
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 14 March 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1145083
This article is part of the Research Topic The Transmission and Prevention of Infectious Diseases in Aquatic Animals View all 7 articles
Turbot (Scophthalmus maximus) is an important commercial fish in China that can be infected by a series of bacterial pathogens, leading to great economic losses. In this study we focused on the epidemiology of turbot bacterial diseases in the major farming areas in China for three years. A total of 155 cases with 446 diseased turbots were investigated, and dominant bacterial pathogens were isolated from 137 cases (344 turbots). Thus, bacteria are the major threat to farming turbot in China. Edwardsiella piscicida was the major pathogen, which isolated as the dominant colony in 62 cases (40.00%) with 151 turbots (33.85%). Aeromonas salmonicida was isolated in 57 cases (36.77%) with 116 turbots (26.01%). Vibrio anguillarum was isolated in nine cases (5.81%), and Streptococcus parauberis in five cases (3.23%). Photobacterium damselae and Mycobacterium marinum were also isolated from one or two diseased fish. Other Vibrio spp. were isolated in 15 cases (9.68%). Two species of pathogen were isolated in 13 cases, and three species (E. piscicida, A. salmonicida, and S. parauberis) in one case. In 19 cases, no bacteria were isolated. Based on the annual disease analysis, we found that the E. piscicida infection proportion of total cases was greatly decreased, which may be caused by the attenuated vaccine inoculated in 2018. The antibiotic resistance of E. piscicida strains isolated in Weifang city was also determined. We found that the resistance to ceftriaxone, doxycycline, and SMZ/TMP were significantly increased from October 2016 to June 2018, and all the E. piscicida isolates exhibited resistance to SMZ/TMP in June 2018. These results indicated that E. piscicida is the major threat to turbot farming in China, and the attenuated E. piscicida vaccine exhibits effective protection. The usage of antibiotics may induce resistance quickly. Thus, development of vaccines is an important work for sustainable development of turbot farming in the future.
Turbot (Scophthalmus maximus), which is cultured in Liaoning, Shandong, Hebei, and Jiangsu provinces, is an important commercial fish and the leader of industrial aquaculture in China. Considering the growth of yield and density, fish diseases have become a limiting factor of turbot farming, which leads to great economic losses. According to the China Fishery Statistical Yearbook, 480.84 million juvenile flatfish (mainly turbot) were breeding in 2019, but only 110.984 thousand tons of flatfish were produced in 2020. The commercial size of flatfish is about 500 g, thus 480.84 million juveniles provide more than 200 thousand tons of fish. Only one-half of the flatfish grow to commercial size, indicating that diseases may be serious in turbot farming.
At present, several pathogens of turbot have been reported in China, including Vibrio anguillarum (Zou et al., 2004), V. harveyi (Fan et al., 2005), V. alginolyticus (Zhang et al., 2006), Edwardsiella piscicida (Li et al., 2006), Aeromonas salmonicida (Lv et al., 2009), Mycobacterium marinum (Li et al., 2019), Streptococcus parauberis (Gao et al., 2021), iridovirus (Shi et al., 2005), and scuticociliate (Wang et al., 2005). The variety of pathogens increases the complexity of disease control in turbot farming. Vaccination has been shown as an effective method to control fish diseases and is widely used in Salmo salar (Adams, 2019), while only two turbot monovalent vaccines have been approved in China (E. piscicida in 2015 and V. anguillarum in 2019), which rarely used until 2018. Most of the S. salar vaccines are multivalent, which protect fish against a variety of diseases with a single dose.
Epidemiologic investigation is essential for vaccine development, especially multivalent vaccine. In this study we focused on the epidemiology of turbot bacterial diseases in China from October 2016 to December 2019. A total of 155 cases with 446 diseased turbots were investigated and analyzed to determine the major pathogens. Antibiotic resistance of E. piscicida isolated in Weifang city was also tested in this study. This investigation could provide important information for turbot disease control, and some evidences for antimicrobial resistance.
The samples of diseased turbot were collected from the turbot farm located in the major farming area of 8 cities in Liaoning, Shandong, and Jiangsu provinces, as shown in Figure 1, with 96.77% production in China. The moribund fish or fish dead less than six hours were sampled, and the liver, spleen, and kidneys were collected and cultured on brain-heart infusion (BHI), 2216e, and trypticase soy agar (TSA) plates at 20°C. If nodules were found in the viscera, the sample was also cultured on Middlebrook 7H10 containing 10% OADC. If numerous homogeneous colonies were observed on the plates, the colonies were purified. The isolates were stored at − 80°C with glycerol.
