
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 15 May 2023
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1134844
This article is part of the Research TopicIntegration of Sustainability, Preservation of Biodiversity and Conservation Goals in AquacultureView all 8 articles
 Sara E. Boles1,2,3
Sara E. Boles1,2,3 Laura Rogers-Bennett1,2,4,5
Laura Rogers-Bennett1,2,4,5 Wendy K. Bragg6
Wendy K. Bragg6 Jessica Bredvik-Curran7
Jessica Bredvik-Curran7 Suzanne Graham7
Suzanne Graham7 Jackson A. Gross1,2,3*
Jackson A. Gross1,2,3*Introduction: Black (H. cracherodii) and white abalone (H. sorenseni) are federally listed as endangered species in the United States. Conservation efforts include captive breeding programs; however, determination of the reproductive state of individual abalone is notoriously difficult using traditional visual assessments. Ultrasonography is a well-recognized technology used to assess gonad reproductive condition accurately and nonlethally in cultured and wild fish, and more recently cultured red abalone (H. rufescens). Here, we implemented the use of ultrasound imaging technology to monitor the gonad condition of endangered black and white abalone.
Methods: Repeated ultrasound assessments of the gonad were used to assess seasonal changes in reproductive development in wild black (n=20), and captive white abalone (n=25).
Results: A modified ultrasound gonad index score was developed to incorporate multiple species of abalone. The ultrasound index scores ranged from one to five, with an index score of one being the lowest (gonad margin is thinly wrapped around the digestive gland or not present) and an index score of five being the highest (gonad margin is thick and significantly compressing the digestive gland).
Conclusion: We show that non-lethal ultrasound imaging technology is useful for tracking cyclical changes in the gonad reproductive condition as well as a more precise selection of individuals that are in peak reproductive condition for captive breeding programs.
Over the past 50 years, populations of California abalone (Haliotis spps.) have dramatically declined due to a suite of factors that include overharvesting, disease, and starvation (Altstatt et al., 1996; Hobday and Tegner, 2000; Karpov et al., 2000; Hobday et al., 2001; Rogers-Bennett et al., 2010), with all seven species being listed as endangered or critically endangered by the International Union for Conservation of Nature (Peters and Rogers-Bennett, 2021a; Peters and Rogers-Bennett, 2021b; Peters and Rogers-Bennett, 2021c; Peters and Rogers-Bennett, 2021d; Peters and Rogers-Bennett, 2021e; Peters et al., 2021). Moreover, black (H. cracherodii) and white abalone (H. sorenseni) were listed under the United States Endangered Species Act in 2009 and 2001, respectively (Federal Register 66 [103]; Federal Register 74 [9]). Current efforts to conserve and restore wild populations of black and white abalone are in place, such as the Final Recovery Plan for Black Abalone (National Marine Fisheries Service, 2020) developed by the Black Abalone Recovery Team and the White Abalone Captive Breeding Program that includes over a dozen partner institutions (Rogers-Bennett et al., 2016). The White Abalone Captive Breeding Program seeks to enhance gametogenesis through the implementation of a wide range of tools that include hormone therapy, molecular biology, nutritional physiology, and other techniques used to increase the reproductive output of this endangered species. Abalone are dioecious, herbivorous gastropods that release sperm and egg into the water column for external fertilization and become reproductive between the age of four and seven years (Hahn, 1989). One of the challenges associated with spawning the limited number of endangered white abalone broodstock used in the captive breeding program, is that gonad score by visual assessment is often an unreliable predictor of spawning success (K. Aquilino and A. Frederick, unpublished data). The lack of predictability in spawning abalone is not specific to white abalone as visual abalone gonad assessment methods have long failed to reliably detect gonad condition and sexually dimorphic traits (Ebert and Houk, 1984).
Ultrasound technology has been used by biologists for decades as a non-lethal technique to assess the reproductive health in finfish conservation (Evans et al., 2004; Swanson et al., 2008; Chiotti et al., 2016; Brizendine et al., 2018; Carim et al., 2021) and for commercial aquaculture purposes (Bonar et al., 1989; Blythe et al., 1994; Colombo et al., 2004; Naeve et al., 2018). While ultrasonography had previously been used in pāua (H. iris) to identify shell lesions that diminish the ability of the adductor muscle to properly adhere to the shell, leading to increased mortality (Nollens et al., 2002), only recently has ultrasound technology been successfully used to characterize abalone reproductive state, specifically red abalone gonad condition (Boles et al., 2022). The goal of this study is to assess the use of non-lethal ultrasonography as a technique to monitor the reproductive condition of endangered black and white abalone and to evaluate the applicability of the technology for use in conservation programs. In this research, we seek to utilize non-lethal ultrasonography to (1) develop a ultrasound scoring index to assess the gonad condition in multiple species of California abalone, (2) characterize the reproductive state of individual endangered captive white abalone during scheduled spawning events during peak reproductive season, and (3) evaluate the reproductive state of rescued wild black abalone brought into captivity following post-fire debris flows. We discuss the application of these methods to support the captive breeding and monitoring of endangered abalone in California.
Under authorization from the National Oceanic and Atmospheric Administration National Marine Fisheries Service and the California Department of Fish and Wildlife in February and March of 2021, wild black abalone (n=213) were rescued from post-fire debris flows that occurred following heavy rain that coincided with the 2020 Dolan Fire scar along the Big Sur Coast, California. Rescued black abalone were transported to the University of California, Santa Cruz’s Coastal Science Campus, Santa Cruz, California where they were processed for a temporary period of captivity. Weights and lengths for each individual abalone was recorded and the animals were tagged (Floy® Shellfish Tag, Seattle, Washington), housed in 623 L flow-through totes, and fed ad libitum kelp (Macrocystis pyrifera) until their release back into the wild in July 2021. Forty seven individuals died from post-fire debris flow injuries, and the remaining abalone were released back into the wild. One abalone received an injury during removal from the tanks prior to release and was transported to the California Department of Fish and Wildlife's Shellfish Health Lab at the University of California, Davis' Bodega Marine Laboratory for treatment and recovery.
Abalone reproductive anatomy was imaged with a SonoSite Edge II Ultrasound System (FUJIFILM SonoSite Bothell, Washington) using a HFL50 15-6-MHz transducer probe (Mechanical Index=0.7; Thermal Index=0.1; Read Depth=6; exam type: breast). To reduce handling stress, ultrasound examinations were conducted by immersing abalone in seawater affixed to transparency copier film (3M #PP2950, Austin, Texas) shell side down to expose the muscular foot and categorically ranked using established methods (Boles et al., 2022). Abalone gonadal tissue wraps around the outer portion of the cone-shaped digestive gland and is located between the shell and the foot on the right-hand side of the mantle cavity (Figure 1). For each abalone, the tag identification was recorded into the ultrasound, and two to three replicate still images were taken. Mean gonad thickness was calculated by using three replicate measurements from each still image and then calculating the mean gonad thickness of the three images using Image J software v1.50i. (Figure 1B) (Schneider et al., 2012). The established ultrasound index score ranked on a scale from one to five, with one being the lowest score and an ultrasound index score of five being the highest score when the thickness of the gonad margin has grown to a thickness whereas the digestive gland coelom is partially or completely compressed (Figure 2).
