- 1Hellenic Centre for Marine Research (HCMR), Institute of Marine Biological Resources and Inland Waters, (IMBRIW), Crete, Greece
- 2ECOSEAS, Université Côte d’Azur, CNRS, Nice, France
- 3Department of Biology, University of Naples Federico II, Naples, Italy
- 4National Biodiversity Future Center (NBFC), Palermo, Italy
- 5Ecologia Marina, Centre d’Estudis Avançats de Blanes, Consejo Superior de Investigaciones Científicas (CEAB-CSIC), Blanes, Spain
- 6Dipartimento di Scienze della Vita e dell’Ambiente, Università Politecnica delle Marche, Ancona, Italy
- 7European Topic Centre on Spatial Analysis and Synthesis (ETC-UMA), University of Malaga, Malaga, Spain
- 8MedGardens program, Cleanwave Foundation, Palma de Mallorca, Spain
- 9Agence de l’eau Rhône Méditerranée Corse, Marseille, France
- 10Stazione Zoologica Anton Dohrn, Calabria Marine Centre, Amendolara (CS), Area Marine Protetta Porto Cesareo, Porto Cesareo, Italy
- 11SUEZ Consulting, Aix-en-Provence, Provence-Alpes-Côte d’Azur, France
- 12Natura2000 Baie et Cap d’Antibes-Iles de Lérins, Antibes, France
- 13Fisheries Research Institute, Hellenic Agricultural Organization-Demeter, Kavala, Greece
Macroalgal forests characterised by species of the genus Cystoseira sensu lato form important shallow coastal rocky habitats in the Mediterranean Sea. These forests support a high biodiversity and provide important ecosystem services and societal benefits. Currently these habitats are often in a poor condition in many areas, due to loss and degradation from both anthropogenic and climate stressors. Restoration has recently moved to the forefront of the United Nations and European Union agendas to reverse this trend, particularly in the last decade with the implementation of various international policies. However, this has been in the form of generic targets (e.g., restoration of 30% of degraded habitats by 2030) and has not been linked to specifically what habitat or species to restore, where and how. Initial targets have been missed, new targets are expected through the proposed EU Nature Restoration Law, but overall guidance is still lacking. There are few specific guides to marine habitat restoration limited to mostly seagrass, corals and shellfish. As a priority action for the recovery of coastal marine ecosystems a decision-support framework has been developed for the restoration of Mediterranean macroalgal forests, comprising a stepwise decision tree with additional descriptions of key elements to be considered for a restoration action. The decision tree includes steps concerning current and historical forest presence, site local condition assessment and choice of actions. Key considerations include restoration implementation (competence, society and support, finance and governance), success evaluation (at the target species and the ecosystem level) and long-term management. The framework builds on existing work on Cystoseira s.l. restoration, the work carried out in the EU AFRIMED project, but also on principles and guidelines in place for both generic and specific marine habitats. The work reported here has involved the expertise of scientists and information from stakeholders. Gaps were identified and recommendations were made, dealing with stressors, coordinating and networking stakeholders, integrating top down policy and bottom up initiatives, funding of restoration actions, establishing synergies between restoration, conservation and marine spatial planning and finally communication and publicity.
1 Introduction
Mediterranean macroalgal forests are typically dominated by canopy-forming Cystoseira sensu lato species, including the genera Cystoseira, Gongolaria and Ericaria (Sauvageau, 1912; Feldmann, 1937; Ercegović, 1952; Molinari Novoa and Guiry, 2020) (Figures 1A, B). They are generally considered as the ‘Mediterranean kelps’ (Mangialajo et al., 2008a). Cystoseira s.l. species form dense canopies and create complex three-dimensional structures in rocky coastal ecosystems (Bulleri et al., 2002; Rodríguez-Prieto et al., 2013) providing habitat, food and shelter for many other associated species (Giaccone, 1973; Giaccone and Bruni, 1973; Ballesteros, 1988, Ballesteros, 1990a; Ballesteros, 1990b; Ballesteros et al., 1998; Cheminée et al., 2017; Piazzi et al., 2018; Sant and Ballesteros, 2021a). Cystoseira s.l. forests occur from the upper infralittoral down to the upper circalittoral zone (reported to 50 m depth in Hereu et al., 2008). Their distribution is dependent on a variety of environmental factors such as light intensity, hydrodynamics, temperature and nutrient availability, among others (Feldmann, 1937; Giaccone and Bruni, 1973; Ballesteros, 1989; Ballesteros and Zabala, 1993; Delgado et al., 1995; Arévalo et al., 2007; Hereu et al., 2008; Sales and Ballesteros, 2009; Vergés et al., 2009; Chappuis et al., 2014; Sant and Ballesteros, 2021a; Sant and Ballesteros, 2021b; Ballesteros and Sant, 2022). Cystoseira s.l. species represent one of the most productive and biodiversity-rich habitats of the Mediterranean Sea and underpin important ecosystem services, functions and benefits (e.g., carbon burial and nutrient cycling) (Boudouresque, 1972; Verlaque, 1987; Ballesteros, 1988; Ballesteros, 1989; Ballesteros, 1990a; Ballesteros, 1990b; Sales and Ballesteros, 2012; Piazzi et al., 2018; Pinna et al., 2020). Cystoseira s.l. provides nursery services for fish stocks which in turn support commercial and recreational fisheries, thereby delivering both economic and cultural values (Costa-Domingo et al., 2022; Friedrich et al., 2022). Cystoseira s.l. also provides service as bioindicator for water quality (Ballesteros et al., 2007; Orfanidis et al., 2011).

Figure 1 Healthy forest of Gongolaria barbata (A), healthy forest of Ericaria crinita (B), degraded rocky bottom (C, D). Ex situ recruitment enhancement restoration technique: growth of Cystoseira recruits in laboratory culture on mobile substrates (E) and placement of seeded substrates with Cystoseira recruits in the restoration site (F). Photo credits (A): Stéphan Jamme; (B), (E) and (F): Jana Verdura; (C): Emma Cebrian; (D): Xavi Calsina.
During the last three decades, most of the Cystoseira s.l. forests have been progressively lost in the Mediterranean Sea (Bellan-Santini, 1965; Munda, 1993; Cormaci and Furnari, 1999; Thibaut et al., 2005; Mangialajo et al., 2008a; Pinedo et al., 2013; Iveša et al., 2016), as with other macroalgal forests around the globe (Steneck et al., 2002; Airoldi and Beck, 2007; Filbee-Dexter and Wernberg, 2018; Bernal-Ibáñez et al., 2021; Martín García et al., 2022). Habitat destruction (loss of suitable substrate from coastal development or other direct seabed contact), changes in water quality following sedimentation, eutrophication and pollution, as well as overgrazing have been the main causes of their decline (Thibaut et al., 2005; Arévalo et al., 2007; Mangialajo et al., 2008b; Sala et al., 2011, Sala et al., 2012; Vergés et al., 2014; Pinedo et al., 2015; Piazzi and Ceccherelli, 2019; Orfanidis et al., 2021) (Figures 1C, D). This is expected to be exacerbated by impacts of climate change (such as marine heatwaves; Lejeusne et al., 2010; Celis-Plá et al., 2017; Verdura et al., 2021). Currently, all Cystoseira s.l. species except C. compressa are included in the Annex II of the Barcelona Convention (United Nations Environment Programme/Mediterranean Action Plan) and the establishment of dedicated Marine Protected Areas (MPAs) has been encouraged (Gianni et al., 2013).
Despite a few populations exhibiting natural recovery after a decline (e.g., Iveša et al., 2016), the natural re-establishment of Cystoseira s.l. forests is extremely rare (Chapman, 1995; Soltan et al., 2001; Sales et al., 2011; Capdevila et al., 2018a; Riquet et al., 2021). The lack of a nearby source of propagules and the low dispersal capacity of these species hinder their natural recovery (Perkol-Finkel and Airoldi, 2010). Consequently, active restoration methodologies have become one of the few feasible alternatives to promote the re-establishment of lost Cystoseira s.l. forests, following mitigation of the factors responsible for the decline.
The first records of macroalgal restoration projects go back to 1959, and have substantially been increasing since the 1990s (Eger et al., 2022a). However, these efforts have not been homogeneously distributed across the globe since most projects have been performed in Japan and the USA (Ueda et al., 1963; North, 1976; Wilson and McPeak, 1983; Arai, 2003; Japanese Fisheries Agency, 2009; Japanese Fisheries Agency, 2015; Japanese Fisheries Agency, 2021; see also Fraschetti et al., 2021 Eger et al., 2022a and references therein). Macroalgal restoration efforts targeting Cystoseira species in the Mediterranean Sea only started in 2006 (see Gianni et al., 2013 for a review). Since 2011, collaborative efforts generated knowledge on restoration techniques, protocols and trials (Figures 1E, F), as well as complementary actions (Sales et al., 2015; Falace et al., 2018; Verdura et al., 2018; De La Fuente et al., 2019; Tamburello et al., 2019; Medrano et al., 2020; Orlando-Bonaca et al., 2022), roadmaps (Cebrian et al., 2021) and spatial prioritisation (Fabbrizzi et al., 2020; Fabbrizzi et al., 2023). Most of these efforts have been led and developed by academic researchers from public research institutions and universities, at small scales, reflecting the relatively incipient stage of macroalgal restoration (Eger et al., 2022a). In parallel, practitioners have been researching and refining methodologies, exploring the effectiveness of large-scale restoration interventions (e.g., Thibaut et al., 2021). The contribution of other stakeholders, such as governments, private companies, non-governmental organisations (NGOs) or community groups, is now critically needed to go forward with restoration upscaling. Large-scale solutions in restoration actually arise from small-scale successes. These successes inject social values and optimism needed for global investment (McAfee et al., 2021).
The degree of Cystoseira s.l. restoration knowledge is now robust enough to scale up restoration projects (Tamburello et al., 2019). Restoration upscaling requires baseline information (e.g., historic distribution), biological and ecological features (e.g., reproductive phenology, population connectivity), knowledge of mechanisms that promote and dampen the recolonisation process, and indicators for the evaluation of the restoration success (e.g., target species and ecosystem level long-term success). Restoration upscaling also requires a better understanding of the benefits that the restored habitats can deliver to people and the local economy, as well as the costs involved in implementation, monitoring and maintenance. The objective of scaling up restoration actions in the Mediterranean Sea is driven by new ambitious initiatives of the United Nations and the EU: the UN Decade on Ocean Science for Sustainable development (2021-2030), the UN Decade on Ecosystem Restoration (2021-2030) aiming to accelerate restoration of marine ecosystems (UN, 2019) and the proposed EU Nature Restoration Law (EU NRL) (EC, 2022) aiming to repair damage done to European nature by 2050. It is therefore urgent to identify robust guiding principles and practices on macroalgal forest restoration in order to foster stakeholder engagement within science-based restoration interventions.
Restoration in the marine realm is gaining recognition globally, however, it still lags behind terrestrial work due to science gaps, implementation scale, and the appropriate restoration reporting framework to better support decisions on marine restoration (Elliott et al., 2007; Suding, 2011; Blignaut et al., 2013; Bayraktarov et al., 2016; Bayraktarov et al., 2020; Eger et al., 2022b). The importance and increasing practice of marine restoration has driven the need to guide restoration projects towards the best possible outcomes. This has resulted in an increasing number of experience-based publications, particularly in the last few years, with clear guidelines. Whilst large coordinating organisations have taken the role of providing high level generic restoration approaches (IUCN – Keenleyside et al., 2012; SER – Gann et al., 2019; FAO et al., 2021), these are still based primarily on terrestrial restoration. In the Mediterranean, a best practices guide has been developed for site specific case studies, collaboratively with the FAO led Task Force on Ecosystem Restoration (MBPC, 2022). However, on the whole, marine species and habitat specific guidelines have only recently appeared, targeting macroalgae (Gianni et al., 2013; Cebrian et al., 2021; Eger et al., 2022c), seagrasses (van Katwijk et al., 2009; UNEP-Nairobi Convention/USAID/WIOMSA, 2020a; Beheshti and Ward, 2021; Gamble et al., 2021), saltmarshes (Hudson et al., 2021), mangroves (ICRI, 2018; UNEP-Nairobi Convention/USAID/WIOMSA, 2020b), corals (Edwards and Gómez, 2007; Goergen et al., 2020; Hein et al., 2020; Shaver et al., 2020; Quigley et al., 2021; Escovar-Fadul et al., 2022), shellfish (MIT Sea Grant, n.d.; Leonard and Macfarlane, 2011; Fitzsimons et al., 2019; Preston et al., 2020) and multiple habitats (Leocadie et al., 2020). These documents are a mixture of principles, best practices and guidelines for successful restoration and share important key considerations around a restoration action.
Moving further from previous works (Gianni et al., 2013; Cebrian et al., 2021; best principles and guides mentioned above), the aim of the current work was to provide a framework to assist in the restoration decision-making process and to address key considerations of restoration implementation (society, competence, governance and finance). It also completes and improves a previous version of the decision tree proposed by Gianni et al. (2013), appropriately modified to meet new specific considerations for Cystoseira s.l. restoration in the Mediterranean. It simplifies complex decision making and helps to decrease uncertainty in restoration initiatives.
2 Decision-support framework
The Cystoseira s.l. decision-support framework (Figure 2) aims at avoiding the initiation of restoration actions where the chances of success are very low and increasing the overall likelihood of restoration success. Those interested in performing a restoration action will have easy-to-follow steps that make the decision-making process smoother whilst science-based.
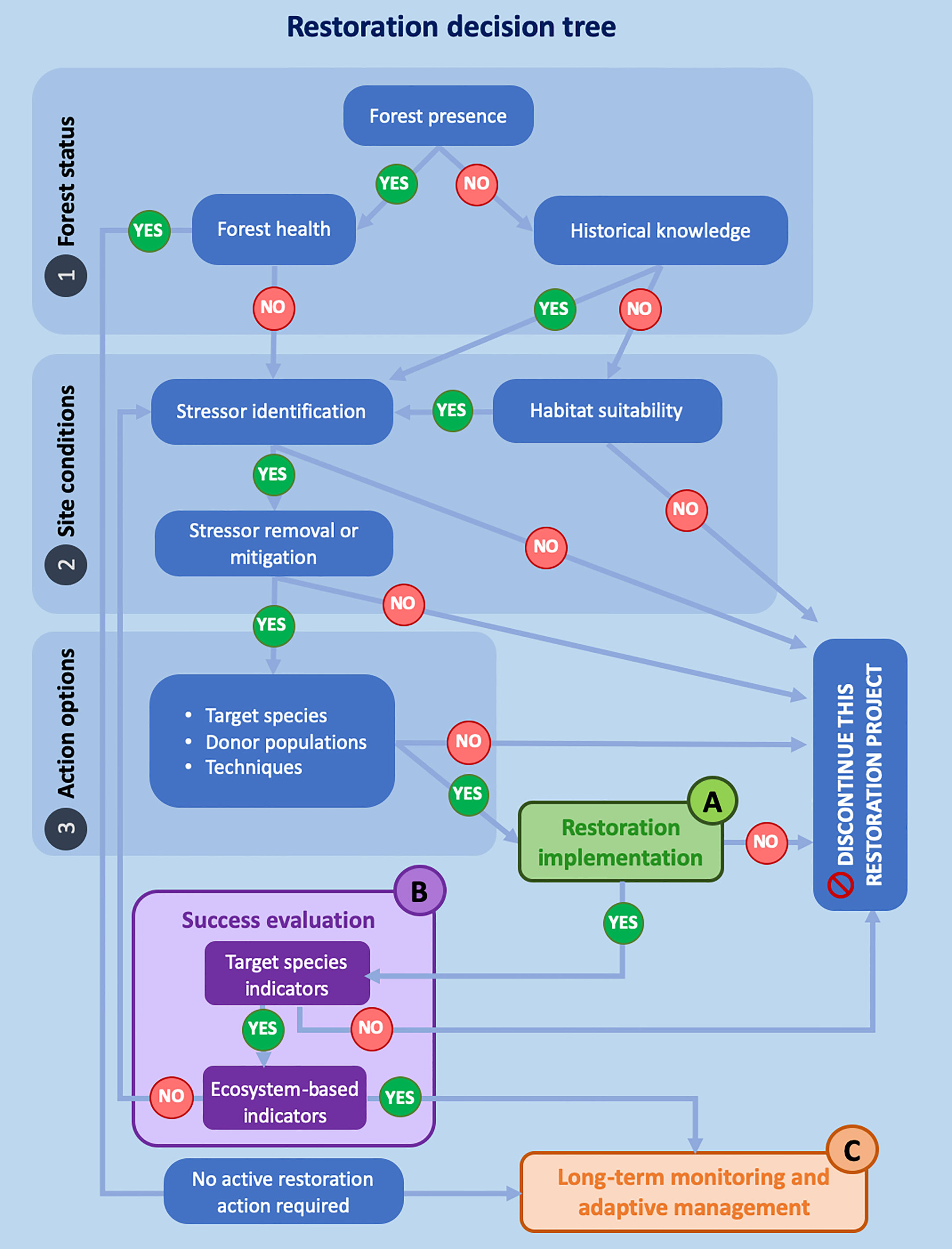
Figure 2 Decision-support framework to assist the Cystoseira s.l. restoration decision-making process and management. Restoration decision tree (modified from Gianni et al., 2013) highlighting critical steps in assessment and decision-making process. Decision steps; 1) Forest Status, 2) Site Conditions and 3) Action Options; with critical project steps; (A) Restoration implementation, (B) Success evaluation and (C) Long-term monitoring and adaptive management. See Figure 4 for the detail of identified critical project steps (A–C).
The framework consists of a sequential decision process with nested elements, giving details on what is needed to be considered when implementing a restoration project, and the steps to follow in the evaluation of success at species and ecosystem levels and long-term monitoring. The decision tree gives insight as to whether an active restoration project should take place or not. The framework also addresses non-academic stakeholders involved in the process of restoration. The following sections give further details on the framework elements.
2.1 Restoration decision tree
2.1.1 Forest status
2.1.1.1 Introduction – establishing site suitability
Conservation of Mediterranean marine forests should be based on the protection and correct management of already existing forests and restoration of forests that are already lost or in danger of disappearance (Gianni et al., 2013; Cebrian et al., 2021; Eger et al., 2022a).
Where to restore is a key question to be solved. The first step in a restoration intervention is to establish if the site is suitable for the presence of Cystoseira s.l. (Gianni et al., 2013; Fraschetti et al., 2021; Fabbrizzi et al., 2023). This involves understanding whether any forest is present, its level of degradation, and the availability of related historical data. Key steps, knowledge and variables that must be considered to determine site suitability are detailed below and in Figure 2. We use the generic term ‘site’ (or area) without specifying any spatial scale, as a restoration action may be planned from a few square metres scale (i.e., within a rockpool) in order to guarantee the connectivity of a rare species, to dozens of kilometres of coastline to guarantee ecosystem services at the regional level.
