- 1School of Life Sciences, Arizona State University, Tempe, AZ, United States
- 2Beneath The Waves, Herndon, VA, United States
- 3College of Liberal Arts and Sciences, Arizona State University, Tempe, AZ, United States
- 4School of Mathematical and Natural Sciences, Arizona State University-West, Glendale, AZ, United States
Effective management and conservation of threatened species biodiversity requires knowledge of reproductive biology, such as cyclicity, mode, and age at maturity. We combined reproductive endocrinology and in-situ ultrasonography to examine reproductive characteristics of female Caribbean reef sharks Carcharhinus perezi, a widely distributed, threatened marine predator which remains largely understudied throughout its range. Unique to this study was the opportunity to conduct longitudinal assessments of two individuals, recaptured across multiple seasons during sampling in The Bahamas. Within-individual, paired hormone analyses and in-situ ultrasounds of female sharks that were confirmed as either pregnant, non-pregnant, or reproductively active, suggest a biennial reproductive cycle for Carcharhinus perezi. This unique opportunity to assess the reproductive biology of the same individuals over time underscore the importance of repeated sampling for elucidating population reproductive cyclicity of highly mobile sharks in the wild.
1 Introduction
Elasmobranch fishes exhibit conservative life-history traits including slow growth, late maturity, and low reproductive output, rendering them highly vulnerable to overfishing (Stevens et al., 2000; Dulvy et al., 2021). As a result, approximately one-third of all living elasmobranch species are listed as “Vulnerable” to “Critically Endangered” and 14% considered as “Data Deficient” by the International Union for the Conservation of Nature (IUCN) 2022 Red List (IUCN, 2022). Understanding components of a species’ reproductive biology (i.e., age-at-maturity, gestation period, reproductive mode, reproductive cyclicity) and life-history strategies related to reproduction are essential for effective management of wild populations (Hammerschlag and Sulikowski, 2011; Natanson et al., 2019). For example, such information can assist stock assessments thereby informing management decisions related to protected area designation (Awruch, 2013). This is particularly true for elasmobranch fishes who employ a suite of reproductive strategies, thereby challenging management without species-specific, population-specific, or even region-specific data (Musick et al., 2005; Hamlett et al., 2011; Natanson et al., 2019).
The Caribbean reef shark (Carcharhinus perezi, Poey, 1876) is a medium-bodied requiem shark found throughout the sub-tropical latitude band of the western Atlantic Ocean, with a range extended from the southern North America to South America (Castro et al., 1999; Compagno, 2002; Tavares, 2009; Carlson et al., 2021). This species exhibits a conservative life history including an estimated slow growth of 23.5 cm yr-1 in Venezuela (Tavares, 2009) and 8.8 cm yr-1 in Belize (Bond et al., 2017), late maturation around 14.8 year for males and 16.4 years for females (Tavares, 2009; Talwar et al., 2022), and a small litter size of approximately 4 pups (Talwar et al., 2022). Coupled with fisheries exploitation throughout parts of their distribution (i.e., Belize, Brazil; Carlson et al., 2021) these traits have exposed Caribbean reef sharks to population declines in parts of their range (~50-80%), thus elevating the risk assessment of this species as globally “Endangered” by the IUCN Red List (Carlson et al., 2021; Gallagher et al., 2021). Information linking the reproductive biology, spatial movement, and habitat use in Caribbean reef sharks remains poor, thereby precluding species-specific management efforts (Carlson et al., 2021).
Caribbean reef sharks exhibit a placental viviparous reproductive strategy (i.e., placental connection formed between mother and offspring; live-bearing) with an assumed biennial reproductive cycle (Carrier et al., 2004). This species is thought to exhibit low reproductive output (3-6 pups) and a relatively long gestational period (~1 year; Rangel et al., 2022). Yet, the general understanding of Caribbean reef shark reproductive biology remains extremely poor and, to date, no published studies have specifically addressed the reproductive physiology or cyclicity of the species (see Brooks et al., 2013; Talwar et al., 2022). Here, the first ever empirical reproductive hormone concentrations and in-situ ultrasonography for female Caribbean reef sharks was investigated by presenting data collected from The Bahamas. This analysis included multiple reproductive assessments from two individuals recaptured across various seasons, a unique opportunity given the low recapture rate of this species (~6.97%, Talwar et al., 2022).