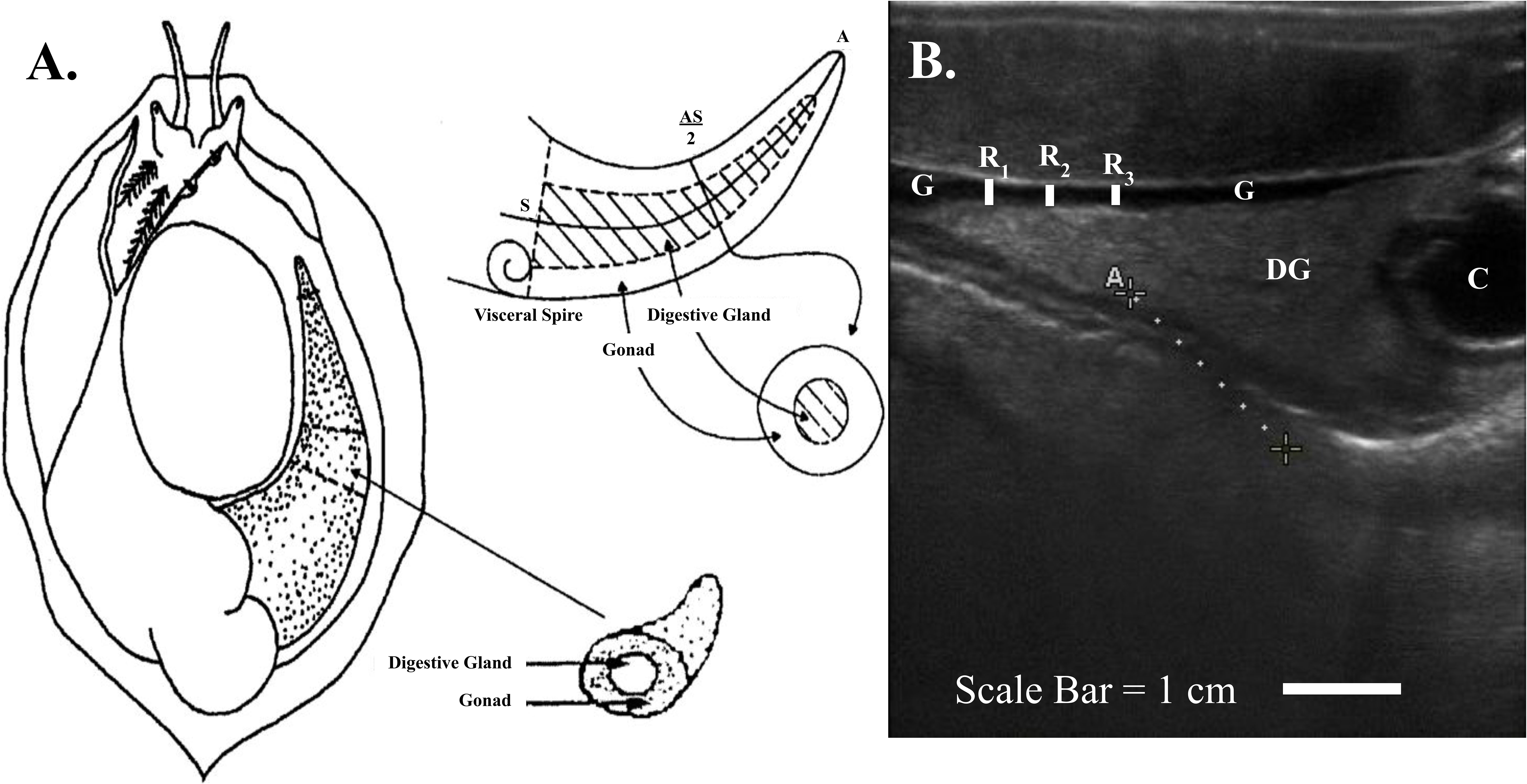
Figure 1 (A) Schematic of abalone (Haliotis spps.) gonad and digestive gland (Rogers-Bennett et al., 2004). (B) White abalone (H. sorenseni) ultrasound image with the gonad (G) (black band) enveloped around the grey cone shaped digestive gland (DG) and coelom (C). Replicate measurements (Rx) for each replicate image were taken to estimate gonad thickness for each individual abalone.
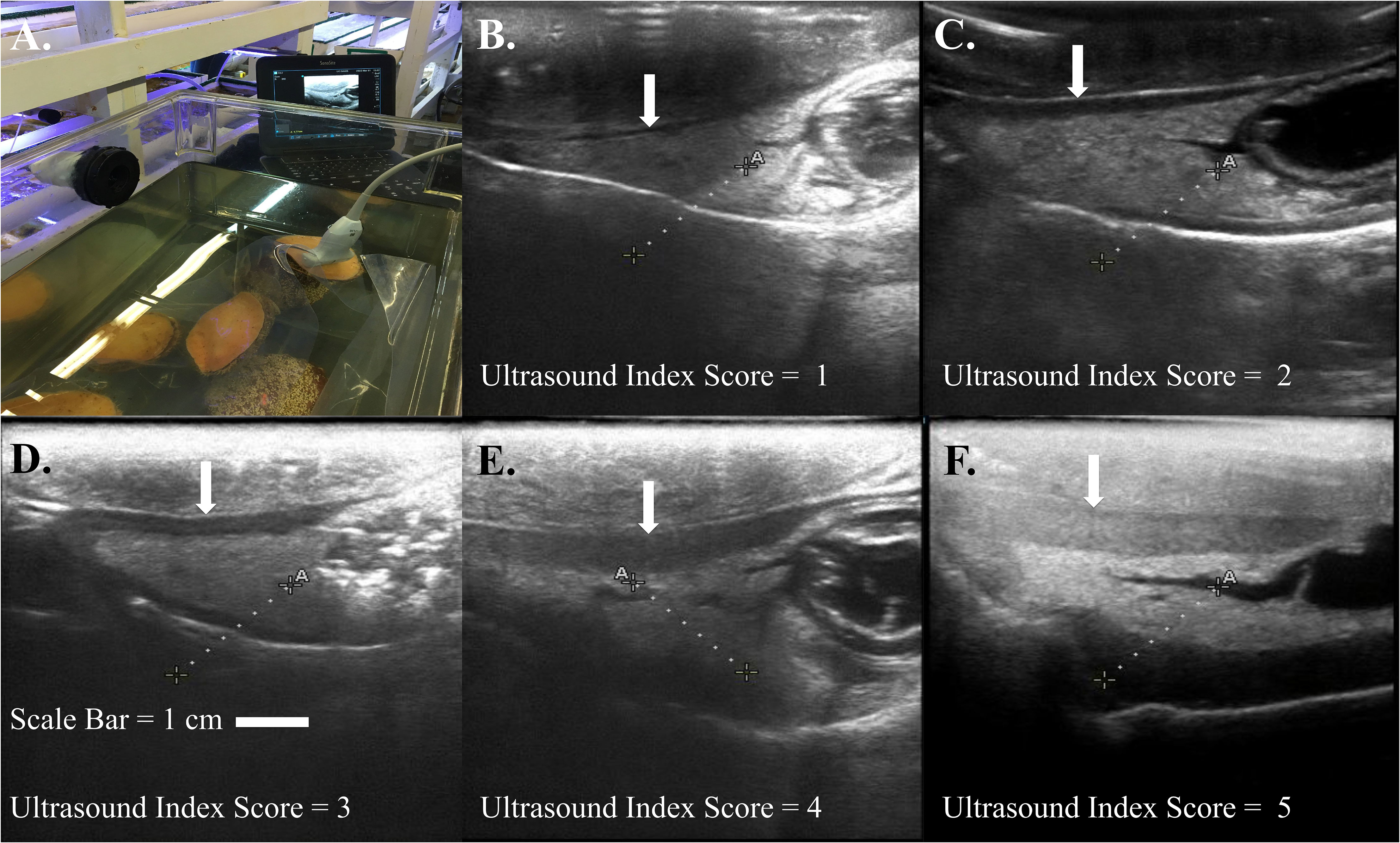
Figure 2 (A) White abalone (Haliotis sorenseni) ultrasound examination conducted through muscular foot attached to clear vinyl sheet. Ultrasound visual index classification of black (H. cracherodii) and white abalone (H. sorenseni) (B) index = 1, (C) index = 2, (D) index = 3, (E) index = 4, (F) index = 5. Scale bar = 1 cm. White arrows indicate progressive thickening of abalone gonad enveloped around conical shaped digestive gland.
Ultrasound reproductive and health assessments were conducted on rescued endangered black abalone during facility intake just prior to their return to suitable ocean habitat. Upon intake, only 20 of the 213 rescued black abalone were assessed for mean gonad thickness due to logistical constraints. Prior to their release, surviving black abalone (n=145) were imaged (Supplementary Data) with all 20 of the initially black abalone initially assessed, receiving a second ultrasound examination. Ultrasound examinations were conducted in March 2021 and again in July 2021.
