
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 12 July 2023
Sec. Aquatic Physiology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1176976
This article is part of the Research TopicFunctional Feed Additives and Intestinal Health in Aquatic AnimalsView all 11 articles
Introduction: Although carbohydrates and lipids are important energy substances for Chinese mitten crab (Eriocheir sinensis), little is known about their synergistic effect on the growth, energy utilization characteristics and mechanisms involved in this process.
Methods: A 58-d feeding experiment was conducted to investigate the effects of dietary carbohydrate to lipid ratio (C/L) on the growth performance, biochemical indices, and metabolism-related differential gene expression of juvenile E. sinensis in both intermolt (InM) and premolt (PrM) stages. Five experimental diets were formulated with increasing dietary C/L (1.34, 2.39, 3.59, 5.52 and 9.42).
Results: The results showed that the weight growth rate of juvenile E. sinensis was highest in dietary C/L3.59 group, which was significantly higher than that in the other groups. As dietary C/L increased, the hepatic glycogen contents increased, but triglyceride contents decreased in the hepatopancreas of E. sinensis in the InM. In both two molting stages, the activities of glycogen synthase and fatty acid synthase paralleled with their contents, respectively. Crabs in the InM showed higher contents of triglyceride and the activities of glycolytic rate-limiting enzymes but lower contents of hepatic glycogen than those in the PrM, especially in the C/L 1.34 and C/L 3.59 groups. In all dietary groups, the activities and transcription of gluconeogenesis and fatty acid synthesis related enzymes were significantly higher in the InM than those in the PrM. KEGG analysis showed that differential genes were enriched in fatty acid biosynthesis, fatty acid metabolism, oxidative phosphorylation pathway, pentose phosphate pathway, pyruvate metabolism and steroid biosynthesis between different dietary groups and molting stages.
Discussion: To conclude, the optimal dietary C/L was estimated to be 3.59 for juvenile E. sinensis based on the survival and growth performance. Compared to PrM, E. sinensis in the InM was more active in the carbohydrate metabolism (glycolysis and gluconeogenesis) and fatty acid synthesis, with more triglyceride and less glycogen accumulated in the hepatopancreas.This study could contribute to better understanding the carbohydrate and lipid metabolism between different molting stages, and optimizing the precise feed formulation for juvenile E. sinensis.
Because of high nutritional value and delicious taste, Chinese mitten crab (Eriocheir sinensis) has been very popular among Chinese consumers for centuries. In recent years, there is a rapid development of crab aquaculture in China, the production of Chinese mitten crab has been steadily increasing, with the China Fisheries Yearbook reporting that it has reached 800 thousand tons per year (Song et al., 2019). Molting is necessary for the growth, development, and reproduction of E. sinensis Panganiban et al., 1995; Jung et al., 2013; Huang et al., 2015).
Protein is an important nutrient for sustaining the normal growth and physiological process of aquatic animals (Johnston et al., 2003). The ever-increasing price of protein ingredients such as fish meal and soybean meal seriously limits the sustainable development of aquaculture industry (Moreira et al., 2008; Lee et al., 2012). As non-protein energy sources, carbohydrates and lipids have the characteristics of low price, easy access and low nitrogen pollution (Gao et al., 2010; Wang et al., 2014). Furthermore, carbohydrates and lipids are closely related to the nutritional metabolism and immunological regulation of aquatic animals (Nakano et al., 1998; Borba et al., 2006; Dong et al., 2018). However, excessive dietary lipids and carbohydrates can cause metabolic disorder, reduced growth rate and even threaten the health status of aquatic animals (Borges et al., 2009; Zhang et al., 2013; Qiang et al., 2017; Li et al., 2020).
It is considered that carbohydrates and lipids have an inseparable close relationship between each other, and an imbalance may negatively affect the growth, feed conversion, and body composition of aquatic animals (Chen et al., 2021; Miller et al., 2023). Carbohydrates are converted into lipid and stored in the body when their contents beyond the optimal requirement for energy supply (Chen et al., 2021). Similarly, lipids can replace carbohydrates for energy supply when the carbohydrate content is insufficient (Meng et al., 2013). Therefore, the steady state of carbohydrates and lipids metabolism is particularly important. It was previously found that the supplementation of carbohydrates or lipids can improve the growth performance and disease resistance of E. sinensis (Chen et al., 2016; Wen et al., 2021). Molting is an important biological process closely related to the growth of crustaceans. The molting cycle could be divided into three vital stages including intermolt (InM), premolt (PrM) and postmolt (PoM) (Gao et al., 2015). The premolt (PrM) is a preparation stage for upcoming molting and energy consumption, and the intermolt (InM) is the longest period in a molting cycle during which accumulates energy for next molting (Huang et al., 2015). However, to the best of our knowledge, little is known about the synergistic effect of carbohydrates and lipids on the growth and dietary administration and mechanisms involved in this process. Thus, this study was conducted to investigate the effects of the dietary carbohydrate to lipid ratio (C/L) on the survival, growth, biochemical indices in E. sinensis. Furthermore, digital gene expression (DGE) analysis was used as a transcriptome sequencing method to measure high-throughput relative gene expression, and to identify genes related to glucose metabolism (glycolysis, gluconeogenesis and glycogen synthesis) and lipid metabolism (fatty acid synthesis and fatty acid oxidation) in E. sinensis at different molting stages.
The goals of this study were to determine: i) the optimal C/L for juvenile E. sinensis; ii) the characteristics of energy utilization at different molting stages; and iii) preliminary mechanisms involved in carbohydrates and lipids metabolism in E. sinensis.
In this study, all the operational procedures were granted by ethical rules of Dalian Ocean University and relevant rules of China.
The ratios of different C/L in the diets were designed according to Li et al. (2022). Five isoproteic and isoenergetic feeds with different ratios of C/L were formulated by adjusting the amounts of soybean oil and corn starch in the formulation (Table 1) (), which were named C/L1.34, C/L2.39, C/L3.59, C/L5.52, and C/L9.42, respectively.
The feeds were manufactured by following the procedures described by Luo et al. (2008). The solid ingredients (<150 μm) were first mixed evenly, which were then mixed well with the oil and water. After that, a twin screw granulator (Jinan Dingrun Machinery Company, Jinan, China) was used to produce feed pellets (1.5 mm ×1.0 mm). After dying, the feeds were cooled, packed, and stored at -20°C.
Crabs were purchased from Jiangsu Haitong Aquatic Products Co. Ltd. (Nantong, China) and transported to the experimental base of Dalian Ocean University. After two weeks of acclimation, healthy and intact crabs (initial body weight: 1.09 ± 0.01 g) were randomly allocated to 15 plastic tanks (96L). Each tank was stocked with 25 individuals. Each diet was assigned to three tanks (25 crabs/tank) at random. Plastic tubes and nets were used as shelters to avoid cannibalism between individuals.
E. sinensis juveniles were fed to apparent satiation at 9:00 and 18:00 every day. At the beginning of feeding, a small number of feeds were thrown into the tanks to attract the attention of crabs. Crabs gathered quickly and ingest the feeds. When most of them dispersed, it indicated that crabs approached to the state of satiation.The residual feeds, feces, shells, and carcass in the tanks were cleaned up every day by syphoning. In total, 2/3 of the water was exchanged every two days. The following water conditions were maintained during the 58d-feeding experiment: temperature, 18–22°C; dissolved oxygen, above >8 mg/L; and ammonia-N, below 0.05 mg/L.
