
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 31 March 2023
Sec. Aquatic Microbiology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1160585
 Shanshan Liu1,2,3,4
Shanshan Liu1,2,3,4 Zhiming Yu1,2,3,4*
Zhiming Yu1,2,3,4* Zaixing Wu1,2,4
Zaixing Wu1,2,4 Xihua Cao1,2,3,4
Xihua Cao1,2,3,4 Ruihong Cheng1,2,3,4
Ruihong Cheng1,2,3,4 Xiuxian Song1,2,3,4
Xiuxian Song1,2,3,4Herein, the algicidal effects of Brevibacillus laterosporus on typical red tide organisms were investigated. Through comparative analysis of the fermentation solution, sterile filtrate and bacterial body, it was found that this strain mainly exerts its algicidal function by secreting algicidal substances. In this paper, we established a method for extracting the algicidal substances of B. laterosporus and systematically investigated their features and effect on Heterosigma akashiwo. The results showed that the algicidal substances are a mixture of compounds all with molecular weights less than 3500 Da, with those below 100 Da producing 45% of the algicidal effect; these are more polar and best extracted with methanol. The algicidal substances are stable over a range of temperatures (-80.0 to 70.0°C) and pH values (4.0-8.0). The algicidal substances caused most H. akashiwo cells to rupture within 24 h. In the remaining cells, the algicidal substances activated the antioxidant system and reduced their metabolic activity, leading to apoptosis, as observed by cell crumbling and a reduction in membrane potential. The responses of different algal cell surface structures to the algicidal substances were also compared and analysed. It was concluded that these algicidal substances can act on the cell membrane and change its permeability, allowing entry of the algicidal substances to produce an algicidal effect.
Red tide is an ecological anomaly caused by the proliferation or accumulation of algae in water bodies that poses a serious threat to human survival and health (NOAA, 2016). In recent years, red tide has been expanding worldwide, destroying marine ecosystems and impacting human health (Yu et al., 2017). The effective prevention and control of red tide has become an important issue for coastal livelihoods.
It has been demonstrated recently that numerous genera of marine bacteria have algicidal effects against red tide organisms and most of them belong to the Cytophaga/Flavobacterium/Bacteroidetes (CFB) group or to the γ-proteobacteria group, e.g. the genera Alteromonas, Bacillus, Cellulophaga, Cytophaga, Flavobacterium, Micrococcus, Planomicrobium, Pseudoalteromonas, Pseudomonas, Saprospira, Vibrio, and Zobellia (Wang et al., 2020). These findings have accordingly raised the possibility for the bacteria to be used to control red tides (Pal et al., 2020).
Generally, the bacteria have the algicidal action directly or indirectly. Direct action means that bacterial functions such as cytokinesis and cellular respiration depend on direct contact between bacterial and algal cells, with some bacteria even being able to invade the algal cells. Indirect action means that the bacteria inhibit algal growth by secreting algicidal substances or competing with algal cells for nutrients, in which 70% of the bacteria have the algicidal action by secreting extracellular substances (Inaba et al., 2017; Sun et al., 2018). At present, the algicidal substances that have been found include amino acids, biosurfactants, peptides, proteins, pigments and fatty acids etc. Zhang et al. (2020) found that prodigiosin secreted by the bacteria Hahella sp. KA22 can lyse Heterosigma akashiwo. The rhamnolipid biosurfactants (the mixture of Rha-Rha-C-10-C-10 and Rha-C-10-C-10) produced by Pseudomonas aeruginosa also have potential algicidal effects on Gymnodinium sp. and Prorocentrum dentatum (Wang et al., 2005). Li (2015) found that the algicidal mode of Bacillus sp. Lzh-5 was to attack Microcystis aeruginosa cells by releasing algicidal compounds S-5A (hexahydropyrrolo[1,2-a]pyrazine-1,4-dione) and S-5B (3-isopropyl-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione). Although more and more algicidal bacteria have been found and isolated, the study of algicidal substances is still quite backward. Due to the difficulty in separation, purification and enrichment of algicidal substances, only a small number of algicidal substances have been identified.
In this study, a strain of Brevibacillus laterosporus, which is commonly used in aquaculture, was found for the first time that it has the algicidal effect. This strain was found to secrete algicidal substances to inhibit the growth of red tide organisms. In this paper, a method to extract these algicidal substances was developed, the characteristics of the substances were analysed, and the mechanism of their algae-suppressing effect was explored, providing an important reference for the development of new red tide prevention and control technologies.
The B. laterosporus strain used in the experiments was provided by Qingdao GBW Group Company Limited. The strain was cultured in LB liquid medium (peptone 10 g, yeast extract 5 g, NaCl 10 g, pH 7.2-7.4 in a volume of 1 L of water) and fermented at 30°C for 24 h with shaking at 160 rpm.
All of the red tide algal species used in the experiments (Table 1) were obtained from the Key Laboratory of Marine Ecology and Environmental Science, Institute of Oceanography, Chinese Academy of Sciences. The medium used was L1, the incubation temperature was (20 ± 1) °C, the light intensity was 65 µmol of photons/(m2·s), and the light-dark cycle was L:D=12 h:12 h. The seawater used was obtained offshore of Huiquan Bay, Qingdao, filtered through a 0.45 µm mixed fibre membrane, and autoclaved before use.
Fifty millilitres of red tide organisms in the exponential growth phase were placed in a colorimetric tube, subjected to algae suppression treatment (described in detail later), mixed upside down and incubated for a certain period of time. The algal solution was fixed with Lugo’s reagent after totally mixed, and the number of algal cells was counted by cell counting board (100μL) under a microscope (Olympus IX71, Japan). Three parallels were set up for each experiment.
The algicidal rate was calculated as
Algicidal rate (%) = (1 – experimental algae density/control algae density) ×100% (Liu et al., 2017).
The algicidal effects against Heterosigma akashiwo by different components of B. laterosporus were investigated separately to determine the algicidal mode of B. laterosporus on red tide organisms. Method refers to (Guan et al., 2014).
Fifteen millilitres of the B. laterosporus fermentation broth (bacterial density of 107 cells/mL) described in 2.1 was used as the culture group (A). Then, the same volume of the above fermentation broth was centrifuged at high speed (8000 rpm, 15 min), and the supernatant was collected and passed through a 0.22 μm filter membrane to obtain the supernatant group (B). The bacteria obtained by the above centrifugation were collected and resuspended in an equal volume of sterile seawater as a bacterial cell suspension (group C). The algicidal rate was calculated by comparing the algicidal rates of the supernatant (B) and the cell suspension (C) to determine whether the extracellular secretion of B. laterosporus and the bacterium itself have algicidal activity.
To determine whether the algicidal effect of B. laterosporus on H. akashiwo cells depends on direct contact or an algicidal substance, 2 mL of cell suspension (C) was placed in a 3500 Da dialysis bag (substances larger than 3500Da can penetrate, while other substances cannot), sealed and placed in 100 mL of H. akashiwo (bacterial density of approximately 105 cells/mL) as a dialysis group (C-dialysis), and an equal volume of bacterial suspension (C) without dialysiswas used as a control (C-control). The algal fluid was fixed at different times (0.5, 4, 8, 12, 24 and 48 h), and the algicidal rate was calculated.
Referring to Liu’s method (Liu et al., 2016), methanol, ethanol, ethyl acetate, dichloromethane, and petroleum ether were used to extract the B. laterosporus cells. Ten grams of cells was added to 100 mL of extractant. After shaking in a constant temperature shaker (20°C, 160 rpm) for 3 h and centrifuging (8000 rpm, 10 min), the supernatant was evaporated using a rotary evaporator (40°C). The algicidal activity of the crude extracts obtained with different extractants was investigated after 24h. The algicidal rate of the crude extract obtained with methanol as the extractant was the highest, reaching more than 95% that of the control group (using the same mass of bacteria after sonication). Therefore, methanol was used as the extractant for all subsequent experiments.
