Abstract
The knowledge held by local and indigenous communities has been recognized as an invaluable yet underused resource for understanding how the environment, local conditions, and fish populations change over time. Empirical information on population sizes, ecology, and threats to fish species and their habitats can be complemented with local knowledge to better guide management and conservation efforts, particularly for understudied species. Here, we investigated the habitat preferences, population status, fishing, sightings patterns, threats, and habitat characteristics of juveniles of the cubera snapper, the largest snapper in the Atlantic Ocean. We used a combination of empirical evidence from underwater surveys by using small-action cameras and an ethnological perspective based on fishers’ perceptions who are cubera-snapper fishing specialists to have a comprehensive understanding of the species and habitat use during its early life stages. A range of estuarine habitats was examined over a year to assess the association between cubera snapper juveniles and different estuarine characteristics and conservation scenarios. Both sources of data indicated that cubera snapper juveniles heavily rely on mangrove habitats, exhibiting a clear temporal pattern of residence within these habitats. However, the probability of occurrence varied based on the level of coastal development in each estuarine system. Estuaries with small drainage areas, directly connected to the ocean, and presenting larger mangrove areas accounted for the highest abundances of juveniles. Factors such as mangrove removal, overfishing, and water pollution significantly reduced the occurrence and reliance of cubera snapper juveniles in the studied estuaries. The study represents the first attempt to shed light on the ecological aspects of cubera snapper juveniles, addressing a gap in their life cycle. It underscores the importance of integrating complementary sources of evidence to understand the relationships between the crucial life stage of cubera snappers, their habitats, and the threats they face. The ecological and ethnographic knowledge gained from this research should be incorporated into biomonitoring and conservation policy to effectively preserve this vulnerable top predator.
1 Introduction
The relationships between animals and their habitats are intricate and dynamic, as adult and juvenile individuals often occupy distinct habitat types across their life cycles (Bradley et al., 2020). Consequently, fauna-habitat relationships exhibit variability both spatially and temporally, making them complex. Depending on the specific location, a species may demonstrate varying degrees of reliance on a particular habitat feature, ranging from obligatory to facultative, temporary, or even permanent. Animal-habitat relationships are not rigid or consistent across space; instead, they exhibit plasticity. Therefore, assigning absolute values to habitat features is not appropriate, and generalizations may not always hold (Baker and Sheaves, 2009; Chamberlain et al., 2014). Without a comprehensive understanding of the limitations within this paradigm, it is challenging to determine the validity of animal-habitat relationships, integrate them into our general understanding of ecosystem function, and adequately address their implications for human society (Carpenter et al., 2009). This is particularly relevant for coastal fish and fishery systems supported by them, upon which millions of people rely worldwide (Gilby et al., 2017; Sheaves et al., 2020). Given the relatively small scale of several estuarine systems, crucial for the survival of small-scale fishery communities (Kaliloski and Satterfield, 2004; Kurien and Willmann, 2009), the significance of fishery resources and their contextual, place-based relationships becomes even more pronounced.
Many complex factors determine the role of estuarine habitats and landscapes in fish growth and survival that ultimately contribute individuals to adult populations (Able et al., 2022). Ontogenetic shifts in habitat utilization can serve to decrease intraspecific competition for resources, allow for the fulfillment of distinct dietary needs, provide size-specific shelter from predators, and support reproductive activities (Claydon et al., 2015). This phenomenon can be observed in several species of marine fish that inhabit reefs and other coastal deep habitats as adults but rely on estuarine ecosystems and mangrove habitats as critical environments during their juvenile stages (Dorenbosch et al., 2004a; Dorenbosch et al., 2004b). A continuum exists ranging from species that primarily inhabit reefs throughout their post-settlement lives, to species that rely on nurseries habitats (such as mangroves) during early life stages and then migrate to off-shore habitats when adults (Adams et al., 2006; Dahlgren et al., 2006). These ‘ontogenetic habitat shifters’ (sensu Adams et al., 2006), including groupers (Serranidae), snappers (Lutjanidae), grunts (Haemulidae), and barracudas (Sphyraenidae), are targeted by fisheries worldwide as adults in many tropical and subtropical waters (Faunce and Serafy, 2008).
Along this ontogenetic shift continuum, certain reef species are referred to as “nursery species” due to the predominance of their juveniles in non-reef habitats (Nagelkerken and van der Velde, 2002). However, there is limited knowledge about the relative importance or value of nursery habitats, particularly mangrove-dominated estuaries, for species whose adult stages inhabit offshore habitats. This knowledge gap can be attributed to the perception of shoreline mangroves as homogenous units; neglecting variations in size or area among locations (see Faunce and Serafy, 2008). Studies that compare the availability of a habitat type or patch to its use by animals, especially the “nursery species”, offer a means toward achieving habitat valuation under the assumption that animals will occupy sites best suited for their needs (Thomas and Taylor, 2006). Although the connectivity between mangroves and reefs has been established for numerous coastal fish species, there is still limited evidence regarding the reliance on non-reef nursery grounds, especially for commercially or artisanally important species. Furthermore, there are substantial differences between estuarine and marine mangroves. Estuarine mangroves are found in the transition zones between rivers and the sea, experiencing fluctuation in salinity and freshwater input. Marine mangroves, on the other hand, thrive in areas with stable saline conditions along the coastlines, lacking the freshwater influence seen in estuarine settings (Hogarth, 2015).
Snappers are well-known coastal predators in the West Atlantic Ocean and face significant fishing pressure along the continental shelf. The dependency of some species, such as the dog snapper Lutjanus jocu, for non-reef nursery grounds during their early life stages is well-established (Moura et al., 2011). Conversely, the obligate dependency of others, such as the cubera snapper Lutjanus cyanopterus, lacks further empirical evidence (Lindeman and De Maria, 2005). Cubera snapper fisheries primarily target spawning aggregations, highlighting their importance for conservation since these aggregations are transient, occur during short periods of the year, and are concentrated at specific locations, sometimes far from their home range (Baisre, 2017; Malafaia et al., 2020). Currently, the Red List of Threatened Species, maintained by the International Union for the Conservation of Nature (IUCN), classifies L. cyanopterus as vulnerable (IUCN Red List of Threatened Species, 2017). In the Caribbean Sea, historical landing data reveals a decline in snappers’ contribution to the total finfish catch, dropping from approximately 35% in the 1970s to 18% in 1999 (Claro et al., 2009). Recent studies in Brazilian waters further suggest that overfishing, particularly targeting spawning aggregations, could have adverse effects on cubera snapper populations (Malafaia et al., 2020; Motta et al., 2022) and also other fish species, such as permits and pompanos (Reis-Filho et al., 2021).
Despite the availability of reasonable information on cubera snapper adult stages and their associated habitats, which largely rely on fishery landings, our understanding of the habitat preferences of juvenile individuals remains limited. In addition, there are no studies that describe the residency/occurrence or have tracked the density patterns of early stages of the cubera snapper considering their nursery habitats. In the pursuit of empirical approaches to address this knowledge gap in cubera snapper ecology, it is crucial to avoid the intentional selective use of only one source of evidence to support a position (Cooke et al., 2023). Understanding how different scientific approaches engage with environmental evidence is therefore a valid question. For example, fisheries-dependent data from logbooks, landings monitoring, and web-based information pose challenges in determining reference points and the stock status of exploited species (Reis-Filho et al., 2021). Conversely, approaches that consider social and ecological attributes enable the elaboration of diagnostic assessments and the generations of information for the development of management strategies (Carvalho Costa et al., 2023). Therefore, integrating alternative research approaches, such as Local Ecological Knowledge (LEK) to generate evidence can help fill gaps in coastal fish studies and provide complementary and diverse sources of information. Indigenous and local communities, with their deep connection to the environment, possess a wealth of knowledge passed down through generations (Renk et al., 2023), including observation of fish behavior (Leduc et al., 2023), fishing practices, and the dynamics of environmental changes over time (Reis-Filho et al., 2020). Consequently, integrating LEK with other established scientific approaches can enhance the comprehensiveness of data collection in ecological studies. In this sense, underwater footage obtained through small-action cameras has emerged as a cost-effective method for assessing ecological aspects of coastal fish fauna, particularly in complex and diverse environments like mangroves (Reis-Filho et al., 2016; Reis-Filho et al., 2020). These recordings offer valuable visual documentation of fish species, their behavioral patterns, and their habitats, offering a non-intrusive and comprehensive perspective (Borland et al., 2022). By combining LEK and underwater footage, researchers can establish a robust and multidimensional approach to fish data records (Fogliarini et al., 2021; Tengo et al., 2021; Schmid et al., 2022). This integrated methodology not only strengthens the scientific understanding of fish ecology aspects but also acknowledges and respects the knowledge systems of local communities, fostering collaboration and co-management of coastal resources for sustainable conservation and management efforts (Armitage et al., 2008; Berkes, 2012).
The cubera snapper is classified as data-poor due to infrequent capture in sampling programs (Farmer et al., 2016; Malafaia et al., 2020), resulting in the limited availability of fundamental biological and ecological information. Therefore, a better understanding of the ecological requirements influencing fish population, their life stages, and their spatial distribution warrants further investigation. Thus, this study aims to examine the association between cubera snapper juveniles and mangrove-dominated estuaries considering spatially-distinct environments. Using a complementary approach belonging to different scientific fields: the assessment of Local Ecological Knowledge from fishers’ interviews and analysis of underwater footage in mangrove habitats, we investigated fish density dependence and ontogenetic structure patterns exhibited by cubera snapper juveniles. This research intends to enhance our understanding of the importance of estuarine and mangrove habitats as essential nursery areas for the early life stages of cubera snappers. Furthermore, it seeks to provide recommendations for the effective management and conservation of this important fishery species by involving fishers in the data collection through their shared knowledge, paving the way for management strategies and making them part of the process.
2 Material and methods
2.1 Study area
The study encompassed eight estuaries along a 500-km stretch of the Brazilian central coast (Figure 1). The Itariri, Inhambupe, Pojuca, and Joanes estuaries have a smaller drainage basin and direct connections to the ocean. Conversely, the Paraguaçu, Jaguaripe, Aratu Bay, and Serinhaém estuaries have larger areas and flow into an inner bay before reaching the ocean. All of these estuaries are characterized by symmetric diurnal tides ranging between 2.1 and 2.4 m (Cirano and Lessa, 2007). The study region experienced an average annual rainfall of approximately 210 mm, with a rainy season from March to August (mean monthly precipitation ~280 mm) and a dry season from September to February (~110 mm per month). Three estuaries (Pojuca, Joanes, and Aratu Bay) underwent significant coastal development pressures, including urbanization, pastureland use, reduced mangrove cover, port development, or industrialization, while the other five (Itariri, Inhambupe, Paraguaçu, Jaguaripe, and Serinhaém) remained relatively little affected by coastal development.