In March and April 2021, repeated ultrasound examinations of white abalone broodstock (n=25) from the White Abalone Captive Breeding Program were conducted during spawning induction attempts at the BML. Floy® tags were used to identify individual abalone. White abalone broodstock were induced to spawn using established techniques with hydrogen peroxide (Moss et al., 1995; Boles, 2020; Swezey et al., 2020). In March 2021, white abalone did not release gametes when induced to spawn. White abalone were again induced to spawn in April 2021, select gametes were fertilized, and the resulting larvae contributed to the five genetic crosses that constitute the White Abalone Captive Breeding Program cohort of 2021. To quantify changes in gonad thickness, ultrasound examinations were conducted on individual white abalone before and after spawning.
Data visualizations were performed using R Studio (version 1.1.463) (Wickam, 2016). The mean and standard deviation were determined for maximum shell length and total weight.
Ultrasound imaging allowed for non-lethal gonad reproductive assessments of 20 black abalone during the initial intake in March 2021 and before animals were returned to the wild in July 2021 (Figure 2). The mean length and weight of the 20 abalone returned to the wild was 100.09 mm ( ± 21.29) and 179.76 g ( ± 133.84), respectively. Repeated ultrasound images showed black abalone gonad thickness increased for eleven abalone by July 2021, whereas four individual abalone displayed a decline in gonad thickness, and the remaining five animals showed little to no change in gonad thickness (Figure 3).
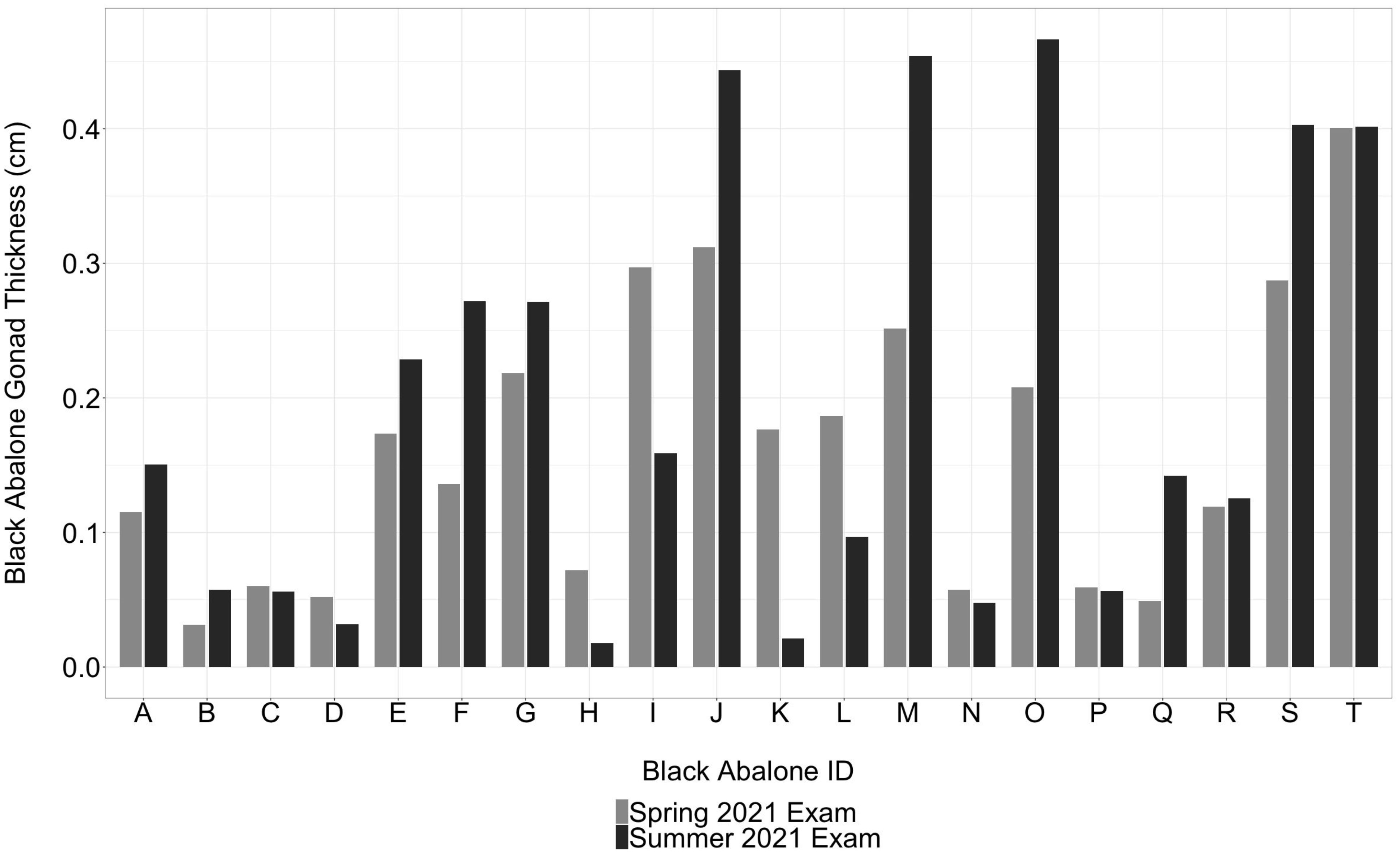
Figure 3 Changes in gonad thickness in black abalone (Haliotis cracherodii) rescued post-debris flow from the Dolan Fire (grey bars) and before being released back into the wild during summer 2021 (black bars).
