
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 03 May 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1144448
This article is part of the Research Topic The Transmission and Prevention of Infectious Diseases in Aquatic Animals View all 7 articles
The parasitism by Polyascus gregaria on Eriocheir sinensis induces feminization of the appearance of male crabs, misleading fishermen to bring them to the breeding ponds as female crabs to cultivate broodstock selection. However, there are few studies on whether P. gregaria feminizes the male germ cells, resulting in a decline in the fecundity of male crabs. Therefore, this study aims to clarify the changes in gene expression levels of male crab testes after being parasitized by P. gregaria through transcriptome sequencing to evaluate the change in fecundity. We selected parasitized and healthy male crabs from a pond culture for comparison of gene expression in germ cells. The results showed that, compared with healthy male crabs, there were 104 genes with significantly different expressions, of which 79 were up-regulated and 25 were down-regulated. These genes are mainly focused on the cytoskeleton pathway in cell components and cellular protein complex assembly in biological processes. Several spermatogenesis-related genes, such as Kazal-type protease inhibitor, which inhibits gelatinolytic activities of sperm proteases, and juvenile hormone esterase 6, which degrades methyl farnesoate, were up-regulated; while the down-regulated expression of certain heat shock proteins may lead to spermatogenic dysfunction. In addition, some immune-related genes, such as double whey acidic protein domain-containing protein and serine proteinase inhibitor 3, were significantly up-regulated. These results indicated that P. gregaria changed the development process and cell structure of male host germ cells to inhibit sperm proliferation and maturation, while multiple immune pathways in the hosts were activated to resist P. gregaria invasion.
Sacculinidae is a family of parasitic barnacles belonging to the infraclass Rhizocephala and Kentrogonida (Arthropoda, Crustacea, Cirripedia). They live as crustacean parasites in their adult stage, and in their juvenile stage, they live in seawater as plankton (Alvarez et al., 1995; Shukalyuk, 2002). These parasites infest their specific crustacean hosts, such as the swimming crab (Charybdis longicollis) parasitized by Heterosaccus dollfusi (Innocenti et al., 2003), the Chinese mitten crab Eriocheir sinensis infested by Polyascus gregaria(Tang et al., 2005; Li et al., 2011), the coastal crab (Hemigrapsus sanguineus and Hemigrapsus longitarsis) infested by Polyascus polygenea (Shukalyuk et al., 2007). Grapsid hosts Metopograpsus thukuhar are parasitized by Polyascus plana (Noever et al., 2016), the green crab (Carcinus maenas) is infested by the parasitic barnacle Sacculina carcini (Kristensen et al., 2012), and Sacculina Spp. infestations occur in Portunus sanguinolentus (Raffi et al., 2012).
The Chinese mitten crab, E. sinensis, is one of the most important cultivated crustacean species in China. The adult crab is integral to the rice-crab culture, and the production of E. sinensis not only enhances farm productivity and the income of farmers, but also balances the health of the ecosystem and agricultural production(Bashir et al., 2021). In 2020, the production of E. sinensis reached to 775,887 tons, with a commercial value of approximately US$ 7.8 billion in China (Dan Wang, 2021). The rhizocephalan parasite P. gregarious is the most common parasite found in E. sinensis, potentially harming aquaculture crabs in various aspects, including broodstock selection, culture, and growth. Parasitized crabs are significantly smaller than healthy crabs living concurrently in ponds or rice fields. Male parasitized crabs display more evident alterations in traits that are collectively referred to as “morphologic feminization” (Kristensen et al., 2012; Short et al., 2014; Waiho et al., 2017), including widening of the abdominal carapace from a narrow triangle to a semicircular shape, the development of setae on the edges, and copulatory appendages that are shorter and thinner. A previous investigation discovered that the frequency of infested female crabs and feminized males was higher than that of unmodified males. It is possible that morphologic feminization allows male crabs to hold more externae of P. gregarious in the wider abdominal carapace to better hatch the offspring, which is similar to the hatching behavior of female crabs (Rees and Glenner, 2014; Nagler et al., 2017; Mouritsen et al., 2018).
At present, partial broodstock of E. sinensis still depends on fishing, and strongly feminized males are often mistaken for females by fishermen seeking to replenish broodstock, which leads to the spread of parasites and resulting economic losses (Waiho et al., 2021). The changes in germ cells of infected males have yet to be determined, and thus the risk of production reduction and the spread of parasites cannot be ascertained. In this study, we compared the gene expression profiles of testes between parasitized and healthy crabs using transcriptome sequencing. We aimed to analyze the changes in male germ cells in response to P. gregaria infestations to evaluate the hosts’ fertility and resistance. These differentially expressed genes (DEGs) related to reproduction and immunity will provide us with a more in–depth understanding of the mechanisms of sex regulation and immunity to parasitic diseases in crabs.
In this experiment, 10 healthy male E. sinensis were collected as the control group (CG), while 10 male E. sinensis parasitized by P. gregaria were selected as the experimental group (EG). They were all 2 years old and obtained from a pond culture in Yingkou City, Liaoning Province, China, in September. There were no records of rhizocephalan parasites on cultured crabs in this pond prior to this study. The abdomens of the parasitized male crabs were broader and carried 1–8 externae each. The pair of copulatory organs were thinner than those of healthy male crabs. The two groups were housed in two separate laboratory ponds for 1 week, and the temperature was maintained at 16°C. The light time was consistent with normal weather. The crabs were anesthetized on ice whereafter the profile data was measured and they were dissected. Since the testis of parasitized crabs had atrophied and the mature spermatozoa existed primarily in the vas deferens, both the testis and vas deferens were dissected and combined as one sample in each crab. The samples were separately flash frozen in liquid nitrogen and then kept at −80°C until RNA isolation and sequencing. Three or four samples were pooled as one sequencing sample. A total of six samples were sequenced.
The externae of P. gregarious and testes of infected and healthy crabs were peeled off and fixed in 4% paraformaldehyde, then dehydrated using an alcohol series, embedded in paraffin, and cut into 5 µm sections. Tissue sections were then deparaffinated, rehydrated, and stained with hematoxylin-eosin staining following established protocols (Eddy et al., 2007). The sections were observed using an OLYMPUS CX43 microscope with an Mshot MsX2 camera.
Total RNA was extracted using a Trizol reagent kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNA quality was assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and checked using RNase free agarose gel electrophoresis. After total RNA was extracted, eukaryotic mRNA was enriched using Oligo(dT) beads (NEBNext), while prokaryotic mRNA was enriched by removing rRNA using Ribo-Zero™ Magnetic Kit (Epicentre, Madison, WI, USA). The enriched mRNA was then fragmented using a fragmentation buffer and reverse transcribed into cDNA with random primers. Second-strand cDNA was synthesized using DNA polymerase I, RNase H, dNTP, and a buffer. The cDNA fragments were then purified using a QiaQuick PCR extraction kit (Qiagen, Venlo, The Netherlands), end repaired, poly(A) added, and ligated to Illumina sequencing adapters. The ligation products were size selected by agarose gel electrophoresis, PCR amplified, and sequenced using Illumina HiSeq™ 4000 (Genedenovo Biotechnology Co., Ltd., Guangzhou, China). The final sample was filtered using fastp (version 0.18.0) to obtain high quality clean reads. Transcriptome denovo assembly was carried out using the short reads assembling program-Trinity.