Experimental animals were counted and weighed following a 24h period of starvation. Before sampling, food intake and activity of animals were monitored every day. Crabs with vigorous food intake were thought to be at the intermolt (InM). When the food intake gradually decreased and then stopped, they were thought to be at the intermolt (InM).
In each tank, three crabs in intermolt (InM) and premolt (PrM) were chosen out and placed in an ice box for anesthesia. Subsequently, hepatopancreas were dissected and pooled into the sterile centrifuge tube. The hepatopancreas were used to determine the contents of biochemical indices, activities of metabolic enzymes, and high-throughput relative gene expression. All tubes with samples were frozen by liquid nitrogen and then stored at -80°C.
The contents of moisture, crude protein, lipid and ash were analyzed following the AOAC (1995). All the samples were dried to constant weight at 105°C to calculate moisture contents. Then, Kjeldahl method was used to determine the protein contents. Soxhlet method was used to determine the lipid contents. Ash was determined by calculating the remaining weight of samples after they were burned at 550°C. Finally, the contents of carbohydrates in a sample were calculated by subtracting the weight of moisture, protein, lipid and ash.
The hepatopancreas was mixed with freezing saline (0.85% NaCl) at a ratio of 1/9, which was then homogenated under the ice-water bath. Then, the homogenate was centrifuged (9000 g) at 4°C for 10 min. After that, the supernatant was separated and transferred into new centrifuge tubes. The supernatant was then analyzed for the biochemical indices and metabolic enzymes.
The concentration of biochemical indices including hepatic glycogen (HG) and triglyceride (TG), and the activities of metabolic enzymes including glycogen synthase (GS), hexokinase (HK), pyruvate kinase (PK), fatty acid synthesis (FAS), acetyl-CoA carboxylase (ACC), phosphoenolpyruvate carboxykinase (PEPCK), and carnitine palmitoyltransferase (CPT) were measured by following the instructions of the kits of Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
The transcriptome sequencing of the hepatopancreas of E. sinensis at the InM and PrM stages in the C/L1.34, C/L3.59 and C/L9.42 groups was performed by using the Illumina Nova seq 6000 (Biomarker Technologies, Beijing, China). The transcriptome assembly was done with DIAMOND, and the assembled unigenes were then annotated based on multiple databases, including Nr (NCBI non-redundant protein sequences), Swiss-Prot (a manually annotated and reviewed protein sequence database), KOG/COG (clusters of orthologous groups of proteins), GO (Gene Ontology) and Pfam (a large collection of protein families). Q30 was used as an indicator to measure the quality of sequencing data (Kozich et al., 2013). The unigenes were mapped to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database to annotate their potential metabolic pathways. Differentially expressed gene sets were obtained from the different samples using the DESeq2 software. To identify differentially expressed genes (DEGs) across samples, the fold change (the ratio of expression levels between two samples) ≥1.5 and p value<0.05 were set to be the thresholds.
Trizol (TIANGEN, China) was used to extract total RNA from the hepatopancreas of E. sinensis, and then the gDNA Eraser (Takara, Japan) was used to remove gDNA contamination in the first reaction of cDNA synthesis. The first strand of cDNA was synthesized by using 1 μg total RNA as template and oligo dT-adaptor as primers according to the protocol of manufacturer (TaKaRa, China). The synthesis reaction was performed at 37°C for 15 min, and terminated by heating at 85°C for 5 s. After the integrity was checked, total RNA was reverse transcribed to cDNA, which was used for the templates of RT-PCR. Fast Start Essential DNA Green Master was used to prepare the reaction system by following the instructions. The primer sequences can be referred in Table 2. A LightCycler®96 (Roche group, Basel, Switzerland) was used to perform the RT-PCR, which was programmed as follows: 95°C (10 min); 95°C (15 s), 60°C (60 s) for 40 cycles; 95°C (10 s), 65°C (60 s); and 97°C, 1 s. The 2-ΔΔCT method (Dhanasekaran et al., 2010) was used to calculate the relative mRNA expression levels.
Where Wi and Wf are the initial and final average weights of crabs in each tank, respectively. Ni and Nf are the initial and final numbers of crabs in each tank, respectively.
The interaction effects between dietary C/L and molting stage were analyzed by a two-way analysis of variance (ANOVA) in SPSS 23.0 (Redmond, WA, USA) for Windows. All data was presented in the form of means ± standard error (n=3). If a statistical significance (P < 0.05) was detected, Tukey’s multiple range test was applied to compare the means between dietary groups. Statistical significance was considered when P values <0.05.
The SR of E. sinensis was higher than 90%, with no statistical significance observed between dietary groups (P > 0.05). The WGR was significantly affected by dietary C/L (P < 0.05). The highest WGR was observed in the C/L3.59 group (131%), which was significantly higher than that in the C/L1.34, C/L2.39, C/L5.52 and C/L9.42 groups (P < 0.05) (Table 3).
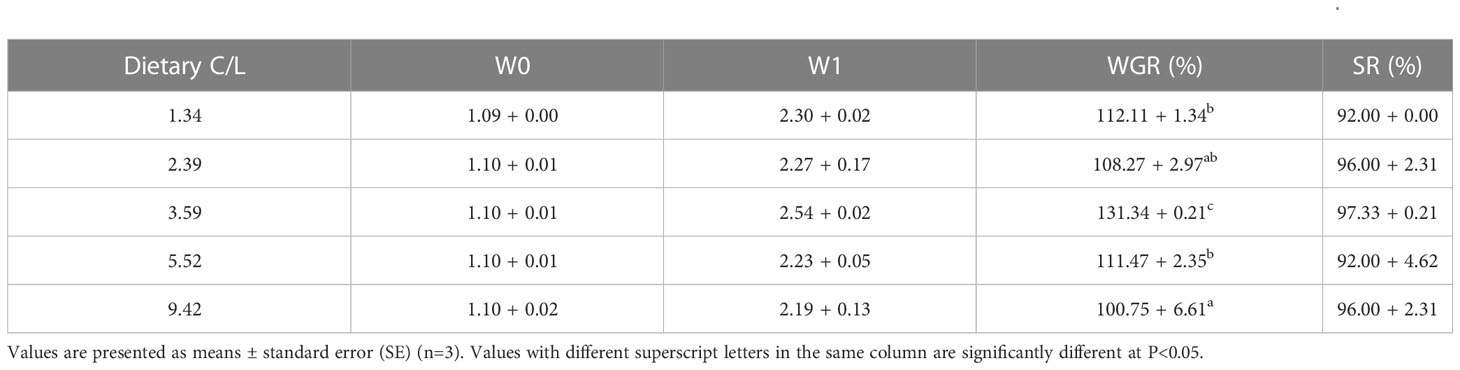
Table 3 Effects of different dietary carbohydrate to lipid ratio (C/L) on weight gain rate (WGR) and survival rate (SR) of juvenile E. sinensis.