The above methanol-extracted algicidal substances were dissolved in seawater and subjected to the following analyses. For the following experiments, three biological replicates were prepared for each sample. Method refers to (Tang et al., 2011).
(1) Temperature stability
To investigate the temperature stability of the algicidal substances, they were treated at -80°C, 20°C, 60°C, 70°C, 80°C and 90°C for 20 min and then returned to room temperature (20°C). H. akashiwo was added in the index growth period to make algicidal substance concentrations of 10, 25 and 50 mg/L. The algicidal efficiencies of the algicidal substances at different temperatures were compared.
(2) pH tolerance
To investigate pH tolerance, the pH values of algicidal substance aqueous solutions (algicidal substance concentration of 10 g/L, initial pH=6.0, 20°C) were adjusted to 3.0, 4.0, 5.0, 6.0, 8.0, 9.0, 10.0 and 11.0 with NaOH or HCl (1 mol/L), and returned to pH=6.0 after 4 h of treatment. The algicidal substances were added to H. akashiwo in the index growth period to make crude extract concentrations of 5, 10 and 20 mg/L, and the algicidal efficiencies of the crude extracts after different pH treatments were compared after 24 h.
(3) Molecular weight range
To determine the molecular weight distribution of the algicidal substances, their solutions were dialyzed in dialysis bags of different molecular weight cut-offs (MWCOs). Previous studies have performed preliminary analyses of the molecular weights of algicidal substances using dialysis bags. For example, Tang et al. (2011) isolated an algicidal substance from the fermentation broth of the actinomycete Actinomyces L74 and found that the molecular weight of the substance was less than 3 kDa when measured by dialysis bags. Algicidal substances (0.1 g) were dissolved in 10 mL of phosphate-buffered saline (PBS) buffer, placed in dialysis bags with MWCOs of 100 Da, 1.0 kDa and 3.5 kDa, and dialyzed against PBS with magnetic stirring at 100 rpm at room temperature (20°C). Substances smaller than the MWCO will be outside the dialysis bag, while substances larger than the MWCO will remain inside the bag. The dialysis solutions were changed every 12 h, and after 48 h, the liquid in the dialysis bag was collected and added to H. akashiwo in the index growth period so that the final concentrations of algicidal substances were 10, 15 and 20 mg/L. Finally, the algicidal rates were calculated after 24 h to compare the algicidal effects of substances with different molecular weight ranges. The algicidal effect by the algicidal substances <100Da is obtained by subtracting the algicidal effect of substances >100Da from the algicidal effect of un-dialyzed algicidal substances.
The algicidal substances obtained by the method in 2.3.2 were added to H. akashiwo at final concentrations of 10, 20 and 50 mg/L, and samples were taken at different times (0.5, 4, 8, 12, 24, 48 and 72 h) to determine the algicidal efficiency of the algicidal substances. The treatment group without adding any substance was set as a control, and each group was set with three parallels.
The algicidal substances were added to an algal solution of H. akashiwo during the index growth period to a final concentration of 20 mg/L. The morphological changes in the algal cells were observed and photographed under a microscope (Olympus IX71, Japan) at 0.5, 4 and 24 h.
Algal cells were centrifuged (3000 × g, 5 min), the supernatant was discarded, and the samples were completely immersed in 5% glutaraldehyde and fixed for 1 h. Next, the samples were rinsed three times (10 min each time) with 0.1 mol/L phosphate buffer at room temperature and then dehydrated in an ethanol gradient before placing the cells in isoamyl acetate. After 3 h of drying in a critical point drier, the samples were fixed using conductive adhesive and sprayed with gold. A scanning electron microscope (S-3400N, Hitachi, Japan) was used to observe the morphological changes in the algal cells at greater depths.
In the following sections, the experimental treatment is described in 2.4.1. Reactive oxygen species (ROS) are the most sensitive physiological and biochemical response parameters to external environmental stresses in algal cells, and excess ROS can cause irreversible damage to nucleic acids, membrane lipids and proteins through peroxidation. MDA, SOD, CAT and GSH are the four most representative ROS parameters. Therefore, these four indicators were chosen to study the effects of the algicidal substances on the physiological and biochemical characteristics of the algal cells in this study.
(1) Response of the algal cell antioxidant system
MDA, SOD, CAT and GSH were measured according to the method of (Liu et al., 2017). Algal cells were washed three times with sterilized PBS and then resuspended in an ice water bath using an ultrasonic cell crusher at 5 s intervals. After centrifugation (12000 rpm, 10 min, 4°C), the supernatant was used as the crude enzyme solution, and the contents of malondialdehyde (MDA) and glutathione (GSH) and the enzymatic activities of superoxide dismutase (SOD) and catalase (CAT) were determined by kits purchased in Nanjing Jiancheng Institute of Biological Engineering (China). The operation was carried out according to the instructions.
(2) Changes in metabolic activity and apoptosis-like phenomena in algal cells
Changes in oxidative stress in algal cells can induce a series of apoptotic phenomena, such as cell shrinkage and reduced membrane potential (Bidle, 2015). In addition, esterases are hydrolytic enzymes found in many organisms, and their activity is considered to be an indicator of cellular metabolic activity and is thus commonly used to assess the toxicity of environmental factors to algae (Lin et al., 2020).
Changes in metabolic activity was measured according to the method of (Bidle, 2015). The algae were stained with fluorescein diacetate (FDA) at 4 h and 24 h, and the changes in green fluorescence (FL1) intensity with treatment time were recorded by flow cytometry to analyse the changes in H. akashiwo esterase activity as influenced by the algae-inhibiting crude extract.
As the method in (Zhang et al., 2013), the cell size and membrane potential of the residual H. akashiwo at different times (1, 4, 12, 24 and 48 h) were recorded and analysed by flow cytometry (BD FACS Calibur, USA). The former was characterized by the ratio of forwards scattered light (FSCsample/FSCcontrol), and the latter was characterized by the green fluorescence (FL1) of the dye 3,3’-dihexyloxy-phthalocyanine (DiOC6(3)).
As mentioned earlier, algicidal effects produced by bacteria can be classified as direct or indirect effects, and 70% of algicidal bacteria inhibit algae through the secretion of extracellular substances (Boruszko, 2017). To determine the mode of algal inhibition by B. laterosporus against H. akashiwo, the effects of the culture (A), supernatant (B) and cell suspension (C) on the growth of H. akashiwo were investigated to determine the algicidal mode.
The algicidal effects of different components of the culture against H. akashiwo are displayed in Figure 1A, which shows that the algicidal rate of the supernatant (B) is closer to that of the culture (A) at the same fraction volume, indicating that B. laterosporus mainly exerts its algicidal effects through the secretion of extracellular substances.
