
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 19 May 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1199524
This article is part of the Research TopicEnvironmental Regulation and Restoration of Aquaculture SystemsView all 5 articles
 Xiaopeng Cheng1
Xiaopeng Cheng1 Zhenhua Wang1,2*
Zhenhua Wang1,2* Shouyu Zhang1,2*
Shouyu Zhang1,2* Xu Zhao1,2
Xu Zhao1,2 Jun Lin1,2
Jun Lin1,2 Hong Huang1,2
Hong Huang1,2 Yihui Chen1
Yihui Chen1 Qindong Zou1
Qindong Zou1Understanding the changes in community structure of fishery resources in island reef waters is crucial for effective fishery resource management, as these waters constitute a critical link in maintaining the sustainable development of offshore fishery resources. The study analyzed the structure of the fish community in the waters surrounding Dachen Island by using data collected during four voyage surveys conducted between September 2020 and April 2021. The study utilized co-occurrence network analysis, multiple regression trees, and functional diversity analysis to investigate the short-term and local scale changes in fish community structure. The results showed that the fish community in the waters adjacent to Dachen Island can be categorized into two groups: annual and stage-use species. The stage-use species include Scoliodon laticaudus, Trichiurus lepturus, Tetraodontidae, and others, which are used during the summer and autumn stages of bait migration. Additionally, Dasyatidae and others, which used during the winter and spring stages of reproductive migration. Furthermore, the study found that the habitats dominated by nearshore rocky habitats and distant deep habitats formed the local scale variation in community structure in island waters. The integrated analysis of species α-diversity and functional diversity provided a basis for understanding the mechanisms of biodiversity action. Our study aims to provide a theoretical framework for the development of fishery resource management strategies based on the life cycle of fish, and to serve as a reference for the prioritization of key protection areas for fishery resources in island and reef environments. Overall, the study’s findings can promote the understanding of community structure dynamics in island waters. Additionally, these findings can offer valuable insights into the restoration of habitats and the implementation of marine ranching activities in Dachen Island. Moreover, the outcomes can serve as a theoretical foundation for the development of appropriate management and conservation strategies for fisheries resources.
Island waters serve as a crucial intermediary zone between estuaries and offshore ecosystems, and fulfill important ecological functions as spawning, baiting, and nursery grounds for offshore fishery resources (Borges et al., 2007; Wang, 2011; Henriques et al., 2013; Jiang et al., 2022; Riofrío-Lazo et al., 2022). As a pivotal component of the offshore ecosystem, island waters constitute a critical link in maintaining the sustainable development of offshore fishery resources (Seitz et al., 2014). However, anthropogenic activities, climate change, and environmental pollution have adversely impacted the island waters, resulting in overfishing, habitat degradation, and dwindling fishery resources (Buchsbaum et al., 2005; Liang et al., 2014; Nichols, 2019.). Therefore, it is imperative to undertake restoration and management efforts to address these pressing issues. To achieve this goal, a comprehensive understanding of the structure of the fisheries resource community and its dynamic patterns in island is essential, as it provides a reference basis for the development of effective fisheries resource management strategies (Rice, 2005). In view of the above, it is imperative to study and elucidate the short-term and local scale variation patterns of the structure of the fish community in island waters to facilitate the implementation of enhanced fishery resource management practices.
To gain insight into the structure of the fisheries resource community structure and its variability in island waters, numerous studies have been undertaken in this domain (Mustamaki et al., 2015; Jiang et al., 2019; Riofrío-Lazo et al., 2022). Hu et al. conducted an investigation on the alterations in the structure of the fish community during spring and summer periods along the Zhejiang coast. Their findings revealed significant spatio-temporal variations in fish biomass and diversity in coastal waters, with disparate environmental factors exerting influence on fish diversity and abundance during spring and summer (Hu et al., 2018). Likewise, Rui Yin et al. analyzed the community structure of fishery resources during spring and autumn in the spawning ground vicinity of Dachenyang. The authors identified Harpadon nehereus, Benthosema pterotum, and Thryssa kammalensis as the primary divergent species responsible for seasonal changes in community structure (Rui et al., 2022). Furthermore, the water depth, temperature, and salinity were identified as crucial factors influencing the spatial and temporal changes in fish species composition and community structure in the sea. Additionally, several interspecific connectivity analyses based on ecological niche theory have been conducted to explore the connectivity among different species, providing a foundation for comprehending interspecific interactions (Han et al., 2020).
The environmental conditions and structure of the fishery resource community in the mid-latitude reef waters of the island exhibit notable patterns of seasonal variation. Thus, comprehending the seasonal variation patterns of the structure of the fish community that arise from environmental changes is imperative for further exploring the differential impacts of environmental factors on fish community structure across distinct seasons (Mustamaki et al., 2015; Funk et al., 2020; Xu et al., 2022). Nonetheless, the continuous seasonal monitoring of the ecological environment and fish community at a local scale remains unreported, and there is a lack of comprehensive understanding of the intra-annual cyclical changes of fish community structure in island waters. Continuous seasonal monitoring constitutes a fundamental prerequisite for apprehending the seasonal variation of fishery resources and for analyzing the mechanism of construction of fish communities in island waters. Such monitoring is of vital importance for the subsequent investigation of ecosystem connectivity.
Dachen Island is the second largest fishing ground in Zhejiang Province, boasting abundant and diverse habitat types and ample bait resources. As a spawning, nursery, and baiting ground for a variety of marine organisms, the island offers a unique opportunity for research on the changes in the structure of the fish community. Our working hypothesis posits that the fish community structure at local scale exhibits both short-term and local scale variation. The research objectives are twofold: (1) to investigate potential short-term differences in the fish community structure of the Dachen Island Waters. This will involve an examination of the fish species present during different short-term periods and a discussion of the cyclic utilization of fishery resources within the island waters. (2) to explore possible local scale differences in scale. To achieve this, we will develop a multiple regression tree model based on four quarters of fish type composition and non-biological factors, thereby analyzing differences between the environmental factors of the fish community structure and those of the Dachen Island Waters. The primary aim of this study is to provide a theoretical foundation for establishing fishing resource management measures based on the historical life of fish and to offer guidance for prioritizing critical protection areas within the island waters.
Dachen Island, the largest island in the Taizhou Islands, is located in the outer sea of southeastern Zhejiang Province, consisting of two main parts: “Upper Dachen Island” (6.6 km2) and “Lower Dachen Island” (5.2 km2). The latter is situated southeast of the mouth of Taizhou Jiaojiang, with the nearest point to the mainland being 21.6 km away (Figure 1). The waters surrounding Dachen Island are the convergence of the low-saline and high-saline water systems, and are influenced by the intersection of the coastal current and the Taiwan warm current. Such factors render it a suitable location for various fish species to spawn, grow, migrate, and find bait, forming the second largest fishing ground in Zhejiang Province, known as “Dachen Fishing Ground”. Nevertheless, excessive fishing and human activities have seriously impacted the ecological condition of Dachen Island over the past few decades, resulting in a significant decline of fishery resources in the Dachen Fishing Ground. In recent years, the relevant departments have undertaken a series of habitat restoration and marine ranching construction activities in the waters surrounding Dachen Island, with the aim at restore the fishery resources.