There was no significant interactive effect (P > 0.05) between dietary C/L and molting stage on the contents of hepatic glycogen and triglyceride in the hepatopancreas of E. sinensis. In the two molting stages, the hepatic glycogen contents significantly increased with increasing dietary C/L (P < 0.05). The highest hepatic glycogen contents were observed in dietary C/L9.42 groups in the two molting stages, which were significantly higher than that in the C/L1.34 group (P < 0.05). In dietary C/L3.59 groups, hepatic glycogen contents in the InM were significantly higher than that in the PrM (P < 0.05) (Figure 1A).
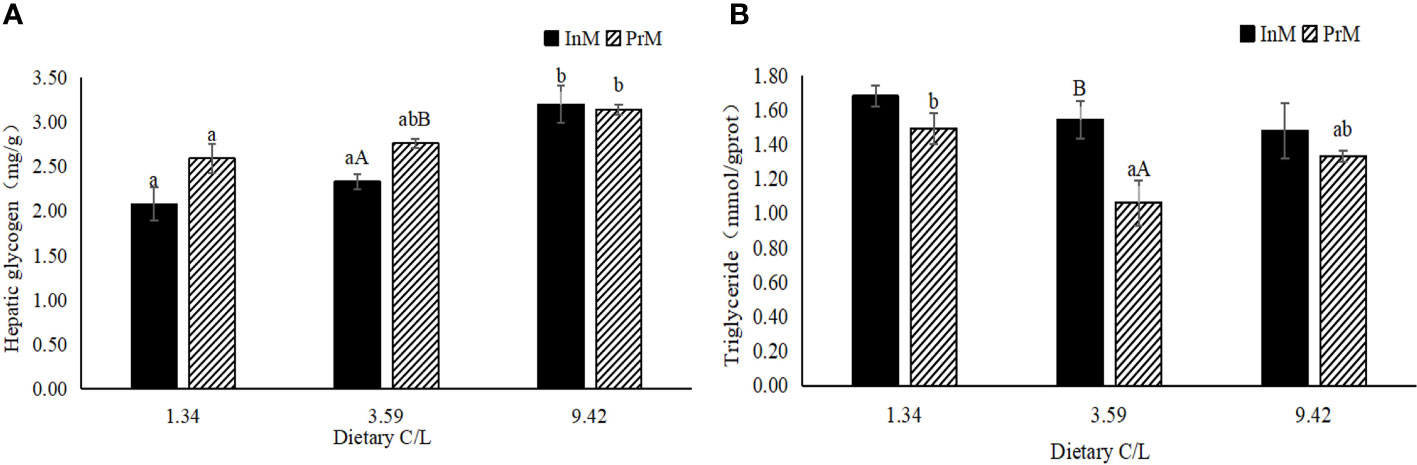
Figure 1 Effects of different dietary carbohydrate to lipid ratio on biochemical criterion of hepatopancreas of juvenile E. sinensis in different molting stage. Values are presented as means ± standard error (SE) (n=3). Bars with different upper-case letters differ significantly from each other in the same dietary C/L groups (P<0.05). Bars with different lower-case letters differ significantly from those of other dietary C/L groups in the same molting stage (P<0.05). Hepatic glycogen contents (A), Triglyceride contents (B).
As the dietary C/L increased, triglyceride contents in the InM showed a decreased tendency (P > 0.05). In all dietary C/L groups, triglyceride contents in InM were all higher than that in PrM, with statistical significance only observed in the C/L3.59 group (P < 0.05) (Figure 1B).
There was a significant interaction between dietary C/L and molting stage on the activities of HK, PK and FAS in juvenile E. sinensis (P < 0.05). At both molting stages, the activities of GS significantly increased with increasing dietary C/L (P < 0.05), but no statistical significance was observed between InM and PrM in all dietary groups (P > 0.05) (Figure 2A).

Figure 2 Effects of different dietary carbohydrate to lipid ratio on metabolic enzymes activities of hepatopancreas of juvenile E. sinensis in different molting stage. Values are presented as means ± standard error (SE) (n=3). Different upper-case letters on the bars represent a significance in the values between the two molting stages within the same dietary group (P<0.05). Different lower-case letters on the bars represent a significance in the values between dietary groups with the same molting stage (P<0.05). GS: glycogen synthase (A), HK, hexokinase (B), PK, pyruvate kinase (C), FAS, fatty acid synthesis (D), ACC, acetyl-CoA carboxylase (E), PEPCK, phosphoenolpyruvate carboxykinase (F), CPT, carnitine palmitoyltransferase (G).
As the dietary C/L increased, the activities of HK significantly increased (P < 0.05) in the PrM but decreased in the InM (P > 0.05) (Figure 2B). The highest activities of PK in the InM were observed in the C/L3.59 group, which were significantly higher than those in the other groups (P < 0.05) (Figure 2C). In dietary C/L1.34 and C/L3.59 groups, HK and PK activities in the InM were higher than those in the PrM (Figures 2B, C).
As the dietary C/L increased, the activities of FAS significantly decreased in the InM (P < 0.05). FAS activities in the C/L9.42 group in the InM were significantly lower than those in the PrM (Figure 2D).
The activities of ACC, PEPCK and CPT were not significantly affected by different dietary C/L, with higher values observed in the InM than those in the PrM in all dietary groups (Figures 2E–G).
A total of 118.16 Gb of Clean Data was obtained from transcriptome analysis of 18 samples. Q30 base percentage of all samples in this study were above 91.86%, which showed that all data were qualified. The DEG number between each two different groups at the same dietary C/L level in different molting stages was analyzed, and the results were shown in Table 4. In the comparison among these nine groups, the DEG number between C/L1.34 of InM and C/L1.34 of PrM was the most, and the DEG number between C/L3.59 of PrM and C/L9.42 of PrM was the least. Feeding with dietary C/L1.34 has more DEGs between InM and PrM than that feeding with dietary C/L3.59 and C/L9.42. The number of DEGs was higher between feeding dietary C/L3.59 and C/L9.42 in InM, and higher between C/L1.34 and C/L3.59 in PrM. To further assign the putative functions to DEGs, KEGG analysis was performed. KEGG enrichment results showed that the DEGs were mainly enriched in biological processes, such as metabolic process (GO:0008152), cellular process (GO:0009987) and biological regulation (GO:0065007). With the enrichment of KEGG, fatty acid biosynthesis (ko00061), fatty acid metabolis (ko01212), moxidative phosphorylation (ko00190), pentose phosphate pathway (ko00030) and pyruvate metabolism (ko00620) have enriched more DEGs. For C/L 1.34, C/L 3.59 and C/L 9.42, the DEGs between InM and PrM were enriched in oxidative phosphorylation process (ko00190), steroid biosynthesis (ko00100) and pyruvate metabolism (ko00620), respectively. For In InM, the more DEGs were found between C/L 1.34 and C/L 3.59, which were mostly enriched in oxidative phosphorylation process (ko00190), and lower DEGs were found between C/L 1.34 and C/L 9.42. While, the C/L 1.34 and C/L 3.59 group had more DEGs in PrM, which were enriched in starch and sucrose metabolism (ko00500) and fatty acid metabolism (ko01212) (Table 4).
