
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 30 November 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1290879
 Morteza Yousefi1*
Morteza Yousefi1* Seyyed Morteza Hoseini2*
Seyyed Morteza Hoseini2* Maryam Ghadamkheir3
Maryam Ghadamkheir3 Heba H. Mahboub4
Heba H. Mahboub4 Yury Anatolyevich Vatnikov1
Yury Anatolyevich Vatnikov1 Evgeny Vladimirovich Kulikov1
Evgeny Vladimirovich Kulikov1 Elena Dmitriyevna Sotnikova1
Elena Dmitriyevna Sotnikova1Nano-encapsulation protects essential oils and increases their efficiency, compared to bulk forms. Hence in the present study, four diets (328 g/kg crude protein and 4402 kcal/kg gross energy) containing 0 (CTL), 25 mg/kg (25TV), 50 mg/kg (50TV), and 100 mg/kg (100TV) thyme, Thymus vulgaris, essential oil nano-liposomes (TV-NP) were offered to Nile tilapia fingerlings (initial weight of 4.27 ± 0.05 g) at water temperature of 26.46 ± 0.43°C, followed by intraperitoneal infection by Aeromonas hydrophila. Three hundred and sixty healthy fish were stocked in 12 tanks (60 L), 30 fish per tank, with daily water renewal rate of 40%. Each diet was offered to three tanks for 70 days. The fish were sampled at the end of feeding period and 12 h after the bacterial challenge. Compared to CTL, 50TV and 100TV treatments exhibited significant elevations in growth rate (14-17%; P<0.001), intestinal activities of amylase (9-19%; P=0.004), lipase (13-26%; P<0.001), protease (20-23%; P=0.001), and post-challenge survival (26-27%; P=0.001). Plasma lysozyme (14-15% P<0.001) and complement (5.1-5.4%; P=0.004) activities significantly increased in 25TV and 50TV, but decreased (lysozyme: 19%, complement 5.9%) in 100TV before the challenge; however, all TV-NP treatments showed similar lysozyme and complement activities after the challenge that were higher than CTL. 50TV and 100TV treatments also showed a decrease in lipid peroxidation (23-26%; P<0.001) and highest glutathione peroxidase activity (17-18%; P=0.001) and pre-challenge superoxide dismutase (21%; P=0.046) and catalase (15-17%; P=0.001) activities. Expression of tumor necrosis factor-alpha (11-fold, P<0.001), inerleukin-1 beta (5-fold, P<0.001), and transforming growth factor-beta (31-fold; P=0.001) in head kidney significantly increased in 100TV before the challenge. After the challenge, the transcripts of the cytokines significantly increased in all treatments and the highest expressions were observed in 50TV and 100TV treatments (62-148-fold). In conclusion, dietary 50-100 mg/kg TV-NP can be considered as a new feed additive in tilapia culture, as it improves growth rate, antioxidant capacity, and disease resistance in the fish.
Due to the ever-increasing population of the world, food security and protein consumption per capita are one of the most important global challenges. In this regard, aquaculture is one of the important food production industries with rapid growth during the recent decades (Subasinghe et al., 2009). Because of rapid growth and tolerating captivity, Nile tilapia, Oreochromis niloticus, is one of the important aquaculture species that is reared in many countries (Gabriel, 2019). Modern aquaculture is based on increasing the density of cultivation and production. But the increase in density causes stress in fish and the outbreak of various diseases (Akdemir et al., 2017). Aeromonas hydrophila is a gram-negative bacteria that can cause various diseases in fish, including Nile tilapia (Shirajum Monir et al., 2020). It is one of the most common pathogens in tilapia aquaculture and can cause significant economic losses. Some of the diseases caused by A. hydrophila in tilapia include hemorrhagic septicemia, fin rot, and skin ulcers. These diseases can lead to decreased growth rates, increased mortality, and reduced marketability of the fish (Noga, 2011).
The use of antibiotics to control livestock diseases is very limited due to environmental pollution, consumer health (Zargar et al., 2020; Yilmaz et al., 2022), and the emergence of resistant strains of various bacteria, including A. hydrophila (Li et al., 2019; Zdanowicz et al., 2020). For this reason, the main strategy in aquaculture is to increase the health and immunity of fish and prevent the occurrence of diseases. Improve in feed quality and using functional feeds can increase fish health and disease resistance (Favero et al., 2020). Various feed additives have been found to improve growth performance, antioxidant capacity, immune strength, and disease resistance in aquaculture fish (Lee et al., 2015). Among them, herbal additives have gained great attentions, because of their natural origin, growth promotion, immunostimulation, and pathogen elimination, which can augment fish health and decrease the use of antibiotics (Hoseinifar et al., 2020; Zhu, 2020).
Thyme, Thymus vulgaris, essential oil has been shown to have potential benefits in aquaculture and fish nutrition. It contains a range of bioactive compounds, including thymol, carvacrol, and p-cymene, which have antimicrobial, antioxidant, and anti-inflammatory properties (Silva et al., 2021). Studies have demonstrated that adding thyme essential oil/extract to fish feed can improve growth performance, antioxidant capacity, immune function, and resistance against diseases, including Aeromonas septicemia (Navarrete et al., 2010; Hoseini and Yousefi, 2019; Zargar et al., 2019; Khalil et al., 2020; Ghafarifarsani et al., 2022). One of the limitations of using essential oils in aquaculture is their stability, high light sensitivity and strong organoleptic characteristic (Luis et al., 2019). Nanotechnology is one of the approaches to resolve these problems. Various nanoparticles of essential oils have been found to improve fish growth performance, health, and disease resistance (Souza et al., 2017a; Souza et al., 2017b; Abdel-Tawwab et al., 2018; Sheikh Asadi et al., 2018; Gheytasi et al., 2021). It has been demonstrated that nano-emulsion of thyme essential oil can excrete anti-bacterial effects against A. hydrophila, both in vitro and in vivo in Nile tilapia, Oreochromis niloticus, concurrent with elevation of serum immunoglobulin M, lysozyme, and interleukin-1 beta (il-1b) and preventing mortality after an experimental bacterial challenge (Salam et al., 2021). Another studies revealed protection of Nile tilapia against Streptococcus iniae infection by dietary administration of nano-emulsion of thyme essential oil, which was accompanied by lower inflammation and stress (Korni et al., 2023). Hence, nanoparticle of thyme essential oil is a promising prophylactic agent against opportunistic bacterial pathogens.
Type of nanoparticle substantially affects its functions. Nano-liposomes are known for their protection of essential oils and slow release, which increase the essential oils anti-bacterial activity (Fajardo et al., 2022). Moreover, slow releasing results in the release of essential oil along the gastrointestinal tract, when nano-liposomes are orally administered. Thus, the present study aimed to extend the knowledge regarding the benefits of nanoparticles of thyme essential oil in Nile tilapia by applying nano-liposomes over a long time (70 d) and monitoring the fish growth performance, humoral innate immunity responses, hepatic antioxidant status, and head kidney immune-related genes’ expression, before and after infection with A. hydrophila.
First, 0.5 g of whey powder was poured into a 100-mL beaker and 7 mL of distilled water were added to it and placed in a bain-marie at a temperature of 50°C for 12 h and after cooling with phosphate buffer was adjusted to pH=6 (Lutz et al., 2009).
The organic phase was prepared by adding 0.1 g of lecithin to 1 mL of olive oil and mixing. The aqueous phase was prepared by adding 0.2 g of glycerol in 2 mL of distilled water and incubating for 2 h in a Bain-Marie (55°C). The organic phase was added drop-wise to the aqueous phase; then 60 ppm of thyme essential oil (purchased from Dr. Soleymani pharmacy, Gorgan, Iran) was added to it and the pH of the solution was adjusted to pH=6 with phosphate buffer (Mohammadi et al., 2016). Finally, the obtained solution was added to the whey powder solution and placed in an ultrasonic homogenizer for 5 min to prepare TV-NP. The product was powdered using a freeze-dryer. The characteristics of nano-liposomes were examined by scanning electron microscope (KYKY, EM-3200, China) and particle size analyzer (Malvern model MS1002, UK) and shown in Figure 1.
