
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Mar. Sci. , 19 December 2023
Sec. Marine Ecosystem Ecology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1304000
This article is part of the Research Topic Degradation, Ecological Restoration and Adaptive Management of Estuarine Wetlands under Intensifying Global Changes, volume II View all 11 articles
Studies examining the relationship between seaweeds and the diversity of associated organisms have been attempted far less than those focusing on the influence of terrestrial plants. That is troublesome considering the growing number of studies reporting the decline or local loss of macroalgae because of ocean warming and climate change. Since the fate of macroalgae will have an influence on associated organisms, this brief overview examined the different roles played by macroalgae, making the distinction between morphological features associated with individual seaweed species and those associated with populations or habitats. Most studies at both (individual and population) levels have identified positive relationships between morphological features such as structural complexity (including fractal dimensions) and invertebrate biodiversity, and the abundance of various faunistic groups. Some of these relationships are stronger than others, often with complex outcomes, suggesting that the current and future ecological benefits provided by macroalgae are strongly species- and habitat-dependent. While the displacement or local-scale loss of seaweeds may continue because of climate change, the features identified here may become useful in light of conservation and restoration efforts.
Many macroalgal species function as ecosystem engineers or foundation species (sensu Marzinelli et al., 2016) and are therefore central to restoration and sustainability goals (Ellison et al., 2005; Marzinelli et al., 2016). These macroalgae create direct or indirect food sources (Tano et al., 2016), space suitable for attachment, growth, or reproduction (Gallardo et al., 2021), refuge from predators (Best et al., 2014), and altered (often ameliorated) physical conditions for marine invertebrates (Brawley and Adey, 1981; Christie et al., 2003; Schmidt and Scheibling, 2006; Best et al., 2014; Schaal et al., 2016; Tano et al., 2016; Gallardo et al., 2021; El-Khaled et al., 2022) and vertebrates (e.g., Norderhaug et al., 2005), including seabirds and mammals (Lorentsen et al., 2010; Christensen-Dalsgaard et al., 2017). Despite their importance, studies addressing the biodiversity associated with marine macroalgae have been attempted considerably less than those associated with terrestrial plants (Parker et al., 2001; Goodsell et al., 2004; Best et al., 2014; Gallardo et al., 2021). However, there is a need for these studies given ongoing climate events and anthropogenic disturbances, which are resulting in accelerated loss of species across coastal habitats worldwide (Bates and DeWreede, 2007). In this changing scenario, quantifying the contribution of macroalgae to biodiversity, both as individual algae or as clumps, beds, or populations, is undeniably important (Bates and DeWreede, 2007; El-Khaled et al., 2022).
Several macroalgal morphological or structural features have been directly or indirectly associated with the colonization and use of macroalgae by infaunal and epifaunal organisms (Bates and DeWreede, 2007; Duarte et al., 2020). However, these features have not been thoroughly explored in the context of biodiversity levels and their change in a warming ocean (Chemello and Milazzo, 2002). An examination of these features at the individual and population levels is not only important from a research perspective. It may also help conservation efforts, particularly in the case of macroalgal species that are threatened or are experiencing long-term declines (Lilley and Schiel, 2006; Marzinelli et al., 2014; Tummon Flynn et al., 2019). For those species, evidence showing their contribution to biodiversity and the features that enhance biodiversity levels, can support claims calling for their further protection and restoration (Marzinelli et al., 2014; Buršić et al., 2019; El-Khaled et al., 2022). Therefore, the goal of this minireview was to examine whether macroalgae influence biodiversity, and to identify the characteristics of individual seaweeds, or seaweed populations, that have an influence on any aspect of biodiversity. For this review, “biodiversity” was quantified using various measures, including species richness, diversity and/or species evenness, and biomass and abundance of organisms, among other metrics.
The influence of macroalgae on biodiversity is outlined in Table 1 and includes a compilation of 62 published studies (as of November 2023) deemed representative rather than comprehensive of those addressing seaweed-biodiversity relationships. We focused primarily on physical or structural features, including fractal dimensions (e.g. Davenport et al., 1996; Tokeshi and Arakaki, 2012) when measured, but did not account for a distinct branch of studies on algal chemical products (e.g. Hay and Fenical, 1996). These chemical products have evolved from the interaction between seaweeds, consumers, hosts, or colonizers (often as defenses against herbivory) at every coastal region and latitude (see e.g., Amsler, 2008 and Amsler et al., 2014). Their influence on species interactions and biodiversity has been comprehensively reviewed by other authors (Amsler, 2008 and chapters and references therein; Ianora et al., 2006; Hay, 2009; and Sotka et al., 2018, among others). Studies focusing primarily on aquatic plants outside of the realm of marine macroalgae were not considered either. For instance, species such as Zostera marina often overlap and interact with various marine macroalgae (Richard and Quijon, 2023). However, they belong into a distinct group of flowering plants with root systems, which has been examined by other authors (e.g., Hansen et al., 2010) and was deemed beyond the scope of this minireview. The articles examined here were obtained using search engines such as OneSearch and Google Scholar, networks (e.g., ResearchGate), and cross-listed references within articles and other online resources. Key words used in these searches included “seaweed” or “algae” or “kelp,” “rhodoliths”, “biodiversity” or “diversity” “invertebrates” or “epifauna” or “infauna”, and various combinations among them. Our review uses rather loosely the terms algae and seaweeds, but in all instances, we are referring to marine macroalgae. Likewise, our review includes studies on algae identified as “foundation species” or “ecosystem engineers”, but these terms were not used as keywords given their widely known importance (e.g., Dayton, 1985; Stachowicz, 2001; Ellison et al., 2005) and much broader influence, often above the level of communities.