2.1.1.2 Forest presence
The macroalgal decision tree begins with the question of whether there is an existing forest in the area of restoration interest. In many areas of the Mediterranean, answering this question is often challenging, as the current distribution of Cystoseira s.l. forests is mostly unavailable in the literature (Rehues et al., 2021). As a result, researchers often must explore alternative sources such as grey literature, local or expert knowledge, or rely on their own first-hand observations. In several European countries a huge effort has been made to map different habitats in Natura2000 sites. Unfortunately, Cystoseira s.l. forests are not differentiated from other macroalgal communities (e.g., erect algae, turfs and even barren grounds). Therefore, existing cartography, while valuable for habitats such as for Posidonia oceanica meadows, cannot fill this knowledge gap and further mapping is needed. Mapping, however, can now be supported by novel technologies, such as Unmanned Aerial Vehicles (UAVs) over shallow waters and Remotely Operated Vehicles (ROVs). UAVs in particular, can produce high-definition maps of the distribution of benthic assemblages, together with the collection of several environmental variables, at large-scale extents.
2.1.1.3 Forest health
If the site is forested, and the existing forest is healthy (i.e. more than 50% of cover, Fraschetti et al., 2021), practitioners should consider the set-up of a regular monitoring programme of the forest. The health (status in relation to reference populations) and trajectory of health (established though monitoring), will define the needs for restoration. Indicators of good condition include but are not limited to; macroalgal species density, population size-structure, presence of reproductive individuals and recruits, population extension (m2, hectares), biomass, and associated biodiversity (Cheminée et al., 2013; Bianchelli and Danovaro, 2020). While aiming to preserve the forest itself, healthy populations can be used as donors for restoring other degraded populations in the future.
2.1.1.4 Historical knowledge
If the site is not forested the next step is to search for historical data to determine whether a Cystoseira s.l. forest existed there previously, and which species formed it.
The growing attention on the conservation and restoration of habitat-forming species, has led to the recent increase in knowledge acquisition relating to Cystoseira s.l. distribution and abundance. Several outputs have been provided from the Mediterranean coasts including France (Thibaut et al., 2005; Sales and Ballesteros, 2009; Thibaut et al., 2014; Thibaut et al., 2015; Blanfuné et al., 2016; Thibaut et al., 2016; Thibaut et al., 2017), Spain (Catalonia; Mariani et al., 2019), Italy (Lucia et al., 2020; Tamburello et al., 2022) and Istria (Iveša et al., 2016). Further information may be available in grey literature, monitoring programs, unpublished data from experts and local/traditional ecological knowledge (Ballesteros et al., 2014; Mariani et al., 2019; Tamburello et al., 2022). Unfortunately, these data are generally available for easy-to-identify species in limited locations. As a result, our knowledge on the distribution of Cystoseira s.l. is globally incomplete and often biased (Rehues et al., 2021). Based on the published data, Figure 3 indicates areas in the North-Western Mediterranean with some of the reported Cystoseira s.l. regression or loss.
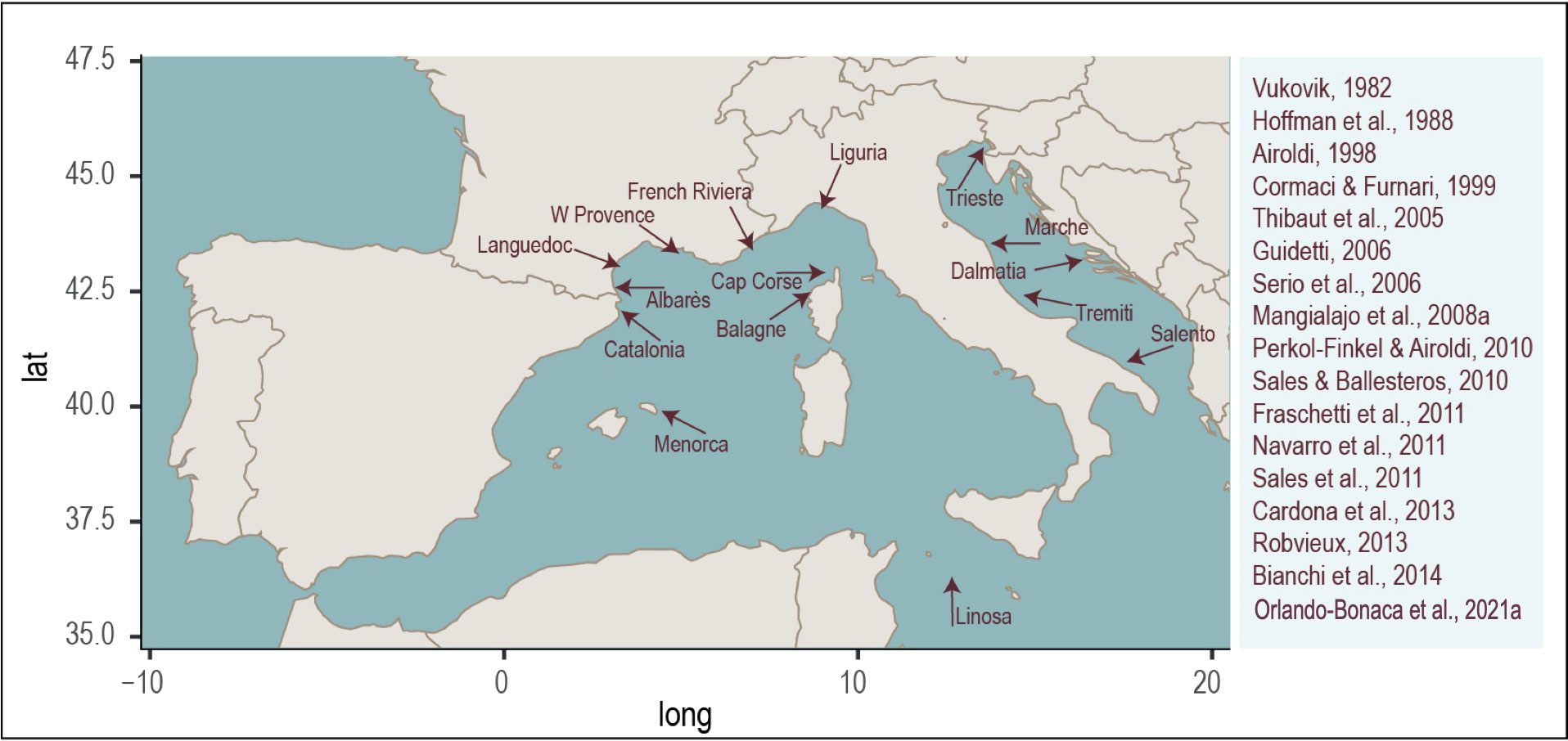
Figure 3 Reported areas of regression or loss of Cystoseira s.l. forest in the North-Western Mediterranean region.
2.1.2 Site conditions
The assessment of the target site local conditions should include: i) the likelihood of habitat suitability for Cystoseira s.l. (in the lack of historical data), ii) the identification of causes of forest degradation or loss and iii) the removal or mitigation of such causes.
2.1.2.1 Habitat suitability
A prerequisite for achieving higher restoration success is whether site conditions match the habitat requirements of the target species (depth, substrate, exposure, turbidity, temperature, etc.). The use of Habitat Suitability Models (HSMs) can be critical where areas lack historical data (Kearney and Porter, 2009). Modelling is a cost-effective approach to identify suitable and unsuitable areas for species and habitats, predict their possible shifts in distribution under global climate change (Fabbrizzi et al., 2020; Santiago et al., 2023) and provide insights about potential causes of habitat loss (Catucci et al., 2022). Modelling combines multiple predictor variables (e.g. coastline geomorphology, temperature, human pressures; see Cefalì et al., 2016; Cefalì et al., 2018; Fabbrizzi et al., 2020) and target species occurrence data. The quality of data feeding HSMs is of paramount importance, and planning large-scale restoration interventions in the absence of fine-scale information may seriously compromise output accuracy.
2.1.2.2 Stressor identification
Where stressors are present that impact macroalgal forests, the success of the restoration action is unlikely (Cebrian et al., 2021). All causes of forest regression or loss must be identified, removed or mitigated at a satisfactory level. If this is not possible and relevant impacts are still present, the active restoration program should be discontinued.
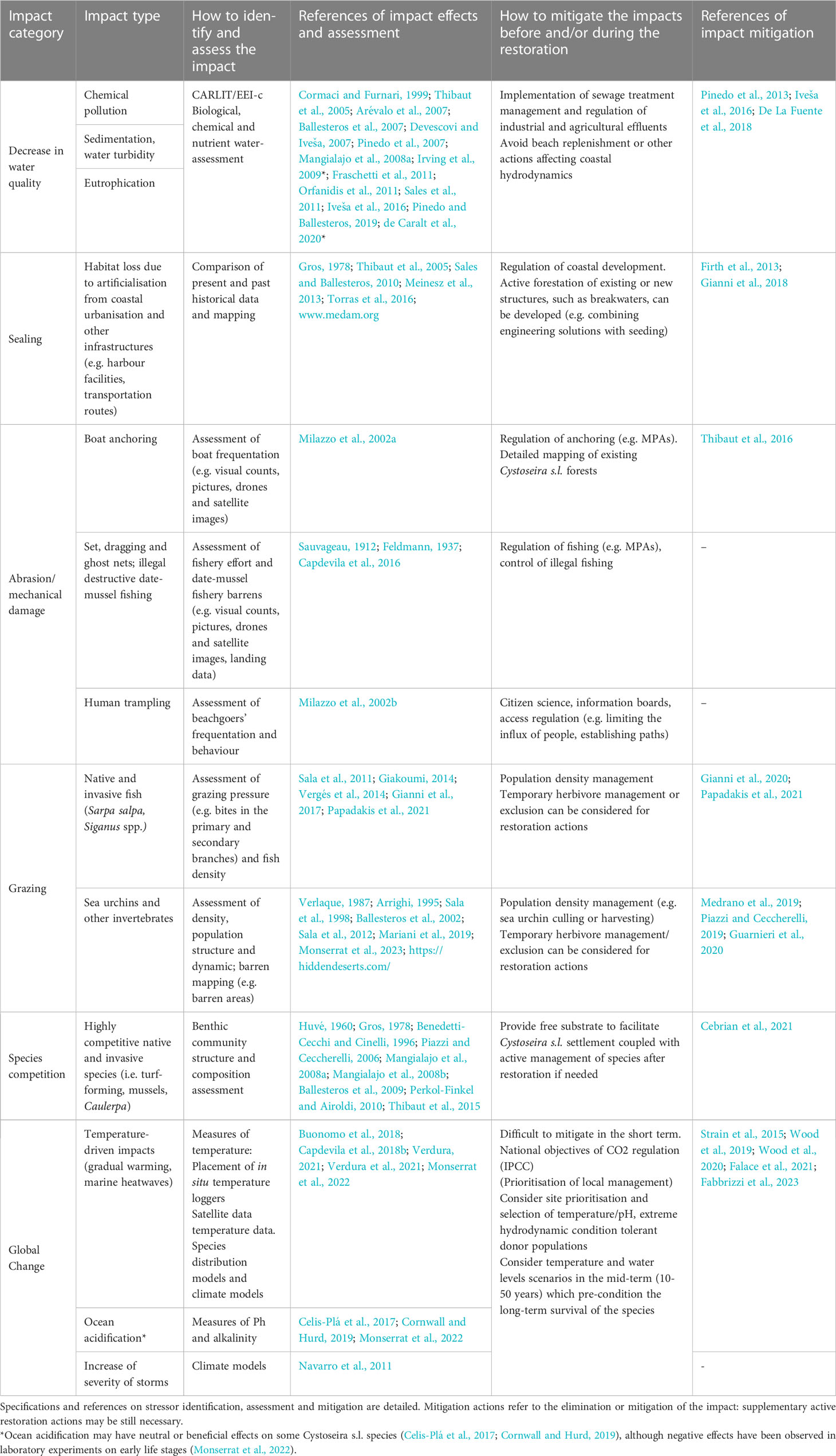
Cystoseira s.l. populations have been threatened by multiple stressors operating from local (e.g., changes in water quality, overgrazing; de Caralt et al., 2020; Papadakis et al., 2021) to global scales (e.g., marine heatwaves; Thibaut et al., 2015; Gianni et al., 2017; Verdura et al., 2021). Although various stressors have been identified across the Mediterranean basin, in most cases, the causal stressors involved in local population declines have not been identified (Tamburello et al., 2022), and therefore, the relationship between stressors and the disappearance of Cystoseira s.l. species remains largely unknown (Hillebrand et al., 2020). Stressor identification and prioritisation (particularly in the presence of multiple stressors) is necessary for local intervention planning (Gann et al., 2019). Table 1 summarises the main reported stressors of Cystoseira s.l., with suggestions and references on how to identify and mitigate their impacts.
2.1.2.3 Stressor removal or mitigation
Some local stressors may be, relatively easy to remove or minimise through local management and interventions (see Table 1 for examples), such as herbivore management (Ballesteros et al., 2002; Guarnieri et al., 2020. Mitigation of other local issues such as improving water quality (e.g. wastewater management), will require the involvement of local governments, making these interventions more complex and time-consuming to address. MPAs can present ideal areas for restoration activities if habitat requirements are present as some anthropogenic stressors will already be removed or strictly managed (Pogoda et al., 2020).
In contrast, global stressors, such as ocean warming and marine heatwaves require collaboration among countries or regional bodies and may take centuries to be mitigated. However, previous studies have underscored the importance of local management in order to foster the resilience of the macroalgal populations in the face of global stressors (O'Leary et al., 2017; Morris et al., 2020).
Besides current stressors, the consideration of potential future impacts, and their mitigation potential, is of high relevance when assessing site suitability. HSMs can inform on shifts of habitat suitability in response to environmental changes, such as future global warming scenarios including large-scale range shift predictions (Pearson and Dawson, 2003; Peterson, 2006; Kearney and Porter, 2009). This may help to identify both sites predicted to be most impacted by future stressors and sites acting as possible future refugia (e.g. climatic refugia; Verdura et al., 2021; Fabbrizzi et al., 2023). As model predictions become more robust (Martínez et al., 2015), their use is highly recommended, to give an insight into where a restoration effort may fail or succeed in future expected conditions.
2.1.3 Choice of actions for restoration implementation: technical feasibility
Choice of action and feasibility include crucial steps related to selecting the target Cystoseira species, donor site and technique as detailed below.
2.1.3.1 Target species and donor populations
The criteria for target species selection should be based on species ecological relevance and status (Swan et al., 2016; Cebrian et al., 2021), but also should match both project-specific restoration objectives and local site requirements (Thomas et al., 2017; Atkinson et al., 2021).
Targeting habitat-forming species such as macroalgal forests is a first step in whole ecosystem restoration. Given they are long-lived and play a central role in the functioning of the ecosystem, species selection needs also to be tailored to maximise the persistence under current and future conditions of the site (Fremout et al., 2021a; Fremout et al., 2021b). In-depth knowledge is required for the appropriate selection of the target species (Montero-Serra et al., 2018). This knowledge should include information concerning life history traits, ecological interactions, environmental requirements, and vulnerability to different stressors. It should also consider their differential implications at the distinct life stages of the species (Cebrian et al., 2021).
When faced with two equally optimal species to restore a given site, selection should be prioritised for the species for which there is a greater degree of knowledge, covering for example, optimal restoration techniques (e.g., Verdura et al., 2018), optimal culture protocols (e.g., Falace et al., 2018) or information on the reproductive phenology and early-life stages development (e.g., Savonitto et al., 2019; Lardi et al., 2022).
The use of wild donor populations for restoration purposes may compromise the persistence of such populations. While there is a need to restore degraded or lost populations, the conservation of remnant wild populations should be prioritised. Therefore, the conservation status of the donor population is paramount in deciding whether the removal of material is sustainable. Thus, only well-preserved, extensive, and healthy populations, able to recover from collection of material without being compromised, should be selected as donors.
Whenever possible, it is suggested to select donor populations as close as possible to the restoration area, as well as from comparable environments. This minimises the specimen manipulation and may optimise action cost-effectiveness (Tamburello et al., 2019). It may also help short-term restoration success, since new individuals will have the appropriate traits (e.g., pre-adaptation to high sedimentation) necessary to survive and expand in the selected restoration site (van Katwijk et al., 2009; Wood et al., 2019; Orlando-Bonaca et al., 2022). Another criterion is that donor populations should display sufficient genetic variation to be able to adapt to environmental changes and avoid inbreeding (van Katwijk et al., 2009; Wood et al., 2021). Despite limited research on how donor population selection affects the success of Cystoseira s.l. restoration, recent findings have highlighted significant differences in reproductive potential and success among different and geographically proximate populations (Orlando-Bonaca et al., 2022). This underscores the importance of implementing appropriate monitoring programs and protocols to characterise potential donor populations and enable the optimal selection of the most appropriate donors.
To date, the objective of macroalgal restoration has mostly been the re-establishment of the native ecosystem in pre-disturbed conditions by actively restoring the dominant habitat-forming species. However, the success of marine forest restoration can be especially at risk due to stressful novel ecological conditions, such as increasing grazing pressure and seawater temperatures. Moderate and recurrent stress conditions during the ex-situ cultivation period of recruits have been suggested to foster the resilience and productivity of juveniles in the short term, possibly led by an increased capacity for acclimation (Clausing et al., 2023). However, under predicted climate change context, research has also focused on ways to enhance the chance of long-term survival of restored populations and ecosystems (Wood et al., 2019; Wood et al., 2021; Fabbrizzi et al., 2023), particularly for those locations predicted to be more affected by global warming. Besides predictive models for site prioritisation, restoring future-proof populations is becoming an increasingly relevant approach, especially in environments subjected to rapid anthropogenic change (using, for example, more thermo-tolerant genotypes or species; Wood et al., 2019). On the other hand, repairing ecosystem functions (e.g., rehabilitation) rather than restoring native ecosystems is an argument that is increasingly discussed (Coleman et al., 2020), especially when restoring “pristine” habitats that have not been predicted to cope well with future environmental conditions. All these approaches are still under development in the macroalgal restoration field, especially in the Mediterranean. Therefore, further research on these lines is advocated, in order to aid decision-making processes for future cost-feasible and effective restoration programs.
