- 1Projeto Baleia à Vista (ProBaV), Ilhabela, SP, Brazil
- 2Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo, São Paulo, SP, Brazil
- 3Department of Fisheries, Wildlife and Conservation Sciences, Marine Mammal Institute, Oregon State University, Newport, OR, United States
- 4Orca Behavior Institute, Friday Harbor, WA, United States
- 5Instituto Argonauta para Conservação Costeira e Marinha, Ubatuba, SP, Brazil
- 6Departamento de Ciências Biológicas, Escola Nacional de Saúde Pública/ Fiocruz and Grupo de Estudos de Mamíferos Marinhos da Região dos Lagos (GEMM-Lagos), Rio de Janeiro, RJ, Brazil
Killer whales (Orcinus orca) are cosmopolitan apex predators that occupy important ecological roles and show some variations in feeding and social habits in coastal and pelagic environments worldwide. Although they have been regularly reported along the Brazilian coastline, their natural history in these tropical and subtropical waters remains poorly understood. Here, we provide new information on group size, behavior, movements and the first assessment of their social structure in Brazilian coast. From 2005 to 2021, 57 new records of sightings were opportunistically observed with estimated group sizes ranging from 1 to 11 individuals (mean = 5.61; SD = 2.91), and 47 individuals were photo-identified—28% adult females, 19% adult males, 19% juveniles, 17% calves and 17% adults of unknown sex. Thirty-one individuals (66%) were sighted just once and sixteen (34%) were resighted more than once (resighting rate = 0.30 ± 0.30 SD). Killer whales were observed feeding on rays four times (two out of which on butterfly rays Gymnura altavela), twice on an unidentified fish school of fish, while attacks on marine mammals were recorded. Between 2020 and 2021, photo-identification results of 11 specific individuals revealed both long and short-distance movements from the southeastern and southern Brazilian coasts to the coast of Uruguay. Individuals seem to be resighted together over time, as suggested by the average half-weight association index (HWI = 0.29 ± 0.19 SD) and a permutation test rejecting the null hypothesis of random association (CVreal = 0.67 > CVmean = 0.01, pCV = 1.00), forming small groups of mixed age-sex that engage in both short- and long-term associations. These patterns suggest that they could form stable social units that also experience some degree of fission-fusion dynamics. While the nature of the opportunistic data hinders a definitive portrayal of the social structure of killer whales using the Brazilian coastal waters, these novel insights contribute to mapping the socio-ecology and behavioral diversity of one of the most widely distributed mammals.
Introduction
Killer whales (Orcinus orca, Linnaeus, 1758) have a wide distribution throughout the world’s oceans. They are most commonly found in higher latitudes in temperate and sub-polar waters with high marine productivity but also occur in offshore and less productive tropical waters (Leatherwood and Dalheim, 1978; Forney and Wade, 2006). They are considered top ocean predators, feeding on a variety of different prey, from invertebrates, fishes, and other marine mammals (Ford, 2002). Within their monotypic genus (Rice, 1998), killer whales from the both hemispheres form several ecotypes with distinguished external morphology, habitat use, behavior, social structure, acoustic repertoire, prey preferences, and genetics (Ford et al., 1998; Baird and Whitehead, 2000; Pitman and Ensor, 2003; Visser, 2007; LeDuc et al., 2008; Foote et al., 2009; Pitman, 2011; Durban et al., 2016; Foote et al., 2016). In the south-western Atlantic, killer whales were recorded along all Argentinean coast, mainly in Peninsula Valdés (Iñíguez, 2001). Small groups have been sighted around the Falkland (Malvinas) Islands (Yates et al., 2007) and in Uruguay, were observed in coastal waters and also related with depredation on catches of the pelagic longline fishery in offshore waters (Iriarte, 2006; Passadore et al., 2015).
Along most of the Brazilian coast, between latitudes 03°N to 32° S, killer whales have been reported as opportunistic sightings or stranding events of single individuals (Lodi and Hetzel, 1998; Siciliano et al., 1999; Dantas, 2007). Capture records by ex-soviet pelagic fleets during the whaling period (1969/70-1978/79) were also recorded in the region (Mikhalev et al., 1981). Their occurrence in coastal waters off south-eastern Brazil was associated with upwellings conditions and prey availability (Siciliano et al., 1999) and records have increased in the last two decades as more search efforts were applied (Pinedo et al., 2002; Santos and Netto, 2005; Meirelles et al., 2009; Santos and Silva, 2009; Santos et al., 2010; Batista et al., 2012; Ott et al., 2017; Lodi and Tardin, 2018; Santos et al., 2019; Renault-Braga et al., 2019). Few specimens had been studied in detail since fresh strandings are rare (Lemos et al., 2013; Laeta et al., 2019; Groch et al., 2020) and Morin et al. (2015) cite the stranding of an Antarctic killer whale type C in Brazil but without providing detailed information. The depredation by killer whales in the tuna longline fishery that operates from the north to south of Brazil to Uruguay indicates the regular presence of killer whales in offshore waters (Secchi and Vaske, 1998; Dalla Rosa and Secchi, 2007; Dantas, 2007; Passadore et al., 2012; Wedekin et al., 2014). Further evidence of their occurrence in offshore waters off Brazil comes from dedicated surveys and technical reports (Ramos et al., 2010; Andriolo et al., 2015; Di Tullio et al., 2016; PMC-BS, 2017).
Despite these observations off Brazil, the natural history of killer whales in these tropical and subtropical waters remains poorly understood, and data deficiency precludes the assessment of their conservation status (ICMBio, 2023). The first published record of killer whale movements in the region described a solitary male resighted over 11 years along the coast of Rio de Janeiro and Parana (22°S - 25°S) (Santos and Silva, 2009; Lodi and Farias-Junior, 2011). In 2016 and 2017, the PMC-BS (Santos Basin Cetacean Monitoring Project) that monitors the cetacean populations in coastal and oceanic areas of the Santos Basin offshore sedimentary basin to assess possible impacts of oil activities and gas on these animals, tagged four individuals in southeastern Brazil. Their data showed animals that remained approximately 225 km from the coast for 3 to 13 days, and a solitary male that moved from southern Brazil to the southern coast of Uruguay and Argentina during 33 days (PMC-BS, 2017). In addition, Santos et al. (2019) reported on a group of killer whales that travelled between Rio de Janeiro and São Paulo over a period of 27 days and Durban and Pitman (2012), through satellite telemetry, identified movements of five Type B Antarctic killer whales between the Antarctic Peninsula and the oceanic waters of southern Brazil and Uruguay. Such scattered reports on movements and behavior, although precious, hinders our understanding of the complex relationships of killer whale groups of this region. In this paper, new observations including group size, behavior and movements are presented contributing with more pieces of this puzzle in order to have a better understanding of the whole picture. Also, considering that social structure synthesizes a vital class of ecological relationships (Whitehead, 2008), can affect population dynamics, genetics (Wilson, 1975; Strier, 1997) and, is an important factor for conservation (Sutherland, 1998), using traditional photo-identification techniques, we present the first assessment of social structure of killer whales off the Brazilian coast.
Materials and methods
Data collection and study area
The sighting data and images of killer whales in this study were recorded opportunistically from 2005 to 2021 by 3 different contributors: (i) from Baleia à Vista Project (ProBaV), a citizen science project that monitors the waters off the north coast of São Paulo, Brazil, around the region of Ilhabela Archipelago (23° 48.735' S, 45° 22.019' W) (Cardoso et al., 2019a; Cardoso et al., 2019b; Athayde et al., 2020; Siciliano et al., 2020; Marcondes et al., 2021; Athayde et al., 2022), (ii) from Argonauta Institute, an institution dedicated to the conservation of coastal and marine ecosystems that also monitors the same region as ProBaV since 1998; and (iii) from occasional contributors: wildlife photographers and enthusiasts, divers, sailors, and others who had opportunistically encountered killer whales during their professional or recreational activities. Most of these contributor’s records were reported from southern and southeastern Brazil, except for five records that were made in La Paloma, on the coast off Uruguay (Figure 1). The authors were contacted directly and the following information were collected: additional media (video/ photos), geographical coordinates, group size estimate and, species behavior observed during the sightings (travelling, feeding, courtship and resting). Permission for the academic use of their images and data were obtained.
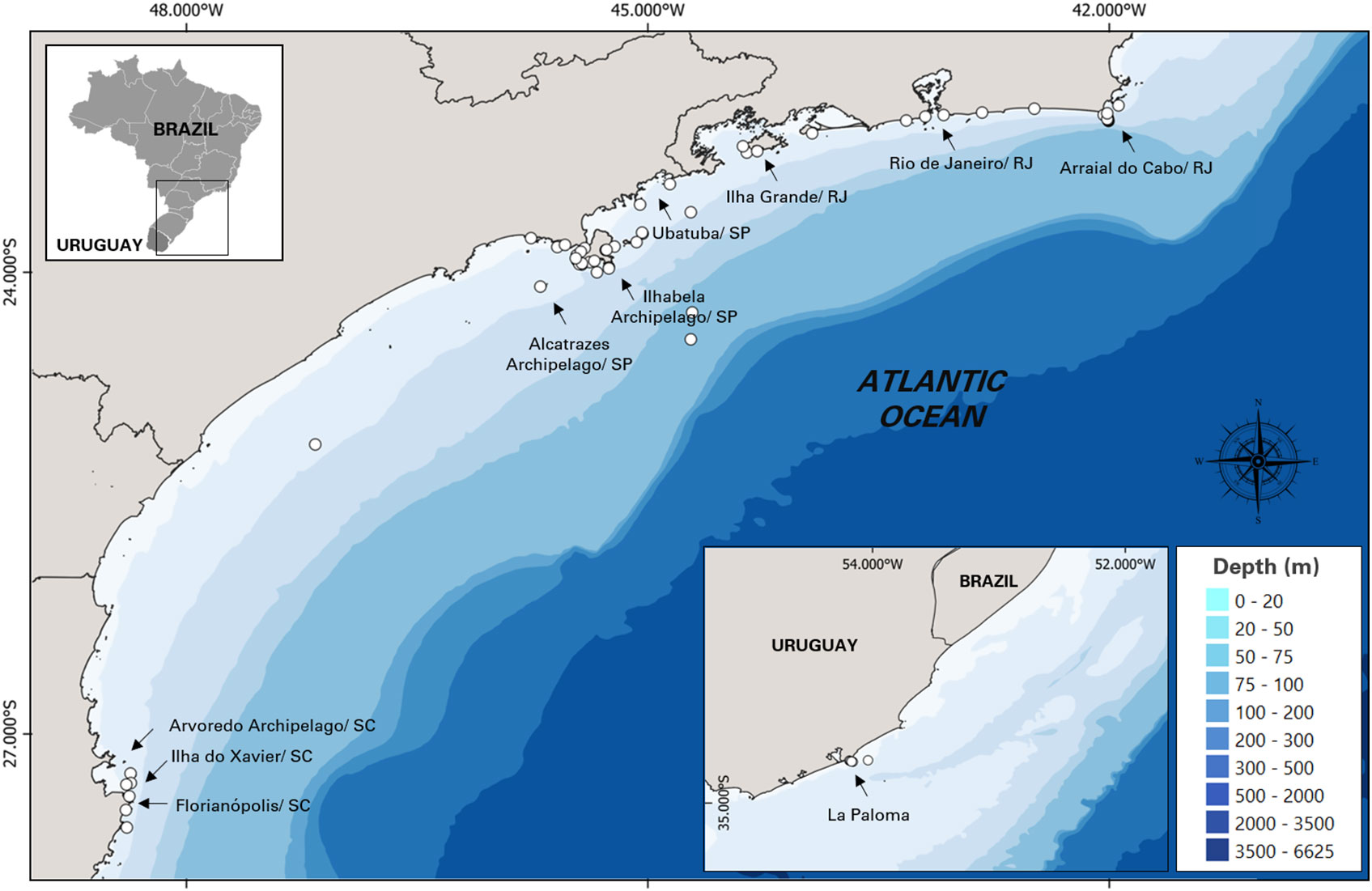
Figure 1 Study area in southeastern coast of Brazil and the 5 additional sightings of Uruguay showing the location of killer whale sightings (n = 57).