Fish samples were mainly collected using single-vessel bottom trawl from 2020 to 2021. Sampling sites were selected systematically, with a total of 21 designated sampling stations in the Dachen Island Waters, spaced at intervals of 4.89 km (east-west) and 3.69 km (north-south). Specifically, the study utilizes a short-term temporal scale spanning several months and a local spatial scale within kilometers. The survey area had an average water depth of 20.1 m, ranging from 9.3 to 29.7 m. The fishery resource investigation was conducted during four voyages: September (summer), November (autumn), January (winter), and April (spring). Due to maritime control measures during the fishing ban period in the East China Sea, the September survey results were used to represent the summer fishery resources in the Dachen Island Waters. We conducted trawl surveys at each designated station for a duration of 20 minutes. During the September 2020 survey, the 21st sampling site was not surveyed due to vessel interference; however, trawl surveys were conducted at all other designated stations during the remaining three voyages, resulting in a total of 83 trawl surveys. The total length of single trawl was 36 m, comprising a sleeve length of 16 m, mesh specification of 6 cm+7 cm+16 cm (cuff); a body length of 18 m, mesh specification of 3.5 cm+4.5 cm+5.5 cm (at the connection with the sleeve); and a capsule length of 2 m, mesh specification of 2 cm. The average effective expansion height and width of the net opening during towing were approximately 1.5 m and 5 m, respectively. The Latin names, and codes involved in the text are shown in Appendix S1.
Environmental factor monitoring was conducted simultaneously with trawl surveys, which involved monitoring various physical and chemical parameters of the water. The monitored parameters included temperature, salinity, depth, chlorophyll a, dissolved oxygen, and pH, and were measured using a multi-parameter water quality meter (Seabird 19plus, USA). The distance of the survey site from the island was determined based on its actual latitude and longitude and the nearest shoreline. In accordance with the “Marine Survey Specifications”, the chemical indices of phosphate, biochemical oxygen demand, ammonium, nitrate, and nitrite were monitored to assess the water quality of the Dachen Island area.
The fish community data were subjected to analysis using various parameters, namely the Shannon-Wiener index (H’), richness index (d), evenness index (J’), and relative importance index (IRI) (Margalef, 1958; Pielou, 1966; Pinkas et al., 1970). The computation of each parameter is as follows:
In the formula, Pi represents the number of individuals of the i-th species, N represents the total number of individuals of all species at the station, and S represents the number of species present at the station.
In the present study, the relative importance index (IRI) was computed using three variables, namely, the percentage occurrence (F), the percentage numerical abundance (N), and the percentage of biomass (W) of each species. The IRI provides a comprehensive assessment of the importance of a given species in the ecosystem, taking into account its abundance, distribution, and biomass. Specifically, Ni, Wi, and Fi represent the numerical abundance, biomass, and frequency of occurrence of the i-th species, respectively, expressed as a percentage of the total values of these variables across all stations surveyed.
The quarterly community turnover index and migration index are computed as follows.
A represents the number of species observed in each quarter, C and B denote the number of immigrating and emigrating species, respectively, and R denotes the number of resident species present throughout the year. The value of AI represents the rate of species turnover in different quarters, while MI reflects the proportion of migrating and emigrating species relative to the total community in the study area (Wang et al., 2019).
In this study, the functional traits of collected fish species were classified into five categories: feeding, locomotion, behavior, reproduction, and population dynamics, encompassing 11 indicators such as habitat layer, egg ecotype, food type, body size, mouth position, maximum body length, asymptotic body length, growth coefficient K, life span, generation time, and age at first sexual maturity. To collect functional trait data, the Fishbase website was utilized (https://fishbase.se/search.php)1. The functional diversity index (FD) was computed using the least convex polygon method, following the methods of Cornwell et al. and Villeger et al. for computing functional diversity (Cornwell, 2006; Villéger et al., 2008). Functional richness (FRic) denotes the degree of ecological space utilization, with a higher index signifying greater space utilization. The functional evenness index (FEve) signifies the uniformity of the distribution of functional traits across ecological space, with a larger index indicating more effective resource utilization. The functional differentiation index (FDiv) denotes the variability of characteristic values of organisms within the community, with a higher FDiv index indicating stronger complementarity of ecological niches of organisms within the community (Villéger et al., 2008).
In this study, fish population information from four trawl survey cruises was used to analyze fish species information in different seasons and draw a Venn diagram of fish distribution in Dachen Island waters during different seasons, using the Origin 2020 software (https://www.originlab.com)2. Additionally, an adjacency matrix was constructed from the trawl survey fish abundance data, and network processing was performed using the R software igraph package (Csardi and Nepusz, 2006). The picante package was utilized to calculate coeval distance (Kembel et al., 2010).
This study used a multiple regression tree model to analyze the local scale differences in fish community structure in the Dachen Island Waters fishery resources. The fish species composition at each sampling station served as the response variable, while environmental factors were used as explanatory variables to construct multiple regression tree models for four voyages. Classification of fish community groups for 21 sampling stations was conducted by selecting classification nodes based on environmental factors. environmental factors were primarily used as the basis for classification analysis. This study constructed multiple regression trees using both non-biological and biological factors, and conducted classification analysis on the results of the four voyages, thus exploring the local scale differences in fish community structure in the Dachen Island Waters.
The mvpart package and MVPARTwrap package R software were employed for multiple regression trees analysis (MRT) (De’ath, 2002). The MRT computation involved the constrained partitioning of data and grouping of outcomes. The size of the regression tree was determined by prediction error (CVRE) by transforming the species data using Euclidean distance and conducting cross-validation of the data. The R package linkET was used to examine the correlations between the diversity indices of the fish community and environmental factors. A one-way ANOVA analysis of variance was performed to determine the variability of species diversity and functional diversity between seasons, and the Tukey comparison method was used to evaluate the variability of the diversity index between seasons.
During the four voyage trawl surveys in the waters of Dachen Island, a total of 110 species of fish belonging to 14 orders and 51 families were collected. The highest number of species (59) was surveyed in autumn, while the lowest number was collected in spring. Figure 2 illustrates the common and unique situation of fish species in different seasons. Specifically, 19 species appeared in all four seasons, 11 appeared only in summer and autumn, and 6 appeared only in winter and spring. Notably, 18 species occurred only in summer. The turnover and migration of species between seasons indicated that the migration of fish occurred mainly in spring-summer, while the migration of species occurred mainly in autumn-winter. Table 1 shows the ranking of relative importance indices for fish for the four seasons, with Harpadon nehereus and Amblychaeturichthys hexanema as the main dominant species with absolute dominance in all four seasons. Muraenesox cinereus and Pampus echinogaster had high relative importance indices mainly in summer and autumn, while Lophius litulon had high relative importance indices in winter and spring.
The present study investigates the fish co-occurrence network in the adjacent waters of Dachen Island at different times. The resulting co-occurrence networks for each season are presented in Figures 3A–D, and they are found to vary significantly in terms of their network properties, as detailed in Table 2. The summer co-occurrence network analysis comprises 47 nodes and 255 connections, with four main modules. The M2 module is found to be the widespread summer species module, dominated by Harpadon nehereus and Collichthys niveatus (Figure 3B). Similarly, the fall network analysis includes a total of 40 nodes and 175 connections, with module M9 representing the winter widespread species collection module. The spring network analysis comprises 35 nodes and 113 connections, with the M13 and M14 modules representing the widely distributed species aggregation modules.