2.1.3.2 Restoration techniques
Defining the optimal strategy and the use of state-of-art techniques is of paramount importance for the success of the restoration intervention. Different techniques have been used (see Gianni et al., 2013; Cebrian et al., 2021) with individual transplants from wild donor populations being the early suggested mode of restoration (Falace et al., 2006; Sales et al., 2011). However, considering the threatened or endangered status of the remaining Cystoseira s.l. populations, non-invasive techniques should be prioritised. Recruitment enhancement methods, which take advantage of the high reproductive potential of these species, have proven to be cost-feasible in the restoration of Cystoseira s.l. populations, while at the same time having limited effects on donor populations (e.g., Verdura et al., 2018; De La Fuente et al., 2019; Tamburello et al., 2019; Medrano et al., 2020). Recruit enhancement can be achieved through different techniques: obtaining new recruits directly at sea (in situ) or culturing new recruits in aquaria (ex situ) (Falace et al., 2018; Verdura et al., 2018). Hybrid methods combining ex situ cultivation and suspended cultures in the field have been also tested and proposed as a potential approach to reduce the cost and time required for cultivation (Orlando-Bonaca et al., 2022). Aspects related to the target species (e.g., dispersal ability, species-specific culture protocols), conditions at the target site (e.g., hydrodynamic conditions, herbivory pressure, accessibility), as well as other aspects related to logistics and budget (e.g., availability of cultivation facilities and their proximity to the destination site, costs associated to each technique), must be carefully considered. Each technique has its advantages and disadvantages and the different interplaying factors must be considered carefully. Cebrian et al. (2021) have provided detailed considerations on the selection of appropriate restoration techniques, which are complemented by more recent works (e.g., Orlando-Bonaca et al., 2022; Clausing et al., 2023).
2.1.3.3 Complementary techniques
Once the restoration action has been carried out, ecological interactions in the restored area can also hinder success. High densities of herbivores, mainly the sea urchins Paracentrotus lividus and Arbacia lixula and the herbivorous fish Sarpa salpa and Siganus spp. can hinder survival and growth of the introduced individuals (Tamburello et al., 2019; Gianni et al., 2020). Other smaller invertebrate species, such as gastropods and decapods (e.g., marine snails and hermit crabs) can also graze the different life stages of canopy-forming species (Arrontes et al., 2004; Gunnarsson and Berglund, 2012; Hong et al., 2021; Monserrat et al., 2023; Navarro-Barranco et al., 2023). After a preliminary identification of herbivorous species and the assessment of grazing pressure on canopy-forming species, complementary actions of herbivory management should be integrated into the restoration program (Ballesteros et al., 2002; Cebrian et al., 2021). Combining the restoration actions with the deployment of different types of devices (e.g., cages or fish-deterrents) can prevent access to grazers (Tamburello et al., 2019; Gianni et al., 2020; Orlando-Bonaca et al., 2021b; Savonitto et al., 2021). Alternatively, herbivore removal or decreasing the density of herbivores to certain density thresholds (e.g., sea urchin culling or harvesting), has also been shown as a feasible action to reduce herbivory pressure (Ballesteros et al., 2002; Medrano et al., 2019; Guarnieri et al., 2020), although this may not be sustainable in the long-term and different strategies may be required or preferred. Further research on establishing herbivore density thresholds and undesired (or collateral) effects of some devices is needed. Finally, while the effects of MPAs on macroalgal restoration success are not yet fully understood, some restoration programs combining passive (MPAs) and active restoration strategies (e.g., recruitment enhancement) have shown a synergistic positive effect on restoration success (Medrano et al., 2019).
Competition with, and over-growth by opportunistic species (e.g., native turf-forming algae, exotic invasive species) may also hinder success (e.g., settlement, survival or growth) of the restoration action (Airoldi, 2000; Ballesteros et al., 2009). Complementary actions such as removal of competing algal species and provision of available substrate can substantially increase the chances of success of the new individuals (Capdevila et al., 2015; Medrano et al., 2019).
3 Restoration implementation
The key considerations for successful restoration implementation are grouped in four framework pillars concerning; society and support, competence, finance, and governance (Figure 4, Box A).
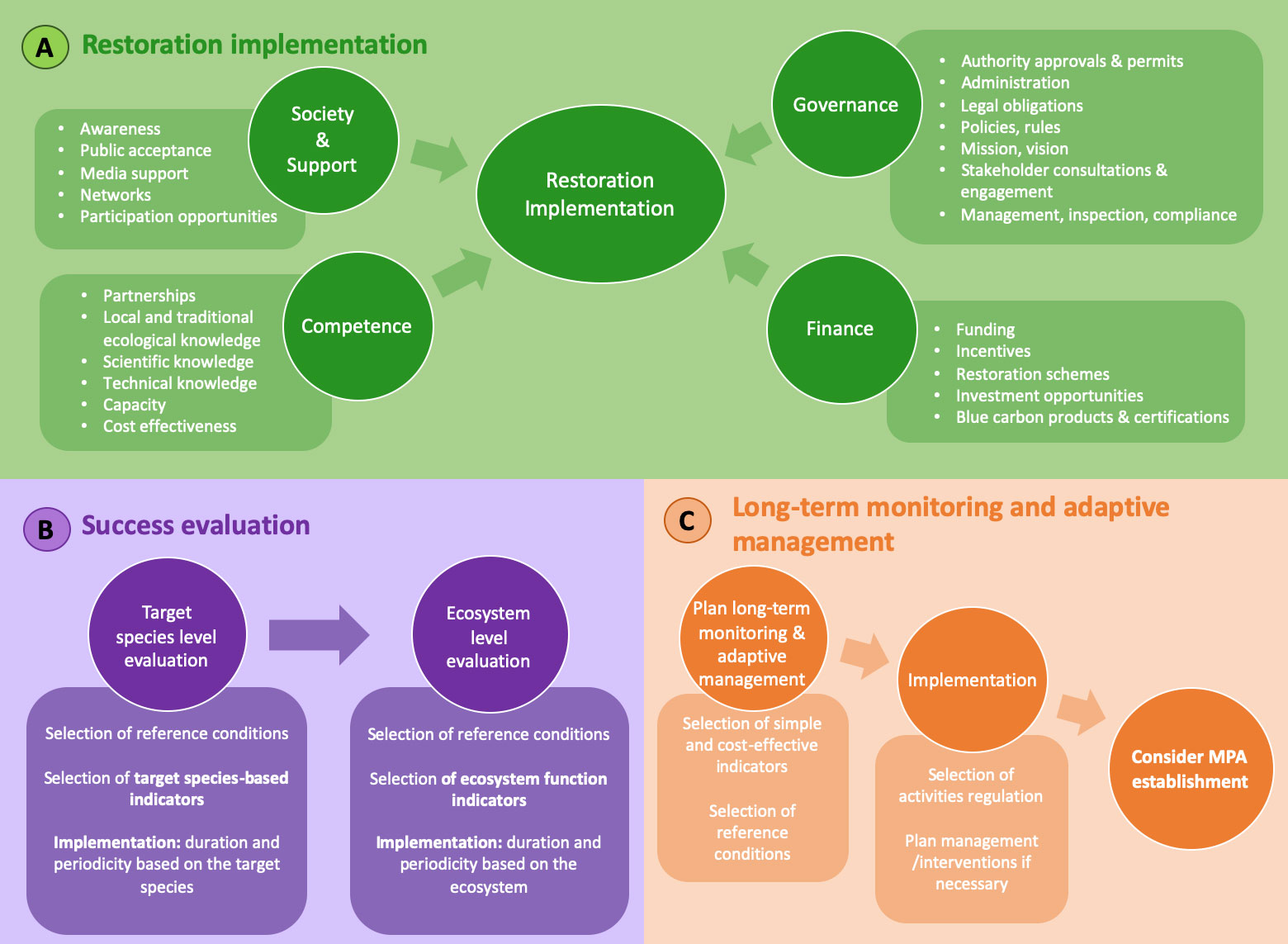
Figure 4 Critical steps of a restoration program: key considerations (around the four pillars; society, competence, governance and finance) that need to be addressed before implementing a restoration project (A), the key elements of restoration success evaluation (B), and long-term monitoring and adaptive management (C).
3.1 Society and support
Societal support is crucial in restoration success, in its uptake and acceptance. The various pathways to ensure this support include:
3.1.1 Awareness
Awareness mostly concerns the spread of the message communicating the Cystoseira s.l. restoration action. The message of awareness should include aspects of the cultural value, natural heritage and environmental value of the habitat, causes of degradation, needs for restoration, restoration success stories and the benefits of successful restoration. Spreading awareness can be realised through outreach, typically disseminating information to the general public. Effective outreach can raise the public interest and involve them as important stakeholders. At the local level, this should actively engage, for example, the tourism and shallow water recreational sectors that support awareness through both employees and recreational users. Key parts of effective communication are media engagement (in all forms from mainstream press to social media), programmes of ocean literacy, and developing networks (particularly at the local level).
3.1.2 Public acceptance
Public acceptance is an important step towards growing public support. It implies an acknowledgement of the existence of a problem and subsequent need of reparatory action, which is facilitated by the existence of an emotional experience of marine ecosystems (van Putten et al., 2018). ‘Buying in’ provides tacit support strengthening the role of the public as a stakeholder, particularly if they understand the value of Cystoseira s.l. habitats and the relation they have with their wellbeing. Promoting the feeling that they are part of a consultative process also adds to public support. This will apply even more to local coastal communities and local influencers.
3.1.3 Media support
Nowadays media can easily be self-created and self-disseminated to wide audiences, particularly through social media (e.g., Facebook, Instagram, Twitter, YouTube, LinkedIn, ResearchGate). Environmental NGOs and professional media (private companies) are best trained to provide informative, visually attractive, and balanced dissemination materials across many platforms. Interest in an action can also be garnered from local, and national news reporting, again through different media (print, on-line, television). Happy and hopeful stories capturing underwater images in front of us, but out of sight, are often attractive for audiences, generating interest and inspiring conservation action (Cvitanovic and Hobday, 2018; McAfee et al., 2019). Dissemination of the activity would benefit from engaging reporters interested in conservation, wildlife, environmental journalism, as well as the use of innovative tools such as storytelling or artistic collaborations (Vergés et al., 2020).
3.1.4 Networks
Networks should be developed with the project. They provide the opportunity for effective communication and coordination, whether this is between Cystoseira s.l. restoration practitioners, outside the project to higher bodies (e.g. restoration groups and organisations, national and regional authorities) that might be being advised, providing advice or further coordination, sideways to other restoration actions and activities (e.g. seagrass or coralligenous habitats), or around the particular project for stakeholders (including increasing local awareness). Networking can also provide some degree of security to the project in linking, advising and coordinating, which may help with risk management. Linking to existing thematic networks (e.g., for seagrass https://seagrassrestorationnetwork.com/, https://medposidonianetwork.com/, or to citizen scientist networks https://www.marineforests.com/), supports broader sharing of knowledge and approaches and long-term-large scale projects and interventions.
3.1.5 Participation opportunities
Participation opportunities include those directly involved in the Cystoseira s.l. restoration action comprising local authorities and councils, practitioners, supporting personnel and commercial companies providing equipment or services. All of which provide at least experience opportunities, and at most livelihood possibilities. There are opportunities for the public, local community or students to participate as volunteers or visitors to the site, (especially as restoration will be in shallow, easily visible waters). The participation of these groups showcases the project and can be achieved by fostering participatory sessions that bring science and users closer in a reciprocal relationship, for example, promoting marine citizen science (Cigliano et al., 2015; Kelly et al., 2020), photo contests or other artistic events (Vergés et al., 2020). Local businesses, companies, organisations and individuals can participate in restoration through greening activities or companies/organisations/individuals through charitable/altruistic motives. Participation elicits a sense of connection and stewardship and can offer rich opportunities for individuals to explore and experience the potential to reverse ecological degradation in shallow waters, and be inspired (Keenleyside et al., 2012). For example, Marine Stewardship processes are participatory tools with high potential to achieve persistence and replicability of conservation and restoration actions (McAfee et al., 2022), where all stakeholders (local authorities, councils, scientific community, local businesses and citizens) coordinate for the co-management of a natural space.
3.2 Competence
Competence includes the resources of expertise and knowledge, and the pathways to ensure these include:
3.2.1 Partnerships
The success of restoration programs typically requires multidisciplinary involvement and collaborative partnerships (Eger et al., 2022a). The partnership constitutes a closer relationship than project networking, leading to improved collaborative decision-making and strengthening both capacity and empowerment. Partnerships may be within the partners of the Cystoseira s.l. restoration project or through bringing particular competencies into the project (e.g. agencies, organisations, industry, universities, research institutes) and with local communities. It is also essential to consider the local authorities as part of the networking, to ensure that the project complements existing or future management plans.
3.2.2 Local and traditional ecological knowledge
Local Ecological Knowledge (LEK) is defined as place-based knowledge of the land and its processes applied by humans to create more productive and healthier ecosystems, increasing biodiversity and improving ecosystem resilience. Traditional Ecological Knowledge (TEK) is defined as knowledge and practice passed on from generation to generation and informed by strong cultural memories, sensitivity to change, and values that include reciprocity (both definitions from Gann et al., 2019). These knowledge resources can help to define Cystoseira s.l. restoration sites and reference conditions, and can be obtained from interviews and questionnaires, particularly from recreational snorkelers, divers, recreational and professional fishermen, as well as local environmentalist groups. Canvassing sources of local knowledge may also raise awareness and engage stakeholders.
3.2.3 Scientific knowledge
Scientific knowledge is derived from observation, measurements and analysis. This knowledge can be gathered directly from scientists or bibliographic searches (on-line, libraries, museums) concerning the status of the environment, or the environment required, the Cystoseira species to be restored, their ecological relationships and indicators of success.
3.2.4 Technical knowledge
Technical knowledge pertains to the techniques to be used in the restoration activity; including survey area, collection of samples and their maintenance in aquaria, collection and nursery of zygotes, transportation and planting in the field, protection, and monitoring. The techniques should ensure optimal survival at all steps and an overall cost-effectiveness. Technical knowledge comes from Cystoseira s.l., or related, bibliographic sources, and personnel experience, where the latter can be imported into the project through effective networking.
3.2.5 Capacity
Human capacity concerns people with the expertise, know-how, experience, and commitment to undertake actions. The project may need to build or strengthen capacity by training or recruiting for particular skills (e.g. handling, zygote collection, or aquaria know-how), which should be able to cope with changing circumstances during the Cystoseira s.l. restoration activity. Capacity can pertain to other resources such as facilities and equipment. Capacity also includes management, communication and stakeholder engagement. Capacity constraints should be identified and understood for proper conduction of the project under changing conditions.
3.2.6 Cost effectiveness
Cost-effectiveness concerns how practitioners may restore the highest Cystoseira s.l. cover per unit of currency spent, ensuring that limited funds are spent in the best manner (Kimball et al., 2015). This depends on streamlining restoration techniques, methodologies and protocols for the highest possible success, where success is defined by specific goals with defined metrics (e.g. area covered, biodiversity value, specific ecosystem function returned, etc.). A number of recent works (Bayraktarov et al., 2016; Fraschetti et al., 2021; Friedrich et al., 2022) have stressed that future restoration projects should use standardised protocols for reporting restoration costs as well as integrating long-term monitoring to improve understanding of ecosystem restoration benefits.
3.3 Governance
There are many aspects of governance that the restoration practitioner or group should be aware of at different levels. It is necessary to have contact with, follow procedures or seek advice, particularly on legal matters.
3.3.1 Authority approvals and permitting
Explicit consent and permitting may be needed from a variety of different competent authorities depending on national and local rules. Jurisdictions may include marine licensing and marine planning, protected species licence, seabed owners, habitat regulation assessment, and water quality boards (Gamble et al., 2021). This may include application and approval of the Cystoseira s.l. restoration plan, authorisation with specific permits or licences, or locally having permission to access or make interventions in an area that is not specifically related to the restoration activity. This includes scuba diving, boating, coastguard permissions (access, activity or notices for other users) or biosecurity licences (using non-native stock). Permitting and licences may have specific associated costs and may take time to achieve. It is important that the restoration activity has a leader that bears the legal responsibility of the restoration action (e.g., licence compliance).
3.3.2 Administration
It is important to work closely with the local administrations, councils and authorities and have them involved in the partnership or network. This is also important during the project design phase, and always before issuing the formal request. This will also ensure that the Cystoseira s.l. restoration project is compatible and beneficial to current coastal management plans, adding further support and acceptance to the restoration activity.
3.3.3 Legal
In addition to top-down legal approvals and permitting mentioned above, governments have legal restoration obligations to commitments from international treaties as well as under domestic legislation. There will also be legal obligations to compensate for planned environmental impacts (e.g., offsetting and compensatory habitat) or accidental impacts (polluter pays principle), both of which may be sources of funding for restoration work. Government or other authorities benefit from restoration works as these may be counted against national or regional targets. The EU recently chose a legislative approach (with the proposed Nature Restoration Law) to ensure the long-term objective of ecological restoration of terrestrial and marine ecosystems, that will include habitats characterised by different species of Cystoseira (EC, 2022). The law will be directly applicable and EU Member States are expected to draw national restoration plans to meet targets and obligations.
3.3.4 Policies
International policies may promote restoration action, but may not be either translated into national legislation or be specific. Examples of this may be the EU Biodiversity Strategy for 2030 that directs towards scheduled target values (percent restoration of overall or degraded habitats) by a specific date, but does not state what, neither where, nor how to restore. Achieving UN Sustainable Development Goals will also drive restoration through the need for mitigation of coastal erosion and protection of habitats that sustain fish stocks.
3.3.5 Mission and vision
The mission can be seen as the high-level target of the restoration action (related to the high-level provisions of the Convention on Biological Diversity (CBD)). Vision is the future desired condition of the restoration site we aim to achieve. Both should be clear and agreed within the Cystoseira s.l. restoration project and the network of those involved in it. They should be long-term and may be beyond the timescale of the actual restoration activity. Stakeholders desired outcomes should be translated into short, medium and long-term objectives (Gann et al., 2019).
3.3.6 Stakeholders
A stakeholder is a person, organisation or group with an interest (professional or societal), or an influence on the marine environment, or who is influenced directly or indirectly by activities and management decisions (Newton and Elliott, 2016). Stakeholder engagement helps define ecological goals, objectives, and methods of implementation and ensures that social needs are also being met (Gann et al., 2019). Stakeholders should be included at an early point into the participatory process. They may not have equal interests nor voices; hence, it is important to understand their aspirations and values, and balance them objectively (Wells et al., 2021), e.g., through Marine Stewardship processes (McAfee et al., 2022). Cystoseira s.l. restoration stakeholders may include amongst others, recreational users (beach/shore users, swimmers, snorkelers, divers, boaters, fishers), amateur and professional fishermen, funders, local businesses and hotels, local authorities, research institutions, universities, schools, restoration practitioners, conservation groups, local community groups and the general public.
3.3.7 Management
The restoration project should have a management board. Its job is to define the Cystoseira s.l. restoration project and implementation plans, seek planning approval or permits, undertake risk assessment, oversee the project work and budgets (including tendering and purchasing, employment and contracting, running costs), ensure networking, public engagement and communication. It should also ensure that the on-going work is checked, permits and approvals are compliant and that monitoring is completed to measure success (see sections “Success evaluation” and “Long-term monitoring and adaptive management”).
3.4 Finance
Projects require financing and this can be obtained from a number of different sources.
