- 1Consiglio Nazionale delle Ricerche (CNR) - Istituto per lo studio degli impatti Antropici e Sostenibilità in ambiente marino (IAS) S.S. di Capo Granitola, Campobello di Mazara, TP, Italy
- 2Consiglio Nazionale delle Ricerche (CNR) - Istituto di Scienze Marine (ISMAR) S.S. di Napoli, Napoli, Italy
- 3Fondazione COISPA ETS, Bari, Italy
Data on Atlantic horse mackerel Trachurus trachurus (Linneus, 1758) were collected along 8-year acoustic surveys (2011-2018). Age and growth variability of horse mackerel from the Central Mediterranean Sea were investigated within different, contrasting habitats, from the south of Sicily to the north Tyrrhenian (Ligurian Sea). Data from satellite provided the habitat features along the study period over the whole surveyed area. For comparison purposes, according to the ecosystems difference the study area has been split into four subareas: Strait of Sicily (SoS), North of Sicily (NS), south Tyrrhenian Sea (ST) and north Tyrrhenian Sea (NT). In terms of the FAO Geographical Sub-Area definition the SoS corresponding to GSA15 and 16, NS to GSA 10 south, ST to GSA10 north and NT to GSA 9. Results showed a growth homogeneity in the study area, suggesting a unique stock inhabiting these waters. The only exception was recorded for juveniles (Age 0 class) in the SoS, where a lower size at age was detected compared to other areas. A multiple linear modelling analyses suggested that variability in length at age 0 was mainly linked to the oceanographic differences between an upwelling driven system (Strait of Sicily) and the other ecosystems, where enrichment processes are mainly due to river runoff (relevant in ST and NT). Namely, Absolute Dynamic Topography (ADT) and body condition factor (Kn) were significantly related to differences in length at age 0. Results revealed that currents (and gyres) are among the principal abiotic factors controlling Atlantic horse mackerel growth in its first year of life, suggesting that circulation and food-related processes (i.e., zooplankton concentration) are of major importance for this species. Finally preliminary observations suggest the Strait of Sicily may be the main spawning area among those analyzed.
Introduction
Atlantic horse mackerel Trachurus trachurus (Linneus, 1758) is a schooling (Iglesias, 2003; D’Elia et al., 2014), zooplanktivorous fish species (Rumolo et al., 2017), which forms large shoals that occur in bottom waters and midwater during the day, whereas T. trachurus individuals disperse and form a layer just off the seabed during the night (Macer, 1977). The range of these vertical migrations decreases during winter, when activity is low (Chuksin and Nazarov, 1989). The species mainly occupies shelf seas, down to 200 m, although in Mediterranean Sea T. trachurus has been reported up to 500 m depth (Milisenda et al., 2018).
This species has a wide latitudinal coverage in the northeast Atlantic Ocean (from the West African Cape Verde Islands to Norwegian Sea and North Sea), as well as in the Mediterranean and Black Sea (Abaunza et al., 2003a; ICES, 2020). T. trachurus plays also a relevant role in the marine ecosystem, because of its trophic position in the marine food web as both a prey for large pelagic and a predator of other important commercial species, such as anchovy (Rumolo et al., 2017; Milisenda et al., 2018). T. trachurus is one of the highly exploited species, however it represents around 2% of total production of the Mediterranean and Black Sea in the period 2014-2016, although since early 2000 the mean landings per year drastically declined (FAO, 2018). Such decline in landings, together with the transition from single-species to ecosystem-based fishery management adopted by the Marine EU policy (EU, 2008; EU, 2013), required precise consideration also for non-target or minor species (such as T. trachurus) to be assessed (EU, 2008; EU, 2017). This transition has led to a need for greater understanding of detailed information on the biological processes and the spatial distribution of a broad spectrum of fish species across large spatial scales, such as large marine ecosystems or eco-regions (Kelley and Sherman, 2018), mainly based on fishery independent surveys data (Moriarty et al., 2019). Therefore, many species, usually not involved in the management plans, are assuming renewed interest by the scientific community. In the North Sea and north-eastern Atlantic, stocks of horse mackerel have been defined for management and assessment purposes since late ‘70s (ICES, 2020), contrary to the Mediterranean Sea, where this species has received little attention regarding sustainable exploitation and monitoring plans (Rückert, 2002; Abaunza et al., 2003a).
Knowledge on the fish growth is fundamental for fishery sciences and such aspect directly enter in age-structured models used to assess the status of fishery resources. Growth may vary seasonally or along a time period due to a number of factors such as temperature (Taylor, 1958; Pauly, 1980), food availability (Jones, 1986), salinity (Bœuf and Payan, 2001), light (Boeuf and Le Bail, 1999), and fishing pressure (Carbonara et al., 2022). Studies comparing different stocks or populations of the same species from different ecosystems/areas have demonstrated that growth is largely affected by temperature (Basilone et al., 2004; Brunel and Dickey-Collas, 2010), and that changes in growth rate may modify the sustainability of the fishery (Brander, 2007). Moreover, it is well-known that individuals of a species grow in low latitudes at a faster rate and mature earlier than those of the same species in high latitudes. Although for T. trachurus results were not conclusive (Laevastu and Favorite, 1988), differences attributable to the ability to adapt to large-scale patterns in environmental conditions (Winton et al., 2014) have already observed for this species in central Mediterranean Sea (Ferreri et al., 2019). Understanding the relationship between growth and environmental factors could allow to evaluate the variability of potential fishing yields based on habitat characteristics (DeVries and Frie, 1996).
Furthermore, knowledge on age and size structures still contributes to the comprehension of how fish populations fluctuate in abundance and the awareness of their health condition (Brunel and Piet, 2013). The life history of small pelagic species – including clupeids, engraulids, and carangids – is poorly known, and the highly variable recruitment complicated standard stock assessment models (Abaunza et al., 2003a; Barange et al., 2009). However, a considerable knowledge gap still persists largely due to the difficulties in monitoring multiple processes interaction over the full life cycle of pelagic fish species in nature, as much as from the high uncertainty associated with field measurements (Rose et al., 2001). Despite the importance of comparative growth studies, there is still a great deal of uncertainty due to the age assignment variability. Indeed, individual growth of different stocks (areas) are assessed by different sampling procedures and by different reading protocols, as well as by different readers (Carbonara et al., 2019), which subjectivity and/or experience may affect the observed variability among areas, more than the environmental variations. T. trachurus may grow to about 60 cm length, but the size range of 15-40 cm is more common in Mediterranean waters (Smith-Vaniz, 1986). They grow rapidly during the first years of life and much more slowly after age 3. They are reported to reach 40 years of age (Abaunza et al., 2003b), although ageing methods are somewhat uncertain.
Even though all physical and physiological features of an organism are interrelated, the body size is probably the most important characteristic, as it represents an integrated outcome of different physical, physiological and environmental stressors; consequently, size is the main life history parameters also in the case of fishes (Calder, 1984). Thus, among the parameters describing the fish growth, the mean size at age represents a key information, which generally provides more robust data for comparative studies on life histories than the parameters obtained by fitting growth models (i.e., von Bertalanffy, Gompertz, logistic etc.). Indeed, latter data could be highly influenced by problems in sampling procedures, such as an unbalanced number of individuals in specific age/length classes. Although the mean size at age is generally assumed constant through time (Lorenzen, 2016), in several fish species it differs considerably among populations and it can vary over time for a given population (Brander, 1995; Neuheimer et al., 2008). Existing literature evidenced a great variability and plasticity of this species in the time and space, deserving major consideration, as in the case of growth parameters estimation, which may affect the possibility to establish a reliable relationship between fish length and age (Abaunza et al., 2003a). However, T. trachurus in Mediterranean areas still presents important gaps of information from biological, spatial and ecological point of view (Giannoulaki et al., 2013; Milisenda et al., 2018; Ferreri et al., 2019).
This paper investigated the spatial variability of length at age of Atlantic horse mackerel along three different central Mediterranean Sea sectors, which mainly differ in terms of coastline complexity, riverine input, continental shelf extension, enrichment processes (i.e., upwelling or river runoff driven) and primary productivity levels (Bonanno et al., 2014b; Bonanno et al., 2016). Moreover, environmental variability and other biological traits (i.e. physiological status) were analyzed to evaluate their effect on growth.
Material and methods
Study areas
Samples of Atlantic horse mackerel were collected in the framework of acoustic surveys (carried out to monitoring anchovy and sardine population; MEDIAS, 2019) over the continental shelf (depth<200m) in different geographical areas, from the Strait of Sicily to the Tyrrhenian and Ligurian Seas (Figure 1). Each area is characterized by distinct hydrographic conditions, seabed morphology and productivity. The area stratification also corresponds to the GFCM geographical sub-areas 9, 10, 15 and 16, as defined by General Fisheries Commission for the Mediterranean (GFCM, 2009).
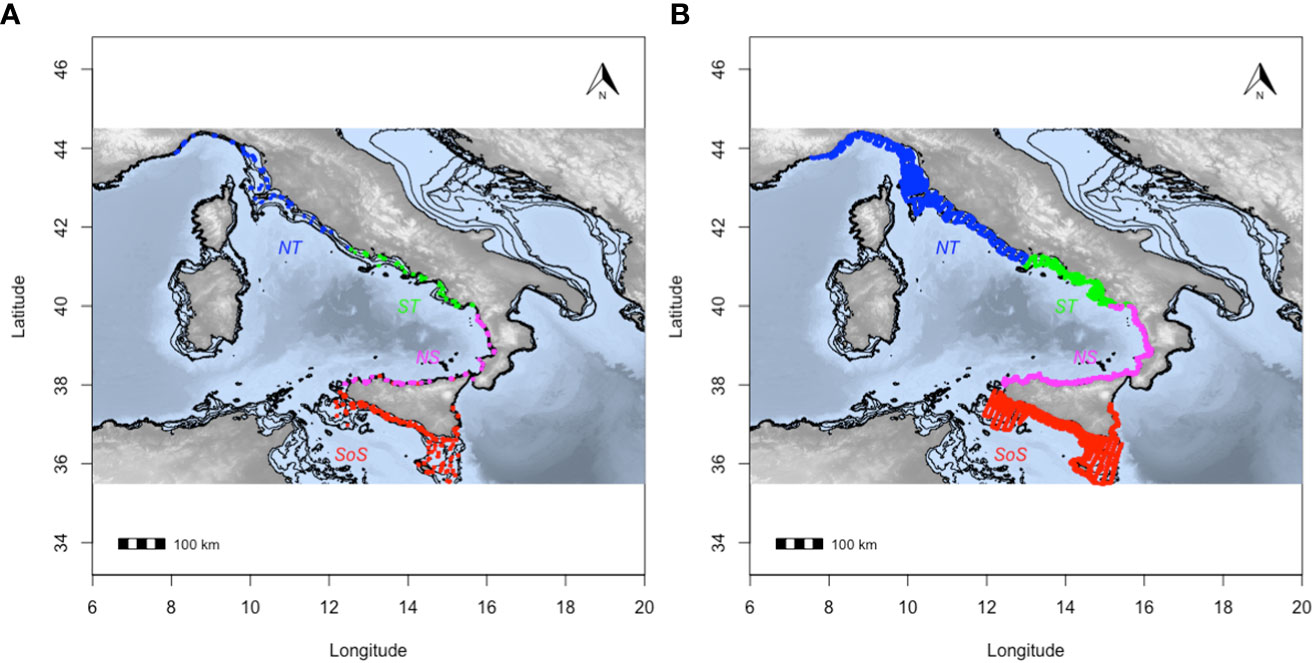
Figure 1 (A) Trawl sampling carried out in the study area along the survey period: the black line represents the 200m isobath; (B) areas stratification for comparative purposes: Strait of Sicily (SoS) in red, North of Sicily and Calabria (NS) in magenta, south Tyrrhenian Sea (ST) in green and north Tyrrhenian Sea (NT) in blue.
