Abstract
Increasing human pressure has strongly impacted the littoral environment, altering the habitats and population parameters of some species. The Mediterranean Sea is one of the regions that have been highly affected by these events. In response to these events, marine protected areas (MPAs) have emerged as one of the main conservation tools for marine habitats and species. In this regard, harvesting of limpets (Patella spp.) is a good example of the impact of human activity on the coast. Limpets are mollusks that are collected as food or fishing bait, and their exploitation causes an immediate decrease in the density and size structure of individuals. In the Mediterranean Sea, the genus Patella is represented by five species: Patella rustica, P. caerulea, P. ulyssiponensis, P. ferruginea, and P. depressa. To verify the effectiveness of the MPAs, a deep review of 75 studies on Patella spp. in the Mediterranean Sea was conducted to analyze the spatial and temporal distribution of studies. Data extracted from these articles were used to assess the effect of protection on the density and size of P. ferruginea. Regarding spatial distribution, the studies reviewed were performed at 67 sites in 13 countries, of which 23 were in MPAs and 44 in non-protected or control areas. The findings of this study show that P. ferruginea is the species most studied, because it is one of the most threatened marine invertebrates in the Mediterranean Sea. This explains the temporal distribution of the studies, which coincides with the establishment of the conservation status and the concern about the population status of P. ferruginea. Analysis of the effect of protection on the density and mean size of P. ferruginea individuals revealed no significant difference in mean density between the control sites and MPAs. However, mean size between individuals was significantly higher in MPAs than control sites. This pattern may be related to intra- and interspecific competition between individuals.
1 Introduction
Human settlements have long been attracted to coastal areas, which provide a wealth of resources, commercial opportunities, and leisure activities (McGranahan et al., 2007). Coastal areas and marine ecosystems are being pressured by population development, resulting in accelerated habitat loss and degradation (Burak et al., 2004), mainly because of stressors such as industrial expansion, overfishing, and tourism (Stanchev et al., 2015).
Tourism has become a growing trend in coastal regions worldwide. Globally, marine and coastal tourism is one of the major economic activities (Smith, 2000; Zahedi, 2008). Therefore, their impact on the coastal environment is significant and extremely difficult to manage (Davenport and Davenport, 2006). Overexploitation, pollution, coastal destruction, biological invasion by non-indigenous species, and climate change have major environmental impact on marine ecosystems (Jackson et al., 2001; Probert, 2017). Shellfishing, if not properly managed, can lead to serious alterations in the ecosystem, such as biodiversity loss, serial depletion, habitat degradation, and changes in the food chain (Murawski, 2000; Scheffer et al., 2005).
The Mediterranean region is not an exception and suffers the consequences of human activities and climate change. For many years, the coastal areas of the Mediterranean region have attracted attention for their environment and culture (Burak et al., 2004). Due to its peculiar characteristics and history, the Mediterranean Sea can be considered a miniature ocean (Bianchi and Morri, 2000), whose reduced extent and volume help that even minor modifications can cause major changes in the climate and functioning of the Mediterranean, which makes it a region potentially vulnerable to climate change and human activities (Lionello et al., 2006; Garrabou et al., 2022). In fact, it is considered one of the most prominent “hot spots” in future projections of climate change (Giorgi, 2006).
The problems caused by factors such as human activity and climate change have led to initiatives for the protection of marine and coastal ecosystems. This is how the term “marine protected area” or “MPA” was adopted by Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008. According to this law, MPAs are natural spaces whose objective is to protect ecosystems, communities, and biological or geological elements of the marine environment that, depending on their rarity, fragility, or importance, deserves special protection. In total, 1,062 MPAs are distributed throughout the coast of the Mediterranean Sea (by 2019, according to Claudet et al., 2020). The main objective of MPAs is the conservation of marine life and its intrinsic processes (Ballantine and Langlois, 2008). By protecting these areas from damage and degradation, MPAs facilitate the recovery of disturbed habitats, especially those that are vulnerable and highly diverse (Selig and Bruno, 2010). Numerous studies have shown that the main direct effects of protection of marine areas are positive, such that biological variables (e.g., biomass, density of individuals, species richness, or body size) are increased within the limits of the MPA (Claudet et al., 2008; Lester et al., 2009). Despite all these positive effects, the effectiveness of MPAs is questioned. This depends on various factors, such as the design of the MPA and the levels of protection established (Halpern, 2003). In addition, MPAs may provide insufficient protection on their own, because they are not isolated from environmental impacts (Allison et al., 1998). For this reason, the effectiveness of MPAs depends on the protection provided outside their boundaries (Agardy, 1994).
Harvesting has been a major threat to the conservation of intertidal species worldwide (Kingsford et al., 1991; Keough et al., 1993; Thompson et al., 2002). Mollusk species have evolved survival strategies, adapting their life cycles, reproduction rates, behavioral mechanisms, and so forth, to survive the challenges of their physical and biotic environment (Hockey, 1994). Human predation through shellfishing is a relatively recent phenomenon in evolutionary terms that has not been able to predict (Mannino and Thomas, 2002). Patella is one of the main genera that are affected by shellfishing in the Mediterranean Sea. These limpets are collected as food or bait for fishing, and their exploitation causes an immediate decrease in density, change in size structure of individuals, an imbalance in the sex ratio, and a decrease in reproduction (Paracuellos et al., 2003; Rivera-Ingraham et al., 2011). These actions lead to the decline of the species, as well as alter the ecological succession that occurs in this habitat, because this herbivore plays an important ecological role in community structuring, regulating the recruitment of macroalgae through consumption (Guerra and Gaudencio, 1986). Therefore, these limpets act as key species providing space for many others, thereby enhancing biodiversity (Power et al., 1996; Raffaelli and Hawkins, 1996).
In the Mediterranean Sea, the genus Patella is represented by four species: P. rustica Linnaeus, 1758; P. caerulea Linnaeus, 1758; P. ulyssiponensis Gmelin, 1791; and P. ferruginea Gmelin, 1791 (Mauro et al., 2003), to which P. depressa Pennant, 1777—recorded in the Alboran Sea—must be added (Gofas et al., 2011). Although all species can inhabit intertidal rocky areas, they avoid overlapping their distribution by placing themselves at different vertical levels on rocks: P. rustica is usually located at the supralittoral level, above the upper mesolittoral occupied by P. ferruginea; P. ulyssiponensis is usually located in the lower mesolittoral; and P. caerulea can be found in both intertidal and upper infralittoral habitats (Guallart and Templado, 2012). Of the five species, P. ferruginea should be highlighted, because it is one of the most threatened marine invertebrates according to Directive 92/43/EEC of the European Council, as well as in “danger of extinction” according to the Barcelona Convention (UNEP/MAP-SPA/RAC, 2018). The state of this species is mainly due to human activities on the Mediterranean coast and its excessive collection.
Although marine protection figures tend to encompass as many habitats as possible within their boundaries, not all are given the same importance. An intertidal rocky area is one of these habitats, whose visibility is usually lower, but importance in the ecosystem is very high. Rocky shores are likely to be more vulnerable than other marine habitats due to their susceptibility to terrestrial and marine disturbances (Schmitt and Osenberg, 1996). The creation of MPAs covering intertidal rocky areas aims to remedy and prevent the decline of threatened species of intertidal mollusks, thus improving their population density, average size, and population viability (Keough et al., 1993). In fact, the effects of protection or harvesting on limpets are a global concern (Branch and Odendaal, 2003; Lasiak, 2006; Sagarin et al., 2007; Espinosa et al., 2009; Martins et al., 2017; Sousa et al., 2019a; Sousa et al., 2019b; Sousa et al., 2020; Baliwe et al., 2022).
To verify the effectiveness of Mediterranean MPAs on limpets and analyze the spatial and temporal distribution of studies, a deep review of studies on the genus Patella in the Mediterranean was performed. With the data extracted from these articles, the effect of protection was assessed on the density and size of P. ferruginea. In addition, correlations between the density and size of individuals were evaluated.
2 Materials and methods
2.1 Literature review
A bibliographic review of existing studies (scientific papers, “gray literature”) on the genus Patella in the Mediterranean Sea until October 2022 was performed following the scheme of Moher et al. (2009). A recompilation of articles was made through a search in all databases of the Web of Science (WoS) entering the keywords: “Patella + Mediterranean + density” (n = 68), “Patella + Mediterranean + size” (n = 110), “limpet + Mediterranean + density” (n = 79), and “limpet + Mediterranean + size” (n = 108) in the TOPIC field. Additionally, for each one of the species of the genus Patella present in the Mediterranean Sea, a specific search entering species name + Mediterranean was performed (n = 334). Because some works not published in the journals included in WoS (including the gray bibliography) may contain interesting evidence for this review of the genus Patella, additional studies were identified through a Google Scholar search. The information has been supplemented with data from authors’ colleagues and includes theses, technical reports, working papers, and so forth. These searches produced n = 410 articles after duplicate removal. After a first review of all the studies collected, studies without information on the density or size of Patella were discarded. The final number of studies selected for analysis was n = 75. The information collected from each study included year of publication, site and country of study, level of protection (differentiating between MPAs and non-protected or control areas), species studied, and information about density and size in as raw format as possible (at least the mean values of these variables were extracted). The sites identified as artificial habitats (port infrastructures) with prohibited access (frequently protected with metal fences) were considered MPAs, because they act as Artificial Marine Micro-Reserves (AMMR) for these species (Espinosa et al., 2009; García-Gómez et al., 2011; García-Gómez et al., 2015; Ostalé-Valriberas et al., 2022).
In this review, information about the density and size was found for the following species: P. rustica, P. caerulea, P. ulyssiponensis, and P. ferruginea, considering as Patella spp. those information that could not be identified at the species level. No studies were found on the density and size of P. depressa. The complete list of studies compiled can be found in Supplementary Data.