2 Materials and methods
All protocols for capture and sampling were approved by the Arizona State University Institutional Animal Care & Use Committee (IACUC; #20-1745) as well as the Government of the Bahamas annual fishing permits granted to Beneath the Waves Non-profit Research Organization (BTW; BS-2021-991344 and BS-2022-348632).
Female Caribbean reef sharks were opportunistically captured between August 2021 and July 2022 using scientific drumlines (see Gallagher et al., 2014) in coastal waters of Great Exuma, The Bahamas (research permit BS-2021-765539). Individuals were measured (total length, cm), tagged with conventional dart tags, and a 10 mL blood sampled was taken via caudal venipuncture and stored in sodium heparin-lined vacutainers. Reproductive state of females was identified in-situ using an Ibex EVO II portable ultrasound (E.I. Medical Imaging) with a 60 mm curved linear array 5-2.5 MHz transducer (model 290470) capable of a 30 cm scan depth. Briefly, in-situ ultrasound scanning was performed on the ventral surface from the pectoral to the pelvic fin in both a transverse and longitudinal orientation to obtain cross sectional and lengthwise images of the reproductive tract, ovaries and follicles (Sulikowski et al., 2016). On return to the lab, blood was separated into primary constituents (red blood cells, plasma, and platelets) and ~2mL plasma was stored at -20°C. Samples were shipped to Arizona State University (Glendale, AZ) for processing of reproductive hormones.
Following Sulikowski et al. (2004) 17β-estradiol (E2) and progesterone (P4) concentrations were quantified using standard radioimmunoassay techniques. The average hormone extraction recovery was 72.5% for E2 and 55.7% for P4. The mean inter-assay coefficients of variation for E2 and P4 were 3.44% and 24.4%, and the mean intra-assay coefficients of variation were 9.61% and 7.95%, respectively. Samples that fell below the detectable limits of the assay were concentrated and re-assayed. Final concentrations were corrected for procedural loss during the extraction using individual sample recoveries.
3 Results
Two mature females (IDs 00546, 00543) were captured on August 13th, 2021, in Great Exuma, The Bahamas (Table 1). In-situ ultrasonography indicated that neither female was pregnant based on the appearance of an empty uterus (Figure 1B; Sulikowski et al., 2016). Hormone concentrations were measured (Sulikowski et al., 2004) for one female (ID 00543) which revealed E2 and P4 concentrations of ~36.5 pg ml-1 and ~306.1 pg ml-1, respectively (Table 1). Both females (IDs 00546, 00543) were subsequently recaptured on December 14th, 2021 (Table 1). In-situ ultrasonography confirmed for a second time that neither female was pregnant at time of recapture. E2 and P4 concentrations for the same female (ID 00543) were ~64.6 pg ml-1 and ~72.8 pg ml-1 P4, respectively; Table 1). On July 19th and 21st, 2022, two additional females (IDs 00521, 00767; Table 1) were sampled in Great Exuma, The Bahamas with obvious, healed but recent mating scars (~1 month old) along the axis of the body (Figure 1A). These scars, coupled with candle-like structures in the uterus detected via in-situ ultrasonography (ID 00521; Figure 1C) allowed us to confirm pregnancy at capture for both females (IDs 00767, 00521). However, due to technical difficulties with equipment in the field, the ultrasound footage of one female (ID 00767) was not preserved. Hence, in Table 1, we refer to this female as “presumably pregnant” instead of “pregnant”. Further, E2 and P4 concentrations for one female (ID 00521) were ~33.4 pg ml-1 and ~146.6 pg ml-1, respectively, and for the second female (ID 00767) were ~241.6 pg ml-1 and ~1349.0 pg ml-1, respectively (Table 1).