Ultrasonography provides researchers with a quantitative metric to indirectly assess gonad maturation state in abalone. This project specifically illustrates the applied use of a non-lethal method to monitor the reproductive condition of endangered black and white abalone through the use of high-quality images of the gonad for qualitative and quantitative evaluations. Furthermore, the methodology of maintaining the abalone on plastic sheets successfully avoided injury in endangered abalone thereby reducing handling stress associated with probing and manipulation of the muscular foot.
Ultrasound technology has been successfully used to evaluate the gonad maturation of various species of fish, including those that are cultured or endangered (Moghim et al., 2002; Albers et al., 2013). This non-lethal technique has the potential to enhance abalone husbandry practices by allowing researchers and program managers to monitor the gonad maturation status of abalone over the course of their reproductive cycle. This information can inform broodstock selection and spawning arrangements, which are critical components of captive breeding programs that supplement dwindling populations, restore extirpated areas, or preserve genetic materials (Rytwinski et al., 2021). Successful outcomes from these programmatic goals are determined by metrics for specific management actions. The use of ultrasound technology can help conservation managers avoid relying solely on a spawning success metric, which requires a large sample size, usually with an unknown reproductive state, and with good genetic diversity to achieve a successful outcome for restoration purposes. A spawning event can be particularly challenging for programs that have limited number of abalone in the wild or in captivity, or when the gonad status of the abalone is unknown.
While abalone stress response was not a focus of the current study, for endangered abalone species, ultrasound technology also represents a potential breakthrough for reducing animal handling stress and broodstock selection. Implementing the use of non-lethal ultrasound image technology in endangered abalone captive breeding and recovery programs increases animal welfare by eliminating the need to sacrifice animals, reducing potential risk of injury from repeated removal and handling, and decreasing incidental stress associated with chemically induced spawning of abalone that are not ready to spawn. Moreover, research on the potential stress response in abalone from prolonged ultrasound exposure should be explored (Barnett et al., 1994; Duck, 2007; Hanson, 2010), in addition to using ultrasound to assess other stressors such as nutrition restriction, (Rogers-Bennett et al., 2010); Meusel et al., 2022), pathogens and parasites (Nollens et al., 2002; Crosson et al., 2014), this new tool should prove beneficial in management and research programs.
Ultrasonography in captive breeding and recovery programs could potentially increase fertilization success by reducing the probability of spawning undersized or immature ova which may contribute to poor survival due to reduced maternally derived nutrients available during the free swimming larval phase or settling period (Moran and McAlister, 2009; Santella et al., 2020). It is currently unknown what the long-term impacts of suboptimal gamete fertilization are on energy reserves (abalone larvae are lecithotrophic), growth, survival, and fitness in juvenile and adult abalone. Clearly, non-lethal tools that aid in determining reproductive condition will be useful for monitoring and managing abalone broodstock conditioning. For instance, the usage of ultrasonography during gametogenic cycles may be critical to understanding broodstock culture and dietary energy mobilization required to improve spawning success and subsequent larval survival.
While white abalone gonad maturation has been demonstrated in captivity through successful annual spawning events, black abalone have historically been difficult to spawn. Fortunately, in this study we were able to evaluate and visualize gonad maturation on a number of black abalone at intake and again prior to release in July when black abalone are said to spawn (Webber and Giese, 1969). We noted several abalone did undergo gonad maturation in captivity with an increase of gonad thickness by 55% (i.e., Black abalone ‘O’: 0.21 cm gonad thickness in March 2021/0.46 cm in July 2021). Whereas individual ‘T’ had an increase in gonad thickness of only 0.2% during the same period. In contrast, some individuals, such as individual ‘I’, displayed a marked decline by 43% in gonad thickness (0.28 cm in March 2021 to 0.16 cm in July 2021). While ultrasonography is sensitive enough to measure small changes in black abalone gonad thickness, without regular assessments, we are not able to verify whether animals that presented with no change or reduced gonad thickness, experienced gonad atresia or spawned in captivity.
Since endangered white abalone used in the current study are involved in a captive breeding program, white abalone were available for repeated ultrasound imaging. Ultrasound imaging was successful in monitoring changes in gonadal thickness in white abalone over a 30-day period (Figure 4). Ultrasound technology also detected changes in male and female white abalone gonad thickness before and after spawning on April 21, 2021 (Figure 5). Male white abalone ‘A’ had a pre-spawn gonad thickness of 0.37 cm (± 0.04) and post-spawn gonad thickness of 0.15 cm (± 0.02), representing a 58.9% decrease in gonad thickness. Female ‘C’ white abalone had a pre-spawn gonad thickness of 1.13 cm (± 0.08) and a post-spawn thickness of 0.92 cm (± 0.07), representing an 18.3% change in gonad thickness. White abalone female ‘L’ had a pre-spawn gonad thickness of 0.96 cm (± 0.02) and a post-spawn gonad thickness of 0.79 cm (± 0.05), also representing an 18.3% decrease in gonad thickness.