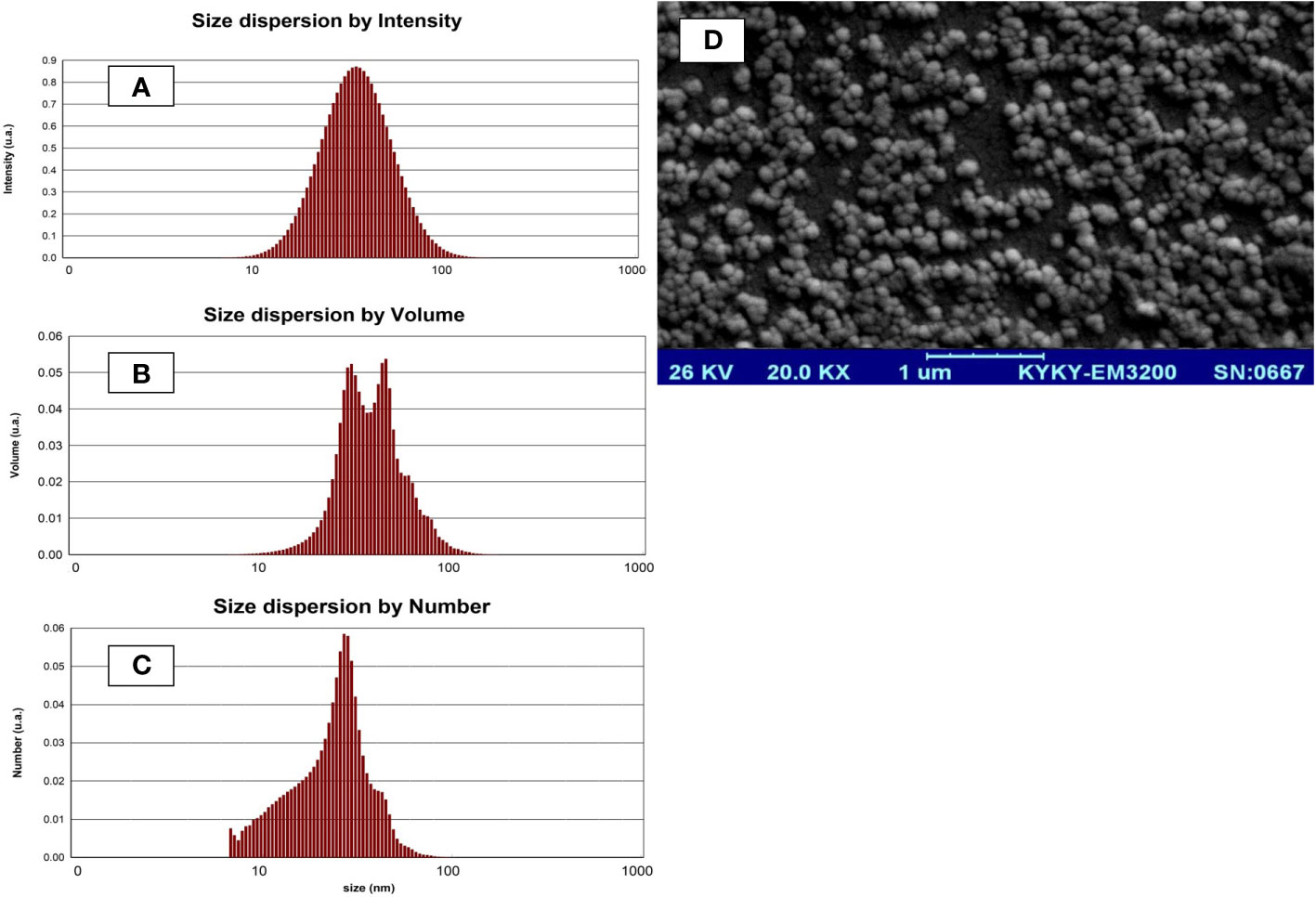
Figure 1 Particle size analysis (A-C) and scanning electron microscope photograph (D) of the nano-liposomes.
A control diet without TV-NP (CTL) and three diets containing 25 (25TV), 50 (50TV), or 100 (100TV) mg/kg TV-NP were prepared in this study (Besharat et al., 2021). Feedstuffs (Table 1) were mixed and pasted after moisturizing with 400 mL/g water. The paste was pelleted using a meat grinder and dried against a fan blow. The diets were analyzed according to AOAC (2005).
In this study, 360 Nile tilapia (initial weight of 4.27 ± 0.05 g) were purchased from a private sector (Kashan, Iran) and transferred to the laboratory (University of Gonbad-e-Kavoos, Gonbad, Iran). The fish were healthy, approved by no morphological abnormality and macroscopic signs of diseases (no wounds and patchy redness, normal coloration, and healthy fins). After 7 d acclimation in a 1 m3 tank and feeding with CTL diet, the fish were allocated into 12 tanks filled with 60 L water. Each of the above-mentioned diet was offered to three tanks for 70 d at a daily ration of 4% of biomass. All tanks were continuously aerated and their water was daily renewed by 40%. Water temperature, pH, dissolved oxygen, total alkalinity, and un-ionized ammonia were 26.46 ± 0.43°C, 7.64 ± 0.15, 6.70 ± 0.38 mg/L, 401.80 ± 9.31mg CaCO3/L, and 0.059 ± 0.016 mg/L, respectively. Feed amounts were adjusted every other week by measuring the tanks’ biomasses.
And the end of the feeding trial, fish final weight, specific growth rate (SGR), weight gain (WG), and feed conversion ratio (FCR) were calculated according to the following formula:
A. hydrophila (ATCC 7966) was obtained from the Iranian Biological Resource Center (Tehran, Iran) and cultured on TSA medium and suspended in 0.85% NaCl solution. Thirteen fish per tank were used for the bacterial challenge, and remaining fish were removed from the tanks and stocked in another 2000-L tank. The fish were anesthetized by eugenol (50 mg/L) and injected intraperitoneally. Each fish was injected by 250 µL of bacterial suspension with a cell density of 1 × 107 cell/mL. A batch of 10 fish from the CTL tanks were injected by 250 µL of phosphate buffer as negative control. The injected fish were returned to their corresponding tanks and mortality was daily observed at 6.00, 14.00, and 22.00 for 14 d.
At the end of the feeding trial, three fish from each tank were caught and anesthetized (50 mg/L eugenol). Blood samples were collected from the fish by caudal puncture, using heparinized syringes. Then, the fish were killed by spinal cord dissecting and pieces of anterior/middle intestine, liver, and head kidney were sampled and immediately frozen in liquid nitrogen. Then the remaining fish were challenged by A. hydrophila and blood, liver, and head kidney were sampled again 12 h after challenge.
The intestine samples were homogenized in cold buffer (phosphate buffer, pH 7.0) at a ratio of 1:3 (w:v). Enzyme extract was obtained by centrifugation at 4°C (13000 g; 15 min). Protease activity was determined based on AZO-casein method using a spectrophotometer as described before (Iversen and Jørgensen, 1995). Amylase activity was determined according to Winn-Deen et al. (1988), based on the decomposition of starch. Lipase activity was determined using 1, 2-O-dilauryl-rock-glycero-3-glutaric acid- (6-methyl-resorophine)-ester emulsion as substrate (Iijima et al., 1998). Soluble protein concentration was determined based on the Bradford method (Bradford, 1976).
The whole blood samples were centrifuged (5000 g, 7 min) to separate plasma; the plasma kept at -70°C until analysis. Plasma lysozyme activity was measured using Micrococcus luteus as the target. To 1 mL of the bacterium suspension (in phosphate buffer, pH 6.2) was added 30 µL of the plasma samples and decrease in optical density (550 nm) was recorded for 5 min. Each 0.001 decrease in optical density was considered as one unit of lysozyme activity (Ellis, 1990). Plasma alternative complement (ACH50) activity was determined as hemolytic capacity of the samples. Serially diluted plasma samples were mixed with sheep erythrocyte in EGTA-magnesium-veronal buffer containing gelatin. After 90 min incubation in room temperature, hemolysis rate was determined at 412 nm. ACH50 activity was calculated according to Yano (1992).
The hepatic samples were homogenized using a mortar and mixed with three volumes of cold phosphate buffer (pH 7.0). The mixture was then centrifuged at 4°C (13000 g; 15 min) and the supernatant was used for analysis. Commercial kits (Zellbio Co., Deutschland, Germany) were used for superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and malondialdehyde (MDA). SOD activity was determined according to hydroxylamine method. CAT activity was determined according to decomposition of hydrogen peroxide over time. GPx activity was determined based on the decomposition of hydrogen peroxide using reduced glutathione (GSH) and the reaction on the remaining GSH with dinitrobenzoic acid. Malondialdehyde (MDA) content was determined based on the reaction with thiobarbituric acid at 95°C. Soluble protein concentration was determined based on the Bradford method (Bradford, 1976).
A commercial kit was used for extraction of RNA (Denazist Co., Tehran, Iran), which was then treated by DNase I (Thermo Fisher Scientific, Waltham, MA, USA). The RNA quality was confirmed by agarose gel. Then, cDNA was synthetized using a commercial kit. Specific primers of tumor necrosis factor-alpha (tnf-a), il-1b, and transforming growth factor-beta (tgf-b) were used (Table 2) for gene expression assessments. RT-PCR method and a SYBER GREEN kit was used for quantification of RNA in the samples. Beta-actin (b-actin) was used as the housekeeping gene. Ct of the target and housekeeping genes were determined and used for gene expression analysis. The expressions of the genes were presented as fold changes relative to the CTL treatment, based on Livak and Schmittgen (2001).
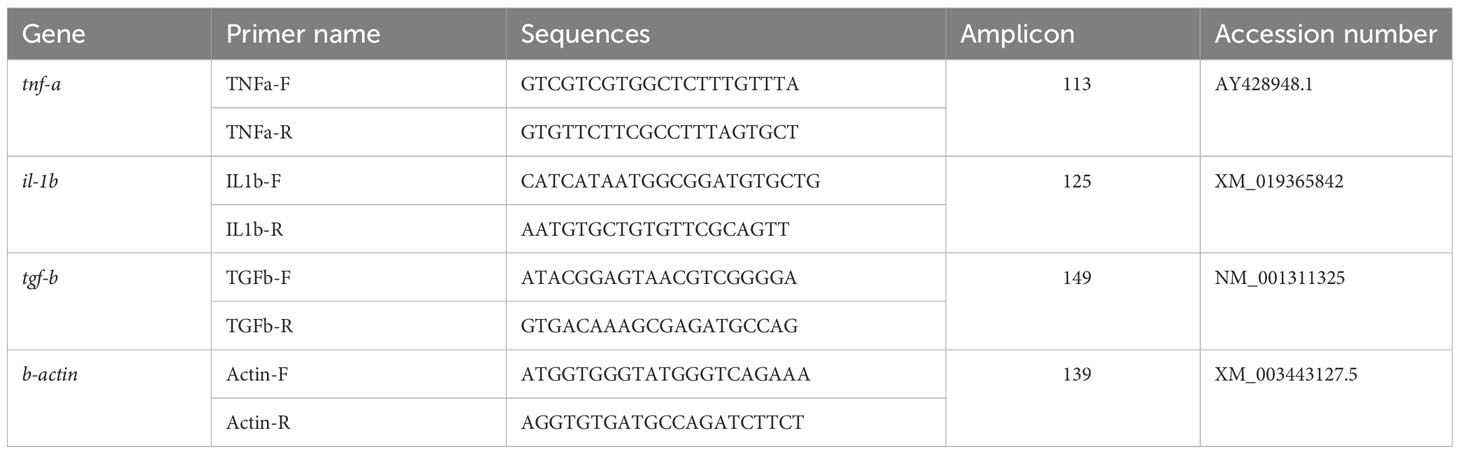
Table 2 Sequence, amplicon, and accession number of specific primers used for transcriptomic analysis.
