- 1College of Economics and Management, Taishan University, Taian, China
- 2CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 3Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao, China
- 4Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao, China
- 5College of Biology and Brewing Engineering, Taishan University, Taian, China
Marine planktonic Ciliophora serve as a key component of the plankton food web. The formation of cysts is of common occurrence among planktonic Ciliophora, and encystment plays an important role in the persistence and diffusion of population. However, studies on the seasonal pattern of encystment of planktonic Ciliophora in natural environments were limited. Here, we investigated the sedimentation of Ciliophora cysts, and explored the seasonal differences of encystment between aloricate Oligotrichea and Tintinnina in Bohai Bay. Ciliophora cysts were collected monthly with a sediment trap from July 2019 to June 2020 at a fixed station, and identified according to the morphological characteristics of cysts by fluorescence microscopy. Ten types of aloricate Oligotrichea cysts were identified and only three species of Tintinnina cysts were recognized, namely, Favella sp., Helicostomella longa and Tintinnopsis sp. There were obvious seasonal differences of encystment between aloricate Oligotrichea and Tintinnina. Encystment of Tintinnina mainly occurred in summer, while encystment of aloricate Oligotrichea was found at all seasons and the seasonal patterns varied among species. The production rate of several types of cysts showed significant positive correlations with water temperature and Chlorophyll a concentration, and a significant negative correlation with salinity. Our study exhibited that the seasonal pattern of encystment of Ciliophora varied greatly from species to species, and assessing seasonal patterns of encystment will aid our ability to understand the mechanism of vegetative population dynamics.
1 Introduction
Planktonic Ciliophora, mainly including aloricate Oligotrichea and Tintinnina, comprise a major part of microzooplankton in various marine environments (Yang et al., 2008; Montagnes et al., 2010; Romano et al., 2021) and they play an important role in material recycling and energy flow through pelagic marine ecosystems (Pierce and Turner, 1992; Calbet, 2008). Similar to Bacillariophyceae, Dinophyceae and other microorganisms, the formation of cysts is of common occurrence among planktonic Ciliophora. Generally, encystment is considered as a protection strategy against ‘unfavorable environmental conditions’, and therefore playing an important role in the persistence and diffusion of population (Lennon and Jones, 2011; Verni and Rosati, 2011; Li et al., 2022).
Knowledge on cysts of planktonic Ciliophora in marine environments was limited, although Ciliophora serve as a key component of the plankton food web (Calbet, 2008). To the best of our knowledge, about 17 species of aloricate Oligotrichea and 61 species of Tintinnina (Belmonte and Rubino, 2019; Ganser et al., 2022; Yu et al., 2022) were recorded to produce cysts in marine environments. Assessing seasonal patterns of encystment will aid our ability to understand the mechanism of vegetative population dynamics. At present, few studies focused on the seasonal pattern of encystment in natural waters. Mass encystment of Strombidium crassulum (Reid, 1987) and Cyrtostrombidium boreale (Kim et al., 2002) were both found in spring. Mass encystment of Strombidium capitatum occurred in spring in Onagawa Bay and in autumn in Masan Bay (Kim et al., 2008). Encystment of Tintinnina was primarily a late summer/autumn event (Paranjape, 1980; Reid and John, 1981; Davis, 1985). These previous studies showed that the seasonal pattern might vary from species to species, and from region to region. More investigations are required to further study the seasonal patterns of encystment of planktonic Ciliophora.
Bohai Bay is a typical temperate semi-closed bay (mean depth of 12 m) on the continental shelf of the western Pacific Ocean. Seasonal variation of cyst bank of planktonic Ciliophora was investigated in surface sediments of Bohai Bay (Yu et al., 2022). However, the seasonal patterns of the formation of cysts in the water column are not clear. Here, we reported annual variation of encystment through continuous recording the production rate of cysts in the waters, and explored the seasonal differences of encystment between aloricate Oligotrichea and Tintinnina in Bohai Bay. Consequently, assessing seasonal patterns of encystment will aid our ability to understand the mechanism of vegetative population dynamics.
2 Materials and methods
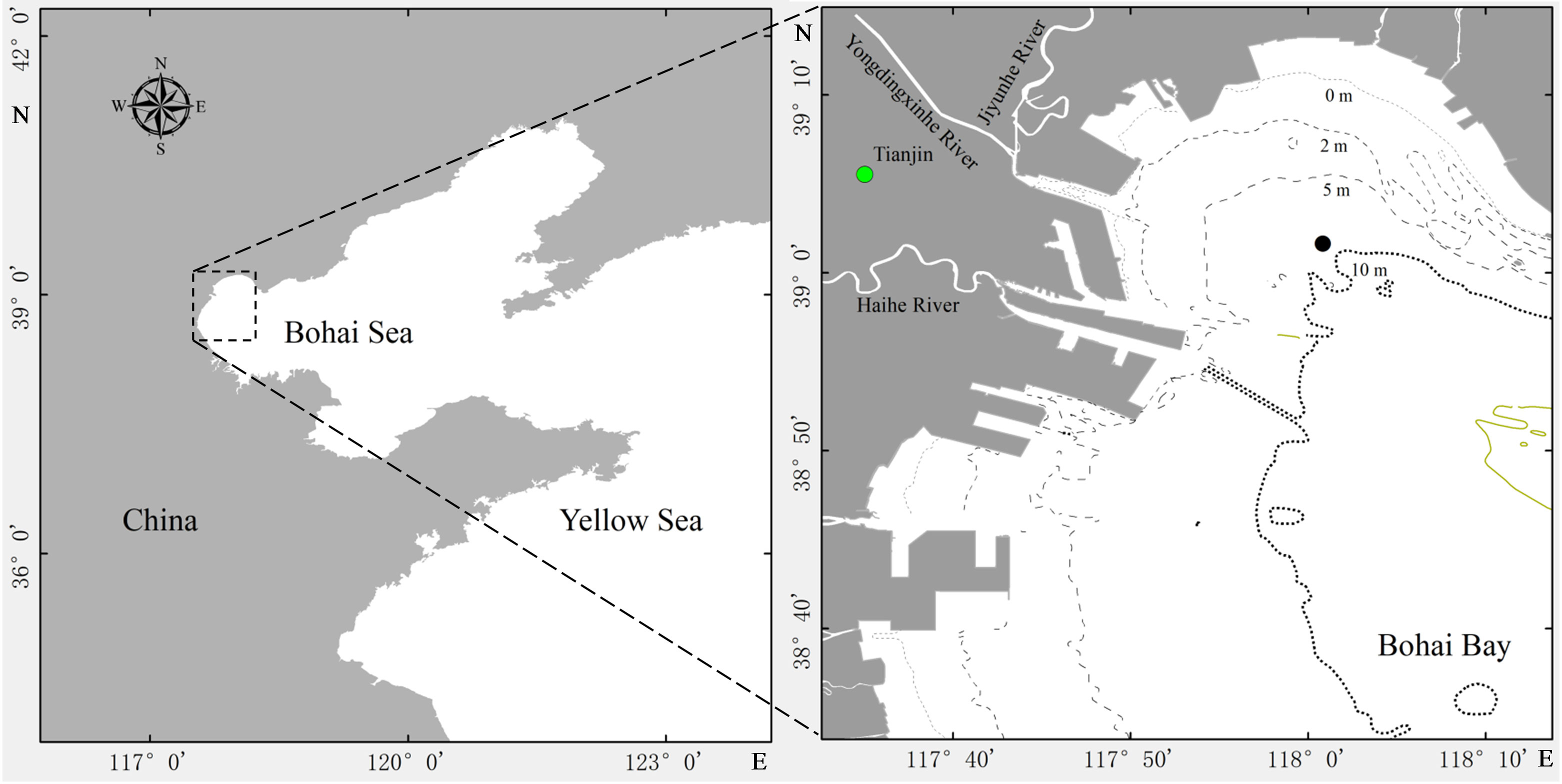
Ciliophora and the sedimentation of their cysts were investigated monthly from July 2019 to June 2020 at a fixed station (118°02′ E, 39°02′ N, with less human disturbance due to far from marine ranching and seaway) in Bohai Bay (Figure 1). Water depth was 8 to 9 m according to tide. Surface water temperature and salinity were measured by YSI Professional Plus instrument. For total Chlorophyll a (Chl a) determination, 250 ml of surface water was filtered (GF/F) and filters were frozen at -20°C. Chl a was extracted with 90% acetone at -20°C in the dark for 24 h. Chl a concentration was determined using a spectrophotometer.
For sampling of Ciliophora vegetative cells, 1 L of surface water was fixed (1% acid Lugol’s iodine, Harris et al., 2000) and stored in dark until analysis. Samples were concentrated and examined following Utermöhl method (Utermöhl, 1958) under inverted microscope (Olympus IX71). Due to the lack of infraciliature, aloricate Oligotrichea were only identified to the genus level according to the morphological characteristics and the common species recorded in Bohai Bay. For Tintinnina, species were identified based on lorica morphology (Hada, 1937; Yin, 1952; Zhang et al., 2012).
