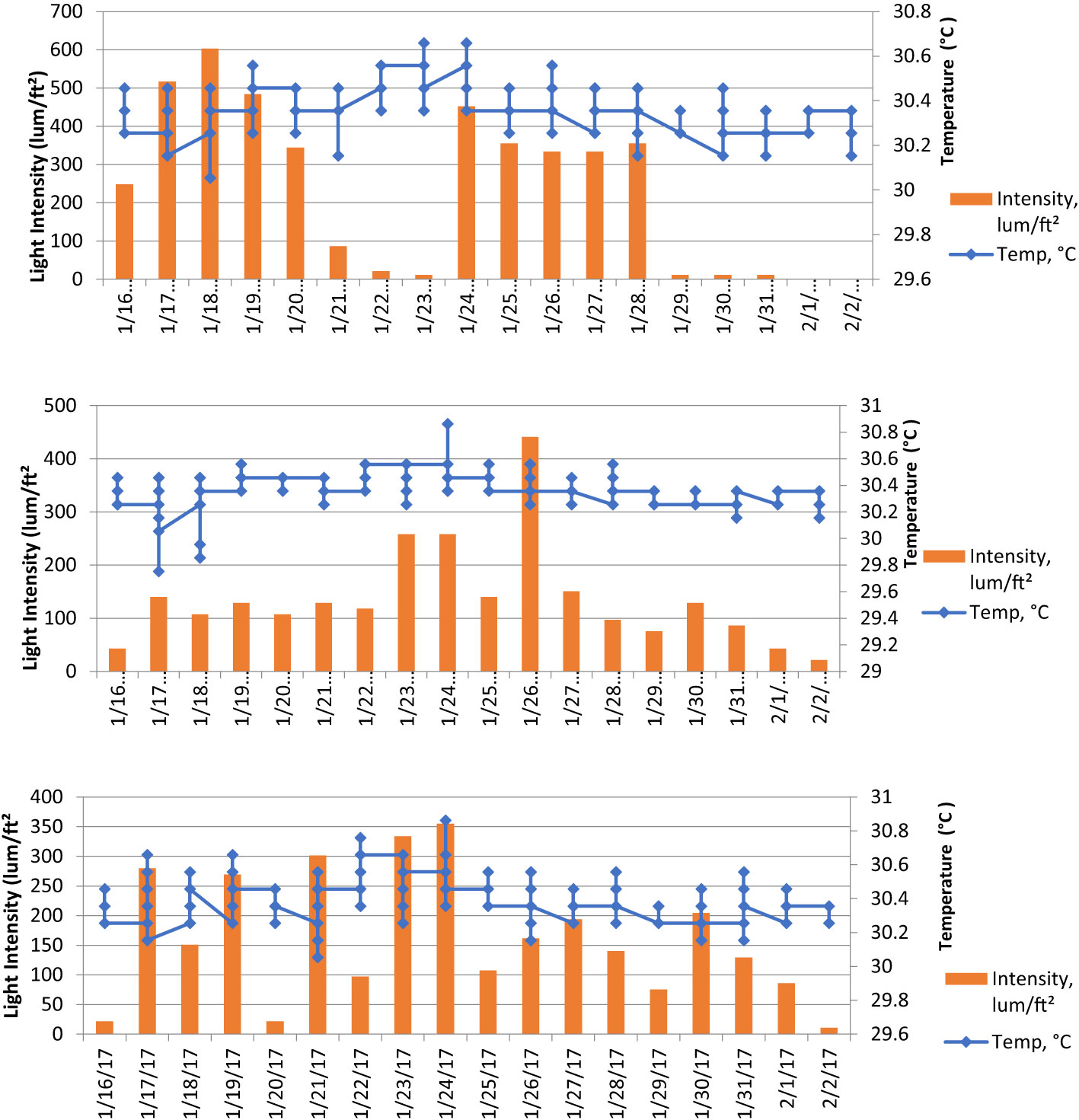
Figure 3 Temperature and light intensity parameters at a depth of 5 m (top), 10 m (center), and 15 m (bottom) using the Hobo logger data tool for ± 2 weeks (17 days) in Banyuwangi waters as one of the ornamental coral propagation locations in Indonesia.
Maximum growth rates for corals have been reported in the temperature range of 25°C–28°C (Kajiwara et al., 1995). In this study, temperature was above 28°C; therefore, the calcification rates might already decline.