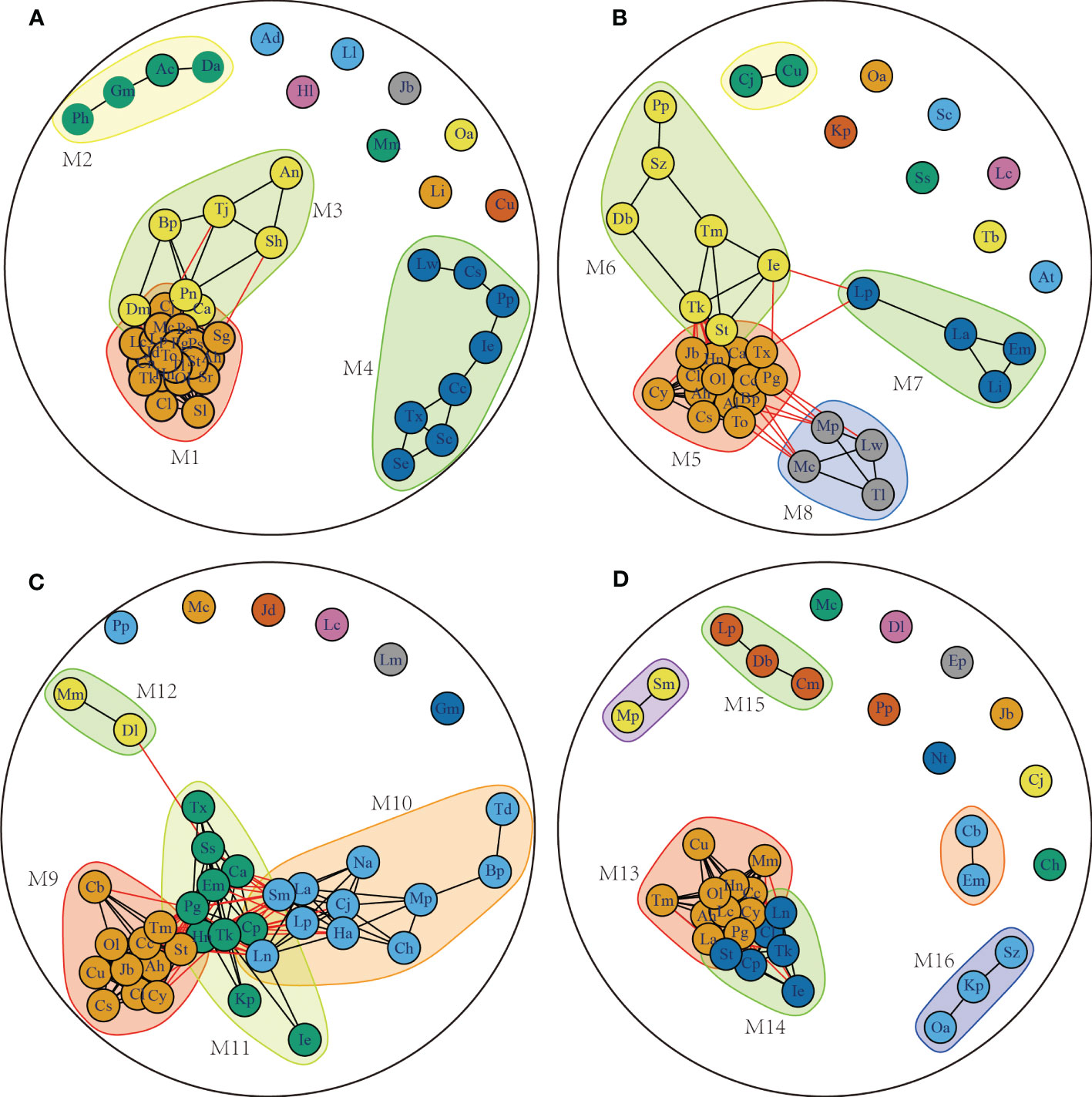
Figure 3 Fish symbiosis map of Dachen Island waters in different seasons based on Jaccard similarity. Picture (A–D) are Summer, autumn, winter, and spring. Different modules are distinguished by color. The black line indicates positive intra-module association, and the red line indicates positive inter-module association Species codes are listed in Appendix S1.
The network properties of different seasons are found to be distinct. In summer and autumn, the fish co-occurrence network is tightly connected within each module, with partial connections between modules and less nested structures between modules. In contrast, the fish co-occurrence network in winter shows low intra-module connectivity, low inter-module independence, and obvious nesting between modules. Finally, the spring fish co-occurrence network has a simpler structure with more fragmented nodes, a single intra-module connectivity path, and a lower average connectivity path between nodes. The findings of this study provide insights into the structural composition of fish communities in the Dachen Island waters at different times.
The correlation analysis between environmental factors is presented in Figure 4. The patterns of correlation among the environmental factors varied between seasons. Specifically, the relationships between environmental factors and spatial factors, such as depth of water and offshore distance, were investigated for each season. The results are summarized as follows: during summer, offshore distance displayed a significant negative correlation with water temperature, chlorophyll a, and dissolved oxygen, while water depth showed a significant negative correlation with nutrient and . In contrast, during winter, the depth of the water was positively correlated with water temperature, salinity, and , but negatively correlated with dissolved oxygen and BOD.
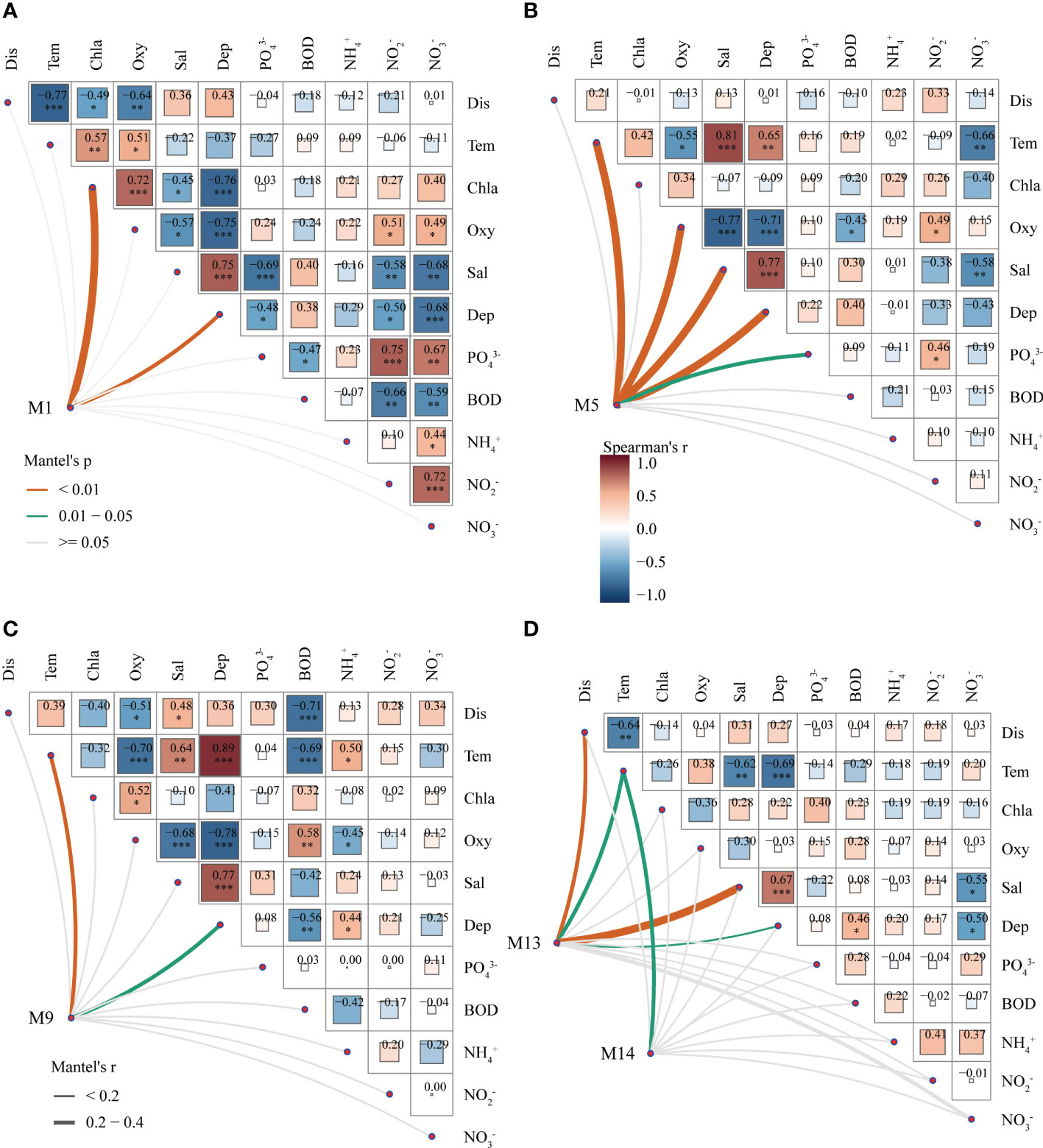
Figure 4 Correlation analysis of environmental factors and the main coexistent fish in different seasons. Picture (A–D) are Summer, Autumn, Winter, and Spring. (*p<0.05, **p<0.01, ***p<0.001).