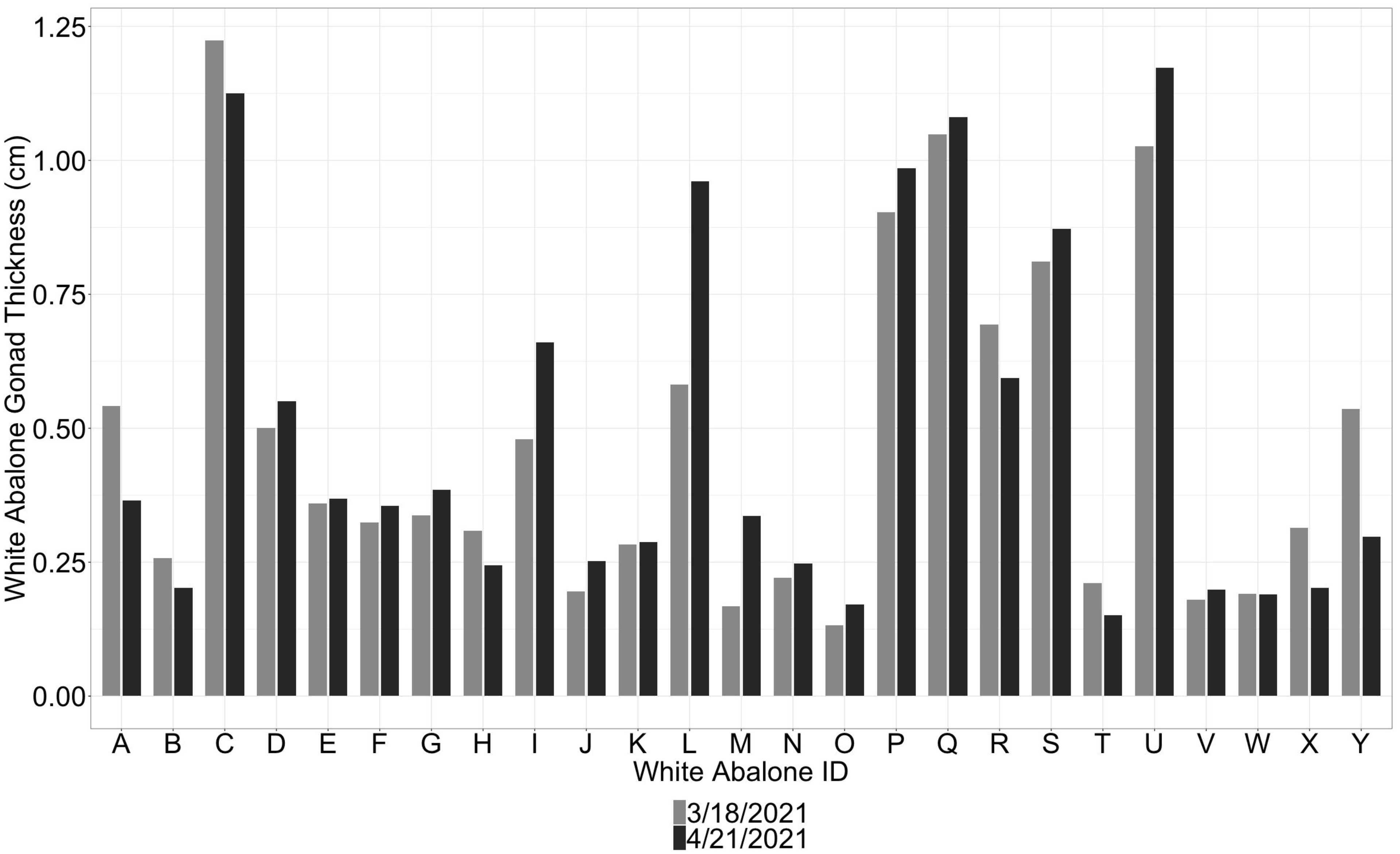
Figure 4 Ultrasound imaging detects changes in gonad thickness in endangered white abalone (Haliotis sorenseni). Grey bars represent ultrasound examinations in March 2021, where black bars indicate ultrasound examinations conducted in April 2021.
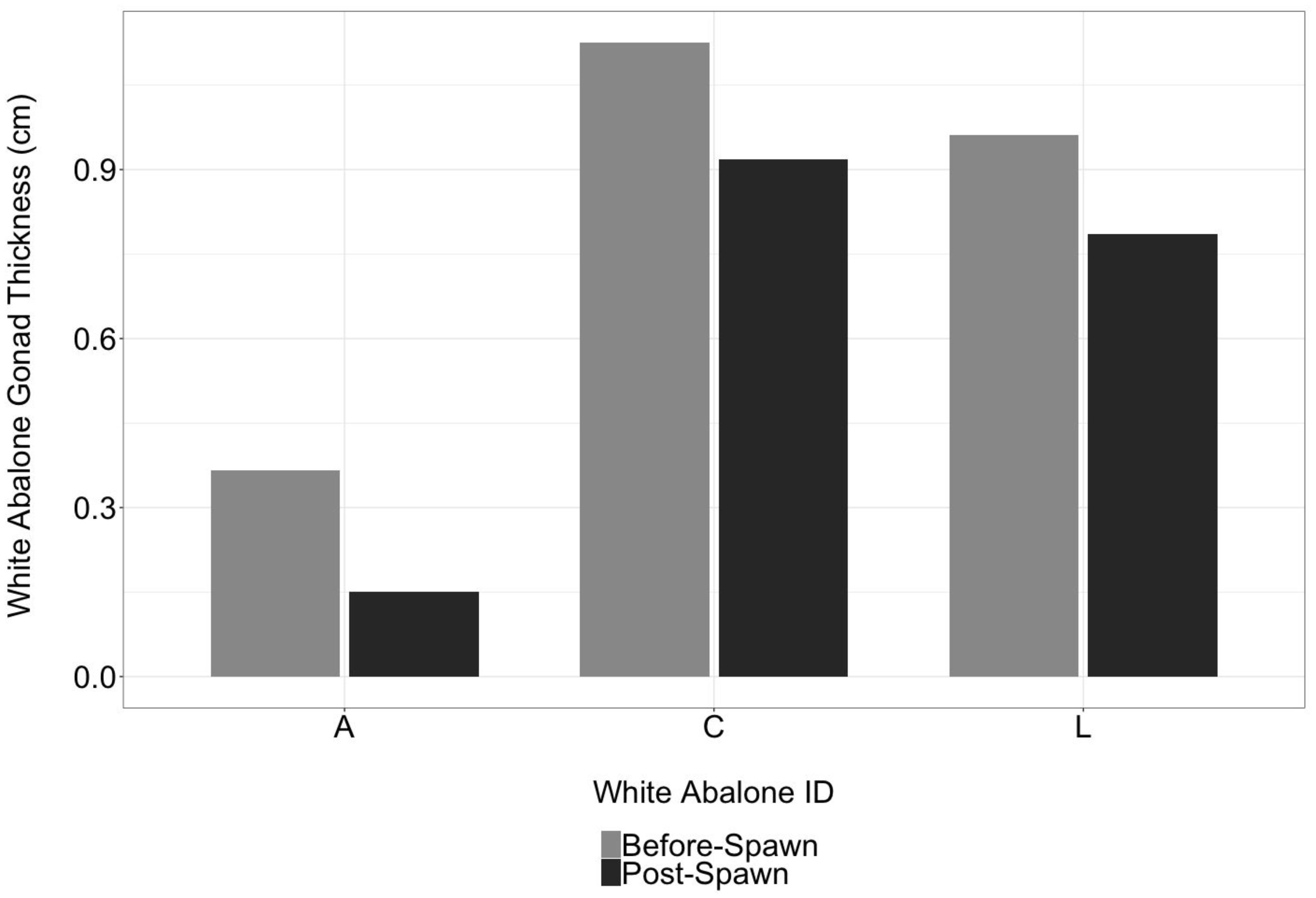
Figure 5 Ultrasonography detects changes in endangered white abalone (Haliotis sorenseni) gonad thickness before spawning and post-spawning. Changes in gonad thickness before spawning (grey bars) and post-spawning (black bars) in April 2021.
