
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 29 March 2023
Sec. Marine Biology
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1128819
This article is part of the Research Topic The Biology and Conservation of Elasmobranchs and Chimaeras View all 12 articles
Manta rays (Mobula alfredi and M. birostris) are poorly understood in South Africa, despite their ecological importance and charismatic appeal. This study analyzed a 41-year dataset from the KwaZulu-Natal bather protection program to investigate catch per unit effort between 1981-2021. We used Generalized Additive Models and the probability of encounter to assess annual and seasonal trends, as well as the effect of location and moon phases on catch rates. We also evaluated the size composition and demographics of caught manta rays using the same dataset. Our analysis revealed a significant decline in overall manta ray catches since the late 1990s (p<0.0001), with increased catch rates during summer, suggesting seasonal visitation to South African waters. We found that manta rays were caught at least once in all 46 netted beaches along the 350 km span of coastline, but with significantly more catches in the Central Area, between Anstey’s beach in the north and Mtwalume in the south. We also observed that moon phase had an effect on manta ray presence, with significantly more catches during spring tides at new and full moon phases. Over half of the caught individuals were juveniles, and a total of 841 individuals (52% of the total catch) belonged to the confirmed juvenile size class (1400-2500 mm disc width). We further found that a greater proportion (70%) of juveniles were caught in the southernmost sampled area, from Hibberdene in the north to Mzamba in the south. These findings highlight the importance of South African waters as a seasonal habitat for manta rays along the southern African coastline. The significant decline and spatial-temporal patterns we observed have critical implications for management and conservation efforts. Our study provides valuable baseline data for future research and underscores the need for continued monitoring and protection of these iconic marine species.
Manta rays (Family Mobulidae) are pelagic planktivores that aggregate in regions supporting high zooplankton densities and cleaning stations, where symbiotic fish remove parasites from them (Feder, 1966; Couturier et al., 2012; Stevens, 2016; White et al., 2017). Being large filter feeders, manta rays spend their lives in proximity to where plankton blooms occur, these being elicited by temporal and spatial environmental cues (Sims et al., 2005; Armstrong et al., 2021). The great variability and transience of regional plankton likely drive their foraging behavior, prey sources, and habitat use (Stewart et al., 2017; Barr and Abelson, 2019; Putra et al., 2020).
The oceanic manta ray, Mobula birostris (Walbaum, 1792), has circumglobal distribution, and generally occurs more offshore than the smaller, more coastal reef manta ray, Mobula alfredi, (Kreft, 1868), which is semi-circumglobal and restricted to tropical and subtropical waters (Marshall et al., 2009; Burgess et al., 2016; Armstrong et al., 2020). These are the two largest of all ray species (M. birostris; 8 m maximum disc width (DW); M. alfredi; 5.5 m maximum DW) and are both slow-growing, with late maturation and low fecundity (Marshall et al., 2009; Marshall and Bennett, 2010; Stevens et al., 2018). Due to these life history characteristics, as well as the exploitation of mobulids for the gill plate trade, both manta ray species are listed on the IUCN’s Red List of Threatened Species (M. birostris as Endangered and M. alfredi as Vulnerable) (Marshall et al., 2009; O’Malley et al., 2016; Marshall et al., 2018a; Marshall et al., 2018b). Although directly fished and caught as bycatch in Mozambique (Couturier et al., 2012; Croll et al., 2016), one of the sources of fishing mortality for M. birostris and M. alfredi in the south-west Indian Ocean is the KwaZulu-Natal (KZN) bather protection program in South Africa. Although not a fishery in the conventional sense, this is the only shark fishing operation in South Africa documented to catch these species as a means to protect public bathers (Dudley and Cliff, 1993; Marshall et al., 2008; Croll et al., 2016).
Both M. birostris and M. alfredi are known to migrate, with current recorded ranges of >1400 km for oceanic manta rays (Hearn et al., 2014) and 1150 km for reef manta rays (Armstrong et al., 2019). Despite such extensive horizontal movements, manta rays display affinity to certain locations such as inshore reefs, seamounts, or foraging sites, for example, which the same individuals have been found frequenting for up to 30 years (Dewar et al., 2008; Couturier et al., 2014; Couturier et al., 2018; Venables et al., 2020).
Manta ray movement patterns in southern Africa may be driven by temporal and spatial patterns of zooplankton abundance (Sims et al., 2006; Rohner et al., 2017; Stewart et al., 2019). On the east coast of South Africa, the narrow continental shelf (Martin and Flemming, 1988) and shifting seasonal water temperatures and currents (Walker, 1990; Roberts et al., 2010) allow numerous elasmobranch species, such as the tiger shark, Galeocerdo cuvier, and the diamond ray, Gymnura natalensis, to exploit a wide range of habitat and area (Connell, 2001; Wetherbee, 2004; Dicken et al., 2006; Daly et al., 2018; Daly et al., 2022). Acoustic telemetry revealed a reef manta ray that traveled up to 90 km in a single day in Mozambique (Venables et al., 2020). At monitored locations in southern Mozambique, manta ray habitat use is seasonal; sightings increase in Tofo during austral summer (November to February), (Marshall et al., 2011) while more sightings occur from July to November in Závora, which is 90 km further south (Carpenter et al., 2022). Oceanic manta ray sightings peak in April in Tofo (Rohner et al., 2013). Despite contrasting temporal patterns, oceanic and reef manta rays in southern Mozambique overlap in their use of cleaning and foraging habitats, which may be a result of resource availability in the area (Kashiwagi et al., 2011).
While manta rays have been studied for two decades in Mozambique, they remain relatively understudied in South Africa, despite sightings from KwaZulu-Natal (KZN) and the availability of suitable habitat, including cleaning stations (Carpenter, unpublished data). Genetic analysis and photo identification studies suggest that there is a single breeding population of reef manta rays common between the two countries (Venables et al., 2021; Marshall et al., 2022), and it is likely that KZN coastal waters may serve as critical habitat for southern African manta ray populations.
Given the migratory nature of manta rays and limited information about the species in South African waters, we evaluate baseline trends in encounters, similar to other studies on ray species in KZN (Daly et al., 2021; Daly et al., 2022). We use 41 years of catch data from the KZN bather protection program to investigate long-term trends in manta ray occurrence, body size and demographic composition. We determine the influence of environmental variables on manta ray occurrence using Generalized Additive Models (GAMs), and describe patterns of temporal and spatial habitat use.
The marine environment of the KwaZulu-Natal (KZN) Province, on the east coast of South Africa, is subtropical and dominated by the southward-flowing Agulhas Current (Lutjeharms et al., 2000). Two ecoregions have been described by Sink et al. (2019) within KZN borders: ‘Maputaland’, which extends from the Mozambique border southwards to Cape Vidal, and ‘Natal’, from south of Cape Vidal to the Eastern Cape (Sink et al., 2005; Griffiths et al., 2010). However, within the Natal region there is variation in the flow of the Agulhas Current and how it interacts with the continental shelf (Lutjeharms et al., 2000; Roberts et al., 2010). This variation is largely due to the presence of the Natal Bight, a 160 km long and 50 km wide coastal offset located between Cape St. Lucia and immediately south of Durban (160 km), which interrupts the strong, stable flow of the Agulhas Current evident along most of the coast (Fennessy et al., 2016). South of the Natal Bight, the continental shelf break becomes narrower and closer to shore, extending southwards to the Eastern Cape (Fennessy et al., 2016). Therefore, for the occurrence analysis in this study, the Natal region is further divided into three areas to allow for the possibility of the heterogeneity of ocean processes along the coastline. The study area extended approximately 350 km from Richard’s Bay in the North, to Mzamba Beach in the South (Figure 1 and Table S1). The three areas (North, Central, and South) from North to South measure 84.9 km, 84.6 km, and 86.1 km, respectively (Figure 1) and are broadly consistent with designated regions defined by previous local studies (Dicken et al., 2006; Dudley and Cliff, 2010).