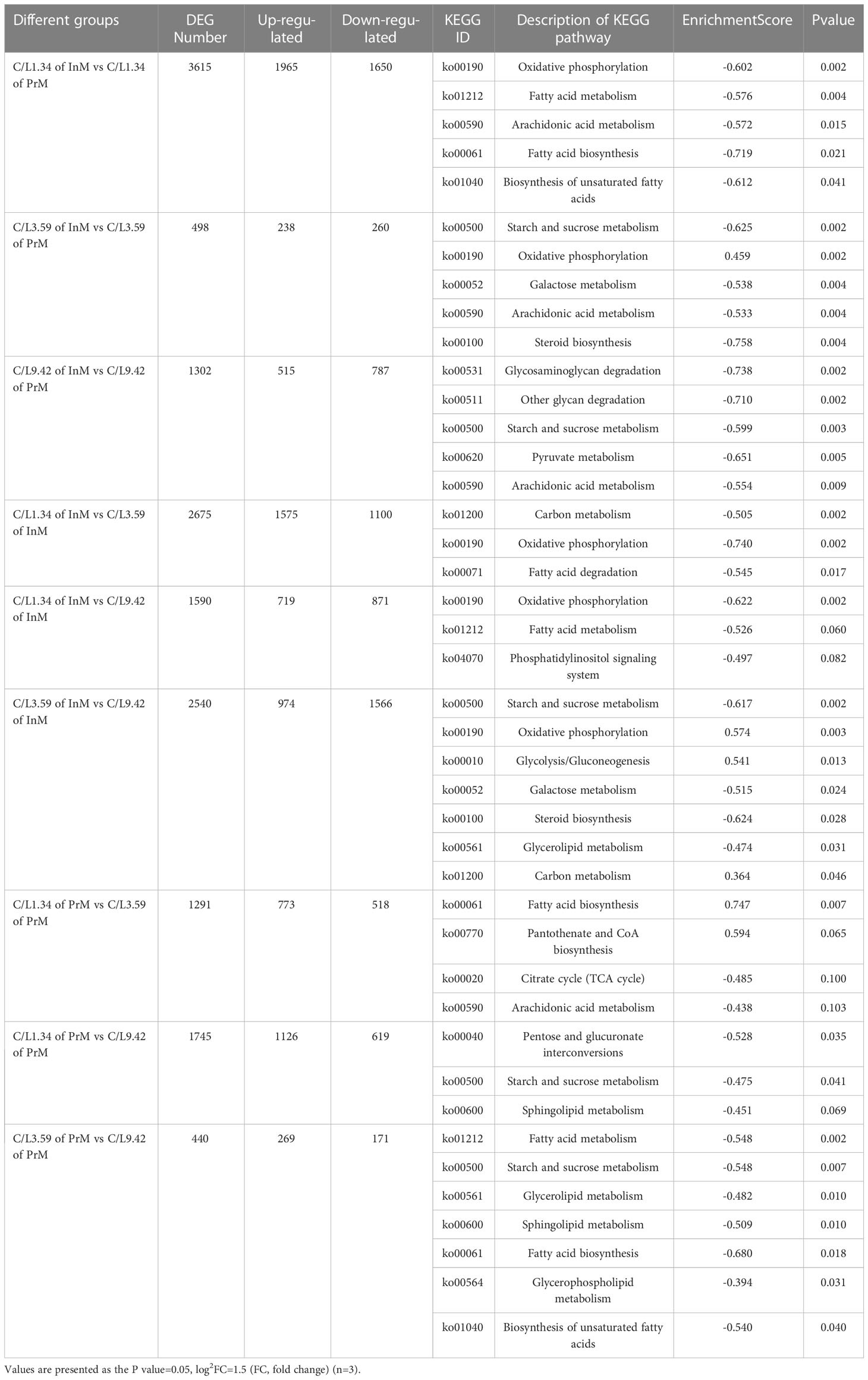
Table 4 Effects of different dietary carbohydrate to lipid ratio and molting cycle on DEG number and analysis of KEGG pathway in juvenile E. sinensis.
There was a significantly interactive effect (P< 0.05) between dietary C/L and molting stage on the mRNA expression levels of all selected genes in E. sinensis (Figure 3). It appears that the mRNA expression levels of FAS, G6PD, PEPCK, Ndufa6, CPT, ACAA2, Elovl6, Aco and Acly vary significantly depending on the dietary C/L ratio. The expression levels of Fas, G6PD, PEPCK, Ndufa6 and Acly at the InM were higher than that at the PrM (Figures 3A-D, I). The expression levels of CPT at the InM post the dietary C/L1.34 and C/L3.59 treatment were lower than that in the PrM stage, and the trend in the C/L9.42 group was the opposite (Figure 3E). In each dietary group, the expression level of Aco at the InM was lower than that at the PrM (Figure 3H).
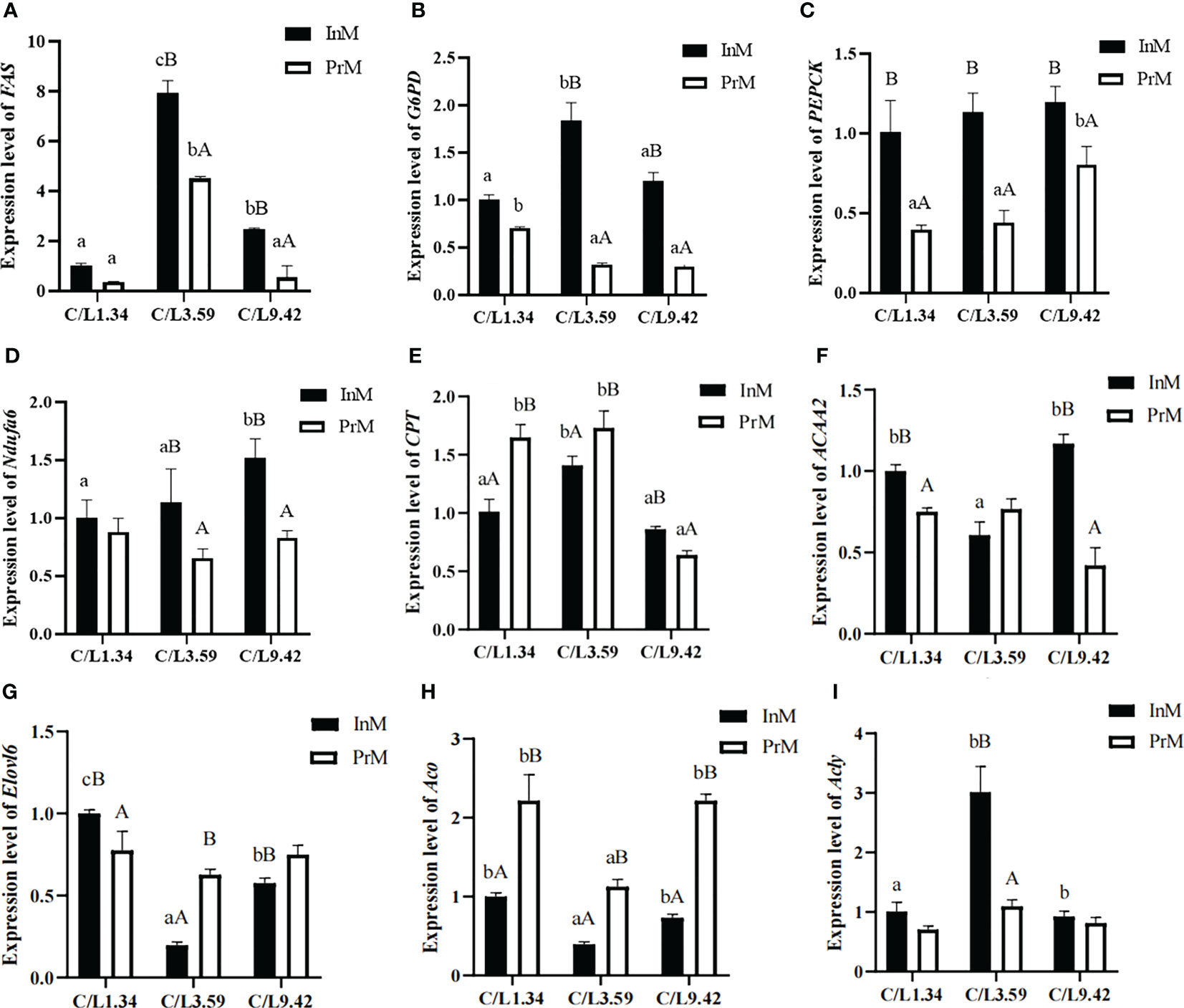
Figure 3 Effects of different dietary carbohydrate to lipid ratio on the mRNA expression of antioxidative genes of juvenile E. sinensis in different molting stage. Values are presented as means ± standard error (SE) (n=3). Bars with different upper-case letters differ significantly from each other in the same dietary C/L groups (P<0.05). Bars with different lower-case letters differ significantly from those of other dietary C/L groups in the same molting stage (P<0.05). FAS relative mRNA expression (A), G6PD relative mRNA expression (B), PEPCK relative mRNA expression (C), Ndufa6 relative mRNA expression (D), CPT relative mRNA expression (E), ACAA2 relative mRNA expression (F), Elovl6 relative mRNA expression (G), Aco relative mRNA expression (H), Acly relative mRNA expression (I).
