
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 07 September 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1234667
 Suresh Babu P. P.1*
Suresh Babu P. P.1* Anuraj A.1
Anuraj A.1 Shilta M. T.2
Shilta M. T.2 Sanal Ebeneezar3
Sanal Ebeneezar3 Shinoj P.3
Shinoj P.3 Raghu Ramudu K.1
Raghu Ramudu K.1 Praveen N. D.1
Praveen N. D.1 Vaidya N. G.1
Vaidya N. G.1 Mahendra Pal1
Mahendra Pal1 Boby I.3
Boby I.3 Anikuttan K. K.4
Anikuttan K. K.4 Gopalakrishnan A.3
Gopalakrishnan A.3The effect of stunting by feed and space deprivation on compensatory growth (CG) in Silver pompano, Trachinotus blochii, was investigated. A commercial pellet feed (45% protein and 10% fat) was fed two times a day, throughout the entire experiment. The 270-day experiment consisted of an initial 60-day stunting phase and a 60-day post-stunting phase carried out in 4 × 2 × 2 m3 galvanized iron (GI) rectangular cages, and a 150-day grow-out phase carried out in 3-m diameter circular GI cages. During the stunting phase, the normal fish (in triplicates) were stocked at lower stocking density (17 fish/m3) and fed at 10% of body weight (BW), while stunted fish (one replication) were stocked at about three times higher stocking density (56 fish/m3) and fed at a three times lower feeding rate (3% of BW). The stunted and normal fish were reared in triplicates during the post-stunting phase, at uniform stocking density (15 fish/m3) with feeding at a higher rate (10% of BW) for stunted fish and normal feeding rate (8% of BW) was adopted for normal fish. During the grow-out stage, each replication from the post-stunting phase was shifted to 3-m circular cages with the same feeding rates. The lag in growth in stunted fish (5.56 g against 9.43 ± 0.13 g of normal) during the stunting phase was compensated during the post-stunting phase (36.88 ± 2.23 g against 38.13 ± 1.48 g of normal) by higher feeding rate. There were no significant (p > 0.05) differences in final harvest, biometry, morphometry, dressing yield, carcass nutritional composition, and serum biochemical markers at the end of grow-out stage. Because of the significant difference (p < 0.05) in the total feed provided (5.2 kg for stunted fish against 22.8 kg for normal fish) and the lesser unit cost for the production of stunted fingerling (USD 0.087 for stunted fish against USD 0.106 for normal), the farming of stunted fish brought about a higher net operational revenue and benefit:cost ratio.
Stunted fingerlings are better stocking material for fish farming. Several studies have reported that stunted fingerling production ensures prolonged seed availability, especially during off seasons, and production of healthy and hardy fingerlings with higher survival, and also helps to maintain fish seed in transportable size (Radheysham and Saha, 2009; Das et al., 2016; Lingam et al., 2019; Suresh Babu et al., 2022a). Lingam et al. (2019) reported better growth and meat quality for stunted fish compared to normal fish in an on-farm trial for milkfish. The practice of stunting Indian Major Carps seed by Indian farmers is presumed to be for removing weak and sick fry and also to produce healthy larger fish seed as a stocking material for grow-out culture (Abraham et al., 2010).
Compensatory growth (CG) in fish is a phase of augmented growth when favorable conditions are restored, after a period of growth deprivation or stunting (Ali et al., 2003). The ability of stunted fingerlings to attain CG has been demonstrated in several commercial fishes such as Nile tilapia (Bhujel et al., 2007), rohu (Das et al., 2016), milkfish (Lingam et al., 2019), Atlantic cod (Jobling, 2010) and Atlantic salmon (Hvas et al., 2022). The CG pattern varies with species farmed (Jobling et al., 1994; Foss et al., 2009; Lingam et al., 2019), duration of stunting (Das et al., 2016; Anikuttan et al., 2020; Suresh Babu et al., 2022a), stunting protocol adopted (Ali et al., 2003), and re-feeding strategies implemented (Llameg and Serrano, 2014; Hvas et al., 2022). Won and Borski (2013) opined that endocrine regulation during the catabolic and hyper-anabolic phases contributes immensely for CG. The degree of CG varies from over-compensation, full compensation, partial compensation, and no compensation based on the rate of regaining of the growth rate (Ali et al., 2003). Furthermore, CG depends on the duration of stunting (Lingam et al., 2019; Anikuttan et al., 2020), which may be short-term spanning from a few weeks to 2 to 3 months (Anikuttan et al., 2020) or long-term lasting for even more than 6 months (Das et al., 2016; Lingam et al., 2019; Suresh Babu et al., 2021) depending on the purpose of stunting. Short-term stunting is ideal for maintaining the fish in confined conditions during adverse climatic conditions while long-term stunting can be adopted for prolonging the seed availability.
Silver pompano, Trachinotus blochii (Lacepede, 1801), is a recently introduced marine fish for aquaculture practices in the Indian subcontinent. Ever since the standardization of its commercial hatchery production (Abdul Nazar et al., 2012), this fish is considered as a good candidate for aqua-farming in ponds (Jayakumar et al., 2014; Damodaran et al., 2019), cages (Kalidas et al., 2020; Suresh Babu et al., 2022a), and Recirculating Aquaculture Systems (Suresh Babu et al., 2022b). Furthermore, it is considered as an ideal candidate species for farming in both marine and low-saline conditions (Kalidas et al., 2012). The fish can be reared even in salinity up to 5 ppt (Kalidas et al., 2012).
Anikuttan et al. (2020) reported the potential for achieving CG in stunted fingerlings of Silver pompano when reared under indoor marine conditions. CG in stunted Silver pompano in low saline indoor conditions (<15 ppt) was estimated, and its field-level application was validated by our team (Suresh Babu et al., 2022a) and noted that short-term (60 days) stunting is ideal for elucidating CG in Silver pompano. In these studies, stunted fingerling production was achieved through a combination of feed and space deprivation following Das et al. (2016) and Lingam et al. (2019). The present study is designed to evaluate the growth and production performance of short-term (60 days) stunted Silver pompano with that of normally reared fish in low saline cage conditions with special emphasis on biometry, morphometry, carcass nutritional composition, serum biochemical markers, and economic benefits.
The manuscript followed all the ethical guidelines for conducting experiments on food fish in India as per the guidelines of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India) for experimentation on fishes. The research work was approved under the Institutional funded project (MDN/HCY/18) of ICAR-Central Marine Fisheries Research Institute, Kochi, India.
Details of the experimental design are given in Figure 1. The field trial was done in the coastal water area near Karwar Regional Station of Indian Council of Agricultural Research (ICAR)-Central Marine Fisheries Research Institute, Karwar, Karnataka, India following Suresh Babu et al. (2022a). The experiment was carried out in two stages, viz, with a nursery stage having two phases (a stunting phase and a post-stunting phase) and a grow-out stage. A commercial floating pellet feed (Nutrila, Growel India Pvt. Ltd; 45% crude protein; 10% crude fat; 2.5% crude fiber; 11% moisture) with a pellet size of 0.8 mm and 1.2 mm for nursery rearing phase and 3 mm for grow-out phase were used for feeding the fish following Damodaran et al. (2019).
The nursery rearing stage with stunting and post-stunting phases (with 60 days duration each) was carried out in 4 × 2 × 2 m (16 m3) galvanized iron (GI) floating rectangular cages. During stunting, the normal fish were reared in triplicate by stocking 280 fish (17 fish/m3) in each cage, and the fish for stunting was maintained in one cage stocked with 900 fish (56 fish/m3) for space deprivation. Only one replication was maintained for stunting since the number of fish stocked is sufficient for stocking during the post-stunting phase. The growth pattern of stunted fish has been evaluated during our previous report (Suresh Babu et al., 2022a). During the 60 days of stunting phase, the fish for stunting was fed with feed only at 3% of body weight (BW) for feed deprivation and the normal fish were fed at 10% of BW (normal feeding rate). The methodology adopted for stunting of fish is a combination of space and feed deprivation, which is adopted by other authors in carps (Das et al., 2016) and milkfish (Lingam et al., 2019) and the feeding percentage is also determined based on these reports.
During the post-stunting phase, the stunted and normal fish were harvested from the respective cages and restocked in 6 similar cages for making uniform stocking density for farming. During this phase, the stunted and normal fish were reared in triplicate at a uniform stocking density of 240 fish per cage (15 fish/m3). The stunted fish were fed at 10% of BW (higher feeding for compensation) and the normal fish were fed at 8% of BW during this phase.
After the post-stunting phase, the fish were harvested and shifted to circular GI floating cages (with 3 m diameter and 3 m depth) for grow-out culture after noting the survival. During the grow-out phase, both the stunted and normal fish were fed at 6% of BW during the first 2 months followed by 5% of BW in the third month, 4% of BW in the fourth month, and 3% of BW in the fifth month.
To assess the growth of the fish, a monthly sampling approach was employed, involving the measurement of length and weight for individual fish in the stock. To measure the total length of the fish accurately, a 1-m wooden scale with a precision of 1 mm was used, measuring from the tip of the snout to the tip of the caudal fin. For smaller fish weighing less than 100 g, an electronic weighing balance (Sartorius, Germany) with a precision of 0.001 g was utilized, while a pan balance with a precision of 1 g was employed for larger fish weighing 100 g or more. To calculate the specific growth rate per day (SGR/Day %), the method described by De Silva and Anderson (1995) was followed. The formula used was as follows: Specific growth rate (%/day) = [ln (Final weight) − ln (Initial weight)]/[Experimental days] × 100, where “ln” denotes the natural logarithmic value. The total feed provided based on the feeding rate was noted daily for feed calculation and the actual feed consumed by the fish was difficult to note in the experimental cages. Growth efficiency (%) was calculated as per Yengkokpam et al. (2014) as GE = 100 × (Final weight − Initial weight)/total feed given in grams. Daily weight gain (DWG) was calculated as per Damodaran et al. (2019) as DWG = Final weight (g) − Initial weight (g)/days of culture. The food conversion ratio (FCR) was calculated as feed given (g)/weight gain (g).
