
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 30 May 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1193405
This article is part of the Research Topic Functional Feed Additives: Current Trends View all 8 articles
 Cristina Velasco1
Cristina Velasco1 Daniela Resende1,2,3
Daniela Resende1,2,3 Beatriz Oliveira1,2
Beatriz Oliveira1,2 Paula Canada1,4
Paula Canada1,4 Miguel Pereira3
Miguel Pereira3 Carlos Pereira5
Carlos Pereira5 Manuela Pintado3
Manuela Pintado3 Luisa M. P. Valente1,2*
Luisa M. P. Valente1,2*Dietary supplementation with hydrolysates has been suggested to influence muscle protein synthesis and fish growth. This study assessed the impact of including 3% swine blood hydrolysates (BH) in a plant-based diet on muscle cellularity and the expression of molecular markers related to muscle fibre proliferation and hypertrophic growth of European seabass. Three BH fractions were obtained by two different processes, autohydrolysis (AH-H) and enzymatic hydrolysis followed by micro- (RMF-H) and nanofiltration (RNF-H). Each BH was added to a commercial-based diet, where 50% of fishmeal was replaced by vegetable proteins (negative control, NC). A fishmeal-based diet was used as positive control, PC. The diets were fed to juveniles (12 g) during 74 days. The RMF group showed down-regulation of myod1 and fgf4, essential to myoblast proliferation and differentiation, and upregulation of mafbx, responsible for protein breakdown, resulting in impairment of muscle hyperplasic growth and the lowest muscle fibres number. However, compensatory growth mechanisms were observed through capn1 downregulation and mymk upregulation, suggesting decreased muscle proteolysis and increased myoblast fusion. Despite this, the compensatory mechanisms were insufficient as RMF group had the worst growth. RNF group had a final weight similar to the NC, but downregulation of fgf4, fgf6 and capn1 may compromise growth potential at long term. The expression of these genes in the AH group was similar to that in the FM-based diet. Despite not having demonstrated growth promotion ability, BH affect muscle growth and cellularity factors, prompting further research on commercial-sized fish to reveal their impact on important commercial traits.
Aquaculture is an activity with great potential to meet the increasing demand for high-quality fish products, whilst also ensuring sustainable use of aquatic resources. The fast growth of this sector, together with the limited availability and increasing prices of marine-based ingredients such as fishmeal (FM) and fish oil, has forced the feed industry to look for alternative and more sustainable sources of dietary protein and lipid (Naylor et al., 2021). Vegetable protein sources have been considered as an alternative to FM but may negatively impact fish feed intake and growth (Torrecillas et al., 2017; Conde-Sieira et al., 2018; Martins et al., 2019; Costa et al., 2020; Fontagné-Dicharry et al., 2020), particularly in carnivorous fish species that have a high protein requirement, ranging from 45 to 55% (NRC, 2011). That is often attributed to poor palatability, lower quality, and anti-nutritional factors of vegetable dietary protein sources (Francis et al., 2001). In this regard, dietary supplementation with bioactive compounds has recently attracted great interest among aquafeed manufacturers to produce functional diets with benefits beyond their nutritional value (Daroit and Brandelli, 2021).
In mammals, dietary supplementation with hydrolysates has long been suggested to increase protein synthesis in skeletal muscle, promoting muscle building in exercise (Andersen et al., 2005; Manninen, 2009; López-Martínez et al., 2022), and resting situations (Shin et al., 2020), which has been associated with the bioactivity potential of such hydrolysates. In rainbow trout (Oncorhynchus mykiss) moderate inclusion of fish protein hydrolysates (FPH) in plant-based diets not only promoted feed intake and growth, but also improved feed efficiency, protein efficiency ratio, and the retention of some essential amino acids (Aksnes et al., 2006a; Aksnes et al., 2006b). Similar results were obtained in Atlantic salmon (Salmo salar) (Refstie et al., 2004; Hevrøy et al., 2005) and red seabream (Pagrus major) (Khosravi et al., 2015). Other studies have shown that the dietary inclusion of certain protein hydrolysates can induce alterations on myogenic processes and impact muscle development and growth in Atlantic salmon (Hevrøy et al., 2005), Senegalese sole (Solea senegalensis) larvae and post-larvae (Canada et al., 2018), and turbot (Scophthalmus maximus) juveniles (Wei et al., 2020). Furthermore, Hevrøy et al. (2005) demonstrated that dietary inclusion of a FPH increased protein accretion and the expression of myosin heavy chains (MyHC) in the muscle of Atlantic salmon juveniles. This increase was correlated with a higher specific growth rate, suggesting that FPH may affect muscle growth regulation and subsequently improve the growth performance of fish juveniles. A recent study from Wei et al. (2020) showed that a moderate inclusion (12%) of FPH in a feed for turbot reduced the expression of a negative regulator of muscle growth (mstn2) and increased muscle cross-sectional area. These positive results have been associated with specific chemical and structural characteristics of the peptides present in the hydrolysates (Carvalho et al., 2004; Swanepoel and Goosen, 2018), such as high digestibility and intestinal absorption (Martínez-Alvarez et al., 2015). It has recently been shown that many of the bioactive properties are attributed to specific functional peptides encoded in the parental protein structure, corresponding to short sequence of approximate 2-20 amino acids in length (Rizzello et al., 2016).
With advances in biotechnology and a push towards circular economy and zero waste, there is growing interest in the use of by-products as sources for functional ingredients in animal nutrition (Pal and Suresh, 2016). Animal and plant by-products can undergo chemical and enzymatic hydrolysis to generate high-quality peptides. These peptides have important nutritional and physiological functions in livestock, poultry, and fish, playing an essential role in the development of functional foods (Himaya et al., 2012). Focusing on the meat processing industry, a significant amount of non-edible by-products such as bones, skin, and blood (Toldrà et al., 2019; European Commission, 2021) are discarded, resulting in considerable environmental impacts and high costs for the animal production sector. Although blood is commonly processed into low-cost blood meal for animal feed or fertilizer (Bah et al., 2013; Adhikari et al., 2018), it is also a source of protein than can be used to produce high value-added products like hydrolysates rich in bioactive peptides (Bernardini et al., 2011). Blood hydrolysates (BH) have already been evaluated in aquafeeds for both larvae (Gisbert et al., 2012) and juveniles (Gisbert et al., 2021; Resende et al., 2022) with promising results. Gisbert et al. (2021) found that a 5% dietary inclusion of BH led to increased feed intake and growth of gilthead seabream (Sparus aurata) juveniles, while Resende et al. (2022) reported improved resistance to Tenacibaculum maritimum infection in European seabass (Dicentrarchus labrax) fed plant-based diets supplemented with 3% BH. Recent studies associated with the development of new hydrolysates suggest that the resulting bioactive properties are linked to specific peptides and the balance between medium and low molecular weight peptides (Offret et al., 2019; Resende et al., 2022). This highlights the importance of the hydrolysis method used in the regulation of fish muscle growth. However, the main physiological mechanisms underlying muscle fiber proliferation and size, as well as flesh textural properties, are still unclear.
To evaluate the potential of innovative BH as a dietary supplement for promoting muscle accretion and growth in European seabass juveniles, this study analyzed the expression of genes related to fish performance, energy metabolism, protein turnover, muscle cell proliferation, and differentiation. The aim is to determine whether including 3% swine BH in a plant-based diet for European seabass juveniles affects the dynamics of skeletal muscle growth.