RNAs differential expression analysis between the two different groups was performed using DESeq2 software (Love et al., 2014). The genes with the parameter of false discovery rate (FDR) below 0.05 and absolute fold change ≥2 were considered DEGs. GO function analysis on the one hand gived the GO function classification annotation of differentially expressed proteins; On the other hand, GO functional significance enrichment analysis of differentially expressed proteins was presented by qvalue<0.05.
Total RNA was extracted from the sequencing samples to verify the accuracy of RNA-Seq. The first-strand cDNA was synthesized using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara) depending on the concentrations of total RNA. The templates of 2 μL cDNA were analyzed by qRT-PCR using the SYBR Green II in an Applied Biosystems 7500 Fast Real-Time PCR System with β -actin as the reference gene. Results were analyzed through a comparative Ct method. Relative expression amounts were calculated based on the equation 2−ΔΔCt. These eight pairs of gene-specific primers were used to amplify the partial cDNA sequences (Table 1). Cycle parameters were 95°C for 30 s followed by 40 cycles of 95°C for 5 s and 60°C for 30 s.
The profile data of EG and CG were measured, including weight, carapace width and length, abdomen width, and copulatory organ width and length. All data was analyzed using Independent-samples T test in SPSS Statistics 18 and considered at P<0.05.
In EG, the average weight of parasitized crabs was 42.02 ± 7.97g, the carapace width was 4.93 ± 0.35cm, the carapace length was 4.56 ± 0.25 cm, and the width and length of the copulatory organ were 0.18 ± 0.05cm and 1.58 ± 0.36cm, respectively. In CG, the average weight of healthy crabs was 84.13 ± 6.80g, the carapace width was 5.66 ± 0.13cm, the carapace length was 5.07 ± 0.10cm, and the width and length of the copulatory organ were 0.31 ± 0.02 and 2.05 ± 0.07, respectively (Table 2). As a result of parasitism, the size of parasitized male crabs was significantly smaller than that of healthy males of the same age, including the weight, carapace width and length, copulatory organ width and length (P<0.05).
The externae of P. gregarius are oval in shape with a smooth external cuticle in the abdomen of crabs. The abdomen shape of parasitized male crabs changed from a narrow triangle to a semicircle (Figures 1A, B). The diameters of these externae were 0.4–1 cm and the number of externae varied from 1–15 on one host. There were two types of externae. One was small, pure white or yellowish in color, and round in shape (Figure 1C). The other was larger in size, color was brown, and shape was oval (Figure 1D). The observation of tissue sections showed that the internal structure of the white externa was compactly packed and the oocytes were arranged orderly. Several to dozens of oocytes formed a cluster, which was separated from other oocytes by a diaphragm. The content of the nucleus was high, suggesting that germ cells in white externae were in a period of rapid proliferation (Figures 1E). However, the internal structure of brown externa was loose. The ovary appeared degenerated and only remnants of germ cells could be found (Figure 1F). The testes sections showed that the numbers of spermatids in parasitized male crabs were less than in healthy crabs, and the distribution was looser (Figures 2A, B). Most of the shapes of spermatids in parasitized male crabs were round, while those of healthy crabs were cup-shaped (Figures 2C, D).
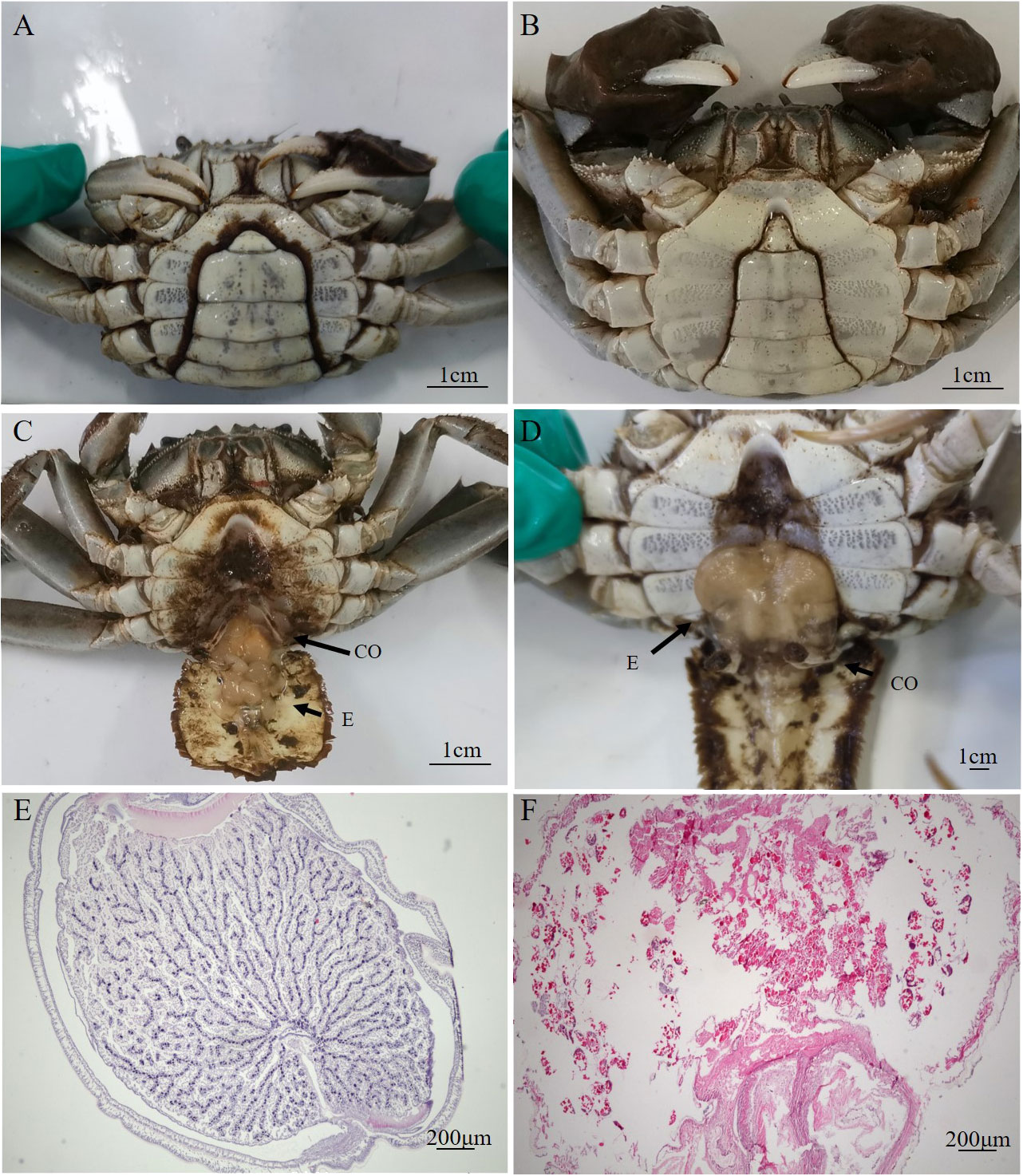
Figure 1 Deformed abdomen and histology of the externae of parasitized E. sinensis (A): the parasitized male crab with semicircular abdomen; (B): the healthy male crab with narrow triangle abdomen (C): the abdomen of parasitized male crab with thinner and shorter copulatory organs and white externae; (D): the abdomen of parasitized male crab with thinner and shorter copulatory organs and brown externae; (E): the internal structure of the white externae by HE staining; (F): the internal structure of the brown externae by HE staining, CO, copulatory organ; E, externae).