Data of growth performance and digestive enzymes activities were analyzed by polynomial contrast analysis to find relationship between the TV concentration and the tested parameters. Among the models, linear was the most significant one, thus was used for all parameters. Significant differences among the treatments were determined by contrast analysis. Post-challenge survival data were arcsin-transformed and analyzed by contrast test to find significant differences among the treatments. Hepatic antioxidant, humoral immune-related, and kidney transcriptomic parameters were analyzed by repeated measure two-way ANOVA (time × dietary TV). Hepatic GPx and MDA exhibited no significant responses to the interaction of time and dietary TV levels, thus main effects of dietary TV were determined by contrast analysis. The other parameters exhibited significant responses to the interaction of time and dietary TV levels, thus pair comparisons were performed by contrast analysis to find significant differences among the treatment combinations. All analysis were performed in SPSS v.22 at the significance level of 0.05.
There were significant relationships between the dietary TV levels and fish final weight, weight gain, and SGR, but not FCR. These parameters were not significantly different between the CTL and 25TV treatments, which showed significantly lower values compared to 50TV and 100TV treatments. Weight gain and SGR were similar between the 50TV and 100TV treatments, but the later exhibited significantly higher final weight (Table 3).
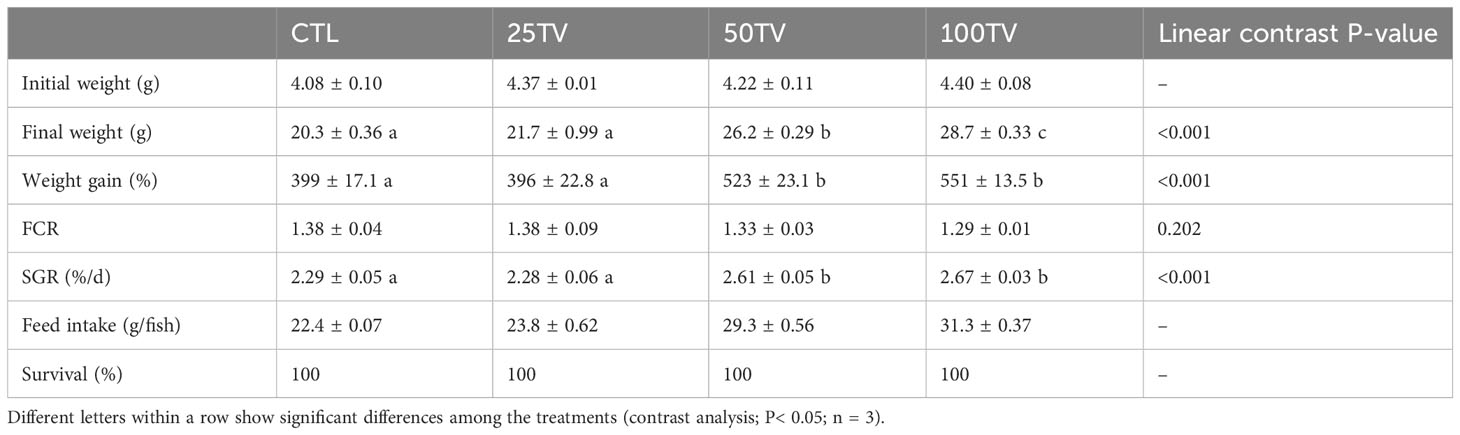
Table 3 Growth performance, feed efficiency, and survival of Nile tilapia after 70-d feeding with diets containing 0 g/kg (CTL), 25 mg/kg (25TV), 50 mg/kg (50TV), or 100 g/kg (100TV) TV-NP.
There were significant relationships between the dietary TV levels and intestinal amylase, lipase, and protease activities. These parameters were not significantly different between the CTL and 25Tv treatments, which showed significantly lower values compared to 50TV and 100TV treatments. 50TV and 100TV treatments exhibited the highest amylase and lipase activities, respectively; but there was no significant difference in protease activity between these treatments (Table 4).
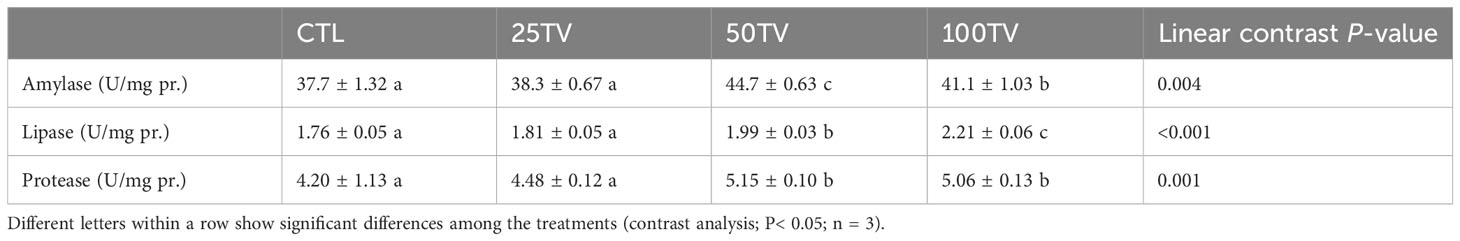
Table 4 Intestinal digestive enzymes’ activities of Nile tilapia after 70-d feeding with diets containing 0 g/kg (CTL), 25 mg/kg (25TV), 50 mg/kg (50TV), or 100 g/kg (100TV) TV-NP.
The negative control groups showed no mortality during the 14-d post-challenge. Post-challenge survival rates were significantly different among the treatments. Survival rates in CTL and 25TV were similar (53.3 and 56.7%, respectively) and significantly lower than 50TV and 100TV treatments (76.7 and 80.0%, respectively). There was no significant difference in the post-challenge survival rate between 50TV and 100TV treatments (Figure 2).
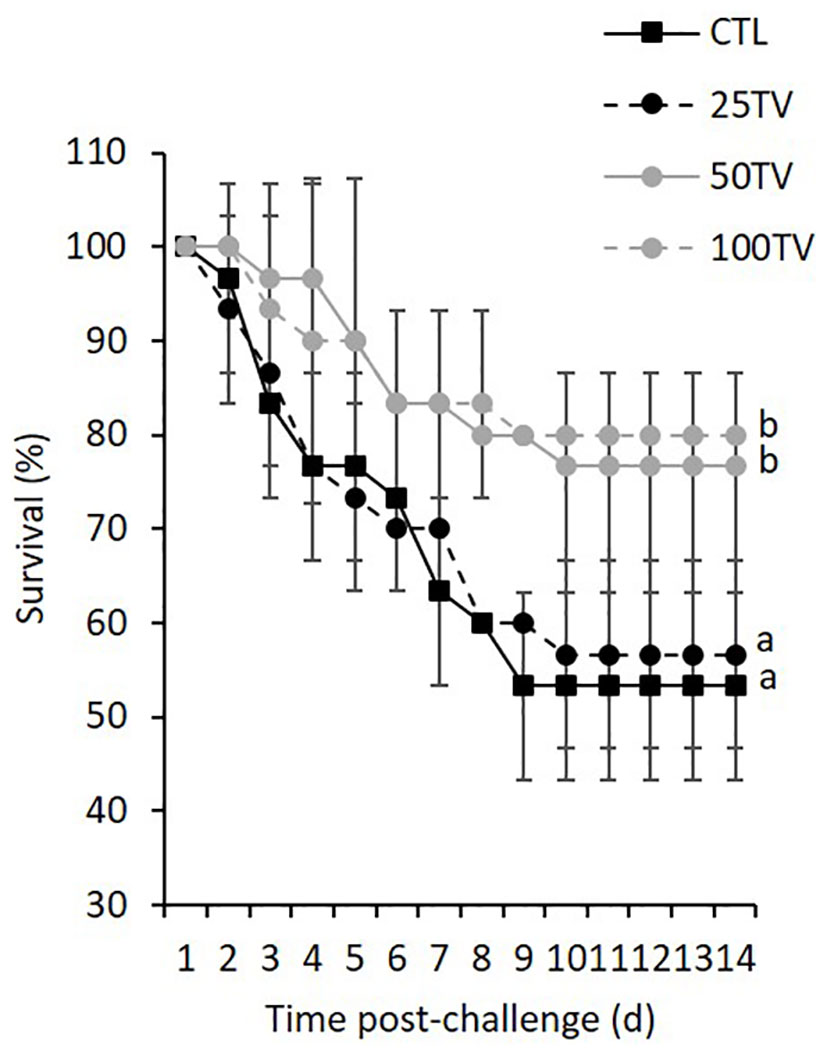
Figure 2 Survival of Nile tilapia after 70-d feeding with diets containing 0 g/kg (CTL), 25 mg/kg (25TV), 50 mg/kg (50TV), or 100 g/kg (100TV) TV-NP and challenged by A. hydrophila. Different letters show significant differences in cumulative mortality among the treatments, 14 day after the infection (contrast analysis; P< 0.05; n = 3).
