- 1Department of Parasitology, Parasite Research Center and School of Medicine, Chungbuk National University, Cheongju, Republic of Korea
- 2Laboratory of Parasitology, College of Veterinary Medicine, Seoul National University, Seoul, Republic of Korea
- 3Cetacean Research Institute, Ulsan, Republic of Korea
- 4Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, Sofia, Bulgaria
The East Asian finless porpoise, Neophocaena asiaeorientalis sunameri, ranks among the most endangered species with rapidly decreasing population in the Northwest Pacific. Trematode parasites of the genus Nasitrema that inhabit the air sinuses, inner ear, and the central nervous system of cetaceans frequently cause equilibrium dysfunction, disorientation, interference with echolocation, incoordination, and nervous system degeneration. Due to their specific location and associated pathologies, they have been recognized as one of the causes of cetacean strandings. Stranding data provides crucial information on the species’ biology, population health, and on the status of entire marine ecosystem. However, published data on parasite-induced standings that include information on the causative parasite pathogens are scarce. As part of a wider survey on the causes of East Asian finless porpoise strandings along the west coast of Korea, herein, we provide novel morphological and molecular data on two sympatric species of Nasitrema, namely, Nasitrema spathulatum and Nasitrema sunameri based on newly collected specimens from a stranded alive East Asian finless porpoise at the West coast of Korea. Our study adds a new distribution record for important parasite pathogens in cetaceans and provides the first molecular data for the parasite species recovered, which enabled us to re-evaluate the species relationships within the family Brachycladiidae, a group of important parasite pathogens of marine mammals.
1 Introduction
The coastal waters of the Korean Peninsula are one of the richest and most diverse areas for cetaceans in the Northwest Pacific. With a total of 35 different species, 29 odontocetes and 8 mysticets having been reported (Sohn et al., 2012; Kim et al., 2000), the area holds nearly half of the known cetacean species worldwide and therefore is of an extremely high natural value in terms of diversity of marine megafauna. This is largely due to its specific location and oceanographic characteristics, having great depths and lack of continental shelf in the east and shallow waters in the west. Of the 37 cetacean species occurring in the area, the East Asian finless porpoise, Neophocaena asiaeorinetalis sunameri Pilleri et Gihr, is the most abundant species with the largest population occurring in Korean waters. Under the current taxonomic treatment, the East Asian finless porpoise is one of the two recognized subspecies of N. asiaeorientalis (Pilleru & Gihr, 1972) distributed throughout the coastal waters of Yellow and South China Seas and the southern part of the Japanese archipelago in the East Sea. In contrast, its congener Ne. a. asiaeorientalis (Pilleri & Gihr, 1972), widely known as the Yangtze finless porpoise, exclusively inhabits freshwater habitats in the lower and middle course of the Yangtze River and its adjacent lakes (Rudolph & Smeenk, 2009).
The East Asian finless porpoise is highly vulnerable to anthropogenic threats. The anthropogenic pressure on the coastal ecosystems throughout its distributional range is immense (Park et al., 2015), being surrounded by the most rapidly industrialized countries with immense population and occurring in the most exploited seas in the world ocean (Yoo et al., 2019). Despite being the most abundant cetacean species in Korean waters, the population of the East Asian finless porpoise is on a decline as a result of artisanal fisheries, frequent bycatch, and habitat disruption (Baker et al., 2006; Kim et al., 2013). Its population has sharply decreased of approximately 70% for a period of just 7 years (2004–2011; Park et al., 2015). This has prompted a concern about the status of the species and led to its declaration as endangered (IUCN Red List of Threatened Species) in 2017 under the criteria A2bcde+3bcde+4bcde that has made the East Asian finless porpoise a species of high priority for conservation.
Whereas a great deal has been learned about the biology and ecology of the East Asian finless porpoise, no evaluation of its parasite fauna has been undertaken so far. Marine mammal standing data provide vital clues on species’ life histories, population health, including the incidence of disease and cause of death, and on the status of the marine ecosystems (Arbelo et al., 2013; Prado et al., 2016; ten Doeschate et al., 2018; Coombs et al., 2019). Although standings are caused by multiple triggers including biological, physical, and social processes (Williams et al., 2011; Peltier et al., 2013), parasite-induced strandings are frequently encountered and have been reported worldwide (Dailey and Walker, 1978; Morimitsu et al., 1986; Morimitsu et al., 1987; Lewis and Berry, 1988; O’Shea et al., 1991; Morimitsu et al., 1992; Degollada et al., 2002; Arbelo et al., 2013; Díaz-Delgado et al., 2018). However, difficulties in obtaining good parasitological material from accidentally stranded animals frequently refer to their quality as determined by the host conditions. Trematodes of the family Brachycladiidae Odhner, 1905 are known as specific parasites of marine mammals, occurring in diverse microhabitats such as the hepatic and pancreatic ducts, the intestine, lungs, and head sinuses (Gibson, 2005). High pathogenicity has been reported for those occurring in the tissues and especially for the members of Nasitrema Ozaki, 1935 that inhabit the air sinuses, respiratory tract, and the central nervous system of odontocete cetaceans (Gibson et al., 1998). Due to their specific location and resulted pathologies, including equilibrium dysfunction, disorientation, interference with echolocation, incoordination, and nervous system degeneration, species of Nasitrema have been frequently associated as causatives of single and mass strandings of odontocetes (Dailey and Walker, 1978; Morimitsu et al., 1986; Morimitsu et al., 1987; Lewis and Berry, 1988; O’Shea et al., 1991; Morimitsu et al., 1992; Degollada et al., 2002; Arbelo et al., 2013; Díaz-Delgado et al., 2018).
Although the cetacean populations, including those of the East Asian finless porpoise, have been extensively monitored and studied in Korean waters (Park et al., 2015; Yim & Lee, 2015; Lee et al., 2018; Oh et al., 2018; Kim et al., 2019; Jeong et al., 2020) only a handful of parasitological surveys have been conducted. So far, a single study has reported a species of Nasitrema, i.e., Nasitrema attenuatum Neiland, Rice & Holden, 1970, in the common dolphin (Delphinus d. delphis L. 1758; Lim et al., 2016) from the east coast of Korea. To date, virtually, no data exist on the parasite fauna of the East Asian finless porpoise in Korean waters. With the aim of bridging this gap, herein, we present a case study detailing on the parasite pathogens of Nasitrema in the East Asian finless porpoise that was initiated during a major survey on the causes of its strandings along the west coast of Korea. We report Nasitrema spp. infection in a stranded Ne. a. sunameri and provide novel morphological and molecular data for the type species Na. spathulatum and its sympatric congener Na. sunameri based on the newly collected specimens. Phylogenetic analyses based on partial mtND3 and 28S rDNA sequences were carried out and led to re-evaluation of the species relationships within the family Brachycladiidae, comprising a group of important parasite pathogens of marine mammals.
2 Materials and methods
A single specimen of Ne. a. sunameri was found stranded alive within a small pool formed at low tide near Woong Island, Daesan-eup, off Seosan-city (36.9201N, 126.3730E) on 25 April 2017 (Figure 1). Despite the attempts to be rescued by the local marine police, the animal died shortly after the transportation to the veterinary care center at the animal hospital of Seoul Zoo. The necropsy examination was performed on the following day. The specimen was a female, 148 cm long. The cranial sinuses and auditory tubes were reached by removing the auditory ossicles, and the sinuses were rinsed with running water at high pressure to expel the parasites. Parasite specimens recovered were rinsed and preserved in two fixatives for subsequent studies, i.e., in 10% neutral buffered formalin for morphological examinations and in 70% ethanol for molecular studies.

Figure 1 East Asian finless porpoise, Neophocaena asiaeorientalis sunameri Pilleri et Gihr (A) found alive on the west coast of Korea in April 2017; (B) illustrative representation of the head anatomy as schematic sagittal reconstruction showing the nasal structures and microhabitat of Nasitrema spp. in the air spaces of the upper respiratory tract. Color codes: blue, air spaces of the upper respiratory tract; gray, digestive system; light gray, cartilages and bones of the skull; yellow, fat bodies (melon and bursae cantantes).
Specimens preserved in 10% neutral buffered formalin were stained with alum-carmine solution, cleared with carbol-xylene and xylene, and prepared as permanent mounts in Canada balsam. Parasites were examined using light microscope BX53, Olympus Co. (Tokyo, Japan) and drawings were made with the aid of a drawing tube attached to the microscope. Measurements were taken from the drawings made and expressed in millimeters unless otherwise stated.
Genomic DNA was extracted from entire specimens using a Gentra Puregene Cell and Tissue Kit (Qiagen Co., Hilden, Germany) according to the manufacturer’s protocol for DNA purification from fixed tissue. Partial fragments of the mtND3 and LSU (D1–D3 regions) rRNA genes were amplified using primer combinations: ND3F (5′-GCT TAA TTK KTA AAG CYT TGR ATT CTT ACT-3′; Fernández et al., 2000) + ND3-4 (5′-CTA GTC CCA CTC AAC GTA ACC TT-3′; Fernández et al., 1998), and CLF28A (5′-AGT AAC GGC GAG TGA ACA GG-3′) + CLF28B (5′-GCA CTG GTC CGA AGA CTA TG-3′; Nakagun et al., 2018), respectively. The amplification reactions were performed in a total volume of 50 μl comprising 1 (mtND3) or 5 (28S rDNA) μl gDNA, 0.4 mM dNTP, 15 μM of each primer, 0.2 µg/µl of BSA, 2.5 mM MgCl2, and 0.5 μl of Taq DNA polymerase. Thermocycling conditions were as follows: 95°C for 5 min, 35 cycles of 95°C for 30 s, 50°C for 30 s (mtND3) or 60°C for 30 s (LSU), 72°C for 50 s, and a final extension at 72°C for 7 min. PCR products were purified and sequenced by Sanger cycle sequencing at the Cosmogenetech Co. (Seoul, Korea) using the same primers as for the amplification reactions.