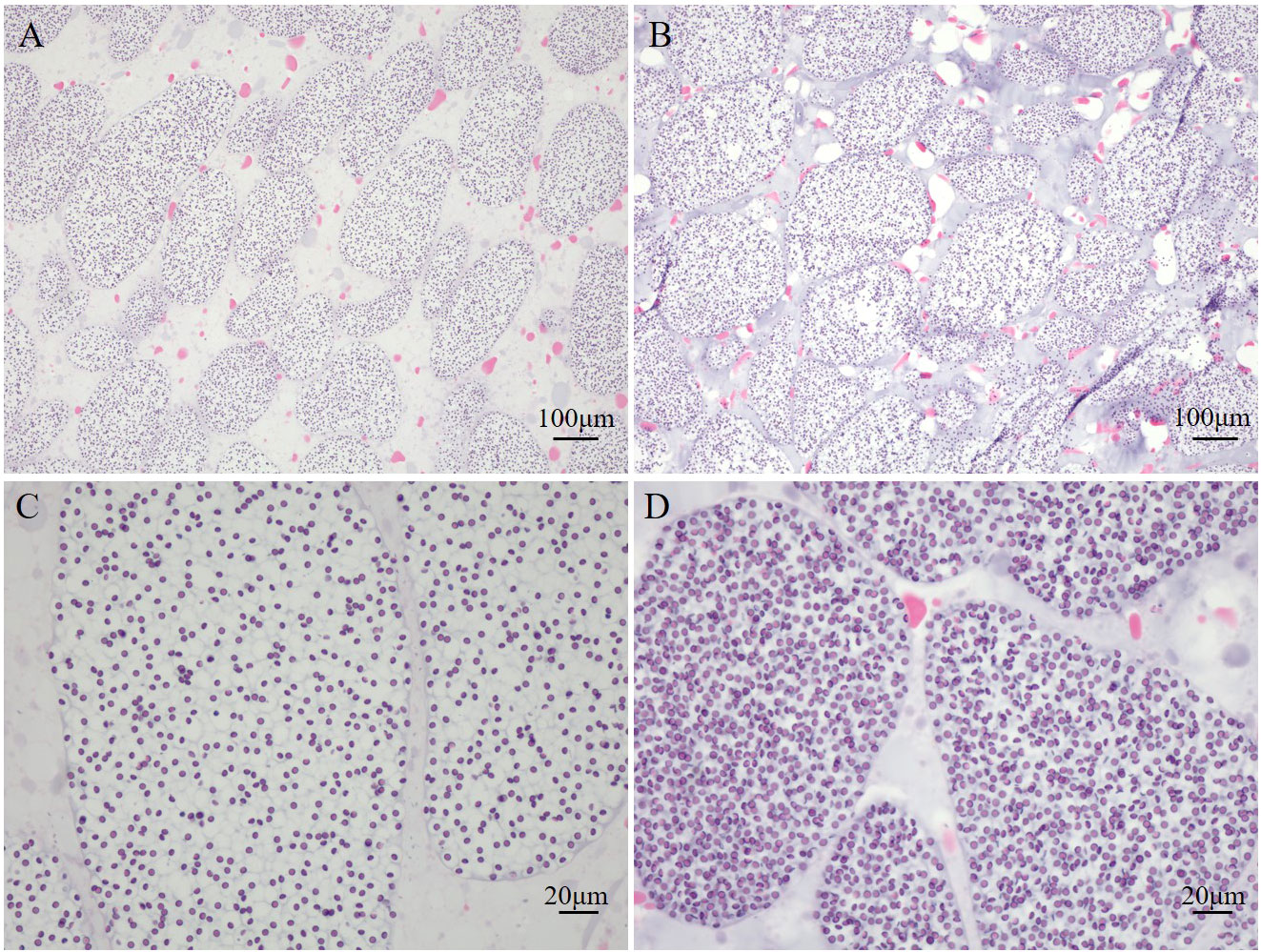
Figure 2 Histology of the testes of parasitized and healthy male E. sinensis (A, C): the internal structure of the testes of parasitized male crab by HE staining; (B, D): the internal structure of the testes of healthy male crab by HE staining).
The purpose of transcriptome sequencing was to analyze the changes in gene expression in the testes of E. sinensis between the EG (parasitized crabs) and CG (healthy crabs). Principal component analysis based on testes transcriptome profiles showed that the repeatability within the group in the sample was good and there was a good degree of distinction between groups (Figure 3). The sequence data reported in this study were archived in the Sequence Read Archive (SRA) with the accession number PRJNA801113.
A total of 90438 unigenes were assembled, among which 34999 genes were annotated and 55439 genes were not annotation. The four databases of Nr, KEGG, KOG, and SwissProt were annotated with 33513, 27274, 19914, and 21911 genes, respectively (Table 3). A large proportion of genes without annotation might be attributed to the limited number of crustacean sequences in public databases.
In the Nr database, the top three species with the highest number of sequence hits were Hyalella azteca (5200, 15.52%), Daphnia magna (1501, 4.48%), and Zootermopsis nevadensis (1170, 3.49%). According to KEGG analysis, these genes were assigned to 145 pathways, most of which belong to metabolism (90/145), Genetic Information Processing (22/145), environmental information processing (16/145), cellular processes (9/145), and organismal systems (6/145) successively. By searching against KOG database, all unigenes were classified into 25 groups. The top three function classifications were general function prediction only (4063/19914), signal transduction mechanisms (3234/19914), and posttranslational modification, protein turnover, and chaperones (2439/19914).
To identify the change in testes of E. sinensis during Sacculina parasitism, we screened the genes with FDR<0.05 and |log2FC|>1 as significantly different genes. In total, 104 genes showed significant differences in genes abundance between EG and CG, including 79 genes that were highly expressed in EG and 25 genes that were highly expressed in CG (Figure 4). Gene Ontology (GO) enriched analysis showed that cytoskeleton, cellular protein complex assembly, protein complex assembly, protein complex biogenesis, cellular macromolecular complex assembly, macromolecular complex assembly, cellular component assembly, cellular component biogenesis, aminoglycan biosynthetic process, regulation of tube size, regulation of tube diameter, and protein complex subunit organization were significantly enriched (Table 4). KEGG pathway enrichment analysis showed that the “Phagosome” (ko04145) was enriched.
