- 1Graduate School of Fisheries and Environmental Sciences, Nagasaki University, Nagasaki, Japan
- 2Atmosphere and Ocean Research Institute, The University of Tokyo, Kashiwa, Chiba, Japan
- 3Faculty of Life and Environmental Sciences, Teikyo University of Science, Uenohara, Yamanashi, Japan
- 4Faculty of Bioindustry, Tokyo University of Agriculture, Abashiri, Hokkaido, Japan
- 5Fisheries Resources Institute, Japan Fisheries Research and Education Agency, Yokohama, Kanagawa, Japan
- 6National Museum of Nature and Science, Tsukuba, Ibaraki, Japan
Male sperm whales are under pressure to grow larger in order to increase their mating opportunities, which could lead them to more efficiently forage in high latitude feeding grounds. Movement patterns of male sperm whales in Nemuro Strait, Japan, were investigated horizontally and vertically using land-based observation and bio-logging methods to determine how they facilitate foraging in the narrow submarine canyon. Eleven tagged whales showed the distinct diel pattern for dive depth, as it was deeper at night than during the day. Five-year data of land-based observation and GPS data from six tagged whales revealed the tendency of whales to change the north-south direction of their horizontal movement every 4–6 h, and this movement direction was not related to the direction of the current. Their periodic heading change is thought to be a consequence of the whales making two round trips each day within the foraging area, one during the day to shallow layers and one during the night to deep layers. These tactics may help the whales to search for prey in this narrow submarine canyon efficiently. Most whales changed their direction of movement in a similar manner, which is probably due to the whales’ tendency to stay close enough to each other to obtain information about the prey environment using the echolocation clicks of other whales. The results emphasize the ability of male sperm whales to adapt their foraging tactics according to the prey environment of their habitat and intense pressure to grow faster may be the drive for this ability. The importance of social cohesion among foraging male sperm whales was also suggested.
Introduction
Sperm whales (Physeter macrocephalus) inhabit pelagic waters of all ocean basins, but their distribution varies by sex and age. Female sperm whales and their calves live in stable social units in low to mid-latitude waters (Whitehead, 2003), and most females remain in the units for their entire lives (Christal et al., 1998). Contrarily, males leave their natal units before reaching sexual maturity, and form groups called “bachelors schools” with individuals of similar size and move to higher latitudes than females (Gaskin, 1970; Ohsumi, 1971; Best, 1979). As they grow older, the male groups gradually shift their habitat to higher latitudes and decrease in number of members (Gaskin, 1970; Ohsumi, 1971; Best, 1979). Eventually, males become solitary at 40 years or older and occasionally migrate to lower latitude waters inhabited by females to mate (Whitehead and Weilgart, 2000).
Sperm whales, which are deep divers that prey on meso- and bathypelagic cephalopods and fish, are known to change their foraging behaviors in relation to prey availability. Predators should decide where and how to feed to enhance foraging success and, ultimately, their fitness (Stephens and Krebs, 1986). Diving predators can maximize their foraging success by tuning their horizontal and vertical movement patterns (Austin et al., 2006; Dragon et al., 2012). The horizontal movement of foraging females and immature groups of sperm whales has been studied in several locations in the Pacific Ocean (Jaquet et al., 2000). The results showed that sperms whales tend to stay in relatively small areas of approximately 20 km across when foraging was successful, as measured by their defecation rates, and moved straight when foraging was less successful.
The diel diving behavior of sperm whales has been investigated using multi-sensor suction cup attached tags or archival tags in a few decades (e.g. Aoki et al., 2007; Teloni et al., 2008; Fais et al., 2015; Guerra et al., 2017; Irvine et al., 2017; Chambault et al., 2021). Sperm whales alter their dive depth, i.e., vertical movement, according to the vertical distribution of their prey. Female sperm whales off the coast of the Ogasawara Islands off Japan have been observed to alter their dive depths between day and night, likely in response to the concentration of vertically migrating prey at the boundary of water masses during the night (Aoki et al., 2007). In contrast, whales off the Kumano coast of Japan, where no such water mass structure is present, did not exhibit this diel pattern (Aoki et al., 2007). Diel changes in sperm whale dive depth in the Indian Ocean have indicated that they may change according to the vertical migration of the prey (Chambault et al., 2021). Male sperm whales off the coast of Norway are known to forage in shallow depths (depths of 17 m) as well as deep depths (depths of > 1000 m), suggesting that they change their foraging behaviors irrespective of it being day or night and thus the vertical migration of their prey (Teloni et al., 2008). Male sperm whales in Kaikoura submarine canyon, New Zealand, mainly forage in three different vertical strata; a demersal and two mid-water layers (Guerra et al., 2017). The plasticity in foraging behavior is facilitated by a long-range sonar system, which enables sperm whales to detect prey or prey patches efficiently at a distance of several hundred meters (Madsen et al., 2002; Teloni et al., 2008; Fais et al., 2015; André et al., 2017; Tønnesen et al., 2020). Whales are thus able to identify foraging layers or areas on a relatively narrow range of a few kilometers or dive-to-dive scale by searching around using a sonar system at depth. However, whales must also decide where and how to forage in the entire foraging area, or on a larger scale.
Sperm whales are the most striking example of sexual dimorphism observed in mammals, where sexual selection favors larger males. Thus, male sperm whales are thought to migrate to higher latitudes than reproductive females to seek prey and grow larger to increase mating opportunities when they return to lower latitudes inhabited by females (Whitehead, 2003). Although the habitat use of foraging male sperm whales is less understood than that of females, it has been reported that they tend to stay in somewhat limited foraging areas, including submarine canyons (Whitehead et al., 1992; Teloni et al., 2008; Sagnol et al., 2014; Kobayashi and Amano, 2020). Because of the limited paternities of calves in a female social unit compared to the number of adult males present in the vicinity of the unit during the breeding season, it has been proposed that male-male competition or female choice is a major part of the sperm whale mating system (Whitehead, 2003). Effective breeding among males is only possible after they reach the age of 30, ten to fifteen years after they reach sexual maturity. Males are thought to gain an advantage in gaining preferential access to mates by growing larger during this period (Whitehead, 1994). Given the increased pressure to grow larger, it is imperative for male sperm whales to effectively search for food and promote their own growth. For efficient foraging, sperm whales should modify foraging behaviors both vertically, that is diving, and horizontally to target high prey density layers and locations (Fais et al., 2015; Irvine et al., 2017). However, to date, there have been no studies which have examined the characteristics of male sperm whales foraging behaviors based on both horizontal and vertical movement data.
Male sperm whales migrate into Nemuro Strait, Hokkaido, Japan, during the summer period (Kobayashi and Amano, 2020). All whales were presumed to be male as no animals were seen with a calf, and none were less than 12 m long [estimated photogrammetrically following the procedures of Gordon (1990) and Jaquet (2006)], the documented maximum length of female sperm whales (Best, 1969). Whales usually dispersed and engaged in foraging dives in the strait. We carried out hill-top theodolite surveys and deployments of depth and GPS data loggers to reveal their horizontal and vertical foraging patterns and understand how they utilize this foraging ground both vertically and horizontally. The results showed the stereotypical horizontal movement pattern of male sperm whales, which was not related to the direction of the current in the strait. We discuss the possibility that the observed diel pattern of dive depth and horizontal movement pattern is whales’ tactics to facilitate their foraging in the narrow submarine canyon. We further suggest that male sperm whales move in synchrony to share information about the prey environment that each whale obtained by echolocation, and this collaboration in foraging may be a key factor of the social bonds among males.
Materials and methods
Theodolite survey
We established a theodolite station at the Whale View Park in Rausu Town, Hokkaido (44°02’N, 145°13’E; 73 m above sea level, Figure 1) between August and September 2010–2014 with an observation range of ~15 nm (27.8 km; 98.3% of 3,339 whale sightings were in this range, Supplementary Figure 1). Sighting was carried out every day from sunrise to 30 min before sunset when the following conditions were met: visibility > 5 nm (determined by measuring the distance to an object on the surface, typically fishing vessels, using a theodolite), Beaufort wind scale < 5, and no rainfall. Four experienced researchers were tasked with searching for whale blows with binoculars (7x and 12x magnifications) on the tripods. They conducted a random search within the entire visibility range, and whales in the area were effectively detected with this method. After a whale was spotted, horizontal and vertical angles to the whale’s blowhole were measured using a DT500S theodolite (Sokkia, Atsugi, Japan), and its location was calculated and recorded by Cyclops Tracker 2.4 software (Eric Kniest, University of Newcastle, Australia). Since the sperm whales dispersed over a few kilometers while foraging (Whitehead, 2003), almost all whales were found alone (98.8% of 3,339 sightings) in our study. This is common for male sperm whales (Whitehead et al., 1992; Whitehead, 1993). We tracked the whale from the discovery to fluke up with the theodolite and only used the first location determined as the position of the whale to avoid pseudo-replication. The water depth of the whale’s location was determined using the 500 m gridded bathymetry data (Japan Oceanographic Data Center, http://www.jodc.go.jp/jodcweb/JDOSS/index.html). We determined the whale’s movement direction based on its surfacing movement between blows recorded as ‘outward’ when the whale moved leftward in the telescope field of the theodolite or ‘inward’ when the whale moved rightward in relation to movement toward the inside or outside of Nemuro Strait. If two or more whales were in a cluster, we only determined the position and direction of one whale in the cluster. When whales changed their movement direction or direction was not able to be defined in the theodolite field during surfacing, it was recorded as ‘unknown’. Once whales dove, we were not able to follow the same individual. Thus, the movement direction was recorded only during the whale was at the surface for respiration.