The association between fish abundance and environmental factors across different modules is illustrated in Figure 4. Module M1 in summer consists of 20 fish species, including Harpadon nehereus (Hn), Collichthys lucidus (Cl), and Amblychaeturichthys hexanema (Ah), where the abundance of fish is mainly linked to chlorophyll a and water depth. In module M5 during autumn, which encompasses 14 species such as Harpadon nehereus (Hn), Amblychaeturichthys hexanema (Ah), and Benthosema pterotum (Bp), species abundance is significantly associated with water temperature, dissolved oxygen, salinity, water depth, and the distance from shore. Module M9 in winter includes 11 fish species such as Amblychaeturichthys hexanema (Ah), Coilia nasus (Cn), and Coilia mystus (Cm), with the abundance of species significantly correlated with water temperature and water depth. Finally, the spring module M13 consists of 11 species, including Harpadon nehereus (Hn), Amblychaeturichthys hexanema (Ah), and Thryssa mystax (Tm), where species abundance is mainly correlated with offshore distance, water temperature, water depth, and salinity. Notably, the abundance of species in all modules is significantly related to distance from shore, water temperature, water depth, and salinity.
Using environmental factors and fish species composition in the Dachen Island Reef Sea, a multiple regression tree model was constructed, as shown in Figures 2–5. Environmental factors served as classification nodes to divide the 21 sampling stations into 3-4 groups, each representing a set of sampling points with similar environmental conditions and fish community compositions. During summer, the fish community was divided into four groups, where water depth, salinity, and water temperature together explained 61.7% of the variation in data (Figures 2–5A). During autumn, the fish community was divided into four groups, where salinity and water depth together explained 80.3% of the variation in data (Figures 2–5B). During winter, the fish community was divided into three groups, where dissolved oxygen and ammonia nitrogen together explained 44.9% of the variation in data (Figures 2–5C). Finally, during spring, the fish community was divided into three groups, where salinity and offshore distance together explained 54.5% of the variation in data (Figures 2–5D).Rank analysis was employed to investigate the classification results of fish community composition in different seasons, and the local scale differences in fish community structure are presented in Figures 2–6. The biplot displays the species vectors representing all the analyzed species, where the first and second principal components altogether account for over 97% of the variability. Based on PCA analysis, significant differences were observed in the classification results of fish community composition in the Dachen Island Waters. When combined with the sampling point compositions of each group, there were evident local scale differences in the fish community structure in this area.
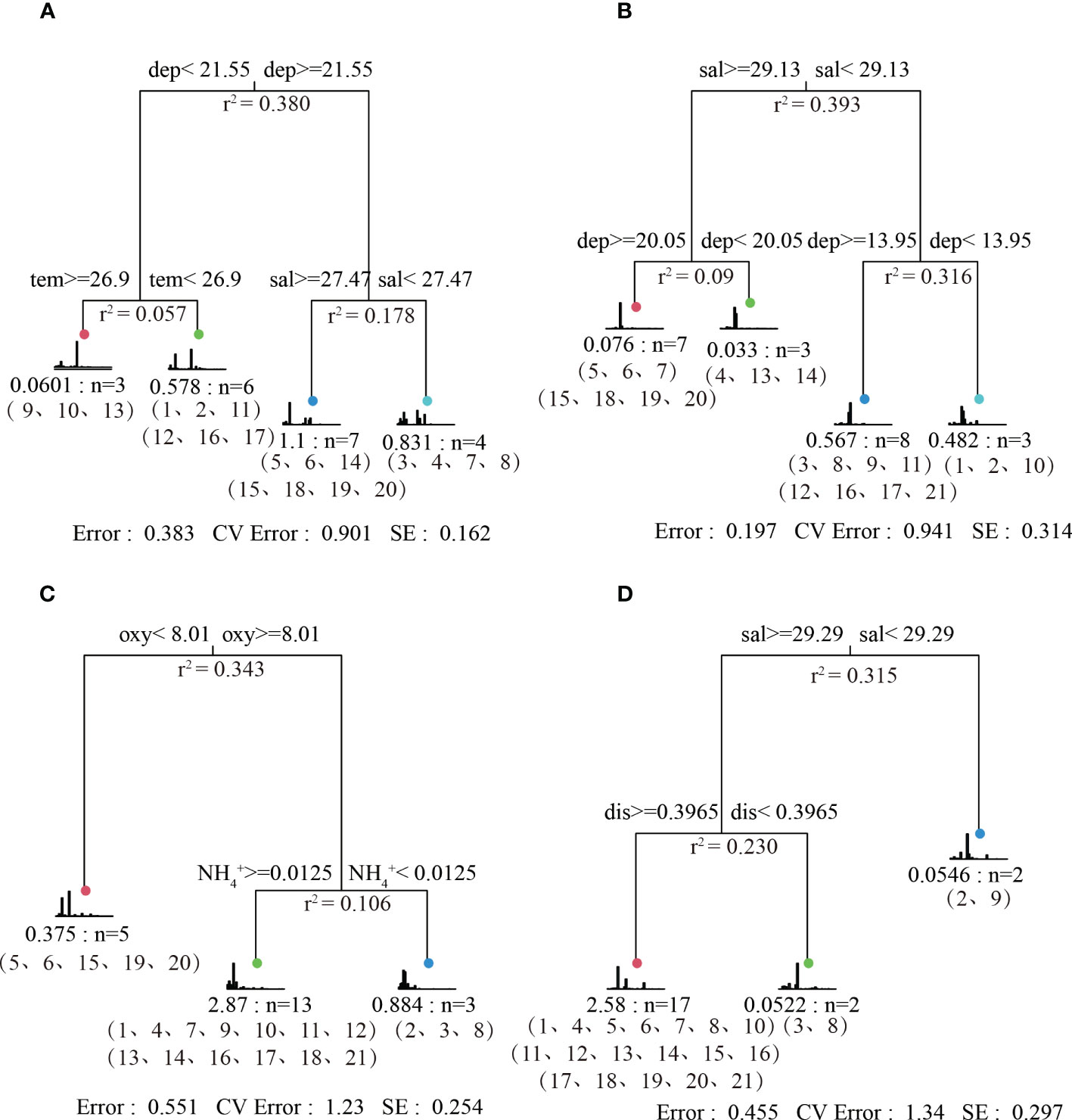
Figure 5 Analysis of the structure of the fish community in the waters of the Dachen Island in different seasons. Picture (A–D) are Summer, Autumn, Winter, and Spring.

