
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci. , 20 October 2023
Sec. Discoveries
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1180219
This article is part of the Research Topic Marine Turtles View all 8 articles
Bycatch is a major global threat to marine megafauna and occurs in nearly all fishing fleets, including small-scale fisheries that use gillnets. Gillnets represent a threat to endangered air-breathing megafauna, who incidentally entangle in bottom-set gillnets and suffocate after being attracted by bait that is secured on fishing gear. We here provide the first evidence that hawksbill turtles feed on trapped fish in gillnets, suggesting that potential prey items trapped in gillnets may act as additional bait, attracting carnivorous sea turtles towards this threat. This overlooked depredating behaviour potentially explains and increases the likelihood of critically endangered hawksbill turtle bycatch in gillnet fisheries, calling for technological and management solutions.
Incidental take, i.e., the bycatch of marine megafauna, occurs in nearly all fishing fleets and is of growing global concern (Baum et al., 2003; Lewison et al., 2004; Lewison and Crowder, 2007; Peckham et al., 2007; Rees et al., 2016). Recent research reveals that marine migratory megafauna, including cetaceans (Brownell et al., 2019), elasmobranchs (Temple et al., 2018) and sea turtles (Wallace et al., 2013a), frequent coastal areas within the range of small-scale fisheries, potentially increasing the likelihood of producing high levels of bycatch (Block et al., 2005; James et al., 2005) and/or depredation, i.e., the predation of fish trapped by fishing gear while the gear is still in the water (Fader et al., 2021). The deployment of gillnets is a typical technique used in small-scale fisheries, yet this technique poses lethal threats to marine mammals (Muir and Kiszka, 2012; Temple et al., 2019), elasmobranchs and batoids (Temple et al., 2018), and sea turtles (Lewison and Crowder, 2007; Peckham et al., 2007). The magnitude of these threats can be seen in the International Union for Conservation of Nature (IUCN) Red List status for sea turtles, listing 6 out of 7 as vulnerable [Olive Ridley Turtle (Abreu-Grobois et al., 2008), Leatherback Turtle (Wallace et al., 2013b), Loggerhead Turtle (Casale and Tucker, 2017)], endangered [Green Turtle, (Seminoff et al., 2004)], or critically endangered [Kemp’s Ridley (Wibbels and Bevan, 2019), Hawksbill Turtle (Mortimer and Donnelly, 2008)]. Despite having historically been hunted for trading purposes, hawksbill sea turtles worldwide are now particularly susceptible to being entangled within marine debris (including fishing gear), as well as mistakenly ingesting it (Mortimer and Donnelly, 2008).
The importance of hawksbill sea turtles as keystone species for reef and seagrass ecosystems has been highlighted before (Jackson, 1997), and reductions to their population have been hypothesised to cause pivotal effects on these ecosystems, due to their role as marine grazers (León and Bjorndal, 2002). E. imbricata is considered an omnivorous species (Bjorndal, 2017), with behavioural observations (Blumenthal et al., 2009), stomach content analyses (Carr and Stancyk, 1975), oesophageal lavage (Forbes, 1999), and stable isotope analyses from blood plasma and skin tissue (Clyde-Brockway et al., 2022) all showing that hawksbill sea turtles feed on benthic (e.g., sponges, tunicates, ascidians; León and Bjorndal, 2002; Blumenthal et al., 2009; Carríon-Cortez et al., 2013) or pelagic (jellyfish; Blumenthal et al., 2009) invertebrates and macroalgae (Carr and Stancyk, 1975; León and Bjorndal, 2002). Only anecdotal reports exist that document hawksbill sea turtle hatchlings or captive adults, where they were found to feed on fish fillet pieces provided in an experimental setup (Witzell, 1983; Mellgren et al., 1994).
