
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 28 November 2023
Sec. Marine Megafauna
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1303961
Bycatch of cartilaginous species is considered one of the main drivers for the dramatic declines observed in many populations. Pelagic longlines and passive nets impact many species depending on their life stage and habitat use. Here, we present an updated list of incidental catches collected through a 4-year fishery-dependent survey. We documented the bycatch of four critically endangered species, particularly 13 individuals of Isurus oxyrinchus, Prionace glauca, and Mobula mobular by longlines and one specimen of Lamna nasus by trammel nets in the Asinara Gulf (Northern Sardinia, Italy). As almost all specimens were juveniles or newborns, we explored and discussed the potential drivers explaining their prevalence in the sample. Despite our low sample size, of the four possible options discussed, the role of the Asinara Gulf as an Important Shark and Ray Area (ISRA) for large pelagic elasmobranch species is one worth considering.
Bycatch is considered one of the main drivers determining many population declines observed for k-selected cartilaginous fish worldwide (Heppell et al., 1999; Musick et al., 2000; Pacoureau et al., 2021), especially for large and highly migratory demersal–pelagic shark and ray species particularly vulnerable to pelagic longlines (Beerkircher et al., 2002; Coelho et al., 2005; Megalofonou et al., 2005; Gilman et al., 2007a; Gallagher et al., 2014; Kroodsma et al., 2018; Queiroz et al., 2019) and set gillnets and trammel nets (Perez and Wahrlich, 2005; Valenzuela et al., 2008; Thorpe and Frierson, 2009; Benjamins et al., 2010; Tiralongo et al., 2018a) in offshore and coastal waters depending on their life stage and habitat use. The shortfin mako shark Isurus oxyrinchus Rafinesque, 1810, the porbeagle shark Lamna nasus (Bonnaterre, 1788), the blue shark Prionace glauca (Linnaeus, 1758), and the spinetail devil ray Mobula mobular (Bonnaterre, 1788) are, at different extents, large pelagic migratory species in the world ocean and semi-enclosed basins (Ebert et al., 2021), such as the Mediterranean Sea. The shortfin mako and the porbeagle sharks, representative of the Lamnidae family (Compagno et al., 2005; Ebert et al., 2021), are typical top predator species on medium–large bony fish and cephalopods (Compagno et al., 1989; Revill et al., 2009); they actively hunt in the water column of open waters, thanks to their high swimming efficiency (Carrier et al., 2022; Waller et al., 2023). In contrast, the blue shark is an opportunistic feeder and has less pelagic habits, like many other requiem sharks (Carcharhinidae) to which it belongs (Compagno et al., 2005; Ebert et al., 2021). As a matter of fact, species belonging to Carcharhinidae are associated with rocky bottoms and shoals (Last and Stevens, 1994; Mundy, 2005), compared with the more pelagic-adapted lamnids. The spinetail devil ray, a pelagic-readapted batoid (McEachran and Capapé, 1984; Notarbartolo di Sciara and Bianchi, 1998; Ebert et al., 2021), is a filter-feeding species on planktonic preys throughout the water column, thanks to its peculiar cephalic fins and gill rakers (Abudaya et al., 2018). The species here considered have a natural low density in the Mediterranean (Tortonese, 1956; Notarbartolo di Sciara and Bianchi, 1998), and fishery-dependent occurrence data are sparse along different fishing gear and fishing zones (Carpentieri et al., 2021). Pelagic longlines are the most threatening fishery (Bartolí et al., 2017), as bycatch of large pelagic sharks has been reported in the Adriatic (Carbonara et al., 2023), Ligurian (Garibaldi, 2015), Ionian Sea (Megalofonou et al., 2005), Sicilian Channel (Burgess et al., 2010; Cattano et al., 2023a), Spanish (Mejuto et al., 2002), Greek (Peristeraki et al., 2008), French (Doherty et al., 2022), and southeastern (Damalas and Megalofonou, 2012) Mediterranean waters. Recreational fishery has also a relevant role, as it shows an important bycatch of many pelagic shark and ray species in the Mediterranean (Panayiotou et al., 2020). Unfortunately, fishing areas often overlap with pelagic shark and ray aggregation sites (Kroodsma et al., 2018; Queiroz et al., 2019) that may meet the criteria to be considered Important Shark and Ray Areas (ISRAs), thanks to the presence of favorable biotic and abiotic parameters. The definition of an ISRA refers specifically to elasmobranch species, i.e., “a discrete, tri-dimensional portions of habitat, important for one or more shark species, that have the potential to be delineated and managed for conservation” (IUCN, 2023a). Within an ISRA, the general criteria used to characterize an Ecologically or Biologically Significant marine Area (EBSA) may apply, i.e., a spatially defined area where aggregations of individuals of species are known to display biologically important behavior such as breeding, foraging, resting, or migration (Convention on Biological Diversity (CBD), 2023). During recent years, increasing effort has been spent by the General Fisheries Commission for the Mediterranean (GFCM) and the European Union (EU) to improve elasmobranch conservation by contrasting underreporting, illegal fishing, and trade of these species, as well as bycatch, within the European Community waters and the Mediterranean basin (EU, 2019; GFCM, 2021). In this context, gathering information on the size structure of large shark populations at the local scale is of paramount importance to a regionally focused conservation management in the Mediterranean Sea. For instance, the juvenile–adult ratio in a fishery bycatch area can provide clues on the presence of potential ISRAs, which can hold nursery, mating, foraging, and/or refuge grounds, particularly where marine environments are favorable, thanks to high biodiversity and optimal hydrological condition (Ward-Paige et al., 2014; Roff et al., 2018). In general, the higher availability of both refuges and food resources that is found in coastal and land-surrounded waters favors particularly the occurrence of the most fragile life stages, such as newborns and, consequently, “parturient” females (Vandeperre et al., 2014). This favorable condition advantages also growing individuals, such as the young of the year (YOY), i.e., individuals aged up to 1 year, and juveniles, i.e., individuals that are older than 1 year and below the size at first maturity (Nakano and Stevens, 2009). Contrastingly, open waters are typical of adult specimens for hunting, mating, and migrations (Branstetter, 1990; Heupel et al., 2007). Indeed, it is suspected that large pelagic elasmobranch species regularly exploit the whole Mediterranean basin to set their nursery and foraging areas for newborns and juveniles, particularly in favorable coastal and slope habitats (Kohler et al., 2002), respectively. In this context, the Asinara Gulf and Bonifacio Mouths are already known for the particularly high biodiversity of marine habitats and peculiar hydrological circulation (Bell and Harmelin-Vivien, 1983; Francour, 1994; CoNiSMA, 2018; Pascucci et al., 2018). In fact, the area benefits from a high protection regime, thanks to the presence of the Asinara National Park and the Marine Protected Area of Punta Falcone Capo Testa, both in the Northern Sardinia and the Bonifacio and the Scandola Marine Reserves in the southern and western Corse, respectively. The Asinara Gulf embraces the Castel Sardo Canyon, one of the most important Mediterranean canyons (Würtz, 2012). Such a deeply incised geological structure has a peculiar morphology and generates upward and downward (turbidites) movements of water masses from the deep western Mediterranean into the Gulf and vice versa, respectively (Kenyon et al., 2002). Thanks to the consequent cascade effect occurring throughout the whole local trophic chain, prey available to top predator species increases in abundance in such an area (Würtz, 2012). Bonifacio Mouths connect the wide northern-central part of the Tyrrhenian Sea to the wider region of the western Mediterranean. This narrow passage, together with the geographical disposition of emerged lands and seas, has peculiar hydrological conditions. Strong and seasonal currents and local wind-induced gyres are generated by the Bernoulli’s effect that takes place in the area, altering wind and seawater speed (Gérigny et al., 2015). Indeed, hydrological constraints are known to model the distribution of migratory species such as large pelagic elasmobranchs (Campana and Joyce, 2004; Riede, 2004; Grose et al., 2020; Swift and Portnoy, 2021). Areas characterized by important interchanges of water masses between basins are attractive for large pelagic migratory sharks and rays (Capapé and Zaouali, 1976; Capapé et al., 1990; Braun et al., 2019). They exhibit highly mobile adult individuals on conservative routes, at the regional (Cox and Francis, 1997; Kohler et al., 2002; Swift and Portnoy, 2021; Gennari et al., 2022) and even at the hemispheric scales (Stevens, 1976; Stevens, 1990; Cox and Francis, 1997; Compagno, 2001). In contrast, juveniles are unable to migrate long distances (Nakano and Stevens, 2009; Vandeperre et al., 2014) due to a less efficient swimming ability they exhibit compared with adults (Sepulveda et al., 2007; Saraiva et al., 2023).
The bycatch size structure can be the result of a size-dependent selectivity of the gear, which depends on the interaction between the fishing gear (type of gear and fishing techniques) and the species’ life-history traits (Ellis et al., 2017). For instance, ontogenic and species-specific differences in resistance to capture (Scacco et al., 2023a) and in the probability of escape (Gilman et al., 2016) can influence the size structure of the bycatch in longlines. The latter can be influenced also by changes in feeding habits during ontogeny of the species such that bait preference can be size-dependent as well. Even though cephalopods and fish are constant components in the diet of some of the species considered, changes are mainly in the size and type of prey (Joyce et al., 2002; Maia et al., 2006; Kubodera et al., 2007). Some data show that the replacement of cephalopods with fish as bait in the swordfish pelagic longlines can reduce the bycatch of the blue shark (Watson et al., 2005; Gilman et al., 2007b; Galeana-Villasenor et al., 2009; Petersen et al., 2009) and also of other shark species, together with the use of circular hooks (Gilman et al., 2007a), but a size effect on bait preference was not observed. Additionally, a size-dependent selectivity of the gear can depend on the interaction between sex and/or size-dependent habitat use, as observed in some large pelagic sharks (Mucientes et al., 2009; Schlaff et al., 2014; González-Andrés et al., 2021; Gennari et al., 2022; Kock et al., 2022), and the operational fishing depth of the gear. For instance, it has been shown that deploying hooks at an increased fishing depth can reduce the bycatch of P. glauca, Carcharhinus falciformis (Müller and Henle, 1839), Carcharhinus longimanus (Poey, 1861), and Carcharhinus obscurus (Lesueur, 1818) though the bycatch size composition of single species is not affected as observed in the pelagic longlines deployed in the Pacific Ocean (Williams, 1999; Hinke et al., 2004; Ward and Myers, 2005; Gilman et al., 2008). Indeed, juveniles and adults of large pelagic sharks are generally distributed along an inshore–offshore gradient (Branstetter, 1990; Heupel et al., 2007), respectively, rather than along a surface–deep water slope in the offshore waters.