Figure 1
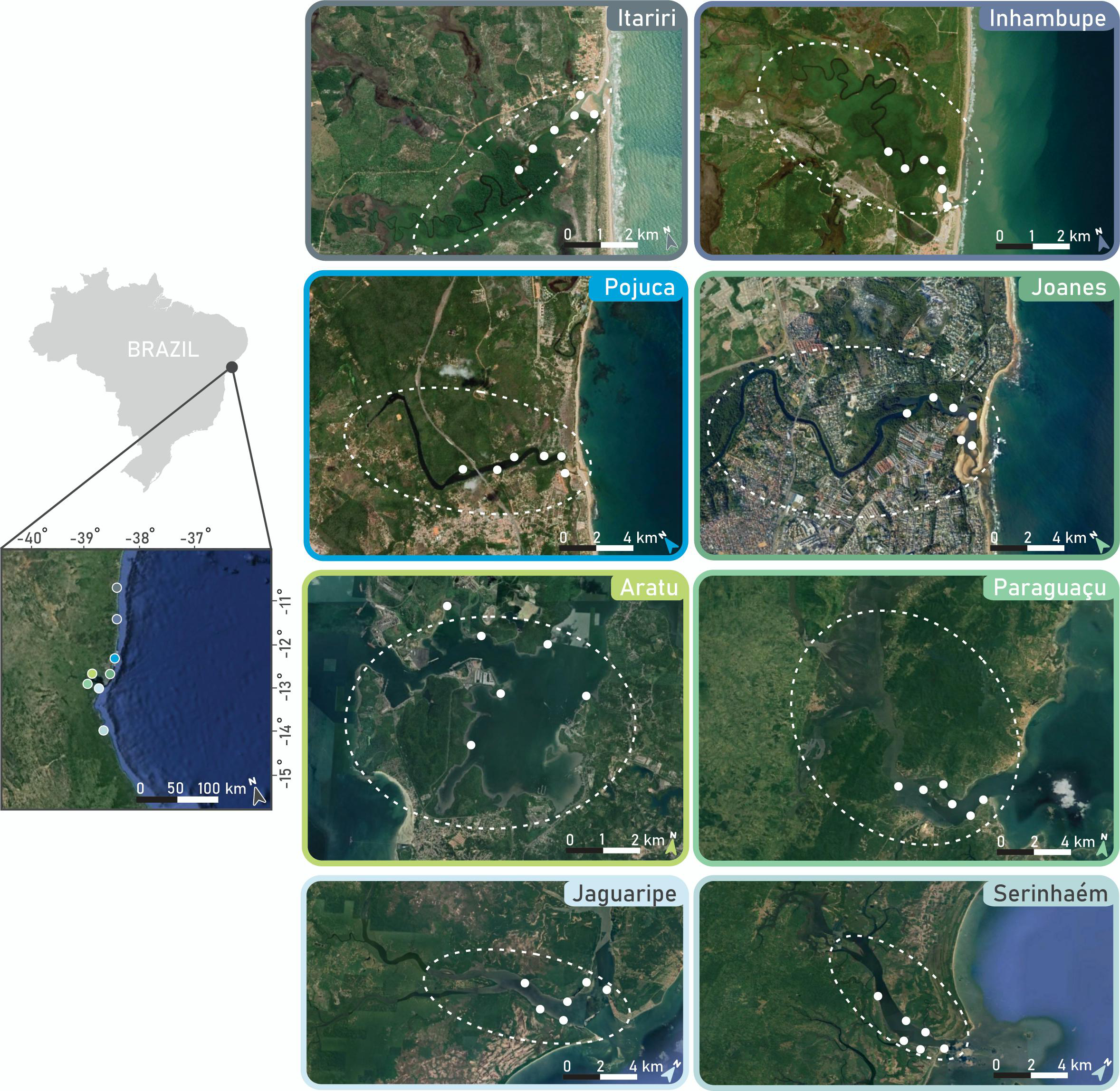
Estuarine regions (dashed ellipses) where the study was conducted using a combination of fisher interviews and deployment of small action cameras (white circles). The individual map images were sourced from Google Earth.
2.2 General aspects of the artisanal fishers and environmental characteristics
Fishers from small villages situated in each of the eight estuarine areas were selected to participate in the study. These fishers engaged in traditional and small-scale fishing activities, which were specific to each estuary and focused on local targets. Recreational fishers in the region are limited and frequently engage in fishing activities in continental shelf areas, several miles away from the fishing grounds. Small action cameras were deployed in specific locations identified by the fishers as preferable for finding snappers. Spatial features, including the drainage area, mangrove cover, and aspects related to the coastal development in each estuarine system, were measured in km2 using Google Earth Pro and ArcMap (ESRI®). To aim was to explore potential relationships between the cubera snapper and these environmental factors, as presented in Table 1.
Table 1
| Estuaries | Drainage area (km2) | Mangrove cover (km2) | Coastal development aspects (km2) |
|---|---|---|---|
| Itariri | 0.29 | 1.32 | 1.52 |
| Inhambupe | 0.43 | 3.32 | 0.31 |
| Pojuca | 0.46 | 1.27 | 2.13 |
| Joanes | 0.55 | 0.59 | 5.66 |
| Aratu | 15.5 | 6.77 | 9.81 |
| Paraguaçu | 90.6 | 55.7 | 0.88 |
| Jaguaripe | 19.7 | 17.3 | 0.49 |
| Serinhaém | 28.1 | 32.2 | 0.63 |
Description of variable metrics derived from the drainage area, mangrove cover, and coastal development aspects in each studied estuary.
2.3 Sampling procedure
We employed a unique approach combining scenario interviews, which provide a local ecological knowledge (LEK) perspective, and underwater footage captured by remotely-operated small-action cameras, ensuring minimal human influence. Scenario interviews are commonly utilized in behavioral sciences and predictive conservation to explore people’s perceptions of potential changes by presenting a series of plausible questions (Cinner et al., 2011). Meanwhile, underwater footage is a well-established stated preference method that reduces human interference, allowing for the assessment of fish fauna, including measures of abundance and habitat use (Reis-Filho et al., 2016). By employing these complementary methodologies, we aimed to gain a comprehensive understanding of the intricate relationships between human users (specifically, fishers) and the animals within a given system, as well as the underlying drivers and mechanisms shaping these relationships.
Initially, we contemplated using smaller estuarine systems as control sites, expecting that these systems, with their lower drainage basin and shallow grounds, would be less suitable for sustaining the population of cubera snapper juveniles. However, due to the lack of comprehensive scientific information on probable habitats for juveniles of this species, coupled with local fishers’ information on the high frequency of sightings, especially in the smaller estuaries, we opted to conduct the study to evaluate the level of importance of each estuarine systems for cubera snapper juveniles. This approach deviates from attempting to attribute differences through a classic experimental design of expected effect vs. control effect.
2.3.1 Fisher selection and perception inquiries
During the year 2012, fisher perceptions of the occurrence and ecology of the cubera snapper were recorded in each one of the study estuaries. The estuarine and nearshore fisheries in this region, as in most parts of the Brazilian coast, are largely artisanal, serving both subsistence needs and local markets (Reis-Filho et al., 2018). A preliminary investigation was done in the coastal region with five fishers per estuary to determine the proportion of them involved in estuarine and coastal fisheries, resulting in 85% of fishers with knowledge of cubera snapper fishing and sightings. The 85% obtained proportion was then used in Dagnelie (1998) formula with a 95% confidence level to estimate the necessary sample size:
,where N represents the total number of fishers to be surveyed. U21-α/2 is the value of the normal random variable for a probability value of 1-α/2 = 0.975, U21-α/2 = 1.95; p is the estimated proportion of fishers who known and catch the cubera snapper; and d represents the margin of error of a parameter estimated from the sample fixed at 0.05. From the calculation, the sample size of 99 fishers was the minimum needed.
Concerning the data collection, fishers were randomly selected within the different estuaries. We aimed to interview fishers from distinct age groups, but always following the main criteria of interviewing those fishers more dedicated to estuarine fisheries. To avoid selecting occasional or recreational fishers, we included only artisanal fishers who cited fishing as their main activity for subsistence. The interviews were conducted face-to-face using a structured questionnaire (see Appendix) from January to December of 2012. We explained the research, including the main goals and methods, to each interviewed fisher and clarified any doubts about methods, potential benefits, and risks, before the interview (following procedure from Ribeiro et al., 2021). A set of information about the selected fishers’ socio-demographic background is summarized in Table S1. The questionnaire was designed to assess the fishers’ knowledge of the species names, estuaries, and habitats where the cubera snapper is caught or found, fish abundance in the past (when the fisher started fishing) and at present, and size estimates from a spatial and temporal perspective. Fishers’ perceptions of a decrease in cubera snapper abundance over the last three decades were also assessed. The questions also covered fishing aspects, such as months when the fish were more caught or sighted, and the fishing technique adopted for catching the cubera snapper. Furthermore, the environmental threats faced by the cubera snapper fish populations on the Brazilian central coast and their aquatic ecosystems due to coastal development were additionally addressed by the questionnaire. The duration of each interview varied between 30 and 60 min.
2.3.2 Underwater cameras deployments
Sampling was carried out in each one of the eight estuarine systems, characterized by the predominant presence of Avicennia schaueriana and Rhizophora mangle prop roots, with relatively lower densities of Laguncularia racemosa. The mangroves in all study sites presented very clear waters and were bordered by low turbidity sandy-muddy flats in the flooded subtidal, allowing for effective underwater video monitoring (Reis-Filho et al., 2016). Fieldwork was conducted bimonthly throughout 2012 during the spring tides, precisely three days during the peak of the full moon. For the deployments, six replicate small-action cameras were placed at each estuary (refer to Figure 1), resulting in a total of 288 recordings. The specific sites remained the same throughout the entire study period due to the consistently good clear water conditions. All sampling activities were carried out between 0600 and 1700 to minimize potential twilight-induced effects on fish fauna dynamics.
The cameras were positioned approximately 20 cm above the substrate, capturing a view that included the mangrove forest (refer to Figure 2). The arrangement of cameras was non-linear, with a minimum spacing of 100 m between each camera, providing a horizontal field view of at least 2 m. All video footage was obtained using small-action cameras (GoPro™ Hero 4+ High Definition camera, USA). These cameras had a battery life of approximately ten hours (4017 mAh lithium batteries) and were redeployed once daily to minimize potential human interference. The first 10 minutes of each footage were discarded following the methodology proposed by Becker et al. (2012) and Nanninga et al. (2017) to reduce the influence of camera setup on fish behavior. The MaxN (maximum number) of cubera snappers and their respective size classes were recorded hourly. For body length estimates, fish were compared to the nearby mangrove prop roots when they were equidistant from the camera and were separated in classes of 5 to 14 cm and 15 to 40 cm. Accurate estimation of body size required the fish to be fully visible in the frame, a method similar to that employed by Reis-Filho et al. (2020). Known age-related variations in coloration patterns, which are characteristics of the cubera snapper, were also taken into consideration during the analysis.
