
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 01 November 2023
Sec. Marine Biology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1247263
The brachyuran crabs Carcinus maenas and Hemigrapsus sanguineus belong to the most ´successful´ invaders along the oceans coasts. In 2009, H. sanguineus appeared at the rocky intertidal of the island of Helgoland in the North Sea, where it encounters the native Green shore crab, C. maenas. H. sanguineus established a self-sustaining population, approaching in numbers and biomass that of C. maenas. Both species are considered to be opportunistic omnivores with variable food preferences and, thus, are potential competitors for food. To evaluate the intrinsic properties of either species to utilize food, we analyzed their stomach content, the morphology of the gastric mills, which shred the ingested food, the activities of digestive enzymes during a seasonal cycle, and the stable isotope ratios. A huge share of the stomach contents was macerated and, thus, could not be identified. The shares of animal food and algae food were almost equal in C. maenas but algae food dominated over animal food in H. sanguineus. The gastric mill of C. maenas shows blunt medial tooth and rounded lateral teeth, which indicates efficient grinding of a carnivorous diet. In contrast, the gastric mill of H. sanguineus shows sharp ridges, which facilitate cutting of algal food. The activities of the proteolytic enzymes trypsin and leucine-aminopeptidase were almost equal in both species with slightly higher activities in C. maenas in summer. The activities of the carbohydrases laminarinase and amylase dominated in H. sanguineus during all seasons. Stable isotope ratios indicate a higher degree of carnivory in C. maenas. The morphological and biochemical features indicate that C. maenas is better suited to utilize animal food and H. sanguineus algal food. Upon scarcity of animal food or severe competition with C. maenas, H. sanguineus may be able to increase the amount of algal food and to utilize it efficiently.
Unintentional dispersal of marine species into foreign regions has drastically increased along with human trading and shipping activities. The proliferation of foreign species may entail various consequences for established ecosystem and is considered to be a serious threat to biodiversity in marine habitats (Grosholz, 2002). Introduced species may compete for space and food and, in the worst case, displace native residents (Bax et al., 2003). Among marine taxa, brachyuran crabs (Crustacea, Decapoda) are common invaders due to their high larval dispersion capability as well as their habitat preference and lifestyle in coastal regions. Well-documented cases are the almost global dispersion of the green shore crab, Carcinus maenas (Linnaeus, 1758), and the Asian shore crab, Hemigrapsus sanguineus (De Haan, 1835) (Epifanio, 2013; Jungblut et al., 2017; Young and Elliott, 2020).
The Asian shore crab Hemigrapsus sanguineus is a newcomer at the western European coasts where it encounters the native green shore crab. Originally, H. sanguineus inhabited the coasts of the northwest Pacific but started in the early 1990s to settle along the North American coast (Williams and McDermott, 1990; Blakeslee et al., 2017). In the late 1990s, H. sanguineus was introduced most likely via ballast water to the French harbor of Le Havre (Breton et al., 2002). From there, it rapidly dispersed along the European Atlantic coasts through the English Channel into the North Sea (Dauvin et al., 2009). In 2009, H. sanguineus appeared at the rocky intertidal of the island of Helgoland, North Sea, where it established a self-sustaining population, rapidly approaching in number and biomass that of the native C. maenas (Jungblut et al., 2017).
Both species show distinctive biological and ecological parallels. Similar to juvenile green shore crabs, H. sanguineus inhabit the intertidal zone where they are strongly affected by the tides. At low-tide, they hide beneath stones, kelp, or mussel beads to protect themselves from desiccation and predators. Therefore, foraging periods are limited to the high-tide which may additionally increase the competition for food. Based on respiration measurements, Jungblut et al. (2018a) estimated that the energy consumption of the H. sanguineus population in the Helgoland intertidal almost equals that of C. maenas.
Both species are considered to be opportunistic onmivorous (Ropes, 1968; Ledesma and O´Connor, 2001). However, C. maenas shows a strong tendency for carnivory (Crothers, 1967; Ropes, 1968), whereas the food preference of H. sanguineus appears to be more variable. Stomach content analysis of wild-caught H. sanguineus revealed predominantly algal food components (Lohrer and Whitlatch, 1997; McDermott, 1999; Tyrrell and Harris, 2000). Other studies report a balanced or carnivorous diet depending on the environmental food availability (Brousseau and Baglivo, 2005). Apparently, H. sanguineus shows high adaptive capacity towards variable food sources.
Given a strong overlap in habitat use and food preferences of both species, a strong competition for both factors appears unavoidable. Comparative studies are scarce and were almost exclusively carried out at the coasts of USA where both species are invasive. Competition experiments for food and shelter showed that H. sanguineus dominated over C. maenas (Jensen et al., 2002). However, these experiments were carried out in the laboratory and direct competition was promoted. In our case, C. maenas is native, facing the invading H. sanguineus, which reduced comparability with previous reports.
To circumvent behavioral and ecological influences, we focused our study on the intrinsic morphological and physiological capabilities of food utilization in C. maenas and H. sanguineus. First, stomach contents of both species from the intertidal of Helgoland were analyzed over a seasonal cycle to estimate the shares of animal and algal food. Then, we described the morphology of the gastric mills and discussed the functional features in view of food preferences (Giddins et al., 1986; Skilleter and Anderson, 1986; Heeren and Mitchell, 1997; Salindeho and Johnston, 2003; Allardyce and Linton, 2010). A set of digestive enzymes from the midgut gland was analyzed, including trypsin and leucine aminopeptidase representing the proteolytic potential, and laminarinase and amylase representing the potential for carbohydrate utilization. Finally, analysis of stable isotopes of C and N was included in the comparison of the trophic position between both species. The results will show the potential of either species to utilize specific food sources and will support interpretations about their trophic spectrum and, to a certain degree, their ecological performance.
Specimens of Carcinus maenas (Linnaeus 1758) and Hemigrapsus sanguineus (De Haan 1853) were collected in April, June, August, and October 2016 in the intertidal of the island of Helgoland at a spot called “Kringel” (54°10´37N 7°53´07E). This area is characterized by boulders of sandstone and coarse gravel. Sampling was performed one hour after the first low tide at daytime. The animals were collected from under stones and macroalgae and from crevices. The crabs were placed in buckets, covered with moist macroalgae, and carried to the laboratories of the Marine Station Helgoland. In the lab, the catch was sorted according to species and sex and kept in aerated seawater, awaiting subsequent measurement and dissection.
The crabs (2.1 to 4.5 g) were sedated on ice for about 10 min before the carapace was rapidly pulled off and the crabs instantly died. The midgut gland was removed, transferred into weighed reaction cups, shock frozen in liquid nitrogen, and transferred on dry ice to Bremerhaven for biochemical analysis. The esophagus was cut close to the mouth opening and the chitinous stomach capsule was withdrawn as a whole with fine surgical forceps. The stomach was dissected and preserved in 70% ethanol for visual stomach content analysis. The stomach was cut ventrally, the content was removed with forceps, and transferred into 5-mL reaction cups. The fullness of the stomach was graded into five categories from empty (0%) to half-filled (50%) and fully filled (100%) with intermediate grades of 25% and 75%, respectively. Ten stomachs were used per species and per season and the values were averaged. The stomach content often consisted of an agglutinated mash. To disperse the mash, 3 mL of ethanol were added and the sample was vortexed or placed for a few seconds in an ultrasonic bath. The dispersed stomach content was transferred into a small petri dish and inspected under a stereo microscope. A definite identification of the stomach content was not possible due to the very small size of the items and the advanced digestion process. Obvious body parts of marine invertebrates as well as chitin residues, mussel shell fragments, or polychaete spines were classified as ´animal´. Greenish and brownish components, derived from green algae and brown algae, were classified as ´alga´. Mashed material without distinct color was classified as ´not identified (n.i.)´.
Scanning-electron-microscopy (SEM) was used to illustrate the morphology and ultrastructure of the gastric mill. The dissected stomach capsules were ventrally cut and opened under a stereo microscope. The stomach content was carefully rinsed out with a syringe. Subsequently, the entire gastric mill or individual lateral and median teeth of the gastric mill were dissected and prepared for SEM. The preparations were dehydrated in an ethanol series of 2 × 15 min in 50% ethanol, 2 × 15 min in 70% ethanol, 2 × 15 min in 90% ethanol. After air-drying overnight in a desiccator, the samples were mounted on SEM stubs with double-sided carbon tape. The stubs were sputter coated with gold-palladium and the samples were inspected and photographed under the SEM (FEI, Quanta FEG 200).
Frozen midgut gland tissue (about 65 mg) was transferred into 2-mL reaction cups and 500 µL of demineralized water was added. The tissue was thoroughly homogenized with a micro-pestle. Demineralized water was added to adjust a tissue concentration of 50 mg·mL-1. The homogenates were centrifuged for 15 min at 13,000 g and 4°C. Aliquots (250 µL) of the aqueous enzyme extract were transferred into new 1.5-mL reaction cups and stored at -80°C.
Samples taken in April were screened for a set of 19 enzymes with the commercial ApiZym test kit (BioMeríeux, Nürtingen, Germany) as per the manufacturer´s instructions. Sixty µL of midgut gland extract (50 mg·mL-1) were given into each of the test wells and incubated in darkness for 4 hours at room temperature. The dye reaction was initiated by the addition of ZymA and ZymB reagents. After 10 min, the intensity of the dye reaction was visually determined and classified from ´0´= no activity to ´5´= full activity.