Consensus sequences were assembled using Geneious v. 2019.1.3. (Kearse et al., 2012) and visually inspected. Single gene alignments were built and aligned together with published sequences for the Brachycladiidae (see Table 1 for the GenBank accession numbers for the taxa included in the analyses) using MAFFT v7 (Kuraku et al., 2013; Katoh et al., 2019; online execution). The mtND3 dataset was aligned with reference to the amino acid translation, using the echinoderm and flatworm mitochondrial code (Telford et al., 2000). Tormopsolus orientalis Yamaguti, 1934 was used as an outgroup in both datasets analyzed (KT180219, and DQ248217, respectively), as informed by previous phylogenies (Fernández et al., 1998b; Bray et al., 2005; Fraija-Fernández et al., 2015). For the reconstruction of phylogenetic relationships, the “best-fitting” models of sequence evolution were selected using jModeltest 2.1.10 (Darriba et al., 2012) under Bayesian Information Criterion.
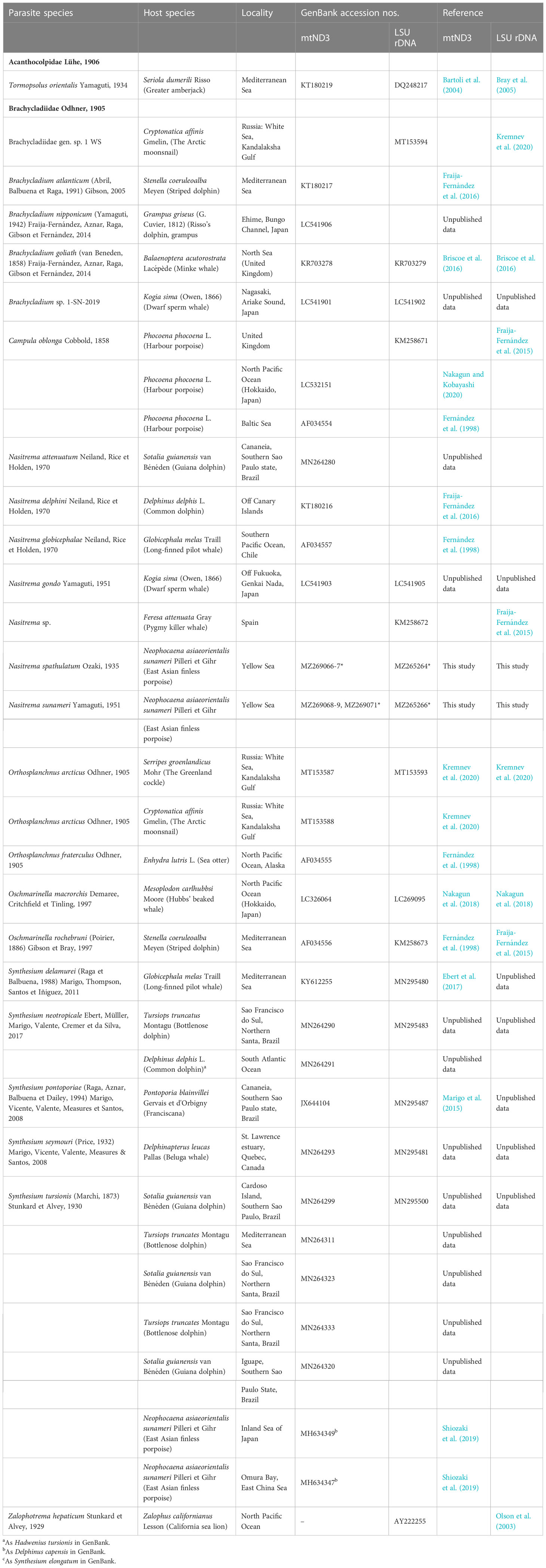
Table 1 List of parasite species included in the phylogenetic analyses with details of provenance and GenBank accession numbers.
Phylogenetic relationships of the individual gene datasets were assessed under Bayesian inference in MrByaes 3.2.6 (Ronquist et al., 2012) executed on the CIPRES Science Gateway v.3.3 (Miller et al., 2011) as two independent runs for 10,000,000 generations and sampled every 1,000th generation. The “burn-in” was set for the first one-fourth of the sampled trees. Parameter convergence and run stationarity were assessed in Tracer v.1.6 (Rambaut et al., 2009). FigTree v.1.4.4. (Rambaut and Drummond, 2012) was used for visualization of the final trees.
3 Results
The single-stranded alive East Asian finless porpoise was infected with 22 specimens of Na. spathulatum Ozaki, 1935 and eight of Na. sunameri Yamaguti, 1951. All specimens recovered from the stranded alive animal were mature and in a good condition for morphological analyses, on which all descriptions, morphometrics, and molecular analyses are done in the present study.
3.1 Species description
Family Brachycladiidae Odhner, 1905
Genus Nasitrema Ozaki, 1935
Nasitrema spathulatum Ozaki, 1935
Locality: Woong Island, Daesan-eup, off Seosan-city (36.9201N, 126.3730E), Korea
Location of infection: cranial sinuses and auditory tubes
Voucher material: eight specimens deposited in Meguro Parasitological Museum, Japan (MPM Coll. No. 21955) and two specimens deposited in the Korean National Institute of Biological Resources (NIBRIV0000895405-406).
Representative DNA sequences: mtND3: MZ269066-7; LSU : MZ265263-4
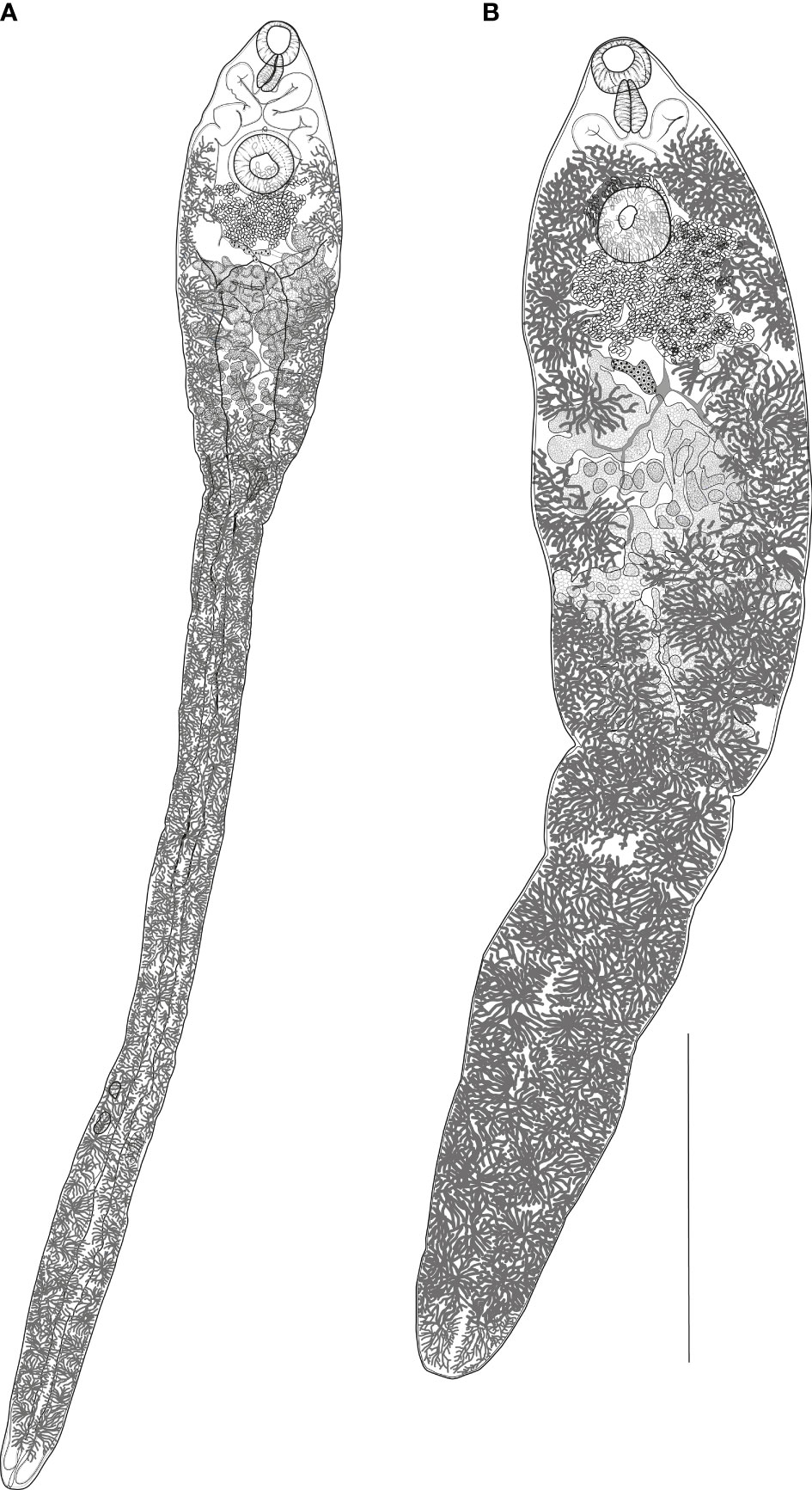
Figure 2 (A) Nasitrema spathulatum ex Neophocaena asiaeorientalis sunameri from the Yellow Sea off Korea, ventral view. (B) Nasitrema sunameri ex Neophocaena asiaeorientalis sunameri from the Yellow Sea off Korea, ventral view. Scale bar: A, 500 µm.