Figure 6 Principal component analysis of the grouping results. Picture (A–D) are Summer, Autumn, Winter, and Spring.
The findings of the analysis of the species diversity of the fish community in the Dachen Island area are presented in Figure 7. The α diversity index and the functional diversity index display significant seasonal fluctuations, with the functional diversity index being more sensitive to ecosystem changes than the α diversity index. Correlation analysis of species diversity and functional diversity in different seasons showed a significant positive correlation between species richness and functional richness across all four seasons (Figure 8). In addition, there was no significant correlation between the Shannon diversity index and functional diversity index in winter and spring, whereas a significant positive correlation was observed in summer and autumn. The distribution frequencies of diversity indices in different seasons revealed that the Shannon diversity index of the structure of the fish community in Dachen Island exhibited an approximately normal distribution.
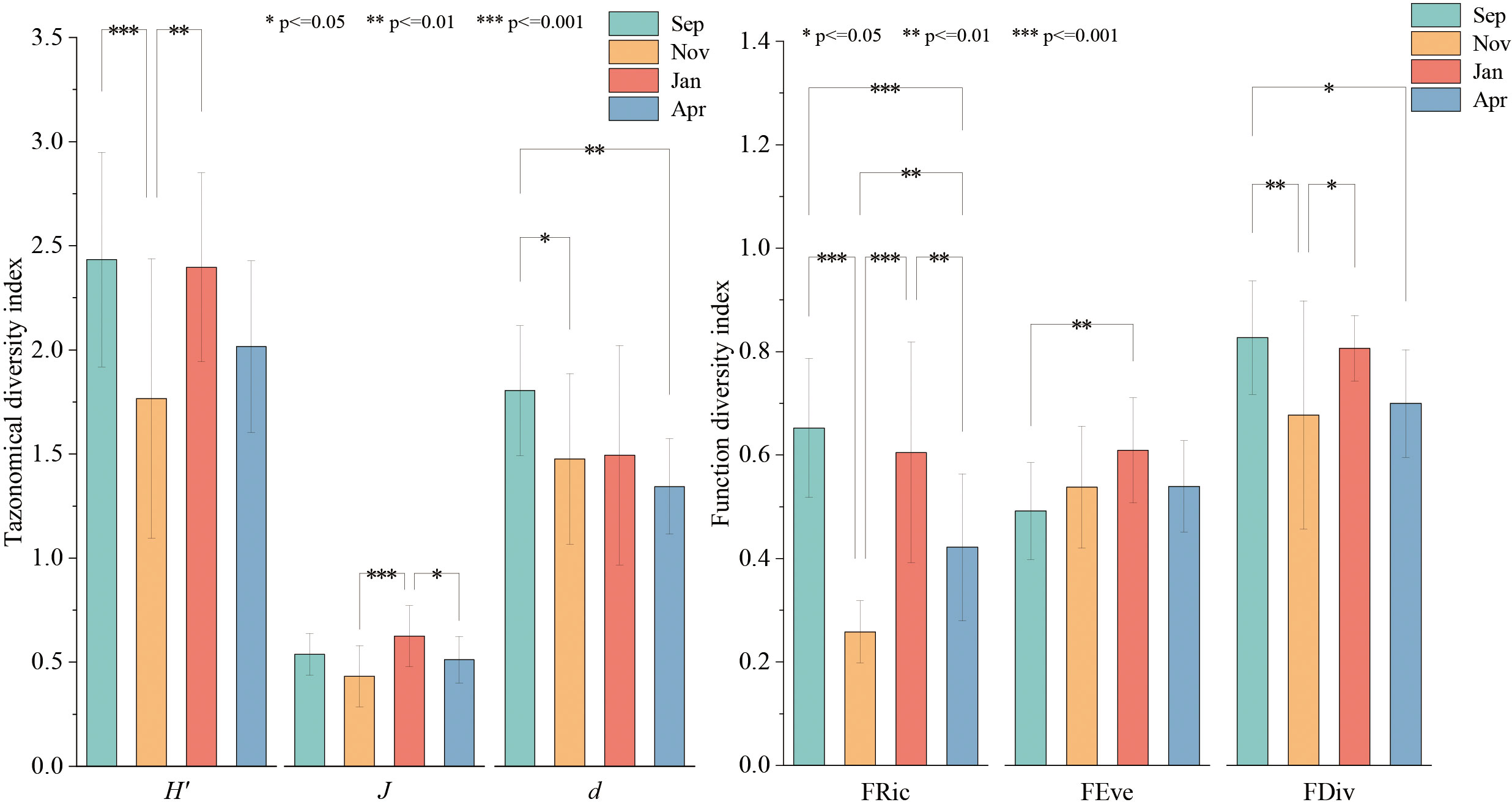
Figure 7 Fish biodiversity in Dachen Islands Reefs adjacent waters (Left: species diversity index, right: functional diversity index). (*p<0.05, **p<0.01, ***p<0.001).
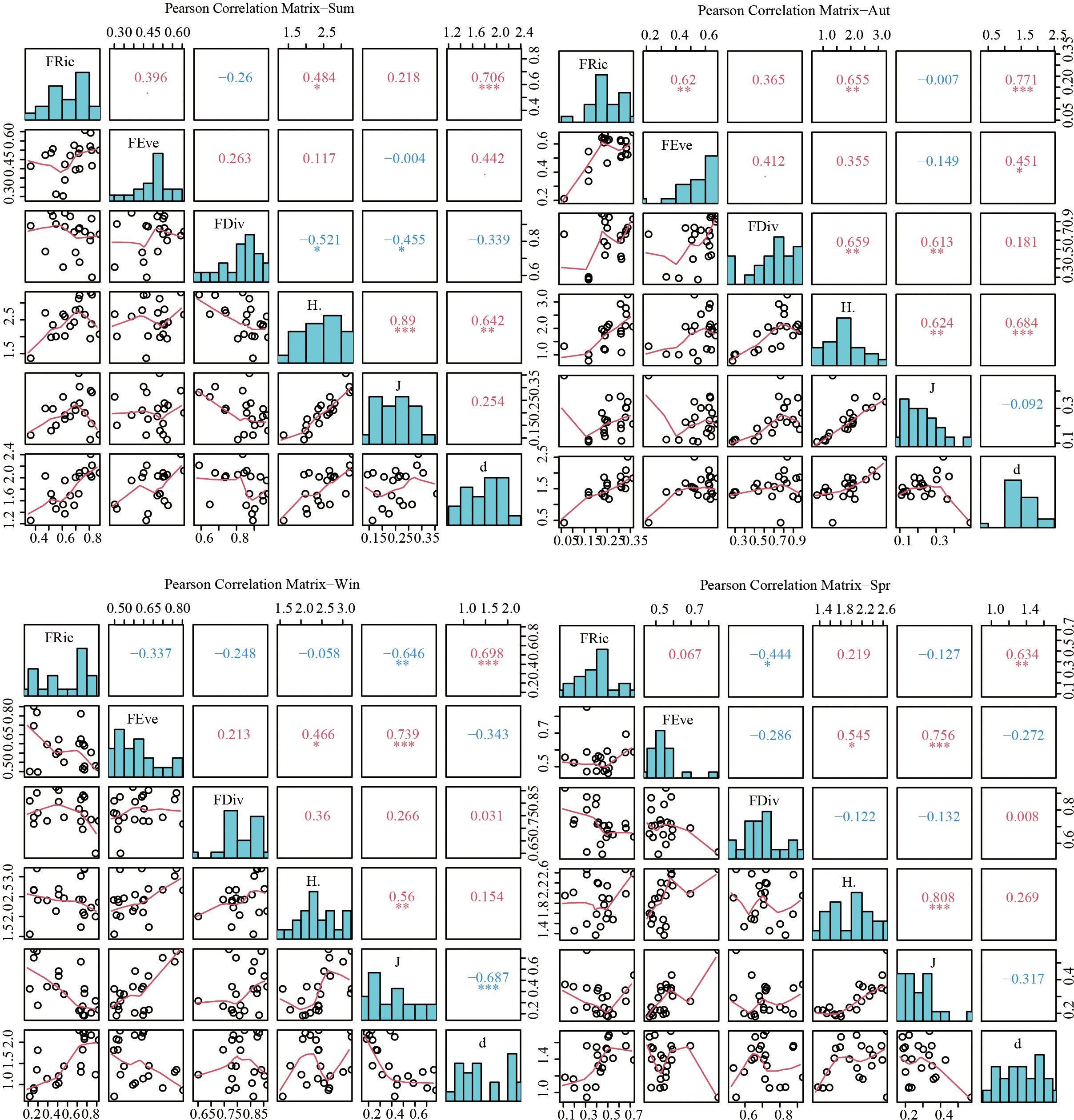
Figure 8 Correlation analysis of fish species diversity and functional diversity in different seasons. (*p<0.05, **p<0.01, ***p<0.001).