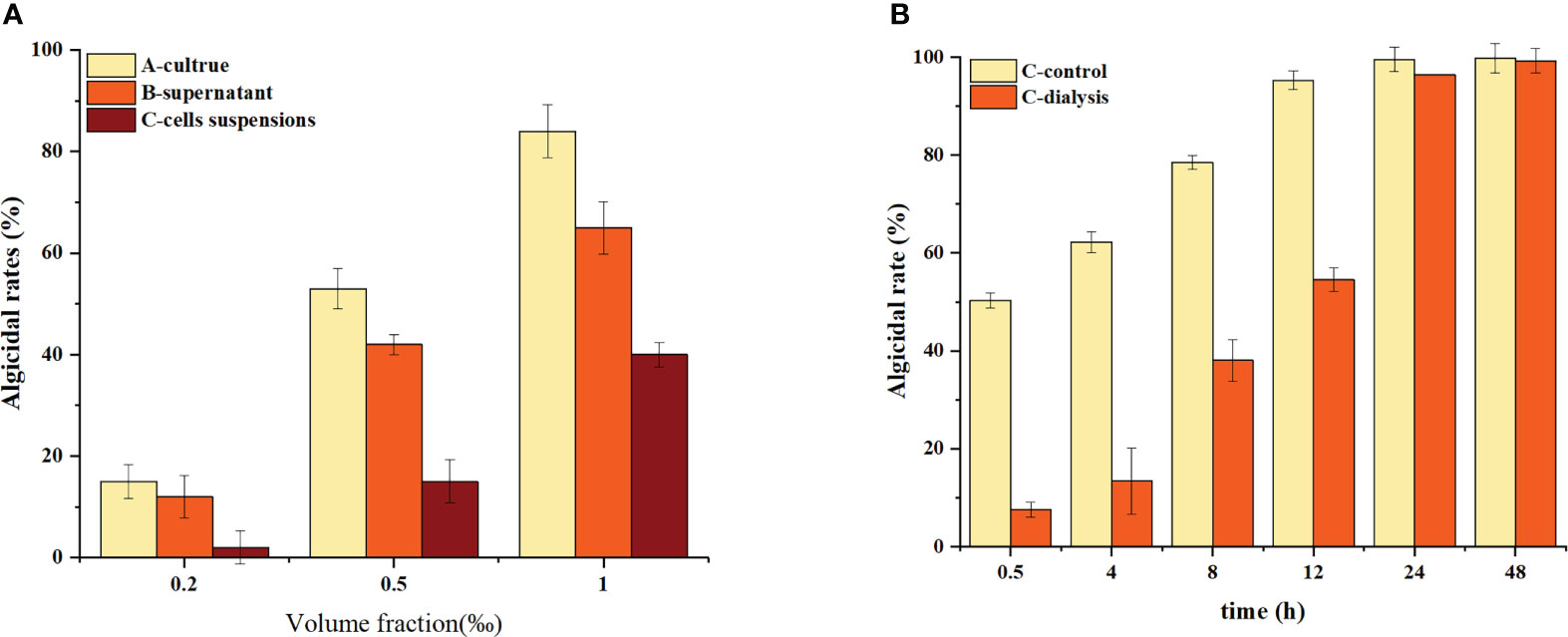
Figure 1 Algicidal characteristics of the crude Bacillus spp. extracts. Three replicates were used for error bars (representing the standard deviation). (A) Algicidal effects of the extracts produced using different polar extractants. (B) The effect of temperature on the algicidal activity of the crude extracts. (C) The effect of pH value on the algicidal activity of the crude extracts. (D) Differences in algicidal activities of the crude extracts with different molecular weight ranges.
To further reveal the mechanism of action of this bacterium, we used dialysis to isolate the bacteria and algal cells and analysed the changes in the algicidal effect. These results are shown in Figure 1B.
The algicidal rate of the C-control group was approximately 50% within 0.5 h and increased to 100% at 12 h. The C-dialysis group showed a slow increase in the initial algicidal rate, which increased to 100% at 12 h. The C-dialysis treatment group displayed a slow increase in the initial algicidal rate, but over time, the algicidal rate increased to 100% at 24 h. Compared to direct addition of the bacterium (C-control) to the algal solution, the algicidal rate of the C-dialysis group increased slowly over time because algae inhibition by the active substance could only be produced by diffusion, which was controlled by the concentration difference due to the dialysis bag. However, there was uniform, direct contact between the bacterium and algal cells in the C-control group, which displayed efficient algicidal activity in a short period of time.
The results are shown in Figure 2A. The algicidal efficiency of the methanol extract was approximately 95% of the control, whereas the ethanol extract retained approximately 25% removal efficiency compared with the control. Ethyl acetate, dichloromethane and petroleum ether could not extract the algicidal components from this bacterium. These data indicate that the algicidal substances secreted by B. laterosporus are more polar, so all subsequent experiments used methanol as the extractant to obtain the algicidal components.
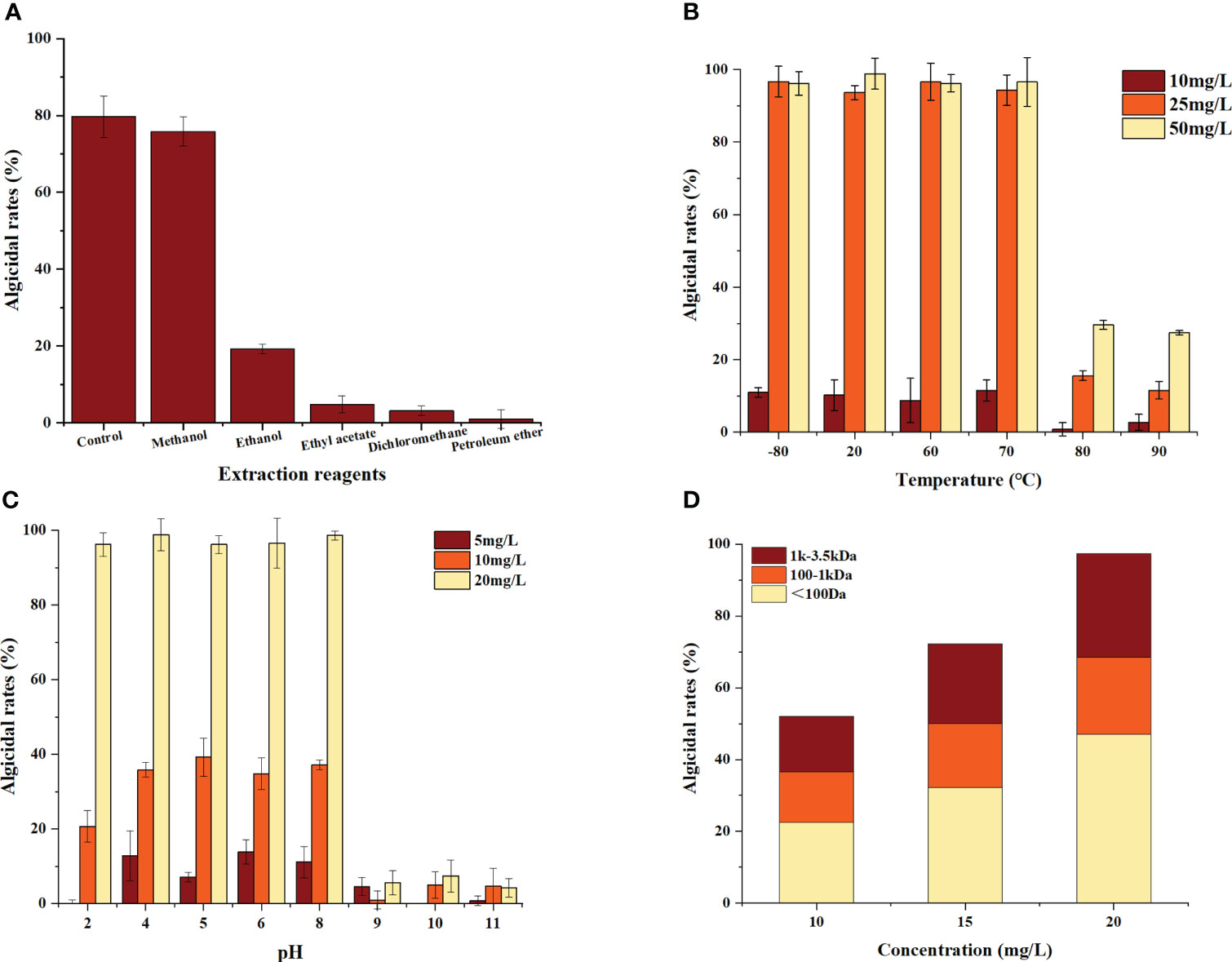
Figure 2 Algicidal characteristics of the crude Bacillus spp. extracts. Three replicates were used for error bars (representing the standard deviation). (A) Algal inhibition effects of the extracts produced using different polar extractants. (B) The effect of temperature on the algae inhibition activity of the crude extracts. (C) The effect of pH value on the algae inhibition activity of the crude extracts. (D) Differences in the algae inhibitory activities of the crude extracts with different molecular weight ranges).