Figure 2
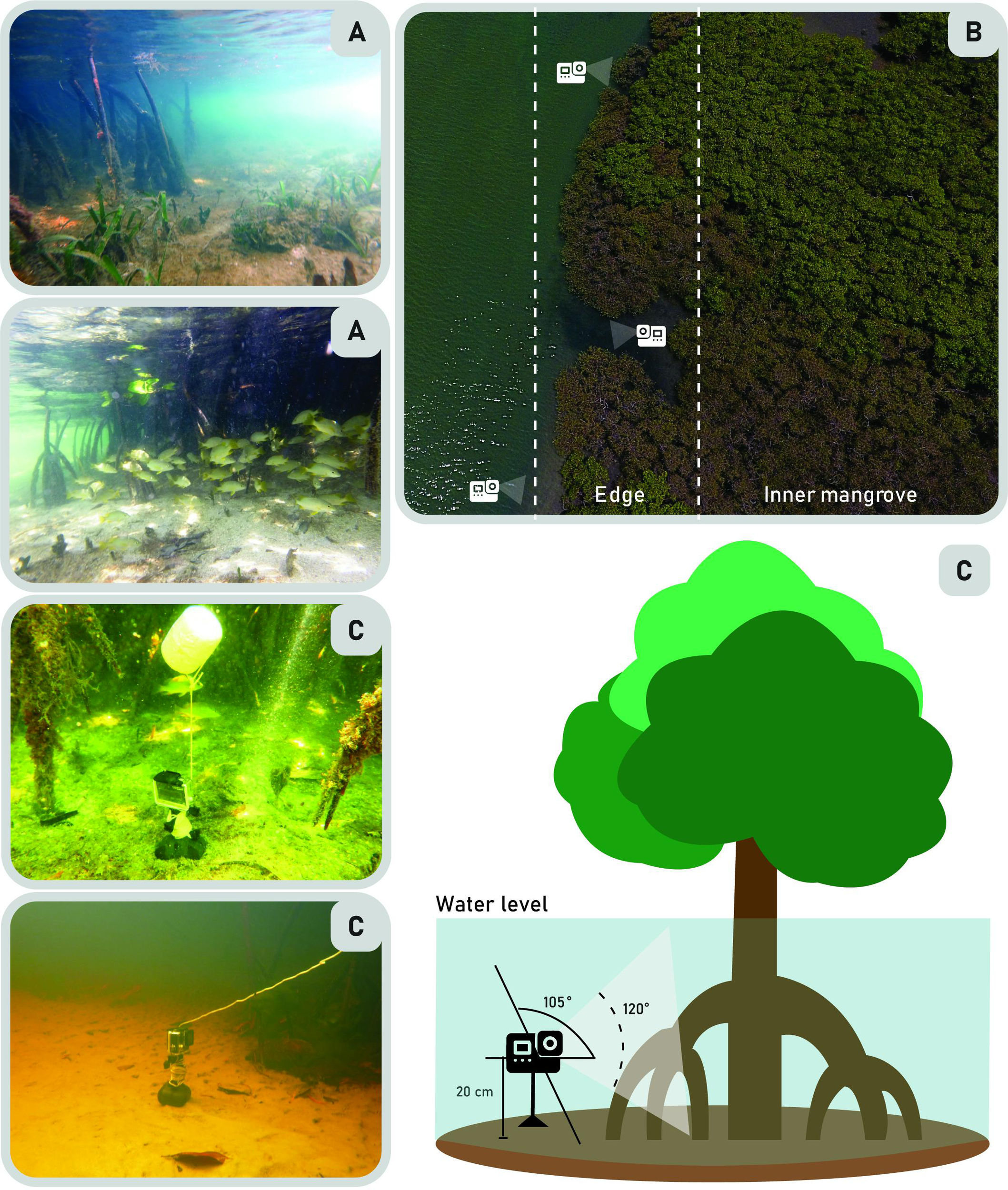
Illustration depicting the arrangement of the wide-angle underwater cameras deployed at the sampling sites. (A) The perspective of the underwater cameras capturing the view of the mangrove edges. (B) A bird’s-view showcasing the experimental set-up in the mangrove ecotone. (C) Submersible camera mounted in the sediment with the lens positioned 20 cm above the substrate.
2.4 Data analysis
Descriptive statistics, including frequency and percentage, were employed to summarize and compare the fishers’ responses regarding their perception and knowledge of cubera snapper. By comparing the results from each estuarine system, we aimed to gain a comprehensive understanding of the local variations in response patterns under different scenarios (Travers et al., 2019). To investigate the relationship between various aspects of cubera snapper ecology and fishing practices (such as preferred habitat, fishing of different life stages, fishing gear for capturing juveniles, months with highest captures or sightings, population decline, and reasons for the decline), we employed mixed-effects logistic regression with multiple and/or binary response variables (Eq. 1). Logistic regression is well-suited for predicting the likelihood of an observation falling into different categories based on independent variables (Garson, 2014). We examined the relationship between fishers’ perception (independent variables) and cubera snapper ecology and fishing data (dependent variables) to determine the best-fit model using backward selection based on the Akaike information criterion. To enable meaningful cross-site comparisons, we excluded site-specific scenarios and treated the “interviewee” as a random effect to address pseudo-replication resulting from survey participants. The perception variables included categories such as (i) occurrence (high, medium, or low) of cubera snapper juveniles in various coastal habitats (e.g., mangrove, seagrass, reef, sand-mud bottoms); (ii) fishing of cubera snapper juveniles; (iii) fishing of cubera snapper sub-adults and adults; (iv) months with highest records of cubera snapper juveniles; and (v) changes in population abundance across different life stages (0 = no decrease; 1 = decrease). We employed the Z-test to assess significant differences in the threats and reasons identified by fishers affecting cubera snapper juveniles within each estuary system. Statistical significance was determined at an alpha level of 0.05. The logistic regression model and Z-test were conducted using the glmer function from the lme4 package in R statistical software (Bates et al., 2015).
Where:
π represents the logarithm probability of the fishing data response variable;
β0 is the intercept;
uZ is the random effect term;
β1, β2, β3, β4, β5, β6, β7, and β8 are the coefficients (parameters) to be estimated, representing the impact of each predictor variable (e.g. occurrence of cubera snapper juveniles in coastal habitats such as mangrove, seagrass, reef, and sand-mud bottoms; fishing of cubera snapper juveniles; fishing of cubera snapper sub-adults and adults; months accounted for the highest records of cubera snapper juveniles; and decreasing of juveniles, sub-adults, and adults of the cubera snapper; respectively) on the log odds of success.
We utilized MaxN (recorded on an hourly basis) as an estimate of the abundance of cubera snapper juveniles in each sampled site and estuary. To investigate the potential influences on the structure of the cubera snapper juvenile population in the estuarine seascape, we employed a multivariate general linear model approach. This analysis aimed to examine the effects of various factors, including month variation, distance from the mouth to the inner estuary, estuary (treated as a random factor), and estuary profile on juvenile’s abundance of cubera snapper. The estuary profile was characterized by categorical factors encompassing coastal development pressures (e.g., urbanization and industrialization), mangrove preservation, drainage area, and direct connection with the ocean (Eq.2). Our goal was to gain a comprehensive understanding of how these factors collectively shape the structure of the cubera snapper juvenile population in the estuarine seascape. To conduct these analyses, we employed the manyGLM function within the mvabund package in R (Wang et al., 2012; Warton et al., 2012). The models identify correlation between a matrix of multivariate abundance data and a suite of explanatory variables and identify models that best explain ecological-level correlations with best-fit variables (Warton et al., 2012). The Person’s correlation coefficient was used to test for co-linearity between the sampling sites within the estuaries and the months. Best-fit models were selected using backward stepwise regression, based on the Akaike information criterion (AIC). The “p.uni” function within the mvabund package was then used to identify the highest cubera snapper juveniles’ abundance that was correlated with significant factors identified by the multivariate GLM model. All models were checked for homogeneity of variance, normally distributed residuals, outliers, and over-dispersion, and were fitted with either Poisson or Negative Binomial (if models were over-dispersed) distributions, with log link functions.
where:
E(Y) represents the expected response variable matrix representing fish abundance, with dimensions n x p, where n is the number of observations (sites) and p is the number of estuaries;
β denote the parameter matrix, with dimensions q x p, representing the coefficients associated with each explanatory variable;
ϵ denote the error term matrix, with dimensions n x p, representing the random variation not explained by the model.
The entire analyses packages describe above were conducted within an R environment (R Core Team, 2020).
3 Results
3.1 Socio-demographic background and fishing characteristics of respondents
In total, 119 questionnaires were successfully completed. All respondents were men between 29 and 71 years old, mostly ranging between 38 and 55 years old. The majority of respondents in all estuarine systems had a low level of education (primary education) or were illiterate (no formal education). The fishing experience ranged from 15 and 49 years, and gillnets (88%) and hooks and lines (76%) were the most used fishing gear.
3.2 Fishers’ perception of cubera snapper
The optimal model for assessing fisher knowledge on the cubera snapper encompassed several key factors, including habitat types, fishing on cubera snapper across various life stages (juveniles and sub-adults/adults), the months during which the highest records of cubera snapper juveniles were observed, and decreasing of juveniles and sub-adults/adults. The model outputs revealed a statistically significant association between mangroves and an increased likelihood of cubera snapper juveniles occurrence compared to other habitats (Figure 3; Table 2). Regarding each estuarine system, there were minor variations observed, with sand-mud habitats exhibiting varying degrees of significance. However, overall, the dominant factor influencing the likelihood of cubera snapper juveniles’ occurrence was associated with mangrove habitats (Figure S1). Fishing of cubera snapper juveniles was not a significant predictor (Table 2) with a negative slope of the logistic regression representing the decreased probability of fishing juveniles as the number of respondents increase (Figure 4A), while fishing of sub-adults and adults revealed marginal significance (Figure 4B). The occurrence of cubera snapper records during specific months (e.g., April to June representing the highest abundance – Figure S2) exhibited a significant positive correlation with the likelihood of obtaining the highest records, as indicated in Table 2. Among the various life stages, juveniles showed a more pronounced association with this likelihood, as depicted in Figure 4C. Additionally, the decline in fish populations across different life stages displayed a significant positive relationship with the increasing number of interviewees, as presented in Table 2. The Akaike’s information criterion (AIC) for the top-ranked logistic regression models can be verified in Table S1. Notably, the perceived decrease in juveniles was particularly evident (Figure 4D), suggesting that it could serve as a proxy for variations in fishing activities among different locations where fishers operate.
Figure 3
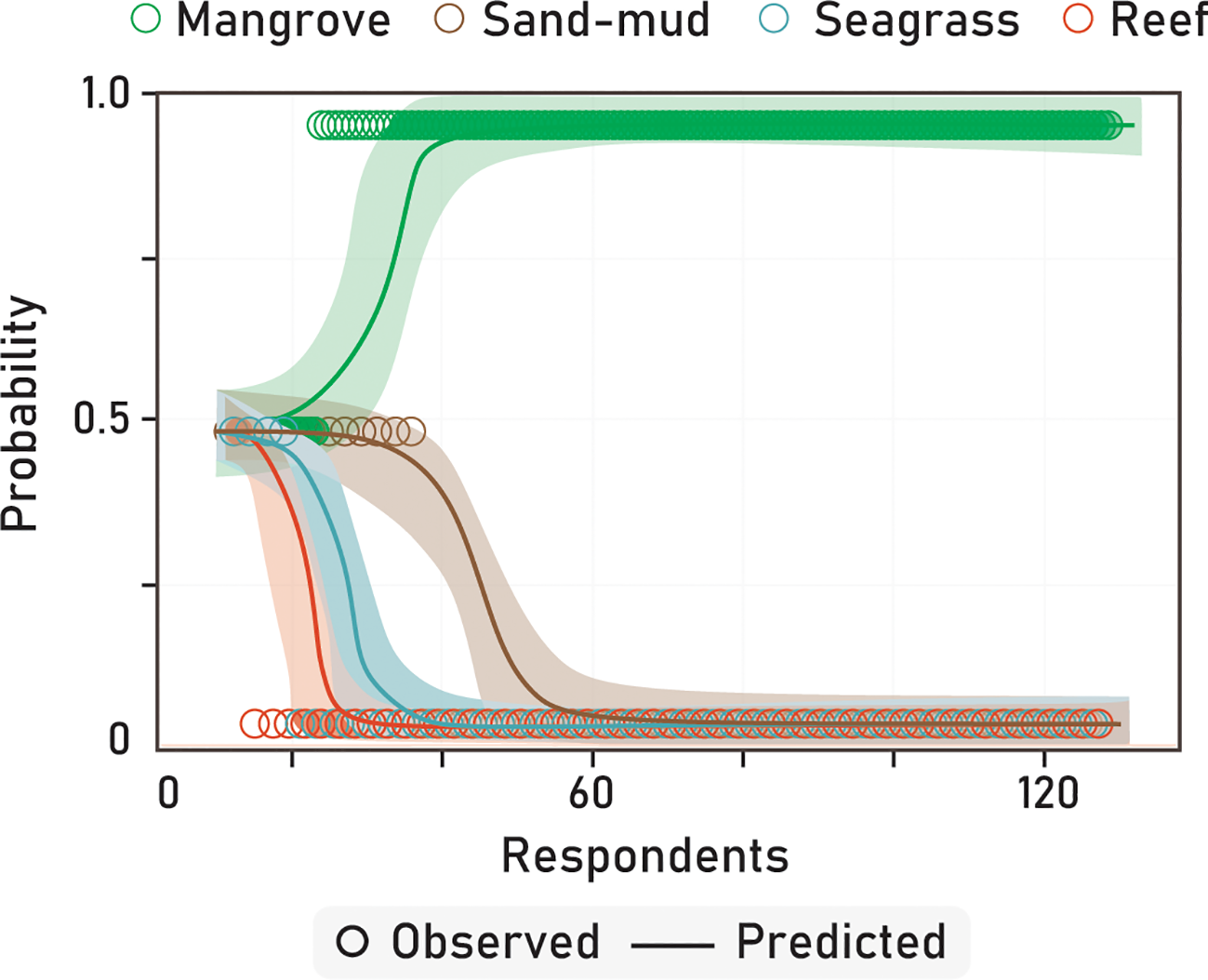
Logistic Regression representations regard to answers of fishers on occurrence habitats for juveniles of the cubera snapper. The slope of the binary logistic regression line indicates the predicted probability of cubera snapper occurrence in each habitat type, taking into account the number of fishers interviewed in all estuarine systems.