Here, we report the first visual evidence of hawksbill turtles engaging in the natural depredation of fish trapped in gillnets (Figure 1A, Supplementary Video 1). Our observation was made at Rabigh beach, Saudi Arabia, in the Central Red Sea on the 20th of January 2023 at 12:20 pm, where a gillnet was deployed by local fishermen on a reef flat (30 to 80 cm water depth covering an area of approx. 30 x 30 m, water temperature: 26°C). The turtle, which was approximately 50 to 70 cm long, was observed an hour after low tide [low tide: 11.12 am (GASGI, 2023)]. This observation is of particular importance for two reasons: firstly, we were able to document that hawksbill turtles have a broader dietary range that exceeds herbivory, spongivory or jellyfish carnivory (Naro-Maciel et al., 2008), with piscivory being part of it. Thus far, only a few anecdotal observations have indicated that E. imbricata may consider fish a food source when provided as size-chopped pieces to captive individuals (Witzell, 1983) or as hatchlings (Mellgren et al., 1994), with no prior knowledge or direct evidence of piscivory of wild individuals. Secondly, we observed that an individual E. imbricata was able to disentangle itself from the gillnet after having fed on a trapped fish (Figure 1B) before swimming along the deployed gillnet to continue its depredation of fish trapped in the net (Figure 1C). Personal communication with the fishermen who deployed the gillnets confirmed that both the feeding on trapped fish and the ability to disentangle itself are well-known for this sea turtle species among local fishermen in the Central Red Sea (El-Khaled 2023, personal communication). Therefore, we suggest that trapped fish are a previously overlooked food source for hawksbill turtles, which is outside of their regular diet, presumably due to the Hawksbill’s limited capacity to hunt them in the wild. We hypothesize that our observations provide a mechanism to explain the active attraction of hawksbill sea turtles to gillnets through depredation opportunities, with its catch acting as additional bait to attract the turtles. Ultimately, this may contribute to hawksbill sea turtle mortality as bycatch, although the significance of fish depredation from gillnets to the diet of hawksbill sea turtles regionally remains unknown and warrants future investigation.
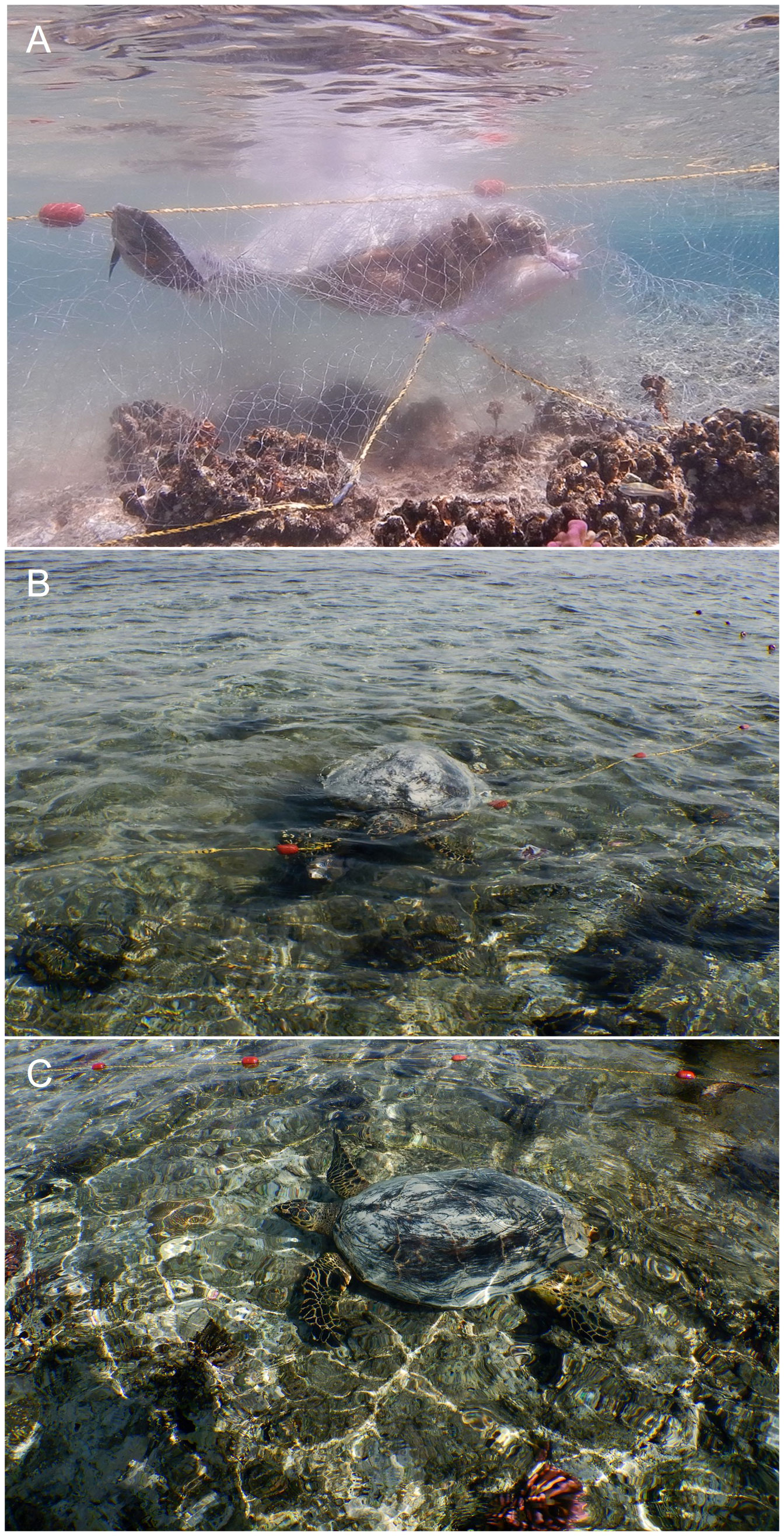
Figure 1 (A) Hawksbill sea turtle (Eretmochelys imbricata) depredating sohal surgeonfish (Acanthurus suhal) trapped in gillnet; (B) E. imbricata disentangling from gillnet; (C) E. imbricata swimming along gillnet to the next caught fish. Photos were taken by Yusuf C. El-Khaled.