Based on the known nutritional requirements of European seabass (NRC, 2011), five isoproteic (54% DM), isolipidic (16% DM) and isoenergetic (22 kJ/g DM) diets were formulated, and subsequently extruded (2 mm) by SPAROS, Lda. (Portugal): a fish meal (FM) based diet (positive control, PC), a commercially-based diet where 50% of FM was replaced by vegetable proteins (negative control, NC) and three experimental diets (RMF, RNF, AH) where 3% of each BH was added to the NC at the expense of wheat gluten (Table 1). These swine blood hydrolysates were obtained by two different processes, enzymatic hydrolysis and autohydrolysis, as described by Resende et al. (2022) and Araújo-Rodrigues et al. (2022). The hydrolysate mixture obtained enzymatically was further fractionated by microfiltration (500 kDa cut-off), with the retentate of the microfiltration being designated RMF-H, while the filtrate product was then subjected to nanofiltration (3 kDa cut-off), obtaining a retentate denoted RNF-H. All information regarding feed ingredients and diets composition are available at Resende et al. (2022).
The fish trial and all animal procedures were subjected to an ethical review process carried out by CIIMAR animal welfare body (ORBEA-CIIMAR_18_2017) and were performed by accredited scientists in laboratory animal science by the Portuguese Veterinary Authority (1005/92, DGAV-Portugal, following FELASA category C recommendations), in compliance with the guidelines of the European Union (directive 2010/63/UE).
Juvenile European seabass were obtained from the commercial fish farm Acuinuga – Acuicultura y Nutrición de Galicia, S.L. (A Coruña, Spain), and transported to the Fish Culture Experimental Unit of CIIMAR (Matosinhos, Portugal). Fish were kept in quarantine, to adapt to the experimental conditions, for two weeks, and hand fed with a commercial diet (AQUASOJA, Portugal; 50% crude protein and 20% crude fat as DM basis).
After the acclimation period, fish were fasted for 24 h, slightly anaesthetized with 60 µL/L of 2-Phenoxyethanol (Sigma-Aldrich, MO, USA) and individually weighed (g) and measured (total length, cm). Fifteen homogeneous groups of 71 fish (initial body weight of 12.3 ± 1.4 g) were randomly distributed into a recirculating aquaculture system (RAS) composed of 15 fiberglass tanks of 250 L each, at a density of 3.5 kg/m3. Each tank was provided with filtered, heated (20 ± 1°C) saltwater (35 ± 1‰) at a flow rate of 16 L/min, and water quality parameters were regularly monitored. Dissolved oxygen level was maintained above 90% saturation and photoperiod was a cycle of 12h light/12h dark. Total ammonium (NH4+), nitrite (NO2-), nitrate (NO3-) and pH levels were monitored twice a week and maintained at levels recommended for this species (Kır et al., 2019). Each experimental diet was randomly allocated to triplicate tanks, and fish were manually fed until apparent visual satiety, three times a day for a period of 74 days. At the end of the growth trial, after a 24 h fasting period, all fish were slightly anesthetized (60 µL/L of 2-Phenoxyethanol) and individually weighed (g) and measured (total length, cm) for evaluation of growth performance. Additionally, three fish per tank (9 fish per treatment) were sacrificed with anesthetic overdose (0.5 mL/L of 2-Phenoxyethanol), for collection of muscle samples. For muscle morphometric analysis, fish were softly scaled on both sides and a cross-sectional fillet with skin (5 mm thick) was taken from the anterior region immediately before the dorsal fin position. Properly labelled, the fillets were quickly photographed with a scale reference for later determination of the white muscle cross-sectional area (CSA). The obtained fillet was divided into two pieces: the left part of the fillet was immediately frozen in isopentane cooled by dry ice and then stored at -80 °C for later determination of muscle cellularity; afterwards, the right side was sampled for gene expression analysis, chopped into smaller pieces, and immediately preserved in RNAlater™ stabilization solution (Thermo Fisher Scientific, Massachusetts, USA) before being stored at -80°C until RNA extraction.
The white muscle CSA was obtained by delimiting the white muscle area on the fillet photos taken at sampling, using the image analysis software Olympus CellSens. For morphometric determination of the number and size of the muscle fibers on each fish fillet, the frozen samples were cut in a cryostat (12 μm) and the membranes of muscle fibers were specifically marked using a primary antibody dystrophin (Santa Cruz Biotechnology, clone MANDRA1) and the Novolink™ Polymer Detection System kit (RE7140-K). The sections were incubated overnight at 4°C with the primary antibody diluted in 1:200 in Phosphate Buffered Saline (PBS) containing 1% of Tween™ 20. The prepared slides were photographed using an Olympus SC50 camera connected to an Olympus BX51 light microscope. Sixteen representative photos of white muscle fields within a CSA were taken per fish, at 100x magnification. The size and number of muscle fibers were determined in each photograph with the aid of the Olympus CellSens software. At least 900 fibers were measured per fish. With this procedure, it was also possible to calculate the following parameters: mean fiber diameter (calculated assuming that all fibers were circular), total number of white fibers in the CSA and fiber density (number of muscle fibers per area).
Total RNA was extracted from 15 mg of skeletal muscle using NZYol reagent (Nyztech, Lisbon, Portugal) following the manufacturer´s recommendations, with some modifications, as previously described by Ferreira et al. (2020). RNA quantity and purity was determined by spectrophotometry and assessed based on the absorbance ratio 260:280 nm, using a Take 3 Micro-Volume plate on a Synergy™ HT Multi-Detection Reader and the Gen5™ software (BioTek Instruments, Winooski, VT, USA). RNA integrity was evaluated based on gel electrophoresis in a 1% (w/v) agarose TAE gel stained with Gel Red™ nucleic acid stain (Biotium, Hayward, CA, USA). Afterwards, a total of 1μg of RNA was reverse transcribed to cDNA using NZY First-Strand cDNA Synthesis Kit (Nyztech, Lisbon, Portugal), following the standard protocol.
Expression of genes related to a) growth (GH/IGF system): growth hormone receptor I and II (ghr-i and ghr-ii), insulin-like growth factor I and II (igf-i and igf-ii), insulin-like binding-protein 3a (igfbp3a), insulin-like binding-protein 5b (igfbp5b) and, insulin-like binding-protein 6b (igfbp6b); b) energy metabolism: citrate synthase (cs); c) muscle cell proliferation and differentiation: myoblast determination protein 1 and 2 (myod1 and myod2), myogenic regulatory factor 4 (mrf4), myogenic factor 5 (myf5), myostatin (mstn), follistatin (fst), fibroblast growth factor 4 and 6 (fgf4 and fgf6), muscle RING-finger protein 1 (murf1), muscle atrophy F-box (mafbx), myomaker (mymk); and d) protein turnover: calpain 1, 2 and 3 (capn1, capn2 and capn3), calpastatin (cpst), was assessed by reverse transcription q-PCR. Quantitative PCR assays were performed with CFX384 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA), with NZYSpeedy qPCR Green Master Mix (2x) (Nyztech, Lisbon, Portugal). Reactions were carried out with 40 – 400 nM of each primer (forward and reverse), 5 μL of Green Master Mix and 2 μL of cDNA, in a total reaction volume of 10 μL. Thermal cycling conditions were 95°C for 2 min, followed by 40 cycles of two steps, 95°C for 5 s followed by primer annealing temperature (60–62°C) for 28 s (annealing temperatures are presented in Table 2). Following the final PCR cycle, post-amplification dissociation curves were systematically monitored (60–95, 0.5°C in each cycle) to ensure reaction specificity. PCR efficiency was analyzed in serial, 2-fold dilutions of cDNA by calculating the slope of the regression line of the cycle thresholds (Ct) vs. the relative concentration of cDNA from a sample pool of all experiments, and only values between 90 and 110% were accepted (The R2 for all genes assessed was higher than 0.985). Each sample was analyzed in duplicate and samples without cDNA were run as negative control. Relative quantification of target gene transcripts was done using elongation factor 1α (eef1a1) and β-actin (β-actin) as housekeeping genes, following the Pfaffl method (Pfaffl, 2001).