Description (based on nine fully mature specimens). Morphometrical data are presented in Table 2. Body elongate, spatulate, slenderer posteriorly, terminates bluntly. Maximum width at level of ovary. Narrow posterior part filled with intestinal caeca and dendritic vitellaria. Tegument thick, spinous.
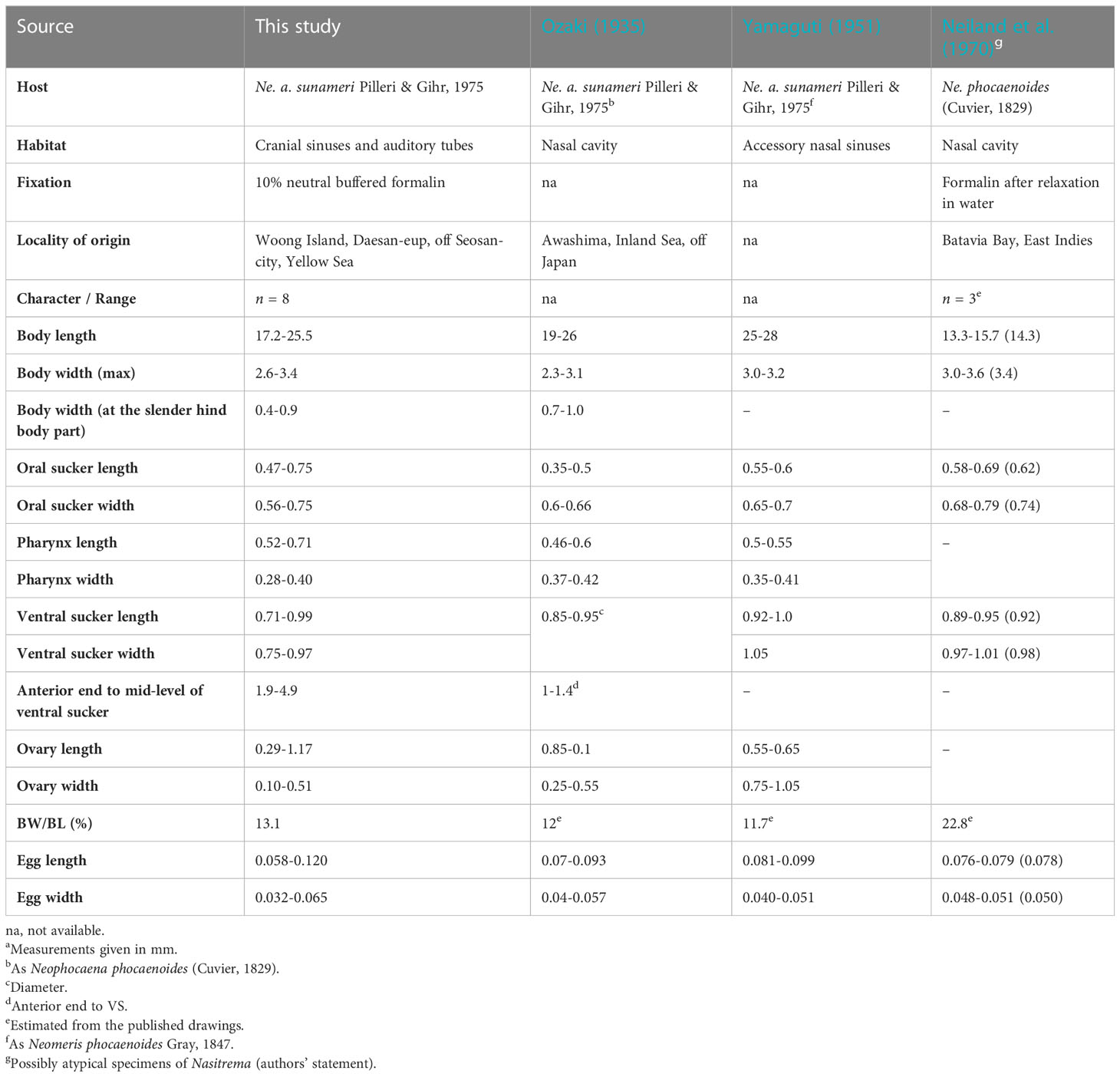
Table 2 Comparative morphometric data for Nasitrema spathulatum Ozaki, 1935a from Neophocena spp.
Oral sucker ventro-subterminal, oval. Prepharynx indistinct. Pharynx muscular, elongate-oval. Esophagus indistinct. Ceca simple, bifurcates just posterior to pharynx, each cecum extends anteriorly to level of mid-pharynx and descends posteriorly, end blindly to posterior extremity. Ventral sucker spherical, slightly bigger than oral sucker, at about anterior 1/11th–1/16th of body length.
Testes two, large, tandem, deeply lobed, appearing follicular at posterior half of expanded body part, overlapping caeca laterally. Cirrus sac absent. Seminal vesicle saccular, extending dorso-laterally to ventral sucker. Pars prostatica tubular. Genital atrium small. Genital pore median, opens anterior to ventral sucker.
Ovary pretesticular, submedian, with irregular lobes. Laurer’s canal and Mehlis’ gland not observed. Uterus wide, convoluted, confined to intercecal field, interjects between seminal vesicle and ovary. Metraterm short, dorsal to pars prostatica. Eggs oval, thick, truncated anteriorly with pointed knob at posterior end; operculum flat; yellow-brown in color; equilateral and transversely triangular. Vitellarium follicular, follicles numerous in tassel-like groups, in two lateral fields distributed from posterior body extremity to level of genital pore. Excretory vesicle tubular, Y-shaped, bifurcates at level of oötype. Excretory pore terminal, opens at median line.
Remarks
Nasitrema spathulatum, the type species of the genus, was described from the East Asian finless porpoise (Ne. a. sunameri Pilleru & Gihr, 1975) from off Awashima island, Japan nearly nine decades ago (Ozaki, 1935). Since then, only two records detailing on the parasite morphology have been published (Yamaguti, 1951; Neiland et al., 1970) so far. All records were originally reported from the Indo-Pacific finless porpoise [Ne. phocaenoides (G. Cuvier, 1829)]. However, under the most recent taxonomic treatment of the finless porpoises, only the record of Neiland et al. (1970) from the Jakarta Bay represents a case from this host species.
Based on the morphological and morphometric observations, the specimens recovered from off Korea agreed well with the diagnosis of Na. spathulatum (Ozaki, 1935). The metrical data for most of the morphological characters of the examined specimens here generally fall within the ranges given by Ozaki (1935) and Yamaguti (1951). Unfortunately, the numbers of the Na. spathulatum specimens observed are not reported in either of the publications, and the individual variation in the morphology is unclear. In contrast, metrical data reported by Neiland et al. (1970) from the Indo-Pacific finless porpoise (Ne. phocaenoides) fall above the ranges for most of the morphometrical characters. Despite the low number of specimens used by these authors (n = 3), the specimens from the Bay of Jakarta (formerly known as the Batavia Bay of the Dutch East Indies) have twice greater body width to body length ratio than the specimens recorded from the East Asian finless porpoise (22.8 vs. 11.7–13.1; Table 2). Although these differences were noted by the authors, the specimens were assumed to represent atypical forms resulting from the fixation procedure applied (i.e., the specimens appeared somehow contracted and obviously not relaxed prior to fixation). However, taking into account the distinct host origin, the specimens of Neiland et al. (1970) may represent distinct species of Nasitrema. Therefore, a novel material from the same host species and locality is needed to resolve the specific status of the material from Jakarta.
Our study provides a new geographical record for Na. spathulatum, extends its known range of metrical features, and provides the first molecular data for the type species of the genus.
Nasitrema sunameri Yamaguti, 1951
Locality: Woong Island, Daesan-eup, off Seosan-city (36.9201N, 126.3730E), Korea
Location of infection: cranial sinuses and auditory tubes
Voucher material: Two specimens deposited in the Meguro Parasitological Museum, Japan (MPM Coll. No. 21956) and two specimens deposited in the Korean National Institute of Biological Resources (NIBRIV0000895399-400).
Representative DNA sequences: mtND3: MZ269068-9, MZ269071; LSU : MZ265265-6
Description (based on two fully mature specimens). Morphometric data are provided in Table 3. Body slender, lanceolate, terminates bluntly with slightly notched end. Maximum width near anterior testis. Tegument densely spinose.