2.2 Data analyses
The temporal distribution of Patella studies was analyzed representing the number of studies performed per year, considering the species included in each study. To assess the spatial distribution of Patella studies, all study sites recorded in the review were georeferenced, and the total number of studies performed at each site was calculated, considering whether the studies analyzed the density, size, or both density and size of each species. Using these data, two maps were generated using QGIS software (QGIS Development Team, 2022), showing the spatial distribution of studies on density and size separately.
Because most data compiled in the review were from P. ferruginea, the evaluation focused on the effect of protection on density and size of this species. For each study site with data on P. ferruginea, the protection status (MPA or control site) was considered, and mean density and mean size were calculated to identify a pattern of protection effect related to a country. Additionally, the effect of protection on the density and size of P. ferruginea at the Mediterranean level was analyzed, considering data as raw as possible for all MPAs and control areas. Due to the unbalanced nature of the analysis, and the lack of normality of the data, univariate PERMANOVA (Anderson, 2001) tests were performed over the untransformed density and size of P. ferruginea to detect the effect of protection, comparing data from MPAs with control areas obtained from the previous review. For density analysis, n = 154 and n = 118, and for size analysis, n = 337 and n = 2,350 were considered in MPAs and control areas, respectively. Euclidean distance matrix and 9,999 permutations were used. Finally, the relationship between the density and mean size of P. ferruginea was evaluated using Pearson’s correlation index for overall data (n = 143) and for data from MPA (n = 105) and control areas (n = 38) separately. Analyses were performed using R statistical computing software (R Core Team, 2020) and the R’s statistical software packages ggplot2 (Wickham, 2016) and Vegan (Oksanen et al., 2019).
3 Results
In this research, a total of 75 studies have been found and reviewed (Supplementary Data), in which four species of the genus Patella have been investigated. P. ferruginea was the most studied species, appearing in 56 studies. P. caerulea and P. rustica appeared in 15 and 13 studies, respectively, and P. ulyssiponensis in 1. Reports on the density or size of P. depressa in the Mediterranean Sea were lacking. Species were not identified in six of the studies reviewed, considered Patella spp.
3.1 Temporal and spatial distribution of studies on Patella in the Mediterranean
Regarding the temporal distribution of studies on the genus Patella (Figure 1), the first studies on the size and density of individuals were performed in 1973 and 1986, respectively. Between 1975 and 1999, studies on this genus were lacking; however, an increasing trend was seen in the number of studies from the early 2000s, reaching the highest number in 2019 (seven studies), followed by 2014 and 2018, with six studies. In general, all species appeared in the studies during all periods, with the exception of P. ulyssiponensis, which was included in a study in 2014.
Figure 1
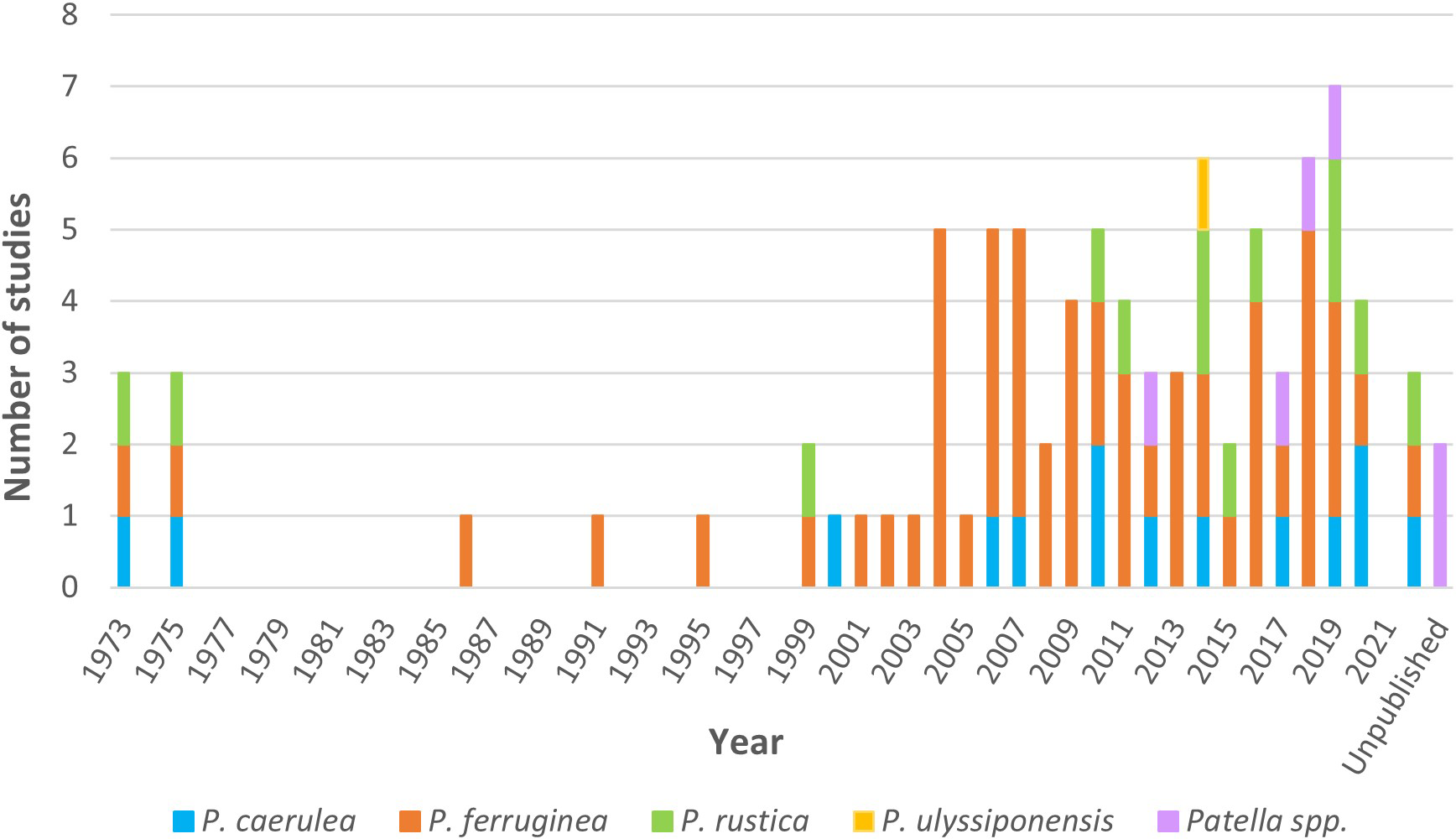
The total number of studies per year on P. caerulea, P. ferruginea, P. rustica, P. ulyssiponensis, and Patella spp. The bar of “unpublished” includes studies with no date of publication.
In terms of spatial distribution, Patella was investigated at 67 sites of the Mediterranean Sea, covering 13 countries. From these sites, 23 were MPAs and 44 were control areas. The countries that contributed the highest number of studies on Patella included Spain (27), Algeria (12), Tunisia (12), and Italy (10).
Focusing on studies that included data on the density of Patella, 39 sites were registered distributed in 10 countries (Figure 2). Most of these sites are located in Spain, Morocco, Algeria, and Tunisia, as well as Corsica (France) and Sardinia (Italy). The sites that contributed the highest number of studies included Ceuta (Spain) and Zembra Island (Tunisia), with eight studies each, followed by Paloma Island (Algeria) with six studies, Tabarca Island (Spain) with five studies, and Mal di Ventre (Sardinia) with four studies. The density of P. ferruginea was studied the most at sites along the Mediterranean Sea (Figure 2). Despite the large number of sites found in the review, more than one species of Patella was studied in only six studies. Notably, studies on the density of P. caerulea, P. ferruginea, and P. rustica were conducted on Paloma Island and the West Coast of Algeria.
Figure 2
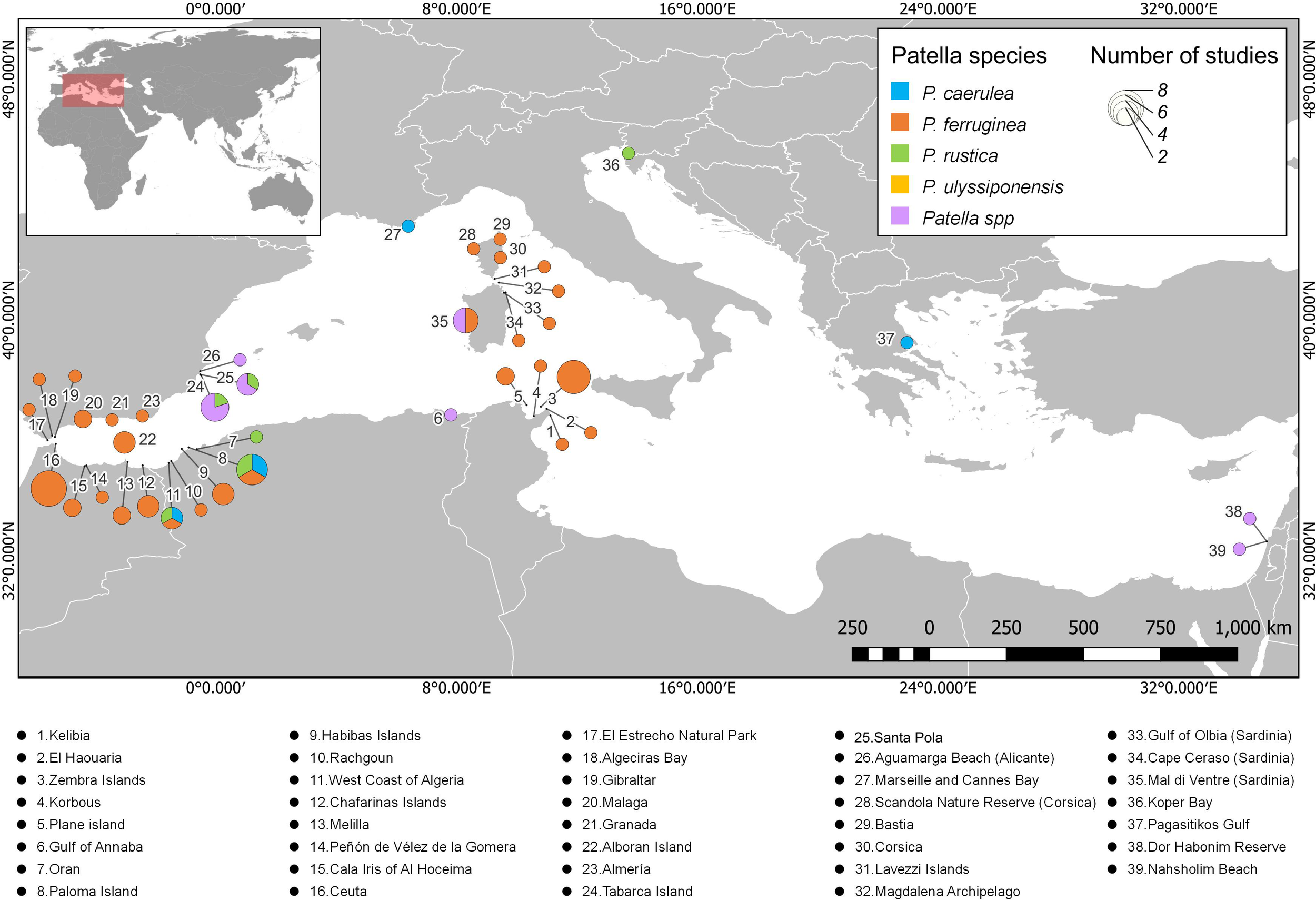
Distribution of studies that include data on densities of Patella individuals along the Mediterranean Sea. Each color represents one of the Patella species, and the diameter of the pie chart indicates the number of studies on density at each site. A list with the names of all the sites registered in the review can be seen below the map.
