
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 02 November 2023
Sec. Marine Biology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1301178
This article is part of the Research TopicCurrent Research on Fish Otoliths and their ApplicationsView all 10 articles
The shape and structure of otoliths play a vital role in studying fish populations because otolith shape indices are often applied to discriminate fish species. This study focused on examining inter- and intra-specific variations in otolith shape and size among three species of rockfish (Sebastes inermis, Sebastiscus marmoratus, and Sebastes zonatus) collected from the Dokdo and Ulleungdo regions of the East Sea in Korea. A total of 35 S. inermis specimens, 19 S. marmoratus specimens, and 59 S. zonatus specimens were collected between April 2021 and August 2022. The otolith shape was visualized using wavelet coefficients in the shapeR package. Otolith size indices, such as length, width, perimeter, and area, and shape indices, including form factor, aspect ratio, ellipticity, circularity, roundness, rectangularity, and squareness, were calculated for each species. Otolith size and shape indices significantly differed among the three rockfish species (p< 0.05). Compared with the other two species, S. marmoratus possessed more elongated otoliths, whereas S. zonatus had the largest otolith area, perimeter, and length. Average shape analysis based on wavelet coefficients revealed significant differences in otolith shape, particularly on the rostrum and posterior sides. A canonical analysis of principal components (CAP) confirmed the complete separation of otolith shapes among the three rockfish species, with 92.5% of the variation explained by the first axis (CAP1). The findings of this study enhance our understanding of the fish species in the Korean East Sea.
In teleost fish, three types of otoliths (sagitta, asteriscus, and lapillus) are found in the inner ear (Das, 1994). Otoliths are important for hearing and maintaining body balance in teleost fishes and are made of calcium carbonate and a small amount of protein material (Poznar et al., 2020). As fish grow, new layers of calcium carbonate are deposited on the surface of otoliths (Edmonds et al., 1999). Owing to their continuous growth and metabolic stability, otoliths are particularly useful as natural markers for studying the ecological characteristics and fisheries aspects of fish species. For example, information stored in otoliths can provide insights into the ecological range, geographic distribution, and stock structure of fish species (Edmonds et al., 1999; Morales et al., 2023). Otoliths are routinely used to determine the age and growth of teleost fish, and their internal structures play a key role in stock management purposes (Hüssy, 2008; Cadrin et al., 2013).
Morphological variations in fish otoliths are often influenced by external factors such as habitat depth, water temperature, salinity, and food availability (Lombarte and Lleonart, 1993; Capoccioni et al., 2011). The utilization of these unique otolith characteristics allows for the successful analysis of large volumes of data within a reasonably short period (Fossen et al., 2003). Otolith shape analysis has the benefit of enhanced effective monitoring of fish stocks when there are statistically significant differences between species because it is relatively inexpensive and requires less work (Christensen et al., 2018), and closely related species can display a wide range of variations in otolith morphologies. Therefore, otolith morphometry is an important approach in taxonomic and ecological research (Zischke et al., 2016). Otolith morphometry is also used as an additional taxonomic tool for identifying fish species, even for distinguishing closely related species that are difficult to identify using body morphometry alone (Sadighzadeh et al., 2012; Park et al., 2018).
Recently, the application of otolith morphometry, such as geometric morphometry, has been developed to facilitate the determination of breeding grounds and fish stocks, as well as the migratory pathways of various commercially valuable species (de Carvalho et al., 2020). Because the shape and size of otoliths vary among fish species, otolith morphometrics can be used to differentiate individual fish populations and determine the impact of environmental factors on their growth (Begg et al., 2001). In addition, the shape and morphometry of the otoliths are valuable for identifying fish species and discriminating fish stocks (Tuset et al., 2008). Otolith morphometry has recently been extensively utilized with the advancements in computing power and image processing. Digital pictures replaced hand-drawn graphics in the 1980s, and image analysis techniques utilizing harmonic expansion and Fourier transform have been employed (Campana and Casselman, 1993; Nikiforidou et al., 2023).
The dark-banded rockfish (Sebastes inermis), false kelpfish (Sebastiscus marmoratus), and banded jacopever (Sebastes zonatus) belong to the family Sebastidae (rockfishes) and are found in various shallow coastal habitats, including rocky reefs, Sargassum and Zostera beds, as well as the Japanese islands (Hokkaido, Kyushu), and the Korean Peninsula (Nakabō, 2002; Kim et al., 2005; Kai and Nakabo, 2008). Among the three rockfish species, S. inermis has long been recognized as a valuable fishery resource along the southern coast of Korea and is important for commercial fishing (Kim et al., 2005) and aquaculture purposes (Oh et al., 2010). However, the annual catch of this species has drastically reduced (An et al., 2011). Although the reason for this could not be determined, it might be related to coastal development, overfishing, and eutrophication (An et al., 2011). S. marmoratus is also considered a commercial fish along the Korean coast because of its availability throughout the year (Lee et al., 2012). Sebastes zonatus belongs to the Sebastes vulpes complex (S. vulpes, S. zonatus, and S. ijimae), which is a group of closely related fish species that are morphologically similar (Muto et al., 2019). Recently, S. zonatus was identified as a distinct species, differentiating it from S. vulpes and S. ijimae based on its body coloration, gill raker number, and squamation patterns within the Sebastes vulpes complex (Kai and Nakabo, 2008).
Otolith morphometry has been used to distinguish between rockfish species worldwide, such as in the Seto Inland Sea, Japan (Deville et al., 2023), Gulf of Alaska (Harris et al., 2019), North Atlantic (Stransky and MacLellan, 2005), Northeastern Pacific (Tuset et al., 2015), Pacific and Atlantic Oceans (Tuset et al., 2016), and Bohai Sea and Yellow Sea (Zhuang et al., 2015). Several ecological and biological studies have been conducted on these three species along the Korean coast (Lee et al., 2012; Jang et al., 2015; Park et al., 2023). However, no prior studies have been conducted on the otolith shapes of these three species along the Korean coast. Limited studies have reported the relationship between otoliths and body sizes of several fish species inhabiting the western Korean coastal regions (Jawad et al., 2017). Therefore, this study aimed to compare the shape and size of otoliths in three rockfishes inhabiting the Korean East Sea and provide significant information regarding the otolith shape of rockfishes found along the Korean coast. This study expands our knowledge of the morphological characteristics and ecological importance of these species in the study area.
Samples of three rockfishes, S. inermis, S. marmoratus, and S. zonatus, were collected from the two offshore islands of Dokdo (37° 14’ 29” N, 131° 51’ 34” E) and Ulleungdo (37° 28’ 32” N, 130° 55’ 22” E) in the Korean East Sea (Figure 1). Sampling was conducted in spring (April) and summer (August) between 2021 and 2022. The fish samples were collected using gill nets by local fishermen. Immediately after capture, fish samples were packed on ice and transferred to the laboratory for further investigation.
