- 1Beneath The Waves, Herndon, VA, United States
- 2Department of Ichthyology and Fisheries Science, Rhodes University, Grahamstown, South Africa
- 3South African International Maritime Institute, Gqeberha, South Africa
Introduction
The spatial distributions of many marine species are rapidly altering in response to anthropogenic impacts such as climate change, with poleward shifts being exhibited by multiple populations of highly mobile consumers (Storrie et al., 2018; Hammerschlag et al., 2022). These population shifts can rearrange food web dynamics and influence species interactions (Pinsky et al., 2020). Ecological data suggest that species with strong behavioural and foraging plasticity are most likely to survive in a rapidly changing ocean. Killer whales Orcinus orca are globally distributed (Forney et al., 2006), travel vast ocean distances whilst hunting, and strongly influence marine food web dynamics through consumptive and non-consumptive effects (Estes et al., 1998). They also influence critical ocean processes, such as the recycling and translocation of nutrients (Kiszka et al., 2021). Additionally, the species displays complex cognitive abilities, with flexible foraging strategies (Paulos et al., 2010; Hill et al., 2022). It is predicted that killer whales will not only be able to adapt more efficiently than other marine predators in a changing ocean environment (Evans and Moustakas, 2018), but they will also play a major role in shaping the trophic interactions of many marine ecosystems in the future.
Often referred to as the ‘wolves of the sea’, killer whales hunt a wide variety of prey species including all marine mammal families, except river dolphins Inia geoffrensis and manatees Trichechus manatus. They have been observed to hunt twenty species of cetaceans, fourteen species of pinnipeds, dugong Dugong dugon and sea otters Enhydra lutris (Jefferson et al., 1991) as well as various marine reptiles (Fertl and Fulling, 2007) and teleosts (Vogel et al., 2021). A review by Fertl and Darby (1996) suggested that elasmobranchs are likely underestimated in the killer whales diet, although records exist globally (Reyes and García-Borboroglu, 2004; Visser, 2005; Williams et al., 2009; Ford et al., 2011, Jorgensen et al., 2019). Many marine predators change their behaviour and distribution in response to the risk of killer whale predation (Pitman and Durban, 2010; Breed et al., 2017) and the decision of ‘fight or flight’ is usually dependent on the trade-off between the risk of mortality and access to, or lack of, prey (Ripple et al., 2014).
Ethograms are a valuable and foundational tool used in the field of ethology and behavioural ecology, as they provide a standardised catalogue and full repertoire of behaviours displayed by a given species in space and time (Lehner, 1987). For captive killer whales, an intraspecific ethogram has been developed (Martinez and Klinghammer, 1978), which has been crucial for animal welfare and husbandry. For wild killer whales, via the use of underwater video, an ethogram outlining the behaviours exhibited towards human snorkellers and divers has been constructed (Pagel et al., 2017). However, a comprehensive ethogram of natural, uninterrupted behaviours for the species, is currently not available. Whilst documenting the behaviour of wild killer whales can present challenges, ethograms should improve our knowledge on their ecological roles, social structures, hunting strategies and responses to environmental changes.
The use of unmanned aerial vehicles (UAVs), more widely observed as drones, are taking flight as an efficient and cost-effective research tool for studying the behaviour marine megafauna (Gallagher et al., 2018; Torres et al., 2018; Barreto et al., 2021). Compared to manned aircrafts, boats, or in water human observations, this non-invasive approach minimises disturbance and allows for more natural behavioural observations (Christiansen et al., 2016; Fettermann et al., 2022). The use of UAVs have been successfully applied to the study of several large marine mammal species including gray whales Eschrichtius robustus in the northwest Pacific (Torres et al., 2018), humpback whales Megaptera novaeangliae in the cenrtal Pacific (Fiori et al., 2020) and Antillean Manatees Trichechus manatus manatus in the Caribbean Atlantic (Landeo-Yauri et al., 2020).
Whilst numerous ecological and behavioural studies have been documented on killer whale populations inhabiting temperate and sub-polar regions of the ocean, information on those populations occupying tropical and sub-tropical regions remain comparatively limited, particularly in the Caribbean Sea, where there have only been a total of 385 records from 1851-2023 (Bolaños-Jiménez et al., 2023). Killer whale densities in The Bahamas, an extensive oceanic archipelago in the subtropical Atlantic, are thought to be very low, despite the first record occurring in 1913 (Murphy, 1948). Dunn and Claridge (2014) presented a robust overview of killer whale occurrence in The Bahamas, performing an extensive review of the literature and collating public reports with their own empirical observations from surveys, resulting in 34 total sightings from 1913-2011. Their results suggested that there were very few (i.e., less than five) independently published scholarly articles documenting killer whales in The Bahamas, and the majority of the previously compiled records/sightings did not contain any photographic or video evidence (Dunn and Claridge, 2014).
Here, we present new empirical observations of killer whales in The Bahamas, by presenting novel aerial video evidence obtained via a UAV. Our ethogram adds to the important work done by colleagues in a data-poor region and advances our knowledge on the behaviour of a highly cryptic population of killer whales. We discuss the potential implications of this sighting within the context of shark survival in The Bahamas shark sanctuary, the ecological implications on the wider marine food web and highlight the importance of citizen science in advancing our ecological knowledge of rare and elusive predators.