To determine the total biomass during different growth phases, the average weight of individual fish was multiplied by the total number of fish harvested at the end of each experimental phase. The total biomass represents the collective weight of all the fish in a given phase. To assess the survival rate at the end of the experiment, the number of fish in each cage was counted. The survival rate was calculated as a percentage using the formula: Survival (%) = (Total number of fish present × 100)/Total number of fish stocked. In the grow-out phase, the survival rate is calculated based on the number of stunted fish stocked during the post-stunting phase. The number of fish surviving until the end of the grow-out phase is taken into account when determining the survival rate. After the grow-out phase, the total harvest weight (in kilograms) was recorded from each cage. This information is used to calculate the total production and productivity, which provide insights into the overall yield and efficiency of the rearing system.
For calculating condition factor (k), length–weight relationship (LWR), and coefficient of variation (CV) in length and CV in weight, data from the replicates were pooled and analyzed. The condition factor (k) of the fish in their natural habitat was determined following the method proposed by Gomiero and Braga (2005). The condition factor is calculated using the formula: k = (W × 100)/L3, where “W” represents the weight of the fish in grams (g), and “L” represents the total length of the fish in centimeters (cm). The length–weight relationship was established by calculating the logarithmically transformed values of the total body length and BW data. The equation used for this relationship is: Log (WEIGHT) = a + (b × log (LENGTH)), where “a” represents the intercept and “b” represents the slope of the linear regression obtained from the log-transformed weight (g) and length (cm) data.
Morphometric measurements were taken for 10 fish from each replicate (total 30 for each treatment) as per Lingam et al. (2019) using a digital Vernier calliper.
Dressed weight and carcass analysis of stunted and normal fish were recorded from the final harvest as per Lingam et al. (2019) by sampling 5 fish from each replicate (15 fish for each treatment), which were in the weight range of 300 to 350 g. The fish were dissected and the offal was weighed using a digital balance (Sartorious, Germany). Dressed fish were cut into three portions as per Lingam et al. (2019), namely, fore cut, mid cut, and hind cut, after which the weight was recorded. The dressing percentage was calculated as the percentage of dressed weight (removing viscera, gills, fins, and head) to the whole wet weight.
The whole-body nutrient composition of fish was assessed following standard methods (AOAC, 1995). For this, the pooled wet weight of fish from each replication (cage-wise) was measured, sliced into pieces, kept in a glass petri plate, and dried in a hot air oven overnight at 105°C until constant dry weight. After drying, the samples were weighed accurately and ground in a grinder to a homogenized form before the analyses of crude lipid, protein, crude fiber, and total ash.
The assessment of crude protein content (N × 6.25) was conducted using the Kjeldahl system (FOSS Kjeltec, 2300). To determine the crude lipid content, the ether-extraction method was employed using the Soxhlet system (FOSS Soxtec, 2043). The ash content was determined by incinerating the samples completely in a muffle furnace at a temperature of 550°C for a duration of 5 h. The moisture content of the sample was calculated using the formula [(wet weight − dry weight) × 100]/wet weight.
Water quality is important for any fish-rearing experiment. Water quality parameters, including dissolved oxygen, pH, temperature, salinity, and NH4+ concentration, were regularly monitored at weekly intervals using established protocols following the guidelines outlined in the APHA (1981). Range of water quality parameters such as dissolved oxygen (4 to 5 ppm), pH (7.6 to 8), temperature (26 to 31°C), salinity (12 to 21 ppt), and NH4+ (0.012 to 0.14 ppm) observed were within the optimum range for the growth of Silver pompano under low-saline conditions (Jayakumar et al., 2014).
In each replication, five fish were anesthetized using 10 ppm Benzocain for a duration of 3 min. Following anesthesia, blood samples were collected by puncturing the caudal vein. The collected blood was then allowed to clot for a period of 1 h at room temperature and subsequently kept overnight at a temperature of 4°C. To obtain the serum, the blood samples were centrifuged at 6,000 rpm for 10 min. Serum samples from each replication were pooled together, categorized based on their respective cages, and stored at a temperature of −20°C for future use.
Serum biochemical parameters, including total proteins, albumins, cholesterol, alkaline phosphatase, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), and triglycerides, were analyzed using diagnostic kits from Tulip Diagnostics Ltd., based in Goa, India. The analysis was conducted following the protocols provided by the manufacturer, as described in the study by Ebeneezar et al. (2020). To measure the absorbance of the samples, a spectrophotometer (Multiskan Sky-High, ThermoFisher Scientific, USA) was used. The absorbance values were recorded to quantify the levels of the respective biochemical parameters in the serum samples.
For the determination of Total protein in serum, the biuret method was followed. The proteins bind with cupric ions to form a blue-violet colored complex, the intensity of which is directly proportional to the protein in the sample. For this, absorbance was measured at 550 nm. Total serum albumin was determined based on the bromocresol green dye binding assay. For this, absorbance was measured at 630 nm. Serum globulin levels were obtained by subtracting serum albumin from total serum protein. Serum cholesterol was determined based on the hydrolysis of esterified cholesterol to free cholesterol. For this, absorbance was measured at 505 nm. Alkaline phosphatase hydrolyzes p-nitrophenyl phosphate to p-nitrophenol and phosphate. The rate of formation of p-nitrophenol is measured as an increase in absorbance, measured at 405 nm. SGOT and SGPT are measured based on the oxidation of NADH to NAD, which is measured as a decrease in the absorbance, which is proportional to the enzyme activity in the sample. For this, absorbance was recorded at 340 nm. Triglycerides were determined based on the hydrolysis of triglycerides to glycerol and free fatty acids by lipoprotein lipase. For this, absorbance was measured at 505 nm.
An economic evaluation of farming of stunted and normal fish was carried out by estimating and comparing the costs and earnings associated with the production of fingerlings as well as experimental farming of fish in both treatments. Various operational cost components such as the cost of fingerlings, feed cost, labor charge, and other miscellaneous expenditures were estimated based on field data and also following the approach adopted by Damodaran et al. (2019). Furthermore, indicative economics of commercial cage farming of stunted fingerlings in comparison with normal fingerlings was also determined by taking a cue from the estimates obtained from experimental farming and also following Aswathy et al. (2020) in the case of fixed cost parameters for scaled-up farming in larger cages. All the values were expressed in USD (1 USD = Rs. 74 INR).
Results are given as mean ± standard error. The mean values were compared using a Student’s t-test to determine if there was a significant difference (p < 0.05) between the stunted and normal fish groups. The statistical analysis was performed using SPSS version 16.0. The normality of the data was tested before t-test visually using the Shapiro–Wilk method wherever applicable.
Information on the growth, feed utilization, and survival characteristics of stunted and normal fish at different phases of farming and the overall performance are given in Table 1. Additionally, Figure 2 illustrates the weight gain pattern during the stunting and post-stunting phases, as well as the grow-out stage. The specific growth rate per day (%) for stunted and normal fish is separately depicted in Figure 3. Successful stunting of fish was achieved under field conditions in low-saline cages, with stunted fish gaining only 4.08 g compared to 7.9 g in normal fish. A comparison of parameters such as final weight, daily weight gain, specific growth rate per day (%), and growth patterns revealed reduced growth in stunted fish compared to normal fish.

Table 1 Growth, production, and biometric characteristics of stunted and normal fish at different phases of the farming.
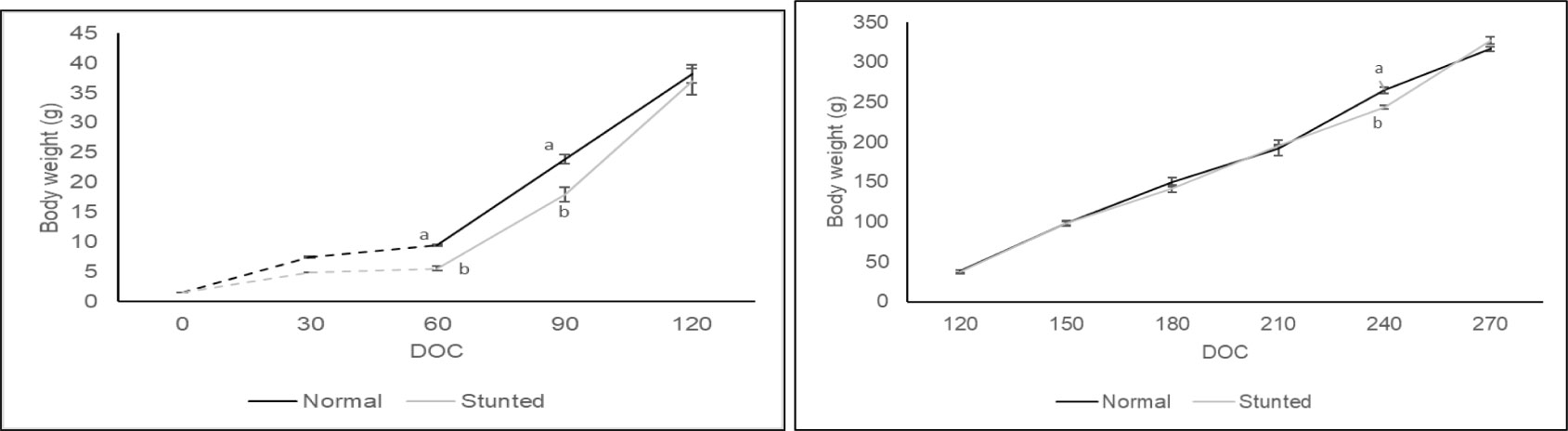
Figure 2 Depiction of the gain in weight in stunted fish and normal fish during the stunting and post-stunting phase (left) and grow-out phase (right). The dotted line indicates the weight (g) during the stunting period and solid the line indicates the weight (g) during the post-stunting and grow-out period. Values with different alphabets on the same DOC differ significantly (p < 0.05).