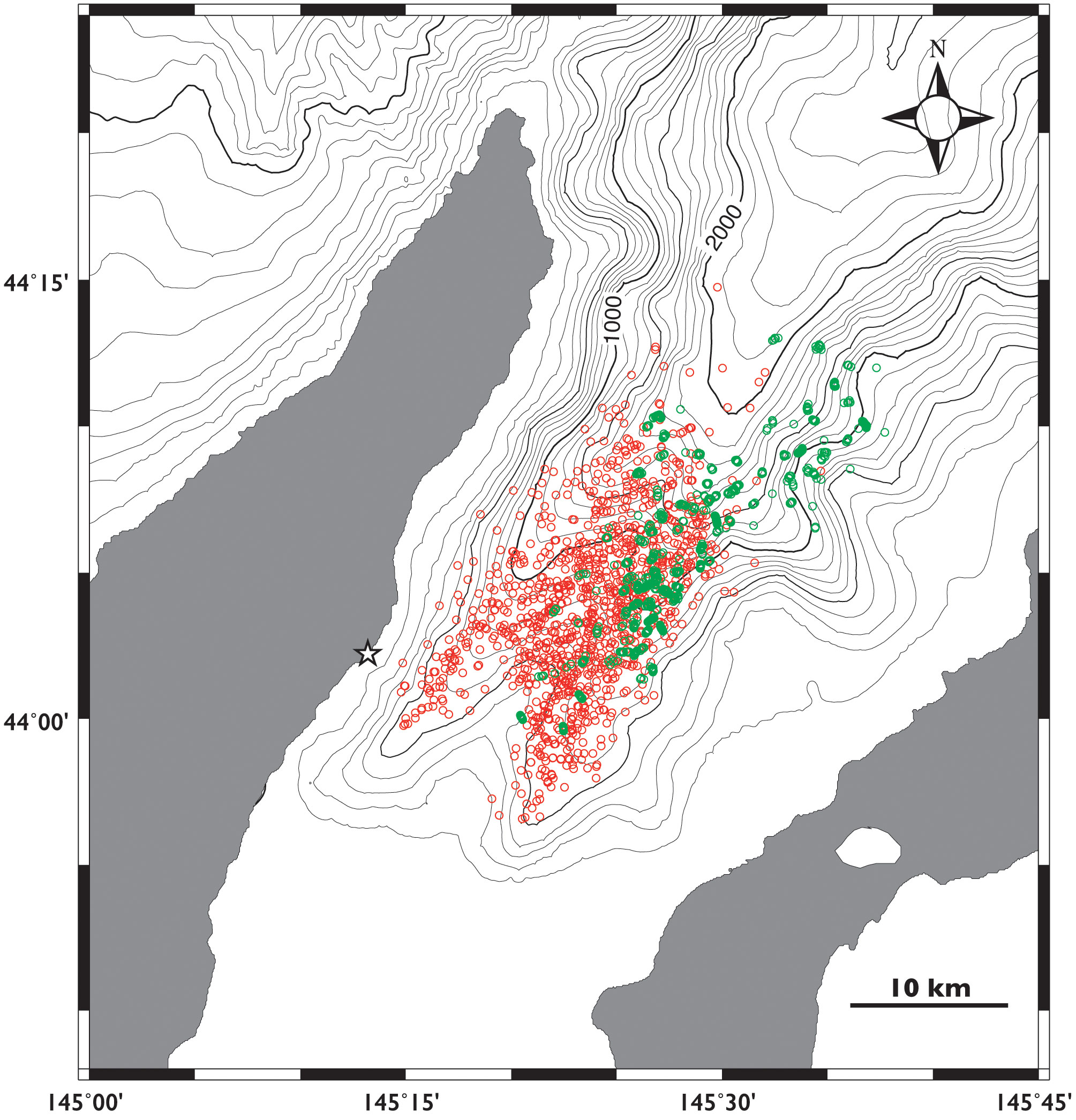
Figure 1 Locations of sperm whales obtained by theodolite tracking in the 2010 season (red circles) and Fastloc GPS on the tagged whales (green circles) in Nemuro Strait, Japan. Star indicates the theodolite station.
We examined whether the current affected their movement direction. The water current in Nemuro Strait is mostly created by the tide and flows inward in flood tide and outward in ebb tide (Japan Coast Guard, http://www1.kaiho.mlit.go.jp/KAN1/soudan/kaikyo.html). The tidal data of Rausu port were obtained from the Japan Metrological Agency (https://www.data.jma.go.jp/kaiyou/db/tide/suisan/index.php). We found that ebb tide was predominant in the morning during the survey period when inward movements of the whales were also predominant (Supplementary Figure 2). Therefore, we calculated the proportion of hours in which the tide was ebb for each hour of the day (5:00 to 17:00) and the proportion of whales moving inward in each hour of the day and examined their correlation using Kendall rank correlation coefficient.
Bio-logging survey
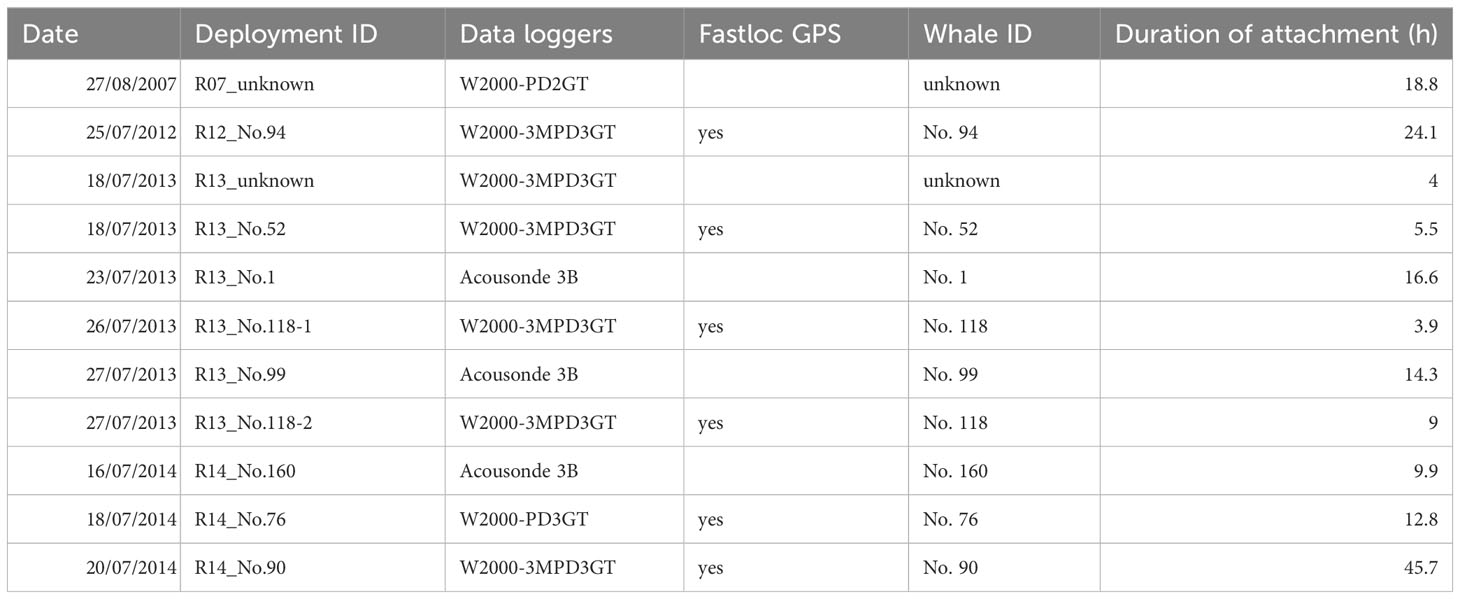
Bio-logging surveys were conducted in Nemuro Strait in July 2007 and 2012-2014. We approached male sperm whales with a fishing boat (length: 16 m) and deployed suction-cup attached tags, including animal-borne devices, using a 13-15 m pole. Two types of suction-cup attached tags were used: tags modified version of the Type B tag (Aoki et al., 2012) and Acousonde 3B tag (229 mm in length, 362 g in the air, Greeneridge Science, Santa Barbra, California, USA). The former tags consisted of a suction-cup (Canadian Tire), a synthetic foam float, a data logger, and a GPS data logger (F2G134A, Fastloc 2, Sirtrack, Havelock North, New Zealand), We used three models of depth data loggers (Little Leonardo, Tokyo, Japan); W2000-PD2GT (23 mm in diameter, 123 mm in length, 90 g in the air), W2000-PD3GT (23 mm in diameter, 123 mm in length, 90 g in the air), or W2000-3MPD3GT (30 mm in diameter, 175 mm in length,140 g in the air). The total weight of the tags ranged from 370 to 410 g. Both types of tags contained a VHF radio transmitter (Advanced Telemetry Systems, Isanti, MN, USA) for the tag recovery after detachment. The Little Leonardo data loggers recorded depth at 1 Hz, while the Acousonde at 10 Hz intervals, which was down-sampled to 1Hz. The depth sensor of each data logger had a measurement range of 0 to 2000 m, with a resolution of 0.5 m. The GPS data loggers were set to record the horizontal location of the tagged whales every 30 sec.