Sinking Ciliophora cysts were collected with a cylindrical sediment trap of 56 cm in height and 14 cm in inner diameter (HYDRO-BIOS, Saarso). The trap was deployed at 7 m depth and retrieved after 70 h, except February 2020 when the trap was deployed for only 50 h because of a strong wind. After retrieval of the trap, supernatant water was gently siphoned after settling at 4°C for 12 h, and the trapped particles were frozen at -20°C until analysis (Figure 2).

Figure 2 Photographs of sediment trap after retrieval. (A): supernatant water was released through the faucet; (B): collecting bottle; (C): trapped particles.
Cyst samples were treated and analyzed in accordance with Ichinomiya et al. (2004). An aliquot (1 g wet weight) of the sample was suspended in ca. 20 ml of filtered seawater and sonicated for 30 s. The fraction between 20 to 125 μm was retained on a sieve, and diluted with 10 ml of filtered seawater. The sieved fraction was treated with glutaraldehyde (2.5% final concentration) and stored in a refrigerator for 24h. For the enumeration of cysts, 1 ml aliquot of the suspension (concentrated to 5 ml) was placed on a counting slide and the whole slide was examined under blue-light excitation (450 to 480 nm) using a fluorescence microscope (Olympus BX53). Cysts of aloricate Oligotrichea were classified according to the shape, size and the characteristics of cyst wall (Kim and Taniguchi, 1995; Kim et al., 2002; Ichinomiya et al., 2004; Agatha et al., 2005; Kim et al., 2008). Tintinnina cysts were identified by the characteristics of lorica, since the cyst was inside the lorica. All the examinations were replicated 3 times.
Annual data were presented as line and bar charts by Grapher (Version 18, Golden Software Inc., USA). Mantel test was performed between cyst compositional and environmental data given geographic distance (9,999 permutations) using the vegan R software package.