A total of 35 S. inermis, 19 S. marmoratus, and 59 S. zonatus specimens were collected. The total length (TL) and wet body weight (BW) were measured to the nearest 0.1 cm and 0.1 g using a measuring board and electric balance, respectively. The right and left sagittal otoliths from each specimen were removed and cleaned using a brush and tap water, left to dry, labeled, and stored in plastic tubes. The right otolith was selected for this study because there were no significant differences in the regression slope between the sizes of the left and right otoliths and the total length of the fish (ANCOVA, P> 0.05).
High-resolution otolith images were captured using a Leica EZ4E microscope, and the Leica image processing tool LAS incorporated in the microscope setup. The microscope was adjusted to ensure a clear background (Figures 2A, D, G). The right sagittal otolith from each specimen was placed on a dark microscope plate with the sulcus facing downward and the rostrum facing the left. Before shape analysis, each otolith image was edited to appear black or white. The otolith shape was represented as high-contrast white against a dark black background using image editing Adobe Photoshop version 23.3.2 (Figures 2B, E, H). This editing process facilitated the ability of the program to detect outlines more easily. The magnification of the microscope was set to 8× for all samples, and images were saved in a.tif format.
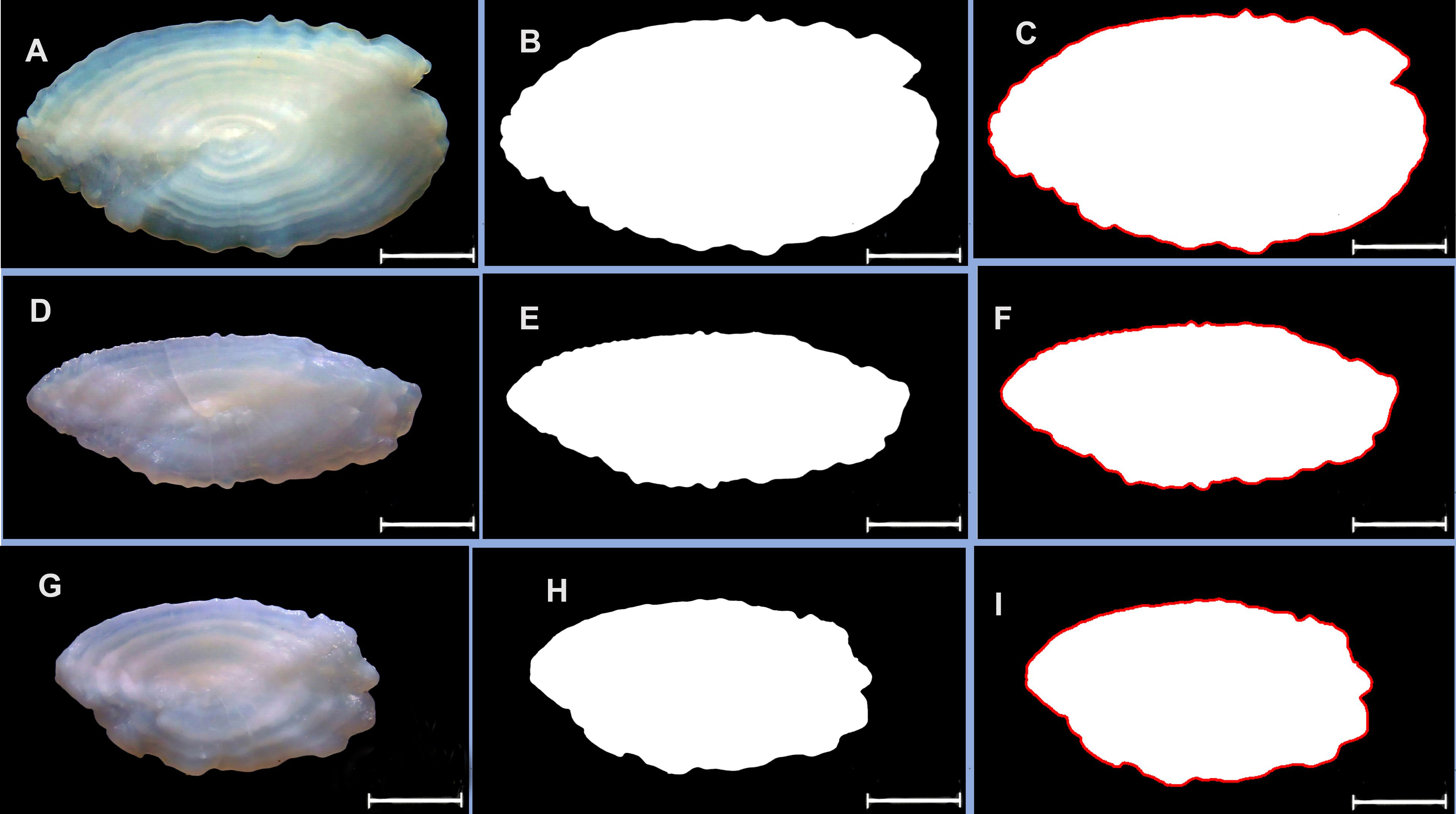
Figure 2 Raw otolith images (left), edited images using Adobe Photoshop (middle), and outlines generated by the outline function of the shapeR package (right). (A–C) represents the otolith of Sebastes zonatus, (D–F) represents Sebastiscus marmoratus, and (G–I) represents Sebastes Inermis. The red line represents the perimeter, while the otolith area is depicted in high-contrast white. The scale bar in each image is 1 mm.
The otolith shapes of the three rockfish were extracted using the ShapeR 1.0-1 package (Libungan and Pálsson, 2015) in RStudio version 4.2.3 (R Core Team, 2023). The outline for each otolith was detected using the ‘detect. Outline’ function, with a threshold level set at 0.2. The underlying principle of ShapeR involves visualizing an otolith image by extracting an outline of the otolith framework. Each outline was automatically saved as.png images, and every file was visually inspected to ensure that the detected outline accurately followed the contour of the otolith edge (Figures 2C, F, I). This outline was then subjected to a smoothing process to generate two coefficients that represent the shape of the otolith, using the wavelet and Fourier coefficients.
Wavelet shape coefficient was used to plot the shape of the otoliths. All outlines were converted into 63 wavelet coefficients and then standardized to the fish’s total length (cm) to eliminate the influence of allometric relationships. Five wavelet coefficients that showed a significant (p< 0.05) relationship were excluded from the analysis. The remaining 58 wavelet coefficients were used for further analyses (Lleonart et al., 2000; Longmore et al., 2010; Libungan and Pálsson, 2015; Libungan et al., 2015). The standardized wavelet coefficients of the three species were compared using canonical analysis of the principal coordinates (Anderson and Willis, 2003).