There were interaction effects of dietary TV and bacterial challenge on the hepatic SOD and CAT activities. Before the challenge, 50TV and 100TV exhibited significantly higher SOD and CAT activities, compared to the CTL. The challenge significantly increased SOD activity in all treatments, but there were no significant differences among the dietary treatments after the challenge. CAT activities significantly increased in CTL and 25TV treatments, after the challenge. 50TV and 100TV exhibited significantly lower CAT activity, compared to CTL, after the challenge (Figure 2).
Dietary TV and bacterial challenge significantly affected the hepatic GPx activity and MDA concentration. Both parameters exhibited significant elevations after the challenge. TV-treated fish presented significantly high GPx activity and lower MDA levels, compared to the CTL. The highest GPx and lowest MDA values were observed in 50TV and 100Tv treatments (Figure 3).
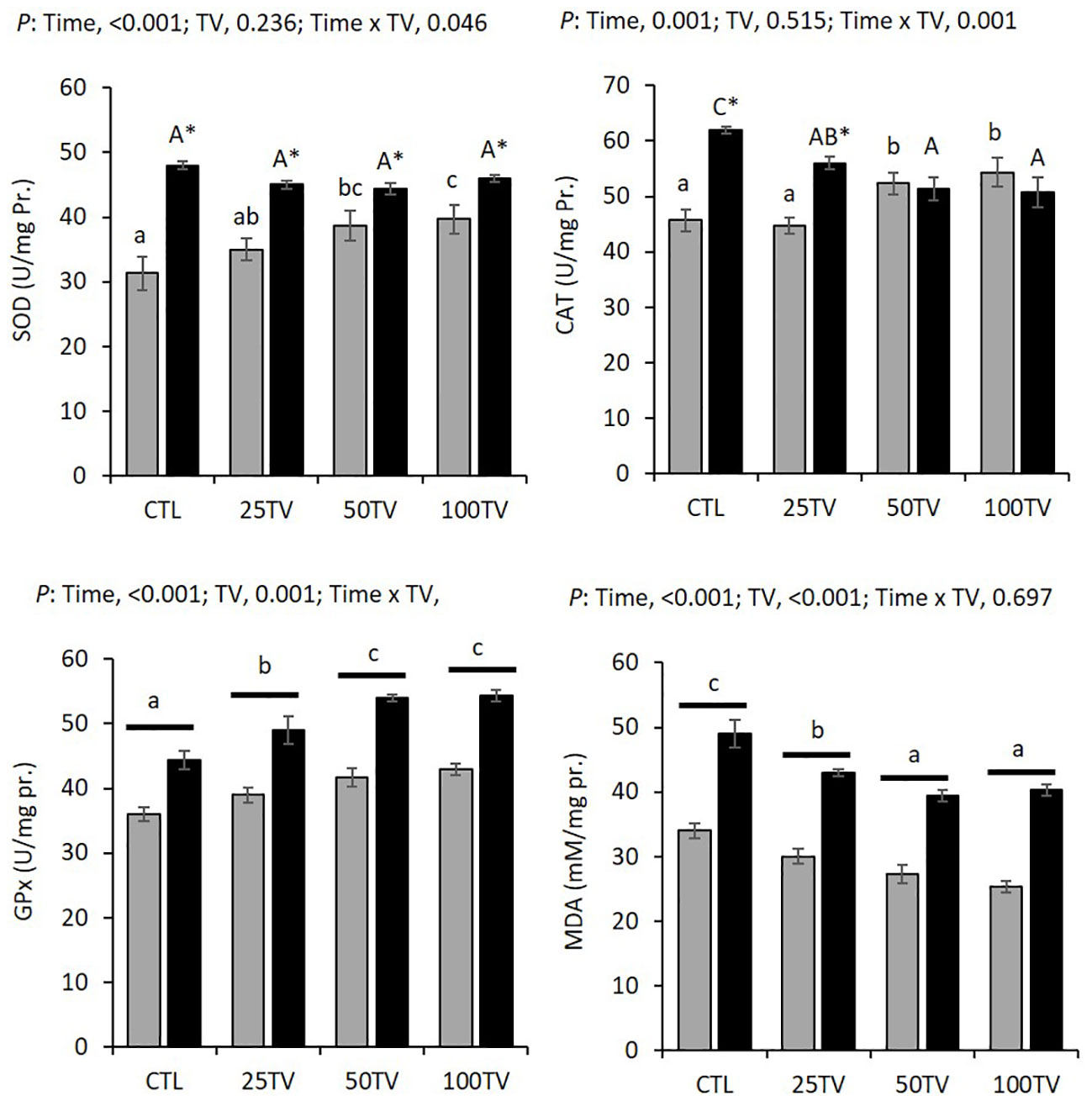
Figure 3 Hepatic antioxidant parameters of Nile tilapia after 70-d feeding with diets containing 0 g/kg (CTL), 25 mg/kg (25TV), 50 mg/kg (50TV), or 100 g/kg (100TV) TV-NP and challenged by A. hydrophila. Grey bars: pre-challenge; black bars: post-challenge. SOD and CAT: Different lowercase and uppercase letters above the bars show significant differences among the treatments, before challenge and after challenge, respectively. Asterisks show significant differences between pre-challenge and post-challenge (contrast analysis; P< 0.05; n = 3). GPx and MDA: Different letters above the bars show significant differences among the treatments (contrast analysis; P< 0.05; n = 3).
There were interaction effects of dietary TV and bacterial challenge on the plasma lysozyme and ACH50 activities. Before the challenge, 25TV and 50TV exhibited significantly higher lysozyme and ACH50 activities, whereas, compared to the CTL. At this time, 100TV exhibited significantly lower lysozyme and ACH50 activities, whereas, compared to the CTL. The challenge significantly decreased lysozyme activity in CTL and 50TV, had no significant effects on lysozyme activity in 25TV, and significantly increased lysozyme activity in 100TV treatments. The challenge induced no significant changes in ACH50 activities in CTL, 25TV, and 50TV, but significantly increased it in 100TV treatment. All TV-treated fish had similar plasma lysozyme and ACH50 activities, which were significantly higher than CTL, after the challenge (Figure 4).
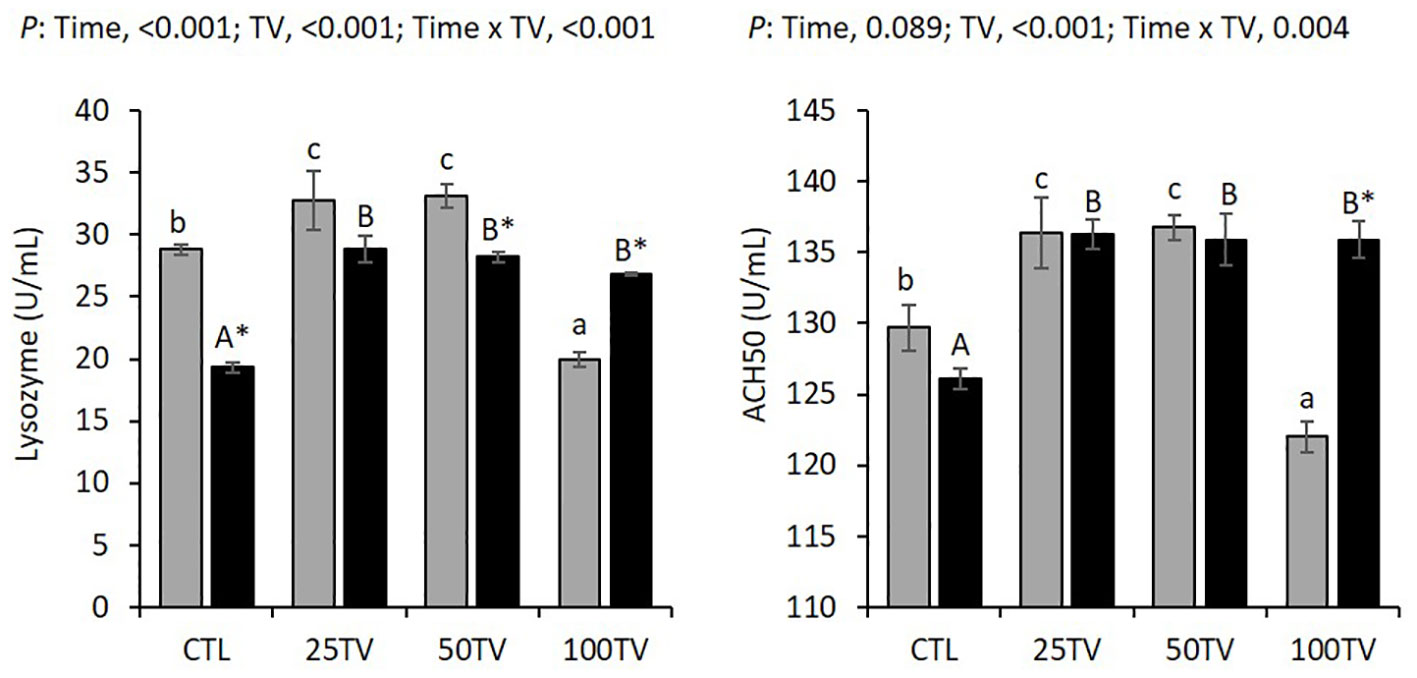
Figure 4 Plasma innate immune-related parameters of Nile tilapia after 70-d feeding with diets containing 0 g/kg (CTL), 25 mg/kg (25TV), 50 mg/kg (50TV), or 100 g/kg (100TV) TV-NP and challenged by A. hydrophila. Grey bars: pre-challenge; black bars: post-challenge. Different lowercase and uppercase letters above the bars show significant differences among the treatments, before challenge and after challenge, respectively. Asterisks show significant differences between pre-challenge and post-challenge (contrast analysis; P< 0.05; n = 3).