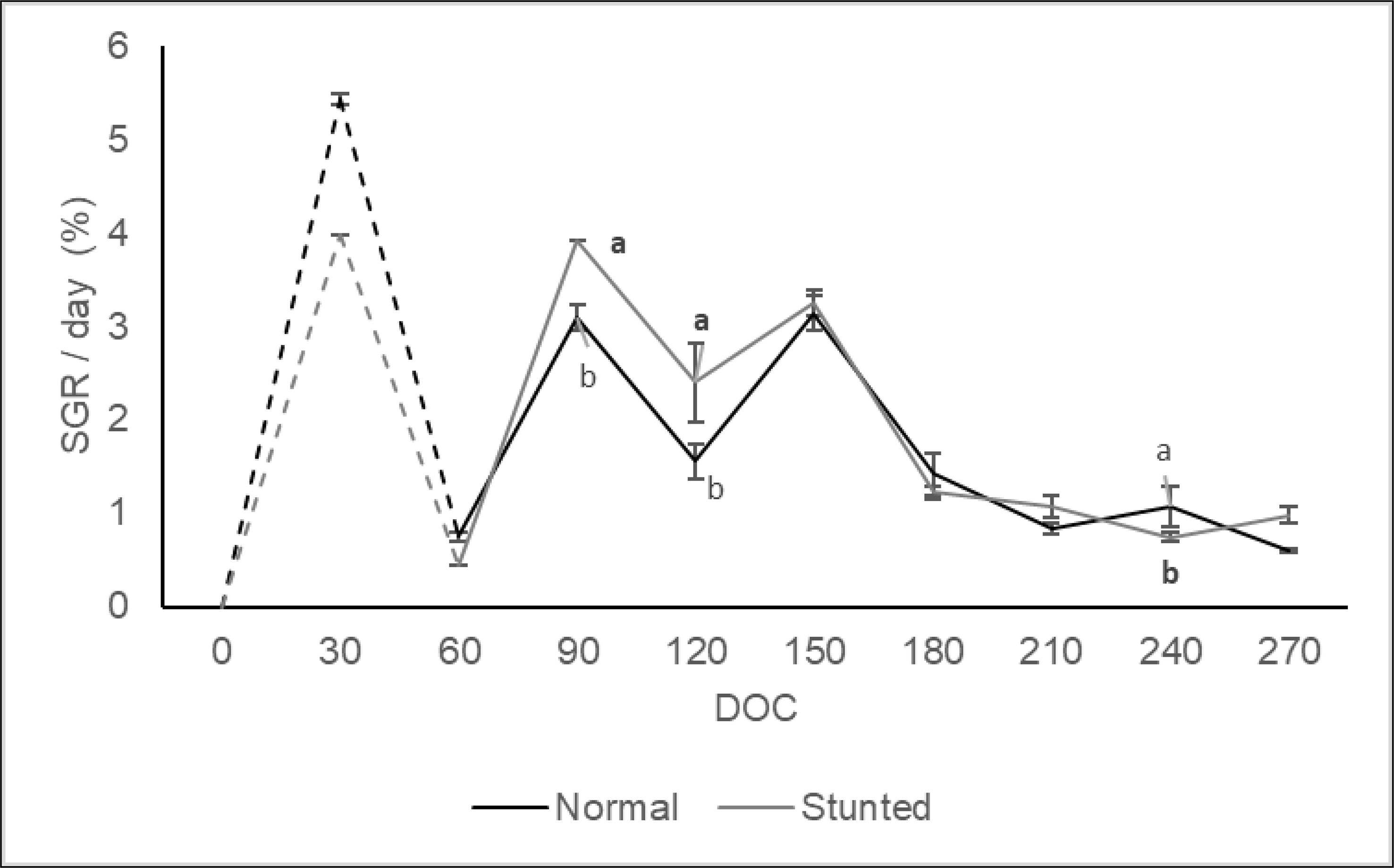
Figure 3 Specific growth rate/day (%) of stunted and normal Silver pompano in low saline cages. The dotted line indicates the growth during the stunting period and the solid line indicates the weight (g) during the post-stunting period. Values with different alphabets on the same DOC differ significantly (p < 0.05).
During the post-stunting phase, stunted fish exhibited rapid growth and reached an average weight of 36.88 ± 2.23 g, similar to that of normal fish (38.13 ± 1.48 g). There was no significant difference (p > 0.05) in the final weight between stunted and normal fish. The weight gain pattern and specific growth rate per day (%) (p < 0.05) indicated significantly accelerated growth in stunted fish when the feeding rate was restored to normal. Although not statistically significant (p > 0.05), stunted fish showed higher daily weight gain. The final average weight of stunted and normal fish did not significantly differ (p > 0.05), suggesting complete compensation in growth.
During the grow-out phase, both stunted and normal fish exhibited similar weight gain, except on the 240th day of culture (DOC) when normal fish showed higher weight gain (p < 0.05). The specific growth rate per day was more or less similar in both stunted and normal fish. Evaluation of the overall performance of growth (across all phases) revealed no significant variation in final weight, daily weight gain, and specific growth rate per day (%). Detailed information on length at different phases of farming in relation to total length was also recorded. Complete catch-up growth in total length also was observed in stunted fish during this study.
Details on feed utilization are also given in Table 1. In this study, the total feed provided to the fish was considered for all calculations due to the difficulty of determining the actual feed consumed in the cage culture system. Despite observing retarded growth, stunted fish exhibited superior feed utilization during the stunting phase in terms of growth efficiency (more than two times) and feed conversion ratio (FCR) (less than two times) compared to normal fish. The individual feed provided for stunted fish was also minimal (one-fifth) in quantity. The feed rate given to stunted fish during the stunting phase was only sufficient to maintain physiological functions and not for promoting growth. Total feed provided and growth efficiency did not significantly differ (p > 0.05) between the two treatments during the post-stunting phase. However, FCR was significantly lower in stunted fish (p < 0.05), indicating better feed utilization. There was no significant difference (p > 0.05) in feed provided and growth efficiency between the treatments during the grow-out phase, but FCR was significantly lower in stunted fish (p < 0.05). Considering the overall performance, the quantity of feed provided for stunted fish was significantly lower (p < 0.05).
During the stunting phase, the survival rate of normal fish was higher compared to stunted fish. However, there was no significant difference (p > 0.05) in survival rate between stunted and normal fish during the post-stunting and grow-out phases. The final production and productivity per unit volume also did not differ significantly (p > 0.05) between stunted and normal fish.
Biometric characteristics of stunted and normal fish at different phases of farming are also given in Table 1. The condition factor (k), which serves as a wellbeing indicator, was above 1 in both treatments, indicating the ideal growth of the fish in the system. In this study, the condition factor (k) decreased as the size of the fish increased in both treatments. Specifically, the condition factor consistently measured above 1.36 for stunted fish and above 1.57 for normal fish. The “b” value for the length (g)–weight (cm) relationship was less than 3 in both treatments throughout all rearing phases, and the regression values for all relationships showed good fitness (R2 above 8). The “b” value was higher in stunted fish during the stunting and post-stunting phases but lower during the final harvest. The coefficient of variation (CV) in weight (ranging from 0.15 to 0.53) was more prominent than that in length (ranging from 0.05 to 0.22) in both treatments, and the variation decreased as the farming progressed. The CV in length was higher in normal fish, while the CV in weight was higher in stunted fish during the stunting phase. However, the variation was nearly equal during the remaining part of the rearing period. Most notably, the size variation was almost equal in both treatments at the final harvest, indicating a uniform harvest at the end.
Table 2 provides the morphometric characteristics of stunted and normal fish at the end of the farming period. The measurements, including total length, standard length, maximum body girth, body width, head length, and mouth length, did not show significant differences between stunted and normal fish. This indicates that the morphometry of fish within the same weight class does not vary significantly (p > 0.05) between stunted and normal fish.