3 Results
3.1 Annual cycle of water temperature, salinity and Chl a concentration
Water temperature was lowest in January (2.35°C) and peaked in July (29.60°C). In contrast, salinity was lower in the summer-autumn and highest in December (32.11). There were two peaks in Chl a concentration: one in March (5.53 μg/L), and another in July (15.29 μg/L, Figure 3).
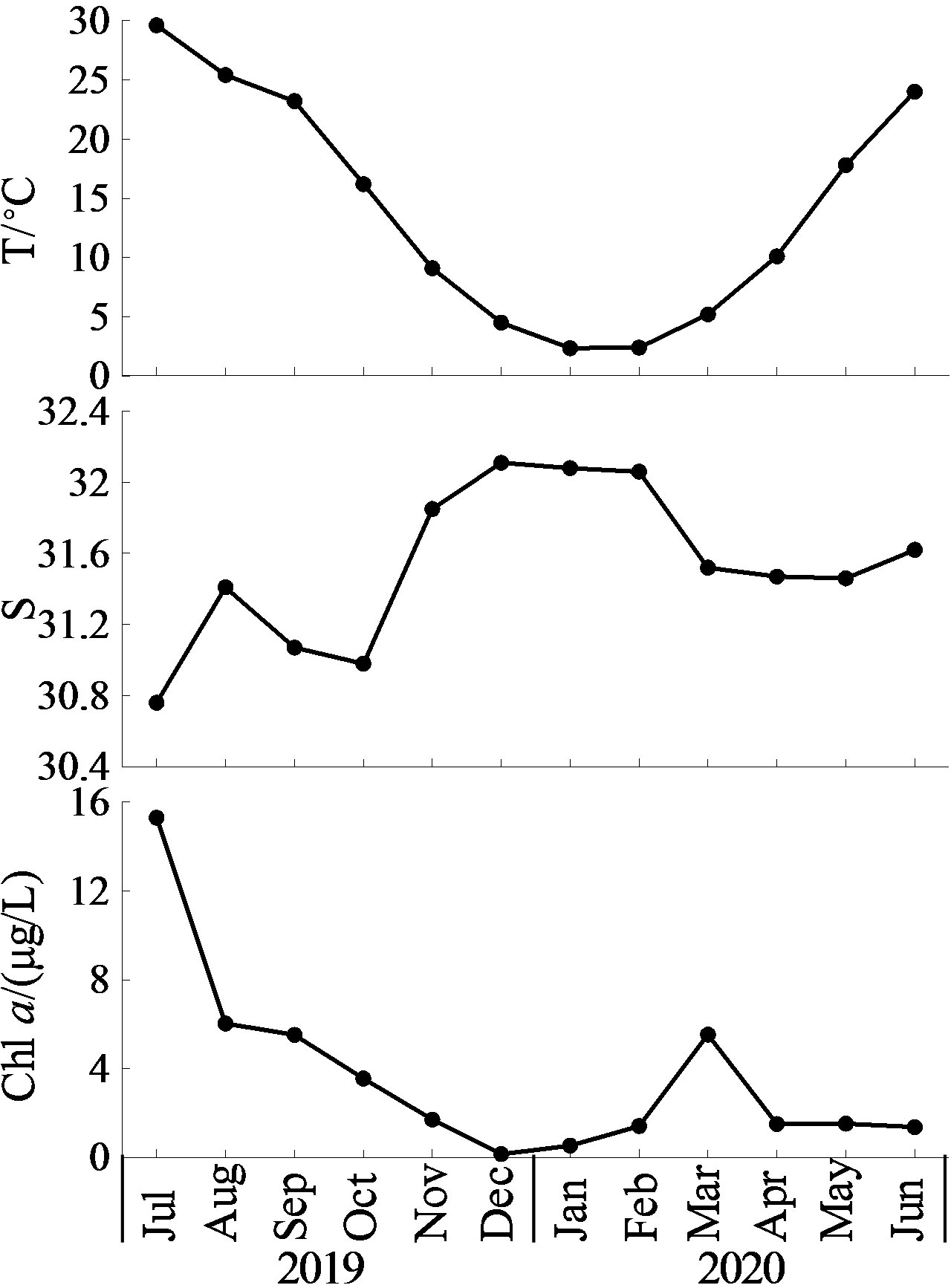
Figure 3 Monthly variation of surface water temperature (T), salinity (S), Chlorophyll a (Chl a) concentration.
3.2 Annual cycle of vegetative population of Ciliophora
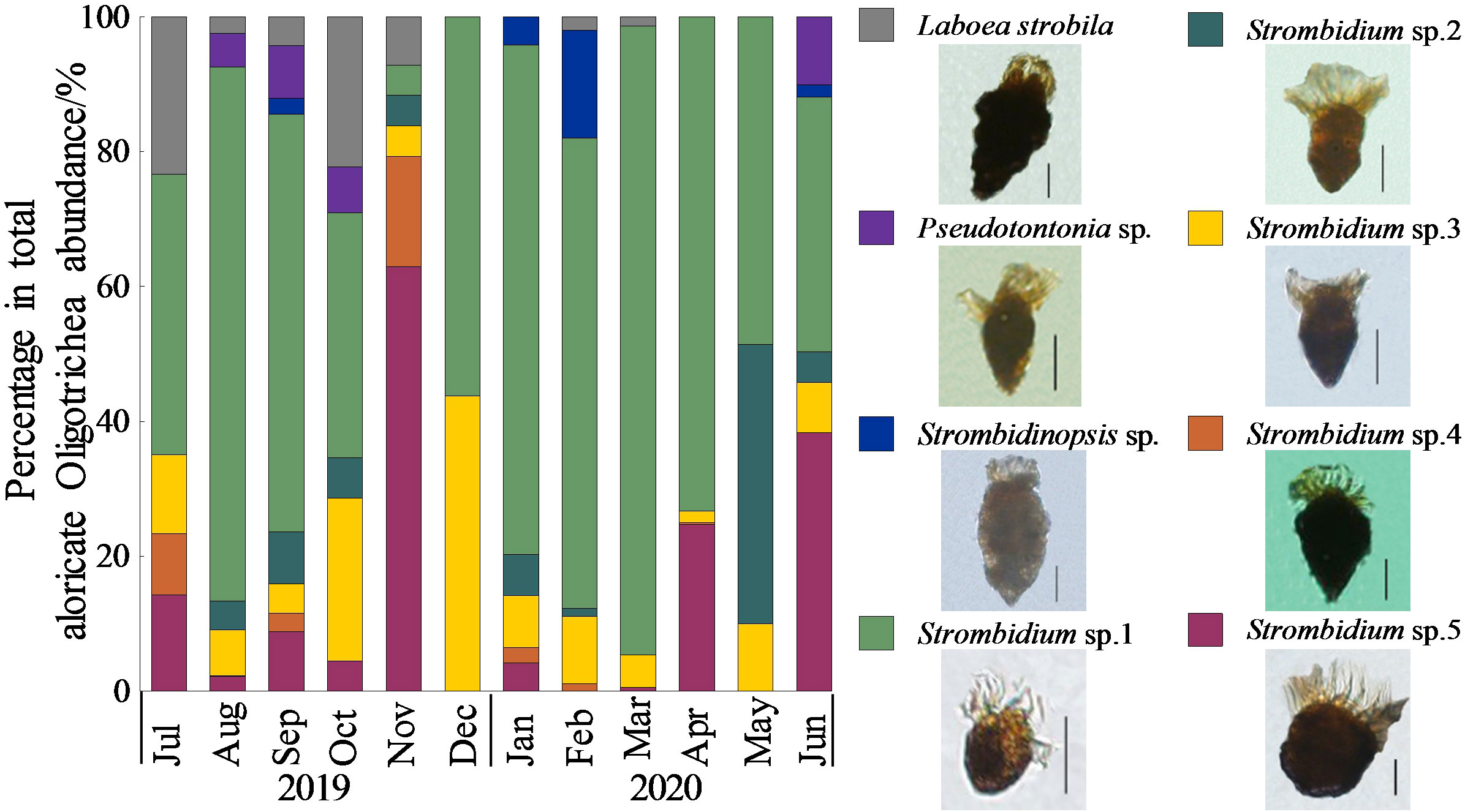
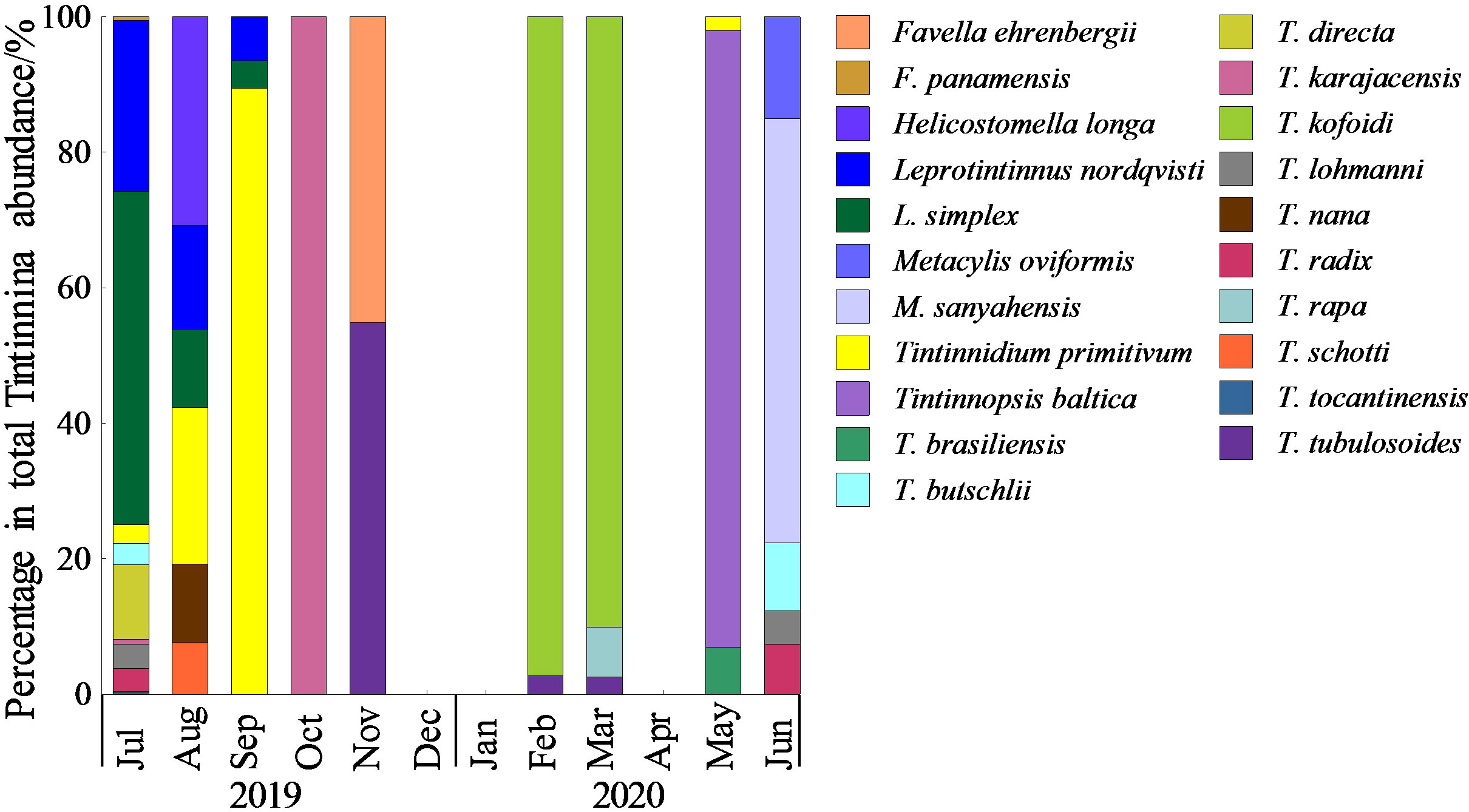
The Ciliophora community was dominated by aloricate Oligotrichea, accounting for an average 71.4 ± 32.1% of the total abundance of Ciliophora. A total of eight taxa of aloricate Oligotrichea were identified, among which Strombidium spp. were dominant with an average abundance ratio of 90.3 ± 10.0% (Figure 4). Twenty-one Tintinnina species were recognized, and the species composition of Tintinnina exhibited obvious seasonal variation (Figure 5). The dominant Tintinnina were Leprotintinnus simplex, Leprotintinnus nordqvisti and Tintinnidium primitivum.
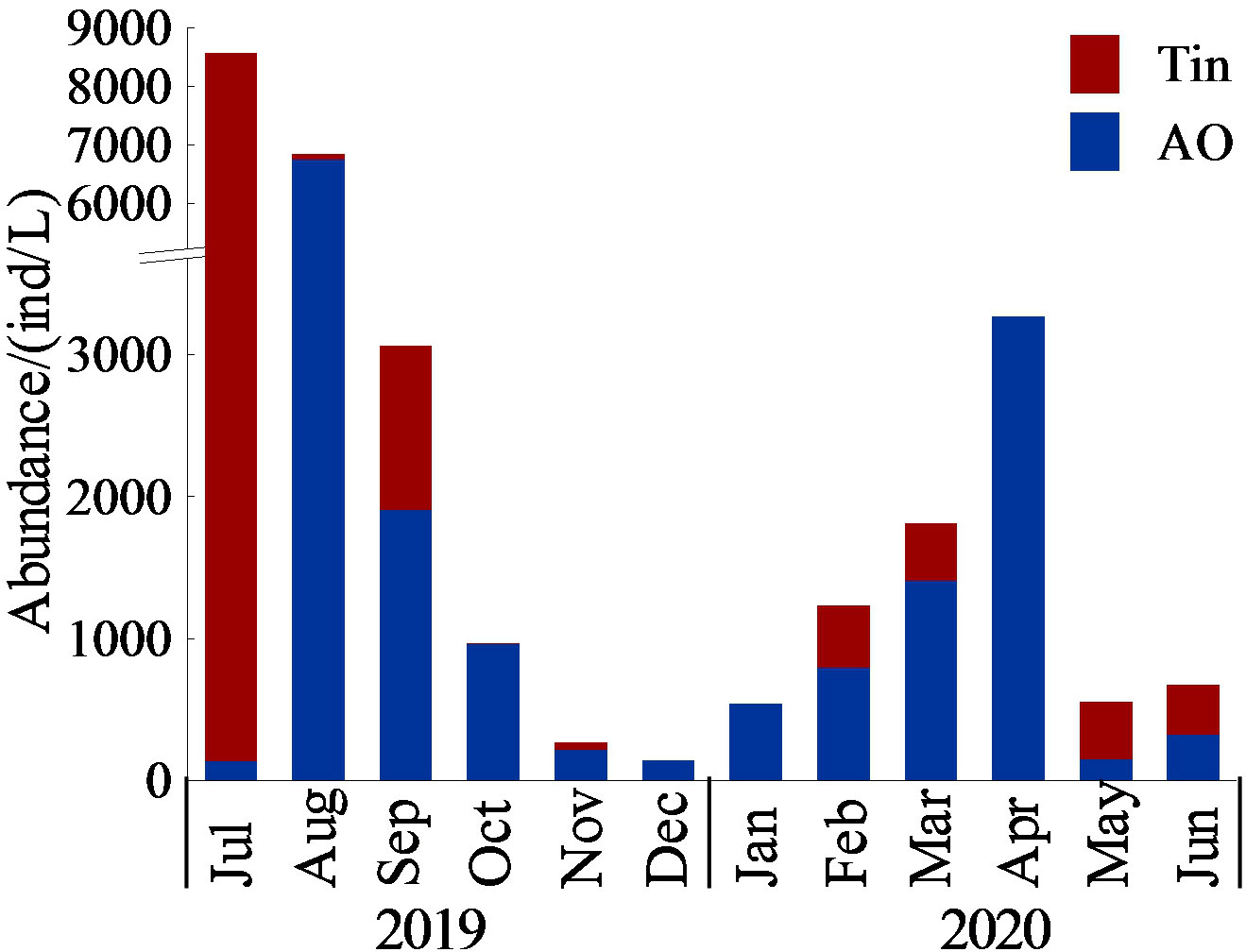
There were two peaks of aloricate Oligotrichea abundance: one in April (3266 ind/L), and another in August (6748 ind/L). One peak of Tintinnina abundance occurred in July (8440 ind/L). There were two peaks of total Ciliophora abundance: one in April (3266 ind/L), and another in July (8577 ind/L). The average contribution of Tintinnina to the total Ciliophora through the whole year was 28.6 ± 32.6%, and they dominated over aloricate Oligotrichea only in May (72.8%), June (53.5%) and July (98.4%, Figure 6).