The isolates were identified by 16S rRNA gene sequencing. The 16S rRNA genes were amplified with primers 27F (5`-AGAGTTTGATCCTGGCTCAG-3`) and 1492R (5`-TACGGCTACCTTGTTACGACTT-3`) as described before (Lane et al., 1991). The PCR products were sequenced and analyzed with Basic Local Alignment Search Tool (BLAST) in GenBank, EzTaxon, and Mega 5 to identify the species. The isolated Vibrio spp. strains were checked by empA gene with primers empAF (5′-CAGGCTCGCAGTATTGTGC-3′) and empAR (5′-CGTCACCAGAATTCGCATC-3′) to identify V. anguillarum (Xiao et al., 2009).
Six kinds of antibiotics (ceftriaxone, doxycycline, ofloxacin, florfenicol, enrofloxacin, and SMZ/TMP) resistance of E. piscicida strains isolated in Weifang city from October 2016 to June 2018, was carried out with K-B methods as described previously (Wang et al., 2021). Briefly, the clinical E. piscicida isolates were cultured in TSB, adjusted to 108 cfu/ml, and coated on TSA plates. The pieces with different antibiotics were placed onto plates. The plates were cultured at 28°C for 24 h and the antibiotics resistances were measured. This experiment was carried out in triplicate and the results are expressed as the mean ± SD. Statistical differences between different groups were tested by the LSD Duncan method (p < 0.05).
From October 2016 to December 2019, a total of 155 cases were investigated with 446 turbots (Table S1). Dominant homogeneous colonies were obtained from 137 cases with 344 turbots. The isolates were identified by 16S rRNA gene analysis and listed in Table 1. No mixed infections in one fish were observed.
Based on our results, pathogens were isolated from 137 of 155 cases (88.39%), indicating that bacterial infections were the major cause of disease in farming turbot from 2016 to 2019. Edwardsiella piscicida infection was detected in 62 cases, accounting for 40.00% of all investigations (Table 2). Aeromonas salmonicida infection was detected in 57 cases (36.77%). In 5.81% farm cases, E. piscicida and A. salmonicida infections were both detected. In our previous study, we found that A. salmonicida chronic infection, which caused cutaneous nodules on the ocular side of the turbot (Coscelli et al., 2014), may facilitate the infection of E. piscicida (Yan et al., 2021). Although no mixed infection in one fish was observed, we supposed that some of the fish detected with E. piscicida infection, may be with A. salmonicida chronic infection, which could not be detected by bacterial isolation (Coscelli et al., 2014). These results indicated that E. piscicida and A. salmonicida infections, which were detected in 70.97% of 155 cases, are a great threat to turbot farming. Both A. salmonicida and E. piscicida are the traditional pathogens in aquaculture, which have been reported to cause disease in fish (Stéphanie et al., 2014; Cao et al., 2021). The E. piscicida live vaccine was approved in China in 2015, and we also developed the A. salmonicida subsp. masoucida vaccine for turbot in our previous study (Yan et al., 2021). Considering there is no commercial vaccine against A. salmonicida for turbot in China, the commercialization of the vaccine is urgently needed.
Vibrio anguillarum has been reported as an important pathogen of turbot for many years (Austin and Austin, 2007). In this investigation V. anguillarum was only detected in nine cases. Based on our experience, V. anguillarum infections in turbot are much easier to control by disinfectants and antibiotics than other pathogens. Other Vibrio spp. were also detected in our investigation with lower outbreak rates and could not be identified by 16S rRNA gene sequence analysis.
Mycobacterium marinum infections were detected in two cases when the water temperature was higher than 20°C. Because the turbot is cultured with underground seawater in China, the water temperature in major farming areas is usually < 20°C. Therefore, M. marinum infections are only found in the southern areas with higher temperatures.
Streptococcus parauberis infection was not detected in our previous 10-year investigation (Lan et al., 2020), and had not been reported to be isolated from fish in China until 2019 in turbots (Gao et al., 2021). In the current study, five cases of S. parauberis infection were detected, accounting for 3.23% of the total number of cases. Interestingly, the S. parauberis from turbots was identified as serotype III, which is different from strains isolated from flounder in South Korea and Japan, but similar to the pathogens from turbot in Spain and striped bass in the USA (Gao et al., 2021). Considering that turbot importation has been eliminated from the list of China customs for 10 years, the origin of S. parauberis may be from freshwater fish importation.