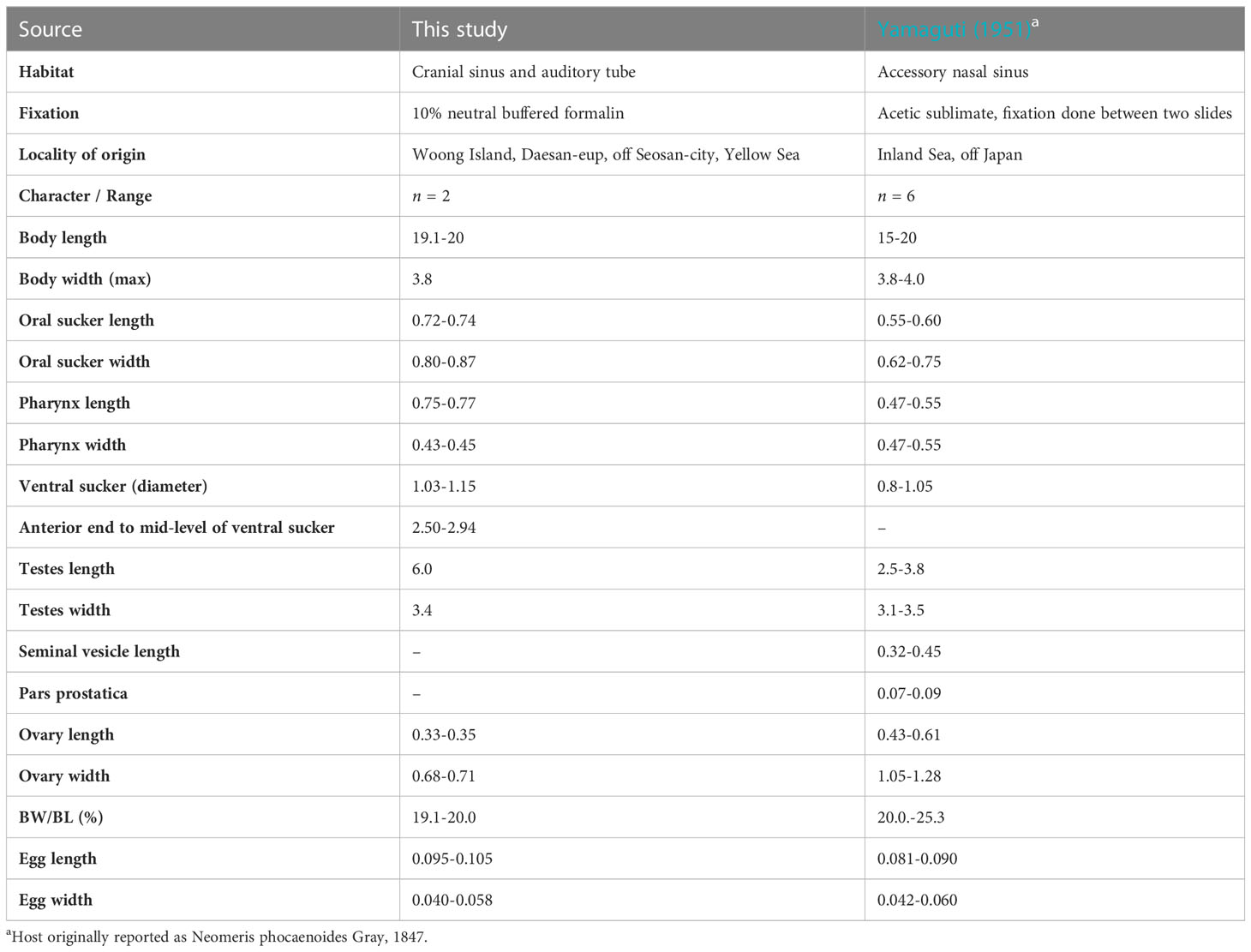
Table 3 Comparative morphometric data for Nasitrema sunameri Yamaguti, 1951 from Neophocaena a. sunameri.
Oral sucker ventral, subterminal, oval. Prepharynx indistinct. Pharynx large, pyriform. Esophagus very short. Ceca bifurcates just posterior to pharynx, sinuous, extends anteriorly to pharynx and turn posteriorly to reach close to posterior extremity. Ventral sucker spherical, at approximately anterior one-seventh of total body length.
Testes two, parallel, located close to each other, consisting of irregular windings. Seminal vesicle sigmoid, extends posteriorly beyond ventral sucker. Pars prostatica cylindrical, surrounded by compact mass of gland cells. Genital pore median, just anterior to ventral sucker.
Ovary slightly dextral, just anterior to testes, forming few blunt lobes. Laurer’s canal and Mehlis’ gland not observed. Uterus convoluted, located between ovary and seminal vesicle. Metraterm short, muscular. Eggs oval or elliptical with knob-like thickening at posterior pole. Vitellarium follicular, arranged in two lateral fields extending anteriorly to ventral sucker to posterior body end; follicular fields comprised dorsal and ventral strings encompassing the uterus, ovary, and testes. Vitelline reservoir dorsal to the ovary.
Excretory vesicle tubular, Y-shaped, sinuous, extends at about level of posterior margin of ovary. Excretory pore terminal.
Remarks
The present material agrees well with the diagnosis of Na. sunameri (Yamaguti, 1951). The most distinct characters comprise the preacetabular distribution of vitellaria, and gradually tapered, not attenuated hindbody. Nasitrema sunameri differs from its sympatric congener Na. spathulatum mainly in respect to the shape and size of the body. The differences consist of a lanceolate body shape (in the case of Na. sunameri) vs. spathulate-shaped body with slenderer and slightly longer hind body (Na. spathulatum). The distribution of the vitellaria, in which the anterior extend surpasses the acetabulum, is a feature characteristic for both species and clearly distinguishes them from all other eight species in the genus. Metrical data obtained in the present study fall somewhat at the upper range or above it when comparing with the only published previous description of the species by Yamaguti (1951).
After the original description on Na. sunameri, seven decades ago, this is the third report of the species and a second study detailing on its morphology. Our study provides new geographical record and the first molecular data for this species.
3.2 Phylogenetic analyses
Sequence data for the novel isolates from off Korea have been submitted to GenBank under the following accession numbers: for mtND3, MZ269066–MZ269069, and MZ269071, and for LSU, MZ265263–MZ265266. No reference sequences for the two species examined were available in the GenBank database. Two alignments were analyzed: (i) the partial mtND3 dataset comprised 331 bp and included representative sequences for 27 taxa of the family Brachycladiidae and (ii) a dataset for the partial LSU rDNA gene comprising 967 bp and included sequence data for 17 representative taxa of the family. Sequence comparisons were performed on aligned and trimmed dataset for both markers including all available sequences for representatives of the genus. Sequence comparison in the LSU rDNA set revealed a single bp difference within the two replicates of both species and a difference of 2–3 bp between the isolates of Na. spathulatum and Na. sunameri. Both species differed by 3–4 bp from an otherwise unidentified isolate of Nasitrema from the Pygmy killer whale (Feresa attenuata) in the Mediterranean. Both intra- and interspecific variations in the two datasets analyzed were low. The intraspecific sequence divergence in the mtND3 set for Na. spathulatum ranged from 0.6% to 1.2% (2–4 bp), and in the case of Na. sunameri, it was 0.3% (1 bp). The interspecific divergence ranged from 5.4% to 6.9% (18–23 bp). The overall interspecific divergence within Nasitrema in the mtND3 gene dataset ranged from 5.7% to 20.8% (19–65 bp).
The tree yielded from the Bayesian analysis of the mtND3 set is presented in Figure 3A. Species of Nasitrema were resolved as strongly supported monophyletic group earlier diverging to the remaining representatives of the Brachycladiidae. The novel sequences for N. spathulatum and N. sunameri were recovered as closely related sister species. All remaining taxa of the Brachycladiidae, representing the genera Brachycladium, Oschmarinella, and Synthesium clustered in a multi-taxon clade, although with lack of significant statistical support. Synthesium, Orthosplanchnus, and Oschmarinella were recovered as paraphyletic with O. articus nested in Synthesium, Campula oblonga nested within Oschmarinella, and O. fraterculus clustered as a sister to Oschmarinella rochebruni.
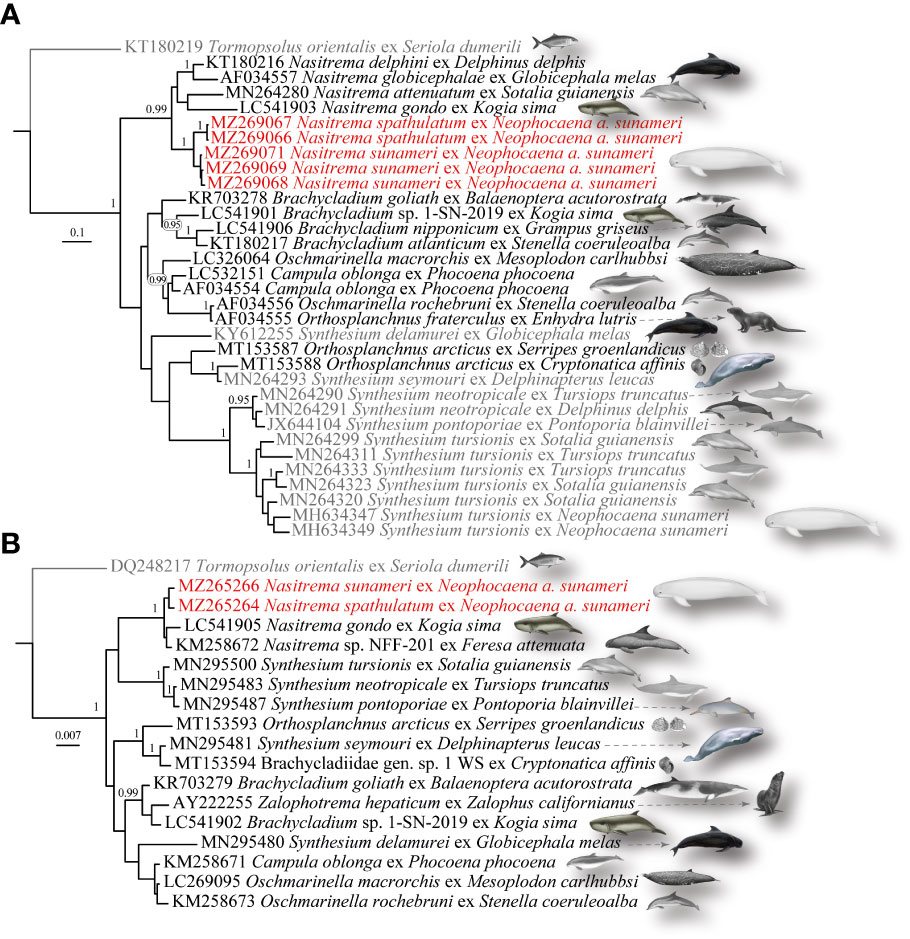
Figure 3 Bayesian inference analyses of the (A) mtND3 and (B) LSU datasets constructed under HKY+I and HKY+I+G models of evolution, respectively. The newly generated sequences are color indicated in red. Posterior probability values (≥0.95) are given on the branches. The branch length scale bars indicate number of substitutions per site. Host origin of the used sequences is provided as a silhouette next to the sequence name.