The start and end of dives were defined as the times when the whales descended below and ascended above a depth of 4 m, respectively (Aoki et al., 2012). We considered dives deeper than 200 m as foraging dives. The bottom phase of the foraging dives was defined from the first time at which the depth change rate was negative to the last time at which the depth change rate was positive (see Aoki et al. (2007) for details). The dives were divided into day and night groups based on the local sunrise and sunset times. The differences in dive depth between day and night were examined using a generalized linear mixed model (GLMM). The model included the average bottom depth of each dive as a dependent variable, day or night as an explanatory variable, and the individual as a random factor, with Gaussian error structure and identity link function. We deployed the tag twice for the same individual, No. 118, in 2013. We treated these two deployments as different random factor values.
The water depth of each dive location was determined from the 500 m gridded bathymetry data (Japan Oceanographic Data Center, http://www.jodc.go.jp/jodcweb/JDOSS/index.html), and the last Fastloc GPS location obtained during the surface time before the dive. If the location was not obtained during the surface time, we did not determine the water depth for that dive. We examined whether the dive depths (maximum depth and average bottom depth; dependent variable) were related to the water depth (explanatory variable) using a GLMM with each individual as a random factor with Gaussian error structure and identity link function.
Fastloc GPS data were used to calculate the whales’ hourly heading and horizontal move distance as the direction and distance between the last GPS location obtained in the previous hour of the day and the last GPS location in the hour being evaluated. We then calculated inward-outward horizontal movement distance as
where θ is the hourly heading in degrees with north as 0°, d is the hourly distance in km, and t is time span in hours between the time when the last GPS location was obtained in each hour. As the direction of the axis of Nemuro Strait is approximately 30° from the north, we subtracted 30° in the formula, and thus positive value indicates outward movement. We did not calculate the movement distances if GPS location was not obtained or a long surface time was included within a certain hour, which happened in 23 of 105 total hours of GPS deployment. To examine whether the tidal current influenced the inward-outward distance, we used the tide height change in each hour of the day as an index of the strength of the tidal current. The effect of the tidal current index (explanatory variable) on the inward-outward movement distance (dependent variable) was examined using the GLMM with each individual as a random factor with Gaussian error structure and identity link function.
All statistical analyses were performed using JMP Pro 15 software (SAS Institute Inc., Cary, NC, USA).
Results
Theodolite survey
We obtained a total of 3,339 sightings of sperm whales in 1,173 hours over 154 days of the survey from 2010 to 2014 (Supplementary Table 1). The sighting locations for the sperm whales were mostly limited to waters with depths of 500–2000 m (94.2% of 3,339 locations recorded, Figure 1). The whales were found to have a general daily movement pattern, as they tended to be found in the deeper waters at depths of deeper than 1000 m in the early morning and then moved to the shallow southern waters at depths of 500–1000 m until noon (Figures 2, 3). Their locations then moved slightly northward as the afternoon progressed. When the data obtained for each year were combined, the direction of movement of the sperm whales in Nemuro Strait showed a typical periodic change every year (Figure 4). In the early morning, most of the whales moved outward (northward), and then the proportion of whales that moved inward (southward) increased until 8 to 9 o’clock. Subsequently, the proportion of whales moved inward gradually decreased in the early afternoon and increased again in the late afternoon. This periodic heading change corresponds to a diurnal change in the location.
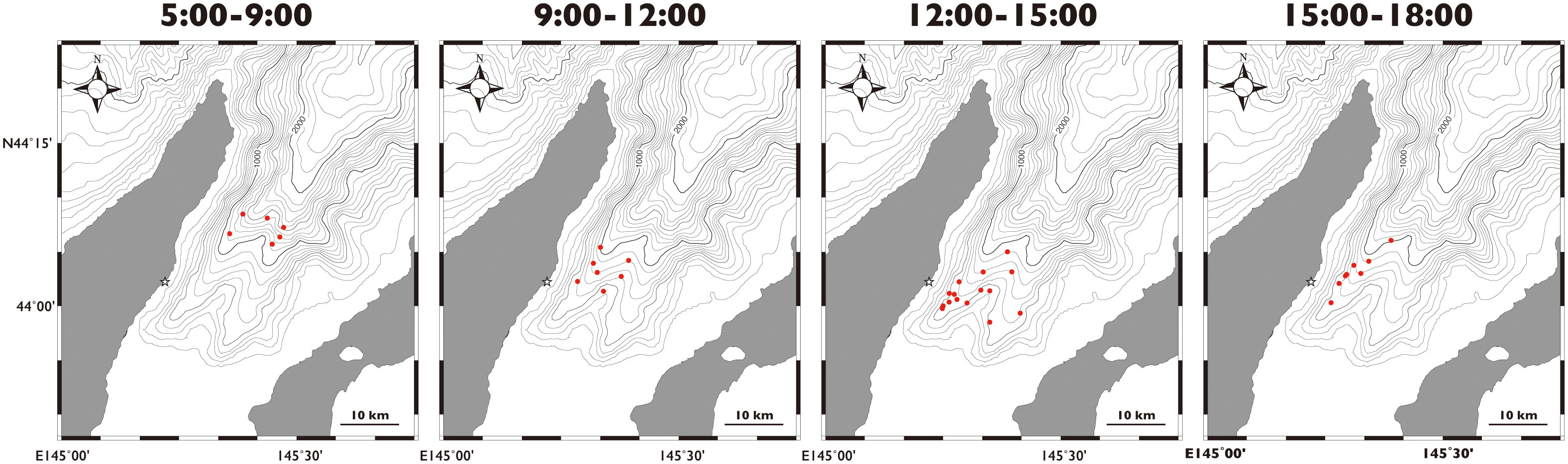
Figure 2 An example location shift of the sperm whales during daytime in Nemuro Strait, Japan. Locations obtained by theodolite survey on August 23, 2010. Star indicates theodolite station.
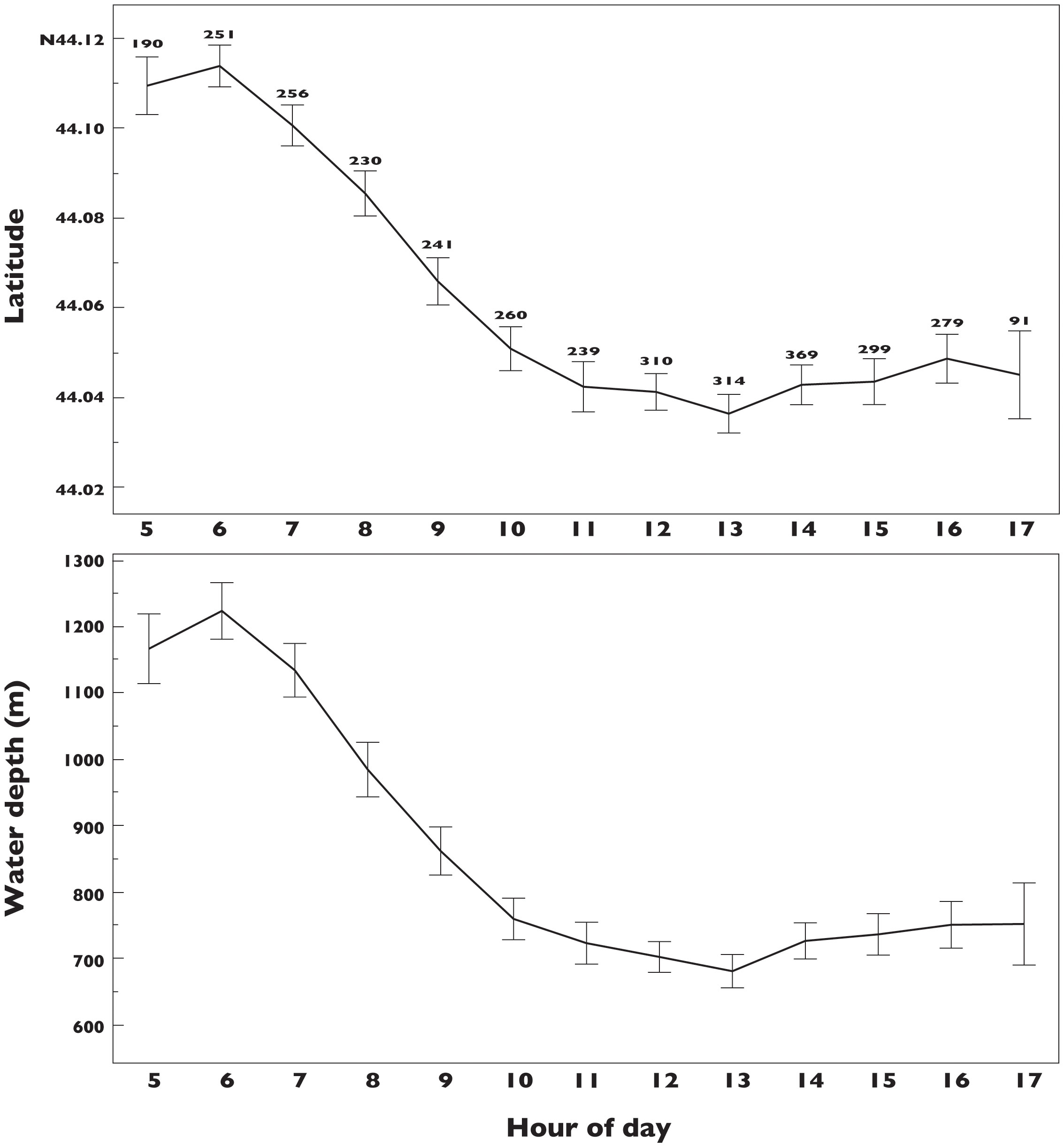
Figure 3 Changes in latitude (upper panel) and water depth (lower panel) of the sighting position of sperm whales (mean ± 95% confidence interval) by hour of the day in Nemuro Strait, Japan. Numbers above bars are number of whales sighted. The data were obtained by the theodolite survey, and all five-year data were combined.
