
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 09 March 2023
Sec. Marine Biology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1147886
This article is part of the Research TopicOcean-Biota System: Integrated Approach to Climate Change Impacts on Plankton Communities in Coastal and Pelagic EnvironmentsView all 8 articles
 Xiaohong Sun1*
Xiaohong Sun1* Minpeng Song1
Minpeng Song1 Zhengguang Li1
Zhengguang Li1 Yan Song2
Yan Song2 Xiaonan Yuan1
Xiaonan Yuan1 Bing Dong3
Bing Dong3 Lu Zhang3
Lu Zhang3 Lixin Zhu1
Lixin Zhu1 Zhenlin Liang1*
Zhenlin Liang1*The mullet Liza haematocheila is widely distributed in low-salinity waters around the world and has high economic value. However, details regarding the foraging ecology of mullet larvae remain unclear. Larvae of L. haematocheila were sampled in Laizhou Bay of the Bohai Sea, China, in May 2016, and diet composition was detected using gut content analysis to compare differences in feeding parameters and diet shift in dominant prey during ontogeny. The results showed no linear relationship between gape size (GS) and standard length (SL) at larva length <7 mm (flexion larvae, FL), but linear increase was observed for SL >7 mm (post-flexion larvae, PFL). Maximum prey size (MPS) overlapped with GS during the FL stage but was never higher than GS during the PFL stage. Trends of increasing MPS and prey size range (PSR) during the PFL stage were lower than those during the FL stage, but prey number (PN) increased significantly during the PFL stage. Diet composition analyses in mullet larvae showed a total of 10 mesozooplankton species (or categories), of which 8 species were copepods (including copepods nauplii and copepodites), and showed the dominance of 4 small copepods (<1 mm). Analyses of the numerical proportion of dominant copepods showed that the largest prey (Paracalanus parvus) gradually increased as GS increased; conversely, the smallest prey (nauplii of Calanus sinicus) decreased. Collectively, these results suggest that PFL tends to exhibit increased PN but not prey size or size range, and diet shifts from smaller to larger prey during ontogeny in mullet larvae. All these indicate that PFL has higher prey selection ability compared with FL, specifically switching the diet to include larger small copepods during the PFL stage and increasing the prey number instead of increasing prey size. These determine the importance of small copepods in mesozooplankton as dominant prey and facilitate predictions of the impact of climate change on mesozooplankton and fish larvae.
Knowledge of the foraging ecology of fishes is fundamental both to understanding the processes that function at the individual, population, and community levels and for the management and conservation of fish populations and habitats (Nunn et al., 2012). Fish food habits research seeks to predict the composition of the fish diet given the size, morphological, and behavioral characteristics of the fish species in order to clearly define their feeding preferences and assess the available food resources and their growth. Feeding and foraging successfully during the larval stage is critical to fish survival (Devries et al., 1998; Skrzypczak et al., 1998). Many factors affect the success of larval predation, including the ability of the predator to consume its prey, morphological constraints during ontogeny, and prey size and abundance (Furgała-Selezniow et al., 2014). It has been reported that fish larvae are gap-limited predators, and they usually swallow prey whole when foraging. Several researchers have documented the relationship between gape size (GS) and prey selection of juvenile fish (Nunn et al., 2007), but few studies have examined this relationship in larval fish. Generally, fish larvae prefer to consume larger prey as their body length and GS increase (Economou, 1991; Pepin and Penney, 1997; Llopiz and Cowen, 2009), and increases in GS along with ontogeny often lead to shifts in diet composition (Nunn et al., 2012). However, prey size is not the only factor that affects prey selection of fish larvae. The taxonomic identity of potential prey, especially copepods, can also affect prey selection by fish larvae (Simonsen et al., 2006; Voss et al., 2006; Catalán et al., 2007). At the onset of exogenous feeding, many fish species are zooplanktivorous, and their survival, growth, and ultimately, their recruitment, is often strongly linked with the availability of zooplankton during early life stages (Mamcarz et al., 1998; Nunn et al., 2007). Calanoid copepods, including their various developmental stages (eggs, nauplii, copepodites, and adults), are important prey for fish larvae, especially in marine ecosystems (Ferrari and Chieregato, 1981; Thiel et al., 1996; Montagnes et al., 2010; Nunn et al., 2012).
Mugilidae species are widely distributed, and they play important ecological roles in coastal systems as well as in fisheries in some parts of the world. These species also have value as biomarkers for monitoring the health of coastal habitats (Ferreira et al., 2004; Ferreira et al., 2005; Waltham et al., 2013; Ouali et al., 2018; Ge et al., 2020). As a typical fish in coastal areas, the foraging ecology of this species, especially larvae and juveniles, can reflect the mesozooplankton situation as prey and environmental conditions in estuarine and coastal zones, as well as the direct/indirect effects of climate change on early recruitment of typical fish resources. Mullet larvae are planktonic feeders with a standard length between 10 and 20 mm (Zismann et al., 1975; Brownell, 1979; Gisbert et al., 1996; Inoue et al., 2005), and they may exhibit changes in diet, initially feeding on small invertebrates and later primarily feeding on benthic organisms (Suzuki, 1965; Blaber and Whitfield, 1977). Calanoid copepods are the first important component of the diet of mugilidae species. Cyclopoid copepods also feature strongly in the diet of late larvae and early juveniles among Mugil species, and Ciripedia nauplii, Polychaeta, and Harpacticoida are other common diet components in juveniles of Liza species (Blaber and Whitfield, 1977; Ferrari and Chieregato, 1981; Whitfield, 1985; Eggold and Motta, 1992; Gisbert et al., 1995). However, knowledge about the foraging ecology of earlier larval stages of these organisms after the exogenous feeding stage remains limited, and whether there are differences in diet between the flexion and postflexion stages is unclear. Apart from the similarity in early diet between mullet fry (Pisarevskaya and Aksenova, 1991), there does not appear to be a universal pattern in predator-prey size relationships for fish larvae (Peck et al., 2012).
The mullet Liza haematocheila (Mugiliformes, Mugilidae) is widely distributed in low-salinity waters around the world (Wang et al., 2018), and it is common in all of the Chinese seas, from the Bohai Sea to the South China Sea (Yang et al., 2012). This species has become an economically important fish in China due to its rapid growth, high survival rate, and economic value. Studies in feeding, breeding and physiology of L. haematochelia were mainly based on aquaculture (Yang et al., 2015; Liu et al., 2021), accordingly, proteomics (Janson et al., 2019) and immunology (Kim et al., 2019; Omeka et al., 2019; Qiao et al., 2019; Sandamalika et al., 2019) were further studied. Studies of the in situ foraging ecology of L. haematocheila, however, are rare and even no research is available regarding the foraging ecology of larvae (Dolganova et al., 2008). Recent research on the egg and larval distribution of L. haematochelia in the Bohai Sea, a region similar to the one we studied, has highlighted the importance of resource conservation and protecting the spawning grounds of this species (Niu et al., 2022).
In this study, the foraging ecology of larvae of mullet L. haematocheila was studied by examining the gut contents. Selective parameters related to feeding during ontogeny were analyzed, and the prey selection was described during the ontogeny of mullet larvae. The aims of this study were to characterize the foraging ecology of L. haematocheila larvae, analyze prey selection during ontogeny, assess the importance of dominant mesozooplankton as prey, and predict the impact of climate change on mesozooplankton and eventually on early replenishment of fishery resources.
Fish larvae were collected at 5 stations in Laizhou Bay of the Bohai Sea, China, in May 2016 (Figure 1). Laizhou Bay is one of the three bays of the Bohai Sea, and also includes the estuary of the Yellow River, Xiaoqing River, Jiaolai River and other rivers. With abundant baits, Laizhou Bay is the main spawning and feeding grounds for various economic fish in the Yellow Sea and the Bohai Sea. Therefore, Laizhou Bay is chosen as the sea area for this study. All fish larvae were captured using a 500-μm mesh zooplankton net (mouth diameter: 50 cm). The net was towed horizontally at a speed of 2 miles per hour for 10 minutes. Samples were preserved in 4% formalin and transported to the laboratory.