Infections by two species of pathogen were detected in 12 cases, which were mainly caused by E. piscicida and A. salmonicida. Infection by three species of pathogens was observed in one case in Yantai city, and E. piscicida, A. salmonicida, and S. parauberis were isolated. No bacterial infections were detected in 16 cases, which may have reflected viral or parasitic infections, or other reasons (Figure 2).
The same results were obtained by analysis of the number of diseased fish. A total of 446 diseased turbots were analyzed in this investigation. According to the bacterial analysis, 151 diseased fish were infected with E. piscicida, which is a major threat accounting for 33.86%. The A. salmonicida infections were detected in 116 fish (26.01%). These results indicated that over 50% of disease were caused by E. piscicida and A. salmonicida. Vibrio anguillarum infections were detected in 29 fish (6.50%), and S. parauberis infections were detected in nine fish (2.02%). In 102 fish (22.87%), no dominant bacteria was isolated, higher than the proportion of farm cases. We speculate that some of the diseased fish may be caused by disease control, such as the use of antibiotics or disinfectants. During disease outbreaks antibiotics or disinfectants may be used by farmers, which may be toxic to fish (Table 1; Figure 3).
The epidemiologic data from different years was also analyzed in this study. Because the investigation was carried out from October 2016, the data from 2016 and 2017 were merged. When focusing on E. piscicida, a very interesting decline was noted. Compared with 2016-2017 (48.39%) and 2018 (51.02%), the E. piscicida infection rate was only 24.18% in 2019, approximately one-half of the last 2 years (Figure 4). The same results were also obtained following an analysis of the number of diseased fish. Edwardsiella piscicida infections affected 44.95% of diseased fish in 2016-2017, 48.78% in 2018, but only 19.63% in 2019 (Figure 5). In 2018, the E. piscicida attenuated vaccine was widely used in breeding farms via the immersion route, and most of the investigated farms received vaccinated juvenile turbot. We tracked a batch of vaccinated juvenile turbot 4 months post-vaccination in 11 farms, and none of these fish were infected by E. piscicida, while the unvaccinated fish from other breeding batches in the same farms had edwardsiellosis. These results indicated that the E. piscicida attenuated vaccine is an effective route to prevent E. piscicida infection, even via the immersion route.
Aeromonas salmonicida infection, which was the second major threat to turbot culture in China, affecting 35.48% of cases in 2016-2017, 28.57% in 2018, and 35.16% in 2019 (Figure 4). Based on the number of diseased fish, A. salmonicida infection affected 22.02% in 2016-2017, 29.27% in 2018, and 26.17% in 2019 (Figure 5). The rate of A. salmonicida infections did not change significantly and was less than the rate of E. piscicida infections in 2016-2017 and 2018, but greater in 2019. Considering the losses caused by A. salmonicida infection, vaccine against A. salmonicida is urgently needed.
The rate of V. anguillarum infection also increased from 3.23% of cases in 2016-2017, to 7.69% of cases in 2019, and 4.59% of diseased fish in 2016-2017, to 9.81% of diseased fish in 2019. The increase of V. anguillarum infection rate may have been caused by the decline in E. piscicida infections. Outbreaks of edwardsiellosis and vibriosis usually occur in summer at high temperature. Edwardsiella piscicida infections were prevented by vaccination, while Vibrio spp. may have had a greater probability to infect fish.
In this study, we found that E. piscicida infection usually occurred when the water temperature was above 16°C and affect fish of all sizes (juveniles to commercial-size fish). Edwardsiellosis was rarely found when the water temperature was below 14°C. Ascites is the main symptom of edwardsiellosis, but is not observed in all diseased fish. In the early stage of E. piscicida infection, hemorrhage occurs in the mouth, eyes, and head, and pus in the eyes could be found (Figures 6A-C). In juveniles, or fish cultured at about 20°C, severe septicemia could be observed during E. piscicida infections, thus causing hemorrhage in fish abdomens, which is similar to vibriosis, and leading to enormous losses during a short period.