In the InM, the mRNA expression levels of Elovl6 and Aco in the dietary C/L1.34 group were significantly higher than those in dietary C/L3.59 and C/L9.42 groups (P < 0.05). The mRNA expression levels of FAS, G6PD, CPT and Acly in the dietary C/L3.59 group were significantly higher than those in the C/L1.34 and C/L9.42 groups (P < 0.05). The expression levels of PEPCK, Ndufa6 and ACAA2 in the dietary C/L9.42 group were significantly higher than those in the dietary C/L1.34 and C/L3.59 groups (P < 0.05) (Figures 3A-H).
In the PrM, the mRNA expression levels of G6PD, Ndufa6, Elovl6 and Aco post dietary C/L1.34 treatment were significantly higher than those in the C/L3.59 and C/L9.42 groups (P < 0.05). The mRNA expression levels of FAS, CPT and ACAA2 post the dietary C/L3.59 treatment were significantly higher than those in the C/L1.34 and C/L9.42 groups (P < 0.05). Besides, the mRNA expression levels of PEPCK post the dietary C/L9.42 treatment were significantly higher than those in the dietary C/L1.34 and C/L3.59 groups (Figures 3A-H).
Carbohydrates and lipids are widely used as non-protein energy sources in the formulated feeds (Xie et al., 2017; Dong et al., 2018; Liu et al., 2020). Survival, growth performance, and feed cost are usually taken into consideration when estimating the optimal dietary lipids and carbohydrates in the diets. The present study showed that, post the 58-day feeding trial, the SR of E. sinensis was above 90% and was hardly affected by dietary C/L. Carbohydrates and lipids can be effectively used to achieve ideal growth performance by most crustaceans, such as Jasus edwardsii, Cherax quadricarinatus (Zhu et al., 2013), E. sinensis (Bao et al., 2020; Wen et al., 2021). In the present study, the optimal dietary C/L for juvenile E. sinensis was estimated to be 3.59 based on WGR. This was close to the optimal requirement of C/L for other aquatic animals, such as blunt snout bream (Megalobrama amblycephala) (Li et al., 2013), large yellow croaker (Larmichthys crocea) (Zhou et al., 2016), bullfrog (Rana (Lithobates) catesbeiana) (Zhang et al., 2016) and hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatus) (Chen et al., 2021), but was higher than that for Jasus edwardsii (Johnston et al., 2003) and Scylla paramamosain (Dong et al., 2018), which was estimated to be 2.0 and 1.39-2.08, respectively. Excessive carbohydrates or insufficient lipids reduced feed palatability (Chen et al., 2021) and negatively affected the normal metabolism of several aquatic animals, such as M. salmoides (Ma et al., 2019) and C. quadricarinatus (Zhu et al., 2013). In this study, the lipid level in the C/L9.42 was only 3.92%, which was lower than the estimated requirement for E. sinensis (Li et al., 2013; Zhang et al., 2016; Wen et al., 2021).
In this study, hepatic glycogen contents in the hepatopancreas significantly increased, while triglyceride (TG) contents decreased with increasing dietary C/L. Similar results have also been reported orange-spotted grouper (E. coioides) (Wang et al., 2017), tilapia (O. niloticus) (Xie et al., 2017) and red swamp crayfish (Procambarus clarkii) (Li et al., 2022). Consistently, activities of glycogen synthase (GS) increased while those of fatty acid synthesis (FAS and ACC) decreased with increasing dietary C/L. CPT located in the mitochondria is the rate-limiting enzyme for fatty acid oxidation, and it plays an important role in the oxidation of fatty acids in E. sinensis (Liu et al., 2018). In this study, the activities of CPT were not significantly affected by the dietary C/L. On one hand, it could be the decreased fatty acid synthesis that accounted for the decreased contents of TG in the hepatopancreas of E. sinensis as observed in this study. On the other hand, the increasing C/L could decrease the lipid transport efficiency that resulted in the decreased retention of TG (Du et al., 2005; Gao et al., 2010).
Molting is an indispensable and ongoing physiological process in the life-history of all crustaceans, especially for sustaining normal growth, development and reproduction (Panganiban et al., 1995; Jung et al., 2013; Huang et al., 2015). E. sinensis accumulates substances and energy in the InM, which are used for the formation of new exoskeletons in the PrM (Huang et al., 2015). In the present study, the glycogen content increased while the TC content decreased in PrM compared with that in InM. Glycolysis and gluconeogenesis are two important activities of glucose metabolism (Zhang et al., 2019). In this study, it was found that the activities of glycolytic rate-limiting enzymes (HK and PK) in the InM were promoted by low or moderate dietary C/L (1.34-3.59) but were inhibited by the highest dietary C/L. This was consistent with the findings of Chen et al. (2021) who found that glycolytic ability of juvenile hybrid grouper was suppressed by excessive carbohydrates in the diets. Notably, more carbohydrates were used for glycolysis in the InM among the two molting stages at low or moderate C/L levels. While in the highest C/L group, more carbohydrates were used for glycolysis in the PrM than InM. This indicated that the amounts of carbohydrates participating into glycolysis were not only affected by dietary C/L, but also were affected by molting stages. Feeding activity decreases and even stops during PrM and molting, and begins again postmolt when the crustaceans are rigid enough to handle food (Li et al., 2022). Thus, crustaceans rely mainly on the internal nutrients reserved in the hepatopancreas during PrM and molting (Niu et al., 2012). The energy released by lipid oxidation is much higher than that of carbohydrates because the relative contents of carbon and hydrogen in lipids are higher than those of carbohydrates. Thus, lipids are more suitable substances for instant and high demand of energy, especially for the premolt and molting crabs. Since crabs in the InM can ingest food normally, thy are prone to utilize glucose through glycolysis and save lipids for later use in PrM stage. It was postulated that steroids may be related to the regulation of glycolysis. The role of PEPCK is to catalyze the conversion of oxaloacetate to phosphoenolpyruvate, which is a key rate-limiting enzyme in the gluconeogenesis pathway (Lu et al., 2018). In this study, PEPCK in the PrM significantly decreased in both mRNA levels and activities than those in the InM in all dietary groups. This indicated that gluconeogenesis is more active in the InM of the E. sinensis. G6PDH is a key enzyme involved in the production of NADPH in the pentose phosphate pathway, and NADPH is necessary for lipogenesis (Enes et al., 2009; Guerrero-Zárate et al., 2019; Liu et al., 2020). In this study, the mRNA levels of G6PDH were higher in the InM, which was consistent with the increased TC contents in this stage of all dietary groups.