3.3 Cyst types
A total of 10 types of aloricate Oligotrichea cysts were identified based on their morphological characteristics under regular light and epifluorescent light.
Type I: similar to Cyrtostrombidium boreale, same as Type I in earlier study of Bohai Bay (Yu et al., 2022).
Type II: similar to Strombidinopsis sp., same as Type II in earlier study of Bohai Bay (Yu et al., 2022).
Type III: similar to Strombidium biarmatum, same as Type III in earlier study of Bohai Bay (Yu et al., 2022).
Type IV: similar to Strombidium capitatum, same as Type IV in earlier study of Bohai Bay (Yu et al., 2022).
Type V: similar to Strombidium conicum, same as Type V in earlier study of Bohai Bay (Yu et al., 2022).
Type VI: same as Type IX in earlier study of Bohai Bay (Yu et al., 2022).
Type VII: same as Type X in earlier study of Bohai Bay (Yu et al., 2022).
Type VIII: an elliptical shape with a short collar at one end, closed by a hyaline, elliptical papula (Figure 7A, a). Size varies from 27–42 μm in total length and 20–32 μm in width. The papula was 7–8 μm in height and 5–6 μm in width. The cyst wall enclosing the cytoplasm of dense granular structure is single and hyaline.
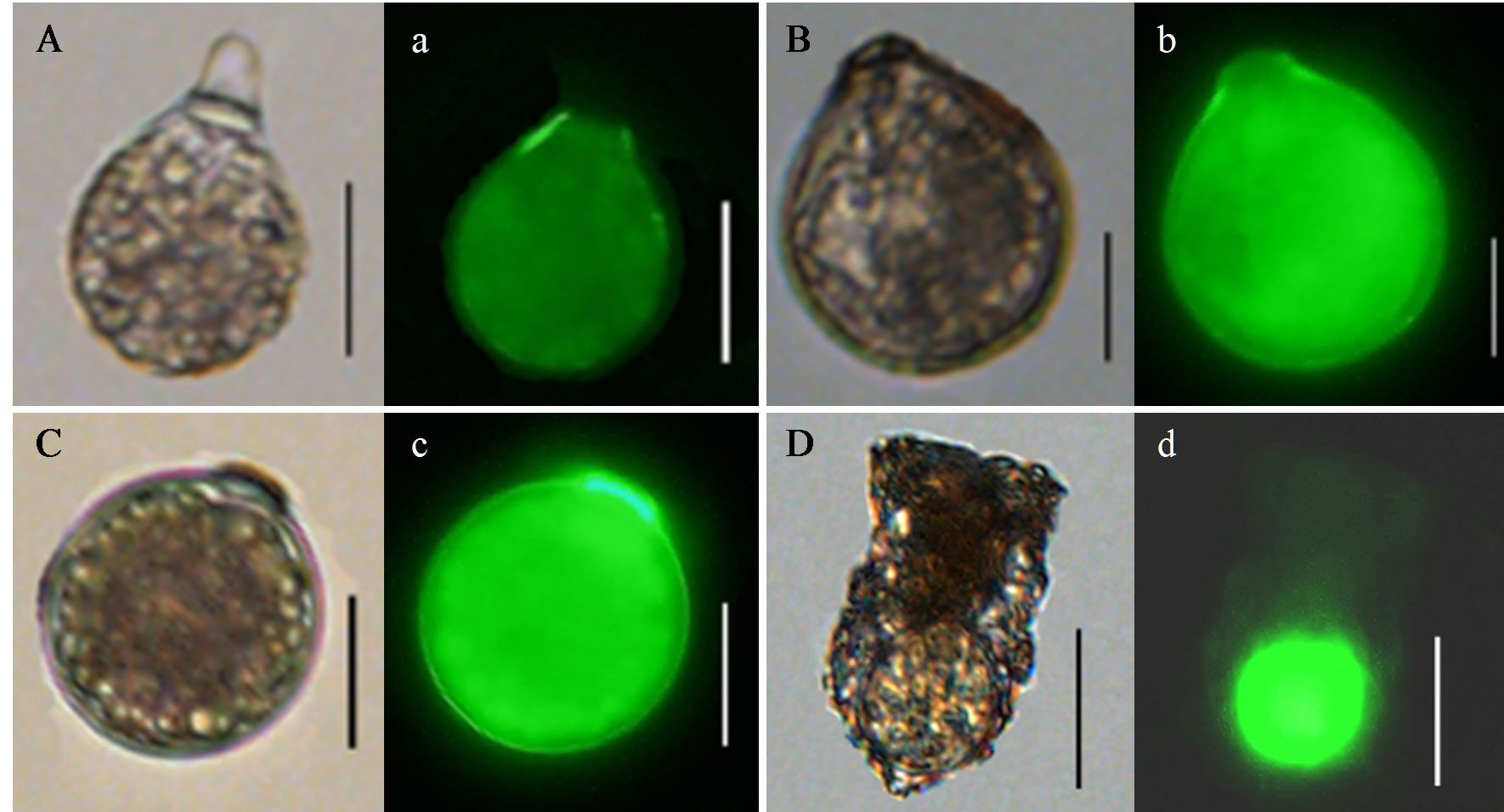
Figure 7 Microphotographs of Ciliophora cysts under regular light (A–D) and epifluorescent light (a–d). (A, a): Type VIII;(B, b): Type IX; (C, c): Type X; (D, d): cyst of Tintinnopsis sp. Scale bar: 20 μm.
Type IX: a nearly spherical body with a short collar at one end, closed by a hyaline papula (Figure 7B, b). Size varies from 45–54 μm in total length and 41–49 μm in width. The papula was 2–3 μm in height and 10–11 μm in width. The cyst wall is composed of two layers, and the inner cyst wall encloses the cytoplasm of dense granular structure.
Type X: a nearly spherical body with a short collar at one end, closed by a hyaline papula (Figure 7C, c). Size varies from 33–45 μm in total length and 30–40 μm in width. The papula was 2 μm in height and 9–10 μm in width. The cyst wall enclosing the cytoplasm of dense granular structure is single and hyaline.
Only three types of Tintinnina cysts were recognized, namely, Favella sp., Helicostomella longa and Tintinnopsis sp.
Cyst of Favella sp.: same as Type VI in earlier study of Bohai Bay (Yu et al., 2022).
Cyst of Helicostomella longa: same as Type VII in earlier study of Bohai Bay (Yu et al., 2022).
Cyst of Tintinnopsis sp.: a spherical cyst, surrounded by a thin layer, within a lorica (Figure 7D, d). Size varies from 16–18 μm in diameter. The cyst wall enclosing the cytoplasm of dense granular structure is hyaline.
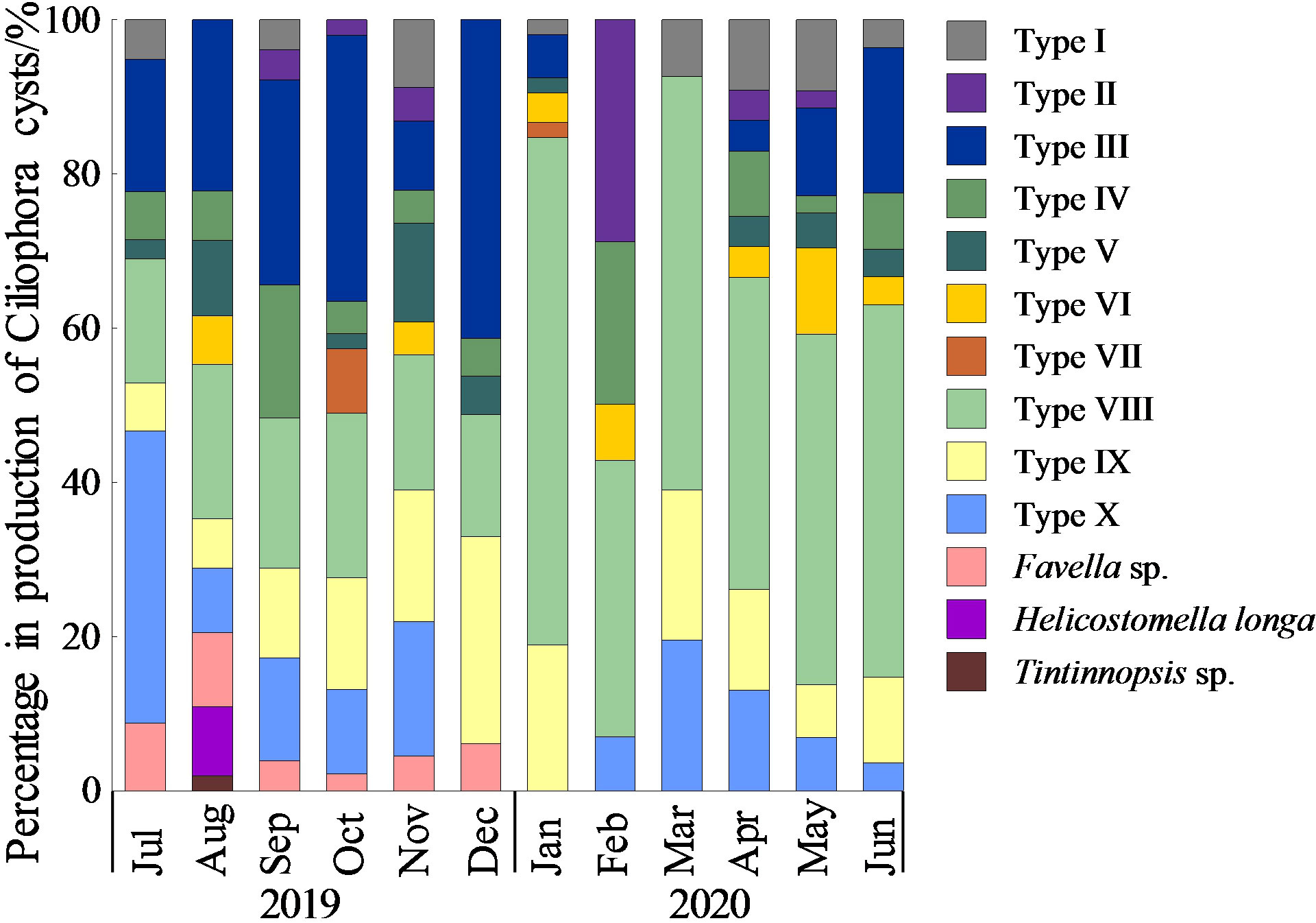
The community of Ciliophora cysts was dominated by aloricate Oligotrichea cysts, with an average contribution of 96.2 ± 6.0% through the whole year. The dominant types were Type VIII, Type III, Type IX and Type X, and they accounted for 33.3 ± 17.2%, 15.9 ± 13.4%, 12.7 ± 7.3% and 11.5 ± 10.4% of the total cyst production through the whole year, respectively (Figure 8).