Coral growth in this study was assumed to be supported by the water current and the tides. Clearly, the placement locations in the Bali strait are suitable. Banyuwangi, which is located between Java and Bali island, near the crossing bridge, has high currents. Such conditions in open straits also allow frequent changes in current patterns. In general, currents can carry food in the form of plankton (Phyto and Zoo), which are needed by corals. In addition to being photo-autotroph, corals also actively use their tentacles to catch food (Nybakken, 1992).
During 17 days of observations, the obtained average temperatures were 30.36°C, 30.38°C, and 30.40°C for depths at <10, 10–15, and >15 m, respectively (Table 2; Figure 2). Statistical testing using one-way ANOVA showed significant differences between the depths (F = 3,748, sig. = 0.024).
Average light intensity is 136.58 lum ft−2 at a depth of 5 m, 63.74 lum ft−2 at a depth of 10 m, and 59.09 lum ft−2 at a depth of 15 m. Statistically, there were very significant differences in light intensity between depths (F = 14.843, sig. = 0.000).
The light intensity is closely related to photosynthesis processes and the coral calcification, and provided irradiation takes place within the full expected spectrum, so the coral growth can be optimal (Cohen et al., 2016). Other studies have shown that the blue light spectrum is more required in the photosynthesis process of Porites lutea and Acropora variabilis, whereas light intensity and exposure length were major factors in coral growth of Platygyra sinensis and Acropora millepora (Kuanui et al., 2020).
Suitable temperature and light intensity are two important environmental conditions that are needed to obtain the color beauty and growth of ornamental corals. Pangaribuan et al. (2013) could show that the density of zooxanthellae is proportional to the nitrate and phosphate concentration. Waters with high nitrate and phosphate value can lead to bright colors in ornamental corals.
The selection of suitable locations will determine the success of the coral propagation, particularly growth and color development. This also includes avoiding bleaching (Johan et al., 2013; Johan et al., 2018). According to Al-Hammady (2013), the same species of coral (in this case Acropora spp.) can have a faster growth rate in the shallow area, compared with that at a depth of 10 m (Al-Hammady, 2013).
All coral exporters have to apply a coral propagation program for coral trade purposes. Beginner exporters can try fast-growing corals with a harvest period of 3–6 months (*) and medium growth rates with a harvest period after 8–12 months (**). For exporters who already have experience and have experts in coral propagation, they can add species and try corals that grow slower, with a harvest time of more than 24 months (***). In Table 5; Figure 4, coral species are grouped on the basis of the harvest period.