The activities of endo- and exopeptidases were determined photometrically after Saborowski et al. (2004) and Saborowski et al. (2006), respectively. Trypsin activity (E.C. 3.4.21.4) was assayed with the chromogenic substrate L-BAPA (Nα-benzoyl-L-arginin-4-nitroanilid-hydrochloride, Applichem A5030). Fifty µL of the extract were pipetted into a glass semi-micro cuvette and 930 µL of Tris/HCl-buffer (0.1 mol·L-1, pH 7.0) were added. The cuvettes were first incubated for 5 min at room temperature (Jena Analytic Specord 200, TempControl). Subsequently, the enzymatic reaction was started with 20 µL of the substrate solution (50 mmol·L-1 in dimethylsulfoxide, DMSO). The final substrate concentration in the reaction mixture was 1 mmol·L-1. The increase of absorbance at 405 nm was recorded for another 5 min. Enzyme activity was expressed as U·g-1FM (= µmol·min-1·g-1FM) using the extinction coefficient ϵ405 = 10.2 L·mmol-1·cm-1.
Leucine aminopeptidase (LeuAP) was determined with the substrate L-leucine-p-nitroanilide (Sigma, L-9125). The substrate was dissolved in dimethylsulfoxide (DMSO) and applied at a final concentration of 1 mmol·L-1 in the reaction mixture. The activity was expressed as U·g-1FM (= µmol·min-1·g-1FM) using an extinction coefficient of 9.9 L·mmol-1·cm-1.
The enzymatic degradation of laminarin and starch were determined by the liberation of reducing sugars from natural substrates. Laminarinase (endo-β-1,3-glucanase) was assayed after Linton and Greenaway (2004) with slight modifications. The reaction mixture contained 20 µL of the tissue extract, 130 µL Na-acetate buffer (0.1 mol·L-1, pH 5.5) and 50 µL of a laminarin solution (1% (w/v) in a. dest.). The sample blank of each sample contained 20 µl of the respective tissue extract and 180 µl Na-acetate buffer and the substrate blank was prepared with 50 µL of laminarin solution. The reaction mixtures were incubated for 10 min in a thermomixer at room temperature and permanent agitation (300 rpm). Thereafter, the reaction was stopped by addition of 50 µL HCl (0.3 mol·L-1), incubated for another 10 min, and neutralized by addition of 10 µL K2CO3-solution (2.5 mol·L-1). Subsequently, the reaction mixture was assayed for reducing sugars with the tetrazolium blue method after Jue and Lipke (1985). Fifty µL of the tests, the blanks, and glucose standard (0.22 to 1.1 mmol·L-1) were transferred into new reaction cups, mixed with 1 mL of the tetrazolium dye reagent (see below), and incubated for 3 min at 100°C in a water bath. Subsequently, the reaction cups were cooled in an ice-water bath to stop the dye-reaction. The absorbance of the tests, blanks, and the glucose standards were read at 660 nm. The tetrazolium dye reagent consisted of equal parts of a tetrazolium blue chloride solution (0.2% w/v) in 0.1 mol·L-1 NaOH and a potassium sodium tartrate solution (0.5 mol·L-1). Amylase activity was assayed as described for laminarinase but with soluble starch as the substrate.
Muscle tissue from the claws of either species was dissected, shock frozen in liquid nitrogen, and lyophilized for 24 hours. Samples of 1 to 2 mg dried muscle tissue were weighed out in tin capsules and send for analysis to Agroisolab GmbH (TÜV Rheinland Group, Jülich, Germany).
Enzyme activity data sets were tested for normal distribution with the Shapiro-Wilk normality test. Statistical comparison was done by 2-way-ANOVA considering the factors ´species´ and ´season´, followed by the Tukey´s multiple comparisons test. The significance level was α = 0.05. These statistical analyses and the graphs were done with the software GraphPad Prism version 7.05 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. Cluster analysis and principal component analysis (PCA) of enzyme activities were performed with the software Primer 7 (ver. 7.0.20) from Primer-e on normalized data. The resemblance measure was the Euclidean distance, and the cluster mode was group average. We also investigated the overall variability in isotopic values for both species. Two metrics were applied: a) the total area (TA), calculated from the convex hull surrounding the outer data points in a δ13C/δ15N biplot and b) the standard ellipses area (SEA). These calculations were done with the package Stable Isotope Bayesian Ellipses in R (SIBER Vers. 2.1.8) (Jackson et al., 2011).
The stomach contents varied considerably in both species over the seasons (Table 1). A huge share of the stomach content was macerated and, thus, could not be identified. The stomach fullness of C. maenas was on average slightly lower than that of H. sanguineus. In C. maenas, it accounted for about 38% in June and 56% in August. Animal items accounted for 17.5 to 31.7%. The amounts of algae items were quite high in April and June and very low in August and October (Table 1). The lowest average stomach fullness in H. sanguineus (44%) appeared in June and the highest of 63% in April. The stomachs of H. sanguineus contained 25.8 to 60% algae items and 7.5 and 28.8% animal items (Table 1). Different to C. maenas, the share of algal items remained high in August and October.
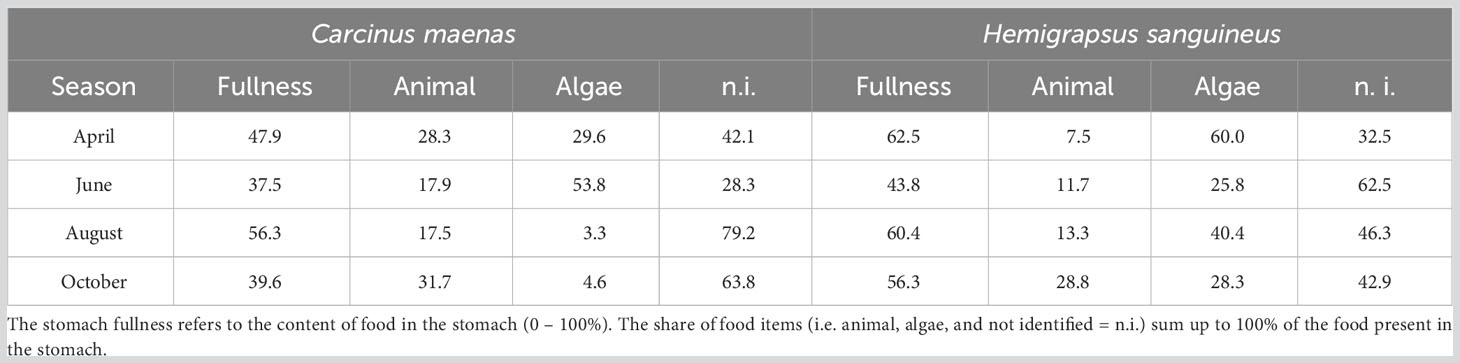
Table 1 Relative values (%) of stomach fullness and shares of stomach content of Carcinus maenas and Hemigrapsus sanguineus from the rocky intertidal of Helgoland (North Sea).
The stomachs of both species showed the typical structures of the brachyuran gastric mill (Figures 1A, B). It consists basically of one median and two lateral robust calcified structures which complete the maceration of ingested material by complex movements facilitating squeezing, cutting, and grinding. A median tooth extends dorsally from the posterior end of the urocadiac ossicle into the lumen of the cardia. The elaborated lateral teeth protrude laterally from the zygocardiac ossicle along the stomach wall.
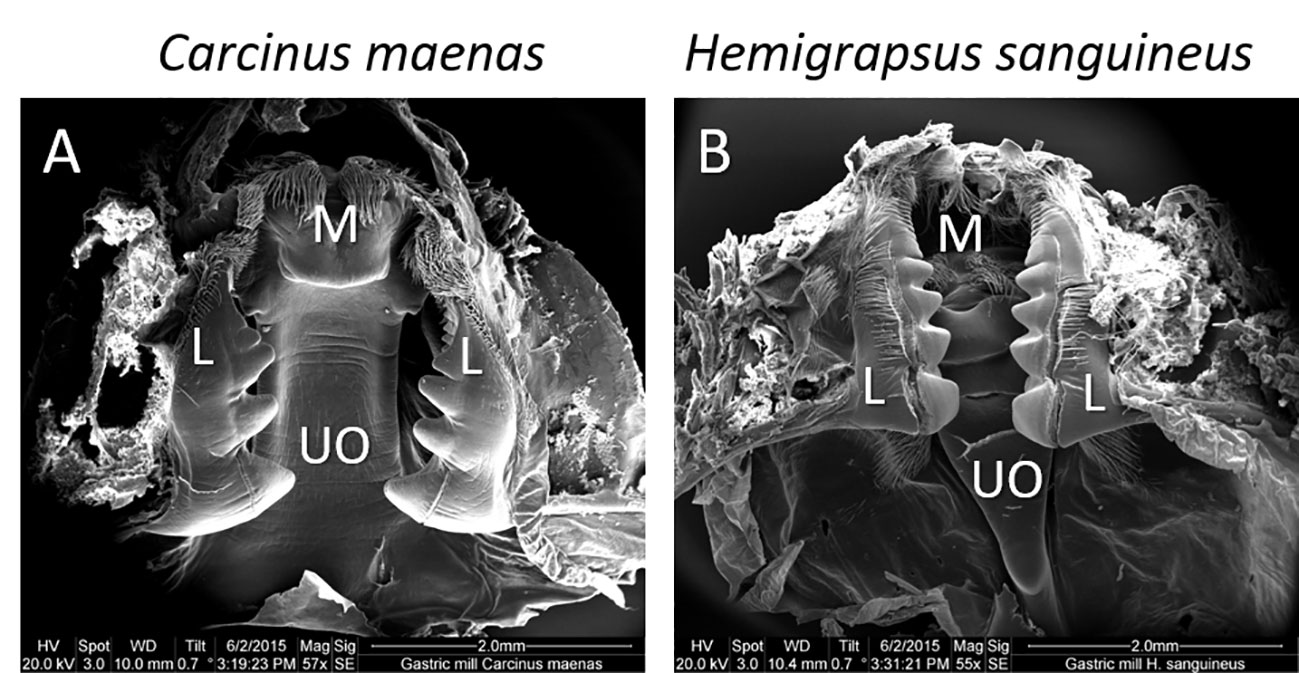
Figure 1 Scanning electron micrograph of the gastric mill of (A) Carcinus maenas and (B) Hemigrapsus sanguines. Overall impression (ventral view) of the gastric mill with the median (M) and lateral (L) teeth and the urocardiac ossicle (UO).