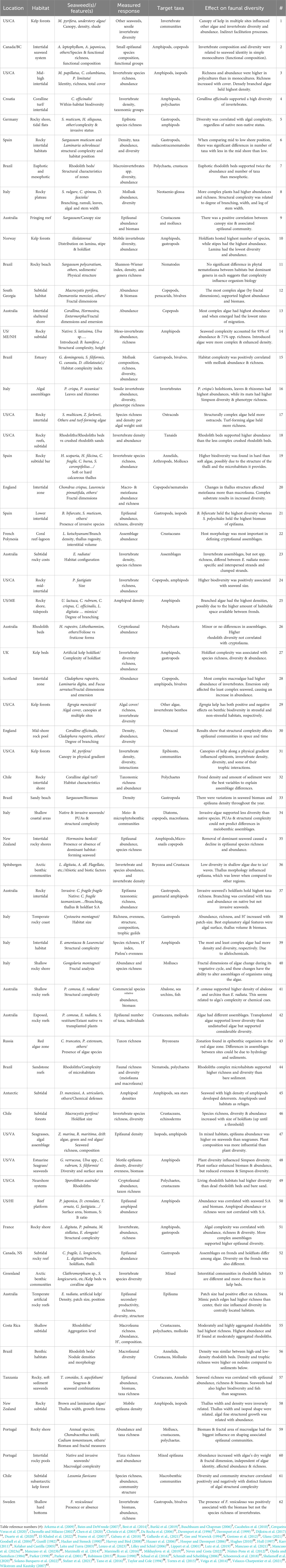
Table 1 A summary of studies identifying relationships between macroalgae or their features and various aspects of coastal organism biodiversity levels. In addition to location and type of habitat, seaweed species and features, responses measured, target taxa (or groups of taxa) and reported effects on biodiversity are presented for 62 studies (reference #s are organized alphabetically by authors’ last names and are identified in the footnote of this Table).
Out of the studies compiled in Table 1, a large majority suggests that seaweed’s structural complexity (measured for instance as branching, physical rugosity or fractal dimensions) contributes to various metrics of invertebrate diversity and abundance. Higher structural complexity has been causally linked with increased invertebrate biodiversity (Chemello and Milazzo, 2002; Frame et al., 2007; Schaal et al., 2016; Mancuso et al., 2023a), species richness (Gee and Warwick, 1994; Duarte et al., 2020), and organisms’ abundance (Russo, 1990; Davenport et al., 1996; Chemello and Milazzo, 2002; Frame et al., 2007; Schaal et al., 2016; Dijkstra et al., 2017; Duarte et al., 2020). The reported influence of complexity is variable, resulting in increases of nearly 58% in Shannon-Wiener diversity (Chemello and Milazzo, 2002) to increases in biodiversity levels ranging between 52% and 115%, depending on the time of year (Schaal et al., 2016). Algal complexity has also resulted in increases that ranged from 40% (Dijkstra et al., 2017) to an almost eight-fold increase in abundance (Chemello and Milazzo, 2002). Consistent with this, seaweed’s fractal dimensions have been also shown to have an influence on invertebrate abundance and taxon richness (Mancuso et al., 2023a) regardless of host identity (Veiga et al., 2014). However, some studies have also found mixed results, with strong positive correlations between community structure and algal complexity features such as holdfast diameter and frond length, and negative correlations with features such as the number of stipes (Velasco-Charpentier et al., 2021).
A few studies have found that algal structural complexity had little influence on e.g. invertebrate species richness (Russo, 1990; Dijkstra et al., 2017) or abundance (Russo, 1990; Lenzo et al., 2023), while others have found that these relationships change temporarily. According to Da Rocha et al. (2006), similar assemblages could be associated with structurally different macroalgae, but complexity still have an influence on the biology of the dominant groups of invertebrates. Due to life cycles, macroalgal morphological features change seasonally (Leite and Turra, 2003; Mancuso et al., 2023b) and this has prompted studies examining the influence of these changes on associated epifaunal assemblages (Hull, 1997; Leite and Turra, 2003; Torres et al., 2015; Mancuso et al., 2023b). For instance, changes in fractal dimensions during the algal vegetative cycle have resulted in changes in associated gastropod communities (Mancuso et al., 2023b) and the timing of peak abundances of ostracod populations (Hull, 1997). The latter author, for example, found that ostracod densities in the most complex alga (Ceramium nodulosum) peaked in February, whereas in the least complex alga (Chondrus crispus) they peaked in December (Hull, 1997). Meanwhile, the seasonal variation in biomass of a different seaweed (Sargassum spp) appeared to be unrelated with the seasonal variation of its own associated epifauna (Leite and Turra, 2003). Yet another study (Torres et al., 2015) found that a combination of two of the macroalgal features cited above (fractal area and biomass) were the best predictors of associated epifaunal assemblages.
While the studies cited above examined seaweed structural complexity at the individual level, others have focused on the complexity of specific traits of seaweeds (e.g., thallus and holdfast morphology). Such traits specifically include blades’ surface area or density (Gunill, 1982; Russo, 1990; Parker et al., 2001; Kelaher and Castilla, 2005), degree of branching or number of blade branches (Hacker and Steneck, 1990; Chemello and Milazzo, 2002; Lutz et al., 2019), thallus characteristics (i.e., the morphology of full seaweeds) (Taylor and Cole, 1994; Schmidt and Scheibling, 2006; Gallardo et al., 2021), and holdfast characteristics (Ojeda and Santelices, 1984; Hauser et al., 2006; Schmidt and Scheibling, 2006; Lutz et al., 2019). The first features (seaweed surface area and density) have been found to have the strongest relationships with invertebrate assemblages (Kelaher and Castilla, 2005). These features have been shown to contribute to significant increases in invertebrate diversity (Gunill, 1982), from two to ten-fold increases in abundance (Russo, 1990; Gunill, 1982), and up to a 15-fold increase in biomass (Parker et al., 2001). Exceptions have been found, though, where surface area was shown to reduce invertebrate diversity and evenness (Parker et al., 2001).
Physiological features of seaweed thalli have also been linked with significant changes in invertebrate biodiversity (Gallardo et al., 2021) and density levels (Taylor and Cole, 1994). For example, comparing soft and hard thalli, Gallardo et al. (2021) reported a 20-50% increase in biodiversity levels in hard thalli species when compared to soft thalli species. Likewise, Taylor and Cole (1994) showed an inverse relationship between thallus width and invertebrate densities, with a density increase of nearly 300% from the widest to the narrowest thallus. Such a surprising relationships can be explained by the finely structured thinner thalli, which led to a higher small-scale structural complexity that favored epifaunal density (Taylor and Cole, 1994). The branching of the thalli also supported a higher number of taxa and individuals (Lutz et al., 2019), and higher overall abundances and biodiversity levels (Hacker and Steneck, 1990; Chemello and Milazzo, 2002). For example, the degree of branching increased the density of amphipods, among the most common epifaunal groups associated with seaweeds (e.g., Tummon Flynn et al., 2021), from ~2 individuals/100mL interstitial volume on seaweeds with large leathery thalli, to ~22 individuals/100mL interstitial volume on coarsely branched thalli (Hacker and Steneck, 1990).