3.4.1 Funding
Much of the Cystoseira s.l. restoration work to date has been funded through local, National, or EU funding. Other possibilities include charitable donations, greening credentials for businesses (e.g., the IBEROSTAR Group responsible tourism initiative, https://www.grupoiberostar.com/en/sustainability/), crowdfunding and investment banks. Restoration is now being increasingly seen as an investment, not a cost, with benefits far outweighing those costs (WWF, 2021; EC, 2022).
3.4.2 Incentives
There is potential for restoration financing for improved ecosystem benefits from payment for ecosystem service (PES) schemes through common asset trusts (Canning et al., 2021). Although this is currently directed towards large scale terrestrial ecosystems, it may also benefit investment in opportunities for stakeholders from successful restoration (e.g., increased visitation for beach operators or income to coastal fisheries from improved fish stocks).
3.4.3 Restoration schemes
Interest may be expressed in future, concerning large-scale schemes for marine restoration following the terrestrial cases, for example, for large-scale reforestation (e.g., the Nature Conservancy’s Plant a Billion Trees campaign). This may apply at the sub-regional or regional level.
3.4.4 Investment opportunities
Investment opportunities might be identified in restoration initiatives e.g., development of new products and engineering solutions to facilitate restoration in shallow waters (e.g., DeFish algal canopy device (Gianni et al., 2020), Mars Assisted Reef Restoration System (www.buildingcoral.com), or Ecocean Biohut (www.ecocean.fr/)).
Marine protection through conservation and restoration is human driven, and a socio-ecological systems perspective is needed to sustainably perform it (Vergés et al., 2020). Social diversity and its relations are as important as species diversity in promoting ecosystem resilience. Only through common work among public administration, social agents, and forward-thinking companies can effective habitat conservation become possible. Industry holds high potential to become a driver for conservation and restoration, as has been proven by many natural capital evaluation exercises, showing that benefits of nature restoration are on average up to ten times higher than costs invested (Interreg-MPA Networks, 2021; WWF, 2021; EC, 2022). In addition, mobilising investment via corporate sponsorships and philanthropy, and understanding the different roles that funders can perform and where they fit into complex conservation networks is key in order to advance conservation goals (Blackwatters et al., 2022).
3.4.5 Blue carbon products and certifications
Blue carbon concerns carbon stored in coastal and marine ecosystems. This is a potential source of funding for blue carbon habitats and a rapidly evolving field with examples for seagrass in the UK and US (Gamble et al., 2021). It should be noted that whilst carbon is fixed and temporarily stored in Cystoseira s.l. forests, it is then exported (grazing, breakdown, physical removal to other areas). Future funding may be available for carbon rich habitats (e.g., in the Mediterranean for seagrasses (IUCN, 2022)).
4 Success evaluation
Usually, restoration initiatives first focus on the development of the target species (e.g. vegetation cover, density and biomass) (Figure 4, Box B). It is broadly assumed that this is the first step for ecosystem recovery, since other species should benefit from increase in structural complexity (Geist and Hawkins, 2016). Recovering the target species population is essential for the potential re-establishment of ecosystem processes and functions (Geist and Hawkins, 2016). However, the relationship between the recovery of a target (e.g., Cystoseira s.l. species) and the processes and functions of the ecosystem has to be empirically tested (Benayas et al., 2009; Moreno-Mateos et al., 2012; Crouzeilles et al., 2016). Therefore, the assessment of ecosystem processes and functions provided by Cystoseira s.l. forests should include quantifiable properties to describe the community in terms of structure alongside ecosystem functions (Montoya et al., 2012). To date, reported successful macroalgal forest restoration has mainly focused on the recovery of the canopy-forming species (Whitaker et al., 2010; Verdura et al., 2018; Fredriksen et al., 2020; Layton et al., 2020; Gran et al., 2022), and only a few studies have evaluated the re-establishment of associated species (Ling, 2008; Marzinelli et al., 2016; Galobart et al., 2023).
Forest restoration projects need to have clear, time-bound and meaningful objectives, based on which the indicators of restoration success can be specified (Stanturf et al., 2001). Monitoring of the selected indicators is based on rigorous sampling (the duration and periodicity of which will be species- and ecosystem-dependent) and reference conditions. The inclusion of multiple control sites is needed to assess the outcomes of the restoration action. Reference sites should be ecologically similar to the site selected for restoration interventions, except for the absence of anthropogenic pressures. In the case of Cystoseira s.l. forests, finding control sites in the same region may not be an easy task due to their important loss at the local scale. Reference sites may be available in MPAs, effectively protected and largely intact, but this is not generally the case for macroalgal forests in the Mediterranean Sea. In the absence of proper reference sites, reference conditions can be established on the basis of historical data or models (e.g. Thibaut et al., 2005; Fabbrizzi et al., 2023).
In general, success evaluation of marine restoration interventions is based on short-term periods (Bayraktarov et al., 2016; Kollmann et al., 2016; Fraschetti et al., 2021). Bearing in mind that the recovery of many marine ecosystems can take up to 15-25 years (Jones and Schmitz, 2009; Borja et al., 2010; Bekkby et al., 2020) and that canopy-forming macroalgae are mid- to long-lived species (Schiel and Foster, 2006; Smale et al., 2013), longer evaluations should be considered for reliable outputs (e.g., Gran et al. (2022) revisiting a site 10 years after re-introduction, reporting a 3 orders-of-magnitude increase in the extension of the forested area). The life span of most Cystoseira s.l. species is still unknown, and likely highly variable. More long-term ecological studies are needed to establish common protocols and indicators of Mediterranean forest restoration actions.
Restoration occurs as a succession of achievements. In the short-term, this involves the success of the action implemented (e.g., recovery of the target species and population). In the long-term, success is assessed through high level restoration goals, usually through ecosystem level indicators, such as ecosystem functions and services.
4.1 Success evaluation at target species level
The way in which a restoration intervention is considered “successful” is extremely heterogeneous. Bayraktarov et al. (2016) define a highly successful ecological restoration project as one where the restoration target was monitored for 5 years and achieved at least 85% survival of restored organisms for the entire mitigation area (Roebig et al., 2012). A restoration intervention is defined as a failure when the outcome corresponds to 10% or less survival of restored organisms. Fraschetti et al. (2021) identified three categories: success, partial success and failure. A highly successful ecological restoration project was defined as one where the restoration target achieved 50% survival of restored organisms for the entire intervention area. They defined restoration failure as an outcome of 10% survival of restored organisms. Partial success was assigned if the outcomes of the intervention were not consistent across the different metrics and species considered in the study. It is suggested to apply a threshold for restoration success over time, aiming for 50% after a short interval from the restoration actions, while expecting higher recovery rate over a longer monitoring period.
The type and quantity of indicators needed to assess the success are species- and context-dependent and considerable effort may be needed to measure them, particularly during the first phases after the restoration action. In the case of Cystoseira s.l. species, it is suggested that success should encompass the first reproductive cycle of the restored individuals, which constitutes the first step towards a self-sustainable population (Verdura et al., 2018). Based on commonly used indicators (De La Fuente et al., 2019; Tamburello et al., 2019; Orlando-Bonaca et al., 2021a; Medrano et al., 2020; Savonitto et al., 2021; Orlando-Bonaca et al., 2022; Clausing et al., 2023) and knowledge gained by the longest successful restoration action in the Mediterranean (restoration of Gongolaria barbata in Cala Teulera, Menorca, Verdura et al., 2018; Gran et al., 2022), a list of indicators is proposed in Table 2. Different attributes to be monitored or sampling periods may be needed for different Cystoseira s.l. species (e.g., different monitoring for deep, or wave-exposed species) and different sites (i.e., with shifted reproductive times at different localities), supported by both species- and site-specific pilot studies. Comparisons of adequate response variables before and after the restoration action, with respect to analogous comparisons in control populations are also needed (Table 2). If the restoration action is successful, as proven by the target-species level indicators, then the assessment of ecosystem-based indicators has to be implemented. If restoration cannot be considered successful, the potential cause of failure (Table 1), should be assessed and the possibility of mitigating them considered (see Figure 2, feedback to Step 2). If the cause of failure cannot be identified, cannot be mitigated or solved, the active restoration project should be discontinued.
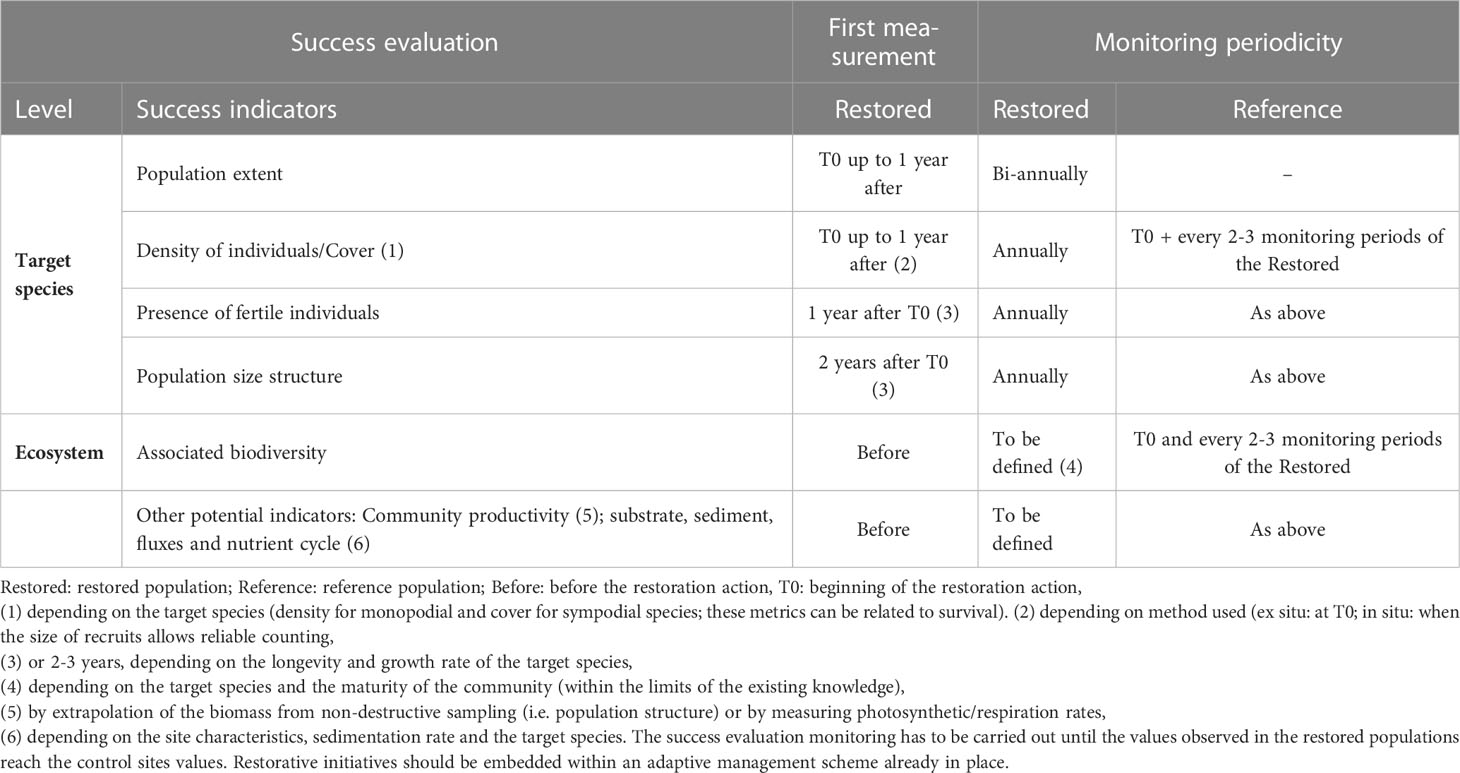
Table 2 Success evaluation indicators: Proposed indicators and monitoring periodicity have been mainly based on existing literature and on the Gongolaria barbata restoration performed in the Balearic Islands (Verdura et al., 2018; Gran et al., 2022; Galobart et al., 2023), but other short-term studies have also been considered (De La Fuente et al., 2019; Tamburello et al., 2019; Medrano et al., 2020; Orlando-Bonaca et al., 2021b; Savonitto et al., 2021; Orlando-Bonaca et al., 2022; Clausing et al., 2023).
4.2 Ecosystem level success evaluation
Assessing the success of a restoration action allows us to check if the ecosystem is on a trajectory towards full recovery. The need for mid- and long-term success assessment and monitoring must be acknowledged before the start of the project. Standard protocols should be developed so that different teams work consistently over time (Keenleyside et al., 2012).
Ecosystem level success evaluation reflects biodiversity and the delivery of goods and services (Table 2). Unfortunately, the goods and services provided by most Cystoseira s.l. forests have not been quantified yet. Indicators at the ecosystem level cannot be measured on a short term as measurable returns can only be expected after a certain time (species- and context-dependent) for the recovery of functions, while matching the analogous values in line with those from reference sites might take even longer.
The Society of Ecological Restoration provides a list of key attributes to support the identification of appropriate indicators, including six key ecosystem attributes to measure progress along a trajectory of recovery (Gann et al., 2019). Possibly due to the early developmental stage of marine restoration, success is still typically reported in terms of target species recovery. Recovery indicators, as stressed before, should be uniquely used for assessing the correct implementation of the restoration action and are not adequate to represent the overall project fulfilment, where success criteria are linked to the recovery of ecosystem function and services (Ruiz-Jaen and Aide, 2005; Bayraktarov et al., 2016). Fraschetti et al. (2021) found that survival of transplanted organisms, followed by growth measurements, were the most commonly-used metrics across marine studies. Ecological processes, for example, productivity, are not measured as frequently as measures of structure or diversity (but see Marzinelli et al., 2016). Conversely, in terrestrial environments, assessment techniques are predominantly based on variables such as biodiversity, vegetation structure, or ecological functions that can provide reliable information on ecosystem functioning services (Ruiz-Jaen and Aide, 2005; but see Marzinelli et al., 2016).
Increasing species diversity affects ecosystem processes, including (but not limited to) greater and more efficient use of limiting resources, higher stability against of disturbances, enhancement of primary and secondary production, and nutrient-cycling feedbacks that lead to larger nutrient storage (Tilman et al., 2014; Lefcheck et al., 2015; Strong et al., 2015). Another way to link biodiversity and ecosystem functions is provided by using functional traits (Garnier et al., 2004; McGill et al., 2006). Functional traits are determined by morphological, physiological, and biological characteristics of the different species and are considered relevant to ecosystem properties and services (Violle et al., 2007; Díaz et al., 2013). Using functional diversity indices (Mouillot et al., 2013; Teixidó et al., 2018) together with traditional taxonomic-based indices can provide a comprehensive evaluation of restoration projects (Cadotte et al., 2011; Montoya et al., 2012).
A further option for the assessment of functional recovery is the study of the different processes and fluxes that occur within the system, such as community productivity (i.e., biomass) and respiration (i.e., oxygen fluxes), carbon balance and nutrient cycling (Table 2; Ballesteros, 1989; Boyer et al., 2009; Sala et al., 2012; Miyajima and Hamaguchi, 2019; Peleg et al., 2020). There is limited research available that assesses the recovery capacity of Cystoseira s.l. forests, and among the available studies, their duration is at most 3.5 years (Milazzo et al., 2004; Piazzi and Ceccherelli, 2006; Sales et al., 2011; Bulleri et al., 2017). Similarly, the restoration efforts for Cystoseira s.l. forests in the Mediterranean Sea are still in their early stages, with insufficient restoration cases to enable long-term evaluations of success (but see Galobart et al., 2023). Consequently, our current knowledge is still insufficient to accurately determine the most appropriate indicators and their evaluation frequency for a comprehensive long-term restoration assessment. In spite of this, based on the first long-term success evaluation of a Cystoseira s.l. forest restoration (10 years; Galobart et al., 2023), the authors personal knowledge, and on indicators used from other benthic marine habitats (e.g., Christensen et al., 2004; Gamble et al., 2021), we propose in Table 2, potential indicators (e.g., associated biodiversity) that can be considered for the long-term success evaluation of Cystoseira s.l. forest restoration.
5 Long-term monitoring and adaptive management
When success is achieved at the ecosystem level (Table 2), the next step in the framework is long-term monitoring and adaptive management (Figure 4 Box C). Here, participatory monitoring should be implemented with the involved stakeholders and, as much as possible, in the framework of an ad hoc long-term program involving citizen science (Gann et al., 2019). Such monitoring, if based on scientific knowledge and robust yet simple methods, is often more beneficial and relevant for stakeholders than conventional scientific approaches (Gann et al., 2019).
An adaptive management approach, suggested in case of uncertainty about which management action is the more appropriate (and this is the case for several Cystoseira s.l. forests) could be based on timely monitoring and an iterative evaluation of results, as well as funding for ongoing restoration (Gann et al., 2019).
The indicators selected for monitoring should not be destructive or invasive, and this is particularly true for restored populations. If possible, they also have to be easy and rapid to assess (including by non-scientists), as well as time- and cost-effective. Target species and ecosystem level indicators (Table 2) and other biotic and abiotic factors potentially threatening the forest (Table 1) should be considered for long-term monitoring in the framework of adaptive management. As an example, the proliferation of herbivores (i.e. sea urchins or herbivorous fish) should be monitored, in order to anticipate the depletion of the restored forest.
Long-term monitoring will contribute to the knowledge of the functioning and evolution of the ecosystem and can point out where further interventions may be required. If during the implementation phase, long-term monitoring shows early warning signals such as a decrease in forest cover or density, an assessment of the causes of degradation (Table 1) should be immediately performed and intervention, mitigation or regulation actions considered.
In order to have a favourable restoration outcome but also to preserve Cystoseira s.l. forests in a good-moderate conservation status, the establishment of an MPA is a proper management tool, prompting increased awareness and better communication of the actions carried out, and the possibility to regulate those activities that could threaten the restored forests (e.g., fishing, trampling, beach management, anchoring). If well managed, MPAs reduce levels of human pressures, allowing long-term stabilisation of essential ecosystem processes, and fostering the resilience of marine communities, such as Cystoseira s.l. forests, to future disturbances (e.g., climate change) (Bevilacqua et al., 2022). MPAs usually offer important support services (e.g., video surveillance monitoring, patrolling, vessels, trained personnel), but their creation should not replace long-term monitoring: disturbance factors may also be present in protected zones, due to fluctuation of other species potentially interacting with the restored Cystoseira s.l. forests. MPA establishment may take considerable time from planning to implementation, and this should be taken into consideration when assessing the priority needs for action towards degraded area recovery.