Hence, the (regular) deployment of gillnets may have unintended consequences. Several studies indicate that multiple sea turtles species, such as hawksbill (Wood et al., 2017), loggerhead (Hawkes et al., 2011) and green turtles (Christiansen et al., 2017), can navigate within familiar reaches of their home range where their prey distribution is well-known. As such, hawksbill sea turtles could regularly engage in this depredation behaviour by collecting fish from bottom-set gillnets, despite the high risk of entanglement and ending up as bycatch. Deploying gillnets outside of regular hawksbill habitats could minimise the risk of sea turtle bycatch.
In conclusion, the disentanglement behaviour observed here represents a first observation, adding to recent evidence of limb use by hawksbill turtles when feeding (Fujii et al., 2018), in this case, to catch the entangled prey while disentangling themselves from the gillnet. The extent to which these patterns could apply to further sea turtle species remains to be determined. Ultimately, the observed behaviour contributes to the urgent need for i) practical and affordable solutions (i.e., bycatch reduction technologies, BRTs; Lucas and Berggren, 2023) and ii) management approaches to reduce the risk of bycatch in gillnet fisheries. Bycatch reduction may be achieved using selective deterrent devices (reviewed in Lucas and Berggren, 2023). For instance, BRTs that have been evaluated and discussed recently involve the deployment of illuminated gillnets with light-emitting diodes (LEDs), chemical lightsticks, or ultraviolet light (Wang et al., 2010; Wang et al., 2013), which may selectively deter unwanted bycatch, such as sea turtles. Recent studies demonstrate that gillnets equipped with green or ultraviolet LEDs can significantly reduce loggerhead and green turtle bycatch (Wang et al., 2010; Wang et al., 2013; Ortiz et al., 2016; Virgili et al., 2018) and seem to be particularly promising for future investigations and management implementations (Lucas and Berggren, 2023). However, knowledge about the potential benefitting effect of LED-equipped gillnets for hawksbill sea turtles is particularly scarce. The extent to which illuminated gillnets are beneficial, or having the undesired effect of attracting hawksbill sea turtles is not known, despite their overall successes in a few studies (Cox et al., 2007; Lucas and Berggren, 2023).