The European side of the Strait of Sicily (SoS), including the GSAs 15 and 16, presents a complex circulation pattern, which heavily affects pelagic resources (Basilone et al., 2013; Bonanno et al., 2014a). Here, the upper layer circulation is mainly controlled by the movement of the Atlantic Ionian Stream, AIS (Robinson et al., 1999), which induces a permanent coastal upwelling along the south-western coast of Sicily especially during summer, when this current is stronger (Bonanno et al., 2014b). The presence of the thermohaline front in the eastern part of the SoS lead also to a longitudinal gradient in terms of temperature and salinity. In addition, an important spatio-temporal variability exists in terms of shape, position, and strength of the permanent or quasi-permanent sub-basin gyres (and their unstable lobes), AIS path, transient eddies and filaments, making this area highly dynamic from an oceanographic point of view (Bonanno et al., 2014b).
The Tyrrhenian study area is located on the continental shelf along the western Italian coast. The surface circulation is characterized by the presence of a fresher water vein of Atlantic origin (Atlantic Water - AW) entering the Tyrrhenian Sea northward directed according to a seasonal-dependent pattern, (Millot and Taupier-Letage, 2005; Bonanno et al., 2014a). The central Tyrrhenian (i.e. the northern part of GSA10) is strongly influenced by the outflow of numerous medium-sized rivers (Rinaldi, 2012), the effect of which is enhanced by the complexity of the coastal morphology (Bonanno et al., 2016), characterized by the presence of enclosed areas (i.e., gulfs). Based on outflow, morphology and continental shelf extension, the south Tyrrhenian Sea (GSA 10) could be split into two subareas: the north Sicilian and Calabrian waters (NS) - characterized by a narrow continental shelf (~3 NM), low coastline complexity and few small-sized rivers - and the southern Tyrrhenian waters - mainly characterized by wider continental shelf (8 - 10 NM) high coastline complexity and a number of medium-sized rivers (ST) (Figure 1). Finally, the north Tyrrhenian waters (NT), corresponding to the Tuscany and Ligurian coasts (GSA 9), present a wider continental shelf (>10 NM), low coastline complexity and some large rivers, such as Tiber and Arno.
Sample collection and analyses
T. trachurus samples for growth studies were obtained within the framework of the hydroacoustic surveys carried out in the summer period during 8 years, from 2011 to 2018 (Table 1). Combining all the data from the 8 year samplings allowed to obtain a more robust dataset (i.e., statistically appropriate number of ages per area and size class). Although the different growth between consecutive year classes could represent a possible confounding factor, the main hypothesis in this work is that most fluctuation in growth among the areas was mainly driven by ecosystem characteristics, that remained well distinct even considering the interannual variability. Experimental fishing hauls were performed according to the MEDIAS protocol (MEDIAS, 2019) on board the research vessel “G. Dallaporta” by means of a pelagic trawl net (vertical opening of 8 m, cod-end mesh size of 18 mm; Figure 1A), towed at 4.0 knots and equipped with a net monitoring system (Simrad ITI). The latter instrumentation was used to monitor the fishing efficiency by checking the trawl position in the water column and net mouth opening (vertical and horizontal). A random sample of maximum 2 kg per haul was immediately frozen on board at -20°C. In the CNR-IAS laboratory, each fish was gently thawed and total length (TL, ± 1mm), total weight (TW, ± 0.01g), somatic weight (i.e. ovary-free weight, SW, ± 0.01g), and gonad weight (GW, ± 0.01g) were measured. Atlantic horse mackerel were sexed, and the sexual maturity was assigned based on a six-phase scale (Ferreri et al., 2019). Otoliths (sagittae) were removed from a sub-sample of five to ten individuals per size class (1cm length intervals), except in the highest and lowest size classes, where often the individuals were one or two per size class. The otoliths were cleaned, dried, and stored in black-plastic labelled moulds.
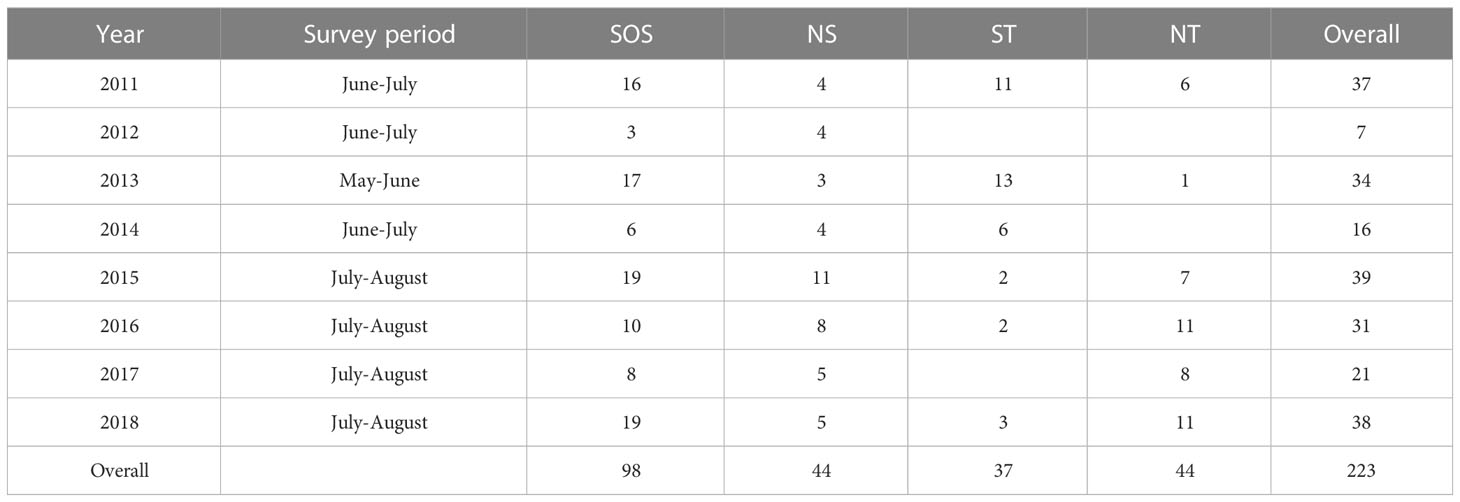
Table 1 Survey period and relative number of trawl hauls per each area: Strait of Sicily (SoS), North of Sicily and Calabria (NS), south Tyrrhenian Sea (ST), north Tyrrhenian Sea (NT).
In order to evaluate variability in maturity pattern or body status among age classes, the somatic condition index (Kn; Le Cren, 1951) and the gonadosomatic index (GSI) were respectively obtained according to Basilone et al. (2021).
Age estimation
The whole time series of age readings has been re-evaluated accounting to the latest ageing rules agreed in international procedure, in order to provide a new, standardized (among surveys) dataset allowing to consistently evaluate growth differences. The age was assigned according to the protocols and guidelines defined within “WKARHOM” international expert working groups on Atlantic horse mackerel (ICES, 2018). The whole otolith was immersed in alcohol 30% solution and analyzed under a dissecting microscope at 16x magnification (Figure 2). To avoid underestimations of older age classes due to hyaline overlapping on the edge of the otolith, a higher magnification was used in case of larger/older specimens only on the edge (Abaunza et al., 2003b). Examination of subsequent growth zones indicated that false rings and annuli often showed a similar appearance and the true annuli can only be identified, if concurrent measurements of growth zone widths are available (Waldron and Kerstan, 2001). Therefore, the measurements of total radius (TR) were also used as a gauge to assign the proper age class, and to exclude faint rings linked to later spring spawning or migration movements (Abaunza et al., 2003b). To avoid differences due to the alternation of readers over time, the re-evaluation of age readings was carried out by an expert reader on the entire historical series. Finally, age assignment was made according to the conventional birthday (set at 1 of January for this species) and the date of capture, by following the interpretation of the growth development of the annual zones over the course of a year (ICES, 2018).