Methods and results
On December 15th 2020 at 11:00 AM, a group of nine killer whales were filmed by a hobbyist drone pilot using an aerial drone (DJI magic pro 2) from a 15m catamaran yacht in the deep waters (>2,000m) of the Northwest Providence Channel off Nassau, New Providence, The Commonwealth of The Bahamas, (25.154460°, -77.501113°) (Figure 1). The authors were not on board when the recording was made, the footage was sent to the authors following the trip for review and analysis.
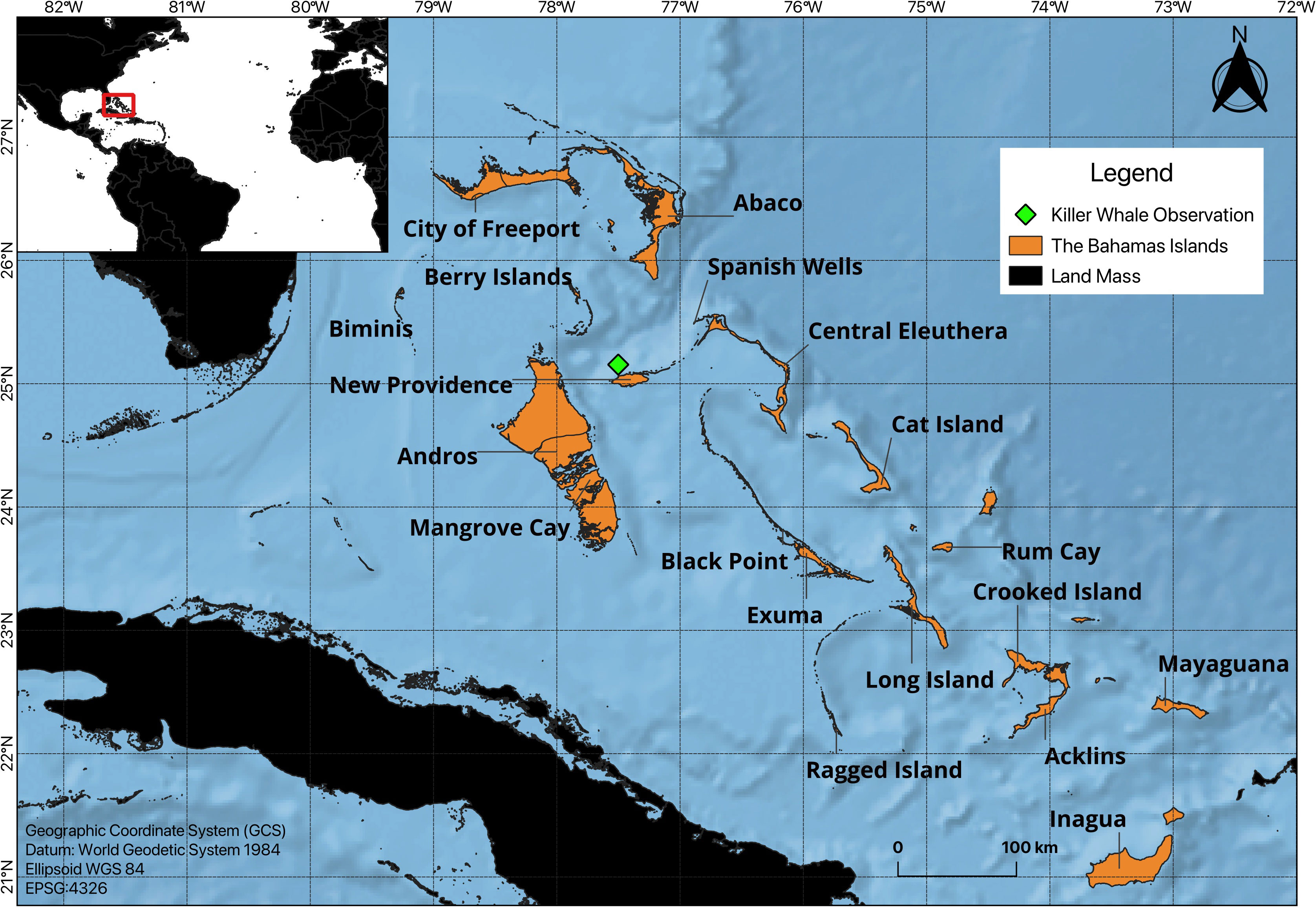
Figure 1 Study map of The Bahamas. Green diamond indicates where the drone footage of the killer whale group was captured.
A total of 14 minutes and 36 seconds of killer whale footage was obtained. The group comprised of 5 adult females, 2 sub-adult females and 2 adult males, led by a female, as the group followed this individual when her swimming direction changed throughout the observation. Throughout the footage, the killer whales exhibited milling and surface active behaviours (SABs) (Noren et al., 2009) which were characterised into five categories outlined in the ethogram (Table 1). A total of 23 synchronous surfacing (Figure 2A) events were recorded, seven of which involved >3 individuals surfacing simultaneously. There appeared to be some sexual segregation during surfacing events, with females surfacing together and the two males surfacing together, which is behaviour that has been reported in other populations (Weiss et al., 2021). Overall, 63 observations of lobtailing (Figure 2B) were observed, with 22 of these displayed as inverted lobtailing (Figure 2C). Lunging behaviour was also observed on two occasions (Figure 2D).