The dynamics of feeding success and diet composition of fish larvae were almost exclusively evaluated based on gut content analysis, which is essentially the only technique available for investigating the specific types of prey that fish larvae consume (Peck et al., 2012). In the laboratory, L. haematocheila larvae were sorted from captured samples. Mullet larvae that had a gastrointestinal tract full of food were examined, as they could be used to detect freshly ingested zooplankton. Standard length (SL) of each sample was measured under a microscope (ZEISS Stemi 508) equipped with a camera and measurement software. Gape size (GS) was measured from the anterior-most tip of the premaxilla to the anterior-most tip of the dentary with the mouth opened so the upper jaw formed a 90° angle with the lower jaw. The gut was dissected using fine needles, and prey zooplankton in the gut contents were identified; copepods were identified to the species level if possible. The body length of prey zooplankton (including copepod nauplii) and the prosome length of copepodites were measured.
Many methods can be used to examine fish stomach contents, such as the occurrence method, numerical method, volumetric method, and gravimetric method (Hyslop, 1980). In this study, the numerical method was used, in which F% and N% as prey were determined to assess the prey importance. N% refers to the percentage of prey number, and F% refers to the frequency at which each prey species occurs:
where Ai is the number of fish in which the stomach contained type i prey item, N is the number of fish containing food in the stomach, Ni is the number of prey type i, and Ntotal is the total number of prey items in all food categories.
For the purpose of comparing prey selection during the ontogeny of L. haematocheila, various feeding parameters were studied, such as maximum prey size (MPS), prey size range (PSR, calculated as the maximum prey size minus minimum prey size in each fish larva), prey number (PN), as well as diet composition and shifts in dominant prey types.
According to the developmental stage criteria of larval and juvenile fish (Kendall and Vinter, 1984), there have three larval stages in the early life of fish: periods-preflexion larva, flexion larva (FL), and postflexion larva (PFL). Studies of L. haematocheila larvae shown that the standard length of FL is approximately 3-7 mm, and the PFL is approximately 7-14 mm (Jiang et al., 2007). Accordingly, the SL of mullet larvae captured in our study area was sorted to FL and PFL.
All statistical analyses were performed using IBM SPSS 22.0. The relationships between larval SL and fish larval GS and feeding parameters were determined by regression analysis. Differences in size of prey copepod species were analyzed using ANOVA. Levene’s test was used to test the assumption of homogeneity of variance. When this assumption was violated, square root transformation was performed to obtain homogeneity of variance. For multiple comparisons of means, Tukey’s test was used. All data were presented as the mean ± standard deviation, and statistical significance was defined as P<0.05.
A total of 142 fish larvae collected from Laizhou Bay were measured; the SL of L. haematocheila ranged from 3.95-13.03 mm, and the GS ranged from 0.34-1.48 mm. A positive linear correlation was found between GS and SL of mullet larvae (Figure 2); moreover, a significant difference was found between FL stage (<7 mm) and PFL stage (>7 mm). There was no significant positive linear correlation when SL was less than 7 mm (R2 = 0.076); however, the GS increased significantly with SL after ~7 mm (R2 = 0.508), and the regression equation showed that GS increased approximately 0.075 mm with each 1-mm increase in SL.
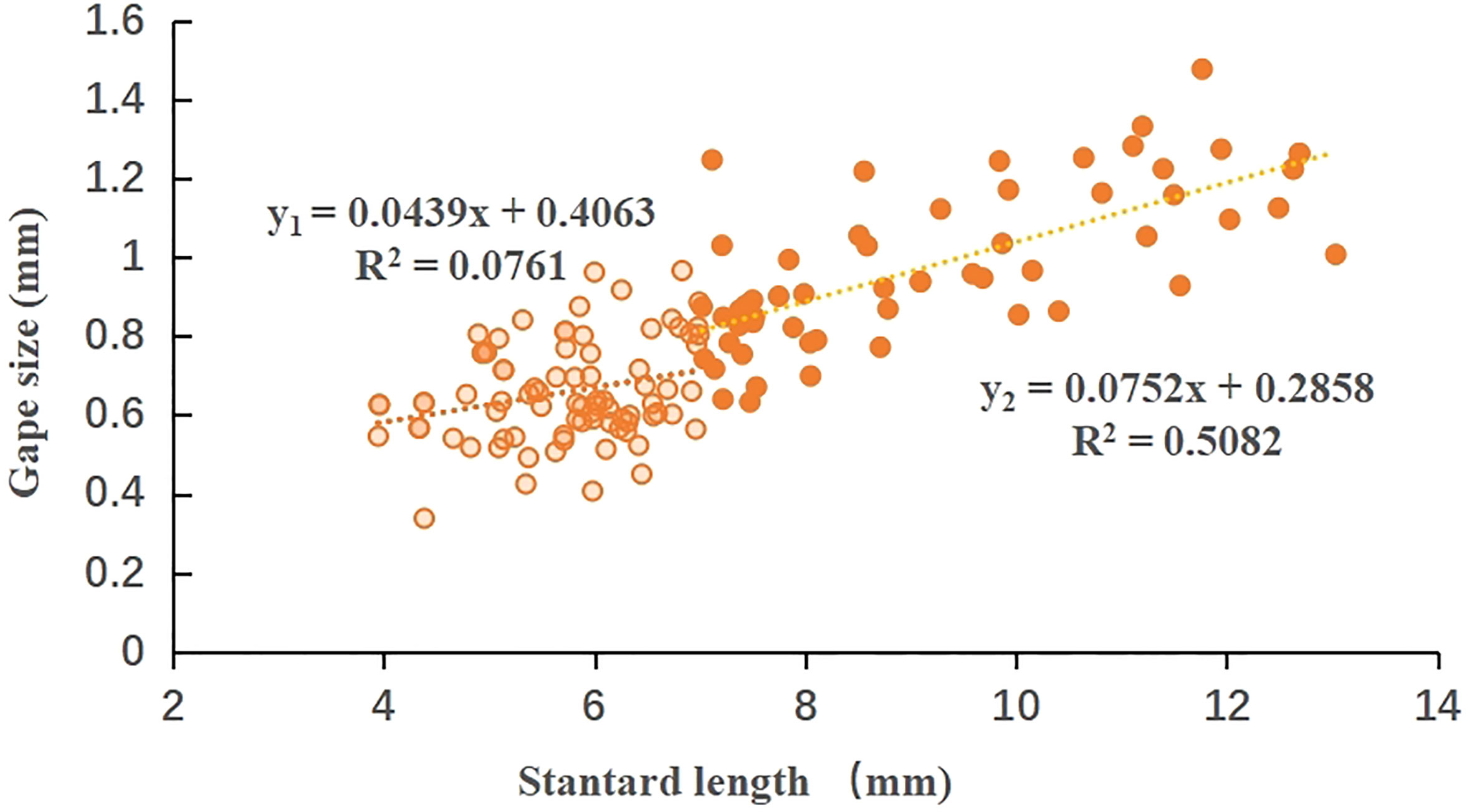
Figure 2 Relationship between standard length and gape size of L. haematocheila larva during the flexion larvae stage and post-flexion larvae stage, collected in Laizhou Bay, Bohai Sea, China.
A total of 103 mullet larvae that had full contents were sorted out, and a total of 2896 zooplankton prey items were identified and measured. Feeding parameters, including MPS, PSR, and PN, were examined for each mullet larva. The MPS in each fish larva varied from 0.37 mm to 0.96 mm, and the PSR varied from 0.02 mm to 0.74 mm. The largest PN in a single fish larva was 116 individual items per fish larva (ind. FL−1).
The relationship between MPS and SL in mullet larvae is shown in Figure 3; the relationship between the GS and SL of the 103 dissected fish larvae is also shown in parallel. Both GS and MPS increased with SL, but with different rates. The MPS overlapped with GS when SL was less than ~7 mm (FL stage), but there was a statistically significant separation with SL increase when SL was greater than 7 mm (PFL stage). Accordingly, the MPS was generally less than the GS and never exceeded 65% of the GS, as indicated by the observation that the maximum value of y4 was approximately 65% of that of y3. Moreover, the rate of increase in MPS varied during ontogeny (Figure 3B). The rate of the increase in MPS declined during the PFL stage (y6) compared with that in the FL stage(y5).
