
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 03 May 2023
Sec. Marine Pollution
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.1124952
This article is part of the Research TopicModel Organisms in Marine PollutionView all 4 articles
Long-term programmes like the UK Clean Seas Environmental Monitoring Programme (CSEMP) rely on biological effects techniques, including biomarkers, to assess if chemical pollutants are affecting sentinel species in the aquatic environment. In this study, we have applied mixed and fixed effect linear models to the long-term CSEMP dataset (2005-2018) to evaluate if factors such as region (location), sex, age, gonadosomatic index (GSI) and condition factor (CF), contribute to the variability observed in the levels of 3 well established biomarkers (inhibition of acetylcholinesterase in muscle-AChE, induction of hepatic 7-ethoxyresorufin-O-deethylase-EROD and presence of biliary 1-hydroxypyrene-1-OH pyrene) used to monitor biological effects of contaminants in dab (Limanda limanda) around UK waters. Regional differences (location) were a significant explanatory variable for the 3 biomarkers. Substantial differences were found in regional average muscle AChE activity, overall indicating the East Coast regions as likely more impacted by acetylcholinesterase inhibitors (e.g. organophosphates and carbamates). We report for the first time that, while accounting for region, sex and gonadosomatic index are significant predictors of muscle acetylcholinesterase isoform in dab (R2 = 0.13-0.15), and therefore advise the future analysis of this marker should be done by sex when reporting for marine environmental purposes. Dab condition factor is also a significant predictor for both enzymatic markers (AChE and EROD) but not for 1-OH pyrene. Sex and age, and their interaction, were the strongest variables (R2 = 0.46) influencing hepatic EROD, showing that although overall females had higher hepatic EROD, male dab hepatic EROD increased with age. Correlations between the three biomarkers might assist in discerning types of prevalent regional contamination over the studied period. Our results highlight the factors to account for when assessing if environmental contamination is contributing to the responses of these 3 biomarkers in dab, and other flatfish species, used in marine environmental monitoring programmes.
Monitoring and regulation of chemical pollutants in the marine environment is achieved by several national and international regulatory agreements such as the EU Marine Strategy Framework Directive (MSFD) and the Co-ordinated Environmental Monitoring Programme (CEMP) and Joint Assessment and Monitoring Programme (JAMP) of the Oslo and Paris convention for the Protection of the North East Atlantic (OSPAR) (European Comission, 2020; OSPAR Comission, 2020). These are the drivers for the UK Clean Seas Environmental Monitoring Programme (CSEMP) which has been collecting data on contaminant levels and assessing their effects in biota at offshore and coastal designated stations around the UK waters for over 30 years. Common flatfish species such as dab (Limanda limanda) and the European flounder (Platichthys flesus) are used as sentinel species, due to their wide distribution and abundance throughout the North Sea (Askem et al., 2018; Sleiderink et al., 1995b).
Conventionally the chemical analysis of sediment and water for specific contaminants allowed to infer potential biological effects in biota such as survival, growth and reproduction, however, this does not allow to detect and assess effects of low concentration pollution, nor complex mixtures of contaminants. This has led to the development of biomarkers, a wide range of sub-lethal indicators of exposure to, and effects of, contaminants and other environmental stressors (Livingston et al., 1994), and these markers can be specific (monitoring for the presence/effect of specific chemical groups) or general.
Biomarker assays are commonly used in marine monitoring programmes to assess biological effects of contaminants in fish. These include induction of hepatic 7- ethoxyresorufin-O-deethylase (EROD) activity, indicative of the CYP1A enzyme pathway activation through the aryl hydrocarbon receptor (AhR) in the fish liver, after exposure to AhR agonists such as polycyclic aromatic hydrocarbons (PAHs), planar polychlorinated biphenyls (PCBs) and dioxins. The presence of 1-hydroxypyrene (1-OH pyrene), a polycyclic aromatic hydrocarbon (PAH) metabolite found in the bile following a recent exposure to PAHs; and the inhibition of acetylcholinesterase (AChE) enzyme, measured in the muscle, indicating exposure to neurotoxins such as organophosphorus and other carbamate insecticides (Bocquene & Galgani, 1998; Ariese et al., 2005; Davies and Vethaak, 2012; Stagg et al., 2016). In the UK, monitoring data for each biomarker, and other contaminant indicators, are analyzed to deliver the UK trend and status assessments for each indicator, on a regional basis (https://moat.cefas.co.uk/pressures-from-human-activities/contaminants/) however there is currently no UK assessment for the inhibition of acetylcholinesterase (AChE). Spatial and temporal analysis of the risks posed by some of the chemical indicators, as well as for the three referred biomarkers, around the UK waters, have also been published (Nicolaus et al., 2015; Nicolaus et al., 2016; Askem et al., 2018).
Well established biomarkers, such as hepatic EROD and biliary 1-OH pyrene have been shown to allow discriminating between distinctly impacted sampling sites, however factors such as season (sexual maturity), gender, nutritional status (condition factor) and age can affect these parameters in fish species used in monitoring programmes (Sleiderink et al., 1995b; Kammann et al., 2005; Kammann, 2007; Dévier et al., 2013; Vethaak et al., 2016). There is however less work reported for other established biomarkers, like acetylcholinesterase inhibition for flatfish species (Kirby et al., 2000).
To assure robust data is generated the consistency of the CSEMP sampling programme is determined by the Green Book, including the preferred time of the year for sampling (season) to account for sentinel species breeding cycle. Furthermore, supporting metrics are also obtained for the sampled fish, such as morphometrics, age, sex and gonadosomatic index (gonad size reported as a percentage of the somatic body weight) (Davies and Vethaak, 2012; MARG, 2020). This large biomarker and supporting metrics dataset for dab, available through the UK monitoring programme, presented an opportunity to assess the interaction between the factors mentioned above and the three biomarkers commonly used in monitoring progammes. For this purpose, the analysis was envisaged making use of the entire data set available for these variables, but excluding the temporal component, and therefore not accounting for year variation.
The aim of this study was to make use of the CSEMP long-term dataset to evaluate and identify if and which factors affect the levels of 3 well established biomarkers (AChE, EROD and 1-OH pyrene) used to monitor biological effects of contaminants in dab in the UK. By using a larger dataset, including 14 years of data, we aimed to evaluate if/how region, age, sex, condition factor and gonadosomatic index are influencing the level of the 3 biomarkers in this indicator species. Furthermore, we aimed to assess for correlations between the three markers. This study will help to identify and advise on improvements to monitoring programmes using similar markers in flatfish species and assist in the interpretation of the outputs for these biomarkers in dab used for marine environmental monitoring.
Data relating to the sentinel species Limanda (Dab) from the Clean Seas Environmental Monitoring Programme (CSEMP) was obtained from the Marine Environment Monitoring and Assessment National database (MERMAN). These data are a snapshot of the data held within MERMAN on the 11th of May 2020. Data was provided by the British Oceanographic Data Centre on behalf of the Clean Safe Seas Evidence Group. Data were collected by the Centre for Environment, Fisheries and Aquaculture Science (CEFAS) and contain public sector information licensed under the Open Government Licence v3.0. The data were funded by the Department for Environment, Food and Rural Affairs. Data refers to dab collected from the sampling stations within eight sub-regions, nested within the eight larger UK Biogeographic Regions (adopted by MERMAN) as shown in Figure 1. The sampling stations are primarily considered to be open sea; however a few coastal stations are included (BDOC, 2016). The CSEMP sampled all sites annually from 1999-2011 and, beginning in 2011, Eastern and Western regions were sampled every other year on a rotating basis (biennial sampling cycle).
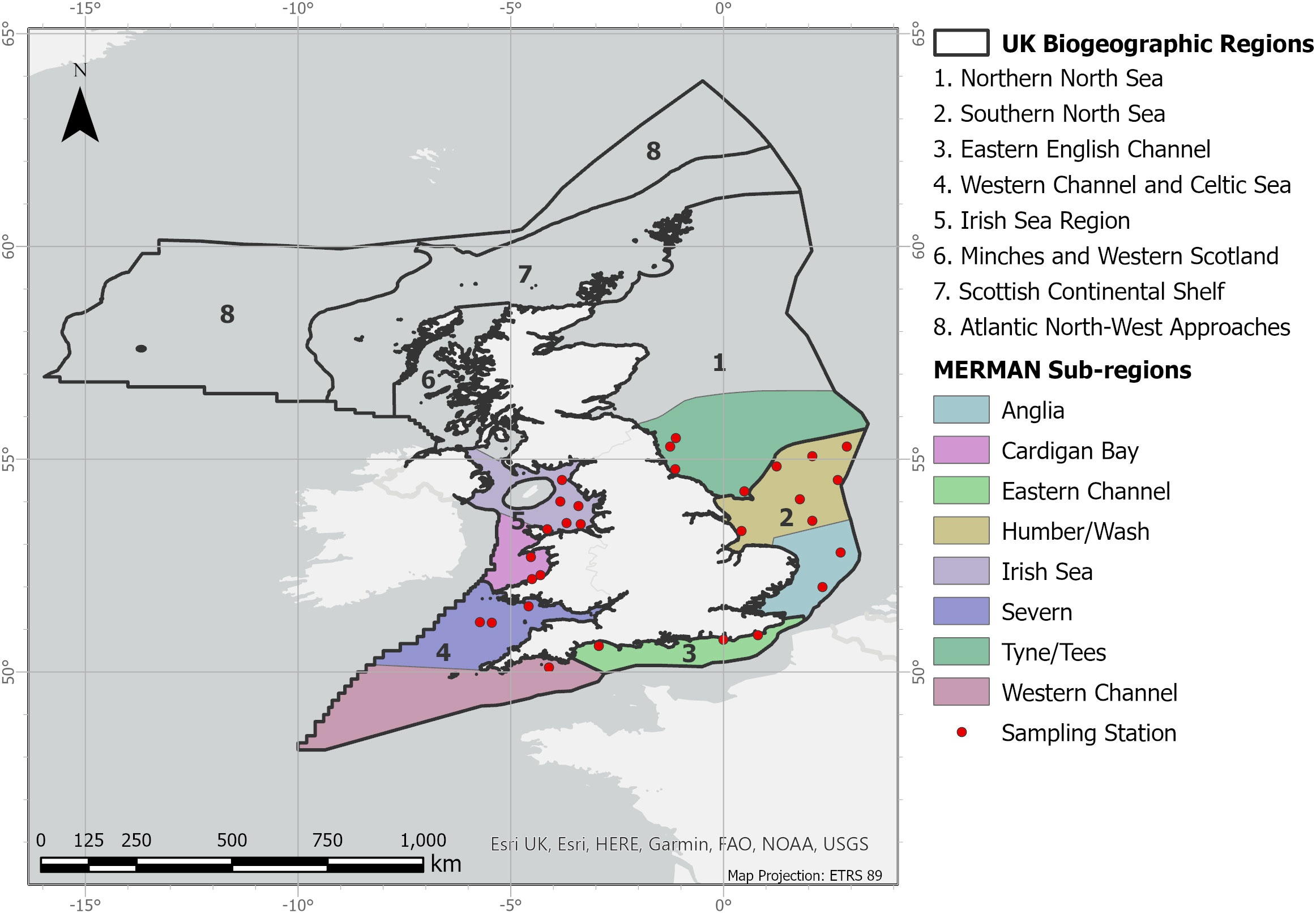
Figure 1 Map of the UK biogeographic regions, depicting the sub-regions adopted by MERMAN and the location of the sampling stations used in the CSEMP programme (for more detail please consult: https://www.bodc.ac.uk/projects/data_management/uk/merman/project_specific/documents/mermanuserguide.pdf).