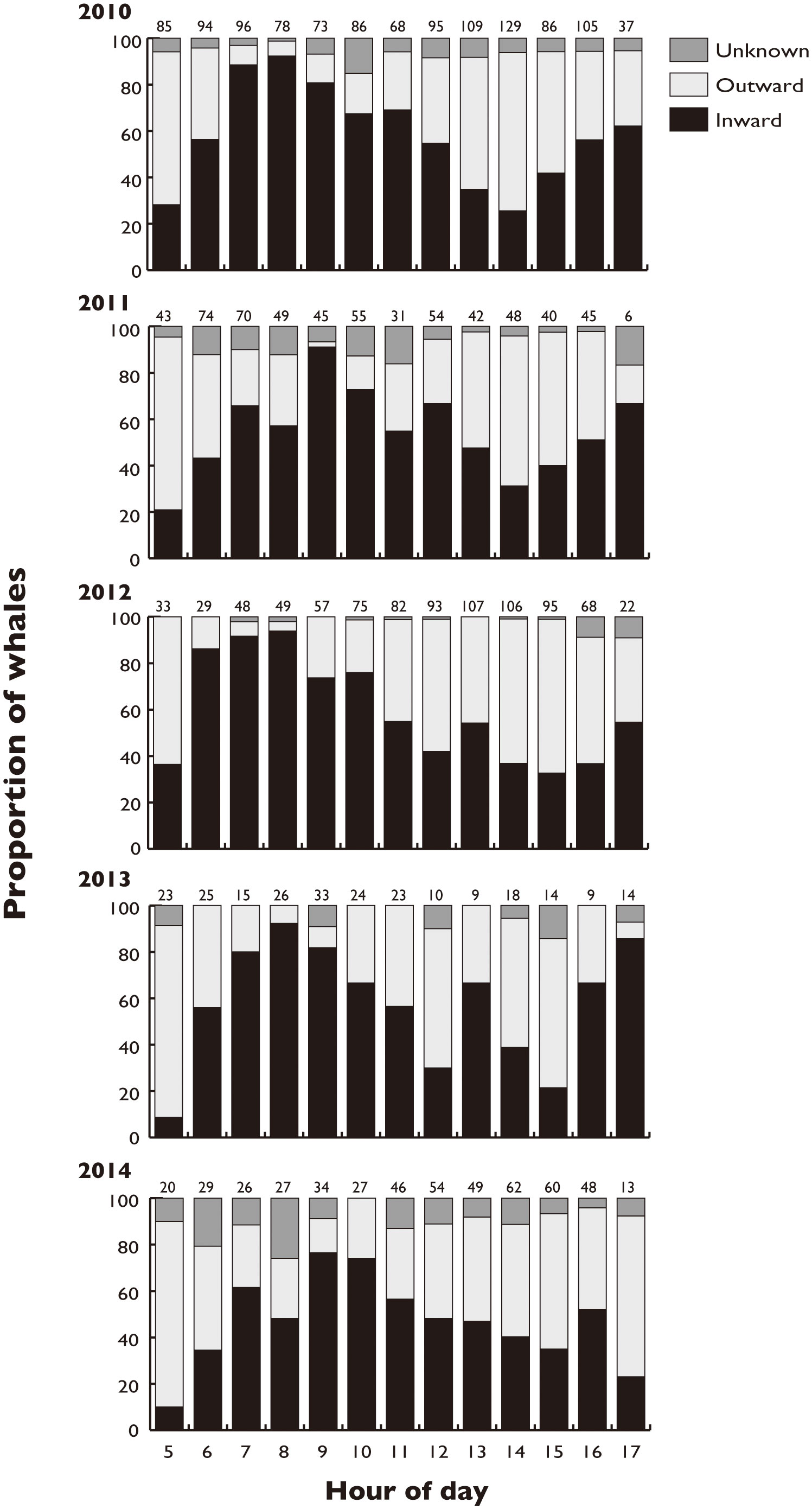
Figure 4 Proportion of sperm whales moving inward and outward Nemuro Strait, Japan in each hour of the day. The data were obtained by the theodolite survey, and those within the same year were combined. Numbers above the bar indicate the number of observed whales in the hour of the day.
There was no correlation between the proportion of whales moving inward and the proportion of ebb tide in each hour of the day (Kendall’s tau = 0.0827, P = 0.341. This indicates that the number of whales moving inward was not large in the hour of the ebb tide.
Bio-logging survey; dive depth
We deployed data loggers on 11 whales and obtained 164.6-hour dive data, with the duration of each deployment ranging from 3.9–45.7 h (Table 1). All whales made regular foraging dives to depths of 300–1000 m for approximately 45 min, and there were long durations of time spent at the surface in five whales (2.01–6.85 hours), which is commonly observed in tagged sperm whales (Figure 5). The average bottom depth of the dives differed significantly between night and day (GLMM, P < 0.001, Supplementary Table 2). Whales dove to depths of 300–400 m during the day and 500–800 m at night (Figure 5; Table 2). Although the whales seemed to dive close to the bottom at night, the water depth at the start of the dive was related to neither the maximum dive depth of the dive nor the average bottom depth during the day and night (Figure 5, GLMM; maximum depth daytime P = 0.192, night P = 0.133; bottom depth day P = 0.288, night P = 0.577, Supplementary Table 3).
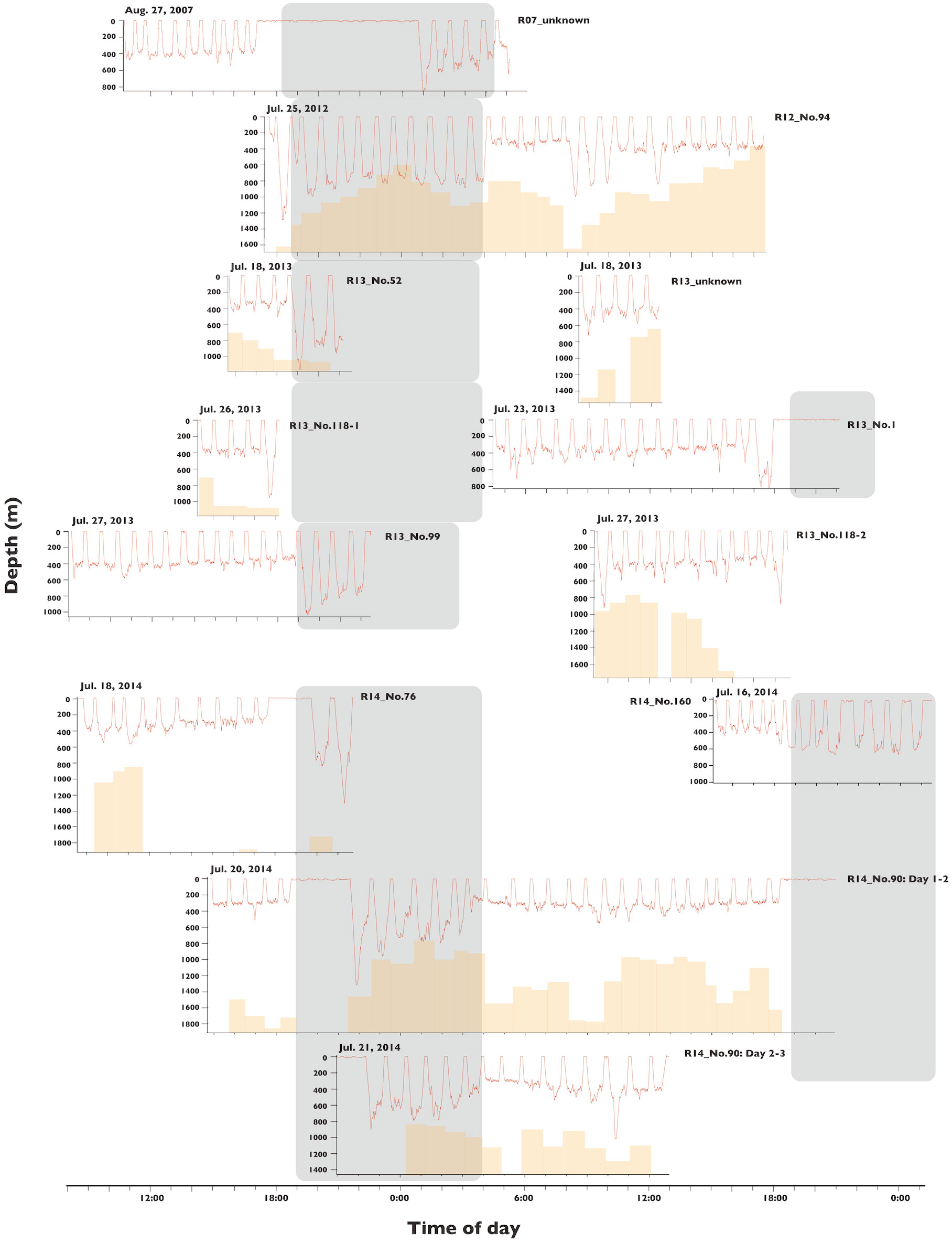
Figure 5 Dive depth profiles of all tagged sperm whales in Nemuro Strait, Japan. Dark shades indicate nighttime, brown bar is bottom depth of the locations of whales before the dive obtained by Fastloc GPS and the ‘500 m gridded bathymetry data’ provided by the Japan Oceanographic Data Center. Position data were unavailable for hours without brown bars.