Otolith size indices, including otolith length (OL; mm), otolith width (OH; mm), otolith perimeter (OP; mm), and otolith area (OA; mm2), were automatically estimated using the ShapeR package. The otoliths were weighed using an Ohaus SPX223 Scout analytical balance. In addition, otolith shape-related indices, including form factor (FF), aspect ratio (AR), ellipticity (E), circularity (C), roundness (RO), rectangularity (RE), and squareness (SQ), were estimated using the equations presented in Table 1 (Tuset et al., 2003; Moore et al., 2022).
Because otolith morphometric indices are generally correlated with fish size, we considered the influence of size variation on otolith indices. Without correcting for allometry, it would be difficult to confidently determine whether the observed differences in otolith shape and measurements within the conspecific group were truly indicative of interspecific variations or simply a consequence of collecting fish of different sizes. Therefore, adjusting for allometric effects was necessary to ensure accurate otolith data interpretation. To remove the allometric influence of fish length on otolith size and shape, all the variables were standardized using the following equation (Elliott et al., 1995; Lleonart et al., 2000; Zischke et al., 2016).
where,
Ms = standardized (size-adjusted) measurement.
Mo = original parameter (size and shape indices).
= average size parameter (TL) for all datasets.
x = size parameter (TL) of each fish species.
b = slope of the regression between log Mo and log x.
The standardized otolith shape and size indices were first subjected to the Shapiro–Wilk normality test. As the data were not normally distributed (p< 0.05), A non-parametric Kruskal-Wallis test was applied to compare the size and shape factors of otoliths among the three rockfish species. If significant differences were in the otolith data, the Kruskal–Wallis Dunn’s test was performed for post hoc comparisons. An ANOVA-like permutation test and a canonical analysis of principal components test were performed on the wavelet coefficients to further compare the mean shape of the three rockfish species. Before performing the principal component analysis (PCA), the Kaiser–Meyer–Olkin (KMO) test was performed. The KMO test is a statistical measure used to determine the suitability of data for factor analysis (Shrestha, 2021). The results showed a value of 0.62, suggesting a moderate level of adequacy for the sampled variables, thereby justifying the inclusion of these variables in the subsequent analysis.
All statistical analyses and otolith shape visualization were performed using the R programming language. The following packages were utilized: shapeR (1.0-1), rstatix (0.7.2), ggpubr (0.6.0.999), ggplot2 (3.4.2), gridExtra (2.3), Vegan (2.6-4), stats (4.2.3), FactoMineR (2.8), factoextra (1.0.7), psych (2.3.3), GGally (2.1.2), gplot (3.1.3), and wavethresh (4.7.2).
In total, Sebastes inermis (N=35) ranging from 22.4 cm to 31.5 cm TL, Sebastiscus marmoratus (N=19) from 15.7 cm to 30.7 cm TL, and Sebastes zonatus (N=59) from 20.3 cm to 36.8 cm TL were used for otolith analyses.
Descriptive statistics for otolith size and shape indices of the three species are shown in Table 2. The otoliths of S. inermis were heavier (OW = 0.09 g ± 0.01), wider (OH = 4.45 mm ± 0.41), rounder (0.51 ± 0.02), and more squared (0.29 ± 0.08) than S. marmoratus and S. zonatus. S. marmoratus otoliths had higher circularity (18.3 ± 0.96) and aspect ratio (2.34 ± 0.20) values than the other two species, indicating a more elongated otolith shape. Furthermore, S. zonatus had the largest otolith area (27.4 mm2 ± 2.15), perimeter (21.6 mm ±0.90), and otolith length (8.54 mm ±0.39) among the three species.
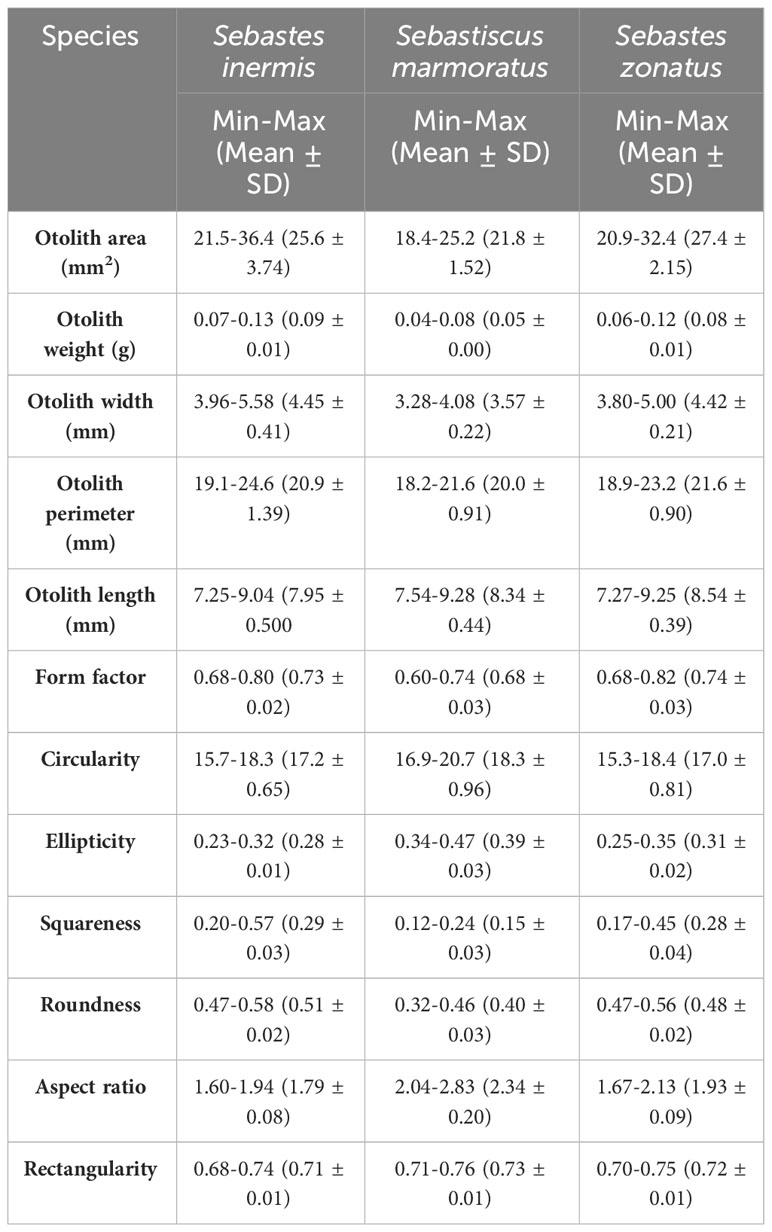
Table 2 Descriptive statistics for otolith size and shape indices for the three rockfishes from the East Sea, Korea.