In addition to use in black and white abalone culturing programs, these techniques will prove useful when and if other species need conservation efforts. Special consideration should be made when applying this scoring metric based on gonad thickness (Table 1) because globally, abalone vary in size with some adult abalone only reaching 80 mm (Poutiers, 1998). The current multi-species gonad score was created using gonad thickness from large abalone found on the western coast of North America. The red abalone, for example, is the largest of all abalone species and was included in the score determination as red abalone populations, once abundant in northern California, have continued to decline since the recreational fishery was closed in 2017, and have recently been declared an endangered species (Peters et al., 2021). Red abalone starvation has been associated with low gonad scores (Rogers-Bennett et al., 2021), and before future fisheries are reopened, there will need to be evidence of healthy gonad development which should be assessed with ultrasound rather than through lethal sampling. The use of ultrasound should prove beneficial in their assessment as starving red abalone have been shown to require feeding for more than one year before they can spawn (Boles, 2020). Thus, red abalone may also be in need of restoration as their recent decline has been associated with mass die-offs, marine heatwaves, and the extreme sea urchin herbivory of kelp forests (De Wit et al., 2014; Rogers-Bennett and Catton, 2019; McPherson et al., 2021). The recent International Union for Conservation of Nature Red List of Threatened Species reports that 37% of all abalone populations worldwide for which data exists are currently endangered (24%) or vulnerable (13%) for extinction (IUCN, 2022). With impacts from climate change projected to increase in intensity and frequency, abalone conservation tools such as non-lethal ultrasound will become invaluable for restoration practitioners worldwide.
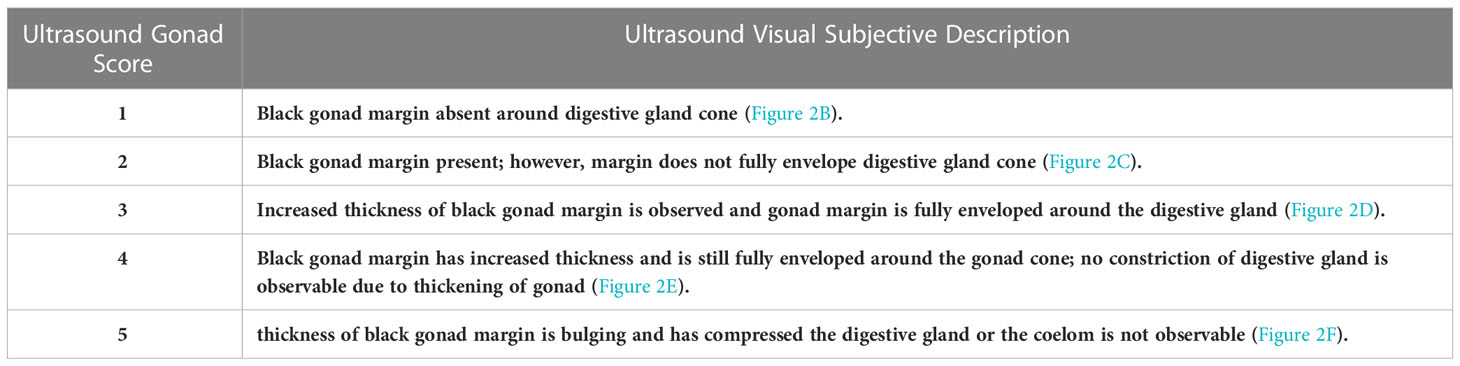
Table 1 Ultrasound index categorical scoring system (Boles et al., 2022).
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the animal study because the University of California, Davis, does not require ethics approval for gastropod species.
SB and JG designed the research. SB, LR-B, WB, and JG performed the research. SB analyzed the data. SB, LR-B, WB, JB-C, SG, and JG wrote the manuscript. All authors contributed to the manuscript and approve the submitted version.
Funding for a portion of this research was provided by the United States Navy, Commander Pacific Fleet N62473-19-0023 and N62473-22-2-0007. Additional funding was also provided by the National Oceanic and Atmospheric Administration Section 6 Grant NA19NMF4720103 to the California Department of Fish and Wildlife through subcontract No. P197003 to the University of California, Davis. Funding for black abalone rescue work was provided by the National Marine Sanctuary Foundation, the Bureau of Ocean Energy Management, National Oceanic and Atmospheric Administration, the National Marine Fisheries Service, and the California Ocean Protection Council.
We would like to extend our gratitude to K. Aquilino and A. Frederick for access to captive white abalone and for their valuable comments and suggestions on this manuscript. We also thank N. Frank, R. Patton, G. Lin, J. Mann, I. Neylan, N. Rizzo, C. Souza for ultrasound technical assistance. We thank the California Department of Fish and Wildlife for support for LR-B. We thank the following for support during the post-fire debris flow rescues and husbandry of black abalone: the National Oceanic and Atmospheric Administration’s National Marine Fisheries Service, the California Department of Fish and Wildlife, the Multi-Agency Rocky Intertidal Network, P. Raimondi, the California Department of Fish and Wildlife Marine Wildlife Veterinary Care and Research Center, the Black Abalone Recovery Team, Monterey Bay National Marine Sanctuary (especially S. Lonhart), D. Richards, Big Sur private landowners, Caltrans, and University of California Santa Cruz undergraduate and graduate student volunteers.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1134844/full#supplementary-material
Albers J. L., Wildhaber M. L., DeLonay A. J. (2013). Gonadosomatic index and fecundity of lower Missouri and middle Mississippi river endangered pallid sturgeon estimated using minimally invasive techniques. J. Appl. Ichthyol. 29 (5), 968–977. doi: 10.1111/jai.12231
Altstatt J. M., Ambrose R. F., Engle J. M., Haacker P. L., Laferty K. D., Raimondi P. T. (1996). Recent declines of black abalone Haliotis cracherodii on the mainland coast of central California. Mar. Ecol. Prog. Series. 142, 185–192. doi: 10.3354/meps142185
Barnett S. B., Ter Haar G. R., Ziskin M. C., Nyborg W. L., Maeda K., Bang J. (1994). Current status of research on biophysical effects of ultrasound. Ultrasound. Med. Biol. 20 (3), 205–218. doi: 10.1016/0301-5629(94)90060-4
Blythe B., Helfich L. A., Beal W. E., Bosworth B., Libey G. S. (1994). Determination of sex and maturational status of striped bass (Morone saxatilis) using ultrasonic imaging. Aquaculture 125 (1-2), 175–184. doi: 10.1016/0044-8486(94)90294-1
Boles S. E. (2020). Effects of ocean acidification on the growth and survival in early-stage red abalone (Haliotis rufescens): adaptive strategies for conservation and production aquaculture (Davis: University of California).