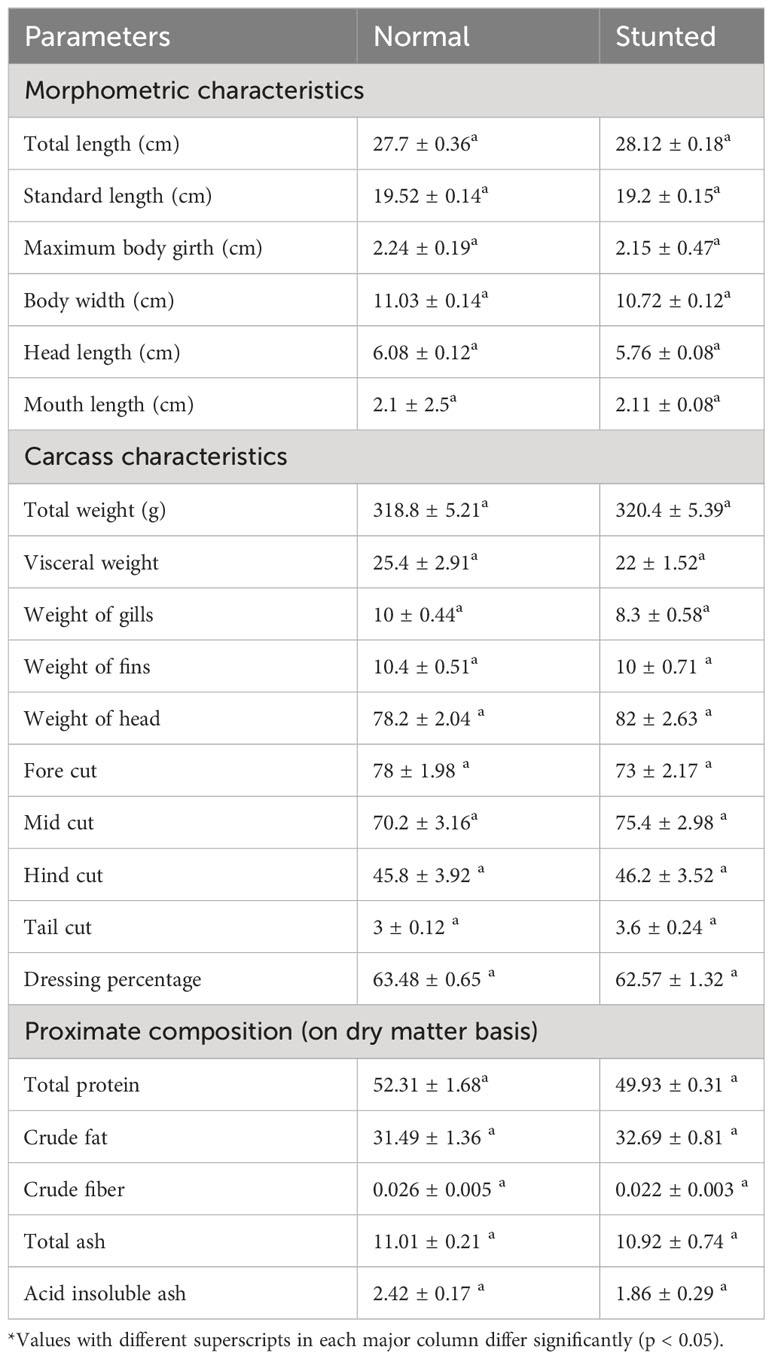
Table 2 Morphometric, carcass characteristics, and proximate composition of whole body of stunted and normal fish at the end of the experimental farming.
The carcass analysis of stunted and normal fish during harvest is also presented in the table. In this study, the weight of the head, viscera, gills, fins, fore cut, head cut, and tail cut for fish within the same weight class did not differ significantly (p > 0.05) between stunted and normal fish at the time of harvest. The dressing percentage, which represents the proportion of edible meat, did not show variation (p > 0.05) between stunted and normal fish. Table 2 also provides the proximate composition of the meat of stunted and normal fish. The parameters, including moisture content, total protein, crude fat, crude fiber, total ash, and acid-insoluble ash composition, did not show significant differences (p > 0.05) between stunted and normal fish.
The results of serum biochemical indices of stunted and normal pompano at the end of the grow-out period are given in Table 3. The feeding strategies had no significant effects on indices such as SGOT, SGPT, and alkaline phosphatase, indicating that the stress and metabolic status of stunted fish were similar to that of normal fish at the end of the grow-out phase.
In this study, significantly lower (p < 0.05) total albumin content and significantly higher (p < 0.05) total globulin content were observed in stunted fish. However, the total albumin-to-globulin ratio did not show significant differences (p > 0.05) between the two groups. Regarding serum total protein, no significant difference (p > 0.05) was found between stunted and normal fish. However, the serum lipid index showed that cholesterol levels were significantly lower (p < 0.05) while triglyceride levels were significantly higher (p < 0.05) in the serum of stunted fish.
Table 4 provides the results of the economic evaluation, specifically the input cost for the production of stunted and normal fingerlings. The unit cost of production for stunted fingerlings was calculated to be USD 0.11, which was lower than the unit cost of production for normal fingerlings, amounting to USD 0.87. Furthermore, an economic analysis of experimental farming comparing stunted fingerlings with normal fish is presented in the table. Only the operational expenditure associated with experimental farming was considered at this stage. The results indicated higher net operational revenue (profit) and a higher benefit–cost ratio (B:C ratio) for the farming of stunted fingerlings compared to normal fingerlings.
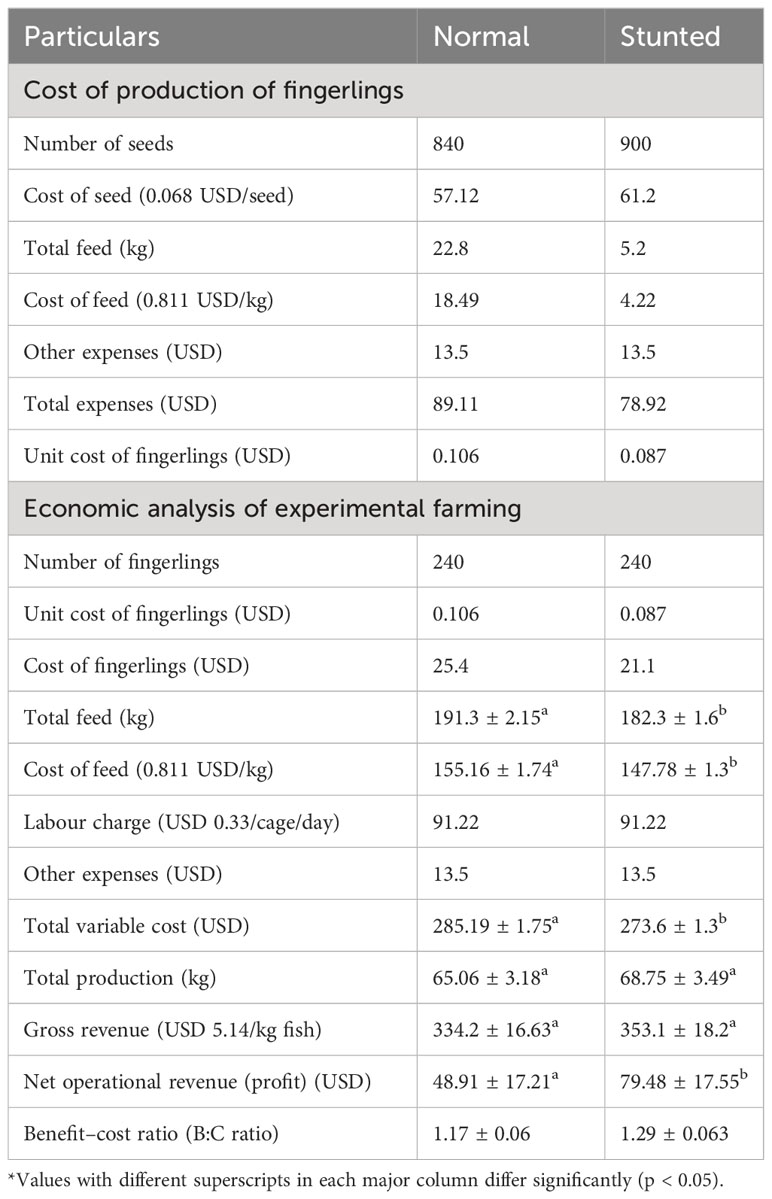
Table 4 Cost of production of fingerlings and economic analysis of experimental farming (based on operational cost) of stunted fish compared to normal fish.
Indicative economics of commercial farming for stunted fingerlings in 6-m-diameter high-density polyethylene (HDPE) cages, in comparison with normal fish farming, is given in Table 5. The calculations took into account biological and economic parameters such as survival rate, the unit price of fingerlings, feed requirement per unit fish, final average BW, and other factors obtained from the experimental farming. The net revenue and benefit–cost (B: C) ratio for farming stunted fish were observed to be higher than those for normal fish.
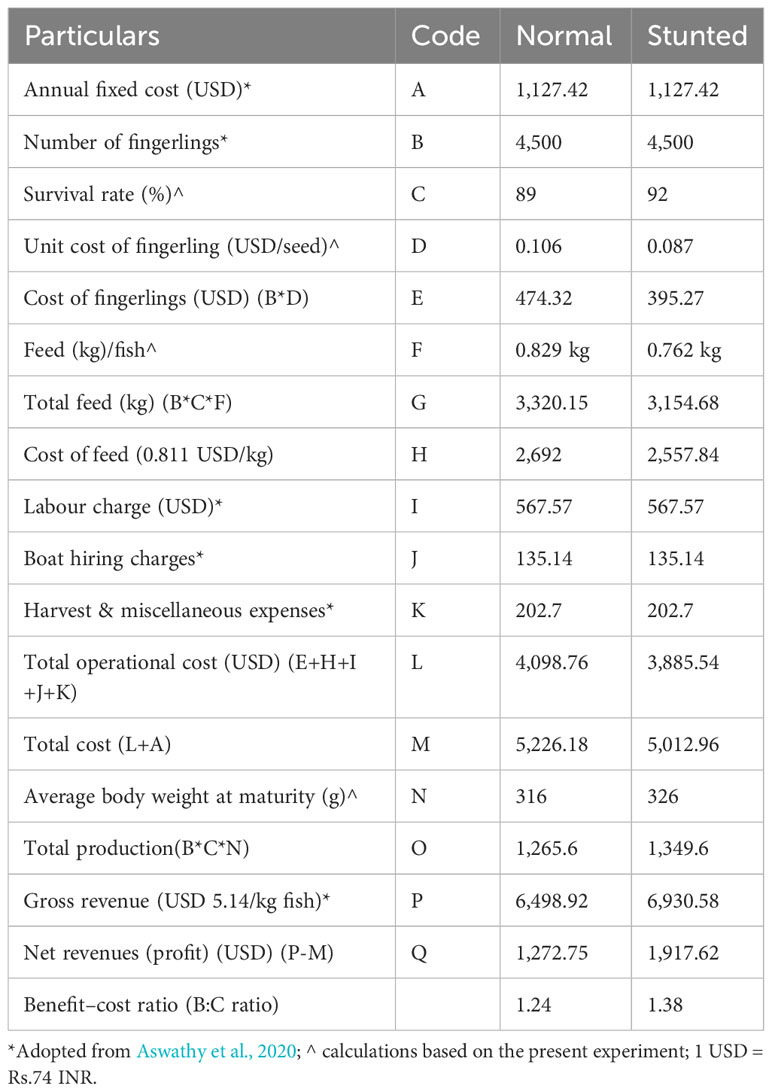
Table 5 Indicative economics of commercial cage farming of stunted fingerlings vis-a-vis normal fish.