Finally, the size structure of a fishery bycatch may be the result of the populations’ demographic condition of the species caught. According to the general principles in population dynamics, multiple different-aged cohorts of individuals are expected to coexist in a healthy elasmobranch population, with the frequency of occurrence of individuals steadily decreasing with the increasing age of the cohorts, i.e., the larger the specimens, the rarer they are (Cortés, 1998; Cortés, 2002). It follows that the population in which only the cohorts of juveniles are present raises concern (Froese et al., 2017). Just like most elasmobranchs, the species that we considered have particularly low resilience against number depletion induced by fishing activity (Ferretti et al., 2008; Froese et al., 2017). This is due to general low fecundity, late maturing, and marked longevity (Cortés, 1998; Cortés, 2002). Such characteristics, typical of k-selected species, and cumulated anthropic pressures act synergically in determining the high extinction risk observed in the populations of several elasmobranch species across different geographical scales (Walls and Dulvy, 2020; Pacoureau et al., 2021), as assessed by the International Union for the Conservation of the Nature (IUCN, 2023b). On the other hand, the globally increased implementation of conservation actions in recent years seems to be inked with some signs of population recovery actually observed for a limited number of elasmobranch species, such that a green list, complementary to the IUCN red list for elasmobranchs, has been proposed (Grace et al., 2021). For Mediterranean elasmobranch species, Scacco et al. (2023b) recently suggested that the severity of IUCN assessments depends on the interaction between the species-specific characteristics of the life history traits (Cheung et al., 2005; Cheung et al., 2007) and the variation in the intensity of the fishing threats posed by different fishing gear on different species at a given geographic scale. The aim of this work is to report on the prevalence of juveniles and newborns of endangered elasmobranchs observed in the bycatch of pelagic longlines and trammel nets of the Asinara Gulf. Despite the boundaries associated with such a limited data collection, the potential drivers explaining the reported occurrences are explored and discussed based on the present 4-year fishery-dependent data and available information from the literature.
The fishery-dependent monitoring survey covered a 4-year period (2018–2021) during the fishing activities of a vessel (vessel length 12 m, gross tonnage 15 tons, engine power 300 kW). The boat is permitted for professional small-scale long line targeting Xiphias gladius Linnaeus, 1758, and trammel net fishery targeting high-commercial value fish (Sparidae, Sciaenidae, and Scorpaenidae), crustaceans (Palinuridae), and cephalopods (Octopodidae and Loliginidae) in the offshore and inshore waters of Asinara Gulf and Bonifacio Mouths (Figure 1), respectively. The Asinara Gulf shows a high heterogeneity of seabed, strong variations in bathymetry (presence of shoals and canyons), and the relevant presence of important habitats, such as posidonia meadows (Telesca et al., 2015), coralligenous (Cocito and Ferdeghini, 2001; Tonin, 2018), sandy, muddy, and rocky grounds (Cossu and De Luca, 2016), favoring high species richness (Cossu et al., 2009; Interreg technical report, 2013). The gear commonly used in each fishing trip by the vessel was a pelagic longline set using a monofilament both for the leading rope (diameter 1.50 mm) and the armrests (diameter 1.20 mm). Armrests were usually 10 m in length, each mounted with a traditional J hook (measure 2/0–3/0), baited with fresh fish of several bony fish and cephalopods (principally Sardina pilchardus (Walbaum, 1792) and Loligo vulgaris Lamarck, 1798), and spaced 30 m apart from each other for a total of 500 hooks along the leading rope (15,000 m). The fishing gear was lowered at sunset and retrieved at dawn, for a total of approximately 8 h for each fishing trip. The pelagic longline was dropped to an operational fishing depth of approximately 20–30 m, within a water column ranging between 50 and 400 m depth. Coastal trammel-fixed nets were also used though secondarily in terms of the annual number of boat’s fishing days per gear. For this type of gear, a fixed trammel net 1,000 m in length was usually set at sunset and hauled at dawn in coastal waters between 15 and 40 m depth, with a mesh size of 3 cm for the internal panel and 10–15 cm for the external one.
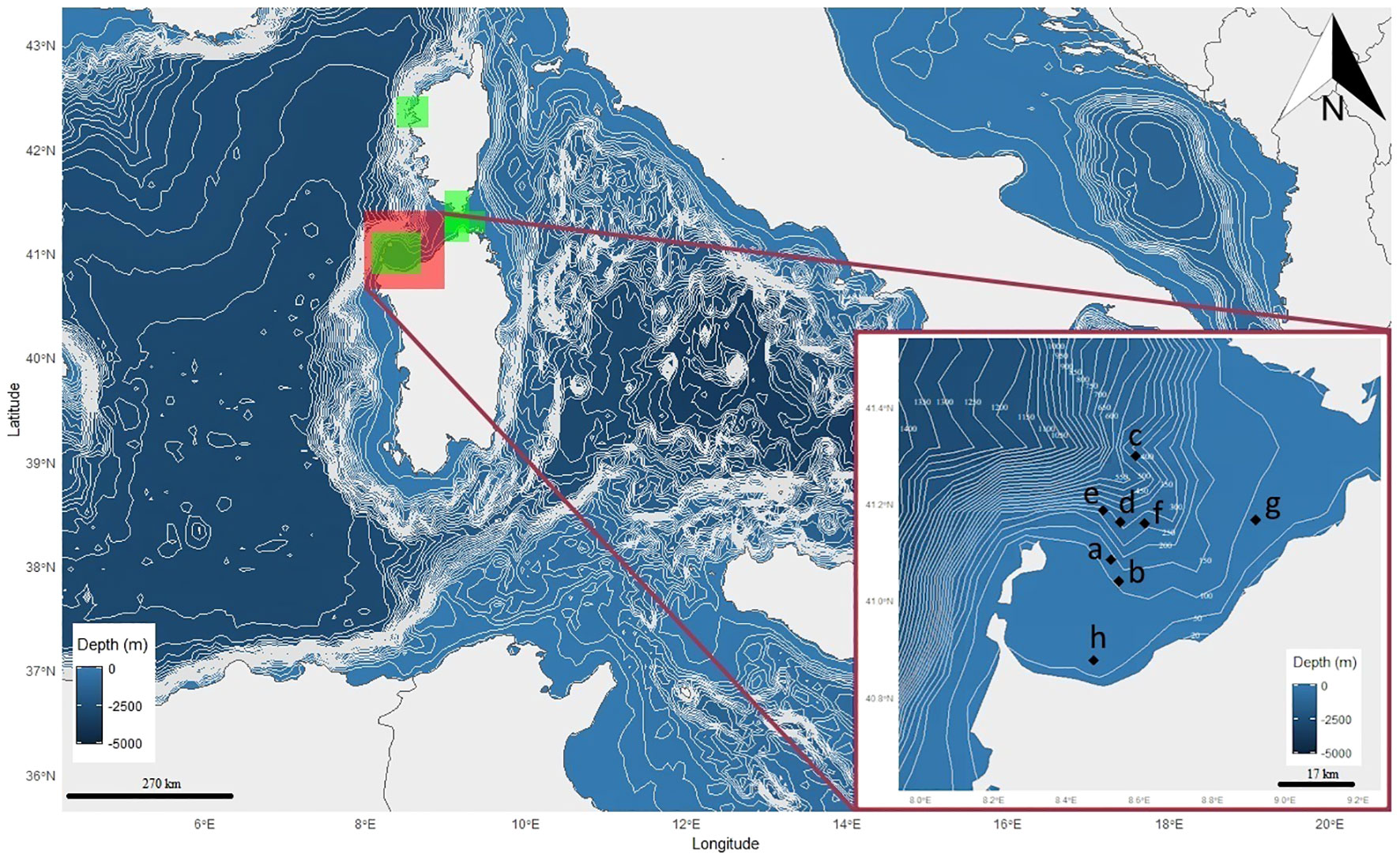
Figure 1 Map of the study area (Asinara Gulf and Bonifacio’s mouths, Northern Sardinia, Italy) provided with the positions (letters) where bycatch occurred in the longline sets deployed at sea during the observational period (2018–2021). Letters (a to h) denote correspondence with specimens and fishery information provided in Table 2. Green shadowed areas inside the large panel indicate the position of MPAs surrounding the study area (North: La Scandola Marine Reserve; East: Punta Falcone Capo Testa Marine Protected Area and Bonifacio Marine Reserve; West: Isola dell’Asinara National Park). Latitude and longitude are reported as decimal degrees, depth in metres and scale bars in kilometres. North (N) arrow is represented.
Compatible with their professional fishing activity, the captain and crew were instructed to take all possible biological data, fishing coordinates, pictures, and/or videos of the bycatch specimens as best as they could. To reduce bias related to the lack of on-board scientific observers, fishermen were also provided with field guides (Serena, 2005) for shark and ray species identification, together with general biological information on species potentially present in the bycatch of the gear they used, such as pelagic longlines and trammel nets. When in safe condition with an animal on board, the crew took or estimated biometric measures such as total length (TL; cm) by a tape measure, weight (W; kg) by a dynamometer, and sex (M or F, male or female, respectively) based on the presence/absence of claspers. Fishery data (date, latitude, and longitude) were collected for each bycatch occurrence as well. Fishers were also instructed to adopt the best manipulating handling practices when facing alive animals at recovery on board to collect biometric measures and to facilitate its release, according to the “Good practice guide for the handling of sharks and rays caught incidentally in Mediterranean pelagic longline fisheries” (FAO and ACCOBAMS, 2018). All carcasses of dead animals were discarded in compliance with updated fishery regulations of the Common Fisheries Policy (EU Regulation 2017/2107; EU, 2017).
CPUEs were calculated based on the effort calculated over the number of hooks per positive set (relative, informing on the bycatch intensity by species) and in all sets (absolute, informing on bycatch rate by species). To calculate total effort, we considered the eight longline fishing campaigns performed during the entire observational period. For CPUE in the trammel net, we considered the number of caught individuals caught per meter of net deployed, considering the 10 net sets deployed at sea by the boat during the entire period. Statistical significance of the difference in the number between sexes was checked through a chi-square test with Yates correction for continuity in species having more than two specimens in the corresponding sample. The life stages of the specimens sampled were assessed as newborn, juvenile, and adult based on the observed size and corresponding information available from the literature for each species separately (Table 1). Also, in this case, a chi-square test and a 2 × 3 contingency table with Yates correction were used to check for the difference in number between stages (newborn plus juveniles vs. adults) within single (one species) and across species, respectively. Finally, a chi-square test was used to check for differences in the number of bycatch events between years.
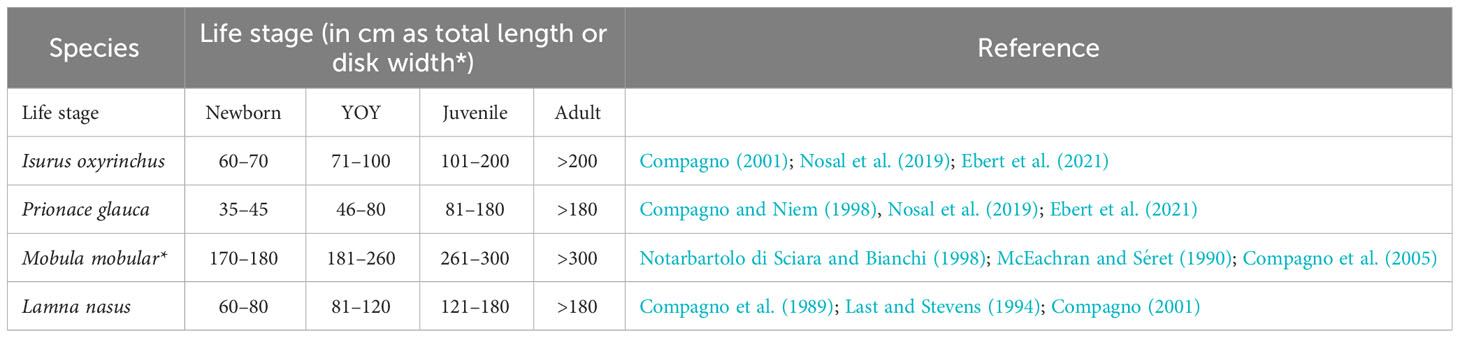
Table 1 Data and references used to assess the life stage based on the size of the specimens of four critically endangered elasmobranch observed in the bycatch of pelagic longline and trammel net fisheries through a 4-year fishery-dependent survey (2018–2021) in the Asinara Gulf.