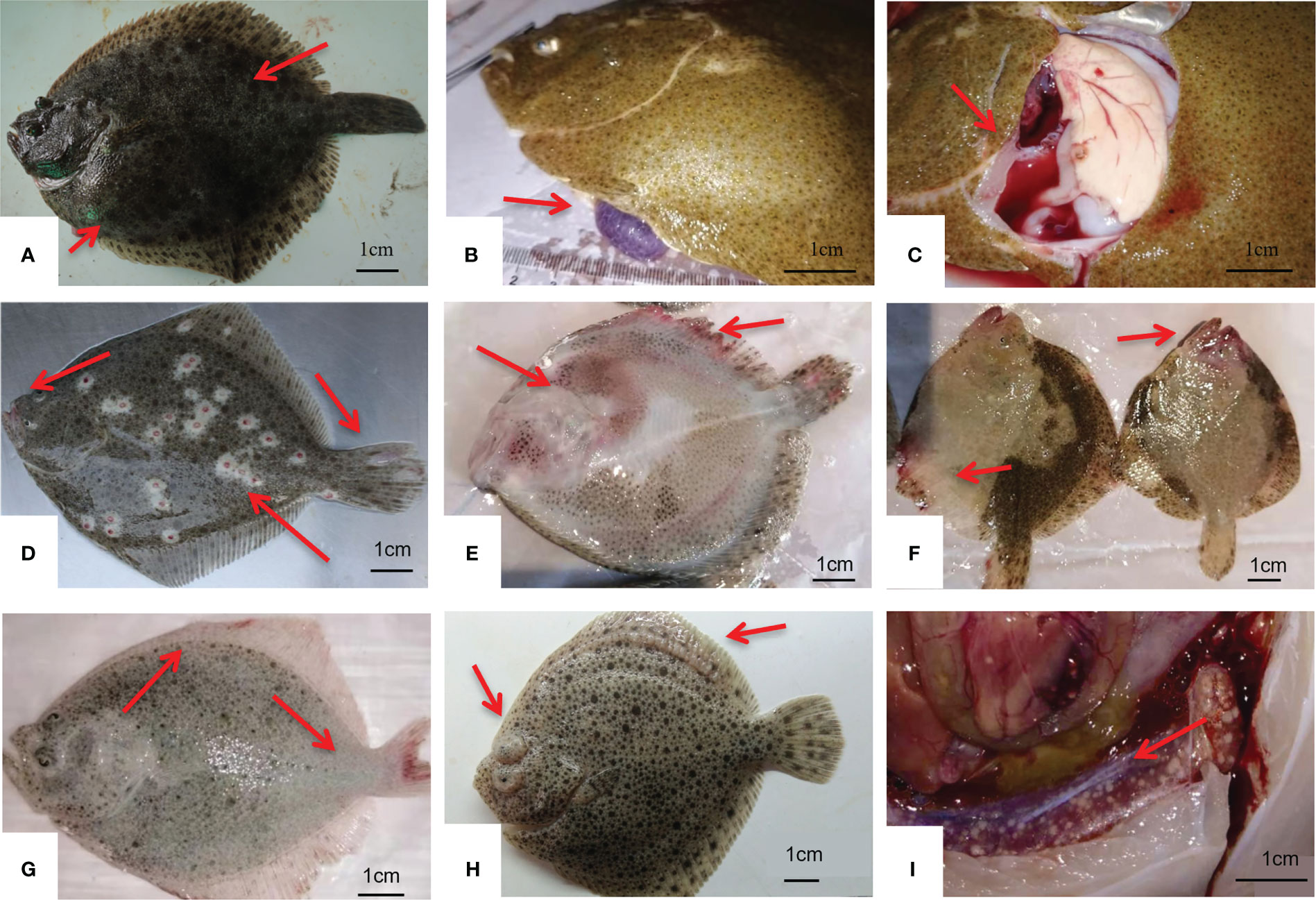
Figure 6 Symptoms of diseased fish. (A, B, C) show turbot infected with Aeromonas salmonicida, with symptoms of cutaneous nodules ((A); Wang et al., 2020), hemorrhage in the abdomen and rotten fins (B), hemorrhage in the mouth (C); (D, E, F) show turbot infected with Edwardsiella piscicida with symptoms of ascites (D), rectal prolapse (E), and pale livers (F); (G) shows turbot infected with Vibrio anguillarum with rotten tail and fins; (H) shows turbot infected with Mycobacterium marinum with renal granulomas (Li et al., 2019).(I) shows turbot infected with Streptococcus parauberis with bilateral exophthalmus and pus in the eyes and pus in fins (Gao et al., 2021). The red arrows mean typical symptom.