The activity time and spatial distribution of fish are determined by their physiological adaptations and life history strategies, and the seasonal patterns of fish community utilization at local scales are more pronounced than those at larger scales (Perry et al., 2005; Mustamaki et al., 2015). Fish communities in mid-latitude island waters exhibit significant seasonal variation due to the annual migration and reproductive strategies of fish. In this study, fish in the island waters of the Dachen Island showed substantial inter-seasonal turnover and migration changes. Based on the differences in the habitat use cycles of fish during different seasons, the fish communities in Dachen Island waters were classified into two primary categories: local species and stage use species.
The fish species that exhibit local species utilization in Dachen Island waters include Harpadon nehereus, Collichthys lucidus, Amblychaeturichthys hexanema, and Muraenesox cinereus, which is consistent with the findings of Du et al. (Du et al., 2018). These species have ecological attributes that enable them to adapt to a wide range of temperature and salinity conditions with high physiological adaptability. The relative importance analysis revealed that local species dominate the island waters and are the primary carriers of energy flow and material circulation. Additionally, co-occurrence network analysis showed that they play a critical role in information connectivity throughout the year. However, some fish species exhibit season-dependent ecological roles, such as the high trophic level aggressive predator, Muraenesox cinereus. During summer and autumn, the Muraenesox cinereus serves as an important connector of intra- and inter-module organismal material and energy flow exchange (Xiao et al., 2022). In contrast, during winter and spring, it does not form significant interaction connections with other species. Considering the results of fish relative importance analysis in Dachen Island waters, the Muraenesox cinereus holds high relative importance during the summer and autumn levels and is also an important catch in local waters. Its fierce feeding habits and high biomass are responsible for its significant ecological linkage role, while its connection role in winter and spring is not significant, which may be due to the low relative importance of Muraenesox cinereus in winter and spring and the replacement of its connection role by high-trophic-level predators such as otoliths.
The present study categorizes stage-use fish into two groups: solicitous migratory fish and reproductive migratory fish. Bait-run fish, which are typically high-trophic-level carnivorous fish, exhibit significant seasonal utilization patterns that are determined by individual physiological adaptations and bait organism abundance. During the summer and autumn seasons, the bait migratory fishes include Scoliodon laticaudus, Trichiurus lepturus, and Takifugu oblongus. While Trichiurus lepturus, and Takifugu oblongus are found throughout the survey area, Scoliodon laticaudus is limited to the inshore reef area. This finding indicates that the habitat selection and utilization pattern of bait migratory fishes may be influenced by the abiotic habitat environment, in addition to biological factors. During the winter and spring seasons, Lophius litulon are the main fish involved in solicitous migrations. These fish are primarily found in muddy and sandy substrates, and less commonly in rocky reef habitats, with bottom Amblychaeturichthys hexanema serving as the primary bait (Zhang et al., 2010). Reproductive migratory fishes exhibit specific habitat requirements for reproduction, such as Myliobatiformes fishes that reproduce as ovoviviparous and only occur in winter and spring in island areas, showing significant habitat selection preferences. Additionally, some reef-loving fishes, such as Engraulidae and Carangidae, exhibit important reproductive migration patterns, often spawning in nearshore bays during the spring season (Wang, 2011).
Understanding the selective utilization pattern of fish in island habitats provides a crucial reference basis for fisheries management departments to develop practical and efficient measures to manage fish fisheries resources. Furthermore, this knowledge is essential to understand the functions of the ecosystem service of island waters and to utilize critical habitats to their fullest potential.
Community assembly is recognized as a process that involves both biotic and abiotic factors, wherein species from regional gene pools undergo screening by environmental conditions and biotic interactions. This results in only select species that meet the environmental requirements being able to colonize local communities, which contributes to local scale variations in community structure (Keddy, 1992; Chu et al., 2017). In this study, we conducted a correlation analysis between the abundance of fish species and environmental factors in the waters surrounding Dachen Island. We categorized fish assemblages into groups and found that the structure of the fish community of the island displayed significant seasonal fluctuations. Furthermore, the structure of the fish community showed local scale differences, with the most pronounced variations in the patterns of the fish community observed during summer and autumn. Using multiple regression tree analysis, we identified water depth and offshore distance as significant explanatory variables for differences in fish community structure. Based on these results, we divided the Dachen Island area into two primary habitat types, the inshore island area and distant deep-water area. We analyzed the characteristics of fish communities in each habitat and demonstrated that the structure of the fish community exhibits significant local scale variability. Our findings are consistent with the conclusion of Ford et al. (2017). that habitat is the primary determinant of fish assemblage.
The impact of habitat on the structure of the fish community primarily occurs through abiotic and biotic mechanisms. The abiotic effect of habitat is primarily exhibited through its filtering effect on fish communities, resulting in distinct community structures in local habitats compared to larger regional backgrounds (Niu et al., 2009). This leads to the formation of fish assemblages with similar ecological attribute requirements, such as the Sciaenidae family in inshore reef areas (Wang et al., 2013) and the Gobiidae family in distant deep-water areas. Additionally, the role of habitat in the structure of the fish community is also evident in the availability of bait. The complex and diverse habitats in nearshore reef areas provide a suitable environment for the settlement of fish larvae and palatable bait, which indirectly influences the structure of the fish community. Distant deep-water areas, dominated by muddy and sandy substrates, also provide a good feeding environment for fish with similar bait requirements, such as Benthosema pterotum and Apogon lineomaculatus. The different action processes of various habitats give rise to local scale variations in the structure of the fish community, which are further influenced by seasonal changes and the complexity of the sea ecosystem of the island. Consequently, this leads to seasonal differences in the patterns of the fish community.
Comprehending the local scale dissimilarities in fish community structure in island waters is critical for fishery resource management planning, and is indispensable for safeguarding key habitats and managing important ecological corridors. Habitat-mediated fish community patterns exhibit noteworthy seasonal variations, and these variations offer insights into the short-term and local scale dynamics of fishery resources, facilitating the establishment of a reference framework for spatial planning of fishery resources and the judicious arrangement of protected areas.