In C. maenas, the gastric mill appears quite massive. The urocardiac ossicle is consistently broad, forming a parallel-edged plate (Figure 2A). The posterior tip forms the medial tooth bearing a head-like structure (protrusion) which is slightly smaller in width than the urocardiac ossicle. The posterior protrusion possesses a small lip-like bulge at the anterior edge. A pair of cusps, pointing out ventrally, is located anterio-lateral to the protrusion. The lateral teeth of C. maenas possess four massive cusps, decreasing in size from anterior to posterior and merging into a smaller crest-like structure (Figure 2C). The cusps of both lateral teeth form a pair of claws and a basket-like space in which the median tooth may move. In an about rectangular median directed position from the huge cusps, the lateral teeth possess a series of seven about equally-sized blunt ridges (Figure 2E). Theses ridges may form within the putative basket a complement for the median tooth in shredding food.
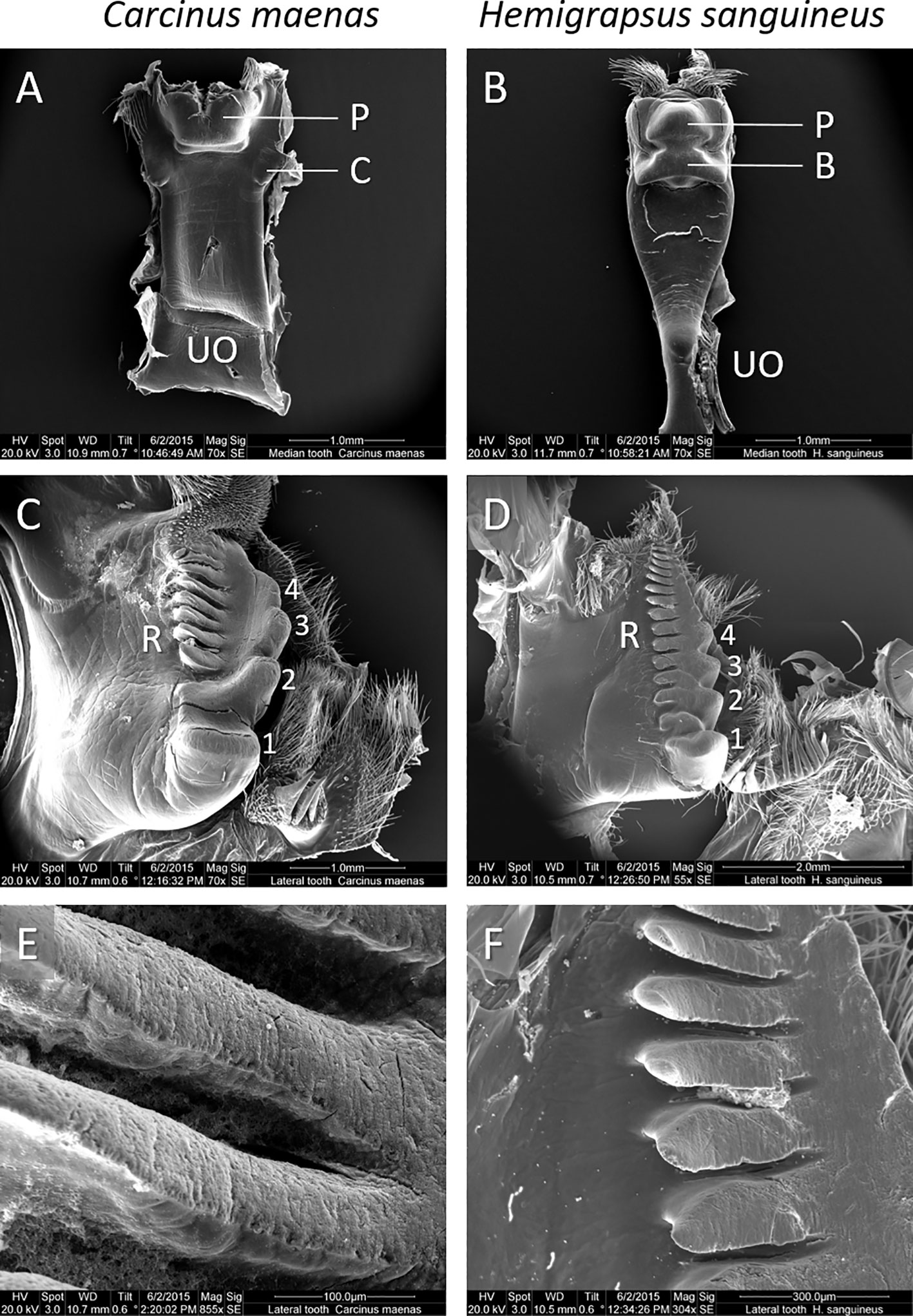
Figure 2 Details of the gastric mill of C. maenas (C.m., left panel) and H. sanguineus (H.s., right panel). The median teeth of (A) C. m. and (B) H. s. (UO: urocardiac ossicle, P: protrusion, C: cusps, B: bulge). The lateral teeth of either specie (C, D) showing massive cusps (1 – 4) and ridges (R). Magnification of (E) blunt ridges of C.m. and (F) sharp-edged ridges of H.s.
The gastric mill of H. sanguineus appears more elaborated. The median tooth arises from a waist urocardiac ossicle at the anterior side which extends to a broad structure at the posterior side bearing the quite massive and complex median tooth (Figure 2B). The median tooth consists of well-defined protrusion with two posterio-lateral arches. Anterior to the main protrusion, the median tooth possesses a wide and sharply contoured bulge which occupies the width of the median tooth. The lateral teeth of H. sanguineus show four cusps (Figure 2D). These cusps are less massive than those of C. maenas and, except the smaller posterior cusp, of about equal size. The cusps appear sharper and more edged than those of C. maenas. Another difference show the series of 14 ridges at the median side of the lateral teeth. These are located closer to the row of cusps, apparently forming a functional unit. The ridges show sharp edges with signs of wear from grinding at their flattened surfaces (Figure 2F).
The ApiZym enzyme screening revealed high activities of most enzymes in the midgut glands of both species (Table 2). Only the esterase (C4), lipase (C14), cysteine arylamidase, and α-galactosidase showed low activities. The substrate for chymotrypsin is not sensitive to hydrolysis by crustacean chymotrypsin as already previously noted (Saborowski, pers. obs.).

Table 2 ApiZym semi-quantitative enzyme screening of midgut gland extracts of Carcinus maenas and Hemigrapsus sanguineus from the rocky intertidal of Helgoland (North Sea).
The trypsin activities of individual C. maenas ranged from 0.20 to 1.44 U·mgFM-1. The lowest average activity of 0.37 ± 0.04 U·mgFM-1 appeared in October and the highest of 0.77 ± 0.18 U·mgFM-1 in August (Figure 3A). The span of trypsin activities in H. sanguineus was in the same range from 0.22 to 1.64 U·mgFM-1. Minimum average trypsin activities appeared in April (0.40 ± 0.08 U·mgFM-1) and the maximum in October (0.54 ± 0.23 U·mgFM-1, Figure 3A). Two-way ANOVA showed no significant effect of season, no difference between species, and no interaction between season and species (Table 3).
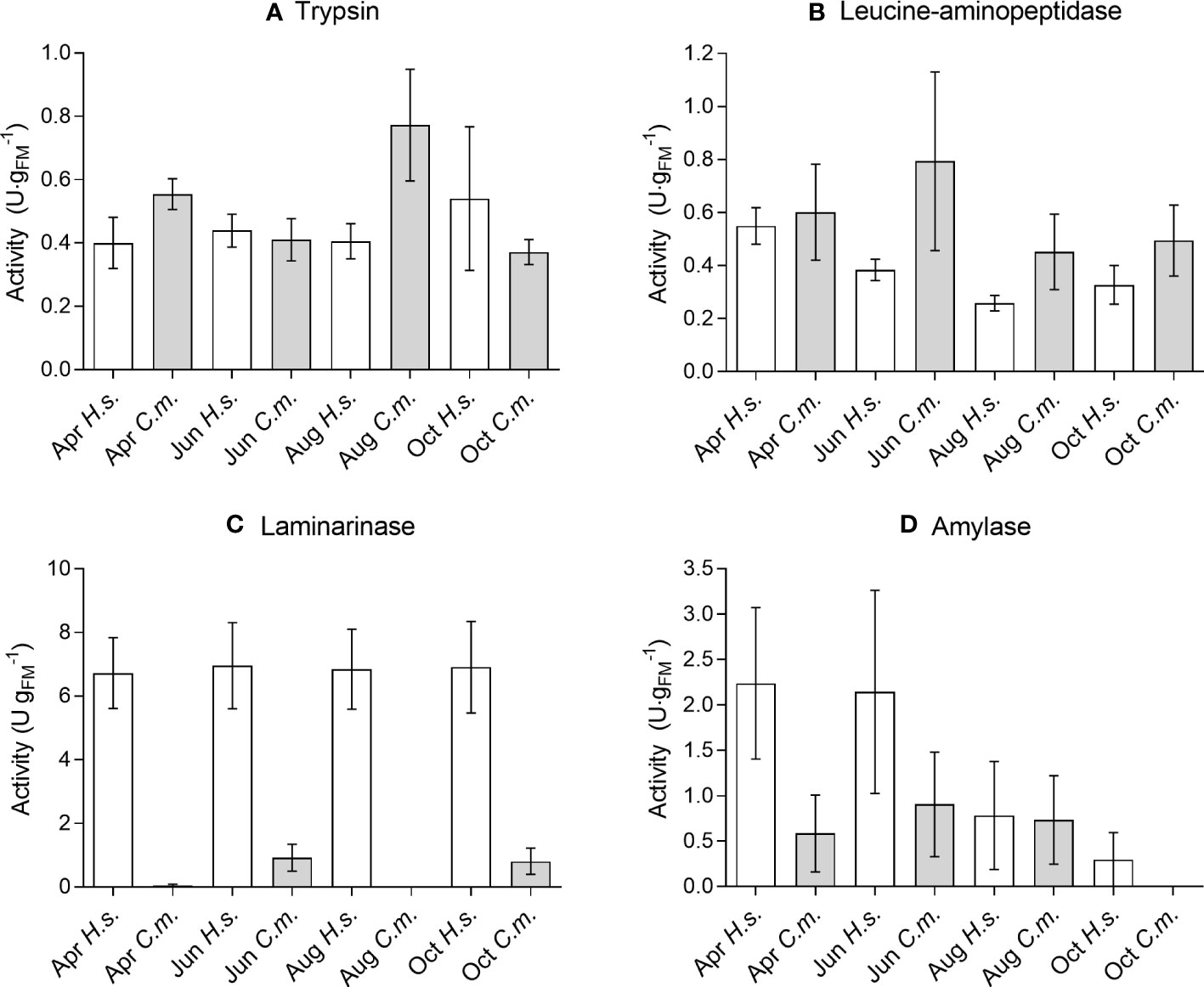
Figure 3 Seasonal variation of digestive enzyme activities (A) Trypsin, (B) Leucine-aminopeptidase, (C) Laminarinase, (D) Amylase in the midgut gland of Carcinus maenas (C.m.) and Hemigrapsus sanguineus (H.s.) samples in April (Apr), June (Jun), August (Aug), and October (Oct). Means ± SEM, n = 6-15.