The phenomenon of CG is an innovative concept that can be applied in aquaculture practices using stunted fingerling as stocking material (Santiago et al., 2004; Cho and Cho, 2009; Lingam et al., 2019; Anikuttan et al., 2020). CG in fish refers to a phase of enhanced growth that occurs when favorable conditions are restored after a period of growth depression (Ali et al., 2003). CG has been demonstrated in many marine candidate fishes for aquaculture such as Sunfish (Hayward et al., 1997), Asian seabass (Tian and Qin, 2003), Oliver flounder (Cho and Cho, 2009), seabream (Bavcevic et al., 2010), milkfish (Lingam et al., 2019), and Atlantic salmon (Hvas et al., 2022). The gradation of compensation varies with the duration of fasting, fish age, the age at sexual maturity, the pattern of re-feeding (Ali et al., 2003), and more importantly the species used for the trial. Studies related to CG in marine finfish in cage farming are rare.
Before the beginning of the experiment, a series of indoor and outdoor experiments for delineating the CG pattern in Silver pompano in both marine and low saline conditions were conducted. Based on our previous reports (Anikuttan et al., 2020), Silver pompano exhibits CG in short-duration stunting in indoor marine conditions. As per our indoor studies on Silver pompano (Suresh Babu et al., 2022a) for 30-, 60-, and 90-day stunting, a better compensation was observed in 60-day stunting under low-saline conditions, and the results were further validated in a field study in low-saline cages. The present work is the first report on the evaluation of the field-level application of stunting and growth compensation for both operational and economic benefits. The protocol for nursery rearing stages was adopted from Anikuttan et al. (2020), which was composed of a 60-day stunting phase followed by a 60-day post-stunting phase, and a 150-day grow-out stage was added for performance evaluation. Since the work was carried out completely in outdoor conditions, a short-term stunting protocol was adopted.
In general, stunting in fish can be achieved through various methods such as ration restriction, density manipulation, thermal and salinity manipulation, and other resource restrictions (Ali et al., 2003). In the present study, a combination of feed and space restriction was employed to induce stunting in fingerlings similar to Anikuttan et al. (2020). This technique has been experimentally demonstrated in carps (Das et al., 2016) and the same is widely adopted by farmers in carps (Radheysham and Saha, 2009; Abraham et al., 2010; Charan et al., 2014). Studies conducted by Lingam et al. (2019) on milkfish and Suresh Babu et al. (2021) on rohu have reported that stunting can be intentionally induced in fish culture systems by implementing a combination of increasing stocking density and providing sub-optimal feeding conditions.
Patterns of gain in weight and specific growth rate/day (%) revealed slower growth in stunted fish. Anikuttan et al. (2020) and Suresh Babu et al. (2022a) also have reported a retarded growth in 60 days of stunted Silver pompano following a similar protocol in indoor conditions. Natural occurrences of stunted growth in fish during unfavorable conditions have been documented in earlier studies (Burrough and Kennedy, 1979). Ylikarjula et al. (1999) identified two possible factors contributing to natural stunting: resource limitation and size- or age-dependent survival probabilities. Limitations of the feed and the living space in the present study induced stunted growth in the treated fish. The stunted fish in the present work attained complete growth compensation during the post-stunting phase itself. In contrast, Anikuttan et al. (2020) and Suresh Babu et al. (2022a) have reported only a partial compensation in 60 days for stunted Silver pompano in indoor conditions. These results reveal that in Silver pompano, CG can be attained faster in cages than in the indoor system, which is advantageous for coastal cage farmers. This may be because open cages have a more conducive environment for growth than indoor systems. Hvas et al. (2022) have reported CG in feed-restricted Atlantic salmon when fed sufficiently in indoor conditions and the same fish had shown an almost similar growth rate to normal fish when shifted to marine floating cages. Interestingly, during the grow-out phase, the stunted fish also exhibited a similar growth rate to that of the normal fish till the end of the present work.
Evaluation of the overall performance of growth (inclusive of all phases) indicates no significant variation in the final weight, daily weight gain, and SGR/day (%). These results reveal that even though a growth lag was observed during the stunting phase, the restoration of the feeding rate during the post-stunting phase compensated the same. It indicates that short-term stunting (60 days) does not have much negative impact on the growth performance of Silver pompano and also shows that stunted fish can be used as stocking material for farming in estuarine and coastal cages without any growth impairment.
Based on the period of stunting, the stunting process can be either long term (from 6 to 12 months) or short term (less than 3 months). In the present work, the short-term stunting (60 days) could induce stunting and the fish could achieve CG in 60 days (post-stunting) itself in Silver pompano. Das et al. (2016) also reported complete CG in rohu stunted for less than 6 months and only partial compensation in fish stunted for more than 6 months. Lingam et al. (2019) reported partial compensation for 4 months stunted milkfish in pond conditions. Since the stunting of Silver pompano is mainly done for maintaining the fingerlings in nursery confinement in either indoor tanks or nearshore nursery cages, a 60-day stunting is ideal to develop strategies to overcome unfavorable climate conditions.
In the past, the study of CG in aquaculture primarily focused on monitoring fish weight, which posed challenges in drawing general conclusions due to the declining specific growth rate (SGR) with increasing animal size (Jobling, 2010). However, Bavcevic et al. (2010) observed that while the CG phase compensated for weight, it did not necessarily compensate for length in the case of Gilthead seabream (Sparus aurata). As a result, they suggested that studying fish length should also be considered when characterizing CG. Interestingly, in the present work, CG in length followed a similar trend as that of the weight, further confirming growth compensation in all dimensions.
Studies on the impact of the combination of ration restriction and space deprivation on CG are rare in marine aquaculture. Ration restriction is one of the most widely used methods for obtaining retarded growth in fish (Ali et al., 2003). Higher stocking densities will also indirectly lead to competition for feed and other resources in confinement. Costa-Bomfim et al. (2014) reported that over-crowding due to higher stocking densities in Cobia in confinements leads to competition for feed.
In our study, throughout the experiment, the FCR was higher compared to other feed studies conducted in Silver pompano. Several authors reported varying ranges of FCR for Silver pompano in different farming conditions. Chavez et al. (2011) reported FCR ranging from 1.67 to 1.85 for Silver pompano reared in marine cages. Damodaran et al. (2019) also reported an FCR of 1.94 for Silver pompano grown in pond conditions. The FCR of the present work cannot be compared with these results since the farming condition (low saline), method of farming, and initial stocking size vary considerably from these works.
Among the treatments, in the stunting and post-stunting phase of the experiment, higher FCR was recorded for normal fish. This may be because more feed was provided for the normal fish compared to stunted fish during the stunting phase. In the indoor stunting experiment, the stunted Silver pompano exhibited higher FCR ranging from 3.3 to 3.6 (Anikuttan et al., 2020). During the post-stunting phase, the fish were provided adequate feed (feeding rate more than normal fish) to attain CG. The stunted fish need to be fed at higher feeding rates in the post-stunting phase to obtain CG (Das et al., 2016; Lingam et al., 2019; Anikuttan et al., 2020; Hvas et al., 2022). During CG, the food-restricted group shows greater food consumption, a hyperphagic response, and lower expenditure for metabolism and locomotion (Jobling et al., 1994; Ali et al., 2003). Moreover, the stunted fish showed high nutrient intake and minimum metabolic energy strategy, adopted from the stunting phase, leading to better feed utilization (Turano et al., 2007; Won and Borski, 2013; Lingam et al., 2019). FCR during the post-stunting phase was significantly lower in stunted fish indicating better feed utilization.
During the grow-out phase, stunted and normal fish were fed with the same feeding rate and thus the feed availability and growth efficiency were not differing much, but FCR was significantly lower in stunted fish indicating optimum utilization of the feed by stunted fish than normal fish, which is highly appreciable for commercial aquaculture practices.
The total quantity of feed provided for stunted fish during the entire experiment was found to be significantly lower than that of the normal fish. A study conducted on barramundi, L. calcarifer, investigated the effects of ration restriction and subsequent re-alimented feeding on growth efficiency, revealing an improvement in growth efficiency during the refeeding phase (Tian and Qin, 2003). However, in a study involving channel catfish, growth efficiency did not differ between the control group and the group that underwent a period of starvation followed by refeeding (Kim and Lovell, 1995). These findings suggest that with minimum feed application, the stunted fish could be raised to marketable size with better FCR.
Survival was higher for normal fish during the stunting period, which may be due to the fact that sub-optimal feeding affected survival due to competition for food. Costa-Bomfim et al. (2014) documented that in commercial farming operations, the presence of a high number of fish within a single-rearing structure can result in aggressive interactions that ultimately affect feeding behavior and survival. Ylikarjula et al. (1999) identified resource limitation and size- or age-dependent survival probabilities as the two possible factors contributing to the occurrence of natural stunting. Inadequate nutrition in the early stage hinders the growth and survival of fish (Kiron, 2012).
In the present study, the survival rate was not differing (p > 0.05) considerably among stunted and normal fish during the post-stunting as well as grow-out phase due to the fact that the stocking density was made equal for both groups during this phase. Lingam et al. (2019) reported a similar survival for stunted and normal fish but a drastic reduction in survival in long-term stunted milkfish.