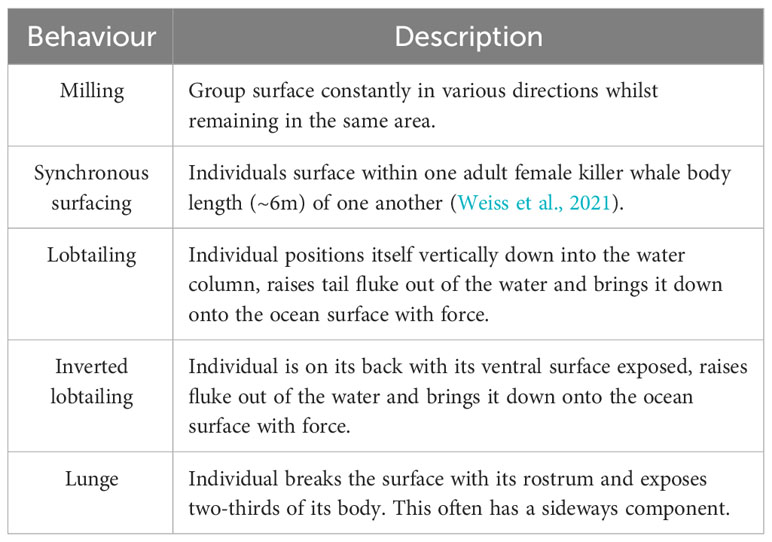
Table 1 Ethogram outlining the various behaviours observed by killer whales in the drone footage from The Bahamas.
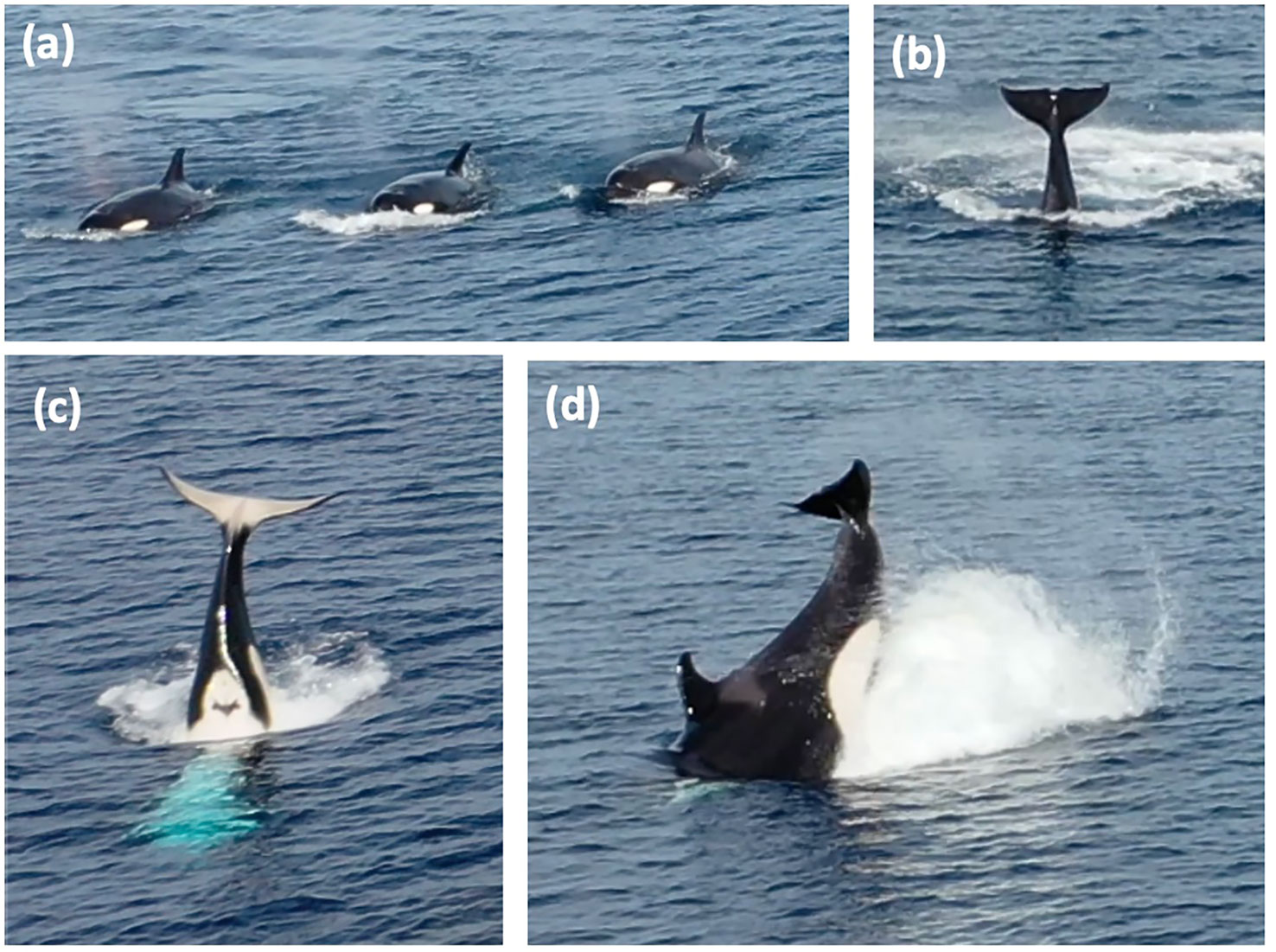
Figure 2 Screenshots from video taken via drone of the killer whale group in The Bahamas displaying various surface-active behaviours: (A) Synchronous surfacing; (B) Lobtailing; (C) Inverted lobtailing; (D) Lunge.