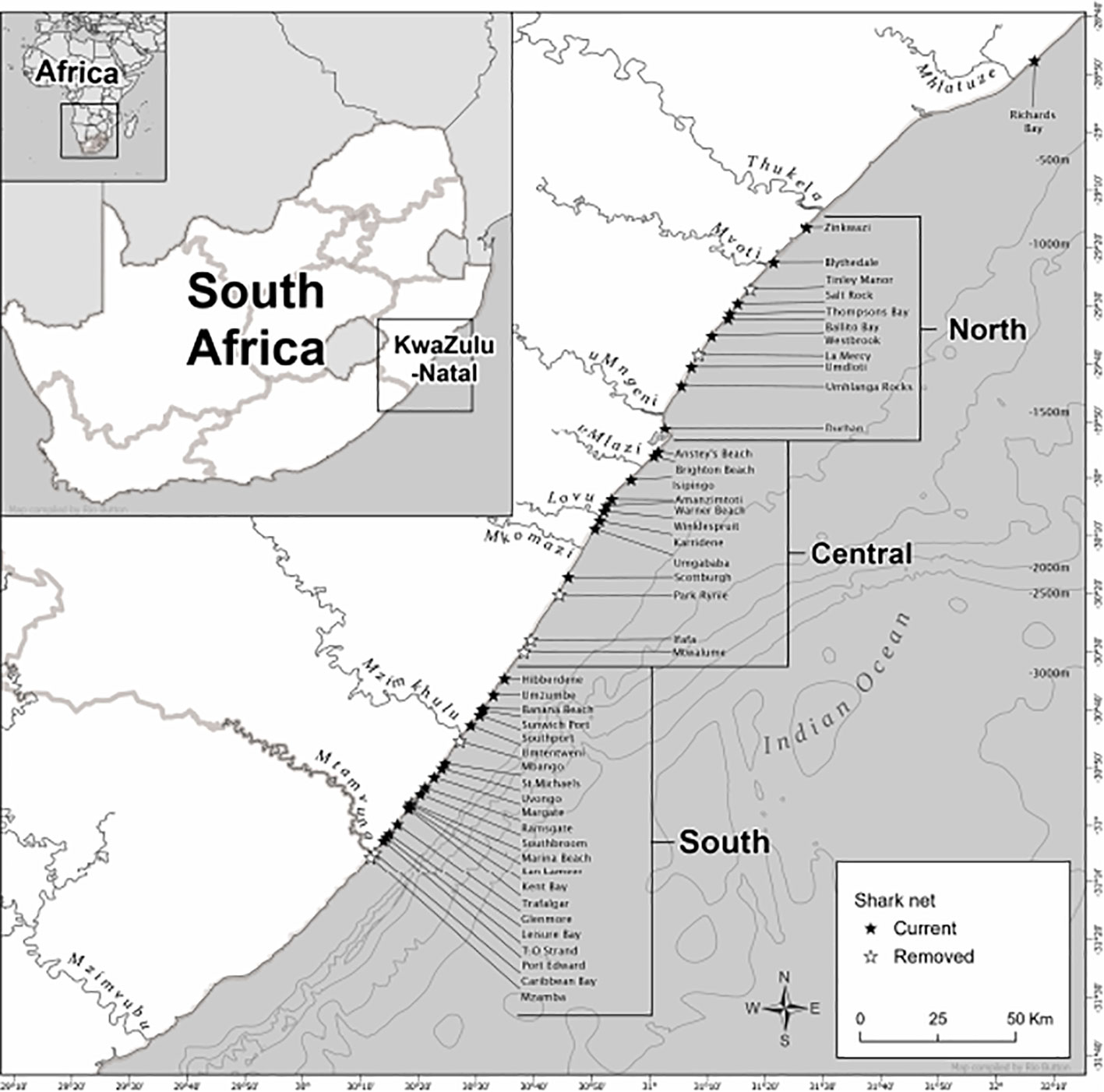
Figure 1 Map of KwaZulu-Natal showing sites where bather protection nets were deployed and defining the three designated coastal areas that were used in the study between 1981-2021. Also shown are depth contour (500 m intervals) and defined major river systems. Black stars indicate nets that remain as of 2022 and white starts indicate currently removed nets.
The KwaZulu-Natal bather protection nets are large-mesh gill nets installed year-round at public recreational beaches since 1952 to mitigate shark-human interaction (Cliff and Dudley, 1992). The nets are 214 m long, 6.3 m deep, and set parallel to, and 300-500 m from the shore, in a water depth of 10-14 m (Cliff and Dudley, 1992; Daly et al., 2022). The nets were deployed at a maximum of 46 fixed locations throughout the study, and are currently installed at 37 locations along the KZN coastline (Table 1 and Figure 1). The deployed nets are regularly inspected, whereby trained field staff visit each net by boat, a process called ‘meshing’. Meshing usually occurs at first light, between 17-19 times per month (Dudley and Cliff, 2010). The monthly average number of nets per day per location multiplied by the average net length was used as a measure of the unit effort. Statistically reliable bycatch data (in this case mobulids) from the bather protection nets began in 1981, therefore data prior to that were excluded. Observers were trained to distinguish between devil ray and manta ray species; but we excluded individuals with a Disc Width (DW) less than 1.4 m from the analysis, as these could be Mobula kuhlii or Mobula eregoodoo specimens (Cliff, pers. comm.). Due to the relatively recent speciation of manta rays (Marshall et al., 2009) and limited access to training, observers could not distinguish between manta ray species. Therefore, the two manta ray species were pooled together. All individuals used in the study were measured in the field. When an individual manta ray was caught, the individual was sexed using the presence or absence of claspers, and the DW was measured to the nearest mm as the straight-line distance between pectoral fin tips.
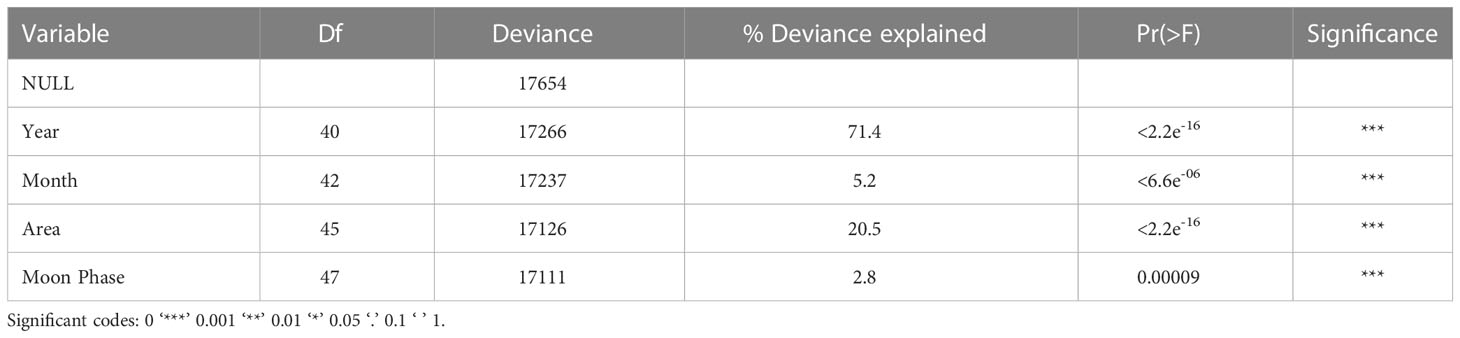
Table 1 Deviance table documenting the relative importance of the explanatory variables included in the GAM model to assess manta ray catch trends from the KwaZulu-Natal Bather protection net dataset in South Africa between 1981-2021.
In an effort to reduce bycatch of non-target species there was substantial removal of nets at 34 of the 37 beaches in the early 2000’s, which were replaced by drumlines (Cliff and Dudley, 2011; Dicken et al., 2016; Dicken et al., 2018). Each drumline is anchored adjacent to the nets and consists of a single Mustad 4480DT 14/0 J hook (Gjøvik, Norway) suspended 4 m beneath a large float (Dudley et al., 1998; Cliff and Dudley, 2011). The hooks were baited and checked every weekday (weather permitting) and re-baited, as necessary. In 2007, a total of 79 drumlines replaced almost half (4 km) of the nets at 17 of the 18 protected beaches along the Hibiscus Coast (Hibberdene, beach 25, to Port Edward, beach 44; Figure 1), An additional 28 drumlines were installed between Zinkwazi and Ballito in 2015, and an additional 70 drumlines between Tongaat and Umgababa in 2019. The 177 drumlines currently in operation were deployed at a replacement ratio of four drumlines to one net. Specifics of the drumline deployments are given in Dicken et al. (2016).
Overall catch per unit effort (CPUE) was measured at each beach by calculating the total catch divided by the total of the monthly average number of nets multiplied by the average net length used at each location between 1981-2021. This is because the number of nets and net length varied at each beach throughout the study period (Table S1). Means (± Standard deviation, or, ‘SD’) were calculated to assess the following: the average annual number killed as a result of catch, the average number of nets and net length at each location, and the average size of each individual caught.
We used Generalized Additive Models (GAMs) to examine the relationships between the Probability of Encounter (PE) (0 = absent, 1 = present) of manta rays and predictor variables assuming a binomial error distribution. All analyses were conducted in R software (R Core Team, 2021). Probability of encounter is preferred over count distributions when a species is rarely captured, as overdispersion is accounted for. Furthermore, simulation testing has shown that if PE decreases below a certain threshold, the information provided by non-zero observations is minimal and the relationship between PE and abundance becomes approximately linear (Parker et al., 2016; Kerwath et al., 2019). Daily moon phase data were extracted from the ‘suncalc’ package (Thieurmel and Elmarhraoui, 2019). Effort was treated as an offset in natural logarithmic scale which included the average number of nets and net length and each location.
The full GAM included the smoothing functions for the variables month and moon phase as follows:
where logit denotes the binomial link function, p is the probability of catching at least one individual per net deployment, α is the intercept, s1-2 denotes cyclic cubic smoothing functions for Month and Moon phase (Wood, 2006). Year and Area were treated as categorical variables. Sequential F-tests were used to determine the covariates that contributed significantly (p < 0.001) to the deviance explained and GAMs were fitted in R statistical software using the ‘mgcv’ and ‘nlme’ (Wood, 2006). The annual value of PE was standardized by fixing all covariates other than Year in the prediction dataset. Drumline data were excluded from GAM analysis because of the short time-frame of their deployment and low catches of manta rays compared to the nets.
All manta rays caught were sexed based on the presence or absence of external claspers, and measured, using DW (Marshall and Bennett, 2010). While a threshold of ≥6 m DW was used to identify oceanic manta rays, the majority of catch data included unknown manta species. Nevertheless, detection of juveniles versus adults was possible using known sizes of maturity for both reef and oceanic manta rays (Table S2). Juvenile and adult maturity status for an individual was determined by a DW between 1400-2500 mm and 3801-8000 mm, respectively (Table S2). Individuals that had a DW between 2501-3800 mm were recorded as being of unknown maturity. Sex ratios were calculated using an exact binomial test in the ‘stats’ package in R (R Core Team, 2021) with a significant difference in sex ratio accepted at p < 0.05.