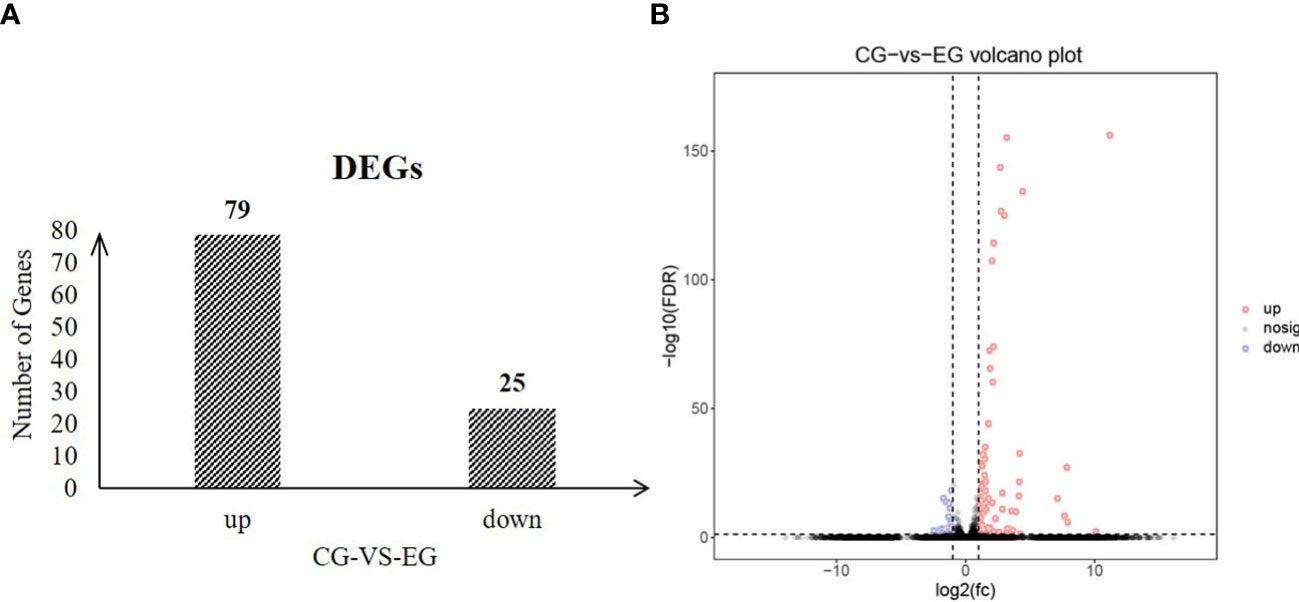
Figure 4 Numbers of significantly differentially expressed genes between CG and EG (FDR<0.05 and |log2FC|>1) (A) number of up and down regulated genes; (B) volcano plot.
The expression levels of some crucial sex determination and reproduction genes were shown to be changed through the transcriptome analysis (Table 5). Tissue gene expression profiles in parasitized E. sinensis showed that the Kazal-type protease inhibitor (Es-KPI) was up-regulated. In addition, seven genes were annotated from the transcriptome by blast searching of all the transcripts against the public database that were related to germ cell proliferation. Among these, five genes, including Es-tubulin, 60S ribosomal protein L38-like (Es-RPL38), cytochrome c oxidase subunit 6B2-like isoform X2-like (Es-Cox6b2), and heat shock proteins (Es-hsp70 and Es-hsp90) were down-regulated. While both juvenile hormone esterase 6 (Es-JHE6) and endonuclease and reverse transcriptase-like protein (Es-RTase) were up-regulated.
We screened and identified a series of up-regulated immune-related genes. The expression level of double whey acidic protein domain-containing protein (Es-DWD) and venom allergen 5 (Es-VA5) of E. sinensis in EG was higher than that in the CG. Multiple sequence alignment revealed that predicted protein serine proteinase inhibitor 3 (Es-serpin 3) showed a sequence similarity of 69.55% with serpin 3 from Penaeus monodon, and a similarity of 68.76% with serpin 3 from Litopenaeus vannamei. The homology between annotated zinc proteinase Mpc1 (Es-ZP) sequence and that of Portunus trituberculatus was 68.08%. Moreover, Vacuolar protein sorting-associated protein 37A (Es-VP37A), annotated from the transcriptome, showed a sequence similarity of 58.76% with VP37A from Amphibalanus amphitrite and a similarity of 43.37% with VP37A from Pollicipes. Compared with the CG, the expression of Es-serpin 3, Es-ZP, and Es-VP37A of EG were similarly significantly up-regulated.
The expression patterns of all eight tested genes showed good consistency between qRT-PCR and RNA-Seq, which verified the reliability of RNA-Seq. As seen in Figure 5, the tested genes that related to sex regulation included Es-KPI, Es-JHE6, Es-tubulin, and Es-RPL38, while those related to immunity included Es-DWD, Es-VA5, Es-serpin 3, and Es-VP37A.
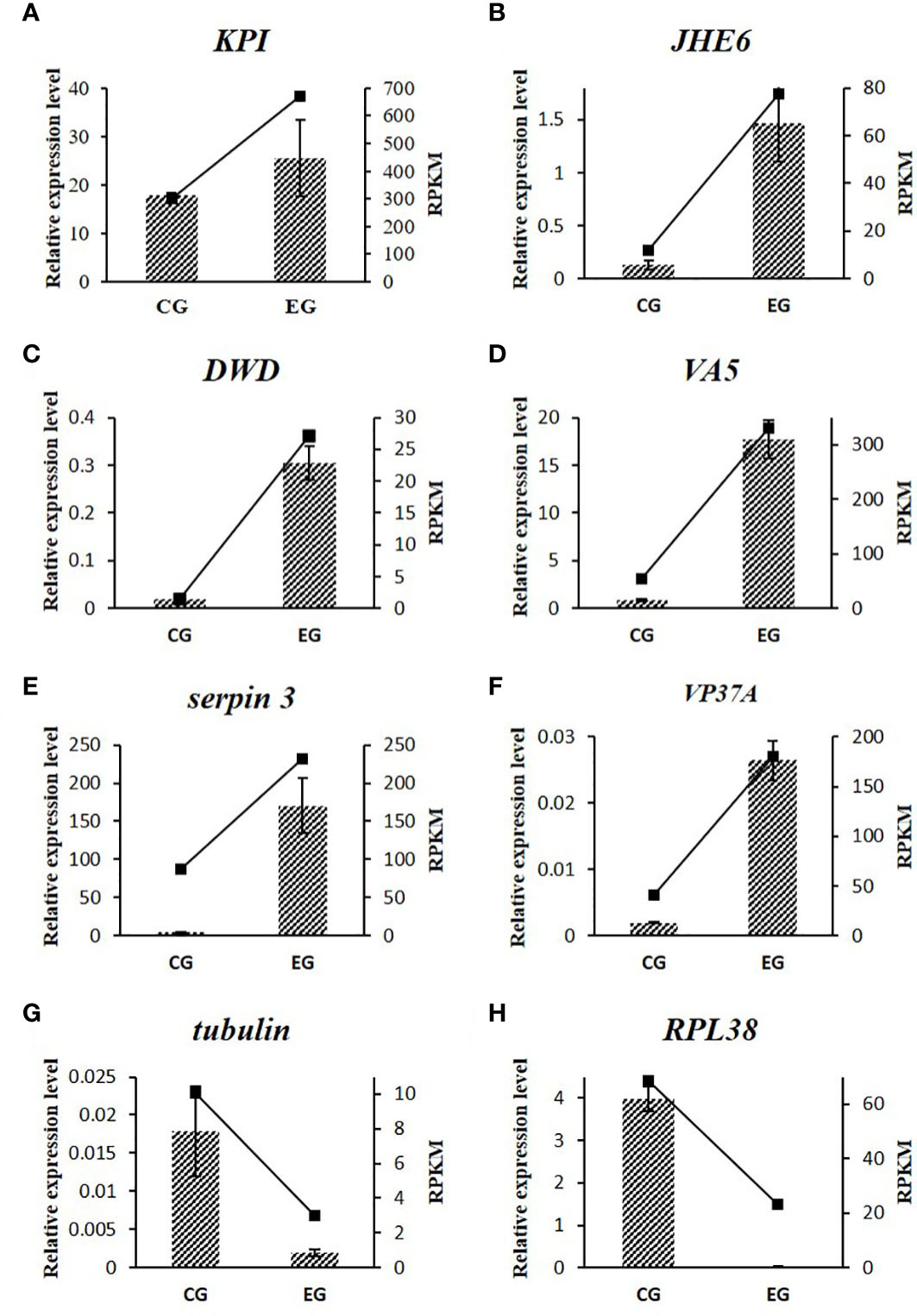
Figure 5 Relative expression profiles of eight DEGs by qRT-PCR and RNA-Seq. The qRT-PCR results were represented with the column diagram and primary axis, and the RNA-Seq results were represented with the line chart and secondary axis.