Killer whales also displayed two interactions with brown macroalgae identified as pelagic sargassum Sargassaceae. In both interactions, the focal individual investigated the sargassum and then, using its mouth, dragged the sargassum underwater. In one interaction, a bubble ring was blown by the same individual prior to taking the sargassum underwater, suggestive of investigative behaviour (Hill et al., 2022). At the end of the drone footage, all killer whales were submerged underwater to the port side of the vessel..
To validate the novelty of UAVs as a tool for documenting the occurrence and behaviour of killer whales in The Bahamas, we also performed a brief social media content analysis of publicly-available footage of killer whale reports in The Bahamas. We used the terms “killer whale Bahamas” and “orca Bahamas” with the search function on Facebook (Meta) to reveal any posts from public pages or profiles (e.g., fishing groups, photographers) from 2018 - present (2023). Results were collated, and scored according to their type (photo/video), location, date, estimated group size, sex ratio, behaviour and whether a UAV was used. An additional eight sightings were obtained (Table 2), revealing killer whale group sizes ranging from 3 to 100 (estimated, in 2023), yet none of these were documented via the use of an UAV.
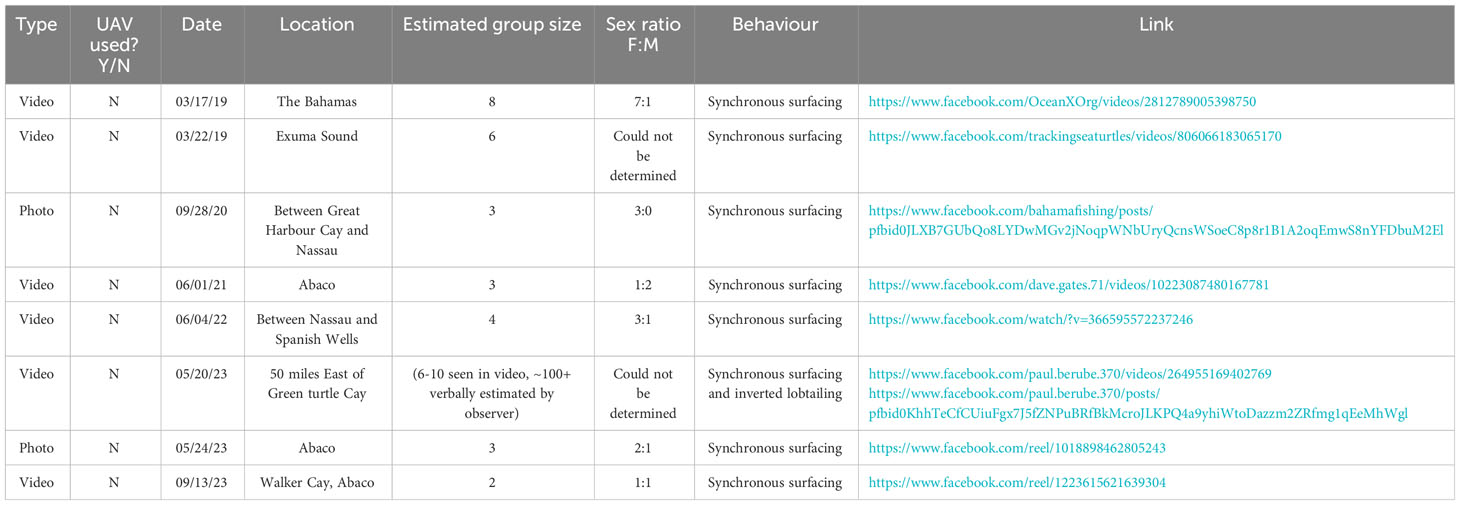
Table 2 Summary of Facebook content analysis of killer whale sightings from The Bahamas from the last 5 years.
Discussion
Via novel aerial drone footage, our study documented an elusive group of killer whales in The Bahamas, thereby supporting previous work, enhancing existing databases (Dunn and Claridge, 2014; Bolaños-Jiménez et al., 2023) and helping to advance our knowledge on the understudied Bahamian population. The highest percentage of killer whale records in the Caribbean Sea (39.5%) are from the eastern Caribbean, with The Bahamian region only contributing 13% of all killer whale records (Bolaños-Jiménez et al., 2023), highlighting the significance of our drone-recorded sighting for this region. This new sighting, which occurred in the winter (where previous sightings were rare, Dunn and Claridge, 2014), adds to the overall Bahamas sighting database, and the body of regional records with photo/video evidence by ~3%, respectively.