We recorded the bycatch of seven specimens of I. oxyrinchus (Figures 2A–F), five of P. glauca (Figures 2G, H, 3J–L), and one newborn of M. mobular (Figure 3N; Supporting Video 1) by pelagic longlines and one juvenile of L. nasus (Figure 3M) by trammel nets (Tables 1, 2). Total length varied between 110 and 180 cm with a median value of 155 cm for the shortfin mako sharks and between 100 and 200 cm with a median value of 170 cm for the blue sharks (Table 2). The sex ratio was balanced in both the shortfin mako and the blue sharks (χ2 ≈ 0, d.f. = 1, p ≈ 1). Juveniles of shortfin makos were exclusive in the sample, whereas the number of juvenile blue sharks appeared higher compared with adults (two specimens, the smaller one presumably a maturing individual, Table 1), yet not significantly (χ2 = 0.8, d.f. = 1, p > 0.05). When cumulating all the species, the young life stage (newborn plus juveniles) had more individuals than the adult one, with the shortfin mako shark showing the highest percentage of juveniles between the species sampled (χ2 = 9.98, d.f. = 3, p < 0.001). Most of the incidental catches occurred in open waters in the proximity of the Castelsardo submarine canyon, where the target species X. gladius is usually caught (Figure 3O). Differently, the bycatch of the porbeagle shark and the spinetail devil ray occurred in coastal waters in the southwestern and northeastern parts of the Asinara Gulf, respectively (Figure 1, Table 2). Occurrences were concentrated in 2018 with eight records, followed by 2021 with four records (χ2 = 9.71, d.f. = 3, p < 0.01) (Table 2). The blue and the shortfin mako sharks showed the highest relative and absolute CPUEs, respectively, and the porbeagle shark had the lowest values (Table 3), compared with the other species sampled. Considering all incidental catches in longline, it is worth noting that a bycatch–entanglement event had a very high (87.5%) probability per set.

Figure 2 Pictures documenting some of the shark bycatch that occurred during the 4-year opportunistic survey on board a longliner in the Asinara Gulf: six shortfin makos Isurus oxyrinchus (A1, B–F) and mouth’s details (A2); two blue sharks Prionace glauca (G, H). Specimens are juvenile or subadult individuals in all the reported occurrences.

Figure 3 Pictures documenting some of the shark and ray bycatch that occurred during the 4-year opportunistic survey on board a longliner-netter in the Asinara Gulf: photos of three blue sharks Prionace glauca [J, K1, L1 (head), L2 (trunk), L3 (tail), K2 (injuring hook)]; one spinetail devil ray Mobula mobular (N, a likely entanglement rather than a bycatch), and one porbeagle shark Lamna nasus (M1 whole individual, M2 details of the lower jaw with teeth) in coastal fixed trammel net. Specimens are newborns, juveniles, or subadult individuals. Example of the main targeted species (O, swordfish Xiphias gladius) usually fished during a longline trip.
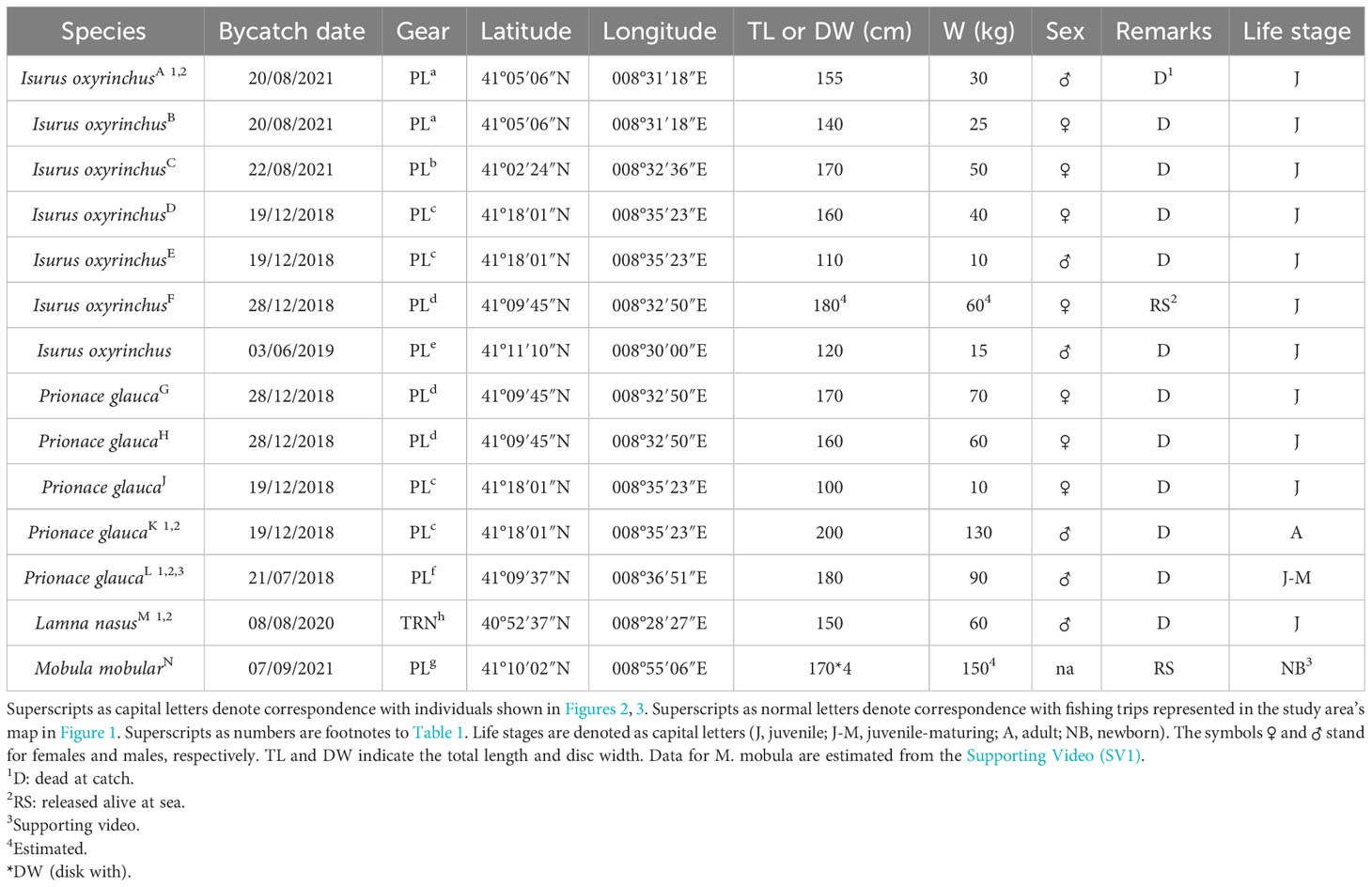
Table 2 Bycatch data of four large elasmobranch species collected during a 4-year (2018–2021) fishery-dependent survey on board a longliner (PL)-netter (TRN) fishing boat in the Asinara Gulf.
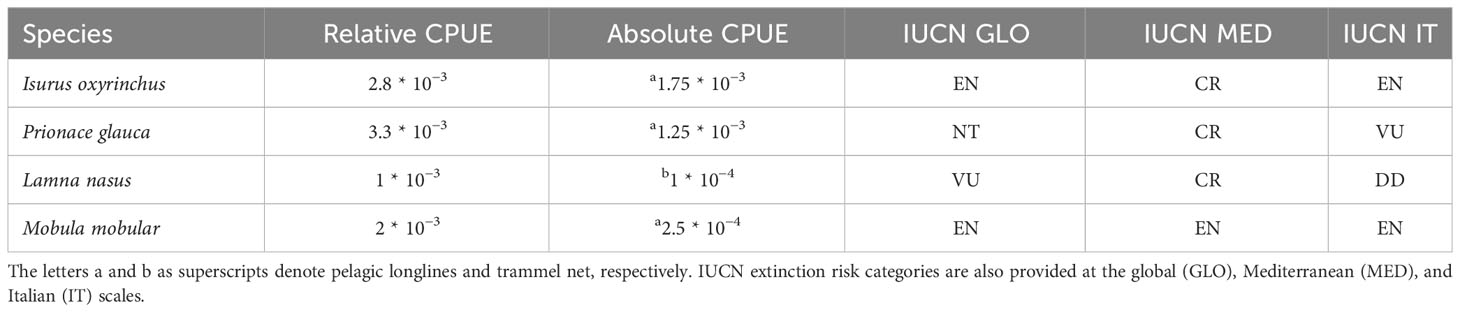
Table 3 Calculated CPUE (relative and absolute as number of specimens/hooks or/net length) of four large demersal–pelagic shark and ray species present in the bycatch of a small-scale longliner-netter in the waters of Northern Sardinia during a 4-year fishery-dependent monitoring survey.
According to the IUCN, the extinction risk of the species here reported varies as the geographical scale of the assessment varies (Table 3). In fact, the shortfin mako and the porbeagle sharks are currently evaluated as both Critically Endangered (CR) in the Mediterranean waters and at high extinction risk (Endangered: EN and Vulnerable: VU, respectively) in the world ocean (Table 3). The shortfin mako and the porbeagle sharks are assessed as EN and Data Deficient (DD) on the Italian scale (Table 3). The conservation status of the blue shark is Near Threatened (NT) at the global scale, whereas the assessments are worse when global is compared with the regional scales (CR and VU at the Mediterranean and Italian scales, respectively) (Table 3). The IUCN extinction risk of the spinetail devil ray is similarly high-leveled (EN) across all the IUCN assessment scales (Table 3).
Our results highlighted the almost exclusive presence of juvenile specimens among the large, critically endangered elasmobranchs collected as bycatch of the pelagic longline and trammel net monitored through a 4-year fishery-dependent survey in the Asinara Gulf. In fact, most of the recorded individuals can be classified as the juveniles of I. oxyrinchus, P. glauca, and L. nasus based on the size observed (Last and Stevens, 1994; Compagno, 2001; Nosal et al., 2019; Ebert et al., 2021). Interestingly, the individual of M. mobular recorded was a newborn, according to its size (Notarbartolo di Sciara and Serena, 1988; McEachran and Séret, 1990). The prevalence of juveniles of different species in a bycatch, as found in this study, could be linked to multiple and interacting drivers. Three main hypotheses can be drawn to explain the present data, based on the limitations and available information, these are related to the role of the Asinara Gulf as a favorable habitat provided with peculiar hydrological constraints, size selectivity of the gear, and population condition.