To investigate the temperature and pH tolerance of the algicidal substances secreted by B. laterosporus, these substances were exposed to a variety of temperature and pH conditions. The results showed that the algicidal substances were more than 95% effective at temperatures ranging from -80.0 to 70.0°C and were not significantly different from the room temperature group (P>0.05). However, after 20 min of incubation at 80.0°C, the algicidal rate decreased to 35.1% (Figure 2B). This indicated that the algicidal substances can be stored at low temperatures, but high temperatures (≥80°C) will significantly reduce their activity (P<0.01).
As shown in Figure 2C, the algicidal rates of the substances remained stable in the pH range of 4.0-8.0, while the algicidal rate decreased significantly at pH ≥ 9 (P<0.01). This result indicated that the performance of these algae-inhibiting crude extracts was influenced by changes in pH, showing acid but not alkali tolerance.
The extracts obtained in this study were mixtures. Therefore, a more detailed analysis of the molecular weight distribution was carried out using dialysis bags with MWCOs of 100 Da, 1 kDa and 3.5 kDa (Figure 2D). The results showed that the algicidal substances were complex, with algicidal substances of <100 Da accounting for approximately 45% of the extract, those between 100 Da and 1 kDa accounted for 25% and those between 1 kDa and 3.5 kDa accounted for 30%.
Algicidal mechanisms are often accompanied by impairment of the physiological and biochemical functions of algal cells (Pal et al., 2020). In this study, the effects of different concentrations of algicidal substances on the algicidal rate, morphological structure and physiological and biochemical functions of algal cells were investigated, and the differences in the impacts on algal cells with different surface structures were also comparatively analyzed.
After the addition of 50 mg/L algicidal substances, all of the cells died within 0.5 h. After the addition of 20 mg/L algicidal substances, 52% of the cells died within 0.5 h, and the algicidal rate peaked at 75% at 12 h. The 10 mg/L group also peaked at 36% after 12 h and remained stable. These results are shown in Figure 3.
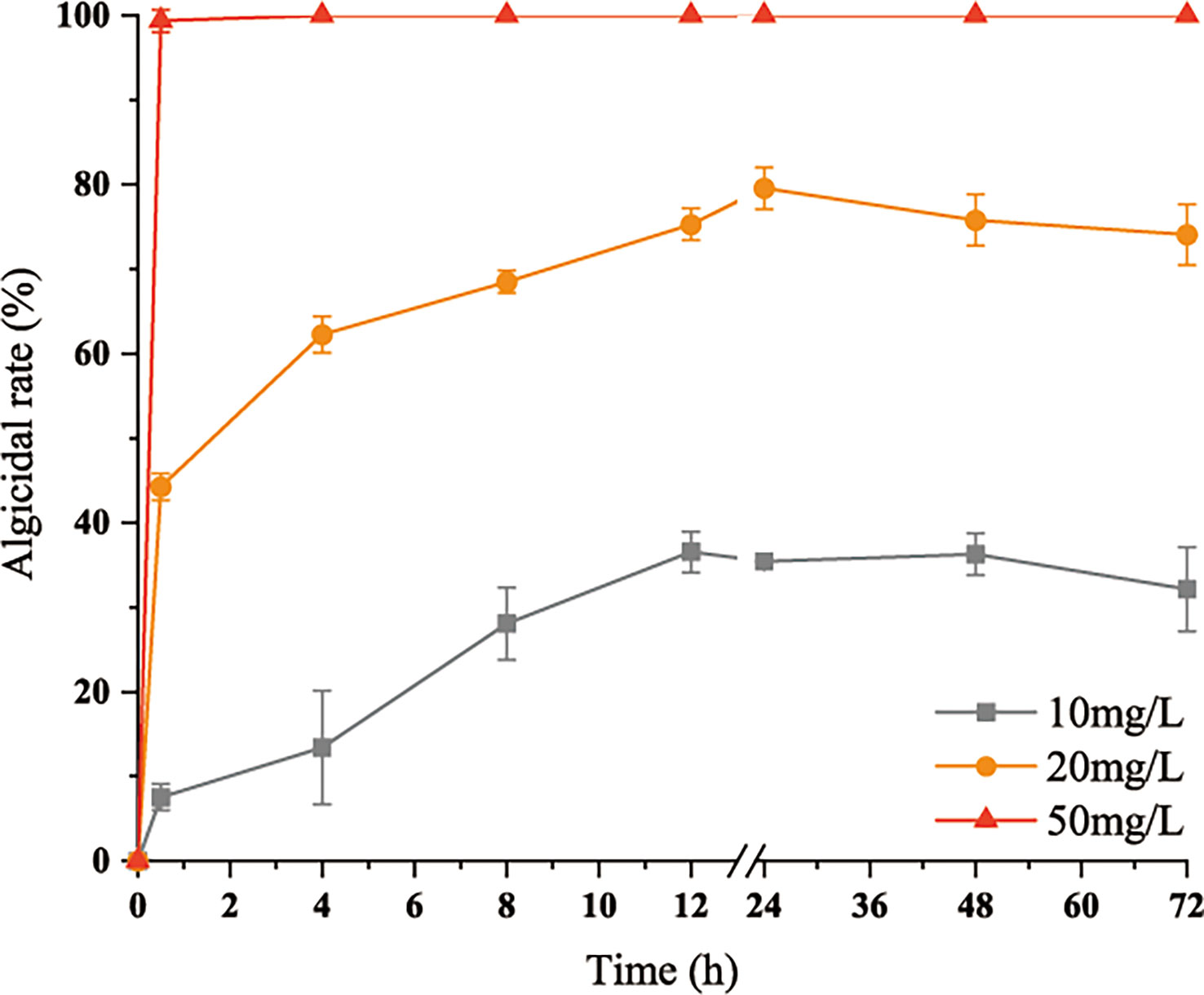
Figure 3 Variations in inhibitory rates of the algicidal substances with time. Three replicates were used for error bars (representing the standard deviation).
The morphological changes in the treated algal cells were observed by light microscopy with scanning electron microscopy (SEM) tracking, as shown in Figures 4A, 5A. The H. akashiwo cells in the control group were plump, with intact structures and smooth surfaces. After 0.5 h of treatment, some cells showed a loss of membrane integrity and rupture (Figure 4B), and the intact cells began to shrink (Figure 5B). After 4 h of exposure to the crude extract, more algal cells ruptured and died, the cell surfaces were rough and dull, cell integrity was severely compromised, and intracellular components or cell debris could be observed in the background (Figures 4C, 5C). After 24 h of treatment (Figures 4D, 5D), almost all of the algal cells had died.