The activities of the exopeptidase (leucine-AP) showed strong variation in C. maenas and ranged from 0.09 to 2.33 U·mgFM-1. The seasonal minimum appeared in August (0.45 ± 0.14 U·mgFM-1) and the maximum in June (0.79 ± 0.34 U·mgFM-1, Figure 3B). The activities of leucine-AP in H. sanguineus were in the same range from 0.09 to 0.71 U·mgFM-1. Minimum average activities appeared in August (0.26 ± 0.03 U·mgFM-1) and the maximum in April (0.55 ± 0.07 U·mgFM-1, Figure 3B). Similar to trypsin, the leucine-aminopeptidase showed no significant variation between season and between species (Table 3).
The ability of laminarin-degradation (laminarinase, endo-1,3-β-glucanase) was lower in C. maenas than in H. sanguineus. In many specimens, the activity was below the detection limit. So it ranged from 0 to 2.51 U·mgFM-1. The lowest seasonal average appeared in August, showing no activity and the maximum appeared in June (0.92 ± 0.42 U·mgFM-1). The laminarinase activity in H. sanguineus was significantly higher than in C. maenas and quite similar between seasons with lowest average values of 6.73 ± 1.11 U·mgFM-1 in April and 6.96 ± 1.36 U·mgFM-1 in June (Figure 3C). Two-way ANOVA showed no effect of season but a significant difference between species. There was no interaction between season and species (Table 3).
Amylase activities of C. maenas ranged from 0 to 2.95 U·mgFM-1. The lowest average activities appeared in October (0 U·mgFM-1) and the highest in June (0.91 ± 0.58 U·mgFM-1). H. sanguineus showed amylase activities from 0 to 8.32 U·mgFM-1. The average activities decreased continuously from April (2.24 ± 0.84 U·mgFM-1) to October (0.30 ± 0.30 U·mgFM-1, Figure 3D). The statistical analysis showed a significant effect of season, species, and interaction (Table 3).
The principal component analysis (PCA, Figure 4) revealed that 38.5% of the variation were covered by PC 1 which was primarily determined by laminariase and amylase. PC2 (27.3%) was determined by leucine aminopeptidase and PC3 (23.6%) by trypsin. The cluster analysis (dendrogram) of enzyme activities shows a clear separation between C. maenas and H. sanguineus. Only 3 of 24 specimens were grouped among the other species (Figure 5).
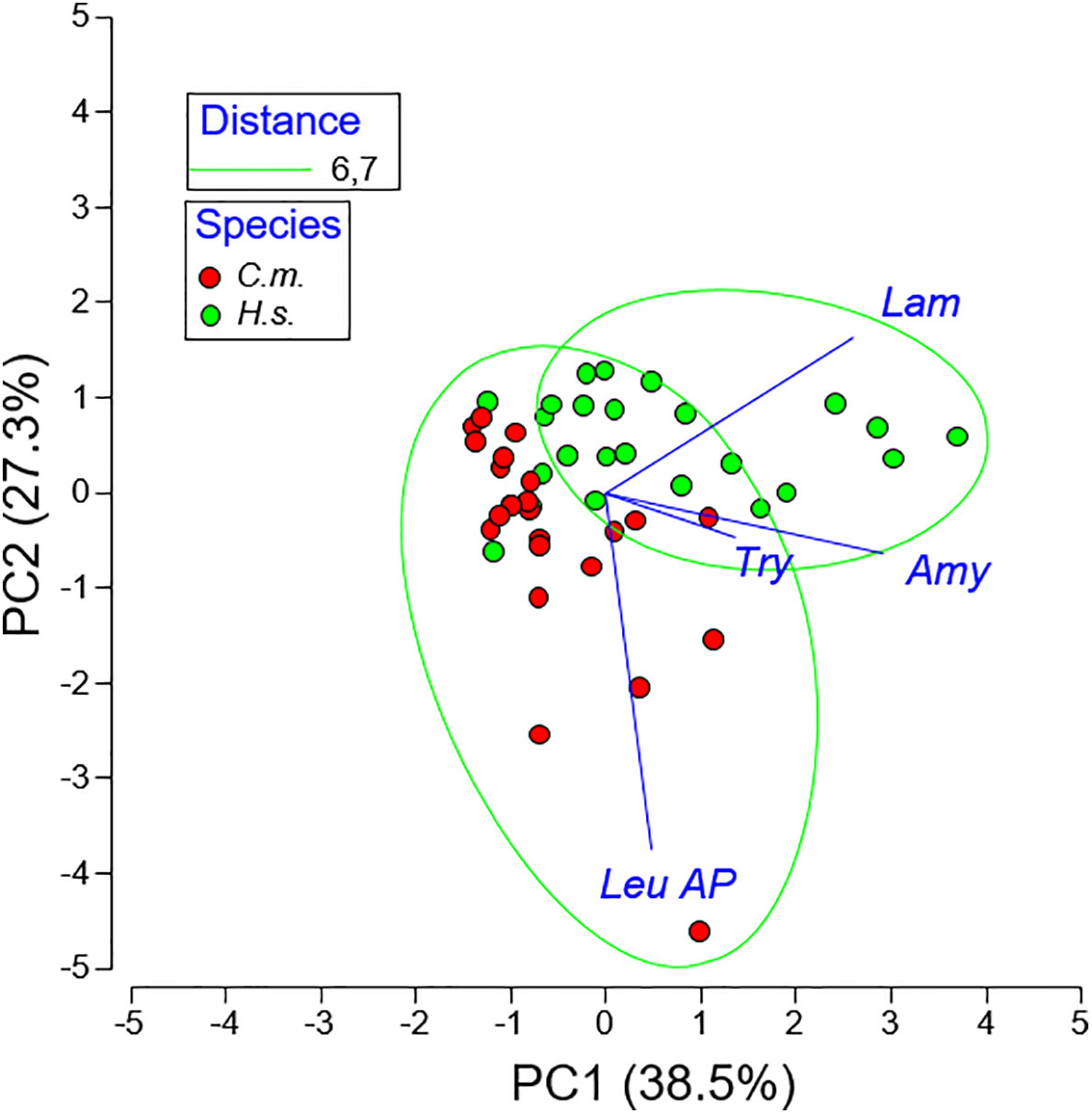
Figure 4 Principal Component Analysis (PCA) of four digestive enzyme activities of Carcinus maenas (C.m.) and Hemigrapsus sanguineus (H.s.).