Aeromonas salmonicida infection usually occurs in winter when the water temperature is below 16°C, especially below 14°C. For all sizes of fish, the main symptom of A. salmonicida infection was hemorrhage in the mouth (Figures 6D–F). For commercial-size fish, cutaneous nodules were observed in A. salmonicida-infected fish, which may be caused by chronic infection (Coscelli et al., 2014), especially when the water temperature is below 10°C during the winter in northeast China. In our investigation we found that the mortality associated with A. salmonicida infection was usually less than E. piscicida. But cutaneous nodules on the fish surface reduces the commercial value of turbot by approximately 50%. We have developed an inactivated vaccine which could protect turbot against A. salmonicida for 24 weeks (Yan et al., 2021). The commercialization of A. salmonicida vaccine is our following work.
Vibriosis, which is a traditional disease in aquaculture, was also detected during our investigation, but was much less than E. piscicida and A. salmonicida infections. The low water temperature of turbot culturing may reduce the morbidity associated with vibriosis. Vibriosis usually occurred in juvenile turbot in the summer when the water temperature was above 18°C, with hemorrhage in fins and abdomen (Figure 6G). The pathogens included V. anguillarum and other Vibrio spp. that could not be identified by 16S rRNA gene sequence analysis. Culture management is also an important factor of vibriosis. During our investigation we found that vibriosis usually occurred in small farms with lower water supplies or dissolved oxygen. In 2019, with the reduction of edwardsiellosis, the rate of vibriosis increased. We supposed that the vibriosis may be obscured by highly-virulent pathogens, such as E. piscicida and A. salmonicida. If these pathogens were prevented by vaccines or other special treatments, vibriosis may become the dominant disease in turbot culture.
Streptococcus parauberis infection had not been reported in China until 2021. The isolates were classified as serotype III by cps gene analysis (Gao et al., 2021). The sick fish exhibited hemorrhage and pus in the fins, bilateral exophthalmus with hemorrhage, and pus in the eyes (Figure 6H). Streptococcus parauberis infection can be found in all sizes of fish, including commercial size. Usually, the daily mortality of S. parauberis-infected fish was not high, but could last months with high cumulative loss. Streptococcus parauberis infections were first observed in Shandong province in 2019, and we also found S. parauberis infections in Liaoning in the winter of 2020 with water temperatures below 10°C. The symptom of turbot infected with M. marinum is showed as Figure 6I.
Antibiotic resistance of E. piscicida strains isolated in Weifang city from October 2016 to June 2018 was assessed with ceftriaxone, doxycycline, ofloxacin, florfenicol, enrofloxacin, and SMZ/TMP (Figure 7). Antibiotic resistances to ceftriaxone, doxycycline, ofloxacin, and SMZ/TMP were significantly increased from October 2016 to June 2018 (p < 0.05). All of the E. piscicida isolates had complete resistance to SMZ/TMP from March 2018. Edwardsiellosis became a major threat in this area from 2016, and the veterinary pharmacy in this area sold antibiotics according to our results. The farms we sampled were customers of the pharmacy. Therefore, our results may have reflected the relationship between the use of antibiotics and the resistances of pathogens. SMZ/TMP is the cheapest antibiotic and was widely used from 2016. The continuous use of SMZ/TMP may results in significant antibiotic resistance.
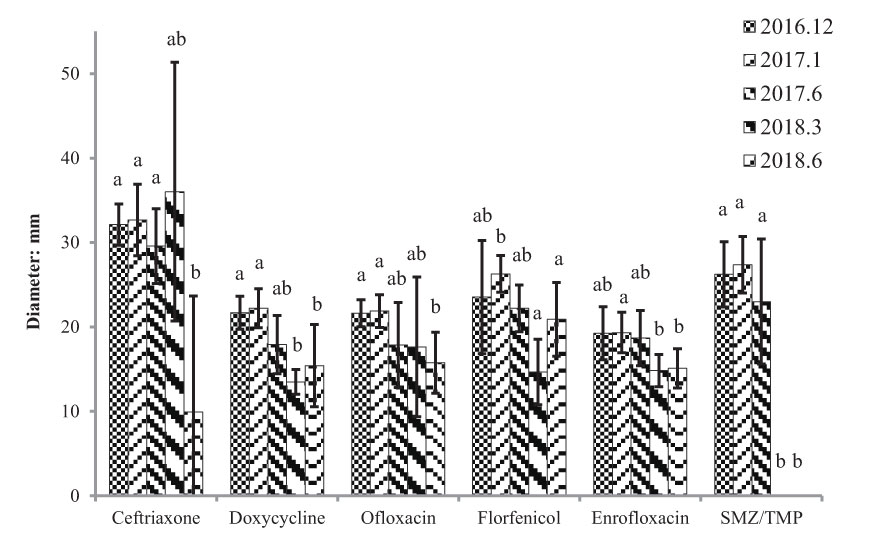
Figure 7 Antibiotic resistance of Edwardsiella piscicida strains isolated in Weifang city from October 2016 to June 2018. a, b: Differences letters indicate significant differences between different groups (P < 0.05).