Photo and data analysis
Photos from ProBaV and Argonauta Institute were taken using various combinations of DSLR cameras and lenses and, in attempt to increase the chances of identifying individuals, when possible, pictures of both sides of the dorsal fins, eyepatches and saddle patches were taken in different angles. In some encounters, it was not achievable to photograph all individuals of the group. The images shared by contributors were added to this unified database. A total of 8.754 images were analyzed and for the entire data set, a quality rating between 1 (lowest) and 3 (highest) was assigned based on sharpness, contrast and angle of the dorsal fin and the eyepatch in relation to the camera (Jourdain et al., 2017).
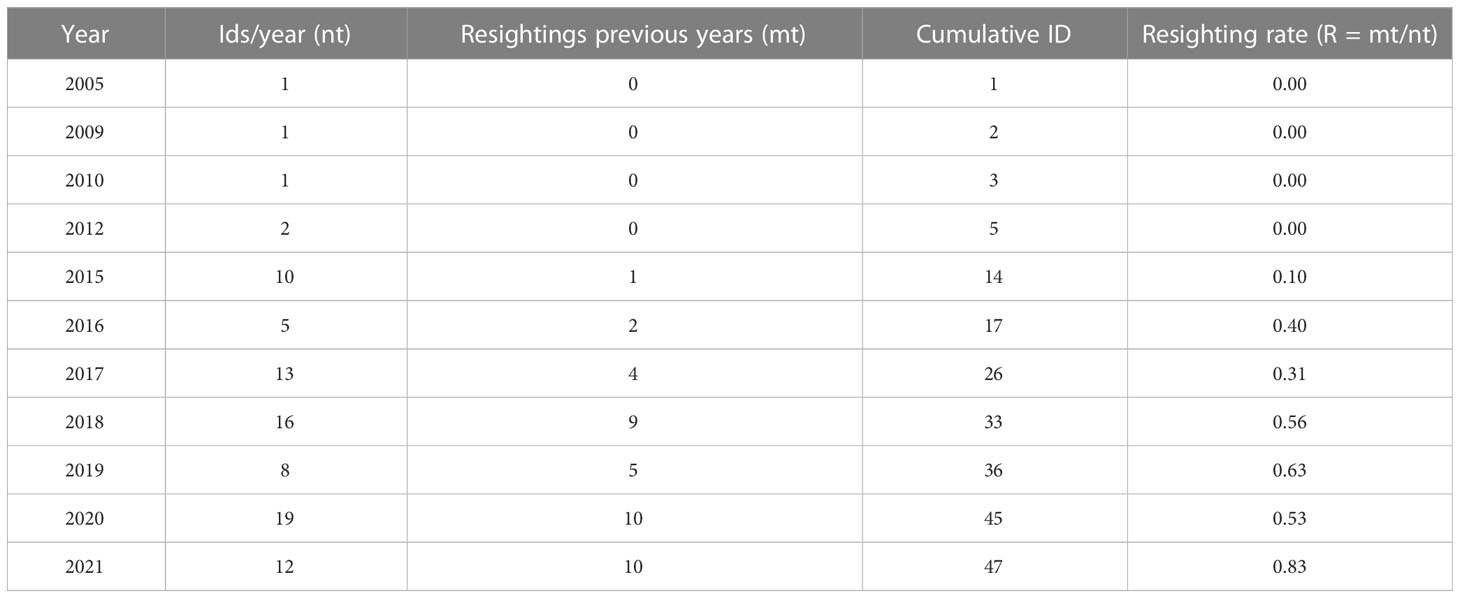
For the photo-identification analysis, only qualified images of a quality ≥ 2 were used. The individuals were photo-identified and cross compared by their natural marks, nicks, notches and scars and were included in the catalog (Figure 2). Sex and stage of maturity were based on the external morphology as previously determined by Bigg et al. (1987) and classified in five different categories: 1) Adult males - males that have reached sexual maturity and clearly present a taller dorsal fin; 2) Adult females - individuals with smaller dorsal fin, where no development of secondary sexual characteristics over the years or by close and consistent association with the calf; 3) Juveniles - individuals of both sexes that have not reached mature size, measuring between 50% to 75% the size of an adult female, but larger than calves; 4) Calves - young killer whales measuring less than 50% the size of an adult female; 5) Unknow - when the distance of the image only allows identifying those animals were killer whales but not classifying the sex/ stage of maturity (Visser, 2000b; Tavares et al., 2016) or when it was not possible to differentiate females from males that had not yet had dorsal fin sprouting. Photo-identified individuals were assigned to alphanumeric labels (prefix BR followed by a number); calves accompanying females were assigned the same label plus a sequential letter. So, if the mother is BR01, the calf will be BR01A. If she is seen again at another time with a new calf, that one will be given the name BR01B and so on. This is a similar method used to catalog the transient killer whales of the west coast of the United States and Canada in Pacific waters (Ford and Ellis, 1999) and for the Bryde’s whales (Balaenoptera brydei) studied in the same region (Athayde et al., 2020). The resighting rate of identified killer whales was calculated by dividing individuals re-sighted in previous years (mt) by individuals identified each year (nt) (Denkinger et al., 2020).

Figure 2 Individuals BR05 and BR16 photo-identified by their natural marks through the dorsal fin and eyepatch.
Group size, behavior, and seasonality analysis were recorded for all sightings from 2005 to 2021 from the three sources. The movement analysis considered only records of re-sightings of individuals to demonstrate their displacements. Maps for analysis and representation of movements and sightings were produced with QGIS 3.10 software using bathymetric data from GEBCO Compilation Group, (2021).
Data social analysis
For the social analysis, we defined groups as two or more different individuals that were in visual range of the observers. To minimize spurious associations, we analyzed only individuals sighted at least three times and in encounters where it was possible to photo-identify at least two individuals in the group. Associations were estimated using the half-weight index (HWI), which represents the proportion of times individuals were seen together, attempting to minimize biases when not all individuals within each group have been identified (Cairns and Schwager, 1987; Whitehead, 2008). The resulting association matrix was projected as a social network, in which numbered nodes representing the individuals were linked by weighted lines whose thicknesses were proportional to their association indices (Whitehead and Dufault, 1999; Whitehead, 2009). A Monte Carlo permutation test was performed by permuting groups within samples (Whitehead, 2009) to test the null hypothesis that individuals associate at random, using the coefficient of variation (CV) of the association matrix as a benchmark. The null hypothesis was rejected when the CV of the observed data was significantly greater than the CV of the permuted data (Bejder et al., 1998; Whitehead, 2009), indicating that there were disproportionately larger and smaller observed indices than expected by chance, which are suggestive of preferred and avoided associations, respectively. The group data were permutated four times (1000, 5000, 10000 and 20000 iterations) until the overall p-value stabilized (Bejder et al., 1998); we reported results generated with 20.000 permutations. To infer social units, if any, we projected the association matrix with hierarchical clustering analysis and used the maximum modularity Q, calculated by the leading eigenvector method (Newman, 2006), to identify a suitable partition of the dendrogram. To test the statistical significance of this partition, we used a null model approach (Farine and Whitehead, 2015), equivalent to the Monte Carlo permutations above (Bejder et al., 1998), to generate an ensemble of 20000 permuted association matrices and build a benchmark distribution of Q-values; the observed Q-value was considered significant if falling outside of the 95% confidence intervals (CI) of the benchmark distribution (Farine and Whitehead, 2015). Finally, we investigate the temporal patterns of associations. We calculated the standardized lagged association rate (SLAR) as the probability of two animals that are associated at a given time to be associated again after different time lags (Whitehead, 1995). As a benchmark, we calculated the null association rates (NAR) in which individuals are assumed to associate at random. The standard errors of SLAR were estimated using the jackknife procedure (Whitehead, 1995). Then, we fitted four exponential models to SLAR (Whitehead, 1995) and selected the most parsimonious with quasi-Akaike Information Criterion (QAIC; Whitehead, 2007), to describe how associations decay over time. The first model, SLAR1, has no decay in association rates, suggesting permanent associations; the second model SLAR2, represents association rates that decay to zero, suggesting associations that happened for a given time but never again; SLAR3 represents rates that decay and level off after a given lag, suggesting both long-lasting and temporary associations; SLAR4 represents two exponential decays to zero, suggesting a level of disassociation at a shorter lag and another at longer time lag (Whitehead, 1995). Social analyses were conducted using SOCPROG 2.9 (Whitehead, 2009).
Results
Group size, behavior and, seasonality
The opportunistic sightings from 2005 to 2021 yielded 57 new observations of killer whales, in groups from one to 11 individuals (mean = 5.61, SD = 2.91). Forty-seven individuals were photo-identified including: 28% adult females (n = 13), 19 % adult males (n = 9), 19% juveniles (n = 9; where 7 of unknow sex and 2 males), 17% calves (n = 8; where 7 of unknow sex and 1 male), and 17% of adults of unknown sex (n = 8). Travelling was the most common behavior observed (88%), followed by feeding (10%). In two occasions (July 20, 2019 and December 30, 2020), killer whales were observed hunting and sharing butterfly rays (Gymnura altavela) in the south of the Ilhabela Archipelago, São Paulo state, in association with frigatebirds (Fregata magnificens) and brown boobies (Sula leucogaster) (Figure 3). In a third occasion (November 24, 2021), the group was hunting a stingray (unidentified species) in Arraial do Cabo, Rio de Janeiro. On January 10, 2021 and December 15, 2021, they were spotted surrounding a school of fish (unidentified species) around the region of the Arvoredo Archipelago in Santa Catarina state and Marambaia Island, Rio de Janeiro, respectively. On November 30, 2018, in the northeast of Ilhabela Archipelago, Bryde’s whales were observed by ProBaV feeding on a school of fish when killer whales approached the whales. No attempt to attack Bryde’s whales was observed and despite a brief interaction, killer whales seemed more interested in attacking a stingray (unidentified species) in the same area. After feeding on the stingray, they left the area. Only in one occasion (2%) it was possible to watch orcas in a video socializing in a possible courtship behavior off Ilhabela on December 7, 2012. Resting was not observed. killer Whales were sighted all year round on the coast, and the number of sightings seem to increase in the austral spring and summer (spring: 39%, summer: 33%, winter: 26%, fall: 2%; n=57 sightings).