Fish were collected for sampling by beam trawling on research vessels, between around June 12th-July 15th, 1999-2018. Fish selection and tissue sampling followed OSPAR Convention for the protection of the North East Atlantic guidelines (OSPAR Comission, 2013) and fishes were assessed for three biomarker assays for biological effects of contaminants, namely, acetylcholinesterase activity (AChE), 7-ethoxyresorufin-O-deethylase (EROD) activity and bile 1-hydroxypyrene equivalent (1-OH pyrene). Detailed information about sample selection, tissue sampling and processing, standardized biomarker analysis and Quality Control and Quality Assurance procedures followed within the CSEMP programme can be found in the CSEMP Green Book (MARG, 2020) and Askem et al. (2018). It should be noted that the CSEMP programme is currently using a modified AChE method. The ICES adopted method measures the combined activity of the two inducible cholinesterase forms, acetylcholinesterase and butyrylcholinesterase (BChE), with AChE having the highest affinity to the neurotransmitter acetylcholine (Bocquene and Galgani, 1998). In dab the highest AChE activity has been shown in the muscle (after the brain), and BChE is also present in this tissue (Galgani et al., 1992). The modified method used for the CSEMP programme includes inhibition of BChE, therefore only measures activity of the AChE form in dab muscle (Askem et al., 2018).
R software version 3.6.2 was used for all data preparation and statistical analysis (R Core Team, 2019). Data were presented as eight files, one for each MERMAN sub-region and each region was handled separately but following the same parameters. The species Limanda, dab, was filtered, as were the chosen biomarkers (EROD, AChE, and 1-OH pyrene) relevant biological data (age, sex, length, weight, and gonadosomatic index-GSI) and abiotic information (location and year). These parameters restricted the data to a 14-year time frame (2005-2018), as not all biomarkers were tested every year. In addition, data relating to the AChE assay was only available from 2014-2016 and 2018. Parameters of 15 to 30 cm were chosen for length and 30 to 350 grams were chosen for weight, to eliminate extreme high or low values occasionally found in the data set; these were chosen in agreement with the recommendations from the CSEMP Green book (MARG, 2020). Fulton’s K condition factor (CF) was calculated when data were available, (De Raedemaecker et al., 2012). Weight, length, (components of CF), age, and GSI were not represented in the data for all fish sampled, which restricted sample size, therefore within each biomarker, separate data subsets were created for age, GSI, and CF. Regional data sets were then combined to form a master data set for each biomarker.
Normal logarithmic transformation of EROD, AChE, and 1-OH pyrene values were conducted to approximate the datasets to normal distribution (Flammarion and Garric, 1999). The Bartlett test of homogeneity of variance was used to check assumptions of equal variance between samples for all independent variables (region, sex, age, GSI and CF). ANOVA was applied to compare means for all independent variables, and Post-Hoc Tukey multiple pairwise-comparisons tests were used to examine differences between groups in categorical variables (sex and region). Extreme GSI and CF values were observed in the Cardigan Bay and the Anglia regions. After testing their influence, they were removed. Linear mixed models were run for EROD and 1-OH pyrene starting with sub-region (hereafter named as region) and controlling for year (random factor). To examine the effect of region, sex, age, GSI and CF, on the different biomarkers, we had to use separate models due to limited data availability for some of these variables (MERMAN database). Hence, to examine the effects of all predictor (independent) variables, while not losing sample size, separate models were run with different sub-sets of the data. Within each model run, sex, age, GSI, and CF were added stepwise, in that order, to find the best fit, with the Akaike’s Information Criterion (AIC) used to determine the best fit model. This approach was used to take advantage of the full data set.
The same procedure was applied to AChE however the year was not included in the model runs since the data for Eastern and Western regions was obtained in alternate years. Mixed effect linear regression (LMM) using the lme4 package was applied to multivariate models when data allowed (EROD and 1-OH pyrene). If two or fewer years of data were available the lm() function was applied rather than lmer() (AChE) (LM). When using the lme4 package restricted maximum likelihood estimation (REML) was set to FALSE for a standard maximum likelihood estimation (Bates et al., 2014). A significance level of 0.05 and 95% confidence intervals using the confint() function were used for all models. Interactions were examined between GSI and sex and CF and sex when data were available. Graphical visualization of significant models was conducted through ggplot regression modeling. Several data points graphed in the significant CF models showed potential as possible outliers. Rosner’s Test for Outliers was conducted and 22 data points were identified as outliers in the effect of CF on EROD data set and 7 points in the effect of CF on AChE data set. Potential outliers were removed and models re-run to determine best fit. Pearson’s Correlation was also examined, between AChE, EROD and 1-OH pyrene per region.
For the AChE data the linear model indicated region as a significant explanatory variable, with all regions except Severn (p=0.328), showing significantly different lower levels than the reference region, Cardigan Bay (p<0.045 for Irish Sea and p<0.001 for the remaining regions); the best model for our data pool also indicated sex as significant variable (p<0.001, R2 = 0.13, n= 837) (Table 1). As shown in Figure 2, the average values of AChE in dab muscle varied amongst the 7 regions, with dab from the west side regions, namely Irish Sea, Cardigan Bay, Severn and Western Channel, showing higher mean values. The lowest values, therefore indicating more pronounced inhibition of this marker, were from dab collected in the Eastern channel stations, Tyne and Tees and Humber Wash. No AChE data was available for the Anglian region. Overall regional average AChE values were higher in males then females (p<0.001).
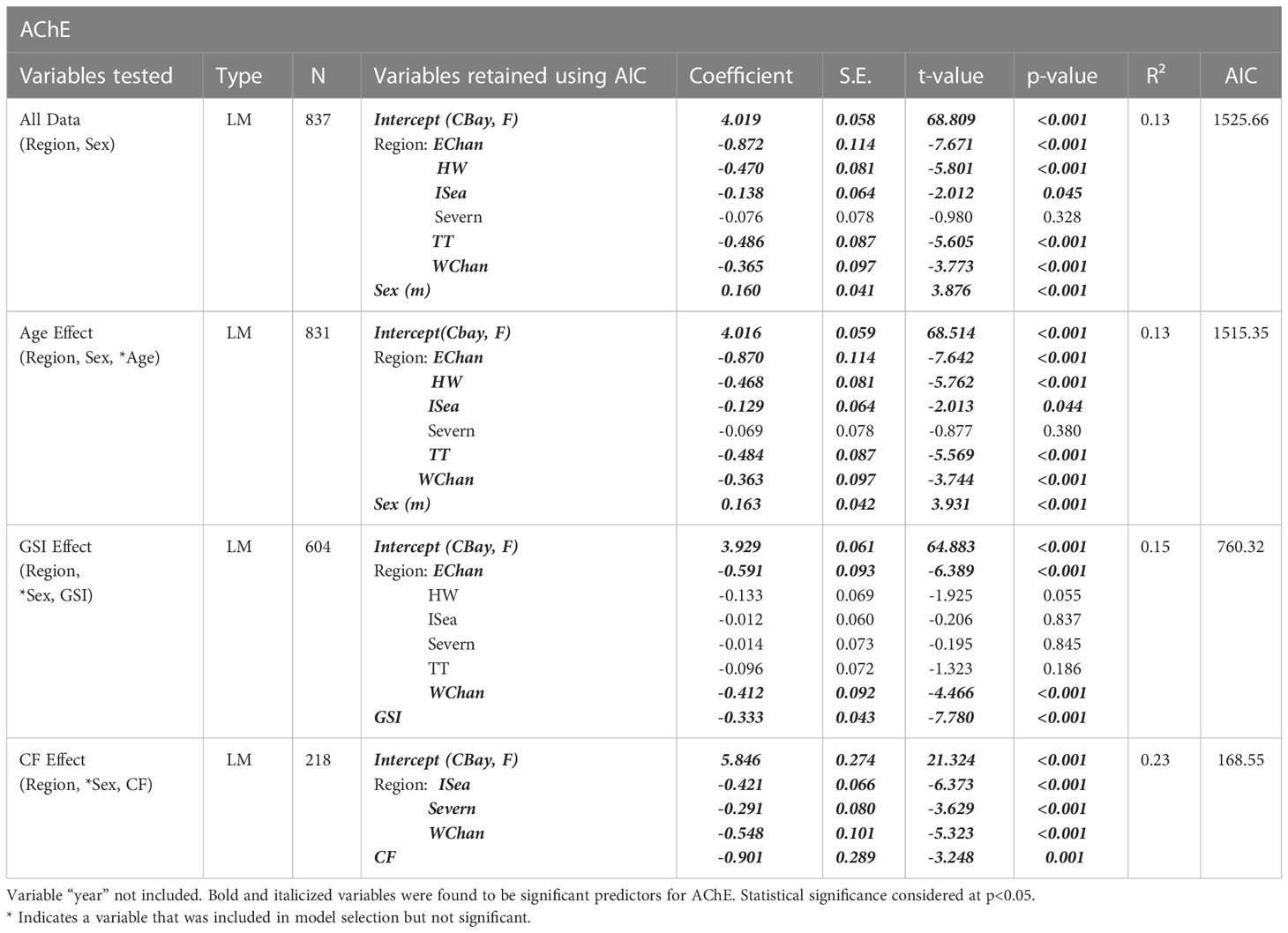
Table 1 Models explaining variability for the acetylcholinesterase (AChE) biomarker. Model sample size varies according to data availability for different variables.