In this study we found that the bacteria were still the major pathogens affecting turbot farming in China, and E. piscicida and A. salmonicida caused substantial losses compared to other pathogens, which accounted for approximately 70%. We also found that E. piscicida live vaccine significantly reduced the percentage of edwardsiellosis in production. The antibiotics could only control the disease for a short period, but led to antibiotic resistance. Based on our results, we suggest that the multivalent vaccine, which prevented E. piscicida, A. salmonicida, V. anguillarum, and S. parauberis, should be an effective route for the sustainable development of turbot farming in China.
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Experimental Animal Care, Ethics and Safety Inspection Form Yellow Sea Fisheries Research Institute, CAFS. Written informed consent for participation was not obtained from the owners because The sample of this research were all diseased dead fish. The owner give the sample to us for free, and they want to know the reason of the disease.
YG: Investigation, Formal analysis, Writing - Original Draft. QW, YL, YM, HJ, JL, HW and YY: Investigation. JL: Investigation, Writing - Review & Editing, Supervision. All authors contributed to the article and approved the submitted version.
This work was funded by the National Key R&D Program of China (2022YFD2400402), the NFSC-Shandong Joint Fund (U1706205), Ministry of Agriculture and Rural Affairs (CARS-47) and the Central Public-interest Scientific Institution Basal Research Fund, CAFS (NO. 2020XT0406).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1145083/full#supplementary-material
Adams A. (2019). Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 90, 210–214. doi: 10.1016/j.fsi.2019.04.066
Austin B., Austin D. (2007). “Characteristics of the pathogens: Gram-negative bacteria,” in Bacterial fish pathogens. springer praxis books (Dordrecht: Springer).
Cao M., Yan X., Li Q., Fu Q., Yang N., Song L., et al. (2021). Genome- wide identification and analysis of NOD- like receptors and their potential roles in response to Edwardsiella tarda infection in black rockfish (Sebastes schlegelii). Aquaculture. 541. doi: 10.1016/J.AQUACULTURE.2021.736803
Coscelli G. A., Bermúdez R., Silva A. R. S., Ocenda M. V. R., Quiroga M. I. (2014). Granulomatous dermatitis in turbot (Scophthalmus maximus l.) associated with natural Aeromonas salmonicida subsp. salmonicida infection. Aquaculture 428-429, 111–116. doi: 10.1016/j.aquaculture.2014.02.038
Fan W. H., Huang J., Wang X. H., Shi C. Y., Liu L. (2005). Identification and phylogenetic study of pathogenic bacteria causing ulcer disease of cultured turbot (Scophthalmus maximus). Acta Microbiologica Sinica. 45 (5), 665–670. doi: 10.3321/j.issn:0001-6209.2005.05.002
Gao Y., Liu Y. K., Wang P. M., Mo Z. L., Li J., Liu S. L. (2021). Isolation, identification and vaccine development of serotype III Streptococcus parauberis in turbot (Scophthalmus maximus) in China. Aquaculture. 538, 736525. doi: 10.1016/j.aquaculture.2021.736525
Lan X., Li J., Li G. Y., Xiao P., Mo Z. L. (2020). Sequencing and phylogenetic analysis of the 16S rRNA genes of bacterial strains isolated from diseased flatfish. Prog. In Fishery Sci. 41, 142–150. doi: 10.19663/j.issn2095-9869.20190128001
Lane D., Stackebrant E., Goodfellow M. (1991). Nucleic acid techniques in bacterial systematics (New York: John Wiley & Sons), 115–175.