6 Discussion, gaps and recommendations
New methodologies and techniques for the restoration of the Cystoseira s.l. have been developed within the last decade through viable programmes and with the know-how for all major steps now in place (Cebrian et al., 2021; Fabbrizzi et al., 2023). Improvements are still necessary in restoration protocols to ensure optimal success, particularly the refinement of propagule handling, the conditions for ensuring their viability and procedures for transplanting them into the field. Restoration upscaling is possible when the environment and the intensity of human impacts is compatible with restoration goals (e.g., Gran et al., 2022). Current knowledge gaps concern some of the less ‘well-known’ Mediterranean macroalgal species and their requirements for optimal survival and growth, as well as their historical and potential distributions. There are also some gaps on how to deal with stressors that hinder restoration success. All present and potential future impacts have to be assessed (Cebrian et al., 2021), particularly natural ones such as extreme climatic conditions and climate change, which may be solved with identification of future potential restoration areas, and the use of different species strains or more tolerant species (Verdura et al., 2021). More efforts to understand the role of grazers (fish and invertebrates, including mesograzers, Monserrat et al., 2023) in the control of macroalgal forests are also needed to define strategies for reducing grazing pressure to an adequate level, over which the restoration programme would not be viable.
One of the key restoration gaps is in linking existing top-down policy requirements and bottom-up initiatives (Ramírez-Monsalve et al., 2021). Policies give target percentages of degraded habitat to be restored, but do not state what, where and how to restore. In contrast, bottom-up initiatives are often promoted by scientists who have specific research interests and find an opportunity for science-based action (Smith et al., 2021). The proposed EU Nature Restoration Law is expected to partially fix this issue by specifying the need for Member States to restore target percentages of selected habitats that include various Cystoseira s.l. habitats. However, there need to be bodies that can coordinate, prioritise, facilitate and fund actions, whether this is part of a regional organisation (e.g. UNEP PAP-RAC, GFCM, Barcelona Convention), national or local authority. Funding could also be secured through these or other groups (e.g. EU LIFE projects including new projects in support of the EU Nature Restoration Law, national bodies), the prioritisation of the Global Environmental Facility (GEF) or industry, with the premise that restoration is an investment, rather than a cost. Funding also needs to consider the scale of the action and the length of time required to maintain a monitoring cycle covering long-term goals. Standardised restoration elements need to be listed and their cost realistically estimated to depict appropriate budgets enabling complete restoration action and for communicating the level of investment required for recovery of ecosystem services (Verdura et al., 2018; Friedrich et al., 2022). Restoration interacts synergistically with conservation (passive restoration) and can easily be linked to, for example, MPAs or other area-based measures. These help to fulfil the removal of human stressors. The difference between conservation and restoration sits within the intervention framework, with the main distinction between passive and active restoration lying primarily in the timing and extent of human interventions (Chazdon et al., 2021). In the EU, restoration should also be linked to Maritime Spatial Planning, and at the Mediterranean regional scale to the Integrated Coastal Zone Management Protocol and the United Nations Mediterranean Action Plan (UNEP-MAP and UNEP PAP/RAC) where restoration areas are allocated within spatial plans and highlighted for protection. Another mechanism that could be used in the region to prioritise restoration actions on Cystoseira s.l. concerns the designation of “Other Effective area-based Conservation Measures (OECMs)”, agreed under the 14th Conference of Parties of the Convention on Biological Diversity.
Publicity is important in providing a common understanding of the problems posed by Cystoseira s.l. degradation and loss. Understanding, valuing and communicating the societal benefits arising from healthy Cystoseira s.l. forests are essential to raise public awareness about their importance. Good and widespread publicity will drive public support, which in turn can drive institutional support for further actions. A coordination group could provide the backbone for efficient information. The message of restoration should be very clear, what is meant, what is feasible, what can be done and what is expected. Although restoration is about helping nature recover for the benefit of people and nature, the language of restoration has not always been clear. This has led to ambiguity and misunderstandings among stakeholders, whether restoration is achieved through protection and natural regeneration only, or restoration may also require various direct interventions (e.g., direct removal of grazers, substrate creation, transplantation). In most sites however, a range of protective and restorative actions will be required for the Cystoseira s.l. restoration (Gann et al., 2019; Chazdon et al., 2021).
In a broader context, Fabbrizzi et al. (2023) demonstrated that introducing systematic conservation planning principles and tools in restoration projects is crucial to understanding and defining how much and where an ecosystem or habitat can be recovered. These conservation planning principles and tools allow to effectively manage efforts and assess possibilities for setting region-specific targets. Adopting marine spatial planning leads to accounting for environmental constraints and socio-economic implications affecting restoration activities. The use of prioritisation software (e.g., MARXAN, Zonation 5) informs the allocation of restoration targets identified a priori, by combining spatial information from different sources. Future efforts should be directed to better integrate site prioritisation into marine spatial plans, accounting for ecological, social and economic objectives to enhance system resilience.
Author contributions
CS, JV, NP, LM, SF, EC, EF, MM, MD and SB contributed to the conception and design of the study. CS, JV, NP and LM led the writing of the manuscript with major contributions from SF, EC, EF, MM, MD and RD. LM, JV and MD provided the original decision-support schematic. JV prepared the manuscript figures and tables. All authors contributed to the revising of the manuscript and approved the submitted version.
Funding
The development of the framework was supported by the EU CINEA (ex-EASME) and EMFF agencies through funding of the project AFRIMED “Algal Forest Restoration in the Mediterranean Sea” (Grant Agreement No. 789059). CG was supported by FoRestA, Spanish Ministry of Science and Innovation (Grant/Award No. PID2020-112985GB-I00). MM was supported by a PhD grant funded by the Région Provence-Alpes-Côte D’Azur (Contract Emplois Jeunes Doctorants 2019–2022).
Acknowledgments
The authors would like to thank all the AFRIMED project participants through many discussions that led to the decision-support framework, in particular Laura Friedrich, Guilia Costa-Domingo and Megan Critchley of UNEP-WCMC, Jose Escaño Roepstorff of MEDGARDENS and Rosalba Giugni of MAREVIVO for useful discussions and critically reading the manuscript as well as Fabrizio Gianni of Université Côte d’Azur for useful exchanges on an early version of the decision tree. The authors would also like to thank the EU CINEA Project Officer Rocío Suárez Jiménez for fostering the work and insightful discussions. The manuscript was much improved during the reviewing process by the two reviewers and the topic editor Bernadette Pogoda, who are all gratefully acknowledged.
Conflict of interest
Author FJ is employed by Suez Consulting, an environmental consultancy company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Airoldi L. (1998). Roles of disturbance, sediment stress, and substratum retention on spatial dominance in algal turf. Ecology 79, 2759–2770. doi: 10.1890/0012-9658
Airoldi L. (2000). Responses of algae with different life histories to temporal and spatial variability of disturbance in subtidal reefs. Mar. Ecol. Prog. Ser. 195, 81–92. doi: 10.3354/meps195081
Airoldi L., Beck M. W. (2007). Loss, status and trends for coastal marine habitats of Europe. Oceanogr. Mar. Biol.: Annu. Rev. 45, 345–405. doi: 10.1201/9781420050943
Arai S. (2003). Eisenia bicyclis and Ecklonia cava. In Seaweeds and Marine Forests and its Developmental Technology. Ed. Notoya M. (Tokyo, Japan: Seizando-Shoten), 100–113.
Arévalo R., Pinedo S., Ballesteros E. (2007). Changes in the composition and structure of Mediterranean rocky-shore communities following a gradient of nutrient enrichment: Descriptive study and test of proposed methods to assess water quality regarding macroalgae. Mar. Pollut. Bull. 55, 104–113. doi: 10.1016/j.marpolbul.2006.08.023
Arrighi F. (1995). “Étude de la structure démographique des communautés de Cystoseira spinosa et d’un facies de surpâturage dans la Reserve Naturelle de Scandola (Corse),” in DEA Chimie de l’Environnement et Santé (Marseille:Université d’Aix-Marseille Université). 36pp.
Arrontes J., Arenas F., Fernández C., Rico J. M., Oliveros J., Martínez B., et al. (2004). Effect of grazing by limpets on mid-shore species assemblages in northern Spain. Mar. Ecol. Prog. Ser. 277, 117–133. doi: 10.3354/meps277117
Atkinson R. J., Thomas E., Roscioli F., Cornelius J. P., Zamora-Cristales R., Franco Chuaire M., et al. (2021). Seeding resilient restoration: An indicator system for the analysis of tree seed systems. Diversity 13, 367. doi: 10.3390/d13080370
Ballesteros E. (1988). Estructura y dinámica de la comunidad de Cystoseira mediterranea Sauvageau en el Mediterráneo noroccidental. Investigación Pesquera 52, 313–334.
Ballesteros E. (1989). Production of seaweeds in Northwestern Mediterranean marine communities: its relation with environmental factors. Sci. Mar. 53, 357–364.
Ballesteros E. (1990a). Structure and dynamics of the community of Cystoseira zosteroides (Turner) C. Agardh (Fucales, Phaeophyceae) in the Northwestern Mediterranean. Sci. Mar. 54, 217–229.
Ballesteros E. (1990b). Structure and dynamics of the Cystoseira caespitosa Sauvageau (Fucales, Phaeophyceae) community in the North-Western Mediterranean. Sci. Mar. 54, 155–168.
Ballesteros E., Garrabou J., Hereu B., Zabala M., Cebrian E., Sala E. (2009). Deep-water stands of Cystoseira zosteroides C. Agardh (Fucales, Ochrophyta) in the Northwestern Mediterranean: Insights into assemblage structure and population dynamics. Estuar. Coast. Shelf Sci. 82, 477–484. doi: 10.1016/j.ecss.2009.02.013
Ballesteros E., Hereu B., Zabala M., Alcoverro T., Garrabou J., Sala E. (2002). Rapport mission scandola 2000. Travaux Scientifiques du Parc Naturel Régional Corse 60, 95–115.
Ballesteros E., Mariani S., Cefalì M. E., Terradas M., Chappuis E. (2014). Manual dels hàbitats litorals de Catalunya. Generalitat de Catalunya (Barcelona: Departament de Territori i Sostenibilitat), 251.
Ballesteros E., Sala E., Garrabou J., Zabala M. (1998). Community structure and frond size distribution of a deep water stand of Cystoseira spinosa (Phaeophyta) in the Northwestern Mediterranean. Eur. J. Phycol. 33, 121–128. doi: 10.1080/09670269810001736613
Ballesteros E., Sant N. (2022). Homogeneity of photosynthetic features in canopy-forming macroalgae of the order Fucales from shallow and sheltered environments. Crypto. Algo. 43, 107–115. doi: 10.5252/cryptogamie-algologie2022v43a6
Ballesteros E., Torras X., Pinedo S., García M., Mangialajo L., de Torres M. (2007). A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Mar. Pollut. Bull. 55, 172–180. doi: 10.1016/j.marpolbul.2006.08.038
Ballesteros E., Zabala M. (1993). “El bentos: el marc físic. In: Història Natural de l'arxipèlag de Cabrera,” in Monografies de la Societat d’Història Natural de Balears, vol. 2 . Eds. Alcover J. A., Ballesteros E., Fornós J. J., 663–685. CSIC-Ed. Moll. Palma de Mallorca. (University of the Balearic Islands, Palma de Mallorca)
Bayraktarov E., Brisbane S., Hagger V., Smith C. S., Wilson K. A., Lovelock C. E., et al. (2020). Priorities and motivations of marine coastal restoration research. Front. Mar. Sci. 7, 484. doi: 10.3389/fmars.2020.00484
Bayraktarov E., Saunders M. I., Abdullah S., Mills M., Beher J., Possingham H. P., et al. (2016). The cost and feasibility of marine coastal restoration. Ecol. Appl. 26 (4), 1055–1074. doi: 10.1890/15-1077
Beheshti K., Ward M. (2021). Eelgrass Restoration on the U.S. West Coast: A Comprehensive Assessment of Restoration Techniques and Their Outcomes. Prepared for the Pacific Marine and Estuarine Fish Habitat Partnership Available at: http://honu.psmfc.org/media/PMEP/Eelgrass_Restoration_Synthesis/Documents/PMEP_Beheshti_Ward_2021_EelgrassSynthesisReport.pdf.
Bekkby T., Papadopoulou N., Fiorentino D., McOwen C. J., Rinde E., Bostrom C., et al. (2020). Habitat features and their influence on the restoration potential of marine habitats in Europe. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00184
Bellan-Santini D. (1965). Étude quantitative du peuplement a Mytilus galloprovincialis Lamarck en eau moyennement polluée. Rapports Commission Int. la Mer Méditerranée 18, 85–89.
Benayas J. M. R., Newton A. C., Diaz A., Bullock J. M. (2009). Enhancement of biodiversity and ecosystem services by ecological restoration: a meta-analysis. Science 325 (5944), 1121–1124. doi: 10.1126/science.1172460
Benedetti-Cecchi L., Cinelli F. (1996). Patterns of disturbance and recovery in littoral rock pools: non-hierarchical competition and spatial variability in secondary succession. Mar. Ecol. Prog. Ser. 135, 145–161. doi: 10.3354/meps135145
Bernal-Ibáñez A., Gestoso I., Wirtz P., Kaufman M., Serrão E. A., Canning-Clode J., et al. (2021). The collapse of marine forests: drastic reduction in populations of the family Sargassaceae in Madeira Island (NE Atlantic). Regional Environ. Change 21 (3), 71. doi: 10.1007/s10113-021-01801-2
Bevilacqua S., Vellani V., Fabbrizio P., Falace A., Ciriaco S., Segarich M., et al. (2022). Multidecadal monitoring highlighted long-term stability of protected assemblages within a Mediterranean marine reserve. Estuar. Coast. Shelf Sci. 274, 107946. doi: 10.1016/j.ecss.2022.107946
Bianchelli S., Danovaro R. (2020). Impairment of microbial and meiofaunal ecosystem functions linked to algal forest loss. Sci. Rep. 10 (1), 19970. doi: 10.1038/s41598-020-76817-5
Bianchi C. N., Corsini-Foka M., Morri C., Zenetos A. (2014). Thirty years after; dramatic change in the coastal marine habitats of Kos Island (Greece), 1981-2013. Mediterr. Mar. Sci. 14, 482–497. doi: 10.12681/mms.678
Blackwatters J. E., Betsill M., Enrici A., Le Cornu E., Basurto X., Gruby R. L. (2022). More than funders: The roles of philanthropic foundations in marine conservation governance. Conserv. Sci. Pract. 5, e12829. doi: 10.1111/csp2.12829
Blanfuné A., Boudouresque C. F., Verlaque M., Thibaut T. (2016). The fate of Cystoseira crinita, a forest-forming Fucale (Phaeophyceae, Stramenopiles), in France (North Western Mediterranean Sea). Estuar. Coast. Shelf Sci. 181, 196–208. doi: 10.1016/j.ecss.2016.08.049
Blignaut J., Esler K. J., de Wit M. P., Le Maitre D., Milton S. J., Aronson J. (2013). Establishing the links between economic development and the restoration of natural capital. Curr. Opin. Environ. Sustain. 5 (1), 94–101. doi: 10.1016/j.cosust.2012.12.003
Borja Á., Dauer D. M., Elliott M., Simenstad C. A. (2010). Medium-and long-term recovery of estuarine and coastal ecosystems: patterns, rates and restoration effectiveness. Estuaries Coasts 33 (6), 1249–1260. doi: 10.1007/s12237-010-9347-5
Boudouresque C. F. (1972). Recherches de bionomie analytique, structurale et expérimentale sur les peuplements benthiques sciaphiles de Méditerranée Occidentale: le sous-strate sciaphile d’un peuplement photophile de mode calme, le peuplement à Cystoseira crinita. Bull. du Muséum d’histoire naturelle Marseille 32, 253–263.
Boyer K. E., Kertesz J. S., Bruno J. F. (2009). Biodiversity effects on productivity and stability of marine macroalgal communities: the role of environmental context. Oikos 118 (7), 1062–1072. doi: 10.1111/j.1600-0706.2009.17252.x
Bulleri F., Benedetti-Cecchi L., Acunto S., Cinelli F., Hawkins S. J. (2002). The influence of canopy algae on vertical patterns of distribution of low-shore assemblages on rocky coasts in the northwest Mediterranean. J. Exp. Mar. Biol. Ecol. 267, 89–106. doi: 10.1016/S0022-0981(01)00361-6
Bulleri F., Benedetti-Cecchi L., Ceccherelli G., Tamburello L. (2017). A few is enough: a low cover of a non-native seaweed reduces the resilience of Mediterranean macroalgal stands to disturbances of varying extent. Biol. Invasions 19, 2291–2305. doi: 10.1007/s10530-017-1442-0
Buonomo R., Chefaoui R. M., Bermejo R., Engelen A. H., Serrão E. A., Airoldi L. (2018). Predicted extinction of unique genetic diversity in marine forests of Cystoseira spp. Mar. Environ. Res. 138, 119–128. doi: 10.1016/j.marenvres.2018.04.013
Cadotte M. W., Carscadden K., Mirotchnick N. (2011). Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 48 (5), 1079–1087. doi: 10.1111/j.1365-2664.2011.02048.x
Canning A. D., Jarvis D., Costanza R., Hasan S., Smart J. C. R., Finisdore J., et al. (2021). Financial incentives for large-scale wetland restoration: Beyond markets to common asset trusts. One Earth 4 (7), 937–950. doi: 10.1016/j.oneear.2021.06.006
Capdevila P., Hereu B., Riera J. L., Linares C. (2016). Unravelling the natural dynamics and resilience patterns of underwater Mediterranean forests: Insights from the demography of the brown alga Cystoseira zosteroides. J. Ecol. 104, 1799–1808. doi: 10.1111/1365-2745.12625
Capdevila P., Hereu B., Salguero-Gómez R., Rovia G., Medrano A., Cebrian E., et al. (2018b). Warming impacts on early life stages increase the vulnerability and delay the population recovery of a long-lived habitat-forming macroalga. J. Ecol. 107, 1129–1140. doi: 10.1111/1365-2745.13090
Capdevila P., Linares C., Aspillaga E., Navarro L., Kersting D. K., Hereu B. (2015). Recruitment patterns in the Mediterranean deep-water alga Cystoseira zosteroides. Mar. Biol. 162 (6), 1165–1174. doi: 10.1007/s00227-015-2658-0
Capdevila P., Linares C., Aspillaga E., Riera J. L., Hereu B. (2018a). Effective dispersal and density-dependence in mesophotic macroalgal forests: Insights from the Mediterranean species Cystoseira zosteroides. PloS One 13 (1), e0191346. doi: 10.6084/m9.figshare.5743221
Cardona L., Moranta J., Reñones O., Hereu B. (2013). Pulses of phytoplanktonic productivity may enhance sea urchin abundance and induce state shifts in Mediterranean rocky reefs. Estuar. Coast. Shelf Sci. 133, 88–96. doi: 10.1016/j.ecss.2013.08.020
Catucci E., Buonocore E., Franzese P. P., Scardi M. (2022). Assessing the natural capital value of Posidonia oceanica meadows in the Italian seas by integrating habitat suitability and environmental accounting models. ICES J. Mar. Sci. 2022, 34. doi: 10.1093/icesjms/fsac034
Cebrian E., Tamburello L., Verdura J., Guarnieri G., Medrano A., Linares C., et al. (2021). A roadmap for the restoration of Mediterranean macroalgal forests. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.709219
Cefalì M. E., Ballesteros E., Riera J. L., Chappuis E., Terradas M., Mariani S., et al. (2018). The optimal sampling design for littoral habitats modelling: a case study in the north-western Mediterranean. PloS One 13 (5), e0197234. doi: 10.1371/journal.pone.0197234
Cefalì M. E., Cebrian E., Chappuis E., Pinedo S., Terradas M., Mariani S., et al. (2016). Life on the boundary: environmental factors as drivers of habitat distribution in the littoral zone. Estuar. Coast. Shelf Sci. 172, 81–92. doi: 10.1016/j.ecss.2016.01.043
Celis-Plá P. S. M., Martínez B., Korbee N., Hall-Spencer J. M., Figueroa F. L. (2017). Ecophysiological responses to elevated CO2 and temperature in Cystoseira tamariscifolia (Phaeophyceae). Climatic Change 142, 67–81. doi: 10.1007/s10584-017-1943-y
Chapman A. R. O. (1995). Functional ecology of fucoid algae: twenty-three years of progress. Phycologia 34, 1–32. doi: 10.2216/i0031-8884-34-1-1.1
Chappuis E., Terradas M., Cefalì M. E., Mariani S., Ballesteros E. (2014). Vertical zonation is the main distribution pattern of littoral assemblages on rocky shores at a regional level. Estuar. Coast. Shelf Sci. 147, 113–122. doi: 10.1016/j.ecss.2014.05.031
Chazdon R. L., Falk D. A., Banin L. F., Wagner M., Wilson S. J., Grabowski R. C., et al. (2021). The intervention continuum in restoration ecology: rethinking the active–passive dichotomy. Restor. Ecol., e13535. doi: 10.1111/rec.13535
Cheminée A., Pastor J., Bianchimani O., Thiriet P., Sala E., Cottalorda J. M., et al. (2017). Juvenile fish assemblages in temperate rocky reefs are shaped by the presence of macro-algae canopy and its three dimensional structure. Sci. Rep. 7, 14638. doi: 10.1038/s41598-017-15291-y
Cheminée A., Sala E., Pastor J., Bodilis P., Thiriet P., Mangialajo L., et al. (2013). Nursery value of Cystoseira forests for Mediterranean rocky reef fishes. J. Exp. Mar. Biol. Ecol. 442, 70–79. doi: 10.1016/j.jembe.2013.02.003
Christensen P. B., Diaz Almela E. D., Diekmann O. (2004). “Can transplanting accelerate the recovery of seagrasses,” in European seagrasses: an introduction to monitoring and management. Eds. Borum J., Duarte C. M., Krause-Jensen D., Greve T. M. (The M&MS project), 77–82.