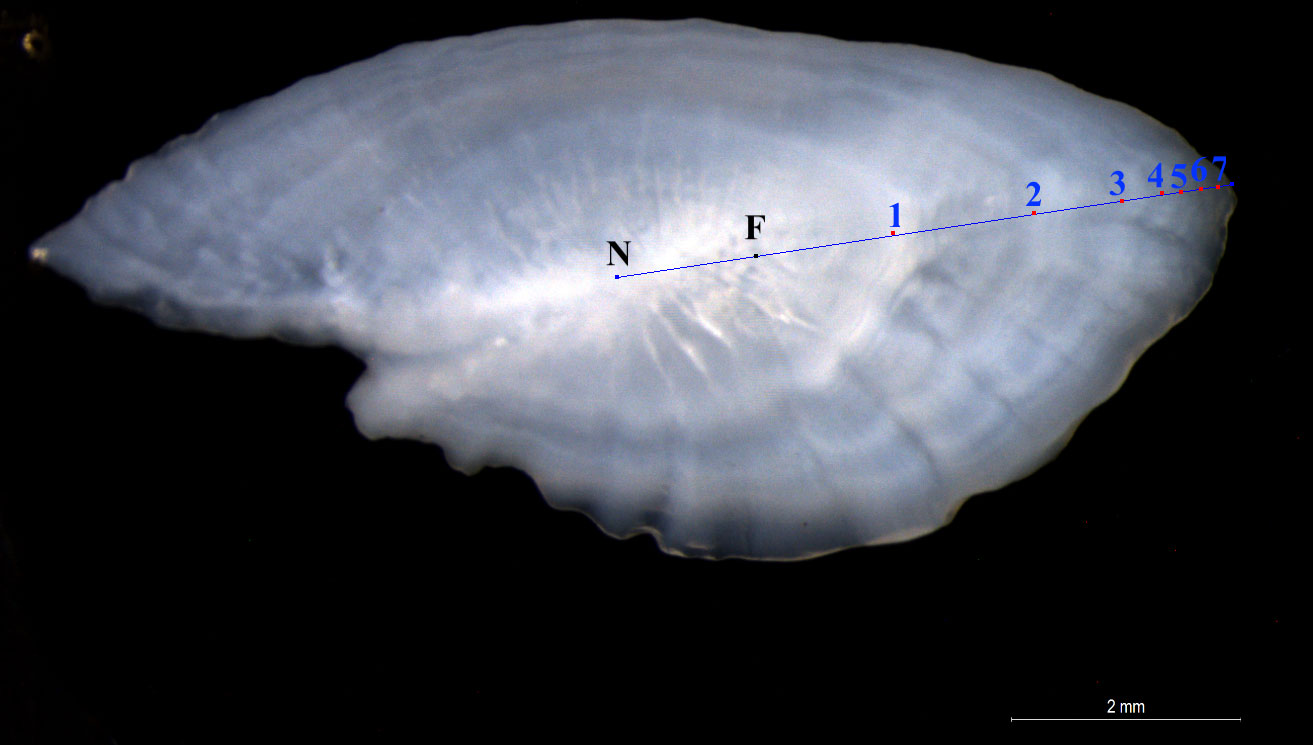
Figure 2 T. trachurus Otolith image with annuli interpretation from 1 to 7 years old. Also the first check is showed: N = nucleus, F= false ring or check.
Length at age and growth models
Age data for each sub area were analyzed for the entire study period (pooling data from 2011 to 2018), to reduce the bias due to low or not equally represented number of fishes for length interval (Abaunza et al., 2008). Non-linear least square regression was used to estimate the von Bertalanffy Growth model (VBG) parameters for each area: the asymptotic length (L∞), the body growth coefficient (k), and the theoretical length at age zero (t0). Differences in the VBG parameters among areas were evaluated by a Likelihood ratio test, according to Kimura (1980). All computations were performed in R environment (R Core Team, 2022), using “FSA” (Ogle, 2016) and “nlstools” (Baty et al., 2015) packages.
T. trachurus growth studies in literature are mostly based on the von Bertalanffy model, as useful tool for comparison purposes, but according to the several limitations in obtaining all size groups in each area, the resulting von Bertalanffy growth function parameter estimates often may represent large extrapolations beyond the range of the sampling data, and therefore it is recommended using simple, more precise approaches, such us the body lengths at age or the absolute annual increments for comparing fish growth rates (Živkov et al., 1999). Therefore, the length at age distributions were also tested to highlight growth differences among areas. To assess such differences, an ANOVA was carried out on each age class, after having verified the basic assumptions. The homogeneity of variance was evaluated by means of Levene’s test, and the significance of fixed effect was tested by means of Likelihood Ratio test (i.e., comparing the model accounting for the fixed effect and the null model for each case). Furthermore, the presence of patterns in the residuals as well as their normality were checked for each model.
Environmental data
In order to investigate the possible habitat effects on T. trachurus growth, satellite and model-derived measurements, available through Copernicus data portal, of water temperature (surface and bottom), salinity (surface and bottom), Absolute Dynamic Topography (ADT), Kinetic Energy (KE), and chlorophyll concentration (CHL - as proxy of primary production) were obtained for the investigated areas. For each parameter, the daily images were downloaded and averaged, to obtain the average conditions during the spring-summer season (from May to September), which represents the period of the year where most part of seasonal growth takes place (Abaunza et al., 2008; ICES, 2018). Depth was extracted by using the EMODnet dataset. All the considered variables have been found to be important variables in previous habitat suitability studies of these species (Giannoulaki et al., 2013; Rumolo et al., 2017; Milisenda et al., 2018).
Effects on growth of physical and biological variables
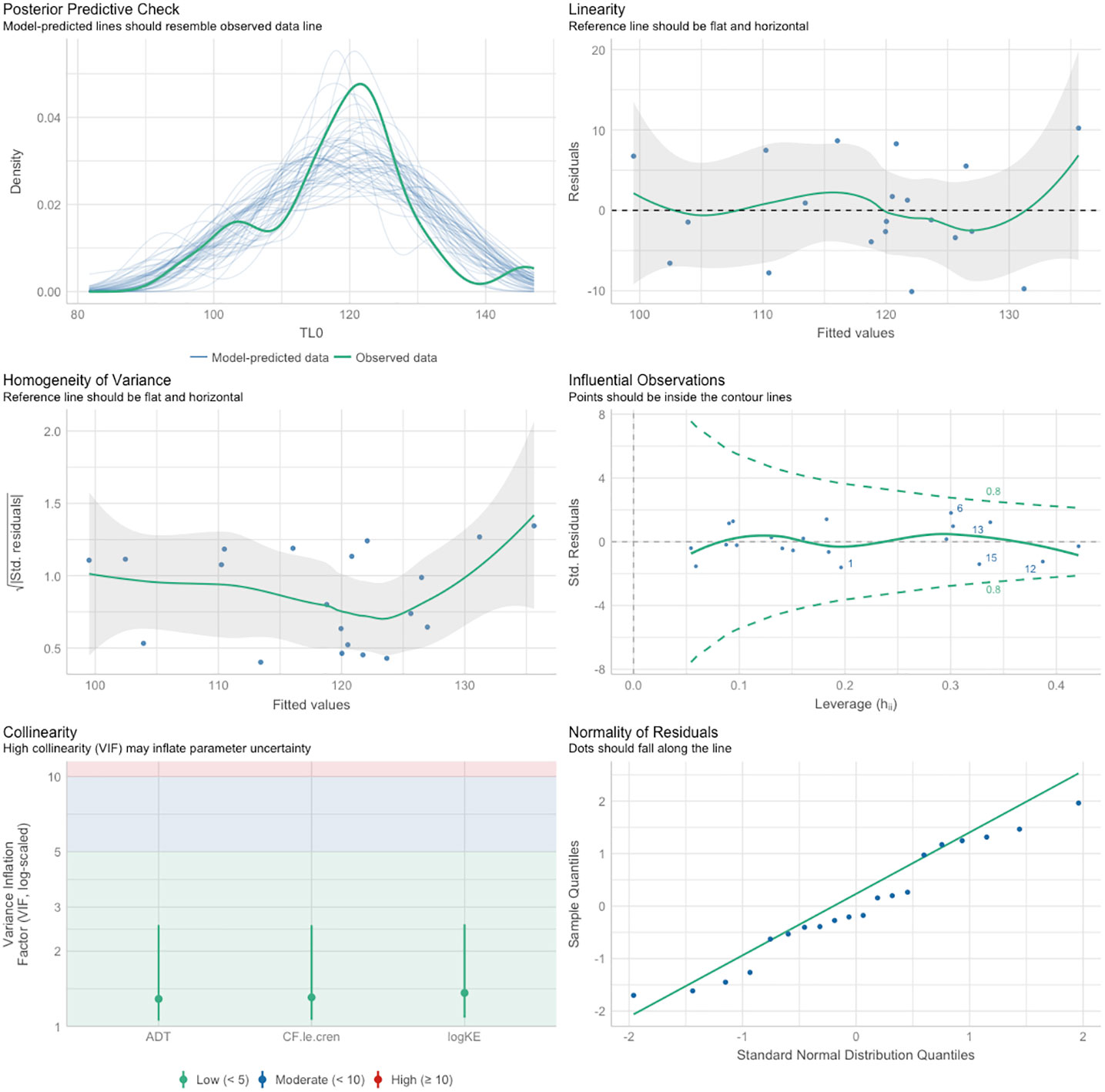
Multiple linear models were fitted in order to assess the effect of multiple predictors (fish packing density, CHL, and physical variables) on the response variable length at age as proxy for growth. The “best model” was selected according to a backward stepwise procedure, in order to identify the most parsimonious model (i.e. the model explaining the largest amount of variance with the lowest number of independent predictors). Standardized coefficients were also extracted in order to evaluate the relative importance of predictors. For each final model, the main statistical assumptions were checked. In particular, specific tests were carried out to evidence possible problems related to multicollinearity, independence of residuals, as well as their distribution and homogeneity of variance. For each model, all the following assumptions were checked: average of residuals should be close to zero, normality of residuals (Shapiro-Wilk and Anderson-Darling tests), homoscedasticity (by means of Breusch Pagan Test for Heteroskedasticity), presence of influential data points (by means of Cook’s distance and diagnostic plot). For a given predictor (p), multicollinearity has been assessed by computing the variance inflation factor (VIF), which measures how much the variance of a regression coefficient is inflated due to multicollinearity in the model. The smallest possible value of VIF is one (absence of multicollinearity). As a rule of thumb, a VIF value that exceeds 5 or 10 indicates a problematic amount of collinearity (James et al., 2014). In the present study, only variables having a VIF>5 were considered problematic (Legendre and Legendre, 2012). Presence of outliers was checked by means of Studentized residuals plots.
Finally, differences among areas for the obtained variables (both biological and physical) were firstly tested by an ANOVA and, in the case of significant differences, pair-wise post-hoc tests were carried out by means of Tukey’s ‘Honest Significant Difference’ method (HSD). All statistical analyses were performed in R statistical environment (R Core Team, 2022).
Results
Body conditions and reproductive status
A total of 5051 T. trachurus individuals were retained for age reading purposes representing 96% of collected otoliths in 223 trawls along the study period (Table 1). The size range of samples spanning between 4 and 35 cm total length (TL) (Table 2).