From the footage, 23 observations of synchronous surfacing events were observed. Within killer whale groups, evidence suggests that synchrony is imperative for social bonding (Connor et al., 2006). Additionally, synchrony is beneficial for cooperative behaviours such as hunting, as seen when displaying wave-wash attacks to prey on pinnipeds (Visser et al., 2008; Pitman and Durban, 2012); and, the carousel method - the formation of bubble rings to trap and herd prey into a dense bait ball towards the surface for ease of consumption (Similä and Ugarte, 1993; Nøttestad et al., 2002). Moreover, although the 63 counts of lobtailing behaviour in this footage appeared to be used in a playful and social (potentially sociosexual) manner (Horback et al., 2012), lobtailing is also commonly used to stun or incapacitate prey (Domenici et al., 2000b) and inverted lobtailing (also seen in this study), is used as a visual cue when hunting to force prey into bait balls (Domenici et al., 2000a). Additionally, it has been noted that killer whales display complex cognitive abilities, are extremely curious and known for manipulating objects, particularly in a novel setting (Paulos et al., 2010; Hill et al., 2022), as reflected through the observed interactions with the pelagic sargassum in the present study. The described repertoire of behaviours displayed by this group in The Bahamas are suggestive of a healthy, functioning unit which will have large metabolic and energetic needs.
Like that of many apex predators, a key driver of habitat selection for killer whales is prey quality and availability. Whilst ocean warming has varying effects on prey availability, evidence suggests that when prey populations are altered, apex predators will shift their distribution to seek the prey elsewhere or shift to an alternative prey item (Shields et al., 2018). Killer whales exhibit strong top-down predator control on large-scale marine ecosystems, as seen in their role in driving one of the most significant marine mammal population declines in modern history - the drastic decline in the sea otter population on the Aleutian Islands (Alaska, USA) in the 1990s (Kuker and Barrett-Lennard, 2010). In this example, observations of killer whales preying on sea otters increased during the study period, giving researchers confidence in the strong influence of orcas in the prey species’ crash. However, there is still much to be learned about these interactions and many complexities we do not yet understand.
Several killer whale populations specialise on a narrow range of prey and can be morphologically and genetically distinct. A rare offshore morphotype of killer whale was described off South Africa by Best et al. (2014). The following year, a distinctive pair of adult males of this morphotype, appeared in the region, and preyed upon various shark species, including white sharks Carcharodon carcharias, extracting and consuming their lipid rich livers (Engelbrecht et al., 2019; Towner et al., 2022a). It is noted that the liver of an adult white shark can match the daily energetic needs of an adult male killer whale (Reisinger et al., 2011). These predations not only caused large scale displacement of sharks from coastal sites, they induced mesopredator release and trophic changes (Towner et al., 2022b). While killer whales have been documented preying on different shark species world-wide (Williams et al., 2009) the predations in South Africa gave insights into how rapidly killer whales can change coastal ecosystems, specifically when they return to the same sites to prey on apex sharks, over several years. The displacement of a top predator such as the white shark, could have far-reaching impacts on ecosystem function at the local and regional level, as well as substantial socio-economic and bather safety impacts (Estes et al., 2011; Towner et al., 2022a).
According to survey work done by Dunn and Claridge (2014), odontocetes appear to comprise a main portion of killer whale diet in The Bahamas, and foraging observations of four species have been documented including the Atlantic spotted dolphin Stenella frontalis, Frasers dolphin Lagenodelphis hosei, the Pygmy sperm whale Kogia breviceps and the Dwarf sperm whale Kogia sima (Dunn and Claridge, 2014). Similarly, through the use of stable isotopes and mixing models, killer whales off St Vincent and The Grenadines were found to mostly feed on teuthophageous odontocetes such as short finned pilot whales Globicephala macrorhynchus and sperm whales, with Oceanic sharks only contributing 17% (SD = 0.13), to the Caribbean killer whale diet (Kiszka et al., 2021). The Bahamas contains healthy and abundant shark populations (Gallagher et al., 2021; Shipley et al., 2023) which have been afforded protections via a de-facto large-scale protected area (e.g., a shark sanctuary) (Haas et al., 2017). Thus, sharks and other chondrichthyan species could present themselves as a potentially profitable foraging item for killer whales in the region, particularly in the absence of other preferred prey.