3.4 Annual cycle of sedimentation of Ciliophora cysts
The production rate of aloricate Oligotrichea cysts ranged from 23.2×103–263.9×103 cysts m-2 d-1 (on average 92.9×103 ± 72.5×103 cysts m-2 d-1). The production rate was lowest in April and higher in January, July and October. The production rate of Tintinnina cysts ranged from 0–20.0×103 cysts m-2 d-1 (on average 4.2×103 ± 6.5×103 cysts m-2 d-1). No production of Tintinnina cysts was detected from January to June, and the production peaked in July and August. In the case of total Ciliophora cysts, the production rate ranged from 23.2×103–269.8×103 cysts m-2 d-1 (on average 97.1×103 ± 74.4×103 cysts m-2 d-1), and the production exhibited the same annual trend with aloricate Oligotrichea cysts (Figure 9).
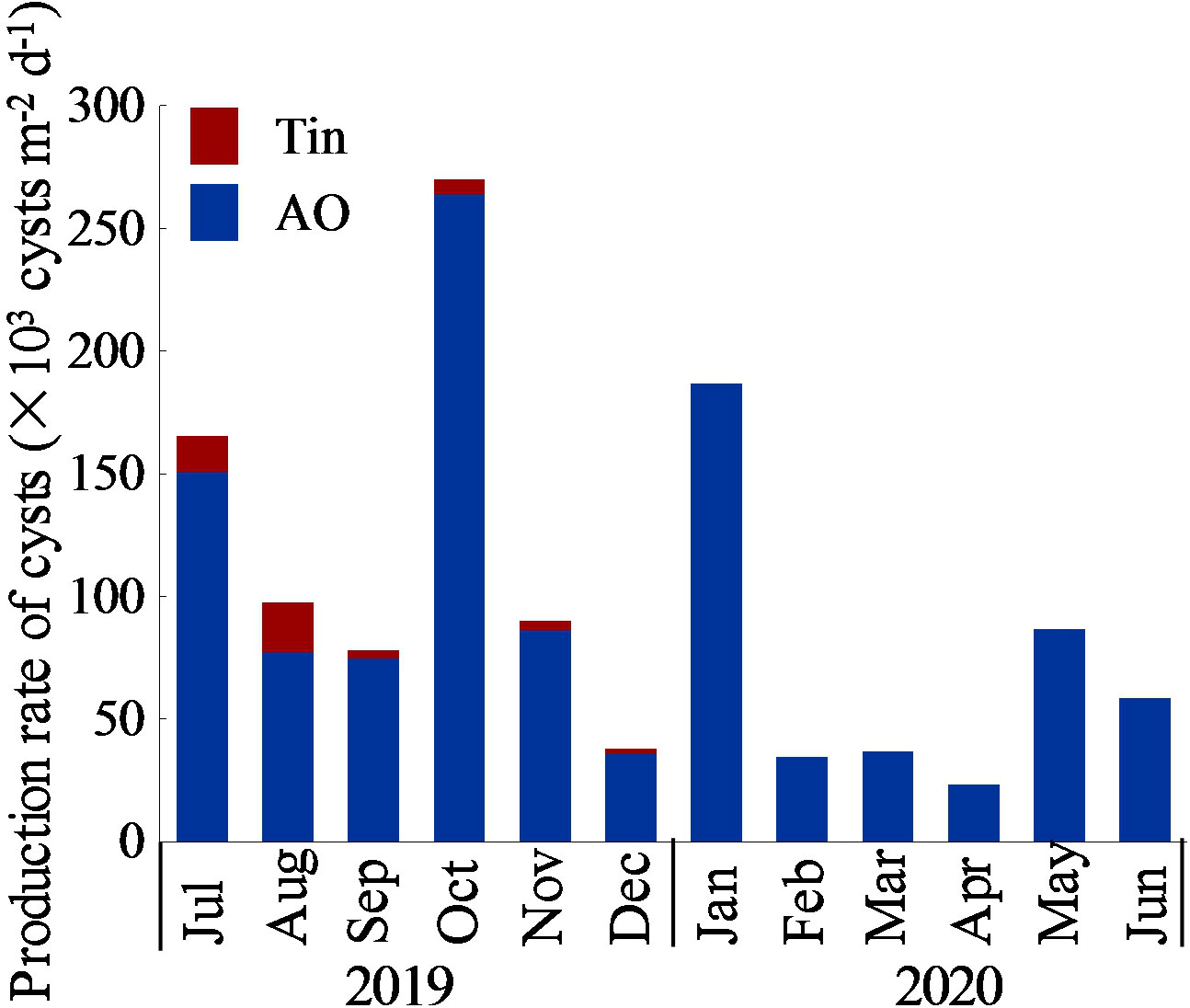
Figure 9 Monthly variation of production rate of aloricate Oligotrichea (AO) and Tintinnina (Tin) cysts.
The production of different types of cysts exhibited different seasonal patterns, and could be dived into three classes. In Class I, the production peaked in one single season. For instance, the production of three types of Tintinnina cysts all peaked in summer (Figures 10A–C); the production of Type III and VII both peaked in autumn (Figures 10D, E); the production of Type VIII peaked in winter (Figure 10F). In Class II, the production peaked in two seasons. For example, the production of Type IV, V and X peaked in both summer and autumn (Figures 10G–I); the production of Type II and IX peaked in both autumn and winter (Figures 10J, K). In Class III, the production occurred throughout the year without obvious seasonal pattern, such as Type I and VI (Figures 10L, M).