All data were tested for normality and homogeneity of variances by Kolmogorov-Smirnov and Levene’s tests, respectively, and adequately transformed whenever required. One-way ANOVA was applied to analyze data, using the SPSS (IBM SPSS Statistics 26, IL, USA) software. Whenever significant effects of treatments were detected, means were compared through the pairwise Tukey multiple comparison test. In all cases, the minimum level of significance was set at P < 0.05 for all analysis. Furthermore, Pearson’s correlations were evaluated for the data and considered a two-tailed analysis of 0.05 and 0.01.
After 10 weeks of feeding the experimental diets, all groups at least tripled their initial weight, except for fish fed RMF (Table 3). Fish fed RNF had similar BW and BL as those fed the NC, but differed significantly from the PC group that had the best performance. Fish fed RMF diet had the lowest body weight and size. White muscle CSA varied between 265 and 352 mm2, total number of fibers ranged from 73 to 97 thousand, and fiber density from 272 to 289 n/mm3. Fish fed the RMF diet showed significantly smaller white muscle CSA when compared to all other groups, and the lowest number of fibers. However, there were no differences among dietary treatments for fiber density values (Table 3). Fibers’ average diameter varied between 60 and 65 µm (Table 3), with only 5-7% of them being lower than 20 µm and 3-4% higher than 120 µm (Figure 1). As shown in Figure 1, fish fed RMF diet had the lowest percentage of both small (< 20 µm) and large sized-fibers (>120 µm), but the highest percentage of intermediate size fibers (40-60 µm). The larger fiber size and reduced number of small-sized fibers in RMF group (Figure 2C) compared to the other groups (Figures 2A–E) can be depicted in the muscle cross sectional sections.
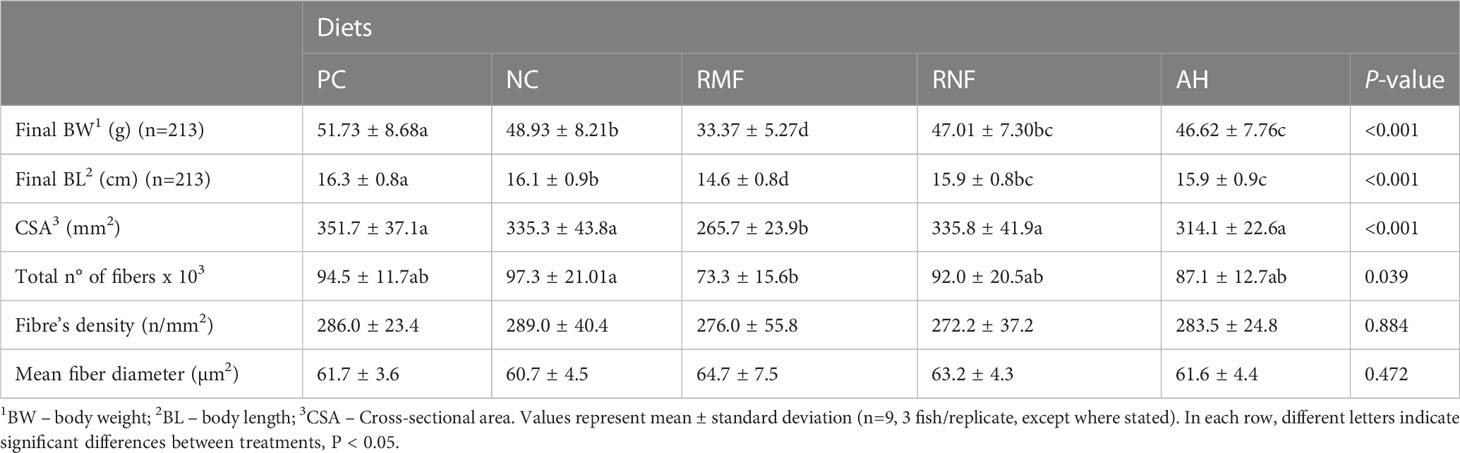
Table 3 Growth performance indicators and muscular cellularity parameters of European seabass fed experimental diets.
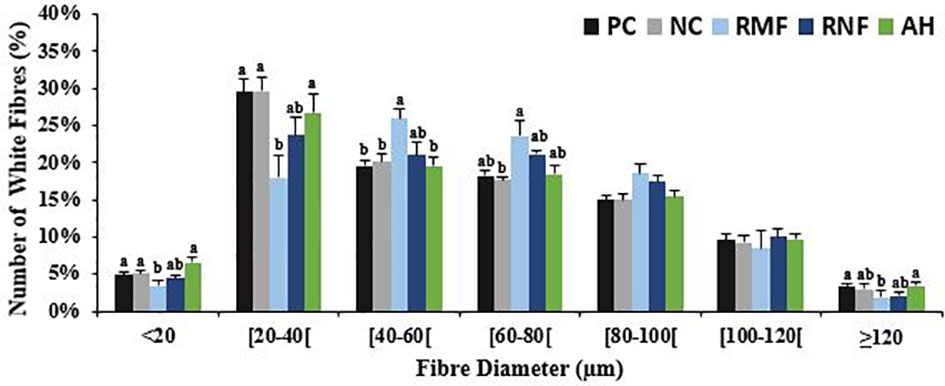
Figure 1 White muscle fibers distribution per size classes of European seabass fed experimental diets. Values represent mean ± SEMs (n=9, 3 fish/replicate). In each diameter range, different letters indicate significant differences between treatments, P < 0.05.