The prevalence of juveniles and newborns of different species of large pelagic sharks and rays here reported adds new insights to the environmental importance of the Asinara Gulf and Bonifacio Mouths for elasmobranchs. As recently shown, the same area exhibits also a high species diversity for the small- and medium-sized demersal sharks and skates distributed in coastal and deep grounds of the Gulf (Scacco et al., 2023b). Referring to the present sample, the bycatch of juveniles of the shortfin mako and blue sharks occurred all in the proximity of the Castelsardo Canyon, which is also the local fishing area for the target species: the swordfish X. gladius (A.G.C.I, 2009). The juveniles of the shortfin mako and blue shark might take advantage of such a prey-productive area, thanks to its favorable hydrological regime, both as a refuge and/or foraging area. In general, fishing areas with a high density of tunas and swordfish overlap with a high density of top predator sharks (Schindler et al., 2002; Romeo et al., 2009; Moro et al., 2019) due to competition for common prey or reciprocal predation (Revill et al., 2009; Li et al., 2016). Although the incidental catches that occurred in the coastal waters of the Gulf are limited to single individuals, coastal marine-rich environments are known to serve as a nursery ground for newborn spinetail devil ray (Notarbartolo di Sciara and Serena, 1988; Notarbartolo di Sciara and Bianchi, 1998), similar to what is presently observed. Otherwise, the same environment could function as a preferential habitat for the hunting activity of juvenile porbeagle shark, as suggested by the size of the captured specimen (Compagno et al., 1989; Last and Stevens, 1994; Compagno, 2001) and as also reported in other favorable coastal areas of the Mediterranean (Orsi Relini and Garibaldi, 2002; Keramidas et al., 2019). On the other hand, the combined action of huge water masses that exchange between the western Mediterranean and the Tyrrhenian Sea through the Asinara Gulf and Bonifacio Mouths could concentrate mostly juveniles and newborns of different large pelagic elasmobranchs in the area. Despite the different bioecological traits of the species, juveniles and newborns are more constrained by hydrological regimes than adults are, as the latter can take advantage of a higher swimming performance compared with former individuals. In addition to this study, the prevalence of juveniles in pelagic longline bycatch was recently observed in other potential favorable shark and ray areas, such as the Gulf of Gabes (Saidi et al., 2019), the southern Adriatic Sea (Carbonara et al., 2023), and the Pelagie Archipelago (Cattano et al., 2023a). A similar bycatch size structure was also observed in coastal passive nets deployed in the Ligurian and northern Tyrrhenian Seas for several species of large elasmobranchs (Mancusi et al., 2023), as well as in Mediterranean recreational fisheries, particularly blue shark (Panayiotou et al., 2020).
Current bycatch rates, as relative and absolute estimates, of shortfin mako and blue shark were found to be generally lower or at most similar to other data for these species in the pelagic longlines of the Mediterranean basin (Garibaldi, 2015; Bartolí et al., 2017; Saidi et al., 2019; Panayiotou et al., 2020; Carbonara et al., 2023; Cattano et al., 2023a; Doherty et al., 2022). Differently, the bycatch size composition and the at-vessel mortality rate obtained were similar. At-vessel mortality was severe for juveniles, all caught dead, except the largest shortfin mako observed (the only shark alive, and released into the sea, at the time of capture) and the newborn spinetail devil ray (disentangled from the gear and left at sea). Species identity was not ascertained in the limited number of catch escapes observed, as the larger specimens of other marine vertebrates are generally able, like sharks and rays, to escape capture by longline (Gilman et al., 2016; Piovano and Gilman, 2017; Papageorgiou et al., 2022). The high uncertainty on species identity in escapes, plus the limited number of the latter, suggests that escapes by the larger individuals of the elasmobranch species sampled may have occurred on a few occasions, i.e., the size structure observed has a very low probability to be affected by a size-related resistance-dependent selectivity of the gear. Differently, no conclusion can be argued about the effects of variation in both bait and operational fishing depth of the longlines on the size structure of the sample. In fact, fish and cephalopods were used in similar quantities as baits, and hook size and fishing depth were kept constant during the observational period of this study. The bycatch of the porbeagle shark by trammel nets is noteworthy, as it is emblematic of the rarity of this species in the Mediterranean Sea. In fact, the porbeagle shark is seldom recorded in the bycatch of the Mediterranean trammel net fishery (Scacco et al., 2012; Mancusi et al., 2020), and the species almost disappeared from the Mediterranean fishery statistics, particularly in pelagic longlines (Bartolí et al., 2017). A few scattered reports appear in the dated elasmobranch checklists of the North Tyrrhenian and Ligurian Sea (Vacchi and Serena, 1997; Orsi Relini and Garibaldi, 2002) and in updated checklists of Calabria (Leonetti et al., 2020) in the Italian waters and of Croatia (Balàka et al., 2023) in the Adriatic Sea, where records of the porbeagle shark appear particularly concentrated (Marconi and De Maddalena, 2001; Soldo and Jardas, 2002; Storai et al., 2005; Lipej et al., 2015). The record of the spinetail devil ray was likely an entanglement rather than bycatch, as already observed in pelagic longlines from other areas (Ceyhan and Akyol, 2014; Mas et al., 2015). Hooks are dangerous to this species not as bait but because of the large size, dorsal-ventrally compressed shape of the species, and its habit to travel across epipelagic waters (Notarbartolo di Sciara and Serena, 1988; Notarbartolo di Sciara and Bianchi, 1998; Abudaya et al., 2018; Lezama-Ochoa et al., 2019) where longlines are usually deployed (FAO, 2016; JRC, 2020) and/or other pelagic gear poses a threat to this species (Scacco et al., 2009). In the present occasion, the specimen was released alive at sea cutting the fishing line without recovery of the animal on board, following the recommendations on handling practices of elasmobranchs in longline bycatch (FAO and ACCOBAMS, 2018).
As observed during the 4-year fishery-dependent survey in the study area, the prevalence of juveniles of k-selected critically endangered large elasmobranchs in the bycatch of longlines and trammel nets is a constant feature of the bycatch size composition recently observed in other areas of the Mediterranean (Saidi et al., 2019; Panayiotou et al., 2020; Carbonara et al., 2023; Doherty et al., 2022; Cattano et al., 2023a; Mancusi et al., 2023). Aside from large elasmobranch species, coastal passive nets are also responsible for a relevant bycatch of juveniles of several small- and medium-sized endangered sharks and rays, as observed in the trammel net fishery in the southeastern Sicily (Tiralongo et al., 2018a; Tiralongo et al., 2018b). Inferring population condition-related explanations for such common evidence is difficult as justification is twofold and paradoxically opposite, i.e., a sign either of a general recovery of large shark populations (an increase in the number of breeding individuals and thus of juveniles) or of a poor population status (overfishing, limited number of adult breeding individuals) in Mediterranean waters. On the one hand, the high extinction risk assessed by the IUCN for the elasmobranchs considered here suggests that the prevalence of juveniles observed in the bycatch area, as recently in other areas, may likely be a sign of a generalized poor population condition, with few reproductive individuals and reduced resilience. On the other hand, the increased attention and commitment to shark and ray species conservation by the public audience and stakeholders (Hind, 2015; Giovos et al., 2016) could have recently contributed significantly to the increase in reports of bycatch or sightings of large sharks and rays. Although most of the conservation effort still needed is far from being complete, the actions actually implemented seem to entail some signs of population recovery that have recently been detected, albeit for a limited number of elasmobranch species, both globally (Grace et al., 2021) and locally (Serena and Silvestri, 2018). However, the information lacking at the subregional scale is still relevant for several species, such as the porbeagle shark, which remains a data-deficient species (DD) in the updated Italian IUCN assessment (Rondinini et al., 2022). Indeed, the populations of lamnid sharks have been decreasing dramatically in the Mediterranean Sea due to the collapse in the number of individuals existing during the last century (Ferretti et al., 2008), as specifically observed for the great white shark (Moro et al., 2019). Newly elaborated metrics suggest the porbeagle shark, like several other DD species, should be assessed as EN or worst in the corresponding IUCN assessment (Scacco et al., 2023a).
Even though considering the boundaries of the fishery-dependent data here presented, our results give strength to two of the hypotheses made. On the one hand, the results are in line with current information about the size structure of the bycatch in pelagic longlines and trammel nets in other Mediterranean areas, suggesting that a poor population condition is very likely for the local populations of large pelagic elasmobranchs in the Asinara Gulf, as well as in other Mediterranean areas. Measuring the kinship of sampled individuals will be crucial to understand the actual population condition and its time trajectory under anthropic pressure. The literature gives several examples about the limited contact between shark and ray populations at both large and small scales. For example, recent studies applying the molecular approach have highlighted the main role of water circulation and temperature in sharks (Di Crescenzo et al., 2022; Melis et al., 2023a) and rays (Catalano et al., 2022; Melis et al., 2023b). Similarly, evidence based on molecular data showed how biotic and abiotic features such as bathymetry, hydrological constraints, and prey abundance can influence the presence, movement, and dispersion of sharks and rays (Catarino et al., 2015; Di Crescenzo et al., 2022). The continued evolution and development of new and more resolutive techniques are allowing the possibility to deeply understand the biology and ecology of marine species, such as P. glauca for which only recently the absence of panmixia worldwide was observed (Nikolic et al., 2023). On the other hand, the presence of juveniles and newborns of different species in the study area can be linked to its high environmental value and the peculiar hydrological condition. Such an environment could satisfy the multiple ecological requirements that are functional to the different reproductive strategies of species composing the assemblage sampled (Nakano and Stevens, 2009; Vandeperre et al., 2014). These characteristics can meet the criteria established for consideration of the Asinara Gulf and Bonifacio Mouths as an ISRA. Suggesting the study area as potential ISRA will need future investigations, for instance, based on interdisciplinary approaches such as dedicated surveys flanked by citizen science initiative (Cattano et al., 2023b), molecular investigation (barcoding and eDNA metabarcoding; Bakker et al., 2017; Cariani et al., 2017; Albonetti et al., 2023; Jenrette et al., 2023), specimen tracking and monitoring using BRUVs (Cattano et al., 2021; Liu et al., 2022; Prat-Varela et al., 2023) and satellite tags and acoustic telemetry (Williamson et al., 2019; Renshaw et al., 2023), multiboat fishery-independent surveys (Scacco et al., 2023a), local ecological knowledge-based investigations (Colloca et al., 2017), and spatially explicit population models (Lauria et al., 2015). These tools will be crucial to disentangle the hypotheses made on the prevalence of juveniles observed in the bycatch area. As a complementary low-cost and at the same time high-informative tool, the effort and collaboration of the fishermen are decisive for obtaining documented fishery-dependent observations. In turn, the latter is important as it represents a first approach to data, above all suggesting research avenues and methods to be implemented in future activities aimed at verifying the hypotheses made. Knowledge of the population abundance and size and sex-related structure at the subregional scale is of paramount importance for the local conservation management and the accuracy of the red list’s assessments at the greater scale, particularly when referring to migratory species that unfortunately stand a few steps to extinction.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements. The specimens described in the paper were handled following the recommendations provided by the “Good practice guide for the handling of sharks and rays caught incidentally in Mediterranean pelagic longline fisheries” (FAO and ACCOBAMS, 2018). All carcasses of dead animals were discarded in compliance with the updated fishery regulations of the Common Fisheries Policy (EU Regulation 2017/2107; EU, 2017). The fishermen did their best to release individuals accidentally caught at sea in healthy condition.