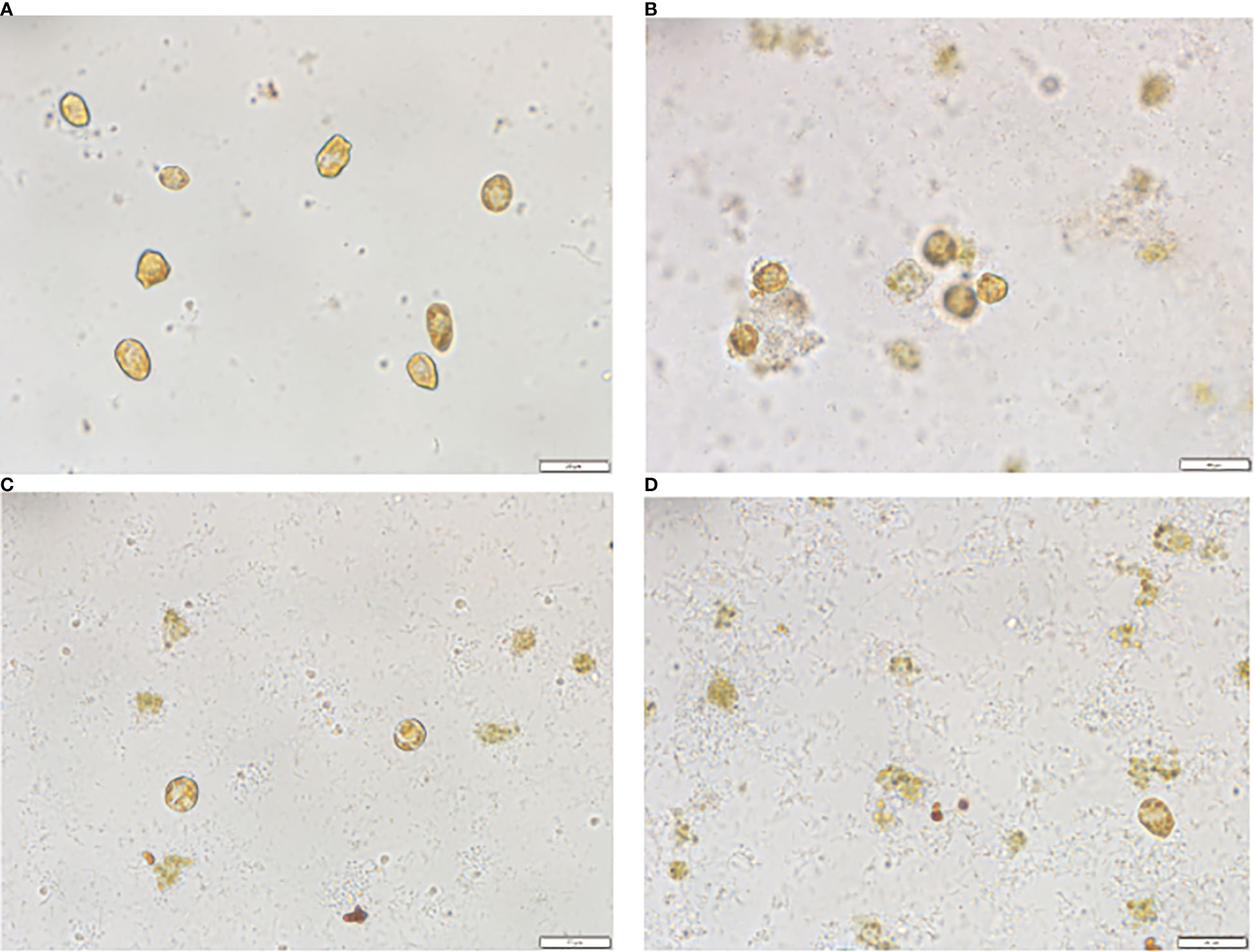
Figure 4 Morphological changes in Heterosigma akashiwo treated with algicidal substances observed by light microscopy after 0h(A), 0.5h(B), 4h(C) and 24h(D).

Figure 5 Morphological changes Heterosigma akashiwo treated with algicidal substances observed by scanning electron microscopy after 0h(A), 0.5h(B), 4h(C) and 24h(D).
(1) Effect of the algicidal substances on oxidation systems
The MDA content reflects the degree of peroxidation and, indirectly, the degree of damage to the membrane system caused by the crude extracts. The data in Figure 6A show that the initial MDA content in H. akashiwo was approximately 1.92 nmol/mg prot. The MDA content in the 20 mg/L treatment group increased to 9.58 nmol/mg prot at 0.5 h and peaked at 28.91 nmol/mg prot at 12 h. Then, the MDA content decreased rapidly, but after 72 h, it was still higher than that in the control group. The MDA content in the remaining algal cells in the 10 mg/L treatment group also showed an increasing and then decreasing trend, but reached the same level as that in the control group after 48 h.
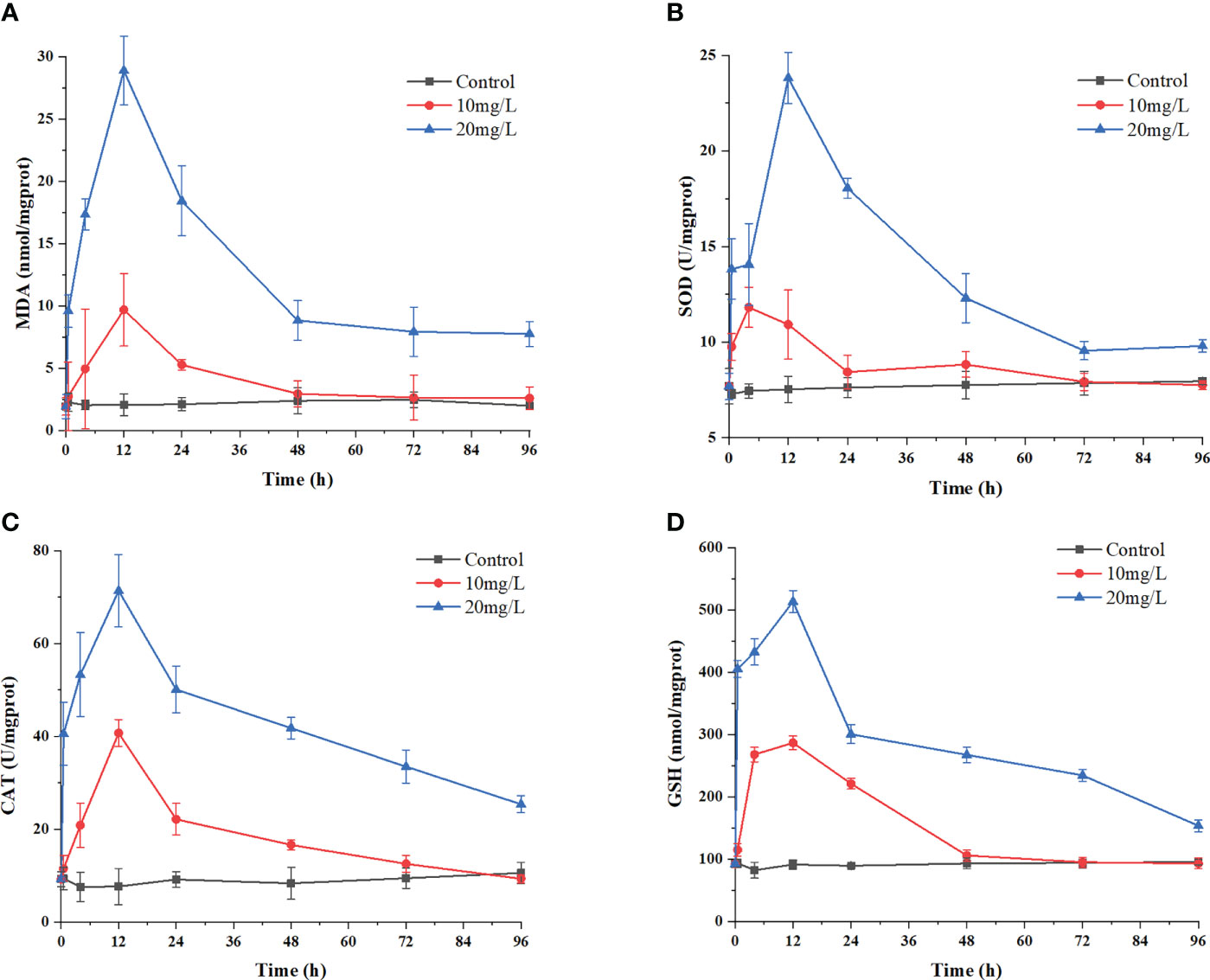
Figure 6 Effects of the algicidal substances on oxidation systems. Treatment with the algicidal substances determined by (A) MDA content, (B) SOD activity, (C) CAT activity and (D) GSH content. Three replicates were used for error bars (representing the standard deviation).