Figure 3 Killer whales hunting a butterfly ray (Gymnura altavela) off the Ilhabela Archipelago, São Paulo, on (A) July 20 2019 and on (B, C) December 30 2020 in association with Fregata magnificens.
Re-sightings
Out of the 47 photo-identified individuals, 31 (66%) were sighted just once and 16 (34%) were resighted more than once, where: 1 (2%) was resighted thirteen times, 1 (2%) eleven times, 1 (2%) ten times, 1 (2%) nine times, 3 (6%) eight times, 1 (2%) seven times, 2 (4%) four times, 4 (8%) three times and, 2 (4%) twice. The mean of resighting rate for the cataloged killer whales was 0.30 (SD = 0.30), noting that in the last 4 years more than 50% of individuals had been sighted in previous years (2018 - 56%; 2019 - 63%; 2020 - 53%; 2021 – 83%) (Table 1).
Two animals were resighted equal to or greater than 10 years apart. The adult female BR22, where the first sighting was November, 22 2010 and last on November 22, 2020 (10 years) and the adult male BR14 where the first sighting was July, 04 2005 and last on August 10, 2018 (13 years). A previously known adult male was sighted once traveling alone (BR01). Published articles reported 14 sightings of this male (Santos and Silva, 2009; Lodi and Farias-Junior, 2011) over 11 years and here we report one additional sighting on July 7, 2009.
Movements
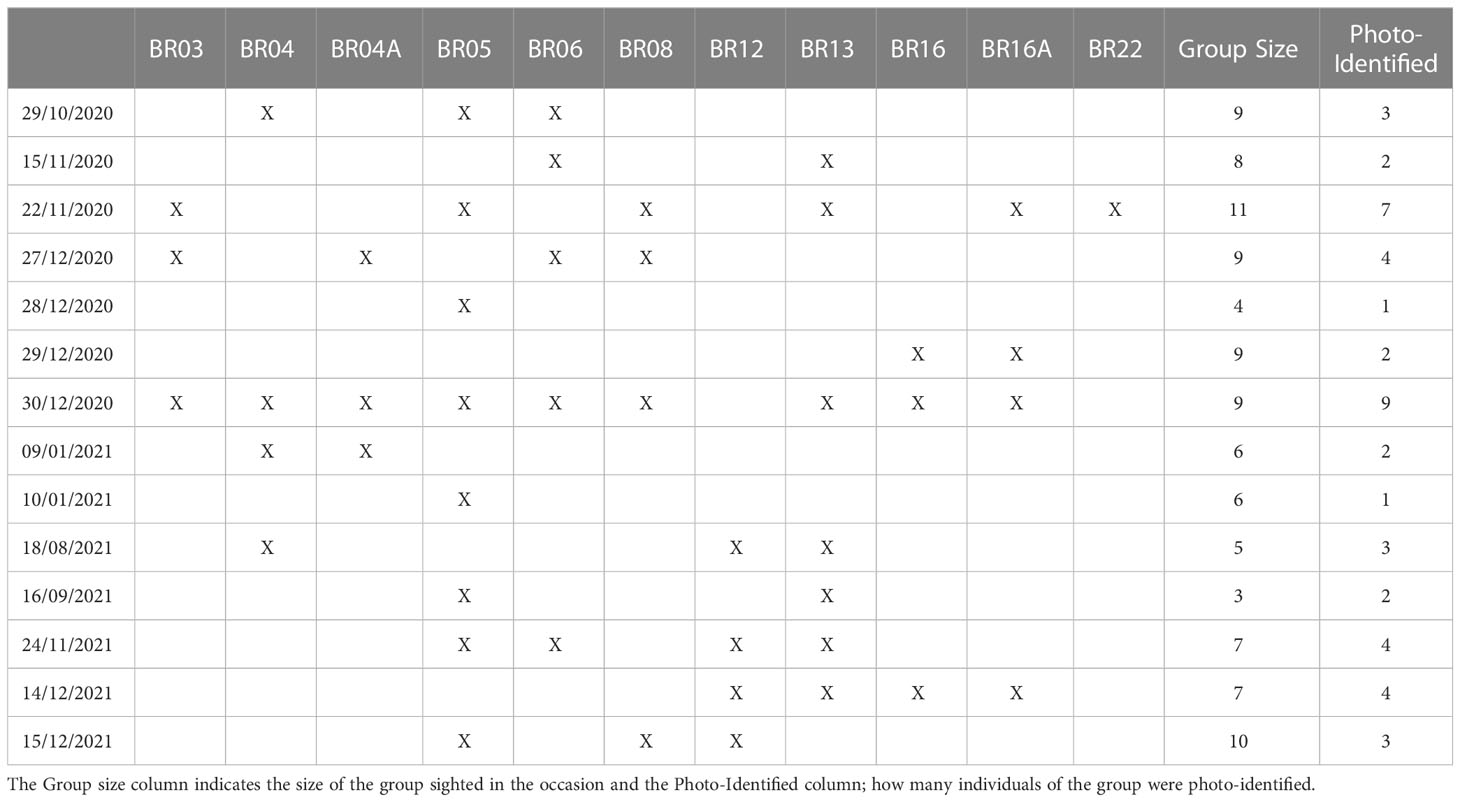
Based on these re-sightings, between 2020 and 2021, it was possible to track the movements of 11 specific individuals: BR03, BR04, BR04A, BR05, BR06, BR08, BR12, BR13, BR16, BR16A and BR22 in different regions (Figure 4). On October 29, 2020, individuals BR04, BR05 and BR06 were sighted with other non-photo-identified individuals near to Ilha do Xavier in the state of Santa Catarina. Seventeen days later, between November 15 and November 22 of 2020, BR03, BR05, BR06, BR08, BR13 and BR16A were sighted in Arraial do Cabo in Rio de Janeiro. On this last occasion, these individuals were seen together with the adult female BR22, which was swimming with 4 other non-photo-identified individuals, in a group of 11 animals. On December 27 of 2020, a group of killer whales were reported in Ilha Grande in Rio de Janeiro and the individuals BR03, BR04A, BR06, and BR08 were identified. On December 28 and 29, BR05, BR16 and, BR16A were sighted between the region of Ubatuba, São Paulo and Ilhabela Archipelago. On December 30, all the killer whales of the group were photo-identified by ProBaV in the Ilhabela Archipelago (BR03, BR04, BR04A, BR05, BR06, BR08, BR12, BR13, BR16 and BR16A). On January 9 and 10, 2021, BR04, BR04A, and BR05, were sighted in Santa Catarina, in the region of Ilha do Arvoredo. Seven months later, on August 18 of 2021, a group of 5 killer whales were sighted in Marica, Rio de Janeiro, where was possible to identify 3 individuals: BR04, BR12 and BR13. The individuals BR05 and BR13 were sighted in La Paloma on September 16, in the coast of Uruguay and 69 days later, on November 24, BR05, BR06, BR12 and BR13 were sighted again on Arraial do Cabo, RJ (approximately 920nm of distance). On December 14, 2021, BR12, BR13, BR16, BR16A were sighted swimming close to the Ipanema beach, Rio de Janeiro and on December 15, individuals BR05, BR08, BR12 were sighted around Marambaia Island. It is important to note that, except for the record of December, 30 of 2020, in all these occasions, more animals were part of the group, but only some of them were photo-identified (Table 2).
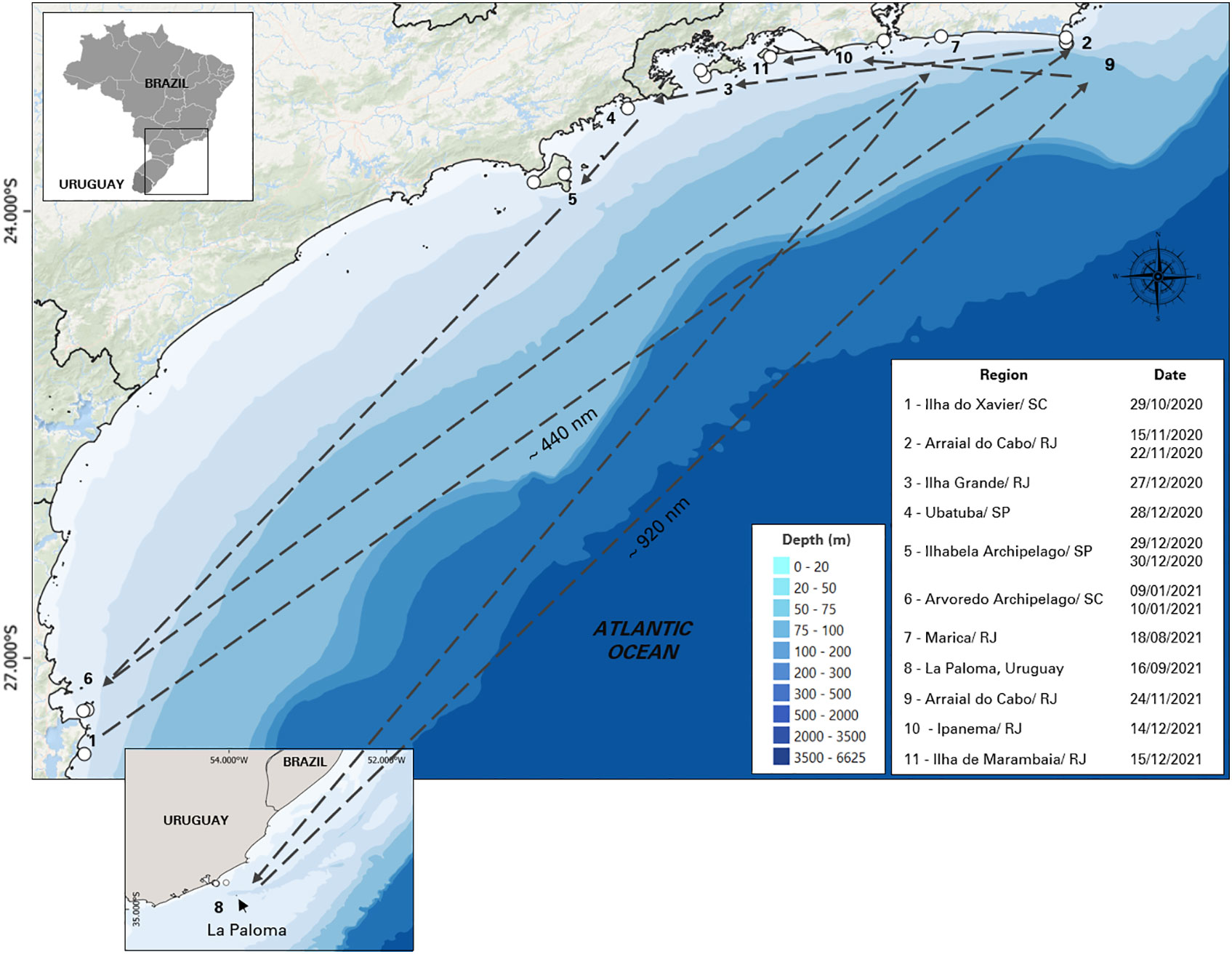
Figure 4 Movements of 11 photo-identified individual killer whales (Orcinus orca) between October 2020 and December 2021 along the southeastern-south Brazilian coast and Uruguay.