The rhizocephalan Sacculina carcini leads to a decrease in the population of many species of crabs, including the ecologically important shore crab C. maenas (Rowley et al., 2020), highly valuable king crab species (Lithodidae) (Noever et al., 2016), family Portunidae (Raffi et al., 2012), and mud crabs (Scylla spp.) (Fazhan et al., 2020). These parasitized crabs receive damage to two essential organs, namely the hepatopancreas and gonads. Since the parasites absorb the nutrition of the hosts and the externae hinder the crabs from molting, the parasitized crabs were less active than the healthy crabs and did not molt during the laboratory breeding period. Some parasitized crabs faced up and their appendages trembled. The infested crabs were smaller than healthy crabs. The tissue sections showed that the number of spermatids in the testis decreased and the shape of spermatids was mostly round in parasitized crabs, which suggest that parasitism suppresses spermatogenesis of crabs. However, there is no effective treatment for the parasitized crabs, and the fishermen kill the crabs found with externae through manual screening. However, it is not possible to ascertain whether the crabs were parasitized with no externae present. Therefore, the transmission of P. gregarious cannot be completely suppressed at present.
Many economically important crustacean species that are infected with rhizocephalan parasites exhibit feminization of morphological characters, including Anomura (Lithodidae), Brachyura (Cancridae), and Brachyura (Platyxanthidae) (Brockerhoff et al., 2010; Noever et al., 2016; Nagler et al., 2017; Boyko and Williams, 2020; Waiho et al., 2021). The abdomen of parasitized male crabs becomes wider, while the copulatory organ becomes shorter and thinner (Kristensen et al., 2012; Waiho et al., 2017). However, it is not clear whether the reproductive system of male crabs is feminized or not. In this study, we examined the molecular changes in the testes of hosts after infestation. From the 90438 unigenes obtained, 109 genes were significantly differentially expressed. Interestingly, Es-JHE6 and Es-KPI were up-regulated. JHE6 is a key enzyme in methyl farnesoate (MF) degradation, which plays a significant role in regulating the MF titer (Xu et al., 2017). MF has been regarded as the crustacean equivalent to the juvenile hormone (JH) in insects (Nagaraju, 2007; Miyakawa et al., 2014), and is involved in regulating reproduction and molting. In the male spider crab (Libinia emarginata), high levels of MF enhance reproductive behavior (Sagi et al., 1994). While in the crayfish (Procambarus clarkii), MF stimulates ovarian maturation (Laufer et al., 1998). Therefore, we speculate that P. gregarious decreases the level of MF by inducing an increase in JHE6 expression in the host, which in turn affects the molting and testes development of the host, resulting in the feminization of certain characteristics. Es-KPI inhibits the gelatinolytic activities of E. sinensis sperm proteases (Qian et al., 2012). Hence, the up-regulated expression of Es-KPI may be one of the reasons the spermatid of parasitized crabs does not mature.
During spermatogenesis, tubulin functions as a platform for proteins to correct transport and assembly in the formation of mature sperm. The expression of tubulin in the testes determines the quality and function of the spermatozoa in mammals (Lehti and Sironen, 2016; Amargant et al., 2019). In the rat testis, destruction of the actin and microtubule-based cytoskeletons induced elongated spermatid exfoliation (Su and Cheng, 2019). As microtubular structures in sperm are a fertility biomarker in Cambaroides japonicas and Cambarus sp. (Aquino et al., 2021), the down-regulated expression of tubulin may be one of the reasons the spermatid of parasitized crabs do not mature.
Proteins containing whey acidic protein (WAP) domains are highly conserved and serve in proteinase inhibition and bacterial killing processes, not only in mammals but also in invertebrates(Smith, 2011). The single WAP domain-containing protein (SWD) and double WAP domain-containing proteins (DWDs) are identified in many species of crustaceans, including Fenneropenaeus chinensis (Du et al., 2009), Penaeus monodon (Suthianthong et al., 2011), Litopenaeus vannamei (Visetnan et al., 2017; Sakunwattana et al., 2020), and E. sinensis (Li et al., 2012; Li et al., 2013). Both SWD and DWDs expressions are up-regulated to perform immune functions against the bacterial infection. Hence, the up-regulated DWDs found in our study may be associated with defense against the parasites.
The serine proteinase inhibitors (SPIs) were classified into several families, such as serpin, Kazal, alpha-2-macroglobulin, and pacifastin, based on their structures, topological similarities, and inhibition mechanisms in invertebrates (Laskowski and Qasim, 2000). In shrimp, Pm-serpin 3 decreases the clearance rate of bacteria by regulating the PO activating system in P. monodon (Wetsaphan et al., 2013). As there was a 69.55% similarity between the serpin 3 obtained through sequencing and Pm-serpin 3, we speculate that the up-regulated serpin 3 is induced by parasites to overcome the activity of the PO system when E. sinensis resists the parasites. However, several serpins and Kazal-type SPIs suppress the proteolytic cascade to control the phenoloxidase (PO) system as the unique immune system of the crustacean (Cerenius et al., 2008; Cerenius and Soderhall, 2021). The serpin and Kazal-type SPIs prevent severe damage to the hosts that is caused by the overreaction of the PO system in crustaceans or inhibit extracellular proteinase secretion by specific pathogens (Cerenius et al., 2010; Apitanyasai et al., 2020). In Penaeus vannamei, serine proteinase inhibitor 7 (LvSerpin7) reduces the toxic effects that result from unregulated activation of the PO defense system by acute hepatopancreatic necrosis disease (AHPND). In P. monodon, Kazal-type serine proteinase inhibitor SPIPm2 resists the yellow head virus (YHV) and Bacillus subtilis infection (Donpudsa et al., 2009; Visetnan et al., 2018). Therefore, the function of Es-serpin 3 in crustaceans requires further study.