Final production also did not differ greatly (p > 0.05) among stunted and normal fish, since there was no significant variation in final average weight and survival. Productivity per unit volume also did not differ significantly (p > 0.05) even though stunted fish have yielded slightly higher productivity. Finally, the present results indicate that the stunted fish performed on par with normal fish in terms of survival, total production, and productivity. Similar observations were reported in milkfish regarding total production when complete growth compensation was observed (Lingam et al., 2019).
CG is also reported to change the body composition of the recovering fish (Oh et al., 2008). The condition factor (k), the “wellbeing indicator” (Gomiero and Braga, 2005; Datta et al., 2013), was above 1 in both treatments, indicating that the fish have grown ideally in the system (Datta et al., 2013). Stunting is having an impact on the condition factor of the fish and the same could be used as an indicator of growth compensation (Turano et al., 2007). In the present work, the “k” value decreased as the size of the fish increased in both treatments. Luo et al. (2009) and Hvas et al. (2022) have reported a significant reduction in condition factors in starved fish. In the present study, the condition factor in stunted fish was consistently measured above 1.36, while for normal fish, it was consistently above 1.57. This finding aligns with the results reported by Damodaran et al. (2019), who conducted pond-rearing experiments with Silver pompano and identified an ideal condition factor value (“k” value) of above 1.5. The condition factor obtained in the present study is closer to the recommended “k” value reported by Damodaran et al. (2019), further supporting the validity of the findings. The “k” value was lower for stunted fish than for normal fish during the stunting and post-stunting phase but was restored by the end of the grow-out phase similar to other reports (Jobling, 2002; Caruso et al., 2012).
The “b” value for the length (g)–weight (cm) relationship was less than 3 in both the treatments in all the rearing phases and the regression value for all the relationships shows good fitness. The “b” value was higher in stunted fish during the stunting and post-stunting phase but was lower during final harvest. Cherif et al. (2008) mentioned that a higher calculated “b” value indicates that, at a given length, fish have more growth potential. LWR, the indicator of weight gain per unit length, was allometric in the present study similar to the other report on Silver pompano by Damodaran et al. (2019).
CV is an index of size variation to assess the size uniformity of harvested fish (Yu and Ueng, 2007; Costa-Bomfim et al., 2014). CV in size of Silver pompano differs considerably in various rearing practices (Suresh Babu et al., 2022b; Suresh Babu et al., 2022c). Contrary to the findings reported by Anikuttan et al. (2020), the present study observed that stunted fish exhibited a higher degree of size variation compared to normal fish during stunting. However, the variation was nearly equal during the remaining part of the rearing period.
Biometric analysis of stunted fish in comparison with normal fish revealed that the fish have grown in good condition at par with the normal fish and the increase in weight per unit length was allometric for both groups. The size of the fish at the time of final harvest was uniform with minimum variation in size for both stunted and normal fish, which indicates that the marketability of the stunted fish was not affected by stunting.
To find out the impact of stunting on meat yield and composition, the proximate composition and carcass analysis was done along with the morphometry of the harvested fish. Initially, the morphometric parameters were compared to find out the external asymmetry, if any, in the stunted fish since it impacts the market value. Total length, standard length, maximum body girth, body width, head length, and mouth length did not differ significantly between stunted and normal fish, indicating that the morphometry of fish belonging to a particular weight class does not vary much (p > 0.05) between stunted and normal fish.
Carcass characteristics were studied mainly to understand the variation in processing yield and nutrient distribution in various parts of the body such as the fore, mid, and hind parts (Ali et al., 2004; Dempson et al., 2004; Ali et al., 2006; Lingam et al., 2019). In fish, the weight of the head, visceral organs, scale, and fins affects the final dressed output (Fauconneau et al., 1995). Previous studies on carcass characteristics suggest that processing yield and carcass composition vary among body parts such as fore, mid, and hind parts (Sahu et al., 2000; Ali et al., 2004; Sahu et al., 2012; Sahu et al., 2013; Sahu et al., 2014). In the present study, the weight of the head, viscera, gills, fins, fore cut, head cut, and tail cut for fish in the same weight class did not differ significantly (p > 0.05) between stunted and normal fish at the time of harvest. In contrast, Lingam et al. (2019) have reported a higher dressing yield in long-term stunted milk fish when compared to normal fish. The dressing percentage did not vary (p > 0.05) between stunted and normal fish in the present work. According to Fauconneau and Laroche (1996), it is generally reported that the final dressed output of farmed fish is approximately 60%. In the present work, both stunted and normal fish yielded more than 60% dressing yield, which indicates that stunting of Silver pompano does not have any negative impact on the meat yield of the fish. Also, the proximate composition result indicates that the stunted fish regained body nutrients during the course of farming. This is in line with the observation of Jobling (2010) who opined that the alterations in the proximate composition of fish during stunting could be regained during the post-stunting phase depending on the degree of compensation.
The feeding strategies had no effects on indices such as SGOT, SGPT, and alkaline phosphatase, indicating that the stress and metabolic status of stunted fish were similar to that of the normal fish at the end of the grow-out phase. Similar results were reported by Azodi et al. (2014) in rainbow trout (Oncorhynchus mykiss) exposed to short-term starvation. Serum protein content especially albumin and globulin levels are the indirect indicators of immunity in fish (Yengkokpam et al., 2014). In the present work, significantly lower (p < 0.05) total albumin content and significantly higher (p < 0.05) total globulin content were observed in stunted fish. However, the total albumin-to-globulin ratio was not significantly different (p > 0.05) among the two groups. Lingam et al. (2019) have also reported significantly higher globulin content in stunted milkfish at the end of the grow-out period. Serum total protein did not differ (p > 0.05) significantly between the stunted and normal fish. Several studies indicate that, during starvation, the serum protein level decreases considerably (Azodi et al., 2014; Yengkokpam et al., 2016; Lingam et al., 2019). The prolonged rearing period after stunting might have helped the stunted fish to regain a total protein level similar to that of normal fish.
Cholesterol, the major serum lipid index, was significantly lower (p < 0.05), but triglycerides were significantly higher (p < 0.05) in the stunted fish. This may perhaps be due to the utilization of reserved lipid sources for metabolism and growth in the case of stunted fish even after the growth compensation phase. Fasting and feed restriction have a lowering effect on the liver’s cholesterol production (West et al., 1966; Kalpana, 1979). During the early stages of food deprivation, triglycerides are recognized to be the most readily available lipid reserve (Navarro and Gutierrez, 1995). Investigations in European sea bass (Perez-Jimenez et al., 2012), sturgeon (Acipenser naccarii), rainbow trout (Furne et al., 2012), and dentex (Dentex dentex) found constant plasma triglyceride levels during re-feeding after starvation. In contrast, multiple studies have shown that when fish species are deprived of food, their plasma triglyceride levels drop (Costas et al., 2011; Falahatkar, 2012). During CG in Nile tilapia, the hematological parameters were found to return to normal levels after refeeding, as reported by Abdel-Tawwab et al. (2006). A holistic approach to find out the lipid metabolism in stunted and normal fish in the controlled condition in stunted and post-stunted fish is planned as a follow-up work.
For evaluating the economic benefits of farming stunted fingerlings vis-à-vis normal fish, the cost of production of fish during the stunting phase was estimated and compared to that of normal fingerlings. The unit cost of production for stunted fingerling (USD 0.11) was found to be lower than that of the normal fingerlings (USD 0.87) mainly due to the difference in the quantity of feed used for the fingerling production and the resultant reduction in feed cost. Only the operational expenditure associated with experimental farming is considered at this stage. Results indicated higher net operational revenue (profit) and B:C ratio for the farming of stunted fingerlings in comparison with normal fingerlings. This can be mainly attributed to the significantly lower (p < 0.05) cost associated with total feed used, besides the lower unit cost of production for stunted fingerlings.
Based on the economic parameters obtained from the experiment, indicative economics of commercial farming of stunted fingerlings for 6-m-diameter HDPE cages in comparison with normal fish farming was calculated. Net revenue and B:C ratio for farming stunted fish were observed to be higher than the normal fish. Moreover, the estimated B:C ratio in the present case was higher than that reported by Damodaran et al. (2019) for pompano farming in ponds, as well as that for commercial cage farming reported by Aswathy et al. (2020).
Silver pompano fingerlings can be stunted in low-saline cages by depriving feed and overcrowding. The stunted fish could completely compensate for the growth within a short duration of post-stunting (60 days) with lower FCR and similar survival to that of the normal fish. Farming of the post-stunted fish in low-saline cages yielded similar growth, survival, and production to that of normal fish with lower FCR. Morphometric and biometric indices, carcass quality, dressing yield, and proximate composition of the stunted fish harvested were similar to that of normal fish, indicating that the stunting process has less impact on the general wellbeing and meat quality of the fish in the long run. Biochemical analysis of serum factors indicated better immunity, protein, and lipid utilization and a similar level of stress tolerance for the stunted fish as compared to normal fish. Owing to better utilization of feed for growth, the stunted fish yielded higher economic returns than that of normal fish. These results suggest that the technology can be widely applied for popularization of the farming of Silver pompano, and the same technique may be adopted for other marine candidate species for aquaculture. Stunted fingerling production has the added advantage of maintaining the fish in smaller transportable size for a longer duration so that the nursey-reared fish can be made available for a longer duration with minimum input cost.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Institutional funded project (MDN/GRO/22) of ICAR- Central Marine Fisheries Research Institute, Kochi, India.