Boles S. E., Neylan I. P., Rogers-Bennett L., Gross J. A. (2022). Evaluation of gonad reproductive condition using non-invasive ultrasonography in red abalone (Haliotis rufescens). Front. Mar. Sci. 9. doi: 10.3389/fmar.2022.784481
Bonar S. A., Thomas G. L., Pauley G. B., Martin R. W. (1989). Management briefs: use of ultrasonic images for rapid nonlethal determination of sex and maturity of pacific herring. J. North Am. Fish. Management. 9 (3), 364–366. doi: 10.1577/1548-8675(1989)009<0364:MBUOUI>2.3.CO;2
Brizendine M. E., Ward D. L., Bonar S. A. (2018). Effectiveness of ultrasonic imaging for evaluating presence and maturity of eggs in fishes in remote field locations. J. North Am. Fish. Management. 38 (5), 1017–1026. doi: 10.1002/nafm.10200
Carim J. E., Relyea S., Barfoot C., Ebay L. A., Kronenberger J. A., Whiteley A. R., et al. (2021). Ultrasound imaging identifies life history variation in resident cutthroat trout. PloS One 16 (2), e0246365. doi: 10.1371/journal.pone.0246365
Chiotti J. A., Base J. C., Hondrop D. W., Briggs A. S. (2016). Assigning sex and reproductive state to adult lake sturgeon using ultrasonography and common morphological measurements. North Am. J. Fish. Management. 36, 21–29. doi: 10.1080/02755947.2015.1103823
Colombo R. E., Wills P. S., Garvey J. E. (2004). Use of ultrasound imaging to determine sex of shovelnose sturgeon. North Am. J. Fish. Management. 24, 322–326. doi: 10.1577/M03-016
Crosson L. M., Wight N., Van Blaricom G. R., Kiryu I., Moore J. D., Friedman C. S. (2014). Abalone withering syndrome: distribution, impacts, current diagnostic methods and new findings. Dis. Aquat. Organisms. 108, 261–270. doi: 10.3354/dao02713
De Wit P., Rogers-Bennett L., Kudela R. M., Palumbi S. R. (2014). Forensic genomics as a novel tool for identifying the causes of mass mortality events. Nat. Commun. 5, 3652. doi: 10.1038/ncomms4652
Duck F. A. (2007). Medical and non-medical protection standards for ultrasound and infrasound. Prog. Biophys. Mol. Biol. 93 (1-3), 176–191. doi: 10.1016/j.pbiomolbio.2006.07.008
Ebert E. E., Houk J. L. (1984). Elements and innovations in the cultivation of red abalone. Haliotis. Rufescens. Aquacult. 39, 375–392. doi: 10.1016/0044-8486(84)90279-5
Endangered Status for Black Abalone (2009). National oceanic and atmospheric administration. [Docket no. 071128765-81658-02] RIN 0648-AW32. federal register 74 (9). 1937–1946.
Endangered Status for White Abalone (2001). National oceanic and atmospheric administration. [Docket no. 990910253-1120-03; ID no. 041300B] RIN 0648-AM90. federal register 66 (103). 29047–29055.
Evans A. F., Fitzpatrick M. S., Siddens L. K. (2004). Use of ultrasound imaging and steroid concentrations to identify maturation state in adult steelhead. North Am. J. Fish. Management. 24 (3), 967–978. doi: 10.1577/M03-112.1
Hahn K. O. (1989). Handbook of culture of abalone and other marine gastropods (Boca, Raton, FL: CRC Press).
Hanson M. A. (2010). Health effects of exposure to ultrasound and infrasound: report of the independent advisory group on non-ionising radiation. Chilton, GB. Health Prot. Agency., 180pp.
Hobday A. J., Tegner M. J. (2000). “Status review of white abalone throughout its range in California and Mexico,” in NOAA Technical memorandum NOAA-TM-NMFS-SWR-035. National Oceanic and Atmospheric Administrations Technical Memorandum
Hobday A. J., Tegner M. J., Haaker P. L. (2001). Over-exploitation of a broadcast spawning marine invertebrate: decline of the white abalone. Rev. Fish. Biol. Fisheries. 10, 493–514. doi: 10.1023/A:1012274101311
IUCN (2022) The IUCN red list of threatened species. version 2022-2. Available at: https://www.iucnredlist.org (Accessed 28 December 2022).
Kainz B., Heinrich M. P., Makropoulos A., Oppenheimer J., Mandegran R., Sankar S., et al. (2021). Non-invasive diagnosis of deep vein thrombosis from ultrasound imaging with machine learning. Nat. Partner. J. Digital. Med. 4 (137). doi: 10.1038/s41746-021-00503-7
Karpov K. A., Haaker P. L., Tanigichi I. K., Rogers-Bennett L. (2000). Serial depletion and the collapse of the California abalone (Haliotis spp.) Fishery. Can. J. Fish. Aquat. Sci. 130, 11–24.
McPherson M. L., Finger D. J. I., Houskeeper H. F., Bell T. W., Carr M. H., Rogers-Bennett L., et al. (2021). Large-Scale shift in the structure of a kelp forest ecosystem co-occurs with an epizootic and marine heatwave. Commun. Biol. 4. doi: 10.1038/s42003-021-01827-6
Meusel E., Menanteau-Ledouble S., Naylor M., Kaiser H., El-Matbouli M. (2022). Gonad development in farmed male and female south African abalone, Haliotis midae, fed artificial and natural diets under a range of husbandry conditions. Aquacult. Int. 30, 1279–1293. doi: 10.1007/s10499-022-00850-6
Moghim M., Vajhi A. R., Veshkini A., Masoudifard M. (2002). Determination of sex and maturity in Acipenser stellatus by using ultrasonography. J. Appl. Ichthyol. 18, 325–328. doi: 10.1046/j.1439-0426.2002.00423.x
Moran A. L., McAlister J. S. (2009). Egg size as a life history character of marine invertebrates: is it all it’s cracked up to be? Biol. Bull. 216 (3), 226–242. doi: 10.1086/BBLv216n3p226
Moss G. A., Illingworth J., Tong L. J. (1995). Comparing two simple methods to induce spawning in the new Zealand abalone (paua), Haliotis iris. New Z. J. Mar. Freshw. Res. 29, 329–333. doi: 10.1080/00288330.1995.9516667
Naeve I., Mommens M., Arukwe A., Kjørsvik E. (2018). Ultrasound as a non-invasive tool for monitoring reproductive physiology in female Atlantic salmon (Salmo salar). Physiol. Rep. 6 (9), e13640.