Between 1981-2021, 1,602 manta rays were caught in the nets. Between 2007-2021, 10 were caught in the drumlines and therefore excluded from statistical analysis. Manta rays were caught throughout the year, with more caught in austral summer (Dec-Feb; n=534), accounting for 33% of the total catch, compared to the austral winter (Jun-Aug; n=302), which accounted for 19% of the total catch. The size of mantas ranged from 1400-8000 mm Disc Width (DW). An average of 40 rays (±29 SD) were caught per year of which approximately one third (n=527) were found dead, the remaining 70% being released, thus resulting an average of 13 (±11 SD) confirmed mortalities per year. Annual mortality ranged from one (9% of annual catch) in 2017 to 38 (49% of annual catch) in 2001. The majority of catches were single individuals, with a maximum of two manta rays caught in a single deployment. The total net length (Figure 2A) and manta ray catches (Figure 2B) exhibited considerable variation, but there was an overall decrease across the 41-year period.
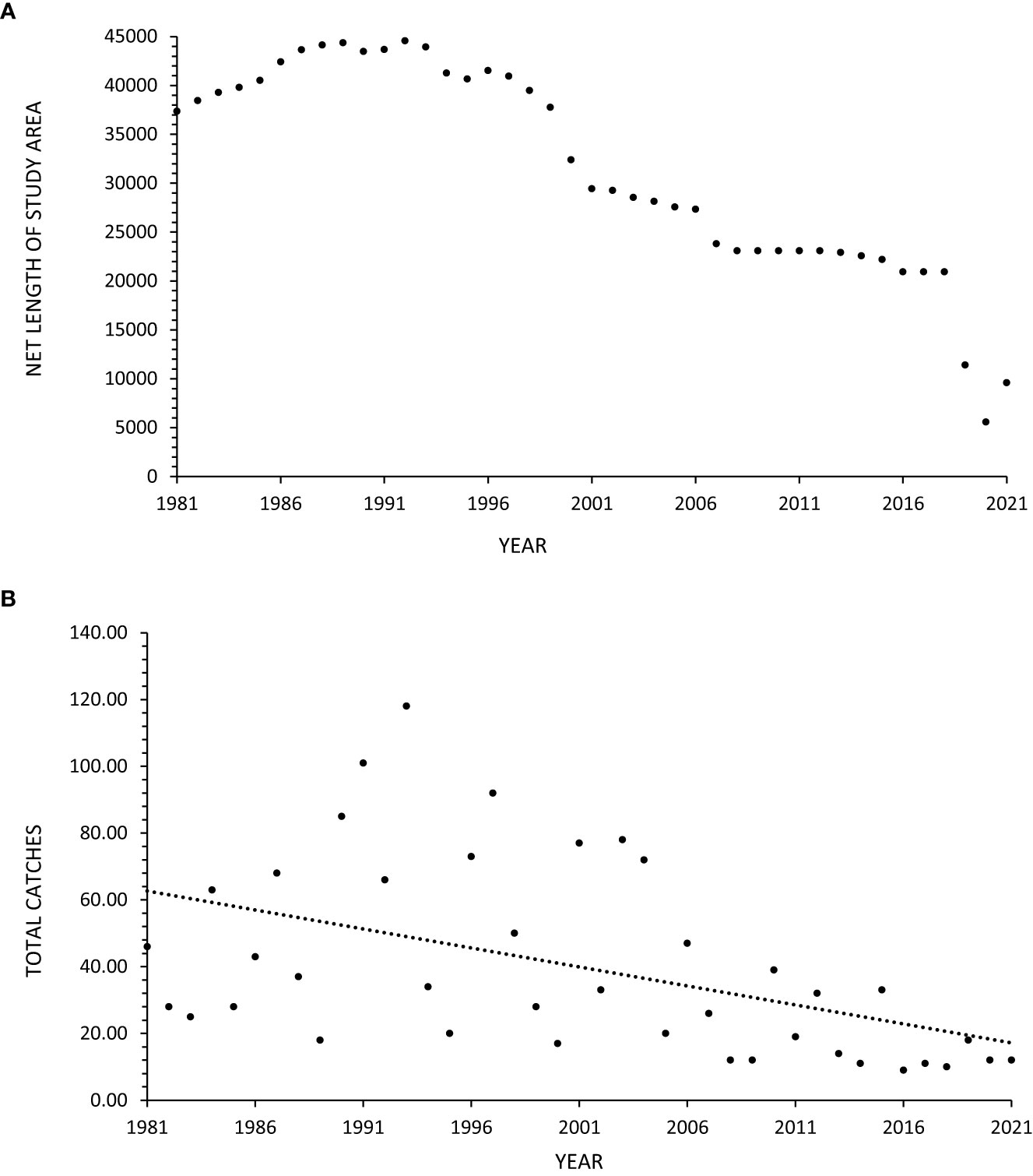
Figure 2 (A, B) Total annual number of bather protection nets (A) and total annual manta ray catches (B) in these nets in KwaZulu-Natal, South Africa, between 1981-2021. The dashed lines represent linear regression fitted to the data.
Spatially, the Central Area had the largest number of catches throughout the study period (n=649), followed by the South (n=528) and then the North Area (n=414), with 11 additional catches at Richard’s Bay (R.B). Amanzimtoti beach (AMA), within the Central Area (Figure 1), had the highest total catch over the entire period (n=120; 7% of total catch) (Figure 3). Only two other beaches reported total catches exceeding 100, these being Scottburgh (SCO) and Zinkwazi (ZIN). AMA and SCO are within approximately 35 km of each other in the Central Area, whereas Zinkwazi is the northernmost beach in the North Area (Figure 3). When incorporating the unit of effort (the total of the monthly average number of nets multiplied by the average net length), the highest CPUE occurred at Winklespruit (0.0046) followed by Park Rynie (0.0036), Caribbean Bay (0.0035) and Ifafa (0.0034), three of these beaches being in the Central Area (Figure 3).
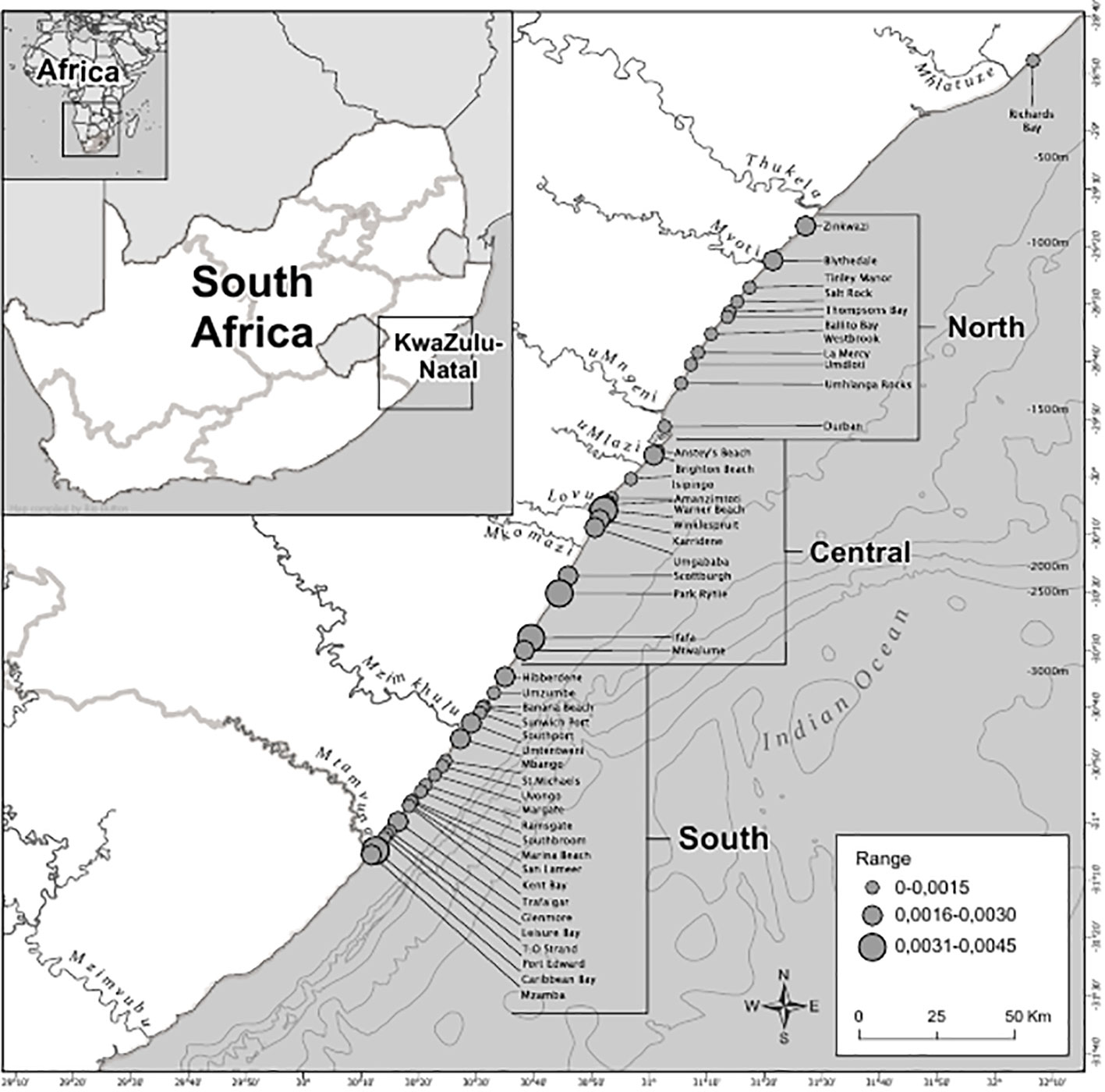
Figure 3 Average manta rays caught and standardized by the average net length at the particular beach (catch per unit effort, or CPUE) in the bather protection nets, KwaZulu-Natal, South Africa between 1981-2021. Catch per unit effort was divided into three ranges, the lowest being between 0.000-0.0015, up to the highest being 0.0031-0.0045.