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving animals with the local legislation and institutional requirements as the individuals were observed in their natural environment with fishing devices being used by local fishermen without intentional preparation or placement by the authors.
YE-K wrote the manuscript with significant contributions of CD and RP. All authors contributed to the article and approved the submitted version.
This research was funded through Kaust grant number BAS/1/1095-01-01.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor AG and reviewer BS declared a past co-authorship with the author CD.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1180219/full#supplementary-material
Abreu-Grobois A., Plotkin P., IUCN SSC Marine Turtle Specialist Group (2008). Lepidochelys olivacea. The IUCN red list of threatened species. IUCN Red List Threat. Species 2008, 8235. doi: 10.2305/IUCN.UK.2008.RLTS.T11534A3292503.en
Baum J. K., Myers R. A., Kehler D. G., Worm B., Harley S. J., Doherty P. (2003). Collapse and Conservation of Shark Populations in the Northwest Atlantic. Science 299 (5605), 389–392. doi: 10.1126/science.1079777
Bjorndal K. A. (2017). “Foraging ecology and nutrition of sea turtles,” in The biology of sea turtles (Boca Raton, Florida, USA: CRC Press), 199–231.
Block B. A., Teo S. L. H., Walli A., Boustany A., Stokesbury M. J. W., Farwell C. J., et al. (2005). Electronic tagging and population structure of Atlantic bluefin tuna. Nature 434, 1121–1127. doi: 10.1029/2002PA000862
Blumenthal J. M., Austin T. J., Bell C. D. L., Bothwell J. B., Broderick A. C., Ebanks-Petrie G., et al. (2009). Ecology of hawksbill turtles, Eretmochelys imbricata, on a western caribbean foraging ground. Chelonian Conserv. Biol. 8, 1–10. doi: 10.2744/CCB-0758.1
Brownell R. L., Reeves R. R., Read A. J., Smith B. D., Thomas P. O., Ralls K., et al. (2019). Bycatch ingillnet fisheries threatens critically endangeredsmall cetaceansand other aquatic megafauna. Endanger. Species Res. 40, 285–296. doi: 10.3354/ESR00994
Carr A., Stancyk S. (1975). Observations on the ecology and survival outlook of the hawksbill turtle. Biol. Conserv. 8, 161–172. doi: 10.1016/0006-3207(75)90060-9
Carríon-Cortez J., Canales-Cerro C., Arauz R., Riosmena-Rodríguez R. (2013). Habitat use and diet of juvenile eastern pacific hawksbill turtles (Eretmochelys imbricata) in the north Pacific coast of Costa Rica. Chelonian Conserv. Biol. 12, 235–245. doi: 10.2744/CCB-1024.1
Casale P., Tucker A. D. (2017). Loggerhead turtle caretta caretta. IUCN Red List Threat. Species 8235, e.T3897A119333622.
Christiansen F., Esteban N., Mortimer J. A., Dujon A. M., Hays G. C. (2017). Diel and seasonal patterns in activity and home range size of green turtles on their foraging grounds revealed by extended Fastloc-GPS tracking. Mar. Biol. 164, 1–11. doi: 10.1007/s00227-016-3048-y
Clyde-Brockway C. E., Heidemeyer M., Paladino F. V., Flaherty E. A. (2022). Diet and foraging niche flexibility in green and hawksbill turtles. Mar. Biol. 169, 1–18. doi: 10.1007/s00227-022-04092-1
Cox T. M., Lewison R. L., Žydelis R., Crowder L. B., Safina C., Read A. J. (2007). Comparing effectiveness of experimental and implemented bycatch reduction measures: The ideal and the real. Conserv. Biol. 21, 1155–1164. doi: 10.1111/j.1523-1739.2007.00772.x
Fader J. E., Elliott B. W., Read A. J. (2021). The challenges of managing depredation and bycatch of toothed whales in pelagic longline fisheries: two U.S. Case studies. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.618031
Forbes G. (1999). “Diet sampling and diet component analysis,” in Research and management techniques for the conservation of sea turtles, vol. 144 . Eds. Eckert K., Bjorndal K. A., Abreu A., Donnelly M. (Pennsylvania: SSC/IUCN Marine Turtle Specialist Group), 1–5.