Structural complexity has also been linked to a prominent group of red coralline algae, the Rhodoliths (Harvey and Bird, 2008; Stelzer et al., 2021; Neves and Costa, 2022). They create a porous habitat that enhances individual and bed physical complexity (Gabara et al., 2018, Cerqueira Veras et al., 2020), supporting significantly higher diversities than non-living beds or adjacent bare sediments (Robinson, 2015; Stelzer et al., 2021; Neves and Costa, 2022), or even some kelp beds (Schoenrock et al., 2018). Along with complexity, the depth (shallow or deep, in tropical or polar latitudes, respectively; Mikhaylova et al., 2019; Cerqueira Veras et al., 2020) and density of these beds also contribute to biodiversity. Counter examples include studies where different rhodolith forms are only weakly associated with species assemblages (Harvey and Bird, 2008), or host morphology was the most important in determining crytofaunal assemblages associated with crustose coralline algae (Glanz, 2021). Lastly, regarding macroalgae that have holdfasts, it has been found that taxa richness (Lutz et al., 2019), number of species, species diversity, and abundance (Ojeda and Santelices, 1984; Hauser et al., 2006) all significantly increased in relation with holdfast structural complexity. For example, Hauser et al. (2006) found increases of nearly 170%, 250% and 260% in invertebrate species richness, diversity, and abundance, respectively, from the least to the most complex holdfasts of artificial kelps under comparison. Similarly, Lutz et al. (2019) found that a species of non-indigenous green alga (Codium fragile spp. Fragile), which possessed more complex holdfasts than native conspecifics (C. fragile), held twice the species richness. However, it is noteworthy that at least two studies have found no differences in invertebrate taxa (Schmidt and Scheibling, 2006) and assemblages (Lutz et al., 2019) in relation to the holdfast complexity, for kelp and green algae, respectively.
In addition to features associated with individual seaweeds, several studies have focused on how the structure or makeup of entire seaweed communities (or habitats) contribute to invertebrate diversity. Various aspects of invertebrate communities, including biodiversity, have been associated with seaweed diversity and/or species richness (Parker, 1998; Bates and DeWreede, 2007; Best et al., 2014; Tano et al., 2016), seaweed identity and species composition (Parker, 1998; Lilley and Schiel, 2006; Wikstrom and Kautsky, 2007; Bates and DeWreede, 2007; Marzinelli et al., 2016), seaweed assemblage structure (Goodsell et al., 2004; Frame et al., 2007), seaweed habitat size (Shelamoff et al., 2020; Chen et al., 2020; Mancuso et al., 2021), and habitat position in relation to tide level (Davenport et al., 1999; Hooper and Davenport, 2006; Cacabelos et al., 2010; Loke and Todd, 2016). The first of these features, seaweed species richness, has often been correlated with higher invertebrate species richness (Parker, 1998; Best et al., 2014). For instance, Best et al. (2014) found that while habitats with higher algal species richness had ~9% higher animal species richness, they tend to have lower animal abundance. At least two articles have found this association to be weak (Parker, 1998; Bates and DeWreede, 2007) and claimed that seaweed species composition (rather than richness) correlated better with increases in invertebrate diversity. With regards to the structure of seaweed assemblages, Goodsell et al. (2004) did not find causal effects on species richness, but the makeup of invertebrate communities was significantly different among distinct algal habitat configurations. Likewise, Frame et al. (2007) found that the abundance of ostracods was closely associated with the structural complexity of various algae, while their highest species richness was associated with turf-forming algae.
Macroalgae often form patches, beds, or habitats of limited dimensions. The size of these patches has been positively correlated with invertebrate species richness, abundance, and biomass (Shelamoff et al., 2020; Mancuso et al., 2021). The former author found that a decrease in seaweed patch size reduced animal species richness by 50%, while reducing Shannon-Wiener diversity by 20%, from the largest to the smallest patch (Shelamoff et al., 2020). Similarly, Chen et al. (2020) found that canopy volume in conjunction with algal weight, had a strong correlation with invertebrate abundance and biomass. Variation in biotic and abiotic factors due to differences in geographic location often accounts for part of these relationships. However, studies conducted in high (including polar) latitudes, follow the general trend observed in warmer coasts: bed size and structural complexity favors higher invertebrate diversity (Lippert et al., 2001; Schoenrock et al., 2018). Harsh physical conditions (including ice and wave stress) frequently limit overall diversity in shallow compared to deep habitats, increasing the likelihood of unstable, more fluctuating communities and favoring the development of barren habitats (Lippert et al., 2001; Mikhaylova et al., 2019). Despite that, most evidence suggests that the food, habitat, and refuge associated with complex seaweed beds also favors stability and ultimately local biodiversity (e.g., Lippert et al., 2001; Núñez-Pons et al., 2012; Mikhaylova et al., 2019). Locally, large seaweed beds, particularly kelp forests, display interesting (complex) relationships between canopies, physical conditions and species interactions and diversity. Karr (2011) found that Macrocystis pyrifera kelp forests most directly exposed to swell, supported larger density and diversity (and different assemblages) than less exposed forests. The same author suggested that kelp canopy’s functional role was a nursery habitat for fish, that indirectly altered trophic interactions (Karr, 2011).
The role played by seaweed beds has also been associated with the presence or absence of certain species, including the giant kelp referred to above (Karr, 2011). For example, large canopies of giant kelp have strong direct and indirect effects on other seaweeds, and ultimately on invertebrate diversity and community structure (e.g., Arkema et al., 2009). As stated above, the influence of “foundation species” (Dayton, 1985; Stachowicz, 2001) exceeds community-level metrics such as biodiversity. Such influence is strong, but as described by Hughes (2010) for the brown alga Egregia menziesii, is also complex and varies with local physical conditions and spatial scale. When these key species are artificially removed or lost, they cause strong changes over multiple species and trophic levels (Ellison et al., 2005; Castorani et al., 2018; Montie and Thomsen, 2023). For example, Lilley and Schiel, 2006 found that when a dominant habitat forming seaweed species was removed, the period following removal (up to two years afterwards), was characterized by a significant decrease in the number of invertebrate taxa. In fact, two years after these experiments, removal plots had ~40% less taxa than control (non-removal) plots (Lilley and Schiel, 2006). Effects on different trophic levels resulting from kelp harvesting in Norway have been reported by Lorentsen et al. (2010) and Christensen-Dalsgaard et al. (2017). Likewise, Perälä et al. (2023) have highlighted the negative influence of the harvesting of Laminaria hyperborea forests from the Northeast Atlantic on the survival of Atlantic cod and European lobsters. All those results are closely related with those documented by Wikstrom and Kautsky (2007), who showed that in the absence of a common seaweed, the biomass of associated invertebrates was significantly lower. In addition, Marzinelli et al. (2016) focused on a related aspect with practical implications for biodiversity: these authors found that when transplanted seaweed species are brought into an area, they often support less diversity than the same species in their “undisturbed” condition.