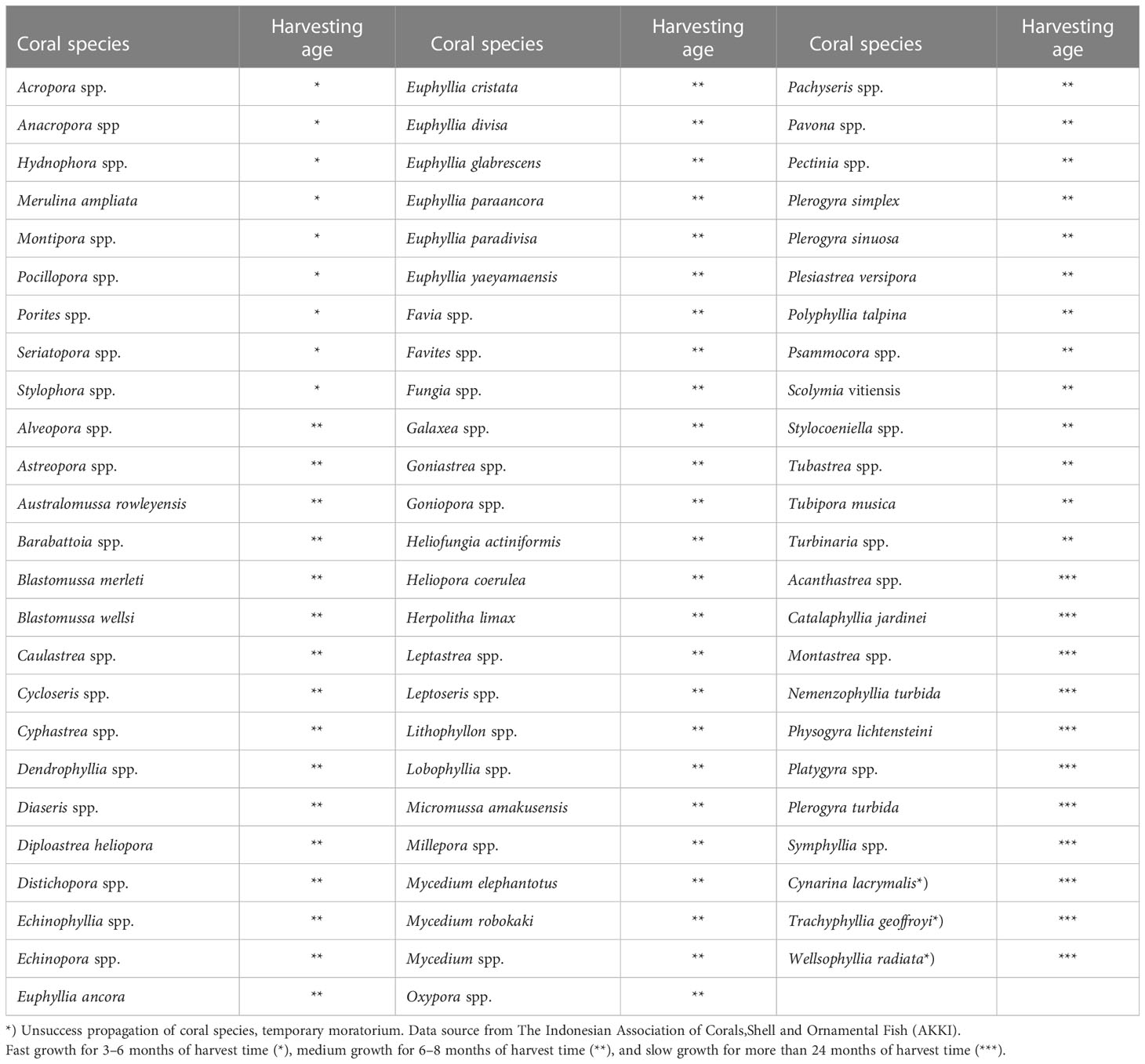
Table 5 The coral growth rate is adjusted to the recommended harvest age for cultivated ornamental corals.

Figure 4 Coral propagation for ornamental trade purposes in Banyuwangi, East Jawa. Acropora sp. with a fast growth rate (A), Goniopora sp. with medium growth rate (B), and Cynarina lacrymalis, Nemenzophyllia tubida (C) and Acanthastrea spp. (D), all with slow growth rate.
The following lessons were learnt from commercial farmers: To obtain low mortality rates and at the same time high-quality ornamental corals, the selection of the transplantation location is crucial. Various propagation locations from these farmers are specific for certain species only. For example, some specific locations, such as Serangan-Bali, Binuangeun-Banten, and Seribu Islands, DKI Jakarta, are only suitable for fast-growing corals and a few corals species with moderate growth. With completely different conditions, the transplant sites in Kendari and Banyuwangi are more likely suitable for growing also fast-growing coral species. These farmers choose depths between 1.5 and 23 m and found out that deep locations (>20 m) are not very adequate for many coral culturists. One of the reasons is that coral farms require regular care and maintenance such as cleaning the racks from algae or other biota, which is more difficult in greater depths.
All exporters in Indonesia are expected to perform coral propagation. In the process, first, exporters’ staff (fishermen) have to prepare mother stock corals, obtained with official agreements of the Scientific Authority and Management Authority, which are issued annually. The “broodstock” has to be maintained for 3–6 months according to the growth rates described in Table 3. Then, farmers are allowed to fragment the mother colony by leaving at least half to one-third of the original size. Fragmented corals are fixed to an artificial substrate that has been provided. Each artificial substrate is tagged with information about the company code, coral name, fragment numbers, and month of propagation (Figure 5). Propagation activities must be documented and then reported to the Natural Resources Conservation Center (KSDA) under the Ministry of Environment and Forests unit, as a representative of the Management Authority. There were 84 exporters in 2018, who were active with propagation of ornamental corals, and these were decreased to 66 and 62 exporters in 2020 and 2022, respectively (Table 4).