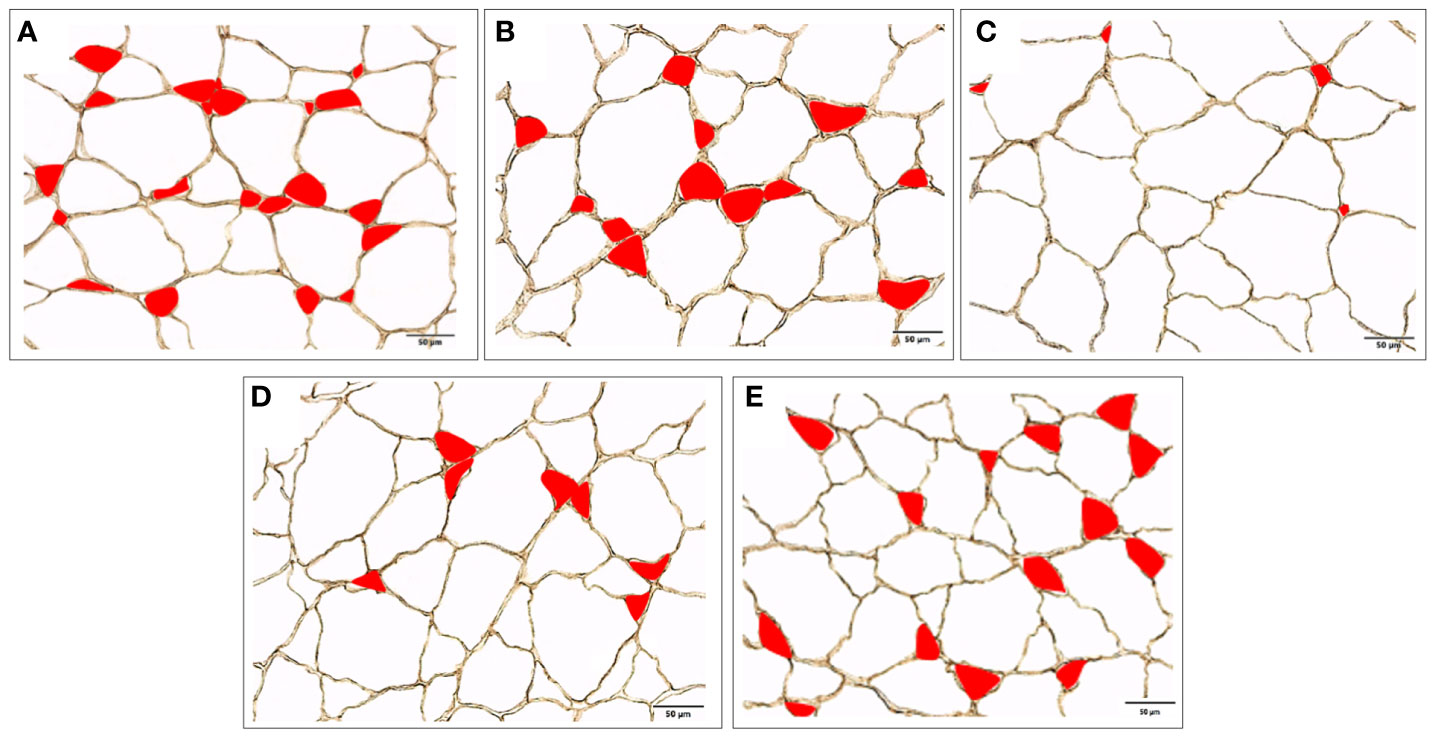
Figure 2 White muscle cross section from European seabass fed with (A) PC, (B) NC, (C) RMF, (D) RNF, (E) AH diets, where small size fibers (≤ 40 µm), are represented in red (200x magnification).
The relative gene expression of key selected markers in muscle is shown in Table 4. Among all the analyzed genes, five markers of cell proliferation and differentiation and one marker of protein turnover were differentially expressed with the inclusion of the various BH in the diet. Regarding markers related to the growth performance, the expression of ghr-ii showed significant differences among groups, with an upregulation in fish fed RMF diet when compared to the groups feeding upon diets including hydrolysates (i.e., RNF and AH groups), but without differences from the control groups.
Although fish fed the NC were smaller than those fed the PC, no differences could be observed in either muscle cellularity or muscle proliferation and differentiation-related genes. The only exception was a downregulation of fgf4. Genes related to cell proliferation were the most affected by BH inclusion: RMF diet was associated with a down-regulation of myod1 and fgf4 expression, and an up-regulation of mymk, compared to the PC; RNF diet down-regulated both fgf4 and fgf6 in relation to the PC and the AH. Fish fed the AH diet did not show differences in the expression of any of these muscle growth markers as compared to the PC group. In terms of protein turnover, most genes remained unaffected by the dietary treatments, with the exception of cpn1 expression that was down-regulated in fish fed either RMF and RNF diets in relation to the PC.
The total number of fibers displayed strong correlations with muscle CSA, BL and BW (Table 5). Furthermore, both BL and BW were strongly correlated with intermediate size fibers (20-40 µm, 40-60 µm and 80-100 µm). The expression of fgf4 was positively correlated with fgf6, myod1 and calpain family genes. Moreover, capn1 in particularly was correlated negatively with fibers of intermediate size and myod1 showed negative correlation with BW and the number of fibers. Mymk expression was correlated with mafbx and fibers of 80-100 µm, and was also negatively correlated with CSA, total number of fibers, fibers of 20-40 µm, BL and BW. While mafbx were correlated with CSA and the number of fibers.
Muscle development and growth relies on protein deposition, as the net result between the in-situ protein synthesis and protein breakdown. White skeletal muscle constitutes the bulk of fish axial locomotor muscle and a major part of body mass in most fish. Therefore, protein deposition in the white muscle is a determining factor to the overall growth of larvae and juvenile fish (Houlihan et al., 1995; Carter and Houlihan, 2001). While protein quality (dietary amino acid profile) directly influences protein deposition and growth performance through the delivery of all the amino acids required for protein synthesis in muscle, dietary protein complexity influences its digestibility and thus amino acids bioavailability (Canada et al., 2016). The use of protein hydrolysates promotes muscle building in mammals, as they provide highly digestible protein and readily available amino acids that being rapidly delivered to the muscular tissue should promote protein accretion in the muscle (Poullain et al., 1989; Kanda et al., 2013; Nakayama et al., 2019). The aquafeed industry has recently shown a great interest in hydrolysates as a source of bioactive peptides, which can improve the nutritional value of end products and contribute to fish welfare. The current study demonstrates that the addition of novel swine BH to plant-based diets has a detrimental impact on the growth of European seabass muscle by myogenic processes.
The growth of fish fed the NC diet was worse than that of those fed the PC diet, which confirms the negative impact of conventional plant protein sources in aquafeeds on the growth and health of carnivorous fish due to their detrimental characteristics (Francis et al., 2001). Growth impairment has also been reported in European seabass (Costa et al., 2020), salmonids (Aksnes et al., 2006b; Alami-Durante et al., 2010; Wong et al., 2013) and gilthead seabream (Gómez-Requeni et al., 2004; Martínez-Llorens et al., 2012) with similar levels of FM-replacement. Additionally, the substitution of 50% FM by plant proteins (NC diet) negatively affected European seabass myogenesis regulation. This was associated with the downregulation of the fibroblast growth factor fgf4, although no differences were observed in muscle cellularity. Fish fed the NC diet also showed a decreasing trend in the expression of other myogenesis regulatory genes such as fgf6, myod1 and calpain family genes, when compared to those fed the PC diet. Indeed, we have found a strong positive correlation (P<0.001) between fgf4 and these genes. In rainbow trout, Alami-Durante et al. (2010) described changes in muscle fiber size distribution when FM was replaced by vegetable protein, but only when the substitution exceeded 75%. Significant differences were also observed in the expression of genes involved in protein degradation, such as cathepsin, in fish fed diets completely devoid of FM.