With 43% of all killer whale records in the Caribbean attributed to citizen science (Bolaños-Jiménez et al., 2023), UAVs and utilising publicly available drone footage can be a valuable data collection tool moving forward, as they can help put current and future observations into context and may reveal new predation threats that killer whales present to local prey populations. Moreover, the ethogram presented in this study, provides the first documented repertoire of behaviours displayed by wild killer whales, providing a baseline to guide future behavioural studies on these top ocean predators.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
OD: Conceptualization, Formal Analysis, Methodology, Writing – original draft. AG: Conceptualization, Writing – review & editing. AT: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful to Dominic Baldzuhn and the crew of the M/Y Mucho Gusto for obtaining the drone footage. We thank J. Kiszka for his comments on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barreto J., Cajaiba L., Teixeira J. B., Nascimento L., Giacomo A., Barcelos N., et al. (2021). Drone-monitoring: Improving the detectability of threatened marine megafauna. Drones 5, 14. doi: 10.3390/drones5010014
Best P. B., Meÿer M., Thornton M., Kotze P., Seakamela S., Hofmeyr G., et al. (2014). Confirmation of the occurrence of a second killer whale morphotype in South African waters. Afr. J. Mar. Sci. 36, 215–224. doi: 10.2989/1814232X.2014.923783
Bolaños-Jiménez J., Kiszka J. J., Bouveret L., Ferrer G. R., Ramos E. A., Henriquez A., et al. (2023). The killer whale in the caribbean sea: an updated review of its ecology, exploitation, and interactions with fisheries. Aquat. Mammals 49, 184–194. doi: 10.1578/AM.49.2.2023.184
Breed G. A., Matthews C. J., Marcoux M., Higdon J. W., Leblanc B., Petersen S. D., et al. (2017). Sustained disruption of narwhal habitat use and behavior in the presence of Arctic killer whales. Proc. Natl. Acad. Sci. 114, 2628–2633. doi: 10.1073/pnas.1611707114
Christiansen F., Rojano-Doñate L., Madsen P. T., Bejder L. (2016). Noise levels of multi-rotor unmanned aerial vehicles with implications for potential underwater impacts on marine mammals. Front. Mar. Sci. 3, 277. doi: 10.3389/fmars.2016.00277
Connor R. C., Smolker R., Bejder L. (2006). Synchrony, social behaviour and alliance affiliation in Indian Ocean bottlenose dolphins, Tursiops aduncus. Anim. Behav. 72, 1371–1378. doi: 10.1016/j.anbehav.2006.03.014
Domenici P., Batty R. S., Similä T. (2000a). Spacing of wild schooling herring while encircled by killer whales. J. Fish Biol. 57, 831–836. doi: 10.1111/j.1095-8649.2000.tb00278.x
Domenici P., Batty R. S., Simila T., Ogam E. (2000b). Killer whales (Orcinus orca) feeding on schooling herring (Clupea harengus) using underwater tail-slaps: kinematic analyses of field observations. J. Exp. Biol. 203, 283–294. doi: 10.1242/jeb.203.2.283
Dunn C., Claridge D. (2014). Killer whale (Orcinus orca) occurrence and predation in the Bahamas. J. Mar. Biol. Assoc. United Kingdom 94, 1305–1309. doi: 10.1017/S0025315413000908
Engelbrecht T. M., Kock A. A., O'Riain M. J. (2019). Running scared: when predators become prey. Ecosphere 10 (1), e02531. doi: 10.1002/ecs2.2531
Estes J. A., Terborgh J., Brashares J. S., Power M. E., Berger J., Bond W. J., et al. (2011). Trophic downgrading of planet Earth. science 333, 301–306. doi: 10.1126/science.1205106
Estes J. A., Tinker M. T., Williams T. M., Doak D. F. (1998). Killer whale predation on sea otters linking oceanic and nearshore ecosystems. science 282, 473–476. doi: 10.1126/science.282.5388.473
Evans M. R., Moustakas A. (2018). Plasticity in foraging behaviour as a possible response to climate change. Ecol. Inf. 47, 61–66. doi: 10.1016/j.ecoinf.2017.08.001
Fertl D. A. A.-G., Darby F. L. (1996). A report of killer whales (Orcinus orca) feeding on a carcharhinid shark in Costa Rica. Mar. Mammal Sci. 12, 606–611. doi: 10.1111/j.1748-7692.1996.tb00075.x
Fertl D., Fulling G. (2007). Interactions between marine mammals and turtles. Mar. Turtle Newslett. 115, 4–8.
Fettermann T., Fiori L., Gillman L., Stockin K. A., Bollard B. (2022). Drone surveys are more accurate than boat-based surveys of bottlenose dolphins (Tursiops truncatus). Drones 6, 82. doi: 10.3390/drones6040082
Fiori L., Martinez E., Bader M. K. F., Orams M. B., Bollard B. (2020). Insights into the use of an unmanned aerial vehicle (UAV) to investigate the behavior of humpback whales (Megaptera novaeangliae) in Vava'u, Kingdom of Tonga. Mar. Mammal Sci. 36, 209–223. doi: 10.1111/mms.12637
Ford J. K., Ellis G. M., Matkin C. O., Wetklo M. H., Barrett-Lennard L. G., Withler R. E. (2011). Shark predation and tooth wear in a population of northeastern Pacific killer whales. Aquat. Biol. 11, 213–224. doi: 10.3354/ab00307
Forney K. A., Wade P. R., Estes J. (2006). Worldwide distribution and abundance of killer whales. Whales Whaling Ocean Ecosyst. 145, 162.