A total of 1,423 captures were included in the Generalized Additive Models. Month, moon phase, area, and year were significant predictors for manta ray capture. This model was offset with the logarithmic of effort. Year explained 71.4% of total deviation, followed by area (20.5%), month (5.2%), and moon phase (2.8%) (Table 1). There was an increase in manta ray catches up until the year 2000 where there was a significant temporal decline (p<2.2e-16) (Figure 4A). This is especially true when viewing year in numerical form, whereby the probability of capture is lowest in 2015-2021 (Figure S1). The probability of catching manta rays peaked in the summer months of December-February and was lowest in winter, between June-August (Figure 4B). The probability of capture was highest in the Central Area and lowest in the North Area (Figure 4C). Moon phase had a significant effect on the probability of manta ray capture, with increased catch during new and full moon phases (Figure 4D).
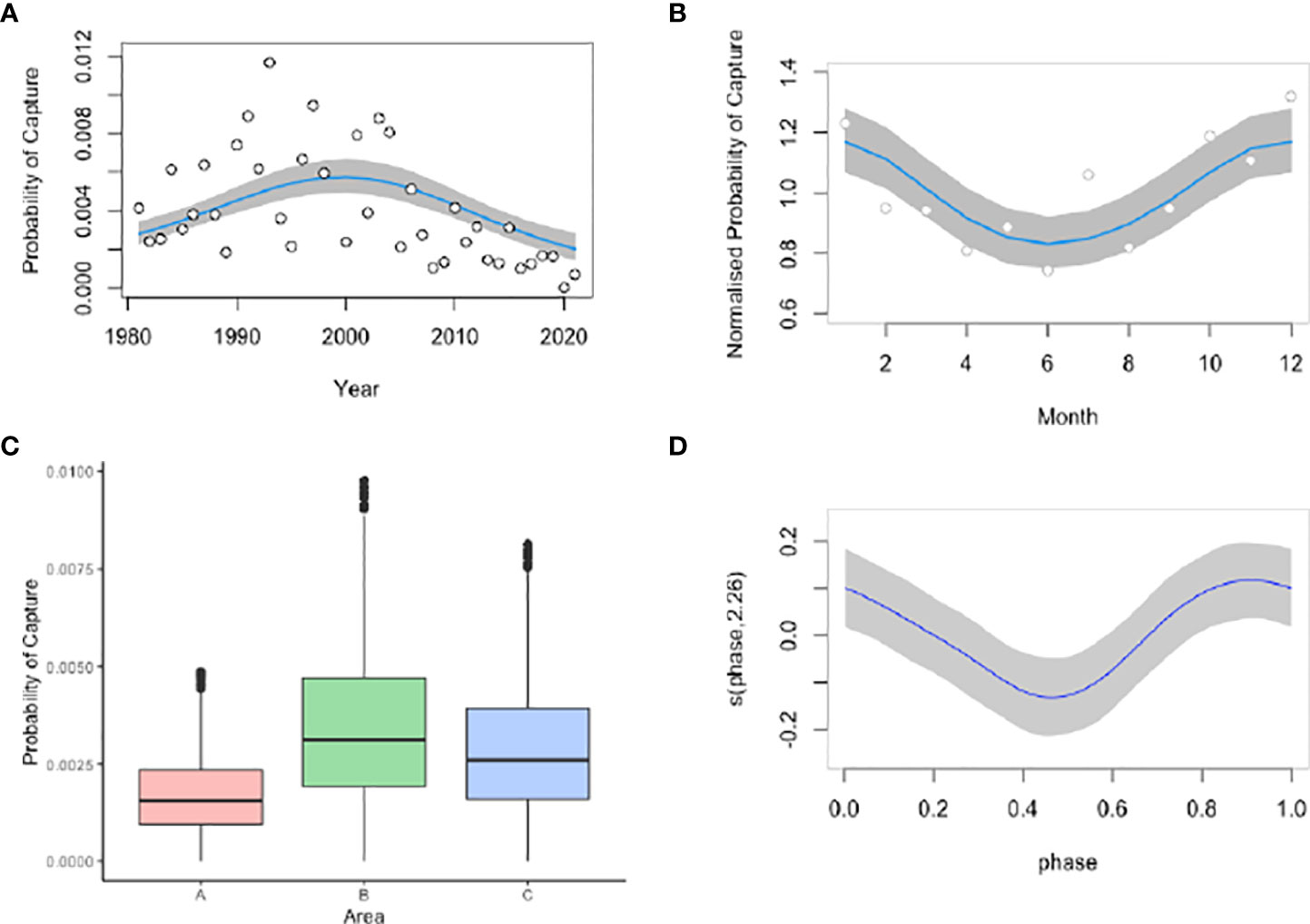
Figure 4 Significant predictors for the probability of manta ray capture in the KwaZulu-Natal bather protection nets between 1981-2021 including year (A), month (B), area (C), and moon phase (D). Year and month plots include both numerical and factor models. South Africa austral summer occurs between December-February and winter between June-August.
Most of the caught rays were sexed (62%, n=997) and of these, 56% were female (n=563) and 44% male (n=434), while 38% were recorded as unknown sex (n=605) and hence were excluded from the analysis of sex ratio. There was no significant difference in sex ratio (p=0.67, exact binomial test). This sex ratio with slightly more females remained similar when assessed by area (F:M North Area 1.28:1.0; Central Area 1.38:1.0; South Area 1.26:1.0).
Averaged across the entire study period, caught rays had an average DW of 2796 mm (± 1368 SD). More than half (52%, n=841) of caught individuals belonged to the juvenile size class, between 1400-2500 mm DW (Figure 5). There was an overall ratio of 1:1.8 adults (n=474) to juveniles (n=841) captured, although 18% (n=287) were recorded at unknown maturity due to the overlap in maturity sizes between oceanic and reef manta rays (a size range of 2501-3800 mm) (Figure 5). The South Area had the highest proportion of juveniles in the catch (70%) (Figure 6), comprising 44% of all juveniles caught throughout the study. Of these, 145 individuals were between 1400-1600 mm in size, the known size at birth. Confirmed adult manta rays of both species (3800-8000 mm) were caught in the highest numbers in Amanzimtoti (AMA; n=43), followed by Zinkwazi (ZIN; n=39), Scottburgh (SCO; n=38), Park Rynie (PAR; n=36), Winklespruit (WIN; n=36), and Durban (DUR; n=31); four of these locations (AMA, SCO, PAR, WIN) being within 35 km of one another. A total of 70 rays were 5501-8000 mm DW, confirming that they could only have been oceanic manta rays, and more than half of these were caught within the Central Area (53%, n=37) (Figure S2). Three individuals were measured to be 8000 mm DW: two from Hibberdene (HIB) in 1987 and 2019, and one from Brighton (BRI) in 1981 (Figure S2). This confirms the maximum size of oceanic manta rays in South Africa to reach at least 8000 mm DW. Both adult and juvenile manta rays were caught throughout the year, with numbers for both peaking in summer, between December-February (Figure 7).
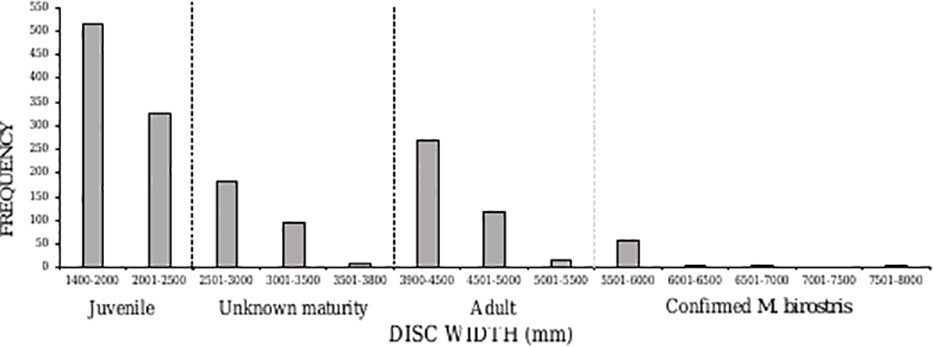
Figure 5 Disc width frequency distribution of manta rays caught in the KwaZulu-Natal shark nets between 1981–2021. Dashed lines indicate the division between juvenile, unknown maturity, adult, of unknown species, and confirmed Mobula birostris individuals based on size.