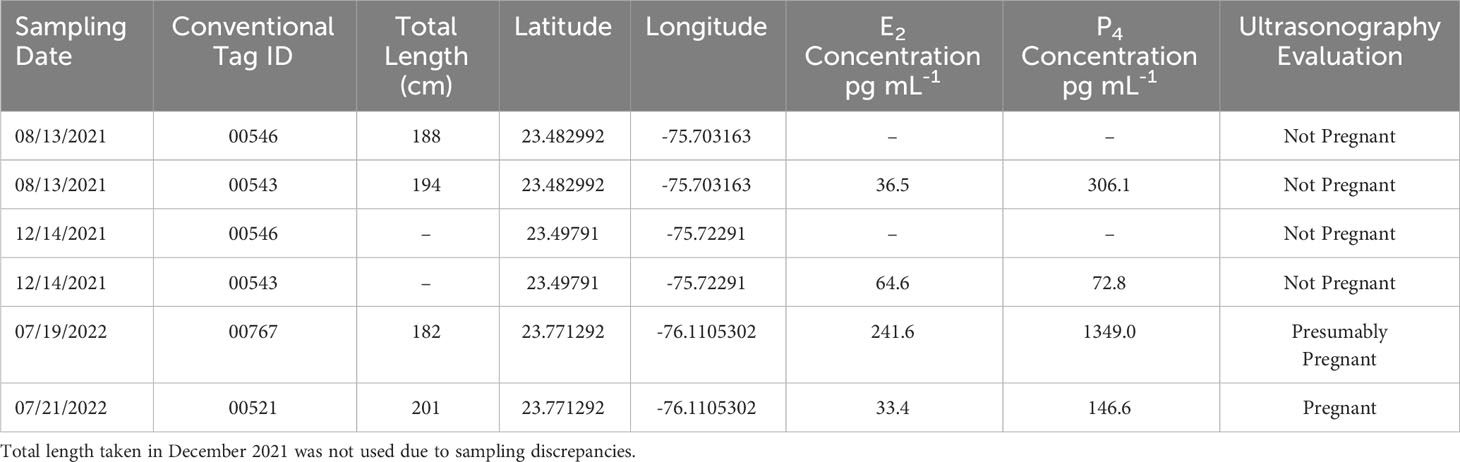
Table 1 Summary data of female C. perezi sampled in Great Exuma, The Bahamas from August 2021 through July 2022 including blood plasma estradiol (E2) and progesterone (P4) concentrations as well as field ultrasonography evaluations.
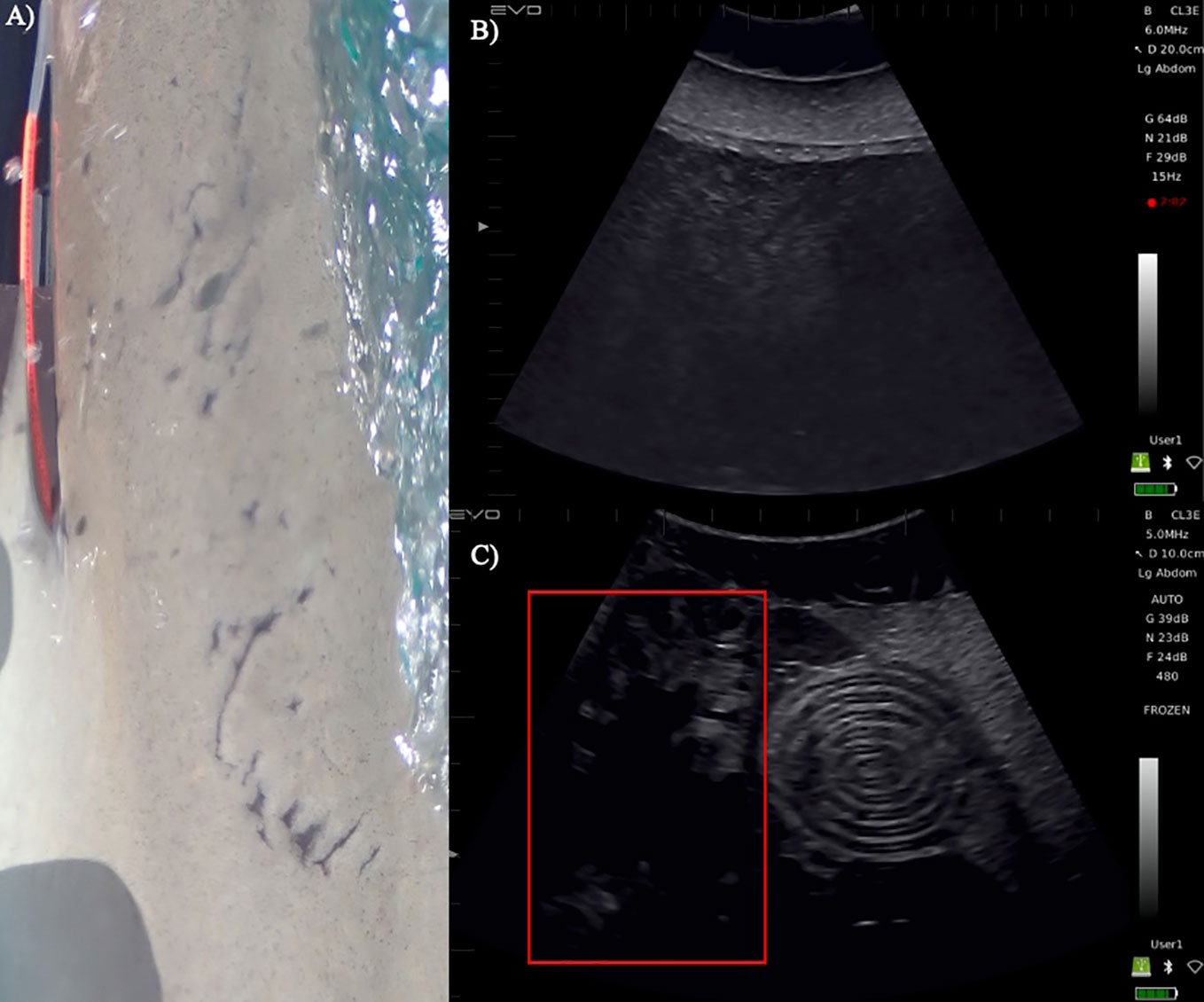
Figure 1 (A) An enlarged photo example of mating scars seen on both females caught in July 2022 (IDs 00767, 00521), (B) in-situ ultrasounds performed on non-pregnant 2021 female (ID 00543) with empty uterus and (C) pregnant female (ID 00521; bottom) captured in 2022. Candle-like structures are highlighted in red. These structures are thin, pleated egg envelopes seen in placental sharks (see Hamlett et al., 2011). The presence of these envelopes indicate that this female had completed ovulation.
4 Discussion
Interpretation of hormone concentrations (i.e., estradiol (E2), progesterone (P4)) in the context of elasmobranch reproduction has been investigated since the mid-1900s (Becerril-García et al., 2020). Generally, it has been shown that high levels of E2 are associated with preparing the female reproductive tract for ovulation through stimulating the production of vitellogenin by the maternal liver (Awruch, 2013). Conversely, high levels of P4 have been hypothesized to play an antagonistic role toward E2, downregulating the production of vitellogenin and prompting ovulation (Verkamp et al., 2022). In this sense, the increase in E2 and decrease in P4 concentrations from August to December demonstrated by individual #00543 is presumed to be indicative of vitellogenesis (Awruch, 2013), suggesting that this female was entering a pre-ovulatory state and preparing for the next mating season (Awruch, 2013). This observation is supported by previous work on the placental viviparous blue shark, where high concentrations of circulating E2 were directly linked to the synthesis of vitellogenin by the maternal liver during the follicular phase (Fujinami and Semba, 2020). Given that Caribbean reef sharks are thought to follow a biennial cycle with a long gestation (~11-12 months; Rangel et al., 2022), it is presumed that this female (ID 00543) had pupped earlier in the year before the first time she was sampled. Furthermore, the absence of soft tissue structure inside the uterus of this female, observed