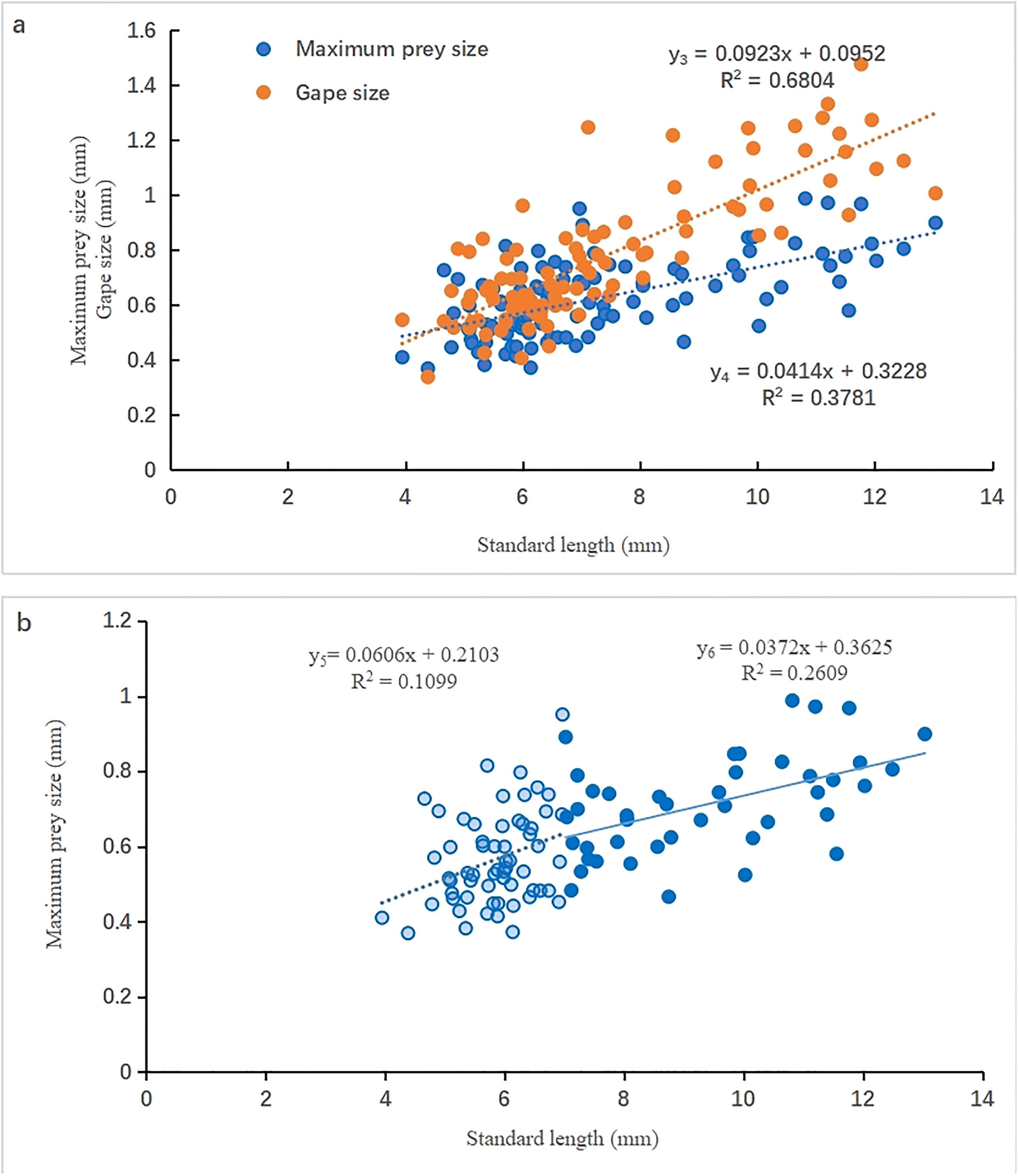
Figure 3 Relationship between maximum prey size (mm) and standard length (mm) of L. haematocheila larvae. (A): scatter diagram of maximum prey size (y3) and gape size(y4); (B): scatter diagram of maximum prey size during the flexion larvae stage (y5) and post-flexion larvae stage (y6).
The relationship between PSR and SL in mullet larvae is shown in Figure 4. The rate of increase in PSR was lower during the PFL stage (y8) than in the FL stage (y7), similar to the trend of increasing MPS.
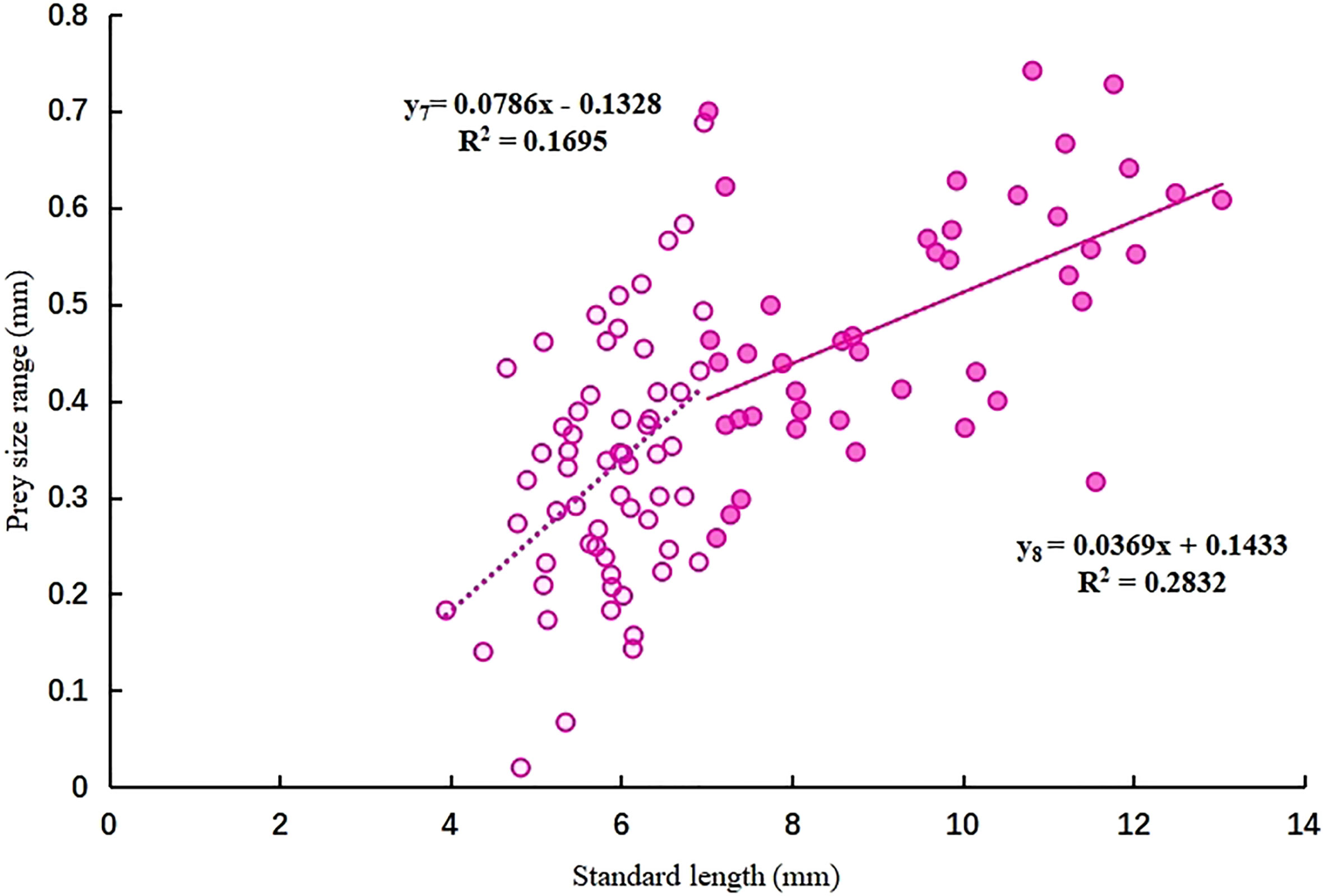
Figure 4 Relationship between prey size range (mm) and standard length (mm) of L. haematocheila larvae during the flexion larvae stage (y7) and post-flexion larvae stage (y8).