The life cycle and developmental characteristics of the rhizocephalan parasite were studied, including Sacculina carcini Thompson, 1836 parasitizing the shore crab C. maenas (Lutzen et al., 2018; Jensen et al., 2019), Sacculina yatsui Boschma, 1936 infecting the Asian shore crab Hemigrapsus sanguineus (Kobayashi et al., 2018), and Polyascus polygenea infecting the coastal crab Hemigrapsus sanguineus (Korn et al., 2004). The developmental stages of the externae were divided into separate stages, including the virgin stage, sexual maturity stage, and degradation stage. In our study, we found two types of externae in P. gregarious. The internal structure of externa shows that the white externa is in the virgin stage and the brown externa is in the degradation stage. These results indicate that P. gregarious is able to be fertilized, reproduce, and spread in cultured E. sinensis of both pond cultures and rice fields. Therefore, careful attention must be paid to prevent this parasite from spreading further.
Apart from clarifying the characterizations of P. gregarious, we need to understand the resistance mechanisms of the hosts. A more in-depth understanding of the interaction mechanism between parasite and host will aid in the development of preventative strategies for Sacculinidae infestations in crabs. Two rhizocephalan species from the families Peltogastridae (Peltogaster paguri) and Peltogasterellidae (Peltogasterella gracilis) invade the nervous systems of hermit crabs Pagurus pubescens, Pagurus ochotensis, and Pagurus pectinatus, which suggests that the parasite interacts with the host on the humoral level via neuromediators, hormones, attractants, and trophic factors(Miroliubov et al., 2020). As eyestalk and thoracic ganglion are the most important neuroendocrine organs of nervous system for various biological functions in E. sinensis (Rotllant et al., 2018; Chen et al., 2019; Jiang et al., 2020), we speculate that P. gregarious induces these two organs to secrete neurotransmitters that change the appearance and behavior of the male crabs, including the male crab hatching externae of Sacculina similar to the female crab hatching eggs. The enriched GO pathways also showed that P. gregaria changed the development process and cell structure of germ cells of male E. sinensis. Further, the molecular mechanisms should be investigated to determine a prevention or treatment.
This is the first transcriptomic analysis of the testes of E. sinensis infested with P. gregaria, which is useful for helping us to understand the change in germ cells after the crabs became parasitized. The parasite induces lower expression of genes related to sperm proliferation and maturation, thus destroying the structure and function of germ cells to reduce the fertility of infected crabs. Meanwhile, the increased expression of several immune genes indicates that the infected crabs adjust multiple immune pathways to resist the parasites. These results will facilitate our understanding of infection pathogenesis and the life cycle progression of P. gregarious which are useful to formulate a control strategy.
The datasets presented in this study can be found in online repositories. The name of the repository and accession number can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA801113.
CF, JZ and QC were involved in designing the experiment and performing the experiments. JB, DL and NJ assisted in sample collection and data analysis. CF and JZ wrote the manuscript. Then, QC and NJ reviewed and edited of the paper. All authors contributed to the article and approved the submitted version.
This work was supported by China Agriculture Research System of MOF and MARA (CARS-48); Liaoning Province Department of Education fund (LJKQZ20222356); Shenyang Agricultural University PhD research fund (880418061); Liaoning Province “The Open Competition Mechanism to Select the Best Candidates” Project (2021JH1/10400040); Liaoning Provincial Science and Technology Mission Project (2022-MS-253).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alvarez F., Hines A. H., Reaka-Kudla M. L. (1995). The effects of parasitism by the barnacle loxothylacus panopaei (Gissler)(Cirripedia: rhizocephala) on growth and survival of the host crab rhithropanopeus harrisii (Gould)(Brachyura: xanthidae). J. Exp. Mar. Biol. Ecol. 192 (2), 221–232. doi: 10.1016/0022-0981(95)00068-3
Amargant F., Barragan M., Vassena R., Vernos I. (2019). Insights of the tubulin code in gametes and embryos: from basic research to potential clinical applications in humans. Biol. Reprod. 100 (3), 575–589. doi: 10.1093/biolre/ioy203
Apitanyasai K., Chang C.-C., Ng T. H., Ng Y. S., Liou J.-H., Lo C.-F., et al. (2020). Penaeus vannamei serine proteinase inhibitor 7 (LvSerpin7) acts as an immune brake by regulating the proPO system in AHPND-affected shrimp. Dev. Comp. Immunol. 106, 103600. doi: 10.1016/j.dci.2019.103600
Aquino J. I. L., Elliott L., Zeng C., Paris D. B. B. P. (2021). Recent developments in male fertility evaluation, sperm cryopreservation and artificial fertilisation, and their potential application to decapod crustacean aquaculture. Rev. Aquaculture 14 (2), 848–889. doi: 10.1111/raq.12627
Bashir M. A., Wang H. Y., Sun W. T., Zhai L. M., Zhang X. S., Wang N., et al. (2021). The implementation of rice-crab co-culture system to ensure cleaner rice and farm production. J. Cleaner Production 316, 128284. doi: 10.1016/j.jclepro.2021.128284
Boyko C. B., Williams J. D. (2020). A new species of parthenopea kossmann 1874 (Cirripedia: rhizocephala: parthenopeidae) from Florida: the first record of a rhizocephalan from a lobster (Decapoda: nephropoidea). Proc. Biol. Soc. Washington 133 (1), 1–6. doi: 10.2988/19-00010
Brockerhoff A. M., McLay C. L., Rybakov A. V. (2010). Occurrence of heterosaccus (Cirripedia: rhizocephala) in the new Zealand crab metacarcinus novaezelandiae (Decapoda: cancridae) and distribution of other rhizocephala in the south pacific. J. Crustacean Biol. 30 (3), 377–383. doi: 10.1651/09-3270.1
Cerenius L., Lee B. L., Soderhall K. (2008). The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 29 (6), 263–271. doi: 10.1016/j.it.2008.02.009