The information obtained from transcriptome analysis can provide some molecular basis for crustaceans (Hu et al., 2015). In this study, DEGs was used to perform transcriptome analysis on the expression profile in the hepatopancreas of E. sinensis fed diets with increasing C/L. The results showed that metabolic process terms were over-represented in the InM and PrM, for instance, glycogen biosynthetic process (GO:0005978) and fatty acid biosynthetic process (GO:0006633). KEGG analysis demonstrated the top enriched pathways include fatty acid biosynthesis (ko00061), fatty acid metabolism (ko01212), oxidative phosphorylation (ko00190), pentose phosphate pathway (ko00030) and pyruvate metabolism (ko00620). Energy metabolism has become an indispensable part of studying ion exchange and osmotic regulation in organisms (Hu et al., 2015). In this study, glycolysis/gluconeogenesis, citric acid cycle (TCA cycle) and fatty acid synthesis/degradation are abundant pathways related to energy metabolism. Our research has found some significant differentially expressed genes related to energy metabolism. The tendency of nine genes (Figure 3) was basically consistent with the transcriptome information after identification by RT-PCR. FAS controls the synthesis of fatty acids which catalyzes the lipid synthesis pathway by converting carbohydrates into fatty acids (Chirala and Wakil, 2004; Mashima et al., 2009). Compared with the PrM, the mRNA expression levels of FAS at the InM were significantly up-regulated, indicating that E. sinensis in the InM needs to accumulate more energy for utilization in the PrM (Huang et al., 2015). At the same time, FAS was also clearly responding to changes in the dietary C/L in the diets. This showed that the dietary C/L3.59 was more conducive to the accumulation of lipid for E. sinensis. It has also confirmed that lipids stored at the InM play an important role in the energy supply of other non-eating molting stages of E. sinensis. G6PD is a key gene involved in the pentose phosphate pathway (Yilmaz et al., 2006). The role of PEPCK is to catalyze the conversion of oxaloacetate to phosphoenolpyruvate, which is a key gene in the gluconeogenesis pathway (Lu et al., 2018). We have observed that these two genes involved in the conversion of carbohydrates and lipids were highly expressed at the InM. It is worth noting that as the dietary C/L increased, the expression levels of PEPCK showed an increasing trend, while G6PD was the opposite at the PrM. Combined with the content of hepatic glycogen, this showed that E. sinensis accumulated more carbohydrates at the PrM, in other words, carbohydrates were not used as the main energy source. The expression level of PEPCK was higher at the InM than at the PrM. This corresponds to the PEPCK activities, which indicates that gluconeogenesis is more active at the InM than at the PrM, with more glucose generated and then converted into glycogen. Both Aco and Acly are involved in the regulation of the Citrate cycle (TCA cycle) (ko00020). The CPT gene participates in the metabolic pathway of AMPK/ACC/CPT, by degrading fatty acids to avoid excessive liver lipid deposition (Liu et al., 2018; Tobita et al., 2018; Fang et al., 2019). Within the appropriate range of dietary C/L (1.34-3.59), the expression levels of CPT were significantly up-regulated at the PrM of E. sinensis. This indicated that lipid was broken down at the PrM when E. sinensis needs a lot of energy to prepare for molting (Huang et al., 2015). However, the mRNA expression level of CPT could be restricted by dietary high carbohydrate or low lipid. Taken together, these results indicated that E. sinensis utilizes carbohydrates as an energy source in the InM, while fatty acids and lipids are used in the PrM.
A moderate dietary C/L (3.59) achieved the best growth performance of juvenile E. sinensis. Dietary C/L increased glycogen synthesis but decreased lipid synthesis in the hepatopancreas. Compared to PrM, E. sinensis in the InM was more active in the carbohydrate metabolism (glycolysis and gluconeogenesis) and fatty acid synthesis, with more triglyceride and less glycogen accumulated in the hepatopancreas. This may indicate that juvenile crabs are prone to utilize carbohydrates for energy supply through glycolysis in the InM and store lipids for later energy use in the PrM. Moreover, the transcriptomic analysis showed that compared with C/L 1.34 and C/L 9.42, the differentially expressed genes between InM and PrM were enriched not only in energy metabolism, but also in steroid biosynthesis in C/L 3.59, which indicated that C/L 3.59 might promote the steroid biosynthesis at PrM stage contributing to growth performance. These results could be helpful for optimizing the feed formulation for this species in different molting stages.
The original contributions presented in the study are publicly available. This data can be found here: NCBI, PRJNA977214.
RZ: conceptualization, methodology, writing. BW: formal analysis, writing. YJ: manuscript revision. SH: data analysis. QY: supervision, project administration. All authors contributed to the article and approved the submitted version.
This work was supported by National Key Research and Development Program of China (2018YFD0900400), Project for Marine Economy Development in Liaoning Province and General Program of Educational Department of Liaoning Province (20220078).
We are grateful to all the laboratory members for their technical advice and helpful discussions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AOAC. (1995). Official methods of analysis (16th ed.). (Washington D.C., USA: Association of Official Analytical Chemists, Inc.).