US: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EG: Conceptualization, Writing – review & editing. SD: Writing – review & editing. EF: Conceptualization, Funding acquisition, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Special thanks to the captain and crew of the fishing vessel involved in the study. We are particularly grateful to the referees who reviewed this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1303961/full#supplementary-material
Supplementary Video 1 | Gear disentanglement of a newborn Mobula mobular in pelagic longlines deployed in the Asinara Gulf.
Abudaya M., Ulman A., Salah J., Fernando D., Wor C., Notarbartolo di Sciara G. (2018). Speak of the devil ray (Mobula mobular) fishery in Gaza. Rev. Fish. Biol. Fish. 28, 229–239. doi: 10.1007/s11160-017-9491-0
A.G.C.I (2009). Federazione Provinciale di Sassari- Stima sforzo di pesca Sardegna Nord Occidentale Prot.
Albonetti L., Maiello G., Cariani A., Carpentieri P., Ferrari A., Sbrana A., et al. (2023). DNA metabarcoding of trawling bycatch reveals diversity and distribution patterns of sharks and rays in the central Tyrrhenian Sea. ICES J. Mar. Sci. 80 (4), 664–674. doi: 10.1093/icesjms/fsad022
Bakker J., Wangensteen O. S., Chapman D. D., Boussarie G., Buddo D., Guttridge T. L., et al. (2017). Environmental DNA reveals tropical shark diversity in contrasting levels of anthropogenic impact. Sci. Rep. 7, 16886. doi: 10.1038/s41598-017-17150-2
Balàka P. F., Ugarković P., Türtscher J., Kriwet J., Niedermüller S., Krstinić P., et al. (2023). Updated checklist of chondrichthyan species in Croatia (Central mediterranean sea). Biology 12, 952. doi: 10.3390/biology12070952
Bartolí A., Polti S., Niedermüller S. K., García R. (2017). Sharks in the Mediterranean: A review of the literature on the current state of scientific knowledge, conservation measures and management policies and instruments (WWF internal report).
Beerkircher L. R., Cortés E., Shivji M. (2002). Characteristics of shark bycatch observed on pelagic longlines off the south-eastern United States 1992-2000. Mar. Fish. Rev. 64, 40–49.
Bell J. D., Harmelin-Vivien M. L. (1983). Fish fauna of French Mediterranean Posidonia oceanica seagrass meadows. II - Feeding habits. Téthys 11, 1–14.
Benjamins S., Kulka D. W., Lawson J. (2010). Recent incidental catch of sharks in gillnet fisheries of Newfoundland and Labrador, Canada. Endanger. Species Res. 11, 133–146. doi: 10.3354/esr00268
Branstetter S. (1990). “Early life-history implications of selected carcharhinoid and lamnoid sharks of the northwest Atlantic,” in Elasmobranchs as living resources: advances in biology, ecology, systematics and the status of the fisheries. Eds. Pratt H. L., Gruber S. H., Taniuchi T. (Silver Spring, MD USA: National Marine Fisheries Service), 17–28. NOAA Technical Report 90.
Braun C. D., Gaube P., Sinclair-Taylor T. H., Thorrold S. R. (2019). Mesoscale eddies release pelagic sharks from thermal constraints to foraging in the ocean twilight zone. PNAS 116 (35), 17187–17192. doi: 10.1073/pnas.1903067116
Burgess E., Dimech M., Caruana R., Darmanin M., Raine H., Harrison A., et al. (2010). Non-target bycatch in the Maltese blue fin tuna (Thunnus thynnus) longline fishery (Central Mediterranean). Col. Vol. Sci. Pap. ICCAT 65, 2262–2269. [SCRS/2009/059]. Available at: http://www.iccat.int/Documents/CVSP/CV065_2010/no_6/CV065062262.pdf.
Campana S. E., Joyce W. N. (2004). Temperature and depth associations of porbeagle shark (Lamna nasus) in the northwest Atlantic. Fish. Oceanogr. 13 (1), 52–64. doi: 10.1111/j.1365-2419.2004.00236.x
Capapé C., Bouchereau J. L., Tomasini J. A. (1990). Pre´sence du diable de mer, Mobula mobular (Bonnaterre 1788) (Pisces, Rajiformes, Mobulidae) dans le golf d’Aigues-Mortes. Anatomie de la ceinture pelvienne et des pte´rygopodes. Mesogee 50, 9–14.
Capapé C., Zaouali J. (1976). Note sur la pre´sence de la mante de mer Mobula mobular (Bonnaterre 1788) (Se´laciens, Rajiformes) dans les eaux tunisiennes. Doriana 5, 1–8.
Carbonara P., Prato G., Niedermüller S., Alfonso S., Neglia C., Donnaloia M., et al. (2023). Mitigating effects on target and bycatch species fished by drifting longlines using circle hooks in the South Adriatic Sea (Central Mediterranean). Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1124093
Cariani A., Messinetti S., Ferrari A., Arculeo M., Bonello J. J., Bonnici L., et al. (2017). Improving the conservation of mediterranean chondrichthyans: the ELASMOMED DNA barcode reference library. PloS One 12 (1), e0170244. doi: 10.1371/journal.pone.0170244
Carpentieri P., Nastasi A., Sessa M., Srour A. (2021). Incidental catch of vulnerable species in Mediterranean and Black Sea fisheries: A review (Rome: FAO). GFCM Studies and Reviews No. 101.
Carrier J. C., Simpfendorfer C. A., Heithaus M. R., Yopak K. E. (2022). Biology of sharks and their relatives. 3rd Edition. Eds. Carrier J. C., Simpfendorfer C. A., Heithaus M. R., Yopak K. E. (Boca Raton, FL, USA: CRC Press), 840.
Catalano G., Crobe V., Ferrari A., Baino R., Massi D., Titone A., et al. (2022). Strongly structured populations and reproductive habitat fragmentation increase the vulnerability of the Mediterranean starry ray Raja asterias (Elasmobranchii, Rajidae). Aquat. Cons. Mar.: Freshw. 32 (1), 66–84. doi: 10.1002/aqc.3739
Catarino D., Knutsen H., Veríssimo A., Olsen E. M., Jorde P. E., Menezes G., et al. (2015). The Pillars of Hercules as a bathymetric barrier to gene flow promoting isolation in a global deep-sea shark (Centroscymnus coelolepis). Mol. Ecol. 24, 6061–6079. doi: 10.1111/mec.13453
Cattano C., Calò A., Aglieri G., Cattano P., Di Lorenzo M., Grancagnolo D., et al. (2023b). Literature, social media and questionnaire surveys identify relevant conservation areas for Carcharhinus species in the Mediterranean Sea. Biol. Cons. 277. doi: 10.1016/j.biocon.2022.109824
Cattano C., Gambardella C., Grancagnolo D., Principato E., Aglieri G., Turco G., et al. (2023a). Multiple interannual records of young-of-the-year identify an important area for the protection of the shortfin mako, Isurus oxyrinchus. Mar. Environ. Res. 192, 106217. doi: 10.1016/j.marenvres.2023.106217
Cattano C., Turco G., Di Lorenzo M., Gristina M., Visconti G., Milazzo M. (2021). Sandbar shark aggregation in the central Mediterranean Sea and potential effects of tourism. Aquat. Cons.: Mar. Freshw. 31, 1420–1428. doi: 10.1002/aqc.3517
Ceyhan T., Akyol O. (2014). On the turkish surface longline fishery targeting swordfish in the Eastern Mediterranean Sea. Turk. J. Fish. Aquat. Sci. 14, 825–830. doi: 10.4194/1303-2712-v14325
Cheung W. W. L., Pitcher T. J., Pauly D. (2005). A fuzzy logic expert system to estimate intrinsic extinction vulnerabilities of marine fishes to fishing. Biol. Cons. 124 (1), 97–111. doi: 10.1016/j.biocon.2005.01.017
Cheung W. W. L., Watson R., Morato T., Pauly D. (2007). Intrinsic vulnerability in the global fish catch. Mar. Ecol. Progr. Ser. 333, 1–12. doi: 10.3354/meps333001
Cocito S., Ferdeghini F. (2001). Carbonate standing stock and carbonate production of the bryozoan Pentapora fascialis in the northwestern Mediterranean. Facies 45, 25–30. doi: 10.1007/BF02668102
Coelho R., Erzini K., Bentes L., Correia C., Lino P. G., Monteiro P., et al. (2005). Semi-pelagic longline and trammel net elasmobranch catches in southern Portugal: Catch composition, catch rates, and discards. J. North. Atl. Fish. Sci. 37, 531–537. doi: 10.2960/J.v35.m482
Colloca F., Enea M., Ragonese S., Di Lorenzo M. (2017). A century of fishery data documenting the collapse of smooth-hounds (Mustelus spp.) in the Mediterranean Sea. Aquat. Cons.: Mar. Freshw. 27, 1145–1155. doi: 10.1002/aqc.2789
Compagno L. J. V. (2001). Sharks of the world. An annotated and illustrated catalogue of shark species known to date Vol. 2 (Rome: FAO).
Compagno L. J. V., Dando D., Fowler S. (2005). Sharks of the world. Princeton field guides (London: Harper Collins Publishing Ltd.). 368 p.
Compagno L. J. V., Ebert D. A., Smale M. J. (1989). Guide to the sharks and rays of southern Africa (London: New Holland (Publ.) Ltd.). 158 p.
Compagno L. J. V., Niem V. H. (1998). “Carcharhinidae. Requiem sharks,” in The living marine resources of the western central pacific. Eds. Carpenter K. E., Niem V. H. (Rome: FAO), 1312–1360. FAO Identification Guide for Fishery Purposes.
CoNiSMA (2018). Contabilità ambientale nelle aree marine protette italiane Area Marina Protetta Isola dell’Asinara Interventi realizzati a valere sulle specifiche risorse assegnate per l’implementazione della rendicontazione naturalistica (ecorendiconto), Report finale.
Convention on Biological Diversity (CBD) (2023). Available at: https://www.cbd.int/ebsa/ (Accessed September 20, 2023).
Cortés E. (1998). Demographic analysis as an aid in shark stock assessment and management. Fish. Res. 39, 199–208. doi: 10.1016/S0165-7836(98)00183-0
Cortés E. (2002). Incorporating uncertainty into demographic modelling: Application to shark populations and their conservation. Cons. Biol. 16, 1048–1062. doi: 10.1046/j.1523-1739.2002.00423.x
Cossu A., Cressa L., Gazale V., Ragazzola F. (2009). On the circalittoral benthic communities in the asinara marine park. Biol. Mar. Medit. 16 (I), 256–257.
Cossu A., De Luca M. (2016). Indagine sui fondi duri ai fini della attuazione della direttiva della Strategia Marina - Sardegna Settentrionale. Biol. Mar. Medit. 23 (1), 178–181.
Cox G., Francis M. (1997). Sharks and rays of New Zealand (New Zealand: Canterbury Univ. Press, Univ. of Canterbury). 68 p.