Cerenius L., Lu H. P., Zhang Y. J., Rimphanitchayakit V., Tassanakajon A., Andersson M. G., et al. (2010). High sequence variability among hemocyte-specific kazal-type proteinase inhibitors in decapod crustaceans. Dev. Comp. Immunol. 34 (1), 69–75. doi: 10.1016/j.dci.2009.08.005
Cerenius L., Soderhall K. (2021). Immune properties of invertebrate phenoloxidases*. Dev. Comp. Immunol. 122, 12. doi: 10.1016/j.dci.2021.104098
Chen X. W., Wang J., Hou X., Yue W. C., Huang S., Wang C. H. (2019). Tissue expression profiles unveil the gene interaction of hepatopancreas, eyestalk, and ovary in the precocious female Chinese mitten crab, eriocheir sinensis. BMC Genet. 20, 12. doi: 10.1186/s12863-019-0716-1
Donpudsa S., Tassanakajon A., Rimphanitchayakit V. (2009). Domain inhibitory and bacteriostatic activities of the five-domain kazal-type serine proteinase inhibitor from black tiger shrimp penaeus monodon. Dev. Comp. Immunol. 33 (4), 481–488. doi: 10.1016/j.dci.2008.09.009
Du Z.-Q., Ren Q., Zhao X.-F., Wang J.-X. (2009). A double WAP domain (DWD)-containing protein with proteinase inhibitory activity in Chinese white shrimp, fenneropenaeus chinensis. Comp. Biochem. Physiol. B-Biochemistry Mol. Biol. 154 (2), 203–210. doi: 10.1016/j.cbpb.2009.06.004
Eddy F., Powell A., Gregory S., Nunan L. M., Lightner D. V., Dyson P. J., et al. (2007). A novel bacterial disease of the European shore crab, carcinus maenas molecular pathology and epidemiology. Microbiol. (Reading England) 153 (Pt 9), 2839–2849. doi: 10.1099/mic.0.2007/008391-0
Fazhan H., Waiho K., Glenner H., Moh J. H. Z., Hassan M., Lkhwanuddin M. (2020). Gonadal degeneration and hepatopancreas alteration in orange mud crab Scylla olivacea infected with sacculina beauforti (Crustacea; rhizocephala; sacculinidae). Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.534443
Innocenti G., Pinter N., Galil B. S. (2003). Observations on the agonistic behavior of the swimming crab charybdis longicollis leene infected by the rhizocephalan barnacle heterosaccus dollfusi boschma. Can. J. Zoology 81 (1), 173–176. doi: 10.1139/z02-226
Jensen A. R., Schneider M. R., Hoeg J. T., Glenner H., Lutzen J. (2019). Variation in juvenile stages and success of male acquisition in Danish and French populations of the parasitic barnacle sacculina carcini (Cirripedia: rhizocephala) parasitizing the shore crab carcinus maenas. Mar. Biol. Res. 15 (2), 191–203. doi: 10.1080/17451000.2019.1612071
Jiang Q., Lu B., Lin D., Huang H., Chen X., Ye H. (2020). Role of crustacean female sex hormone (CFSH) in sex differentiation in early juvenile mud crabs, Scylla paramamosain. Gen. Comp. Endocrinol. 289, 113383. doi: 10.1016/j.ygcen.2019.113383
Kobayashi M., Wong Y. H., Oguro-Okano M., Dreyer N., Høeg J. T., Yoshida R., et al. (2018). Identification, characterization, and larval biology of a rhizocephalan barnacle, sacculina yatsui boschma 1936From northwestern Japan (Cirripedia: sacculinidae). J. Crustacean Biol. 38 (3), 329–340. doi: 10.1093/jcbiol/ruy020
Korn O., Shukalyuk A., Trofimova A., Isaeva V. (2004). Reproductive stage of the life cycle in the rhizocephalan barnacle polyascus polygenea (Crustacea: cirripedia). Russian J. Mar. Biol. 30 (5), 328–340. doi: 10.1023/b:rumb.0000046552.07712.02
Kristensen T., Nielsen A. I., Jorgensen A. I., Mouritsen K. N., Glenner H., Christensen J. T., et al. (2012). The selective advantage of host feminization: a case study of the green crab carcinus maenas and the parasitic barnacle sacculina carcini. Mar. Biol. 159 (9), 2015–2023. doi: 10.1007/s00227-012-1988-4
Laskowski M., Qasim M. A. (2000). What can the structures of enzyme-inhibitor complexes tell us about the structures of enzyme substrate complexes? Biochim. Biophys. Acta 1477 (1-2), 324–337. doi: 10.1016/s0167-4838(99)00284-8
Laufer H., Biggers W. J., Ahl J. S. (1998). Stimulation of ovarian maturation in the crayfish procambarus clarkii by methyl farnesoate. Gen. Comp. Endocrinol. 111 (2), 113–118. doi: 10.1006/gcen.1998.7109
Lehti M. S., Sironen A. (2016). Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 151 (4), R43–R54. doi: 10.1530/rep-15-0310
Li S., Jin X. K., Guo X. N., Yu A. Q., Wu M. H., Tan S. J., et al. (2013). A double WAP domain-containing protein Es-DWD1 from eriocheir sinensis exhibits antimicrobial and proteinase inhibitory activities. PloS One 8 (8), e73563. doi: 10.1371/journal.pone.0073563
Li F. M., Wang L. L., Qiu L. M., Zhang H., Gai Y. C., Song L. S. (2012). A double WAP domain-containing protein from Chinese mitten crab eriocheir sinensis with antimicrobial activities against gram-negative bacteria and yeast. Dev. Comp. Immunol. 36 (1), 183–190. doi: 10.1016/j.dci.2011.07.003
Li H., Yan Y., Yu X., Miao S., Wang Y. (2011). Occurrence and effects of the rhizocephalan parasite, polyascus gregarius, in the Chinese mitten crab, eriocheir sinensis, cultured in a freshwater pond, China. J. World Aquaculture Soc. 42 (3), 354–363. doi: 10.1111/j.1749-7345.2011.00474.x
Love M. I., Huber W., Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 (12), 550. doi: 10.1186/s13059-014-0550-8
Lutzen J., Jensen K. H., Glenner H. (2018). Life history of sacculina carcini thompson 1836 (Cirripedia: rhizocephala: sacculinidae) and the intermoult cycle of its host, the shore crab carcinus maenas (Linnaeu 1758) (Decapoda: brachyura: carcinidae). J. Crustacean Biol. 38 (4), 413–419. doi: 10.1093/jcbiol/ruy044
Miroliubov A., Borisenko I., Nesterenko M., Lianguzova A., Ilyutkin S., Lapshin N., et al. (2020). Specialized structures on the border between rhizocephalan parasites and their host's nervous system reveal potential sites for host-parasite interactions. Sci. Rep. 10 (1), 1128. doi: 10.1038/s41598-020-58175-4
Miyakawa H., Toyota K., Sumiya E., Iguchi T. (2014). Comparison of JH signaling in insects and crustaceans. Curr. Opin. Insect Sci. 1, 81–87. doi: 10.1016/j.cois.2014.04.006
Mouritsen K. N., Geyti S. N. S., Lutzen J., Hoeg J. T., Glenner H. (2018). Population dynamics and development of the rhizocephalan sacculina carcini, parasitic on the shore crab carcinus maenas. Dis. Aquat. Organisms 131 (3), 199–211. doi: 10.3354/dao03290
Nagaraju G. P. C. (2007). Is methyl farnesoate a crustacean hormone? Aquaculture 272 (1), 39–54. doi: 10.1016/j.aquaculture.2007.05.014