SP: conceptualization, methodology, writing -original draft. AA, methodology, data analysis. ST, resources, writing-review & editing, SE, methodology, data analysis. SP, data analysis and economic evaluation. RK methodology, data analysis. PD, investigation, methodology. VG, resources. MP, investigation, methodology. BI, Resources. AK, methodology, data analysis. GA, visualization, overall supervision, acquisition of the funds. All authors contributed to the article and approved the submitted version.
We would like to express our gratitude to the Director of the Central Marine Fisheries Research Institute, Kochi, for the valuable support and encouragement throughout the research work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor SM declared a shared affiliation with the authors at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Tawwab M., Khattab Y. A. E., Ahmad M. H., Shalaby A. M. E. (2006). Compensatory growth, feed utilization, whole body composition and hematological changes in starved juvenile Nile tilapia, Oreochromis niloticus (L.). J. Appl. Aquacult 18, 17–36. doi: 10.1300/J028v18n03_02
Abdul Nazar A. K., Jayakumar R., Tamilmani G., Sakthivel M., Sirajudeen S., Balamurugan V., et al. (2012). Larviculture and seed production of the Silver pompano, Trachinotus blochii (Lacepede,1801) for the first time in India. Indian J. Fish 59, 83–87.
Abraham T. J., Sil S. K., Vineetha P. (2010). A comparative study of the aquaculture practices adopted by fish farmers in Andhra Pradesh and West Bengal. Indian J. Fish 57, 41–48.
Ali M., Iqbal F., Salam A., Sial F., Atha M. (2006). Comparative study of body composition of four fish species in relation to pond depth. Int. J. Environ. Sci. Technol. 2, 359–364. doi: 10.1007/BF03325897
Ali M., Nicieza A., Wootton R. J. (2003). Compensatory growth in fishes: a response to growth depression. Fish Fish 4, 147–190. doi: 10.1046/j.1467-2979.2003.00120.x
Ali M., Salam A., Goher S., Tassaduque K., Latif M. (2004). Studies on fillet composition of freshwater farmed Labeo rohita in relation to body size, collected from Govt. Fish seed hatchery Mianchannu, Pakistan. J. Biol. Sci. 4, 40–46. doi: 10.3923/jbs.2004.40.46
Anikuttan K. K., Jayakumar R., Suresh Babu P. P., Abdul Nazar A. K., Tamilmani G., Sakthivel M., et al. (2020). Assessment of compensatory growth in stunted fingerlings of Snubnose pompano, Trachinotus blochii (Lacepede 1801), in marine conditions. Aquac Res. 52, 403–409. doi: 10.1111/are.14879
AOAC (1995). Official Methods of Analysis of AOAC International (Arlington, Virginia, USA: Association of Official Analytical Chemists), 1230.
APHA (1981). Standard methods of the examination of water (Washington, D.C: American Public Health Association), 1134.
Aswathy N., Joseph I., Ignatius B., Joseph S. (2020). Economic viability of cage fish farming in India CMFRI Special Publication No. 134 (Kochi: ICAR-Central Marine Fisheries Research Institute), p36.
Azodi M., Ebrahimi E., Motaghi E., Vahid M. (2014). Metabolic responses to short starvation and re-feeding in rainbow trout (Oncorhynchus mykiss). Ichthyol Res. 62, 177–183. doi: 10.1007/s10228-014-0421-z
Bavcevic L., Klanjscek T., Karamarko V., Anicic I., Legovic T. (2010). Compensatory growth in gilthead sea bream (Sparus aurata) compensates weight, but not length. Aquaculture 301, 57–63. doi: 10.1016/j.aquaculture.2010.01.009351
Bhujel R. C., Little D. C., Hossain A. (2007). Reproductive performance and the growth of pre-stunted and normal Nile tilapia (Oreochromis niloticus) broodfish at varying feeding rates. Aquaculture 273, 71–79. doi: 10.1016/j.aquaculture.2007.09.022
Burrough R. J., Kennedy C. R. (1979). The occurrence and natural alleviation of stunting in a population of roach, Rutilus (L.). J. Fish Biol. 5, 93–109. doi: 10.1111/j.1095-8649.1979.tb03574.x
Caruso G., Denaro M. G., Caruso R., Genovese L., Mancari F., Maricchiolo G. (2012). Short fasting and re-feeding in red porgy (Pagrus pagrus, Linnaeus 1758): Response of some haematological, biochemical and nonspecific immune parameters. Mar. Environ. Res. 81, 18–25. doi: 10.1016/j.marenvres.2012.07.003
Charan R., Suresh Babu P. P., Venugopal G., Chadha N. K., Sreeramamurthy K. B. (2014). Effect of aromatase inhibitors on the ovarian development of stunted yearlings of rohu (Labeo rohita): a preliminary study. Aquac Int. 22, 689–697. doi: 10.1007/s10499-013-9697-7
Chavez H. M., Fang A. L., Carandang A. A. (2011). Effect of stocking density on growth performance, survival and production of Silver pompano, Trachinotus blochii, (Lacépède 1801) in marine floating cages. Asian Fish Sci. 24, 321–330. doi: 10.33997/j.afs.2011.24.3.005
Cherif M., Zarrad R., Gharbi H., Missaoui H., Jarboui O. (2008). Length-weight relationships for 11 fish species from the Gulf of Tunis (SW Mediterranean Sea, Tunisia). Pan-Am J. Aquat. Sci. 3, 1–5.
Cho Y. J., Cho S. H. (2009). Compensatory growth of olive flounder, Paralichthys olivaceus, fed the extruded pellet with different feeding regimes. J. World Aquac Soc. 40, 505–512. doi: 10.1111/j.1749-7345.2009.00270.x
Costa-Bomfim C. N., Pessoa W. V. N., Oliveira R. L. M., Farias J. L., Domingues E. C., Hamilton S., et al. (2014). The effect of feeding frequency on growth performance of juvenile cobia, Rachycentron canadum (Linnaeus 1766). J. Appl. Ichthyol 30, 135–139. doi: 10.1111/jai.12339
Costas B., Araga˜o C., Ruiz-Jarabo I., Vargas-Chacoff L., Jesu´s Arjona F., Dinis M. T., et al. (2011). Feed deprivation in Senegalese sole (Solea Senegalensis Kaup 1858) juveniles: effects on blood plasma metabolites and free amino acid levels. Fish Physiol. Biochem. 37, 495–504. doi: 10.1007/s10695-010-9451-2
Damodaran D., Mojjada S. K., Vase V. K., Sukhdhane K., Abdul Aziz P., Kumar R., et al. (2019). Intercropping of marine finfish in shrimp ponds: A maiden feasibility study. PloS One 14, e0216648. doi: 10.1371/journal.pone.0216648
Das P. C., Mishra S. S., Mishra B., Jayasankar P. (2016). Influence of juvenile stunting on grow-out performance of rohu, Labeo rohita (Hamilton 1822). J. Appl. Ichthyol 32, 848–858. doi: 10.1111/jai.13131
Datta S. N., Kaur V. I., Dhawan A., Jassal G. (2013). Estimation of length-weight relationship and condition factor of spotted snakehead Channa punctata (Bloch) under different feeding regimes. Retrieved Springerplus. doi: 10.1186/2193-1801-2-436
Dempson J. B., Schwarz C. J., Shears M., Furey G. (2004). Comparative proximate body composition of Atlantic salmon with emphasis on parr from fluvial and lacustrine habitats. J. Fish Biol. 64, 1257–1271. doi: 10.1111/j.0022-1112.2004.00389.x
De Silva S. S., Anderson T. A. (1995). Fish nutrition in aquaculture (London: Chapman & Hall), p319.
Ebeneezar S., Vijayagopal P., Srivastava P. P., Gupta S., Varghese T., Linga Prabu D., et al. (2020). Optimum dietary methionine requirement of juvenile Silver pompano, Trachinotus blochii (Lacepede 1801). Anim. Feed Sci. Technol. 268, 114592. doi: 10.1016/j.anifeedsci.2020.114592
Falahatkar B. (2012). The metabolic effects of feeding and fasting in beluga Huso huso. Mar. Environ. Res. 82, 69–75. doi: 10.1016/j.marenvres.2012.09.003
Fauconneau B., Alami-Durante H., Laroche M., Marcel J., Vallot D. (1995). Growth and meat quality relations in carp. Aquaculture 129, 265–297. doi: 10.1016/0044-8486(94)00309-C
Fauconneau B., Laroche M. (1996). Characteristics of the flesh and quality of products of catfishes. Aquat Living Resour 9, 165–179. doi: 10.1051/alr:1996051
Foss A. K., Imsland A., Vikingstad E., Stefansson S. O., Norberg B., Pedersen S., et al. (2009). Compensatory growth in Atlantic halibut: Effect of starvation and subsequent feeding on growth, maturation, feed utilization and flesh quality. Aquaculture 290, 304–310. doi: 10.1016/j.aquaculture.2009.02.021
Furne M., Morales A. E., Trenzado C. E., Garcıa-Gallego M., Hidalgo M.C., Domezain A., et al. (2012). The metabolic effects of prolonged starvation and refeeding in sturgeon and rainbow trout. J. Comp. Physiol. 182, 63–76. doi: 10.1007/s00360-011-0596-9
Gomiero L. M., Braga F. M. S. (2005). The condition factor of fishes from two river basins in Sao Paulo state, Southeast of Brazil. Acta Sci. 27, 73–78. doi: 10.4025/actascibiolsci.v27i1.1368
Hayward R. S., Noltie D. B., Wang N. (1997). Use of compensatory growth to double hybrid sunfish growth rates. Trans. Am. Fish Soc. 126, 316–322. doi: 10.1111/j.1095-8649.1979.tb03574.x
Hvas M., Nilsson J., Vågseth T., Nola V., Fjelldal P.G., Hansen T.G., et al. (2022). Full compensatory growth before harvest and no impact on fish welfare in Atlantic salmon after an 8-week fasting period. Aquaculture 546. doi: 10.1016/j.aquaculture.2021.737415
Jayakumar R., Abdul Nazar A. K., Tamilmani G., Sakthivel M., Kalidas C., Ramesh Kumar P., et al. (2014). Evaluation of growth and production performance of hatchery produced Silver pompano Trachinotus blochii (Lacepede 1801) fingerlings under brackish water pond farming in India. Indian J. Fish 61, 58–62.