Regarding studies that included data on the size of Patella individuals, 48 sites were identified in the Mediterranean, distributed in 12 countries (Figure 3). The places where the studies are more concentrated include Corsica (France), Sardinia (Italy), and Tunisia. In most cases, only one study on the size of individuals was performed at each site; however, Ceuta (Spain) and Zembra Island (Tunisia) hosted eight studies each. Although P. ferruginea was the most notable species in studies on the size of individuals along most of the sites, P. caerulea was also studied at several sites, mainly located in the central and eastern Mediterranean. The size of more than one species of Patella was studied at only eight sites.
Figure 3
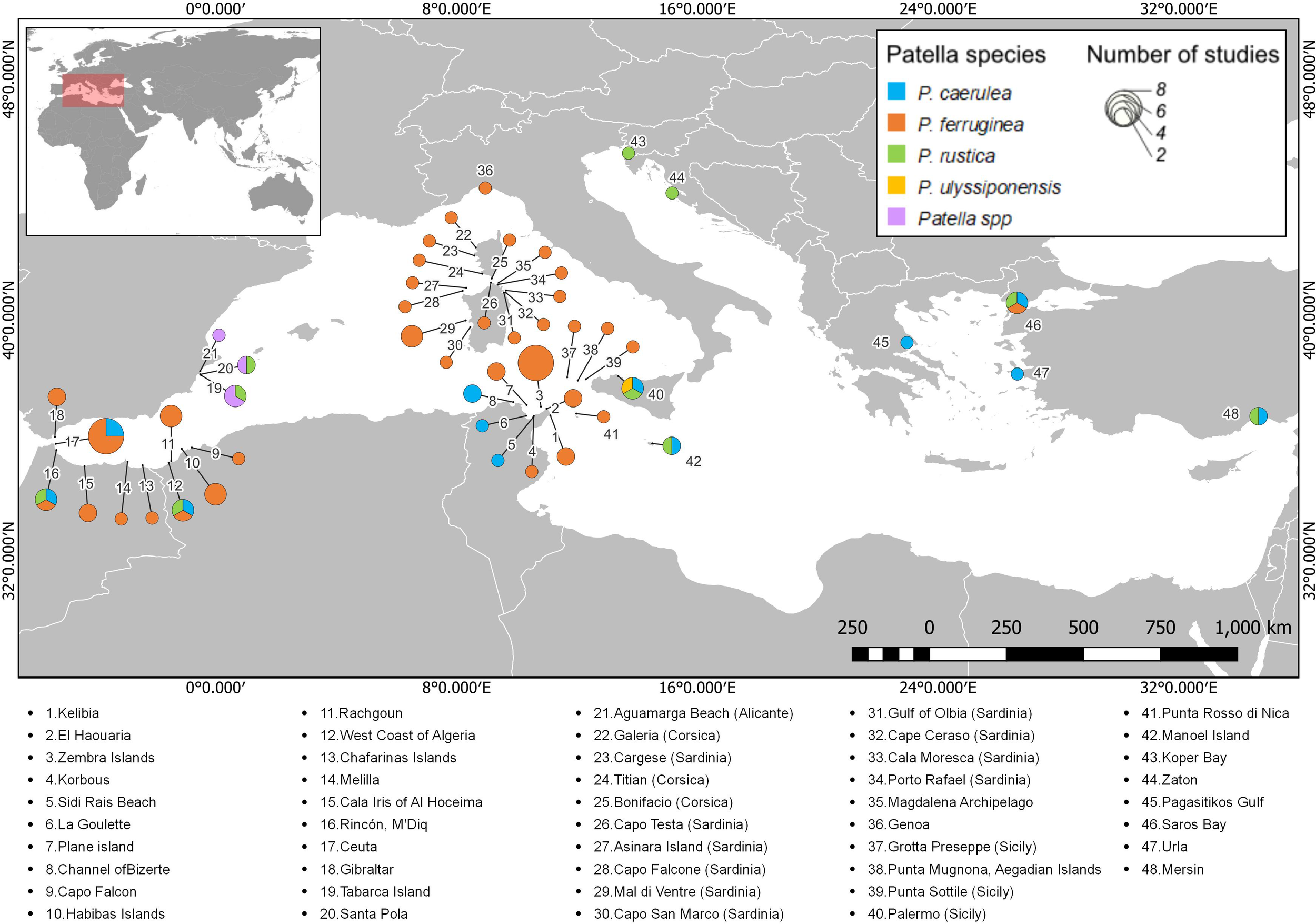
Distribution of studies that include data on the size of Patella individuals along the Mediterranean Sea. Each color represents one of the Patella species, and the diameter of the pie chart indicates the number of studies on size at each site. A list with the names of all the sites registered in the review can be seen below the map.
3.2 Effect of protection on P. ferruginea
Mean density values of P. ferruginea for each site recorded in the review are shown in Figure 4. Although most countries had MPA sites that were studied, the number of control sites studied was greater; specifically, there were included in the studies reviewed 14 MPAs and 17 control sites. Regarding the mean density of individuals, a wide variety of values is appreciated, generally between 0.1 and 10 individuals/m. The mean density of individuals was slightly higher in MPAs than control areas only in Spain, France, and Italy. The highest mean density was observed at the control sites of Plane Island (Tunisia) (15.920 ± 9.790 individuals/m) (s.e., standard error) and Melilla (Spain) (14.154 ± 5.766 individuals/m) and in the MPAs of Chafarinas Island (Spain) (8.865 ± 1.668 individuals/m) and Melilla (Spain) (10.000 individuals/m).
Figure 4
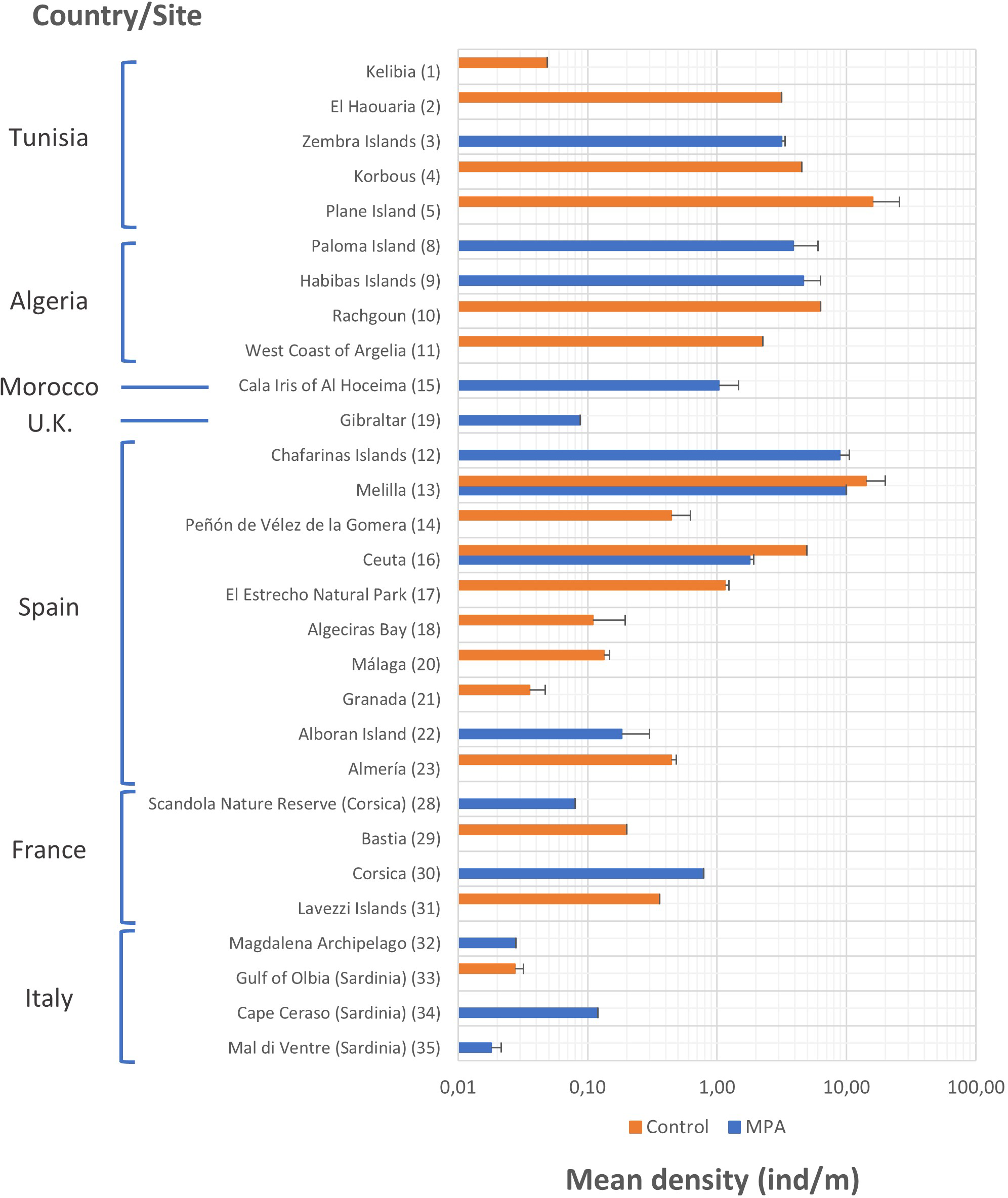
Mean densities and standard error (s.e.) of Patella ferruginea (individuals/m) according to the study site and level of protection. In bars where s.e. is not indicated means that it was not available in the studies or could not be calculated. The numerical code on the right side of the site name corresponds to the list of sites represented in the distribution map (Figure 2). Data are represented on a logarithmic scale.