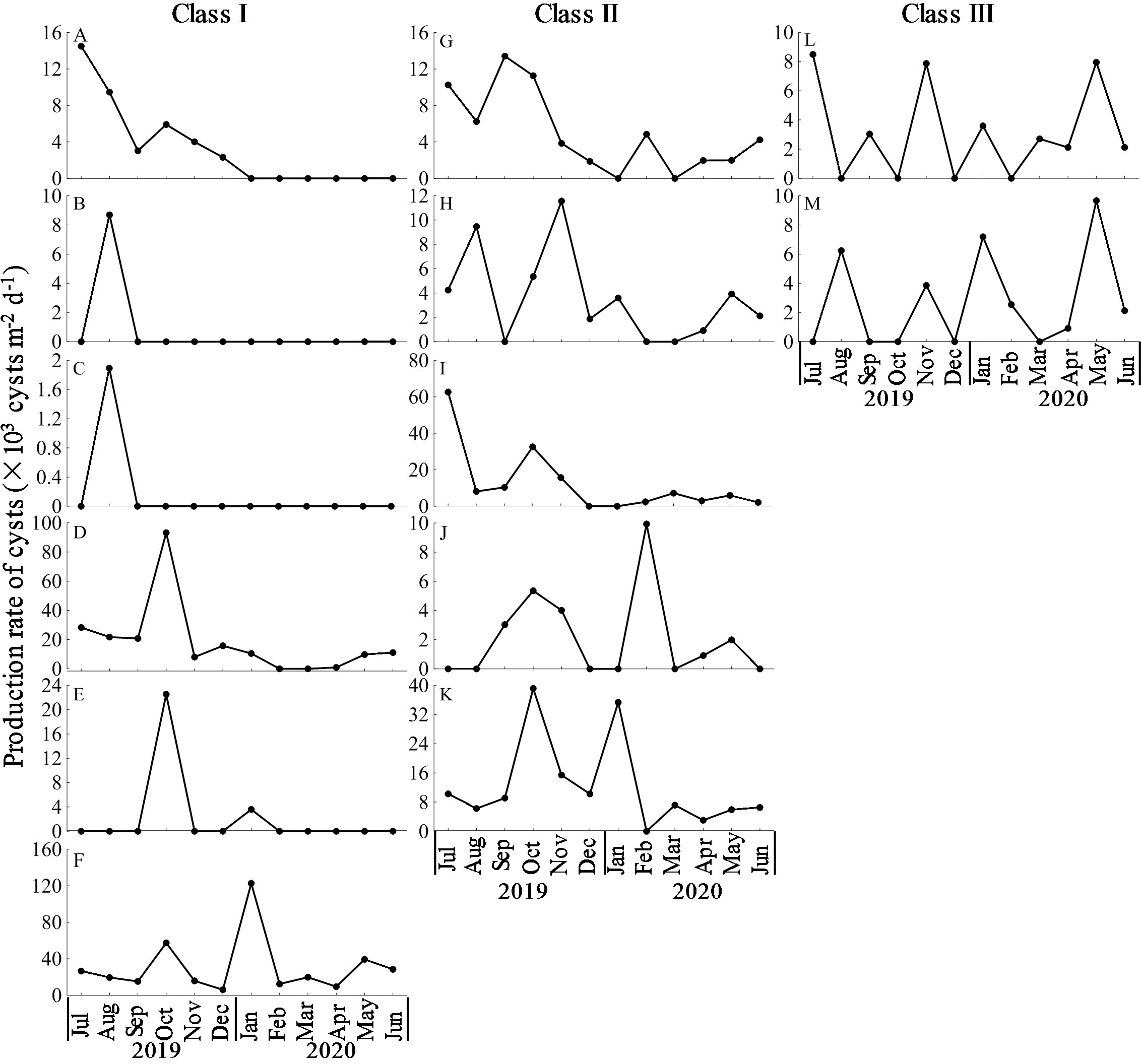
Figure 10 Monthly variation of production rate of Ciliophora cysts. (A): cyst of Favella sp.; (B): cyst of Helicostomella longa; (C): cyst of Tintinnopsis sp.; (D): Type III; (E): Type VII; (F): Type VIII; (G): Type IV; (H): Type V; (I): Type X; (J): Type II; (K): Type IX; (L): Type I; (M): Type VI.
3.5 Relationships between production rate of Ciliophora cysts and environmental factors
The production rate of two types of aloricate Oligotrichea cysts, including Type IV and Type X, showed significant positive correlations with water temperature and Chl a concentration. The production rate of Type III, Type IV and Type X showed a significant negative correlation with salinity. The production rate of Type II, tentatively identified as the cyst of Strombidinopsis sp., showed a significant positive correlation with the abundance of Strombidinopsis sp. (Table 1).
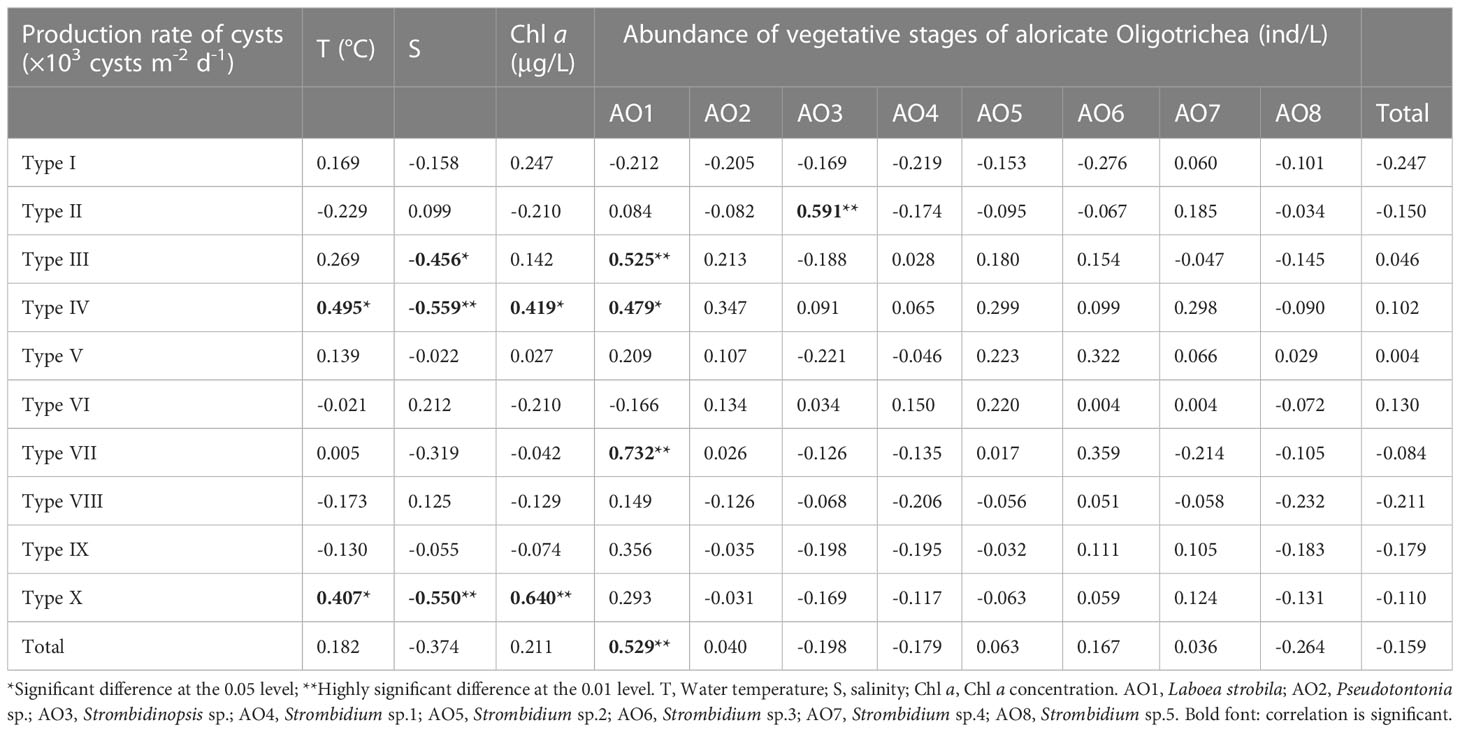
Table 1 Correlation between the production rate of aloricate Oligotrichea cysts and water temperature, salinity, Chlorophyll a (Chl a) concentration, and abundance of vegetative stages of aloricate Oligotrichea.
In Tintinnina cysts, the production rate of cyst of Favella sp. showed significant positive correlations with water temperature and Chl a concentration, and a significant negative correlation with salinity. The production rate of cyst of Favella sp. showed a significant positive correlation with the abundance of Favella panamensis. The production rate of cyst of Tintinnopsis sp. showed a significant positive correlation with the abundance of Tintinnopsis nana (Table 2).
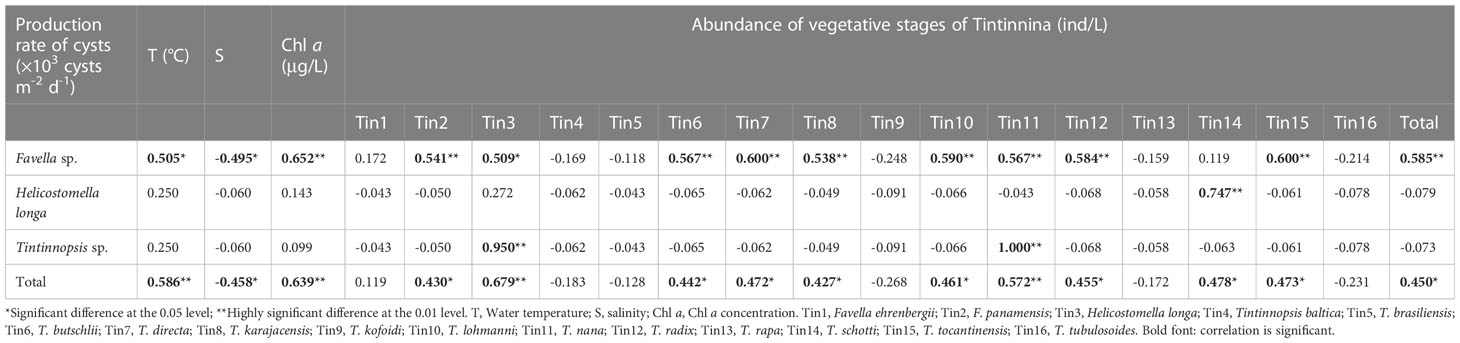
Table 2 Correlation between the production rate of Tintinnina cysts and water temperature, salinity, Chlorophyll a (Chl a) concentration, and abundance of vegetative stages of Tintinnina.
Mantel test showed that there was no significant correlation between aloricate Oligotrichea cyst community and environmental factors. In the case of Tintinnina cysts, Mantel test showed that water temperature (R=0.184, P=0.024) and Chl a concentration (R=0.331, P=0.025) were the main environmental factor driving the annual change of Tintinnina cyst community (Table 3).