Figure 5 Tagging of propagated coral attached to artificial substrate. Examplary: 01, KSDA area; 04, exporter; 21, year; Gafa, coral species; 02, generation; 0001, fragment number; 1–12, month of fragmentation.
The evaluator or auditor as representative of the Scientific Authority performs the task of inspecting whether the coral propagation procedure has been followed such as giving a correct name on tags, coral species, amount of fragments per species, and survival. This includes checking the area eligibility for coral propagation to prevent damage toward coral habitats and other ecosystems in the surrounding location (Figure 5). The evaluators come from the Indonesian Science Center (LIPI, now BRIN), Ministry of Marine Affairs and Fisheries (KKP), and University and NGO teams. The report of the auditors will be the basis for issuing the production and export quota that is approved every year after checking in the field. Companies that do not pass in auditing, because they do not follow the established propagation procedures, can submit an audit again after making improvements according to the advice of the previous auditor. There are three types of audits: first, the feasibility audit to determine the feasibility level of an ornamental coral propagation unit effort following established procedures; second, for adding ornamental coral species if there are additional species from the previous years; and third, every 2 years for monitoring and evaluating coral propagation activities, whether exporters and business actors still follow procedures so as not to damage coral biodiversity.
Coral broodstock can release larvae according to the coral reproduction cycle. Even coral mother stocks for fragmentation (if not being utilized for fragmenting) can release larvae, when reaching the mature size. Many coral recruits can be found within the propagation area and attached to the iron frames and tables, used for propagation. A new policy requires the return of 10% of the propagation results to nature to contribute in the improvement of the coral reef ecosystem at the farm sites (Johan and Suharsono, 2018).
An increase in reef fish population was also observed at the propagation locations, because the locations were monitored and coral shelves and substrates were cleaned of competing biotas such as sponges, ascidians, algae, and predatory animals such as Drupella sp. Naturally, ornamental coral propagation sites are free from destructive fishing practices such as the use of bombs and cyanide. Propagation workers are required to come from the local area to enhance the positive impact on improving the economy of the community in that location.
During 2019, first problems arose when the Ministry of Marine Affairs and Fisheries did not issue health certificates for the export of corals. This led to a situation, where corals were neglected and many competing biotas grow on the transplant racks such as algae, soft corals, sponges, and other biota (see examples shown in Table 4; Southeast Sulawesi BKSDA, Bali BKSDA, East Java BBKSDA, and a few other locations).
In 2008, there were 84 business units in the ornamental propagation sector, and, by 2020, the number decreased to 66 business units only. The government agency that assists and regulates the ornamental coral business uses 11 Natural Resources Conservation Centers (BKSDA), which are widely spread in Indonesia, under the surveillance of the Ministry of Environment and Forestry (Table S1).
Each of those business units had 50 to 500 shelves before the export constraints arose. In 2020, these units were re-evaluated by a team of auditors, consisting of LIPI and ICRWG staff. On the basis of recent data, there were only 58 transplant business units left in Indonesia.
Nowadays, ornamental coral culturists are facing some new problems, such as the decrease in market demand, the difficulties of high transportation costs, and numerous constraints in management due to pandemic situations of COVID-19, when auditing and the evaluation were not possible. Therefore, alternative solutions are required, e.g., through video and photo assessment.
Recently, ornamental coral propagation with fragments has been successfully carried out in two land-based farms (PT. Golden Marindo and CV. Bivaria) and a third farm has just started (CV. Cahaya Baru). These kinds of innovative approaches need to be supported as alternative solutions for satisfying market demand.
The successful development of ornamental coral propagation was demonstrated at several locations in Indonesia. It was related to the suitability of the species at each location and the development of ornamental coral trade management in Indonesia.
The demand for ornamental corals is increasing because of more hobby aquarists and increasing value, providing foreign exchange for developing countries. To satisfy the market, countries such as Indonesia have introduced propagation rules for exporting companies. From 14 commercial farmers, the following lessons were learned:
a) The highest growth rate was found on three branched E. glabrencens (2.50 cm) after 5 months and the lowest growth rate was found on one branched E. paraancora. Corals with more branches (size) with two or three branches of E. glabrescens and E. paraancora have increased the number of branches to three branches as average after 5 months. Although, small colonies also survive with a mortality of 20% during the study.