The relationship between PN and SL of mullet larva is shown in Figure 5. The rate of increase in PN was lower during the FL stage (y9) than the PFL stage (y10), in contrast to the increasing trends in MPS and PSR. There was no significant linear increase (R2 = 0.068) in PN during the FL stage, and the PN remained low (<46 ind. FL−1); however, the PN increased at a significantly higher rate (R2 = 0.4015) during the PFL stage.
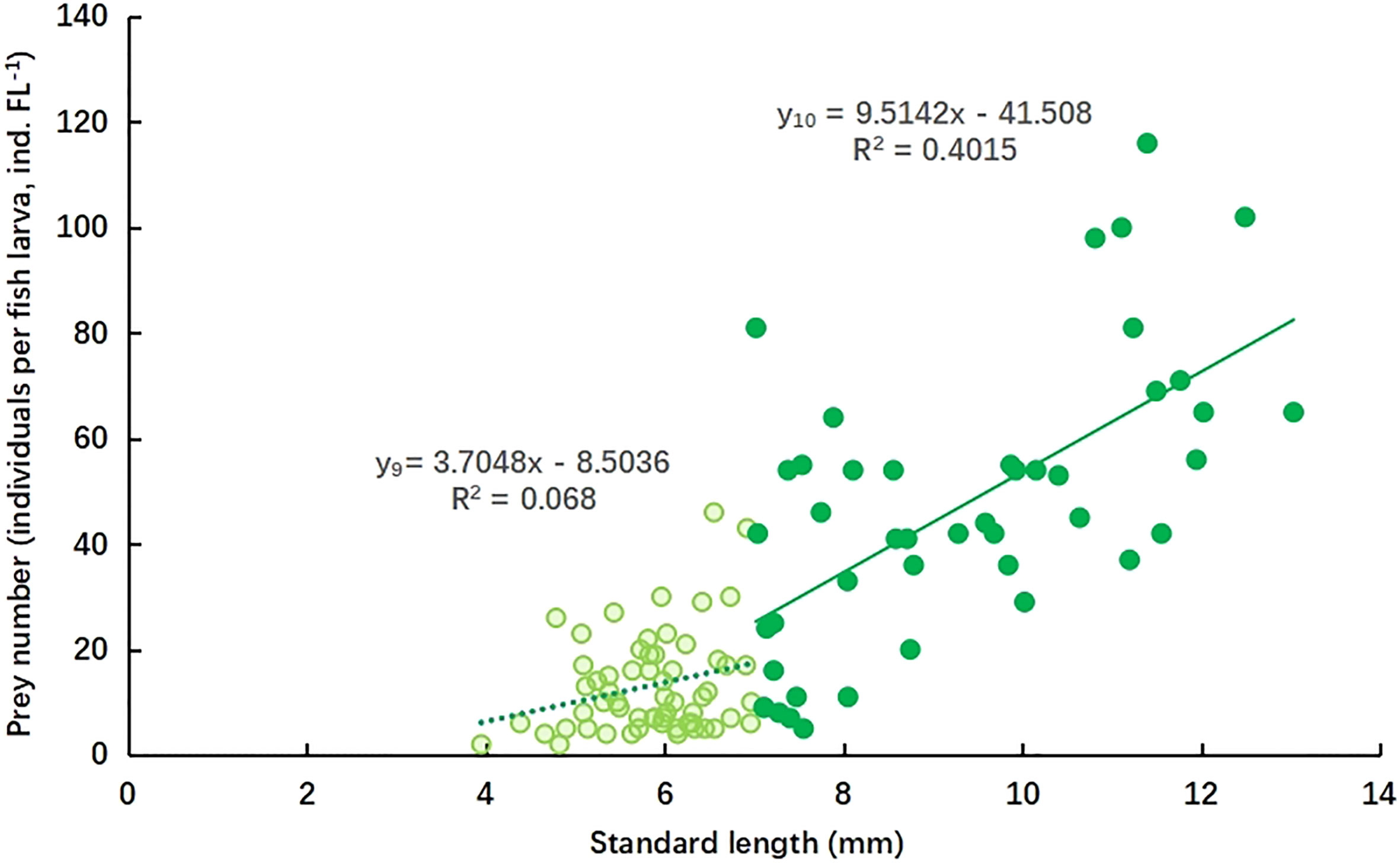
Figure 5 Relationship between prey number (individuals per fish larva, ind. FL−1) and standard length (mm) of L. haematocheila larvae during the flexion larvae stage (y9) and post-flexion larvae stage (y10).
The prey species consumed by the mullet larva are listed in Table 1. In total, 10 zooplankton prey species were consumed, of which eight species were copepods (including copepodites and nauplii). According to the F% and N% values, four copepod species constituted the primary prey items: Paracalanus parvus, Acartia bifilosa, Oithona similis, and Calanus sinicus nauplius. These four copepod species represented 98.1% of the total N%, and the total F% was >66%.
The prosome length of P. parvus, A. bifilosa, O. similis, and total body length of C. sinicus nauplius consumed by mullet larvae were determined, and the data are shown in Figure 6. The mean size of P. parvus was 0.50 ± 0.14 mm, and statistically, this was the largest prey item for mullet larvae (Tukey’s test, P<0.0001). The mean size of C. sinicus nauplius was 0.25 ± 0.08 mm, and statistically, this was the smallest prey item for mullet larvae (Tukey’s test, P<0.0001). The mean prosome length of A. bifilosa and O. similis varied from 0.34-0.38 mm, but the difference was not significant (Tukey’s test, P = 0.14). The numerical percentages of the four primary copepods consumed as prey during ontogeny were compared (in terms of different GS), and the results are shown in Figure 7. According to the original data, four GS groups could be distinguished (0.5-0.7 mm, 0.7-0.9 mm, 0.9-1.1 mm, and 1.1-1.3 mm) in the mullet larvae.