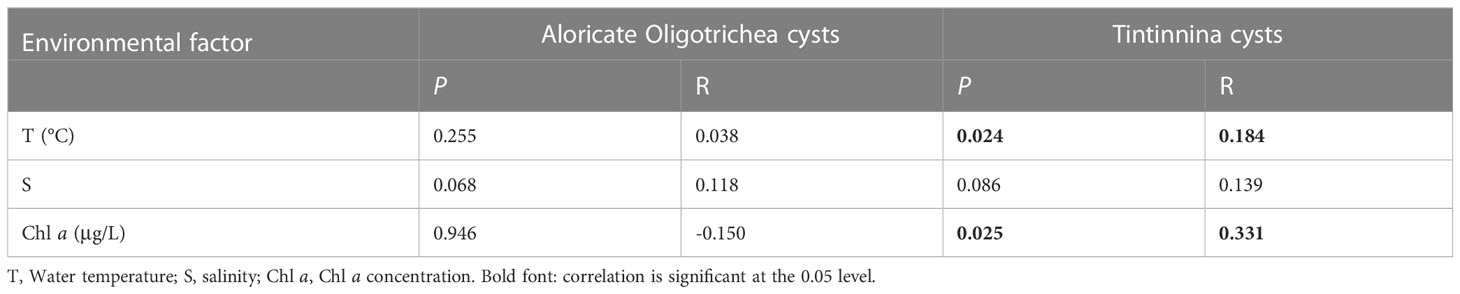
Table 3 Mantel test between Ciliophora cyst community (measured as Bray-Curtis dissimilarity) and environmental factors.
4 Discussion
In our study, a total of 13 types of Ciliophora cysts were identified by morphological characteristics using fluorescence microscopy. This method may lead to inaccuracy in species identification, which has been mentioned in previous papers (Yu et al., 2022). It would be necessary to apply molecular biology techniques, such as DNA metabarcoding, to ecological studies of Ciliophora cysts in the future.
Water depth was shallower than 10 m and only surface water was sampled in our study. No significant difference was observed in water temperature and salinity between surface and bottom layers in coastal waters of Bohai Bay (Shen, 1999; Song, 2009), and thus water temperature and salinity in surface water could be used as a reference for bottom layer in our study.
4.1 Production rate of Ciliophora cysts
To the best of our knowledge, investigations on the seasonal pattern of encystment of Ciliophora in natural waters were limited, except for few studies in Bedford Basin (Paranjape, 1980), the North Atlantic and North Sea (Reid and John, 1981), Conception Bay and Placentia Bay (Davis, 1985), the western English Channel (Reid, 1987), Onagawa Bay (Kim et al., 2002), Masan Bay (Kim et al., 2008) and north coast of Taiwan island (Chao et al., 2013). In early study, Paranjape (1980) collected water samples directly to observe tintinnid cysts. Tintinnid cysts were found from plankton samples by CPR (Continuous Plankton Recorder, Reid and John, 1981) or closing net hauled vertically (Davis, 1985). Later, sediment traps were applied to collect sinking cysts in the water, which are the most convenient tool to obtain qualitative information, such as production rate of cysts and timing of encystment (Kim et al., 2008).
In Bohai Bay, the production rate of Ciliophora cysts varied greatly from species to species, and the maximum value ranged from 1.9×103 cysts m-2 d-1 (cysts of Tintinnopsis sp.) to 1.2×105 cysts m-2 d-1 (Type VIII). Our data is similar to that in previous studies (Reid, 1987; Kim et al., 2002; Kim et al., 2008). The maximum production rate of cysts of Strombidium crassulum was almost 3.5×104 cysts m-2 d-1 in the western English Channel (Reid, 1987). Peak production rate of cysts of Cyrtostrombidum boreale was 5.7×103 cysts m-2 d-1 in Onagawa Bay (Kim et al., 2002). The production of cysts of Strombidium capitatum reached a maximum of 1.4×105 cysts m-2 d-1 in Onagawa Bay, and about 9.0×103 cysts m-2 d-1 in Masan Bay (Kim et al., 2008). The maximum production of two dominant cysts was 1.8×104 and 2.4×104cysts m-2 d-1, respectively in north coast of Taiwan island (Chao et al., 2013).
4.2 Seasonal differences of encystment between aloricate Oligotrichea and Tintinnina
The seasonality of encystment could be speculated by observing sinking cysts in the water periodically and continuously. Most previous studies focused on the seasonal pattern of encystment of single species (Paranjape, 1980; Davis, 1985; Reid, 1987; Kim et al., 2002; Kim et al., 2008). Reid and John (1981) recorded seasonal occurrence of several types of Tintinnina cysts. Chao et al. (2013) concerned about the seasonal dynamics of the entire Ciliophora cyst community (including 5 types of aloricate Oligotrichea cysts and one type of Tintinnina cysts). In this study, we carried out annual change study in production of 10 types of aloricate Oligotrichea cysts and 3 types of Tintinnina cysts, and compared seasonal differences of encystment between aloricate Oligotrichea and Tintinnina in Bohai Bay.
In Bohai Bay, the encystment of Favella sp. mainly occurred in July–August, with minor occurrence from September to December. The sedimentation of cysts of Helicostomella longa and Tintinnopsis sp. were only found in August. Our data is consistent with previous studies that encystment of Tintinnina was primarily a late summer/autumn event (Paranjape, 1980; Reid and John, 1981; Davis, 1985). The abundance of cysts of Helicostomella subulata was high in September, October and November in Bedford Basin (Paranjape, 1980). Tintinnid cysts were most abundant in the late summer/autumn in the North Atlantic and North Sea (Reid and John, 1981). Cysts of Acanthostomella norvegica were mainly found in October and November in Placentia Bay (Davis, 1985). Cysts of these species, which may function as “overwinter stages”, could help Tintinnina to survive in “unfavorable” environmental conditions of winter (Paranjape, 1980; Reid, 1987). The encystment in late summer/autumn, which could provide a means of hibernating through the winter, may have important implications for population persistence (Lennon and Jones, 2011).
In terms of aloricate Oligotrichea, encystment was found at all seasons and the seasonal patterns varied among species in Bohai Bay. Most species could encyst in late summer/autumn, which is similar to Strombidium capitatum whose encystment peaked in autumn in Masan Bay (Kim et al., 2008). These cysts formed in late summer/autumn, similar to Tintinnina cysts, could help ciliates survive “unfavorable” conditions in winter (Müller and Wünsch, 1999). In addition, there were three types of aloricate Oligotrichea (Type II, Type VIII and Type IX) with mass encystment of cysts in January/February. The mass encystment in winter, to our knowledge, has not been recorded in previous investigations. Further studies should be carried out to figure out the role of the winter cysts in population persistence of Ciliophora.
Also, the production of two types of aloricate Oligotrichea (Type I and Type VI) occurred throughout the year without obvious seasonal pattern in our study. Cyst formation of these ciliates maybe part of the regular life cycle and does not require a specific environmental stimulus (Verni and Rosati, 2011). Combination of field investigations and laboratory culture experiments would be needed to confirm this assumption.
4.3 Encystment of Ciliophora and environmental factors
Ciliophora cysts are probably produced in response to environmental stresses, like food shortage, water temperature and salinity, tidal rhythm, etc. However, factors acting in natural environments are still largely unknown (Verni and Rosati, 2011; Kamiyama, 2013). In this study, we found production rate of several types of cysts (Type IV, Type X and Favella sp.) exhibited a significant positive correlation with water temperature, and a significant negative correlation with salinity. This suggested that the variation of temperature and salinity might play important roles in cyst production of Ciliophora, which was consistent with previous studies. The encystment of Cyrtostrombidium boreale in early spring may be triggered by a rapid increase of temperature in Onagawa Bay (Kim et al., 2002). High temperature could impede cyst production of Pelagostrombidium fallax during the summer in freshwater Lake Constance (Müller and Wünsch, 1999). Chao et al. (2013) suggested that variations of salinity might excite Ciliophora to enhance encystment in a coastal ecosystem of subtropical western Pacific. All of these speculations were based on data observed in natural environments, and have not been tested experimentally. Culture experiments in laboratory are needed for a better understanding of the relationships between temperature and salinity and encystment of Ciliophora.
In our study, most species exhibited no significant correlation between cyst production and the presence of vegetative stages, which was consistent with the study in the Mar Piccolo of Taranto (Rubino and Belmonte, 2021). On the one hand, this absence of correspondence was due to identification difficulties of cysts (which cysts belong to which species). A combination of microscopic morphology observation and single-cell sequencing would assist in linking the identity of cysts to vegetative stages (Rubino and Belmonte, 2021). Another reason for this lack of connection was that vegetative stages were active in the past and temporarily absent at the time of cyst study (Rubino and Belmonte, 2021). This time-lag between vegetative stages and cysts was found in both laboratory culture (Müller, 1996) and field investigation (Müller and Wünsch, 1999; Kim et al., 2002; Kim et al., 2008). Cyst abundance of the freshwater ciliate Pelagostrombidium fallax began to increase after the vegetative cells reached a high abundance value in laboratory culture (Müller, 1996). Highest production of Limnostrombidium viride cysts (Müller and Wünsch, 1999), Cyrtostrombidium boreale cysts (Kim et al., 2002) and Strombidium capitatum cysts (Kim et al., 2008) were all recorded shortly after maximum development of the motile population in field investigation. A combination of laboratory culture experiment and field investigation is needed to find out the correspondence between vegetative stages and cysts, and explore the mechanism of vegetative population proliferation on Ciliophora encystment.