There were interaction effects of dietary TV and bacterial challenge on the head kidney tnf-a, il-1b, and tgf-b expressions. Before the challenge, 100TV treatment exhibited significantly higher expression of tnf-a, il-1b, and tgf-b, compared to CTL. The bacterial challenge significantly up-regulated the expression of these genes in all treatments and the highest expressions were observed in 50TV and 100TV treatments (Figure 5).
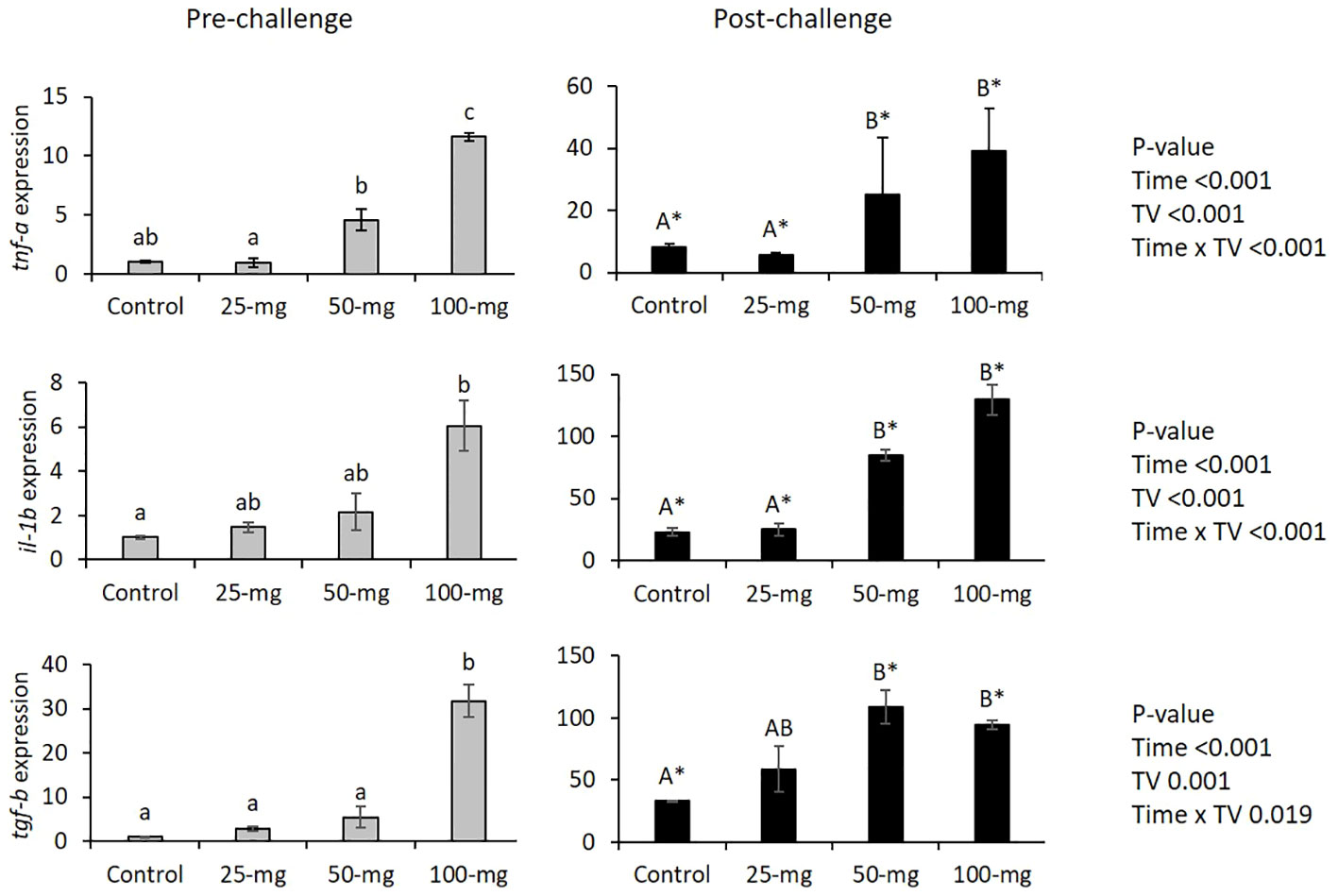
Figure 5 Head kidney expression of immune-related genes of Nile tilapia after 70-d feeding with diets containing 0 g/kg (CTL), 25 mg/kg (25TV), 50 mg/kg (50TV), or 100 g/kg (100TV) TV-NP and challenged by A. hydrophila. Grey bars: pre-challenge; black bars: post-challenge. Different lowercase and uppercase letters above the bars show significant differences among the treatments, before challenge and after challenge, respectively. Asterisks show significant differences between pre-challenge and post-challenge (contrast analysis; P< 0.05; n = 3).
Nano-encapsulation is a reliable method to protect essential oils against degradation and enhance their efficiency in fish nutrition. This study showed that dietary 50-100 mg/kg TV-NP can improve growth rate in Nile tilapia. There is no study on Nile tilapia assessing the effects of nanoparticles of thyme essential oil on growth performance of this species. However, 10 g/kg bulk essential oil of thyme was shown to improve growth performance of Nile tilapia under normal conditions or exposed to waterborne copper (Ahmed et al., 2022). Furthermore, adding 10-20 g/kg thyme meal to diet resulted in no change in growth performance of Nile tilapia (Zaki et al., 2012; Khalil et al., 2020) and Mozambique tilapia, Oreochromis mossambicus (Yılmaz et al.). Considering the essential oil yield of thyme (Alavi-Samani et al., 2015), these concentrations of thyme meal correspond to 83-166 mg/kg thyme essential oil, which are close to the concentrations used in this study. Thus, it is speculated that nano-liposome technique can improve the benefits of thyme in Nile tilapia. However, other differences between the TV-NP and thyme meal should not be neglected and further studies are needed to address this topic. The present results are in line with a study assessed the effects of cinnamon nanoparticle on growth performance of Nile tilapia, which found growth promotion at 3 g/kg dietary nanoparticle (Abdel-Tawwab et al., 2018).
Increase in digestive enzymes’ activity can be a reason for growth promotion in fish fed supplemented diets. It is supposed that increase in the activity of these enzymes leads to better digestion. It has been shown that thyme essential oil improves growth performance concurrent with increase in the intestinal activity of digestive enzymes in common carp, Cyprinus carpio (Ghafarifarsani et al., 2022), which supports the present finding. The exact mechanisms of elevation of digestive enzymes are not clear, but it could be a direct effects of TV-NP on enzyme synthesis and release from pancreas, which needs further investigations. Moreover, intestinal microbes are another source of digestive enzymes and TV-NP might change the composition of microbiota, considering the antimicrobial effects of thyme ingredients (Sienkiewicz et al., 2012). But this topic needs to be investigated in fish.
Bacterial infections induce severe oxidative stress caused by reactive oxygen/nitrogen species. This leads to activation of the antioxidant system to protect host cells. The nuclear factor erythroid 2-related factor 2 (Nrf2)/Kelch-like ECH-associated protein 1 (Keap1) signaling pathway responds to oxidative stress and regulates the antioxidant system (Elbialy et al., 2023). Infection of fish with A. hydrophila has been reported to down-regulate nrf2 transcript and increase reactive oxygen species concentrations in the kidney (Zang et al., 2020). A study on Nile tilapia has reported decreases in SOD and GPx activities accompanied by an increase in MDA (lipid peroxidation) concentration after A. hydrophila infection (Moustafa et al., 2020), suggesting the induction of oxidative stress due to weakness of the enzymatic antioxidant system. However, lipid peroxidation has been observed in Nile tilapia and largemouth bass, Micropterus salmoides, infected with A. hydrophila, along with increase in SOD and/or CAT; increase in these enzymes’ activities have been accompanied by higher post-infection survival (Gong et al., 2019; Kuebutornye et al., 2020). Thyme essential oil mainly contains thymol and carvacrol, two well-known antioxidants (Giannenas et al., 2012; Hashemipour et al., 2013). Dietary supplementation of 10-20 g/kg of this essential oil has been found to improve antioxidant capacity and reduce lipid peroxidation in rainbow trout, Oncorhynchus mykiss (Sönmez et al., 2015), and common carp (Ghafarifarsani et al., 2022); the later also exhibited improved survival after A. hydrophila infection. This suggests TV-NP may be more efficient than non-protected essential oil in strengthening the antioxidant system. In the present study, the highest post-infection survivals were accompanied by lower lipid peroxidation (MDA levels), and elevations in GPx, andpre-infection SOD/CAT activities. Thus, antioxidant-stimulating capacity of TV-NP can be considered as one of the mediators of improved resistance against A. hydrophila infection. Interestingly, CAT activities in the 50TV and 100TV treatments were lower than CTL, despite lower lipid peroxidation in these treatments. CAT decomposes hydrogen peroxide at high concentrations, but low concentrations of hydrogen peroxide are detoxified by GPx (Spolarics and Wu, 1997; Rocha et al., 2015; Djordjević et al., 2022); thus, it can be speculated that 50TV and 100TV experienced lower concentration of hydrogen peroxide that led to lower lipid peroxidation.
Plasma lysozyme is a component of the soluble humoral immune system that attacks both gram-positive (directly) and gram-negative (in contribution with other actors) bacteria (Song et al., 2021). The decrease in the plasma lysozyme activity after the challenge may be as a result of the production of lysozyme inhibitors by A. hydrophila. Gram-negative bacteria have an ability to escape from lysozyme attack by producing periplasmic proteins that inactivate lysozyme (Liu et al., 2015) and A. hydrophila has been found possessing periplasmic lysozyme inhibitors (Leysen et al., 2011). Similar to the present results, El-Houseiny et al. (2021) found decrease in lysozyme activity in Nile tilapia seven days after an experimental infection with A. hydrophila. Wuchang bream, Megalobrama amblycephala, showed similar decrease in lysozyme activity 48 h after an experimental infection with A. hydrophila (Liu et al., 2012). It was shown that thyme essential oil can increase serum and mucosal lysozyme activities in rainbow trout (Zargar et al., 2019) and common carp (Ghafarifarsani et al., 2022), which support the present results. However, the present results show that pre-challenge plasma lysozyme activity is not a suitable predictor of survival after A. hydrophila infection, as shown in 100TV treatment. This result is similar to that obtained in rainbow trout (Zargar et al., 2019).