Table 2
| Predictor | Model coefficient | Probability estimate | 95% Confidence interval | p-Value | Signif. |
|---|---|---|---|---|---|
| (Intercept) | 2.193 | 0.891 | 0.512 - 0.984 | 0.004 | ** |
| Habitat type | 10.23 | 0.975 | 0.859 - 1.000 | 0.0001 | *** |
| Fishing juveniles | 2.311 | 0.357 | 0.243 - 0.504 | 0.958 | |
| Fishing sub-adults and adults | 0.876 | 0.658 | 0.459 - 0.887 | 0.01 | * |
| Months for catches or sightings | 4.841 | 0.883 | 0.701 - 0.956 | 0.01 | * |
| Decreasing of cubera snapper life stages | 6.058 | 0.901 | 0.841 - 0.997 | 0.001 | ** |
Model outputs of fishers’ perception for the influence of habitat type, fishing of juveniles, sub-adults, and adults, months for catches or sightings, and decreasing of cubera snapper life stages variables.
Signif. Codes:< 0.0001 ***;< 0.005 **;< 0.05 *; > 0.05 no signif.
Logit model coefficients from log odds to probabilities with 95% confidence intervals using as per probability = exp.(coeff)/1 + exp.(coeff).
Figure 4
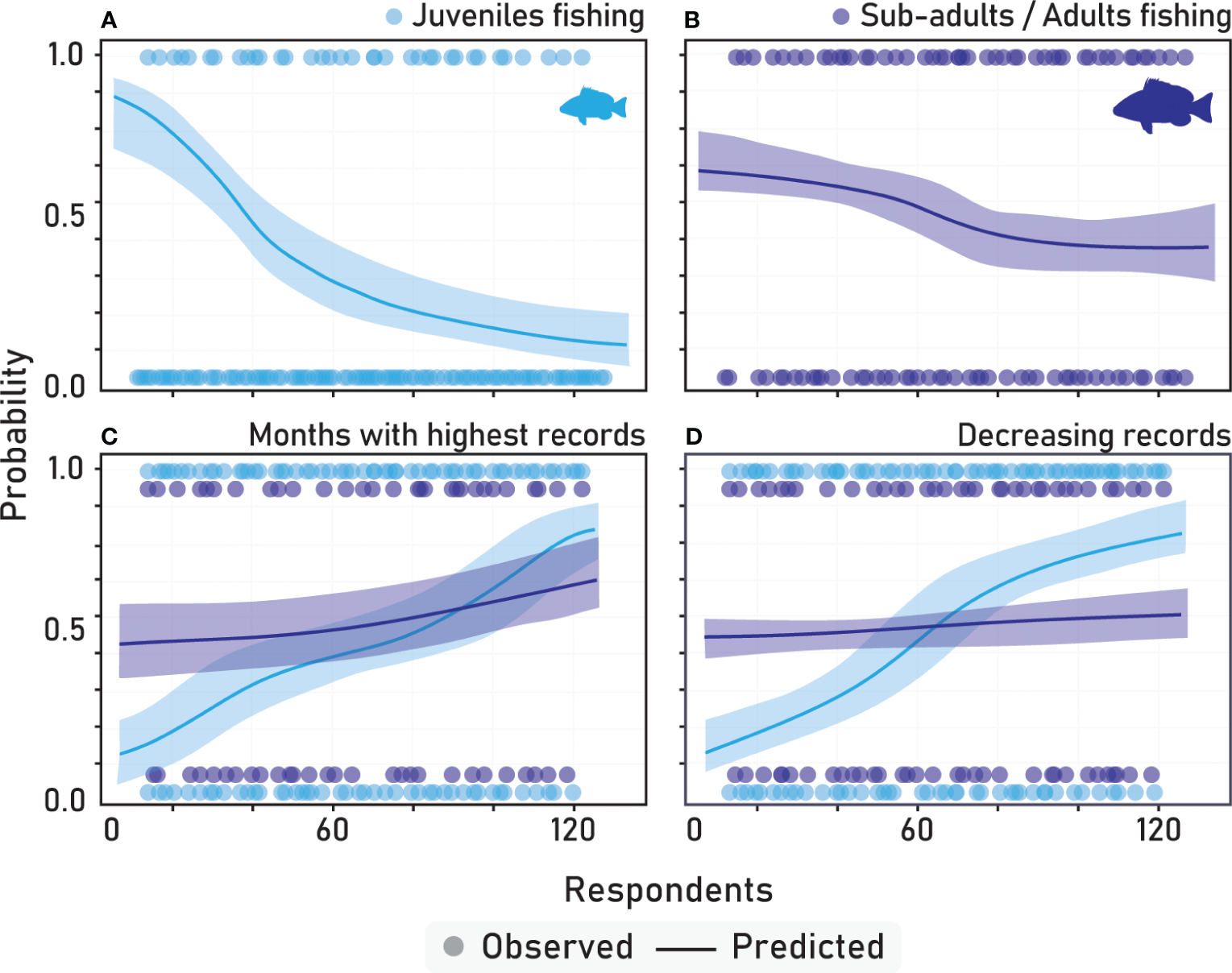
Logistic Regression representations regard answers of fishers on fishing of juveniles (A), sub-adults/adults (B), months for highest records (C), and decreasing records (D) of the cubera snapper. The slope of the binary logistic regression line indicates the predicted probability of fishing cubera snapper taking into account the number of fishers interviewed in all estuarine systems.
Fishers identified several major threats to cubera snapper juveniles in estuarine systems. The primary reasons cited included mangrove removal (62.3%), overfishing (61.1%), water pollution resulting from urban and industrial waste (50.1%), destructive fishing practices (42%), reduction of river flow (31.8%), and fishing during spawning periods (20.3%) (Figure 5). The specific threats reported varied across estuaries, influenced by their conservation status and coastal development pressure. Notably, there were significant differences (p< 0.01; Table 3) in the proportion of each threat mentioned during the interviews among fishers from different estuaries (Figure 5).
Figure 5
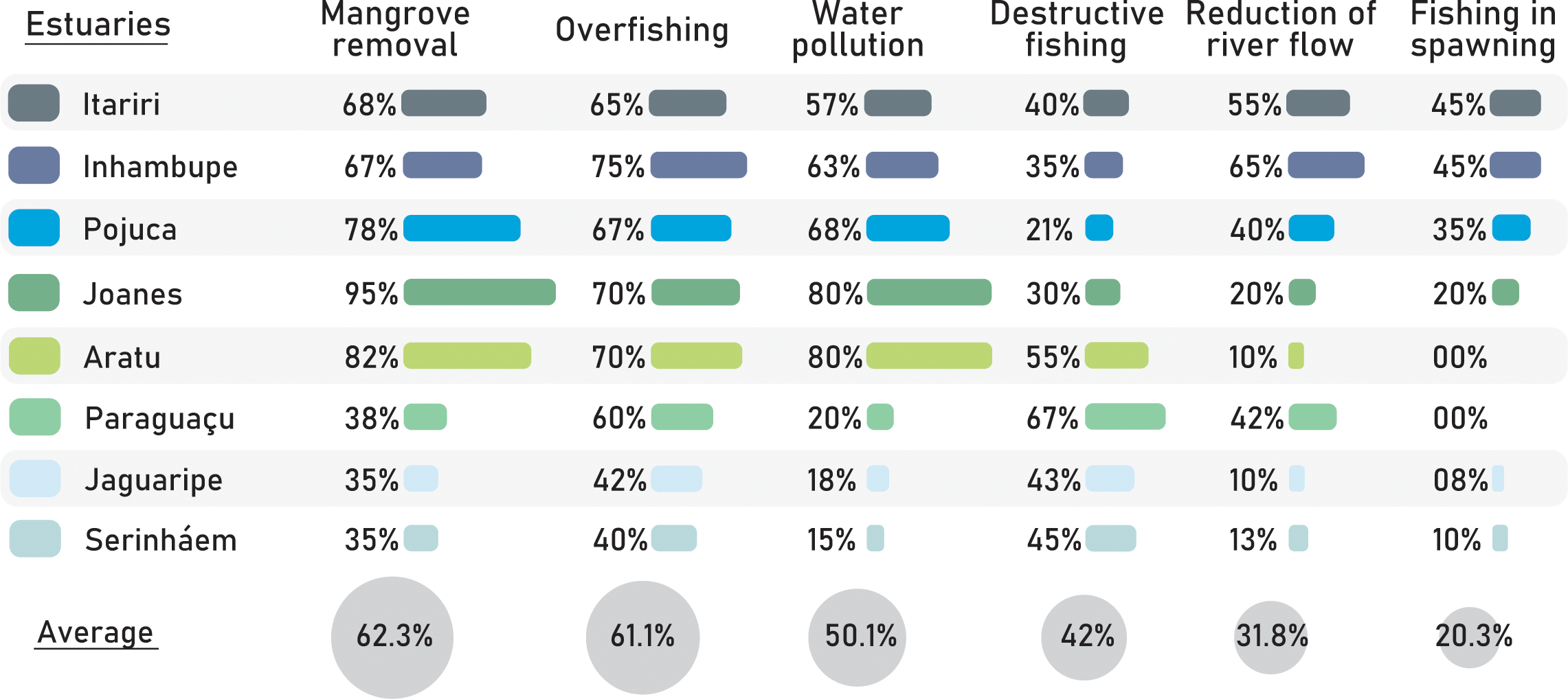
Perception of fishers about the types of threats to the cubera snapper juveniles among the estuarine systems.
Table 3
| Threats to cubera snapper juveniles | χ2 | df | p-value |
|---|---|---|---|
| Mangrove removal | 95.5 | 7 | < 0.001 |
| Overfishing | 65.2 | 7 | < 0.01 |
| Water pollution | 88.3 | 7 | < 0.001 |
| Destructive fishing | 60.4 | 7 | < 0.05 |
| Reduction of river flow | 85.6 | 7 | < 0.001 |
| Fishing during the spawning period | 58.7 | 7 | < 0.05 |
Result of Z-test comparing proportions of perceptions of fishers about each type of threat to the cubera snapper juveniles among the estuarine systems.