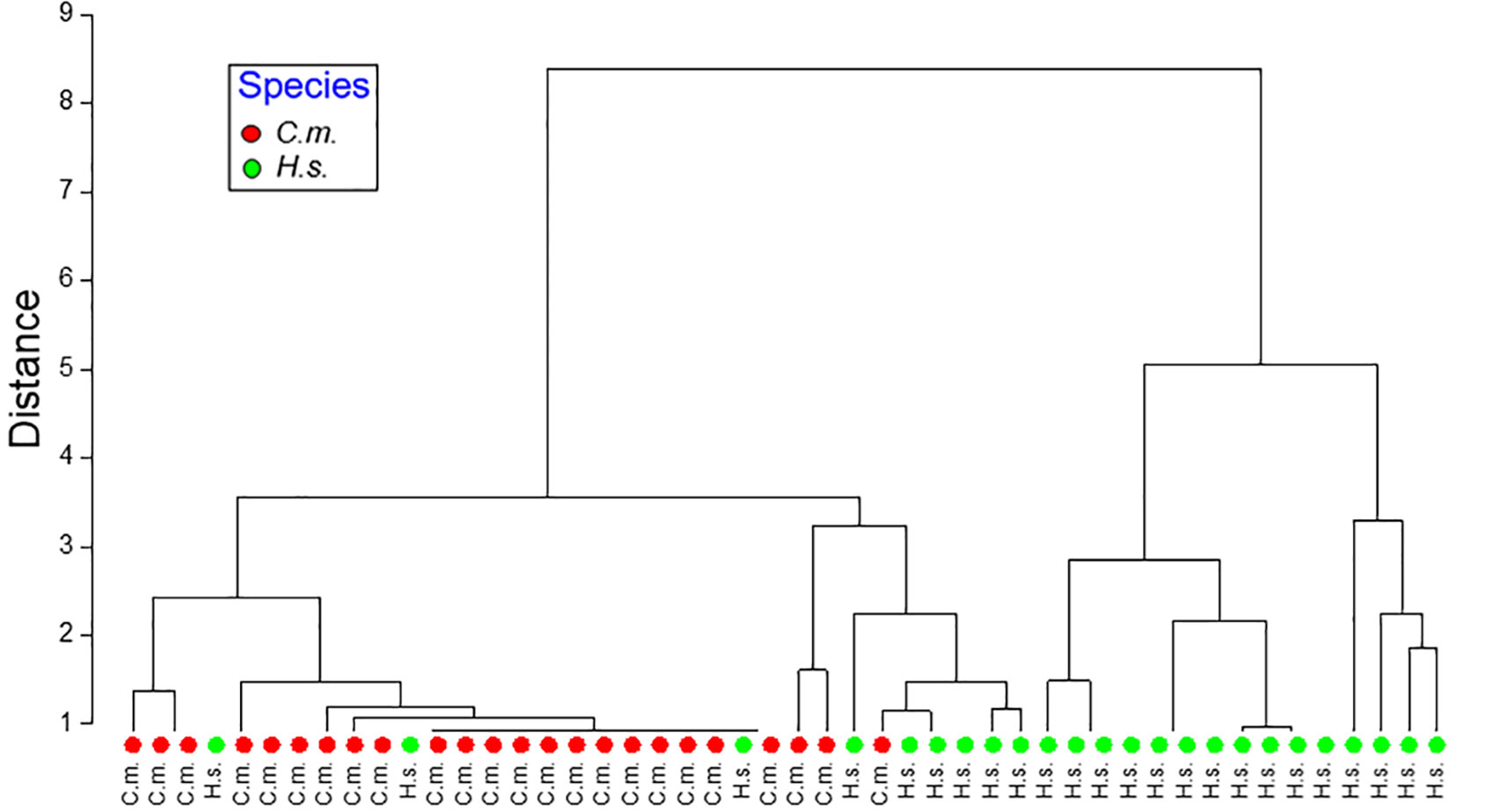
Figure 5 Hierarchical cluster analysis (Euclidian Distance) of digestive enzyme activities of Carcinus maenas (C.m.) and Hemigrapsus sanguineus (H.s.).
There were no statistically significant differences in the N- and C-isotope ratios between females and males within either species (unpaired t-tests, two-tailed p-values > 0.05, n = 5). Therefore, data of females and males of either species were combined to one data set (n = 10, Figure 6). The mean stable isotope value of δ13C was significantly higher (p < 0.0025) in C. maenas (-14.6 ± 0.3) than in H. sanguineus (-15.4 ± 0.6). Similarly, the δ15N-value was significantly higher (p < 0.0001) in C. maenas (16.9 ± 0.3) than in H. sanguineus (15.6 ± 0.5). The average difference in the δ13C-values between species was 0.75 and that in the δ15N values was 1.24. The convex hull total areas (TA) was 0.56 for C. maenas and 1.7 for H. sanguineus. The TAs of both species did not overlap (Figure 6). The standard ellipses area (SEA) of C. maenas was 0.32 and that of H. sanguineus 0.80.
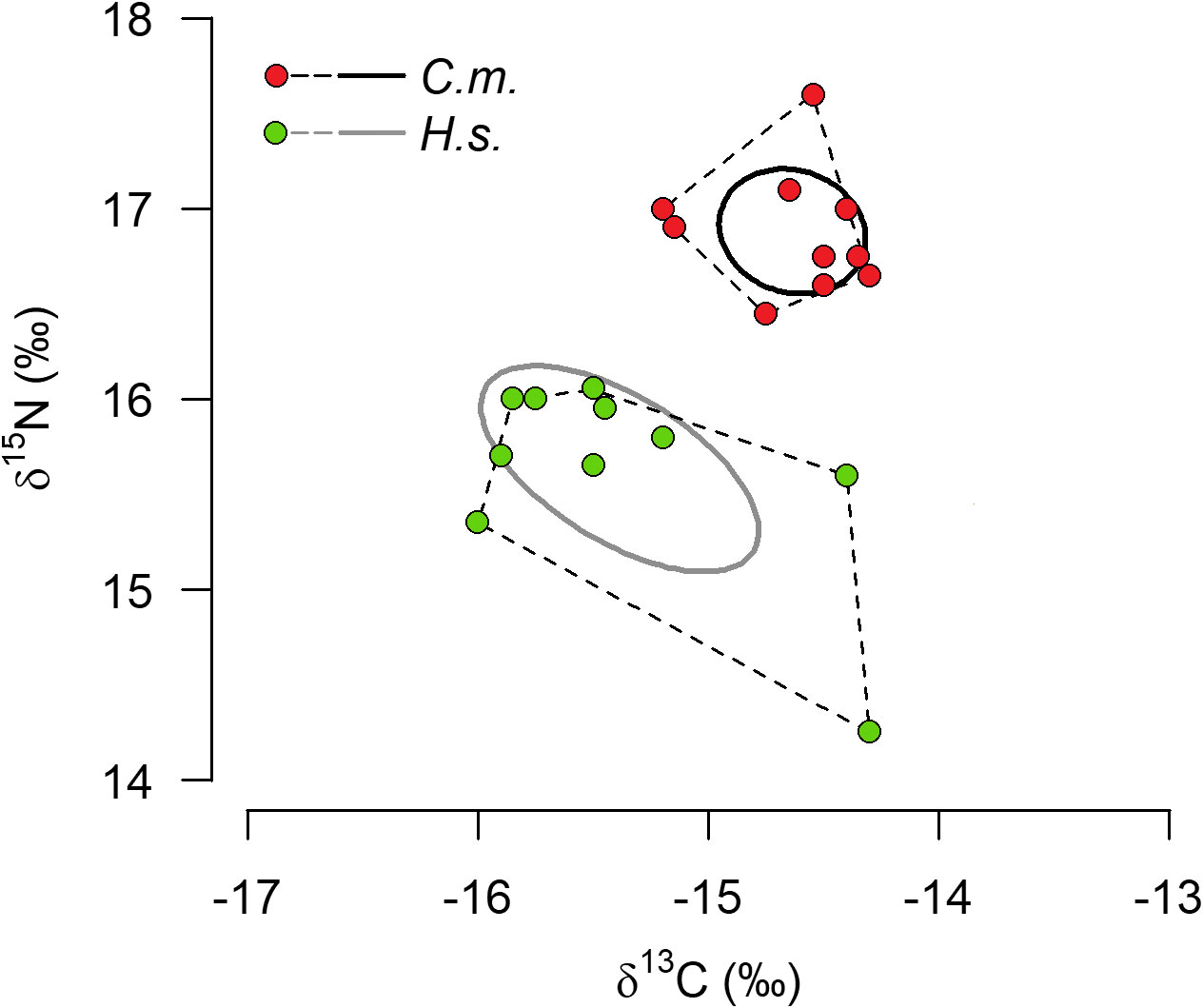
Figure 6 Isotope niche widths of Carcinus maenas (C.m) and Hemigrapsus sanguineus (H.s.). Display of Standard ellipses areas (SEA, solid lines) and total area from convex hull (TA, dotted lines) of 10 individulas of either species using Stable Isotope Bayesian Ellipses in R (SIBER).
Both species, the native Carcinus maenas and the invasive Hemigrapsus sanguineus, ingested animal as well as algal material, confirming omnivorous feeding. However, our studies revealed distinct intrinsic features, such as the morphology of the gastric mill and the activities of digestive enzymes indicating a predisposition for the prevalent food spectrum of either species.
The stomach contents of both species consisted of greenish algae and marine invertebrates, as deduced from fragments of bivalves, gastropods, polychaetes, and crustaceans. Carrion is probably ingested as well. Unfortunately, identification of food items was strongly hampered, because the stomach content was severely macerated and partially digested. Stomach fullness and appearance of the content varied strongly between individuals. Plant material was present in both species, but distinct differences were evident as well. Overall, C. maenas showed a higher share of animal food items than H. sanguineus. H. sanguineus, in turn, contained more algal items than C. maenas. Compared to the plant material, the amount of animal tissue may be underestimated because only the solid parts of animals remained recognizable in the stomach whereas the soft tissue is mostly macerated and not identifiable. Accordingly, it appears reasonable that a high share of unidentified material derived from animal soft tissue.
Our observations agree with previous studies. C. maenas from the Portuguese Mondego estuary showed opportunistic feeding behavior with local differences. Overall, the food of C. maenas was dominated by brown shrimp, Crangon crangon, the polychaete Hediste diversicolor, and fish or fish remains. Algae were present as well (Baeta et al., 2006). Green shore crab from Nova Scotia preferred bivalves over algae, gastropods, and crustaceans and showed seasonal differences (Elner, 1981). Field studies on H. sanguineus confirmed omnivorous feeding and reported a variety of algae and vascular plant remains in the stomachs (Ledesma and O´Connor, 2001). Griffen et al. (2012) reported that H. sanguineus from New England were omnivorous, consuming macroalgae and a variety of animal prey. The amount of 56.3% algal diet in April closely matches our results. Laboratory investigations, in turn, showed that H. sanguineus are opportunistic omnivores. The crabs showed well-developed predatory tendencies and a preference for animal food items over algae (Brousseau and Baglivo, 2005; Bleile and Thieltges, 2021) or no clear preference for mollusks or algae (Bourdeau and O´Connor, 2003). The laboratory studies may have biased the feeding behavior because carnivore diet was readily available. Brousseau and Baglivo (2005) suggested that starvation and competition for food can alter the food selection of H. sanguineus. Crabs that starved for 5 days consumed both food types (algal and animal) more often than those that starved for one day only, which preferred animal food. Moreover, increased crab density lead to increased diet spectrum, suggesting that H. sanguineus is prepared to switch to algal diet in case of food scarcity or competition for food.