The changes in the activities of the antioxidant enzymes SOD and CAT reflect the antioxidant defence response of H. akashiwo to the algicidal substances. These results are shown in Figures 6B, C. The enzymatic activities of SOD and CAT increased significantly in each group after 12 h of treatment with the algae-inhibiting substances, reaching a maximum after 12 h. Compared with the control group, the activities of these two enzymes were 2.85 and 5.5 times higher in the 20 mg/L treatment group, respectively. Notably, the SOD and CAT activities in the treated groups decreased slightly after 48 h but were still higher than those in the control group after 72 h.
As a nonenzymatic antioxidant, GSH can reduce and degrade toxic substances due to its redox properties. Figure 6D shows that during treatment with the crude algae-suppressing extract, the GSH level increased from 0 h to 12 h, reaching its peak at 12 h, with a value 5.2 times higher in the 20 mg/L treatment group than in the control group. This indicates that GSH was produced in large quantities by the H. akashiwo in response to administration of the crude extract to neutralize excess ROS.
(2) Changes in metabolic activity and apoptosis phenomena in algal cells under stress
The changes in the metabolic activity of algal cells after the addition of the crude algicidal extract at a concentration of 10 mg/L are shown in Figure 7. The peak area is the total number of cells (Y-axis), and the FL1 fluorescence intensity (X-axis) indicates the strength of esterase inhibition. The results showed that the number of viable cells decreased over time and H. akashiwo esterase activity was inhibited as the proportion of metabolically inactivated cells increased.
As shown in Figure 8A, there was no significant change in the size of the cells in the low concentration of crude extract group (10 mg/L), while the size of the cells in the 20 mg/L treatment group decreased significantly in a time-dependent manner.
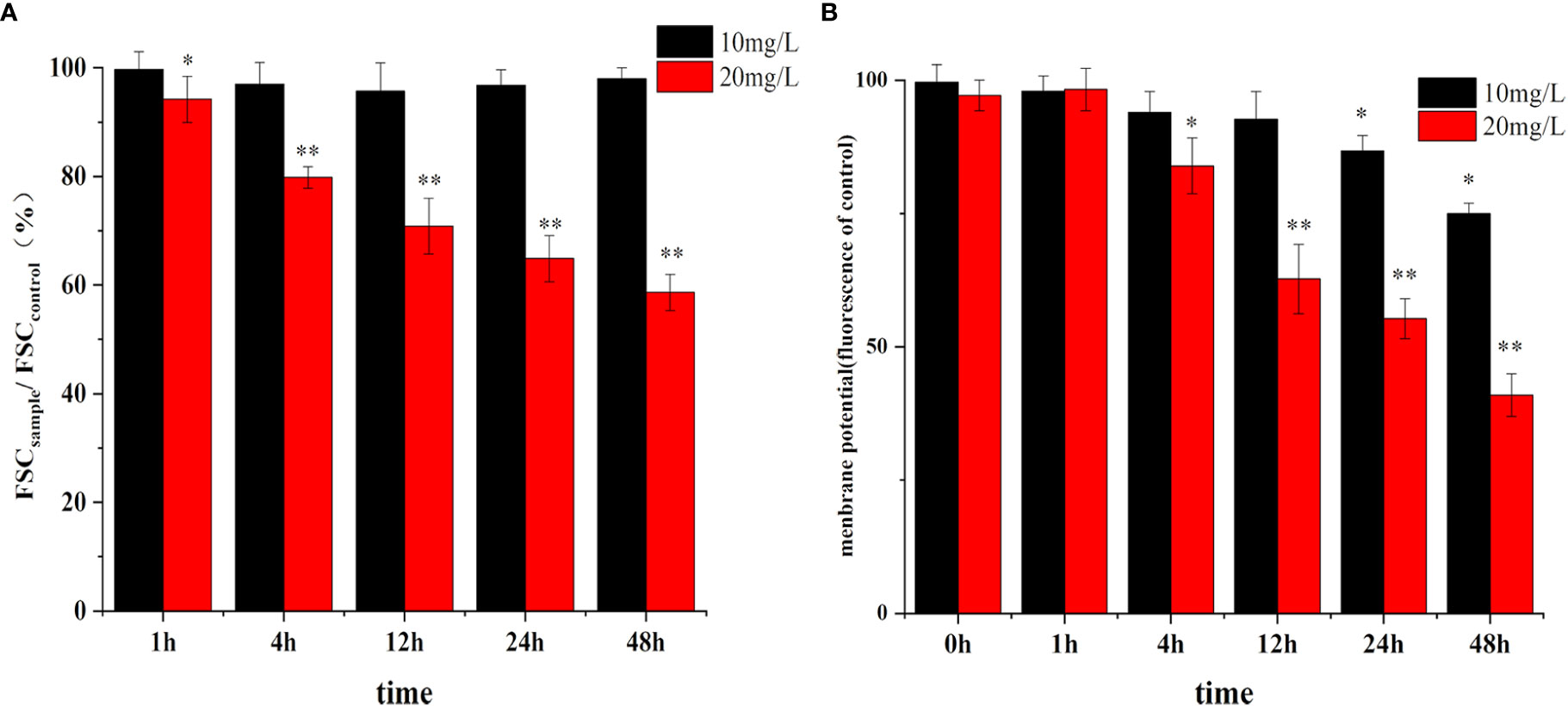
Figure 8 Effect of algicidal substance treatment on cell size (A) and membrane potential (B). (* represents p<0.1, ** represents p<0.05).
Changes in the cell membrane potential are shown in Figure 8B. The cell membrane potential did not change rapidly with the addition of different concentrations of the crude extracts. There was no significant difference between the membrane potential in the treatment group and the control group after 1 h, but the potential decreased significantly after 4 h of treatment and showed more significant changes at 24 h and 48 h. Moreover, the inhibition of membrane potential was higher with increasing extract concentration, decreasing by 63% compared to the control. These changes in membrane potential were consistent with the trend of in cell morphology changes, indicating that the clustering of cell membranes due to apoptosis affected the normal difference in membrane potential to some extent.
The current study demonstrate that this B. laterosporus strain exerts its algicidal effect against red tide organisms in seawater mainly through the secretion of algicidal substances. The results also confirm that B. laterosporus can survive stably in red tide algal fluids, continuously secrete algicidal substances and exert algae-inhibiting effects, overcoming the shortcomings of some bacteria that cannot survive in algal fluids and showing good application prospects.
As research on bacterial-algal interactions continues to progress, an increasing number of algae-suppressing bacteria are being isolated and identified. However, the algicidal substances secreted by these bacteria are usually a multicomponent mixtures with different properties, making them difficult to purify and identify. Structural analysis of algicidal substances has been a hot but difficult area in recent years (Talaat, 2019). A few algicidal substances have been isolated, mainly including macromolecules such as peptides, chitinase (Wang et al., 2016) and luciferin (Zhang et al., 2016b), triterpene saponins (Luo et al., 2013), rhamnolipids (Xu et al., 2020), amino acids (Quan et al., 2021) and tryptamine (Zhang et al., 2016a).
As described in 3.1, the C-dialysis group had an algicidal effect (Figure 1B), indicating that the algicidal function of the bacterium did not depend on the direct contact between the bacterium and the algal cells but instead it depended on the algicidal substances secreted by the bacterium. This result is the same as the algicidal effect against Cochlodinium polykrikoides produced by Bacillus SY-1 reported by Jeong and Son (2021). However, unlike previous reports on algicidal Bacillus spp. (Shunyu et al., 2006; Guan et al., 2014), the cell suspension of B. laterosporus (C) also showed algicidal activity, indicating that the bacterium could survive stably and exert algicidal effects after entering the algal solution, further demonstrating the superiority of B. laterosporus.