Protein hydrolysates added to aquafeeds have been reported to increase feed intake and feed utilization (Refstie et al., 2004; Zheng et al., 2012; Zheng et al., 2013; Bui et al., 2014; Khosravi et al., 2015), resulting in satisfactory somatic growth in juvenile red seabream, Atlantic salmon, Japanese flounder (Paralichthys olivaceus), and turbot. For instance, Khosravi et al. (2015) demonstrated that a diet with 50% FM replaced by soy protein and supplemented with hydrolysates from shrimp coproducts outperformed FM-based diets in red seabream. Similarly, Costa et al. (2020) and Leduc et al. (2018) supplemented plant-based diets for seabass with marine hydrolysates at 3% and 5-10%, respectively, achieving the same fish final weight as a high FM diet. However, in the present study, supplementation of a low FM diet (NC) with 3% of BH was not able to improve European seabass growth in juveniles. Fish fed the RNF diet reached a similar body weight and length as those fed the plant-protein rich diets (NC), but still lower than those fed the FM-based diet (PC). Furthermore, fish fed the RMF and AH diets showed even poorer growth performance compared to both controls. Variations in the effects of hydrolysates on fish growth observed in different studies in literature suggest that the origin of raw materials, enzymatic processing, and inclusion level are determining factors in the functional properties of hydrolysates obtained (Chotikachinda et al., 2013; Opheim et al., 2015; García-Moreno et al., 2017; Lajmi et al., 2019; Costa et al., 2020). Most studies testing the inclusion of marine hydrolysates in a diet evidenced an improvement in fish growth. However, a recent study reported impaired growth in meagre (Argyrosomus regius) when fed diets with FM replacement and 5% inclusion of porcine plasma hydrolysates (Fernández-Alacid et al., 2021). Similarly, Xu et al. (2017) reported reduced growth in turbot when fed a diet containing 20% plant protein and 8% pig BH, compared to a 30% FM-based diet. Despite this, there is growing evidence suggesting that protein hydrolysates derived from swine blood could be a functional ingredient for aquafeeds. Resende et al. (2022) showed for the first time in fish that the same BH used in this study had a beneficial effect against tenacibaculosis in juvenile seabass. In addition, Gisbert et al. (2021) observed a reduced incidence of malformations and an accelerated development of intestinal digestion.
The muscle cross-sectional area of European seabass fed the different BH generally exhibited trends similar to those observed in final body size and length, which in turn reflected differences in white muscle cellularity, specifically in the total number of fibers and fiber size distribution. While fiber density remained relatively constant among the experimental diets, significant differences were detected in the total number of fibers and fiber size distribution. Fish fed the RMF diet showed reduced muscle growth, mainly due to a lower total number of fibers. This was supported by a positive correlation found between the number of fibers and CSA, BL and BW. The size-class distribution of fibers in fish fed RMF, particularly the lower percentage of small-sized fibers (< 20 µm), indicates a decreased capacity for fiber recruitment compared to the other groups. Similarly, Voss et al. (2021) have also observed that Nile tilapia (Oreochromis niloticus) fed diets containing an enzymatically produced okara hydrolysate showed a decrease in the percentage of small-sized fibers (≤ 30 µm) and promoted muscle hypertrophy.
In this study, the reduced total number of fibers and lower percentage of small-sized fibers observed in European seabass fed the RMF diet can be partially attributed to the downregulation of myod1 (myoblast determination factor) compared to the PC group. Myod1 is involved in the commitment of myoblasts to form the population of myogenic progenitor cells (Rescan, 2001) and is associated with the formation of new muscle fibers. The observed positive correlation between myod1 and final BW supports the direct impact of downregulated myod1 expression on muscle CSA and fish final BW. Furthermore, fish fed RMF showed slight upregulation of mafbx, responsible for protein breakdown (Bodine et al., 2001), which negatively correlated with muscle CSA and the total number of fibers. However, compensatory growth mechanisms seem to have occurred in these fish, as evidenced by the concomitant downregulation of capn1 and up-regulation of mymk, suggesting a reduction in muscle proteolysis and increased myoblast fusion (Salem et al., 2005; Honda et al., 2008; Macqueen et al., 2010). These results can partially explain the higher percentage of intermediate fibers in the RMF group. Specifically, a negative correlation was observed between capn1 and fibers size-classes between 40-60 µm and 60-80 µm, and a positive correlation between mymk expression and 80-100 µm fibers. Nevertheless, these compensatory mechanisms were insufficient to support adequate muscle growth in these group of fish.
Contrary to the RMF group, fish fed either the RNF or the AH diet showed no differences on CSA, total number of fibers, and fiber size distribution when compared to the NC or PC. However, a downregulation in the expression of fibroblast growth factors (fgf4 and fgf6) and calpain1 could be perceived in the RNF group, suggesting both a lower muscle proliferative and differentiation capacity, as well as lower protein breakdown. In contrast, the group fed the AH diet showed transcript levels of fgf 4 and fgf6 that were similar to the PC group and tended to be higher than the NC group. This might explain the increasing trend for a high percentage of small-sized fibers, although it did not result in overall growth. Nevertheless, the increased percentage of small-sized fibers suggests a higher growth potential in the long-term. A longer trial is required to assess the full potential of dietary supplementation with AH on white muscle building and overall growth in European seabass. Additionally, fractionation of the AH-H may be of interest as selecting smaller-sized peptides from this hydrolysate (Resende et al., 2022) may enhance the higher growth potential. The downregulation of ghr-ii in the RNF and AH groups compared to both control groups is somewhat puzzling considering the weight gain results. An opposite effect, as observed in the RMF, would be expected in the RNF and AH groups, although not as pronounced since the final weight was closer to that of NC fed fish.
The effects of dietary inclusion of hydrolysates on muscle growth-related genes vary greatly depending on the hydrolysate properties, source, and processing. For example, Wei et al. (2020) found that a 12% inclusion of a FPH (at the expense of FM) in diets for turbot maximized muscle CSA and reduced myostatin mRNA levels, while no impact was observed in myf5 or myod. In contrast, a plant protein hydrolysate and a FPH with different peptide profiles, included in diets for Senegalese sole larvae, had varying effects on muscle-related genes. The plant hydrolysate resulted in a down-regulation of myf5, myod2 and myogenin (Canada et al., 2018). In mice, oral administration of a whey protein hydrolysate mixed with Panax ginseng berry extract did not alter myod expression, but reduced myostatin and increased myogenin mRNA levels, as well as muscle CSA. To the best of our knowledge, the only work that has evaluated the impact of porcine BH on muscle properties has focused on muscle fiber type conversion (from fast-twitch to slow-twitch) using mice as a model. The authors claimed that the hydrolysate improved exercise performance through the promotion of slow-twitch fiber expression (Jin et al., 2022).
Integrating fundamental and applied research allows us to accurately characterize the modulatory potential of certain hydrolysates for specific functions, helping us make more informed selections of ingredients based on the physiological functions we aim to improve. The results presented in this study are an important and innovative evaluation of the impact of BH on essential metabolic pathways for fish growth and muscle development. Previous research has shown that BH can enhance infection resistance (Resende et al., 2022). While they may not have demonstrated the ability to promote juveniles’ growth, BH can influence the dynamics of skeletal muscle growth, potentially affecting flesh quality traits. Therefore, additional research on the impact of incorporating these hydrolysates into the diet of commercially-sized fish over varying time periods would be valuable in evaluating their effects on muscle cellularity and texture. This research would aid in assessing how the hydrolysates affect the desired flesh properties of consumers.