Both species from Helgoland showed seasonal variation of their diet, which differed more in the amount of algae than in the amount of animal diet. C. maenas ingested a high amount of algae in spring but ceased feeding on algae in summer and autumn. This seems to reflect, on one hand, the high productivity and availability of algae in spring but, on the other hand, also the preference for animal food when the biomass increased during the seasonal course of succession (e.g. Munda and Markham, 1982; Janke, 1990 and references cited therein). Moreover, the palatability of young and fresh algae in spring may be better than that of older algae in summer and autumn. In H. sanguineus, we observed a very similar seasonal course of herbivory as Griffen et al. (2012) in crabs from New England with a decrease from April to June, an increase in August and again a decrease in October. The authors ascribed this to the seasonal occurrence and recruitment pulses of prey organisms, such as barnacles or bivalves, which are preferred by H. sanguineus upon appearance. Such behavior in food selection appears reasonable in the Helgoland intertidal as well.
The gastric mill of decapod crustaceans facilitates the internal maceration of ingested food items and mix the food mash with gastric fluid from the midgut gland, initiating the first steps of extracellular digestion (Icely and Nott, 1992; Saborowski, 2015). The gastric mill is a complex calcified structure, basically consisting of two lateral and one median tooth movable against each other. The gastric mill of brachyuran decapods shows features which are characteristic for their preferred diet. A detailed description of the morphology of brachyuran decapods, the properties of the gastric mills of carnivorous, omnivorous, and herbivorous species, as well as their putative function is given elsewhere (e.g. Heinzel, 1988; Heinzel et al., 1993; Linton et al., 2009; Allardyce and Linton, 2010; Brösing, 2010; Allardyce and Linton, 2011).
Unlike in mammals, molar-like processes and rounded shapes of the gastric mill in crabs are indicative for the grinding of soft animal tissue as well as hard structures such as chitin or calcareous mollusk shells (Skilleter and Anderson, 1986). Characteristics of the gastric mills of herbivorous crabs are raised transverse ridges which facilitate cutting of fibrous food (Giddins et al., 1986). Both characteristics appear in omnivorous species, though with different manifestation (Salindeho and Johnston, 2003).
The gastric mills of C. maenas and H. sanguineus revealed differences. The gastric ossicles of C. maenas appear blunt and smooth. Such blunt and smooth surfaces are suitable to grind soft food items, which are typical for animal food. The huge cusp of the median tooth and the few ridges of the lateral teeth function like a mortar and pestle. Such structure coincide with carnivorous feeding and were correspondingly described in various carnivorous crustacean (Heeren and Mitchell, 1997; Salindeho and Johnston, 2003; Allardyce and Linton, 2010).
The gastric mill of H. sanguineus has a different appearance. It is characterized by well-defined sharp edges which form many cutting surfaces. As a result of the masticatory movement, the edges of the median tooth slide along those of the lateral teeth, capable of cutting fibrous material. Therefore, gastric mills with such structures are suitable to chop plant material and are generally ascribed to herbivorous or omnivorous crustacea (e.g. Giddins et al., 1986; Allardyce and Linton, 2010).
These results indicate, that the gastric mills of both species are capable of processing carnivore as well as herbivore food, however with apparent preferences for carnivore diet in C. maenas and herbivore diet in H. sanguineus. Accordingly, these results support the previous observations of higher animal content in the stomach of C. maenas and more algae in H. sanguineus.
The midgut gland (syn. hepatopancreas) is the principal organ of biochemical food utilization (comprehensively reviewed by Vogt, 2019). The relation between feeding mode and digestive enzyme activities in decapod crustaceans is ambiguous. Generally, it is accepted that the major enzymes represent the ingested food, meaning that carnivorous species possess high proteolytic activities and herbivorous species high carbohydrase activities. Omnivorous species show intermediate activities (e.g. Sather, 1969; Lee et al., 1984; Lovett and Felder, 1990; Johnston and Yellowlees, 1998; Figueiredo and Anderson, 2009). The preliminary screening of digestive enzymes, comprising esterases, peptidases, and glucosidases showed quite high activities in both species. For our inter-specific comparison, we chose two protein degrading enzymes and two carbohydrate degrading enzymes, representing the principal constituents of food organisms.
Animal food contains higher amounts of protein than algal food and, thus, is a valuable source for nitrogen and essential amino acids. For example, the crude protein content of the green algae Ulva lactuca, a potential food for both crab species, accounts for 23 to 26% of the dry mass (Bikker et al., 2016). Compared with this, the protein content of blue mussel (Mytilus edulis) flesh and that of Macoma balthica can exceed 60 to 70% of the dry mass (Dare and Edwards, 1975; Beukema and de Bruin, 1977).
The metabolic utilization of proteins is facilitated by proteases, syn. peptidases. Trypsin is a common endopeptidase in brachyuran crabs which cleaves proteins and peptides within the amino acid chain at the carboxyl side of arginine and lysine, thus generating peptides for further degradation (Muhlia-Almazán et al., 2008). Leucine-aminopeptidase is an exopeptidase, preferably liberating leucine at the N-terminus of peptides but often shows a wider substrate specificity (Matsui et al., 2006). Both enzymes were selected as representative for the concerted potential to utilize protein as the principal sources of dietary nitrogen and essential amino acids.
C. maenas as well as H. sanguineus show almost similar proteolytic activities which may indicate an equal ability to digest protein and, thus, supports their omnivorous feeding mode. Apparently, there is no need for H. sanguineus to increase proteolytic activity to compensate for nitrogen deficiency as a consequence of extended herbivorous feeding. Our findings are in agreement with Johnston and Freeman (2005) who found intermediate proteolytic activities in omnivorous species, indicative of their wide food spectrum. Although not statistically significant, the trypsin activity of C. maenas increased markedly in August, which coincides with the minimum of ingested algal material. It may be suggested that C. maenas increase their digestive efficiency to accumulate energy reserves.
Laminarinases (β-1,3-glucanase) are common digestive enzyme in many invertebrates where they hydrolyze the algal storage product laminarin (Piavaux, 1977). Likewise, they are present in marine, terrestrial, and fresh-water decapods (Johnston and Freeman, 2005; Linton et al., 2015). α-amylases facilitate the hydrolysis of α-1,4-glycosidic bound carbohydrates such as starch and glycolgen. They are common in herbivorous and omnivorous crustaceans but also show high activities in some carnivorous species (Rodríguez-Viera et al., 2016). In the marine environment, starch and starch-type polysaccharides are present as storage product of cellular and filamentous green algae including the order Ulvales (Love et al., 1963; Busi et al., 2013; Dominguez and Loret, 2019; Prabhu et al., 2019). Green algae like Ulva spp. or Enteromorpha spp. are common in the rocky intertidal of Helgoland and the greenish food items in the stomach of both species strongly suggest that these algae were ingested by both C. maenas and H. sanguineus.
The consistently higher laminarinase and amylase activities in H. sanguineus indicate that the respective carbohydrates present in algae will be better utilized by H. sanguineus than by C. maenas. Consequently, H. sanguineus can fall back on a rich algal food source and evade competition with other species for carnivore diet.
Other biochemical markers support the macroscopic and microscopic observations of the stomach contents. Jungblut et al. (2018b) showed in both species from Helgoland that trophic fatty acid indices for diatoms (Bacillariophyceae), green algae (Chlorophyta), and especially brown algae (Phaeophyceae) were higher in H. sanguineus than in C. maenas, suggesting a higher share of herbivorous feeding by the invader than by the native crab. Our stable isotope data do not allow for the assignment of the trophic level due to the lack of baseline data (Post, 2002). They are in the same range as reported for other crustacean species and different tissues (e.g. Rudnick and Resh, 2005; Bodin et al., 2007) but higher than stable isotope data previously reported for C. maenas and H. sanguineus (e.g. Watts et al., 2011; Wahyudi et al., 2013; Guo et al., 2016; Bordeyne et al., 2017). Nevertheless, the direct comparison clearly show higher δ15N- and δ13C-values in C. maenas than in H. sanguineus, indicating a higher degree of carnivory in C. maenas. Moreover, C. maenas occupied a smaller isotopic niche than H. sanguineus. The latter species appears to consume more carbon-depleted food (i.e., with more negative values). This may suggest that H. sanguineus consumes different algal species than C. maenas, which, in turn, may reduce competition for algal food in the rocky intertidal of Helgoland.
Our results draw a coherent picture about the trophic preference of both species and support previous studies about their feeding ecology. C. maenas shows distinct morphological and physiological adaptation for animal food and also seem to prefer it in the rocky intertidal of Helgoland. H. sanguineus, in contrast, shows clear morphological and biochemical adaptations for utilizing algal diet. If animal diets becomes scarce or competition with C. maenas or other species for animal food increases, H. sanguineus can efficiently utilize algal diet as well. Although both species occupy the same habitat, they are flexible to exploit different trophic niches and, thus, may widely coexist in their Helgoland habitat.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on animals in accordance with the local legislation and institutional requirements.
RS: conceptualization, data curation, writing – original draft preparation, reviewing and editing, supervision. PB: investigation, methodology, data curation, writing, reviewing and editing. MK: methodology, data curation, writing –reviewing and editing. SJ: conceptualization, data curation, writing – original draft preparation, reviewing and editing, supervision.