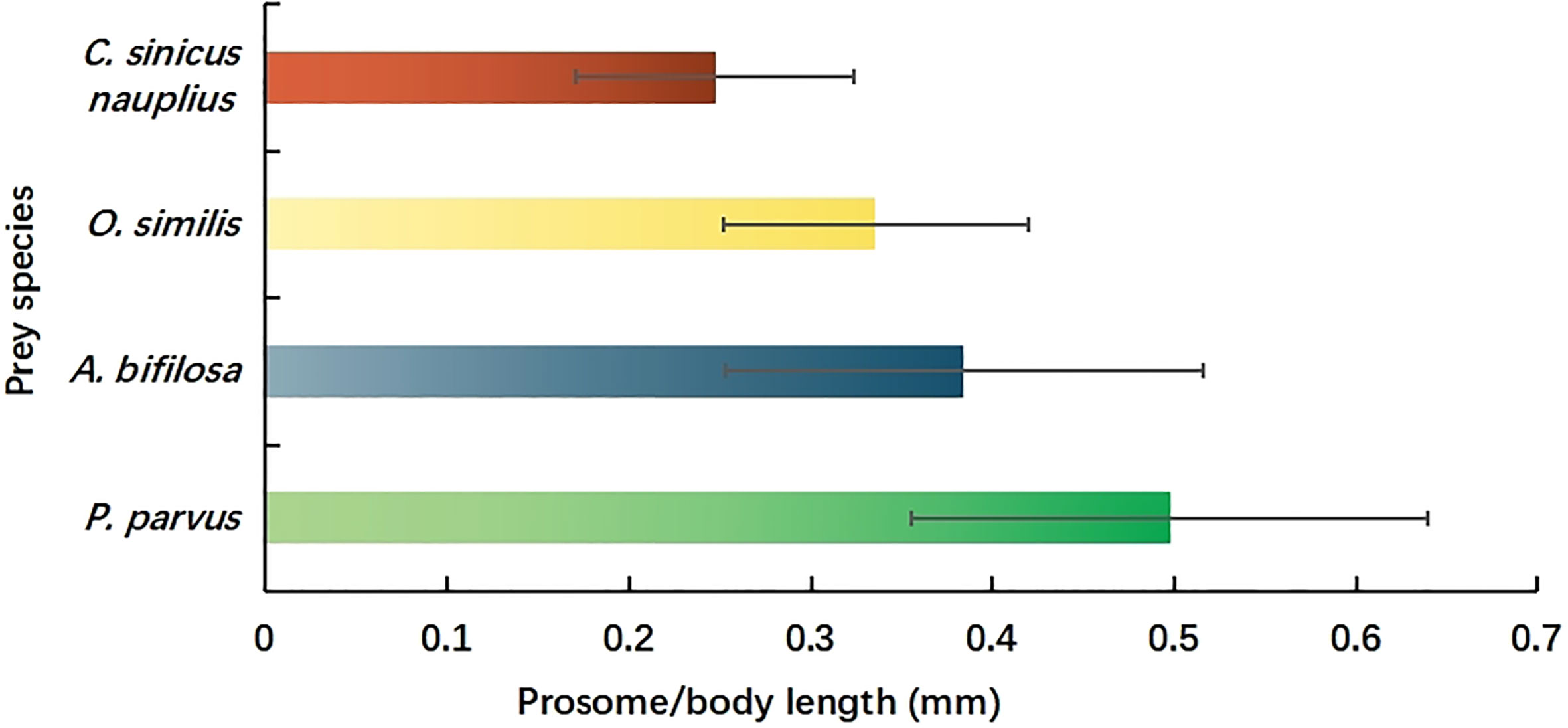
Figure 6 Prosome length of Paracalanus parvus, Acartia bifilosa, and Oithona similis and body length of Calanus sinicus nauplius consumed by L. haematocheila larvae in Laizhou Bay, Bohai Sea, China.
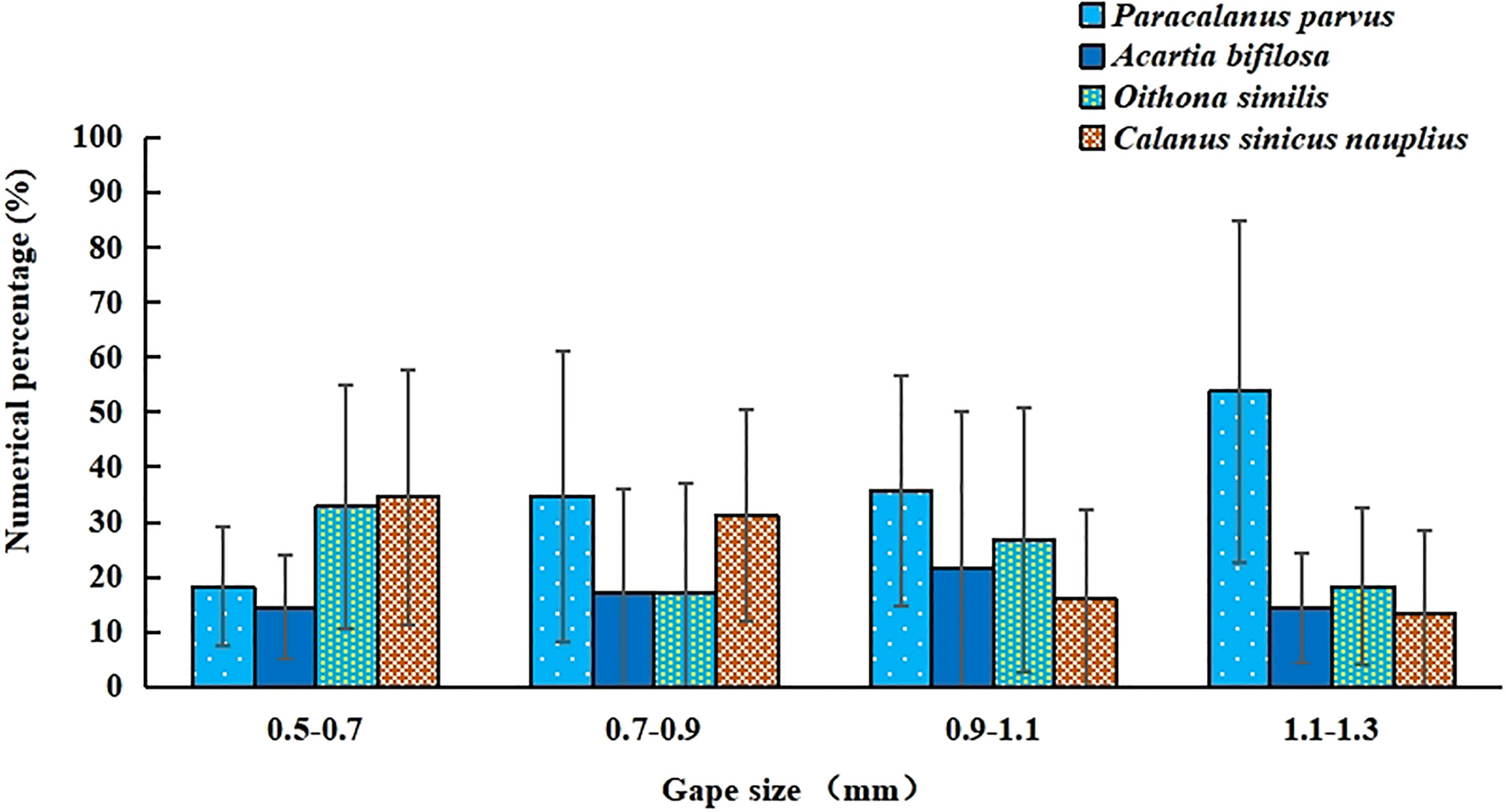
Figure 7 Numerical percentage (%) of four dominant copepod species consumed as prey in different gape-size groups.
Inter-comparisons of the four prey copepods in each GS group revealed that the percentage of C. sinicus nauplius was significantly greater than that of P. parvus in the 0.5-0.7 mm GS group (P< 0.001), but opposite results were obtained for both the 0.9-1.1 mm and 1.1-1.3 mm GS groups (P<0.001, respectively). No significant differences in percentages were found between A. bifilosa and C. sinicus nauplius in each GS group, except for the 0.5-0.7 mm GS group, in which the percentage of A. bifilosa was significantly lower than that of C. sinicus nauplius (P<0.001). Highly similar results were found when comparing the percentages of A. bifilosa and O. similis. There were no significant differences between O. similis and C. sinicus nauplius among all four GS groups (P>0.05).
When comparing the change in each prey copepod during larval ontogeny, the percentage of C. sinicus nauplius significantly decreased in the latter two GS groups (meaning the PFL stage); conversely, the percentage of P. parvus significantly increased during larval ontogeny. No significant trends were found in terms of the percentages of A. bifilosa and O. similis with respect to increasing GS. The percentages of F% and N%, four copepods were the primary prey items, such as Paracalanus parvus, Acartia bifilosa, Oithona similis and Calanus sinicus nauplius. These four copepods contented 98.1% in total N%, and total F% was larger than 66%.
Fish larvae are considered gape-limited consumers that typically swallow their prey whole. How prey size changes during larval ontogeny are important to both the population ecology of fish larvae and aquaculture practices. Feeding studies commonly relate prey size to some metric associated with ontogenic development, usually either a proxy for consumption capacity (e.g., mouth width or jaw length) or simply larval fish length (Nunn et al., 2012). The mean size of prey ingested by larval fish increases with larval growth (Economou, 1991; Pepin and Penney, 1997; Llopiz and Cowen, 2009). Similarly, in our study in L. haematocheila, GS also increased with increasing SL (Figures 2, 3A), and the MPS, PSR, and PN all increased during larvae ontogeny (Figures 3, 4), suggesting that mullet larvae have a higher capacity for prey selection during ontogeny.