In addition to the sighting of individuals BR05 and BR13 on 16 September, 2021 in La Paloma, Uruguay, a third individual sighted on January 22, 2017 in Florianópolis, Santa Catarina, Brazil, in a group with 5 other animals, was also sighted 3 times in Uruguay: the adult male BR34 was sighted alone on September 1 2015, on 26 November, 2017 with another individual non-photo identified and on October 9 2021, with individuals BR38 and BR39.
Social associations
The social analysis was conducted for 12 out of the 47 photo-identified individuals that were sighted at least three times, and during encounters when it was possible to photo-identify at least 2 individuals in the group. The mean half-weight association index was 0.29 ± 0.19 SD, with values ranging from 0 to 0.75 (Table 3). Among the sex and maturity classes, on average, associations with juveniles (0.38 ± 0.03 SD) and with adult females (0.30 ± 0.10 SD) tended to be higher than those with calves (0.22 ± 0.03 SD), and adult males (0.17 ± 0.07 SD).
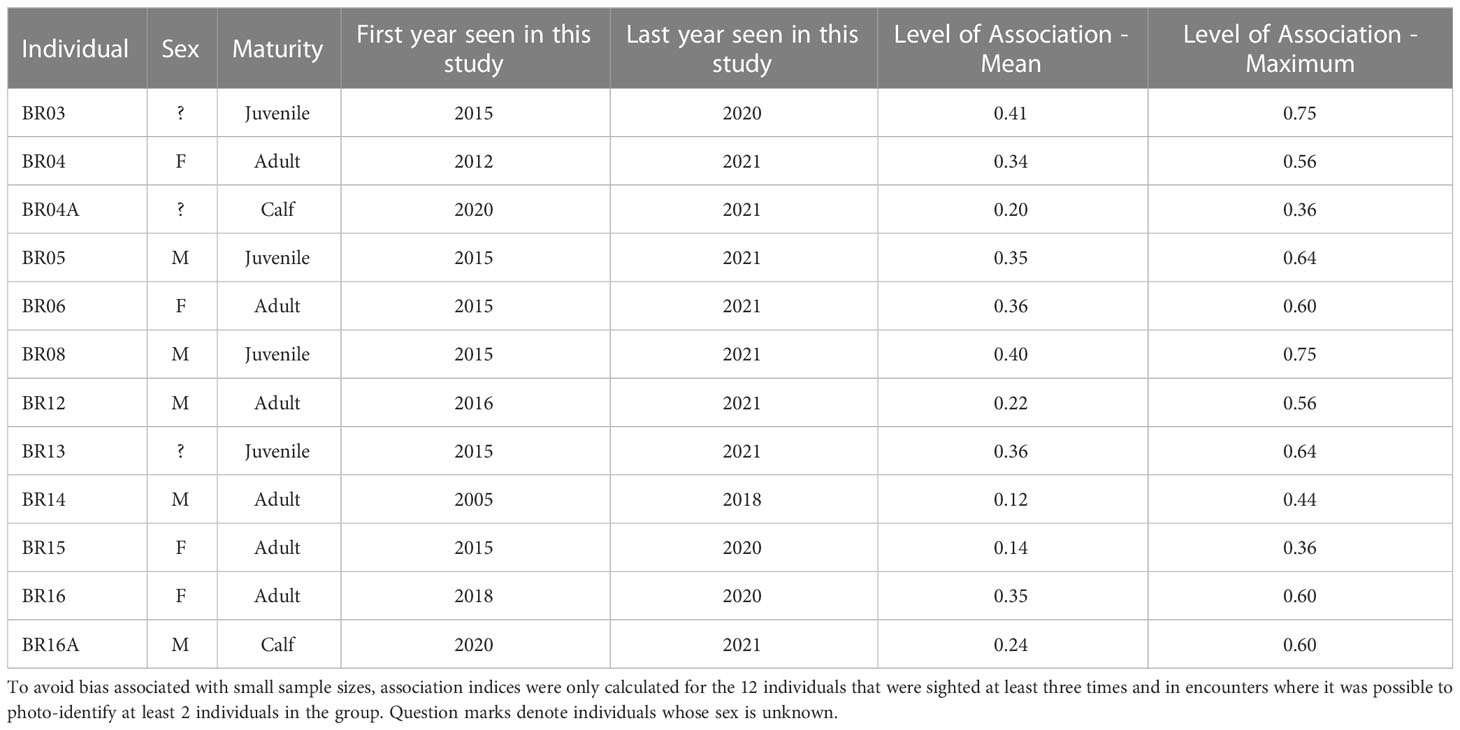
Table 3 Sighting history and associations among killer whale individuals in the Brazilian coastal waters.
The Monte Carlo permutation test allowed us to reject the null hypothesis that individuals associated randomly (CVreal = 0.67 > CVmean = 0.01, pCV = 1.00); therefore there are some preferred or avoided associations among these subset of 12 individuals. Social preferences may happen among individuals of different sexes and maturity classes. For example, the adult male BR14 and the adult female BR15 were seen associated with BR03, BR04, BR16 and BR08 on August 10 2018, and individuals BR16 and BR14 were photographed together on November 7, 2018. The hierarchical cluster analysis produced a dendrogram with a cophenetic correlation coefficient value of 0.82592 (Figure 5A) visually suggesting that there could be different social units with mixed sexes and maturity classes. Values above 0.8 are considered a good representation of social matrix (Bridge, 1993). However, the social network depicting associations among individuals (Figure 5B) was highly connected (proportion of realized links and possible links = 0.833) and showed no reliable partitions since the modularity was low (<0.3; Newman, 2004) and nonsignificant (Q = 0.034, 95% CI = 0.008 –0.088).
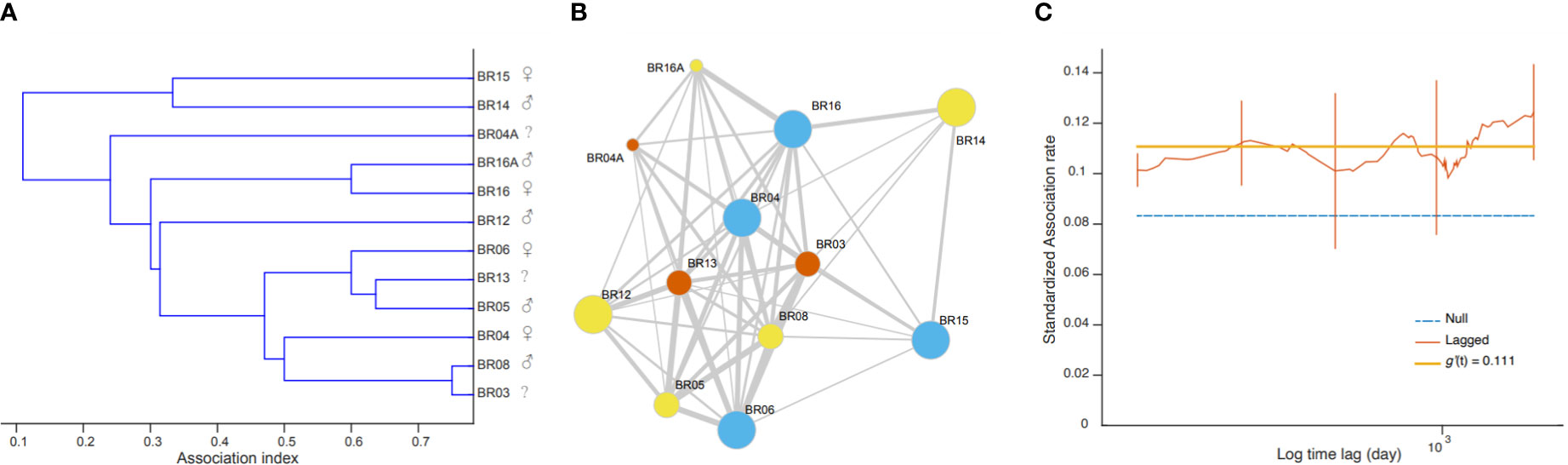
Figure 5 Depiction of the social structure of killer whales off the coast of Brazil. (A) The dendrogram from a hierarchical cluster analysis with the average linkage method. Sexes are indicated when known. (B) The social network in which nodes representing individuals are linked by their association indices with no clear partition in social units. Node colors denote sex (orange = females, blue = males, yellow = unknown), sizes denote maturity (large = adult, medium = juvenile, small = calf). (C) Standardized lagged association rates were, for most part, above the null rate and relatively stable over time, as suggested by the best-fitting model.
The lagged association rate (SLAR) was, for the most part, greater than the null association rate (NAR), suggesting that, overall, individuals continue to associate over time (Figure 5C). The best fitting model was SLAR 1, with association rates of 0.111 ± 0.018 SE and no decay over time (Table 4), reinforcing that there are some long-lasting associations within this subset of 12 individuals. SLAR2 also received some support of the data (ΔQAIC< 2; Whitehead 2007), which indicate that association rates could decay down to zero. This suggest that there are some casual acquaintances, that is, associations that eventually happen among some individuals then never again. Taken together, the social analyses suggest that the most re-sighted individuals can engage in both long- and short-term associations, that are not defined by the sex or maturity classes.
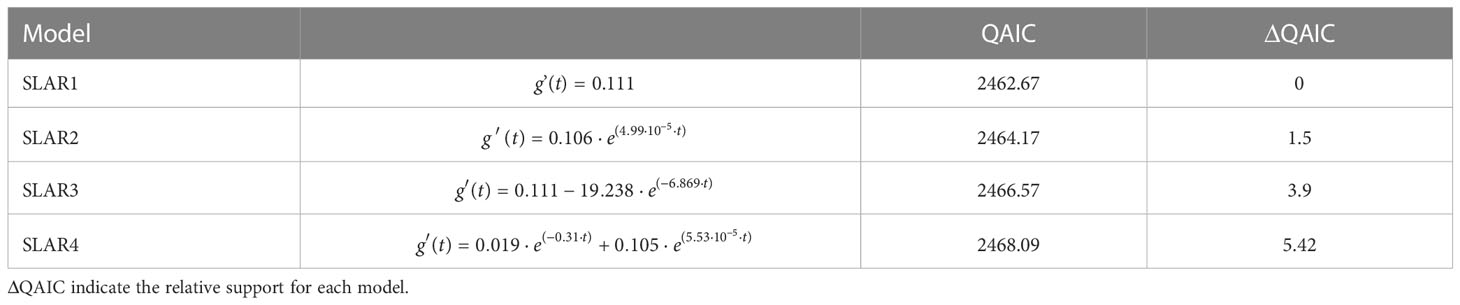
Table 4 Exponential decay models fit to Standardized Lagged Association Rates (SLAR) ranked by the lowest quasi-Akaike Information Criteria (QAIC).