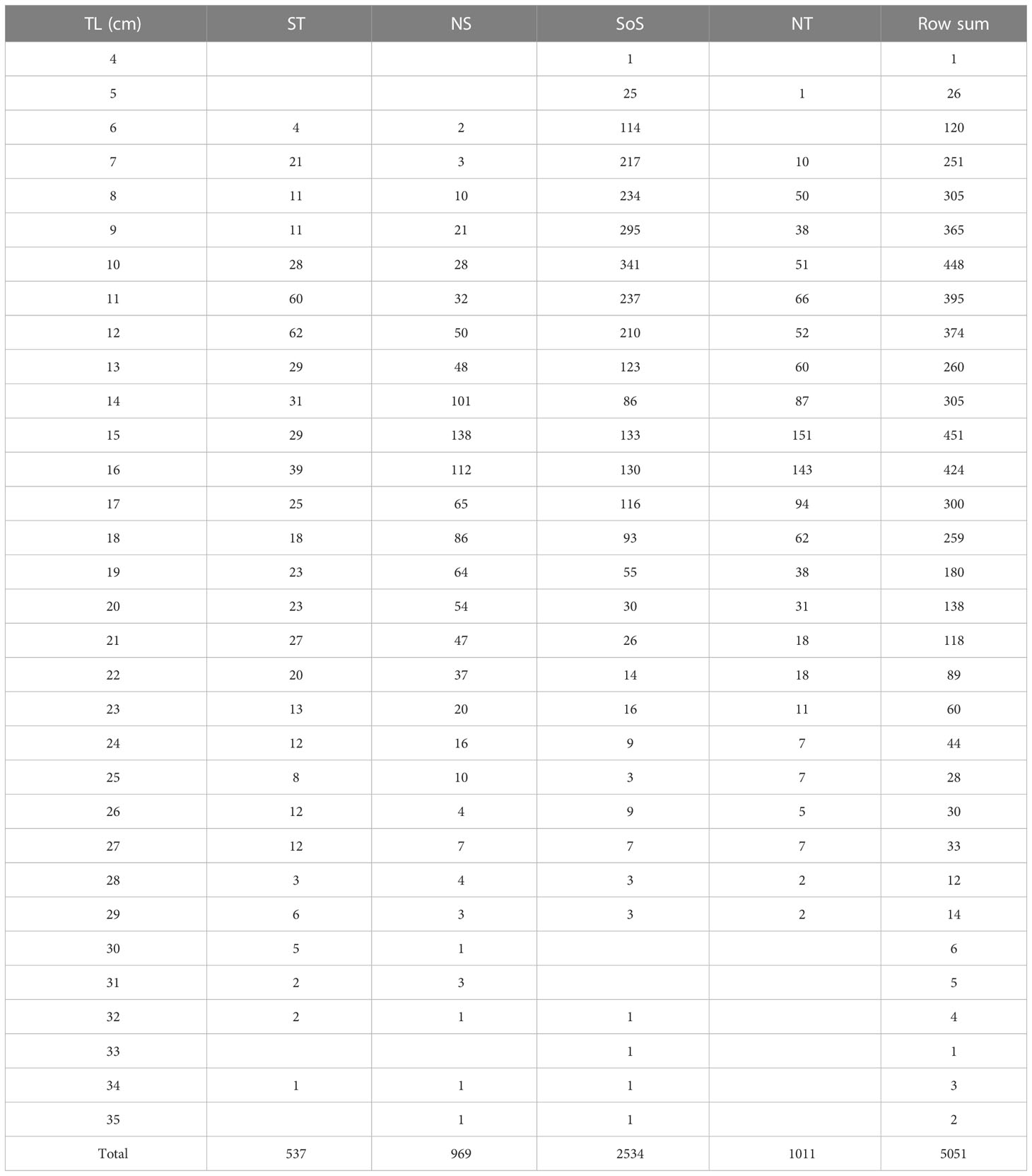
Table 2 Atlantic horse mackerel sampling size distribution range: amount of individuals per length class (1cm, TL) and area - Strait of Sicily (SoS), North of Sicily and Calabria (NS), south Tyrrhenian Sea (ST), north Tyrrhenian Sea (NT).
The Le Cren condition factor (Kn) showed the presence of significant differences among the groups (ANOVA: F(3, 5046) = [189.3], p<0.001). The post-hoc tests evidenced that SoS was significantly different from all the other areas (Table 3), with the higher Kn values recorded (Figure 3A). On the contrary, no significant differences were found among ST, NT and NS. (Table 3). According to age assignment the Kn recorded higher values in the SoS from Age 0 class until Age 5 (Figure 4A).
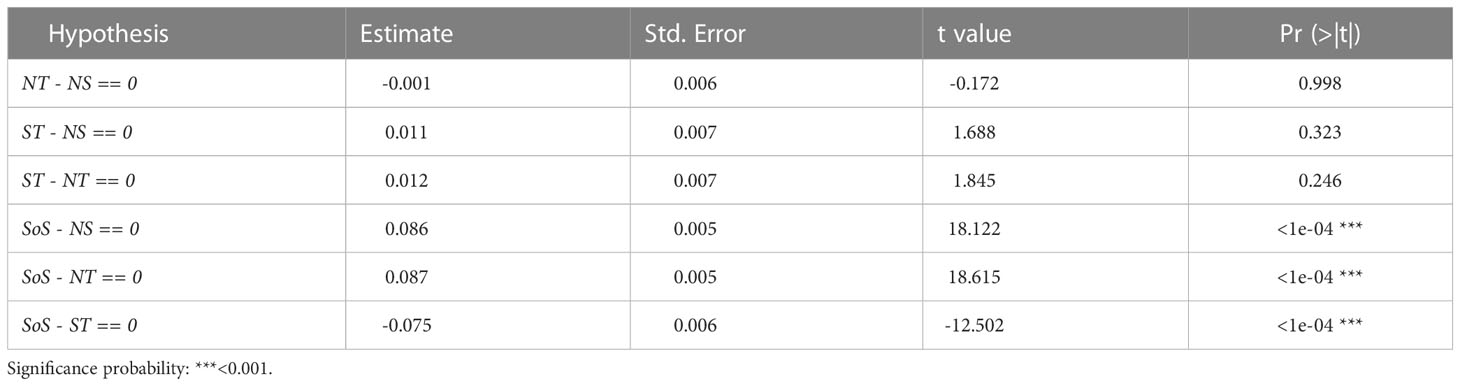
Table 3 Post-hoc test carried out on the body condition factor (Kn), showing the significance of differences between areas (Pr(>|t|)): Strait of Sicily (SoS), North of Sicily and Calabria (NS), south Tyrrhenian Sea (ST), north Tyrrhenian Sea (NT).
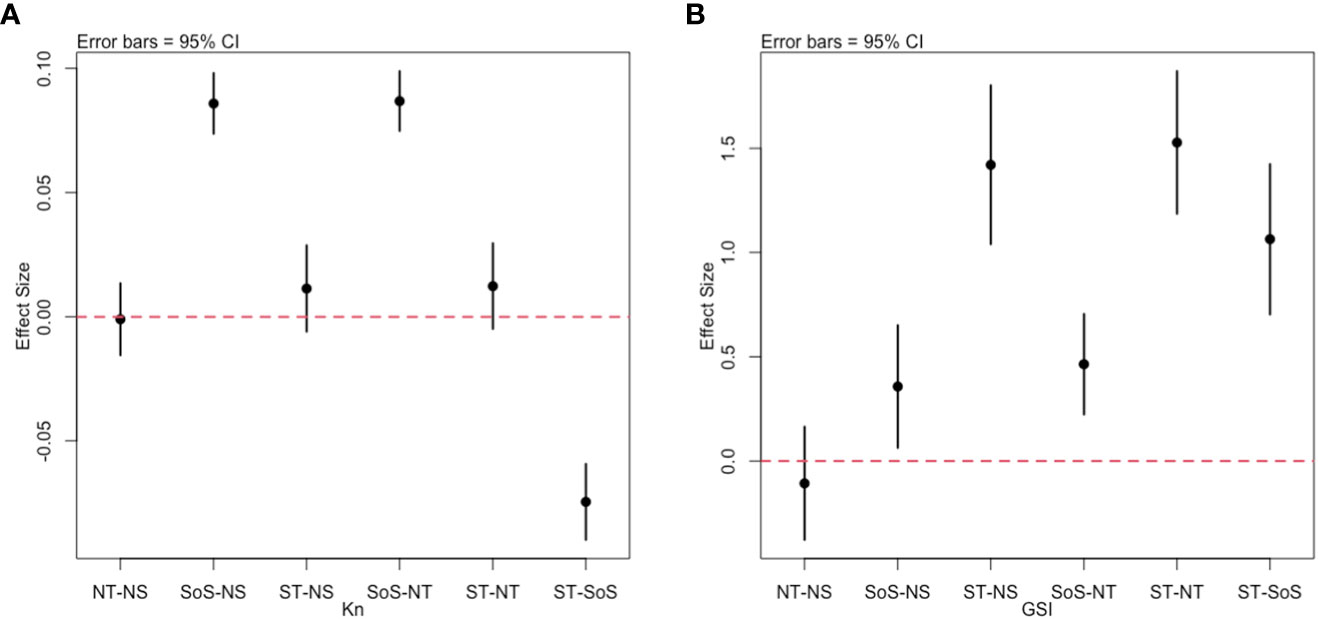
Figure 3 Tukey HSD Post-hoc tests for Kn (A) and GSI (B). On the y axis the differences between group pairs (x axis label) are reported. For each comparison, the error bars of the difference are reported; error bars crossing the red dotted line indicate non-significant differences.

Figure 4 (A) Boxplot of Kn per age class and area for the study period; (B) Boxplot of length at age per age class and area for the study period.
The reproductive status of females, as indicated by the GSI, showed significant differences (ANOVA: F(3, 1416) = [47.3], p<0.001) mainly between the ST and other areas, while the NT was similar to the NS which in turn appears closer to the SoS (Figure 3B).
Length at age
Otolith data displayed ages spanning from 0 to 7, even if the oldest age class (7 years) was not represented in all the areas (Figure 4B). The age distribution along the study period showed the proportions of Age 0 individuals was constantly higher in the SoS compared to others areas. Indeed, in SoS most part of the sampled population (66%) is constituted by juveniles (Table 4).

Table 4 Proportion (%) of sampled individuals for each year and age class by area, along the study period.