via in-situ ultrasonography (Figure 1B) further supports the presence of this pre-ovulatory state suggested by hormone concentrations. Because these observations occurred during an odd year, this could suggest a biennial reproductive cycle for Caribbean reef sharks, at least for The Bahamas population. The subsequent capture of two reproductively active females (IDs 00521, 00767), with evidence of pregnancy in both (ID 00767, 00521; Figure 1C), during an even year provides additional support for a biennial reproductive cycle for this sub-population.
High individual-level variation in hormone concentrations was observed across individuals sampled in this study (Table 1). The levels of E2 in female 00767 were 4-7 times greater than those observed in the other females. In addition, the P4 levels of this female were very high (Table 1) which is similar to levels observed in other ovulated/early post-ovulatory females of different species (Sulikowski et al., 2016). These hormonal observations coupled with recent mating scars and early-stage pregnancy assessments via ultrasound support the notion that this was a recent pregnancy. The female from August 2021 (ID 00543) that was not pregnant had similar levels of E2 and P4 as the pregnant female sampled in July 2022 (ID 00521). This has been observed in other species such as tiger sharks (Galeocerdo cuvier; Sulikowski et al., 2016; Hammerschlag et al., 2018) where hormone concentrations were found to be of similar levels between females in different reproductive stages. The low levels of E2 concentrations exhibited by the pregnant female in this study (ID 00521) is consistent with the completion of ovulation (Awruch, 2013). However, P4 concentrations for this individual were lower than expected, especially when compared to the other reproductively active female from July 2022 (ID 00767). Since the sampling efforts represent a temporal snapshot of the hormonal profiles of these sharks, this variation in this small window of time is expected based on previous studies and can most likely be attributed to individual differences in the exact timing of the reproductive cycle (Verkamp et al., 2022). To provide population-level resolution across seasons and major reproductive events, we recognize the need to obtain a larger sample size (e.g., 10 or more individuals).