National Marine Fisheries Service (2020). Final endangered species act recovery plan for black abalone (Haliotis cracherodii) (Long Beach, California: National Marine Fisheries Service, West Coast Region, Protected Resources Division), 1–112.
Nollens H. H., Schofield J. C., Keogh J. A., Probert P. K. (2002). Evaluations of radiography, ultrasonography and endoscopy for detection of shell lesions in live abalone Haliotis iris (Mollusca: Gastropoda). Dis. Aquat. Organisms. 50 (2), 145–152. doi: 10.3354/dao050145
Peters H., Rogers-Bennett L. (2021a). Haliotis sorenseni. The IUCN red list of threatened species 2021. The International Union for Conservation of Nature (IUCN), e. T7877169A78772593. doi: 10.2305/IUCN.UK.2021-3.RLTS.T7877169A78772593.en
Peters H., Rogers-Bennett L. (2021b). Haliotis cracherodii. the IUCN red list of threatened species 2021. The International Union for Conservation of Nature (IUCN), e.T41880A78775277. doi: 10.2305/IUCN.UK.2021-3.RLTS.T41880A78775277
Peters H., Rogers-Bennett L. (2021c). Haliotis corrugata. the IUCN red list of threatened species 2021. The International Union for Conservation of Nature (IUCN), e.T78763727A8772418. doi: 10.2305/IUCN.UK.2021-3.RLTS.T78763727A78772418.en
Peters H., Rogers-Bennett L. (2021d). Haliotis fulgens. the IUCN red list of threatened species 2021. The International Union for Conservation of Nature (IUCN), e.T78768961A78772463. doi: 10.2305/IUCN.UK.2021-3.RLTS.T78768961A78772463.en
Peters H., Rogers-Bennett L. (2021e). Haliotis walallensis. the IUCN red list of threatened species 2021. e.T78772302A78772648. doi: 10.2305/IUCN.UK.2021-3.RLTS.T78772302A78772648.en
Peters H., Rogers-Bennett L., De Shields R. M. (2021). Haliotis rufescens. the IUCN red list of threatened species 2021. E. T78771583A8772573. doi: 10.2305/IUCN.UK.2021-3.RLTS.T78771583A78772573.en
Poutiers J. M. (1998). “Gastropods,” in FAO species identification guide for fishery purposes. the living marine resources of the Western central pacific. volume 1: seaweeds, corals, bivalves, and gastropods. Eds. Carpenter K. E., Niem V. H. (Rome: FAO), 363–648.
Rogers-Bennett L., Dondanville R. F., Kashiwada J. V. (2004). Size specific fecundity of red abalone (Haliotis rufescens): Evidence for reproductive senescence?. J. Shellfish Res. 23, 553–560.
Rogers-Bennett L., Aquilino K. M., Catton C. A., Kawana S. K., Walker B. J., Ashlock L. W., et al. (2016). Implementing a restoration program for the endangered white abalone (H. sorenseni) in California. J. Shellfish. Res. 35 (3), 611–618. doi: 10.2983/035.035.0306
Rogers-Bennett L., Catton C. A. (2019). Marine heatwave and multiple stressors tip bull kelp forests to sea urchin barrens. Sci. Rep. 9 (1), 1–9. doi: 10.1038/s41598-019-51114-y
Rogers-Bennett L., Dondanville R. F., Moore J. D., Vilchis L. I. (2010). Response of red abalone reproduction to warm water, starvation, and disease stressors: implications of ocean warming. J. Shellfish. Res. 29 (3), 599–611. doi: 10.2983/035.029.0308
Rogers-Bennett L., Klamt R., Catton C. A. (2021). Survivors of climate driven abalone mass mortality exhibit declines in health and reproduction following kelp forest collapse. Frontiers in Marine Science. 8, 725134. doi: 10.3389/fmar.2021.725134
Rytwinski T., Kelly L. A., Donaldson L. A., Taylor J. J., Smith A., Drake D. A. R., et al. (2021). What evidence exists for evaluating the effectiveness of conservation-oriented captive breeding and release programs for imperilled freshwater fishes and mussels? Can. J. Fish. Aquat. Sci. 78 (9), 1332–1346. doi: 10.1139/cjfas-2020-0331
Santella L., Limatola N., Chun J. T. (2020). Cellular and molecular aspects of oocytes maturation and fertilization: a perspective from the actin cytoskeleton. Zool. Lett. 6 (5). doi: 10.1186/s40851-020-00157-5
Schneider C. A., Rasband W. S., Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. doi: 10.1038/nmeth.2089
Swanson P., Campbell B., Shearer K., Dickey J., Beckman B., Larsen D., et al. (2008). Application of reproductive technologies to captive breeding for conservation of imperiled stocks of pacific salmon. Cybium 32 (2), 279–282.
Swezey D. S., Boles S. E., Aquilino K. M., Stott H. K., Bush D., Whitehead A., et al. (2020). Evolved differences in energy metabolism and growth dictate the in pacts of ocean acidification on abalone aquaculture. Proc. Natl. Acad. Sci. 117, 26513–26519. doi: 10.1073/pnas.2006910117
Webber H. H., Giese A. C. (1969). Reproductive cycle and gametogenesis in the black abalone Haliotis cracherodii (Gastropoda: prosobranchiata). Mar. Biol. 4, 152–159. doi: 10.1007/BF00347041
Keywords: animal welfare, conservation aquaculture, reproductive biology, shellfish health, gastropod physiology
Citation: Boles SE, Rogers-Bennett L, Bragg WK, Bredvik-Curran J, Graham S and Gross JA (2023) Determination of gonad reproductive state using non-lethal ultrasonography in endangered black (Haliotis cracherodii) and white abalone (H. sorenseni). Front. Mar. Sci. 10:1134844. doi: 10.3389/fmars.2023.1134844
Received: 30 December 2022; Accepted: 14 April 2023;
Published: 15 May 2023.
Edited by:
Gregor Reid, Gregor Kyle Reid, CanadaReviewed by:
Joshua Patterson, University of Florida, United StatesCopyright © 2023 Boles, Rogers-Bennett, Bragg, Bredvik-Curran, Graham and Gross. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jackson A. Gross, amFncm9zc0B1Y2RhdmlzLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.