The relationships within the Brachycladiidae estimated by the Bayesian inference (BI) approach based on the LSU dataset comprised eight taxa of the family and are presented in Figure 3B. The monophyly of Nasitrema, the closer sister species relationships between N. spathulatum and Na. sunameri, and the paraphyly of Synthesium were further confirmed. However, Nasitrema was not resolved as the earliest diverging taxon but as a sister to a clade comprised of species of Synthesium, namely, S. tursionis, S. neotropicale, and S. pontoporiae. The remaining brachycladiids clustered in a major multitaxon clade, albeit with lack of significant support for most of the nodes. Brachycladium was recovered as paraphyletic with Zalophotrema nested within it. Synthesium seymori clustered in a strongly supported subclade with Orthosplanchnus articus, and an otherwise unidentified brachycladiid from the gastropod Cryptonatica affinis, while Synthesium delamueri was recovered as a closely related to Campula oblonga, and Oshmarinela spp., although with low nodal support.
4 Discussion
Herein, we provide the first report of Nasitrema infection in a stranded East Asian finless porpoise, Ne. a. sunameri, in the Northwest Pacific based on novel molecular data along detailed morphological descriptions. Given that cetacean bycatch is one of the main causes of mortality in small odontocetes (Spencer et al., 2000), our results represent an important contribution to the stranding epidemiology, especially in respect to the limited number of studies available. Stranding data are widely acknowledged as inexpensive to collect and valuable source indicators of mortality at sea (Jefferson and Curry, 1994; Tregenza et al., 1997; Evans and Hammond, 2004; Hoyt, 2005).
Parasites inhabiting cetacean cranial sinuses have been associated as causatives of mass standings worldwide (Parker et al., 1977; Dailey and Walker, 1978; Cowan et al., 1986; Morimitsu et al., 1986; Morimitsu et al., 1987; Lewis and Berry, 1988; O’Shea et al., 1991; Morimitsu et al., 1992). Aberrant migrations from the air sinus–inner ear complex to the subdural space has been reported in previous studies of single and mass cetacean strandings (Cowan et al., 1986; O’Shea et al., 1991). Trematodes of the genus Nasitrema have been recognized as culprits in various odontocete cetacean strandings causing neuropathy in the eight cranial nerve (Morimitsu et al., 1986; Morimitsu et al., 1987; Morimitsu et al., 1992), brain lesions (O’Shea et al., 1991; Arbelo et al., 2013; Degollada et al., 2002), and cerebral necrosis (Dailey and Walker, 1978; Lewis and Berry, 1988). Pathologies caused are recognized to be determined by the intensity of infection ranging from moderate sinusitis to severe encephalitis (Degollada et al., 2002) that can imply cognitive disruption and equilibrium dysfunction and thus induce standing (Dailey and Walker, 1978; Morimitsu et al., 1992; Degollada et al., 2002). Neuropathy in the central nervous system caused by the trematodes and released eggs leads to equilibrium dysfunction, disorientation, interference with echolocation, incoordination, and nervous system degeneration (Arbelo et al., 2013). Species of Nasitrema have been reported to occur at high numbers and prevalence exclusively in odontocetes of the families Phocoenidae Gray and Delphinidae Gray (Fernández et al., 1998). To date, confirmed cases have been reported from different marine areas, the southern Californian Pacific (Cowan et al., 1986), Mexico (O’Shea et al., 1991), the Caribbean (Phillips & Suepaul, 2017), the Northeast Atlantic (Degollada et al., 2002), and the Northwest Pacific off Japan (Morimitsu et al., 1986; Morimitsu et al., 1987; Morimitsu et al., 1992). Further studies including pathological examinations that determine the exact cause of cetacean deaths are largely needed.
Despite the great diversity of cetaceans in the Northwest Pacific, scarce data on their helminth fauna exist. To date, a total of 13 species of helminths have been reported from the East Asian finless porpoise (Shiozaki and Amano, 2017 and references therein). Unfortunately, the records on its helminth diversity come from sporadic studies and restricted geographical areas. Despite being the most abundant cetacean species in Korean waters, no records on its helminth fauna existed prior to our study.
Brachycladiid trematodes are specific parasites of marine mammals (Dailey & Brownell, 1972; Fraija-Fernández et al., 2016; Shiozaki & Amano, 2017) that are acquired through the local food web. Colonization has been recognized as the key driver of brachycladiid evolution (Fraija-Fernández et al., 2016). Despite that, little is known on their life cycles and intermediate host associations (Delyamure, 1955; Adams et al., 1998; Gibson et al., 1998). A recent study matching sequence data from larval and adult isolates (Kremnev et al., 2020) revealed that gastropods of the family Naticidae Guilding, 1834 serve as first intermediate hosts, while bivalves, cephalopods, and fishes served as second intermediate hosts. This is in line with the feeding ecology of the East Asian finless porpoises known as an opportunistic feeder preying on numerous species of fish and cephalopods (Jefferson & Hung, 2004; Shirakihara et al., 2008; Park et al., 2011). Parasite diversity of cetaceans is positively correlated with the diversity of their hosts’ prey (Aznar et al., 1994). However, the intermediate hosts involved in the life cycle of Nasitrema spp. are not known yet. Species of Neophocaena have been reported as the only definitive hosts of the two species, Na. spathulatum and Na. sunameri, reported herein. This calls for further studies with more holistic approach on revealing the parasite fauna in the North Pacific cetaceans and assessing their impact on systems’ function and energetics.
Integration of host–parasite associations and phylogenetic information in disease ecology is of prime importance because closely related species tend to have more similar niches through phylogenetic conservatism (Filion et al., 2022). Members of Brachycladiidae have been subject to numerous studies; however, the taxonomy of the group remains problematic (Fernández et al., 1994; Gibson, 2005). Ozaki (1935) erected the subfamily Nasitrematinae Ozaki, 1935 in the family Fasciolidae Railliet, 1895 and proposed the genus Nasitrema (Ozaki, 1935). Subsequently, Yamaguti (1958) erected Nasitrematidae based on the absence of a cirrus pouch and different characteristics of the eggs, i.e., thick-shelled, truncated at the opercular end, and triangular in cross-section. Nevertheless, Fernández et al. (1998) suggested to revoke the family status of Nasitrematidae based on molecular genetic analysis and proposed that the type genus Nasitrema belongs to the family Campulidae Odhner, 1926. In the most recent revision of the group, the family name was changed to Brachycladiidae following the principle of coordination (ICZN Article 36, Gibson, 2005), and the current taxonomic status of the genus Nasitrema has been retained.
Of the 10 species currently recognized within Nasitrema, six, namely, Na. spathulatum, Na. gondo Yamaguti, 1951, Na. sunameri, Nasitrema dalli Yamaguti, 1951, Na. attenuatum, and Nasitrema lagenorhynchus Kikuchi, Okuyama & Nakajima, 1987, have been reported from the Pacific (Ozaki, 1935; Yamaguti, 1951; Neiland et al., 1970; Kikuchi et al., 1987). Of these, only a single species, Na. attenuatum, found in the common dolphin (Delphinus delphis), has been reported in Korean waters so far (Lim et al., 2016). Nasitrema spathulatum, the type species of the genus, was originally described by Ozaki (1935) from the nasal cavity of Neophocaena phocenoides from Awashima, Inland Sea of Japan. Neophocaena phocenoides has been previously considered as a species with widespread distribution and three geographic populations that have colonized freshwater (Yangtze River) and marine environments along the coast of the Indo-Pacific Ocean (Reeves et al., 2003). Under the current taxonomic treatment of the Marine Mammal Species, the Indo-Pacific finless porpoise (Ne. phocenoides) is considered a distinct species based on characteristic morphological features, genetic differences, and specific geographical distribution in the Indian Ocean to the South China Sea, that occurs sympatrically with Ne. asiaeorientalis only around the Taiwan Strait (Wang et al., 2010). On the other hand, Ne. asiaeorientalis is recognized to comprise two subspecies, Ne. a. asiaeorientalis inhabiting exclusively freshwater habitats (Yangtze River and adjacent water bodies), and Ne. a. sunameri distributed off the coasts of the Taiwan Strait, Korean Peninsula, and Japan. A recent study on the population genomics of the finless porpoises suggested a reproductive isolation between the two subspecies of Ne. asiaeorientalis (Zhou et al., 2018).
Given the current taxonomic treatment of the finless porpoises (Committee on Taxonomy, 2022), the type host of Nasitrema should be considered the East Asian finless porpoise, Na. a. sunameri. After the original description, only two reports including a detailed morphology of the species are known (Yamaguti, 1951; Neiland et al., 1970). The latter one represents a case from the Indo-Pacific finless porpoise, which was provided along the amendment of the generic diagnosis and description of five additional species of Nasitrema. The sympatric Na. sunameri, was described by Ozaki (1935) from the same host species and has been reported only twice after its first report (Kuramochi et al., 2000; Shiozaki & Amano, 2017). This could partially result from the difficulties in obtaining good specimens from frequently decomposed or frozen hosts that reflect the quality of the respective parasites and especially the helminths making the interpretation of their morphology difficult (Gibson, 2005). Additionally, the nasal sinuses are not routinely examined post-mortem, and this could be another reason for the scarce reports of Nasitrema spp. It is worth noting that species of Nasitrema have been reported to infect the respiratory tract and in nasal discharge of live captive dolphins (Kumar et al., 1975; Forrester et al., 1980; O’Shea et al., 1991; Ebert and Valente, 2013). However, we recovered infections with Nasitrema only inside the cranial sinuses and the inner ears.