b) Fast-growing corals are suitable to be propagated at depths of about 5 m, whereas moderate growth rates are suitable for growing at a depth of 5–15 m. Corals with slow growth are suitable to grow in areas with a depth of above 15 m.
c) The coral color is determined by light intensity and spectrum, and brightly colored corals prefer low light intensity. The development of ornamental coral propagation has advanced in several locations in Indonesia, whereas ex situ coral propagation has been started by a small number of coral business companies. Conservation of corals and their habitats can be achieved by cultivating ornamental corals, so in the future, the fulfillment of coral demand can be accomplished without destructing natural habitats.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
OJ designed the research and did the coral transplantation research, OJ and AB collected the data to identify species of transplanted corals. OJ, RG, IA, AP, AK wrote, revision and data analysis. AK made sure and final checked the acquisition, analysis, interpretation of data before final approval of the version to be published.
We would like to thank the Indonesian Association of Corals, Shell and Ornamental Fish, National Association of Ornamental Coral Transplantation, Indonesian Coral Reefs Working Group. The Indonesian Coral Reefs Foundation (TERANGI) and special for CV. Sri Kandi Akuarium who have facilitated this research activity, and all those who have assisted in the successful implementation of research in the field. Thank also to Hatim Albasri who help to improve the English in proof reader.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.928538/full#supplementary-material
Al-Hammady M. A. M. (2013). The effect of zooxanthellae availability on the rates of skeletal growth in the red Sea coral Acropora hemprichii. Egyptian J. Aquat. Res. 39 (3), 177–183. doi: 10.1016/j.ejar.2013.10.005
Best B. A. (2002). “Trade in coral reef animals, algae and products: an overview,” in Implications for coral reef management and policy: relevant findings from the 9th international coral reef symposium. Eds. Best B. A., Pomeroy R. S., Balboa C. M., 73–77. the U.S. Agency for International Development, in collaboration with, the World Resources Institute, Conservation International, and the International Society for Reef Studies (The Environment Information Clearinghouse, PADCO, Inc. The United States of America).
Biondo M. V., Calado R. (2021). The European union is still unable to find nemo and dory-time for a reliable traceability system for the marine aquarium trade. Animals 11 (6), 1668. doi: 10.3390/ani11061668
Bruckner A. W., Borneman E. H. (2010). Implications of coral harvest and transplantation on reefs in northwestern Dominica. Rev. Biol. Trop. 58 (SUPPL. 3), 111–127. doi: 10.15517/rbt.v58i0.20060
Cannas R., Follesa M. C., Cau A., Cau A., Friedman K. (2019). Global report on the biology, fishery and trade of precious corals. FAO Fish. Aquacul. Circular C1184), I–254.
Carpenter K. E., Abrar M., Aeby G., Aronson R. B., Banks S., Bruckner A., et al. (2008). One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563. doi: 10.1126/science.1159196
Carvalho L. M., Mies M., Inagaki K. Y., Sanches E. G., Souza M. R., Longo G. O., et al. (2022). The marine ornamental market in Brazil (Southwestern Atlantic) frequently trades prohibited and endangered species, and threatens the ecosystem role of cleaning mutualism. Mar. Policy 146, 105305. doi: 10.1016/j.marpol.2022.105305
Cohen I., Dubinsky Z., Erez J. (2016). Light enhanced calcification in hermatypic corals_ new insights from light spectral responses _ enhanced reader.pdf. Front. Mar. Sci. 2 (122). doi: 10.3389/fmars.2015.00122
Dee L. E., Horii S. S., Thornhill D. J. (2014). Conservation and management of ornamental coral reef wildlife: successes, shortcomings, and future directions. Biol. Conserv. 169, 225–237. doi: 10.1016/j.biocon.2013.11.025
Harriott V. J. (2001). The sustainability of queensland’s coral harvest fishery (Townsville: CRC Reef Research Centre), 33. CRC Reef Research Centre Technical Report No. 40.
Johan O., Bengen D. G., Zamany N. P., Suharsono (2013). Distribusi dan kelimpahan penyakit karang sabuk hitam secara spasial di kepualan seribu, Jakarta. Jurnal Riset Akuakultur 8 (3), 439–451. doi: 10.15578/jra.8.3.2013.439-451
Johan O., Budianto A., Priono B. (2007). Study of relationship between types of ornamental corals with habitat characteristics in several locations in Indonesia. In Proceeding of Maritime National Seminar lll. Hang Tuah University Surabaya, p. 37–42.