Considering all the available data, although the mean density of P. ferruginea individuals was slightly higher in the control areas (4.192 ± 0.662 individuals/m) than MPAs (3.950 ± 0.434 individuals/m) (Figure 5), statistical analysis of the effect of protection on density with PERMANOVA revealed no significant differences between MPA and control densities (pseudoF1,270 = 0.101, P > 0.05).
Figure 5
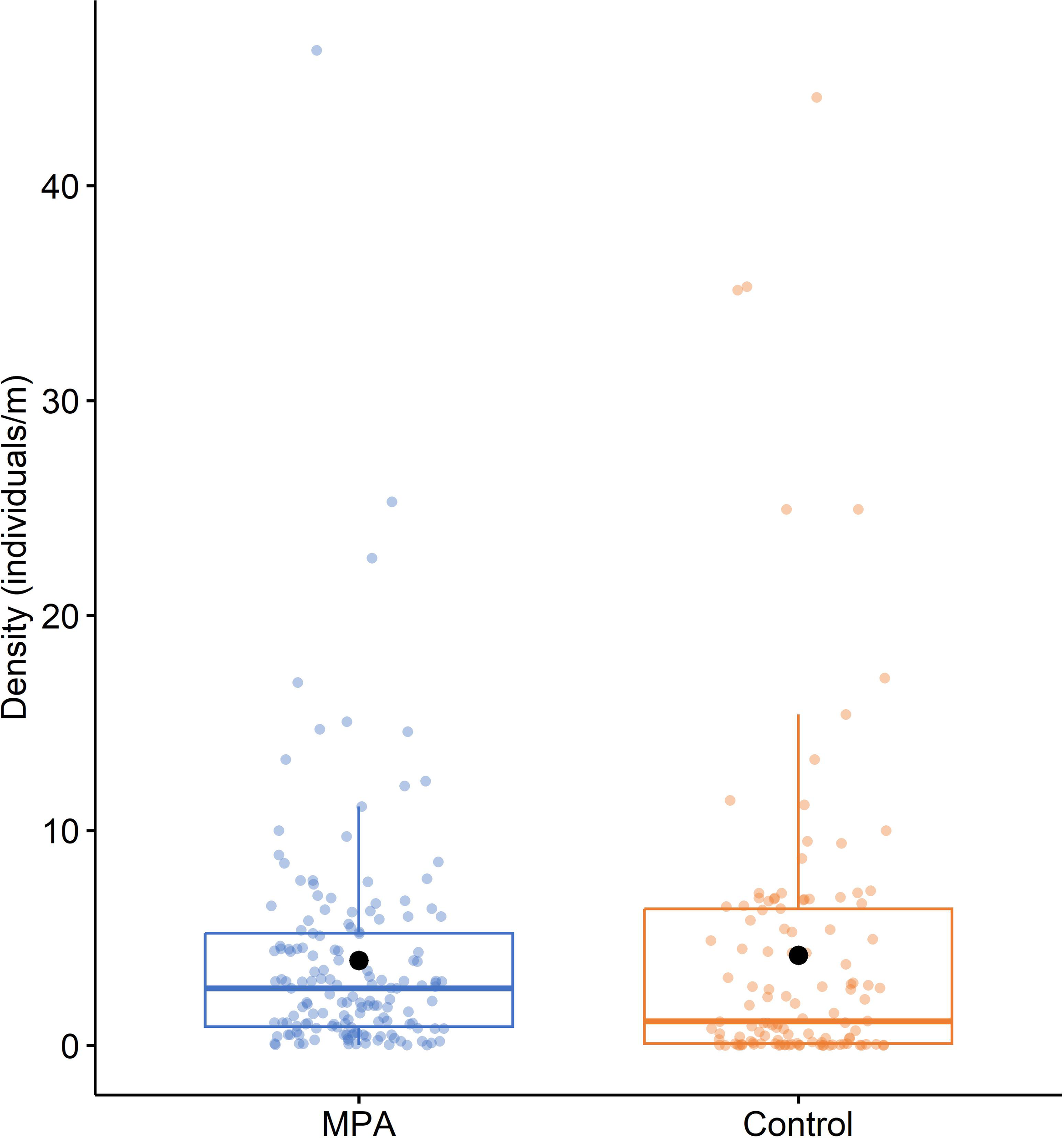
Boxplot of the density of Patella ferruginea (individuals/m) in MPAs and control areas. Within each box, central lines denote median values, boxes extend from the 25th to 75th percentile, and whiskers denote values within 1.5 interquartile range of the 25th and 75th percentiles. Dots above or below the whiskers are outliers. Black dots show mean values.
The mean size of P. ferruginea individuals (Figure 6) was studied in a greater number of control sites than MPAs. This variable was measured at 20 and 17 sites, respectively. In fact, in three of the eight countries that hosted studies on P. ferruginea size, MPAs were not included in the sites studied. The mean size of P. ferruginea generally ranged between 2 and 6 cm at most sites. This variable showed greater values for MPAs than control sites in Tunisia, Algeria, Spain, and Italy; however, the opposite trend was seen in Morocco. The MPAs with the highest mean sizes of P. ferruginea were located in Habibas Islands (Algeria) (6.193 ± 0.115 cm) and Asinara Island (Italy) (6.140 ± 2.030 cm). In the control sites, the highest mean size values were recorded in Rincón/M’Diq (Morocco) (6.080 cm; size range from 3.8 to 8.9 cm) and Genoa (Italy) (5.367 ± 1.124 cm).
Figure 6
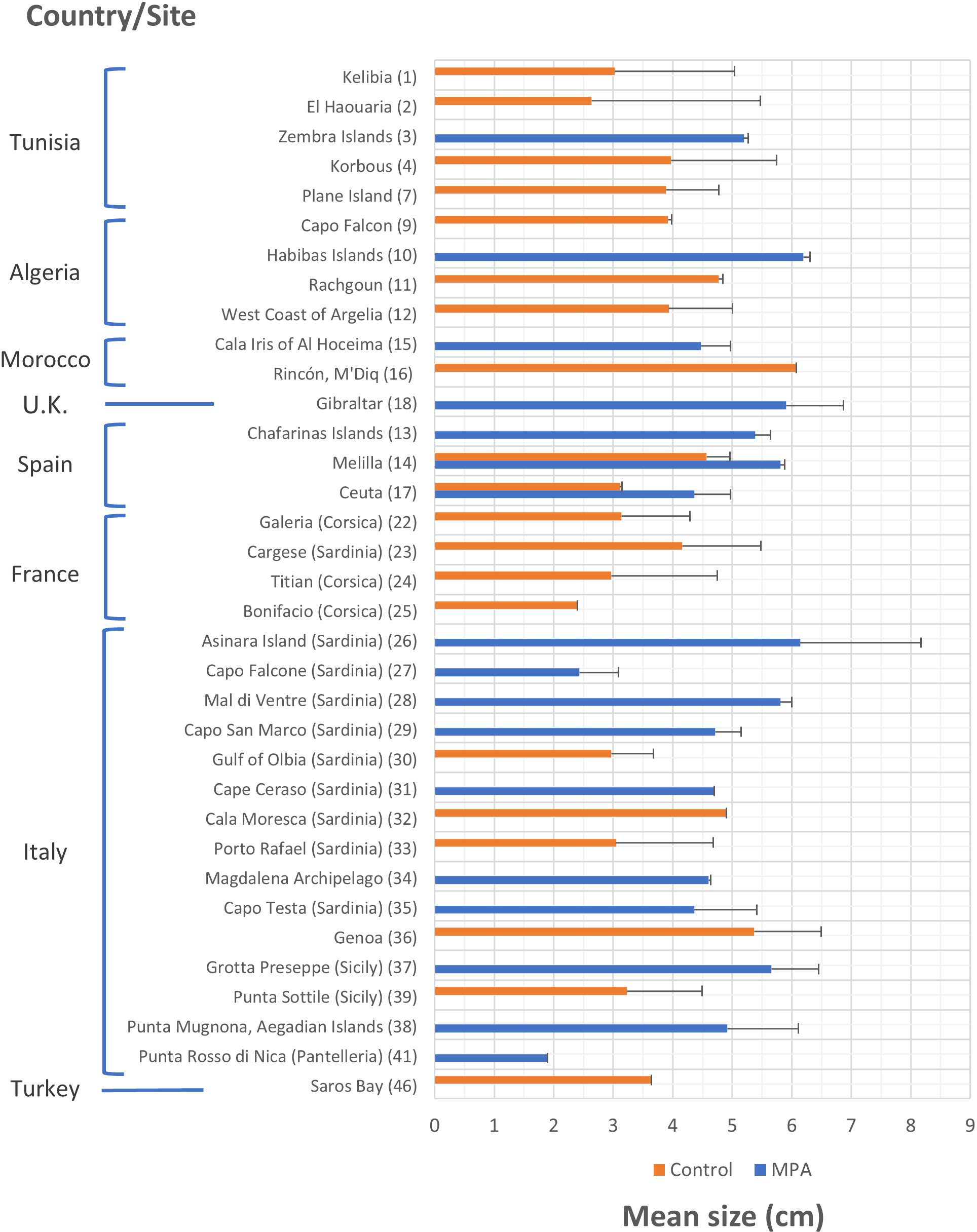
Mean size and standard error (s.e.) of Patella ferruginea individuals (cm) according to the study site and level of protection. Bars where s.e. is not indicated means that it was not available in the studies or could not be calculated. The numerical code on the right side of the site name corresponds to the list of sites represented in the distribution map (Figure 3).