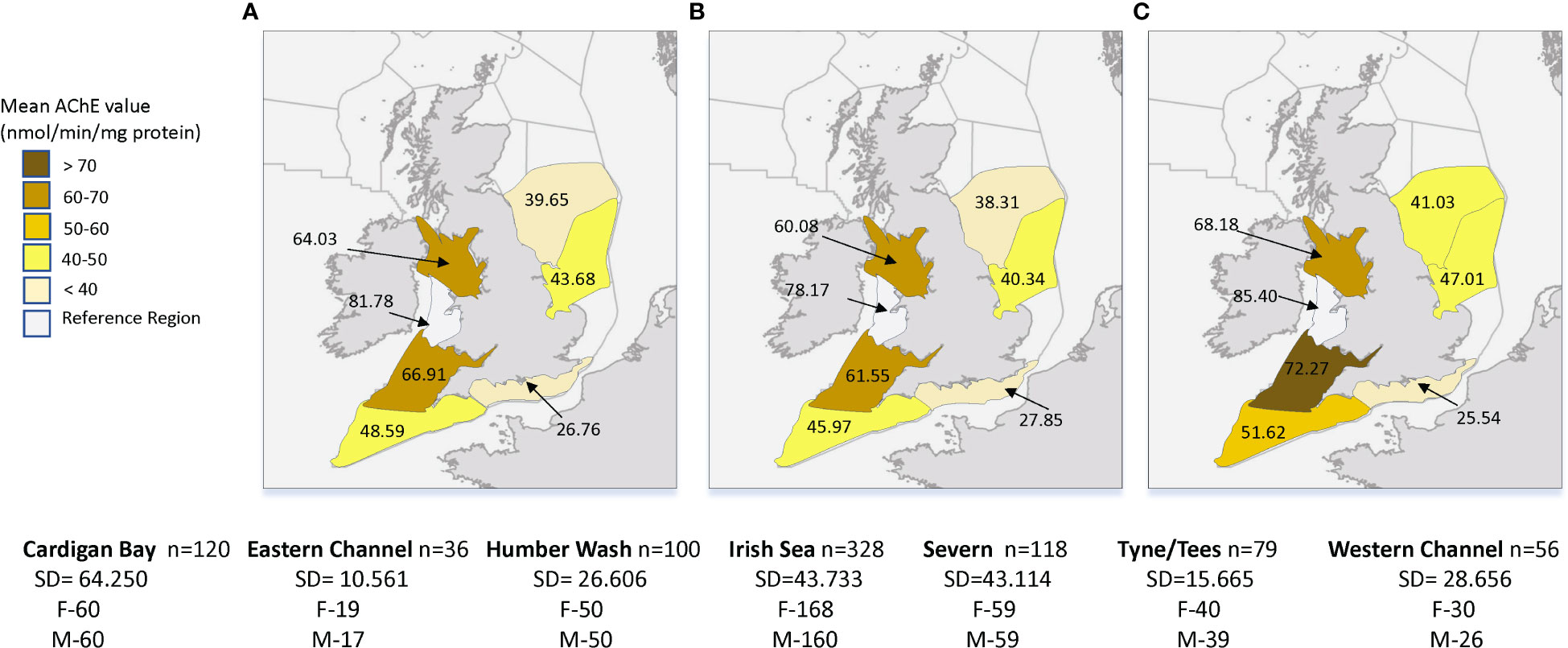
Figure 2 Average values of muscle acetylcholinesterase (AChE) in dab (Limanda limanda), per region (2014-2018) with (A) mean value for all fish, (B) mean value for females and (C) mean value for males. n-sample size per region, SD-standard deviation, F-number of females and M-number of males.
Age did not significantly account to explain AChE variability when added to the model, however region and sex remained significant predictors (p<0.001; R2 = 13, n=831). Gonadosomatic index (GSI) was also a significant predictor of muscle AChE, giving a better model fit then sex (p<0.001; R2 = 15, n=604) (Figure 3). Interactions between sex and GSI were tested but did not improve the model. Condition factor (CF) was also an important predictor for AChE, showing that dab with lower CF scores had higher AChE levels (p=0.001, R2 = 0.23, n=218) (Table 1 and Figure 4), however cautious interpretation is recommended due to the sample size reduction. Interaction between CF and Age and CF and Sex were also tested but did not improve the model.

Figure 3 Effect of gonadosomatic index (GIS) on acetylcholinesterase (AChE) values per sex in dab (Limanda limanda).
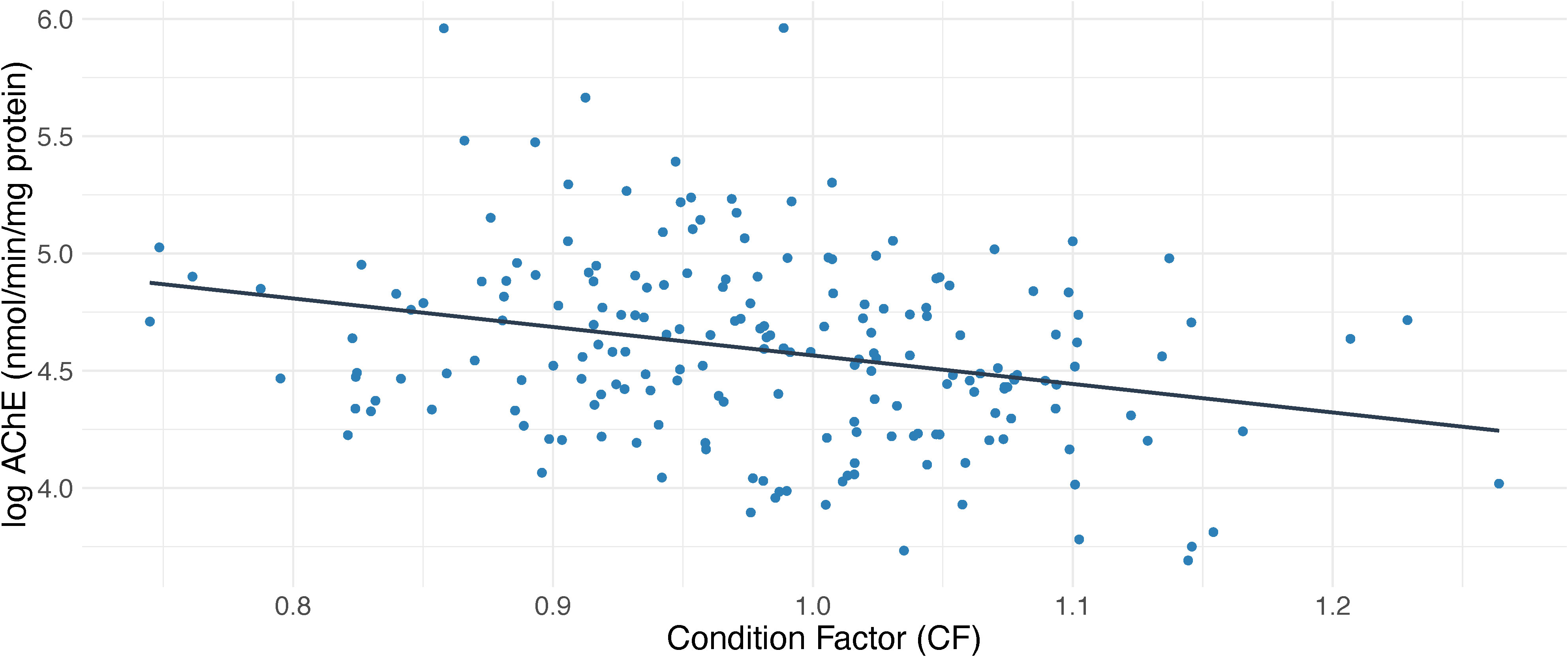
Figure 4 Effect of condition factor (CF) on acetylcholinesterase (AChE) values in dab (Limanda limanda).
Location (region) was an important predictor of hepatic EROD activity in dab for all mixed models (Table 2), with all regions showing significant differences, when compared to the reference region (Anglia), for the period of 2005 to 2018 (p<0.001 to p<0.05, R2 = 0.31, n=4298). The mean regional EROD values for dab for this period were highest at Tyne and Tees region, followed by Humber Wash and Irish Sea (Figure 5), therefore indicating the most pronounced induction of this marker in dab collected from these stations. To note that for both males and females from those regions the mean EROD values were above the background assessment criteria (BAC) (male BAC = 147 pmol/min/mg protein and female BAC = 178 pmol/min/mg protein) (Davies and Vethaak, 2012). The lowest values were from dab collected in the Western channel and Anglian region stations.