Complement proteins have various roles in the fish innate and adaptive immune defenses such as opsonization, cell lysis, B cell activation, and inflammation (Bavia et al., 2022). Pathogens possess adaptive mechanisms to escape from the complement system. A. hydrophila has metaloproteins that degrade the C3 component of the complement system in fish, thus inhibit alternative complement responses (Chen et al., 2019). Such decrements in ACH50 activity have been reported in Nile tilapia El-Houseiny et al. (2021) and olive barb, Systomus sarana (Das et al., 2011), respectively, 7 and 2 d after infection with A. hydrophila. Similar results have been reported for other gram negative pathogens;<24 h post-infection with Flavobacterium columnare (Ravindra et al., 2019) or 48 h post-infection with Edwardsiella tarda (Mohanty and Sahoo, 2010). In the present study, there was a decrease in ACH50 activity in CTL treatment after the infection, but not statistically significant. This might be related to the sampling time, as the above studies show that ACH50 response to infection is time-dependent. Thus, it is speculated that A. hydrophila may suppress ACH50 activity in Nile tilapia, but further studies are needed for confirmation. Essential oil of thyme has been found to increase mucosal ACH50 in common carp (Ghafarifarsani et al., 2022) and C3 mRNA in rainbow trout (Zargar et al., 2019), but none of them were predictor of survival after A. hydrophila infection. The present results also confirmed this, as 100TV had the lowest ACH50 activity before challenge but expressed the highest survival after the challenge.
Inflammation is critical for proper immune function and pathogen clearance in fish. Pro-inflammatory cytokines are the mediators of inflammation with a wide range of effects. Tumor necrosis factor-alpha and il-1b are two major pro-inflammatory cytokines that respond at early stages on infection (reviewed by Zou and Secombes (2016)). They have diverse but overlapping functions in fish including stimulating resting macrophages to actively explore foreign germs and engulf them. Interleukin-1 beta acts as a chemo-attractant for leukocyte and tnf-a increases macrophage survival after phagocytosis (Zou and Secombes, 2016). Studies have shown that increase in these two cytokines (protein or transcript) is necessary for resistance against A. hydrophila in fish (Das et al., 2011; Gong et al., 2019; Zhang et al., 2020), including Nile tilapia (Moustafa et al., 2020; El-Houseiny et al., 2021). Furthermore, it has been shown that A. hydrophila-resistance Nile tilapia expresses higher tnf-a and il-b transcript, compared to non-resistance individuals (El-Magd et al., 2019), suggesting the importance of these cytokines in Aeromonas septicemia control. Transforming growth factor-beta has been traditionally known as an anti-inflammatory and immunosuppressive cytokine (Maehr et al., 2013). But recent findings have demonstrated that this cytokine has active roles in immune responses and disease resistance (Qi et al., 2016). Head kidney leukocytes of Nile tilapia have exhibited up-regulations in tgf-b transcript, following lipopolysaccharide and poly I:C stimulations (Zhan et al., 2015). Macrophages isolated from goldfish, Carassius auratus, head kidney exhibited up-regulations in tgf-b transcript in response to lipopolysaccharide and recombinant TNF-a stimulations (Haddad et al., 2008). It seems that tgf-b controls inflammation induced by pro-inflammatory cytokines like tnf-a and il-1b, which has been confirmed in Nile tilapia (Han et al., 2020) and other species such as common carp (Zhang et al., 2017), obscure puffer, Takifugu obscurus (Liu et al., 2020), and largemouth bass (Gong et al., 2019). The present results are supported by these studies. Thyme essential oil has been found up-regulating il-1b transcript in head kidney of rainbow trout that resulted in higher post-infection (A. hydrophila) survival, suggesting that the essential oil may improve immune response against the disease by inducing inflammation. But, this has not been always the case, as administration of thyme essential oil and its nano-emulsion resulted in lower transcript of tnf-a and higher tgf-b in head kidney, accompanied by lack of mortality after an experimental streptococcusis in Nile tilapia. Thus, the present results show that TV-NP improves survival of Nile tilapia by stimulating pro-inflammatory cytokines that improve cell-mediated immunity, but at the same time, induces anti-inflammatory cytokine (tgf-b) to control detrimental effects inflammation on host cells.
In conclusion, TV-NP at 50-100 mg/kg successfully promoted growth rate of Nile tilapia, which seems to be related to increase in the intestinal digestive enzymes. Moreover, these concentrations improved the fish survival after challenge with A. hydrophila. Based on the results, improved disease resistance was likely related to improved antioxidant function and cytokine responses after infection.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by Bioethics Commission of Agrarian and Technological Institute, RUDN University (Protocol № 12 dated November 14, 2022. The study was conducted in accordance with the local legislation and institutional requirements.
MY: Conceptualization, Data curation, Funding acquisition, Supervision, Writing – review & editing. SMH: Conceptualization, Formal Analysis, Project administration, Writing – original draft. MG: Formal Analysis, Methodology, Resources, Writing – original draft. HHM: Conceptualization, Data curation, Methodology, Writing – review & editing. YAV: Conceptualization, Supervision, Writing – original draft. EVK: Data curation, Methodology, Visualization, Writing – original draft. EDS: Formal Analysis, Methodology, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This publication has been supported by the RUDN University Scientific Projects Grant System, project № “202196-2-000”.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Tawwab M., Samir F., Abd El-Naby A. S., Monier M. N. (2018). Antioxidative and immunostimulatory effect of dietary cinnamon nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to hypoxia stress and Aeromonas hydrophila infection. Fish Shellfish Immunol. 74, 19–25. doi: 10.1016/j.fsi.2017.12.033
Ahmed S. A. A., Ibrahim R. E., Elshopakey G. E., Khamis T., Abdel-Ghany H. M., Abdelwarith A. A., et al. (2022). Immune-antioxidant trait, growth, splenic cytokines expression, apoptosis, and histopathological alterations of Oreochromis niloticus exposed to sub-lethal copper toxicity and fed thyme and/or basil essential oils enriched diets. Fish Shellfish Immunol. 131, 1006–1018. doi: 10.1016/j.fsi.2022.11.013
Akdemir F., Orhan C., Tuzcu M., Sahin N., Juturu V., Sahin K. (2017). The efficacy of dietary curcumin on growth performance, lipid peroxidation and hepatic transcription factors in rainbow trout Oncorhynchus mykiss (Walbaum) reared under different stocking densities. Aquac. Res. 48, 4012–4021. doi: 10.1111/are.13223
Alavi-Samani S. M., Kachouei M. A., Pirbalouti A. G. (2015). Growth, yield, chemical composition, and antioxidant activity of essential oils from two thyme species under foliar application of jasmonic acid and water deficit conditions. Hortic. Environ. Biotechnol. 56, 411–420. doi: 10.1007/s13580-015-0117-y
AOAC (2005). Official methods of analysis of the Association of Official Analytical Chemists (Washington, DC, USA: Association of Official Analytical Chemists).
Bavia L., Santiesteban-Lores L. E., Carneiro M. C., Prodocimo M. M. (2022). Advances in the complement system of a teleost fish, Oreochromis niloticus. Fish Shellfish Immunol. 123, 61–74. doi: 10.1016/j.fsi.2022.02.013
Besharat M., Rajabi Islami H., Soltani M., Abdolmajid Mousavi S. (2021). Effect of different levels of nanoliposome-coated astasxanthin on growth performance, body proximate composition, liver enzyme activity and pigmentation of rainbow trout (Oncorhynchus mykiss). Aquac. Res. 52, 5069–5077. doi: 10.1111/are.15378
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Chen D.-D., Li J.-H., Yao Y.-Y., Zhang Y.-A. (2019). Aeromonas hydrophila suppresses complement pathways via degradation of complement C3 in bony fish by metalloprotease. Fish Shellfish Immunol. 94, 739–745. doi: 10.1016/j.fsi.2019.09.057
Das A., Sahoo P. K., Mohanty B. R., Jena J. K. (2011). Pathophysiology of experimental Aeromonas hydrophila infection in Puntius sarana: Early changes in blood and aspects of the innate immune-related gene expression in survivors. Vet. Immunol. Immunopathol. 142, 207–218. doi: 10.1016/j.vetimm.2011.05.017
Djordjević V. V., Kostić J., Krivokapić Ž., Krtinić D., Ranković M., Petković M., et al. (2022). Decreased activity of erythrocyte catalase and glutathione peroxidase in patients with schizophrenia. Medicina. 58, 1491. doi: 10.3390/medicina58101491
Elbialy Z. I., Salah A. S., Elsheshtawy A., Elkatatny N. M., Fouad A. M., Abo-Al-Ela H. G. (2023). Differential tissue regulation of nrf2/keap1 crosstalk in response to Aeromonas infection in Nile tilapia: a comparative study. Aquac. Int. doi: 10.1007/s10499-023-01175-8
El-Houseiny W., Mansour M. F., Mohamed W. A. M., Al-Gabri N. A., El-Sayed A. A., Altohamy D. E., et al. (2021). Silver nanoparticles mitigate Aeromonas hydrophila-induced immune suppression, oxidative stress, and apoptotic and genotoxic effects in Oreochromis niloticus. Aquaculture 535, 736430. doi: 10.1016/j.aquaculture.2021.736430
Ellis A. E. (1990). “Lysozyme assays,” in Techniques in fish immunology. Ed. Stolen J. S. (Fair Haven: SOS publication), 101–103.