The length at age showed similar values among sub-areas over the whole age range except for the first age class (Table 5), where the differences appeared more relevant, especially in the case of SoS which was characterized by the lowest values (Figure 4B). The ANOVA (F(3, 5046) = [367], p<0.001) performed together with post-hoc pairwise comparison confirmed that the mean length at age differences among areas are mainly linked to the first year of life (i.e., Age 0), while differences in mean length for older ages were not significant among areas (Table 6). The results of age assignment showed average length at age in agreement with observed values in the relevant literature for the Mediterranean Sea (Figure 5) (Abaunza et al., 2008; ICES, 2018).

Table 5 Average length at age values (mm) per area with their respective confidence intervals (95% prob.).
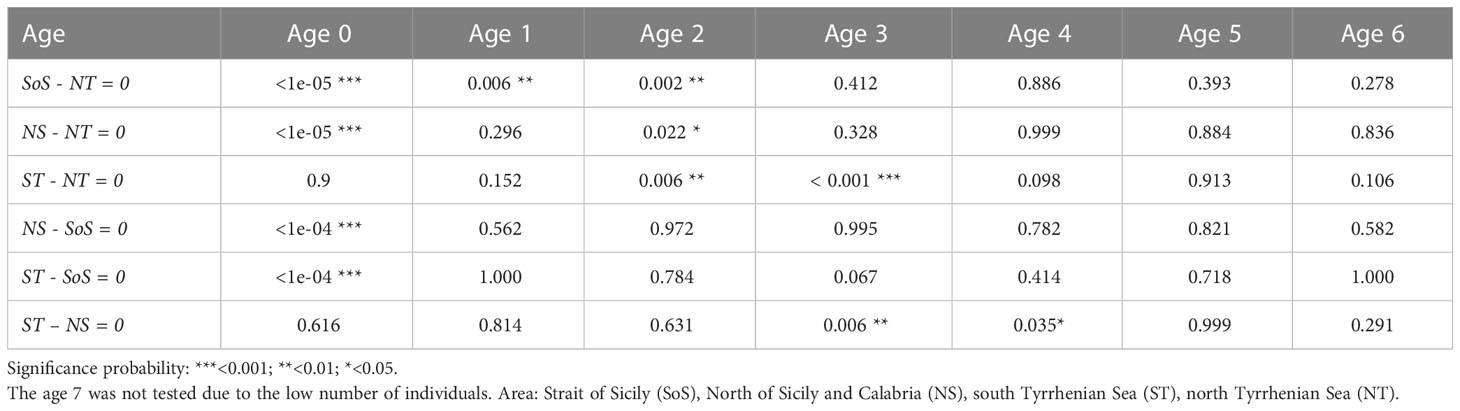
Table 6 Post-hoc test carried out on the total length by age class, showing the significance of differences between areas (Pr(>|t|)) for each age class.

Figure 5 Average length at age from Atlantic and Mediterranean waters obtained by literature (Abaunza et al., 2008). The results from the present study by area are plotted and highlighted by bigger markers (see legend for more details on the areas included).
VBG models
The VBG model parameters showed higher asymptotic length in ST (410 ± 32.8 mm) and lowest in the SoS (275± 32.8 mm), the higher K in SoS (0.36± 0.02 days) and lowers (0.14± 0.02 days) in ST and (0.16± 0.02 days) in NS (Table 7). However, the Likelihood Ratio Test performed on the VBG estimated parameters revealed not significant differences among areas, therefore an overall VBG model was also obtained describing the whole population sampled (Table 8).

Table 7 VBG model estimated parameters for Atlantic horse mackerel sampled in the four areas: estimates are provided with their standard errors and significance probability values.
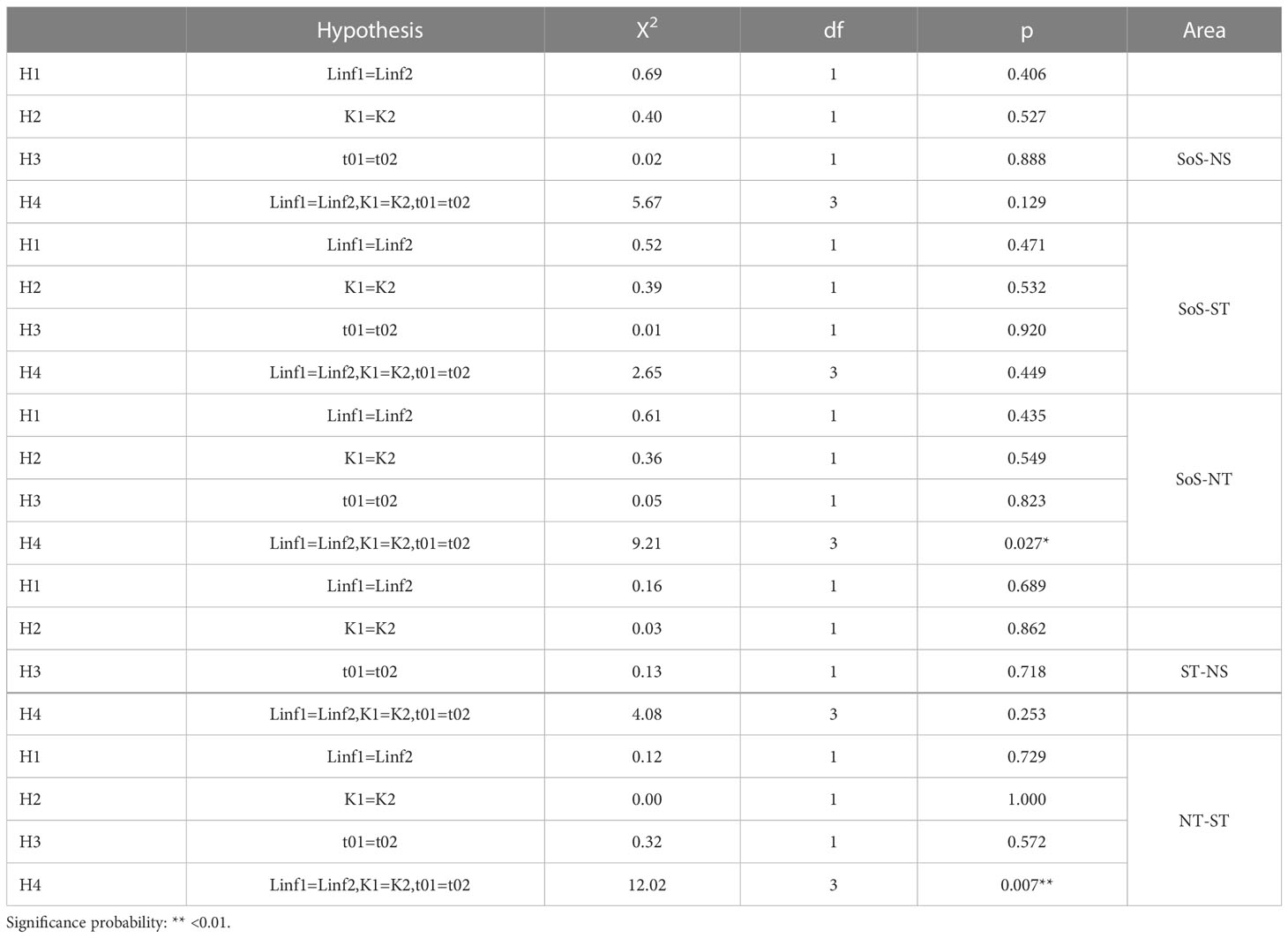
Table 8 Likelihood ratio test comparing pairs of VBG models by area: Strait of Sicily (SoS), North of Sicily and Calabria (NS), south Tyrrhenian Sea (ST), north Tyrrhenian Sea (NT).
Ecological analyses with multi linear models
The environmental observations highlighted differences among the areas in almost all the analyzed variables (Figure 6), with few exceptions: bottom depth, which showed similarities between NT and SoS, salinity (bottom) and temperature which were similar between ST and NT.
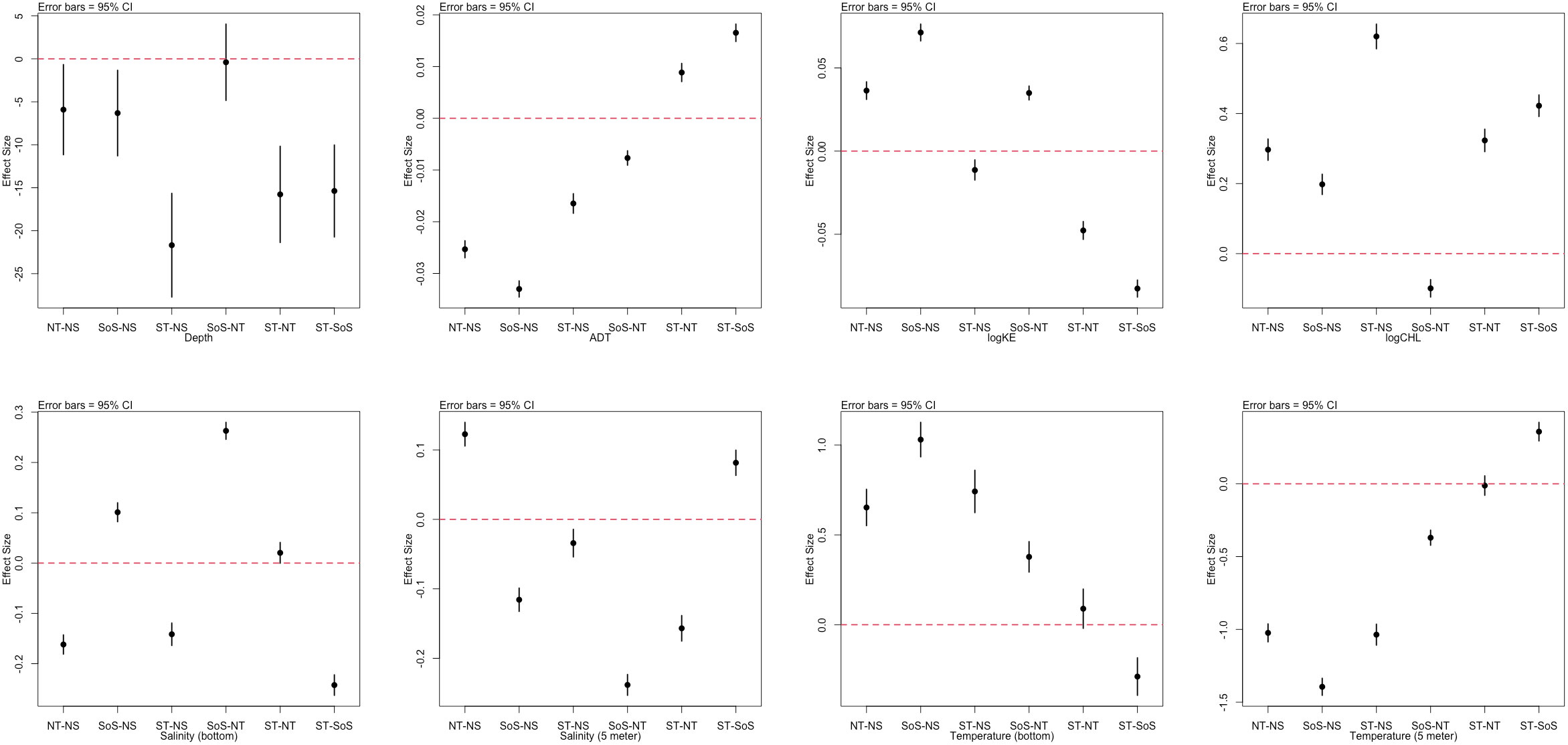
Figure 6 Tukey HSD Post-hoc tests for environmental factors. On the y axis the differences between group pairs (x axis label) are reported. For each comparison, the error bars of the difference are reported; error bars crossing the red dotted line indicate non-significant differences.