Studies focused on brachycladiid phylogenetic relationships have reported lack of well-resolved interrelationships (Fernández et al., 1998a; Fraija-Fernández et al., 2016; Shiozaki et al., 2019). Our phylogenetic reconstructions based on both genetic markers were largely consistent with previous surveys. Worth noting is that previous studies were based on limited sampling across the brachycladiids. Under our taxon sampling, Nasitrema was resolved as a strongly supported monophyletic group in both individual gene phylogenetic reconstructions. The earliest diverging position of Nasitrema recovered in the mtND3 phylogeny is consistent with the current subfamilial status of the group. However, Synthesium and Oschmarinella were recovered as paraphyletic in both individual gene phylogenies, while Orthosplanchnus and Brachycladium were recovered as paraphyletic in mtND3 and LSU, respectively. The paraphyletic clustering was supported with high levels of sequence divergence between the respective congeners. This was not unexpected, as lack of clear species delimitation based especially on the mtND3 sequence data have been reported in the case of Synthesium in previous studies (Ebert et al., 2017; Shiozaki et al., 2019). However, despite the considerably high levels of intraspecific divergence observed in Synthesium from Ne. a. asiaeorientalis, the lack of a more conservative marker sequenced, and morphological differences reported, Shiozaki et al. (2019) have synonymized S. elongatus with S. tursionis. Given the inferred species relationships and assessed genetic divergences in the present study, we conclude that further efforts are needed incorporating thorough morphological evaluation along robust phylogenetic reconstructions to achieve taxonomic clarity within the family.
As an opportunistic feeder and top predator, the East Asian finless porpoise diet includes various species of fish, cephalopods, and crustaceans (Park et al., 2005; Shirakihara et al., 2008; Lu et al., 2016). It is a residential species with seasonal movements between the southern Yellow Sea and the southern coast of Korea in summer and rarely in winter (Jo et al., 2018). It inhabits shallow coastal waters with half of the finless porpoises in Korean waters occurring at a depth of approximately 20 m, and approximately 10 m from the coast is favored (Jo et al., 2018). Although this species is the highest incidentally caught cetacean in Korean waters, the mortality rate is estimated to be approximately 25% (Jo et al., 2018). The most recent estimates for the East Asian finless porpoise abundance account for approximately 13,000 individuals (Park et al., 2015). The East Asian finless porpoise is one of the most vulnerable cetaceans in this region due to its preferred habitat—shallow coastal areas—where it is easily affected by anthropogenic factors such as bycatch, ship collisions, habitat disturbance, and pollution. The species has recently experienced a sharp decline throughout its range, including Korean, Japanese, and Chinese waters (Kasuya et al., 2002; Mei et al., 2012; Park et al., 2015). Since the change in its species status from “vulnerable” to “endangered” in 2017, there has been great interest for its conservation. Despite the fact that the Korean government declared it a marine protected species in 2016 and designated necropsies have been increased as part of the conservation strategy, no reports on the diseases and infection causatives in the Korean populations are available yet. Comparative studies on the helminth fauna of different populations of the East Asian finless porpoise are needed to shed light on the preferences and availability of the local prey species. In this respect, our study represents a small but important contribution towards this aim.
5 Conclusions
This study constitutes an important contribution to the parasite-induced cetacean strandings in the Northwest Pacific with the air sinuses dwelling trematodes of genus Nasitrema, as culprits. Our study adds to the known diversity of cetacean parasite pathogens as causatives of death of non-anthropogenic origin. The results presented herein extend the geographical distribution of these two species of Nasitrema reported in Korean waters, including the Yellow Sea, and contribute to the morphological characterization and genetic data of the two species recovered. It is an important outcome for community ecology given the significant role of parasites on the ecosystem health, as parasites and disease spread are major regulating force with impact on species interactions and density-dependent population processes.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Ethical review and approval was not required for the animal study because the investigations were performed on a dead stranded cetacean. No live animals were involved.
Author contributions
SK: conceptualization, investigation, formal analysis, methodology, writing—original draft. HY: conceptualization and investigation. SC: funding acquisition, writing—reviewing and editing. KL, HL, MK and YK: investigation, and writing—reviewing and editing. SG: conceptualization, investigation, formal analysis, supervision, methodology, and writing—original draft, reviewing, and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Institute of Fisheries Science of the Republic of Korea (R2023004), the National Research Foundation of Korea (Grant no. s2020R1C1C1013563), the National Institute of Biological Resources (NIBR), and the Ministry of Environment (MOE) of the Republic of Korea (NIBR202102203).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams A. M., Hoberg E. P., McAlpine D. F., Clayden S. L. (1998). Occurrence and morphological comparisons of Campula oblonga (Digenea: campulidae), including a report from an atypical host, the thresher shark, Alopias vulpinus. J. Parasitol. 84, 435–438. doi: 10.2307/3284507
Arbelo M, Monteros AELL, Herráez P, Andrada M, Sierra E, Rodríguez F, et al. (2013). Pathology and causes of death of stranded cetaceans in the canary island. Dis. Aquat organisms. 103 (2), 87–99. doi: 10.3354/dao02558
Aznar F. J., Balbuena J. A., Raga J. A. (1994). Helminth communities of Pontoporia blainvillei (Cetacea: pontoporiidae) in Argentinian waters. Can. J. Zool. 72, 702–706. doi: 10.1139/z94-094
Baker C. S., Lukoschek V., Lavery S., Dlebout M. L., Yong-un M., Endo T., et al. (2006). Incomplete reporting of whale, dolphin and porpoise ‘bycatch’ revealed by molecular monitoring of Korean markets. Anim. Conserv. 9, 474–482. doi: 10.1111/j.1469-1795.2006.00062.x
Bartoli P., Bray R. A., Montero F. E. (2004). Tormopsolus orientalis yamaguti 1934 (Digenea: acanthocolpidae) from Seriola dumerili (Risso) (Perciformes: carangidae) in the western Mediterranean Sea. Syst. Parasitol. 57, 201–209. doi: 10.1023/B:SYPA.0000019081.36573.0f
Bray R. A., Webster B. L., Bartoli P., Littlewood D. T. J. (2005). Relationships within the acanthocolpidae lühe 1906 and their place among the digenea. Acta Parasitol. 50, 281–291.
Briscoe A. G., Bray R. A., Brabec J., Littlewood D. T. J. (2016). The mitochondrial genome and ribosomal operon of Brachycladium goliath (Digenea: brachycladiidae) recovered from a stranded minke whale. Parasitol. Int. 65, 271–275. doi: 10.1016/j.parint.2016.02.004
Committee on Taxonomy (2022) List of marine mammal species and subspecies. society for marine mammalogy. Available at: www.marinemammalscience.org.
Coombs EJ, et al. (2019). What can cetacean sranding records tell us? a study of UK and irish cetacean diversity over the past 100 years. Mar. Mamm. Sci. 35, 1527–1555.
Cowan D. F., Walker W. A., Brownell R.L. Jr (1986). “Pathology of small cetaceans stranded along southern California beaches,” in Research on dolphins.Bryden M. M., Harrison R. eds. (Oxford England: Oxford University Press), 323–367.
Dailey M. D., Brownell R. L. (1972). “A checklist of marine mammal parasites,” in Mammals of sea: biology and medicine. Ed. Ridgway S. (New Jersey: Thomas Springfield), 528–589.
Dailey M. D., Walker W. A. (1978). Parasitism as a factor in single strandings of southern California cetaceans. J. Parasitol. 64, 593. doi: 10.2307/3279939
Darriba D., Taboada G. L., Doallo R., Posada D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772. doi: 10.1038/nmeth.2109
Degollada E., André M., Arbelo M., Fernández A. (2002). Incidence, pathology and involvement of Nasitrema species in odontocete strandings in the canary islands. Vet. Rec. 150, 81–82. doi: 10.1136/vr.150.3.81
Delyamure S. L. (1955). Helminthfauna of marine mammals (ecology and phylogeny) (MoscowIzd. Akademii Nauk SSSR), 522.