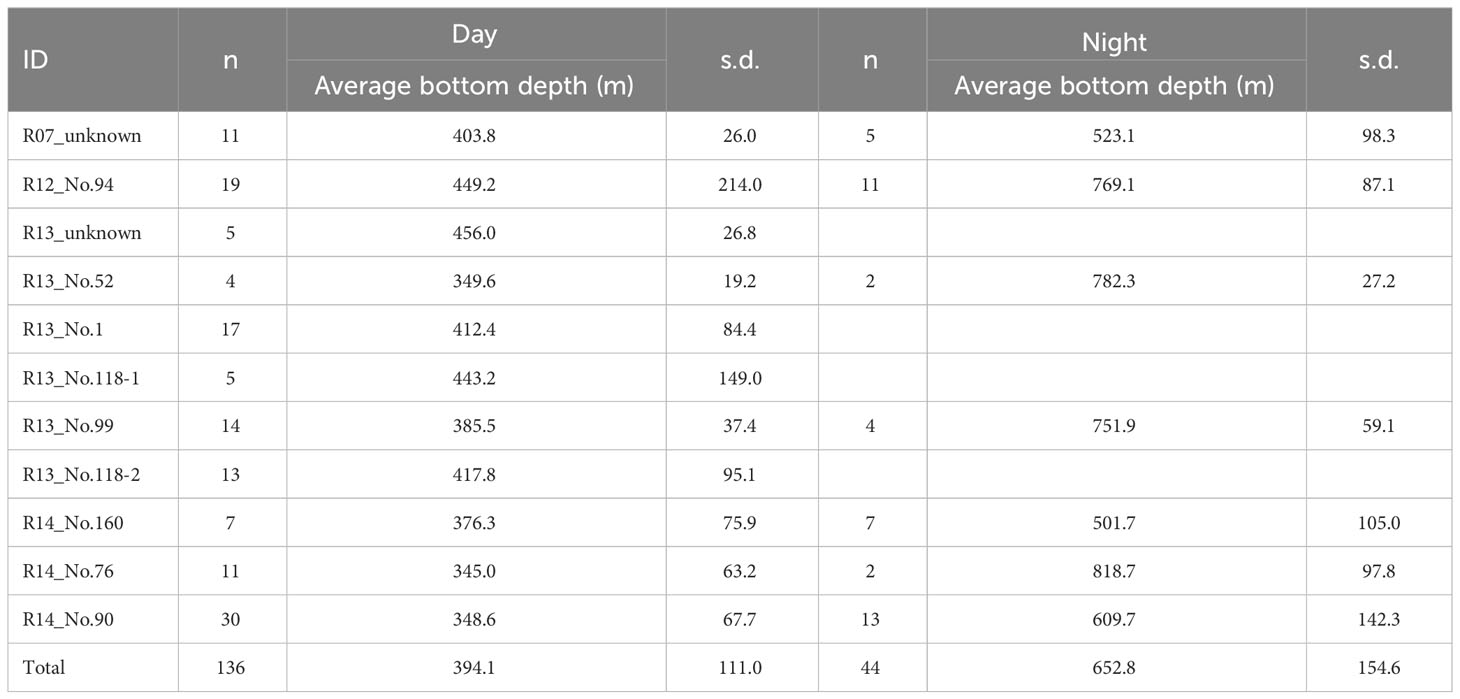
Table 2 Comparison of bottom depths of dive between day and night for male sperm whales in Nemuro Strait.
Bio-logging survery: GPS location
None of the GPS-tagged whales were found to enter the waters deeper than 2000 m, corresponding to an area mostly outside the theodolite observation range (Figure 1). We obtained over 24-hour GPS tracking data from two whales (Figure 6). The whale ID R12_No.94_moved inward after deployment from 17:55 until 23:33, outward until 3:14 (Figure 6A), inward until 4:54, and inward until 16:46 (Figure 6B). The whale ID R14_No.90 moved outward a little after deployment from 15:40 until 18:44, and then it stayed near the surface for two hours and outward until 0:34, inward until 2:34, outward until 6:15, inward until 13:06, outward until 18:35 for the first day of deployment (Figure 6C). On the second day, after a long surface time the whale moved inward from 1:40 until 2:10, outward until 5:52, and inward until 12:02 (Figure 6D). These data demonstrated that whales undertook one long round trip during the day and one short round trip during the night, though they used different areas in the strait.
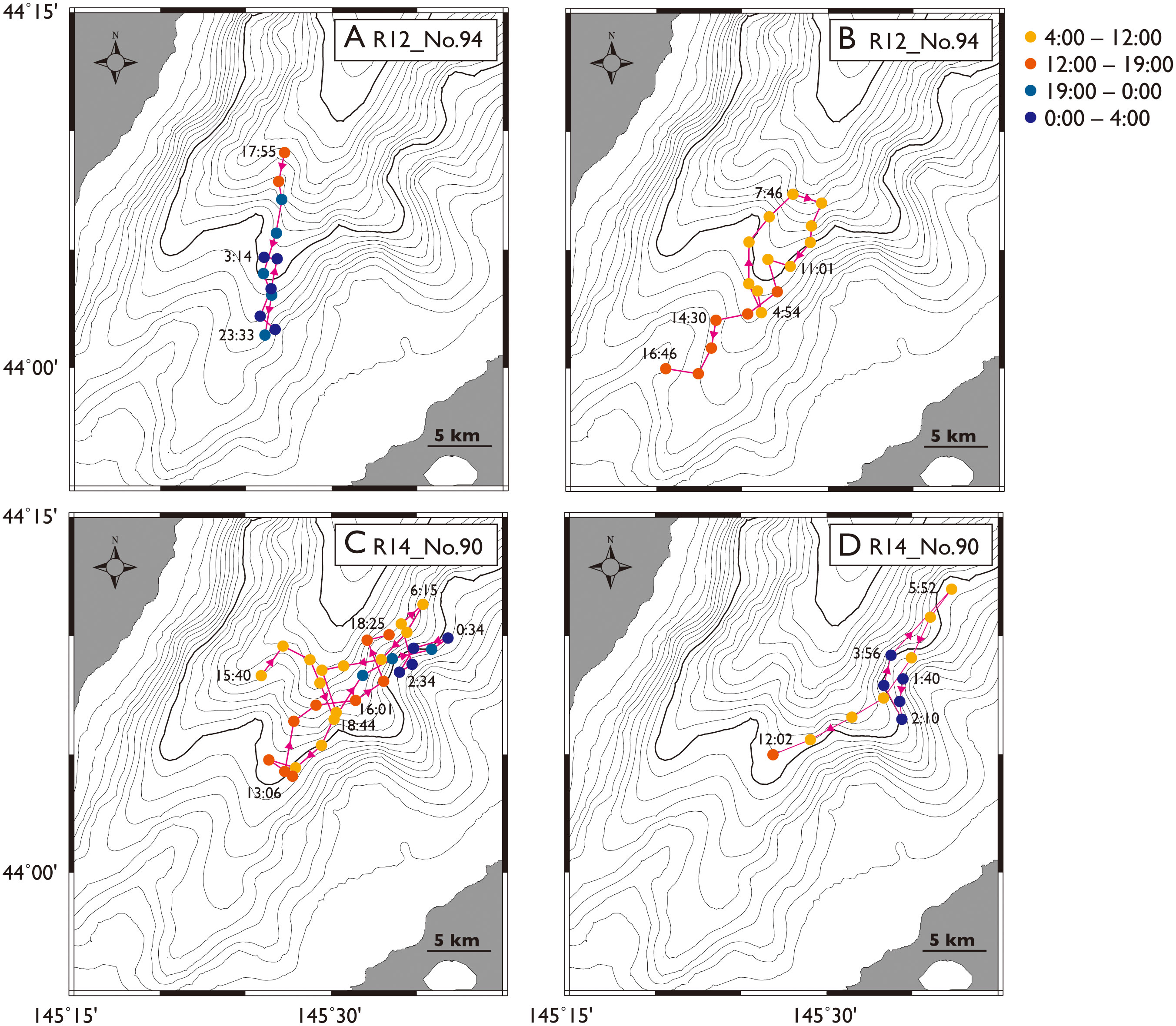
Figure 6 GPS tracks of two tagged sperm whales longer than 24 hours in the Nemuro Strait, Japan. Track from whale ID R12_No.94 and that from ID R14_No.90 is divided into two panels, (A, B) and (C, D) respectively, to avoid complexity.