Comparing all available data on the size of P. ferruginea individuals in MPAs and control sites (Figure 7) revealed that although big individuals were observed at control sites (some reaching > 8 cm), mean size was clearly higher in MPAs (5.778 ± 0.080 cm) than control areas (3.531 ± 0.031 cm). This pattern was statistically confirmed after analyzing the effect of protection on mean size with PERMANOVA, which showed that the mean size of P. ferruginea was significantly larger in MPAs than control areas (pseudoF1,2685 = 676.180, P < 0.001).
Figure 7
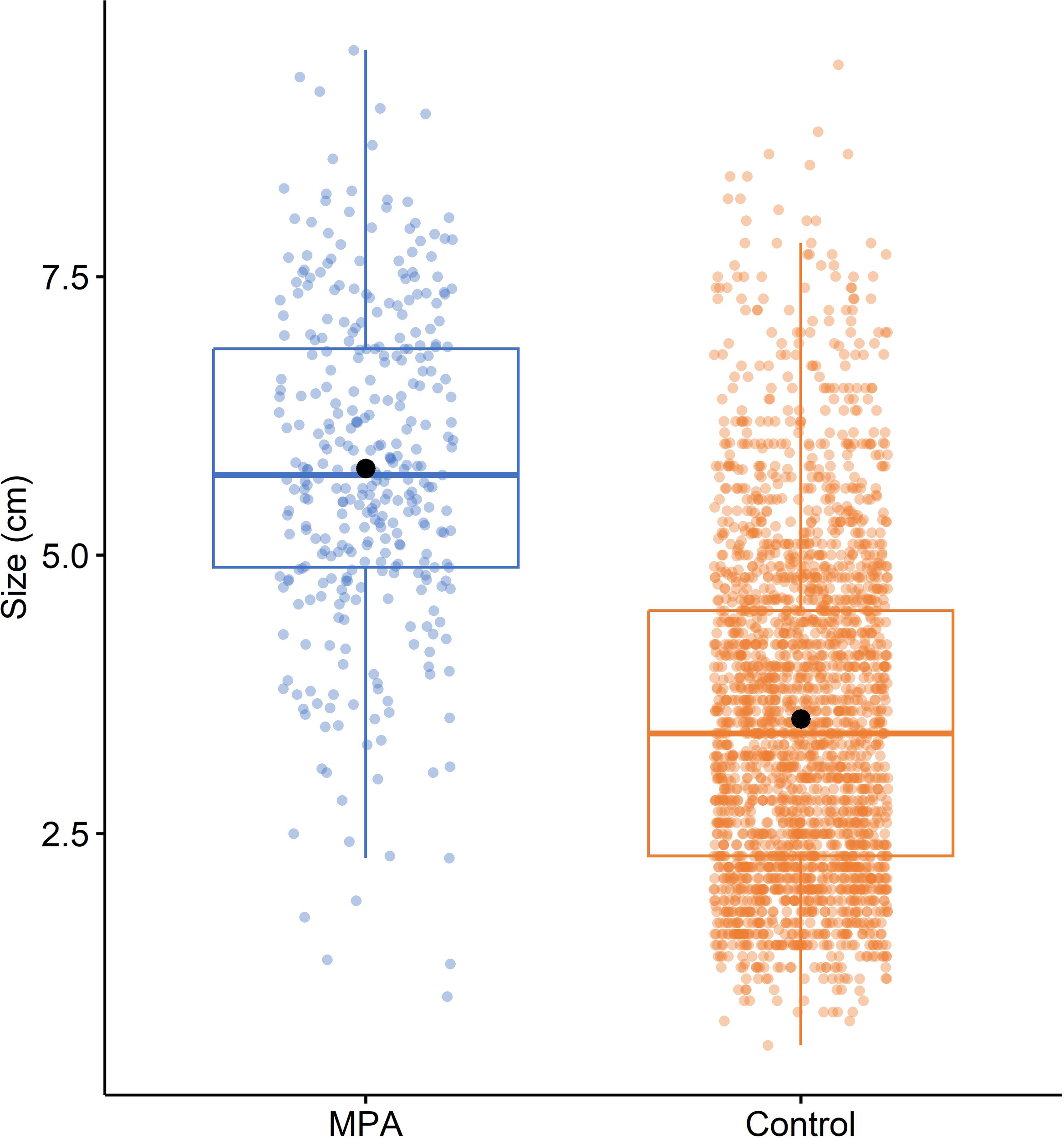
Boxplot for the size of Patella ferruginea (cm) in MPAs and control areas. Within each box, central lines denote median values, boxes extend from the 25th to 75th percentile, and whiskers denote values within 1.5 interquartile range of the 25th and 75th percentiles. Dots above or below the whiskers are outliers. Black dots show mean values.
After extracting data from the reviewed studies for sites where both the density and size of P. ferruginea individuals were sampled (18 studies), the relationship between these two variables was evaluated. Although a slight negative correlation was seen between the density and mean size of P. ferruginea individuals in both MPAs and control sites, Pearson’s correlation index was not statistically significant in any case (Figure 8).
Figure 8
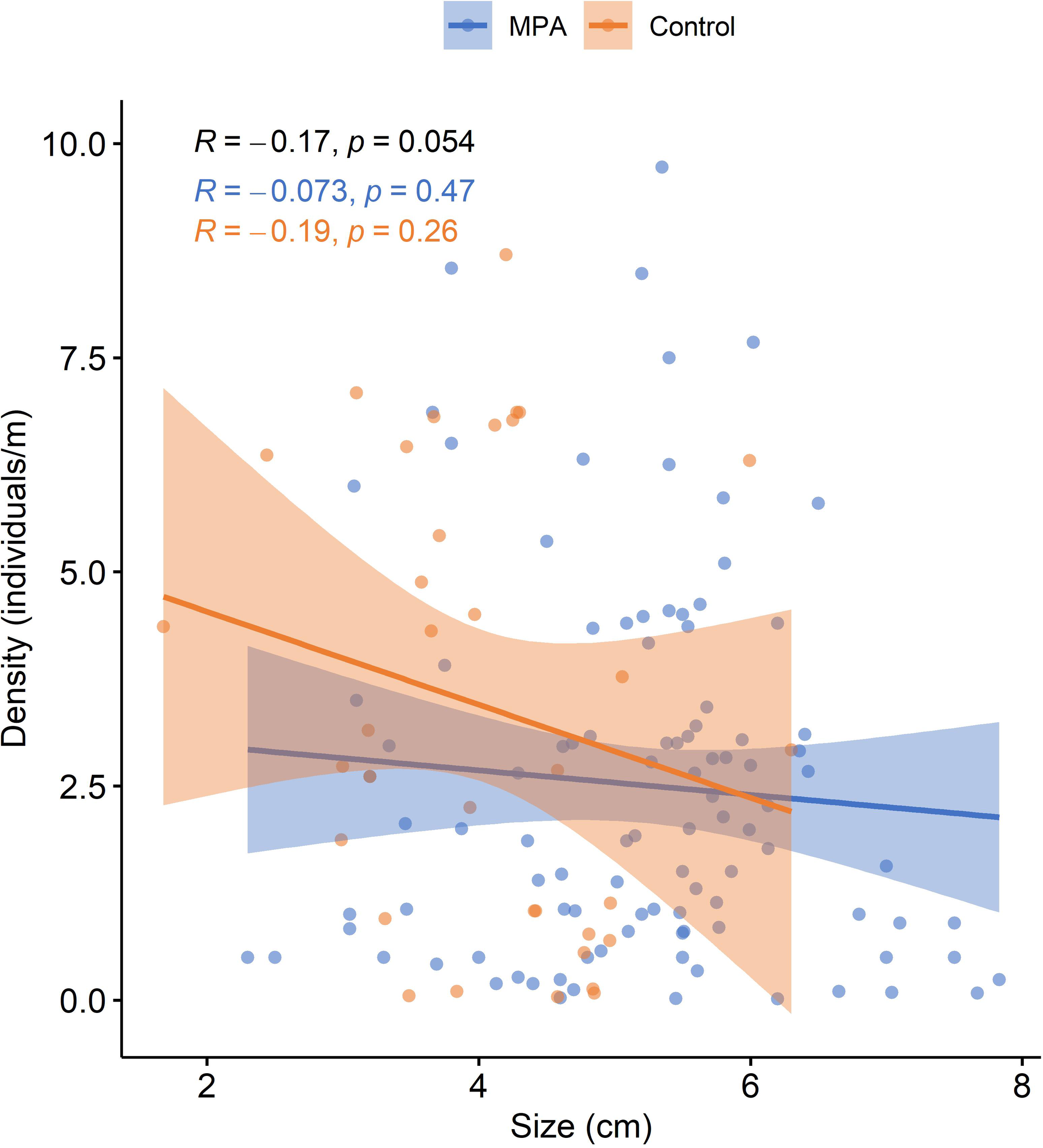
Mean density as a function of mean size of Patella ferruginea individuals in MPAs and control areas. Fitted lines from linear regression are presented with 95% confidence interval for MPAs (blue) and control areas (orange). R: Pearson’s correlation index for MPA data (blue), control area data (orange), and all data (black).