The standardized otolith shape and size indices significantly differed across species according to the Kruskal–Wallis test (p< 0.05; Table S1). Additionally, the post hoc Dunn’s test results showed significant differences in the otolith area between all pairwise comparisons among all groups (p< 0.05; Figure 3; Table S2). Otolith length also significantly differed between S. inermis and S. zonatus (p< 0.0001). Significant differences (p< 0.001) were observed in squareness, circularity, form factor, otolith weight, and otolith width between S. inermis and S. marmortaus, as well as between S. marmortaus and S. zonatus. Furthermore, there were significant differences in roundness, aspect ratio, and ellipticity indices between all three fish species (p< 0.0001 for all species comparisons) (Figure 4; Table S2).

Figure 3 Box plot for otolith size indices with a non-parametric Kruskal–Wallis test, and post hoc Dunn test. Significance: **** (p< 0.0001), *** (p< 0.001), ** (p< 0.01), * (p< 0.05), no asterisks (p > 0.05). SIN, S. Inermis; SMA, S. marmoratus; SZO, S. zonatus.

Figure 4 Box plot for otolith shape indices with a non-parametric Kruskal–Wallis test, and post hoc Dunn test. Significance: **** (p< 0.0001), *** (p< 0.001), * (p< 0.05), no asterisks (p > 0.05). SIN, S. Inermis; SMA, S. marmoratus; SZO, S. zonatus.
The principal component analysis (PCA) results indicated that the first principal component (PCA1), which included otolith width, weight, squareness, area, roundness, and form factor, explained 57.3% of the total variation in the dataset. The second principal component (PCA2), which contained otolith length, perimeter, circularity, aspect ratio, and ellipticity, accounted for 23.7% of the variation (Figure 5). The clusters of S. marmoratus were present along the negative side of PCA1, whereas the clusters of S. zonatus and S. inermis showed overlapping patterns, indicating some similarities in the morphology of the otoliths.
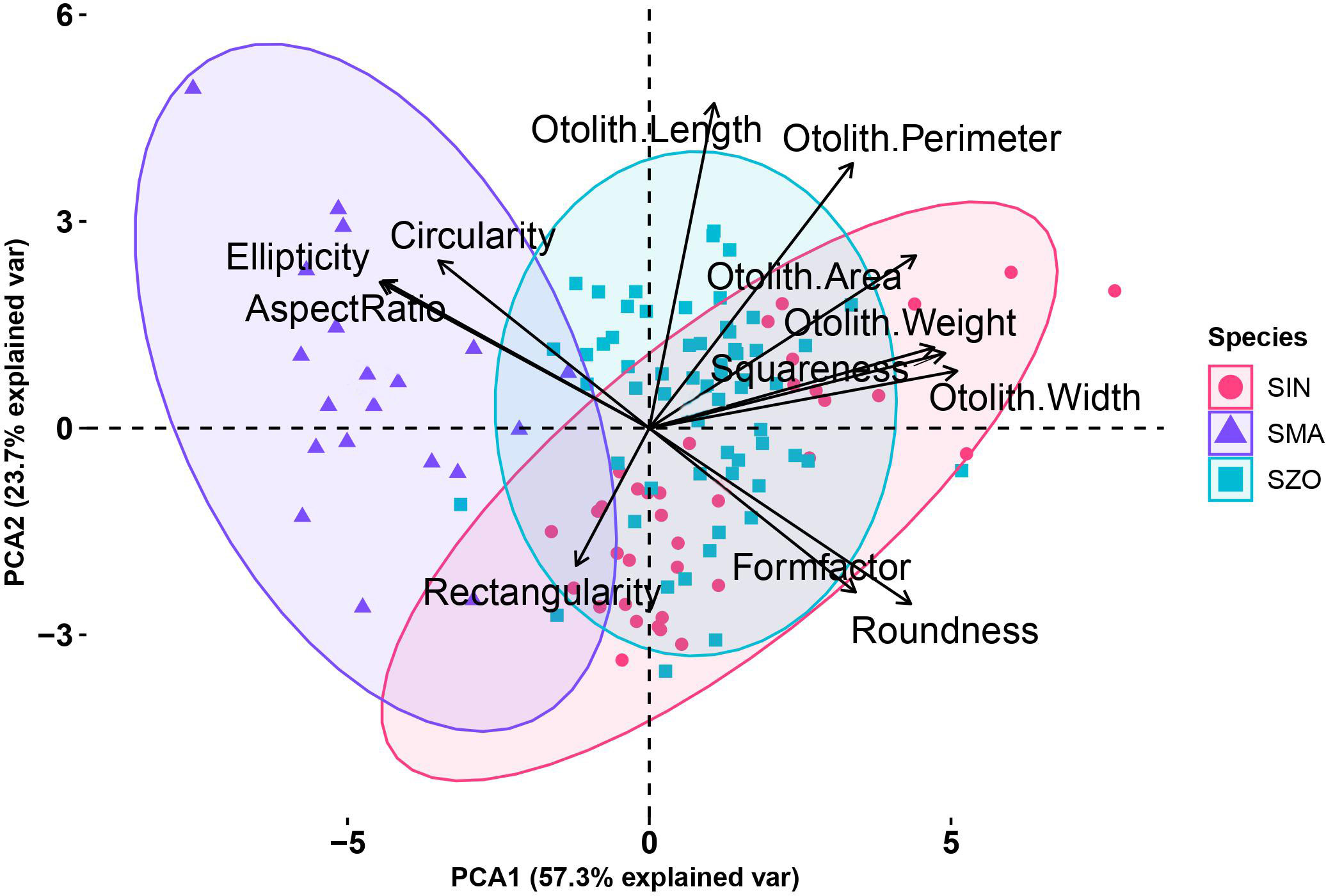
Figure 5 Principal component analysis (PCA) plot for otolith size and shape indices of the three rockfishes. SIN, Sebastes inermis; SMA, Sebastiscus marmoratus; SZO, Sebastes zonatus.
The average otolith shape analysis based on wavelet coefficients at 0° to 360° revealed differences in variation among the three rockfish (Figure 6). The three rockfish species had significantly different otolith shapes according to the permutation-based analysis of variance (ANOVA; p<0.001). The otoliths of S. marmoratus exhibited a more elongated pattern between 0° and 180° and were narrower than those of S. inermis and S. zonatus at 90–270°. The otoliths of S. inermis were wider and less elongated, indicating a rounded shape. Further examination of S. inermis and S. zonatus on the rostrum revealed an overlapping pattern. They also appeared to be similar on the dorsal side (270°). All groups showed a similar pattern between 300° and 320° and 50° and 60°. In addition, the otoliths of S. marmoratus and S. inermis had the greatest distance in the mean shapes.