Since the significant differences in length at age among areas were evidenced mainly for Age 0 class, the multilinear modelling approach considered this class only (i.e., a proxy of growth during the first year). Such choice was also justified considering that the largest amount of growth take place during the first year (Abaunza et al., 2008). The development of candidate linear models used all the environmental variables, as environmental predictors. Among the biological predictors, TR and TW (obviously having a strong link with TL) were excluded, as the focus of the analysis was to highlight the effects of environmental factors and body condition on length at age 0. The models were fitted on the whole time series and areas, in order to evaluate the general variability pattern of T. trachurus growth in the central Mediterranean Sea.
The “full” model was:
The final model for TL0 (i.e., the reduced model after the stepwise backward selection of predictors) revealed a positive effect of ADT and a negative effect of Kn (Table 9). The overall model explained 57% of variation and the standardized coefficients evidenced the effect of the ADT was much stronger than the one of Kn. The model diagnostic showed the absence of multicollinearity problems and outliers, as well as the absence of strong patterns in the residuals or strong deviates from normality (Figure 7).
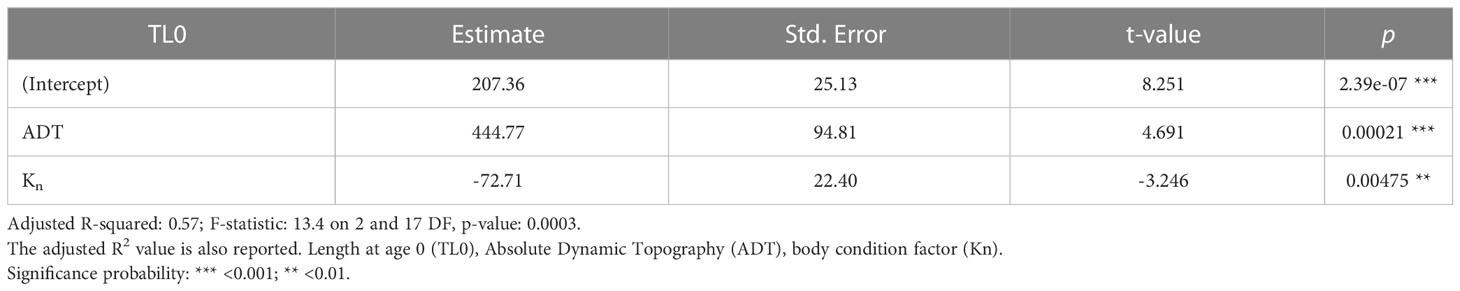
Table 9 Standardized coefficients, standard errors and significance for the estimated multi linear models.
Discussions
The analysis of length at age values in four different areas of the central Mediterranean Sea showed the presence of significant differences only for Age 0 class. In particular, the TL0 in the SoS was lower than in all the remaining Tyrrhenian areas, which resulted similar each other. Such evidences were also confirmed by the comparisons carried out among the area-specific VBG that showed no significant differences in terms of Linf, K and asymptotic length. The multi-linear modelling approach on TL0 also highlighted the difference in terms of TL0, observed among the four areas, was linked to difference in circulation patterns determining habitat differences (as summarized by the ADT) rather than to a specific set of environmental factors. The higher TL0 values were also linked to lower Kn, probably highlighting different investment strategies in terms of the acquired energy.
The energy acquired (ingestion) by fish is intended for basic metabolic maintenance (i.e., respiration, specific dynamic action, etc.), lost to excretion or available as surplus energy. Surplus energy was allocated to direct growth (either somatic or reproductive) or storage as fat or body mass (McBride et al., 2015).
In the present study, GSI (as proxy of energy allocated to reproductive status) and Kn (as proxy of energy storage as fat or body mass) found different among some of the considered areas. According to the pairwise comparison (Figure 3B), two different maturity levels were evident in the GSI: one characterizing the south Tyrrhenian (ST) and another one common to the north Tyrrhenian (NT) and the waters around Sicily (SoS and NS). Although past studies on the reproductive characteristics of T. trachurus within the same study areas reported similar GSI values in Tyrrhenian and Sicilian waters, they have also recorded differences in maturation occurred over a narrower size range and at a smaller size at first maturity in the SoS. The observed differences likely reflected the differences in habitat conditions (e.g., primary production or temperature) between the two areas (Ferreri et al., 2019 and references therein).
In several fish species, variability in Kn values were related to environmental factors, food availability, and fish physiology (Lloret, 2002; De Giosa et al., 2014; McBride et al., 2015), suggesting that similar mechanisms were responsible for the observed Kn variability among the SoS and the other sampling areas, confirmed by pairwise comparison (Figure 3A). In the SoS, enrichment processes are linked to AW advection and wind driven upwelling phenomena (Rinaldi et al., 2014), whilst in ST and NT they are mainly associated to river runoff, coastline complexity and continental shelf extension (Bonanno et al., 2016). In upwelling driven ecosystem (such as the SoS), water temperatures vary and sporadic nutrient enrichment of the euphotic zone occurs, promoting the development of phytoplankton populations and consequently zooplankton. In these ecosystems, copepods are able to discriminate between living phytoplankton cells and not living detritus (Paffenhöfer and Van Sant, 1985). On the contrary, in waters enriched by river run-off, often characterized by high turbidity levels (such as in ST and in NT), non-living matter can represent the bulk of the suspended matter pool (Tackx et al., 2003). The nutritional quality of non-living components of suspended particulate matter is low compared to living phytoplankton cells, and this difference in quality has been shown to affect the productive success of copepods consumers, that are the main food source of small pelagic fish (Burdloff et al., 2002). Although in some specific sectors of the Tyrrhenian Sea (e.g., ST) the net primary production is higher than in the SoS (Rinaldi et al., 2014; Bonanno et al., 2016), the higher Kn values observed in the latter area seems to be mainly related to food quality. The feeding mode of smaller size pelagic fish is favourited in upwelling ecosystems (Rumolo et al., 2016). Similar behavior was also observed in T. trachurus within the SoS, with a clear preference of small sizes for upwelling areas (Rumolo et al., 2017). A clear separation between the trophic niches of different size classes of T. trachurus highlighting a lower intra-specific competition for food resources that contribute to increase the species survivor, and in turn the condition of the first life stages (Rumolo et al., 2017).
The pairwise comparison of T. trachurus length at age, considered as a proxy of somatic growth, showed significant differences among areas, especially in the first year of life, with lower TL0 values in the SoS. Such pattern appeared to be confirmed by literature, since higher length at age values were reported in the Tyrrhenian waters compared to the Strait of Sicily, suggesting higher productivity as the reason for these differences (e.g., Abaunza et al., 2008). Generally, individuals of a particular fish species grow at a faster rate in low latitudes than those of the same species in high latitudes (Laevastu and Favorite, 1988), and this difference is attributable to the ability to adapt to large-scale patterns in environmental conditions (Winton et al., 2014). Therefore, according to the cited literature, the lower size at Age 0 in the SoS together with higher Kn values can be linked to the different quality/availability of food in upwelling areas. Such characteristic in the SoS would also affect older T. trachurus individuals, since their Kn was still higher up to 5 years old (Figure 4), thus this area appeared to be more suitable for storing reserves along the first years of life (i.e., Age 0 to Age 5) than other analyzed areas.
In the Strait of Sicily, it has been observed that food habits change considerably with fish growth, leading to a spatial distribution driven by food composition and, in turn, by specific environmental patterns (Rumolo et al., 2017). Abaunza et al. (2008) showed that differences in length at age in the northeast Atlantic could respond to length-dependent migratory movement between the southern spawning area to the feeding and wintering northern areas. Therefore, it is plausible that a similar migration takes place for T. trachurus also between the South of Sicily (which could constitute the main spawning area) and the Tyrrhenian waters, specially according to the following considerations: (1) along the whole study period the number of Age 0 individuals were much more abundant in the SoS (Table 3); (2) along the first 5 years of life the fish body status (Kn) was better in SoS (Figure 4); (3) along the whole study period no T. trachurus older than 6 years were caught in the SoS (Figure 4). Certainly, the higher number of young individuals in SoS may suggest the existence of a preferred spawning/nursery area for Atlantic horse mackerel, although further data (i.e., ichthyoplanktonic data) are necessary to better support such hypothesis. The obtained final model demonstrated that the body conditions significantly affect the TL0 (here considered as a proxy of fish growth rate along the first year of life). However, this latter factor appeared less important than physical forcing represented by the ADT (Table 8). Water circulation and vorticity measured by ADT are known as structuring factors in the pelagic environment by affecting early stages survivorship (Ruiz et al., 2013). In the SoS, the seasonal phytoplankton variability is mainly driven by the Atlantic water advection, rich in CHL and nutrients from the North Africa coasts as well as in minor part by the vertical nutrient flux associated with coastal upwelling, which affect the temperature field (Rinaldi et al., 2014). The influence of water masses circulation was documented in several marine habitats in the central Mediterranean Sea, both in surface layers and deeper waters (Abella et al., 2008; Tugores et al., 2011; Basilone et al., 2013; Zarrad et al., 2013; Bonanno et al., 2014a; Bonanno et al., 2015). Previous studies in the Strait of Sicily already revealed that currents (and gyres) are among the principal abiotic factors controlling small pelagic fish distribution, suggesting that circulation and food-related processes (i.e., zooplankton concentration) are of major importance for this species (Giannoulaki et al., 2013; Bonanno et al., 2014a).