Many macroalgae and the habitats they create are associated with intertidal systems, and therefore, are exposed to periodic emersion. Not surprisingly, several studies have examined how macroalgal structural complexity influences associated assemblages in relation with their position across the intertidal gradient (Davenport et al., 1999; Hooper and Davenport, 2006; Cacabelos et al., 2010). In sheltered shorelines of South Australia, the periodic emersion of three species of macroalgae caused a significant reduction in associated epifaunal numbers. However, macroalgal structural complexity ameliorated these reductions: for organisms associated with the simplest alga (Enteromorpha) 44 times as many animals were present when submerged than when emersed, a ratio strikingly higher than the one measured in animals associated with the most complex algae (Corallina) for which the submerged to emersed ratio was only 1.8 (Davenport et al., 1999). The reduced losses observed in complex algae was partially attributed to water retention which likely prevents desiccation potential effects. However, trends like these are not necessarily consistent across studies. Hooper and Davenport (2006) compared three macroalgae of distinct structural complexity (as measured by fractal dimensions) and although the most complex alga had the richest associated communities, there were no differences in terms of epifaunal emigration levels during low tide emersion.
As the bulk of the information summarized in Table 1 indicates, most research to date shows that macroalgae and the habitats they create contribute to an increase in at least some measure of biodiversity. While those increases range widely from weak to very strong, and the influence of non-native seaweeds or other factors add an additional level of complexity (e.g., Veiga et al., 2016; Lutz et al., 2019; Ndhlovu et al., 2021; Lenzo et al., 2023), seaweeds generally improve rather than hinder associated communities. The most consistent promoter of faunal diversity seems to be the complexity of identifiable features within a seaweed itself (e.g., branching or holdfast morphology; Hacker and Steneck, 1990; Hauser et al., 2006; Lutz et al., 2019: Velasco-Charpentier et al., 2021) or the complexity achieved by the presence of several individuals or species coming together into a discernable habitat (Best et al., 2014; Tano et al., 2016). Algal structural complexity-biodiversity relationships have also been examined using fractal dimensions, and the outcome of several key studies have provided added support to these relationships (e.g., Mancuso et al., 2023b). However, some of this research has also identified exceptions and highlighted outcome variations, partly due to the strong influence of organisms’ size and spatial scale on fractal dimensions (e.g., Gee and Warwick, 1994). Among the above refereed studies, tide distribution or seasonal variation associated with life cycles, in addition to various concurrent factors (e.g., Ndhlovu et al., 2021) suggest that algal structural complexity is one but not the sole predictor of associated biodiversity levels (Torres et al., 2015).
From a conservation and restoration point of view, such features gain relevance in coastal areas where seaweeds and seaweed habitats are in decline, or otherwise where lasting populations of rare, threatened, or endangered species of seaweeds can be found. Given the importance of these species, some of them coined foundation species (sensu Wikström and Kautsky, 2007; Dijkstra et al., 2017; El-Khaled et al., 2022) or ecosystem engineers (Schmidt and Scheibling, 2006; Schaal et al., 2016; Shelamoff et al., 2020), their loss likely entails escalating changes on associated communities. Based on most of the evidence reviewed here, such changes are most likely detrimental to diversity or abundance metrics (Ellison et al., 2005; Norderhaug et al., 2005; Marzinelli et al., 2016). Conserving or restoring by protecting, planting, or transplanting key seaweed species is complex, resource intensive, and provides variable levels of success due to many unaccounted factors (Marzinelli et al., 2016; Whitaker et al., 2023). However, when facing the accelerated loss or displacement of seaweed species caused by climate change (see Bindoff et al., 2019), they represent the best approach for protecting the ecological services they provide and a diversity of reliant organisms that otherwise may be lost as well. The results of this review also suggest that the monitoring of estuarine or coastal fauna should focus heavily on the sampling of seaweed habitats, particularly those holding complex species- or bed-related features. As highlighted above, such features generally contribute to richer associated communities (Christie and Kraufvelin, 2004; Frame et al., 2007; Shelamoff et al., 2020; Mancuso et al., 2023a) by providing shelter against harsh physical conditions (e.g., the brown alga Phyllospora comosa in exposed rocky reefs of Australia; Marzinelli et al., 2016), or refuge against predators (e.g., a variety of the red alga Chondrus crispus in sedimentary bottoms of Atlantic Canada; Tummon Flynn et al., 2020).
Our call for surveys that include the careful examination of seaweed habitats applies to every type of organism. However, our review incidentally found that meiofaunal groups warrant further attention (see e.g., Da Rocha et al., 2006; Veiga et al., 2016), and a particular group among macrofaunal organisms, the amphipods, has been the most recurrently found, sampled, and studied in relation to seaweed characteristics (Table 1). Similar conclusions were highlighted in Christie et al. (2009)’s review of organisms associated with macrophytes, including macroalgae. These authors showed that amphipod abundances were dynamic (due to dispersal, colonization, and behavior) but consistently among the highest. Likewise, complex interactions between chemically defended macroalgae and amphipods have been documented in multiple regions, including Antarctic habitats (Brawley, 1992; Núñez-Pons et al., 2012). It follows that in a scenario of further seaweed declines due to climate change, amphipods (and the consumers relying on these small crustaceans for food) will be among the most directly affected. Considering the plausibility of monitoring, we argue that among the macrobenthic taxa associated with macroalgae, amphipods may be among the species to be studied as recurrent users of rugose habitats such as those provided by many morphologically complex algae.