Bao J., Li X., Xing Y., Feng C., Jiang H. (2020). Effects of hypoxia on immune responses and carbohydrate metabolism in the Chinese mitten crab, Eriocheir sinensis. Aquac. Res. 51, 2735–2744. doi: 10.1111/are.14612
Borba M. R., Fracalossi D. M., Pezzato L. E. (2006). Dietary energy requirement of piracanjuba fingerlings, Brycon orbignyanus, and relative utilization of dietary carbohydrate and lipid. Aquacult. Nutr. 12 (3), 183–191. doi: 10.1111/j.1365-2095.2006.00401.x
Borges P., Oliveira B., Casal S., Dias J., Conceição L., Valente L. M. P. (2009). Dietary lipid level affects growth performance and nutrient utilisation of Senegalese sole (Solea senegalensis) juveniles. Br. J. Nutr. 102 (7), 1007–1014. doi: 10.1017/S0007114509345262
Chen Y., Chen L., Qin J. G., Ding Z., Li M., Jiang H., et al. (2016). Growth and immune response of Chinese mitten crab (Eriocheir sinensis) fed diets containing different lipid sources. Aquac. Res. 47, 1984–1995. doi: 10.1111/are.12654
Chen G., Qian J., Liu H., Tan B., Dong X., Yang Q., et al. (2021). Dietary carbohydrate-to-lipid ratios modulate juvenile hybrid grouper (♀Epinephelus fuscoguttatus×♂E. lanceolatus): effects on growth, serum biochemistry, intestinal digestion and hepatic metabolism of glucose and lipid. Aquacult. Nutr. 27 (5), 1370–1382. doi: 10.1111/anu.1327
Chirala S. S., Wakil S. J. (2004). Structure and function of animal fatty acid synthase. Lipids 39 (11), 1045–1053. doi: 10.1007/s11745-004-1329-9
Dhanasekaran S., Doherty T. M., Kenneth J., Group T. T. S. (2010). Comparison of different standards for real-time PCR-based absolute quantification. J. Immunol. Methods 354 (1-2), 34–39. doi: 10.1016/j.jim.2010.01.004
Dong L. F., Tong T., Zhang Q., Wang Q. C., Xu M. Z., Yu H. R., et al. (2018). Effects of dietary carbohydrate to lipid ratio on growth, feed utilization, body composition and digestive enzyme activities of golden pompano (Trachinotus ovatus). Aquacult. Nutr. 24 (3), 341–347. doi: 10.1111/anu.12565
Du Z. Y., Liu Y. J., Tian L., Wang J., Wang Y., Liang G. Y. (2005). Effect of dietary lipid level on growth, feed utilization and body composition by juvenile grass carp (Ctenopharyngodon idella). Aquac. Nutr. 11, 139–146. doi: 10.1111/j.1365-2095.2004.00333.x
Enes P., Panserat S., Kaushik S., Oliva-Teles A. (2009). Nutritional regulation of hepatic glucose metabolism in fish. Fish. Physiol. Biochem. 35 (3), 519–539. doi: 10.1007/s10695-008-9259-5
Fang K., Wu F., Chen G., Dong H., Li J., Zhao Y., et al. (2019). Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC complement. Altern. Med. 19, 255. doi: 10.1186/s12906-019-2671-9
Gao W., Liu Y. J., Tian L. X., Mai K. S., Liang G. Y., Yang H. J., et al. (2010). Effect of dietary carbohydrate-to-lipid ratios on growth performance, body composition, nutrient utilization and hepatic enzymes activities of herbivorous grass carp (Ctenopharyngodon idella). Aquacult. Nutr. 16 (3), 327–333. doi: 10.1111/j.1365-2095.2009.00668.x
Gao Y., Zhang X., Wei J., Sun X., Yuan J., Li F., et al. (2015). Whole transcriptome analysis provides insights into molecular mechanisms for molting in Litopenaeus vannamei. PloS One 10 (12), e0144350. doi: 10.1371/journal.pone.0144350
Guerrero-Zárate R., Álvarez-González C. A., Jesus-Contreras R., Peña-Marín E. S., Martínez-García R., Galaviz M. A., et al. (2019). Evaluation of carbohydrate/lipid ratios on growth and metabolic response in tropical gar (Atractosteus tropicus) juvenile. Aquac. Res. 50, 1812–1823. doi: 10.1111/are.14060
Hu D., Pan L., Zhao Q., Ren Q. (2015). Transcriptomic response to low salinity stress in gills of the pacific white shrimp, Litopenaeus vannamei. Mar. Genomics 24 Pt 3, 297–304. doi: 10.1016/j.margen.2015.07.003
Huang S., Wang J., Yue W., Chen J., Gaughan S., Lu W., et al. (2015). Transcriptomic variation of hepatopancreas reveals the energy metabolism and biological processes associated with molting in Chinese mitten crab, Eriocheir sinensis. Sci. Rep. 5, 14015. doi: 10.1038/srep14015
Johnston D. J., Calvert K. A., Crear B. J., Carter C. G. (2003). Dietary carbohydrate/lipid ratios and nutritional condition in juvenile southern rock lobster, Jasus edwardsii. Aquaculture 220, 667–682. doi: 10.1016/S0044-8486(02)00562-8
Jung H., Lyons R. E., Hurwood D. A., Mather P. B. (2013). Genes and growth performance in crustacean species: a review of relevant genomic studies in crustaceans and other taxa. Rev. Aquac. 5 (2), 77–110. doi: 10.1111/raq.12005
Kozich J. J., Westcott S. L., Baxter N. T., Highlander S. K., Schloss P. D. (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120. doi: 10.1128/AEM.01043-13
Lee D. H., Ra C. S., Song Y. H., Sung K. I., Kim and J. D. (2012). Effects of dietary garlic extract on growth, feed utilization and whole body composition of juvenile sterlet sturgeon (Acipenser ruthenus). Asian-Australa. J. Anim. Sci. 25, 577–583. doi: 10.5713/ajas.2012.12012
Li W. F., Li S., Wang X. F., Chen H. Y., Hao H., Wang K. J. (2022). Internal carbohydrates and lipids as reserved energy supply in the pubertal molt of Scylla paramamosain. Aquaculture 549, 737736. doi: 10.1016/j.aquaculture.2021.737736
Li X. F., Wang Y., Liu W. B., Jiang G. Z., Zhu J. (2013). Effects of dietary carbohydrate/lipid ratios on growth performance, body composition and glucose metabolism of fingerling blunt snout bream Megalobrama amblycephala. Aquacult. Nutr. 19 (5), 701–708. doi: 10.1111/anu.12017
Li L., Wang W., Yusuf A., Zhu Y., Zhou Y., Ji P., et al. (2020). Effects of dietary lipid levels on the growth, fatty acid profile and fecundity in the oriental river prawn, Macrobrachium nipponense. Aquac. Res. 51, 1893–1902. doi: 10.1111/are.14539
Liu L., Long X., Deng D., Cheng Y., Wu X. (2018). Molecular characterization and tissue distribution of carnitine palmitoyltransferases in Chinese mitten crab Eriocheir sinensis and the effect of dietary fish oil replacement on their expression in the hepatopancreas. PloS One 13, e0201324. doi: 10.1371/journal.pone.0201324
Liu H., Yang J. J., Dong X. H., Tan B. P., Zhang S., Chi S. Y., et al. (2020). Effects of different dietary carbohydrate-to-lipid ratios on growth, plasma biochemical indexes, digestive, and immune enzymes activities of sub-adult orange-spotted grouper Epinephelus coioides. Fish. Physiol. Biochem. 46 (4), 1409–1420. doi: 10.1007/s10695-020-00799-4
Lu S., Wu X., Gao Y., Gatlin D. M., Wu M., Yao W., et al. (2018). Effects of dietary carbohydrate sources on growth, digestive enzyme activity, gene expression of hepatic GLUTs and key enzymes involved in glycolysis-gluconeogenesis of giant grouper Epinephelus lanceolatus larvae. Aquaculture 484, 343–350. doi: 10.1016/j.aquaculture.2017.07.033