We wish to thank Ms. Kristine Reuter for laboratory support at the Alfred-Wegener-Institute in Bremerhaven and the technical staff of the Department of Marine Zoology at the University of Bremen, the Marine Station Helgoland.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Allardyce B. J., Linton S. M. (2010). Functional morphology of the gastric mills of carnivorous, omnivorous, and herbivorous land crabs. J. Morphology 271 (1), 61–72. doi: 10.1002/jmor.10781
Allardyce B. J., Linton S. M. (2011). Characterisation of cellulose and hemicellulose digestion in land crabs with special reference to Gecarcoidea natalis. Aust. J. Zoology 59, 380–391. doi: 10.1071/ZO11054
Baeta A., Cabral H. N., Marques J. C., Pardal M. A. (2006). Feeding ecology of the green crab, Carcinus maenas (L., 1758) in a temperate estuary, Portugal. Crustaceana 79 (10), 1181–1193. doi: 10.1163/156854006778859506
Bax N., Williamson A., Aquero M., Gonzalez E., Geeves W. (2003). Marine invasive alien species: a threat to global biodiversity. Mar. Policy 27 (4), 313–323. doi: 10.1016/S0308-597X(03)00041-1
Beukema J. J., de Bruin W. (1977). Seasonal changes in dry weight and chemical composition of the soft parts of the tellinid bivalve Macoma balthica in the Dutch Wadden Sea. Netherlands J. Sea Res. 11 (1), 42–55. doi: 10.1016/0077-7579(77)90020-5
Bikker P., van Krimpen M. M., van Wikselaar P., Houweling-Tan B., Scaccia N., van Hal J. W., et al. (2016). Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. J. Appl. Phycology 28, 3511–3525. doi: 10.1007/s10811-016-0842-3
Blakeslee A. M. H., Kamakura Y., Onufry J., Makino W., Urabe J., Park S., et al. (2017). Reconstructing the invasion history of the Asian shore crab, Hemigrapsus sanguineus (De Haan 1835) in the Western Atlantic. Mar. Biol. 164, 47. doi: 10.1007/s00227-017-3069-1
Bleile N., Thieltges D. W. (2021). Prey preferences of invasive (Hemigrapsus sanguineus, H. takanoi) and native (Carcinus maenas) intertidal crabs in the European Wadden Sea. J. Mar. Biol. Assoc. United Kingdom 101 (5), 811–817. doi: 10.1017/S0025315421000655
Bodin N., Le Loc´h F., Hily C. (2007). Effects of lipid removal on carbon and nitrogen stable isotope ratios in crustacean tissues. J. Exp. Mar. Biol. Ecol. 341 (2), 168–175. doi: 10.1016/j.jembe.2006.09.008
Bordeyne F., Davoult D., Migné A., Bertaud du Chazaud E., Leroux C., Riera P. (2017). Trophic structure of two intertidal Fucus spp. communities along a vertical gradient: similarity and seasonal stability evidenced with δ13C and δ15N. J. Sea Res. 120, 50–59. doi: 10.1016/j.seares.2016.12.004
Bourdeau P. E., O´Connor N. J. (2003). Predation by the nonindigenous Asian shore crab Hemigrapsus sanguineus on macroalgae and molluscs. Northeastern Nat. 10 (3), 319–334. doi: 10.1656/1092-6194(2003)010[0319:PBTNAS]2.0.CO;2
Breton G., Faasse M., Noël P., Vincent T. (2002). A new alien crab in Europe: Hemigrapsus sanguineus (Decapoda: Brachyura: Grapsidae). J. Crustacean Biol. 22 (1), 184–189. doi: 10.1163/20021975-99990221
Brösing A. (2010). Recent developments on the morphology of the brachyuran foregut ossicles and gastric teeth. Zootaxa 2510 (1), 1–44. doi: 10.11646/zootaxa.2510.1.1
Brousseau D. J., Baglivo J. A. (2005). Laboratory investigations of food selection by the Asian shore crab, Hemigrapsus sanguineus: algal versus animal preference. J. Crustacean Biol. 25 (1), 130–134. doi: 10.1651/C-2530
Busi M. V., Barchiesi J., Martín M., Gomez-Casati D. F. (2013). Starch metabolism in green algae. Starch 66 (1-2), 28–40. doi: 10.1002/star.201200211
Crothers J. H. (1967). The biology of the shore crab Carcinus maenas (L.) 1. The background – anatomy, growth and life history. Field Stud. 2 (4), 407–434.
Dare P. J., Edwards D. B. (1975). Seasonal changes in flesh weight and biochemical composition of mussels (Mytilus edulis L.) in the Conwy estuary, North Wales. J. Exp. Mar. Biol. Ecol. 18 (2), 89–97. doi: 10.1016/0022-0981(75)90066-0
Dauvin J.-C., Tous Rius A., Ruellet. T. (2009). Recent expansion of two invasive crab species Hemigrapsus sanguineus (de Haan, 1835) and H. takanoi Asakura and Watanabe 2005 along the Opal Coast, France. Aquat. Invasions 4 (3), 451–465. doi: 10.3391/ai.2009.4.3.3
Dominguez H., Loret E. P. (2019). Ulva lactuca, a source of troubles and potential riches. Mar. Drugs 17 (6), 357. doi: 10.3390/md17060357
Elner R. W. (1981). Diet of Green crab Carcinus maenas (L.) from Port Hebert, southwestern Nova Scotia. J. Shellfish Res. 1 (1), 89–94.
Epifanio C. E. (2013). Invasion biology of the Asian shore crab Hemigrapsus sanguineus: a review. J. Exp. Mar. Biol. Ecol. 441, 33–49. doi: 10.1016/j.jembe.2013.01.010
Figueiredo M. S. R. B., Anderson A. J. (2009). Digestive enzyme spectra in crustacean decapods (Palaemonidae, Portunidae, Penaeidae) feeding in the natural habitat. Aquaculture Res. 40 (3), 282–291. doi: 10.1111/j.1365-2109.2008.02087.x
Giddins R. L., Lucas J. S., Neilson M. J., Richards G. N. (1986). Feeding ecology of the mangrove crab Neosarmatium smithi (Crustacea: Decapoda: Sesarmidae). Mar. Ecol. Prog. Ser. 33, 147–155. doi: 10.3354/meps033147
Griffen B. D., Altman I., Bess B. M., Hurley J., Penfield A. (2012). The role of foraging in the success of invasive Asian shore crabs in New England. Biol. Invasions 14, 2545–2558. doi: 10.1007/s10530-012-0251-8
Grosholz E. (2002). Ecological and evolutionary consequences of coastal invasions. Trends Ecol. Evol. 17 (1), 22–27. doi: 10.1016/S0169-5347(01)02358-8
Guo K., Zhao W., Wang S., Liu B., Zhang P. (2016). Food web structure and trophic levels in a saltwater pond sea cucumber and prawn polyculture system. Acta Oceanologica Sin. 35 (4), 58–62. doi: 10.1007/s13131-016-0834-9
Heeren T., Mitchell D. B. (1997). Morphology of the mouthparts, gastric mill and digestive tract of the Giant crab, Pseudocarcinus gigas (Milne Edwards) (Decapoda: Oziidae). Mar. Freshw. Res. 48 (1), 7–18. doi: 10.1071/MF96026
Heinzel H. G. (1988). Gastric mill activity in the lobster. I. Spontaneous modes of chewing. J. Neurophysiol. 59 (2), 528–550. doi: 10.1152/jn.1988.59.2.528
Heinzel H. G., Weimann J. M., Marder E. (1993). The behavioral repertoire of the gastric mill in the crab, Cancer pagurus: an in situ endoscopic and electrophysiological examination. J. Neurosci. 13 (4), 1793–1803. doi: 10.1523/JNEUROSCI.13-04-01793.1993
Icely J. D., Nott J. A. (1992). “Digestion and absorption: digestive system and associated organs,” in Microscopic Anatomy of Invertebrates. Eds. Harrison F. W., Humes A. G. (New York: Wiley-Liss, Inc.), 147–201.