Hourly movement distances along the axis of the strait varied among whales (Figure 7). However, with the exception of ID R12_No.94, most whales moved inward in the late morning and outward in the early afternoon. Although the timing deviated in ID R12 No.94, this whale generally moved outward in the early morning and inward in the late morning. The change in the direction of movement was similar to that observed in the theodolite survey. Hourly movement directions and distances were not related to the direction and index of the tidal currents (Figure 8; GLMM, P = 0.396, Supplementary Table 4).
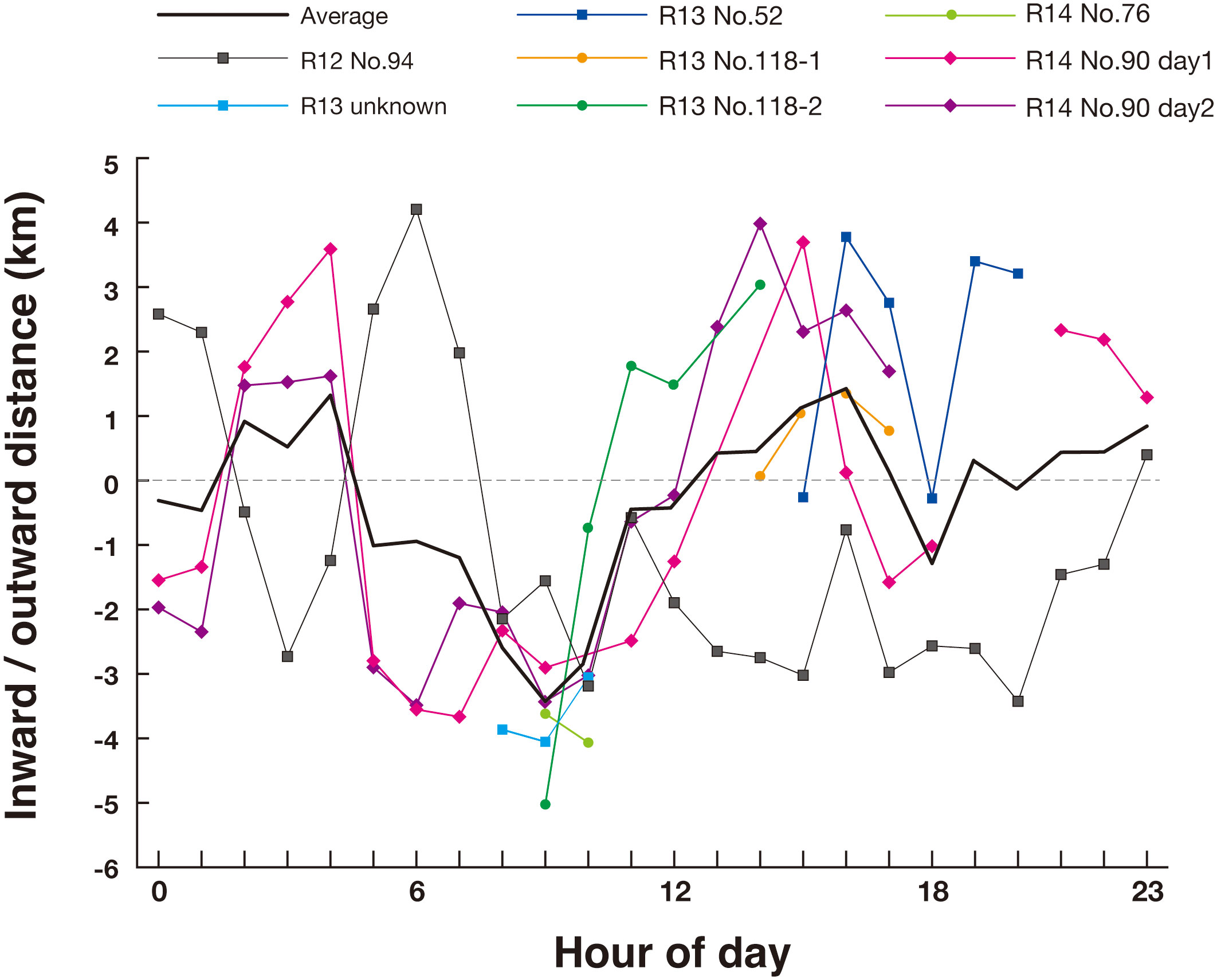
Figure 7 Change of inward and outward movement distance (see Materials and Methods) of tagged sperm whales with hour of the day in Nemuro Strait, Japan.
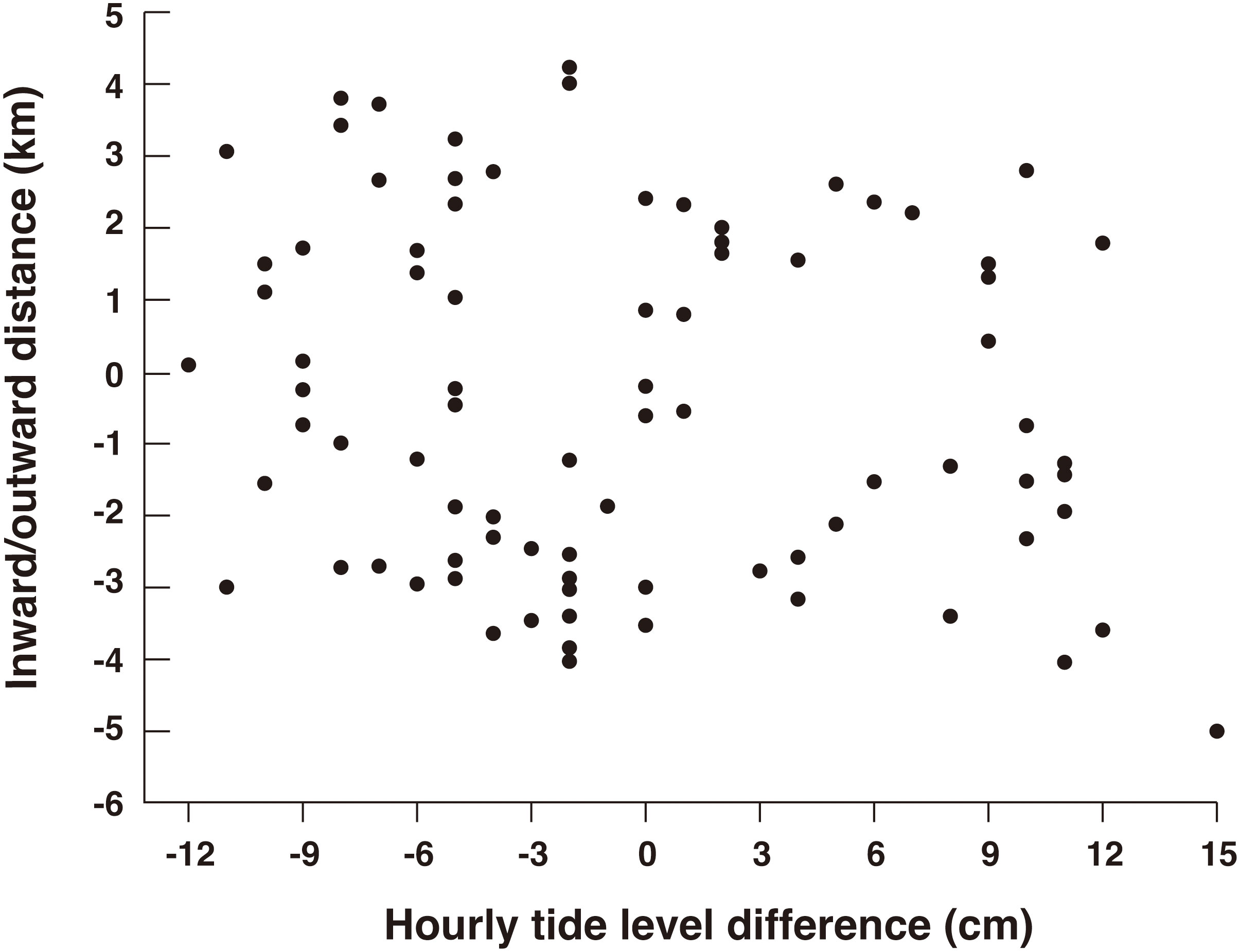
Figure 8 Relationship between the inward and outward movement distance of tagged sperm whales and tidal current index (see Materials and Methods) in each hour of the day in Nemuro Strait, Japan. Positive value of tidal current index indicates inward current in the strait.
Discussion
Habitat
The theodolite and GPS data showed that the foraging area of sperm whales is mostly restricted to a water depth of 500–2000 m in Nemuro Strait. This is consistent with other reported foraging grounds for male sperm whales in areas such as Kaikoura, New Zealand (Jaquet et al., 2000; Sagnol et al., 2014); Scotian Shelf, Canada; (Whitehead et al., 1992); the Andøya (Teloni et al., 2008) and Bleik Canyons in Norway (Rødland and Bjørge, 2015); Gulf of Alaska (Straley et al., 2014). Male sperm whales are likely to utilize prey at a similar water depth range in deep canyons, and the shared oceanographic features among these canyons contribute to the prey environment (Moors-Murphy, 2014; Fernandez-Arcaya et al., 2017).