3.3 Relationships between cubera snapper juveniles and estuary seascape
A total of 1715 cubera snapper juveniles were recorded from underwater sampling. The size classes of cubera snapper exhibited a range of 5 to 40 cm across all studied estuaries. Notably, five estuaries, Itariri (25.3%), Inhambupe (20.1%), Pojuca (14.5%), Jaguaripe (11%), and Serinhaém (9.6%) displayed the highest abundances of cubera snapper, with a pronounced peak in MaxN observed between March to June (Figure 6). In contrast, some estuaries did not exhibit a clear temporal pattern, and there were no discernible signals of peak fish abundance consistently associated with specific months. Estuary types significantly influence the abundance of cubera snapper juveniles; smaller individuals (Figure 6A) and overall fish abundance (Figure 6B) were both highest in small and directly connected to the ocean estuaries. However, estuary and mangrove preservation factors did not emerge as the main predictors in explaining the observed patterns (Table 4). Coastal development did not have a significant effect on records of cubera snapper juveniles (Table 4).
Figure 6
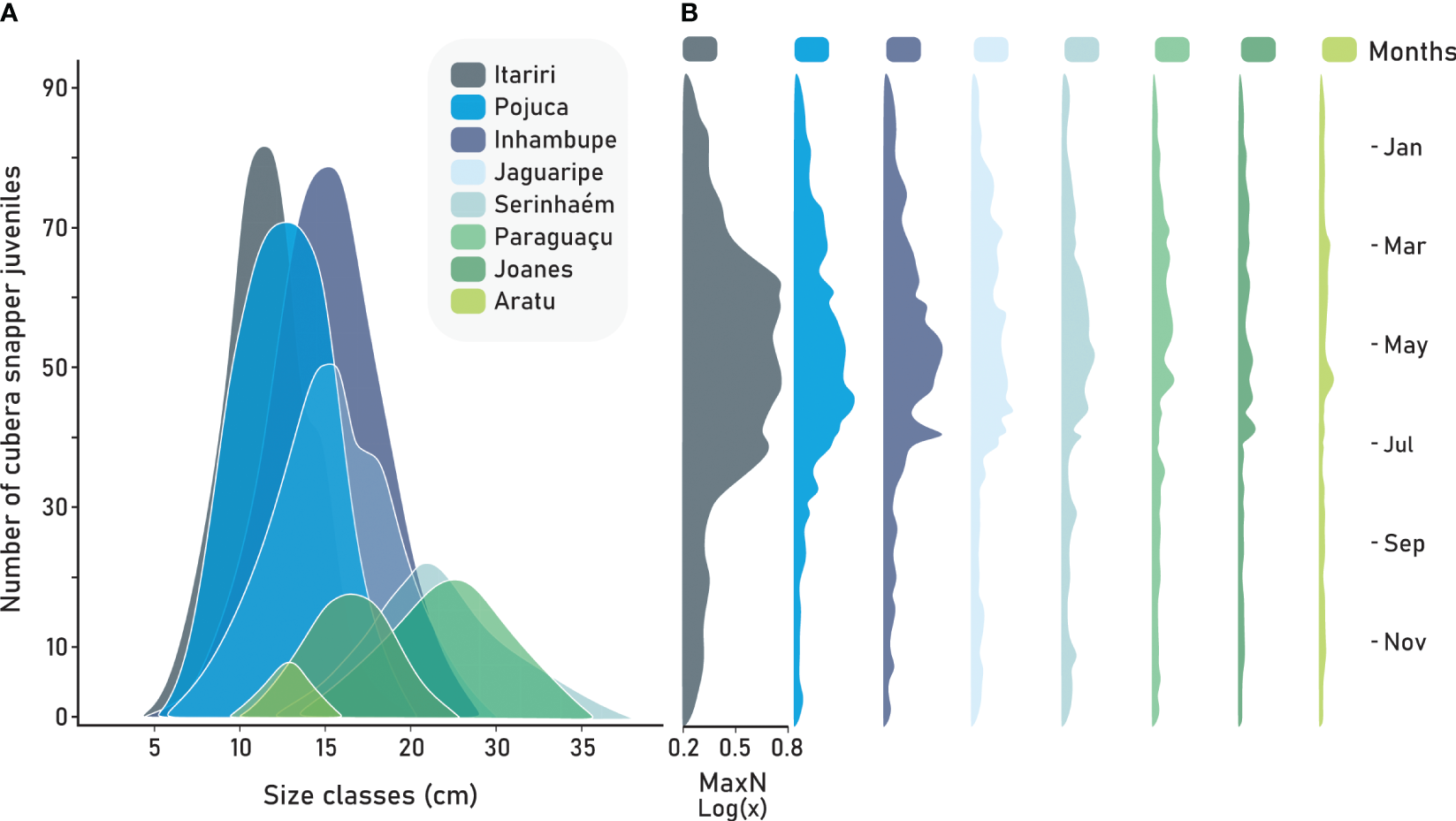
Variation in the number of cubera snapper juveniles based on size classes (cm) (A) and the variation in MaxN of cubera snapper throughout the year (B) across each study estuarine system.
Table 4
| Variable | Dev | p-value |
|---|---|---|
| Best fit model | ||
| Months | 85.89 | 0.003 |
| Estuary | 55.17 | 0.03 |
| Distance from the estuarine mouth (all sizes) | 101.15 | 0.002 |
| Distance from estuarine mouth (15-40 cm) | 110.85 | 0.001 |
| Distance from estuarine mouth (5-14 cm) | 115.16 | 0.001 |
| Direct connection with the ocean | 119.82 | 0.001 |
| Estuary profile × coastal development | 39.05 | 0.07 |
| Estuary profile × mangrove preservation | 58.71 | 0.03 |
| Estuary profile × drainage area (all sizes) | 95.04 | 0.001 |
| Estuary profile × drainage area (15-40 cm) | 81.44 | 0.004 |
| Estuary profile × drainage area (5-14 cm) | 100.48 | 0.001 |
Summary of multivariate generalized linear models (manyGLM) testing for the effects of estuary characteristics and sampling places on the presence and abundance of cubera snapper juveniles.
Values in bold indicate statistical significance (p< 0.05).
Furthermore, the total abundance of cubera snapper based on MaxN was also shaped by specific deployment positions of underwater records, with greater abundance observed in mangrove areas near the estuarine mouth (Figure 7A). Similarly, specific size classes (15-40 cm and 5-14 cm) also evidenced the same pattern (Figures 7C, E, respectively). Additionally, small estuaries with comparably smaller drainage areas exhibited the highest abundances for all size classes of cubera snapper (Figures 7B, D, F), and the monthly variation factor (Figure 6B) revealed that the period from March to June corresponded to the highest abundance, proving to be significant in the model. A summarized overview of the multivariate generalized linear model is provided in Table 4.
Figure 7
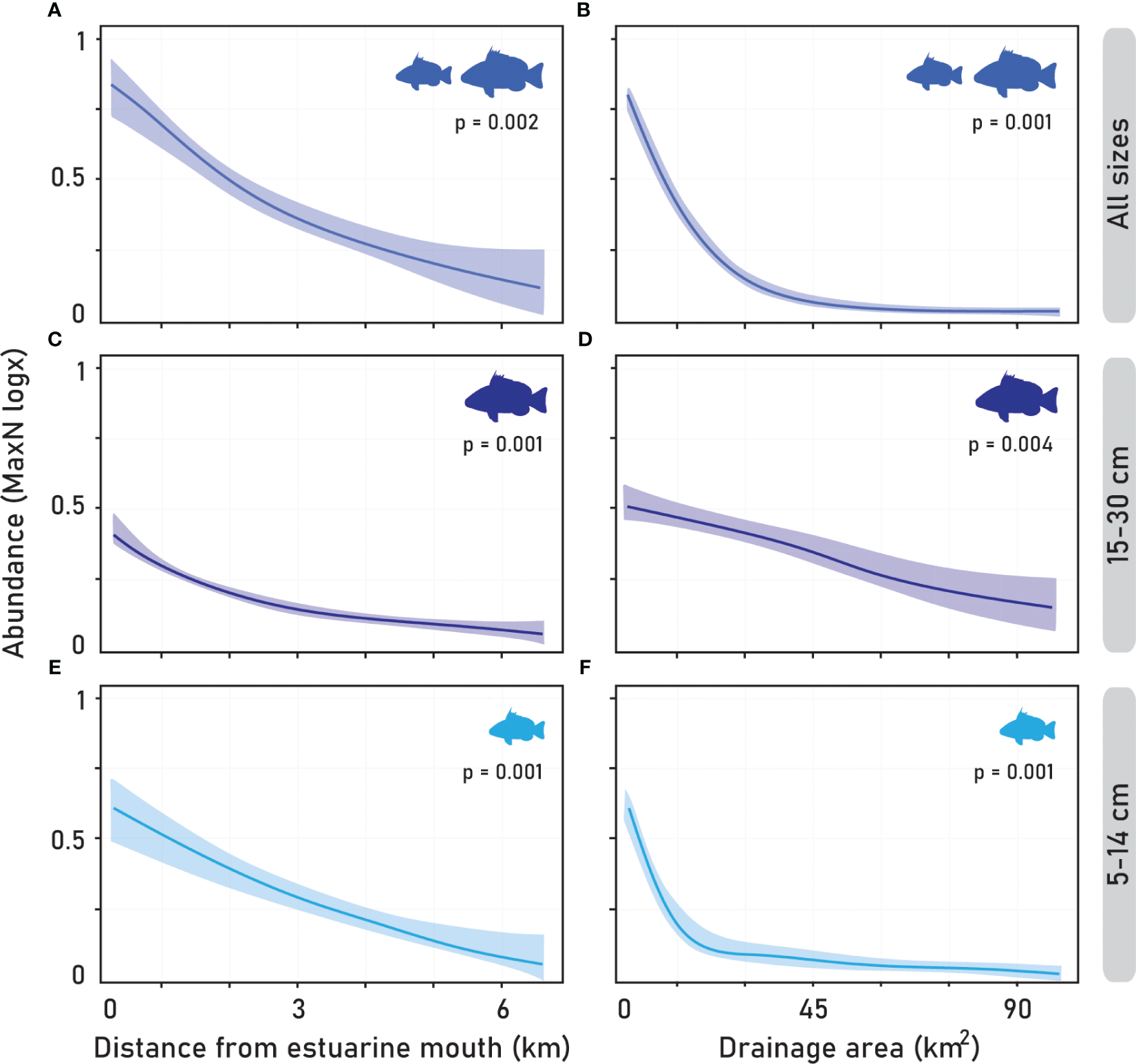
Multivariate generalized linear models (manyGLMs) using a negative binomial distribution with log link functions were used to show the effects of distance from the estuarine mouth regarding underwater deployments and drainage area on the abundance of cubera snapper juveniles. Shaded areas illustrate the 95% confidence intervals. (A, C, E) represent the relationships between size class abundance and distance from the estuarine mouth, while (B, D, F) represent the relationships with the drainage area of estuarine systems.
4 Discussion
Through an analysis of fishers’ perceptions and underwater records, our study combines knowledge from interviews with traditional fishers and aquatic footage to gain a comprehensive understanding of the significance of mangrove habitats and various estuarine systems to the early stages of cubera snapper. Although mangroves are gaining increased recognition as habitats that can enhance the growth and survival of immature reef fishes (e.g. Faunce and Serafy, 2006), few studies have been conducted at sufficient spatial and temporal scales to examine variation in reef fish utilization across different habitat types (but see Igulu et al., 2014). In this context, our empirical approaches afford a more nuanced examination of species reliance, using the cubera snapper as a case study within estuarine ecosystems. This analysis is guided by fisher knowledge and corroborated by underwater footage. Additionally, our findings indicate that both the outcomes derived from LEK and the assessment of various mangrove systems with respect to multiple stressors shed light on the perceived threats to populations of cubera snapper juveniles. The knowledge of local and indigenous communities worldwide has been considered an extremely rich and underused resource of information on how the environment, biodiversity, and local conditions are changing over time (Sobral et al., 2017; Turner et al., 2022). Herein, fisher respondents strongly expressed the presence of the cubera snapper in mangroves and their intensive use of this estuarine habitat.