El-Magd M. A., El-Said K. S., El-Semlawy A. A., Tanekhy M., Afifi M., Mohamed T. M. (2019). Association of MHC IIA polymorphisms with disease resistance in Aeromonas hydrophila-challenged Nile tilapia. Dev. Comp. Immunol. 96, 126–134. doi: 10.1016/j.dci.2019.03.002
Fajardo C., Martinez-Rodriguez G., Blasco J., Mancera J. M., Thomas B., De Donato M. (2022). Nanotechnology in aquaculture: Applications, perspectives and regulatory challenges. Aquac. Fish. 7, 185–200. doi: 10.1016/j.aaf.2021.12.006
Favero L. M., Nuez-Ortín W. G., Isern-Subich M.-M., de Padua Pereira U. (2020). Use of a functional feed additive to reduce mortality from franciselosis and streptococcosis. Aquafeed: Adv. Process. Formulation 12, 23–26.
Gabriel N. N. (2019). Review on the progress in the role of herbal extracts in tilapia culture. Cogent Food Agric. 5, 1619651. doi: 10.1080/23311932.2019.1619651
Ghafarifarsani H., Hoseinifar S. H., Sheikhlar A., Raissy M., Chaharmahali F. H., Maneepitaksanti W., et al. (2022). The effects of dietary thyme oil (Thymus vulgaris) essential oils for common carp (Cyprinus carpio): growth performance, digestive enzyme activity, antioxidant defense, tissue and mucus immune parameters, and resistance against Aeromonas hydrophila. Aquacult. Nutr 2022. doi: 10.1155/2022/7942506
Gheytasi A., Hosseini Shekarabi S. P., Islami H. R., Mehrgan M. S. (2021). Feeding rainbow trout, Oncorhynchus mykiss, with lemon essential oil loaded in chitosan nanoparticles: effect on growth performance, serum hemato-immunological parameters, and body composition. Aquac. Int. 29, 2207–2221. doi: 10.1007/s10499-021-00741-2
Giannenas I., Triantafillou E., Stavrakakis S., Margaroni M., Mavridis S., Steiner T., et al. (2012). Assessment of dietary supplementation with carvacrol or thymol containing feed additives on performance, intestinal microbiota and antioxidant status of rainbow trout (Oncorhynchus mykiss). Aquaculture 350, 26–32. doi: 10.1016/j.aquaculture.2012.04.027
Gong Y., Yang F., Hu J., Liu C., Liu H., Han D., et al. (2019). Effects of dietary yeast hydrolysate on the growth, antioxidant response, immune response and disease resistance of largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 94, 548–557. doi: 10.1016/j.fsi.2019.09.044
Haddad G., Hanington P. C., Wilson E. C., Grayfer L., Belosevic M. (2008). Molecular and functional characterization of goldfish (Carassius auratus L.) transforming growth factor beta. Dev. Comp. Immunol. 32, 654–663. doi: 10.1016/j.dci.2007.10.003
Han Z., Zhou Y., Zhang X., Yan J., Xiao J., Luo Y., et al. (2020). Ghrelin modulates the immune response and increases resistance to Aeromonas hydrophila infection in hybrid tilapia. Fish Shellfish Immunol. 98, 100–108. doi: 10.1016/j.fsi.2020.01.006
Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. (2013). Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 92, 2059–2069. doi: 10.3382/ps.2012-02685
Hoseini S. M., Yousefi M. (2019). Beneficial effects of thyme (Thymus vulgaris) extract on oxytetracycline-induced stress response, immunosuppression, oxidative stress and enzymatic changes in rainbow trout (Oncorhynchus mykiss). Aquacult. Nutr. 25, 298–309. doi: 10.1111/anu.12853
Hoseinifar S. H., Sun Y.-Z., Zhou Z., Van Doan H., Davies S. J., Harikrishnan R. (2020). Boosting immune function and disease bio-control through environment-friendly and sustainable approaches in finfish aquaculture: herbal therapy scenarios. Rev. Fisheries Sci. Aquac. 28, 1–19. doi: 10.1080/23308249.2020.1731420
Iijima N., Tanaka S., Ota Y. (1998). Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream, Pagrus major. Fish Physiol. Biochem. 18, 59–69. doi: 10.1023/A:1007725513389
Iversen S. L., Jørgensen M. H. (1995). Azocasein assay for alkaline protease in complex fermentation broth. Biotechnol. Techniques 9, 573–576. doi: 10.1007/BF00152446
Khalil S. R., Elhakim Y. A., Abd El-fattah A. H., Ragab Farag M., Abd El-Hameed N. E., El-Murr A. E. (2020). Dual immunological and oxidative responses in Oreochromis niloticus fish exposed to lambda cyhalothrin and concurrently fed with Thyme powder (Thymus vulgaris L.): Stress and immune encoding gene expression. Fish Shellfish Immunol. 100, 208–218. doi: 10.1016/j.fsi.2020.03.009
Korni F. M. M., Mohammed A. N., Moawad U. K. (2023). Using some natural essential oils and their nano-emulsions for ammonia management, anti-stress and prevention of streptococcosis in Nile tilapia, Oreochromis niloticus. Aquac. Int. 31, 2179–2198. doi: 10.1007/s10499-023-01076-w
Kuebutornye F. K. A., Wang Z., Lu Y., Abarike E. D., Sakyi M. E., Li Y., et al. (2020). Effects of three host-associated Bacillus species on mucosal immunity and gut health of Nile tilapia, Oreochromis niloticus and its resistance against Aeromonas hydrophila infection. Fish Shellfish Immunol. 97, 83–95. doi: 10.1016/j.fsi.2019.12.046
Lee C.-S., Lim C., Webster C. D. (2015). Dietary nutrients, additives, and fish health (NJ, USA: Wiley-Blackwell).
Leysen S., Van Herreweghe J. M., Callewaert L., Heirbaut M., Buntinx P., Michiels C. W., et al. (2011). Molecular basis of bacterial defense against host lysozymes: X-ray structures of periplasmic lysozyme inhibitors PliI and PliC. J. Mol. Biol. 405, 1233–1245. doi: 10.1016/j.jmb.2010.12.007
Li Z., Wang Y., Li X., Lin Z., Lin Y., Srinivasan R., et al. (2019). The characteristics of antibiotic resistance and phenotypes in 29 outer-membrane protein mutant strains in Aeromonas hydrophila. Environ. Microbiol. 21, 4614–4628. doi: 10.1111/1462-2920.14761
Liu Z., García-Díaz B., Catacchio B., Chiancone E., Vogel H. J. (2015). Protecting Gram-negative bacterial cell envelopes from human lysozyme: Interactions with Ivy inhibitor proteins from Escherichia coli and Pseudomonas aeruginosa. Biochim. Biophys. Acta (BBA) Biomembr. 1848, 3032–3046. doi: 10.1016/j.bbamem.2015.03.024
Liu B., Ge X., Xie J., Xu P., He Y., Cui Y., et al. (2012). Effects of anthraquinone extract from Rheum officinale Bail on the physiological responses and HSP70 gene expression of Megalobrama amblycephala under Aeromonas hydrophila infection. Fish Shellfish Immunol. 32, 1–7. doi: 10.1016/j.fsi.2011.02.015
Liu Q., Xu M., Sun Z., Ye H., Mai K., Tan X., et al. (2020). Effects of dietary monocalcium phosphate supplementation on the anti-oxidative capacity, anti-bacteria function in immune organs of obscure puffer (Takifugu obscurus) after infection with Aeromonas hydrophila. Fish Shellfish Immunol. 98, 843–852. doi: 10.1016/j.fsi.2019.11.043
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2– ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Luis A. I. S., Campos E. V. R., de Oliveira J. L., Fraceto L. F. (2019). Trends in aquaculture sciences: from now to use of nanotechnology for disease control. Rev. Aquacult. 11, 119–132. doi: 10.1111/raq.12229
Lutz R., Aserin A., Wicker L., Garti N. (2009). Release of electrolytes from W/O/W double emulsions stabilized by a soluble complex of modified pectin and whey protein isolate. Colloids Surf. B: Biointerf. 74, 178–185. doi: 10.1016/j.colsurfb.2009.07.014
Maehr T., Costa M. M., González Vecino J. L., Wadsworth S., Martin S. A. M., Wang T., et al. (2013). Transforming growth factor-β1b: A second TGF-β1 paralogue in the rainbow trout (Oncorhynchus mykiss) that has a lower constitutive expression but is more responsive to immune stimulation. Fish Shellfish Immunol. 34, 420–432. doi: 10.1016/j.fsi.2012.11.011
Mohammadi A., Jafari S. M., Assadpour E., Faridi Esfanjani A. (2016). Nano-encapsulation of olive leaf phenolic compounds through WPC–pectin complexes and evaluating their release rate. Int. J. Biol. Macromol. 82, 816–822. doi: 10.1016/j.ijbiomac.2015.10.025
Mohanty B. R., Sahoo P. K. (2010). Immune responses and expression profiles of some immune-related genes in Indian major carp, Labeo rohita to Edwardsiella tarda infection. Fish Shellfish Immunol. 28, 613–621. doi: 10.1016/j.fsi.2009.12.025
Moustafa E. M., Dawood M. A. O., Assar D. H., Omar A. A., Elbialy Z. I., Farrag F. A., et al. (2020). Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquaculture 515, 734589. doi: 10.1016/j.aquaculture.2019.734589