Although preliminary, the findings offer support for a biennial reproductive cycle in the Caribbean reef sharks captured in Exuma. However, given the high individual-level variation in hormonal concentrations, and low sample size, the need for additional non-lethal samples are needed to further support this conclusion. Despite these short comings, we believe these preliminary findings carry conservation value given the paucity of existing information for this species’ overall biology as well as proof-of-concept for future research on elasmobranch reproduction.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Arizona State University Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by private philanthropic donations to Beneath The Waves.
Acknowledgments
We thank members of Beneath The Waves for field assistance, specifically S. Gray, D. Harris, J. Fitzgerald, B. Shea, J. Garvey, E. Lester, and N. Perisic, as well as many student volunteers who provided field assistance in sampling. Additionally, we thank Arizona State University undergraduate students that assisted in laboratory analysis for this study, specifically T. Jones and B. Krause.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Awruch C. A. (2013). Reproductive endocrinology in chondrichthyans: the present and the future. Gen. Comp. Endocrinol. 192, 60–70. doi: 10.1016/j.ygcen.2013.05.021
Becerril-García E. E., Arellano-Martínez M., Bernot-Simon D., Hoyos-Padilla E. M., Galván-Magaña F., Godard-Codding C. (2020). Steroid hormones and chondrichthyan reproduction: Physiological functions, scientific research, and implications for conservation. PeerJ 8, e9686. doi: 10.7717/peerj.9686
Bond M. E., Valentin-Albanese J., Babcock E. A., Abercrombie D., Lamb N. F., Miranda A., et al. (2017). Abundance and size structure of a reef shark population within a marine reserve has remained stable for more than a decade. Mar. Ecol. Prog. Ser. 576, 1–10. doi: 10.3354/meps12241
Brooks E. J., Sims D. W., Danylchuk A. J., Sloman K. A. (2013). Seasonal abundance, philopatry and demographic structure of Caribbean reef shark (Carcharhinus perezi) assemblages in the north-east Exuma Sound, The Bahamas. Mar. Biol. 160, 2535–2546. doi: 10.1007/s00227-013-2246-0
Carlson J., Charvet P., Blanco-Parra M. P., Briones Bell-lloch A., Cardenosa D., Derrick D., et al. (2021). Carcharhinus perezi. IUCN Red List Threatened Species 2021. doi: 10.2305/IUCN.UK.2021-1.RLTS.T60217A3093780.en
Carrier J. C., Pratt H. L., Castro J. I. (2004). Reproductive biology of elasmobranchs (Florida: CRC Press LLC).
Castro J. I., Woodley C. M., Brudek R. L. (1999). A Preliminary evaluation of the status of shark species (Rome: Food & Agriculture Org).
Compagno L. J. V. (2002). Carcharhinidae: Carpenter KE (ed) The Living marine resources of the Western Central Atlantic. Vol 1: Introduction, molluscs, crustaceans, hagfishes, sharks, batoid fishes, and chimaera. Am. Soc. Ichthyol Herpetol Spec Publ 5, 1–486.
Dulvy N. K., Pacoureau N., Rigby C. L., Pollom R. A., Jabado R. W., Ebert D. A., et al. (2021). Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 31 (21), 4773–4787. doi: 10.1016/j.cub.2021.08.062
Fujinami Y., Semba Y. (2020). Non-lethal assessment of reproductive stage for female blue sharks Prionace glauca using sex steroid hormones. J. fish Biol. 96 (6), 1501–1504. doi: 10.1111/jfb.14312
Gallagher A. J., Serafy J. E., Cooke S. J., Hammerschlag N. (2014). Physiological stress response, reflex impairment, and survival of five sympatric shark species following experimental capture and release. Mar. Ecol. Prog. Ser. 496, 207–218. doi: 10.3354/meps10490
Gallagher A. J., Shipley O. N., van Zinnicq Bergmann M. P., Brownscombe J. W., Dalghren C. P., Frisk M. G., et al. (2021). Spatial connectivity and drivers of shark habitat use within a large marine protected area in the Caribbean, the Bahamas shark sanctuary. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.608848
Hamlett W. C., Kormanik G., Storrie M., Stevens B., Walker T. I. (2011). “Chondrichthyan parity, lecithotrophy and matrotrophy,” in Reproductive biology and phylogeny of Chondrichthyes (Boca Raton: CRC Press LLC), 405–421.