Alpha species diversity indicators based on taxonomy utilize information on species abundance and multiplicity to characterize community structure, establishing a logical correlation between an organism’s presence or absence, its frequency, and its size within the ecosystem. However, these indicators neglect the functional traits of species and their role in the ecosystem (McGill et al., 2006). On the other hand, functional diversity indicators based on species’ functional characteristics consider differences in functional traits, while incorporating abundance and multiplicity information of community species. These indicators focus primarily on the services provided by species to the ecosystem, offering a heightened sensitivity to reflect the ecosystem’s state, and are currently the most reliable predictors of response to ecosystem function (Villéger et al., 2017; Zeng et al., 2022). Analysis of the interrelationship between species diversity and functional diversity indicators serves as a fundamental approach for comprehending the association between biodiversity and ecosystem function (Zhang et al., 2019).
In this research, we investigated the association between species diversity and functional diversity in island waters during different seasons. The results showed a significant positive correlation between the species richness index and the functional richness index of the structure of the fish community in all four seasons, which is consistent with the findings reported by Chen et al. (Chen et al., 2022). However, the diversity of species exhibited seasonal fluctuations. The structure of the fish community in winter and spring in the waters of the Dachen Island was characterized by high evenness and low richness, with fish species richness exhibiting an approximately uniform distribution, and species evenness and functional evenness demonstrating a significant positive correlation. Based on the field survey data, it was found that fishery resources were low during winter and spring, with species having limited local scale utilization in the ecosystem, minimal competition among species, and a species composition dominated by small, local fish. The structure of the fish community of Dachen Island area during summer and autumn, on the other hand, was characterized by high richness, with significant local scale variability in the functional differentiation index, with some sites exhibiting low functional differentiation values and others exhibiting high functional differentiation values. Based on the correlation analysis of the species diversity index and functional richness index, it was determined that the functional richness index of fish in the sea area of the Dachen Island increased significantly with an increase in species diversity index during summer and autumn, and an increase in species diversity led to a higher degree of fish utilizing ecological space. In managed and restored ecosystems, high functional diversity results in high stability, as multiple functional characteristics aid the system in resisting changes in abiotic factors (Chen, 2017). Additionally, the fish functional richness index showed a trend of increasing and then decreasing with increasing species diversity index in Dachen Island waters during summer, compared to autumn. The structure of the fish community appeared functionally redundant from the perspective of functional characteristics during summer, and related studies have shown that functionally redundant ecosystems are considered more resilient, resistant, and stable (McLean et al., 2019).
The comprehensive evaluation of fish α-diversity and functional diversity plays a crucial role in comprehending the impacts of environmental stressors and perturbations on community structure (McLean et al., 2019), and serves as a foundation for further investigations into the mechanisms of complementary and competitive effects of ecological niches on biodiversity (Henseler et al., 2019). In addition to conserving biodiversity, the ultimate goal of habitat restoration, ecosystem conservation, and management is to attain the sustainable exploitation of resources while preserving the services and functions provided by ecosystems. Thus, the analysis of α-diversity and functional diversity is essential for understanding the correlation between species diversity and ecosystem functions.
In this study, we conducted continuous monitoring in the waters of Dachen Island to analyze the seasonal variation patterns of the fish community using co-occurrence network analysis. Our results revealed significant seasonal changes in the composition of fish species, indicating that fish migration occurred in and out during summer and spring, respectively. Based on the pattern of fish habitat use, the fish community in Dachen Island waters can be classified into local species with broad temperature and salinity ranges and migratory species with phased use. We employed multiple regression trees analysis to examine the short-term and local scale differences in the structure of the fish community driven by habitats in the waters of the Dachen Island. Moreover, we integrated fish α-diversity and functional diversity analysis to gain insights into dynamic changes in community structure. Based on the findings of our study, we recommend gaining an understanding of the seasonal utilization patterns of fish habitats as a basis for developing fisheries resource management strategies based on fish life history cycles. Furthermore, comprehending the ecological significance of different habitats can aid in establishing prioritized conservation strategies for key regions.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
XC and ZW contributed to the conception and design of the study. XZ conducted the experiments. XC contributed to the analysis and wrote the manuscript. ZW and SZ contributed to the manuscript revision. All authors contributed to the article and approved the submitted version.
This work was supported by National key R&D Program of China (Grants 2019YFD0901303, 2018YFD0900704, 2020YFD0900804), and National Natural Science Foundation of China (Grants 41876191).
We gratefully acknowledge Cui Xiao for the helpful discussion and proofreading in the course of writing articles.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1199524/full#supplementary-material.
Borges R., Ben-Hamadou R., Chícharo M. A., Ré P., Gonçalves E. J. (2007). Horizontal spatial and temporal distribution patterns of nearshore larval fish assemblages at a temperate rocky shore. Estuarine Coast. Shelf Sci. 71, 412–428. doi: 10.1016/j.ecss.2006.08.020
Buchsbaum R., Pederson J., Robinson W. E. (2005). The decline of fisheries resources in new England: evaluating the impact of overfishing, contamination, and habitat degradation. (United States: National Oceanic and Atmospheric Administration).
Chen Y. (2017). Functional diversity: a new view point in the relationship between biodiversity and ecosystem functioning research. J. Yunnan Univ. 39, 1082–1088. doi: 10.7540/jynu.20170175
Chen K., Meng Z., Li X., Hu F., Du H., Liu L., et al. (2022). Community structure and functional diversity of fishes in zhelin reservoir, Jiangxi province. Acta ecologica Sin. 42, 4592–4602. doi: 10.5846/stxb202106031466
Chu C., Wang Y., Liu Y., Jiang L., He F. (2017). Advances in species coexistence theory. Biodiversity Sci. 25, 345–354. doi: 10.17520/biods.2017034
Cornwell W. (2006). Causes and consequences of functional trait diversity: plant community assembly and leaf decomposition. (State of California, United States: Doctoral dissertation, Stanford University).
Csardi G., Nepusz T. (2006). The igraph software package for complex network research. Interjournal Complex Syst. 1695–1704.