The results of this study indicate that BH have the potential to influence the mechanisms involved in regulating white muscle cellularity, which may have implications for important economic traits such as growth potential. This study also provides the first evidence that swine BH may module the expression of muscle growth markers and, thus, fish growth dynamics. Future studies should take into account various factors such as diverse processing methods, inclusion levels of BH in aquafeeds, and feeding periods, in order to enhance the production performance of seabass from both immunological and physiological perspectives. Furthermore, integrative studies are needed to more confidently link the roles of genes explored and the underlying mechanisms, with a focus on growth up to a commercial size, and muscle texture, which is a major factor in assessing the perceived quality of fish by consumers.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by ORBEA-CIIMAR_18_2017.
Conceptualization, LV, MPi, CP, CV. Methodology, LV, MPi, CV, DR, BO, MPe, CP. Validation, CV, DR, LV, MPi, PC. Formal analyses, CV, DR, BO. Data curation, CV, BO. Writing – original draft preparation, CV. Writing – review and editing, all co-authors. Supervision, LV and MPi. Project administration and funding, LV. All authors contributed to the article and approved the submitted version.
This work was supported by Project “FISHCOLBOOSTER - Desenvolvimento de péptidos colagénicos de peixe em sistema integrado com obtenção de frações de elevado valor para alimentação humana, aquacultura e cosmética” with references: POCI-01-0247-FEDER-049636 and supported by Operational Program for Competitiveness and Internationalization (COMPETE2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). CIIMAR acknowledges funds provided by FCT – Foundation for Science and Technology (UIDB/04423/2020, UIDP/04423/2020). DR PhD grant was funded by FCT and SenseTest (PD/BDE/150524/2019) within the scope of SANFEED doctoral program.
The authors acknowledge Cristina M.R. Rocha and Bianca Marques from CEB, U. Minho, for providing the AH hydrolysate.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1193405/full#supplementary-material
Adhikari B. B., Chae M., Bressler D. C. (2018). Utilization of slaughterhouse waste in value-added applications: recent advances in the development of wood adhesives. Polymers (Basel) 10 (2), 176. doi: 10.3390/polym10020176
Aksnes A., Hope B., Høstmark Ø., Albrektsen S. (2006a). Inclusion of size fractionated fish hydrolysate in high plant protein diets for Atlantic cod, Gadus morhua. Aquaculture 261, 1102–1110. doi: 10.1016/j.aquaculture.2006.07.038
Aksnes A., Hope B., Jönsson E., Björnsson B. T., Albrektsen S. (2006b). Size-fractionated fish hydrolysate as feed ingredient for rainbow trout (Oncorhynchus mykiss) fed high plant protein diets. I: growth, growth regulation and feed utilization. Aquaculture 261, 305–317. doi: 10.1016/j.aquaculture.2006.07.025
Alami-Durante H., Médale F., Cluzeaud M., Kaushik S. J. (2010). Skeletal muscle growth dynamics and expression of related genes in white and red muscles of rainbow trout fed diets with graded levels of a mixture of plant protein sources as substitutes for fishmeal. Aquaculture 303, 50–58. doi: 10.1016/j.aquaculture.2010.03.012
Andersen L. L., Tufekovic G., Zebis M. K., Crameri R. M., Verlaan G., Kjær M., et al. (2005). The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metabolism 54, 151–156. doi: 10.1016/j.metabol.2004.07.012
Araújo-Rodrigues H., Coscueta E. R., Pereira M. F., Cunha S. A., Almeida A., Rosa A., et al. (2022). Membrane fractionation of Cynara cardunculus swine blood hydrolysate: ingredients of high nutritional and nutraceutical value. Food Res. Int. 158, 111549. doi: 10.1016/j.foodres.2022.111549
Bah C. S. F., Bekhit A. E. D. A., Carne A., Mcconnell M. A. (2013). Slaughterhouse blood: an emerging source of bioactive compounds. Compr. Rev. Food Sci. Food Saf. 12, 314–331. doi: 10.1111/1541-4337.12013
Bernardini R., Harnedy P., Bolton D., Kerry J., O’Neill E., Mullen A. M., et al. (2011). Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 124, 1296–1307. doi: 10.1016/j.foodchem.2010.07.004
Bodine S. C., Latres E., Baumhueter S., K-M Lai V., Nunez L., Clarke B. A., et al. (2001). Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708. doi: 10.1126/science.1065874
Bui H. T. D., Khosravi S., Fournier V., Herault M., Lee K. J. (2014). Growth performance, feed utilization, innate immunity, digestibility and disease resistance of juvenile red seabream (Pagrus major) fed diets supplemented with protein hydrolysates. Aquaculture 418–419, 11–16. doi: 10.1016/j.aquaculture.2013.09.046
Canada P., Engrola S., Mira S., Teodósio R., Fernandes J. M. O., Sousa V., et al. (2016). The supplementation of a microdiet with crystalline indispensable amino-acids affects muscle growth and the expression pattern of related genes in Senegalese sole (Solea senegalensis) larvae. Aquaculture 458, 158–169. doi: 10.1016/j.aquaculture.2016.03.010
Canada P., Engrola S., Mira S., Teodósio R., Yust M., del M., et al. (2018). Larval dietary protein complexity affects the regulation of muscle growth and the expression of DNA methyltransferases in Senegalese sole. Aquaculture 491, 28–38. doi: 10.1016/j.aquaculture.2018.02.044
Carter C. G., Houlihan D. E. (2001). Protein synthesis. Fish Physiol. 20, 31–75. doi: 10.1016/S1546-5098(01)20003-X
Carvalho A. P., Sá R., Oliva-Teles A., Bergot P. (2004). Solubility and peptide profile affect the utilization of dietary protein by common carp (Cyprinus carpio) during early larval stages. Aquaculture 234, 319–333. doi: 10.1016/j.aquaculture.2004.01.007
Chotikachinda R., Tantikitti C., Benjakul S., Rustad T., Kumarnsit E. (2013). Production of protein hydrolysates from skipjack tuna (Katsuwonus pelamis) viscera as feeding attractants for Asian seabass (Lates calcarifer). Aquac. Nutr. 19, 773–784. doi: 10.1111/anu.12024
Conde-Sieira M., Gesto M., Batista S., Linares F., Villanueva J. L. R., Míguez J. M., et al. (2018). Influence of vegetable diets on physiological and immune responses to thermal stress in Senegalese sole (Solea senegalensis). PLoS One 13 (3), e0194353. doi: 10.1371/journal.pone.0194353
Costa M., Costas B., Machado M., Teixeira C., Fernández-Boo S., Sá T., et al. (2020). Anchovy and giant squid hydrolysates can enhance growth and the immune response of European seabass (Dicentrarchus labrax) fed plant-protein-based diets. Aquaculture 523, 735182. doi: 10.1016/j.aquaculture.2020.735182
Daroit D. J., Brandelli A. (2021). In vivo bioactivities of food protein-derived peptides – a current review. Curr. Opin. Food Sci. 39, 120–129. doi: 10.1016/j.cofs.2021.01.002
European Commission (2021) Pigmeat statistics. Available at: https://ec.europa.eu/info/food-farming-fisheries/farming/facts-and-figures/markets/overviews/market-observatories/meat/pigmeat-statistics_en.