This overview made also evident a potential geographic gap: most of the studies examined were conducted in warm, low-mid latitudes, such as southern U.S.A., the Mediterranean coasts or Australia (Hacker and Steneck, 1990; Russo, 1990; Taylor and Cole, 1994; Lutz et al., 2019; Shelamoff et al., 2020), although a representative number of studies from high (including polar) latitudes have been also considered (e.g., Lippert et al., 2001; Núñez-Pons et al., 2012; Velasco-Charpentier et al., 2021). Often, the diverse complement of seaweeds found in low-mid latitude regions support rich associated communities, making the influence of features such as physical complexity potentially more evident. The lower representation of higher latitude (potentially less diverse) seaweed-invertebrate communities may mask additional relationships in those regions, and merits further examination. Similarly, there was a relatively high number of studies conducted in rocky shores (e.g., Taylor and Cole, 1994; Buschbaum and Chapman, 2006; Schaal et al., 2016; Dijkstra et al., 2017) in comparison to those conducted in sedimentary habitats. Despite obvious differences, physical complexity of seaweeds or seaweed morphological features remained consistently related with biodiversity levels. Still, distinct ecosystems warrant further attention and open a potential venue for future comparative studies to help us better understand how these relationships operate. As forecasted (Bindoff et al., 2019), climate events will continue to displace and locally exclude seaweed species, so knowledge on the seaweed features that contribute the most to the biodiversity of associated organisms should quickly transition to practice. Ultimately, ongoing seaweed losses should be matched by further monitoring, conservation, and restoration efforts.
EG: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review and editing. PQ: Conceptualization, Formal analysis, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. A CGS-M fellowship (EG) and a Discovery grant (PQ) both from the Natural Sciences and Engineering Research Council of Canada (NSERC) provided support during the preparation of this Minireview.
We thank Paula Tummon Flynn and K. Devon Lynn (UPEI), and two reviewers for their feedback on earlier versions of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amsler C. D., McClintock J. B., Baker B. J. (2014). Chemical mediation of mutualistic interactions between macroalgae and mesograzers structure unique coastal communities along the western Antarctic Peninsula. J. phycology 50, 1–10. doi: 10.1111/jpy.12137
Arkema K. K., Reed D. C., Schroeter S. C. (2009). Direct and indirect effects of giant kelp determine benthic community structure and dynamics. Ecology. 90, 3126–3137. doi: 10.1890/08-1213.1
Bates C. R., DeWreede R. E. (2007). Do changes in seaweed biodiversity influence associated invertebrate epifauna? J. Exp. Mar. Biol. Ecology. 344, 206–214. doi: 10.1016/j.jembe.2007.01.002
Best R. J., Chaudoin A. L., Bracken M. E. S., Graham M. H., Stachowicz J. J. (2014). Plant-animal diversity relationships in a rocky intertidal system depend on invertebrate body size and algal cover. Ecology. 95, 1308–1322. doi: 10.1890/13-1480.1
Bindoff N. L., Cheung W. W. L., Kairo J. G., Aristegui J., Guinder V. A., Hallberg R., et al. (2019). “Changing ocean, marine ecosystems, and dependent communities,” in IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. Eds. Portner H.-O., Roberts D. C., Masson-Delmotte V., Zhai P., Tignor M. (Cambridge, UK and New York, NY, USA: Cambridge University Press), 447–587.
Brawley S. H. (1992). “Mesoherbivores,” in Plant-Animal Interactions in the Marine Benthos. Eds. John D., Hawkins S., Price J. (Oxford: Oxford University Press), 235–263.
Brawley S. H., Adey W. H. (1981). The effect of micrograzers on algal community structure in a coral reef microcosm. Mar. Biol. 61, 167–177. doi: 10.1007/BF00386656
Buršić M., Iveša L., Jaklin A., Arko Pijevac M. (2019). A preliminary study on the diversity of invertebrates associated with Corallina officinalis Linnaeus in southern Istrian peninsula. Acta Adriatica. 60, 127–136. doi: 10.32582/aa.60.2.2
Buschbaum C., Chapman A. S. (2006). How an introduced seaweed can affect epibiota diversity in different coastal systems. Mar. Biol. 148, 743–753. doi: 10.1007/s00227-005-0128-9
Cacabelos E., Olabarria C., Incera M., Troncoso J. S. (2010). Effects of habitat structure and tidal height on epifaunal assemblages associated with macroalgae. Estuarine Coastal Shelf Science. 89, 43–52. doi: 10.1016/j.ecss.2010.05.012
Castorani M. C., Reed D. C., Miller R. J. (2018). Loss of foundation species: disturbance frequency outweighs severity in structuring kelp forest communities. Ecology 99, 2442–2454. doi: 10.1002/ecy.2485
Cerqueira Veras P., Pierozzi-Jr I., Lino J. B., Amado-Filho G. M., Senna A. R., Santos C. S. G., et al. (2020). Drivers of biodiversity associated with rhodolith beds from euphotic and mesophotic zones: Insights for management and conservation. Perspect. Ecol. Conserv. 18, 37–43. doi: 10.1016/j.pecon.2019.12.003
Chemello R., Milazzo M. (2002). Effect of algal architecture on associated fauna: some evidence from phytal molluscs. Mar. Biol. 140, 981–990. doi: 10.1007/s00227-002-0777-x
Chen Y. Y., Cooper P., Fulton C. J. (2020). Sargassum epifaunal communities vary with canopy size, predator biomass and seascape setting within a fringing coral reef ecosystem. Mar. Ecol. Prog. Series. 640, 17–30. doi: 10.3354/meps13282
Christensen-Dalsgaard S., Mattisson J., Bekkby T., Gundersen H., May R., Rinde E., et al. (2017). Habitat selection of foraging chick-rearing European shags in contrasting marine environments. Mar. Biol. 164, 1–12. doi: 10.1007/s00227-017-3227-5
Christie H., Jorgensen N. M., Norderhanug K. M., Waage-Nielsen E. (2003). Species distribution and habitat exploitation of fauna associated with kelp (Laminaria hyperborea) along the Norwegian coast. J. Mar. Biol. Assoc. United Kingdom. 83, 1–13. doi: 10.1017/S0025315403007653h