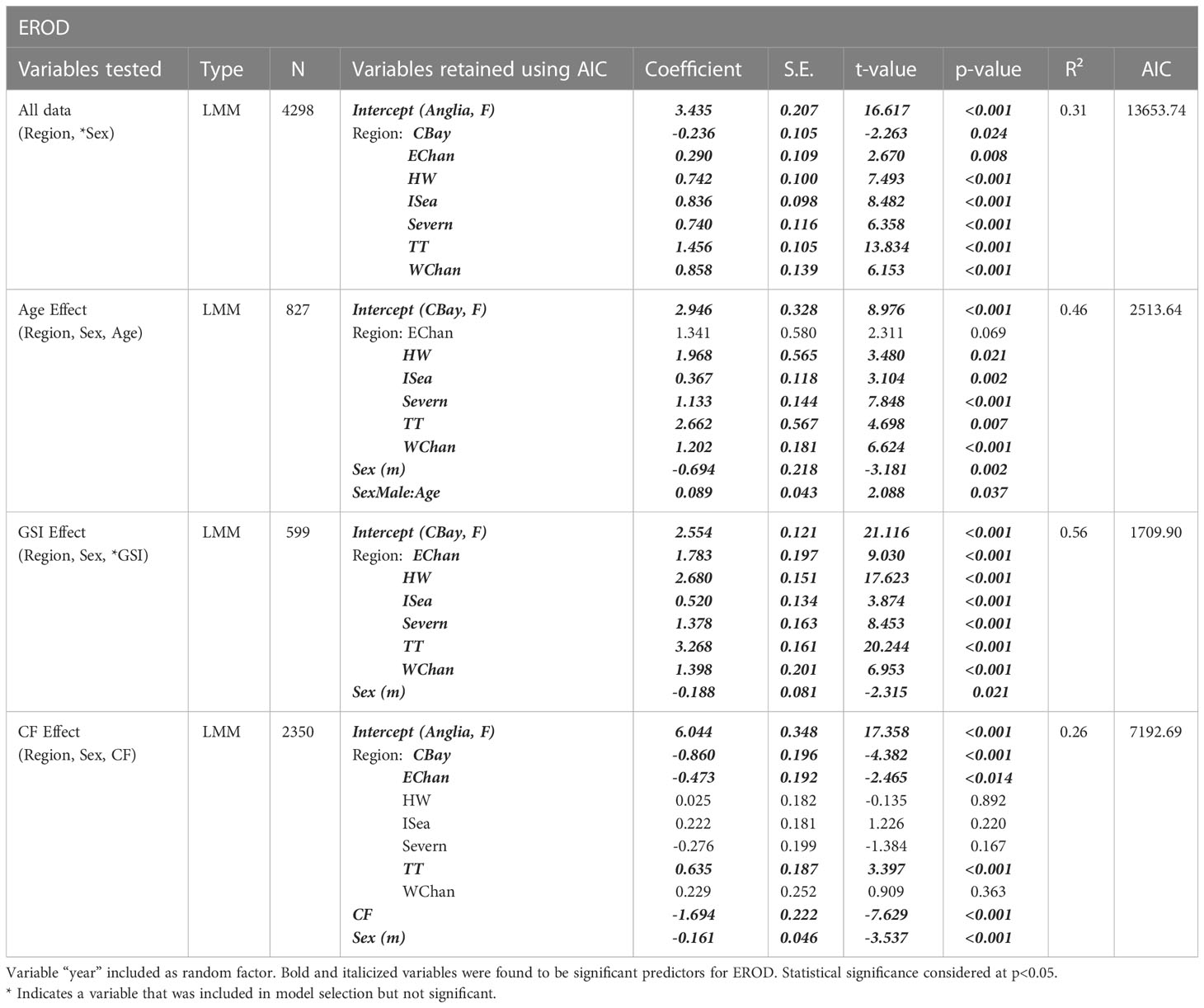
Table 2 Models explaining variability for the 7- ethoxyresorufin-O-deethylase (EROD) biomarker. Model sample size varies according to data availability for different variables.
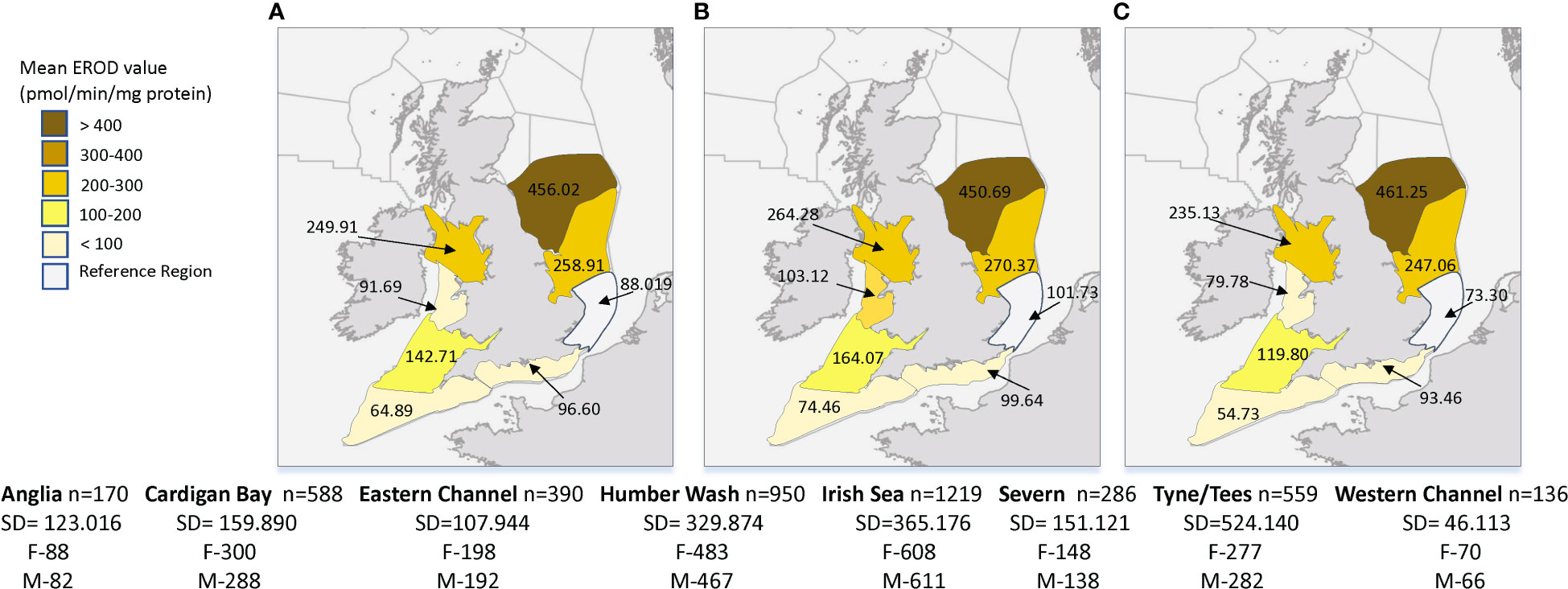
Figure 5 Average values of hepatic 7-ethoxyresorufin-O-deethylase (EROD) in dab (Limanda limanda), per region (2005-2018) with (A) mean value for all fish, (B) mean value for females and (C) mean value for males. n-sample size per region, SD-standard deviation, F-number of females and M-number of males.
For the subset of data where age, region and sex were tested there was a significant effect of age and an interaction effect was observed between age and sex with older fish having significantly higher EROD values than the reference age of 2 years (p=0.04, R2 = 0.46, n=827) (Table 2; Figure 6). Including age, however, also resulted in a reduction of sample size. Sex was also significant in this model with males having lower EROD values than females (p=0.002, R2 = 0.46, n=827) (Table 2). An interaction effect was also observed between age and sex indicating that male dab, aged above 6, had higher EROD levels than females (Table 2; Figure 6). CF was significant with values decreasing as EROD values increased (p<0.001, R2 = 0.26, n=2350) (Table 2; Figure 7). Interactions with sex were also examined but did not improve the model. GSI and interactions between GSI and age were also examined but were not significant (Table 2).
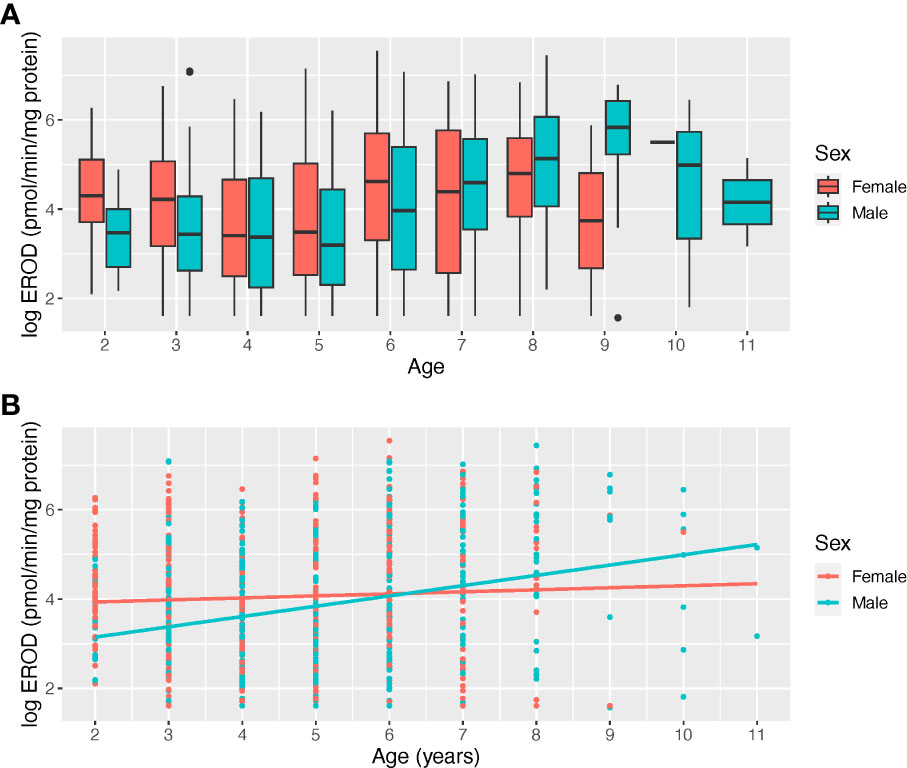
Figure 6 Boxplot of 7-ethoxyresorufin-O-deethylase (EROD) by age and sex (A) and interaction between the age and sex effect (B) in EROD in dab (Limanda limanda).