Díaz-Delgado J., Fernández A., Sierra E., Sacchini S., Andrada M., Vela A. I., et al. (2018). Pathologic findings and causes of death of stranded cetaceans in the canary islands, (2006-2012). PloS One 13, e0204444. doi: 10.1371/journal.pone.0204444
Ebert M. B., Mülller M. I., Marigo J., Valente A. L., Cremer M. J., da Silva R. J. (2017). A new Synthesium species (Digenea: brachycladiidae) from the bottlenose dolphin Tursiops truncatus (Cetacea: delphinidae) in southwestern Atlantic waters. Parasitol. Res. 116, 1443–1452. doi: 10.1007/s00436-017-5421-2
Ebert M. B., Valente A. L. S. (2013). New records of Nasitrema atenuatta and Nasitrema globicephalae (Trematoda: brachycladiidae) neiland, rice and Holden 1970 in delphinids from south Atlantic. Check List. 9 (6), 1538–1540. doi: 10.15560/9.6.1538
Evans P. G. H., Hammond P. S. (2004). Monitoring cetaceans in European waters. Mammal Review. 34, 131–156. doi: 10.1046/j.0305-1838.2003.00027.x
Fernández M., Aznar F. J., Latorre A., Raga J. A. (1998). Molecular phylogeny of the families campulidae and nasitrematidae (Trematoda) based on mtDNA sequence comparison. Int. J. Parasitol. 28, 767–775. doi: 10.1016/S0020-7519(98)00027-7
Fernández M., Aznar F. J., Raga J. A., Latorre A. (2000). The origin of Lecithodesmus (Digenea: campulidae) based on ND3 gene comparison. J. Parasitol. 86, 850–852. doi: 10.1645/0022-3395(2000)086[0850:TOOLDC]2.0.CO;2
Fernández M., Balbuena J. A., Raga J. A. (1994). Hadwenius tursionis (Marchi 1873) n. comb. (Digenea, campulidae) from the bottlenose dolphin Tursiops truncatus (Montagu 1821) in the western Mediterranean. Syst. Parasitol. 28, 223–228. doi: 10.1007/BF00009519
Fernández M., Littlewood D. T. J., Latorre A., Raga J. A., Rollinson D. (1998b). Phylogenetic relationships of the family campulidae (Trematoda) based on 18S rRNA sequences. Parasitol. 117, 383–391. doi: 10.1017/S0031182098003126
Filion A, Doherty JF, Poulin R, Godfrey SS (2022). Building a comprehensive phylogenetic framework in disease ecology. Trends Parasitol 38 (6), 424–427. doi: 10.1016/j.pt.2022.01.008
Forrester D. J., Odell D. K., Thompson N. P., White J. R. (1980). Morphometrics, parasites, and chlorinated hydrocarbon residues of pygmy killer whales from Florida. J. Mammal. 61 (2), 356–360. doi: 10.2307/1380067
Fraija-Fernández N., Aznar F. J., Fernández A., Raga J. A., Fernández M. (2016). Evolutionary relationships between digeneans of the family brachycladiidae odhner 1905 and their marine mammal hosts: a cophylogenetic study. Parasitol. Int. 65, 209–217. doi: 10.1016/j.parint.2015.12.009
Fraija-Fernández N., Olson P. D., Crespo E. A., Raga J. A., Aznar F. J., Fernández M. (2015). Independent host switching events by digenean parasites of cetaceans inferred from ribosomal DNA. Int. J. Parasitol. 45, 167–173. doi: 10.1016/j.ijpara.2014.10.004
Gibson D. I. (2005). “Family brachycladiidae odhne,” in Keys to the trematoda, vol. 2 . Eds. Jones A., Bray R. A., Gibson D. I. (Wallingford: CABI Publishing and The Natural History Museum), 641–652.
Gibson D. I., Harris E. A., Bray R. A., Jepson P. D., Kuiken T., Baker J. R., et al. (1998). A survey of the helminth parasites of cetaceans stranded on the coast of England and Wales during the period 1990–1994. J. Zool. 244, 563–574. doi: 10.1111/j.1469-7998.1998.tb00061.x
Jefferson T. A., Curry B. E. (1994). A global review of porpoise (Cetacea: phocoenidae) mortality in gillnets. Biol. Conserv. 67, 167–183. doi: 10.1016/0006-3207(94)90363-8
Jeong Y., Lee Y., Park K. J., An Y., Moon H. (2020). Accumulation and time trends, (2003-2015) of persistent organic pollutants (POPs) in blubber of finless porpoises (Neophocaena asiaeorientalis) from Korean coastal waters. J. Hazard. Mater. 385, 121598. doi: 10.1016/j.jhazmat.2019.121598
Jo Y.-S., Baccus J. T., Koprowski J. L. (2018). Mammals of Korea. 1st edition (Incheon, Korea: National Institute of Biological Resources).
Kasuya T., Yamamoto Y., Iwatsuki T. (2002). Abundance decline in the finless porpoise population in the inland Sea of Japan. Raffles Bull. Zool. 10, 57–65.
Katoh K., Rozewicki J., Yamada K. D. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20, 1160–1166. doi: 10.1093/bib/bbx108
Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., et al. (2012). Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Kikuchi S., Okuyama Y., Nakajima M. (1987). Nasitrema lagenorhynchus n. sp. from the larynx and lungs of a pacific striped dolphin (Nasitrematidae, trematoda). jpn. J. Parasitol. 36, 42–48.
Kim S., Eo K., Oh J., Lee Y., Yoo M., Kitamura S., et al. (2019). First record of ginkgo-toothed beaked whale (Mesoplodon ginkgodens) stranded in Korea. J. Vet. Med. Sci. 81 (8), 1223–1228. doi: 10.1292/jvms.19-0001
Kim Z. G., Choi S. G., An Y. R,, Kim H. W., Park K. J. (2000). National Whales and dolphins off Korean peninsula. Busan: Hanguel graphics. 10.
Kim D., Sohn H., An Y.-R., Park K. J., Kim H. W., Ahn S. E., et al. (2013). Status of the cetacean bycatch near Korean waters. Kor. J. Fish. Aq. Sci. 46 (6), 892–900.
Kremnev G., Gonchar A., Krapivin V., Knyazeva O., Krupenko D. (2020). First elucidation of the life cycle in the family brachycladiidae (Digenea), parasites of marine mammals. Int. J. Parasitol. 50, 997–1009. doi: 10.1016/j.ijpara.2020.05.011
Kumar V., Vercruysse J., Kageruka P., Mortelmans J. (1975). Nasitrema attenuata (Trematoda) infection of Tursiops truncatus and its potentialities as an aetiological agent of chronic pulmonary lesions. J. Helminthol. 49, 289–292. doi: 10.1017/S0022149X00026298
Kuraku S, Zmasek CM, Nishimura O, Katoh K (2013). aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res 41, W22–W28.
Kuramochi T., Kikuchi T., Okamura H., Tatsukawa T., Doi H., Nakamura K., et al. (2000). Parasitic helminth and epizoit fauna of finless porpoise in the inland Sea of Japan and the western north pacific with a preliminary note on faunal difference by host’s local population. Mem. Natn. Sci. Mus. Tokyo 33, 83–95.
Lee S., Park K. J., Kim B., Min M., Lee H. (2018). Genetic diversity and population demography of narrow-ridged finless porpoise from south Korea on the basis of mitochondrial DNA variation: implications for its conservation on East Asia. Mar. Mamm. Sci. 35 (2), 574–594. doi: 10.1111/mms.12563
Lewis R. J., Berry K. (1988). Brain lesions in a pacific white-sided dolphin (Lagenorhynchus obliquidens). J. Wildl. Dis. 24, 577–581. doi: 10.7589/0090-3558-24.3.577
Lim C. W., Han S. J., Kim B. S., Alexander W., Lee Y. R., Park T. G., et al. (2016). Nasitrema attenuata (Digenia: nasitrematidae) infection of long-beaked common dolphin (Delphius capensis) in the East Sea, Korea. Kor. J. Vet. Clin. 33 (3), 151–154. doi: 10.17555/jvc.2016.06.33.3.151
Lu Z., Xu S., Song N., Gao T., Tian J., Han J. (2016). Analysis of the diet of finless porpoise (Neophocaena asiaeorientalis sunameri) based on prey morphological characters and DNA barcoding. Cons. Gen. Res. 8, 523–531. doi: 10.1007/s12686-016-0575-2
Marigo J, Cunha HA, Bertozzi CP, Souza SP, Rosas FCW, Cremer MJ, et al. (2015). Genetic diversity and population structure of Synthesium pontoporiae (Digenea, brachycladiidae) linked to its definitive host stocks, the endangered franciscana dolphin, Pontoporia blainvillei (Pontoporiidae) off the coast of brazil and argentina. J Helminthol. 89, 19–27. doi: 10.1017/S0022149X13000540
Mei Z., Huang S.-L., Hao Y., Turvey S. T., Gong W., Wang D. (2012). Accelerating population decline of Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis). Biol. Conserv. 153, 192–200. doi: 10.1016/j.biocon.2012.04.029
Miller M. A., Pfeiffer W., Schwartz T. (2011). “The CIPRES science gateway: a community resource for phylogenetic analyses,” in Proceedings of the 2011 TeraGrid Conference: Extreme Digital Discovery. 1–8.
Morimitsu T., Kawano H., Torihara K., Kato E., Koono M. (1992). Histopathology of eighth cranial nerve of mass stranded dolphins at goto islands, Japan. J. Wildl. Dis. 28, 656–658. doi: 10.7589/0090-3558-28.4.656
Morimitsu T., Nagai T., Ide M., Ishii A., Koono M. (1986). Parasitogenic octavus neuropathy as a cause of mass stranding of odontoceti. J. Parasitol. 72 (3), 469–472. doi: 10.2307/3281689
Morimitsu T., Nagai T., Ide M., Kawano H., Naichuu A., Koono M., et al. (1987). Mass stranding of odontoceti caused by parasitogenic eighth cranial neuropathy. J. Wildl. Dis. 23, 586–590. doi: 10.7589/0090-3558-23.4.586
Nakagun S., Shiozaki A., Ochiai M., Matsuda A., Tajima Y., Matsuishi T., et al. (2018). Prominent hepatic ductular reaction induced by Oschmarinella macrorchis in a hubbs’ beaked whale Mesoplodon carlhubbsi, with biological notes. Dis. Aquat. Organ. 127, 177–192. doi: 10.3354/dao03201
Nakagun S, Kobayashi Y. (2020). Histochemical and immunohistochemical characterizations of hepatic trematodiasis in odontocetes. Front Vet Sci 7, 336. doi: 10.3389/fvets.2020.00336
Neiland K. A., Rice D. W., Holden B. L. (1970). Helminths of marine mammals, i. the genus Nasitrema, air sinus flukes of delphinid cetacea. J. Parasitol. 56 (2), 305–316.