5 Conclusions
For now, studies on encystment of planktonic Ciliophora in natural environments were limited. Our study indicates that the production of Ciliophora cysts exhibited obvious seasonal variation, and there were obvious seasonal differences of encystment between aloricate Oligotrichea and Tintinnina in Bohai Bay. The variation of temperature and salinity might play important roles in encystment of various types of Ciliophora. Culture experiments in lab would be required for a better understanding of the role of environmental factors on Ciliophora encystment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YY: ciliate taxonomy and counting, data analysis, writing original draft. WZ: conceptualization, ciliate taxonomy, writing original draft. ZL: field sampling, figure drawing, writing original draft. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by Natural Science Foundation of China (NSFC No. 41806205 and 41576164) and Chineseisch-Deutsches Mobilitatsprogramm: CHESS-Chinese and European Coastal Shelf Seas Ecosystem Dynamics-a Comparative Assessment (M-0053).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agatha S., Struder-Kypke M. C., Beran A., Lynn D. H. (2005). Pelagostrobilidium neptuni (Montagnes and Taylor 1994) and Strombidium biarmatum nov spec. (Ciliophora, Oligotrichea): hylogenetic position inferred from morphology, ontogenesis, and gene sequence data. Eur. J. Protistol. 41 (1), 65–83. doi: 10.1016/j.ejop.2004.09.005
Belmonte G., Rubino F. (2019). Resting cysts from coastal marine plankton. Oceanogr. Mar. Biol. 57, 1–88. doi: 10.1201/9780429026379-1
Calbet A. (2008). The trophic roles of microzooplankton in marine systems. ICES J. Mar. Sci. 65 (3), 325–331. doi: 10.1093/icesjms/fsn013
Chao C. F., Tsai A. Y., Ishikawa A., Chiang K. P. (2013). Seasonal dynamics of ciliate cysts and the impact of short-term change of salinity in a eutrophic coastal marine ecosystem. Terr. Atmos. Ocean. Sci. 24 (6), 1051–1061. doi: 10.3319/TAO.2013.07.18.01(Oc)
Davis C. C. (1985). Acanthostoella norvegica (Daday) in insular Newfoundland waters, Canada (Protozoa: Tintinnina). Internationale Rev. Der Gesamten Hydrobiologie 70 (1), 21–26. doi: 10.1002/iroh.19850700103
Ganser M. H., Bartel H., Weißenbacher B., Andosch A., Lütz-Meindl U., Radacher P., et al. (2022). A light and electron microscopical study on the resting cyst of the tintinnid Schmidingerella (Alveolata, Ciliophora) including a phylogeny-aware comparison. Eur. J. Protistol. 86, 125922. doi: 10.1016/j.ejop.2022.125922
Hada Y. (1937). The fauna of akkeshi bay IV. the pelagic ciliata. J. Fac. Sci. Hokkaido Imp. Univ. Ser. VI. Zoology 5 (3), 143–216.
Harris R., Wiebe P., Lenz J., Skjoldal H., Huntley M. (2000). ICES zooplankton methodology manual (San Diego: Academic Press).
Ichinomiya M., Nakamachi M., Taniguchi A. (2004). A practical method for enumerating cysts of ciliates in natural marine sediments. Aquat. Microb. Ecol. 37 (3), 305–310. doi: 10.3354/ame037305
Kamiyama T. (2013). “Comparative biology of tintinnid cysts,” in The biology and ecology of tintinnid ciliates: models for marine plankton. Eds. Dolan J. R., Montagnes D. J. S., Agatha S., Coats D. W., Stoecker D. K. (Oxford: Wiley-Blackwell), 171–185.
Kim Y. O., Ha S., Taniguchi A. (2008). Morphology and in situ sedimentation of the cysts of a planktonic oligotrich ciliate, Strombidium capitatum. Aquat. Microb. Ecol. 53 (2), 173–179. doi: 10.3354/ame01241
Kim Y. O., Suzuki T., Taniguchi A. (2002). A new species in the genus Cyrtostrombidium (Ciliophora, Oligotrichia, Oligotrichida): its morphology, seasonal cycle and resting stage. J. Eukaryotic Microbiol. 49 (4), 338–343. doi: 10.1111/j.1550-7408.2002.tb00380.x
Kim Y. O., Taniguchi A. (1995). Excystment of the oligotrich ciliate Strombidium conicum. Aquat. Microb. Ecol. 9 (2), 149–156. doi: 10.3354/ame009149
Lennon J. T., Jones S. E. (2011). Microbial seed banks: the ecological and evolutionary implications of dormancy. Nat. Rev. Microbiol. 9 (2), 119–130. doi: 10.1038/nrmicro2504
Li Y., Wang Y., Zhang S., Maurer-Alcalá X. X., Yan Y. (2022). How ciliated protists survive by cysts: some key points during encystment and excystment. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.785502
Montagnes D. J. S., Allen J., Brown L., Bulit C., Davidson R., Fielding S., et al. (2010). Role of ciliates and other microzooplankton in the Irminger Sea (NW Atlantic ocean). Mar. Ecol. Prog. Ser. 411, 101–115. doi: 10.3354/meps08646
Müller H. (1996). Encystment of the freshwater ciliate Pelagostrombidium fallax (Ciliophora, Oligotrichida) in laboratory culture. Aquat. Microb. Ecol. 11 (3), 289–295. doi: 10.3354/ame011289
Müller H., Wünsch C. (1999). Seasonal dynamics of cyst formation of pelagic strombidiid ciliates in a deep prealpine lake. Aquat. Microb. Ecol. 17 (1), 37–47. doi: 10.3354/ame017037
Paranjape M. A. (1980). Occurrence and significance of resting cysts in a hyaline tintinnid, Helicostomella subulata (Ehre.) Jorgensen. J. Exp. Mar. Biol. Ecol. 48 (1), 23–33. doi: 10.1016/0022-0981(80)90004-0
Pierce R. W., Turner J. T. (1992). Ecology of planktonic ciliates in marine food webs. Rev. Aquat. Sci. 6 (2), 139–181.
Reid P. C. (1987). Mass encystment of a planktonic oligotrich ciliate. Mar. Biol. 95 (2), 221–230. doi: 10.1007/BF00409009
Reid P. C., John A. W. G. (1981). A possible relationship between chitinozoa and tintinnids. Rev. Palaeobot. Palyno. 34 (2), 251–262. doi: 10.1016/0034-6667(81)90043-9
Romano F., Symiakaki K., Pitta P. (2021). Temporal variability of planktonic ciliates in a coastal oligotrophic environment: mixotrophy, size classes and vertical distribution. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.641589
Rubino F., Belmonte G. (2021). Habitat shift for plankton: the living side of benthic-pelagic coupling in the Mar Piccolo of Taranto (southern Italy, Ionian Sea). Water 13, 3619. doi: 10.3390/w13243619
Shen Z. L. (1999). Hydrochemical elements in Bohai Bay and its eastern part waters. Stud. Mar. Sin. 41, 51–59.
Song W. P. (2009). The analysis of the structure of T-s and the current characteristics in bohai Sea during winter and summer. Master’s thesis (Qingdao, China: Ocean University of China).
Utermöhl H. (1958). Zur vervollkommnung der quantitativen phytoplankton methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 9, 1–38. doi: 10.1080/05384680.1958.11904091
Verni F., Rosati G. (2011). Resting cysts: a survival strategy in Protozoa ciliophora. Ital. J. Zool. 78 (2), 134–145. doi: 10.1080/11250003.2011.560579
Yang E. J., Choi J. K., Hyun J. H. (2008). Seasonal variation in the community and size structure of nano- and microzooplankton in Gyeonggi Bay, yellow Sea. Estuar. Coast. Shelf Sci. 77 (3), 320–330. doi: 10.1016/j.ecss.2007.09.034
Yin G. D. (1952). Preliminary investigation of tintinnopsis in Jiaozho Bay. J. Shandong Univ. 2, 36–56.
Yu Y., Zhang W. C., Feng M. P., Xu X. F. (2022). Seasonal variation of vegetative stages and cysts of planktonic ciliates in a temperate coastal bay. J. Plankton Res. 44 (6), 923–934. doi: 10.1093/plankt/fbac047
Keywords: planktonic Ciliophora, cyst, sedimentation, seasonal variation, Bohai Bay
Citation: Yu Y, Zhang W and Lin Z (2023) Annual variation of in situ sedimentation of planktonic Ciliophora cysts in a temperate bay. Front. Mar. Sci. 10:1186034. doi: 10.3389/fmars.2023.1186034
Received: 14 March 2023; Accepted: 16 June 2023;
Published: 05 July 2023.
Edited by:
Jun Sun, China University of Geosciences Wuhan, ChinaReviewed by:
Genuario Belmonte, University of Salento, ItalyHenglong Xu, Ocean University of China, China
Weiwei Liu, South China Sea Institute of Oceanology, Chinese Academy of Sciences (CAS), China
Copyright © 2023 Yu, Zhang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wuchang Zhang, d3VjaGFuZ3poYW5nQDE2My5jb20=
 Ying Yu
Ying Yu Wuchang Zhang
Wuchang Zhang Zhenxian Lin5
Zhenxian Lin5