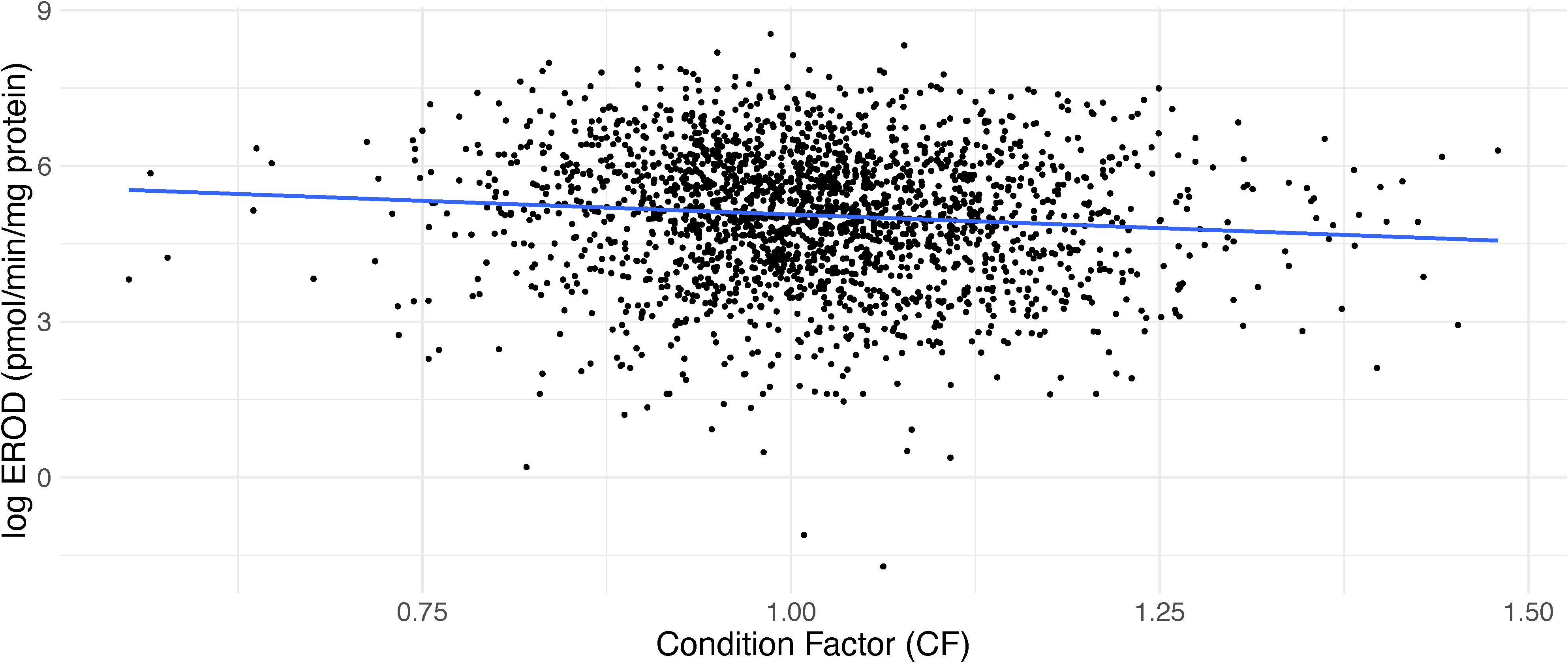
Figure 7 Effect of condition factor (CF) on 7-ethoxyresorufin-O-deethylase (EROD) values in dab (Limanda limanda).
Region was the only significant predictor of 1 OH-pyrene levels in dab bile, for all mixed models with higher average levels in all regions when compared to the reference region, Anglia (p<0.001, R2 = 0.39, n=2511) (Table 3; Figure 8). The BAC for 1 OH-pyrene in dab bile is 150 ng/ml, therefore the mean regional levels for the period of 2005-2018 were above the BAC for all regions except for Humber Wash and Anglian. They were all however below the Environmental Assessment Criteria for this marker (EAC = 22,000 ng/ml). The effect of sex, age, GSI and CF were all examined but were not significant (Table 3).
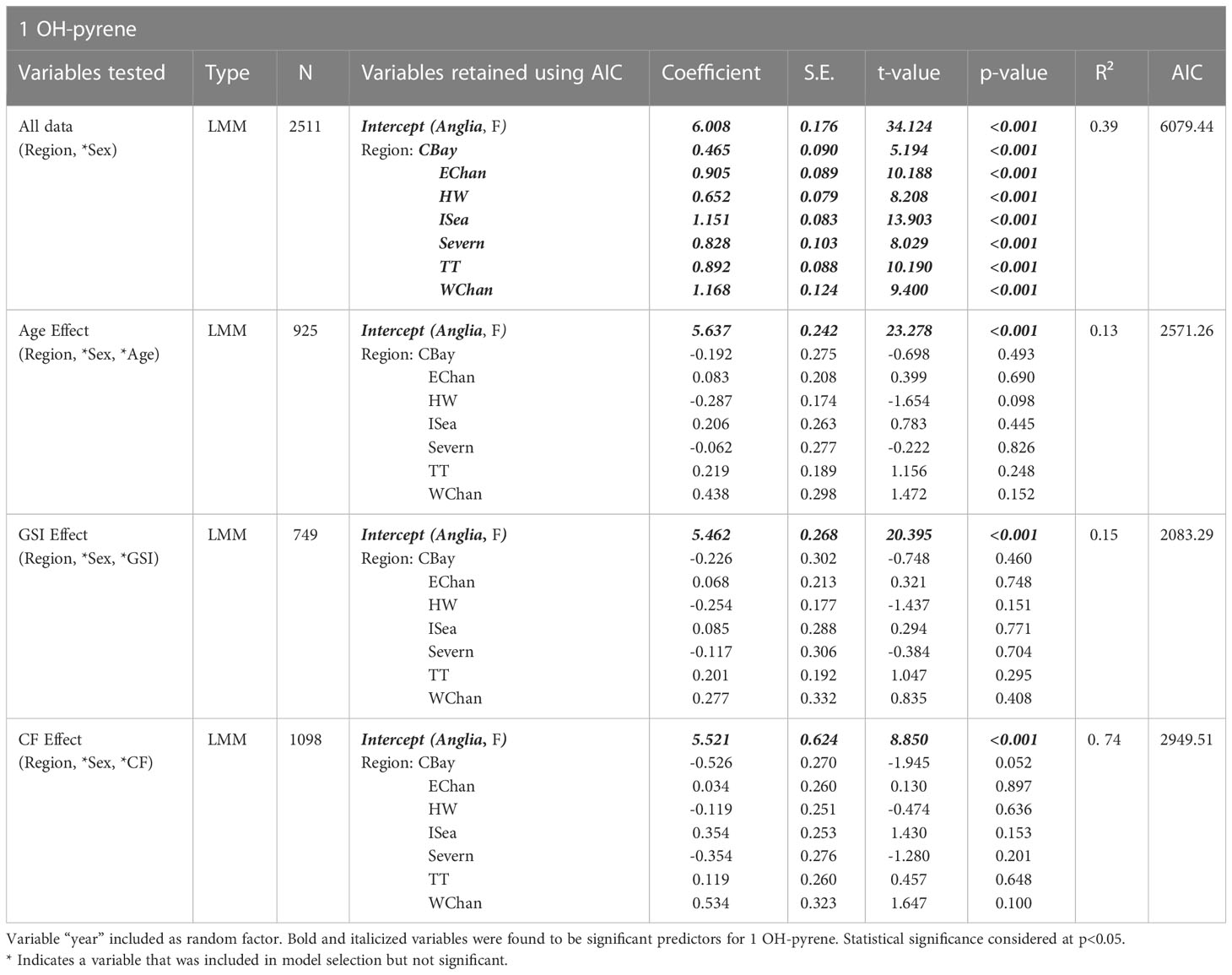
Table 3 Models explaining variability for the 1 OH-pyrene biomarker. Model sample size varies according to data availability for different variables.
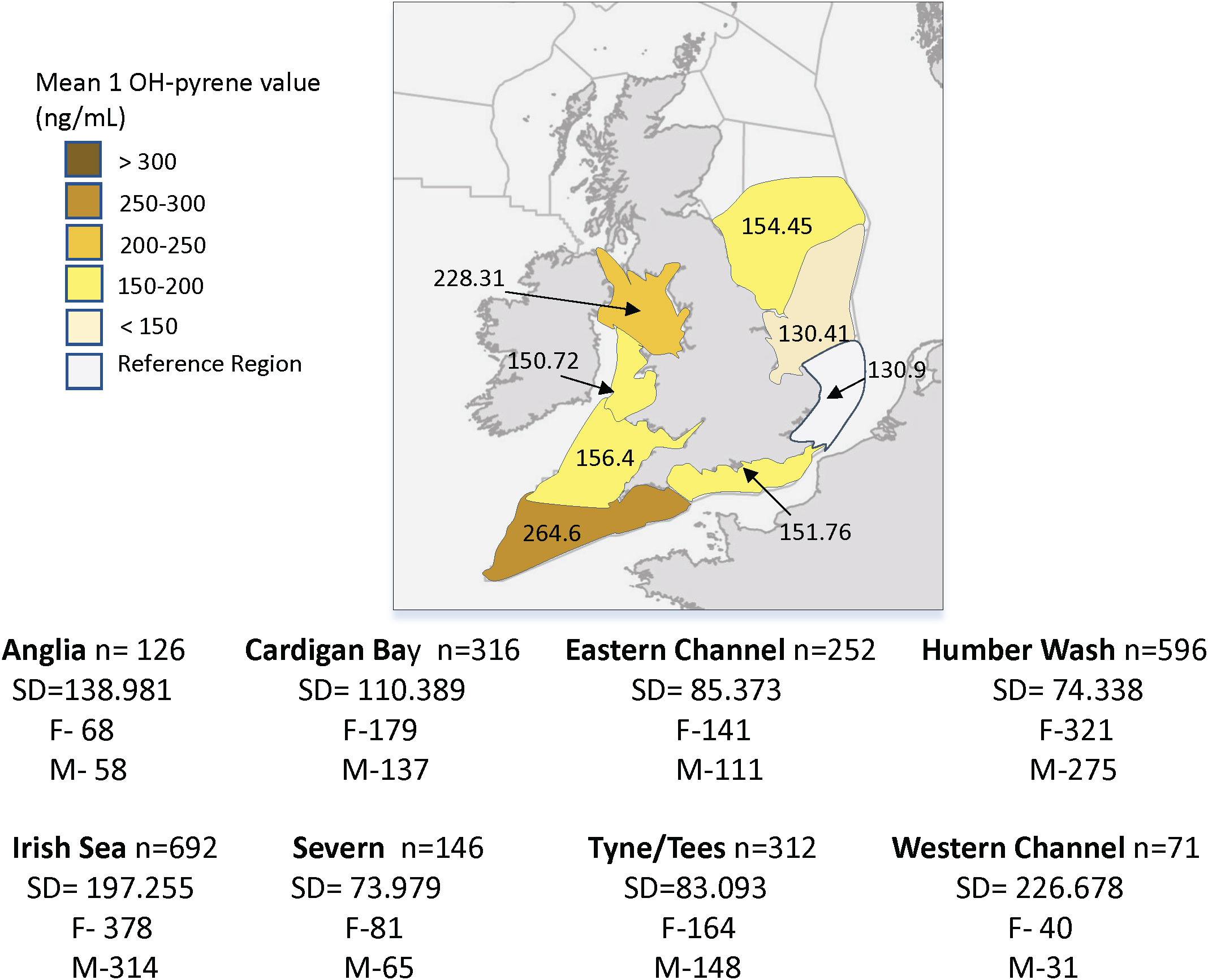
Figure 8 Average values of bile 1-hydroxypyrene equivalent (1-OH pyrene) in dab (Limanda limanda), per region (2005-2018). n, sample size per region; SD, standard deviation; F, number of females and M, number of males.