Damalas D., Megalofonou P. (2012). Occurrences of large sharks in the open waters of the Southeastern Mediterranean Sea. J. Nat. Hist. 46(43-44), 2701–2723. doi: 10.1080/00222933.2012.716864
Di Crescenzo S., Ferrari A., Barría C., Cannas R., Cariani A., Drewery J., et al. (2022). First evidence of population genetic structure of the deep-water blackmouth catshark Galeus melastomus Rafinesque 1810. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.953895
Doherty P. D., Enever R., Omeyer L. C. M., Tivenan L., Course G., Pasco G., et al. (2022). Efficacy of a novel shark bycatch mitigation device in a tuna longline fishery. Curr. Biol. 22 (32), 1260–1261. doi: 10.1016/j.cub.2022.09.003
Ebert D. A., Dando M., Fowler S. (2021). Sharks of the world: a complete guide Princeton NJ U.S.A: Princeton University Press). 2021, 624.
Ellis J. R., Mccully S. R., Poisson P. F. (2017). A review of capture and post-release mortality of elasmobranchs. J. Fish Biol. 90, 653–722. doi: 10.1111/jfb.13197
EU (2017) Regulation (EU) 2017/2107 of the European Parliament and of the Council of 15 November 2017 laying down management, conservation and control measures applicable in the Convention area of the International Commission for the Conservation of Atlantic Tunas (ICCAT), and amending Council Regulations (EC) No 1936/2001, (EC) No 1984/2003 and (EC) No 520/2007. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R2107 (Accessed September 10, 2023).
EU (2019) REGULATION (EU) 2019/833 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 20 May 2019 laying down conservation and enforcement measures applicable in the Regulatory Area of the Northwest Atlantic Fisheries Organisation, amending Regulation (EU) 2016/1627 and repealing Council Regulations (EC) No 2115/2005 and (EC) No 1386/2007. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0833&from=ES (Accessed September 10, 2023).
FAO (2016) International standard statistical classification of fishing gear (ISSCFG 2016). (Coordinating Working Party on Fishery Statistics (CWP). Available at: https://www.fao.org/3/a-bt988e.pdf (Accessed June 5, 2023). Handbook of Fishery Statistics.
FAO and ACCOBAMS (2018) Good practice guide for the handling of sharks and rays caught incidentally in Mediterranean pelagic longline fisheries. Available at: https://www.fao.org/3/i9152en/I9152EN.pdf (Accessed September 20, 2023).
Ferretti F., Myers R. A., Serena F., Lotze H. K. (2008). Loss of large predatory sharks from the Mediterranean Sea. Cons. Biol. 22, 952–964. doi: 10.1111/j.1523-1739.2008.00938.x
Francour P. (1994). Pluriannual analysis of the reserve effect on ichthyofauna in the Scandola natural reserve (Corsica, Northwestern Mediterranean). Oceanol. Acta 17, 309–317.
Froese R., Demirel N., Coro G., Kleisner K. M., Winker H. (2017). Estimating fisheries reference points from catch and resilience. Fish Fish. 18 (3), 506–526. doi: 10.1111/faf.12190
Galeana-Villasenor I., Galvan-Magana F., Santana-Hernandez H. (2009). Fishing by hooks in longliners from the Mexican Pacific Ocean: Effects in the catch rate and weight of sharks and other species. Rev. Biol. Mar. Oceanogr. 44 (1), 163–172.
Gallagher A. J., Orbesen E. S., Hammerschlag N., Serafy J. E. (2014). Vulnerability of oceanic sharks as pelagic longline bycatch. Glob. Ecol. Cons. 1, 50–59. doi: 10.1016/j.gecco.2014.06.003
Garibaldi F. (2015). Bycatch in the mesopelagic swordfish longline fishery in the Ligurian Sea (western Mediterranean). Col. Vol. Sci. Pap. ICCAT 71 (3), 1495–1498. [SCRS/2014/155]. Avaialable at: https://www.iccat.int/Documents/CVSP/CV071_2015/n_3/CV071031495.pdf.
Gennari E., Dylan I., Cowley P. D. (2022). Active acoustic telemetry reveals ontogenetic habitat-related variations in the coastal movement ecology of the white shark. Anim. Biotelemetry 10 (25). doi: 10.1186/s40317-022-00295-x
Gérigny O., Coudray S., Lapucci C., Tomasino C., Bisgambiglia P. A., Galgani F. (2015). Small-scale variability of the current in the Strait of Bonifacio. Ocean Dyn. 65 (8), 1165–1182. doi: 10.1007/s10236-015-0863-5
GFCM (2021)Decision GFCM /44/2021/16. In: Compendium of GFCM decisions Revised version 6.0 2022. Available at: https://gfcmsitestorage.blob.core.windows.net/website/Decisions/GFCM_Compendium_2022-e.pdf (Accessed October 21, 2023).
Gilman E., Chaloupka M., Swimmer Y., Piovano S. (2016). A cross-taxa assessment of pelagic longline bycatch mitigation measures: conflicts and mutual benefits to elasmobranchs. Fish Fish. 17, 748–784. doi: 10.1111/faf.12143
Gilman E. L., Clarke S., Brothers N., Alfaro-Shigueto J., Mandelman J., Mangel J., et al. (2007a). Shark depredation and unwanted bycatch in pelagic longline fisheries: Industry practices and attitudes, and shark avoidance strategies (Honolulu, HI: Western Pacific Regional Fishery Management Council).
Gilman E., Clarke S., Brothers N., Alfaroshigueto J., Mandelman J., Mangel J., et al. (2008). Shark interactions in pelagic longline fisheries. Mar. Pol. 32, 1–18. doi: 10.1016/j.marpol.2007.05.001
Gilman E., Kobayashi D., Swenarton T., Brothers N., Dalzell P., Kinan-Kelly I. (2007b). Reducing sea turtle interactions in the Hawaii based longline swordfish fishery. Biol. Cons. 139, 19–28. doi: 10.1016/j.biocon.2007.06.002
Giovos I., Ganias K., Garagouni M., Gonzalvo J. (2016). Social media in the service of conservation: a case study of dolphins in the Hellenic Seas. Aquat. Mamm. 42 (1), 12–19. doi: 10.1578/AM.42.1.2016.12
González-Andrés C., Sánchez-Lizaso J. L., Cortés J., Pennino M. G. (2021). Predictive habitat suitability models to aid the conservation of elasmobranchs in Isla del Coco National Park (Costa Rica). J. Mar. Syst. 224, 103643. doi: 10.1016/j.jmarsys.2021.103643
Grace M. K., Akçakaya H. R., Bennett E. L., Brooks T. M., Heath A., Hedges S., et al (2021). Testing a global standard for quantifying species recovery and assessing conservation impact. Cons. Biol. 35, 1833–1849. doi: 10.1111/cobi.13756
Grose S. O., Pendleton L., Leathers A., Cornish A., Waitai S. (2020). Climate change will re-draw the map for marine megafauna and the people who depend on them. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00547
Heppell S. S., Crowder L. B., Menzel T. R. (1999). “Life table analysis of long lived marine species with implications for conservation and management,” in Life in the Slow Lane: Ecology and conservation of long-lived marine animals, vol. 23 . Ed. Musick J. A. (Bethesda, MD, USA: American Fisheries Society Symposium), 137–148.
Heupel M. R., Carlson J. K., Simpfendorfer C. A. (2007). Shark nursery areas: concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 337, 287–297. doi: 10.3354/meps337287
Hind E. J. (2015). A review of the past, the present, and the future of fishers' knowledge research: a challenge to established fisheries science. ICES J. Mar. Sci. 72 (2), 341–358. doi: 10.1093/icesjms/fsu169
Hinke J. T., Kaplan I. C., Aydin K., Watters G. M., Olson R., Kitchell J. F. (2004). Visualizing the food web effects of fishing for tunas in the Pacific Ocean. Ecol. Soc 9, 10. doi: 10.5751/ES-00626-090110
Interreg technical report (2013). Progetto strategico MARTE+, SOTTOPROGETTO SB, Modelli di governance e Monitoraggio per la salvaguardia e valorizzazione delle risorse ittiche, Componente 4: MONITORAGGIO DELLE RISORSE ITTICHE E ACQUACOLTURA SOSTENIBILE. Risultati del monitoraggio integrativo: LA PESCA ARTIGIANALE NEL TERRITORIO TRANSFRONTALIERO: CAPACITA', SFORZO DI PESCA E CARATTERIZZAZIONE DEI PRINCIPALI METIERS".
IUCN (2023a) Important shark and ray areas. Available at: https://sharkrayareas.org/isra/ (Accessed September 25, 2023).
IUCN (2023b) The IUCN (International Union for the Conservation of the Nature) red list of threatened species. Available at: https://www.iucnredlist.org/search?query=elasmobranchs&searchType=species (Accessed September 10, 2023).
Jenrette J. F., Jenrette J. L., Truelove N. K., Moro S., Dunn N. I., Chapple T. K., et al. (2023). “Detecting Mediterranean white sharks with environmental DNA,” in Frontiers in ocean observing: emerging technologies for understanding and managing a changing ocean, vol. 36 . Eds. Kappel E. S., Cullen V., Costello M. J., Galgani L., Gordó-Vilaseca C., Govindarajan A., Kouhi S., Lavin C., McCartin L., Müller J. D., Pirenne B., Tanhua T., Zhao Q., Zhao S., 87–89. (Rockville, MD, USA: The official magazine of the Oceanography Society). doi: 10.5670/oceanog.2023.s1.28
Joyce W. N., Campana S. E., Natanson L. J., Kohler N. E., Pratt H. L. Jr, Jensen C. F. (2002). Analysis of stomach contents of the porbeagle shark (Lamna nasus Bonnaterre) in the Northwest Atlantic. ICES J. Mar. Sci. 59, 1263–1269. doi: 10.1006/jmsc.2002.1286
JRC (2020). Available at: https://datacollection.jrc.ec.europa.eu/wordef/fleet-segment-dc (Accessed June 12, 2023).
Kenyon N. H., Klaucke I., Millington J., Ivanov M. K. (2002). Sandy submarine canyon-mouth lobes on the western margin of Corsica and Sardinia, Mediterranean Sea. Mar. Geol. 184, 69–84. doi: 10.1016/S0025-3227(01)00282-1
Keramidas I., Ugarković P., De Maddalena A., Giovos I. (2019). An additional record of Lamna nasus (Bonnaterre 1788) from Croatia, Adriatic Sea. J. Black Sea/Medit. Environ. 25 (1), 87–92.
Kock A. A., Lombard A. T., Daly R., Goodall V., Meÿer M., Johnson R., et al. (2022). Sex and size influence the spatiotemporal distribution of white sharks, with implications for interactions with fisheries and spatial management in the Southwest Indian Ocean. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.811985
Kohler N. E., Turner P. A., Hoey J. J., Natanson L. J., Briggs R. (2002). Tag and recapture data for three pelagic shark species, blue shark (Prionace glauca), shortfin mako (Isurus oxyrinchus), and porbeagle (Lamna nasus) in the North Atlantic Ocean. Col. Vol. Sci. Pap. ICCAT 54 (4), 1231–1260.
Kroodsma D. A., Mayorga J., Hochberg T., Miller N. A., Boerder K., Ferretti F., et al. (2018). Tracking the global footprint of fisheries. Science 359, 904–908. doi: 10.1126/science.aao5646
Kubodera T., Watanabe H., Ichii T. (2007). Feeding habits of the blue shark, Prionace glauca, and salmon shark, Lamna ditropis, in the transition region of the Western North Pacific. Rev. Fish Biol. Fish. 17, 111–124. doi: 10.1007/s11160-006-9020-z
Lamarck J. B. P. A. M. (1798). Sur les genres de la séche, du calmar et du poulpe, vulgairement nommés polypes de mer. - Mémoires de la Société d'Histoire Naturelle de Paris 1 [an VII]: 1-25, Pl. 1-II [= 1-2]. Paris.