Discussion
There is a remarkable lack of consistent data on killer whales’ population characteristics, social structure and behavior in tropical and subtropical waters worldwide. Despite the opportunistic nature of our sighting data, our findings suggest that killer whales are present regularly along the coast of western south Atlantic, and provide novel insights on the occurrence, movements, foraging and social behavior.
These data suggest that killer whales are apparently more frequently sighted off southeastern Brazil during the austral spring and summer (see also Siciliano et al., 1999)—although caution is needed when interpreting seasonality because nautical activity, thus opportunistic sightings, increase in the region during these seasons. Remarkably, the re-sightings of the same 11 individuals in the same region over the years, including re-sightings of 3 individuals off the coast of Brazil and Uruguay, suggest that killer whales are widely distributed in this region and yet can show some degree of site fidelity to specific locations along this coast. Similar wide-ranging movements have been observed across the world. For example, between Mexico and Peru (Guerrero-Ruiz et al., 2005; Pacheco et al., 2019), Antarctic and New Zealand (Eisert et al., 2015), and between Alaska and California (Goley and Straley, 1994; Dahlheim et al., 2008). In the latter, some social units range more widely and transit hundreds of kilometers along the coast, while other units how higher fidelity to certain regions (Ford and Ellis, 1999).
These movements and occurrence of killer whales are typically associated to prey preferences and availability. In the southeastern coast of Brazil, there are upwelling systems (such as the rich waters of the Cabo Frio) that attract a variety of seasonally abundant sharks, rays, fish, and cetaceans (Siciliano et al., 1999). Previous studies on killer whales off Brazil have shown both predation and the presence in the stomach contents of a wide range of prey, such as rays, sharks, teleosts, salps, penguins, Burmeister’s porpoise (Phocoena spinipinnis), franciscana dolphins (Pontoporia blainvillei) and minke whale (Balaenoptera sp.) (See Table S1 on Supplementary Data) (Castello, 1977; Bittencourt, 1983; Castello and Pinedo, 1986; Dalla Rosa, 1995; Lodi and Hetzel, 1998; Secchi and Vaske Jr., 1998; Ott and Danilewicz, 1998; Fernandes, 2001; Santos and Haimovici, 2001; Santos and Netto, 2005; Dalla Rosa and Secchi, 2007, Monteiro, 2008; Troina et al. 2020). Two videos released on the internet, one on September 11, 2019 in Bahia and the other on November 5, 2021, in Arraial do Cabo – Rio de Janeiro, show respectively, killer whales in a potential hunt and an attack on humpback whales (unpublished data). This region is also regularly used by Bryde’s Whales (Gonçalves et al., 2015; Lodi et al., 2015; Athayde et al., 2020) and as a migration route for humpback whales (Megaptera novaeangliae) and southern right whales (Eubalaena australis) (Groch et al., 2005; Siciliano et al., 2019; Renault-Braga et al., 2019)—all of which could be potential targets of killer whales. For instance, in 2008, a newborn female southern right whale was found dead with several tooth marks of adult killer whale in the body (Ott et al., 2017) and in 2012, killer whales were observed harassing a group of sperm whales (Physeter macrocephalus) to prey on a sperm whale calf (Andriolo et al., 2015; Sucunza et al., 2022). By contrast, on September 19, 2019, while conducting regular monitoring of southern right whales in the south of Brazil, researchers observed a group of at least four killer whales in direct interaction with a mother-calf pair of southern right whales. Despite the close proximity of the killer whales to the whales, no attack occurred (Renault-Braga et al., 2019). In our observations, killer whales were only seen feeding on elasmobranchs and fish: four times on rays (such as Gymnura altavela) and twice on a school of unidentified fish. No attacks on marine mammals have been recorded and just as observed with the mother-calf pair in 2019, despite the opportunity to prey on two Bryde's whales on November 30, 2018, north of the Ilhabela Archipelago, the group of eight killer whales preferred to attack a stingray. The killer whales found in these waters seem to be more generalist or opportunistic foragers (Lodi and Hetzel, 1998; Siciliano et al., 1999), in agreement with suggestions that killer whales in tropical waters tend to be less specialized on certain prey items than those found in temperate waters because diet breadth should increase as the availability of the most highly profitable prey decreases (Baird, 2002).
Prey type and availability typically shape group size and stability (Beck et al., 2012). Corroborating this idea, the mean size of the group of killer whales observed in this study was 5.61 individuals (SD = 2.91), similarly to previous observations off Brazil (4.3 individuals; Lodi and Hetzel, 1998; 3.9 individuals; Siciliano et al., 1999). This average group size is also similar to observations in other sub-tropical and tropical waters where killer whales show generalist feeding behaviors: Caribbean: 4.1 individuals (Bolaños-Jiménez et al., 2023), Galápagos: 4 individuals (Denkinger et al., 2020), Mexico Central Pacific coast: 4.6 individuals (Vargas-Bravo et al., 2020), Peru: 4.5 individuals (Garcia-Godos, 2004) and 4.3 individuals (Testino et al., 2019), Costa Rica: 3.4 individuals (Castro-Azofeifa, 2021), Hawaii: 4.2 individuals (Baird et al., 2006), Tropical West Africa: 5.6 individuals (Weir et al., 2010), Eastern Tropical Pacific: 5.4 individuals (Wade and Gerrodette, 1993), and Mozambique Channel: 6.1 individuals (Terrapon et al., 2021). By contrast, in temperate and polar zones, group size and is more variable and vary with the target prey type. For instance, the Antarctic Type A killer whales feeds mainly on Antarctic Minke whales and form groups of 13.6 individuals on average; the Type B (11.8 individuals) tend to prey upon pinnipeds, whales and penguins; and the Type C, (46.1 individuals) apparently feeds mainly on fish (Pitman and Ensor, 2003). In Norwegian waters, the mean size for herring-feeding killer whale groups is 15 individuals (Simila et al., 1996) while seal-feeding groups are 5 individuals (Jourdain et al., 2017); in addition, the group sizes of killer whales hunting seals off Scotland are smaller (5.8 individuals) than those on the Icelandic herring grounds (14.8 individuals) (Beck et al., 2012). These observations in Antarctic, Norway, Scotland and Icelandic are similar to those from the well-studied North Pacific population, with a proportional smaller more consistent group size of mammal-eating transient Killer whales (4.2 individuals; Baird and Dill, 1996) compared with fish- eating resident killer whales that often occur in groups of dozens to hundreds of individuals (Ford et al., 1998). According to Baird and Dill (1996) the small size of the mammal-eating groups could contribute to the management and maximization of energy intake. Associations with other groups, increasing the size of the group, would be useful to increase the success rates of prey difficult to capture or large prey allowing additional individuals to feed without increasing competition. Still in these zones, it is also possible to find more diverse groups where whales seem to be more generalists, as in Chilean Patagonia (4.2 individuals; Häussermann et al., 2013 and 5 individuals; Capella et al., 2014) and in New Zealand (mean of 12 individuals) where 27 different species of prey have been recorded, being ray the most common type of prey and, the diet varying according to the 3 sub-populations proposed for the region (Visser, 2007). Taken together, our data and this literature suggest that killer whales of southeastern Brazil are generalist foragers; however, one cannot yet discard the possibility that there may be specific groups with a specialized diet in the region.
Despite the paucity of encounters, our data provide novel insights on the social patterns of killer whales in the western south Atlantic waters. The association patterns among the most re-sighted 12 killer whale individuals suggest that they can form social groups of mixed age-sex, and engage in both long-term and more labile social associations. It is possible that these individuals are part of a single or more relatively stable social units in which members occasionally associate with each other with no clear preferences for sex and maturity stage. This pattern resembles the social structure of transient killer whales in the North Pacific (Ford and Ellis, 1999; Baird and Whitehead, 2000), Marion Islands (Reisinger et al., 2017), and Galápagos Islands (Denkinger et al., 2020), Argentinean Patagonia (Iñiguez et al., 2005) and, Mexico Pacific coast (Guerrero-Ruíz, 2013). However, the nature and scarcity of our association data is such that it remains premature to make such a conclusion. An increased and more homogeneous sampling effort in space and time to complement our current opportunistic and citizen science sampling will be crucial to further reveal the structure into social units, and to estimate the rates at which different units associate. Beyond odontocetes, such as killer and sperm whales, social patterns combining permanent and temporary associations can be found in several animal populations with different degrees of fission-fusion dynamics, such as orangutans, bats, elephants and hyenas (Kerth and König, 1999; Van Schaik, 1999; Wittemyer et al., 2005; Smith et al., 2008). Social plasticity in groups formation can reflect the underlying social and ecological conditions that modulate the costs and benefits that individual member experience. As in the abovementioned populations, associations among multiple social units of killer whales, temporarily increasing the size of the group, can be advantageous for mating opportunities, care of the young, or to increase foraging success on large prey that is difficult to capture; on the other hand, individuals in larger groups pay the cost of increased probability of competition for prey access (Hoelzel, 1993; Baird and Dill, 1996). In Galápagos, the average group size was estimated to be 4 animals. However, when attacking baleen whales, the groups appeared to consist of at least 5-10 animals, and even up to 25 animals when they attacked sperm whales (Denkinger et al., 2020). In northern Patagonia, the associations between killer whales are mostly long-term, but individuals from different groups have been observed to leave their maternal groups and form new groups (Iñíguez et al., 2005). This fluidity has also been identified along the Mexico Pacific coast, where individuals have been found to have indirect relationships with each other through key associations (Guerrero-Ruíz, 2013). In our study, we observed solitary adult males (BR01) that were not seen associated with any group and males that in certain sightings were alone or that were associated with different groups on different occasions, as is the case of males BR12, BR14 and, BR34 (Figure 6). Solitary adult males have also been recorded preying in the region, including in association with the tuna longline fishery (Secchi and Vaske, 1998; Santos and Netto, 2005). According to Baird and Dill (1996), dispersal of individuals likely to occur due to the energetic benefits of foraging alone or in small groups and other groups may also allow them for a period of time to increased mating opportunities. In our study, we did not observe male-male pairs as described in Argentina (Hoelzel, 1991) and the Galápagos Islands (Denkinger et al., 2020).

Figure 6 The adult male BR12, sighted in association with a mother-calf pair (BR29 and BR29A) on July, 3 2016 in Florianópolis, SC (A) and on November, 22 2018 in Arraial do Cabo, RJ (B) with a group of other eight killer whales (BR03, BR04, BR05, BR06, BR08 BR13 and BR16), suggesting associations with reproductive purposes or energetic benefits.
This is the first integrated assessment of the movements and social behavior of killer whales in tropical and subtropical waters of Brazil. Our data suggest site fidelity by various groups that experience some degree of fission-fusion dynamics and engage in long and short-term movements in the waters of southern and southeastern Brazilian coast. Such movements seem to be erratic and driven by foraging opportunities, likely related to the productivity of western tropical South Atlantic and subtropical waters. Killer whales exhibit a generalist feeding behavior in this area; however, the possibility of there being groups with specialized diets in the region is not ruled out. More systematic surveys, covering both inshore and offshore waters over longer periods, are required to further clarify the status, social patterns, movements in western south Atlantic as well as an analysis of stable isotopes and fatty acids from biopsies to better characterize the diet of killer whales in the region. Given this, it appears premature to classify the killer whales in this particular region as belonging to any current or identified ecotype or a new yet to be defined.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the animal study because the analysis is based in photos of killer whales opportunistically captured.