Moreover, fish do not necessary consume the largest prey possible (Scott, 1987). For example, juvenile roach and common dace (Lecuiscus leuciscus Cyprinidae) favor prey that is approximately 60% of their maximum GS. In our study, mullet larvae in the PFL stage exhibited similar feeding characteristics, and although there was overlap between MPS and GS during the FL stage, these values clearly separated during the PFL stage, with a lower MPS that never exceeded 65% of GS (Figure 3A), indicating that GS is not a limiting factor for PFL. Furthermore, the rate of increase in both MPS and PSR during the FL stage was higher than that in the PFL stage (Figure 3B), further indicating that PFL have greater ability to select prey size compared with FL. Conversely, the rate of increase in PN was significantly higher in PFL than FL (Figure 5), confirming the higher prey selection ability. Thus, the rate of increase in both MPS and PSR decreases during ontogeny of mullet larvae, and the PN increases during the PFL stage, indicating that PFL have higher prey selection ability than FL. PFL can choose suitable prey items that are smaller than its GS and tend to increase PN instead of prey size.
Studies in other fish larvae also confirm that the size of the prey rarely approaches the maximum capacity for ingestion as indicated by mouth size (Arthur, 1976; Economou, 1991; Pepin and Penney, 1997). Prey size is unrelated to larval length for larger larvae (>16 mm), and larger sprat larvae have shown a smaller average niche breadth (Voss et al., 2009). The reason larvae do not necessarily consume the largest prey during ontogeny is probably related to the increased handling time associated with larger prey (Wanzenböck, 1995). It is becoming clearer that prey consumption by fish larvae is not solely a function of prey size, but rather, a variety of factors that underlie both the size and the taxonomic identity of potential prey play an important role (Peck et al., 2012). Therefore, in addition to prey size and PN, prey type in terms of taxonomic species and prey shifts must be considered in order to clarify the details of prey selection by mullet larvae.
Zooplankton species, especially copepods, occupy an important position as prey of fish larvae, and these organisms are of great significance in terms of the survival and growth of larvae (Rasdi and Qin, 2016). Some studies have examined the diet of mugilidae juveniles but rare in their larvae (Blaber and Whitfield, 1977; Ferrari and Chieregato, 1981; Eggold and Motta, 1992), so our analysis of the feeding habits of L. haematocheila larvae could be useful for ecology studies examining early recruitment of this species. The results of our analysis of diet composition are consistent with those of Lin et al. (1985), who reported that mullet fry<20 mm in total length feed mainly on zooplankton, especially nauplii and small copepods. Moreover, the eight categories of copepods as prey type were all small in size (<1 mm) and included various early developmental stages of larger copepods, indicating the importance of small copepods. Nunn et al. (2012) summarized the diets of larvae in European inshore marine and transitional waters and demonstrated the importance of small copepods as the dominant prey in larval diets.
Both marine and estuary feeding were observed in mullet larvae. Calanoida copepods are normally the first dominant zooplankton category for fish larvae in inshore marine and transitional waters (Nunn et al., 2012). In our study, three-fourths of copepods among mullet larvae prey were calanoida copepods, indicating marine feeding by mullet larvae. Besides Calanoida, another dominant copepod as prey in our study was cyclopoida, among which O. similis was the dominant species (Table 1). The absence of any significant differences between O. similis and C. sinicus nauplius as prey among the four GS groups or differences between O. similis and A. bifilosa, except in the smallest GS group (Figure 7), indicate that O. similis, as a cyclopoida species, is as important as calanoida species during ontogeny of mullet larvae. Cyclopoida copepods are usually important in the diet of fish larvae species in fresh water (Hyslop, 1980; Welton et al., 1983; Easton et al., 1996; Declerck et al., 2002; García-Berthou, 2002); accordingly, the prevalence of cyclopoida copepods in the gut contents of mullet larvae indicates they primarily inhabit estuaries. Indeed, Laizhou Bay is a typical nearshore area of the Bohai Sea and a typical estuary ecosystem affected by fresh water from the Yellow River. The zooplankton community structure in this Bay also confirm the feeding characteristics of mullet larvae, in that calanoida and cyclopoida are both dominant copepods (Sheng et al., 2001).
Diet shifts in fish larvae have a theoretical basis, as although small-size preys are easier to capture than large-size prey, larvae need to catch more small-size prey to meet their nutritional needs; thus, they must expend more energy to catch more prey, which affects larval development (Riley et al., 2012). Therefore, the best choice for mullet larvae is to switch their diet composition from small-size to large-size prey. The results of our analysis of the body length of the dominant prey species showed that the largest dominant prey was P. parvus (Figure 6). The results of our analysis of numerical percentage during ontogeny (Figure 7) showed that the largest prey (P. parvus) gradually increased as GS increased, whereas the smallest prey (nauplii of C. sinicus) decreased, indicating that mullet larvae tend to switch their diet composition from smaller to larger prey. Furthermore, our results also showed that diet shifts mainly occur from the flexion to postflexion stages of ontogeny. The numerical percentage of smaller C. sinicus nauplius decreased significantly beyond the 0.7-0.9 mm GS group (Figure 7), which is the period when mullet larvae develop from flexion to postflexion. These diet shifts also confirm our hypothesis that mullet PFL have higher prey selection ability. These observations confirm that specific larger prey such as P. parvus are selected first by mullet PFL, followed by smaller nauplius of C. sinicus. Another study suggested that fish larvae select specific sizes of prey until the stomach is full, whereafter smaller prey are selected (Gill and Hart, 1998).
A significant positive linear correlation was observed between GS and SL during the PFL stage of mullet larvae, and regression analysis showed that the GS increased approximately 0.075 mm with each 1-mm increase in SL. The MPS overlapped with GS during the FL stage when the SL was less than ~7 mm, but the MPS separated from GS during the PFL stage. The rates of increase in both MPS and PSR slowed during the PFL stage compared with the FL stage, and the MPS was generally less than the GS and never exceeded 65% of the GS during the PFL stage. Instead of consuming prey of larger size, PFL choose to increase the PN, indicating that PFL have greater prey selection ability compared with FL and can choose suitable prey items that are smaller than its gape size, thereby tending to increase the PN instead of prey size during the PFL stage. The composition of the stomach contents of mullet larvae showed that 10 categories of zooplankton constituted the primary prey items, among which eight categories were small copepods. Diet shift analyses showed that the largest prey (P. parvus) gradually increased, whereas the smallest prey (nauplii of C. sinicus) decreased as ontogeny progressed, indicating that mullet larvae tend to switch their diet composition from smaller to larger prey items. This diet shift occurs during ontogeny from the flexion to postflexion stages. Collectively, these diet characteristics confirm that mullet PFL have a greater ability to select prey than FL, and small copepods especially P. parvus are their primary favorite prey items.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
XS contributed in conceptualization, methodology, and writing. MS and ZGL contributed in the application of statistical and writing of the draft. YS, LZ, and BD contributed in conducting the research and investigation process. XY contributed in samples analysis and LXZ contributed in data analysis. ZLL contributed in project administration. All authors contributed to the article and approved the submitted version.
This study was financially supported by Chinese Research Academy of Environmental Sciences (HKHA2022004); China Postdoctoral Science Foundation (2014M561913).
The authors are grateful to Professor Zhenbo Lv, for sharing his program cruise to us, Yanyan Yang for participation in counting samples.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arthur D. K. (1976). Food and feeding of larvae of three fishes occurring in the California current, Sardinops sagax, engraulis mordax, and trachurus symmetricus. Fish. Bull. 74 (3), 517–530.
Blaber S. J. M., Whitfield A. K. (1977). The feeding ecology of juvenile mullet (Mugilidae) in south-east African estuaries. Biol. J. Linn. Soc. 9 (3), 277–284. doi: 10.1111/j.1095-8312.1977.tb00270.x
Brownell C. L. (1979). Stages in the early development of 40 marine fish species with pelagic eggs from the cape of good hope. Ichthyol. Bull. J. Smith Institute Ichthyol. 1979.