Lauria V., Gristina M., Attrill M., Fiorentino F., Garofalo G. (2015). Predictive habitat suitability models to aid conservation of elasmobranch diversity in the central Mediterranean Sea. Sci. Rep. 5, 13245. doi: 10.1038/srep13245
Leonetti F. L., Giglio G., Leone A., Coppola F., Romano C., Bottaro M., et al. (2020). An updated checklist of chondrichthyans of Calabria (Central Mediterranean, Southern Italy), with emphasis on rare species. Medit. Mar. Sci. 21 (3), 794–807. doi: 10.12681/mms.23321
LeSueur C. A. (1818). Descriptions of several new species of North American fishes. J. Acad. Nat. Sci. Philad. 1 (2), 222–235; 359-368.
Lezama-Ochoa N., Hall M. A., Pennino M. G., Stewart J. D., López J., Murua H. (2019). Environmental characteristics associated with the presence of the Spinetail devil ray (Mobula mobular) in the eastern tropical Pacific. PloS One 14 (8), e0220854. doi: 10.1371/journal.pone.0220854
Li Y., Zhang Y., Dai X. (2016). Trophic interactions among pelagic sharks and large predatory teleosts in the Northeast Central Pacific. J. Exp. Mar. Biol. Ecol. 483, 97–103. doi: 10.1016/j.jembe.2016.04.013
Linnaeus C. (1758). Systema naturae per regna tria naturae, secundum classes, ordinus, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. Impensis Direct. Laurentii Salvii, Holmiae. 824 p.
Lipej L., Uhan J., Mavrič B., Vujčić-Karlo S. (2015). A record of porbeagle, Lamna nasus (Bonnaterre 1788), in the Gulf of Trieste with discussion on itsoccurrence in the Adriatic Sea. Acta Adriat 57 (2), 305–314.
Liu Z., Collins R. A., Baillie C., Rainbird S., Brittain R., Griffiths A. M., et al. (2022). ). Environmental DNA captures elasmobranch diversity in a temperate marine ecosystem. Environ. DNA 4, 1024–1038. doi: 10.1002/edn3.294
Maia A., Queiroz N., Correia J. P., Cabral H. (2006). Food habits of the shortfin mako, Isurus oxyrinchus, off the southwest coast of Portugal. Environ. Biol. Fish 77, 157–167. doi: 10.1007/s10641-006-9067-7
Mancusi C., Baino R., Fortuna C., Gil De Sola L., Morey G., Bradai M. N., et al. (2020). MEDLEM database, a data collection on large Elasmobranchs in the Mediterranean and Black seas. Medit. Mar. Sci. 0, 276–288. doi: 10.12681/mms.21148
Mancusi C., Serena F., Neri A., Scacco U., Baino R. T., Voliani A., et al. (2023). Unexpected records of newborn and young sharks in ligurian and north tyrrhenian seas (North-western Mediterranean Basin). Diversity 15, 806. doi: 10.3390/d15070806
Marconi M., De Maddalena A. (2001). On the capture of a young porbeagle, Lamna nasus (Bonnaterre 1788), in the Western Adriatic Sea. Ann. Ser. Hist. Nat. 11, 179–184.
Mas F., Forselledo R., Domingo A. (2015). Mobulid ray by-catch in longline fisheries in the south-western Atlantic Ocean. Mar. Fresh. Res. 66, 767–777. doi: 10.1071/MF14180
McEachran J. D., Capapé C. (1984). “Mobulidae,” in Fishes of the north-eastern atlantic and the mediterranean, vol. 1 . Eds. Whitehead P. J. P., Bauchot M.-L., Hureau J.-C., Nielsen J., Tortonese E. (Paris: UNESCO), 210–211.
McEachran J. D., Séret B. (1990). “Mobulidae,” in Check-list of the fishes of the eastern tropical Atlantic (CLOFETA), vol. 1 . Eds. Quero J. C., Hureau J. C., Karrer C., Post A., Saldanha L. (Paris: UNESCO), 73–76. JNICT, Lisbon; SEI, Paris;.
Megalofonou P., Damalas D., Yannopoulos C. (2005). Composition and abundance of pelagic shark by-catch in the Eastern Mediterranean Sea. Cybium 29, 135–140.
Mejuto J., Garcia-Cortes B., de la Serna J. M. (2002). Preliminary scientific estimations of by-catch landed by the Spanish surface longline fleet in 1999 in the Atlantic Ocean and Mediterranean Sea. Coll. Vol. Sci. Pap. ICCAT 54, 1150–1163.[SCRS/2008/045]. Avaialable at: https://www.iccat.int/Documents/Meetings/Docs/SCRS/SCRS-08-045_Mejuto_et_al.pdf.
Melis R., Vacca L., Cariani A., Carugati L., Cau A., Charilaou C., et al. (2023a). Commercial sharks under scrutiny: Baseline genetic distinctiveness supports structured populations of small-spotted catsharks in the Mediterranean Sea. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1050055
Melis R., Vacca L., Cariani A., Carugati L., Charilaou C., Di Crescenzo S., et al. (2023b). Baseline genetic distinctiveness supports structured populations of thornback ray in the Mediterranean Sea. Aquat. Cons.: Mar. Freshw. 33 (5), 458–471. doi: 10.1002/aqc.3939
Moro S., Jona-Lasinio G., Block B., Micheli F., De Leo G., Serena F., et al. (2019). Abundance and distribution of the white shark in the Mediterranean Sea. Fish Fish. 00, 1–12. doi: 10.1111/faf.12432
Mucientes G. R., Queiroz N., Sousa L. L., Tarroso P., Sims D. W. (2009). Sexual segregation of pelagic sharks and the potential threat from fisheries. Biol. Lett. 5, 156–159. doi: 10.1098/rsbl.2008.0761
Müller J., Henle F. G. J. (1839). Systematische Beschreibung der Plagiostomen. Berlin, Veit (2), 29–102.
Mundy B. C. (2005). Checklist of the fishes of the hawaiian archipelago. Bishop Mus. Bull. Zool. 6), 1–704.
Musick J. A., Burgess G., Cailliet G., Camhi M., Fordham S. (2000). Management of sharks and their relatives. Fisheries 25, 9–13. doi: 10.1577/1548-8446(2000)025<0009:MOSATR>2.0.CO;2
Nakano H., Stevens J. D. (2009). “The biology and ecology of the Blue shark, Prionace glauca,” in Sharks of the Open Ocean: Biology, fisheries and conservation. Eds. Camhi M., Pikitch E., Babcock E. (Oxford, UK: Blackwell Publishing), 140–151. 2008.
Nikolic N., Devloo-Delva F., Bailleul D., Noskova E., Rougeux C., Delord C., et al. (2023). Stepping up to genome scan allows stock differentiation in the worldwide distributed blue shark Prionace glauca. Mol. Ecol. 32, 1000–1019. doi: 10.1111/mec.16822
Nosal D. P., Cartamil N. C., Wegner C. H. L., Hastings P. A. (2019). Movement ecology of young-of-the-year blue sharks Prionace glauca and shortfin makos Isurus oxyrinchus within a putative binational nursery area. Mar. Ecol. Prog. Ser. 623, 99–115. doi: 10.3354/meps13021
Notarbartolo di Sciara G., Bianchi I. (1998). Guida degli squali e delle razze del Mediterraneo. Ed. Muzzio F. (Padova: F. Muzio Editore). 388 pp.
Notarbartolo di Sciara G., Serena F. (1988). Term embryo of Mobula mobular (Bonnaterre 1788) from the Northern Tyrhenian Sea. Atti della Società Italiana di Sci. naturali del Museo civico di Storia naturale di Milano 129, 396–400.
Orsi Relini L., Garibaldi F. (2002). “Pups of Lamnid sharks from the Ligurian Sea: morphological and biometrical characteristics of taxonomic value,” in Proceedings of the 4th elasmobranch association meeting, vol. 2000 . Eds. Vacchi M., La Mesa G., Serena F., Seret B. (Livorno, Italy: ICRAM, ARPAT & SFI), 199.
Pacoureau N., Rigby C. L., Kyne P. M., Sherley R. B., Winker H., Carlson J. K., et al. (2021). Half a century of global decline in oceanic sharks and rays. Nature 589, 567–571. doi: 10.1038/s41586-020-03173-9
Panayiotou N., Biton Porsmoguer S., Moutopoulos D.K., Lloret J. (2020). Offshore recreational fisheries of large vulnerable sharks and teleost fish in the Mediterranean Sea: first information on the species caught. Medit. Mar. Sci. 21 (1), 222–227. doi: 10.12681/mms.21938
Papageorgiou M., Hadjioannou L., Jimenez C., Georgiou A., Petrou A. (2022). Understanding the interactions between cetaceans and other megafauna with the albacore tuna fishery: a case study from the Cyprus’ Pelagic longline fishery. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.868464
Pascucci V., Cossu A., De Luca M., Santonastaso A., Pireddu L. (2018). Accordo di cooperazione con l’università degli studi di SassarI, dipartimento di architettura (DADU) e l’ente parco dell’asinara, amp “isola dell’asinara” D.M 22 del 11 febbraio 2015 “ISOLA DELL’ARSINARA”-strategia marina nelle aree marine protette. Relazione sul montoraggio “habitat a coralligeno nell’amp dell’Asinara (Sassari (SS), Italy: Università di Sassari DADU).
Perez J. A. A., Wahrlich R. (2005). A bycatch assessment of the gillnet monkfish Lophius gastrophysus fishery off southern Brazil. Fish. Res. 72, 81–95. doi: 10.1016/j.fishres.2004.10.011
Peristeraki P., Kypraios N., Lazarakis G., Tserpes G. (2008). By-catches and discards of the Greek swordfish fishery. Col. Vol. Sci. Pap. ICCAT 62 (4) 1070–1073.
Petersen S. L., Honig M. B., Ryan P. G., Underhill L. G., Compagno L. J. V. (2009). Pelagic shark bycatch in the tuna- and swordfish directed longline fishery off southern Africa. Afr. J. Mar. Sci. 31, 215–225. doi: 10.2989/AJMS.2009.31.2.9.881
Piovano S., Gilman E. (2017). Elasmobranch captures in the Fijian pelagic longline fishery. Aquat. Conserv.: Mar. Freshw. 27, 381–393. doi: 10.1002/aqc.2666
Poey F. (1858–61). Memorias sobra la historia natural de la Isla de Cuba, acompañadas de sumarios Latinos y extractos en Francés. Tomo 2. La Habana. v. 2: 1-96 (1858), 97-336 (1860), 337-442, (1861), Pls. 1–19.