Author contributions
AA: first authorship. SS: senior authorship. All authors contributed to the article and approved the submitted version.
Acknowledgments
These sightings and this study would not have been possible without the help of the ProBaV crew: Captain Wagner Braz da Silva, Master Marcone dos Santos and Master Natan Santos. We would like to thank Viva Instituto Verde e Azul and Mar e Vida Ecotrip, Capitão Ximango, Monika Wieland Shields, Sara Hysong-Shimazu and, all ProBaV’s contributors for the partnership. Also, Adrien Caradec, Alex Pretto, Aline Bassi, Bruno Oliveira, Cadu Lonias, Captain Nills, Cibele Sanches, Diana Figueroa, Eduardo Honuma, Fernando Viek, Gabriel Klabin, Gisele Matarozzo, Laihany Jacob, Leandro Borba from Fauna Marina Uruguay, Leandro Matheus, Lilian Fontalba, Luiza Perin, Maria Eduarda Barros, Maria Luciene da Silva, Matias Gomes, Mayara Lays, Mercedes Rios, Marcos Paulo Zettritz, Nilson Mattos, Olivar Bouças, Paulo Magalhães, Rafael Rodrigues, Sergio Lobo, Tycho Fernandes and, Victor Aune for shared with us photos and sightings of killer whales contributing to the science and for a better understanding and conservation of this species. Also, SS is supported by CNPq (Bolsa de Produtividade em Pesquisa: 306076/2019-5).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1206796/full#supplementary-material
References
Andriolo A., Reis S. S., Amorim T. O., Sucunza F., de Castro F. R., Maia Y. G., et al. (2015). Killer whale (Orcinus orca) whistles from the western south Atlantic ocean include high frequency signals. J. Acous. Soc. America. 138 (3), 1696–1701. doi: 10.1121/1.4928308
Athayde A., Cardoso J., Francisco A., Siciliano S. (2020). Bryde's whales (Balaenoptera brydei) off the north coast of São Paulo, Brazil: first photo-identification study. Aquat. Mammals. 46 (5), 1–5. doi: 10.1578/AM.46.5.2020.488
Athayde A., Cardoso J., Francisco A., Siciliano S. (2022). A vessel collision report for Bryde's whales (Balaenoptera brydei) off the northern coast of São Paulo, Brazil. Aquat. Conserv.: Mar. Freshw. Ecosys. 33 (2), 1–5. doi: 10.1002/aqc.3906
Baird R. W. (2002). Killer whales of the world natural history and conservation (Stillwater, Minnesota: Voyageur Press).
Baird R. W., Dill L. M. (1996). Ecological and social determinants of group size in transient killer whales. Behav. Ecol. 7, 408–416. doi: 10.1093/beheco/7.4.408
Baird R. W., McSweeney D. J., Bane C., Barlow J., Salden D. R., Antoine L. K., et al. (2006). Killer whales in Hawaiian waters: information on population identity and feeding habits. Pacific Sci. 60, 523–530. doi: 10.1353/psc.2006.0024
Baird R. W., Whitehead H. (2000). Social organization of mammal-eating killer whales: group stability and dispersal patterns. Can. J. Zool. 78, 2096–2105. doi: 10.1139/z00-155
Batista R. L. G., Schiavetti A., Santos U. A., Reis M. S. S. (2012). Cetaceans registered on the coast of Ilhéus (Bahia), northeastern Brazil. Biota Neotrop. 12 (1), 31–38. doi: 10.1590/S1676-06032012000100003
Beck S., Kuningas S., Esteban R., Foote A. D. (2012). The influence of ecology on sociality in the killer whale (Orcinus orca). Behav. Ecol. 23 (2), 246–253. doi: 10.1093/beheco/arr151
Bejder L., Fletcher D., Bräger S. (1998). A method for testing association patterns of social animals. Anim. Behav. 56, 719–725. doi: 10.1006/anbe.1998.0802
Bigg M. A., Ellis G. M., Ford J. K. B., Balcomb K. C. (1987). Killer whales: a study of their identification, genealogy and natural history in British Columbia and Washington state (Nanaimo, B.C: Phantom Press and Publishers), 79.
Bittencourt M. L. (1983). Orcinus orca "Baleia assassina" (Cetacea, Delphinidae), primeiro registro para o litoral norte catarinense, com notas osteológicas. [Orcinus orca "Killer whale" (Cetacea, Delphinidae), first record for the north coast of Santa Catarina, with osteological notes]. Arq. Biol. Tecnol. 26 (1), 77–103.
Bolaños-Jiménez J., Kiszka J. J., Bouveret B., Ferrer G. R., Ramos E. A., Henriquez A., et al (2023). The Killer Whale in the Caribbean Sea: An Updated Review of Its Ecology, Exploitation, and Interactions with Fisheries. Aquat. Mamm. 49, 184–194. doi: 10.1578/AM.49.2.2023.184
Bridge P. D. (1993). “Classification,” in Biological data analysis. Ed. Fry J. C. (Oxford, UK: Oxford University Press), 219–242.
Cairns S. J., Schwager S. J. (1987). A comparison of association indices. Anim. Behav. 35, 1454–1469. doi: 10.1016/S0003-3472(87)80018-0
Capella J., Abramson J. Z., Vilina Y., Gibbons J. (2014). Observations of killer whales (Orcinus orca) in the fjords of Chilean Patagonia. Polar Biol. 37, 1533–1539. doi: 10.1007/s00300-014-1535-5
Cardoso J., Francisco A., De Souza S. P., Siciliano S. (2019a). Rough-toothed dolphins (Steno bredanensis) along southeastern Brazil: report of an anomalous pigmented juvenile and description of social and feeding behaviors. Aquat. Mamm. J. 45 (1), 30–36. doi: 10.1578/AM.45.1.2019.30
Cardoso J., Francisco A., Siciliano S., Moreira S. C. (2019b). A stop for a snack: apparent humpback whale (Megaptera novaeangliae) feeding behavior and association with gillnets during migration off south-eastern Brazil. Boletim Do Laboratório Hidrobiologia (UFAMA IMPRESSO) 29, 41–49. doi: 10.18764/1981-6421e2019.6
Castello H. P. (1977). Food of a killer whale: eagle sting-ray, Myliobatis, found in the stomach of a stranded Orcinus orca. Sci. Rep. Whales Res. Inst. 29, 107–111.
Castello H. P., Pinedo M. C. (1986). “Sobre unos avistajes en el mar de distintas especies de cetaceos en el sur del brasil [About sightings in the sea of different species of cetaceans in the south of Brazil],” in Actas de la primera reunion de trabajo de expertos en mamiferos acuaticos de America del sur (Buenos Aires), 61–68.
Castro-Azofeifa C. (2021). Avistamientos de Orcinus orca (Linnaeus 1758) (Cetartiodactyla: Odontoceti: Delphinidae) en el pacífico costarricense, (1990- 2020) [Sightings of Orcinus orca (Linnaeus 1758) (Cetartiodactyla: Odontoceti: Delphinidae) in the Costa Rican pacific ocean, (1990-2020)]. Rev. Cienc. Marinas y Costeras 13 (2), 29–47. doi: 10.15359/revmar.13-2.3
Dahlheim M., Schulman-Janiger A., Black N. A., Ternullo R., Ellifrit D., Balcomb K. C. (2008). Eastern Temperate north pacific offshore killer whales (Orcinus orca): occurrence, movements, and insights into feeding ecology. Mar. Mamm. Sci. 24, 719–729. doi: 10.1111/j.1748-7692.2008.00206.x
Dalla Rosa L. (1995). Interações com a pesca de espinhel e informações sobre a dieta alimentar de orca, Orcinus orca (Linnaeus 1758) (Cetacea, Delphinidae), no sul e sudeste do Brasil. [Interactions with longline fisheries and information on the diet of killer whales, Orcinus orca (Linnaeus 1758) (Cetacea, Delphinidae), in southern and southeastern Brazil] (Rio Grande, RS, Brasil: Monografia de Graduação do Curso de Oceanologia. Fundação Universidade de Rio Grande), 40.
Dalla Rosa L., Secchi E. R. (2007). Killer whale (Orcinus orca) interactions with the tuna and swordfish longline fishery off southern and southeastern Brazil: a comparison with shark interactions. J. Mar. Biol. Assoc. United Kingdom 87, 135–140. doi: 10.1017/S0025315407054306
Dantas W. (2007). Interações entre orcas Orcinus orca (Linnaeus 1758) e falsas-orcas Pseudorca crassidens (Owen 1846) com a pesca de espinhel pelágico monofilamento no atlântico oeste tropical. [Interactions between killer whales Orcinus orca (Linnaeus 1758) and false killer whales Pseudorca crassidens (Owen 1846) with pelagic monofilament longline fisheries in the tropical Western Atlantic] (Recife, Pernambuco, Brazil: Universidade Federal Rural de Pernambuco). Master Thesis dissertation.
Denkinger J., Alarcon D., Espinosa B., Fowler L., Manning C., Oña J., et al. (2020). Social structure of killer whales (Orcinus orca) in a variable low-latitude environment, the Galápagos archipelago. Mar. Mamm. Sci. 36, 774–785. doi: 10.1111/mms.12672
Di Tullio J. C., Gandra T. B. R., Zerbini A. N., Secchi E. R. (2016). Diversity and distribution patterns of cetaceans in the subtropical southwestern Atlantic outer continental shelf and slope. PloS One 11 (5), e0155841. doi: 10.1371/journal.pone.0155841
Durban J. W., Fearnbach H., Burrows D. G., Ylitalo G. M., Pitman R. L. (2016). Morphological and ecological evidence for two sympatric forms of type b killer whale around the Antarctic peninsula. Polar Biol. 40, 231−236. doi: 10.1007/s00300-016-1942-x
Durban J. W., Pitman R. L. (2012). Antarctic Killer whales make rapid, round-trip movements to subtropical waters: evidence for physiological maintenance migrations? Biol. Lett. 8, 39–45. doi: 10.1098/rsbl.2011.0875
Eisert R., Ovsyanikova E., Visser I., Ensor P., Currey R., Sharp B. (2015). Seasonal site fidelity and movement of type-c killer whales between Antarctica and new zealand. paper SC/66a/SM/69 (Alaska: International Whaling Commission. Anchorage).
Farine D. R., Whitehead H. (2015). Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84 (5), 1144–1163. doi: 10.1111/1365-2656.12418
Fernandes T. (2001). Ocorrência e monitoramento de cetáceos na região de arraial do cabo - RJ.[Occurrence and monitoring of cetaceans in the region of arraial do cabo – RJ]. Brasil. UFRJ: Master Thesis dissertation. Rio de Janeiro, Brasil: Departamento de Biologia Marinha, UFRJ.