Fujii J. A., McLeish D., Brooks A. J., Gaskell J., Van Houtan K. S. (2018). Limb-use by foraging marine turtles, an evolutionary perspective. PeerJ 2018, 1–11. doi: 10.7717/peerj.4565
GASGI (2023). Monthly Tide Tables - Red Sea and Gulf of Aqaba - January 2023. General Authority for Survey and Geospatial Information, Riyadh, Saudi Arabia
Hawkes L. A., Witt M. J., Broderick A. C., Coker J. W., Coyne M. S., Dodd M., et al. (2011). Home on the range: Spatial ecology of loggerhead turtles in Atlantic waters of the USA. Divers. Distrib. 17, 624–640. doi: 10.1111/j.1472-4642.2011.00768.x
James M. C., Ottensmeyer C. A., Myers R. A. (2005). Identification of high-use habitat and threats to leatherback sea turtles in northern waters: New directions for conservation. Ecol. Lett. 8, 195–201. doi: 10.1111/j.1461-0248.2004.00710.x
León Y. M., Bjorndal K. A. (2002). Selective feeding in the hawksbill turtle, an important predator in coral reef ecosystems. Mar. Ecol. Prog. Ser. 245, 249–258. doi: 10.3354/meps245249
Lewison R. L., Crowder L. B. (2007). Putting longline bycatch of sea turtles into perspective. Conserv. Biol. 21, 79–86. doi: 10.1111/j.1523-1739.2006.00592.x
Lewison R. L., Crowder L. B., Read A. J., Freeman S. A. (2004). Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 19, 598–604. doi: 10.1016/j.tree.2004.09.004
Lucas S., Berggren P. (2023). A systematic review of sensory deterrents for bycatch mitigation of marine megafauna. Rev Fish Biol Fisheries 33, 1–33. doi: 10.1007/s11160-022-09736-5
Mellgren R. L., Mann M. A., Zurita J. C. (1994). “Feeding on novel food in green (Chelonia mydas) and hawksbill (Eretmochelys imbricata) hatchling sea turtles,” in Proceedings of the thirteenth annual symposium on sea turtle biology and conservation 1993. National Marine Fisheries Service, Southeast Fisheries Science Center, Miami, Florida, USA 105–106. Available at: https://repository.library.noaa.gov/view/noaa/6160.
Mortimer J. A., Donnelly M. (2008). Eretmochelys imbricata. The IUCN red list of threatened species 112. doi: 10.2305/IUCN.UK.2008.RLTS.T8005A12881238.en
Muir C., Kiszka J. J. (2012). “Eastern african dugongs,” in Sirenian Conservation: Issues and Strategies in Developing Countries. Eds. Hines E. M., Reynolds J. E., Mignucci-Giannoni A. A., Marmontel M. (Gainesville, Florida, USA: University Press of Florida), 84–90.
Naro-Maciel E., Le M., FitzSimmons N. N., Amato G. (2008). Evolutionary relationships of marine turtles: A molecular phylogeny based on nuclear and mitochondrial genes. Mol. Phylogenet. Evol. 49, 659–662. doi: 10.1016/j.ympev.2008.08.004
Ortiz N., Mangel J. C., Wang J., Alfaro-Shigueto J., Pingo S., Jimenez A., et al. (2016). Reducing green turtle bycatch in small-scale fisheries using illuminated gillnets: The cost of saving a sea turtle. Mar. Ecol. Prog. Ser. 545, 251–259. doi: 10.3354/meps11610
Peckham S. H., Maldonado Diaz D., Walli A., Ruiz G., Crowder L. B., Nichols W. J. (2007). Small-scale fisheries bycatch jeopardizez endangered pacific loggerhead turtles. PLoS One 2, 1–6. doi: 10.1371/journal.pone.0001041
Rees A. F., Alfaro-Shigueto J., Barata P. C. R., Bjorndal K. A., Bolten A. B., Bourjea J., et al. (2016). Are we working towards global research priorities for management and conservation of sea turtles? Endanger. Species Res. 31, 337–382. doi: 10.3354/esr00801
Seminoff J. A., Crouse D., Pilcher N. (2004). 2004 IUCN Red List of Threatened Species - Chelonia mydas. Soutwest Fisheries Science Center, La Jolla, California, USA 8235.