Fernández-Alacid L., Firmino J. P., Sanahuja I., Madrid C., Polo J., de Borba M. R., et al. (2021). Impact of dietary porcine blood by-products in meagre (Argyrosomus regius) physiology, evaluated by welfare biomarkers and the antibacterial properties of the skin mucus. Fish Shellfish Immunol. 118, 241–250. doi: 10.1016/j.fsi.2021.09.011
Ferreira M., Larsen B. K., Granby K., Cunha S. C., Monteiro C., Fernandes J. O., et al. (2020). Diets supplemented with Saccharina latissima influence the expression of genes related to lipid metabolism and oxidative stress modulating rainbow trout (Oncorhynchus mykiss) fillet composition. Food Chem. Toxicol. 140, 111332. doi: 10.1016/j.fct.2020.111332
Fontagné-Dicharry S., Véron V., Larroquet L., Godin S., Wischhusen P., Aguirre P., et al. (2020). Effect of selenium sources in plant-based diets on antioxidant status and oxidative stress-related parameters in rainbow trout juveniles under chronic stress exposure. Aquaculture 529, 735684. doi: 10.1016/j.aquaculture.2020.735684
Francis G., Makkar P. S., Becker K. (2001). Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199, 197–227. doi: 10.1016/S0044-8486(01)00526-9
García-Moreno P. J., Pérez-Gálvez R., Espejo-Carpio F. J., Ruiz-Quesada C., Pérez-Morilla A. I., Martínez-Agustín O., et al. (2017). Functional, bioactive and antigenicity properties of blue whiting protein hydrolysates: effect of enzymatic treatment and degree of hydrolysis. J. Sci. Food Agric. 97, 299–308. doi: 10.1002/jsfa.7731
Gisbert E., Ibarz A., Firmino J. P., Fernández-Alacid L., Salomón R., Vallejos-Vidal E., et al. (2021). Porcine protein hydrolysates (Pepteiva®) promote growth and enhance systemic immunity in gilthead sea bream (Sparus aurata). Animals 11, 2122. doi: 10.3390/ani11072122
Gisbert E., Skalli A., Fernández I., Kotzamanis Y., Zambonino-Infante J. L., Fabregat R. (2012). Protein hydrolysates from yeast and pig blood as alternative raw materials in microdiets for gilthead sea bream (Sparus aurata) larvae. Aquaculture 338–341, 96–104. doi: 10.1016/j.aquaculture.2012.01.007
Gómez-Requeni P., Mingarro M., Calduch-Giner J. A., Médale F., Martin S. A. M., Houlihan D. F., et al. (2004). Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata). Aquaculture 232, 493–510. doi: 10.1016/S0044-8486(03)00532-5
Hevrøy E. M., Espe M., Waagbø R., Sandnes K., Ruud M., Hemre G.-I., et al. (2005). Nutrient utilization in Atlantic salmon (Salmo salar l.) fed increased levels of fish protein hydrolysate during a period of fast growth. Aquac. Nutr. 11, 301–313. doi: 10.1111/j.1365-2095.2005.00357.x
Himaya S. W. A., Ngo D. H., Ryu B., Kim S. K. (2012). An active peptide purified from gastrointestinal enzyme hydrolysate of pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food. Chem. 132, 1872–1882. doi: 10.1016/j.foodchem.2011.12.020
Honda M., Masui F., Kanzawa N., Tsuchiya T., Toyo-oka T. (2008). Specific knockdown of m-calpain blocks myogenesis with cDNA deduced from the corresponding RNAi specific knockdown of m-calpain blocks myogenesis with cDNA deduced from the corresponding. Am. J. Physiol. Cell Physiol. 294, 957–965. doi: 10.1152/ajpcell.00505.2007.-Fusion
Houlihan D. E., Carter C. G., Mccarthy I. D. (1995). Protein synthesis in fish. Biochem. Mol. Biol. Fishes 4, 191–220. doi: 10.1016/S1873-0140(06)80011-1
Jin S. W., Lee G. H., Kim J. Y., Kim C. Y., Choo Y. M., Cho W., et al. (2022). Effects of porcine whole-blood protein hydrolysate on exercise function and skeletal muscle differentiation. Appl. Sci. (Switzerland) 12 (1), 17. doi: 10.3390/app12010017
Kanda A., Nakayama K., Fukasawa T., Koga J., Kanegae M., Kawanaka K., et al. (2013). Post-exercise whey protein hydrolysate supplementation induces a greater increase in muscle protein synthesis than its constituent amino acid content. Br. J. Nutr. 110, 981–987. doi: 10.1017/S0007114512006174
Khosravi S., Rahimnejad S., Herault M., Fournier V., Lee C. R., Dio Bui H. T., et al. (2015). Effects of protein hydrolysates supplementation in low fish meal diets on growth performance, innate immunity and disease resistance of red sea bream Pagrus major. Fish Shellfish Immunol. 45, 858–868. doi: 10.1016/j.fsi.2015.05.039
Kır M., Sunar M. C., Gök M. G. (2019). Acute ammonia toxicity and the interactive effects of ammonia and salinity on the standard metabolism of European sea bass (Dicentrarchus labrax). Aquaculture 511 (1–4), 734273. doi: 10.1016/j.aquaculture.2019.734273
Lajmi K., Gómez-Estaca J., Hammami M., Martínez-Alvarez O. (2019). Upgrading collagenous smooth hound by-products: effect of hydrolysis conditions, in vitro gastrointestinal digestion and encapsulation on bioactive properties. Food Biosci. 28, 99–108. doi: 10.1016/j.fbio.2019.01.014
Leduc A., Zatylny-Gaudin C., Robert M., Corre E., Corguille G., Castel H., et al. (2018). Dietary aquaculture by-product hydrolysates: impact on the transcriptomic response of the intestinal mucosa of European seabass (Dicentrarchus labrax) fed low fish meal diets. BMC Genom. 19, 396. doi: 10.1186/s12864-018-4780-0
López-Martínez M. I., Miguel M., Garcés-Rimón M. (2022). Protein and sport: alternative sources and strategies for bioactive and sustainable sports nutrition. Front. Nutr. 9. doi: 10.3389/fnut.2022.926043
Macqueen D. J., Meischke L., Manthri S., Anwar A., Solberg C., Johnston I. A. (2010). Characterisation of capn1, capn2-like, capn3 and capn11 genes in Atlantic halibut (Hippoglossus hippoglossus l.): transcriptional regulation across tissues and in skeletal muscle at distinct nutritional states. Gene 453, 45–58. doi: 10.1016/j.gene.2010.01.002
Manninen A. H. (2009). Protein hydrolysates in sports nutrition. Nutr. Metab. (Lond) 6, 38. doi: 10.1186/1743-7075-6-38
Martínez-Alvarez O., Chamorro S., Brenes A. (2015). Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: a review. Food Res. Int. 73, 204–212. doi: 10.1016/j.foodres.2015.04.005
Martínez-Llorens S., Baeza-Ariño R., Nogales-Mérida S., Jover-Cerdá M., Tomás-Vidal A. (2012). Carob seed germ meal as a partial substitute in gilthead sea bream (Sparus aurata) diets: amino acid retention, digestibility, gut and liver histology. Aquaculture 338–341, 124–133. doi: 10.1016/j.aquaculture.2012.01.029