Conclusions
Although significant differences in the length at age 0 have been recorded between the Strait of Sicily and the Tyrrhenian waters, Atlantic horse mackerel growth patterns along the central Mediterranean appeared quite similar, suggesting a common growth pattern for the whole population investigated. The differences observed in the first year of life resulted mainly linked to physical variability, but also body condition showed a relevant role in structuring the population growth, at least for the first year.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
GB, RF, MB, and AB: data curation. PR, PC, MB and GB: formal analysis and validation. AB: funding acquisition and project administration. RF and GB: investigation, methodology, and writing—original draft. SA and SG: software. GB: supervision. AB, MB, SA, and SG: writing—review and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
This study was supported by the Consiglio Nazionale delle Ricerche and the European Union through the Data Collection Framework (DCF – Reg. Ce. No 199/2008, No 665/2008 and Commission Decision No 949/2008; MEDIAS – Mediterranean International Acoustic Surveys). The Master of the R/V G. Dallaporta and all his crew are thanked for their work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abaunza P., Fariña A. C., Murta A. (2003a). Applying biomass dynamic models to the southern horse mackerel stock (Atlantic waters of Iberian peninsula). a comparison with VPA-based methods. Sci. Mar. 67, 291–300. doi: 10.3989/scimar.2003.67s1291
Abaunza P., Gordo L., Karlou-Riga C., Murta A., Zimmermann C., Hammer C., et al. (2003b). Growth and reproduction of horse mackerel, Trachurus trachurus (carangidae). Rev. Fish Biol. Fish. 13, 27–61. doi: 10.1023/A:1026334532390
Abaunza P., Gordo L. S., Santamaría M. T. G., Iversen S. A., Murta A. G., Gallo E. (2008). Life history parameters as basis for the initial recognition of stock management units in horse mackerel (Trachurus trachurus). Fish. Res. 89, 167–180. doi: 10.1016/j.fishres.2007.09.021
Abella A., Fiorentino F., Mannini A., Orsi Relini L. (2008). Exploring relationships between recruitment of European hake (Merluccius merluccius l. 1758) and environmental factors in the ligurian Sea and the strait of Sicily (Central Mediterranean). J. Mar. Syst. 71, 279–293. doi: 10.1016/j.jmarsys.2007.05.010
Barange M., Bernal M., Cercole M. C., Cubillos L. A., Cunningham C. L., et al. (2009). “Current trends in the assessment and management of stocks,” in Climate change and small pelagic fish. Eds. Checkley D., Roy D. M. C. Jr., Oozeki Y., Alheit J. (Cambridge, UK: Cambridge University Press), 191–255.
Basilone G., Bonanno A., Patti B., Mazzola S., Barra M., Cuttitta A., et al. (2013). Spawning site selection by European anchovy (Engraulis encrasicolus) in relation to oceanographic conditions in the strait of Sicily. Fish. Oceanogr. 22, 309–323. doi: 10.1111/fog.12024
Basilone G., Ferreri R., Aronica S., Mazzola S., Bonanno A., Gargano A., et al. (2021). Reproduction and sexual maturity of European sardine (Sardina pilchardus) in the central Mediterranean Sea. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.715846
Basilone G., Guisande C., Patti B., Mazzola S., Cuttitta A., Bonanno A., et al. (2004). Linking habitat conditions and growth in the European anchovy (Engraulis encrasicolus). Fish. Res. 68, 9–19. doi: 10.1016/j.fishres.2004.02.012
Baty F., Ritz C., Charles S., Brutsche M., Flandrois J.-P., Delignette-Muller M.-L. (2015). A toolbox for nonlinear regression in R : the package nlstools. J. Stat. Software 66, 1–21. doi: 10.18637/jss.v066.i05
Boeuf G., Le Bail P.-Y. (1999). Does light have an influence on fish growth? Aquaculture 177, 129–152. doi: 10.1016/S0044-8486(99)00074-5
Bœuf G., Payan P. (2001). How should salinity influence fish growth? 130, 411–423. doi: 10.1016/S1532-0456(01)00268-X
Bonanno A., Barra M., Basilone G., Genovese S., Rumolo P., Goncharov S., et al. (2016). Environmental processes driving anchovy and sardine distribution in a highly variable environment: the role of the coastal structure and riverine input. Fish. Oceanogr. 25, 471–490. doi: 10.1111/fog.12166
Bonanno A., Giannoulaki M., Barra M., Basilone G., Machias A., Genovese S., et al. (2014a). Habitat selection response of small pelagic fish in different environments. two examples from the oligotrophic Mediterranean Sea. PloS One 9, e101498. doi: 10.1371/journal.pone.0101498
Bonanno A., Placenti F., Basilone G., Mifsud R., Genovese S., Patti B., et al. (2014b). Variability of water mass properties in the strait of Sicily in summer period of 1998–2013. Ocean Sci. 10, 759–770. doi: 10.5194/os-10-759-2014
Bonanno A., Zgozi S., Basilone G., Hamza M., Barra M., Genovese S., et al. (2015). Acoustically detected pelagic fish community in relation to environmental conditions observed in the central Mediterranean sea: a comparison of Libyan and Sicilian–Maltese coastal areas. Hydrobiologia 755, 209–224. doi: 10.1007/s10750-015-2234-0
Brander K. M. (1995). The effect of temperature on growth of Atlantic cod (Gadus morhua L.) 52, 1, 1–10. doi: 10.1016/1054-3139(95)80010-7
Brander K. M. (2007). Global fish production and climate change. Proc. Natl. Acad. Sci. 104, 19709–19714. doi: 10.1073/pnas.0702059104
Brunel T., Dickey-Collas M. (2010). Effects of temperature and population density on von bertalanffy growth parameters in Atlantic herring: a macro-ecological analysis. Mar. Ecol. Prog. Ser. 405, 15–28. doi: 10.3354/meps08491
Brunel T., Piet G. J. (2013). Is age structure a relevant criterion for the health of fish stocks? ICES J. Mar. Sci. 70, 270–283. doi: 10.1093/icesjms/fss184
Burdloff D., Gasparini S., Villate F., Uriarte I., Cotano U., Sautour B., et al. (2002). Egg production of the copepod Acartia bifilosa in two contrasting European estuaries in relation to seston composition. J. Exp. Mar. Biol. Ecol. 274, 1–17. doi: 10.1016/S0022-0981(02)00133-8
Carbonara P., Ciccolella A., De Franco F., Palmisano M., Bellodi A., Lembo G., et al. (2022). Does fish growth respond to fishing restrictions within marine protected areas? a case study of the striped red mullet in the south-west Adriatic Sea (central Mediterranean). Aquat. Conserv. Mar. Freshw. Ecosyst. 32, 417–429. doi: 10.1002/aqc.3776
Carbonara P., Zupa W., Anastasopoulou A., Bellodi A., Bitetto I., Charilaou C., et al. (2019). Explorative analysis on red mullet (Mullus barbatus) ageing data variability in the Mediterranean. Sci. Mar. 83, 271. doi: 10.3989/scimar.04999.19A
Chuksin Y. V., Nazarov N. A. (1989). Peculiarities of distribution and behaviour of horse mackerel in the NE Atlantic Vol. 7 (ICES), 18 p.
De Giosa M., Czerniejewski P., Rybczyk A. (2014). Seasonal changes in condition factor and weight-length relationship of invasive Carassius gibelio (Bloc 1782) from leszczynskie Lakeland, Poland. Adv. Zool. 2014, 1–7. doi: 10.1155/2014/678763
D’Elia M., Patti B., Bonanno A., Fontana I., Giacalone G., Basilone G., et al. (2014). Analysis of backscatter properties and application of classification procedures for the identification of small pelagic fish species in the central Mediterranean. Fish. Res. 149, 33–42. doi: 10.1016/j.fishres.2013.08.006
DeVries D. R., Frie R. V. (1996). “Determination of age andgrowth,” in Fisheries techniques. 2nd edn. Eds. Murphy B. R., Willis D. W. (Bethesda, MD: American Fisheries Society), Pages 483–512.
EU (2008). Directive 2008/56/EC of the European parliament and of the council of 17 June 2008 establishing a framework community action in the field of marine environmental policy (Marine strategy framework directive) (Belgium: European Union), 19–40.
EU (2013). Regulation (EU) no 1380/2013 of the European parliament and of the council of 11 December 2013 on the common fisheries policy, amending council regulations (EC) no 1954/2003 and (EC) no 1224/2009 and repealing council regulations (EC) no 2371/2002 and (EC) Vol. 354 (Belgium: European Union), 22–61.
EU (2017). Regulation (EU) 2017/1004 of the European parliament and of the council of 17 may 2017 on the establishment of a union framework for the collection, management and use of data in the fisheries sector and support for scientific advice regarding the common fisheries policy and repealing council regulation (EC) no 199/2008, 2017. l 157/1-21.
FAO (2018). The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. Rome. ed. FAO, Rome (Italy), 227 pp.
Ferreri R., McBride R. S., Barra M., Gargano A., Mangano S., Pulizzi M., et al. (2019). Variation in size at maturity by horse mackerel (Trachurus trachurus) within the central Mediterranean Sea: implications for investigating drivers of local productivity and applications for resource assessments. Fish. Res. 211, 291–299. doi: 10.1016/j.fishres.2018.11.026
GFCM (2009). Establishment of geographical Sub-areas in the GFCM area amending the resolution GFCM/31/2007/2. (Rome Italy: FAO).