Christie H., Kraufvelin P. (2004). Mechanisms regulating amphipod population density within macroalgal communities with low predator impact. Scientia Marina. 68, 189–198. doi: 10.3989/scimar.2004.68s1189
Christie H., Norderhaug K. M., Fredriksen S. (2009). Macrophytes as habitat for fauna. Mar. Ecol. Prog. Series. 396, 221–233. doi: 10.3354/meps08351
Da Rocha C. M. C., Venekev V., Bezerra T. N. C., Souza J. R. B. (2006). Phytal marine nematode assemblages and their relation with the macrophytes structural complexity in a Brazilian tropical rocky beach. Hydrobiologia. 553, 219–230. doi: 10.1007/s10750-005-0923-9
Davenport J., Butler A., Cheshire A. (1999). Epifaunal composition and fractal dimensions of marine plants in relation to emersion. J. Mar. Biol. Assoc. United Kingdom. 79, 351–355. doi: 10.1017/S0025315498000393
Davenport J., Pugh P. J. A., McKechnie J. (1996). Mixed fractals and anisotropy in subantarctic marine macroalgae from South Georgia: implications for epifaunal biomass and abundance. Mar. Ecol. Prog. Series. 136, 245–255. doi: 10.3354/meps136245
Dayton P. K. (1985). Ecology of kelp communities. Annu. Rev. Ecol. Systematics. 16, 215–245. doi: 10.1146/annurev.es.16.110185.001243
Dijkstra J. A., Harris L. G., Mello K., Litterer A., Wells C., Ware C. (2017). Invasive seaweeds transform habitat structure and increase biodiversity of associated species. J. Ecology. 105, 1668–1678. doi: 10.1111/1365-2745.12775
Duarte R. C. S., Mota E. L. S., Dias T. L. P. (2020). Algal complexity positively affects the abundance, richness and diversity of molluscan assemblages of a semiarid hypersaline mangrove. Aquat. Ecology. 54, 1001–1013. doi: 10.1007/s10452-020-09789-3
El-Khaled Y. C., Daraghmeh N., Tilstra A., Roth F., Huettel M., Rossbach F. I., et al. (2022). Fleshy red algae mats act as temporary reservoirs for sessile invertebrate biodiversity. Commun. Biol. 5, 579. doi: 10.1038/s42003-022-03523-5
Ellison A. M., Bank M. S., Clinton B. D., Colburn E. A., Elliott K., Ford C. R., et al. (2005). Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 3, 479–486. doi: 10.1890/1540-9295(2005)003[0479:LOFSCF]2.0.CO;2
Frame K., Hunt G., Roy K. (2007). Intertidal meiofaunal biodiversity with respect to different algal habitats: a test using phytal ostracodes from Southern California. Hydrobiologia. 586, 331–342. doi: 10.1007/s10750-007-0707-5
Gabara S. S., Hamilton S. L., Edwards M. S., Steller D. L. (2018). Rhodolith structural loss decreases abundance, diversity, and stability of benthic communities at Santa Cataline Island, CA. Mar. Ecol. Prog. Series. 595, 71–88. doi: 10.3354/meps12528
Gallardo D., Oliva F., Ballesteros M. (2021). Marine invertebrate epibionts on photophilic seaweeds: importance of algal architecture. Mar. Biodiversity. 51, 16. doi: 10.1007/s12526-020-01151-y
Gee J. M., Warwick R. M. (1994). Metazoan community structure in relation to the fractal dimensions of marine macroalgae. Mar. Ecol. Prog. Series. 103, 141–150. doi: 10.3354/meps103141
Gestoso I., Olabarria C., Troncoso J. S. (2012). Effects of macroalgal identity on epifaunal assemblages: native species versus the invasive species Sargassum muticum. Helgoland Mar. Res. 66, 159–166. doi: 10.1007/s10152-011-0257-0
Glanz J. S. (2021). Relationship between a branch-forming crustose coralline algae, associated small motile invertebrates, and water flow (Northridge: California State University).
Goodsell P. J., Fowler-Walker M. J., Gillanders B. M., Connell S. D. (2004). Variations in the configurations of algae in subtidal forests: Implications for invertebrate assemblages. Austral Ecol. 29, 350–357. doi: 10.1111/j.1442-9993.2004.01372.x
Gunill F. C. (1982). Effects of plant size and distribution on the numbers of invertebrate species and individuals inhabiting the brown alga Pelvetia fastigiata. Mar. Biol. 69, 263–280. doi: 10.1007/BF00397492
Hacker S. D., Steneck R. S. (1990). Habitat architecture and the abundance and body-size dependent habitat selection of a phytal amphipod. Ecology. 71, 2269–2285. doi: 10.2307/1938638
Hansen J. P., Sagerman J., Wikstrom. S. A. (2010). Effects of plant morphology on small-scale distruction of invertebrates. Mar. Biol. 157, 2143e2155. doi: 10.1007/s00227-010-1479-4
Harvey A. S., Bird F. L. (2008). Community structure of a rhodolith bed from cold-temperature waters (southern Australia). Aust. J. Botany. 56, 437–450. doi: 10.1071/BT07186
Hauser A., Attrill M. J., Cotton P. A. (2006). Effects of habitat complexity on the diversity and abundance of macrofauna colonising artificial kelp holdfasts. Mar. Ecol. Prog. Series. 325, 93–100. doi: 10.3354/meps325093
Hay M. E. (2009). Marine chemical ecology: chemical signals and cues structure marine populations, communities, and ecosystems. Annu. Rev. Mar. Science. 1, 193–212. doi: 10.1146/annurev.marine.010908.163708
Hay M. E., Fenical W. (1996). Chemical ecology and marine biodiversity: Insights and products from the sea. Oceanography 9, 10–20. doi: 10.5670/oceanog.1996.21
Hooper G. J., Davenport J. (2006). Epifaunal composition and fractal dimensions of intertidal marine macroalgal in relation to emersions. J. Mar. Biol. Assoc. United Kingdom. 86, 1297–1304. doi: 10.1017/S0025315406014329
Hughes B. B. (2010). Variable effects of a kelp foundation species on rocky intertidal diversity and species interactions in central California. J. Exp. Mar. Biol. Ecology. 393, 90–99. doi: 10.1016/j.jembe.2010.07.003
Hull S. L. (1997). Variable effects of a kelp foundation species on rocky intertidal diversity and species interactions in central California. Marine Ecology Progress Series 161, 71–82. doi: 10.3354/meps161071
Ianora A., Boersma M., Casotti R., Fontana A., Harder J., Hoffmann F., et al. (2006). New trends in marine chemical ecology. Estuaries Coasts. 29, 531–551. doi: 10.1007/BF02784281
Karr K. A. (2011). Patterns, mechanisms and community consequences of variation in kelp forest canopies (Santa Cruz: University of California).