De’ath G. (2002). Multivariate regression trees: a new technique for modeling species–environment relationships. Ecology 83, 1105–1117. doi: 10.1890/0012-9658(2002)083[1105:MRTANT]2.0.CO;2
Du X., Tian S., Wang J., Wang Z., Gao C. (2018). Spatial and temporal variations in fish community off shore southern zhejiang province, East China Sea. J. Dalian ocean Univ. 33, 522–531. doi: 10.16535/j.cnki.dlhyxb.2018.04.018
Ford B., Stewart B., Roberts J. (2017). Species pools and habitat complexity define Western Australian marine fish community composition. Mar. Ecol. Prog. Ser. 574, 157–166. doi: 10.3354/meps12167
Funk S., Krumme U., Temming A., Möllmann C. (2020). Gillnet fishers’ knowledge reveals seasonality in depth and habitat use of cod (Gadus morhua) in the Western Baltic Sea. ICES J. Mar. Sci. 77, 1816–1829. doi: 10.1093/icesjms/fsaa071
Han X., Wang Y., Qiu J., Zhang M., Yu S., Liang H., et al. (2020). Niche and interspecific associations of dominant fisher in southern coastal waters in taizhou, China. J. fisheries China 44, 621–631. doi: 10.11964/jfc.20190411721
Henriques S., Pais M. P., Costa M. J., Cabral H. N. (2013). Seasonal variability of rocky reef fish assemblages: detecting functional and structural changes due to fishing effects. J. Sea Res. 79, 50–59. doi: 10.1016/j.seares.2013.02.004
Henseler C., Nordström M. C., Törnroos A., Snickars M., Pecuchet L., Lindegren M., et al. (2019). Coastal habitats and their importance for the diversity of benthic communities: a species- and trait-based approach. Estuarine Coast. Shelf Sci. 226, 106272. doi: 10.1016/j.ecss.2019.106272
Hu C., Zhang Y., Li D., Zhu W., Jiang R., Li P., et al. (2018). Study on fish resources and community diversity during spring and summer in the coastal spawning ground of zhejiang provinces, China. Acta hydrobiological Sin. 42, 984–995. doi: 10.7541/2018.121
Jiang R., Sun H., Li X., Zhou Y., Chen F., Xu K., et al. (2022). Habitat suitability evaluation of Harpadon nehereus in nearshore of zhejiang province, China. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.961735
Jiang R., Zhang L., Xu K., Li P., Xiao Y., Fan Z. (2019). Characteristics and diversity of nekton functional groups in the coastal waters of south-central zhejiang province. Biodiversity Sci. 27, 1330–1338. doi: 10.17520/biods.2019281
Keddy P. A. (1992). Assembly and response rules: two goals for predictive community ecology. J. Vegetation Sci. 3. doi: 10.2307/3235676
Kembel S. W., Cowan P. D., Helmus M. R., Cornwell W. K., Morlon H., Ackerly D. D., et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. doi: 10.1093/bioinformatics/btq166
Liang J., Wang W., Yu B., Zhang H. (2014). Seasonal variations of fish resources and community diversity of reef habitat in marine protected area of zhongjieshan islands. Oceanologia limnologia Sin. 45, 979–989. doi: 10.11693/hyhz20130800114
Margalef R. (1958). Spatial heterogeneity and temporal succession of phytoplankton. In: Buzzati-Traverso A. (ed.) Perspectives in Marine Biology. (Berkeley: University of California Press) 1958, 323–350. doi: 10.1525/9780520350281-024
McGill B. J., Enquist B. J., Weiher E., Westoby M. (2006). Rebuilding community ecology from functional traits. Trend Ecol. Evolut. 21, 178–185. doi: 10.1016/j.tree.2006.02.002
McLean M., Auber A., Graham N. A. J., Houk P., Villéger S., Violle C., et al. (2019). Trait structure and redundancy determine sensitivity to disturbance in marine fish communities. Global Change Biol. 25, 3424–3437. doi: 10.1111/gcb.14662
Mustamaki N., Jokinen H., Scheinin M., Bonsdorff E., Mattila J. (2015). Seasonal small-scale variation in distribution among depth zones in a coastal Baltic Sea fish assemblage. Ices J. Mar. Sci. 72, 3997–4000. doi: 10.1093/icesjms/fsv068
Nichols R. L. (2019). Sustainable fisheries and habitat-fishery interactions (Shanghai, China: PhD thesis, University of Tasmania). doi: 10.25959/100.00031396
Niu K., Liu Y., Shen Z., He F., Fang J. (2009). Community assembly: the relative importance of neutral theory and niche theory. Biodiversity Sci. 17, 579–593. doi: 10.3724/SP.J.1003.2009.09142
Perry A. L., Low P. J., Ellis J. R., Reynolds J. D. (2005). Climate change and distribution shifts in marine fishes. Science 308, 1912–1915. doi: 10.1126/science.1111322
Pielou E. C. J. (1966). The measurement of diversity in different types of biological collections. J. Theor. Biol. 13, 131–144. doi: 10.1016/0022-5193(66)90013-0
Pinkas L., Oliphant M. S., Iverson I. L. K. (1970). Food habits of albacore, bluefin tuna, and bonito in California waters. [Sacramento] State California Dept. Fish Game. Fish Bulletin 152.
Rice J. C. (2005). Understanding fish habitat ecology to achieve conservation*. J. Fish Biol. 67, 1–22. doi: 10.1111/j.0022-1112.2005.00933.x
Riofrío-Lazo M., Zetina-Rejón M. J., Vaca-Pita L., Murillo-Posada J. C., Páez-Rosas D. (2022). Fish diversity patterns along coastal habitats of the southeastern Galapagos archipelago and their relationship with environmental variables. Sci. Rep. 12, 3604. doi: 10.1038/s41598-022-07601-w
Rui Y., Jiang R., Wang H., Luan H., Yin R., Zhuyudan L., et al. (2022). Characteristics of fish community structure and its relationship with environmental factors in dachenyang spawning ground reserve. J. fisheries China 46, 995–1007. doi: 10.11964/jfc.20200912421
Seitz R. D., Wennhage H., Bergström U., Lipcius R. N., Ysebaert T. (2014). Ecological value of coastal habitats for commercially and ecologically important species. ICES J. Mar. Sci. 71, 648–665. doi: 10.1093/icesjms/fst152
Villéger S., Brosse S., Mouchet M., Mouillot D., Vanni M. J. (2017). Functional ecology of fish: current approaches and future challenges. Aquat Sci. 79, 783–801. doi: 10.1007/s00027-017-0546-z
Villéger S., Mason N. W. H., Mouillot D. (2008). New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301. doi: 10.1890/07-1206.1
Wang Z. (2011). Fish community patterns in meta-habitat: a case study from ma’an archipelago (Shanghai, China: PhD thesis, Shanghai ocean University).
Wang Z., Zhang S., Zhang D., Li X., Kong X. (2019). Species composition and monthly changes of fish tianjin coastal waters from July to November in 2017. J. Dalian ocean Univ. 34, 733–738. doi: 10.16535/j.cnki.dlhyxb.2018-237
Wang Z., Zhao J., Wang K., Zhang S. (2013). Fish community ecology in rocky reef habitat of ma’an archipelago. Acta ecologica Sin. 33, 6218–6226. doi: 10.5846/stxb201306081445
Xiao Y., Jiang R., Yin R., Wang J., Yang F., Wang H., et al. (2022). Trophic niche and interspecific relationship of five eels the waters of the zhoushan islands. J. fisheries China, 1–10.
Xu Y., Wu Y., Xiu P., Ge J., Zhang J. (2022). Unraveling environmental drivers of chlorophyll seasonal and interannual variability in the East China Sea. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.951395
Zeng Z., Cheung W. W. L., Lai H., Yi H., Bi S., Li H., et al. (2022). Species and functional dynamics of the demersal fish community and responses to disturbances in the pearl river estuary. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.921595
Zhang X., Cheng J., Shen W., Liu Z. (2010). Feeding ecology of lophius litulon in the south of yellow Sea. Acta Ecologica Sin. 30, 3117–3125.
Keywords: Dachen Island, fish community structure, functional diversity, cooccurrence network, multiple regression trees
Citation: Cheng X, Wang Z, Zhang S, Zhao X, Lin J, Huang H, Chen Y and Zou Q (2023) Analysis of short-term and local scale variations in fish community structure in Dachen Island waters. Front. Mar. Sci. 10:1199524. doi: 10.3389/fmars.2023.1199524
Received: 03 April 2023; Accepted: 05 May 2023;
Published: 19 May 2023.
Edited by:
Kai Zhang, Chinese Academy of Fishery Sciences, ChinaReviewed by:
Jie Cao, North Carolina State University, United StatesCopyright © 2023 Cheng, Wang, Zhang, Zhao, Lin, Huang, Chen and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhua Wang, emhfd2FuZ0BzaG91LmVkdS5jbg==; Shouyu Zhang, c3l6aGFuZ0BzaG91LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.