Jobling M. (2002). “Environmental factors and rates of development and growth,” in Handbook of Fish Biology and Fisheries, vol. Vol 1 . Eds. Hart P. J. B., Reynolds J. D. (New Jersey, USA: Blackwell), 97–122.
Jobling M. (2010). Are compensatory growth and catch-up growth two sides of the same coin? Aquac Int. 18, 501–510. doi: 10.1007/s10499-009-9260-8
Jobling M., Meloey O. H., Santos J., Christiansen B. (1994). The compensatory growth response of the Atlantic cod: Effects of nutritional history. Aquac Int. 2, 75–90. doi: 10.1007/BF00128802
Kalidas C., Ramesh Kumar P., Linga Prabu D. (2020). Optimizing stocking density for grow-out culture of snubnose pompano Trachinotus blochii (lacépède 1801) in marine floating cages. J. Appl. Aquac 34, 223–233. doi: 10.1080/10454438.2020.1829245
Kalidas C., Sakthivel M., Tamilmani G., Balamurugan V., Ramkumar P., Prem J., et al. (2012). Survival and growth of Juvenile Silver pompano Trachinotus blochii (Lacepede ,1801) (Lacepède 1801) at different salinities in tropical conditions. IJ Fish 59, 95–98.
Kalpana D. S. (1979). Influence of starvation on the brain and liver cholesterol levels of the cat-fish, Heteropneustes fossilis (Bloch). Proc. Indian Acad. Sci. 88, 205–208.
Kim M. K., Lovell R. T. (1995). Effect of restricted feeding regimens on compensatory weight gain and body tissue changes in channel catfish Ictalurus punctatus in ponds. Aquaculture 135, 285–293.
Kiron V. (2012). Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol. 173, 111–133. doi: 10.1016/j.anifeedsci.2011.12.015
Lingam S. S., Sawant P. B., Chadha N. K., Pani Prasad K., Muralidhar A.P., Syamala K., et al. (2019). Duration of stunting impacts compensatory growth and carcass quality of farmed milkfish, Chanos chanos (Forsskal 1775) under field conditions. Sci. Rep. 9, 16747. doi: 10.1038/s41598-019-53092-7
Llameg M., Serrano A. E. Jr (2014). Effect of cyclic feeding on compensatory growth in milkfish Chanos chanos juveniles. Astrobiol 6, 22–28.
Luo Z., Tan X. Y., Wang W. M., Fan Q. X. (2009). Effects of long-term starvation on body weight and body composition of juvenile channel catfish, Ictalurus punctatus, with special emphasis on amino acid and fatty acid changes. J. Appl. Ichthyol 25, 184–189. doi: 10.1111/j.1439-0426.2009.01216.x
Navarro I., Gutierrez J. (1995). “Fasting and starvation,” in Biochemistry and molecular biology of fishes, vol. vol 4 . Eds. Hochachka P. W., Mommsen T. P. (New York: Elsevier), 393–433.
Oh S., Noh C. H., Kang R., Kim C., Cho S.H., Jo J., et al. (2008). Compensatory growth and body composition of juvenile black rockfish Sebastes schlegeli following feed deprivation. Fish Sci. 74, 846–852. doi: 10.1111/j.1444-2906.2008.01598.x
Perez-Jimenez A., Cardenete G., Hidalgo M. C., Garcia-Alcazar A., Abellan E., Morales A. E., et al. (2012). Metabolic adjustments of Dentex dentex to prolonged starvation and re-feeding. Fish Physiol. Biochemist 38, 1145–1157. doi: 10.1007/s10695-011-9600-2
Radheysham H. K., Saha G. S. (2009). Role of community in production of larger and quality fingerlings. Aquaculture Asia Magazine, 16–17.
Sahu B. B., Meher P. K., Mohanty S., Reddy P. V. G. K., Ayyappan S. (2000). Evaluation of carcass and commercial characteristics of carps. Naga ICLARM Q 23, 10–14.
Sahu B. B., Raghunath M. R., Meher P. K., Das P. C., Mishra B., Senapati D.K., et al. (2013). Carcass characteristics of marketable size farmed catla, Catla catla (Hamilton 1822). J. Appl. Ichthyol 29, 854–857. doi: 10.1111/jai.12217
Sahu B. B., Raghunath M. R., Meher P. K., Senapati D. K., Das P.C., Mishra B., et al. (2014). Comparison studies on carcass characteristics marketable size farmed mrigal Cirrhinus mrigala, (Hamilton, 1822) and Silver carp Hypophthalmichthys molitrix, (Val. 1844). J. Appl. Icthyol 30, 195–199. doi: 10.1111/jai.12354
Sahu B. B., Samal R., Meher P. K. (2012). Carcass traits of different marketable sizes of rohu, Labeorohita (Hamilton 1822). J. Appl. Ichthyol 3, 673–677.
Santiago C. B., Gonzal A. C., Aralar E. V., Arcilla R. P. (2004). Effects of stunting of juvenile bighead carp, Aristichthys nobilis (Richardson) on compensatory growth and reproduction. Aquac Res. 35, 836–841. doi: 10.1111/j.1365-2109.2004.01074.x
Suresh Babu P. P., Anikuttan K. K., Anuraj A., Jayakumar R., Nazar A. K. A., Sakthivel M., et al. (2022c). Estimation of Size Variation and Other Biometrics in Silver Pompano Trachinotus blochii (Lacepede, 1801) Reared in Different Farming conditions. Thalassas: Int. J. Mar. Sci. 39 (1), 1–6. doi: 10.1007/s41208-022-00485-7
Suresh Babu P. P., Anuraj A., Loka J., Praveen N. D., Srinivas Rao K., Shilta M. T., et al. (2022a). Impact of duration of stunting on compensatory growth and biometrics of snubnose pompano, Trachinotus blochii (Lacepede,1801) in low saline conditions. Thalassas: an Int. J. Mar. Sci. doi: 10.1007/s41208-022-00426-4
Suresh Babu P. P., Anuraj A., Loka J., Ramudu K. R., Rao K. S., Dube P., et al. (2022b). Investigations on the performance of snubnose pompano Trachinotus blochii (Lacepede,1801) in a lowcost recirculating aquaculture system. Indian J. Fish 69 (2), 139–143.
Suresh Babu P. P., Rao P. S., Krishna Prasad J., Sharma R., Babitha Rani A. M., Biju I. F., et al. (2021). Observations on impact of stunting on breeding performance of farmed rohu Labeo rohita (Hamilton, 1822). Indian J. Fish 68, 117–121. doi: 10.21077/ijf.2021.68.1.109035-08
Tian X., Qin J. G. (2003). A single phase of food deprivation provoked compensatory growth in barramundi Lates calcarifer. Aquaculture 224, 169–179. doi: 10.1016/s0044-8486(03)00224-2
Turano M. J., Borski R. J., Daniels H. V. (2007). Compensatory growth of pond reared hybrid striped bass, Morone chrysops X Morone saxatilis, fingerlings. World Aquac 38, 250–261. doi: 10.1111/j.1749-7345.2007.00094.x
West E. S., Todd W. R., Mason H. S., Van Bruggen J. T. (1966). Text book of biochemistry. 4th ed (London, UK: New York: Macmillan Company; London: Collier Macmillan Ltd), 1017–1023.
Won T. E., Borski R. J. (2013). Endocrine regulation of compensatory growth in fish. FrontEndocrinol 4. doi: 10.3389/fendo.2013.00074
Yengkokpam S., Debnath D., Sahu N. P., Pal A.K., Jain K.K., Kartik B., et al. (2016). Dietary protein enhances non-specific immunity, anti-oxidative capability and resistance to Aeromonas hydrophila in Labeo rohita fingerlings pre-exposed to short feed deprivation stress. Fish Shellfish Immunol. 59, 439–446. doi: 10.1016/j.fsi.2016.10.052
Yengkokpam S., Sahu N. P., Pal A. K. (2014). Short term periodic starvation of Labeo rohita fingerlings: effect on growth and health. Fish Soc. 44, 20–29. doi: 10.1016/j.aquaculture.2013.07.025
Ylikarjula J., Henio M., Dieckmann U. (1999). Ecology and adaptations of stunted growth in fish. Evol. Ecol. 13, 433–453. doi: 10.1023/A:1006755702230
Keywords: carcass analysis, compensatory growth, economic evaluation, proximate composition, snubnose pompano
Citation: P. P. SB, A. A, M. T. S, Ebeneezar S, P. S, K. RR, N. D. P, N. G. V, Pal M, I. B, K. K. A and A. G (2023) Compensatory growth and production economics of Silver pompano, Trachinotus blochii (Lacepede, 1801), fingerlings stunted by feed and space deprivation. Front. Mar. Sci. 10:1234667. doi: 10.3389/fmars.2023.1234667
Received: 05 June 2023; Accepted: 09 August 2023;
Published: 07 September 2023.
Edited by:
Sukham Munilkumar, Central Institute of Fisheries Education (ICAR), IndiaReviewed by:
Chang’an Wang, Chinese Academy of Fishery Sciences, ChinaCopyright © 2023 P. P., A., M. T., Ebeneezar, P., K., N. D., N. G., Pal, I., K. K. and A.. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suresh Babu P. P., c2JhYnVra2RAcmVkaWZmbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.