Kelaher B. P., Castilla J. C. (2005). Habitat characteristics influence macrofaunal communities in coralline turf more than mesoscale coastal upwelling on the coast of Northern Chile. Estuarine Coast. Self Science. 63, 155–165. doi: 10.1016/j.ecss.2004.10.017
Leite F.P.P., Turra A. (2003). Temporal variation in Sargassum biomass, Hypnea epiphytism and associated fauna. Braz. Arch. Biol. Technol. 46, 665–671. doi: 10.1590/S1516-89132003000400021
Lenzo D., Colangelo M. A., Pasteris A., Rindi F., Pistocchi R., Pezzolesi L. (2023). Understanding the role of macroalgal complexity and allelochemicals production in invasive and non-invasive macroalgae in the North-Western Adriatic Sea: effect on the associated communities. Water. 15, 1697. doi: 10.3390/w15091697
Lilley S. A., Schiel D. R. (2006). Community effects following the deletion of a habitat-forming alga from rocky marine shores. Oecologia. 148, 672–681. doi: 10.1007/s00442-006-0411-6
Lippert H., Iken K., Rachor E., Wiencke C. (2001). Macrofauna associated with macroalgae in the Kongsfijord (Spitsbergen). Polar Biol. 24, 512–522. doi: 10.1007/s003000100250
Loke L. H., Todd P. A. (2016). Structural complexity and component type increase intertidal biodiversity independently of area. Ecology. 97, 383–393. doi: 10.1890/15-0257.1
Lorentsen S. H., Sjøtun K., Grémillet D. (2010). Multi-trophic consequences of kelp harvest. Biol. Conserv. 143, 2054–2062. doi: 10.1016/j.biocon.2010.05.013
Lutz M. L., Minchinton T. E., Davis A. R. (2019). Differences in architecture between native and non-indigenous macroalgae influence associations with epifauna. J. Exp. Mar. Biol. Ecology. 514, 76–86. doi: 10.1016/j.jembe.2019.03.006
Mancuso F. P., Lo Brutto S., Chemello R., Sarà G., Mannino A. M. (2023a). Effects of structural complexity on epifaunal assemblages associated with two intertidal Mediterranean seaweeds. Plant Biosystems. 157, 812–820. doi: 10.1080/11263504.2023.2200775
Mancuso F. P., Milazzo M., Chemello R. (2021). Decreasing in patch-size of Cystoseira forests reduces the diversity of their associated molluscan assemblage in Mediterranean rocky reefs. Estuarine Coast. Shelf Science. 250, 107163. doi: 10.1016/j.ecss.2020.107163
Mancuso F. P., Milazzo M., Sarà G., Chemello R. (2023b). Bi- and three-dimensional fractal analysis of the brown seaweed Gongolaria montagnei and their relationship with gastropod molluscs assemblage. Mar. pollut. Bulletin. 186, 114396. doi: 10.1016/j.marpolbul.2022.114396
Marzinelli E. M., Campbell A. H., Verges A., Coleman M. A., Kelaher B. P., Steinberg P. D. (2014). Restoring seaweeds: does the declining fucoid Phyllospora comosa support different biodiversity than other habitats? J. Appl. Phycology. 26, 1089–1096. doi: 10.1007/s10811-013-0158-5
Marzinelli E. M., Leong M. R., Campbell A. H., Steinberg P. D., Verges A. (2016). Does restoration of a habitat-forming seaweed restore associated faunal diversity? J. Soc. Ecol. Restoration. 24, 81–90. doi: 10.1111/rec.12292
Mikhaylova T. A., Aristov D. A., Naumov A. D., Malavenda S. S., Savchenko O. N., Bijagov K. L. (2019). Diversity and structure of epibenthic communities of red algae zone in the White Sea. Polar Biol. 42, 953–968. doi: 10.1007/s00300-019-02488-2
Montie S., Thomsen M. S. (2023). Long-term community shifts driven by local extinction of an iconic foundation species following an extreme marine heatwave. Ecol. Evol. 13, e10235. doi: 10.1002/ece3.10235
Ndhlovu A., Lathlean J. A., McQuaid C. D., Seuront L. (2021). Indirect effects shape macroalgal epifaunal communities. Ecol. Evol. 11, 15141–15152. doi: 10.1002/ece3.8195
Neves S. B., Costa K. G. (2022). Diversity of benthic fauna of rhodoliths and sediments deposited on sandstone reefs in Southeast Brazil. Ocean Coast. Res. 70, e:22010. doi: 10.1590/2675-2824070.21029sbn
Norderhaug K. M., Christie H., Fosså J. H., Fredriksen S. (2005). Fish – macrofauna interactions in a kelp (Laminaria hyperborea) forest. J. Mar. Biol. Assoc. UK. 85, 1279–1286. doi: 10.1017/S0025315405012439
Núñez-Pons L., Rodríguez-Arias M., Gómez-Garreta A., Ribera-Siguán A., Avila C. (2012). Feeding deterrency in Antartic marine organisms: bioassays with the omnivore amphipod Cheirimedon femoratus. Mar. Ecol. Prog. Series. 462, 163–174. doi: 10.3354/meps09840
Ojeda F. P., Santelices B. (1984). Invertebrate communities in holdfasts of the kelp Macrocystis pyrifera from southern Chile. Mar. Ecol. Prog. Series. 16, 65–73. doi: 10.3354/meps016065
Parker J. D. (1998). Does plant diversity control animal diversity? an experimental approach. MSc Thesis. (Virginia, USA: The College of William and Mary). doi: 10.25773/v5-1y50-6034
Parker J. D., Duffy J. E., Orth R. J. (2001). Plant species diversity and composition: experimental effects on marine epifaunal assemblages. Mar. Ecol. Prog. Series. 224, 55–67. doi: 10.3354/meps224055
Perälä T., Pesari S. N. K., Ahti P. A., Lehtinen S. O., Schoenrock K. M., Kuparinen. A. (2023) Non-trophic interactions amplify kelp harvest-induced biomass oscillations and biomass changes in a kelp forest ecological network model. Mar. Ecol. Prog. Ser. 722, 19–36. doi: 10.3354/meps14438
Richard M., Quijón P. A. (2023). Seagrass-macroalgal interactions in a changing ocean. Front. Clim. 5, 1283305. doi: 10.3389/fclim.2023.1283305
Robinson K. M. (2015). Motile cryptofaunal invertebrate assemblages in Catalina Island’s rhodolith beds in relation to physical structure and live rhodoliths (Monterey Bay: California State University).