Hammerschlag N., Skubel R. A., Sulikowski J., Irschick D. J., Gallagher A. J. (2018). A comparison of reproductive and energetic states in a marine apex predator (the tiger shark, Galeocerdo cuvier). Physiol. Biochem. Zoology 91 (4), 933–942. doi: 10.1086/698496
Hammerschlag N., Sulikowski J. (2011). Killing for conservation: the need for alternatives to lethal sampling of apex predatory sharks. Endangered Species Res. 14, 135–140. doi: 10.3354/esr00354
IUCN (2022) The IUCN red List of Threatened Species. Available at: https://www.iucnredlist.org (Accessed 15 October 2022).
Musick J. A., Ellis J. K., Hamlett W. (2005). Reproductive evolution of chondrichthyans. HAMLETT WC Reprod. Biol. phylogeny chondrichthyes sharks batoids chimaeras. 3, 45–71.
Natanson L. J., Deacy B. M., Joyce W., Sulikowski J. A. (2019). Presence of a resting population of female porbeagles (Lamna nasus), indicating a biennial reproductive cycle, in the western North Atlantic Ocean. Fishery Bull. 117, 70–77.
Rangel B. S., Afonso A. S., Garla R. (2022). Female wound records suggest mating periods for the Caribbean reef shark at an insular marine protected area from the Equatorial Atlantic Ocean. J. Fish Biology 101(6), 1591–1594. doi: 10.1111/jfb.15212
Stevens J. D., Bonfil R., Dulvy D. K., Walker P. A. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 57 (3), 476–494. doi: 10.1006/jmsc.2000.0724
Sulikowski J. A., Tsang P. C. W., Huntting Howell W. (2004). An annual cycle of steroid hormone concentrations and gonad development in the winter skate, Leucoraja ocellata, from the western Gulf of Maine. Mar. Biol. 144, 845–853. doi: 10.1007/s00227-003-1264-8
Sulikowski J. A., Wheeler C., Gallagher A., Prohaska B., Langan J., Hammerschlag N. (2016). Seasonal and life-stage variation in the reproductive ecology of a marine apex predator, the tiger shark (Galeocerdo cuvier), at a protected female-dominated site. Aquat. Biol. 24 (3), 175–184. doi: 10.3354/ab00648
Talwar B. S., Bradley D., Berry C., Bond M. E., Bouyoucos I. A., Brooks A. M., et al. (2022). Estimated life-history traits and movements of the Caribbean reef shark (Carcharhinus perezi) in The Bahamas based on tag-recapture data. Mar. Biol. 169 (5), 55. doi: 10.1007/s00227-022-04044-9
Tavares R. (2009). Fishery biology of the Caribbean reef sharks, Carcharhinus perezi (Poe), in a Caribbean insular platform: Los Roques Archipelago National Park, Venezuela. Pan-American J. Aquat. Sci. 4 (4), 500–512.
Keywords: estradiol and progesterone, ultrasound, endangered, non-lethal, elasmobranch
Citation: Campbell BA, Shipley ON, Jones TR, Gallagher AJ and Sulikowski JA (2024) Observations of biennial reproduction in Caribbean reef sharks ‘Carcharhinus perezi’. Front. Mar. Sci. 10:1160199. doi: 10.3389/fmars.2023.1160199
Received: 06 February 2023; Accepted: 20 December 2023;
Published: 11 January 2024.
Edited by:
Elizabeth Grace Tunka Bengil, University of Kyrenia, CyprusReviewed by:
James Gelsleichter, University of North Florida, United StatesŞehriban Çek-Yalnız, Iskenderun Technical University, Türkiye
Copyright © 2024 Campbell, Shipley, Jones, Gallagher and Sulikowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beckah A. Campbell, YmVja2FoLmNhbXBiZWxsODlAZ21haWwuY29t
 Beckah A. Campbell
Beckah A. Campbell Oliver N. Shipley
Oliver N. Shipley Taeler R. Jones3
Taeler R. Jones3 Austin J. Gallagher
Austin J. Gallagher James A. Sulikowski
James A. Sulikowski