Our findings combining both fishers’ perspectives and underwater records highlight the crucial role of mangroves as important nursery grounds for cubera snapper. Mangroves are frequently found in estuarine environments, and there is emerging evidence that estuarine mangroves tend to be more frequented by estuarine fish species compared to juvenile coral reef fishes, whereas the opposite is true for marine mangroves (Igulu et al., 2014; Lugendo et al., 2007). Nevertheless, our research findings concerning cubera snapper juveniles, a species typically associated with reef habitats in its adult stages, provide compelling evidence that estuarine mangrove habitats can be highly attractive and important for this particular species. These habitats offer a suitable environment that provides protection and abundant food resources for juvenile cubera snappers, thereby enhancing their survival and growth, particularly during this vulnerable life stage (Lindeman and De Maria, 2005). The positive correlation observed between the presence of mangroves and the occurrence and increased abundance of cubera snapper juveniles supports the importance of the mangrove habitats for the conservation of this species. These results have practical implications for our study sites and broader implications for methods and approaches to understanding the importance of mangrove and estuarine habitats as crucial nursery habitats for fish (Nagelkerken et al., 2015), including the cubera snapper. Resource selection studies, which compare the availability of a habitat type or patch to its use by animals, offer a means toward achieving habitat valuation under the assumption that animals will occupy sites that are best suited for their fitness (Thomas and Taylor, 2006). Based on the assumption that selective habitat use occurs when animals disproportionately utilize a particular habitat or patch compared to its availability or size (Manly et al., 2002), our study suggests that mangrove habitats can be considered attractive for cubera snapper juveniles. The presence of mangroves is likely to influence predation risk and foraging success in fish, as previously demonstrated (Reis-Filho et al., 2016; Dahlgren & Eggleston, 2000). By selectively utilizing mangrove habitats, cubera snapper juveniles may be seeking favorable conditions that enhance their survival and optimize their feeding opportunities.
Interestingly, our findings reveal that fishers’ fishing activities targeting cubera snapper juveniles were not a significant predictor of their occurrence. This suggests that fishing during the early stages may not have a substantial impact on the abundance of juveniles, although it is important to note that small-scale fisheries have been acknowledged to contribute to the reduction of mangrove fish abundance and richness (see Reis-Filho et al., 2018). However, fishers emphasized a range of major threats to cubera snapper juveniles, including mangrove removal, overfishing, water pollution, destructive fishing, and reduction of river flow, which are believed to contribute to the overall decline of this species, mainly in their early stages. These findings underscore the multifaceted nature of the challenges faced by cubera snapper populations. While these combined impacts may be associated with the broader range of impacts affecting many estuarine-dependent fish species (Airoldi et al., 2020; Bradley et al., 2023), it remains difficult to isolate specific mechanistic causes (Bradley et al., 2023). It is noteworthy that the specific threats reported varied across estuaries, influenced by differences in conservation status and coastal development pressure. However, certain estuarine characteristics, such as the dimension of the drainage areas (i.e., smaller estuaries) and direct connection to the ocean, appear to play a more positive role in the abundance of cubera snapper juveniles.
The unique combination of singular features in estuarine environments, along with dense mangrove forests, can provide a degree of protection from hydrodynamic forces and predation pressure (Pittman et al., 2007; Pavlov et al., 2008). These features may act as refugia for nursery species, including cubera snapper juveniles, allowing them to minimize energy expenditure while facilitating growth before migrating to definitive non-estuarine habitats, such as reefs. Small tropical estuaries and bays are claimed to serve as intermediate nursery areas for juveniles of estuary-associated marine fish which, after less than a year, have to migrate to nearby coastal systems to complete their life cycles (Magoro et al., 2019; Reis-Filho et al., 2019). However, further investigation is needed to fully understand the intricacies of these estuarine-specific relationships and their implications for the conservation and management of cubera snapper populations. The insights gained from such investigations can inform targeted conservation efforts and the development of management strategies aimed at preserving and enhancing the habitat conditions necessary for the long-term persistence of cubera snapper populations.
Landscape features within estuarine systems, such as roughness, plan curvature, and slope, have been found to influence estuarine fish assemblages and have even been associated with increased fish abundance in these systems (Borland et al., 2022). Moreover, urban-industrial estuarine seascapes can serve as abundant and dynamic fish habitats (Bradley et al., 2023), further complicating the association between degraded and depauperate areas and the status of fish communities. This diversity of responses challenges simplistic notions of negative anthropogenic effects and highlights the complex ways in which relationships are reordered across entire seascapes (Plaza and Lambertucci, 2017). For instance, despite facing significant coastal development pressures, the estuaries of Pojuca and Joanes rivers were still found to support substantial abundances of cubera snapper juveniles, indicating their suitability as a habitat for this species. However, predicting how physical-chemical and other coastal development factors might influence fish habitat use within mangroves poses a significant challenge. This prediction is contingent upon a complex interplay of local topographical, environmental, and anthropogenic factors, including the potential effects of climate change (Field, 1995).
Analysis of the data collected using fisher perception and underwater surveys results in very similar conclusions regarding the presence and mainly the dependence of the cubera snapper juveniles in mangrove and estuarine habitats. Both methods indicate the months with the highest abundance (April-July) for the cubera snapper juveniles present at the mangrove and estuarine habitats. However, the underwater surveys collect more specific data than fisher perception interviews about the length classes of the fish. In turn, the post-estuarine habitats and migratory patterns of cubera snapper juveniles remain uncertain, including the distance they travel and their ultimate destinations. However, researchers have recognized their residency as spawners in specific locations on the continental shelf, where significant fish aggregations occur during short-term periods (February - Biggs and Nemeth, 2015; Malafaia et al., 2020; January-Motta et al., 2022). A combination of factors likely influences their behavior, including spawning aggregation, the movement of eggs and larvae toward estuaries, initial growth stages, and subsequent residence of sub-adults and adults in the reef habitats on the continental shelf. This hypothesis is supported by the observed concentration of juveniles within estuarine habitats for a limited period (e.g., March to June), followed by a steeply decreasing in their abundance. Understanding these temporal patterns is crucial for effective conservation strategies, as they inform management measures, such as implementing fishing restrictions during critical early life stages.
It is acknowledged that the growth of the cubera snapper is rapid (see Burton and Potts, 2017). However, there remains a dearth of information regarding the younger stages of this species, mainly due to the limited availability of age-growth studies focusing on sub-adults to adults starting from age-5. This scarcity of young fish is a common issue in studies on cubera snappers, which primarily rely on fishery-dependent samples. Consequently, estimating growth curves for the youngest age groups becomes problematic (Burton and Potts, 2017). To address this concern, our findings help to shed light on the importance of estuaries and mangroves as critical habitats for cubera snapper juveniles and where focus on to obtain them. Nonetheless, it is important to consider variations in the dependence of cubera snapper juveniles on estuarine habitats based on their conservation status when making definitive statements in this regard. Based on our study, which employed a combination of survey methods including fisher perception and underwater records, we demonstrated that the environmental characteristic of each estuarine habitat played a significant role in predicting the occurrence and abundance of cubera snapper juveniles.
The data presented and discussed in this study represent the initial steps towards addressing these complex issues and toward the development of effective conservation and management strategies for the cubera snapper during its critical early life stages. As previously highlighted by Igulu et al. (2014), there appears to be worldwide trend of higher mangrove reliance by various members of the Lutjanidae family during their juvenile stages, regardless of their specific biogeographic locality. This trend may be linked to specific life-history traits exhibited by certain species within this fish family. To further advance our understanding and confirm these patterns, the next steps involve expanding the scope of research to encompass a greater number and variety of estuarine systems. Additionally, it is crucial to engage more fishing communities and recreational fishers in this endeavor to obtain a more comprehensive understanding of the extent of cubera snapper movement during their early growth stages as they transition to other habitats. Our study provides insights into the connections between various estuaries and their significance for cubera snapper juveniles, allowing for a nuanced understanding of the degrees of association with different estuarine systems (see summary in Figure 8). By examining factors such as habitat characteristics, fish abundance, and occurrence patterns, we have shed light on the varying levels of association that cubera snapper juveniles have with different estuarine environments. Although this species is often overlooked in terms of ecological research during its early stages due to the emphasis on larger individuals in commercial and recreational fisheries (Biggs and Nemeth, 2015; Giglio et al., 2020; Malafaia et al., 2020; Motta et al., 2022), it remains crucial to investigate these apex predators during their initial life phases. Moreover, comprehending their presence and association with estuarine and mangrove habitats is essential as it can influence the conservation status of the population during later stages, particularly those residing in reef ecosystems on the continental shelf.
Figure 8
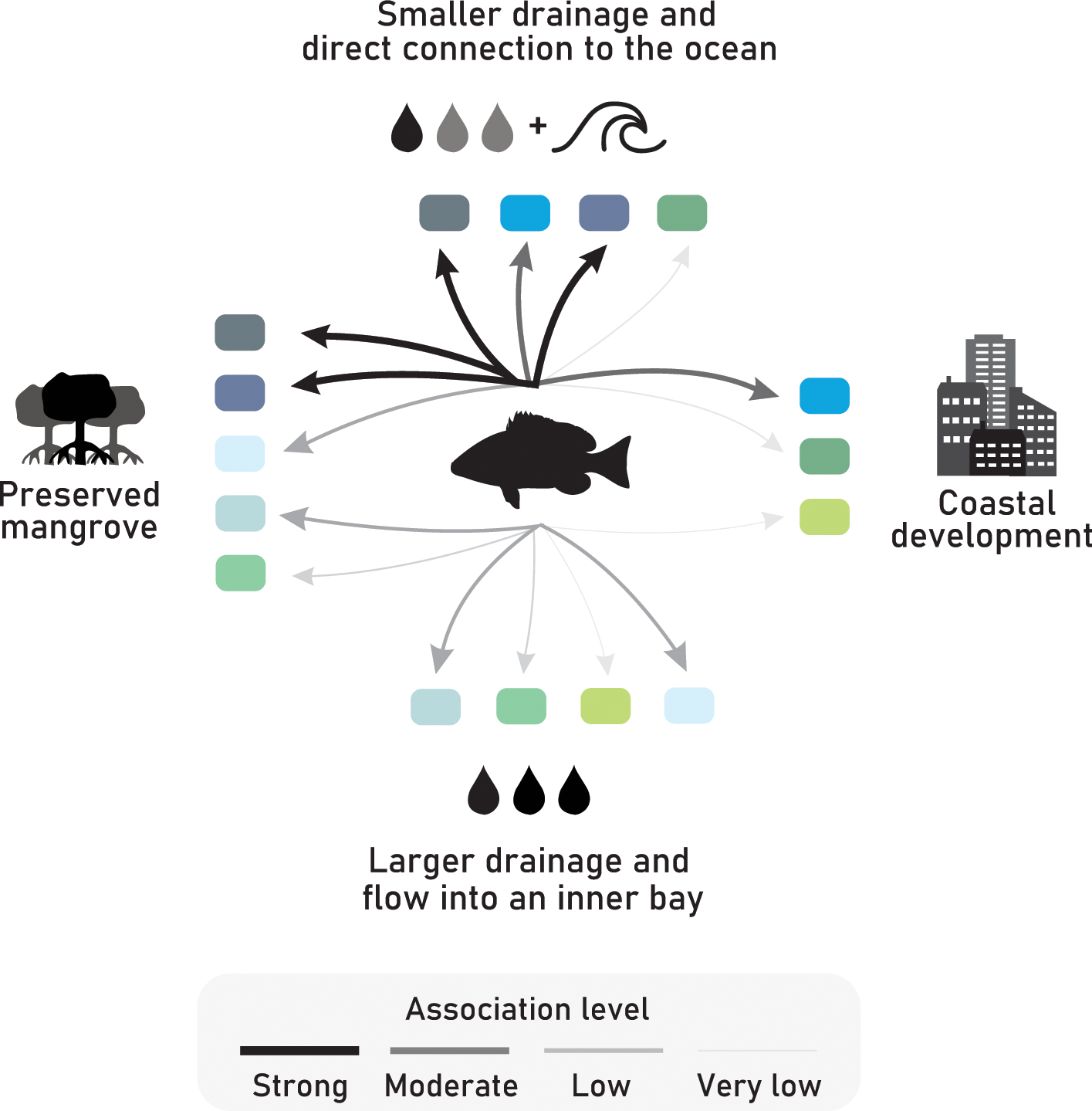
Scheme depicting the association level of cubera snapper juveniles with different estuarine systems based on their main environmental characteristics. Each estuarine system is represented by a rectangular shape, and the colors of the rectangles correspond to the colors used in Figures 5, 6 for easy reference.