Move direction
The theodolite survey revealed that whales tended to move outward of the strait in the early morning, and inward until approximately noon, and then outward in the afternoon. Overall, the predominant headings of whales changed every 4–6 h, and this pattern was observed in all five years of this study. This pattern corresponds to the change in latitude and water depth. In the morning, whales moved inward, resulting in a shift of their positions to the south in shallower waters. In the early afternoon, the whales moving outward increased, resulting in a northward shift of their positions. However, this shift was not pronounced, possibly due to the overlapping of whales moving inward and outward in the southern shallower waters, as they do not enter waters shallower than 500 m. The headings observed at the surface by the theodolite survey was consistent with the overall moving direction of whales, including during dives.
Tagged whales changed their north-south direction of movement every 3–10 h, four times a day. The timing of direction change was different among the tagged whales, but when overlaying all data, the pattern was similar to that obtained from the theodolite survey. In both surveys, the change in heading was not related to the direction of the current in the strait, and other extrinsic factors were unlikely to be involved. These results on the heading change suggest that sperm whales generally follow a simple routine of north-southward movement tactics, modifying the timing and location of the direction changes, possibly according to the local prey availability.
Dive depth difference between day and night
Tagged whales forage at the shallower (about 400 m) layer in the daytime and the deeper layer (about 600-800 m) at night. The difference in the foraging layer was distinct and persistently shown in all whales tagged in four different years.
The diel difference in dive depth was reported for female sperm whale groups in various locations (off Ogasawara Islands, western North Pacific, (Aoki et al., 2007); Gulf of California, (Davis et al., 2007); Mascarene Archipelago, Indian Ocean, (Chambault et al., 2021). In these areas, whales dive to a shallower depth at night than during the day; this pattern is thought to reflect the diel vertical migration of the prey. The diel pattern is the opposite in Nemuro Strait. A similar diel pattern has only been reported for male sperm whales in Kaikoura submarine canyon, where whales were reported to forage in the depths of 450–500 m during the day and in the depth of 850–950 m at night (Guerra et al., 2017). Guerra et al. (2017) suggested that whales forage for squid that prey on mesopelagic myctophid fish associated with the deep scattering layer during the day and demersal fish at night. Although we do not have any information on prey species of sperm whales in Nemuro Strait, the similar diel difference in prey availability could cause the diel pattern of diving behavior. Since we did not find any relationships between water depth and dive depth, sperm whales in Nemuro Strait target prey in deeper layers at night, but it may not necessarily be benthopelagic prey, but they are likely to feed on different prey species distributed in the different depth layers, and the availability or vulnerability differs between day and night. This could be verified in the future by examining the detailed body movement pattern of prey-searching whales using acceleration and geomagnetic data from data loggers.
Foraging tactics
The results of the theodolite and bio-logging surveys suggest that sperm whales in Nemuro Strait follow simple routine foraging tactics, that is, foraging in the shallower (300–400 m) layer during the daytime and deeper layer (500–800 m) during the night, making two round north-south horizontal trips per day.
In the narrow strait, it is natural that the primary direction of movement is in the direction of the long axis of the strait, that is, in- and outward, or south- and northward in Nemuro Strait. The 500–2000 m depth area utilized by the sperm whales is approximately 30 km along the long axis of this strait. As the daytime dive depths of the whales were shallower than 500 m, the north-south distance of the area where whales utilize during the daytime is approximately 30 km. The usual horizontal displacement of foraging sperm whales is approximately 4 km/h (Whitehead, 2003), which agrees with the current GPS logger data. Thus, it takes whales approximately 7.5 h to travel through the foraging range if they move straight along the long axis of the strait. The daytime period in the research area in August is about 15 h, and whales could make one round trip during the daytime in the foraging area. At night, the dive depths were deeper than 500 m, and thus, the travel distance would be shorter than that during the daytime, and whales could make another round trip during the nighttime. This movement pattern is thought to be a foraging tactics that enables whales to explore two different layers efficiently within a day.
The prevailing moving directions changed over time in the cumulative theodolite data in each season. We did not know whether whales actually changed their heading synchronously since only a few location data were usually obtained in a certain hour of the day. However, the change in the heading from the cumulative data accounts for the diurnal change in the location of whales. Sperm whales found in a three-hour time frame were mostly within 10 km range, and these whales are considered to form an aggregation, one of the spatial structures of this species (Whitehead, 2003). It is still unclear whether there are any social relationships among males in an aggregation. Christal and Whitehead (1997) reported that members of the male aggregations displayed heading synchrony, and those found in a day usually showed consistent headings in the breeding ground. They suggested that this coordinated heading results from social cohesion between the whales or individual responses to the environmental conditions, such as prevailing currents. The present results show that the headings of the whales did not correlate with the direction of the tidal current. We cannot deny, however, that social relationships are involved in coordination. Kobayashi et al. (2020) found long-term social associations among foraging males in Nemuro Strait.
Fais et al. (2015) consider that whales obtain prey patch information individually on a dive-to-dive basis by their own echolocation because the whales were dispersed over several kilometers while foraging, and the prey communities should be heterogeneous because of the complex bathymetry of the canyon. In our study, tagged whales foraged in certain layers independent of the water depth, especially in the shallow layer away from the seafloor during the daytime. This implies that the prey community in the strait is not so heterogeneous, at least that targeted during daytime. In this situation, the coordinated movements could benefit the whales. Whales gain the information on prey individually on a small spatial scale of a few kilometers, but that of prey density information can be gained on a broader scale over 10 km from other conspecifics by hearing other whales’ echolocation clicks each other, i.e., local enhancement (Madsen et al., 2002; Whitehead, 2003). Sperm whales are likely to keep a certain distance between individuals that is far enough to avoid direct competition among prey or prey patches, but close enough to gain information on other’s foraging success by hearing buzzes of nearby whales, moving in the strait in a coordinated way. However, each whale should modify its movement pattern according to the information on prey availability obtained by itself, which results in considerable variability in movement patterns among whales and days. Thus, the coordinated changes in the moving direction only become apparent when whale data from many days have been combined.
The typical foraging pattern of the sperm whales in Nemuro Strait is based on biotic reasons (e.g., prey distribution) and abiotic reasons (e.g., extent of foraging area and environments), both of which are specific to this strait. However, considering that the average residency of male sperm whales in Nemuro Strait is 769 days (Kobayashi and Amano, 2020) and some individuals should have been replaced during our study, this invariability of habitat use patterns among years conversely suggests that whales have the ability to adapt their foraging behavior according to the broad geographical prey environment of the habitat, and not only dive-to-dive scale (Teloni et al., 2008). It is believed that the intense pressure to grow faster in male sperm whales that drives this ability (Whitehead, 1994; Teloni et al., 2008).
Among female sperm whales in the Eastern Caribbean, a similar type of habitat specialization is suggested to be transmitted through social learning within units and clans (Vachon et al., 2022). We cannot deny that the stereotypical foraging pattern of male sperm whales in Nemuro Strait may be socially learned from individuals with prolonged affiliations (Kobayashi et al., 2020). For example, the pattern could also be explained by the tendency of young whales naive to the environment to follow the older individuals who decide the direction of movement like the African elephant, Loxodonta africana (Allen et al., 2020). Further studies on the moving pattern of individually identified whales in the strait will shed light on how this typical movement pattern is acquired among male sperm whales.
The current and previous studies on foraging behavior of male sperm whales highlight their remarkable capabilities for prey search, including the use of echolocation to detect prey at considerable distances (Madsen et al., 2002; Fais et al, 2016; Tønnesen et al., 2020), the ability to adapt their foraging behavior in response to the prey environment (Teloni et al., 2008; Isojunno and Miller, 2018), and the social cohesion that possibly enables them to share information about prey (Kobayashi et al., 2020). These capacities likely contributed to their extraordinary success as predators in global mesopelagic waters.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Biologging data are available from the BiP Earth Project database (https://www.bip-earth.com).
Ethics statement
The animal study was approved by Animal Experiment Committee of Nagasaki University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MA coordinated and carried out the research, data analysis, and wrote the manuscript, KA and SM planned and carried out the tagging survey; and HK carried out the theodolite survey. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by JSPS KAKENHI Nos. 21570020, 24370012, and 21K06350.