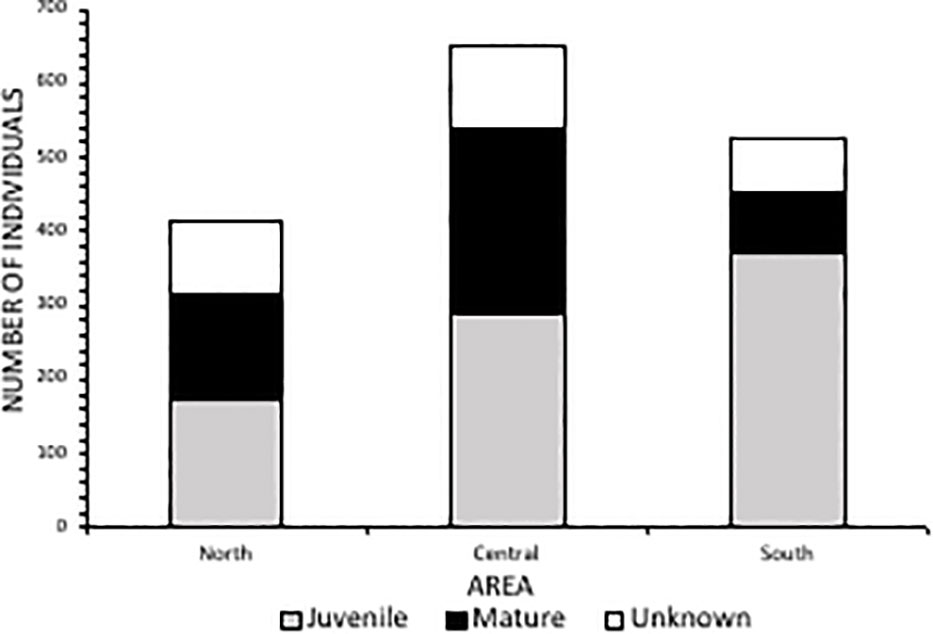
Figure 6 Catch and maturity status of manta rays from each area (North Area=Zinkwazi to Durban; Central Area=Anstey’s Beach to Mtwalume; South Area=Hibberdene to Mzamba) from bather protection net catch data in KwaZulu-Natal, South Africa 1981-2021.
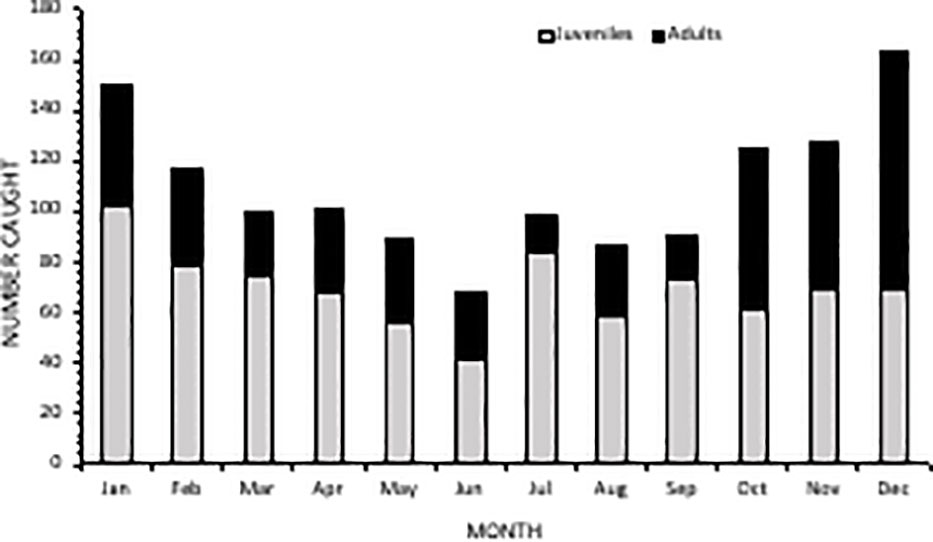
Figure 7 Monthly comparison of catch and maturity status of manta rays from bather protection net catch data in KwaZulu-Natal, South Africa over the period 1981-2021.
Using a 41-year dataset, we describe broad spatial-temporal trends of manta ray distribution and abundance in South Africa for the first time. We found an overall significant decline in catches between 1981-2021 and South Africa to be important habitat for manta rays, especially in summer (December-February), and in the Central and South Areas. When accounting for variation in effort, as well as other possible environmental influences, the standardized probability of capture shows a peak in the late 1990s, followed by a marked decline thereafter. Further, nominal probability of capture has consistently remained below the annual mean since 2007. This supports the majority of studies from Mozambique which report that manta ray encounters have generally declined over time (Rohner et al., 2013; Venables, 2020).
Manta ray populations in the southern African region are of immediate conservation concern (Tibiriçá et al., 2011; Peel, 2019; Venables, 2020). Venables (2020) stated that annual landings in an artisanal fishery of 20-50 individuals per year over 16 years could have resulted in the detected abundance decline in Tofo, Mozambique; from 836 in 2004 to less than 100 since 2013 (Marshall et al., 2011; Temple et al., 2018). The 88% decline in sightings of reef manta rays observed in Tofo between 2003-2011 further supports this (Rohner et al., 2013). Manta ray populations cannot withstand fishing mortality due to their low fecundities (one pup per two years), even from small artisanal fisheries, or as bycatch from destructive fishing practices (Croll et al., 2016; Lawson et al., 2017; Parton et al., 2019). Given that M. alfredi in South Africa and Mozambique comprise a single breeding population, it is crucial to ensure that these mobile, threatened species are adequately safeguarded in both countries.
The catch numbers found in this study suggest that South Africa encompasses important habitat for manta rays, the extent of the visitation to that habitat which differs across seasons. Though manta rays were caught throughout the year, catches were highest in summer (Dec-Feb). In KZN, summer is associated with higher rainfall and north-easterly winds that drive the Durban Eddy, both of which increase upwelling and riverine output, and subsequent primary productivity and abundance and diversity of marine taxa (Woodson et al., 2012; Guastella and Roberts, 2016). Increased copepod and chaetognath abundance occur during summer in KZN, these being known prey of manta rays (Schleyer, 1985; Couturier et al., 2013; Bennett et al., 2016; Peel, 2019). Therefore, the increased manta ray catches may be due to the increased peaks of phytoplankton and subsequent zooplankton blooms during summer. These results indicate that manta rays may be present year-round in the region but with seasonal peaks, which suggests migration from other parts of the coast driven by life stage, reproduction, food availability, or individual movements.
There were significantly higher catches of manta rays from the area between Anstey’s beach and Mtwalume (Central Area), nearby the Aliwal Shoal Marine Protected Area. The Aliwal Shoal Marine Protected Area is an important offshore habitat for elasmobranchs (Dicken et al., 2006; Dicken and Hosking, 2009; Dicken et al., 2016). It was declared a Marine Protected Area in 2004 (Government Gazette No. 26433) with fishing prohibited in the controlled zone, however, bather protection nets are also permitted at Scottburgh Beach, which is located five kilometers southwest. Despite historically high catches, few manta rays have been observed at Aliwal Shoal Marine Protected Area between 2020-2022 (Carpenter, unpublished data).
With at least one catch from every beach, this study provides further evidence that manta rays utilize the expansive continental coastline year-round from the Eastern Cape (approximately 175 km south of Mzamba) (Marshall et al., 2022) northwards into southern Mozambique. However, the intricacies of habitat use remain unknown in KZN, for instance, the specific hotspots for each species, and how often they move in and out of various areas. Full and new moons were significant with manta ray capture, a known predictor of manta ray sightings (Rohner et al., 2013; Fonseca-Ponce et al., 2022). This may be due to tidal effects on zooplankton availability (Rohner et al., 2013; Barr and Abelson, 2019), or the efficacy of nets in capturing manta rays during the full tidal range. The variability in manta ray catches during this study are thus likely consequences of physical processes that drive resource availability and/or net efficacy (Graham et al., 2012; Braun et al., 2014; Jaine et al., 2014; Stewart et al., 2016). Further in-water surveys and telemetry studies would allow for the determination of the possible hotspots for manta rays in KZN, and the visitation patterns associated with these sites.
A greater proportion of juvenile manta rays (DW of 1400-2500) were found in the South Area, from Hibberdene to Mzamba. A total of 9% of individuals (n=145) caught were at the estimated birth size (1400-1600 mm) (Stewart et al., 2018), and most were caught at Mzamba (MZA) (n=14), the most southerly location in the present study. Initial observations in Port St. John’s, Eastern Cape, roughly 93 km south of Mzamba, reported six juvenile individuals sighted during winter (Marshall et al., 2022). Further, 52% of total catches (n=841) were within juvenile size for either manta ray species, with almost half of these (43%; n=365) from the South Area. Aggregations in Mozambique monitored for 11 - 20 years have reported small numbers of juveniles (roughly 5% of the photo-identified population in Tofo and Závora and 3% in Bazaruto) (Venables, 2020; Carpenter et al., 2022). Our results fit two of three of the criteria outlined by Heupel et al. (2007): juveniles were more common in a certain area and the habitat was repeatedly used across multiple years; however, we could not validate one criterion; this being if individuals remain or return to the area for extended periods. In contrast, overall, larger mantas were caught in the North and Central Areas, from Zinkwazi (ZIN) to Mtwalume (MTW), where the most confirmed oceanic manta rays (based on size class) were also caught, which may be reflective of a possible oceanic manta ray aggregation. More research is needed to confirm this as it is possible that the nets are incapable of holding large adults.