As described in 3.2, preliminary analysis and evaluation of the nature of the algicidal substances of B. laterosporus in this paper concluded that these algicidal substances are also a multicomponent mixture, all of which the components have molecular weights below 3500 Da and should not be enzymes, polysaccharides or other large molecules. Extraction with different solvents indicated that these substances were strongly polar, were intolerant to high temperature and basic solutions but not acidic solutions (Figure 2), and positive for ninhydrin. Taking these data together, we believe that these substances are mainly peptides.
Lipopeptide Bacillus metabolites have received much attention in previous studies as common natural antibiotics (Chen et al., 2022). However, the effects of lipopeptides on algae have been less well reported. Jeong inhibited C. polykrikoides with Bacillus subtilis SY-1, which was found to secrete an anti-Mycobacterium lipopeptide with a molecular weight of approximately 1 kDa (Jeong and Son, 2021). The results showed that the algae Prorocentrum dentatum is similar to those described in this paper. This result supports our hypothesis to some extent.
Therefore, the algicidal substances found in this paper are a multicomponent mixture, of which the larger molecular weight component (1000-3500 Da) is probably a lipopeptide, which is also the main type of algicidal substance of this strain. In addition, the experimental results showed that the components of the mixture with molecular weights of less than 1 kDa also have some algae-inhibiting effects. However, whether they include the above-mentioned tyrosine and uronic acid or luciferin, rhamnogalactan or other small molecules needs further research.
Research on algicidal mechanisms is currently focused at the cellular, physiological and molecular levels. At the cellular level, algaecides kill algal cells by destroying the cell structure; at the physiological level, algicidal substances have been found to cause oxidative stress in the cells, leading to lipid peroxidation in organelles with membrane structures and triggering apoptosis; and at the molecular level, algaecides kill algal cells by inhibiting photosynthesis-related genes (Xu et al., 2019).
In this study, the mechanism of the extract was further confirmed. The algicidal substances caused some of the algal cells to rupture and die. In the remaining cells that had not yet ruptured, increases were found in their MDA and GSH contents and SOD and CAT activities, indicating that the antioxidant defence system of the algal cells was activated. Additionally, the normal physiological and biochemical functions of the cells were disrupted and oxidative damage was observed. In a previous related study, Shewanella sp. IRI-160 cells treated with Pseudomonas aeruginosa also revealed oxidative stress and produced apoptotic phenomena such as cycle arrest and reduced membrane potential (Pokrzywinski et al., 2017), similar to the results in this paper. This suggests that the algal inhibitory substances secreted by S. lateralis can also cause apoptotic phenomena in algal cells due to oxidative stress, such as a reduction in membrane potential and cell shrinkage, resulting in an algicidal effect.
The cell membrane is the main site of action for many algicidal substances. Such compounds can alter the permeability of the cell membrane, leading to an imbalance in ion concentrations within the cell, and disrupt cell membrane integrity (Demuez et al., 2015). Cao found that lipopeptides could form conductive ions in fungal cell membranes, thus affecting its permeability and subsequently lysing the cells (Cao et al., 2020). The results of this paper show that the algicidal substances can cause some of the red tide heterocyclic algae cell membranes to rupture and the contents to flow out. Additionally, the unruptured algae cells showed surface wrinkling and reduced membrane potential, also indicating that the site of action of these substances is the cell membrane. To further test this hypothesis, we conducted comparative experiments with red tide species with and without cell walls. The results confirmed that the algae-inhibiting extract had a strong effect on red tide organisms without cell walls, with over 95% inhibition at 25 mg/L, while there was almost no inhibition of the microalgae with cell walls (Table 2). It was therefore assumed that the cell wall may have prevented direct contact between the algae-inhibiting substances and the cell membrane, giving microalgae with different cell structures different tolerances to these algicidal substances.
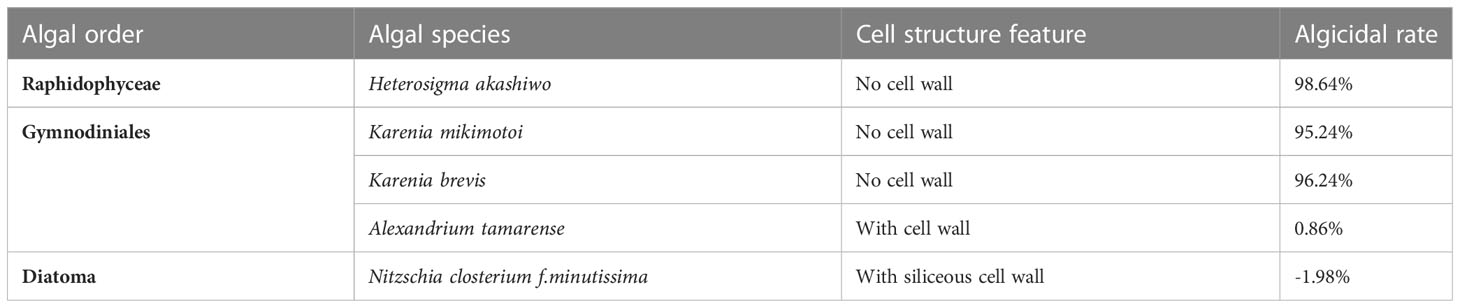
Table 2 Inhibition of different typical red tide organisms by the algicidal substances of B.laterosporus.
We conclude that B. laterosporus interacts with the cell membranes of red tide organisms by secreting algicidal substances. For some poorly resistant algal cells, treatment can directly disrupt the physical defences, causing cell content leakage and death. For more resistant cells, treatment leads to oxidative stress, reduced metabolic activity and apoptosis.
However, the research on algicidal bacteria still can’t explain the action mechanism of algicdal substances on algal cells deeply. The identification and analysis of algicidal substances also need further research. Therefore, the subsequent research should isolate and purify the algicidal substances and enhance the practicability of algicidal bacteria in red tide control.
In this paper, it was discovered for the first time that B. laterosporus has an algicidal effect on typical red tide organisms, which is achieved by the secretion of algicidal substances. These algicidal substances are polar, hydrophilic, heat insensitive and acid resistant but not alkali resistant. These algicidal substances disrupt the cell membrane of red tide organisms, causing the leakage of cell contents and oxidative damage, which reduces the metabolic activity of the cells and leads to apoptosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
ZY conceived and supervised the work. SL designed the experiments and data analysis strategies. SL, RC and ZW carried out the experiments and data analysis. SL wrote the manuscript. ZY, XS and XC provided technological and logistic support and reviewed the manuscript.