Correlations between the three biomarkers were assessed, on a regional basis. As shown in Figure 9 significant moderate positive correlations between hepatic EROD and AChE in dab muscle were found in 4 out of 7 regions studied (Anglia was not included due to the lack of AChE data); namely the Irish Sea, Cardigan Bay and Severn (West Coast regions), and also for the Humber region on the East coast. When assessing hepatic EROD against biliary 1 OH-pyrene, significant moderate positive correlations emerged in the Humber and Anglia region, on the East coast, and a negative weak correlation was found for the Severn region (Figure 10). For muscle AChE and biliary 1 OH-pyrene, a significant moderate negative correlation was found for Cardigan Bay, West Coast (Figure 11).
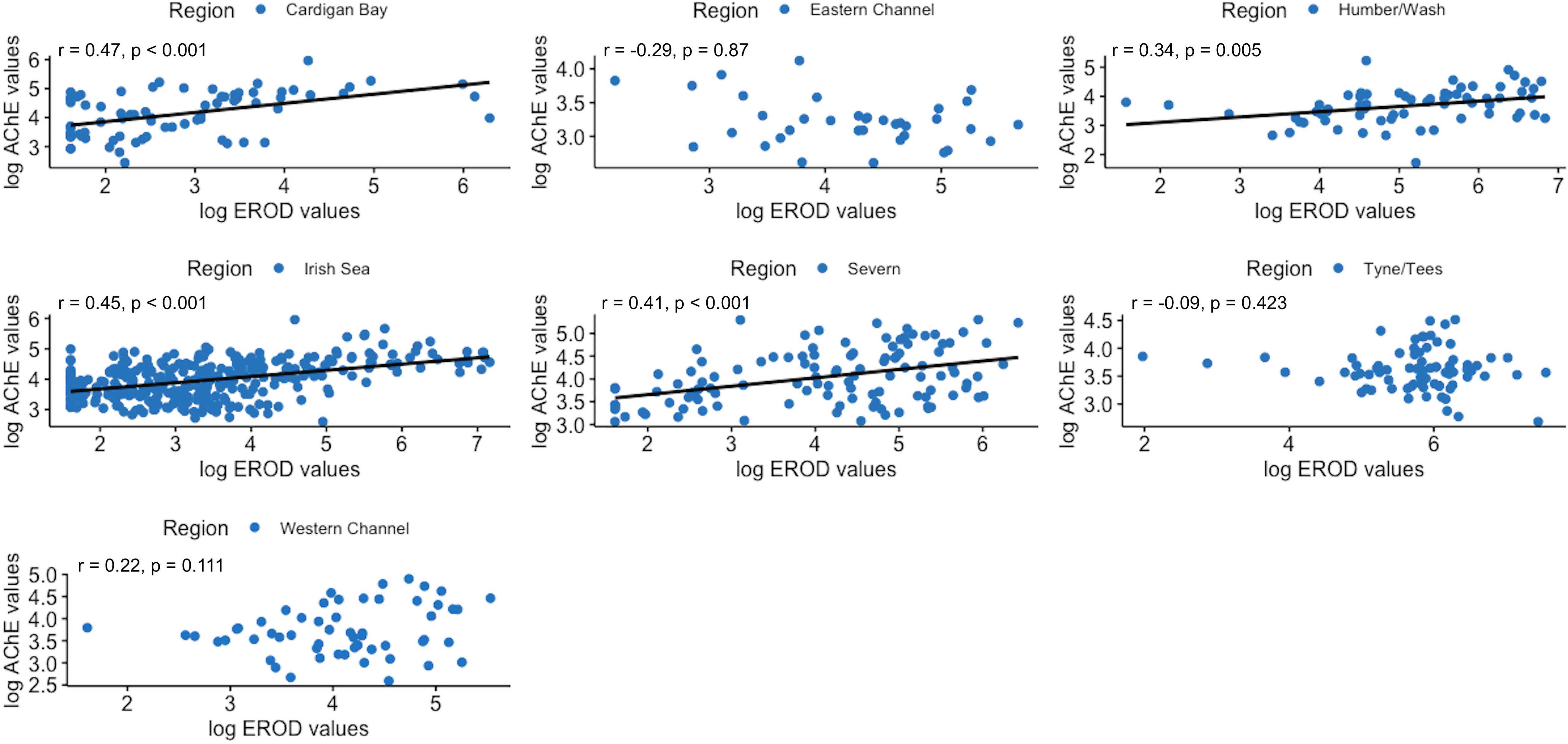
Figure 9 Pearson’s correlation plots between 7-ethoxyresorufin-O-deethylase (EROD) and acetylcholinesterase (AChE) in dab, per region (2014-2018). Data are normal log transformed and statistical significance considered at p<0.05.
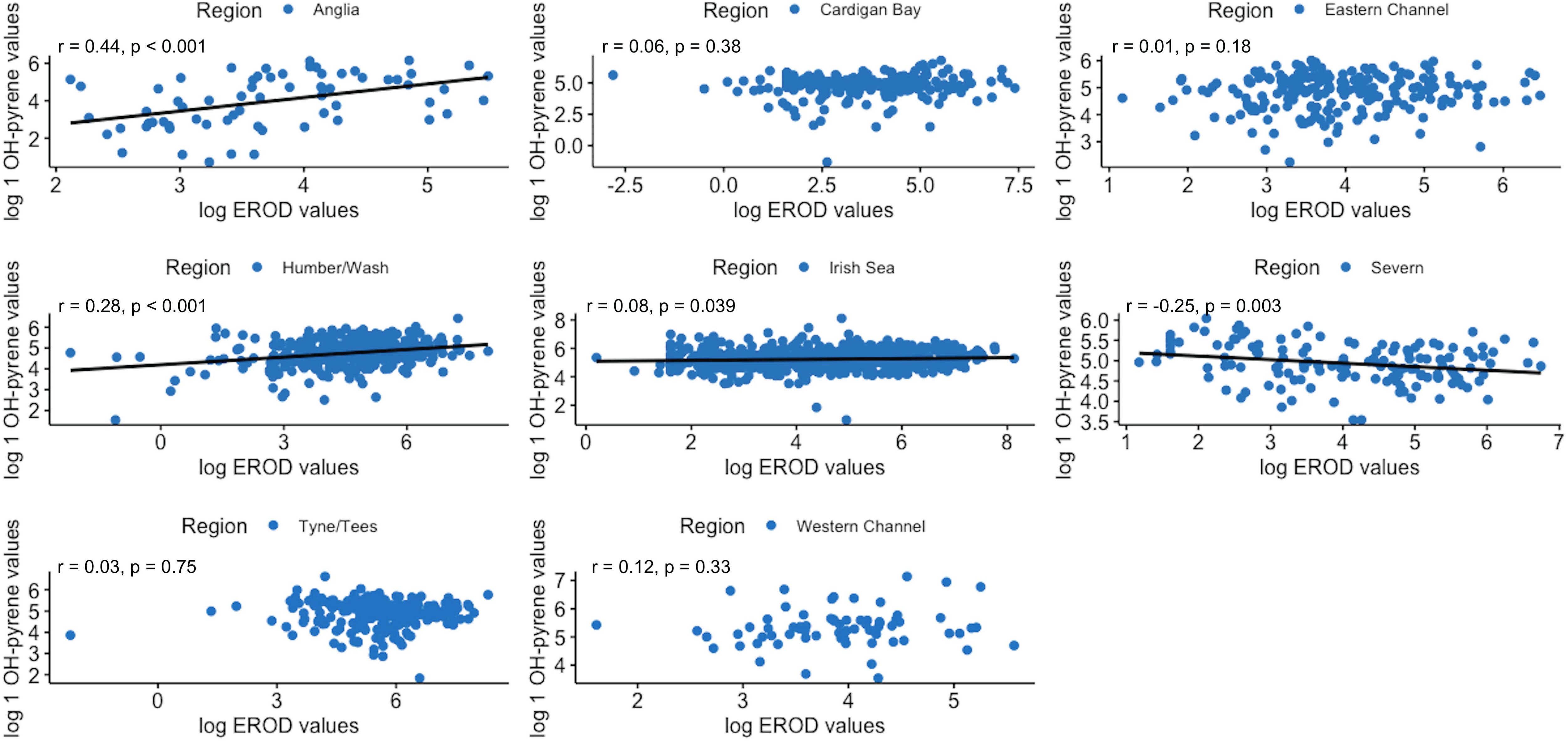
Figure 10 Pearson’s correlation plots between 7-ethoxyresorufin-O-deethylase (EROD) and bile 1-hydroxypyrene equivalent (1-OH pyrene) in dab, per region (2005-2018). Data are normal log transformed and statistical significance considered at p<0.05.