Nagler C., Hornig M. K., Haug J. T., Noever C., Hoeg J. T., Glenner H. (2017). The bigger, the better? volume measurements of parasites and hosts: parasitic barnacles (Cirripedia, rhizocephala) and their decapod hosts. PloS One 12 (7), e0179958. doi: 10.1371/journal.pone.0179958
Noever C., Olson A., Glenner H. (2016). Two new cryptic and sympatric species of the king crab parasite briarosaccus (Cirripedia: rhizocephala) in the north pacific. Zoological J. Linn. Soc. 176 (1), 3–14. doi: 10.1111/zoj.12304
Qian Y.-Q., Li Y., Yang F., Yu Y.-Q., Yang J.-S., Yang W.-J. (2012). Two kazal-type protease inhibitors from macrobrachium nipponense and eriocheir sinensis: comparative analysis of structure and activities. Fish Shellfish Immunol. 32 (3), 446–458. doi: 10.1016/j.fsi.2011.12.006
Raffi S. M., Vadivel E., Selvam D., T.V S., Pravinkumar M., Viswanathan C., et al. (2012). Studies on the prevalence of sacculina spp. infestation in portunus sanguinolentus (Herbst 1783) from parangipettai coastal waters, southeast coast of India. J. Biodiversity Endangered Species 1, 101. doi: 10.4172/2332-2543.1000101
Rees D., Glenner H. (2014). Control region sequences indicate that multiple externae represent multiple infections by sacculina carcini (Cirripedia: rhizocephala). Ecol. Evol. 4 (16), 3290–3297. doi: 10.1002/ece3.1177
Rotllant G., Nguyen T. V., Aizen J., Suwansa-ard S., Ventura T. (2018). Toward the identification of female gonad-stimulating factors in crustaceans. Hydrobiologia 825 (1), 91–119. doi: 10.1007/s10750-017-3497-4
Rowley A. F., Davies C. E., Malkin S. H., Bryan C. C., Thomas J. E., Batista F. M., et al. (2020). Prevalence and histopathology of the parasitic barnacle, sacculina carcini in shore crabs, carcinus maenas. J. Invertebrate Pathol. 171, 8. doi: 10.1016/j.jip.2020.107338
Sagi A., Ahl J. S., Danaee H., Laufer H. (1994). Methyl farnesoate levels in male spider crabs exhibiting active reproductive behavior. Hormones Behav. 28 (3), 261–272. doi: 10.1006/hbeh.1994.1022
Sakunwattana T., Jaree P., Rimphanitchayakit V., Tassanakajon A., Tharntada S. (2020). Antibacterial and antiproteinase activities of a double whey acidic protein domain-containing protein from penaeus vannamei boone 1931 (Decapoda, penaeidae). Crustaceana 93 (1), 51–69. doi: 10.1163/15685403-00003962
Short S., Yang G., Guler Y., Etxabe A. G., Kille P., Ford A. T. (2014). Crustacean intersexuality is feminization without demasculinization: implications for environmental toxicology. Environ. Sci. Technol. 48 (22), 13520–13529. doi: 10.1021/es5050503
Shukalyuk A. (2002). Organization of interna in sacculina polygenea (Crustacea: rhizocephala). Russian J. Mar. Biol. 28 (5), 329–335. doi: 10.63-0740/02/2805
Shukalyuk A. I., Golovnina K. A., Baiborodin S. I., Gunbin K. V., Blinov A. G., Isaeva V. V. (2007). Vasa-related genes and their expression in stem cells of colonial parasitic rhizocephalan barnacle polyascus polygenea (Arthropoda: Crustacea: cirripedia: rhizocephala). Cell Biol. Int. 31 (2), 97–108. doi: 10.1016/j.cellbi.2006.09.012
Smith V. J. (2011). Phylogeny of whey acidic protein (WAP) four-disulfide core proteins and their role in lower vertebrates and invertebrates. Biochem. Soc. Trans. 39 (5), 1403–1408. doi: 10.1042/bst0391403
Su W., Cheng C. Y. (2019). Cdc42 is involved in NC1 peptide-regulated BTB dynamics through actin and microtubule cytoskeletal reorganization. FASEB J. 33 (12), 14461–14478. doi: 10.1096/fj.201900991R
Suthianthong P., Pulsook N., Supungul P., Tassanakajon A., Rimphanitchayakit V. (2011). A double WAP domain-containing protein PmDWD from the black tiger shrimp penaeus monodon is involved in the controlling of proteinase activities in lymphoid organ. Fish Shellfish Immunol. 30 (3), 783–790. doi: 10.1016/j.fsi.2010.12.029
Tang B., Wang Q., Chen L., Yang S. (2005). Features of an intersex Chinese mitten crab, eriocheir japonica sinensis (Decapoda, brachyura). Crustaceana 78 (3), 371–377. doi: 10.1163/1568540054286529
Visetnan S., Donpudsa S., Tassanakajon A., Rimphanitchayakit V. (2018). Silencing of a kazal-type serine proteinase inhibitor SPIPm2 from penaeus monodon affects YHV susceptibility and hemocyte homeostasis. Fish Shellfish Immunol. 79, 18–27. doi: 10.1016/j.fsi.2018.05.004
Visetnan S., Supungul P., Tassanakajon A., Donpudsa S., Rimphanitchayakit V. (2017). A single WAP domain-containing protein from litopenaeus vannamei possesses antiproteinase activity against subtilisin and antimicrobial activity against AHPND-inducing vibrio parahaemolyticus. Fish Shellfish Immunol. 68, 341–348. doi: 10.1016/j.fsi.2017.07.046
Waiho K., Fazhan H., Glenner H., Ikhwanuddin M. (2017). Infestation of parasitic rhizocephalan barnacles sacculina beauforti (Cirripedia, rhizocephala) in edible mud crab, Scylla olivacea. Peerj 5, 20. doi: 10.7717/peerj.3419
Waiho K., Glenner H., Miroliubov A., Noever C., Hassan M., Ikhwanuddin M., et al. (2021). Rhizocephalans and their potential impact on crustacean aquaculture. Aquaculture 531, 13. doi: 10.1016/j.aquaculture.2020.735876
Wetsaphan N., Rimphanitchayakit V., Tassanakajon A., Somboonwiwat K. (2013). PmSERPIN3 from black tiger shrimp penaeus monodon is capable of controlling the proPO system. Dev. Comp. Immunol. 41 (2), 110–119. doi: 10.1016/j.dci.2013.04.022
Xu Y., Zhao M., Deng Y., Yang Y., Li X., Lu Q., et al. (2017). Molecular cloning, characterization and expression analysis of two juvenile hormone esterase-like carboxylesterase cDNAs in Chinese mitten crab, eriocheir sinensis. Comp. Biochem. Physiol. B-Biochemistry Mol. Biol. 205, 46–53. doi: 10.1016/j.cbpb.2017.01.002
Keywords: transcriptome analysis, germ cell, Eriocheir sinensis, Polyascus gregaria, feminization
Citation: Feng C, Zhang J, Bao J, Luan D, Jiang N and Chen Q (2023) Transcriptome analysis of germ cell changes in male Chinese mitten crabs (Eriocheir sinensis) induced by rhizocephalan parasite, Polyascus gregaria. Front. Mar. Sci. 10:1144448. doi: 10.3389/fmars.2023.1144448
Received: 14 January 2023; Accepted: 21 April 2023;
Published: 03 May 2023.
Edited by:
Shihao Li, CAS, ChinaCopyright © 2023 Feng, Zhang, Bao, Luan, Jiang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qijun Chen, cWlqdW5jaGVuNzU5QHN5YXUuZWR1LmNu
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.