Cigliano J. A., Meyer R., Ballard H. L., Freitag A., Phillips T. B., Wasser A. (2015). Making marine and coastal citizen science matter. Ocean Coast. Manage. 115, 77–87. doi: 10.1016/j.ocecoaman.2015.06.012
Clausing R. J., de la Fuente G., Falace A., Chiantore M. (2023). Accounting for environmental stress in restoration of intertidal foundation species. J. Appl. Ecol. 60, 305–318. doi: 10.1111/1365-2664.14334
Coleman M. A., Wood G., Filbee-Dexter K., Minne A. J., Goold H. D., Vergés A., et al. (2020). Restore or redefine: Future trajectories for restoration. Front. Mar. Sci. 7, 237. doi: 10.3389/fmars.2020.00237
Cormaci M., Furnari G. (1999). Changes of the benthic algal flora of the Tremiti Islands (southern Adriatic) Italy. Hydrobiologia 398/399, 75–79. doi: 10.1007/978-94-011-4449-0_9
Cornwall C. E., Hurd C. L. (2019). Variability in the benefits of ocean acidification to photosynthetic rates of macroalgae without CO2-concentrating mechanisms. Mar. Freshw. Res. 71 (3), 275–280. doi: 10.1071/MF19134
Costa-Domingo G. M., Critchley M., Vukelić J., Gosling J., Friedrich L. (2022). Assessment of ecosystem change, recovery success and ecosystem service change following restoration. AFRIMED Project Deliverable 4.3 https://afrimed-project.eu/?page_id=878.
Crouzeilles R., Curran M., Ferreira M. S., Lindenmayer D. B., Grelle C. E., Benayas J. M. R. (2016). A global meta-analysis on the ecological drivers of forest restoration success. Nat. Commun. 7 (1), 11666. doi: 10.1038/ncomms11666
Cvitanovic C., Hobday A. (2018). Building optimism at the environmental science-policy-practice interface through the study of bright spots. Nat. Commun. 9, 3466. doi: 10.1038/s41467-018-05977-w
de Caralt S., Verdura J., Vergés A., Ballesteros E., Cebrian E. (2020). Differential effects of pollution on adult and recruits of a canopy-forming alga: implications for population viability under low pollutant levels. Sci. Rep. 10, 17825. doi: 10.1038/s41598-020-73990-5
De La Fuente G., Chiantore M., Asnaghi V., Kaleb S., Falace A. (2019). First ex situ outplanting of the habitat-forming seaweed Cystoseira amentacea var. stricta from a restoration perspective. PeerJ 7, e7290. doi: 10.7717/peerj.7290
De La Fuente G., Chiantore M., Gaino F., Asnaghi V. (2018). Ecological status improvement over a decade along the Ligurian coast according to a macroalgae based index (CARLIT). PloS One 13 (12), e0206826. doi: 10.1371/journal.pone.0206826
Delgado O., Rodríguez-Prieto C., Frigola L., Ballesteros E. (1995). Drought tolerance and light requirements on high and low sublittoral species of Mediterranean macroalgae of the genus Cystoseira C. Agardh (Fucales, Phaeophyceae). Botanica Marina 38, 127–132. doi: 10.1515/botm.1995.38.1-6.127
Devescovi M., Iveša L. (2007). Short term impact of planktonic mucilage aggregates on macrobenthos along the Istrian rocky coast (Northern Adriatic, Croatia). Mar. Pollut. Bull. 54 (7), 887–893. doi: 10.1016/j.marpolbul.2007.03.009
Díaz S., Purvis A., Cornelissen J. H., Mace G. M., Donoghue M. J., Ewers R. M., et al. (2013). Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecol. Evol. 3 (9), 2958–2975. doi: 10.1002/ece3.601
EC (2022). Proposal for a Regulation of the European Parliament and of the Council on Nature restoration. Brussels, 22.6.2022, COM, (2022) 304 final. (European Commission, Brussels).
Edwards A. J., Gómez E. D. (2007). Reef Restoration Concepts and Guidelines: making sensible management choices in the face of uncertainty (St Lucia, Australia: Coral Reef Targeted Research & Capacity Building for Management Programme), 38.
Eger A. M., Earp H. S., Friedman K., Gatt Y., Hagger V., Hancock B., et al. (2022b). The need, opportunities, and challenges for creating a standardized framework for marine restoration monitoring and reporting. Biol. Conserv. 266, 109429. doi: 10.1016/j.biocon.2021.109429
Eger A. M., Layton C., McHugh T. A., Gleason M., Eddy N. (2022c). Kelp Restoration Guidebook: Lessons Learned from Kelp Projects Around the World (Arlington, VA, USA: The Nature Conservancy).
Eger A. M., Marzinelli E., Christie H., Fagerli D. C., Fujita D., Gonzalez A. P., et al. (2022a). Global kelp forest restoration: Past lessons, status, and future directions. Biol. Rev. 97 (4), 1449–1475. doi: 10.1111/brv.12850
Elliott M., Burdon D., Hemingway K. L., Apitz S. E. (2007). Estuarine, coastal and marine ecosystem restoration: confusing management and science - a revision of concepts. Estuar. Coast. Shelf Sci. 74, 349–366. doi: 10.1016/j.ecss.2007.05.034
Ercegović A. (1952). Sur les Cystoseira adriatiques. Leur morphologie, écologie et évolution (Institut za Oceanografiju i Ribarstvo FNR Jugoslavije publisher). Split: 212 pp + 30 plates +1 map.
Escovar-Fadul X., Hein M. Y., Garrison K., McLeod E., Eggers M., Comito F. (2022). A Guide to Coral Reef Restoration for the Tourism Sector: Partnering with Caribbean Tourism Leaders to Accelerate Coral Restoration (Arlington, VA, USA: The Nature Conservancy).
Fabbrizzi E., Giakoumi S., De Leo F., Tamburello L., Chiarore A., Colletti A., et al. (2023). The challenge of setting restoration targets for macroalgal forests under climate changes. J. Environ. Manage. 326, 116834. doi: 10.1016/j.jenvman.2022.116834
Fabbrizzi E., Scardi M., Ballesteros E., Benedetti-Cecchi L., Emma C., Ceccherelli G., et al. (2020). Modeling macroalgal forest distribution at Mediterranean scale: Present status, drivers of changes and insights for conservation and management. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00020
Falace A., Kaleb S., de la Fuente G., Asnaghi V., Chiantore M. (2018). Ex situ cultivation protocol for Cystoseira amentacea var. stricta (Fucales, Phaeophyceae) from a restoration perspective. PloS One 13, e0193011. doi: 10.1371/journal.pone.0193011
Falace A., Marletta G., Savonitto G., Candotto Carniel F., Srijemsi M., Bevilacqua S., et al. (2021). Is the South-Mediterranean canopy-forming Ericaria giacconei (= Cystoseira hyblaea) a loser from ocean warming? Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.760637
Falace A., Zanelli E., Bressan G. (2006). Algal transplantation as a potential tool for artificial reef management and environmental mitigation. Bull. Mar. Sci. 78, 161–166.
FAO, IUCN, CEM, SER (2021). Principles for ecosystem restoration to guide the United Nations Decade (Rome: FAO), 2021–2030.
Feldmann J. (1937). Recherches sur la végétation marine de la Méditerranée: la côte des Albères. Rev. Algologique 10, 1–340.
Filbee-Dexter K., Wernberg T. (2018). Rise of turfs: A new battlefront for globally declining kelp forests. BioScience 68, 64–76. doi: 10.1093/biosci/bix147
Firth L. B., Mieszkowska N., Thompson R. C., Hawkins S. J. (2013). Climate change and adaptational impacts in coastal systems: the case of sea defences. Environ. Sci.: Processes Impacts 15, 1665. doi: 10.1039/c3em00313b
Fitzsimons J., Branigan S., Brumbaugh R. D., McDonald T., Zu Ermgassen P. S. E. (2019). Restoration Guidelines for Shellfish Reefs (Arlington VA, USA: The Nature Conservancy).
Fraschetti S., McOwen C., Papa L., Papadopoulou N., Bilan M., Boström C., et al. (2021). Where is more important than how in coastal and marine ecosystems restoration. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.626843
Fraschetti S., Terlizzi A., Guarnieri G., Pizzolante F., D’Ambrosio P., Maiorano P., et al. (2011). Effects of unplanned development on marine biodiversity: A lesson from Albania (Central Mediterranean Sea). J. Coast. Res. 10058, 106–115. doi: 10.2112/SI
Fredriksen S., Filbee-Dexter K., Norderhaug K. M., Steen H., Bodvin T., Coleman M. A., et al. (2020). Green gravel: a novel restoration tool to combat kelp forest decline. Sci. Rep. 10 (1), 3983. doi: 10.1038/s41598-020-60553-x
Fremout T., Gutiérrez-MIranda C. E., Briers S., Marcelo-Peña J. L., Cueva-Ortiz E., Linares-Palomino R., et al. (2021b). The value of local ecological knowledge to guide tree species selection in tropical dry forest restoration. Restor. Ecol. 29 (4), e13347. doi: 10.1111/rec.13347
Fremout T., Thomas E., Bocanegra-González K. T., Aguirre-Morales C. A., Morillo-Paz A. T., Atkinson R., et al. (2021a). Dynamic seed zones to guide climate-smart seed sourcing for tropical dry forest restoration in Colombia. For. Ecol. Manage. 490, 119127. doi: 10.1016/j.foreco.2021.119127
Friedrich L. S., King G., Costa Domingo G. M., Critchley M. (2022). Assessment of the social and economic benefits of changing ecosystem services generated by restoration. AFRIMED Project Deliverable, 4.4. 7.1 pp. Available at: http://afrimed-project.eu/?page_id=878.
Galobart C., Ballesteros E., Golo R., Cebrian E. (2023). Addressing marine restoration success: Evidence of species and functional diversity recovery in a ten-year restored macroalgal forest. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1176655
Gamble C., Debney A., Glover A., Bertelli C., Green B., Hendy I., et al. (2021). Seagrass Restoration Handbook (London, UK: Zoological Society of London, UK.).
Gann G. D., McDonald T., Walder B., Aronson J., Nelson C. R., Jonson J., et al. (2019). International principles and standards for the practice of ecological restoration. Second edition. Restor. Ecol. 27, S1–S46. doi: 10.1111/rec.13035
Garnier E., Cortez J., Billes G., Navas M.-L., Roumet C., Debussche M., et al. (2004). Plant functional markers capture ecosystem properties during secondary succession. Ecology 85, 2630–2637. doi: 10.1890/03-0799
Geist J., Hawkins S. J. (2016). Habitat recovery and restoration in aquatic ecosystems: current progress and future challenges. Aquat. Conserv.: Mar. Freshw. Ecosyst. 26 (5), 942–962. doi: 10.1002/aqc.2702
Giaccone G. (1973). Écologie et chorologie des Cystoseira de Méditerranée. Rapports la Communauté Internationale Mer Méditerranée 22, 49–50.
Giaccone G., Bruni A. (1973). Le Cystoseire e la vegetazione sommersa del Mediterraneo. Atti Istituto Veneto Scienze Lettere ed Arti 131, 59–103.
Giakoumi S. (2014). Distribution patterns of the invasive herbivore Siganus luridus (Rüppell 1829) and its relation to native benthic communities in the central Aegean Sea, Northeastern Mediterranean. Mar. Ecol. 35, 96–105. doi: 10.1111/maec.12059
Gianni F., Bartolini F., Airoldi L., Ballesteros E., Francour P., Guidetti P., et al. (2013). Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of Marine Protected Areas. Adv. Oceanogr. Limnol. 4, 83–101. doi: 10.1080/19475721.2013.845604
Gianni F., Bartolini F., Airoldi L., Mangialajo L. (2018). Reduction of herbivorous fish pressure can facilitate focal algal species forestation on artificial structures. Mar. Environ. Res. 138, 102–109. doi: 10.1016/j.marenvres.2018.04.007
Gianni F., Bartolini F., Pey A., Laurent M., Martins G. M., Airoldi L., et al. (2017). Threats to large brown algal forests in temperate seas: the overlooked role of native herbivorous fish. Sci. Rep. 7 (1), 6012. doi: 10.1038/s41598-017-06394-7
Gianni F., Mačić V., Bartolini F., Pey A., Laurent M., Mangialajo L. (2020). Optimizing canopy-forming algae conservation and restoration with a new herbivorous fish deterrent device. Restor. Ecol. 28, 750–756. doi: 10.1111/rec.13143
Goergen E. A., Schopmeyer S., Moulding A. L., Moura A., Kramer P., Viehman T. S. (2020). “Coral reef restoration monitoring guide: Methods to evaluate restoration success from local to ecosystem scales,” in NOAA Technical Memorandum NOS NCCOS 279 (Silver Spring, MD). 145 pp. doi: 10.25923/xndz-h538
Gran A., Movilla J., Ballesteros E., Sales M., Bolado I., Galobart C., et al. (2022). Assessing the expansion and success of a restored population of Gongolaria barbata (Stackhouse) Kuntze (Fucales, Phaeophyceae) using high-precision positioning tools and size distribution frequencies. Mediterr. Mar. Sci. 23, 907–916. doi: 10.12681/mms.30500
Gros C. (1978). Le genre Cystoseira sur la côte des Albères. Répartition, écologie, morphogénèse (Paris: Université Pierre et Marie Curie). PhD Thesis115 pp.
Guarnieri G., Bevilacqua S., Figueras N., Tamburello L., Fraschetti S. (2020). Large-scale sea urchin culling drives the reduction of subtidal barren grounds in the Mediterranean Sea. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00519
Guidetti P. (2006). Marine reserves reestablish lost predatory interactions and cause community changes in rocky reefs. Ecol. Interact. 16 (3), 963–976. doi: 10.1890/1051-0761(2006)016[0963:MRRLPI]2.0.CO;2
Gunnarsson K., Berglund A. (2012). The brown alga Fucus radicans suffers heavy grazing by the isopod Idotea baltica. Mar. Biol. Res. 8, 87–89. doi: 10.1080/17451000.2011.594890
Hein M. Y., McLeod I. M., Shaver E. C., Vardi T., Pioch S., Boström-Einarsson L., et al. (2020). Coral Reef Restoration as a strategy to improve ecosystem services. A guide to coral restoration methods (Nairobi, Kenya: United Nations Environment Program).
Hereu B., Mangialajo L., Ballesteros E., Thibaut T. (2008). On the occurrence, structure and distribution of deep-water Cystoseira (Phaeophyceae) populations in the Port-Cros National Park (northwestern Mediterranean). Eur. J. Phycol. 43, 263–273. doi: 10.1080/09670260801930330
Hillebrand H., Jacob U., Leslie H. M. (2020). Integrative research perspectives on marine conservation. Philos. Trans. R. Soc. B 375 (1814), 20190444. doi: 10.1098/rstb.2019.0444
Hoffman L., Clarisse S., Detienne X., Goffart A., Renard R., Demoulin V. (1988). Evolution of the populations of Cystoseira balearica (Phaeophycae) and epiphytic Bangiophyceae in the Bay of Calvi (Corsica) in the last eight years. Bull. Societé Royale Liège 4–5, 263–273.
Hong S., Kim J., Ko Y. W., Yang K. M., Macias D., Kim J. H. (2021). Effects of sea urchin and herbivorous gastropod removal, coupled with transplantation, on seaweed forest restoration. Botanica Marina 64, 438. doi: 10.1515/bot-2021-0043
Hudson R., Kenworthy J., Best M. (2021). Saltmarsh Restoration Handbook: UK and Ireland (Bristol, UK: Environment Agency).
Huvé H. (1960). Résultats sommaires de l’étude expérimentale de la réinstallation d’un peuplement à Cystoseira stricta (Mont.) Sauv. Rapport procès-verbal Des. réunions Commission internationale pour l’exploration scientifique la Mer Méditerranée 15, 121–125.
ICRI (2018). Mangrove Restoration: the key elements to be considered in any restoration project. Technical guide, Pôle-relais zones humides tropicales (Basse Terre, Guadeloupe: Guadeloupe), 32.
Interreg-MPA Networks (2021) Natural capital accounting pilot study in a protected marine area in the Balearic Islands. Partners: Fundació Marilles, CentroBalear de Biología Aplicada, S. L. (CBBA), Ecoacsa Reserva de Biodiversidad, S. L., eftec, MEDPAN. Available at: https://marilles.org/storage/media/2021/10/917/deliverable-ii-natural-capital-accounting-pilot-study-in-balearic-islands-mpa.pdf.