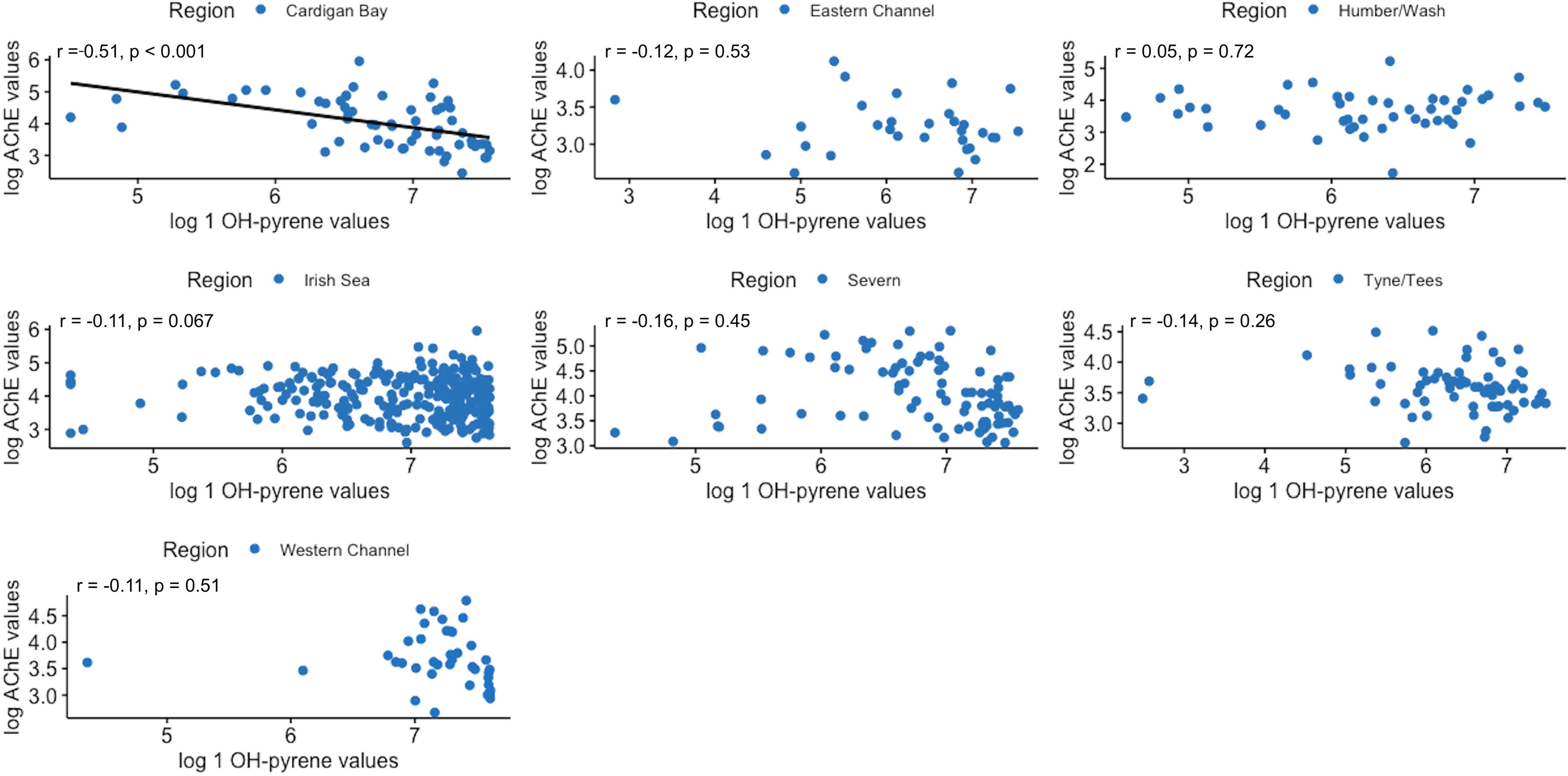
Figure 11 Pearson’s correlation plots between and acetylcholinesterase (AChE) and bile 1-hydroxypyrene equivalent (1-OH pyrene) in dab, per region (2014-2018). Data are normal log transformed and statistical significance considered at p<0.05.
Organophosphates and carbamates, highly toxic non-persistent insecticides primarily used in agriculture and household products, enter the marine environment usually through runoff and urban effluence. Even at low level concentrations they can inhibit the enzyme acetylcholinesterase (AChE). AChE inhibition will result in the build-up of the acetylcholine (ACh), the primary and vital neurotransmitter in the sensory and neuromuscular systems of fish, which will lead to overstimulation of nerves/muscle fibres causing constant firing, paralysis and ultimately the death of the organism (Bocquene and Galgani, 1998; Kirby et al., 2000; Fulton & Key, 2001). Fulton and Key (2001) mentioned that monitoring AChE might be advantageous in relation to running analytical chemistry for insecticides since they degrade rapidly in the environment (hours to days) and AChE inhibition is the primary mechanism by which these compounds produce toxicity and can last days to weeks. Other compounds such as heavy metals, hydrocarbons, detergents, pyrethroids, herbicides, and algal toxins are also associated with inhibited AChE activity (Giltrap et al., 2017, and references within), therefore this biomarker can indicate exposure to neurotoxins without requiring knowledge of the specific contaminant (Davies and Vethaak, 2012).
Our analysis of the available AChE data (2014 to 2018) indicates region and sex as significant predictors with the linear model explaining 13% of the variability (Tabel 1). At regional level, our data shows lower average values of AChE on the Eastern side regions (26.76 to 43.68) and higher values on the Western regions (48.59 to 81.78) (Figure 2), highlighting a potentially higher exposure to AChE inhibitors on the East coast regions of the UK. The Easter regions include more coastal stations but also some open ocean ones (Figure 1), therefore our results agree with previous studies were AChE inhibition in dab muscle was reported both for coastal station but also more offshore stations, and further from riverine runoffs (Galgani et al., 1992). The same authors also reported a 3-fold difference between the highest and lowest activities sampled along a pollution gradient in the North Sea; we have also found a 3-fold difference between the AChE highest and lowest regional average values measured around the UK between 2014 and 2018. The established BAC and EAC reference values for this biomarker in dab muscle, as reported in Davies and Vethaak (2012), do not apply to this data due to the application of the modified AChE analysis method in the CSEMP programme. The same authors refer that a 20% reduction of baseline AChE levels in fish is generally accepted as indication of neurotoxin exposure, with<50% considered to fall just short of lethal and 60-70% causing death. If we were to consider our highest average regional AChE value (Irish Sea) as a baseline (100%), the lowest regional average value measured in the Eastern Channel over the period of 2014 to 2018 corresponds to a 67% reduction in AChE activity; even considering the large scale (regional) used in this study, the reductions in AChE activity reported merit further investigation, namely the inclusion of this biomaker in the reported UK trend and status assessment for biological effects of contaminats (https://moat.cefas.co.uk/pressures-from-human-activities/contaminants/) once the CSEMP dataset allows it.
Although age was not a significant predictor of AChE variability in dab for our data, our model results allow us to report for the first-time sex and gonadosomatic index (GIS) as significant predictors, albeit explaining 13 and 15% of variability. According to our results, on average regional AChE levels are higher in males when compared to females. The model was improved when the variable GSI was added, rendering sex a non-significant variable. Considering our data sampling occurred during the dab “resting period” the significance observed with GSI could be associated with sex; when more data becomes available this potential association should be further assessed. Previous studies analyzing AChE inhibition in flatfish around Ireland and UK estuaries did not find significant differences between sexes (Kirby et al., 2000; Giltrap et al., 2017). However in flounder and cod (Gadus morhua L.) from the Baltic Sea, an area heavily subjected to agricultural and industrial effluents, females had lower muscle AChE levels when compared to males, and these differences were significant for cod (Schneider et al., 2000; Napierska and Podolska, 2003). Based on our results we would recommend for the dab muscle AChE data to be analyzed and reported by sex, when part of marine environmental monitoring programmes.
Dab from the North Sea have been shown to experience cyclic variation in condition factor (CF) and feeding frequency in accordance with their spawning cycle, with highest values noted during the pre and early spawning seasons and the lowest during peak season (Htun-Han, 1978). During the resting period, when the sampling of our data occurred, feeding and CF have been shown to level off for both sexes (Htun-Han, 1978). A reduced sample size of our data was available to assess the effect of CF in dab, and this factor explained 23% of the variability in AChE, indicating that dab with lower CF had higher AChE levels. Considering that, when exposed to its common inducers (e.g. insecticides), AChE inhibition is the immediate mechanism after exposure, we could be seeing a quick inhibition response that has not affected the individual fitness of the dab, therefore not being reflected in its CF. AChE levels in fish species have been shown to recover (de novo synthesis of enzyme protein) once exposure ceases. Furthermore exposure experiments, using freshwater and diadromous species, have shown that some sublethal effects on fish stamina (e.g. swimming performance) have been associated to brain AChE inhibition of around 50%, although the majority indicates that these effects are only observed when brain AChE inhibition is at near-lethal levels (Fulton and Key, 2001).