Figure 6 The average otolith shape of the three rockfishes was generated using the shape R package, which utilizes wavelet coefficients. The numbers represent the angles in degrees. The black cross map shows the position of the otoliths: posterior (P), anterior (A), ventral (V), and dorsal (D). SIN = Sebastes Inermis; SMA, Sebastiscus marmoratus; SZO, Sebastes zonatus.
Wavelet coefficients across populations were compared using canonical analysis of principal coordinates (CAP). Variations in otolith shape were observed in all populations along the first two canonical axes (Figure 7). The results of the CAP analysis showed a complete separation in otolith shape among the three rockfish species, explaining 92.5% of the variation for the first axis (CAP1) and 7.5% for the second axis (CAP2). Sebastiscus marmoratus was mainly found in the positive range of the CAP1 axis, whereas S. inermis species was found in the negative ranges of CAP1. Additionally, the CAP2 axis further separated S. zonatus and S. inermis along the vertical axis.
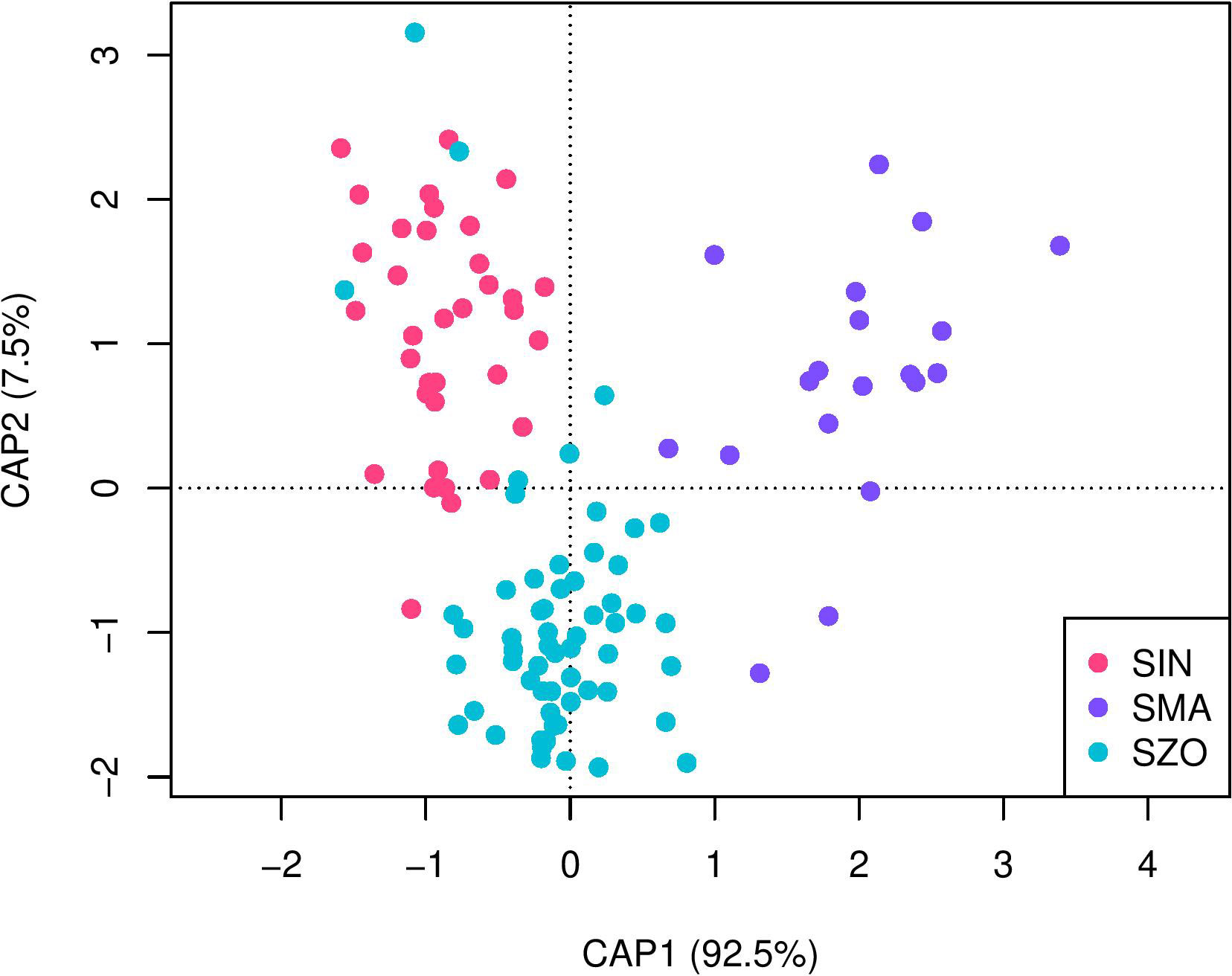
Figure 7 A canonical analysis of principal coordinates (CAP) based on wavelet coefficients. SIN, Sebastes Inermis; SMA, Sebastiscus marmoratus; SZO, Sebastes zonatus.
The present study examined the three rockfishes (Sebastes inermis, Sebastiscus marmoratus, and Sebastes zonatus) from the Korean East Sea for morphological differences in otolith size and shape. The results show that the otoliths of the three rockfish species varied in terms of both shape and size indices. The rostrum side at 180°, posterior side at 0°, and ventral side at 90° showed the greatest variation in average otolith shape. Principal Component Analysis (PCA) provided valuable insights into the overall variability in otolith size and shape, with PCA1 and PCA2 combined explaining 81% of the total variance. The PCA plot further differentiated the otoliths of S. marmoratus from those of the other two species by otolith aspect ratio, circularity, and ellipticity. These results suggest that otolith shape could be a valuable tool for distinguishing between these three rockfish species, providing valuable information about fish evolution and phylogeny (Reichenbacher et al., 2007).
Previous studies on otolith shape and size among three Sebastes species inhabiting the Seto Inland Sea, Japan, revealed that S. cheni inhabiting deeper depths had relatively larger otoliths than the sympatric S. inermis and S. ventricosus (Deville et al., 2023). In addition, among the three flathead species inhabiting the southern Australian Sea, the distributional depth of the deepwater flathead (Platycephalus conatus) extends toward a deeper depth of up to 490 m (Froese and Pauly, 2023), and they show significantly longer otolith length at a given body length compared to the other two flathead species (Park et al., 2018). The present study also found a relatively large otolith size in Sebastes zonatus. This can be attributed to the fact that the general range of distributional depths of Sebastes zonatus is deeper (approximately 50 to 175 m) than S. marmoratus and S. inermis, which are both found mainly at shallower depths of 50 m (Nakabō, 2002). Interspecific variations in otolith morphology are potentially indicative of the habitat preferences of different species in terms of depth or prey resources (Tuset et al., 2016). Consequently, fish species distributed at greater depths are expected to have larger otoliths (Tuset et al., 2003).