4 Discussion
The findings of this study reveal that among the five species of the genus Patella present in the Mediterranean, P. ferruginea is the most studied. This is not surprising, because P. ferruginea is one of the most threatened marine invertebrates in the Mediterranean Sea and is listed as “endangered” according to the Barcelona Convention (UNEP/MAP-SPA/RAC, 2018). However, although P. ferruginea is on the list of European regulations and several Mediterranean countries, it has not been evaluated by the IUCN Red List. Their endangered status can be attributed to human activities on the coast and excessive exploitation as food or fishing bait, as well as for ornamental purposes (Rivera-Ingraham, 2010; Tlig-Zouari et al., 2010). Studies on the density and size of the other four Mediterranean species are few. However, many studies have performed molecular and phylogenetic analyses on these species (Mauro et al., 2003; Casu et al., 2010; Prusina et al., 2014).
Regarding of studies on the density and size of species of the genus Patella, 67 sites were investigated, which most are located in the western Mediterranean Sea. Because P. ferruginea is the most vulnerable member of this genus and had almost completely disappeared from the European coasts (Templado and Moreno, 1997; Guallart et al., 2013), studies were performed in areas where the species persisted due to conservation concerns. The known distribution of P. ferruginea is practically limited to the coasts of North Africa—from the Strait of Gibraltar (Ceuta) to the Zembra Islands (Tunisia)—some parts of southern Spain (coasts of Málaga, Granada, and Almería), the Alboran Island, and the coasts of Corsica and Sardinia (Laborel-Deguen and Laborel, 1991; Templado et al., 2004; Moreno and Arroyo, 2008; Guallart and Templado, 2012). The hot spots of P. ferruginea must be protected as a priority owing to the general regression of its geographic distribution (Templado, 2001; Guerra-García et al., 2004; Templado et al., 2004; Espinosa, 2009; Espinosa et al., 2014).
Regarding the temporal distribution of studies on Patella (Figure 3), an increase was seen in the number of publications since the early 2000s, because most of the studies focused on P. ferruginea, which was listed as an endangered species in Spain in 1999 (BOE, 1999) and considered “threatened” by the EU Habitat Directive (Annex IV) and the Barcelona Convention (Annex II). Thus, the concern for P. ferruginea has increased because of its population decline.
Many studies have compared the densities of individuals in MPAs and control zones, confirming the presence of a positive effect on the density and size of limpets in protected areas. The effects of protection on limpets were observed in South Africa for Helcion concolor (Branch, 1975), Cymbula oculus (Branch and Odendaal, 2003; Baliwe et al., 2022), C. granatina, and Scutellastra argenvillei (Baliwe et al., 2022); in the Madeira archipelago for Patella aspera and P. candei (Martins et al., 2017; Sousa et al., 2019a; Sousa et al., 2019b; Sousa et al., 2020), in the Canary Islands for Patella ulyssiponensis (López et al., 2012); in Chile for Fissurella crassa and F. limbata (Durán and Castilla, 1989; Godoy and Moreno, 1989); and in the USA for Lottia gigantea (Sagarin et al., 2007; Fenberg and Roy, 2012). However, in this study, the mean density of P. ferruginea individuals was slightly higher in the control sites than in MPAs; the statistical analyses revealed no significant differences between the levels of protection, which is consistent with the results of Espinosa et al. (2009) for this species in the Mediterranean. In fact, intra- and interspecific competition effects, related to increase in limpet size and the consequent decrease in population density, were seen, because the distance between nearest neighbors increased as the sum of their sizes increased (Black, 1979; Espinosa et al., 2006; Espinosa et al., 2009). In this study, the populations with the highest average densities were in unprotected areas (Plane Island and Melilla). However, other factors may affect the density of individuals regardless of protection, such as the effect of hydrodynamism, because many studies have shown that limpet species tend to be more abundant in exposed rocks (Laborel-Deguen and Laborel, 1991; Marra et al., 2018). As key species of rocky ecosystems (Menge, 2000), limpets depend on the biota that surrounds them, such as the availability of food or the presence of natural predators, thus altering population densities.
The effect of protection on mean size showed different results than those observed for mean density; in this case, mean size was significantly greater in MPAs than control areas. Generally, larger individuals are the main target of illegal catches (Sagarin et al., 2007; Espinosa, 2009); therefore, in unprotected areas, where they can be easily caught, limpets had a lower mean size. Human pressure can shift the population structure toward higher frequencies of smaller individuals in unprotected areas (Kido and Murray, 2003), reducing the proportion of larger individuals (mostly females), reducing gonadal production, and lowering reproductive success, which is the main cause for population vulnerability (Ostalé-Valriberas et al., 2022). Therefore, if with the implementation of MPAs, big individuals could be preserved, as seems to happen for P. ferruginea in the Mediterranean, can be obtained by indirect effects regarding the reproductive output of these broadcast spawners.
Therefore, the major factor allowing for the coexistence of size classes is probably the effect of very high-intraspecific competition within large sizes, which reduces densities below levels at which small limpets can be eliminated (Boaventura et al., 2003). However, intraspecific competition depends on the species, because it is related to behavior and spatial distribution (Espinosa and Rivera-Ingraham, 2017). In P. ferruginea, intraspecific competition appears to be lower than in other species (Espinosa et al., 2006). By contrast, the recruitment of Patella is lower in sheltered areas (Fernández et al., 2016), which must be compensated with higher survival and growth rates, because lower densities allow for a lower degree of intraspecific competition. Moreover, some experimental studies support the hypothesis that greater growth in sheltered areas is specifically more due to lower density per unit grazeable area, meaning higher food availability (Jenkins and Hartnoll, 2001). However, as the study has shown, harvesting seems to be the main factor affecting the size classes of Patella spp., being significantly higher in MPAs (Espinosa et al., 2008), although the surveillance factor is fundamental (Coppa et al., 2016; Marra et al., 2018).
From the findings of this study, it can be concluded that of the five species of the genus Patella present in the Mediterranean, the most studied is P. ferruginea. This explains the temporal distribution of the studies, because the increase in studies occurred in the early 2000s, which coincides with the establishment of the conservation status and the concern about the population status of P. ferruginea. The analysis of the effects of protection on the density and mean size of P. ferruginea individuals revealed no significant differences in mean density between the control sites and MPAs. However, the mean size of individuals was significantly higher in MPAs than control sites. This pattern may be related to the intra- and interspecific competition between individuals.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AF and AC-S contributed to the conception and design of the study. AC-S and AR-E have collaborated in the bibliographic review. AC-S organized the database. AF and AC-S performed the statistical analysis. AC-S wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
Many thanks are due to the editor and to the reviewers for their comments and constructive criticism of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1158470/full#supplementary-material
References
1
Agardy M. T. (1994). Advances in marine conservation: the role of marine protected areas. Trends Ecol. Evol.9 (7), 267–270. doi: 10.1016/0169-5347(94)90297-6
2
Allison G. W. Lubchenco J. Carr M. H. (1998). Marine reserves are necessary but not sufficient for marine conservation. Ecol. Appl.8 (1), 79–92. doi: 10.2307/2641365
3
Anderson M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecol.26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
4
Baliwe N. G. Pfaff M. C. Branch G. M. (2022). Assessing the effects of no-take zones in a marine protected area spanning two ecoregions and rock substrate types. Front. Mar. Sci.9, 893260. doi: 10.3389/fmars.2022.893260
5
Ballantine W. J. Langlois T. J. (2008). Marine reserves: the need for systems. Challenges to Mar. Ecosyst.202, 35–44. doi: 10.1007/978-1-4020-8808-7_3
6
Bianchi C. N. Morri C. (2000). Marine biodiversity of the Mediterranean Sea: situation, problems and prospects for future research. Mar. pollut. Bull.40 (5), 367–376. doi: 10.1016/S0025-326X(00)00027-8
7
Black R. (1979). Competition between intertidal limpets: an intrusive niche on a steep resource gradient. J. Anim. Ecol.48, 401–411. doi: 10.2307/4169
8
Boaventura D. Da Fonseca L. C. Hawkins S. J. (2003). Size matters: competition within populations of the limpet Patella depressa. J. Anim. Ecol.72 (3), 435–446. doi: 10.1046/j.1365-2656.2003.00713.x
9
BOE (1999). Orden de 9 de junio de 1999 por la que se incluyen en el catálogo nacional de especies amenazadas determinadas especies de cetáceos, de invertebrados marinos y de flora y por la que otras especies se excluyen o cambian de categoría (Madrid, Spain: Boletín Oficial del Estado).