Prat-Varela A., Torres A., Cervantes D., Aquino-Baleytó M., Abril A. M., Clua E. E. G. (2023). Improved baited remote underwater video (BRUV) for 24 h real-time monitoring of pelagic and demersal marine species from the epipelagic zone. J. Mar. Sci. Eng. 11 (6), 1182. doi: 10.3390/jmse11061182
Queiroz N., Humphries N. E., Couto A., Vedor M., da Costa I, Sequeira A. M. M., et al. (2019). Global spatial risk assessment of sharks under the footprint of fisheries. Nature 572, 461–466. doi: 10.1038/s41586-019-1444-4
Renshaw S., Hammerschlag N., Gallagher A. J., Lubitz N., Sims D. W. (2023). Global tracking of shark movements, behaviour and ecology: A review of the renaissance years of satellite tagging studies 2010–2020. J. Exp. Mar. Biol. Ecol. 560, 151841. doi: 10.1016/j.jembe.2022.151841
Revill A. T., Young J. W., Lansdel M. (2009). Stable isotopic evidence for trophic groupings and bio-regionalization of predators and their prey in oceanic waters off Eastern Australia. Mar. Biol. 156, 1241–1253. doi: 10.1007/s00227-009-1166-5
Riede K. (2004). Global register of migratory species - from global to regional scales (Bonn, Germany: Federal Agency for Nature Conservation). Final Report of the R&D-Projekt 808 05 081329 p.
Roff G., Brown C. J., Priest M. A., Mumby P. (2018). Decline of coastal apex shark populations over the past half century. Commun. Biol. 1, 223. doi: 10.1038/s42003-018-0233-1
Romeo T., Consoli P., Castriota L., Andaloro F. (2009). An evaluation of resource partitioning between two billfish, Tetrapturus belone and Xiphias gladius in the central Mediterranean Sea. J. Mar. Biol. Assoc. UK. 89, 849–857. doi: 10.1017/S0025315408002087
Rondinini C., Battistoni A., Teofili C. (2022). Lista Rossa IUCN dei vertebrati italiani 2022 Comitato Italiano IUCN e Ministero dell'Ambiente e della Sicurezza Energetica, Roma.
Saidi B., Enajjar S., Karaa S., Echwikhi K., Jribi I., Nejmed-dine Bradai M. (2019). Shark pelagic longline fishery in the Gulf of Gabes: Inter-decadal inspection reveals management needs. Medit. Mar. Sci. 20 (3), 532–541. doi: 10.12681/mms.18862
Saraiva B. M., Macena B. C. L., Solleliet-Ferreira S., Afonso P., Jorge F. (2023). First insights into the shortfin mako shark (Isurus oxyrinchus) fine-scale swimming behaviour. R. Soc Open Sci. 10(5):230012. doi: 10.1098/rsos.230012
Scacco U., Consalvo I., Di Muccio S., Tunesi L. (2012). On the by-catch of two porbeagle sharks Lamna nasus in the Central Adriatic Sea. Mar. Biodivers. Rec 5, E61. doi: 10.1017/S1755267212000127
Scacco U., Consalvo I., Mostarda E. (2009). First documented catch of the giant devil ray Mobula mobular (Chondrichthyes: Mobulidae) in the Adriatic Sea. Mar. Biodivers. Rec. 2, E93. doi: 10.1017/S1755267209001110
Scacco U., Di Crescenzo S., Sbrana A. (2023b). Exploring fishing threat at fleet segment and subregional scale: Least expert knowledge and a resilience versus disturbance-based approach as conservation's tools for cartilaginous fish. Ecol. Evol. 13, e9881. doi: 10.1002/ece3.9881
Scacco U., Fortibuoni T., Baini M., Franceschini G., Giani D., Concato M., et al. (2023a). Gradients of variation in the at-vessel mortality rate between twelve species of sharks and skates sampled through a fishery-independent Trawl Survey in the Asinara Gulf (NW Mediterranean Sea). Biology 12 (3), 363. doi: 10.3390/biology12030363
Schindler D. E., Essington T. E., Kitchell J. F., Boggs C., Hilborn R. (2002). Sharks and tunas: fisheries impacts on predators with contrasting life histories. Ecol. Appl. 12 (3), 735–748. doi: 10.2307/3060985
Schlaff A. M., Heupel M. R., Simpfendorfer C. A. (2014). Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Rev. Fish Biol. Fish. 24, 1089–1103. doi: 10.1007/s11160-014-9364-8
Sepulveda C., Graham J., Bernal D. (2007). Aerobic metabolic rates of swimming juvenile shortfin mako sharks, Isurus oxyrinchus. Mar. Biol. 152, 1087–1094. doi: 10.1007/s00227-007-0757-2
Serena F. (2005). Field identification guide to the sharks and rays of the Mediterranean and Black Sea (Rome: FAO). FAO Species Identification Guide for Fishery Purposes.
Serena F., Silvestri R. (2018). “Preliminary observations on juvenile shark catches as by-catch of the Italian fisheries with particular attention to the Tuscany coasts,” in Proceedings of 7 Congress of Codice Armonico, 18b/Scientific Section, Rosignano Marittimo. 158–169.
Soldo A., Jardas I. (2002). “Large sharks in the eastern Adriatic,” in Proceedings of the 4th european elasmobranch association, vol. 2000 . Eds. Vacchi M., La Mesa G., Serena F., Seret B. (Livorno, Italy: ICRAM, ARPAT and SFI), 141–155.
Stevens J. D. (1976). First results of shark tagging in the north-east Atlantic 1972-1975. J. Mar. Biol. Ass. U.K. 56, 929–937. doi: 10.1017/S002531540002097X
Stevens J. D. (1990). Further results from a tagging study of pelagic sharks in the north-east Atlantic. J. Mar. Biol. Ass. U.K. 70, 707–720. doi: 10.1017/S0025315400058999
Storai T., Celona A., Zuffa M., De Maddalena A. (2005). On the occurrence of the porbeagle, Lamna nasus (Bonnaterre 1788) (Chondrichthyes: Lamnidae) off Italian coasts (Northern and Central Mediterranean Sea): a historical survey. Ann. Ser. Hist. Nat. 15 (2), 195–202.
Swift D. G., Portnoy D. S. (2021). Identification and delineation of essential habitat for elasmobranchs in estuaries on the texas coast. Estuaries Coasts 44, 788–800. doi: 10.1007/s12237-020-00797-y
Telesca L., Belluscio A., Criscoli A., Ardizzone G., Apostolaki E. T., Fraschetti S., et al. (2015). Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 5, 12505. doi: 10.1038/srep12505
Thorpe T., Frierson D. (2009). Bycatch mitigation assessment for sharks caught in coastal anchored gillnets. Fish. Res. 98, 102–112. doi: 10.1016/j.fishres.2009.04.003
Tiralongo F., Messina G., Cazzolla Gatti R., Tibullo D., Lombardo B. M. (2018b). Some biological aspects of juveniles of the rough ray, Raja radula Delaroche 1809 in Eastern Sicily (central Mediterranean Sea. J. Sea Res. 142, 174–179. doi: 10.1016/j.seares.2018.10.001
Tiralongo F., Messina G., Lombardo B. M. (2018a). Discards of elasmobranchs in a trammel net fishery targeting cuttlefish, Sepia officinalis Linnaeus 1758, along the coast of Sicily (central Mediterranean Sea). Reg. Stud. Mar. Sci. 20, 60–63. doi: 10.1016/j.rsma.2018.04.002
Tonin S. (2018). Economic value of marine biodiversity improvement in coralligenous habitats. Ecol. Ind. 85, 1121–1132. doi: 10.1016/j.ecolind.2017.11.017
Tortonese E. (1956). Fauna d'Italia vol. 02 -leptocardia, ciclostomata, selachii (Bologna: Calderini), 334.
Vacchi M., Serena F. (1997). Squali di notevoli dimensioni nel Mediterraneo centrale. Quad. Civica Stazione Idrobiol. Milano 22, 39–45.
Valenzuela A., Bustamante C., Lamilla J. (2008). Morphological characteristics of five bycatch sharks caught by southern Chilean demersal longline fisheries. Sci. Mar. 72, 231–237. doi: 10.3989/scimar
Vandeperre F., Aires-da-Silva A., Fontes J., Santos M., Serrão Santos R., Afonso P. (2014). Movements of Blue sharks (Prionace glauca) across their life history. PloS One 9 (8), 1–14, e103538. doi: 10.1371/journal.pone.0103538
Walbaum J. J. (1792). Petri Artedi renovati. Part 3. Petri Artedi sueci genera Piscium in quibus systema totum ichthyologiae proponitur cum classibus, ordinibus, generum characteribus, specierum diffentiis, observationibus plumiris. Redactis Speciebus 2. Ichthyologiae, part III. 723 p. Grypeswaldiae
Waller M. J., Queiroz N., da Costa I., Cidade T., Loureiro B., Womersley F. C., et al. (2023). Direct measurement of cruising and burst swimming speeds of the shortfin mako shark (Isurus oxyrinchus) with estimates of field metabolic rate. J. Fish Biol. 103(5):864–883. doi: 10.1111/jfb.15475
Walls R. H. L., Dulvy N. K. (2020). Eliminating the dark matter of data deficiency by predicting the conservation status of Northeast Atlantic and Mediterranean Sea sharks and rays. Biol. Cons. 246, 108459. doi: 10.1016/j.biocon.2020.108459
Ward P., Myers R. (2005). Inferring the depth distribution of catchability for pelagic longlines correcting for variations in the depth of longline fishing. Can. J. Fish. Aquat. Sci. 62, 1130–1142. doi: 10.1139/f05-021
Ward-Paige C. A., Britten G. L., Bethea D. M., Carlson J. K. (2014). Characterizing and predicting essential habitat features for juvenile coastal sharks. Mar. Ecol. (Berl.) 36, 419–431. doi: 10.1111/maec.12151
Watson J. W., Epperly S. P., Shah A. K., Foster D. G. (2005). Fishing methods to reduce sea turtle mortality associated with pelagic longlines. Can. J. Fish. Aquat. Sci. 981, 965–981. doi: 10.1139/F05-004
Williams P. G. (1999). “Shark and related species catch in tuna fisheries of the tropical western and central Pacific Ocean,” in Case studies on the management of elasmobranch fisheries. Ed. Shotton R. (Rome: FAO). 378 Part 1.
Williamson M. J., Tebbs E. J., Dawson T. P., Jacoby D. M. P. (2019). Satellite remote sensing in shark and ray ecology, conservation and management. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00135
Keywords: bycatch, Isurus oxyrinchus, Lamna nasus, Prionace glauca, young, Mobula mobular, newborns
Citation: Scacco U, Gennari E, Di Crescenzo S and Fanelli E (2023) Looking into the prevalence of bycatch juveniles of critically endangered elasmobranchs: a case study from pelagic longline and trammel net fisheries of the Asinara Gulf (western Mediterranean). Front. Mar. Sci. 10:1303961. doi: 10.3389/fmars.2023.1303961
Received: 28 September 2023; Accepted: 02 November 2023;
Published: 28 November 2023.
Edited by:
Branko Dragičević, Institute of Oceanography and Fisheries (IZOR), CroatiaReviewed by:
Bianca Maria Lombardo, University of Catania, ItalyCopyright © 2023 Scacco, Gennari, Di Crescenzo and Fanelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Umberto Scacco, dW1iZXJ0by5zY2FjY29AaXNwcmFtYmllbnRlLml0
†ORCID: Enrico Gennari, orcid.org/0000-0002-4334-727X
Emanuela Fanelli, orcid.org/0000-0002-5358-5159
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.