Gallagher A. J., Papastamatiou Y. P., Barnett A. (2018). Apex predatory sharks and crocodiles simultaneously scavenge a whale carcass. J. Ethology 36, 205–209. doi: 10.1007/s10164-018-0543-2
Gallagher A. J., Shipley O. N., Van Zinnicq Bergmann M. P., Brownscombe J. W., Dahlgren C. P., Frisk M. G., et al. (2021). Spatial connectivity and drivers of shark habitat use within a large marine protected area in the caribbean, the Bahamas shark sanctuary. Front. Mar. Sci. 7, 1223. doi: 10.3389/fmars.2020.608848
Haas A. R., Fedler T., Brooks E. J. (2017). The contemporary economic value of elasmobranchs in The Bahamas: Reaping the rewards of 25 years of stewardship and conservation. Biol. Conserv. 207, 55–63. doi: 10.1016/j.biocon.2017.01.007
Hammerschlag N., Mcdonnell L. H., Rider M. J., Street G. M., Hazen E. L., Natanson L. J., et al. (2022). Ocean warming alters the distributional range, migratory timing, and spatial protections of an apex predator, the tiger shark (Galeocerdo cuvier). Global Change Biol. 28, 1990–2005. doi: 10.1111/gcb.16045
Hill H. M., Weiss M., Brasseur I., Manibusan A., Sandoval I. R., Robeck T., et al. (2022). Killer whale innovation: teaching animals to use their creativity upon request. Anim. Cogn. 25, 1091–1108. doi: 10.1007/s10071-022-01635-3
Horback K. M., Muraco H., Kuczaj S. A. (2012). Variations in interspecific behavior throughout the estrous cycle of a killer whale (Orcinus orca). Aquat. Mammals 38, 428–434. doi: 10.1578/AM.38.4.2012.428
Jefferson T. A., Stacey P. J., Baird R. W. (1991). A review of killer whale interactions with other marine mammals: predation to co-existence. Mammal Rev. 21, 151–180. doi: 10.1111/j.1365-2907.1991.tb00291.x
Jorgensen S. J., Anderson S., Ferretti F., Tietz J. R., Chapple T., Kanive P., et al. (2019). Killer whales redistribute white shark foraging pressure on seals. Sci. Rep. 9 (1), 6153. doi: 10.1038/s41598-019-39356-2
Kiszka J. J., Caputo M., Méndez-Fernandez P., Fielding R. (2021). Feeding ecology of elusive Caribbean killer whales inferred from Bayesian stable isotope mixing models and whalers’ ecological knowledge. Front. Mar. Sci. 8, 423. doi: 10.3389/fmars.2021.648421
Kuker K., Barrett-Lennard L. (2010). A re-evaluation of the role of killer whales Orcinus orca in a population decline of sea otters Enhydra lutris in the Aleutian Islands and a review of alternative hypotheses. Mammal Rev. 40, 103–124. doi: 10.1111/j.1365-2907.2009.00156.x
Landeo-Yauri S. S., Ramos E. A., Castelblanco-Martínez D. N., Niño-Torres C. A., Searle L. (2020). Using small drones to photo-identify Antillean manatees: A novel method for monitoring an endangered marine mammal in the Caribbean Sea. Endangered Species Res. 41, 79–90. doi: 10.3354/esr01007
Lehner P. N. (1987). Design and execution of animal behavior research: an overview. J. Anim. Sci. 65, 1213–1219. doi: 10.2527/jas1987.6551213x
Martinez D., Klinghammer E. (1978). Partial ethogram of the killer whale-(Orcinus orca L). Carnivore 1, 13–27.