10
Branch G. M. (1975). Notes on the ecology of Patella concolor and Cellana capensis, and the effects of human consumption of limpet populations. Zoologica Africana10, 75–85. doi: 10.1080/00445096.1975.11447494
11
Branch G. M. Odendaal F. (2003). The effects of marine protected areas on the population dynamics of a south African limpet, Cymbula oculus, relative to the influence of wave action. Biol. Conserv.114, 255–269. doi: 10.1016/S0006-3207(03)00045-4
12
Burak S. Dogan E. Gazioglu C. (2004). Impact of urbanization and tourism on coastal environment. Ocean Coast. Manage.47 (9-10), 515–527. doi: 10.1016/j.ocecoaman.2004.07.007
13
Casu M. Sanna D. Cristo B. Lai T. Dedola G. L. Curini-Galletti M. (2010). COI sequencing as tool for the taxonomic attribution of patella spp. (Gastropoda): the case of morphologically undistinguishable juveniles settled on a patella ferruginea adult. J. Mar. Biol. Assoc. United Kingdom90 (7), 1449–1454. doi: 10.1017/S0025315409991603
14
Claudet J. Loiseau C. Sostres M. Zupan M. (2020). Underprotected marine protected areas in a global biodiversity hotspot. One Earth2 (4), 380–384. doi: 10.1016/j.oneear.2020.03.008
15
Claudet J. Osenberg C. W. Benedetti-Cecchi L. Domenici P. García-Charton J. A. Pérez-Ruzafa Á. et al . (2008). Marine reserves: size and age do matter. Ecol. Lett.11 (5), 481–489. doi: 10.1111/j.1461-0248.2008.01166.x
16
Coppa S. De Lucia G. A. Massaro G. Camedda A. Marra S. Magni P. et al . (2016). Is the establishment of MPAs enough to preserve endangered intertidal species? the case of Patella ferruginea in mal di ventre island (W Sardinia, Italy). Aquat. Conservation: Mar. Freshw. Ecosyst.26 (4), 623–638. doi: 10.1002/aqc.2579
17
Davenport J. Davenport J. L. (2006). The impact of tourism and personal leisure transport on coastal environments: a review. Estuarine Coast. Shelf Sci.67 (1-2), 280–292. doi: 10.1016/j.ecss.2005.11.026
18
Durán L. R. Castilla J. C. (1989). Variation and persistence of the middle rocky intertidal community of central Chile, with and without human harvesting. Mar. Biol.103, 555–562. doi: 10.1007/BF00399588
19
Espinosa F. (2009). Populational status of the endangered mollusc Patella ferruginea gmelin 1791 (Gastropoda, patellidae) on Algerian islands (SW Mediterranean). Anim. Biodiversity Conserv.32 (1), 19–28. doi: 10.32800/abc.2009.32.0019
20
Espinosa F. Guerra-García J. M. Fa D. García-Gómez J. C. (2006). Effects of competition on an endangered limpet Patella ferruginea (Gastropoda: patellidae): implications for conservation. J. Exp. Mar. Biol. Ecol.330 (2), 482–492. doi: 10.1016/j.jembe.2005.09.020
21
Espinosa F. Rivera-Ingraham G. A. (2017). Biological conservation of giant limpets: the implications of large size. Adv. Mar. Biol.76, 105–155. doi: 10.1016/bs.amb.2016.10.002
22
Espinosa F. Rivera-Ingraham G. A. Fa D. García-Gómez J. C. (2009). Effect of human pressure on population size structures of the endangered ferruginean limpet: toward future management measures. J. Coast. Res.25 (4), 857–863. doi: 10.2112/08-1005.1
23
Espinosa F. Rivera-Ingraham G. García-Gómez J. C. (2008). Seasonal activity and foraging behaviour of the endangered limpet patella ferruginea. Ethology Ecol. Evol.20 (2), 173–181. doi: 10.1080/08927014.2008.9522537
24
Espinosa F. Rivera-Ingraham G. A. Maestre M. González A. R. Bazairi H. García-Gómez J. C. (2014). Updated global distribution of the threatened marine limpet Patella ferruginea (Gastropoda: patellidae): an example of biodiversity loss in the Mediterranean. Oryx48 (2), 266–275. doi: 10.1017/S0030605312000580
25
Fenberg P. B. Roy K. (2012). Anthropogenic harvesting pressure and changes in life history: insights from a rocky intertidal limpet. Am. Nat.180, 200–210. doi: 10.1086/666613
26
Fernández N. Alborés I. Aceña-Matarranz S. (2016). Spatial variability of the reproductive cycle and physiological condition of patella spp. (Mollusca Gastropoda) in the NW of the Iberian peninsula: implications for exploitation. Fisheries Res.179, 76–85. doi: 10.1016/j.fishres.2016.02.010
27
García-Gómez J. C. Guerra-García J. M. Espinosa F. Maestre M. J. Rivera-Ingraham G. Fa D. et al . (2015). Artificial marine micro-reserves networks (AMMRNs): an innovative approach to conserve marine littoral biodiversity and protect endangered species. Mar. Ecol.36 (3), 259–277. doi: 10.1111/maec.12167
28
García-Gómez J. C. López-Fé C. M. Espinosa F. Guerra-García J. M. Rivera-Ingraham G. A. (2011). Marine artificial micro-reserves: a possibility for the conservation of endangered species living on artificial substrata. Mar. Ecol.32 (1), 6–14. doi: 10.1111/j.1439-0485.2010.00409.x
29
Garrabou J. Gómez-Gras D. Medrano A. Cerrano C. Ponti M. Schlegel R. et al . (2022). Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Global Change Biol.28 (19), 5708–5725. doi: 10.1111/gcb.16301
30
Giorgi F. (2006). Climate change hot-spots. Geophysical Res. Lett.33 (8), L08707. doi: 10.1029/2006GL025734
31
Godoy C. Moreno C. A. (1989). Indirect effects of human exclusion from the rocky intertidal in southern Chile: a case of cross-linkage between herbivores. Oikos54, 101–106. doi: 10.2307/3565902
32
Gofas S. Salas C. Moreno D. (2011). Moluscos marinos de andalucía (Vol. 1) (Málaga: Universidad de Málaga, Servicio de Publicaciones e Intercambio Científico), 342.
33
Guallart J. Luque Á.A. Acevedo I. Calvo M. (2013). Distribución y censo actualizado de la lapa ferrugínea (Patella ferruginea gmelin 1791) en el litoral de melilla (Mediterráneo suroccidental). Iberus31 (1), 21–51.
34
Guallart J. Templado J. (2012). Patella ferrugínea. bases ecológicas preliminares para la conservación de las especies de interés comunitario en españa: invertebrados (Madrid: Ministerio de Agricultura, Alimentación y Medio Ambiente), 86.
35
Guerra M. T. Gaudencio M. J. (1986). Aspects of the ecology of patella spp. on the Portuguese coast. Hydrobiologia142, 57–69. doi: 10.1007/BF00026747
36
Guerra-García J. M. Corzo J. Espinosa F. García-Gómez J. C. (2004). Assessing habitat use of the endangered marine mollusc Patella ferruginea (Gastropoda, patellidae) in northern Africa: preliminary results and implications for conservation. Biol. Conserv.116 (3), 319–326. doi: 10.1016/S0006-3207(03)00201-5
37
Halpern B. S. (2003). The impact of marine reserves: do reserves work and does reserve size matter? Ecol. Appl.13 (1), 117–137. doi: 10.1890/1051-0761(2003)013[0117:TIOMRD]2.0.CO;2
38
Hockey P. A. R. (1994). “Man as a component of the littoral predator spectrum: a conceptual overview,” in Rocky shores: exploitation in Chile and south Africa. Ed. SiegfrieldW. R. (Berlin, Heidelberg: Springer). vol 103, 17–31.
39
Jackson J. B. C. Kirby M. X. Berger W. H. Bjorndal K. A. Botsford L. W. Bourque B. J. et al . (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science293 (5530), 629–637. doi: 10.1126/science.1059199
40
Jenkins S. R. Hartnoll R. G. (2001). Food supply, grazing activity and growth rate in the limpet Patella vulgata l.: a comparison between exposed and sheltered shores. J. Exp. Mar. Biol. Ecol.258 (1), 123–139. doi: 10.1016/s0022-0981(01)00211-8
41
Keough M. J. Quinn G. P. King A. (1993). Correlations between human collecting and intertidal mollusc populations on rocky shores. Conserv. Biol.7 (2), 378–390. doi: 10.1046/j.1523-1739.1993.07020378.x
42
Kido J. S. Murray S. N. (2003). Variation in owl limpet Lottia gigantea population structures, growth rates, and gonadal production on southern California rocky shores. Mar. Ecol. Prog. Ser.257, 111–124. doi: 10.3354/meps257111
43
Kingsford M. J. Underwood A. J. Kennelly S. J. (1991). Humans as predators on rocky reefs in new south Wales, Australia. Mar. Ecol. Prog. Ser.72, 1–14. doi: 10.3354/meps072001
44
Laborel-Deguen F. Laborel J. (1991), 91–103. Statut de Patella ferruginea Gmelin en Méditerranée. Les Espèces marines à protéger en Méditerranée, GIS Posidonie.