Foote A. D., Newton J., Piertney S. B., Willerslev E., Gilbert M. T. P. (2009). Ecological, morphological and genetic divergence of sympatric north Atlantic killer whale populations. Mol. Ecol. 18, 5207–5217. doi: 10.1111/j.1365-294X.2009.04407.x
Foote A., Vijay N., Ávila-Arcos M., Baird R., Durban J. W., Fumagalli M., et al. (2016). Genome-culture coevolution promotes rapid divergence of killer whale ecotypes. Nat. Commun. 7, 11693. doi: 10.1038/ncomms11693
Ford J. K. B. (2002). “Killer whale: Orcinus orca. Pp. 669-676,” in Encyclopedia of marine mammals. Eds. Perrin W. F., Wursigand B., Thewissen J. G. M. First Edition. (San Diego: Academic Press), p. 1414.
Ford J. K. B., Ellis G. M. (1999). Transients: Mammal-Hunting Killer Whales. (Vancouver, British Columbia: UBC Press), 96 pp.
Ford J. K. B., Ellis G. M., Barrett-Lennard L. G., Morton A. B., Palm R. S., Balcomb I. K. C. (1998). Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. J. Zool. 76, 1456–1471. doi: 10.1139/z98-089
Forney K. A., Wade P. (2006). “Worldwide distribution and abundance of killer whales,” in Whales, whaling and ocean ecosystems. Eds. Estes J. A., Brownell R. L., DeMaster D. P., Doak D. F., Williams T. M. (Berkeley: University of California Press), 1–25.
Garcia-Godos I. (2004). Killer whale (Orcinus orca) occurrence off Peru 1995–2003. Latin Am. J. Aquat. Mamm. 3, 177–180. doi: 10.5597/lajam00064
Goley P. D., Straley J. M. (1994). Attack on grey whales in Monterey bay, California, by killer whales previously identified in glacier bay, Alaska. Can. J. Zool. 72 (8), 1528–1530. doi: 10.1139/z94-202
Gonçalves L. R., Augustowski M., Andriolo A. (2015). Occurrence, distribution and behavior of Bryde’s whales (Cetacea: Mysticeti) off south-east Brazil. J. Mar. Biol. Assoc. United Kingdom 96 (4), 943–954. doi: 10.1017/S0025315415001812
Groch K. R., Jerdy H., Marcondes M. C., Barbosa A. L., Ramos G. C. H., Pavanelli L., et al. (2020). Cetacean morbillivirus infection in a killer whale (Orcinus orca) from Brazil. J. Comp. Pathol. 181, 26–32. doi: 10.1016/j.jcpa.2020.09.012
Groch K. R., Palazzo J. T. Jr., Flores P. A. C., Adler F. R., Fabian M. E. (2005). Recent rapid increases in the right whale (Eubalaena australis) population of southern Brazil. LAJAM. 4, 41–47. doi: 10.5597/lajam00068
Guerrero-Ruiz M., García-Godos I., Urbán J. (2005). Photographic match of a killer whale (Orcinus orca) between Peruvian and Mexican waters. Aquat. Mammals. 31 (4), 438–411. doi: 10.1578/AM.31.4.2005.438
Guerrero-Ruíz M. E. (2013). Identidad poblacional y estructura social de la orca Orcinus orca (Linnaeus 1758) en el pacífico mexicano [Population identity and social structure of the killer whale Orcinus orca (Linnaeus 1758) in the Mexican pacific] (Tesis de doctoral) (La Paz, México: Universidad Autónoma de Baja California Sur).
Häussermann V., Acevedo J., Försterra G., Bailey M., Aguayo-Lobo. A. (2013). Killer whales in Chilean Patagonia: additional sightings, behavioural observations, and individual identifications. Rev. Biol. Mar. Oceanogr. 48, 73–85. doi: 10.4067/S0718-19572013000100007
Hoelzel A. R. (1991). Killer whale predation on marine mammals at Punta norte, Argentina; food sharing, provisioning and foraging strategy. Behav. Ecol. Sociobiol. 29, 197–204. doi: 10.1007/BF00166401
Hoelzel A. R. (1993). Foraging behaviour and social group dynamics in puget sound killer whales. Anim. Behav. 45 (3), 581–591. doi: 10.1006/anbe.1993.1068
ICMBio (2023) Sistema de avaliação do risco de extinção da biodiversidade - SALVE. Available at: https://salve.icmbio.gov.br.
Iñíguez M. A. (2001). Seasonal distribution of killer whales (Orcinus orca) in northern Patagonia, Argentina. Aquat. Mammals. 27 (2), 154–161.
Iñíguez M. A., Tossenberger V. P., Gasparrou C. (2005). Socioecology of killer whales (Orcinus orca) in northern Patagonia, Argentina. Paper SC/57/SM5 presented to IWC Sci. Committee (Ulsan, Korea), 9.
Iriarte V. (2006). Killer whale (Orcinus orca) occurrence at Isla de Lobos, Uruguay. Latin Am. J. Aquat. Mammals. 5 (1), 73–76. doi: 10.5597/lajam00096
Jourdain E., Vongraven D., Bisther A., Karoliussen R. (2017). First longitudinal study of seal-feeding killer whales (Orcinus orca) in Norwegian coastal waters. PloS One 12, e0180099. doi: 10.1371/journal.pone.0180099
Kerth G., König B. (1999). Fission, fusion and nonrandom associations in female bechstein’s bats (Myotis bechsteinii). Behaviour 136 (9), 1187–1202.
Laeta M., Kompanje E. J. O., Watson A., Souza S. M. F. M., Dittmar K., Cuenca S. C., et al. (2019). Osteochondromatosis (multiple cartilaginous exostoses) in an immature killer whale (Orcinus orca). Dis. Aquat. Organ. 34 (3), 209–213. doi: 10.3354/dao03372
Leatherwood J. S., Dalheim M. E. (1978). Worldwide distribution of pilot whales and killer whales. Nav. Ocean Syst. Cent. Tech. Rep. 443, 1–39.
LeDuc R. G., Robertson K. M., Pitman R. L. (2008). Mitochondrial sequence divergence among Antarctic killer whale ecotypes is consistent with multiple species. Biol. Lett. 4, 426–429. doi: 10.1098/rsbl.2008.0168
Lemos L. S., Moura J. F., Hauser-Davis R. A., Campos R. C., Siciliano S. (2013). Small cetaceans found stranded or accidentally captured in southeastern Brazil: bioindicators of essential and non-essential trace elements in the environment. Ecotoxicol. Environ. Saf. 97, 166–175. doi: 10.1016/j.ecoenv.2013.07.025
Lodi L., Farias-Junior S. (2011). Movements of a solitary adult male killer whale, Orcinus orca (Cetacea, Delphinidae), along the coast of south-eastern Brazil. Pan-Am J. Aquat. Sci. 6, 325–328.
Lodi L., Hetzel B. (1998). Orcinus orca (Cetacea, Delphinidae) em águas costeiras do estado do Rio de Janeiro. Bioikos. 12 (1), 46–r54.
Lodi L., Tardin R. (2018). Citizen science contributes to the understanding of the occurrence and distribution of cetaceans in southeastern Brazil–a case study. Ocean Coast. Manage. 158, 45–55. doi: 10.1016/j.ocecoaman.2018.03.029
Lodi L., Tardin R. H., Hetzel B., Maciel I. S., Figueiredo L. D., Simão S. M. (2015). Bryde’s whale (Cetartiodactyla: Balaenopteridae) occurrence and movements in coastal areas of southeastern Brazil. Zoologia Curitiba. 32 (2), 171–175. doi: 10.1590/S1984-46702015000200009
Marcondes M. C. C., Cheeseman T., Jackson J. A., Friedlaender A. S., Pallin L., Olio M., et al. (2021). The southern ocean exchange: porous boundaries between humpback whale breeding populations in southern polar waters. Sci. Rep. 11, 23618. doi: 10.1038/s41598-021-02612-5
Meirelles A. C. O., Monteiro-Neto C., Martins A. M. A., Costa A. F., Barros H. M. D. R., Alves A. M. D. O. (2009). Cetacean strandings on the coast of Ceará, northeastern Brazil, (1992-2005). J. Mar. Biol. Assoc. United Kindom. 89 (5), 1083–1090. doi: 10.1017/S0025315409002215
Mikhalev Y. A., Ivashin M. V., Sausin V. P., Zelemaya F. E. (1981). The distribution and biology of killer whales in the southern hemisphere. Rep. Int. Whal. Commn. 31, 551–566.
Monteiro D. (2008). Fatores determinantes da captura incidental de aves e tartarugas marinhas e da interação com orcas/falsas-orcas, na pescaria com espinhel pelágico no sudeste-sul do brasil [Determining factors of incidental capture of birds and sea turtles and interaction with killer whales/false killer whales, in the pelagic longline fishery in southeastern-south brazil.] (Rio Grande, Brazil: Universidade Federal do Rio Grande/FURG). Master Thesis dissertation.
Morin P. A., Parsons K. M., Archer F. I., Ávila-Arcos M. C., Barrett-Lennard L. G., et al. (2015). Geographic and temporal dynamics of a global radiation and diversification in the killer whale. Mol. Ecol. 24. doi: 10.1111/mec.13284
Newman M. E. J. (2004). Analysis of weighted networks. Phys. Rev. E. 70, 56131. doi: 10.1103/PhysRevE.70.056131
Newman M. E. J. (2006). Modularity and community structure in networks. Proc. Natl. Acad. Sci. U.S.A. 103, 8577–8582. doi: 10.1073/pnas.0601602103
Ott P. H., Danilewicz D. (1998). Presence of franciscana dolphins (Pontoporia blainvillei) in the stomach of a killer whale (Orcinus orca) stranded in southern Brazil. Mammalia 62 (4), 605–609.
Ott P. H., Sucunza F., Wickert J., Danilewicz D., Tavares M. (2017). Evidences of attack of a killer whale on a calf southern right whale in southern Brazil. Mastozoología Neotropical 24 (1), 235–240. Available at: https://www.redalyc.org/pdf/457/45753369020.pdf.
Pacheco A. S., Castro C., Carnero-Huaman R., Villagra D., Pinilla S., Denkinger J., et al. (2019). Sightings of an adult male killer whale match humpback whale breeding seasons in both hemispheres in the Eastern tropical pacific. Aquat. Mammals. 45, 320–326. doi: 10.1578/AM.45.3.2019.320
Passadore C., Domingo A., Secchi E. R. (2015). Depredation by killer whale (Orcinus orca) and false killer whale (Pseudorca crassidens) on the catch of the Uruguayan pelagic longline fishery in southwestern Atlantic ocean. ICES J. Mar. Sci. 72 (5), 1653–1666. doi: 10.1093/icesjms/fsu251
Passadore C., Domingo A., Szephegyi M., Secchi E. R. (2012). Influence of environmental and longline fishing operational variables on the presence of killer whales (Orcinus orca) in south-western Atlantic. J. Mar. Biol. Assoc. United Kingdom 94 (6), 1267–1276. doi: 10.1017/S002531541200166X
Pinedo M. C., Polacheck T., Barreto A. S., Lammardo M. P. (2002). A note on vessel of opportunity sighting surveys for cetaceans in the shelf edge region off the southern coast of Brazil. J. Cetacean Res. Manag. 4 (3), 323–329. doi: 10.47536/jcrm.v4i3.846
Pitman R. L. (2011). Antarctic Killer whales: top of the food chain at the bottom of the world. J. Am. Cetac. Soc 40 (1), 39–45.