5 Conclusion and remarks
In conclusion, our study offers valuable insights into both the perception of fishers and ecological records of the cubera snapper juveniles. It illuminates the factors influencing awareness and knowledge of this species, highlighting its reliance on mangrove habitats. Wider application of investigative methods such as those presented here could support robust project design in the future (Travers et al., 2019; Travers et al., 2021), and our methods could be easily adapted and scaled to gather context-specific data to inform management across other small-scale fisher systems or resource use contexts. The findings emphasize the crucial role of mangrove habitats in supporting the early life stages of cubera snapper, underscoring their importance as nursery grounds. Additionally, our results highlight the potential impacts of fishing activities on sub-adult and adult populations, suggesting the need for careful management strategies to ensure sustainable fishing practices. The temporal variations in the cubera snapper abundance observed in our study emphasize the dynamic nature of estuarine ecosystems and the need for continuous monitoring and adaptive management approaches. Furthermore, the identified threats, including mangrove degradation, overfishing, and pollution, call for targeted conservation efforts that address these specific challenges in different estuaries. Integrating indigenous and local knowledge with norms-based approaches can contribute to more effective conservation strategies and setting the base for co-management with fishers, enhancing the long-term sustainability of cubera snapper populations and the overall health of estuarine ecosystems.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Brazilian Institute of Environment and Renewable Nature Resources (IBAMA) (n 02001.006831/2008-76) and the Chico Mendes Institute for Biodiversity Conservation (ICMBio) through the Biodiversity Information and Authorization System (n 01/2012). It was also registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen) under n. A068F72. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FS: Investigation, Methodology, Writing – review & editing. EN-F: Formal Analysis, Methodology, Writing – review & editing. MG: Visualization, Writing – review & editing. FK: Formal Analysis, Methodology, Visualization, Writing – review & editing. ML: Writing – review & editing. TG: Conceptualization, Project administration, Supervision, Visualization, Writing – review & editing. JAR-F: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, but some authors (FRMS, EN-F, MLG, FWK, TG, and JAR-F) received scholarships during their education. Publication support was guaranteed from PAPQ/PROPESP 02/2023 program of Universidade Federal do Pará.
Acknowledgments
We thank all the members of the artisanal fishing communities that volunteered their time to this project, to ensure its success. This research wouldn’t have happened without their engagement. Our thanks to the editor and reviewers for the constructive comments and recommendations, which improved quality of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1292788/full#supplementary-material
References
1
Able K. W. Simenstad C. Strydom N. A. Bradley M. Sheaves M. (2022). “Habitat use and concectivity,” in Fish and Fisheries in Estuaries, A global perspective, vol. 1 . Eds. WhitfieldA. K.AbleK. W.BlaberS. J. M.ElliottM. (West Sussex, UK: Wiley Online Library), 188–254. doi: 10.1002/9781119705345.ch4
2
Adams A. J. Dahlgren C. P. Kellison G. T. Kendall M. S. Layman C. A. Ley J. A. et al . (2006). Nursery function of tropical back-reef systems. Mar. Ecol. Prog. Ser.318, 287–301. doi: 10.3354/meps318287
3
Airoldi L. Beck M. W. Firth L. B. Bugnot A. B. Steinberg P. D. Dafforn K. A. (2020). Emerging solutions to return nature to the urban ocean. Annu. Rev. Mar. Sci.13, 445–477. doi: 10.1146/annurev-marine-032020-020015
4
Armitage D. R. Plummer R. Berkes F. Arthur R. I. Charles A. T. Davidson-Hunt I. J. et al . (2008). Adaptive co-management for social–ecological complexity. Front. Ecol. Environ.7, 95–102. doi: 10.1890/070089
5
Baisre J. (2017). An overview of Cuban commercial marine fisheries: the last 80 years. Bull. Mar. Sci.94 (2), 359–375. doi: 10.5343/bms.2017.1015
6
Baker R. Sheaves M. (2009). Overlooked small and juvenile piscivores dominate shallow-water estuarine “refuges” in tropical Australia. Estuarine Coastal Shelf Sci.85, 618–626. doi: 10.1016/j.ecss.2009.10.006
7
Bates D. Mächler M. Bolker B. M. Walker S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software67 (1), 1–48. doi: 10.18637/JSS.V067.I01
8
Becker A. Coppinger C. Whitfield A. K. (2012). Influence of tides on assemblage and behaviors of fishes associated with shallow seagrass edges and bare sand. Mar. Ecol. Prog. Ser.456, 187–199. doi: 10.3354/meps09695
9
Berkes F. (2012). Sacred ecology. 4th Edition (New York: Routledge, Taylor & Francis Group). 392 pp. doi: 10.4324/9780203123843
10
Biggs C. R. Nemeth R. S. (2015). Timing, Size, and duration of a Dog (Lutjanus jocu) and Cubera Snapper (Lutjanus cyanopterus) Spawning Aggregation in the U.S. Virgin Islands. Christ Church, Barbados. Proceeding 67th Gulf Caribbean Fisheries Institute67, 241–245.
11
Borland H. P. Gilby B. L. Henderson C. J. Connoly R. M. Gorissen B. Ortodossi N. L. et al . (2022). Dredging fundamentally reshapes the ecological significance of 3D terrain features for fish in estuarine seascapes. Landscape Ecol.37, 1835–1400. doi: 10.1007/s10980-021-01394-5
12
Bradley M. Nagelkerken I. Baker R. Sheaves M. (2020). Context dependence: A conceptual approach for understanding the habitat relationships of coastal marine fauna. BioScience70 (11), 986–1004. doi: 10.1093/biosci/biaa100
13
Bradley M. Sheaves M. Waltham N. J. (2023). Urban-industrial seascapes can be abundant and dynamic fish habitat. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.1034039
14
Burton M. L. Potts J. C. (2017). Age, growth, and natural mortality of cubera snapper, Lutjanus cyanopterus, from the southeastern United States. Bull. Mar. Sci.93 (3), 815–828. doi: 10.5343/bms.2016.1116
15
Carpenter S. R. Mooney A. Agard J. Capistrano D. DeFries R. S. Díaz S. et al . (2009). Science for managing ecosystem services: beyond the Millennium Ecosystem Assessment. Proc. Natl. Acad. Sci.106, 1305–1312. doi: 10.1073/pnas.0808772106
16
Carvalho Costa H. S. Scachetti R. Vivacqua M. Choueri R. (2023). A framework to assess resilience atributes based on local knowledge. Mar. Policy, 155. doi: 10.1016/j.marpol.2023.105766
17
Chamberlain S. A. Bronstein J. L. Rudgers J. A. (2014). How context dependent are species interactions? Ecol. Lett.17, 881–890. doi: 10.1111/ele.12279
18
Cinner J. E. Folke C. Daw T. Hicks C. C. (2011). Responding to change: using scenarios to understand how socioeconomic factors may influence amplifying or dampening exploitation feedbacks among Tanzanian fishers. Global Environ. Change21 (1), 7–12. doi: 10.1016/j.gloenvcha.2010.09.001
19
Cirano M. Lessa G. C. (2007). Oceanographic characteristics of Baía de Todos os Santos, Brazil. Part I: circulation and seasonal variations described from in situ data. Rev. Bras. Geofísica25 (4), 363–387. doi: 10.1590/S0102-261X2007000400002
20
Claro R. Sadovy de Mitcheson Y. Lindeman K. C. Garca-Cagíde A. (2009). Historical analysis of Cuban commercial fishing effort and the effects of management interventions on important reef fishes from 1960–2005. Fisheries Res.99 (1), 7–16. doi: 10.1016/j.fishres.2009.04.004
21
Claydon J. A. B. Calosso M. C. De Leo G. A. Peachey R. B. J. (2015). Spatial and demographic consequences of nursery-dependence in reef fishes: an empirical and simulation study. Mar. Ecol. Prog. Ser.525, 171–183. doi: 10.3354/meps11245
22
Cooke S. J. Cook C. N. Nguyen V. M. Walsh J. C. Young N. Cvitanovic C. et al . (2023). Environmental evidence in action: on the science and practice of evidence synthesis and evidence-based decision-making. Environ. Evidence12, 10. doi: 10.1186/s13750-023-00302-5
23
Dagnelie P. (1998). Statistiques théoriques et appliquées (Bruxelles, Belgique: de Boeck et Larcier).
24
Dahlgren C. P. Eggleston D. B. (2000). Ecological processes underlying ontogenetic habitat shifts in a coral reef fish. Ecology81 (8), 2227–2240. doi: 10.2307/177110
25
Dahlgren C. P. Kellison T. Adams A. J. Gillanders B. M. Kendall M. S. Layman C. A. et al . (2006). Marine nurseries and effective juvenile habitats: concepts and applications. Mar. Ecol. Prog. Ser.312, 291–295. doi: 10.3354/meps312291
26
Dorenbosch M. Van Riel M. C. Nagelkernen I. van der Velde G. (2004a). The relationship of reef fish densities to the proximity of mangrove and seagrass nurseries. Estuarine Coast. Shelf Sci.60, 37–48. 10.1016/j.ecss.2003.11.018
27
Dorenbosch M. Verweij M. C. Nagelkernen I. Jid-Dawi N. van der Velde G. (2004b). Homing and day-time tidal movements of juvenile snappers (Lutjanidae) between shallow-water nursery habitats in Zanzibar, western Indian ocean. Environ. Biol. Fish70, 203–209. doi: 10.1023/B:EBFI.0000033336.10737.f5
28
Farmer N. A. Malinowski r.P. McGovern M. F. Rubec P. J. (2016). Stock complexes for fisheries management in the Gulf of Mexico. Mar. Coast. Fisheries8, 177–201. doi: 10.1080/19425120.2015.1024359
29
Faunce C. H. Serafy J. E. (2006). Mangroves as fish habitat: 50 Years of field studies. Mar. Ecol. Prog. Ser.318, 1–18. doi: 10.3354/meps318001
30
Faunce C. H. Serafy J. E. (2008). Selective use of mangrove shorelines by snappers, grunts, and great barracuda. Mar. Ecol. Prog. Series.356, 153–162. doi: 10.3354/meps07231
31
Field C. D. (1995). Impact of expected climate-change on mangroves. Hydrobiologia295, 75–81. doi: 10.1007/BF00029113
32
Fogliarini C. O. Ferreira C. E. L. Bornholdt J. Barbosa M. C. Giglio V. J. Bender M. G. (2021). Telling the same story: Fishers and landing data reveal changes in fisheries on the Southeastern Brazilian Coast. PloS One16 (6), e0252391. doi: 10.1371/journal.pone.0252391
33
Garson G. D. (2014). Logistic Regression: Binary and Multinomial (North Caroline, USA: Statistical Associates Publishing).