The primary caveat in this study is that it reports on relatively low sampling coverage over an expansive area. Considering their depth and habitat ranges, both manta ray species are likely to be spending the majority of time outside the limits of bather protection nets or recreational diving in KZN. Further, we did not distinguish between species in the catch data, due to the overlap in size and potential confusion with species identification. Both species are known to overlap in habitat use (Marshall et al., 2009; Kashiwagi et al., 2011) and both have been identified in various locations amongst the KZN coast (Carpenter, unpublished data; Marshall et al., 2022). Therefore, the pooling of species needs to be considered when interpreting the results, as this describes trends of the two species. In further studies species identification and data quality could be improved via team training or the implementation of photographic records for each captured animal, whether dead or alive (and released). Nevertheless, our results are informative for baseline spatial-temporal habitat use, and can be used to inform policymakers on the impacts of bycatch mortality and the need for development of local conservation management plans.
Both manta ray species are protected in South Africa through international agreements; the Conservation of Migratory Species (CMS) and the Convention on the International Trade of Endangered Species (CITES, 2013; Lawson et al., 2017); and national protection including oceanic manta rays under the Biodiversity Act of 2004 in South Africa, and reef manta rays listed under Threatened or Protected Species (TOPS) regulations (Notice No. 40875 under No. 476 of the Biodiversity Act, 10 of 2004, 2017). An increased network of Marine Protected Areas would benefit manta rays and other threatened species, as South Africa has not yet reached the Ocean Economy and Sustainability Goals of the United Nations of 10% by 2020.
Identifying sources of mortality of manta rays within the southern African region is key to mitigating impacts. Though the scientific knowledge gained from the bather protection nets has been unprecedented, including pioneering studies on numerous elasmobranch species in KZN (Cliff and Dudley, 1991; Dudley and Simpfendorfer, 2006; Kock et al., 2022), the current design may impact manta ray populations (an average of 13, up to 38 confirmed annual catch fatalities). Manta rays have one of the lowest reported population growth rates (median rmax of 0.116 year−1 95th percentile [0.089–0.139]; Dulvy et al., 2014) of 106 assessed elasmobranch species. Efforts to reduce bycatch have been implemented by the KZN bather protection program, such as reducing the number of nets and drumlines (Guyomard et al., 2019), and the removal of gear at three of the four beaches with the highest manta ray CPUE. However, due to the bycatch mortality of many vulnerable species, efforts should continue in seeking solutions to mitigate catches even further, especially at beaches installed within already established species refuges (i.e., Marine Protected Areas). This would help reduce impact to the southern African manta ray populations and facilitate their conservation into the future.
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
The KwaZulu-Natal Sharks Board is granted permission to catch animals as part of its operational activities as per its permit conditions listed in OCS/TOPS/STANDINGPERMIT/2022/02. This permit is issued by the Department of Forestry, Fisheries and the Environment in terms of regulation 7(1) of the Threatened or Protected Marine Species Regulations, 2017 (Government Gazette Notice No. 40876 of 30 May 2017) (the Regulations) read with the National Environmental Management: Biodiversity Act, 2004 (Act no.10 of 2004) (the Act).
MC and MD conceived the paper. Data was collected by MD. Data analysis was conducted by MC and DP. Written principally by MC with input and editorial comments from all other authors. All authors contributed to the article and approved the submitted version.
Fieldwork and database management was funded by the KwaZulu-Natal Sharks Board. Analysis and writeup supported by the University of Cape Town Science Fellowship.
Fieldwork and database management were supported by the KwaZulu-Natal Sharks Board. Analysis and write-up were supported by the University of Cape Town Science Faculty Fellowship. We are grateful to the reviewers and S. Venables for their valuable feedback, which greatly improved the manuscript. We thank R. Button for designing the maps used in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1128819/full#supplementary-material
Supplementary Figure 1 | Significant decline in the probability of manta ray capture over time (numerical) in the KwaZulu-Natal bather protection nets between 1981-2021.
Armstrong A. J., Armstrong A. O., Bennett M. B., McGregor F., Abrantes K. G., Barnett A., et al. (2020). The geographic distribution of reef and oceanic manta rays (Mobula alfredi and Mobula birostris) in Australian coastal waters. J. Fish Biol. 96 (3), 835–840. doi: 10.1111/jfb.14256
Armstrong A. O., Armstrong A. J., Bennett M. B., Richardson A. J., Townsend K. A., Dudgeon C. L. (2019). Photographic identification and citizen science combine to reveal long distance movements of individual reef manta rays Mobula alfredi along australia’s east coast. Mar. Biodiversity Records 12 (1), 14. doi: 10.1186/s41200-019-0173-6
Armstrong A. O., Stevens G. M., Townsend K. A., Murray A., Bennett M. B., Armstrong A. J., et al. (2021). Reef manta rays forage on tidally driven, high density zooplankton patches in hanifaru bay, Maldives. PeerJ 9, e11992. doi: 10.7717/peerj.11992
Barr Y., Abelson A. (2019). Feeding–cleaning trade-off: Manta ray “decision-making” as a conservation tool. Front. Mar. Sci. 6 (88), 1–10. doi: 10.3389/fmars.2019.00088
Bennett M. B., Coman F. F., Townsend K. A., Couturier L. I. E., Jaine F. R. A., Richardson A. J. (2016). A historical and contemporary consideration of the diet of the reef manta ray (Manta alfredi) from the great barrier reef, Australia. Mar. Freshw. Res. 68 (5), 993–997. doi: 10.1071/MF16046
Braun C. D., Skomal G. B., Thorrold S. R., Berumen M. L. (2014). Diving behavior of the reef manta raylinks coral reefs with adjacent deep pelagic habitats. PloS One 9 (2), e88170. doi: 10.1371/journal.pone.0088170
Burgess K. B., Couturier L. I., Marshall A. D., Richardson A. J., Weeks S. J., Bennett M. B. (2016). Manta birostris, predator of the deep? Insight into the diet of the giant manta ray through stable isotope analysis. R. Soc. Open Sci. 3 (11), 1–10. doi: 10.1098/rsos.160717
Carpenter M., Cullain N., Venables S. K., Tibiriçá Y., Griffiths C., Marshall A. D. (2022). Evidence of závora bay as a critical site for reef manta rays, Mobula alfredi, in southern Mozambique. J. FishBiol 101 (3), 628–639. doi: 10.1111/jfb.15132
CITES (2013). Notification to the parties no. 2013/012. amendment to appendices I and II of the convention. Convention on the International Trade of Endangered Species
Cliff G., Dudley S. F. J. (1991). Sharks caught in the protective gill nets off natal, south africa. 4. the bull shark Carcharhinus leucas valenciennes. South Afr. J. Mar. Sci. 10 (1), 253–270. doi: 10.2989/02577619109504636
Cliff G., Dudley S. F. (1992). Protection against shark attack in south africa 1952-90. Mar. Freshw. Res. 43 (1), 263–272. doi: 10.1071/MF9920263
Cliff G., Dudley S. F. (2011). Reducing the environmental impact of shark-control programs: A case studyfrom KwaZulu-natal, south Africa. Mar. Freshw. Res. 62 (6), 700–709. doi: 10.1071/MF10182
Connell A. D. (2001). Pelagic eggs of marine fishes from park rynie, KwaZulu-natal, south Africa: Seasonal spawning patterns of the three most common species. Afr. Zool 36 (2), 197–204. doi: 10.1080/15627020.2001.11657138
Couturier L. I., Dudgeon C. L., Pollock K. H., Jaine F. R. A., Bennett M. B., Townsend K. A., et al. (2014). Population dynamics of the reef manta ray Manta alfredi in eastern Australia. Coral Reefs 33 (2), 329–342. doi: 10.1007/s00338-014-1126-5
Couturier L. I. E., Marshall A. D., Jaine F. R. A., Kashiwagi T., Pierce S. J., Townsend K. A., et al. (2012). Biology, ecology and conservation of the mobulidae. J. Fish Biol. 80 (5), 1075–1119. doi: 10.1111/j.1095-8649.2012.03264.x
Couturier L. I. E., Newman P., Jaine F. R. A., Bennett M. B., Venables W. A., Cagua E. F., et al. (2018). Variation in occupancy and habitat use of mobula alfredi at a major aggregation site. Mar. Ecol. Prog. Ser. 599, 125–145. doi: 10.3354/meps12610