Irving A. D., Balata D., Colosio F., Ferrando G. A., Airoldi L. (2009). Light, sediment, temperature, and the early life-history of the habitat-forming alga Cystoseira barbata. Mar. Biol. 156, 1223–1231. doi: 10.1007/s00227-009-1164-7
IUCN (2022). Available at: https://www.iucn.org/news/mediterranean/202201/private-companies-can-now-participate-development-blue-carbon-projects-andalusia-Spain.
Iveša L., Djakovac T., Devescovi M. (2016). Long-term fluctuations in Cystoseira populations along the west Istrian Coast (Croatia) related to eutrophication patterns in the northern Adriatic Sea. Mar. Pollut. Bull. 106, 162–173. doi: 10.1016/j.marpolbul.2016.03.010
Jones H. P., Schmitz O. J. (2009). Rapid recovery of damaged ecosystems. PloS One 4 (5), e5653. doi: 10.1371/journal.pone.0005653
Kearney M., Porter W. (2009). Mechanistic niche modelling: combining physiological and spatial data to predict species' ranges. Ecol. Lett. 12, 334–350. doi: 10.1111/j.1461-0248.2008.01277.x
Keenleyside K. A., Dudley N., Cairns S., Hall C. M., Stolton S. (2012). Ecological Restoration for Protected Areas: Principles, Guidelines and Best Practices (Gland, Switzerland: IUCN), 120.
Kelly R., Fleming A., Pecl G. T., von Gönner J., Bonn A. (2020). Citizen science and marine conservation: a global review. Philos. Trans. R. Soc. B 375 (1814), 20190461. doi: 10.1098/rstb.2019.0461
Kimball S., Lulo M., Sorenson Q., Balazs K., Fang Y.-C., Davis S. J., et al. (2015). Cost-effective ecological restoration. Restor. Ecol. 23 (6), 800–810. doi: 10.1111/rec.12261
Kollmann J., Meyer S. T., Bateman R., Conradi T., Gossner M. M., de Souza Mendonça M. Jr., et al. (2016). Integrating ecosystem functions into restoration ecology - recent advances and future directions. Restor. Ecol. 24 (6), 722–730. doi: 10.1111/rec.12422
Lardi P. I., Varkitzi I., Tsiamis K., Orfanidis S., Koutsoubas D., Falace A., et al. (2022). Early development of Gongolaria montagnei (Fucales, phaeophyta) germlings under laboratory conditions, with a view to enhancing restoration potential in the Eastern Mediterranean. Botanica Marina 65 (4), 279–287. doi: 10.1515/bot-2021-0105
Layton C., Coleman M. A., Marzinelli E. M., Steinberg P. D., Swearer S. E., Vergés A., et al. (2020). Kelp forest restoration in Australia. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00074
Lefcheck J. S., Byrnes J. E., Isbell F., Gamfeldt L., Griffin J. N., Eisenhauer N., et al. (2015). Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats. Nat. Commun. 6 (1), 1–7. doi: 10.1038/ncomms7936
Lejeusne C., Chevaldonné P., Pergent-Martini C., Boudouresque C. F., Pérez T. (2010). Climate change effects on a miniature ocean: the highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 25, 250–260. doi: 10.1016/j.tree.2009.10.009
Leocadie A., Pioch S., Pinault M. (2020). Guide to Ecological Engineering: The restoration of coral reefs and associated ecosystems (Published by IFRECOR), 114. Available at: https://icriforum.org/guide-to-ecological-engineering-the-restoration-of-coral-reefs-and-associated-ecosystems/.
Leonard D., Macfarlane S. (2011). Best management practices for shellfish restoration (ISSC Shellfish Restoration Committee), 90. Available at: https://www.issc.org/Data/Sites/1/media/publications/final%20draft%20bmps-01-23-12.pdf.
Ling S. D. (2008). Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: a new and impoverished reef state. Oecologia 156 (4), 883–894. doi: 10.1007/s00442-008-1043-9
Lucia P., Grech D., Buia M. C. (2020). Long-term changes, (1800–2019) in marine vegetational habitats: Insights from a historic industrialised coastal area. Mar. Environ. Res. 161, 105003. doi: 10.1016/j.marenvres.2020.105003
Mangialajo L., Chiantore M., Cattaneo-Vietti R. (2008a). Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar. Ecol. Prog. Ser. 358, 63–74. doi: 10.3354/meps07400
Mangialajo L., Chiantore M., Cattaneo-Vietti R. (2008b). Changes in marine macroalgal assemblages due to human impacts: general trends and applications in coastal management. Biol. Marina Mediterr. 15 (1), 115–117.
Mariani S., Cefalì M. E., Chappuis E., Terradas M., Pinedo S., Torras X., et al. (2019). Past and present of Fucales from shallow and sheltered shores in Catalonia. Regional Stud. Mar. Sci. 32, 100824. doi: 10.1016/j.rsma.2019.100824
Martínez B., Arenas F., Trilla A., Viejo R. M., Carreño F. (2015). Combining physiological threshold knowledge to species distribution models is key to improving forecasts of the future niche for macroalgae. Global Change Biol. 21 (4), 1422–1433. doi: 10.1111/gcb.12655
Martín García L., Rancel-Rodríguez N. M., Sangil C., Reyes J., Benito B., Orellana S., et al. (2022). Environmental and human factors drive the subtropical marine forests of Gongolaria abies marina to extinction. Mar. Environ. Res. 181, 105759. doi: 10.1016/j.marenvres.2022.105759
Marzinelli E. M., Leong M. R., Campbell A. H., Steinberg P. D., Vergés A. (2016). Does restoration of a habitat-forming seaweed restore associated faunal diversity? Restor. Ecol. 24 (1), 81–90. doi: 10.1111/rec.12292
MBPC (2022) Mediterranean ecosystem restoration sites. Interreg Mediterranean Biodiversity Protection Community Project. Available at: https://planbleu.org/wp-content/uploads/2022/11/catalogue_Mediterranean-ecosystem-Restoration.pdf.
McAfee D., Doubleday Z. A., Geiger N., Connell S. D. (2019). Everyone loves a success story: optimism inspires conservation engagement. BioScience 69, 274–281. doi: 10.1093/biosci/biz019
McAfee D., Drew G., Connell S. D. (2022). Recentering the role of marine restoration science to bolster community stewardship. Earth System Governance 13, 100149. doi: 10.1016/j.esg.2022.100149
McAfee D., Reinhold S. L., Alleway H. K., Connell S. D. (2021). Environmental solutions fast-tracked: Reversing public scepticism to public engagement. Biol. Conserv. 253, 108899. doi: 10.1016/j.biocon.2020.108899
McGill B. J., Enquist B. J., Weiher E., Westoby M. (2006). Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21 (4), 178–185. doi: 10.1016/j.tree.2006.02.002
Medrano A., Hereu B., Cleminson M., Pagès-Escolà M., Rovira G., Solà J., et al. (2020). From marine deserts to algal beds: Treptacantha elegans revegetation to reverse stable degraded ecosystems inside and outside a No-Take marine reserve. Restor. Ecol. 28 (3), 632–644. doi: 10.1111/rec.13123
Medrano A., Linares C., Aspillaga E., Capdevila P., Montero-Serra I., Pagès-Escolà M., et al. (2019). No-take marine reserves control the recovery of sea urchin populations after mass mortality events. Mar. Environ. Res. 145, 147–154. doi: 10.1016/j.marenvres.2019.02.013
Meinesz A., Blanfuné A., Chancollon O., Javel F., Longepierre S., Markovic L., et al. (2013). Côtes méditerranéennes françaises: inventaire et impact des aménagements gagnés sur la mer. Ed. Lab (Nice, France: ECOMERS, Université Nice Sophia Antipolis), 153.
Milazzo M., Badalamenti F., Riggio S., Chemello R. (2004). Patterns of algal recovery and small-scale effects of canopy removal as a result of human trampling on a Mediterranean rocky shallow community. Biol. Conserv. 117 (2), 191–202. doi: 10.1016/S0006-3207(03)00292-1
Milazzo M. P., Chemello R., Badalamenti F. O., Riggio S. (2002b). Short-term effect of human trampling on the upper infralittoral macroalgae of Ustica Island MPA (western Mediterranean, Italy). J. Mar. Biol. Assoc. United Kingdom 82 (5), 745–748. doi: 10.1017/S0025315402006112
Milazzo M., Chemello R., Badalamenti F., Riggio R. C., Riggio S. (2002a). The impact of human recreational activities in marine protected areas: What lessons should be learnt in the Mediterranean Sea? Mar. Ecol. 23, 280–290. doi: 10.1111/j.1439-0485.2002.tb00026.x
MIT Sea Grant. Available at: https://seagrant.mit.edu/shellfish-restoration/.
Miyajima T., Hamaguchi M. (2019). “Carbon sequestration in sediment as an ecosystem function of seagrass meadows,” in Blue carbon in shallow coastal ecosystems (Singapore: Springer), 33–71.
Molinari Novoa E., Guiry M. D. (2020). Reinstatement of the genera gongolaria boehmer and ericaria stackhouse (Sargassaceae, phaeophyceae). Notulae Algarum 172, 1–10.
Monserrat M., Comeau S., Verdura J., Alliouane S., Spennato G., Priouzeau F., et al. (2022). Climate change and species facilitation affect the recruitment of macroalgal marine forests. Sci. Rep. 12, 18103. doi: 10.1038/s41598-022-22845-2
Monserrat M., Verdura J., Comeau S., Cottalorda J. M., Priouzeau F., Romero G., et al. (2023). The role of grazers in early-life stages of Cystoseira sensu lato can be crucial in the restoration of marine forests. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1176780
Montero-Serra I., Garrabou J., Doak D. F., Figuerola L., Hereu B., Ledoux J. B., et al. (2018). Accounting for life-history strategies and timescales in marine restoration. Conserv. Lett. 11 (1), e12341. doi: 10.1111/conl.12341
Montoya D., Rogers L., Memmott J. (2012). Emerging perspectives in the restoration of biodiversity-based ecosystem services. Trends Ecol. Evol. 27 (12), 666–672. doi: 10.1016/j.tree.2012.07.004
Moreno-Mateos D., Power M. E., Comín F. A., Yockteng R. (2012). Structural and functional loss in restored wetland ecosystems. PloS Biol. 10 (1), e1001247. doi: 10.1371/journal.pbio.1001247
Morris R. L., Hale R., Strain E. M., Reeves S. E., Vergés A., Marzinelli E. M., et al. (2020). Key principles for managing recovery of kelp forests through restoration. Bioscience 70 (8), 688–698. doi: 10.1093/biosci/biaa058
Mouillot D., Graham N. A., Villéger S., Mason N. W., Bellwood D. R. (2013). A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28 (3), 167–177. doi: 10.1016/j.tree.2012.10.004
Munda I. M. (1993). Changes and degradation of seaweed stands in the Northern Adriatic. Hydrobiologia 260–261, 239–253. doi: 10.1007/BF00049025
Navarro L., Ballesteros E., Linares C., Hereu B. (2011). Spatial and temporal variability of deep-water algal assemblages in the Northwestern Mediterranean: The effects of an exceptional storm. Estuar. Coast. Shelf Sci. 95, 52–58. doi: 10.1016/j.ecss.2011.08.002
Navarro-Barranco C., Lanza-Arroyo P., Serrano J. G., Rocha J. M. D. (2023). Amphipod assemblages associated with native habitat-forming seaweeds of the Alboran Sea: Influence of environmental protection and biogeographical patterns. Mar. Freshw. Res. 74 (1), 50–64. doi: 10.1071/MF22080
Newton A., Elliott M. (2016). A typology of stakeholders and guidelines for engagement in transdisciplinary, participatory processes. Front. Mar. Sci. 3, 230. doi: 10.3389/fmars.2016.00230
North W. J. (1976). Aquacultural techniques for creating and restoring beds of giant kelp, Macrocystis spp. J. Fish. Res. Board Canada 33, 1015–1023. doi: 10.1139/f76-129
O'Leary J. K., Micheli F., Airoldi L., Boch C., De Leo G., Elahi R., et al. (2017). The resilience of marine ecosystems to climatic disturbances. BioScience 67 (3), 208–220. doi: 10.1093/biosci/biw161
Orfanidis S., Panayotidis P., Ugland K. (2011). Ecological Evaluation Index continuous formula (EEI-c) application: a step forward for functional groups, the formula and reference condition values. Mediterr. Mar. Sci. 12, 199–231. doi: 10.12681/mms.60
Orfanidis S., Rindi F., Cebrian E., Fraschetti S., Nasto I., Taskin E., et al. (2021). Effects of natural and anthropogenic stressors on Fucalean brown seaweeds across different spatial scales in the Mediterranean Sea. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.658417
Orlando-Bonaca M., Pitacco V., Lipej L. (2021a). Loss of canopy-forming algal richness and coverage in the northern Adriatic Loss of canopy-forming algal richness and coverage in the northern Adriatic Sea. Ecol. Indic. 125, 107501. doi: 10.1016/j.ecolind.2021.107501
Orlando-Bonaca M., Pitacco V., Slavinec P., Šiško M., Makovec T., Falace A. (2021b). First restoration experiment for Gongolaria barbata in Slovenian coastal waters. What can go wrong? Plants 10(2), 239. doi: 10.3390/plants10020239
Orlando-Bonaca M., Savonitto G., Asnaghi V., Trkov D., Pitacco V., Šiško M., et al. (2022). Where and how - new insight for brown algal forest restoration in the Adriatic. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.988584
Papadakis O., Tsirintanis K., Lioupa V., Katsanevakis S. (2021). The neglected role of omnivore fish in the overgrazing of Mediterranean rocky reefs. Mar. Ecol. Prog. Ser. 673, 107–116. doi: 10.3354/meps13810
Pearson R. G., Dawson T. P. (2003). Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol. Biogeogr. 12 (5), 361–371. doi: 10.1046/j.1466-822X.2003.00042.x
Peleg O., Guy-Haim T., Yeruham E., Silverman J., Rilov G. (2020). Tropicalization may invert trophic state and carbon budget of shallow temperate rocky reefs. J. Ecol. 108 (3), 844–854. doi: 10.1111/1365-2745.13329
Perkol-Finkel S., Airoldi L. (2010). Loss and recovery potential of marine habitats: An experimental study of factors maintaining resilience in subtidal algal forests at the Adriatic Sea. PloS One 5, e10791. doi: 10.1371/journal.pone.0010791
Peterson A. (2006). Uses and requirements of ecological niche models and related distributional models. Biodivers. Inf. 3, 59–72. doi: 10.17161/bi.v3i0.29
Piazzi L., Bonaviri C., Castelli A., Ceccherelli G., Costa G., Curini-Galletti M., et al. (2018). Biodiversity in canopy-forming algae: Structure and spatial variability of the Mediterranean Cystoseira assemblages. Estuar. Coast. Shelf Sci. 207, 132–141. doi: 10.1016/j.ecss.2018.04.001
Piazzi L., Ceccherelli G. (2006). Persistence of biological invasions effects: recovery of macroalgal assemblages after removal of Caulerpa racemosa var. cylindracea. Estuar. Coast. Shelf Sci. 68 (3-4), 455–461. doi: 10.1016/j.ecss.2006.02.011
Piazzi L., Ceccherelli G. (2019). Effect of sea urchin human harvest in promoting canopy forming algae restoration. Estuar. Coast. Shelf Sci. 219, 273–277. doi: 10.1016/j.ecss.2019.02.028
Pinedo S., Arévalo R., Ballesteros E. (2015). Seasonal dynamics of upper sublittoral assemblages on Mediterranean rocky shores along a eutrophication gradient. Estuar. Coast. Shelf Sci. 161, 93–101. doi: 10.1016/j.ecss.2015.05.004
Pinedo S., Ballesteros E. (2019). The role of competitor, stress-tolerant and opportunist species in the development of indexes based on rocky shore assemblages for the assessment of ecological status. Ecol. Indic. 107, 105556. doi: 10.1016/j.ecolind.2019.105556
Pinedo S., García M., Satta M. P., De Torres M., Ballesteros E. (2007). Rocky shore communities as indicators of water quality: a case study in the Northwestern Mediterranean. Mar. Pollut. Bull. 55, 126–135. doi: 10.1016/j.marpolbul.2006.08.044
Pinedo S., Zabala M., Ballesteros E. (2013). Long-term changes in sublittoral macroalgal assemblages related to water quality improvement. Botanica Marina 56, 461–469. doi: 10.1515/bot-2013-0018
Pinna S., Piazzi L., Ceccherelli G., Castelli A., Costa G., Curini-Galletti M., et al. (2020). Macroalgal forest vs sea urchin barren: Patterns of macro-zoobenthic diversity in a large-scale Mediterranean study: Macro-zoobenthos of barren and macroalgal forests. Mar. Environ. Res. 159, 104955. doi: 10.1016/j.marenvres.2020.104955
Pogoda B., Merk V., Colsoul B., Hausen T., Peter C., Pesch R., et al. (2020). Site selection for biogenic reef restoration in offshore environments: the Natura 2000 area Borkum reef ground as a case study for native oyster restoration. Aquat. Conserv.: Mar. Freshw. Ecosyst. 30 (11), 2163–2179. doi: 10.1002/aqc.3405
Preston J., Gamble C., Debney A., Helmer L., Hancock B., Zu Ermgassen P. S. E. (2020). European Native Oyster Habitat Restoration Handbook (London, UK: The Zoological Society of London).
Quigley K. M., Hein M., Suggett D. J. (2021). Translating the 10 golden rules of reforestation for coral reef restoration. Conserv. Biol. 36 (4), e13890. doi: 10.1111/cobi.13890
Ramírez-Monsalve P., Coelho N. F., Carballo-Cárdenas E., van Tatenhove J. P. M., Papadopoulou N., Smith C. J. (2021). Marine restoration governance arrangements: Issues of legitimacy. Environ. Policy Governance 32 (2), 122–134. doi: 10.1002/eet.1970
Rehues L. L., Verdura J., Fraschetti S., Ballesteros E., Bianchelli S., Blanfuné A., et al. (2021). Present patterns and future trends of Mediterranean forests. What do we know about their conservation status? 9th World Conference on Ecological Restoration SER 2021, Virtual, S-19 Implementing marine ecosystems restoration: new insights from shallow and deep ecosystems (Program and Abstracts Book), 46. Available at: https://www.ser-rrc.org/resource/present-patterns-and-future-trends-of-mediterranean-forests-what-do-we-know-about-their-conservation-status/.
Riquet F., de Kuyper C. A., Fauvelot C., Airoldi L., Planes S., Fraschetti S., et al. (2021). Highly restricted dispersal in habitat-forming seaweed may impede natural recovery of disturbed populations. Sci. Rep. 11, 16792. doi: 10.1038/s41598-021-96027-x
Robvieux P. (2013). “Conservation des populations de Cystoseira en régions Provence-Alpes-Côte-d’Azur et Corse,” in Sciences de la Terre (Français: Université Nice Sophia Antipolis). PhD thesisNNT: 2013NICE4000. tel-00876899.