This work was financially supported by the Marine S & T Fund of Shandong Province for the Pilot National Laboratory for Marine Science and Technology (Qingdao) (grant #2021QNLM040001), Taishan Scholars Climbing Program of Shandong Province of the year 2019 and the Key Research Program of the Center for Ocean Mega-Science, Chinese Academy of Sciences (Grant # COMS2020J02). Sincere thanks to Ding Yu and Zhong Yuxia for their preliminary work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1160585/full#supplementary-material
Bidle K. D. (2015). The molecular ecophysiology of programmed cell death in marine phytoplankton. Ann. Rev. Mar. Sci. 7, 341–375. doi: 10.1146/annurev-marine-010213-135014
Boruszko D. (2017). Research on the influence of anaerobic stabilization of various dairy sewage sludge on biodegradation of polycyclic aromatic hydrocarbons PAHs with the use of effective microorganisms. Environ. Res. 155, 344–352. doi: 10.1016/j.envres.2017.02.019
Cao J., Yu Z., Liu W., Zhao J., Zhang H., Zhai Q., et al. (2019). Probiotic characteristics of bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 64, 103643. doi: 10.1016/j.jff.2019.103643
Chen X., Lu Y., Shan M., Zhao H., Lu Z., Lu Y. (2022). A mini-review: Mechanism of antimicrobial action and application of surfactin. World J. Microbiol. Biotechnol. 38 (8), 143. doi: 10.1007/s11274-022-03323-3
Demuez M., Gonzalez-Fernandez C., Ballesteros M. (2015). Algicidal microorganisms and secreted algicides: New tools to induce microalgal cell disruption. Biotechnol. Adv. 33 (8), 1615–1625. doi: 10.1016/j.biotechadv.2015.08.003
Guan C., Guo X., Cai G., Zhang H., Li Y., Zheng W., et al. (2014). Novel algicidal evidence of a bacterium Bacillus sp. LP-10 killing Phaeocystis globosa, a harmful algal bloom causing species. Biol. Control 76, 79–86. doi: 10.1016/j.biocontrol.2014.05.007
Inaba N., Trainer V. L., Onishi Y., Ishii K. I., Wyllie-Echeverria S., Imai I. (2017). Algicidal and growth-inhibiting bacteria associated with seagrass and macroalgae beds in puget sound, WA, USA. Harmful Algae 62, 136–147. doi: 10.1016/j.hal.2016.04.004
Jeong S. Y., Son H. J. (2021). Effects of mycosubtilin homolog algicides from a marine bacterium, Bacillus sp. SY-1, against the harmful algal bloom species Cochlodinium polykrikoides. J. Microbiol. 59 (4), 389–400. doi: 10.1007/s12275-021-1086-8
Lin Z., Chen B., Zhao L. (2020). Fluorescence-based bioassays with dose-response curve and relative potency in measuring algicidal virulence of Bacillus sp. B1 exudates against heterosigma akashiwo. Sci. Total Environ. 724, 137691. doi: 10.1016/j.scitotenv.2020.137691
Liu J. H., Li L., Shang X. D., Zhang J. L., Tan Q. (2016). Anti-helicobacter pylori activity of bioactive components isolated from Hericium erinaceus. J. Ethnopharmacol. 183, 54–58. doi: 10.1016/j.jep.2015.09.004
Liu S., Yu Z., Song X., Cao X. (2017). Effects of modified clay on the physiological and photosynthetic activities of Amphidinium carterae hulburt. Harmful Algae 70 (dec), 64–72. doi: 10.1016/j.hal.2017.10.007
Luo J., Wang Y., Tang S., Liang J., Lin W., Luo L. (2013). Isolation and identification of algicidal compound from streptomyces and algicidal mechanism to Microcystis aeruginosa. PloS One 8 (10), e76444. doi: 10.1371/journal.pone.0076444
NOAA. (2016). Available at: http://www.noaa.gov/what-is-harmful-algal-bloom.
Pal M., Yesankar P. J., Dwivedi A., Qureshi A. (2020). Biotic control of harmful algal blooms (HABs): A brief review. J. Environ. Manage. 268, 110687. doi: 10.1016/j.jenvman.2020.110687
Pokrzywinski K. L., Tilney C. L., Warner M. E., Coyne K. J. (2017). Cell cycle arrest and biochemical changes accompanying cell death in harmful dinoflagellates following exposure to bacterial algicide IRI-160AA. Sci. Rep. 7, 45102. doi: 10.1038/srep45102
Quan H., Zhang Y., Yin P., Zhao L. (2021). Effects of two algicidal substances, ortho-tyrosine and urocanic acid, on the growth and physiology of Heterosoigma akashiwo. Environ. Pollut. 284, 117004. doi: 10.1016/j.envpol.2021.117004
Shunyu S., Yongding L., Yinwu S., Genbao L., Dunhai L. (2006). Lysis of aphanizomenon flos-aquae (Cyanobacterium) by a bacterium Bacillus cereus. Biol. Control 39 (3), 345–351. doi: 10.1016/j.biocontrol.2006.06.011
Sun R., Sun P., Zhang J., Esquivel-Elizondo S., Wu Y. (2018). Microorganisms-based methods for harmful algal blooms control: A review. Biores. Technol. 248 (Pt B), 12–20. doi: 10.1016/j.biortech.2017.07.175
Talaat N. B. (2019). Effective microorganisms: An innovative tool for inducing common bean (Phaseolus vulgaris l.) salt-tolerance by regulating photosynthetic rate and endogenous phytohormones production. Sci. Hortic. 250, 254–265. doi: 10.1016/j.scienta.2019.02.052
Tang S. S., Lin W. T., Li J. Y., Cai X. L., Li H. Q. (2011). Isolation and algicidal characteristics of the algicidal components from actinomycete strain L 74. Microbiology China. 38(5), 654–659. doi: 10.13344/j.microbiol.china.2011.05.004
Wang H., Butt L., Rooks P., Khan F., Allen M. J., Ali S. T. (2016). Characterisation of algicidal bacterial exometabolites against the lipid-accumulating diatom skeletonema sp. Algal Res. 13, 1–6. doi: 10.1016/j.algal.2015.11.012
Wang M., Chen S., Zhou W., Yuan W., Wang D. (2020). Algal cell lysis by bacteria: A review and comparison to conventional methods. Algal Res. 46. doi: 10.1016/j.algal.2020.101794
Wang X., Gong L., Liang S., Han X., Zhu C., Li Y. (2005). Algicidal activity of rhamnolipid biosurfactants produced by Pseudomonas eruginosa. Harmful Algae 4 (2), 433–443. doi: 10.1016/j.hal.2004.06.001
Xu W., Tan L., Guo X., Wang J. (2020). Isolation of anti-algal substances from Cylindrotheca closterium and their inhibition activity on bloom-forming Prorocentrum donghaiense. Ecotoxicol. Environ. Saf. 190, 110180. doi: 10.1016/j.ecoenv.2020.110180
Xu W., Xu Y., Huang X., Hu X., Xu Y., Su H., et al. (2019). Addition of algicidal bacterium CZBC1 and molasses to inhibit cyanobacteria and improve microbial communities, water quality and shrimp performance in culture systems. Aquaculture 502, 303–311. doi: 10.1016/j.aquaculture.2018.12.063
Yu Z., Song X., Cao X., Liu Y. (2017). Mitigation of harmful algal blooms using modified clays: Theory, mechanisms, and applications. Harmful Algae 69, 48–64. doi: 10.1016/j.hal.2017.09.004
Zhang B. H., Ding Z. G., Li H. Q., Mou X. Z., Zhang Y. Q., Yang J. Y., et al. (2016a). Algicidal activity of Streptomyces eurocidicus JXJ-0089 metabolites and their effects on microcystis physiology. Appl. Environ. Microbiol. 82 (17), 5132–5143. doi: 10.1128/AEM.01198-16
Zhang X., Fan L., Roddick F. A. (2013). Influence of the characteristics of soluble algal organic matter released from microcystis aeruginosa on the fouling of a ceramic microfiltration membrane. J. Membrane Sci. 425-426 (Complete), 23–29. doi: 10.1016/j.memsci.2012.09.033
Zhang H., Peng Y., Zhang S., Cai G., Li Y., Yang X., et al. (2016b). Algicidal effects of prodigiosin on the harmful algae Phaeocystis globosa. Front. Microbiol. 7, 602. doi: 10.3389/fmicb.2016.00602
Keywords: Brevibacillus laterosporus, red tide organisms, algicidal mechanisms, algicidal substances, algicidal bacteria
Citation: Liu S, Yu Z, Wu Z, Cao X, Cheng R and Song X (2023) Algicidal substances of Brevibacillus laterosporus and their effect on red tide organisms. Front. Mar. Sci. 10:1160585. doi: 10.3389/fmars.2023.1160585
Received: 07 February 2023; Accepted: 08 March 2023;
Published: 31 March 2023.
Edited by:
Yunyun Zhuang, Ocean University of China, ChinaReviewed by:
Yida Gao, Florida Fish and Wildlife Research Institute, United StatesCopyright © 2023 Liu, Yu, Wu, Cao, Cheng and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiming Yu, enl1QHFkaW8uYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.