The size and shape of otoliths in fish can be influenced by the type of prey items they consume because the composition of the prey item can affect the number of nutrients and energy that the fish receives and, consequently, fish growth and otolith production (Mille et al., 2016; Qiao et al., 2022). Dietary investigations of S. inermis and S. marmoratus from the Korean coast revealed considerable differences in their prey preferences, with S. inermis largely feeding on amphipods and S. marmoratus consuming teleosts (Kim et al., 2009; Lee et al., 2012). Mille et al. (2016) studied the potential association between diet and otolith shape in five marine fish species and discovered that otolith shape variation was associated with primary and secondary prey groups. In addition, Hüssy (2008) examined the influence of temperature and food availability on the development of otolith shapes in juvenile cod (Gadus morhua). The number and size of otolith lobes were influenced by the amount of food consumed by the fish. Increased food consumption resulted in a greater number of wider lobes, leading to a more rectangular otolith. Although information is not available on the effect of the types of prey items consumed by predatory fishes on otolith morphometrics, the variations in the otolith shape of rockfish in the current study are likely due to the type of prey items they consume. S. inermis, which feeds mainly on amphipods, exhibited heavier and wider otoliths, whereas S. marmoratus, which preys on teleosts, showed more elongated otoliths. These dietary variables may have contributed to the observed variation in otolith size and shape (Mille et al., 2016).
Otolith shape analysis was used to distinguish the fish species. Analyses of otolith shapes among four coexisting Sebastes species in the Bohai and Yellow seas have proven to be valuable tools for identifying species and conducting phylogenetic studies on the Sebastes group (Zhuang et al., 2015). Similarly, otolith shape analysis has been applied as an effective technique to distinguish between Sebastes species in the North Atlantic, North Pacific, and South Atlantic regions (Stransky and MacLellan, 2005).
Otoliths are regarded as a significant source of information for determining the life cycle of fish (Campana and Thorrold, 2001). Otolith morphology has practical implications in fishery management, taxonomy, and migration studies (D’Iglio et al., 2021). The otolith shape is species-specific in some taxa, using these characteristics as a helpful tool for species identification because they offer key information for identifying and classifying different species (Avigliano et al., 2018). Fish biologists, taxonomists, and even archaeologists have been enthralled by the diversity of otolith morphologies, which depend on accurate species categorization and identification within and across various fish species (Pattuinan and Demayo, 2018). For example, sagittal otoliths have specific physical characteristics that differ significantly between fish species and genera. Because these changes are often specific to species and genera, otoliths are valuable tools for ichthyologists in terms of taxonomy and phylogeny (Zarei et al., 2023).
Geometric morphometrics is a taxonomic technique that uses statistical analysis of shape data to identify and distinguish species, reducing misidentification (Stransky and MacLellan, 2005). Landmark- and outline-based geometric morphometric approaches provide various benefits over traditional measurement approaches (Adams et al., 2004). Outline-based geometric morphometric approaches are a better approach for otolith shape analysis than landmark-based methods because they are more comprehensive, precise, and reliable (Lishchenko and Jones, 2021). Therefore, outline-based geometric morphometric approaches were chosen in this study, as well as by many scientists (Lishchenko and Jones, 2021).
This research focuses only on examining the size and shape of otoliths in three rockfish species in the Korean East Sea. It does not take into account other factors that could affect otolith morphology, such as genetic differences, or the impact of environmental conditions. Conducting further studies that consider these additional factors could lead to a more complete understanding of otolith morphology in these species.
Our results highlighted the importance of otolith shape and size analyses for distinguishing and identifying rockfish species. This study showed that the otolith shape and size indices significantly differed among the three rockfish species. In particular, S. marmortaus had a distinct otolith morphometry compared to the other two species, whereas Sebastes zonatus and Sebastes inermis exhibited overlapping patterns in the mean otolith shape. For a better understanding, the characteristics of rockfish populations in the Korean East Sea could be improved through further research on the ecological significance of these variations as well as their correlation with specific environments and dietary patterns. Our study shows that otoliths can provide valuable data for differentiating fish species. Otoliths also provide valuable biological data on fish and require less time and cost than molecular approaches. Furthermore, research on body morphology, otolith microchemistry, and stock assessment of rockfish species in the Korean East Sea will aid in understanding these differences.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because this study utilized samples obtained from commercial bycatch, which were sourced from local fishermen. Korea Institute of Ocean Science and Technology Animal Ethics Committee did not require the study to be reviewed or approved by an ethics committee because the samples were collected as part of routine commercial fishing operations.
JP: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing. MK: Formal Analysis, Resources, Writing – review & editing. JK: Formal Analysis, Resources, Writing – review & editing. LJ: Writing – review & editing. SM: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Korea Institute of Ocean Science & Technology project [grant numbers PEA0111], and “A project on sustainable research and development of Dokdo” [PG53500] funded by the Ministry of Oceans and Fisheries, Korea.
We are thankful to the local fishermen for assistance with sample collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1301178/full#supplementary-material
Adams D. C., Rohlf F. J., Slice D. E. (2004). Geometric morphometrics: Ten years of progress following the ‘revolution’. Ital. J. Zool. 71, 5–16. doi: 10.1080/11250000409356545
An H. S., Kim E. M., Lee J. H., Noh J. K., An C. M., Yoon S. J., et al. (2011). Population genetic structure of wild and hatchery black rockfish Sebastes inermis in Korea, assessed using cross-species microsatellite markers. Genet. Mol. Res. 10, 2492–2504. doi: 10.4238/2011.October.13.6
Anderson M. J., Willis T. J. (2003). Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 84, 511–525. doi: 10.1890/0012-9658(2003)084[0511:CAOPCA]2.0.CO;2
Avigliano E., Rolón M. E., Rosso J. J., Mabragaña E., Volpedo A. V. (2018). Using otolith morphometry for the identification of three sympatric and morphologically similar species of Astyanax from the Atlantic Rain Forest (Argentina). Environ. Biol. Fish. 101, 1319–1328. doi: 10.1007/s10641-018-0779-2
Begg G. A., Overholtz W. J., Munroe N. J. (2001). The use of internal otolith morphometrics for identification of haddock (Melanogrammus aeglefinus) stocks on Georges Bank. Fish. Bull. 99, 1–14.
Cadrin S., Kerr L., Mariani S. (2013). Stock identification Methods: Applications in Fishery Science. 2nd ed. (Cambridge, Massachusetts, United States: Elsevier Academic Press).