Couturier L. I., Rohner C. A., Richardson A. J., Marshall A. D., Jaine F. R., Bennett M. B., et al. (2013). Stable isotope and signature fatty acid analyses suggest reef manta rays feed on demersal zooplankton. PloS One 8 (10), e77152. doi: 10.1371/journal.pone.0077152
Croll D. A., Dewar H., Dulvy N. K., Fernando D., Francis M. P., Galván-Magaña F., et al. (2016). Vulnerabilities and fisheries impacts: The uncertain future of manta and devil rays. Aquat. Conservation: Mar. Freshw. Ecosyst. 26 (3), 562–575. doi: 10.1002/aqc.2591
Daly R., Jordaan G. L., Parker D., Cliff G., Nkabi N., Kyle R., et al. (2022). Movement patterns and catch trends of the diamond ray gymnura natalensis (Dasyatidae) in south African waters. Afr. J. Mar. Sci. 44 (1), 35–48. doi: 10.2989/1814232X.2022.2032826
Daly R., Parker D., Cliff G., Jordaan G. L., Nomfundo N., Bennett R. H., et al. (2021). Long-term catch trends and risk assessment of the critically endangered white-spotted wedgefish (Rhynchobatus djiddensis) from south Africa. Aquat. Conservation: Mar. Freshw. Ecosyst. 31 (4), 777–788. doi: 10.1002/aqc.3483
Daly R., Smale M. J., Singh S., Darrell A., Shivji M., Daly C. A. K., et al. (2018). Refuges and risks: Evaluating the benefits of an expanded MPA network for mobile apex predators. Diversity Distributions 24 (9), 1217–1230. doi: 10.1111/ddi.12758
Dewar H., Mous P., Domeier M., Muljadi A., Pet J., Whitty J. (2008). Movements and site fidelity of the giant manta ray, manta birostris, in the komodo marine park, Indonesia. Mar. Biol. 155 (2), 121. doi: 10.1007/s00227-008-0988-x
Dicken M. L., Cliff G., Winker H. (2016). Sharks caught in the KwaZulu-natal bather protection programme, south africa. 13. the tiger shark galeocerdo cuvier. Afr. J. Mar. Sci. 38 (3), 285–301. doi: 10.2989/1814232X.2016.1198276
Dicken M. L., Hosking S. G. (2009). Socio-economic aspects of the tiger shark diving industry within the aliwal shoal marine protected area, south Africa. Afr. J. Mar. Sci. 31 (2), 227–232. doi: 10.2989/AJMS.2009.31.2.10.882
Dicken M. L., Smale M. J., Booth A. J. (2006). Spatial and seasonal distribution patterns of the ragged- tooth shark carcharias taurus along the coast of south Africa. Afr. J. Mar. Sci. 28 (3-4), 603–616. doi: 10.2989/18142320609504210
Dicken M. L., Winker H., Smale M. J., Cliff G. (2018). Sharks caught in the KwaZulu-natal bather protection programme, south africa. 14. the smooth hammerhead shark sphyrna zygaena (Linnaeus). Afr. J. Mar. Sci. 40 (2), 157–174. doi: 10.2989/1814232X.2018.1470031
Dudley S. F., Cliff G. (1993). Some effects of shark nets in the natal nearshore environment. Environ. Biol. Fishes 36 (3), 243–255. doi: 10.1007/BF00001720
Dudley S. F., Cliff G. (2010). Influence of the annual sardine run on catches of large sharks in the protective gillnets off KwaZulu-natal, south Africa, and the occurrence of sardine in shark diet. Afr. J. Mar. Sci. 32 (2), 383–397. doi: 10.2989/1814232X.2010.502641
Dudley S. F. J., Haestier R. C., Cox K. R., Murray M. (1998). Shark control: Experimental fishing with baited drumlines. Mar. Freshw. Res. 49 (7), 653–661. doi: 10.1071/MF98026
Dudley S. F., Simpfendorfer C. A. (2006). Population status of 14 shark species caught in the protective gillnets off KwaZulu–natal beaches, south Africa 1978–2003. Mar. Freshw. Res. 57 (2), 225–240. doi: 10.1071/MF05156
Dulvy N. K., Pardo S. A., Simpfendorfer C. A., Carlson J. K. (2014). Diagnosing the dangerous demography of manta rays using life history theory. PeerJ 2, e400. doi: 10.7717/peerj.400
Fennessy S. T., Roberts M. J., Paterson A. W. (2016). A brief overview of the ACEP project: Ecosystem processes in the KwaZulu-natal bight. Afr. J. Mar. Sci. 38 (sup1), S1–S6. doi: 10.2989/1814232X.2016.1141116
Fonseca-Ponce I. A., Zavala-Jiménez A. A., Aburto-Oropeza O., Maldonado-Gasca A., Galván-Magaña F., González-Armas R., et al. (2022). Physical and environmental drivers of oceanic manta ray mobula birostris sightings at an aggregation site in bahía de banderas, Mexico. Mar. Ecol. Prog. Ser. 694, 133–148. doi: 10.3354/meps14106
Graham R. T., Witt M. J., Castellanos D. W., Remolina F., Maxwell S., Godley B. J., et al. (2012). Satellite tracking of manta rays highlights challenges to their conservation. PloS One 7 (5), e36834. doi: 10.1371/journal.pone.0036834
Griffiths C. L., Robinson T. B., Lange L., Mead A. (2010). Marine biodiversity in south Africa: An evaluation of current states of knowledge. PloS One 5, e12008. doi: 10.1371/journal.pone.0012008
Guastella L. A., Roberts M. J. (2016). Dynamics and role of the Durban cyclonic eddy in the KwaZulu- natal bight ecosystem. Afr. J. Mar. Sci. 38 (Supplement), S23–S42. doi: 10.2989/1814232X.2016.1159982
Guyomard D., Perry C., Tournoux P. U., Cliff G., Peddemors V., Jaquemet S. (2019). An innovative fishing gear to enhance the release of non-target species in coastal shark-control programs: The SMART (Shark management alert in real-time) drumline. Fisheries Res. 216, 6–17. doi: 10.1016/j.fishres.2019.03.011
Hearn A. R., Acuna D., Ketchum J. T., Penaherrera C., Green J., Marshall A., et al. (2014). “Elasmobranchs of the Galapagos marine reserve,” in The Galapagos marine reserve (Cham: Springer), 23–59.
Heupel M. R., Carlson J. K., Simpfendorfer C. A. (2007). Shark nursery areas: Concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 337, 287–297. doi: 10.3354/meps337287
Jaine F. R. A., Rohner C. A., Weeks S. J., Couturier L. I. E., Bennett M. B., Townsend K. A., et al. (2014). Movements and habitat use of reef manta rays off eastern Australia: Offshore excursions, deep diving and eddy affinity revealed by satellite telemetry. Mar. Ecol. Prog. Ser. 510, 73–86. doi: 10.3354/meps10910
Kashiwagi T., Marshall A. D., Bennett M. B., Ovenden J. R. (2011). Habitat segregation and mosaic sympatry of the two species of manta ray in the Indian and pacific oceans: Manta alfredi and m. birostris. Mar. Biodiversity Records 4 (3), 1–8. doi: 10.1017/S1755267211000479
Kerwath S. E., Parker D., Winker H., Potts W., Mann B., Wilke C., et al. (2019). Tracking the decline of the world’s largest seabream against policy adjustments. Mar. Ecol. Prog. Ser. 610, 163–173. doi: 10.3354/meps12853
Kock A. A., Lombard A. T., Daly R., Goodall V., Meÿer M., Johnson R., et al. (2022). Sex and size influence the spatiotemporal distribution of white sharks, with implications for interactions with fisheries and spatial management in the southwest Indian ocean. Front. Mar. Sci. 9 (2022). doi: 10.3389/fmars.2022.811985
Lawson J. M., Fordham S. V., O’Malley M. P., Davidson L. N., Walls R. H., Heupel M. R., et al. (2017). Sympathy for the devil: A conservation strategy for devil and manta rays. PeerJ 5, e3027. doi: 10.7717/peerj.3027
Lutjeharms J. R. E., Cooper J., Roberts M. (2000). Upwelling at the inshore edge of the agulhas current. Continental Shelf Res. 20 (7), 737–761. doi: 10.1016/S0278-4343(99)00092-8
Marshall A. D., Bennett M. B. (2010). Reproductive ecology of the reef manta ray manta alfredi in southern Mozambique. J. Fish Biol. 77 (1), 169–190. doi: 10.1111/j.1095-8649.2010.02669.x
Marshall A., Bennett M. B., Kodja G., Hinojosa-Alvarez S., Galvan-Magana F., Harding M., et al. (2018a). Mobula birostris (amended version of 2011 assessment). the IUCN red list of threatened species 2018. International Union for Conservation of Nature doi: 10.2305/IUCN.UK.2018-1.RLTS.T198921A126669349.en
Marshall A. D., Compagno L. J., Bennett M. B. (2009). Redescription of the genus manta with resurrection of manta alfredi (Kreff 1868) (Chondrichthyes; myliobatoidei; mobulidae). Zootaxa 2301, 1–28. doi: 10.11646/zootaxa.2301.1.1
Marshall A. D., Dudgeon C. L., Bennett M. B. (2011). Size and structure of a photographically identified population of manta rays manta alfredi in southern Mozambique. Mar. Biol. 158 (5), 1111–1124. doi: 10.1007/s00227-011-1634-6
Marshall A. D., Flam A. L., Cullain N., Carpenter M., Conradie J., Venables S. K. (2022). Southward range extension and transboundary movements of reef manta rays mobula alfredi along the east African coastline. J. Fish Biol. 1–7. doi: 10.1111/jfb.15290
Marshall A., Kashiwagi T., Bennett M. B., Deakos M., Stevens G., McGregor F., et al. (2018b). Mobula alfredi the IUCN red list of threatened species 2018. International Union for Conservation of Nature
Marshall A. D., Pierce S. J., Bennett M. B. (2008). Morphological measurements of manta rays (Manta birostris) with a description of a foetus from the east coast of southern Africa. Zootaxa 1717 (1), 24–23. doi: 10.11646/zootaxa.1717.1.2
Martin A. K., Flemming B. W. (1988). “Physiography, structure, and geological evolution of the natal continental shelf,” in Coastal ocean studies off natal, south Africa, lecture notes on coastal and estuarine studies, vol. 26 . Ed. Schumann E. H., 11–46.