34
Giglio V. J. Suhett A. C. Zapelini C. S. Ramiro A. S. Quimbayo J. P. (2020). Assessing captures of recreational spearfishing in Abrolhos reefs, Brazil, through social media. Regional Stud. Mar. Sci.34, 100995. doi: 10.1016/j.rsma.2019.100995
35
Gilby B. L. Olds A. D. Connolly R. M. Yabsley N. A. Maxwell P. S. Tibbetts I. R. et al . (2017). Umbrellas can work under water: Using threatened species as indicator and management surrogates can improve coastal conservation. Estuar. Coast. Shelf Sci.199, 132–140. doi: 10.1016/j.ecss.2017.10.003
36
Hogarth P. J. (2015). The Biology of Mangroves and Seagrasses. 3rd Edtion (Oxford: Oxford University Press). 289 pp.
37
Igulu M. M. Nagelkerken I. Dorenbosch M. Grol M. G. G. Harborne A. R. Kimirei I. A. et al . (2014). Mangrove habitat use by juvenile reef fish: meta-analysis reveals that tidal regime matters more than biogeographic region. PloS One9 (12), e114715. doi: 10.1371/journal.pone.0114715
38
IUCN Red List of Threatened Species (2017) International Union for Conservation of Nature and Natural Resources. Available at: www.iucnredlist.org.
39
Kaliloski D. C. Satterfield T. (2004). On crafting a fisheries co-management arrangement in the estuary of Patos Lagoon (Brazil): opportunities and challenges faced through implementation. Mar. Policy28, 503–522. doi: 10.1016/j.marpol.2003.12.001
40
Kurien J. Willmann R. (2009). “Special considerations for small-scale fisheries management in developing countries,” in A Fishery Manager’s Guidebook. Eds. CochraneK. L.GarciaM. S. (Singapore: FAO, Singapore).
41
Leduc A. O. H. C. Hussey N. E. Lopes P. F. M. Reis-Filho J. A. (2023). Linking fair participatory monitoring, perception and compliance: A pathway for conserving a critically endangered fish. Biol. Conserv. (In press).
42
Lindeman K. C. De Maria D. (2005). Juveniles of the Caribeean’s largest coral reef snapper do not use reefs. Coral Reefs24, 359. doi: 10.1007/s00338-005-0015-3
43
Lugendo B. R. Nagelkerken I. Kruitwagen G. van der Velde G. Mgaya Y. D. (2007). Relative importance of mangroves as feeding habitats for fishes: A comparison between mangrove habitats with different settings. Bull. Mar. Sci.80, 497–512.
44
Magoro M. L. Perissinotto R. Wooldridge T. H. Whitfield A. K. (2019). Micro-estuaries and micro-outlets as incipient estuarine systems – Does size and coastal connectivity count? Sci. Total Environ.703 (4), 134707. doi: 10.1016/j.scitotenv.2019.134707
45
Malafaia P. N. França A. R. Olavo G. (2020). Spawning aggregation sites of the cubera snapper, Lutjanus cyanopterus, on the continental shelf of Bahia state, Northeastern Brazil. Fisheries Res.242, 106037. doi: 10.1016/j.fishres.2021.106037
46
Manly B. F. J. Mc.Donald L. L. Thomas L. D. Mc.Donald T. T. Erickson W. P. (2002). Resource Selection by animals: Statistical design and analysis for field studies (New York, Boston, Dordrecht, London, Moscow: Kluwer Academic Publishers). 220p.
47
Motta F. S. Freitas M. O. Rolim F. A. Abilhoa V. Pereira Filho G. H. (2022). Direct evidence of a spawning aggregation of cubera snapper (Lutjanus cyanopterus) in southeastern Brazil and its management implications. Fisheries Res.252, 106339. doi: 10.1016/j.fishres.2022.106339
48
Moura R. L. Francini-Filho R. B. Chaves E. M. Minte-Vera C. V. Lindeman. K. C. (2011). Use of riverine through reef habitat systems by dog snapper (Lutjanus jocu) in eastern Brazil. Estuar. Coast. Shelf Sci.95 (1), 274–278. doi: 10.1016/j.ecss.2011.08.010
49
Nagelkerken I. Sheaves M. Baker R. Connolly R. M. (2015). The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fisheries16, 362–371. doi: 10.1111/faf.12057
50
Nagelkerken I. van der Velde G. (2002). Do non-estuarine mangroves harbour higher densities of juvenile fish than adjacent shallow-water and coral reef habitats in Curaçao (Netherlands Antilles)? Mar. Ecol. Prog. Ser.245, 191–204. doi: 10.3354/meps245191
51
Nanninga G. B. Côte I. M. Beldade R. Mills S. C. (2017). Behavioural acclimation to cameras and observers in coral reef fishes. Ethology123 (10), 705–711. doi: 10.1111/eth.12642
52
Pavlov D. S. Mikheev V. N. Lupandin A. I. Skorobogatov M. A. (2008). Ecological and behavioural influences on juvenile fish migrations in regulated rivers: a review of experimental and field studies. Hydrobiologia609, 125–138. doi: 10.1007/s10750-008-9396-y
53
Pittman S. J. Caldow C. Hile S. D. Monaco M. E. (2007). Using seascape types to explain the spatial patterns of fish in the mangroves of SW Puerto Rico. Mar. Ecol. Prog. Ser.348, 273–284. doi: 10.3354/meps07052
54
Plaza P. I. Lambertucci S. A. (2017). How are garbage dumps impacting vertebrate demography, health, and conservation? Global Ecol. Conserv.12, 9–20. doi: 10.1016/j.gecco.2017.08.002
55
R.C. Team (2020). R: A language and environment for statistical computing (Version 4.0. 2) (R Foundation for Statistical Computing). Available at: http://www.r-project.org.
56
Reis-Filho J. A. Giarrizzo T. Barros F. (2016). Tidal migration and cross-habitat movements of fish assemblage within a mangrove ecotone. Mar. Biol.163 (111). doi: 10.1007/s00227-016-2885-z
57
Reis-Filho J. A. Giarrizzo T. Barros F. (2020). Stationary underwater cameras assess more efficiently clear-water mangrove fish assemblages: A comparison of non-extractive techniques. Mar. Ecol.41 (4), e12597. doi: 10.1111/maec.12597
58
Reis-Filho J. A. Harvey E. S. Giarrizzo T. (2018). Impacts of small-scale fisheries on mangrove fish assemblages. ICES J. Mar. Sci.1, 1–12. doi: 10.1093/icesjms/fsy110
59
Reis-Filho J. A. Miranda R. J. Sampaio C. L. S. Nunes J. A. C. C. Leduc A. O. H. C. (2021). Web-based and logbook catch data of permits and pompanos by small-scale and recreational fishers: predictable spawning aggregation and exploitation pressure. Fisheries Res.243, 106064. doi: 10.1016/j.fishres.2021.106064
60
Reis-Filho J. A. Schmid K. Harvey E. S. Giarrizzo T. (2019). Coastal fish assemblage reflect marine habitat connectivity and ontogenetic shifts in an estuary-bay-continental shelf gradient. Mar. Environ. Res.148. doi: 10.1016/j.marenvres.2019.05.004
61
Renk V. Ludwig D. Bolletin P. Reis-Filho J. A. Poliseli L. El-Hani C. N. (2023). Taking fishers’ knowledge and its implications to fisheries policy seriously. Ecol. Soc.28 (2). doi: 10.5751/ES-14104-280207
62
Ribeiro A. R. Damasio L. M. A. Silvano R. A. M. (2021). Fishers’ ecological knowledge to support conservation of reef fish (groupers) in the tropical Atlantic. Ocean Coast. Manage.204, 105543. doi: 10.1016/j.ocecoaman.2021.105543
63
Schmid K. Reis-Filho J. A. Loiola M. Harvey E. S. de Kikuchi R. K. P. Giarrizzo T. (2022). Habitat-specific fish fauna responses to different management regimes in the largest coral reef complex in the South Atlantic. Mar. Environ. Res.178. doi: 10.1016/j.marenvres.2022.105661
64
Sheaves M. Abrantes K. Barnett A. Benham C. Dale P. Mattone C. et al . (2020). The consequences of paradigm change and poorly validated science: The example of the value of mangroves to fisheries. Fish Fisheries21 (5), 1067–1075. doi: 10.1111/faf.12479
65
Sobral A. La Torre-Cuadros M.Á. Alves R. R. N. Albuquerque U. P. (2017). Conservation efforts based on local ecological knowledge: the role of social variables in identifying environmental indicators. Ecol. Indic.81, 171–181. doi: 10.1016/j.ecolind.2017.05.065
66
Tengo M. Austin B. J. Danielsen F. Fernádez-Llamazares A. (2021). Creating synergies between citizien science and indigenous and local knowledge. BioScience71, 503–518. doi: 10.1093/biosci/biab023
67
Thomas D. L. Taylor E. J. (2006). Study designs and tests for comparing resource use and availability. J. Wildlife Manage.70, 324–336. doi: 10.2307/3809050
68
Travers H. Selinske M. Nuno A. Serban A. Mancini F. Barychka T. et al . (2019). A manifesto for predictive conservation. Biol. Conserv.237, 12–18. doi: 10.1016/J.BIOCON.2019.05.059
69
Travers H. Walsh J. Vogt S. Clements T. Milner-gulland E. J. (2021). Delivering behavioural change at scale: what conservation can learn from other fields. Biol. Conserv.257, 109092. doi: 10.1016/j.biocon.2021.109092
70
Turner N. J. Cuerrier A. Joseph L. (2022). Well grounded: Indigenous Peoples’ knowledge, ethnobiology and sustainability. People Nat.4, 627–651. doi: 10.1002/pan3.10321
71
Wang Y. Naumann U. Wright S. T. Warton D. I. (2012). mvabund–an R package for model-based analysis of multivariate abundance data. Methods Ecol. Evol.3 (3), 471–474. doi: 10.1111/j.2041-210X.2012.00190.x
72
Warton D. I. Wright S. T. Wang Y. (2012). Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol. Evol.3 (1), 89–101. doi: 10.1111/j.2041-210X.2011.00127.x
Summary
Keywords
cubera snapper juveniles, indigenous knowledge, underwater footage, integrated knowledge, coastal development
Citation
da Silva FRM, Noleto Filho EM, Gallina ML, Keppeler FW, Loiola M, Giarrizzo T and Reis-Filho JA (2023) From fisher tales to scientific evidence: revealing the significance of estuarine and mangrove habitats as nursery grounds for juveniles of the largest Atlantic Ocean snapper. Front. Mar. Sci. 10:1292788. doi: 10.3389/fmars.2023.1292788
Received
12 September 2023
Accepted
04 October 2023
Published
20 October 2023
Volume
10 - 2023
Edited by
Tomaso Fortibuoni, Istituto Superiore per la Protezione e la Ricerca Ambientale (ISPRA), Italy
Reviewed by
Chiara Mancino, Sapienza University of Rome, Italy; Federica Poli, Anton Dohrn Zoological Station Naples, Italy
Updates
Copyright
© 2023 da Silva, Noleto Filho, Gallina, Keppeler, Loiola, Giarrizzo and Reis-Filho.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: José Amorim Reis-Filho, amorim_agua@yahoo.com.br
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.