Rodríguez-Prieto C., Ballesteros E., Boisset F., Afonso-Carrillo J. (2013). Guía de las macroalgas y fanerógamas marinas del Mediterráneo Occidental. Omega Barcelona. 656 pp.
Roebig J. H., McLaughlin J. K., Feller M. J. (2012). Restoring a salt marsh in a highly urbanized environment of New York City: the alley park restoration project. Environ. Pract. 14, 68–78. doi: 10.1017/S1466046611000512
Ruiz-Jaen M. C., Aide T. M. (2005). Restoration success: how is it being measured? Restor. Ecol. 13 (3), 569–577. doi: 10.1111/j.1526-100X.2005.00072.x
Sala E., Ballesteros E., Dendrinos P., Di Franco A., Ferretti F., Foley D., et al. (2012). The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PloS One 7 (2), e32742. doi: 10.1371/journal.pone.0032742
Sala E., Boudouresque C. F., Harmelin M. (1998). Fishing, trophic cascades, and the structure of algal assemblages: evaluation of and old but untested paradigm. Oikos 82, 425–439. doi: 10.2307/3546364
Sala E., Kizilkaya Z., Yildirim D., Ballesteros E. (2011). Alien marine fishes deplete algal biomass in the Eastern Mediterranean. PloS One 6, e17356. doi: 10.1371/journal.pone.0017356
Sales M., Ballesteros E. (2009). Shallow Cystoseira (Fucales: Ochrophyta) assemblages thriving in sheltered areas from Menorca (NW Mediterranean): relationships with environmental factors and anthropogenic features. Estuar. Coast. Shelf Sci. 84, 476–482. doi: 10.1016/j.ecss.2009.07.013
Sales M., Ballesteros E. (2010). Long-term comparison of algal assemblages dominated by Cystoseira crinita (Fucales, Heterokontophyta) from Cap Corse (Corsica, North Western Mediterranean). Eur. J. Phycol. 45, 404–412. doi: 10.1080/09670262.2010.498585
Sales M., Ballesteros E. (2012). Seasonal dynamics and annual production of Cystoseira crinita-dominated assemblages from the north-western Mediterranean. Sci. Mar. 76, 391–401. doi: 10.3989/scimar.03465.16D
Sales M., Ballesteros E., Vidal E., Tomas F., Moranta J., Cebrian E. (2015). New method for restoring degraded Cystoseira forests. Eur. J. Phycol. 50, 108–109. doi: 10.1080/09670262.2015.1069489
Sales M., Cebrian E., Tomas F., Ballesteros E. (2011). Pollution impacts and recovery potential in three species of the genus Cystoseira (Fucales, Heterokontophyta). Estuar. Coast. Shelf Sci. 92, 347–357. doi: 10.1016/j.ecss.2011.01.008
Sant N., Ballesteros E. (2021a). Depth distribution of canopy-forming algae of the order Fucales is related to their photosynthetic features. Mar. Ecol. 42 (3), e12651. doi: 10.11164/jjsps.8.2_255_5
Sant N., Ballesteros E. (2021b). Photosynthetic performances of two deep-water canopy-forming fucoids across a depth gradient: interspecific variability and short-term adaptation to the light environment. Mar. Ecol. 42 (4), e12666. doi: 10.1111/maec.12666
Santiago E., Pineda-Metz A., Colsoul B., Niewöhner M., Hausen T., Peter C., et al. (2023). Setting the stones to restore and monitor European flat oyster reefs in the German North Sea. Aquat. Conserv.: Mar. Freshw. Ecosyst. 33 (7), 661–667. doi: 10.1002/aqc.3945
Sauvageau C. (1912). À propos des Cystoseira de Banyuls et de Guéthary. Bull. Station Biologique d'Arcachon 12, 133–556.
Savonitto G., Alongi G., Falace A. (2019). Reproductive phenology, zygote embryology and germling development of the threatened Carpodesmia barbatula (= Cystoseira barbatula) (Fucales, phaeophyta) towards its possible restoration. Webbia 74 (2), 317–323. doi: 10.1080/00837792.2019.1692594
Savonitto G., de la Fuente G., Tordoni E., Ciriaco S., Srijemsi M., Bacaro G., et al. (2021). Addressing reproductive stochasticity and grazing impacts in the restoration of a canopy-forming brown alga by implementing mitigation solutions. Aquat. Conserv.: Mar. Freshw. Ecosyst. 31 (7), 1611–1623. doi: 10.1002/aqc.3555
Schiel D. R., Foster M. S. (2006). The population biology of large brown seaweeds: ecological consequences of multiphase life histories in dynamic coastal environments. Annu. Rev. Ecology Evolution Sys. 37, 343–372. doi: 10.1146/annurev.ecolsys.37.091305.110251
Serio D., Alongi G., Catra M., Cormaci M., Furnari G. (2006). Changes in the benthic algal flora of Linosa Island (Straits of Sicily, Mediterranean Sea). Botanica Marina 49, 135–144. doi: 10.1515/BOT.2006.018
Shaver E. C., Courtney C. A., West J. M., Maynard J., Hein M., Wagner C., et al. (2020). A manager’s guide to coral reef restoration planning and design. NOAA coral reef conservation program. NOAA Tech. Memorandum CRCP 36, 128.
Smale D. A., Burrows M. T., Moore P., O'Connor N., Hawkins S. J. (2013). Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol. Evol. 3 (11), 4016–4038. doi: 10.1002/ece3.774
Smith C. J., Papadopoulou N., Carballo-Cárdenas E., van Tatenhove J. P. M. (2021). Marine restoration in the Mediterranean: red coral and fan mussel discourses, uncertainty and reaching restoration targets. Mar. Policy 128, 104488. doi: 10.1016/j.marpol.2021.104488
Soltan D., Verlaque M., Boudouresque C. F., Francour P. (2001). Changes in macroalgal communities in the vicinity of a Mediterranean sewage outfall after the setting up of a treatment plant. Mar. Pollut. Bull. 42, 59–70. doi: 10.1016/S0025-326X(00)00116-8
Stanturf J. A., Schoenholtz S. H., Schweitzer C. J., Shepard J. P. (2001). Achieving restoration success: myths in bottomland hardwood forests. Restor. Ecol. 9 (2), 189–200. doi: 10.1046/j.1526-100x.2001.009002189.x
Steneck R. S., Graham M. H., Bourque B. J., Corbett D., Erlandson J. M., Estes J. A., et al. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 29, 436–459. doi: 10.1017/S0376892902000322
Strain E. M. A., Van Belzen J., Van Dalen J., Bouma T. J., Airoldi L. (2015). Management of local stressors can improve the resilience of marine canopy algae to global stressors. PloS One 10, e0120837. doi: 10.1371/journal.pone.0120837
Strong J. A., Andonegi E., Bizsel K. C., Danovaro R., Elliott M., Franco A., et al. (2015). Marine biodiversity and ecosystem function relationships: the potential for practical monitoring applications. Estuar. Coast. Shelf Sci. 161, 46–64. doi: 10.1016/j.ecss.2015.04.008
Suding K. N. (2011). Toward an era of restoration in ecology: successes, failures, and opportunities ahead. Annu. Rev. Ecology Evol. Sys. 42, 465–487. doi: 10.1017/S0376892902000322
Swan K. D., McPherson J. M., Seddon P. J., Moehrenschlager A. (2016). Managing marine biodiversity: the rising diversity and prevalence of marine conservation translocations. Conserv. Lett. 9 (4), 239–251. doi: 10.1111/conl.12217
Tamburello L., Chiarore A., Fabbrizzi E., Colletti A., Franzitta G., Grech D., et al. (2022). Can we preserve and restore overlooked macroalgal forests? Sci. Total Environ. 806, 150855. doi: 10.1016/j.scitotenv.2021.150855
Tamburello L., Papa L., Guarnieri G., Basconi L., Zampardi S., Scipione M. B., et al. (2019). Are we ready for scaling up restoration actions? An insight from Mediterranean macroalgal canopies. PloS One 14, e0224477. doi: 10.1371/journal.pone.0224477
Teixidó N., Gambi M. C., Parravacini V., Kroeker K., Micheli F., Villéger S., et al. (2018). Functional biodiversity loss along natural CO2 gradients. Nat. Commun. 9, 5149. doi: 10.1038/s41467-018-07592-1
Thibaut T., Blanfuné A., Boudouresque C. F., Personnic S., Ruitton S., Ballesteros E., et al. (2017). An ecosystem-based approach to assess the status of Mediterranean algae-dominated shallow rocky reefs. Mar. Pollut. Bull. 117, 311–329. doi: 10.1016/j.marpolbul.2017.01.029
Thibaut T., Blanfuné A., Boudouresque C. F., Verlaque M. (2015). Decline and local extinction of Fucales in French Riviera: the harbinger of future extinctions? Mediterr. Mar. Sci. 16, 206–224. doi: 10.12681/mms.1683
Thibaut T., Blanfuné A., Javel F., Puissant C. (2021). Is it possible to restore algal forest on large areas? The French experience. 9th World Conference on Ecological Restoration SER 2021, Virtual, S-19 Implementing marine ecosystems restoration: new insights from shallow and deep ecosystems (Program and Abstracts Book), 46. Available at: https://www.ser-rrc.org/resource/is-it-possible-to-restore-algal-forest-on-large-areas-the-french-experience/.
Thibaut T., Blanfuné A., Markovic L., Verlaque M., Boudouresque C. F., Perrret-Boudouresque M., et al. (2014). Unexpected abundance and long-term relative stability of the brown alga Cystoseira amentacea, hitherto regarded as a threatened species, in the north western Mediterranean Sea. Mar. Pollut. Bull. 89 (1-2), 305–323. doi: 10.1016/j.marpolbul.2014.09.043
Thibaut T., Bottin L., Aurelle D., Boudouresque C. F., Blanfuné A., Verlaque M., et al. (2016). Connectivity of populations of the seaweed Cystoseira amentacea within the Bay of Marseille (Mediterranean Sea): Genetic structure and hydrodynamic connections. Crypto. Algo. 37, 233–255. doi: 10.7872/crya/v37.iss4.2016.233
Thibaut T., Pinedo S., Torras X., Ballesteros E. (2005). Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Mar. Pollut. Bull. 50, 1472–1489. doi: 10.1016/j.marpolbul.2005.06.014
Thomas E., Alcazar C., Moscoso H. L. G., Vásquez A., Osorio L. F., Salgado-Negret B., et al. (2017). “The importance of species selection and seed sourcing in forest restoration for enhancing adaptive potential to climate change: Colombian tropical dry forest as a model,” in CBD Technical series N° 89: The Lima declaration on biodiversity and climate change: Contributions from science to policy for sustainable development. Eds. Rodríguez L., Anderson I. (Convention on Biological Diversity, Montreal), 122–132.
Tilman D., Isbell F., Cowles J. M. (2014). Biodiversity and ecosystem functioning. Annu. Rev. Ecology Evolution Sys. 45, 471–493. doi: 10.1146/annurev-ecolsys-120213-091917
Torras X., Pinedo S., García M., Weitzmann B., Ballesteros E. (2016). “Environmental quality of Catalan coastal waters based on macroalgae: the interannual variability of CARLIT index and its ability to detect changes in anthropogenic pressures over time,” in Experiences from ground, coastal and transitional water quality monitoring: The EU Water Framework Directive implementation in the Catalan river basin district (Part II): 183-199. The Handbook of Environmental Chemistry, vol. 43 . Eds. Munné A., Ginebreda A., Prat N. (Switzerland: Springer International Publishing).
UN (2019). United Nations Decade on Ecosystem Restoration, (2021–2030). Resolution adopted by the General Assembly on 1 March 2019 (United Nations, New York), A/RES/73/284.
UNEP-Nairobi Convention/USAID/WIOMSA (2020b). Guidelines on Mangrove Ecosystem Restoration for the Western Indian Ocean Region Vol. 71 (Nairobi: UNEP).
UNEP-Nairobi Convention/WIOMSA (2020a). Guidelines for Seagrass Ecosystem Restoration in the Western Indian Ocean Region (Nairobi: UNEP), 63.
van Katwijk M. M., Bos A. R., de Jonge V. N., Hanssen L. S. A. M., Hermus D. C. R., de Jong D. J. (2009). Guidelines for seagrass restoration: Importance of habitat selection and donor population, spreading of risks, and ecosystem engineering effects. Mar. Pollut. Bull. 58, 179–188. doi: 10.1016/j.marpolbul.2008.09.028
van Putten I. E., Plaganyi E. E., Booth K., Cvitanovic C., Kelly R., Punt A., et al. (2018). A framework for incorporating sense of place into the management of marine systems. Ecol. Soc. 23 (3), 24. doi: 10.5751/ES-10504-230404
Verdura J. (2021). Mediterranean macroalgal forests under threat: The effects of ongoing climate change and design of restoration methods (University of Girona, Girona). Available at: http://hdl.handle.net/10803/673885. PhD Thesis.
Verdura J., Sales M., Ballesteros E., Cefalì M. E., Cebrian E. (2018). Restoration of a canopy-forming alga based on recruitment enhancement: Methods and long-term success assessment. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01832
Verdura J., Santamaría J., Ballesteros E., Smale D. A., Cefalì M. E., Golo R., et al. (2021). Local-scale climatic refugia offer sanctuary for a habitat-forming species during a marine heatwave. J. Ecol. 109, 1758–1773. doi: 10.1111/1365-2745.13599
Vergés A., Alcoverro T., Ballesteros E. (2009). Role of fish herbivory in structuring the vertical distribution of canopy algae Cystoseira spp. in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 375, 1–11. doi: 10.3354/meps07778
Vergés A., Campbell A. H., Wood G., Kajlich L., Eger A. M., Cruz D., et al. (2020). Operation Crayweed: Ecological and sociocultural aspects of restoring Sydney’s underwater forests. Ecol. Manage. Restor. 21 (2), 74–85. doi: 10.1111/emr.12413
Vergés A., Steinberg P. D., Hay M. E., Poore A. G. B., Campbell A. H., Ballesteros E., et al. (2014). The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B: Biol. Sci. 281, 20140846. doi: 10.1098/rspb.2014.0846
Verlaque M. (1987). Contributions à l’étude du phytobenthos d’un écosystème photophile thermophile marin en Méditerranée occidentale : étude structurale et dynamique du phytobenthos et analyse des relations faune - flore (Université d'Aix-Marseille II. Faculté des sciences, Marseille). PhD ThesisNNT: 1987AIX22052.
Violle C., Navas M. L., Vile D., Kazakou E., Fortunel C., Hummel I., et al. (2007). Let the concept of trait be functional! Oikos 116 (5), 882–892. doi: 10.1111/j.0030-1299.2007.15559.x
Vukovic A. (1982). Florofaunistic changes in the infralittoral zone after Paracentrotus lividus population explosion. Acta Adriatica 23, 237–241.
Wells H. B., Kirobi E. H., Chen C. L., Winowiecki L. A., Vågen T. G., Ahmad M. N., et al. (2021). Equity in ecosystem restoration. Restor. Ecol. 29 (5), e13385. doi: 10.1111/rec.13385
Whitaker S. G., Smith J. R., Murray S. N. (2010). Reestablishment of the southern California rocky intertidal brown alga, Silvetia compressa: an experimental investigation of techniques and abiotic and biotic factors that affect restoration success. Restor. Ecol. 18, 18–26. doi: 10.1111/j.1526-100X.2010.00717.x
Wilson K. C., McPeak R. H. (1983). Kelp restoration. The effects of waste disposal on kelp communities. Southern California Coastal Water Restoration Project (USA: Scripps Institute of Oceanography).
Wood G., Marzinelli E. M., Campbell A. H., Steinberg P. D., Vergés A., Coleman M. A. (2021). Genomic vulnerability of a dominant seaweed points to future-proofing pathways for Australia's underwater forests. Global Change Biol. 27 (10), 2200–2212. doi: 10.1111/gcb.15534
Wood G., Marzinelli E. M., Coleman M. A., Campbell A. H., Santini N. S., Kajlich L., et al. (2019). Restoring subtidal marine macrophytes in the Anthropocene: trajectories and future-proofing. Mar. Freshw. Res. 70 (7), 936–951. doi: 10.1071/MF18226
Wood G., Marzinelli E. M., Vergés A., Campbell A. H., Steinberg P. D., Coleman M. A. (2020). Using genomics to design and evaluate the performance of underwater forest restoration. J. Appl. Ecol. 57 (10), 1988–1998. doi: 10.1111/1365-2664.13707
Keywords: algal forest, Mediterranean Sea, decision tree, stressors, management
Citation: Smith CJ, Verdura J, Papadopoulou N, Fraschetti S, Cebrian E, Fabbrizzi E, Monserrat M, Drake M, Bianchelli S, Danovaro R, Malak DA, Ballesteros E, Benjumea Tesouro T, Boissery P, D’Ambrosio P, Galobart C, Javel F, Laurent D, Orfanidis S and Mangialajo L (2023) A decision-support framework for the restoration of Cystoseira sensu lato forests. Front. Mar. Sci. 10:1159262. doi: 10.3389/fmars.2023.1159262
Received: 05 February 2023; Accepted: 31 July 2023;
Published: 24 August 2023.
Edited by:
Chiara Piroddi, Joint Research Centre, ItalyReviewed by:
Valentina Asnaghi, University of Genoa, ItalyTomas Vega Fernandez, Anton Dohrn Zoological Station Naples, Italy
Copyright © 2023 Smith, Verdura, Papadopoulou, Fraschetti, Cebrian, Fabbrizzi, Monserrat, Drake, Bianchelli, Danovaro, Malak, Ballesteros, Benjumea Tesouro, Boissery, D’Ambrosio, Galobart, Javel, Laurent, Orfanidis and Mangialajo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher J. Smith, Y3NtaXRoQGhjbXIuZ3I=
†These authors have contributed equally to this work and share first authorship
 Christopher J. Smith
Christopher J. Smith Jana Verdura
Jana Verdura Nadia Papadopoulou
Nadia Papadopoulou Simonetta Fraschetti
Simonetta Fraschetti Emma Cebrian
Emma Cebrian Erika Fabbrizzi
Erika Fabbrizzi Margalida Monserrat
Margalida Monserrat Matilde Drake2
Matilde Drake2 Roberto Danovaro
Roberto Danovaro Dania Abdul Malak
Dania Abdul Malak Enric Ballesteros
Enric Ballesteros Tatí Benjumea Tesouro
Tatí Benjumea Tesouro Pierre Boissery
Pierre Boissery Cristina Galobart
Cristina Galobart Fabrice Javel
Fabrice Javel Sotiris Orfanidis
Sotiris Orfanidis Luisa Mangialajo
Luisa Mangialajo