Li J., Liu Y. K., Bai L., Chen S. Q., Yan Y. W., Mo Z. L. (2019). Isolation and identification of Mycobacterium marinum associated with splenic and renal granuloma disease of cultured turbot (Scophthalmus maximus). Prog. In Fishery Sci. 40, 195–199. doi: 10.19663/j.issn2095-9869.20180721001
Li Y., Yan X. H., Chen J. X., Wang Y. G., Li Q. F. (2006). Studies on the characteristics of pathogenic Edwardsiella tarda isolated from diseased Scophthalmus maximus. Periodical Ocean Univ. China. 4, 649–654. doi: 10.3969/j.issn.1672-5174.2006.04.028
Lv J. C., Zhang X. H., Wang Y., Han Y., Liu Y. (2009). Isolation and identification of bacterial pathogen: Aeromonas salmonicida subsp. achromogenes in cultured turbot and histopathological study. Periodical Ocean Univ. China. 39 (1), 97–101. doi: 10.3969/j.issn.1672-5174.2009.01.011
Shi C. Y., Wang Y. G., Qin L., Zhang Z., Yang B., Yang S. L. (2005). Investigation of pathogen and epidemiology of a novel disease, ‘Viral reddish body syndrome’, among farmed Scophthalmus maximus in China. Mar. Fisheries Reseaech. 1, 1–6. doi: 10.3969/j.issn.1000-7075.2005.01.001
Stéphanie D. D., Katherine H. T., Mélanie V. T., Andrée L., Steve J. C. (2014). Virulence, genomic features, and plasticity of Aeromonas salmonicida subsp. salmonicida, the causative agent of fish furunculosis. Veterinary Microbiol. 169 (1-2), 1–7. doi: 10.1016/j.vetmic.2013.06.025
Wang Y. G., Chen J. J., Qin L. (2005). Mesanphrys carcini causing severe scuticociliatosis in farmed turbot Scophthalmus maximus in China. J. of Fishery Sci. of China. 5, 594–601. doi: 10.3321/j.issn:1005-8737.2005.05.011
Wang D., Gong C., Gu H., Huang H., Xian J., Hu Y. (2021). Bicistronic operon YhaO-YhaM contributes to antibiotic resistance and virulence of pathogen Edwardsiella piscicida. Aquaculture 541, 736849. doi: 10.1016/j.aquaculture.2021.736849
Wang P., Li J., He T. T., Li N. N., Mo Z. L., Nie P., et al. (2020). Pathogenic characterization of Aeromonas salmonicida subsp. masoucida turbot isolate from China. J. Fish Diseases. 43 (10), 1145–1154. doi: 10.1111/jfd.13224
Xiao P., Mo Z. L., Mao Y. X., Wang C. L., Zou Y. X., Li J. (2009). Detection of Vibrio anguillarum by PCR amplification of the empA gene. J. fish Dis. 32 (3), 293–296. doi: 10.1111/j.1365-2761.2008.00984.x
Yan Y. W., Liu Y. K., Mo Z. L., Li J., Liu S. L., Gao Y., et al. (2021). Development of Aeromonas salmonicida subsp. masoucida vaccine in turbot and evaluation of protection efficacy under field conditions. Aquaculture. 544. doi: 10.1016/J.AQUACULTURE.2021.737035
Zhang W. N., Zhou L., Xing J., Zhan W. B. (2006). Isolation and identification of pathogen SR1 associated with swollen abdomen of cultured turbot (Scophthalmus maximus). J. of Fishery of Sci. of China. 3, 77–82. doi: 10.3321/j.issn:1005-8737.2006.04.015
Keywords: Scophthalmus maximus, epidemiology, Edwardsiella piscicida, Aeromonas salmonicida, antibiotic resistance
Citation: Gao Y, Wang Q, Liu Y, Ma Y, Jin H, Liu J, Wang H, Yan Y and Li J (2023) Epidemiology of turbot bacterial diseases in China between October 2016 and December 2019. Front. Mar. Sci. 10:1145083. doi: 10.3389/fmars.2023.1145083
Received: 15 January 2023; Accepted: 24 February 2023;
Published: 14 March 2023.
Edited by:
Shihao Li, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Jingguang Wei, South China Agricultural University, ChinaCopyright © 2023 Gao, Wang, Liu, Ma, Jin, Liu, Wang, Yan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Li, bGlqaWVAeXNmcmkuYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.