Catalán I. A., Alemany F., Morillas A., Morales-Nin B. (2007). Diet of larval albacore Thunnus alalunga (Bonnaterre 1788) off mallorca island (NW Mediterranean). Sci. Marina 71 (2), 347–354. doi: 10.3989/scimar.2007.71n2347
Declerck S., Louette G., De Bie T., De Meester L. (2002). Patterns of diet overlap between populations of non-indigenous and native fishes in shallow ponds. J. Fish Biol. 61 (5), 1182–1197. doi: 10.1111/j.1095-8649.2002.tb02464.x
Devries D. R., Stein R. A., Bremigan M. T. (1998). Prey selection by larval fishes as influenced by available zooplankton and gape limitation. Trans. Am. Fish. Soc. 127 (6), 1040–1050. doi: 10.1577/1548-8659(1998)127<1040:psblfa>2.0.co;2
Dolganova N., Kolpakov N., Chuchukalo V. (2008). Feeding interactions and foraging of juvenile fish and shrimp in the estuaries of Peter the great bay in the summer-fall period. Russian J. Mar. Biol. 34 (7), 482–489. doi: 10.1134/s1063074008070079
Easton R. S., Orth D. J., Voshell J. R. (1996). Spatial and annual variation in the diets of juvenile smallmouth bass, Micropterus dolomieu. Environ. Biol. Fish. 46 (4), 383–392. doi: 10.1007/bf00005018
Economou A. N. (1991). Food and feeding ecology of five gadoid larvae in the northern north Sea. ICES J. Mar. Sci. 47 (3), 339–351. doi: 10.1093/icesjms/47.3.339
Eggold B. T., Motta P. J. (1992). Ontogenetic dietary shifts and morphological correlates in striped mullet, Mugil cephalus. Environ. Biol. Fish. 34 (2), 139–158. doi: 10.1007/bf00002390
Ferrari I., Chieregato A. (1981). Feeding habits of juvenile stages of Sparus auratus l., Dicentrarchus labrax l. and mugilidae in a brackish embayment of the po river delta. Aquaculture 25 (2-3), 243–257. doi: 10.1016/0044-8486(81)90186-1
Ferreira M., Antunes P., Gil O., Vale C., Reis-Henriques M. (2004). Organochlorine contaminants in flounder (Platichthys flesus) and mullet (Mugil cephalus) from douro estuary, and their use as sentinel species for environmental monitoring. Aquat. Toxicol. 69 (4), 347–357. doi: 10.1016/j.aquatox.2004.06.005
Ferreira M., Moradas-Ferreira P., Reis-Henriques M. (2005). Oxidative stress biomarkers in two resident species, mullet (Mugil cephalus) and flounder (Platichthys flesus), from a polluted site in river douro estuary, Portugal. Aquat. Toxicol. 71 (1), 39–48. doi: 10.1016/j.aquatox.2004.10.009
Furgała-Selezniow G., Skrzypczak A., Kucharczyk D., Kujawa R., Mamcarz A., Żarski D., et al. (2014). Food selection of burbot (Lota lota l.) larvae reared in illuminated net cages in mesotrophic lake maróz (north-eastern Poland). Aquac. Int. 22, 41–52. doi: 10.1007/s10499-013-9707-9
García-Berthou E. (2002). Ontogenetic diet shifts and interrupted piscivoryin introduced largemouth bass (Micropterus salmoides). Int. Rev. Hydrobiol.: A. J. Covering all Aspects Limnol. Mar. Biol. 87 (4), 353–363. doi: 10.1002/1522-2632(200207)87:4<353::aid-iroh353>3.0.co;2-n
Ge M., Liu G., Liu H., Liu Y. (2020). Levels of metals in fish tissues of Liza haematocheila and Lateolabrax japonicus from the yellow river delta of China and risk assessment for consumers. Mar. Pollut. Bull. 157, 111286. doi: 10.1016/j.marpolbul.2020.111286
Gill A., Hart P. (1998). Stomach capacity as a directing factor in prey size selection of three-spined stickleback. J. Fish Biol. 53 (4), 897–900. doi: 10.1111/j.1095-8649.1998.tb01844.x
Gisbert E., Cardona L., Castelló F. (1995). Competition between mullet fry. J. Fish Biol. 47 (3), 414–420. doi: 10.1111/j.1095-8649.1995.tb01910.x
Gisbert E., Cardona L., Castelló F. (1996). Resource partitioning among planktivorous fish larvae and fry in a Mediterranean coastal lagoon. Estuar. Coast. Shelf Sci. 43 (6), 723–735. doi: 10.1006/ecss.1996.0099
Hyslop E. (1980). Stomach contents analysis–a review of methods and their application. J. fish Biol. 17 (4), 411–429. doi: 10.1111/j.1095-8649.1980.tb02775.x
Inoue T., Suda Y., Sano M. (2005). Food habits of fishes in the surf zone of a sandy beach at sanrimatsubara, Fukuoka prefecture, Japan. Ichthyol. Res. 52 (1), 9–14. doi: 10.1007/s10228-004-0246-2
Janson N. D., Jehanathan N., Jung S., Priyathilaka T. T., Nam B.-H., Kim M.-J., et al. (2019). Insight into the molecular function and transcriptional regulation of activator protein 1 (AP-1) components c-Jun/c-Fos ortholog in red lip mullet (Liza haematocheila). Fish Shellfish Immunol. 93, 597–611. doi: 10.1016/j.fsi.2019.08.013Jiang
Jiang R., Tang J. H., Liu P. T., Zhong J. S., Wu L., Wu F. Q. (2007). Horizontal distribution and moving tendency of Liza haematocheila larvae and juveniles in the coastal surface of the south yellow Sea. J. Shanghai Fish. Univ. 4, 323–328. doi: 10.1360/yc-007-1071
Kendall A. W. Jr., Vinter B. (1984). Development of hexagrammids (Pisces: Scorpaeniformes) in the northeastern pacific ocean. Noaa/national Mar. Fish. Service 1–34.
Kim J., Perera N. C. N., Godahewa G. I., Priyathilaka T. T., Lee J. (2019). Characterization of a catalase from red-lip mullet (Liza haematocheila): demonstration of antioxidative activity and mRNA upregulation in response to immunostimulants. Gene 712, 143945. doi: 10.1016/j.gene.2019.143945
Lin C. X., Li W. J., Tang T. D. (1985). A study on feeding habits of mullet fry under culturing environments. J. Fish. of China 9(3), 289–296. (in Chinese with English Abstract).