Campana S. E., Casselman J. M. (1993). Stock discrimination using otolith shape analysis. Can. J. Fish. Aquat. Sci. 50, 1062–1083. doi: 10.1139/f93-123
Campana S. E., Thorrold S. R. (2001). Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 58, 30–38. doi: 10.1139/f00-177
Capoccioni F., Costa C., Aguzzi J., Menesatti P., Lombarte A., Ciccotti E. (2011). Ontogenetic and environmental effects on otolith shape variability in three Mediterranean European eel (Anguilla Anguilla, L.) local stocks. J. Exp. Mar. Biol. Ecol. 397, 1–7. doi: 10.1016/j.jembe.2010.11.011
Christensen H. T., Rigét F., Backe M. B., Saha A., Johansen T., Hedeholm R. B. (2018). Comparison of three methods for identification of redfish (Sebastes mentella and S. norvegicus) from the Greenland east coast. Fish. Res. 201, 11–17. doi: 10.1016/j.fishres.2018.01.003
Das M. (1994). Age determination and longevity in fishes. Gerontology 40, 70–96. doi: 10.1159/000213580
de Carvalho B. M., Volpedo A. V., Fávaro L. F. (2020). Ontogenetic and sexual variation in the sagitta otolith of Menticirrhus americanus (Teleostei; Sciaenidae) (Linnaeus 1758) in a subtropical environment. Pap. Avulsos. Zool. 60, e20206009. doi: 10.11606/1807-0205/2020.60.09
Deville D., Kawai K., Fujita H., Umino T. (2023). Ecomorphology of three closely related Sebastes rockfishes with sympatric occurrence in Seto Inland Sea, Japan. Hydrobiologia 850, 4049–4066. doi: 10.1007/s10750-023-05286-4
D’Iglio C., Albano M., Famulari S., Savoca S., Panarello G., Di Paola D., et al. (2021). Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 11, 16315. doi: 10.1038/s41598-021-95814-w
Edmonds J. S., Steckis R. A., Moran M. J., Caputi N., Morita M. (1999). Stock delineation of pink snapper and tailor from Western Australia by analysis of stable isotope and strontium/calcium ratios in otolith carbonate. J. Fish. Biol. 55, 243–259. doi: 10.1111/j.1095-8649.1999.tb00676.x
Elliott N. G., Haskard K., Koslow J. A. (1995). Morphometric analysis of orange roughy (Hoplostethus atlanticus) off the continental slope of southern Australia. J. Fish. Biol. 46, 202–220. doi: 10.1111/j.1095-8649.1995.tb05962.x
Fossen I., Albert O. T., Nilssen E. M. (2003). Improving the precision of ageing assessments for long rough dab by using digitised pictures and otolith measurements. Fish. Res. 60, 53–64. doi: 10.1016/S0165-7836(02)00063-2
Froese R., Pauly D. (2023). FishBase (World Wide Web electronic publication). Available at: http://www.fishbase.org (Accessed 9/16/2023).
Harris J. P., Hutchinson C., Wildes S. (2019). Using otolith morphometric analysis to improve species discrimination of blackspotted rockfish (Sebastes melanostictus) and rougheye rockfish (S. aleutianus). Fish. Bull. 117, 234–244. doi: 10.7755/FB.117.3.10
Hüssy K. (2008). Otolith shape in juvenile cod (Gadus morhua): Ontogenetic and environmental effects. J. Exp. Mar. Biol. Ecol. 364, 35–41. doi: 10.1016/j.jembe.2008.06.026
Jang Y. S., Kim K. Y., Oh S. Y., Choi H. J., Myoung J. G., Kim S. (2015). The complete mitochondrial genome of the dark-banded rockfish Sebastes inermis (Scorpaenidae, Scorpaeniformes). Mitochondrial. DNA. 26, 895–896. doi: 10.3109/19401736.2013.861448
Jawad L., Park J. M., Kwak S., Ligas A. (2017). Study of the relationship between fish size and otolith size in four demersal species from the south-eastern Yellow Sea. Cah. Biol. Mar. 58, 9–15. doi: 10.21411/CBM.A.645C2013
Kai Y., Nakabo T. (2008). Taxonomic review of the Sebastes inermis species complex (Scorpaeniformes: Scorpaenidae). Ichthyol. Res. 55, 238–259. doi: 10.1007/s10228-007-0029-7
Kim I. S., Choi Y., Lee C. L., Lee Y. J., Kim B. J., Kim J. H. (2005). Illustrated book of Korean fishes (Seoul: Kyo-Hak Publishing Co.), 615pp.
Kim G.-S., Son M.-H., Kwak S.-N., Park J. M., Huh S.-H. (2009). Feeding habits of released black rockfish, Sebastes inermis, in Coastal Waters off Jam Island, Jinhae Bay, Korea. Korean. J. Fisheries. Aquat. Sci. 42, 73–77. doi: 10.5657/kfas.2009.42.1.073
Lee S.-J., Kim B.-Y., Cha H.-K. (2012). Feeding habits of Sebastiscus marmoratus in the coastal waters of Jeju island, Korea. J. Korean. Soc Fish. Technol. 48, 379–386. doi: 10.3796/KSFT.2012.48.4.379
Libungan L. A., Óskarsson G. J., Slotte A., Jacobsen J. A., Pálsson S. (2015). Otolith shape: A population marker for Atlantic herring Clupea harengus. J. Fish. Biol. 86, 1377–1395. doi: 10.1111/jfb.12647
Libungan L. A., Pálsson S. (2015). ShapeR: An R package to study otolith shape variation among fish populations. PloS One 10, e0121102. doi: 10.1371/journal.pone.0121102
Lishchenko F., Jones J. B. (2021). Application of shape analyses to recording structures of marine organisms for stock discrimination and taxonomic purposes. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.667183
Lleonart J., Salat J., Torres G. J. (2000). Removing allometric effects of body size in morphological analysis. J. Theor. Biol. 205, 85–93. doi: 10.1006/JTBI.2000.2043
Lombarte A., Lleonart J. (1993). Otolith size changes related with body growth, habitat depth and temperature. Environ. Biol. Fish. 37, 297–306. doi: 10.1007/BF00004637
Longmore C., Fogarty K., Neat F., Brophy D., Trueman C., Milton A., et al. (2010). A comparison of otolith microchemistry and otolith shape analysis for the study of spatial variation in a deep-sea teleost, Coryphaenoides rupestris. Environ. Biol. Fish. 89, 591–605. doi: 10.1007/s10641-010-9674-1
Mille T., Mahé K., Cachera M., Villanueva M. C., De Pontual H., Ernande B. (2016). Diet is correlated with otolith shape in marine fish. Mar. Ecol. Prog. Ser. 555, 167–184. doi: 10.3354/meps11784
Moore B. R., Parker S. J., Pinkerton M. H. (2022). Otolith shape as a tool for species identification of the grenadiers Macrourus Caml and M. whitsoni. Fish. Res. 253, 106370. doi: 10.1016/j.fishres.2022.106370
Morales C. J. C., Barnuevo K. D. E., Delloro E. S., Cabebe-Barnuevo R. A., Calizo J. K. S., Lumayno S. D. P., et al. (2023). Otolith morphometric and shape distinction of three redfin species under the genus Decapterus (Teleostei: Carangidae) from Sulu Sea, Philippines. Fishes 8, 95. doi: 10.3390/fishes8020095
Muto N., Kai Y., Nakabo T. (2019). Taxonomic review of the Sebastes vulpes complex (Scorpaenoidei: Sebastidae). Ichthyol. Res. 66, 9–29. doi: 10.1007/s10228-018-0641-8
Nakabō T. (2002). Fishes of Japan: With pictorial keys to the species (Tokyo,Japan: Tokai University Press). Available at: https://books.google.co.kr/books?id=QpYWAQAAIAAJ.