Martins N., Magalhães R., Castro C., Couto A., Díaz-Rosales P., Oliva-Teles A., et al. (2019). Taurine modulates hepatic oxidative status and gut inflammatory markers of European seabass (Dicentrarchus labrax) fed plant feedstuffs-based diets. Amino Acids 51, 1307–1321. doi: 10.1007/s00726-019-02769-4
Nakayama K., Tagawa R., Saito Y., Sanbongi C. (2019). Effects of whey protein hydrolysate ingestion on post-exercise muscle protein synthesis compared with intact whey protein in rats. Nutr. Metab. (Lond) 16, 90. doi: 10.1186/s12986-019-0417-9
Naylor R. L., Hardy R. W., Buschmann A. H., Bush S. R., Cao L., Klinger D. H., et al. (2021). A 20-year retrospective review of global aquaculture. Nature 591, 551–563. doi: 10.1038/s41586-021-03308-6
Offret C., Fliss I., Bazinet L., Marette A., Beaulieu L. (2019). Identification of a novel antibacterial peptide from atlantic mackerel belonging to the gapdh-related antimicrobial family and its in vitro digestibility. Mar. Drugs 17 (7), 413. doi: 10.3390/md17070413
Opheim M., Šližyte R., Sterten H., Provan F., Larssen E., Kjos N. P. (2015). Hydrolysis of Atlantic salmon (Salmo salar) rest raw materials - effect of raw material and processing on composition, nutritional value, and potential bioactive peptides in the hydrolysates. Process Biochem. 50, 1247–1257. doi: 10.1016/j.procbio.2015.04.017
Pal G. K., Suresh P. v. (2016). Sustainable valorisation of seafood by-products: recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 37, 201–215. doi: 10.1016/j.ifset.2016.03.015
Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, 45e–445. doi: 10.1093/nar/29.9.e45
Poullain M.-G., Cezard J.-P., Roger L., Mendy F. (1989). Effect of whey proteins, their oligopeptide hydrolysates and free amino acid mixtures on growth and nitrogen retention in fed and starved rats. J. Parenter. Enteral Nutr. 13, 382–386. doi: 10.1177/0148607189013004382
Refstie S., Olli J. J., Standal H. (2004). Feed intake, growth, and protein utilisation by post-smolt Atlantic salmon (Salmo salar) in response to graded levels of fish protein hydrolysate in the diet. Aquaculture 239, 331–349. doi: 10.1016/j.aquaculture.2004.06.015
Rescan P. Y. (2001). Regulation and functions of myogenic regulatory factors in lower vertebrates. Comp. Biochem. Physiol. B 130, 112. doi: 10.1016/s1096-4959(01)00412-2
Resende D., Costas B., Sá T., Golfetto U., Machado M., Pereira M., et al. (2022). Innovative swine blood hydrolysates as promising ingredients for European seabass diets: impact on growth performance and resistance to Tenacibaculum maritimum infection. Aquaculture 561, 738657. doi: 10.1016/j.aquaculture.2022.738657
Rizzello C. G., Tagliazucchi D., Babini E., Sefora Rutella G., Taneyo Saa D. L., Gianotti A. (2016). Bioactive peptides from vegetable food matrices: research trends and novel biotechnologies for synthesis and recovery. J. Funct. Foods 27, 549–569. doi: 10.1016/j.jff.2016.09.023
Salem M., Nath J., Rexroad C. E., Killefer J., Yao J. (2005). Identification and molecular characterization of the rainbow trout calpains (Capn1 and Capn2): their expression in muscle wasting during starvation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 140, 63–71. doi: 10.1016/j.cbpc.2004.09.007
Shin J. E., Park S. J., Ahn S., Choung S.-Y. (2020). Soluble whey protein hydrolysate ameliorates muscle atrophy induced by immobilization via regulating the PI3K/Akt pathway in C57BL/6 mice. Nutrients 12, 3362. doi: 10.3390/nu12113362
Swanepoel J. C., Goosen N. J. (2018). Evaluation of fish protein hydrolysates in juvenile African catfish (Clarias gariepinus) diets. Aquaculture 496, 262–269. doi: 10.1016/j.aquaculture.2018.06.084
Toldrà M., Lynch S. A., Couture R., Álvarez C. (2019). “Blood proteins as functional ingredients,” in Sustainable Meat Production and Processing. Ed. Galanakis C. M. (London: Academia Press), 85–101.
Torrecillas S., Mompel D., Caballero M. J., Montero D., Merrifield D., Rodiles A., et al. (2017). Effect of fishmeal and fish oil replacement by vegetable meals and oils on gut health of European sea bass (Dicentrarchus labrax). Aquaculture 468, 386–398. doi: 10.1016/j.aquaculture.2016.11.005
Voss G. B., Sousa V., Rema P., Pintado M. E., Valente L. M. P. (2021). Processed by-products from soy beverage (Okara) as sustainable ingredients for nile tilapia (O. niloticus) juveniles: effects on nutrient utilization and muscle quality. Animals 11, 1–17. doi: 10.3390/ani11030590
Wei Y., Li B., Xu H., Liang M. (2020). Fish protein hydrolysate in diets of turbot affects muscle fiber morphometry, and the expression of muscle growth-related genes. Aquac. Nutr. 26, 1780–1791. doi: 10.1111/anu.13129
Wong S., Waldrop T., Summerfelt S., Davidson J., Barrows F., Kenney P. B., et al. (2013). Aquacultured rainbow trout (Oncorhynchus mykiss) possess a Large core intestinal microbiota that is resistant to variation in diet and rearing density. Appl. Environ. Microbiol. 79, 4974–4984. doi: 10.1128/AEM.00924-13
Xu H., Mu Y., Liang M., Zheng K., Wei Y. (2017). Application of different types of protein hydrolysate in high plant protein diets for juvenile turbot (Scophthalmus maximus). Aquac. Res. 48, 2945–2953. doi: 10.1111/are.13127
Zheng K., Liang M., Yao H., Wang J., Chang Q. (2012). Effect of dietary fish protein hydrolysate on growth, feed utilization and IGF-I levels of Japanese flounder (Paralichthys olivaceus). Aquac. Nutr. 18, 297–303. doi: 10.1111/j.1365-2095.2011.00896.x
Keywords: zero waste, peptide bioactivity, fiber proliferation, muscle cellularity, fish robustness
Citation: Velasco C, Resende D, Oliveira B, Canada P, Pereira M, Pereira C, Pintado M and Valente LMP (2023) Dietary inclusion of blood hydrolysates affects muscle growth in European seabass (Dicentrarchus labrax). Front. Mar. Sci. 10:1193405. doi: 10.3389/fmars.2023.1193405
Received: 24 March 2023; Accepted: 27 April 2023;
Published: 30 May 2023.
Edited by:
Mohsen Abdel-Tawwab, Agricultural Research Center, EgyptReviewed by:
Fabián Canosa, CONICET Institute of Biotechnological Research (IIB-INTECH), ArgentinaCopyright © 2023 Velasco, Resende, Oliveira, Canada, Pereira, Pereira, Pintado and Valente. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luisa M. P. Valente, bHZhbGVudGVAaWNiYXMudXAucHQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.