Navarrete P., Toledo I., Mardones P., Opazo R., Espejo R., Romero J. (2010). Effect of Thymus vulgaris essential oil on intestinal bacterial microbiota of rainbow trout, Oncorhynchus mykiss (Walbaum) and bacterial isolates. Aquac. Res. 41, e667–e678. doi: 10.1111/j.1365-2109.2010.02590.x
Qi P., Xie C., Guo B., Wu C. (2016). Dissecting the role of transforming growth factor-β1 in topmouth culter immunobiological activity: a fundamental functional analysis. Sci. Rep. 6, 27179. doi: 10.1038/srep27179
Ravindra ,. P. P. K., Pande V., Yadav M. K., Verma D. K., Sood N. (2019). Modulation of the innate immune responses in Indian major carp, Catla catla following experimental infection with Flavobacterium columnare. Aquaculture 510, 22–31. doi: 10.1016/j.aquaculture.2019.05.015
Rocha S., Gomes D., Lima M., Bronze-da-Rocha E., Santos-Silva A. (2015). Peroxiredoxin 2, glutathione peroxidase, and catalase in the cytosol and membrane of erythrocytes under H2O2-induced oxidative stress. Free Radic. Res. 49, 990–1003. doi: 10.3109/10715762.2015.1028402
Salam H. S. H., Mohamed W. M. S., Aziz S. A. A. A., Mohammed A. N., Korni F. M. M. (2021). Prevention of motile Aeromonas septicemia in Nile tilapia, Oreochromis niloticus, using thyme essential oil and its nano-emlusion. Aquac. Int. 29, 2065–2084. doi: 10.1007/s10499-021-00735-0
Sheikh Asadi M., Gharaei A., Mirdar Harijani J., Arshadi A. (2018). A comparison between dietary effects of Cuminum cyminum essential oil and Cuminum cyminum essential oil, loaded with iron nanoparticles, on growth performance, immunity and antioxidant indicators of white leg shrimp (Litopenaeus vannamei). Aquacult. Nutr. 24, 1466–1473. doi: 10.1111/anu.12683
Shirajum Monir M., Yusoff S. M., Mohamad A., Ina-Salwany M. Y. (2020). Vaccination of tilapia against motile aeromonas septicemia: A Review. J. Aquat. Anim. Health 32, 65–76. doi: 10.1002/aah.10099
Sienkiewicz M., Łysakowska M., Denys P., Kowalczyk E. (2012). The antimicrobial activity of thyme essential oil against multidrug resistant clinical bacterial strains. Microb. Drug Resist. 18, 137–148. doi: 10.1089/mdr.2011.0080
Silva A. S., Tewari D., Sureda A., Suntar I., Belwal T., Battino M., et al. (2021). The evidence of health benefits and food applications of Thymus vulgaris L. Trends Food Sci. Technol. 117, 218–227. doi: 10.1016/j.tifs.2021.11.010
Song Q., Xiao Y., Xiao Z., Liu T., Li J., Li P., et al. (2021). Lysozymes in fish. J. Agric. Food Chem. 69, 15039–15051. doi: 10.1021/acs.jafc.1c06676
Sönmez A. Y., Bilen S., Alak G., Hisar O., Yanık T., Biswas G. (2015). Growth performance and antioxidant enzyme activities in rainbow trout (Oncorhynchus mykiss) juveniles fed diets supplemented with sage, mint and thyme oils. Fish Physiol. Biochem. 41, 165–175. doi: 10.1007/s10695-014-0014-9
Souza C. F., Baldissera M. D., Guarda N. S., Bollick Y. S., Moresco R. N., Brusque I. C. M., et al. (2017b). Melaleuca alternifolia essential oil nanoparticles ameliorate the hepatic antioxidant/oxidant status of silver catfish experimentally infected with Pseudomonas aeruginosa. Microb. Pathog. 108, 61–65. doi: 10.1016/j.micpath.2017.05.016
Souza C. F., Baldissera M. D., Santos R. C. V., Raffin R. P., Baldisserotto B. (2017a). Nanotechnology improves the therapeutic efficacy of Melaleuca alternifolia essential oil in experimentally infected Rhamdia quelen with Pseudomonas aeruginosa. Aquaculture 473, 169–171. doi: 10.1016/j.aquaculture.2017.02.014
Spolarics Z., Wu J.-X. (1997). Role of glutathione and catalase in H2O2detoxification in LPS-activated hepatic endothelial and Kupffer cells. Am. J. Physiol Gastrointest. Liver Physiol. 273, G1304–G1311. doi: 10.1152/ajpgi.1997.273.6.G1304
Subasinghe R., Soto D., Jia J. (2009). Global aquaculture and its role in sustainable development. Rev. Aquacult. 1, 2–9. doi: 10.1111/j.1753-5131.2008.01002.x
Winn-Deen E. S., David H., Sigler G., Chavez R. (1988). Development of a direct assay for alpha-amylase. Clin. Chem. 34, 2005–2008. doi: 10.1093/clinchem/34.10.2005
Yano T. (1992). “Assays of hemolytic complement activity,” in Techniques in fish immunology. Ed. J.S. S. (Fair haven: SOS publication), 131–141.
Yilmaz S., Yilmaz E., Dawood M. A. O., Ringø E., Ahmadifar E., Abdel-Latif H. M. R. (2022). Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: A review. Aquaculture 547, 737514. doi: 10.1016/j.aquaculture.2021.737514
Yılmaz S., Ergün S., Soytaş N. (2012) Herbal supplements are useful for preventing streptococcal disease during first-feeding of tilapia fry, Oreochromis mossambicus. Israeli J. Aquac. Bamidgeh 65, 833–837.
Zaki M. A., Labib E. M., Nour A. M., Tonsy H. D., Mahmoud S. H. (2012). Effect some medicinal plants diets on mono sex Nile tilapia (Oreochromis niloticus), growth performance, feed utilization and physiological parameters. APCBEE Procedia 4, 220–227. doi: 10.1016/j.apcbee.2012.11.037
Zang Y., Zheng S., Tang F., Yang L., Wei X., Kong D., et al. (2020). Heme oxygenase 1 plays a crucial role in swamp eel response to oxidative stress induced by cadmium exposure or Aeromonas hydrophila infection. Fish Physiol. Biochem. 46, 1947–1963. doi: 10.1007/s10695-020-00846-0
Zargar A., Rahimi-Afzal Z., Soltani E., Taheri Mirghaed A., Ebrahimzadeh-Mousavi H. A., Soltani M., et al. (2019). Growth performance, immune response and disease resistance of rainbow trout (Oncorhynchus mykiss) fed Thymus vulgaris essential oils. Aquac. Res. 50, 3097–3106. doi: 10.1111/are.14243
Zargar A., Taheri Mirghaed A., Mirzargar S. S., Ghelichpour M., Yousefi M., Hoseini S. M. (2020). Dietary ginger administration attenuates oxidative stress and immunosuppression caused by oxytetracycline in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 51, 4215–4224. doi: 10.1111/are.14763
Zdanowicz M., Mudryk Z. J., Perliński P. (2020). Abundance and antibiotic resistance of Aeromonas isolated from the water of three carp ponds. Vet. Res. Commun. 44, 9–18. doi: 10.1007/s11259-020-09768-x
Zhan X.-l., Ma T.-y., Wu J.-y., Yi L.-y., Wang J.-y., Gao X.-k., et al. (2015). Cloning and primary immunological study of TGF-β1 and its receptors TβR I /TβR II in tilapia (Oreochromis niloticus). Dev. Comp. Immunol. 51, 134–140. doi: 10.1016/j.dci.2015.03.008
Zhang X., Sun Z., Cai J., Wang J., Wang G., Zhu Z., et al. (2020). Effects of dietary fish meal replacement by fermented moringa (Moringa oleifera Lam.) leaves on growth performance, nonspecific immunity and disease resistance against Aeromonas hydrophila in juvenile gibel carp (Carassius auratus gibelio var. CAS III). Fish Shellfish Immunol. 102, 430–439. doi: 10.1016/j.fsi.2020.04.051
Zhang C.-N., Zhang J.-L., Guan W.-C., Zhang X.-F., Guan S.-H., Zeng Q.-H., et al. (2017). Effects of Lactobacillus delbrueckii on immune response, disease resistance against Aeromonas hydrophila, antioxidant capability and growth performance of Cyprinus carpio Huanghe var. Fish Shellfish Immunol. 68, 84–91. doi: 10.1016/j.fsi.2017.07.012
Zhu F. (2020). A review on the application of herbal medicines in the disease control of aquatic animals. Aquaculture 526, 735422. doi: 10.1016/j.aquaculture.2020.735422
Keywords: disease, nano-technology, Thymus vulgaris, aquaculture, cytokine, antioxidant
Citation: Yousefi M, Hoseini SM, Ghadamkheir M, Mahboub HH, Vatnikov YA, Kulikov EV and Sotnikova ED (2023) Nano-liposome of thyme essential oil promotes growth performance, antioxidant and immune responses to aeromonad septicemia in Nile tilapia, Oreochromis niloticus, fingerlings. Front. Mar. Sci. 10:1290879. doi: 10.3389/fmars.2023.1290879
Received: 08 September 2023; Accepted: 15 November 2023;
Published: 30 November 2023.
Edited by:
Bin Xia, Qingdao Agricultural University, ChinaReviewed by:
Yunfei Sun, Shanghai Ocean University, ChinaCopyright © 2023 Yousefi, Hoseini, Ghadamkheir, Mahboub, Vatnikov, Kulikov and Sotnikova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Morteza Yousefi, bXlvdXNlZmk4MUBnbWFpbC5jb20=; Seyyed Morteza Hoseini, c2V5eWVkbW9ydGV6YS5ob3NlaW5pQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.