Oh Y., Sohn H., Lee D., An Y., Kang C., Kang M. G., et al. (2018). Feeding patterns of ‘Finless porpoise (Neophocaena asiaeorientalis) in the yellow Sea as indicated by stable carbon and nitrogen isotope ratios. J. Coast. Res. 85, 386–390. doi: 10.2112/SI85-078.1
Olson P. D., Cribb T. H., Tkach V. V., Bray R. A., Littlewood D. T. J. (2003). Phylogeny and classification of the digenea (Platyhelminthes: trematoda). Int. J. Parasitol. 33, 733–755. doi: 10.1016/S0020-7519(03)00049-3
O’Shea T. J., Homer B. L., Greiner E. C., Leyton A. W. (1991). Nasitrema sp.–associated encephalitis in a striped dolphin (Stenella coeruleoalba) stranded in the gulf of Mexico. J. Wildl. Dis. 27, 706–709. doi: 10.7589/0090-3558-27.4.706
Ozaki Y. (1935). Trematode parasites of Indian porpoise Neophocaena phocaenoides Gray. J. Sci. Hiroshima Univ. 3, 115–138.
Park K., An Y., Lee Y., Park J., Moon D., Choi S. (2011). Feeding habits and consumption by finless porpoises (Neophocaena asiaeorientalis) in the yellow Sea. Fish. Aquat. Sci. 44 (1), 78–84.
Park K. J., Sohn H., An Y. R., Kim H. W., An D. H. (2015). A new abundance estimate for the finless porpoise Neophocaena asiaeorientalis on the West coast of Korea: an indication of population decline. Fish. Aquat. Sci. 18, 411–416. doi: 10.5657/FAS.2015.0411
Park K. J., Zhang C. I., Sohn H., Kim Z. G. (2005). Feeding habits of finless porpoise (Neophocaena phocaenoides) in the west coast of Korea (IWC, Impington, Cambridge, United Kingdom). Paper SC/57/SM17.
Parker G. A., Migaki G., Walker W. A. (1977). Cerebral trematodiasis: case for diagnosis. Mil. Med. 142(11)861, 869–870.
Peltier H., Baagøe H. J., Camphuysen K. C. J., Czeck R., Dabin W., Daniel P., et al. (2013). The stranding anomaly as population indicator: the case of harbour porpoise Phocoena phocoena in north-Western Europe. PloS One 8, e62180. doi: 10.1371/journal.pone.0062180
Phillips A. C. N., Suepaul R. (2017). Nasitrema species: a frequent culprit in melon-headed whale (Peponocephala electra) strandings in Trinidad. Aquat. Mamm. 43 (5), 547–557. doi: 10.1578/AM.43.5.2017.547
Prado JHF, Mattos PH, Silva ,KG, Secchi ,ER (2016). Long-terms easonal and interannual patterns of marine mammals trandings in Subtropical Western South Atlantic. PloS One 11, e0146339.
Rambaut A, Drummond AJ. Tracer v1. 5 (2009). Available at: http://beast.bio.ed.ac.uk/Tracer.
Reeves R. R., Smith B. D., Crespo E. A., Notarbartolo di Sciara G. (2003). Dolphins, whales and porpoises: 2002-2010 conservation action plan for the world’s cetaceans (Switzerland and Cambridge, UK: IUCN, Gland).
Ronquist F., Teslenko M., Mark P. V. D., Ayres D. L., Darling A., Höhna S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Rudolph P., Smeenk C. (2009) “Indo-West pacific marine mammals,” in Encyclopedia of marine mammals, 2nd ed. Eds. Perrin W. F., Würsig B., Thewissen J. G. M. (London: Academic Press), 608–616. doi: 10.1016/B978-0-12-373553-9.00142-5
Shiozaki A., Amano M. (2017). Population- and growth-related differences in helminthic fauna of finless porpoises (Neophocaena asiaeorientalis) in five Japanese populations. J. Vet. Med. Sci. 79 (3), 534–541. doi: 10.1292/jvms.16-0421
Shiozaki A., Amano M., Fernández M., Fraija-Fernández N. (2019). Revision of the taxonomic status of Synthesium elongatum (Ozaki 1935) (Brachycladiidae), an intestinal digenean of narrow-ridged finless porpoise (Neophocaena asiaeorientalis). J. Vet. Med. Sci. 81 (4), 601–607. doi: 10.1292/jvms.18-0636
Shirakihara M., Seki K., Takemura A., Shirakihara K., Yoshida H., Yamazaka T. (2008). Food habits of finless porpoises Neophocaena phocaenoides in western Kyushu, Japan. J. Mammal. 89, 1248–1256. doi: 10.1644/07-MAMM-A-264.1
Sohn H., An D., Kim D. (2012). Review of the Korean vernacular names of cetaceans. Kor. J. Fish Aqua. Sci 15 (3), 513–522. doi: 10.5657/KFAS.2012.0513
Spencer N, Santos MB, Pierce GJ (2000). Evaluation of the state of knowledge concerning by-catches of cetaceans; final report tender no XIV/1999/01 lot 7 (31/12/99–31/10/00). Brussels, Belgium: European Commission. Available at: http://www.eurocbc.org/page345.html (Accessed 10 May 2022).
Telford M. J., Herniou E. A., Russell R. B., Littlewood D. T. J. (2000). Changes in mitochondrial genetic codes as phylogenetic characters: two examples from the flatworms. Proc. Natl. Acad. Sci. 97, 11359–11364. doi: 10.1073/pnas.97.21.11359
ten Doeschate M. T., Brownlow A. C., Davison N. J., Thompson P. M. (2018). Dead useful; methods for quantifying baseline variability in stranding rates to improve the ecological value of the strandings record as a monitoring tool. J. Mar. Biol. Assoc. U.K. 98 (5), 1205–1209. doi: 10.1017/S0025315417000698
Tregenza N. J. C., Berrow S. D., Hammond P. S., Leaper R. (1997). Harbour porpoise (Phocoena phocoena l.) by-catch in set gillnets in the celtic Sea. ICES J. Mar. Sci: J. Cons. 54, 896–904. doi: 10.1006/jmsc.1996.0212
Wang J. Y., Reeves R. (2017) Neophocaena asiaeorientalis. the IUCN red list of threatened species, version 2021-3. Available at: https://www.iucnredlist.org/species/41754/50381766.
Wang J. Y., Yang S. C., Wang B. J., Wang L. S. (2010). Distinguishing between two species of finless porpoises (Neophocaena phocaenoides and N. asiaeorientalis) in areas of sympatry. Mammalia 74, 305–310. doi: 10.1515/mamm.2010.029
Williams R., Gero S., Bejder L., Calambokidis J., Kraus S. D., Lusseau D., et al. (2011). Underestimating the damage: interpreting cetacean carcass recoveries in the context of the deepwater Horizon/BP incident. Conserv. Lett. 4, 1–6. doi: 10.1111/j.1755-263X.2011.00168.x
Yamaguti S. (1951). Studies on the helminth fauna of japan. part 45. trematodes of marine mammals. Arb. Med. Fak. Okayama. 7 (4), 283–294. doi: 10.18926/AMO/31774
Yamaguti S. (1958). Systema helminthum. vol. i. the digenetic trematodes of vertebrates-part II (New York & London: Interscience), 981–1575.
Yim H., Lee J. (2015). Prediction of hypoxia-inducible factor binding site in whale genome and analysis of target genes regulated by predicted sites. J. Mar. Biosci. Biotechnol. 7 (2), 35–41. doi: 10.15433/ksmb.2015.7.2.035
Yoo S., Kong C. E., Son Y. B., Ishizaka J. (2019). A critical re-assessment of the primary productivity of the yellow Sea, East China Sea and Sea of Japan/East Sea large marine ecosystems. Deep Sea Res. II: Topical Stud. Oceanography 163, 6–15. doi: 10.1016/j.dsr2.2018.05.021
Keywords: Trematoda, Nasitrema, parasite-induced standings, disease, East Asian finless porpoise
Citation: Kim S, Youn H, Lee K, Lee H, Kim MJ, Kang Y, Choe S and Georgieva S (2023) Novel morphological and molecular data for Nasitrema spp. (Digenea: Brachycladiidae) in the East Asian finless porpoise (Neophocaena asiaeorientalis sunameri). Front. Mar. Sci. 10:1187451. doi: 10.3389/fmars.2023.1187451
Received: 16 March 2023; Accepted: 02 May 2023;
Published: 24 May 2023.
Edited by:
Ahasan Habib, University of Malaysia Terengganu, MalaysiaReviewed by:
Nur Fadli, Syiah Kuala University, IndonesiaMuhammad Hafiz Borkhanuddin, Universiti Malaysia Terengganu, Malaysia
Copyright © 2023 Kim, Youn, Lee, Lee, Kim, Kang, Choe and Georgieva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sunmin Kim, eHN1bm1pbnhAZ21haWwuY29t; Seongjun Choe, cGFyYXNpdGVAY2h1bmdidWsuYWMua3I=; Simona Georgieva, c2ltb25hLmdlb3JnaWV2YUBnbWFpbC5jb20=
†Deceased
 Sunmin Kim
Sunmin Kim Heejeong Youn2†
Heejeong Youn2† Kyunglee Lee
Kyunglee Lee Hyunjoo Lee
Hyunjoo Lee Min Ju Kim
Min Ju Kim Yeseul Kang
Yeseul Kang Seongjun Choe
Seongjun Choe Simona Georgieva
Simona Georgieva