Johan O., Ginanjar R., Kadarini T. (2018). Budidaya karang hias polip besar pada kedalaman yang berbeda di alam dan sistem resirkulasi. Jurnal Riset Akuakultur 13 (3), 229–237. doi: 10.15578/jra.13.3.2018.229-237
Johan O., Suharsono (2018). “Kontribusi budidaya karang hias dalam mendukung konservasi dan rehabilitasi kondisi terumbu karang di Indonesia,” in Rehabilitasi ekosistem terumbu karang untuk keberlanjutan sumberdaya perikanan, 1st ed. Eds. Suharsono N., Wiadnyana, Edrus I. (Jakarta Pusat. Indonesia: AMAFRAD Press-Badan Riset dan Sumberdaya Manusia Kelautan dan Perikanan), 271–282.
Kajiwara K., Nagai A., Ueno S., Yokochi H. (1995). Examination of the effect of temperature, light intensity, and zooxanthellae concentration on calcification and photosynthesis of scleractinian coral acropora pulchra. Faculty Mar. Sci. Technol. Tokai Univ. 40, 95–103.
Kuanui P., Chavanich S., Viyakarn V., Omori M., Fujita T., Lin C. (2020). Effect of light intensity on survival and photosynthetic efficiency of cultured corals of different ages. Estuarine Coast. Shelf Sci. 235, 106515. doi: 10.1016/j.ecss.2019.106515
Nybakken J. W. (1992). Biologi laut suatu pendekatan ekologis. Eds. Eidman A. b. H. M., Bengen K. D.G., Sukardjo M.H. d. S. (Jakarta: PT Gramedia).
Pangaribuan T. H., Ain C., Soedarsono P. (2013). Hubungan kandungan nitrat dan fosfat dengan densitas zooxanthellae pada polip karang acropora sp. di perairan terumbu karang pulau menjangan kecil, karimun jawa. Diponegoro J. Maquares 2 (4), 136–145. doi: 10.14710/marj.v2i4.4277
Rinkevich B., Shafir S. (2000). Ex-situ culture of colonial marine ornamental invertebrates: concepts for domestication. Aquarium Sci. Conserv. 2 (4), 237–250. doi: 10.1023/A:1009664907098
Schaar S. I., Cox L. J. (2021). The future for hawai‘i’s marine aquarium fishery: a cost benefit analysis compared to an environmental impact assessment. Mar. Policy 127, 104429. doi: 10.1016/j.marpol.2021.104429
Timotius S., Syahrir M. (2009). A review on ornamental coral farming effort in Indonesia 12–14. Int. Ocean Sci. Tech and Policy Symp, World Ocean Conference, Manado. p 12-14.
Wabnitz C., Taylor M., Green E., Razak T. (2003). From ocean to aquarium: the global trade in marine ornamental species (UNEP-WCMC, Cambridge, UK: UNEP World Conservation Monitoring Centre). Available at: http://www.unep-wcmc.org/resources/publications/UNEP_WCMC_bio_series/17.htm.
Williams S. L., Janetski N., Abbott J., Blankenhorn S., Cheng B., Crafton R. E., et al. (2014). Ornamental marine species culture in the coral Triangle: seahorse demonstration project in the spermonde islands, sulawesi, Indonesia. Environmental Management 54, 1342–1355. doi: 10.1007/s00267-014-0343-6
Keywords: coral trade, depths, environmental conditions, propagation, export, growth
Citation: Johan O, Ginanjar R, Budiyanto A, Ardi I, Priyadi A and Kunzmann A (2023) The success of ornamental coral propagation in Banyuwangi East Java, Indonesia: observation of different depths and species. Front. Mar. Sci. 10:928538. doi: 10.3389/fmars.2023.928538
Received: 25 April 2022; Accepted: 27 March 2023;
Published: 07 June 2023.
Edited by:
Shubhadeep Ghosh, Central Marine Fisheries Research Institute (ICAR), IndiaReviewed by:
Biji Xavier, Central Marine Fisheries Research Institute (ICAR), IndiaCopyright © 2023 Johan, Ginanjar, Budiyanto, Ardi, Priyadi and Kunzmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ofri Johan, b2ZyaS5qb2hhbkBicmluLmdvLmlk
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.