Russo A. R. (1990). The role of seaweed complexity in structuring Hawaiian epiphytal amphipod communities. Hydrobiologia. 194, 1–12. doi: 10.1007/BF00012107
Schaal G., Leclerc J. C., Droual G., Leroux C., Riera P. (2016). Biodiversity and trophic structure of invertebrate assemblages associated with understorey red algae in a Laminaria digitata bed. Mar. Biol. Res. 12, 513–523. doi: 10.1080/17451000.2016.1164318
Schmidt A. L., Scheibling R. E. (2006). A comparison of epifauna and epiphytes on native kelps (Laminaria species) and an invasive alga (Codium fragile spp. tomentosoides) Nova Scotia Canada. Botanica Marina. 49, 1–16. doi: 10.1515/BOT.2006.039
Schoenrock K. M., Vad J., Muth A., Pearce D. M., Rea B. R., Schofield J. E., et al. (2018). Biodiversity of kelp forests and coralline algae habitats in Southwestern Greenland. Diversity. 10, 1–20. doi: 10.3390/d10040117
Shelamoff V., Layton C., Tatsumi M., Cameron M. J., Edgar G. J., Wright J. T., et al. (2020). Kelp patch size and density influence secondary productivity and diversity of epifauna. Oikos. 129, 331–345. doi: 10.1111/oik.06585
Solano-Barquero A., Sibaja-Cordero J. A., Cortés J. (2022). Macrofauna associated with a rhodolith bed at an Oceanic Island in the Eastern Tropical Pacific (Isla del Coco National Park, Costa Rica). Front. Mar. Science. 9, 1–16. doi: 10.3389/fmars.2022.858416
Sotka E. E., Jormalainen V., Poore A. G. B. (2018). “The evolution of marine herbivores in response to algal secondary metabolites,” in Chemical ecology: the ecological impacts of marine natural products. Eds. Puglisi M. P., Becerro M. A. (Boca Raton, FL, USA: CRC Press), 193–220.
Stachowicz J. J. (2001). Mutualism, facilitation, and the structure of ecological communities. BioScience. 51, 235–246. doi: 10.1641/0006-3568(2001)051[0235:MFATSO]2.0.CO;2
Stelzer P. S., Mazzuco A. C. A., Gomes L. E., Martins J., Netto S., Bernardino A. F. (2021). Taxonomic and functional diversity of benthic macrofauna associated with rhodolith beds in SE Brazil. PeerJ Life Environ. 9, e:11903. doi: 10.7717/peerj.11903
Tano S., Eggertsen M., Wikstrom S. A., Berkstrom C., Buriyo A. S., Halling C. (2016). Tropical seaweed beds are important habitats for mobile invertebrate epifauna. Estuarine Coast. Shelf Science. 183, 1–12. doi: 10.1016/j.ecss.2016.10.010
Taylor R. B., Cole R. G. (1994). Mobile epifauna on subtidal brown seaweeds in northeastern New Zealand. Mar. Ecol. Prog. Series. 115, 271–282. doi: 10.3354/meps115271
Tokeshi M., Arakaki S. (2012). Habitat complexity in aquatic systems: fractals and beyond. Hydrobiologia. 685, 27–47. doi: 10.1007/s10750-011-0832-z
Torres A. C., Veiga P., Rubal M., Sousa-Pinto I. (2015). The role of annual macroalgal morphology in driving its epifaunal assemblages. J. Exp. Mar. Biol. Ecology. 464, 96–106. doi: 10.1016/j.jembe.2014.12.016
Tummon Flynn P., Lynn K. D., Cairns D., Quijόn P. A. (2019). The role of the non-indigenous green crab (Carcinus maenas) in the decline of a unique strain of Irish moss (Chondrus crispus): direct and indirect effects. ICES J. Mar. Science. 76, 2338–2348. doi: 10.1093/icesjms/fsz130
Tummon Flynn P., Lynn K. D., Cairns D., Quijón P. A. (2021). Mesograzer interactions with a unique strain of Irish moss Chondrus crispus: colonization, feeding, and algal condition-related effects. Mar. Ecol. Prog. Series. 669, 83–96. doi: 10.3354/meps13737
Tummon Flynn P., McCarvill K., Lynn K. D., Quijón P. A. (2020). The positive effect of coexisting ecosystem engineers: a unique seaweed-mussel association provides refuge for native mud crabs against a non-indigenous predator. PeerJ. 8, e10540. doi: 10.7717/peerj.10540
Veiga P., Rubal M., Sousa-Pinto I. (2014). Structural complexity of macroalgae influences epifaunal assemblages associated with native and invasive species. Mar. Environ. Res. 101, 115–123. doi: 10.1016/j.marenvres.2014.09.007
Veiga P., Sousa-Pinto I., Rubal M. (2016). Meiofaunal assemblages associated with native and non-indigenous macroalgae. Continental Shelf Res. 123, 1–8. doi: 10.1016/j.csr.2016.04.007
Velasco-Charpentier C., Pizarro-Mora F., Navarro N. P., Valdivia N. (2021). Disentangling the links between habitat complexity and biodiversity in a kelp-dominated subantarctic community. Ecol. Evol. 11, 1214–1224. doi: 10.1002/ece3.7100
Whitaker S. G., Ambrose R. F., Anderson L. M., Fales R. J., Smith J. R., Sutton S., et al. (2023). Ecological restoration using intertidal foundation species: Considerations and potential for rockweed restoration. Ecosphere. 14, e4411. doi: 10.1002/ecs2.4411
Keywords: seaweeds, biodiversity, abundance, conservation, restoration, climate change
Citation: Gibbons EG and Quijón PA (2023) Macroalgal features and their influence on associated biodiversity: implications for conservation and restoration. Front. Mar. Sci. 10:1304000. doi: 10.3389/fmars.2023.1304000
Received: 28 September 2023; Accepted: 29 November 2023;
Published: 19 December 2023.
Edited by:
Milko Alberto Jorquera, University of La Frontera, ChileReviewed by:
Marcos Rubal, University of Minho, PortugalCopyright © 2023 Gibbons and Quijón. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro A. Quijón, cHF1aWpvbkB1cGVpLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.