45
Lasiak T. (2006). Spatial variation in density and biomass of patellid limpets inside and outside a marine protected area. J. Molluscan Stud.72, 137–142. doi: 10.1093/mollus/eyi058
46
Lester S. E. Halpern B. S. Grorud-Colvert K. Lubchenco J. Ruttenberg B. I. Gaines S. D. et al . (2009). Biological effects within no-take marine reserves: a global synthesis. Mar. Ecol. Prog. Ser.384, 33–46. doi: 10.3354/meps08029
47
Lionello P. Malanotte-Rizzoli P. Boscolo R. Alpert P. Artale V. Li L. et al . (2006). The Mediterranean climate: an overview of the main characteristics and issues. Developments Earth Environ. Sci.4, 1–26. doi: 10.1016/S1571-9197(06)80003-0
48
López C. Poladura A. Hernández J. C. Martín L. Concepción L. Sangil C. et al . (2012). Contrasting effects of protection from harvesting in populations of two limpet species in a recently established marine protected area. Scientia Marina76, 799–807. doi: 10.3989/scimar.03601.15C
49
Mannino M. A. Thomas K. D. (2002). Depletion of a resource? the impact of prehistoric human foraging on intertidal mollusc communities and its significance for human settlement, mobility and dispersal. World Archaeology33 (3), 452–474. doi: 10.1080/00438240120107477
50
Marra S. Coppa S. Camedda A. Massaro G. de Lucia G. A. (2018). The exploitation of limpets in a Mediterranean marine protected area: assessing the effectiveness of protection in the intertidal zone. Mediterr. Mar. Sci.18 (3), 406–423. doi: 10.12681/mms.2087
51
Martins G. M. Borges C. D. G. Vale M. Ribeiro P. A. Ferraz R. R. Martins H. R. et al . (2017). Exploitation promotes earlier sex change in a protandrous patellid limpet, Patella aspera rödin. Ecol. Evol.7, 3616–3622. doi: 10.1002/ece3.2925
52
Mauro A. Arculeo M. Parrinello N. (2003). Morphological and molecular tools in identifying the Mediterranean limpets Patella caerulea, patella aspera and Patella rustica. J. Exp. Mar. Biol. Ecol.295 (2), 131–143. doi: 10.1016/S0022-0981(03)00291-0
53
McGranahan G. Balk D. Anderson B. (2007). The rising tide: assessing the risks of climate change and human settlements in low elevation coastal zones. Environ. Urbanization19 (1), 17–37. doi: 10.1177/0956247807076960
54
Menge B. A. (2000). Top-down and bottom-up community regulation in marine rocky intertidal habitats. J. Exp. Mar. Biol. Ecol.250 (1-2), 257–289. doi: 10.1016/S0022-0981(00)00200-8
55
Moher D. Liberati A. Tetzlaff J. Altman D. G. The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med.6, e1000097. doi: 10.1371/journal.pmed.1000097
56
Moreno D. Arroyo M. C. (2008). “Patella ferruginea gmeli,” in Libro rojo de los invertebrados de andalucía, vol. I . Eds. Barea-AzcónJ. M.Ballesteros-DuperónE.MorenoD. (Junta de Andalucía, Sevilla: Consejería de Medio Ambiente), 308–319.
57
Murawski S. A. (2000). Definitions of overfishing from an ecosystem perspective. ICES J. Mar. Sci.57 (3), 649–658. doi: 10.1006/jmsc.2000.0738
58
Oksanen J. Guillaume Blanchet F. Friendly M. Kindt R. Legendre P. McGlinn D. et al . (2019) Vegan: community ecology package. r package version 2.5-6. Available at: https://CRAN.R-project.org/package=vegan.
59
Ostalé-Valriberas E. Sempere-Valverde J. Pavón-Paneque A. Coppa S. Espinosa F. García-Gómez J. C. (2022). Artificial marine micro-reserves as a new ecosystem-based management tool for marine conservation: the case of Patella ferruginea (Gastropoda, patellidae), one of the most endangered marine invertebrates of the Mediterranean. Mar. Policy136, 104917. doi: 10.1016/j.marpol.2021.104917
60
Paracuellos M. Nevado J. C. Moreno D. Giménez A. Alesina J. J. (2003). Conservational status and demographic characteristics of Patella ferruginea gmelin 1791 (Mollusca, gastropoda) on the alboran island (Western Mediterranean). Anim. Biodiversity Conserv.26 (2), 29–37.
61
Power M. E. Tilman D. Estes J. A. Menge B. A. Bond W. J. Mills L. S. et al . (1996). Challenges in the quest for keystones: identifying keystone species is difficult but essential to understanding how loss of species will affect ecosystems. BioScience46 (8), 609–620. doi: 10.2307/1312990
62
Probert P. K. (2017). Marine conservation (Cambridge, United Kingdom: Cambridge University Press).
63
Prusina I. Ezgeta-Balić D. Ljubimir S. Dobroslavić T. Glamuzina B. (2014). On the reproduction of the Mediterranean keystone limpet Patella rustica: histological overview. J. Mar. Biol. Assoc. United Kingdom94 (8), 1651–1660. doi: 10.1017/S0025315414000976
64
QGIS Development Team (2022). QGIS geographic information system (QGIS Association). Available at: http://www.qgis.org.
65
Raffaelli D. Hawkins S. (1996). Intertidal ecology (London: Chapman and Hall).
66
R Core Team (2020). R: a language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org.
67
Rivera-Ingraham G. A. (2010). Biología de la conservación de especies de patélidos en el umbral atlántico-mediterráneo (Sevilla, Spain: University of Seville). [dissertation/PhD thesis.
68
Rivera-Ingraham G. A. Espinosa F. García-Gómez J. C. (2011). Environmentally mediated sex change in the endangered limpet patella ferruginea (Gastropoda: patellidae). J. Molluscan Stud.77 (3), 226–231. doi: 10.1093/mollus/eyr007
69
Sagarin R. D. Ambrose R. F. Becker B. J. Engle J. M. Kido J. Lee S. F. et al . (2007). Ecological impacts on the limpet lottia gigantea populations: human pressure over a broad scale on island and mainland intertidal zones. Mar. Biol.150, 399–413. doi: 10.1007/s00227-006-0341-1
70
Scheffer M. Carpenter S. de Young B. (2005). Cascading effects of overfishing marine systems. Trends Ecol. Evol.20 (11), 579–581. doi: 10.1016/j.tree.2005.08.018
71
Schmitt R. J. Osenberg C. W. (1996). Detecting ecological impacts: concept and applications in coastal habitats (San Diego: Academic Press).
72
Selig E. R. Bruno J. F. (2010). A global analysis of the effectiveness of marine protected areas in preventing coral loss (5(2: PLoS One).
73
Smith H. D. (2000). The industrialisation of the world ocean. Ocean Coast. Manage.43 (1), 11–28. doi: 10.1016/S0964-5691(00)00028-4
74
Sousa R. Henriques P. Vasconcelos J. Pinto A. R. Delgado J. Riera R. (2020). The protection effects of marine protected areas on exploited molluscs from an oceanic archipelago. Aquat. Conservation: Mar. Freshw. Ecosyst.30, 717–729. doi: 10.1002/aqc.3285
75
Sousa R. Vasconcelos J. Henriques P. Pinto A. R. Delgado J. Riera R. (2019a). Long-term population status of two harvested intertidal grazers (Patella aspera and Patella candei), before, (1996-2006) and after, (2007-2017) the implementation of management measures. J. sea Res.144, 33–38. doi: 10.1016/j.seares.2018.11.002
76
Sousa R. Vasconcelos J. Riera R. Pinto A. R. Delgado J. Henriques P. (2019b). Potential impact of harvesting management measures on the reproductive parameters of the limpets Patella aspera and Patella candei from Madeira island. Estuarine Coast. Shelf Sci.226, 106264. doi: 10.1016/j.ecss.2019.106264
77
Stanchev H. Stancheva M. Young R. (2015). Implications of population and tourism development growth for Bulgarian coastal zone. J. Coast. Conserv.19 (1), 59–72. doi: 10.1007/s11852-014-0360-x
78
Templado J. (2001). “Patella ferruginea (Gmelin 1791),” in Los Invertebrados no insectos de la directiva hábitats en españa. ediciones serie técnica, organismo autónomo parques nacionales, dirección general de conservación de la naturaleza. Eds. RamosM. A.BragadoD.FernándezJ. (Madrid: Ministerio de Medio Ambiente), 41–49.
79
Templado J. Calvo M. Garvía A. Luque A. Maldonado M. Moro L. (2004). Guía de invertebrados y peces marinos protegidos por la legislación nacional e internacional (58-59: M° Medio Ambiente-CSIC).
80
Templado J. Moreno D. (1997). La lapa ferrugínea. Biológica: conocer y conservar la naturaleza6, 80–81.
81
Thompson R. C. Crowe T. P. Hawkins S. J. (2002). Rocky intertidal communities: past environmental changes, present status and predictions for the next 25 years. Environ. Conserv.29 (2), 168–191. doi: 10.1017/S0376892902000115
82
Tlig-Zouari S. Rabaoui L. Fguiri H. Ben Hassine O. K. (2010). Status, habitat and distribution of the endangered limpet Patella ferruginea along the northern and eastern Tunisian coastline: results and implications for conservation. Cahiers Biologie Mar.51 (1), 75.
83
UNEP/MAP-SPA/RAC (2018). Annex II: list of endangered or threatened species (Tunis Cedex, Tunisia: SAP/ RAC, SPA-BD Protocol).
84
Wickham H. (2016). ggplot2: elegant graphics for data analysis (New York: Springer-Verlag).
85
Zahedi S. (2008). Tourism impact on coastal environment. WIT Trans. Built Environ.99, 45–57. doi: 10.2495/CENV080051
Summary
Keywords
Patella spp., Mediterranean Sea, density, size, marine protected areas
Citation
Cascales-Soler A, Ramos-Espla AA and Forcada A (2023) Reviewing the knowledge on the genus Patella in the Mediterranean Sea: testing the effect of protection on the mean abundance and size of Patella ferruginea. Front. Mar. Sci. 10:1158470. doi: 10.3389/fmars.2023.1158470
Received
03 February 2023
Accepted
02 May 2023
Published
19 May 2023
Volume
10 - 2023
Edited by
Diego Castejón Bueno, Centro Interdisciplinar de Investigação Marinha e Ambiental da Madeira (CIIMAR-Madeira), Portugal
Reviewed by
Free Espinosa Torre, Sevilla University, Spain; Salvatore Giacobbe, University of Messina, Italy; José Carlos García-Gómez, University of Seville, Spain
Updates
Copyright
© 2023 Cascales-Soler, Ramos-Espla and Forcada.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aitor Forcada, forcada@ua.es
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.