Jackson A. L., Inger R., Parnell A. C., Bearhop S. (2011). Comparing isotopic niche widths among and within communities: SIBER - Stable Isotope Bayesian Ellipses in R. J. Animal. Ecol. 80 (3), 595–602. doi: 10.1111/j.1365-2656.2011.01806.x
Janke K. (1990). Biological interactions and their role in community structure in the rocky intertidal of Helgoland (German Bight, North Sea). Helgoländer Meeresuntersuchungen 44, 219–263. doi: 10.1007/BF02365466
Jensen G. C., McDonald P. S., Armstrong D. A. (2002). East meets west: comparative interactions between green crab Carcinus maenas, and native and introduced shore crab Hemigrapsus spp. Mar. Ecol. Prog. Ser. 225, 251–262. doi: 10.3354/meps225251
Johnston D., Freeman J. (2005). Dietary preference and digestive enzyme activities as indicators of trophic resource utilization by six species of crab. Biol. Bull. 208 (1), 36–46. doi: 10.2307/3593099
Johnston D. J., Yellowlees D. (1998). Relationship between dietary preferences and digestive enzyme complement of the Slipper lobster Thenus orientalis (Decapoda: Scyllaridae). J. Crustacean Biol. 18 (4), 656–665. doi: 10.1163/193724098X00511
Jue C. K., Lipke P. N. (1985). Determination of reducing sugars in the nanomole range with tetrazolium blue. J. Biochem. Biophys. Methods 11 (2-3), 109–115. doi: 10.1016/0165-022X(85)90046-6
Jungblut S., Beermann J., Boos K., Saborowski R., Hagen W. (2017). Population development of the invasive crab Hemigrapsus sanguinneus (De Haan, 1853) and its potential native competitor Carcinus maenas (Linnaeus, 1758) at Helgoland (North Sea) between 2009 und 2014. Aquat. Invasions 12 (1), 85–96. doi: 10.3391/ai.2017.12.1.09
Jungblut S., Boos K., McCarthy M. L., Saborowski R., Hagen W. (2018a). Invasive versus native brachyuran crabs in a European rocky intertidal: respiratory performance and energy expenditures. Mar. Biol. 165, 54. doi: 10.1007/s00227-018-3313-3
Jungblut S., McCarthy M. L., Boos K., Saborowski R., Hagen W. (2018b). Seasonal lipid storage and dietary preferences of native European versus invasive Asian shore crabs. Mar. Ecol. Prog. Ser. 602, 169–181. doi: 10.3354/meps12712
Ledesma M. E., O´Connor N. J. (2001). Habitat and diet of the non-native crab Hemigrapsus sanguineus in southeastern New England. Northeastern Nat. 8 (1), 63–78. doi: 10.1656/1092-6194(2001)008[0063:HADOTN]2.0.CO;2
Lee P. G., Smith L. L., Lawrence A. L. (1984). Digestive protease of Penaeus vannamei Boone: relationship between enzyme activity, size and diet. Aquaculture 42 (3-4), 225–239. doi: 10.1016/0044-8486(84)90103-0
Linton S. M., Allardyce B. J., Hagen W., Wencke P., Saborowski R. (2009). Food utilization and digestive ability of aquatic and semi-terrestrial crayfishes, Cherax destructor and Engaeus sericatus (Astacidae, Parastacidae). J. Comp. Physiol. B 179, 493–507. doi: 10.1007/s00360-008-0332-2
Linton S. M., Cameron M. S., Gray M. C., Donald J. A., Saborowski R., von Bergen M., et al. (2015). A glycosyl hydrolase family 16 gene is responsible for the endogenous production of β-1,3-glucanases within decapod crustaceans. Gene 569 (2), 203–217. doi: 10.1016/j.gene.2015.05.056
Linton S. M., Greenaway P. (2004). Presence and properties of cellulase and hemicellulase enzymes of the gecarcinid land crabs Gecarcoidea natalis and Discoplax hirtipes. J. Exp. Biol. 207 (23), 4095–4104. doi: 10.1242/jeb.01252
Lohrer A. M., Whitlatch R. B. (1997). “Ecological studies on the recently introduced Japanese shore crab (Hemigrapsus sanguineus) in eastern Long Island Sound,” in Proceedings of the Second Northeast Conference on Nonindigenous Aquatic Nuisance Species, Burlington VT, 18-19 April 1997 Balcom N. (Groton, CT: Connecticut Sea Grant College Program), 49–60.
Love J., Mackie W., McKinnell J. W. (1963). 790. Starch-type polysaccharides isolated from the green seaweeds, Enteromorpha compressa, Ulva lactuca, Cladophora rupestris, Codium fragile, and Chaetomorpha capillaris. J. Chem. Soc., 4177–4182. doi: 10.1039/jr9630004177
Lovett D. L., Felder D. L. (1990). Ontogenetic change in digestive enzyme activity of larval and postlarval White shrimp Penaeus setiferus (Crustacea, Decapoda, Penaeidae). Biol. Bull. 178 (2), 144–159. doi: 10.2307/1541973
Matsui M., Fowler J. H., Walling L. L. (2006). Leucine aminopeptidases: diversity in structure and function. Biol. Chemsitry 387 (12), 1535–1544. doi: 10.1515/BC.2006.191
McDermott J. J. (1999). “The western Pacific brachyuran Hemigrapsus sanguineus (Grapsidae) in its new habitat along the Atlantic coast of the United States: feeding, cheliped morphology and growth,” in Crustaceans and the Biodiversity Crisis, vol. 2 . Eds. Schram F. R., von Vaupel Klein J. C. (The Netherlands: Brill, Leiden), 425–444.
Muhlia-Almazán A., Sánchez-Paz J., García-Carreño F. L. (2008). Invertebrate trypsins: a review. J. Comp. Physiol. B 178, 655–672. doi: 10.1007/s00360-008-0263-y
Munda I. M., Markham J. W. (1982). Seasonal variations of vegetation patterns and biomass constituents in the rocky eulittoral of Helgoland. Helgoländer Meeresuntersuchungen 35, 131–151. doi: 10.1007/BF01997550
Piavaux A. (1977). Distribution and localization of the digestive laminarinases in animals. Biochem. Systematics Ecol. 5 (3), 231–239. doi: 10.1016/0305-1978(77)90009-6
Post D. M. (2002). Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83 (3), 703–718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Prabhu M., Chemodanov A., Gottlieb R., Kazir M., Nahor O., Gozin M., et al. (2019). Starch from the sea: the green macroalga Ulva ohnoi as a potential source for sustainable starch production in marine biorefinery. Algal Res. 37, 215–227. doi: 10.1016/j.algal.2018.11.007
Rodríguez-Viera L., Perera E., Martos-Sitcha J. A., Perdomo-Morales R., Casuco A., Montero-Alejo V., et al. (2016). Molecular, biochemical, and dietary regulation features of α-amylase in a carnivorous crustacean, the Spiny lobster Panulirus argus. PloS One 11 (7), e0158919. doi: 10.1371/journal.pone.0158919
Ropes J. W. (1968). The feeding habits of the green crab, Carcinus maenas (L.). Fishery Bull. 67 (2), 183–203.
Rudnick D., Resh V. (2005). Stable isotopes, mesocosms and gut content analysis demonstrate trophic differences in two invasive decapod crustacea. Freshw. Biol. 50 (8), 1323–1336. doi: 10.1111/j.1365-2427.2005.01398.x
Saborowski R. (2015). “Nutrition and digestion,” in Natural History of the Crustacea. Vol IV Physiological regulation. Eds. Chang E., Thiel M. (New York: Oxford University Press), 285–319.
Saborowski R., Sahling G., Navarrete del Toro M. A., Walter I., García-Carreño F. L. (2004). Stability and effects of organic solvents on endopeptidases from the gastric fluid of the marine crab Cancer pagurus. J. Mol. Catalysis B: Enzymatic 30 (3-4), 109–118. doi: 10.1016/j.molcatb.2004.04.002
Saborowski R., Thatje S., Calcagno J. A., Lovrich G. A., Anger K. (2006). Digestive enzymes in the ontogenetic stages of the southern king crab, Lithodes santolla. Mar. Biol. 149, 865–873. doi: 10.1007/s00227-005-0240-x
Salindeho I. R., Johnston J. D. (2003). Functional morphology of the mouthparts and proventriculus of the rock crab Nectocarcinus tuberculosus (Decapoda: Portunidae). J. Mar. Biol. Assoc. United Kingdom 83 (4), 821–834. doi: 10.1017/S0025315403007859h
Sather B. T. (1969). A comparative study of amylases and proteinases in some decapod crustacea. Comp. Biochem. Physiol. 28 (1), 371–379. doi: 10.1016/0010-406X(69)91350-4
Skilleter G. A., Anderson D. T. (1986). Functional morphology of the chelipeds, mouthparts and gastric mill of Ozius truncates (Milne Edwards) (Xanthidae) and Leptograpsus variegatus (Fabricius) (Grapsidae) (Brachyura). Aust. J. Mar. Freshw. Res. 37 (1), 67–79. doi: 10.1071/MF9860067
Tyrell M. C., Harris L. G. (2000). “Potential impacts of the introduced Asian shore crab, Hemigrapsus sanguineus, in northern New England: diet, feeding preferences, and overlap with the green crab, Carcinus maenas.” in Marine Bioinvasions: Proceedings of the First National Conference, Cambridge MA, 24-27 January 1999 Pederson J. (Cambridge, MA: MIT Sea Grant College Program), 208–220.
Vogt G. (2019). Functional cytology of the hepatopancreas of decapod crustaceans. J. Morphology 208 (9), 1405–1444. doi: 10.1002/jmor.21040
Wahyudi A. J., Wada S., Aoki M., Hama T. (2013). Stable isotope signature and pigment biomarker evidence of the diet sources of Gaetice depressus (Crustacea: Eubrachyura: Varunidae) in a boulder shore ecosystem. Plankton Benthos Res. 8 (2), 55–67. doi: 10.3800/pbr.8.55
Watts A. J. R., McCafferty D. J., Newton J., Bailey D. M. (2011). Does seabird carrion contribute to the diet of the shore crab Carcinus maenas on the Isle of May, Scotland? An isotopic perspective. J. Mar. Biol. Assoc. United Kingdom 91 (7), 1459–1464. doi: 10.1017/S0025315410002286
Williams A. B., McDermott J. J. (1990). An eastern United States record for a western Indo-Pacific crab, Hemigrapsus sanguineus (Crustacea: Decapoda: Grapsidae). Proc. Biol. Soc. Washington 103 (1), 108–109.
Keywords: invasive species, food competition, herbivory, carnivory, gastric mill, digestive enzymes
Citation: Saborowski R, Bartolin P, Koch M and Jungblut S (2023) Trophic ecophysiology of the native green shore crab, Carcinus maenas, and the invasive Asian shore crab, Hemigrapsus sanguineus, in the rocky intertidal of Helgoland (North Sea). Front. Mar. Sci. 10:1247263. doi: 10.3389/fmars.2023.1247263
Received: 25 June 2023; Accepted: 09 October 2023;
Published: 01 November 2023.
Edited by:
Adriana Muhlia, National Council of Science and Technology (CONACYT), MexicoReviewed by:
Patricia Briones-Fourzan, National Autonomous University of Mexico, MexicoCopyright © 2023 Saborowski, Bartolin, Koch and Jungblut. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reinhard Saborowski, UmVpbmhhcmQuU2Fib3Jvd3NraUBhd2kuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.