Liu T., Han T., Wang J., Liu T., Bian P., Wang Y., et al. (2021). Effects of replacing fish meal with soybean meal on growth performance, feed utilization and physiological status of juvenile redlip mullet Liza haematocheila. Aquac. Rep. 20, 100756. doi: 10.1016/j.aqrep.2021.100756
Llopiz J. K., Cowen R. K. (2009). Variability in the trophic role of coral reef fish larvae in the oceanic plankton. Mar. Ecol. Prog. Ser. 381, 259–272. doi: 10.3354/meps07957
Mamcarz A., Kucharczyk D., Kujawa R., Skrzypczak A., Furgala-Selezniow G. (1998). Ontogeny of feeding habits in northern pike, esox lucius (Esocidae), larvae reared in illuminated cages. Ital. J. Zool. 65 (S1), 251–253. doi: 10.1080/11250009809386827
Montagnes D. J., Dower J. F., Figueiredo G. M. (2010). The protozooplankton–ichthyoplankton trophic link: An overlooked aspect of aquatic food webs1. J. Eukaryotic Microbiol. 57 (3), 223–228. doi: 10.1111/j.1550-7408.2010.00476.x
Niu M. X., Zuo T., Wang J., Chen R. S., Zhang J. X. (2022). Egg and larval distribution of Liza haematocheila and their relationship with environmental factors in the coastal waters of the yellow river estuary. J. Fish. Sci. China 29, 377–387. doi: 10.12264/JFSC2021-0532
Nunn A., Harvey J., Cowx I. (2007). The food and feeding relationships of larval and 0+ year juvenile fishes in lowland rivers and connected waterbodies. i. ontogenetic shifts and interspecific diet similarity. J. Fish Biol. 70 (3), 726–742. doi: 10.1111/j.1095-8649.2007.01334.x
Nunn A. D., Tewson L. H., Cowx I. G. (2012). The foraging ecology of larval and juvenile fishes. Rev. Fish Biol. Fish. 22 (2), 377–408. doi: 10.1007/s11160-011-9240-8
Omeka W. K. M., Liyanage D. S., Priyathilaka T. T., Kwon H., Lee S., Lee J. (2019). Characterization of four C1q/TNF-related proteins (CTRPs) from red-lip mullet (Liza haematocheila) and their transcriptional modulation in response to bacterial and pathogen-associated molecular pattern stimuli. Fish shellfish Immunol. 84, 158–168. doi: 10.1016/j.fsi.2018.09.078
Ouali N., Belabed B.-E., Chenchouni H. (2018). Modelling environment contamination with heavy metals in flathead grey mullet Mugil cephalus and upper sediments from north African coasts of the Mediterranean Sea. Sci. Total Environ. 639, 156–174. doi: 10.1016/j.scitotenv.2018.04.377
Peck M. A., Huebert K. B., Llopiz J. K. (2012). Intrinsic and extrinsic factors driving match–mismatch dynamics during the early life history of marine fishes. Adv. Ecol. Res. 47, 177–302. doi: 10.1016/B978-0-12-398315-2.00003-X
Pepin P., Penney R. W. (1997). Patterns of prey size and taxonomic composition in larval fish: are there general size-dependent models? J. Fish Biol. 51, 84–100. doi: 10.1111/j.1095-8649.1997.tb06094.x
Pisarevskaya I., Aksenova E. (1991). Feeding of black Sea mullets during early ontogeny. J. Ichthyol 31 (3), 22–30.
Qiao G., Xu C., Sun Q., Xu D.-H., Zhang M., Chen P., et al. (2019). Effects of dietary poly-β-hydroxybutyrate supplementation on the growth, immune response and intestinal microbiota of soiny mullet (Liza haematocheila). Fish Shellfish Immunol. 91, 251–263. doi: 10.1016/j.fsi.2019.05.038
Rasdi N. W., Qin J. G. (2016). Improvement of copepod nutritional quality as live food for aquaculture: a review. Aquac. Res. 47 (1), 1–20. doi: 10.1111/are.12471
Riley K. L., Binion S. M., Overton A. S. (2012). Estimating the food requirements and prey size spectra of larval American shad. Mar. Coast. Fish. 4 (1), 228–238. doi: 10.1080/19425120.2012.675979
Sandamalika W. M. G., Priyathilaka T. T., Nam B.-H., Lee J. (2019). Two phospholipid scramblase 1–related proteins (PLSCR1like-a &-b) from Liza haematocheila: Molecular and transcriptional features and expression analysis after immune stimulation. Fish shellfish Immunol. 87, 32–42. doi: 10.1016/j.fsi.2018.12.044
Scott A. (1987). Prey selection by juvenile cyprinids from running water. Freshw. Biol. 17 (1), 129–142. doi: 10.1111/j.1365-2427.1987.tb01034.x
Sheng B. H., Sun S., Gao S. W., Zhang G. T. (2001). The ecological characteristics of zooplankton community in the bohai Sea II.The distribution of copepoda abundance and seasonal dynamics. Acta Ecol. Sin. 2001 (2), 177–185. doi: 10.1016/0166-0934(89)90166-3
Simonsen C., Munk P., Folkvord A., Pedersen S. (2006). Feeding ecology of Greenland halibut and sandeel larvae off West Greenland. Mar. Biol. 149 (4), 937–952. doi: 10.1007/s00227-005-0172-5
Skrzypczak A., Mamcarz A., Kujawa R., Kucharczyk D., Furgala-Selezniow G. (1998). Feeding habits of larval Eurasian perch, perca fluviatilis (Percidae). Ital. J. Zool. 65 (S1), 243–245. doi: 10.1080/11250009809386825
Suzuki K. (1965). Biology of striped mullet mugil cephalus linne. i. food content of young. Rep. Faculty Fish. 1965, 295–305.
Thiel R., Mehner T., Kopcke B., Kafemann R. (1996). Diet niche relationships among early life stages of fish in German estuaries. Mar. Freshw. Res. 47 (2), 123–136. doi: 10.1071/mf9960123
Voss R., Clemmesen C., Baumann H., Hinrichsen H. H. (2006). Baltic Sprat larvae: coupling food availability, larval condition and survival. Mar. Ecol. Prog. Ser. 308, 243–254. doi: 10.3354/meps308243
Voss R., Dickmann M., Schmidt J. O. (2009). Feeding ecology of sprat (Sprattus sprattus l.) and sardine (Sardina pilchardus w.) larvae in the German bight, north Sea. Oceanologia 51 (1), 117–138. doi: 10.5697/oc.51-1.117
Waltham N. J., Teasdale P. R., Connolly R. M. (2013). Use of flathead mullet (Mugil cephalus) in coastal biomonitor studies: review and recommendations for future studies. Mar. pollut. Bull. 69 (1-2), 195–205. doi: 10.1016/j.marpolbul.2013.01.012
Wang A., Yang W., Liu F., Wang Z., Cang P., Yin X., et al. (2018). Cloning and characterization of lipoprotein lipase (LPL) and the effects of dietary lipid levels on the expression of LPL in the redlip mullet (Liza haematocheila). Aquac. Nutr. 24 (2), 832–841. doi: 10.1111/anu.12612
Wanzenböck J. (1995). Changing handling times during feeding and consequences for prey size selection of 0+ zooplanktivorous fish. Oecologia 104 (3), 372–378. doi: 10.1007/bf00328373
Welton J., CA M., EL R. (1983). Food and habitat partitioning in two small benthic fishes, noemacheilus barbatulus (L.) and cottus gobio l. Archiv. fur. Hydrobiol. 97 (4), 434–454.
Whitfield A. (1985). The role of zooplankton in the feeding ecology of fish fry from some southern African estuaries. Afr. Zool. 20 (3), 166–171. doi: 10.1080/02541858.1985.11447930
Yang G., Hong Q., Zhang T., Hou J., Zhi Y., Zhuang P., et al. (2012). Fish community structure in intertidal area of acipenser sinensis natural reserve in Yangtze river estuary. J. Ecol. 31, 1194–1201. doi: 10.1007/s11783-011-0280-z
Yang W., Wang A., Gao F., Yu Y., Lv L., Lv F. (2015). Dietary lipid concentrations influence growth and chemical and fatty acid compositions of juvenile redlip mullet, Liza haematocheila. Aquac. Int. 23, 981–996. doi: 10.1007/s10499-014-9856-5
Keywords: mullet larvae, small copepods, standard length, gape size, gut content, diet shift, prey selection
Citation: Sun X, Song M, Li Z, Song Y, Yuan X, Dong B, Zhang L, Zhu L and Liang Z (2023) Selective feeding of the mullet larvae Liza haematocheila during ontogeny in Laizhou Bay, Bohai Sea, China: The importance of small copepods in mesozooplankton as prey. Front. Mar. Sci. 10:1147886. doi: 10.3389/fmars.2023.1147886
Received: 19 January 2023; Accepted: 16 February 2023;
Published: 09 March 2023.
Edited by:
Yunyan Deng, Institute of Oceanology (CAS), ChinaReviewed by:
Alexander Kasumyan, Lomonosov Moscow State University, RussiaCopyright © 2023 Sun, Song, Li, Song, Yuan, Dong, Zhang, Zhu and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohong Sun, c3VueGlhb2hvbmdAc2R1LmVkdS5jbg==; Zhenlin Liang, bGlhbmd6aGVubGluQHNkdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.