Noren D., Johnson A., Rehder D., Larson A. (2009). Close approaches by vessels elicit surface active behaviors by southern resident killer whales. Endangered Species Res. 8, 179–192. doi: 10.3354/esr00205
Nøttestad L., Fernö A., Axelsen B. E. (2002). Digging in the deep: killer whales' advanced hunting tactic. Polar Biol. 25, 939–941. doi: 10.1007/s00300-002-0437-0
Pagel C. D., Scheer M., Lück M. (2017). Swim encounters with killer whales (Orcinus orca) off Northern Norway: interactive behaviours directed towards human divers and snorkellers obtained from opportunistic underwater video recordings. J. Ecotourism 16, 190–200. doi: 10.1080/14724049.2016.1273939
Paulos R. D., Trone M., Kuczaj Ii S. A. (2010). Play in wild and captive cetaceans. Int. J. Comp. Psychol. 23, 701–722. doi: 10.46867/IJCP.2010.23.04.06
Pinsky M. L., Selden R. L., Kitchel Z. J. (2020). Climate-driven shifts in marine species ranges: Scaling from organisms to communities. Annu. Rev. Mar. Sci. 12, 153–179. doi: 10.1146/annurev-marine-010419-010916
Pitman R. L., Durban J. W. (2010). Killer whale predation on penguins in Antarctica. Polar Biol. 33, 1589–1594. doi: 10.1007/s00300-010-0853-5
Pitman R. L., Durban J. W. (2012). Cooperative hunting behavior, prey selectivity and prey handling by pack ice killer whales (Orcinus orca), type B, in Antarctic Peninsula waters. Mar. Mammal Sci. 28, 16–36. doi: 10.1111/j.1748-7692.2010.00453.x
Reisinger R. R., De Bruyn P., Tosh C. A., Oosthuizen W. C., Mufanadzo N. T., Bester M. N. (2011). Prey and seasonal abundance of killer whales at sub-Antarctic Marion Island. Afr. J. Mar. Sci. 33, 99–105. doi: 10.2989/1814232X.2011.572356
Reyes L. M., García-Borboroglu P. (2004). Killer whale (Orcinus orca) predation on sharks in Patagonia, Argentina: a first report. Aquat. Mammals 30, 376–379. doi: 10.1578/AM.30.3.2004.376
Ripple W. J., Estes J. A., Beschta R. L., Wilmers C. C., Ritchie E. G., Hebblewhite M., et al. (2014). Status and ecological effects of the world’s largest carnivores. Science 343 (6167), 1241484. doi: 10.1126/science.1241484
Shields M. W., LINDELL J., WOODRUFF J. (2018). Declining spring usage of core habitat by endangered fish-eating killer whales reflects decreased availability of their primary prey. Pacific Conserv. Biol. 24, 189–193. doi: 10.1071/PC17041
Shipley O. N., Matich P., Hussey N. E., Brooks A. M., Chapman D., Frisk M. G., et al. (2023). Energetic connectivity of diverse elasmobranch populations–implications for ecological resilience. Proc. R. Soc. B 290, 20230262. doi: 10.1098/rspb.2023.0262
Similä T., Ugarte F. (1993). Surface and underwater observations of cooperatively feeding killer whales in northern Norway. Can. J. Zoology 71, 1494–1499. doi: 10.1139/z93-210
Storrie L., Lydersen C., Andersen M., Wynn R. B., Kovacs K. M. (2018). Determining the species assemblage and habitat use of cetaceans in the Svalbard Archipelago, based on observations from 2002 to 2014. Polar Res. 37, 1463065. doi: 10.1080/17518369.2018.1463065
Torres L. G., Nieukirk S. L., Lemos L., Chandler T. E. (2018). Drone up! Quantifying whale behavior from a new perspective improves observational capacity. Front. Mar. Sci. 319. doi: 10.3389/fmars.2018.00319
Towner A. V., Kock A. A., Stopforth C., Hurwitz D., Elwen S. H. (2022b). Direct observation of killer whales preying on white sharks and evidence of a flight response. Ecology 104, e3875. doi: 10.1002/ecy.3875
Towner A., Watson R., Kock A., Papastamatiou Y., Sturup M., Gennari E., et al. (2022a). Fear at the top: killer whale predation drives white shark absence at South Africa’s largest aggregation site. Afr. J. Mar. Sci. 44, 139–152. doi: 10.2989/1814232X.2022.2066723
Visser I. N. (2005). First observations of feeding on thresher (Alopias vulpinus) and hammerhead (Sphyrna zygaena) sharks by killer whales (Orcinus orca) specialising on elasmobranch prey. Aquat. Mammals 31, 83. doi: 10.1578/AM.31.1.2005.83
Visser I., Smith T., Bullock I., Green G., Carlsson O. L., Imberti S. (2008). Antarctic peninsula killer whales (Orcinus orca) hunt seals and a penguin on floating ice. Mar. Mammal Sci. 24, 225–234. doi: 10.1111/j.1748-7692.2007.00163.x
Vogel E. F., Biuw M., Blanchet M.-A., Jonsen I. D., Mul E., Johnsen E., et al. (2021). Killer whale movements on the Norwegian shelf are associated with herring density. Mar. Ecol. Prog. Ser. 665, 217–231. doi: 10.3354/meps13685
Weiss M. N., Franks D. W., Giles D. A., Youngstrom S., Wasser S. K., Balcomb K. C., et al. (2021). Age and sex influence social interactions, but not associations, within a killer whale pod. Proc. R. Soc. B 288, 20210617. doi: 10.1098/rspb.2021.0617
Keywords: killer whale (Orcinus orca), The Bahamas, The Caribbean, UAV (unmanned aerial vehicle), drone, shark sanctuary
Citation: Dixon OFL, Gallagher AJ and Towner AV (2023) Novel aerial observations of a group of killer whales Orcinus orca in The Bahamas. Front. Mar. Sci. 10:1265064. doi: 10.3389/fmars.2023.1265064
Received: 21 July 2023; Accepted: 26 October 2023;
Published: 23 November 2023.
Edited by:
Nathan Jack Robinson, Fundación Oceanográfica, SpainReviewed by:
Rowan Jordaan, University of Pretoria, South AfricaJaime Bolaños-Jiménez, Universidad Veracruzana, Mexico
Copyright © 2023 Dixon, Gallagher and Towner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olivia F. L. Dixon, bGl2QGJlbmVhdGh0aGV3YXZlcy5vcmc=
 Olivia F. L. Dixon
Olivia F. L. Dixon Austin J. Gallagher
Austin J. Gallagher Alison V. Towner
Alison V. Towner