Giannoulaki M., Iglesias M., Tugores M. P., Bonanno A., Patti B., De Felice A., et al. (2013). Characterizing the potential habitat of European anchovy Engraulis encrasicolus in the Mediterranean Sea, at different life stages: Habitat of anchovy in the mediterranean. Fish. Oceanogr. 22, 69–89. doi: 10.1111/fog.12005
ICES (2018). Report of the Workshop on Age reading of Horse Mackerel, Mediterranean Horse Mackerel and Blue Jack Mackerel (Trachurus trachurus, T. mediterraneus and T. picturatus) (WKARHOM3), 5–9 November 2018. Livorno, Italy. ICES CM 2018/EOSG:28. ICES Scientific Reports, Copenhagen (Denmark), 186pp. doi: 10.17895/ICES.PUB.8170
ICES (2020). Working Group on Southern Horse Mackerel, Anchovy and Sardine (WGHANSA). ICES Scientific Reports. Copenhagen (Denmark), 2:41.655 pp. doi: 10.17895/ices.pub.5977
Iglesias M. (2003). Spatio-temporal patterns and morphological characterisation of multispecies pelagic fish schools in the north-Western Mediterranean Sea. Aquat. Living Resour. 16, 541–548. doi: 10.1016/j.aquliv.2003.07.003
James D. A., Mosel K., Chipps S. R. (2014). The influence of light, stream gradient, and iron on Didymosphenia geminata bloom development in the black hills, south Dakota. Hydrobiologia 721, 117–127. doi: 10.1007/s10750-013-1654-y
Jones G. P. (1986). Food availability affects growth in a coral reef fish. Oecologia 70, 136–139. doi: 10.1007/BF00377123
Kelley E., Sherman K. (2018). Trends of the Large marine ecosystem assessment and management approach as reflected in the literature. Ocean Coast. Manage. 155, 104–112. doi: 10.1016/j.ocecoaman.2017.12.008
Kimura D. K. (1980). Likelihood methods for the von Bertalanffy growth curve. U. S. Fish. Bull. 77(4), 765–776.
Laevastu T., Favorite F. (1988). Fishing and Stock Fluctuations ed. Fishing News Books Ltd., Farnham, UK, 239 pp.
Le Cren E. D. (1951). The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 20, 201–221. doi: 10.2307/1540
Legendre P., Legendre L. (2012). “Interpretation of ecological structures,” in Developments in environmental modelling (Elsevier), 521–624. doi: 10.1016/B978-0-444-53868-0.50010-1
Lloret J. (2002). Effects of large-scale habitat variability on condition of demersal exploited fish in the north-western Mediterranean. ICES J. Mar. Sci. 59, 1215–1227. doi: 10.1006/jmsc.2002.1294
Lorenzen K. (2016). Toward a new paradigm for growth modeling in fisheries stock assessments: embracing plasticity and its consequences. Fish. Res. 180, 4–22. doi: 10.1016/j.fishres.2016.01.006
Macer C. T. (1977). Some aspects of the biology of the horse mackerel [Trachurus trachurus (L.)] in waters around Britain. J. Fish Biol. 10, 51–62. doi: 10.1111/j.1095-8649.1977.tb04041.x
McBride R. S., Somarakis S., Fitzhugh G. R., Albert A., Yaragina N. A., Wuenschel M. J., et al. (2015). Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish Fish 16, 23–57. doi: 10.1111/faf.12043
MEDIAS (2019). MEDIAS handbook. common protocol for the pan-MEditerranean acoustic survey (MEDIAS) (Athens, Greece), 24 p. Available at: http://www.medias-project.eu/medias/website.
Milisenda G., Garofalo G., Fezzani S., Rjeibi O., Othman J., Chemmam B., et al. (2018). Erratum to: biomass HotSpot distribution model and spatial interaction of two exploited species of horse mackerel in the south-central Mediterranean Sea. Hydrobiologia 821, 135–150. doi: 10.1007/s10750-017-3336-7
Millot C., Taupier-Letage I. (2005). “Circulation in the Mediterranean Sea,” in The Mediterranean Sea handbook of environmental chemistry. Ed. Saliot A. (Berlin, Heidelberg: Springer Berlin Heidelberg), 29–66. doi: 10.1007/b107143
Moriarty M., Greenstreet S. P. R., Rasmussen J., de Boois I. (2019). Assessing the state of demersal fish to address formal ecosystem based management needs: making fisheries independent trawl survey data ‘Fit for purpose.’. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00162
Neuheimer A., Taggart C., Frank K. (2008). “Size-at-age in haddock (Melanogrammus aeglefinus): application of the growing degree-day (GDD) metric,” in Resiliency of gadid stocks to fishing and climate change (Fairbanks, Alaska, US: Alaska Sea Grant College Program), 111–124. doi: 10.4027/rgsfcc.2008.06
Paffenhöfer G., Van Sant K. (1985). The feeding response of a marine planktonic copepod to quantity and quality of particles. Mar. Ecol. Prog. Ser. 27, 55–65. doi: 10.3354/meps027055
Pauly D. (1980). On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. J. Cons. CIEM 39 (2), 175–192. doi: 10.1093/icesjms/39.2.175
R Core Team (2022). R: a language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Rinaldi E. (2012). Satellite and in situ data integrated analysis to study the upper ocean and coastal environment of the Italian seas (Italy: Alma Mater Studiorum Università di Bologna).
Rinaldi E., Buongiorno Nardelli B., Volpe G., Santoleri R. (2014). Chlorophyll distribution and variability in the Sicily channel (Mediterranean Sea) as seen by remote sensing data. Cont. Shelf Res. 77, 61–68. doi: 10.1016/j.csr.2014.01.010
Robinson A. R., Sellschopp J., Warn-Varnas A., Leslie W. G., Lozano C. J., Haley P. J., et al. (1999). The Atlantic Ionian stream. J. Mar. Syst. 20, 129–156. doi: 10.1016/S0924-7963(98)00079-7
Rose K. A. Jr, Cowan J. H., Winemiller K. O., Myers R. A., Hilborn R. (2001). Compensatory Blackwell Science Ltd density dependence in fish populations: importance, controversy, understanding and prognosis. FISH Fish. 35, 293–327. doi: 10.1046/j.1467-2960.2001.00056.x
Rückert C. (2002). An estimate of horse mackerel biomass in the north Sea 1991–1997. ICES J. Mar. Sci. 59, 120–130. doi: 10.1006/jmsc.2001.1146
Ruiz J., Macías D., Rincón M. M., Pascual A., Catalán I. A., Navarro G. (2013). Recruiting at the edge: kinetic energy inhibits anchovy populations in the western Mediterranean. PloS One 8, e55523. doi: 10.1371/journal.pone.0055523
Rumolo P., Basilone G., Fanelli E., Barra M., Calabrò M., Genovese S., et al. (2017). Linking spatial distribution and feeding behavior of Atlantic horse mackerel (Trachurus trachurus) in the strait of Sicily (Central Mediterranean Sea). J. Sea Res. 121, 47–58. doi: 10.1016/j.seares.2017.01.002
Rumolo P., Bonanno A., Barra M., Fanelli E., Calabrò M., Genovese S., et al. (2016). Spatial variations in feeding habits and trophic levels of two small pelagic fish species in the central Mediterranean Sea. Mar. Environ. Res. 115, 65–77. doi: 10.1016/j.marenvres.2016.02.004
Smith-Vaniz W. F. (1986). “Carangidae,” in Fishes of the north Eastern atlantic and the Mediterranean volume II. Eds. Whitehead P. J. P., Bauchot M. L., Hurreau J. C., Nielsen J., Tortonese E. (Paris: UNESCO), 815–844.
Tackx M. L. M., Herman P. J. M., Gasparini S., Irigoien X., Billiones R., Daro M. H. (2003). Selective feeding of Eurytemora affinis (Copepoda, calanoida) in temperate estuaries: model and field observations. Estuar. Coast. Shelf Sci. 56, 305–311. doi: 10.1016/S0272-7714(02)00182-8
Taylor C. C. (1958). Cod growth and temperature. J. Cons. CIEM 23, 366–370. doi: 10.1093/icesjms/23.3.366
Tugores M., Giannoulaki M., Iglesias M., Bonanno A., Tičina V., Leonori I., et al. (2011). Habitat suitability modelling for sardine Sardina pilchardus in a highly diverse ecosystem: the Mediterranean Sea. Mar. Ecol. Prog. Ser. 443, 181–205. doi: 10.3354/meps09366
Waldron M., Kerstan M. (2001). Age validation in horse mackerel (Trachurus trachurus) otoliths. ICES J. Mar. Sci. 58, 806–813. doi: 10.1006/jmsc.2001.1071
Winton M. V., Wuenschel M. J., McBride R. S. (2014). Investigating spatial variation and temperature effects on maturity of female winter flounder (Pseudopleuronectes americanus) using generalized additive models. Can. J. Fish. Aquat. Sci. 71, 1279–1290. doi: 10.1139/cjfas-2013-0617
Zarrad R., Alemany F., Rodriguez J.-M., Jarboui O., Lopez-Jurado J.-L., Balbin R. (2013). Influence of summer conditions on the larval fish assemblage in the eastern coast of Tunisia (Ionian Sea, southern Mediterranean). J. Sea Res. 76, 114–125. doi: 10.1016/j.seares.2012.08.001
Keywords: age structure, growth models, body condition, Central Mediterranean, Trachurus trachurus
Citation: Basilone G, Ferreri R, Aronica S, Bonanno A, Genovese S, Rumolo P, Carbonara P and Barra M (2023) Growth variability in Atlantic horse mackerel Trachurus trachurus (Linneus, 1758) across the central Mediterranean Sea: contrasting latitudinal gradient and different ecosystems. Front. Mar. Sci. 10:1161552. doi: 10.3389/fmars.2023.1161552
Received: 08 February 2023; Accepted: 02 May 2023;
Published: 22 May 2023.
Edited by:
Athanassios C. Tsikliras, Aristotle University of Thessaloniki, GreeceReviewed by:
M. Cristina Mangano, Anton Dohrn Zoological Station Naples, ItalyDeniz Erguden, Iskenderun Technical University, Türkiye
Copyright © 2023 Basilone, Ferreri, Aronica, Bonanno, Genovese, Rumolo, Carbonara and Barra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gualtiero Basilone, Z3VhbHRpZXJvLmJhc2lsb25lQGNuci5pdA==; Rosalia Ferreri, cm9zYWxpYS5mZXJyZXJpQGNuci5pdA==
 Gualtiero Basilone
Gualtiero Basilone Rosalia Ferreri1*
Rosalia Ferreri1* Pierluigi Carbonara
Pierluigi Carbonara Marco Barra
Marco Barra