Pitman R. L., Ensor P. (2003). Three forms of killer whales (Orcinus orca) in Antarctica. J. Cetacean Res. Manag. 5, 131–139. doi: 10.47536/jcrm.v5i2.813
PMC-BS (2017) 2° relatório anual – ciclos 1 a 4 (Volume unico) [2nd annual report - cycles 1 to 4 (Single volume)]. Available at: https://sispmcprd.petrobras.com.br/sispmc/faces/informacoes/relatorios.xhtml?name=relatorios.
Ramos R. M. A., Siciliano S., Ribeiro R. (2010). Monitoramento da biota marinha em navios de sísmica: seis anos de pesquisa, (2001-2007). [Marine biota monitoring on seismic vessels: six years of research, (2001-2007)] (Vitória, ES, Brasil: Everest Tecnologia em Serviços).
Reisinger R. R., Beukes C., Hoelzel A. R., de Bruyn P. J. N. (2017). Kinship and association in a highly social apex predator population, killer whales at Marion island. Behav. Ecol. 28 (3), 750e759. doi: 10.1093/beheco/arx034
Renault-Braga E. P., Medeiros C. R. M., Leite H. N., Groch K. R., Maciel L. R. L. D., Albernaz T. L. First record of direct interaction between a potential predator – Orcinus orca (Odontoceti, Delphinidae) – and a mother-calf pair of Eubalaena australis (Mysticeti, Balaenidae) in Brazilian calving ground). Pan-American J. Aquat. Sci. 17 (3), 319–325.
Rice D. W. (1998). Marine mammals of the world: systematics and distribution (Lawrence, Kansas, USA: Society for Marine Mammalogy).
Santos M. C. O., Brito J. L., Flach L., Oshima J. E. F., Figueiredo G. C., Carvalho R. R., et al. (2019). Cetacean movements in coastal waters of the southwestern Atlantic ocean. Biota Neotropica 19 (2), e20180670. doi: 10.1590/1676-0611-bn-2018-0670
Santos R. A., Haimovici M. (2001). Cephalopods in the diet of marine mammals caught stranded or incidentally caught along southeastern and southern Brazil (21-34° s). Fish. Res. 52 (1-2), 99–112. doi: 10.1016/S0165-7836(01)00234-X
Santos M. C. O., Netto D. F. (2005). Killer whale (Orcinus orca) predation on franciscana dolphin (Pontoporia blainvillei) in Brazilian waters. Latin Am. J. Aquat. Mamm. 4 (1), 69–72. doi: 10.5597/lajam00072
Santos M. C. O., Siciliano S., Vicente A. F. C., Alvarenga F. S., Zampirolli E., Souza S. P., et al. (2010). Cetacean records along São Paulo state coast, southeastern Brazil. Braz. J. Oceanogr. 58 (2), 123–142. doi: 10.1590/S1679-87592010000200004
Santos M. C. O., Silva E. (2009). Records of a male killer whale (Orcinus orca) off southeastern Brazil. Braz. J. Oceanogr. 57, 65– 68. doi: 10.1590/S1679-87592009000100007
Secchi E. R., Vaske T. Jr. (1998). Killer whale (Orcinus orca) sightings and depredation on tuna and swordfish longline catches in southern Brazil. Aquat. Mammals. 24 (2), 117–122.
Siciliano S., Brito J. L., Azevedo A. F. (1999). Seasonal occurrence of killer whales (Orcinus orca) in waters of Rio de Janeiro, Brazil. Mamm. Biol. 64 (4), 251–255.
Siciliano S., Cardoso J., Francisco A., de Souza S. P., Hauser-Davis R. A., Iwasa-Arai T. (2020). Epizoic barnacle (Xenobalanus globicipitis) infestations in several cetacean species in south-eastern Brazil. Mar. Biol. Res. 16, 1–13. doi: 10.1080/17451000.2020.1783450
Siciliano S., Cardoso J., Francisco A., Moreira S. C. (2019). Stop for a snack: evidence of humpback whale (Megaptera novaeangliae) feeding behavior and association with gillnets during migration off southeastern Brazil. Boletim do Laboratório Hidrobiologia. 29, 41–49. doi: 10.18764/1981-6421e2019.6
Simila T., Holst J. C., Christensen I. (1996). Occurrence and diet of killer whales in northern Norway: seasonal patterns relative to the distribution and abundance of Norwegian spring-spawning herring. Can. J. Fish. Aquat. Sci. 53 (4), 769–779. doi: 10.1139/f95-253
Smith J. E., Kolowski J. M., Graham K. E., Dawes S. E., Holekamp K. E. (2008). Social and ecological determinants of fission–fusion dynamics in the spotted hyaena. Anim. Behav. 76 (3), 619–636. doi: 10.1016/j.anbehav.2008.05.001
Strier K. B. (1997). Behavioral ecology and conservation biology of primates and other animals. Adv. Study Behav. 26, 101–158. doi: 10.1016/S0065-3454(08)60378-2
Sucunza F., Andriolo A., Dalla Rosa L., Castro F. R., Danilewicz D., Zerbini A. N. (2022). Sperm whale, Physeter macrocephalus, harassment by killer whales, Orcinus orca, in the western south Atlantic ocean. Latin Am. J. Aquat. Mammals. 17 (2), 129–132. doi: 10.5597/lajam00286
Sutherland W. J. (1998). The importance of behavioural studies in conservation biology. Anim. Behav. 56, 801–809. doi: 10.1006/anbe.1998.0896
Tavares S. B., Samarra F. I. P., Miller P. J. O. (2016). A multilevel society of herring-eating killer whales indicates adaptation to prey characteristics. Behav. Ecol. 28 (2), 500–514. doi: 10.1093/beheco/arw179
Terrapon M., Kiszka J. J., Wagner J. (2021). Observations of killer whale (Orcinus orca) feeding behavior in the tropical waters of the northern Mozambique channel island of Mayotte, southwest Indian ocean. Aquat. Mammals. 47 (2), 196–205. doi: 10.1578/AM.47.2.2021.196
Testino J. P., Petit A., Acorta B., Pacheco A. S., Silva S., Alfaro-Shigueto J., et al. (2019). Killer whale (Orcinus orca) occurrence and interactions with marine mammals off Peru. Pacific Sci. 73 (2), 261–273. doi: 10.2984/73.2.7
Troina G. C., Botta S., Dehairs F., Di Tullio J. C., Elskens M., Secchi E. R. (2020). Skin 13C and 15N reveal spatial and temporal patterns of habitat and resources use by free-ranging odontocetes from the southwestern Atlantic ocean. Mar. Biol. 167, 186. doi: 10.1007/s00227-020-03805-8
Van Schaik C. P. (1999). The socioecology of fission-fusion sociality in orangutans. Primates 40, 69–86. doi: 10.1007/BF02557703
Vargas-Bravo M. H., Elorriaga-Verplancken F. R., Olivos-Ortiz A., Morales-Guerrero B., Liñán-Cabello M. A., Ortega-Ortiz C. D. (2020). Ecological aspects of killer whales from the Mexican central pacific coast: revealing a new ecotype in the Eastern tropical pacific. Mar. Mam. Sci. 1–16. doi: 10.1111/mms.12748
Visser I. N. (2000b). Orca (Orcinus orca) in new Zealand waters (Auckland: University of Auckland), 193. Ph.D. Dissertation.
Visser I. N. (2007). “Killer whales in new Zealand waters: status and distribution with comments on foraging,” in 59th Annual meeting of the International Whaling Commission Scientific Committee. Paper SC/59/SM19. (Anchorage, Alaska: International Whaling Commission).
Wade P. R., Gerrodette T. (1993). Estimates of cetacean abundance and distribution in the eastern tropical pacific. Rep. Int. Whaling Commun. 43, 477–493.
Wedekin L. L., Rossi-Santos M. R., Baracho C., Cypriano-Souza A. L., Simões-Lopes P. C. (2014). Cetacean records along a coastal offshore gradient in the vitória-trindade chain, western south Atlantic ocean. Braz. J. Biol. 74 (1), 137–144. doi: 10.1590/1519-6984.21812
Weir C. R., Collins T., Carvalho I., Rosenbaum H. C. (2010). Killer whales (Orcinus orca) in Angolan and gulf of Guinea waters, tropical West Africa. J. Mar. Biol. Assoc. United Kingdom 90, 1601–1611. doi: 10.1017/S002531541000072X
Whitehead H. (1995). Investigating structure and temporal scale in social organizations using identified individuals. Behav. Ecol. 6, 199–208. doi: 10.1093/beheco/6.2.199
Whitehead H. (2007). Selection of models of lagged identification rates and lagged association rates using AIC and QAIC. Commun. Stat. Simul. Comput. 36, 1233–1246. doi: 10.1080/03610910701569531
Whitehead H. (2008). Analyzing animal societies: quantitative methods for vertebrate social analysis (Chicago and London: The University of Chicago Press).
Whitehead H. (2009). SOCPROG programs: analysing animal social structures. Behav. Ecol. Sociobiol. 63, 765–778. doi: 10.1007/s00265-008-0697-y
Whitehead H., Dufault S. (1999). Techniques for analyzing vertebrate social structure using identified individuals. Adv. Stud. Behav. 28, 33–74. doi: 10.1016/S0065-3454(08)60215-6
Wittemyer G., Douglas-Hamilton I., Getz W. M. (2005). The socioecology of elephants: analysis of the processes creating multitiered social structures. Anim. Behav. 69 (6), 1357–1371. doi: 10.1016/j.anbehav.2004.08.018
Keywords: killer whales, Orcinus orca, Brazil, photo-identification, social behavior, cetaceans, movements
Citation: Athayde A, Cantor M, Cardoso J, Francisco A, Santos FPd, Crespo H, de Morais MV, Albaladejo MdC, Gallo Neto H and Siciliano S (2023) Movements and social behavior of killer whales (Orcinus orca) off the Brazilian coast. Front. Mar. Sci. 10:1206796. doi: 10.3389/fmars.2023.1206796
Received: 16 April 2023; Accepted: 07 June 2023;
Published: 20 July 2023.
Edited by:
Jose Carlos Báez, Spanish Institute of Oceanography (IEO), SpainReviewed by:
Mingming Liu, Chinese Academy of Sciences (CAS), ChinaLuis Cardona, University of Barcelona, Spain
Copyright © 2023 Athayde, Cantor, Cardoso, Francisco, Santos, Crespo, de Morais, Albaladejo, Gallo Neto and Siciliano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aline Athayde, YWxpbmVhLmF0aGF5ZGVAZ21haWwuY29t
†These authors have contributed equally to this work
 Aline Athayde
Aline Athayde Mauricio Cantor
Mauricio Cantor Júlio Cardoso
Júlio Cardoso Arlaine Francisco1†
Arlaine Francisco1† Heitor Crespo
Heitor Crespo Marcel Vinicius de Morais
Marcel Vinicius de Morais Salvatore Siciliano
Salvatore Siciliano