Molewa B. E. E. (2015). National environmental management: Shark biodiversity management plan. government gazette, republic of south Africa, notice no. 38607 under no. 258 of the biodiversity act, 10 of 2004. Biodiversity Act of 2004 in South Africa. Department of Forest, Fisheries, and the Environment, Republic of South Africa
Molewa B. E. E. (2017). National environmental management: List of marine species that are threatened or protected, restricted activities that are prohibited and exemption from restriction. government gazette, republic of south Africa, notice no. 40875 under no. 476 of the biodiversity act, 10 of 2004. Department of Forest, Fisheries, and the Environment, Republic of South Africa
O’Malley M. P., Townsend K. A., Hilton P., Heinrichs S., Stewart J. D. (2016). Characterization of the trade in manta and devil ray gill plates in China and southeast Asia through trader surveys. Aquat. Conserv. Mar. Freshw. Ecosyst. 27 (2017). doi: 10.3389/fmars.2018.00314
Parker D., Winker H., Attwood C. G., Kerwath S. E. (2016). Dark times for dageraad chrysoblephus cristiceps: Evidence for stock collapse. Afr. J. Mar. Sci. 38 (3), 341–349. doi: 10.2989/1814232X.2016.1200142
Parton K. J., Galloway T. S., Godley B. J. (2019). Global review of shark and ray entanglement in nanthropogenic marine debris. Endangered Species Res. 39, 173–190. doi: 10.3354/esr00964
Peel L. R. (2019). Movement patterns and feeding ecology of the reef manta ray (Mobula alfredi) in Seychelles (University of Western Australia, School of Biological Sciences). PhD thesis.
Putra M. I. H., Setyawan E., Laglbauer B. J., Lewis S., Dharmadi D., Sianipar A., et al. (2020). Predicting mobulid ray distribution in coastal areas of lesser sunda seascape: Implication for spatial and fisheries management. Ocean Coast. Manage. 198, 105328. doi: 10.1016/j.ocecoaman.2020.105328
R Core Team (2021). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Roberts M. J., van der Lingen C. D., Whittle C., Van den Berg M. (2010). Shelf currents, lee-trapped and transient eddies on the inshore boundary of the agulhas current, south Africa: Their relevance to the KwaZulu-natal sardine run. Afr. J. Mar. Sci. 32, 423–447. doi: 10.2989/1814232X.2010.512655
Rohner C. A., Flam A. L., Pierce S. J., Marshall A. D. (2017). Steep declines in sightings of manta rays and devil rays (Mobulidae) in southern Mozambique. PeerJ Preprints 3051 (1), 1–32. doi: 10.7287/peerj.preprints.3051v1
Rohner C. A., Pierce S. J., Marshall A. D., Weeks S. J., Bennett M. B., Richardson A. J. (2013). Trends in sightings and environmental influences on a coastal aggregation of manta rays and whale sharks. Mar. Ecol. Prog. Ser. 482, 153–168. doi: 10.3354/meps10290
Schleyer M. H. (1985). Chaetognaths as indicators of water masses in the agulhas current system. Oceanogr Res. Institute Durban Investigational Rep. 61, 1–20.
Sims D. W., Southall E. J., Tarling G. A., Metcalfe J. D. (2005). Habitat-specific normal and reverse diel vertical migration in the plankton-feeding basking shark. J. Anim. Ecol. 74 (4), 755–761. doi: 10.1111/j.1365-2656.2005.00971.x
Sims D. W., Witt M. J., Richardson A. J., Southall E. J., Metcalfe J. D. (2006). Encounter success of free-ranging marine predator movements across a dynamic prey landscape. Proc. R. Soc. B:Biological Sci. 273 (1591), 1195–1201. doi: 10.1098/rspb.2005.3444
Sink K. J., Branch G. M., Harris J. M. (2005). Biogeographic patterns in rocky intertidal communities in KwaZulu-natal, south Africa. Afr. J. Mar. Sci. 27 (1), 81–96. doi: 10.2989/18142320509504070
Sink K. J., Harris L. R., Skowno A. L., Livingstone T., Franken M., Porter S., et al. (2019). “Chapter 3: Marine ecosystem classification and mapping,” in South African national biodiversity assessment 2018 technical report volume 4: Marine realm. Eds. Sink K. J., van der Bank M. G., Majiedt P. A., Harris L. R., Atkinson L. J., Kirkman S. P., Karenyi N. (South Africa: South African National Biodiversity Institute, Pretoria).
Stevens G. M. W. (2016). Conservation and population ecology of manta rays in the Maldives (University of York, School of Environment). PhD thesis.
Stevens G. M., Hawkins J. P., Roberts C. M. (2018). Courtship and mating behaviour of manta rays mobula alfredi and m. birostris in the Maldives. J. Fish Biol. 93 (2), 344–359. doi: 10.1111/jfb.13768
Stewart J. D., Hoyos-Padilla E. M., Kumli K. R., Rubin R. D. (2016). Deep-water feeding and behavioural plasticity in manta birostris revealed by archival tags and submersible observations. Zoology 119 (5), 406–413. doi: 10.1016/j.zool.2016.05.010
Stewart J. D., Jaine F. R., Armstrong A. J., Armstrong A. O., Bennett M. B., Burgess K. B., et al. (2018). Research priorities to support effective manta and devil ray conservation. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00314
Stewart J. D., Rohner C. A., Araujo G., Avila J., Fernando D., Forsberg K., et al. (2017). Trophic overlap in mobulid rays: Insights from stable isotope analysis. Mar. Ecol. Prog. Ser. 580, 131–151. doi: 10.3354/meps12304
Stewart J. D., Smith T. T., Marshall G., Abernathy K., Fonseca-Ponce I. A., Froman N., et al. (2019). Novel applications of animal-borne crittercams reveal thermocline feeding in two species of manta ray. Mar. Ecol. Prog. Ser. 632, 145–158. doi: 10.3354/meps13148
Temple A. J., Kiszka J. J., Stead S. M., Wambiji N., Brito A., Poonian C. N., et al. (2018). Marine megafauna interactions with small-scale fisheries in the southwestern Indian ocean: A review of status and challenges for research and management. Rev. Fish Biol. Fisheries 28 (1), 89–115. doi: 10.1007/s11160-017-9494-x
Thieurmel B., Elmarhraoui A. (2019) Suncalc: Compute sun position, sunlight phases, moon position and lunar phase. r package version 0.5.0. Available at: https://CRAN.R-project.org/package=suncalc.
Tibiriçá Y., Birtles A., Valentine P., Miller D. K. (2011). Diving tourism in Mozambique: an opportunity at risk? Tourism Mar. Environments 7 (3-4), 141–151. doi: 10.3727/154427311X13195453162732
Venables S. K. (2020). Ecology and conservation of a threatened reef manta ray (Mobula alfredi) population in southern Mozambique (University of Western Australia, School of Biological Sciences). PhD thesis.
Venables S. K., Marshall A. D., Armstrong A. J., Tomkins J. L., Kennington W. J. (2021). Genome-wide SNPs detect no evidence of genetic population structure for reef manta rays (Mobula alfredi) in southernMozambique. Heredity 126 (2), 308–319. doi: 10.1038/s41437-020-00373-x
Venables S. K., van Duinkerken D. I., Rohner C. A., Marshall A. D. (2020). Habitat use and movement patterns of reef manta rays mobula alfredi in southern Mozambique. Mar. Ecol. Prog. Ser. 634, 99–114. doi: 10.3354/meps13178
Walbaum J. J. (1792). “Quibus systema totum ichthyologiae proponitur cum classibus, ordinibus, generum characteribus, specierum differentiis, observationibus plurimis,” in Griefswald: Petri artedi sueci genera piscium (Germany: Grypeswaldiae), 535.
Walker N. D. (1990). Links between south African summer rainfall and temperature variability of the agulhas and benguela current systems. J. Geophys Res. 95, 3297–3319. doi: 10.1029/JC095iC03p03297
Wetherbee B. M. (2004). “Food consumption and feeding habits,” in Biology of sharks and their relatives. Eds. Carrier J. C., Musick J. A., Heithaus M. R. (Boca Raton: CRC Press), 225–246.
White W. T., Corrigan S., Yang L., Henderson A. C., Bazinet A. L., Swofford D. L., et al. (2017). Phylogeny of the manta and devil rays (Chondrichthyes: Mobulidae), with an updated taxonomic arrangement for the family. Zoological J. Linn. Soc. 182 (1), 50–75. doi: 10.1093/zoolinnean/zlx018
Keywords: mobulidae, generalized additive models, fisheries, Southern Africa, marine conservation, probability of encounter, catch analysis
Citation: Carpenter M, Parker D, Dicken ML and Griffiths CL (2023) Multi-decade catches of manta rays (Mobula alfredi, M. birostris) from South Africa reveal significant decline. Front. Mar. Sci. 10:1128819. doi: 10.3389/fmars.2023.1128819
Received: 21 December 2022; Accepted: 13 January 2023;
Published: 29 March 2023.
Edited by:
Elizabeth Grace Tunka Bengil, University of Kyrenia, CyprusReviewed by:
Natascha Wosnick, Federal University of Paraná, BrazilCopyright © 2023 Carpenter, Parker, Dicken and Griffiths. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle Carpenter, Y3JwbWljMDAxQG15dWN0LmFjLnph
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.