Temple A. J., Kiszka J. J., Stead S. M., Wambiji N., Brito A., Poonian C. N. S., et al. (2018). Marine megafauna interactions with small-scale fisheries in the southwestern Indian Ocean: a review of status and challenges for research and management. Rev. Fish Biol. Fish. 28, 89–115. doi: 10.1007/s11160-017-9494-x
Temple A. J., Wambiji N., Poonian C. N. S., Jiddawi N., Stead S. M., Kiszka J. J., et al. (2019). Marine megafauna catch in southwestern Indian Ocean small-scale fisheries from landings data. Biol. Conserv. 230, 113–121. doi: 10.1016/j.biocon.2018.12.024
Virgili M., Vasapollo C., Lucchetti A. (2018). Can ultraviolet illumination reduce sea turtle bycatch in Mediterranean set net fisheries? Fish. Res. 199, 1–7. doi: 10.1016/j.fishres.2017.11.012
Wallace B. P., Kot C. Y., Dimatteo A. D., Lee T., Crowder L. B., Lewison R. L. (2013a). Impacts of fisheries bycatch on marine turtle populations worldwide: Toward conservation and research priorities. Ecosphere 4, 1–49. doi: 10.1890/ES12-00388.1
Wallace B. P., Tiwari M., Girondot M. (2013b). “Dermochelys coriacea, Leatherback,” in IUCN Red List Threat. Species 2013, Vol. 8235. 1–23. Available at: https://www.iucnredlist.org/species/6494/43526147.
Wang J., Barkan J., Fisler S., Godinez-Reyes C., Swimmer Y. (2013). Developing ultraviolet illumination of gillnets as a method to reduce sea turtle bycatch. Biol. Lett. 9, 3–6. doi: 10.1098/rsbl.2013.0383
Wang J. H., Fisler S., Swimmer Y. (2010). Developing Visual deterrents to reduce sea turtle bycatch in gill net fisheries. Mar. Ecol. Prog. Ser. 408, 241–250. doi: 10.3354/meps08577
Wibbels T., Bevan E. (2019). Lepidochelys kempii, Kemp’s ridley. The IUCN red list of threatened species 2019. J. Allergy Clin. Immunol. doi: 10.2305/IUCN.UK.2019-2.RSTS.T11533A155057916.en
Witzell W. N. (1983). Synopsis of biological data on the hawksbill turtle, Eretmochely imbricata (Linaeus 1766) (Rome, Italy: Food & Agriculture Org).
Keywords: incidental bycatch, food source, small-scale fishery, depredation, sea turtle, marine megafauna bycatch, marine megafauna
Citation: El-Khaled YC, Duarte CM and Peixoto RS (2023) Evidence of hawksbill turtle (Eretmochelys imbricata) depredation on fish caught in gillnets. Front. Mar. Sci. 10:1180219. doi: 10.3389/fmars.2023.1180219
Received: 05 March 2023; Accepted: 27 September 2023;
Published: 20 October 2023.
Edited by:
Austin Gallagher, Beneath the Waves, Inc., United StatesReviewed by:
Helene Denise Marsh, James Cook University, AustraliaCopyright © 2023 El-Khaled, Duarte and Peixoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yusuf C. El-Khaled, eXVzdWYua2hhbGVkQGthdXN0LmVkdS5zYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.