Nikiforidou V., Gkikas E., Mytilineou C., Haralabous J., Koutsoubas D., Anastasopoulou A. (2023). Age, growth and otolith morphometrics of Serranus hepatus (L. 1758) in two areas of the Eastern Mediterranean. J. Mar. Biol. Assoc. U.K. 103, e59. doi: 10.1017/S0025315423000474
Oh S.-Y., Kang R.-S., Myoung J.-G., Kim C.-K., Park J., Daniels H. V. (2010). Effect of ration size restriction on compensatory growth and proximate composition of dark-banded rockfish, Sebastes inermis. J. World Aquac. Soc 41, 923–930. doi: 10.1111/j.1749-7345.2010.00435.x
Park J. M., Gaston T. F., Riedel R., Williamson J. E. (2018). Biometric relationships between body and otolith measurements in nine demersal fishes from north-eastern Tasmanian waters, Australia. J. Appl. Ichthyol. 34, 801–805. doi: 10.1111/jai.13612
Park J. M., Rho H. S., Lee H. G., Myoung S. H., Jawad L. A., Lee J. H., et al. (2023). First biometric relationship and seasonal condition factors of Sebastes zonatus Chen and Barsukov 1976 and Thamnaconus modestus (Günther 1877) inhabiting the waters of Ulleung-do and Dokdo. Korean. J. Ichthyol. 35, 50–56. doi: 10.35399/ISK.35.1.7
Pattuinan J. O., Demayo C. G. (2018). Morphometric shape analysis of otolith from selected goby fishes. Trans. Sci. Technol. 5, 190–196.
Poznar M., Stolarski J., Sikora A., Mazur M., Olesiak-Bańska J., Brach K., et al. (2020). Fish otolith matrix macromolecule-64 (OMM-64) and its role in calcium carbonate biomineralization. Cryst. Growth Des. 20, 5808–5819. doi: 10.1021/acs.cgd.0c00413
Qiao J., Zhu R., Chen K., Zhang D., Yan Y., He D. (2022). Comparative otolith morphology of two morphs of Schizopygopsis thermalis Herzenstein 1891 (Pisces, Cyprinidae) in a headwater lake on the Qinghai-Tibet plateau. Fishes 7, 99. doi: 10.3390/fishes7030099
R Core Team (2023). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing).
Reichenbacher B., Sienknecht U., Küchenhoff H., Fenske N. (2007). Combined otolith morphology and morphometry for assessing taxonomy and diversity in fossil and extant killifish (Aphanius, †Prolebias). J. Morphol. 268, 898–915. doi: 10.1002/jmor.10561
Sadighzadeh Z., Tuset V. M., Valinassab T., Dadpour M. R., Lombarte A. (2012). Comparison of different otolith shape descriptors and morphometrics for the identification of closely related species of Lutjanus spp. from the Persian Gulf. Mar. Biol. Res. 8, 802–814. doi: 10.1080/17451000.2012.692163
Shrestha N. (2021). Factor analysis as a tool for Survey Analysis. Am. J. Appl. Math. Stat. 9, 4–11. doi: 10.12691/ajams-9-1-2
Stransky C., MacLellan S. E. (2005). Species separation and zoogeography of redfish and rockfish (genus Sebastes) by otolith shape analysis. Can. J. Fish. Aquat. Sci. 62, 2265–2276. doi: 10.1139/f05-143
Tuset V. M., Imondi R., Aguado G., Otero-Ferrer J. L., Santschi L., Lombarte A., et al. (2015). Otolith patterns of rockfishes from the northeastern pacific. J. Morphol. 276, 458–469. doi: 10.1002/jmor.20353
Tuset V. M., Lombarte A., Assis C. A. (2008). Otolith atlas for the western Mediterranean, north and central eastern Atlantic. Sci. Mar. 72, 7–198. doi: 10.3989/scimar.2008.72s17
Tuset V. M., Lombarte A., González J. A., Pertusa J. F., Lorente M. J. (2003). Comparative morphology of the sagittal otolith in Serranus spp. J. Fish. Biol. 63, 1491–1504. doi: 10.1111/J.1095-8649.2003.00262.X
Tuset V. M., Otero-Ferrer J. L., Gómez-Zurita J., Venerus L. A., Stransky C., Imondi R., et al. (2016). Otolith shape lends support to the sensory drive hypothesis in rockfishes. J. Evol. Biol. 29, 2083–2097. doi: 10.1111/jeb.12932
Zarei F., Esmaeili H. R., Stepien C. A., Kovačić M., Abbasi K. (2023). Otoliths of Caspian gobies (Teleostei: Gobiidae): Morphological diversity and phylogenetic implications. PloS One 18, e0285857. doi: 10.1371/journal.pone.0285857
Zhuang L., Ye Z., Zhang C. (2015). Application of otolith shape analysis to species separation in Sebastes spp. from the Bohai Sea and the Yellow Sea, northwest Pacific. Environ. Biol. Fish. 98, 547–558. doi: 10.1007/s10641-014-0286-z
Zischke M. T., Litherland L., Tilyard B. R., Stratford N. J., Jones E. L., Wang Y. G. (2016). Otolith morphology of four mackerel species (Scomberomorus spp.) in Australia: Species differentiation and prediction for fisheries monitoring and assessment. Fish. Res. 176, 39–47. doi: 10.1016/j.fishres.2015.12.003
Keywords: rockfish, morphometry, otolith, shapeR, East Sea
Citation: Park JM, Kang MG, Kim JH, Jawad LA and Majeed S (2023) Otolith morphology as a tool for stock discrimination of three rockfish species in the East Sea of Korea. Front. Mar. Sci. 10:1301178. doi: 10.3389/fmars.2023.1301178
Received: 24 September 2023; Accepted: 18 October 2023;
Published: 02 November 2023.
Edited by:
Josipa Ferri, University of Split, CroatiaReviewed by:
Nadjette Bourehail, University of Annaba, AlgeriaCopyright © 2023 Park, Kang, Kim, Jawad and Majeed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samroz Majeed, c2Ftcm96bWFqZWVkQGtpb3N0LmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.