Acknowledgments
We would like to thank Rausu Town, Shiratoko Rausu Kankosen Kyogikai, Shiretoko Rausu Tourism Association, Shiretoko Foundation, Noboru and Takako Shimizubata, and Hal Sato for their kind support in the field. We would also like to thank Aya Kourogi, Yukiyo Sueishi, Miku Kusumoto, Yudai Kawano, Shingo Nomura, Wataru Hirayama, Ai Kobayashi, Shino Mitarai, Koko Kusumoto, Keiko Nishimaniwa, Atsuhiro Noda, Sumiko Amano, Mayumi Yogo, and Shigerori Nobata for assisting with the data collection. We are grateful to three reviewers for their constructive and helpful comments. We would like to thank Editage (www.editage.com) for their writing support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1150308/full#supplementary-material
References
Allen C. R. B., Brent L. J. N., Motsentwa T., Weiss M. N., Croft D. P. (2020). Importance of old bulls: leaders and followers in collective movements of all-male groups in African savannah elephants (Loxodonta africana). Sci. Rep. 10, 1–9. doi: 10.1038/s41598-020-70682-y
André M., Caballé A., van der Schaar M., Solsona A., Houégnigan L., Zaugg S., et al. (2017). Sperm whale long-range echolocation sounds revealed by ANTARES, a deep-sea neutrino telescope. Sci. Rep. 7, 45517. doi: 10.1038/srep45517
Aoki K., Amano M., Mori K., Kourogi A., Kubodera T., Miyazaki N. (2012). Active hunting by deep-diving sperm whales: 3D dive profiles and maneuvers during bursts of speed. Mar. Ecol. Prog. Ser. 444, 289–301. doi: 10.3354/meps09371
Aoki K., Amano M., Yoshioka M., Mori K., Tokuda D., Miyazaki N. (2007). Diel diving behavior of sperm whales off Japan. Mar. Ecol. Prog. Ser. 349, 277–287. doi: 10.3354/meps07068
Austin D., Bowen W. D., McMillan J. I., Iverson S. J. (2006). Linking movement, diving, and habitat to foraging success in a large marine predator. Ecology 87, 3095–3108. doi: 10.1890/0012-9658(2006)87[3095:lmdaht]2.0.co;2
Best P. B. (1979). ”Social organization in sperm whales, Physeter macrocephalus.” in Behaviour of marine animals: vol. 3 cetaceans. Eds. Winn H. E., Olla B. L. (New York, NY: Plenum Press), 227–289.
Chambault P., Fossette S., Heide-Jørgensen M. P., Jouannet D., Vely M. (2021). Predicting seasonal movements and distribution of the sperm whale using machine learning algorithms. Ecol. Evol. 11, 1432–1445. doi: 10.1002/ece3.7154
Christal J., Whitehead H. (1997). Aggregations of mature male sperm whales on the Galápagos Islands breeding ground. Mar. Mamm. Sci. 13, 59–69. doi: 10.1111/j.1748-7692.1997.tb00612.x
Christal J., Whitehead H., Lettevall E. (1998). Sperm whale social units: variation and change. Can. J. Zool. 76, 1431–1440. doi: 10.1139/z98-087
Davis R., Jaquet N., Gendron D., Markaida U., Bazzino G., Gilly W. (2007). Diving behavior of sperm whales in relation to behavior of a major prey species, the jumbo squid, in the Gulf of California, Mexico. Mar. Ecol. Prog. Ser. 333, 291–302. doi: 10.3354/meps333291
Dragon A., Bar-Hen A., Monestiez P., Guinet C. (2012). Horizontal and vertical movements as predictors of foraging success in a marine predator. Mar. Ecol. Prog. Ser. 447, 243–257. doi: 10.3354/meps09498
Fais A., Soto N. A., Johnson M., Pérez-González C., Miller P. J. O., Madsen P. T. (2015). Sperm whale echolocation behaviour reveals a directed, prior-based search strategy informed by prey distribution. Behav. Ecol. Soc Biol. 69, 663–674. doi: 10.1007/s00265-015-1877-1
Fernandez-Arcaya U., Ramirez-Llodra E., Aguzzi J., Allcock A. L., Davies J. S., Dissanayake A., et al. (2017). Ecological role of submarine canyons and need for canyon conservation: A review. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00005
Gaskin D. E. (1970). Composition of schools of sperm whales Physeter catodon Linn. east of New Zealand. NZ J. Mar. Freshw. Res. 4, 456–471.
Gordon J. C. (1990). A simple photographic technique for measuring the length of whales from boats at sea. Rep. Int. Whal. Commun. 40, 581–588.
Guerra M., Hickmott L., van der Hoop J., Rayment W., Leunissen E., Slooten E., et al. (2017). Diverse foraging strategies by a marine top predator: Sperm whales exploit pelagic and demersal habitats in the Kaikōura submarine canyon. Deep Sea Res. Part I 128, 98–108. doi: 10.1016/j.dsr.2017.08.012
Irvine L., Palacios D. M., Urbán J., Mate B. (2017). Sperm whale dive behavior characteristics derived from intermediate-duration archival tag data. Ecol. Evol. 7, 7822–7837. doi: 10.1002/ece3.3322
Isojunno S., Miller P. J. O. (2018). Movement and biosonar behavior during prey encounters indicate that male sperm whales switch foraging strategy with depth. Front. Ecol. Evol. 6. doi: 10.3389/fevo.2018.00200
Jaquet N. (2006). A simple photogrammetric technique to measure sperm whales at sea. Mar. Mamm. Sci. 22, 862–879. doi: 10.1111/j.1748-7692.2006.00060.x
Jaquet N., Dawson S., Slooten E. (2000). Seasonal distribution and diving behaviour of male sperm whales off Kaikoura: foraging implications. Can. J. Zool. 78, 407–419. doi: 10.1139/z99-208
Kobayashi H., Amano M. (2020). Residency and abundance of sperm whales (Physeter macrocephalus) in Nemuro Strait, Hokkaido, Japan. Mar. Mamm. Sci. 37, 267–211. doi: 10.1111/mms.12662
Kobayashi H., Whitehead H., Amano M. (2020). Long-term associations among male sperm whales (Physeter macrocephalus). PloS One 15, e0244204. doi: 10.1371/journal.pone.0244204
Madsen P., Wahlberg M., Møhl B. (2002). Male sperm whale (Physeter macrocephalus) acoustics in a high-latitude habitat: implications for echolocation and communication. Behav. Ecol. Sociobiol. 53, 31–41. doi: 10.1007/s00265-002-0548-1
Moors-Murphy H. B. (2014). Submarine canyons as important habitat for cetaceans, with special reference to the Gully: A review. Deep-Sea Res. Part II 104, 6–19. doi: 10.1016/j.dsr2.2013.12.016
Ohsumi S. (1971). Some investigations on the school structure of sperm whale. Sci. Rep. Whales Res. Inst. 23, 1–25.
Rødland E. S., Bjørge A. (2015). Residency and abundance of sperm whales (Physeter macrocephalus) in the Bleik Canyon, Norway. Mar. Biol. Res. 11, 974–982. doi: 10.1080/17451000.2015.1031800
Sagnol O., Richter C., Field L., Reitsma F. (2014). Spatio-temporal distribution of sperm whales (Physeter macrocephalus) off Kaikoura, New Zealand, in relation to bathymetric features. New Zeal. J. Zool. 41, 234–247. doi: 10.1080/03014223.2014.936474
Stephens D. W., Krebs J. R. (1986). Foraging theory (Princeton, NJ, USA: Princeton University Press).
Straley J. M., Schorr G. S., Thode A. M., Calambokidis J., Lunsford C. R. (2014). Depredating sperm whales in the Gulf of Alaska: local habitat use and long distance movements across putative population boundaries. Endang. Species Res. 24, 125–135.
Teloni V., Mark J., Patrick M., Peter M. (2008). Shallow food for deep divers: Dynamic foraging behavior of male sperm whales in a high latitude habitat. J. Exp. Mar. Biol. Ecol. 354, 119–131. doi: 10.1016/j.jembe.2007.10.010
Tønnesen P., Oliveira C., Johnson M., Madsen P. T. (2020). The long-range echo scene of the sperm whale biosonar. Biol. Lett. 16, 20200134–6. doi: 10.1098/rsbl.2020.0134
Vachon F., Hersh T. A., Rendell L., Gero S., Whitehead H. (2022). Ocean nomads or island specialists? Culturally driven habitat partitioning contrasts in scale between geographically isolated sperm whale populations. R. Soc Open Sci. 9, 211737. doi: 10.1098/rsos.211737
Whitehead H. (1993). The behavior of mature male sperm whales on the Galapagos breeding grounds. Can. J. Zool. 71, 689–699.
Whitehead H. (2003). Sperm whales: social evolution in the ocean (Chicago, USA: University of Chicago Press).
Whitehead H., Brennan S., Grover D. (1992). Distribution and behavior of male sperm whales on the Scotian Shelf, Canada. Can. J. Zool. 70, 912–918.
Keywords: sperm whale, movement, diving, habitat use, foraging tactics, submarine canyon
Citation: Amano M, Aoki K, Kobayashi H, Minamikawa S, Sato K and Kubodera T (2023) Stereotypical diel movement and dive pattern of male sperm whales in a submarine canyon revealed by land-based and bio-logging surveys. Front. Mar. Sci. 10:1150308. doi: 10.3389/fmars.2023.1150308
Received: 24 January 2023; Accepted: 24 October 2023;
Published: 09 November 2023.
Edited by:
Leslie New, Ursinus College, United StatesReviewed by:
Natalia Sidorovskaia, University of Louisiana at Lafayette, United StatesLauren Wild, University of Alaska Southeast, United States
Copyright © 2023 Amano, Aoki, Kobayashi, Minamikawa, Sato and Kubodera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masao Amano, bS1hbWFub0BuYWdhc2FraS11LmFjLmpw
 Masao Amano
Masao Amano Kagari Aoki
Kagari Aoki Hayao Kobayashi
Hayao Kobayashi Shingo Minamikawa
Shingo Minamikawa Katsufumi Sato
Katsufumi Sato Tsunemi Kubodera6
Tsunemi Kubodera6