Luo Z., Tan X. Y., Chen Y. D., Wang W. M., Zhou G. (2008). Apparent digestibility coefficients of selected feed ingredients for Chinese mitten crab Eriocheir sinensis. Aquaculture 285, 141–145. doi: 10.1016/j.aquaculture.2008.08.004
Ma H. J., Mou M. M., Pu D. C., Lin S. M., Chen Y. J., Luo L. (2019). Effect of dietary starch level on growth, metabolism enzyme and oxidative status of juvenile largemouth bass, Micropterus salmoides. Aquaculture 498, 482–487. doi: 10.1016/j.aquaculture.2018.07.039
Mashima T., Seimiya H., Tsuruo T. (2009). De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer. 100, 1369–1372. doi: 10.1038/sj.bjc.6605007
Meng S., Cao J., Feng Q., Peng J., Hu Y. (2013). Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evid. Based Complement Alternat. Med. 2013, 801457. doi: 10.1155/2013/801457
Miller J. R., Salze G. P., Stuart K. R., Drawbridge M. A., Davis D. A. (2023). The effect of dietary carbohydrate to lipid ratios on growth performance in juvenile California yellowtail Seriola dorsalis. Aquaculture 563, 738892. doi: 10.1016/j.aquaculture.2022.738892
Moreira I. S., Peres H., Couto A., Enes P., Oliva-Teles A. (2008). Temperature and dietary carbohydrate level effects on performance and metabolic utilisation of diets in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 274, 153–160. doi: 10.1016/j.aquaculture.2007.11.016
Nakano K., Tagawa M., Takemura A., Hirano T. (1998). Temporal changes in liver carbohydrate metabolism associated with seawater transfer in Oreochromis mossambicus. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 119, 721–728. doi: 10.1016/S0305-0491(98)00048-0
Niu J., Lin H. Z., Jiang S. G., Chen X., Wu K. C., Tian L. X., et al. (2012). Effect of seven carbohydrate sources on juvenile penaeus monodon. Anim. Feed Sci. Technol. 174, 86–95. doi: 10.1016/j.anifeedsci.2012.03.003
Panganiban G., Sebring A., Nagy L., Carroll S. (1995). The development of crustacean limbs and the evolution of arthropods. Science 270 (5240), 1363–1366. doi: 10.1126/science.270.5240.1363
Qiang J., He J., Yang H., Sun Y. L., Tao Y. F., Xu P., et al. (2017). Dietary lipid requirements of larval genetically improved farmed tilapia, Oreochromis niloticus (L.), and effects on growth performance, expression of digestive enzyme genes, and immune response. Aquac. Res. 48, 2827–2840. doi: 10.1111/are.13117
Song C., Liu L., Hui M., Liu Y., Liu H., Cui Z. (2019). Primary molecular basis of androgenic gland endocrine sex regulation revealed by transcriptome analysis in Eriocheir sinensis. J. Oceanol. Limnol. 37, 223–234. doi: 10.1007/s00343-019-7254-6
Tobita H., Sato S., Yazaki T., Mishiro T., Ishimura N., Ishihara S., et al. (2018). Alogliptin alleviates hepatic steatosis in a mouse model of nonalcoholic fatty liver disease by promoting CPT1a expression via Thr172 phosphorylation of AMPKα in the liver. Mol. Med. Rep. 17 (5), 6840–6846. doi: 10.3892/mmr.2018.8673
Wang J. T., Jiang Y. D., Han T., Li X. Y., Wang Y., Liu Y. J. (2017). Effects of dietary carbohydrate-to-lipid ratios on growth and body composition of orange-spotted grouper Epinephelus coioides. N. Am. J. Aquac. 79 (1), 1–7. doi: 10.1080/15222055.2016.1194924
Wang L. N., Liu W. B., Lu K. L., Xu W. N., Cai D. S., Zhang C. N., et al. (2014). Effects of dietary carbohydrate/lipid ratios on non-specific immune responses, oxidative status and liver histology of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 426–427, 41–48. doi: 10.1016/j.aquaculture.2014.01.022
Wen B., Jiang Y., Yuan R., He Y., Liu Q., Li X., et al. (2021). Effects of dietary lipid sources on the survival, growth, body composition, antioxidant capacity and expression of antioxidant and pro-inflammatory genes in juvenile Chinese mitten crab (Eriocheir sinensis) reared under three salinities. Aquac. Res. 52, 5307–5320. doi: 10.1111/are.15401
Xie D., Yang L., Yu R., Chen F., Lu R., Qin C., et al. (2017). Effects of dietary carbohydrate and lipid levels on growth and hepatic lipid deposition of juvenile tilapia, Oreochromis niloticus. Aquaculture 479, 696–703. doi: 10.1016/j.aquaculture.2017.07.013
Yilmaz S., Beytut E., Erişir M., Ozan S., Aksakal M. (2006). Effects of additional vitamin e and selenium supply on G6PDH activity in rats treated with high doses of glucocorticoid. Neurosci. Lett. 393 (2-3), 85–89. doi: 10.1016/j.neulet.2005.03.076
Zhang C. X., Huang K. K., Wang L., Song K., Lu K. L., Zhang L., et al. (2016). Optimal dietary carbohydrate to lipid ratio for bullfrog Rana (Lithobates) catesbeiana. Aquac. Res. 47, 3332–3340. doi: 10.1111/are.13109
Zhang S. P., Li J. F., Wu X. C., Zhong W. J., Xian J. A., Liao S. A., et al. (2013). Effects of different dietary lipid level on the growth, survival and immune-relating genes expression in pacific white shrimp, Litopenaeus vannamei. Fish. Shellfish Immunol. 34, 1131–1138. doi: 10.1016/j.fsi.2013.01.016
Zhang W., Liu K., Tan B., Liu H., Dong X., Yang Q., et al. (2019). Transcriptome, enzyme activity and histopathology analysis reveal the effects of dietary carbohydrate on glycometabolism in juvenile largemouth bass, Micropterus salmoides. Aquaculture 504, 39–51. doi: 10.1016/j.aquaculture.2019.01.030
Zhou P., Wang M., Xie F., Deng D. F., Zhou Q. (2016). Effects of dietary carbohydrate to lipid ratios on growth performance, digestive enzyme and hepatic carbohydrate metabolic enzyme activities of large yellow croaker (Larmichthys crocea). Aquaculture 452, 45–51. doi: 10.1016/j.aquaculture.2015.10.010
Zhu H., Jiang Q., Wang Q., Yang J., Dong S., Yang J. (2013). Effect of dietary carbohydrate-to-lipid ratios on growth performance, body composition, hepatic enzyme activities, and digestive enzyme activities of juvenile Australian redclaw crayfish, Cherax quadricarinatus (von martens). J. World Aquac. Soc 44, 173–186. doi: 10.1111/jwas.12024
Keywords: carbohydrate to lipid ratio, Eriocheir sinensis, growth performance, biochemical indices, transcriptomic analysisprofile
Citation: Zuo R, Wen B, Jiang Y, Huang S and Yi Q (2023) Growth, biochemical indices and transcriptomic profile of Chinese mitten crab (Eriocheir sinensis) respond to different ratios of dietary carbohydrates to lipids. Front. Mar. Sci. 10:1176976. doi: 10.3389/fmars.2023.1176976
Received: 01 March 2023; Accepted: 05 May 2023;
Published: 12 July 2023.
Edited by:
Gang Yang, Nanchang University, ChinaReviewed by:
Shan Gong Wu, Institute of Hydrobiology, Chinese Academy of Sciences (CAS), ChinaCopyright © 2023 Zuo, Wen, Jiang, Huang and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qilin Yi, eWlxaWxpbkBkbG91LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.