Many of the EROD inducing chemicals were in heavy use around the mid twentieth century and have since been banned, however decades later they persist in the environment due to their chemical structure and lipophilic nature and can have detrimental effects for years or decades to come (Farrington and Takada, 2014; Askem et al., 2018). Our data showed the average regional values for hepatic EROD in dab, for the period of 2005 to 2018, were higher in the Tyne/Tees region, with Humber Wash and the Irish Sea following, all of them exceeding the Background Assessment Criteria (BAC) for both genders. The Northern regions of the UK are currently and historically the most industrialized and therefore higher contamination levels in marine sediment and biota have been recorded over the years in the adjacent marine regions (Nicolaus et al., 2015; Nicolaus et al., 2016). According to the last published UK assessment for EROD, produced using data from the CSEMP programme and covering the period of 2010 to 2015, despite 42% of EROD assessments exceeding the BAC value in the Northern North Sea biogeographic region, a significant downward trend in EROD values was found for the Northern North Sea, Southern North Sea and Irish Sea regions (Nicolaus et al., 2018b).
The results of our mixed linear model (LMM) suggest that, in addition to region (location), sex, age and condition factor contributed to explain the observed variability of hepatic EROD in dab explaining from 26 to 56% of EROD variability. For the period of 2005 to 2018, females have significantly higher average EROD levels than males in all regions. Differences in hepatic EROD levels between sexes, in relation to sexual maturity and reproductive cycle phase have been established for dab; while spawning CYP1A enzymes can be suppressed in females as estrogen interferes with the transcription of that particular gene; if not taken into consideration this can lead to an underestimation of contaminant induced EROD activity (Sleiderink et al., 1995b; Kammann et al., 2005). In the present study this was controlled for by sampling design, with the CSEMP surveys being conducted in June-July, therefore outside the dab breeding season for the North Sea (Htun-Han, 1978). Furthermore, according to our results the gonadosomatic index was not an explanatory variable to hepatic EROD in both genders between 2005 and 2018, further confirming the fish were sampled in the “resting period”. This sampling design also accounted to control for variations in water temperature, another seasonal exogenous factor known to affect hepatic EROD activity (Sleiderink et al., 1995a; Davies and Vethaak, 2012).
The sensitivity of dab to CYP1A1 induction is considered to be higher in mature males, and separate analysis and reporting of EROD results by gender is advised for monitoring purposes (Sleiderink et al., 1995b; Stagg et al., 2016); our results further confirm this advice since significant sex differences were observed. The interaction effect between age and sex might help to elucidate the overall higher EROD values we observed in females for all regions in our long-term analysis. Our results suggest that while EROD average values in female dab see some increase with age, for males lower hepatic EROD values are observed in younger fish and a clear increase with age is observed (Figure 6). Similar results were reported for dab by Dévier et al. (2013) with higher, and significant differences, in mean hepatic EROD observed between male juvenile and adult fish, when compared to differences in females. As seen in Figure 5 our sample sex ratio per region is balanced, our EROD results therefore point to gender differences in EROD among regions, even when sampling during the “resting period”, except for Tyne and Tees where the highest EROD values were recorded. Although an increase in cytochrome P450-dependent enzymatic activities with age has been found in flatfish (Peters and Livingstone, 1995; Sleiderink et al., 1995b), earlier studies of dab have not reported age as a significant predictor for EROD, however small sample sizes were examined (Lange et al., 1992). Our substantial data set might have helped discern the observed effects and interactions of sex and age in EROD for dab. In order to be able to discriminate potential age effects in hepatic EROD induction from contaminant related induction, in both genders, further analysis should be considered to account for potentially disproportionate numbers of fish within a given year class. Such methodology has been applied by Bignell et al. (2018, In preparation) when assessing the UK trends and status of liver neoplasms (cancer), in flatfish, also part of the CSEMP programme. Other biological effects in flatfish species attributed to exposure to PAHs, DDTs and other contaminants, such as prevalence of liver neoplasms have been shown to be related to age (Johnson et al., 1993; Myers et al., 2003).
Our results confirm a negative correlation between condition factor and hepatic EROD activity for dab. Diet related factors, such as inadequate nutrition, can affect the uptake of organic pollutants and the appropriate functioning of enzymatic systems (Lange et al., 1992; Davies and Vethaak, 2012). In a previous study with dab from the North Sea, Sleiderink et al. (1995a) also reported on the influence of CF in CYP1A levels, recommending care when comparing dab from stations where dab showed different nutritional status.
In most aquatic vertebrates PAHs are quickly metabolized into more soluble components which are collected in the gall bladder before being excreted through bile or urine, therefore fish tissue concentrations remain low. However PAH and toxic intermediate metabolites that can emerge during metabolism via the phase I hepatic cytochrome P450 enzymes (CYP1A1) have been linked to increases in neoplasms and tumors in fish (Myers et al., 2003). 1-OH pyrene, is the main metabolite detected in fish bile, with other metabolites found at considerably lower levels (Kammann, 2007). Their quantification represents the flux of PAH’s going through the fish body, however considering the episodic releases of the gall bladder during the digestion process, the feeding status of the fish is an important factor (Davies and Vethaak, 2012). Due to fish efficient metabolic excretion, PAH metabolites measured in the bile will represent exposure from days to up to 2 weeks (Davies and Vethaak, 2012).
Our result analysis indicates region (location) as the only significant predictor of biliary 1 OH-pyrene in dab, with all regions showing values significantly higher than the reference station (Anglia) for the period of 2005 to 2018. The higher mean regional values are in the Western Channel and Irish Sea, followed by Severn and Tyne/Tees, and although these are above the BAC value for dab, they are also well below the Environmental Assessment Criteria for this marker. Our results mirror the UK status and trend assessment findings for bile metabolite in dab from the UK biogeographic regions, covering the period of 2010 to 2015, where PAHs were found to be unlikely to cause adverse biological effects (Nicolaus et al., 2018a). As also shown by other authors, PAH metabolites analysis in dab and flounder bile allowed to discriminate between distinctly impacted sampling sites (Richardson et al., 2001; Kammann, 2007; Dévier et al., 2013; Vethaak et al., 2016), making it a reliable indicator of dab exposure to PAHs.
When accounting for region, our results showed no obvious relationship between biliary 1 OH-pyrene and sex, age, GSI and CF in dab. Previous studies have reported higher biliary PAH metabolites in male flounder (Platichthys flesus) when compared to females in the Baltic Sea, but the sample size was small and no definite statistically significance was reported (Vuorinen et al., 2006). Adult mixed sex dab (2.5 to 4 years) has been reported to have higher levels of biliary 1 OH-pyrene when compared to juveniles (under 2 years) in the Seine estuary, and season also showed a clear influence in dab biliary 1 OH-pyrene levels, with increased values in winter when compared with summer months (Kammann, 2007; Dévier et al., 2013). Seasonal PAH input due to higher river flow rates have been suggested for coastal sites but season related changes in dietary and physiological status of the fish could also be playing a part (Kammann, 2007).
The sampling design of the CSEMP surveys accounts for reduced variation in season and reproductive annual cycle, by conducting surveys in the time of the year corresponding to the dab “resting period”, as well as age by sampling adult fish. Nevertheless, analysis of our long-term data set indicates that the sex and the condition factor of dab, for the 2005 to 2018 period, in the sampled UK regions did not contribute to explain the variability observed in biliary 1 OH-pyrene levels.
Correlations between biomarkers revealed an interesting trend between hepatic EROD and AChE muscle in dab, with significant positive correlations (“r” varying from 0.41 to 0.47) observed for 3 regions on Western side of the UK, and also Humber region on the East coast. These results contrast with Burgeot et al. (1996) and Kirby et al. (2000) previous studies where these two parameters showed very week negative correlations in flounder from UK estuaries, and red mullet (Mullus barbatus) from coastal sites in the North West Mediterranean. Considering different classes of contaminants, with different modes of action, are the primary inducers of these biomarkers, the observed trend might be reflecting regional prevalent types of contamination for the period studied (2014 to 2018), as also reported for red mullet (Burgeot et al., 1996). The three Western regions are the ones with the highest average levels of muscle AChE, therefore the least impacted regions for common AChE inducers; the positive correlation with EROD could indicate that when dab is responding to hepatic EROD inducers, in general these are not concurrent to AChE inducers, as this marker levels are not reduced, and vice versa. Although co-exposure to inducers of both biomarkers is likely occurring in UK estuaries, the majority of the sampling stations for these 3 Western regions, and also for Humber on the East coast, are offshore, therefore different correlation patterns for dab biomarkers response might emerge. In fact, in the Eastern Channel region, where the lowest AChE average values were obtained, from predominantly more coastal stations, a weak negative and non-significant correlation was found with hepatic EROD levels (Figure 9).
Variable regional correlation patterns were found between hepatic EROD and biliary 1 OH-pyrene levels in dabs for the period of 2005 to 2018. Positive weak correlations emerged for the Anglian region and Humber Wash on the East Coast, a negative correlation in the Severn, and non-significant correlations for the remaining regions. Variable patterns in this correlation have been previously reported for flatfish, including dab (Dévier et al., 2013, and references within). For the Humber and Anglian region, the hepatic EROD induction is likely reflecting in part PAHs induction. However hepatic EROD measured in dab can also be reflecting induction by other co-planar compounds, other than PAHs, with affinity to the aryl hydrocarbon receptor, and therefore capable of inducing CYP1A1. PCBs have been reported in dab (2009 to 2013), and sediments (1999 to 2011), in the CSEMP programme, with PCB 118 (co-planar congener) exceeding the assessment criteria limit (Nicolaus et al., 2015; Nicolaus et al., 2016). Another explanation is the different time scale response of these two markers; after exposure biliary PAHs metabolites formation has been shown to be much quicker (up to 24h after exposure) then hepatic EROD induction (maximum induction achieved between 2 to 5 days), therefore rendering PAH biliary metabolites as the likely more reliable indicator of local contamination in fish (Dévier et al., 2013 and references within).
Lastly, no regional correlation pattern was evident between muscle AChE and biliary 1 OH-pyrene levels in dab, except in Cardigan Bay, where a significant moderate negative correlation was determined (“r”=-0.51). These results suggest that here, higher levels of biliary 1 OH-pyrene in dab are related to inhibition of muscle AChE. However, considering Cardigan Bay was the region with higher average muscle AChE levels, this result might reflect some level of inhibition by PAHs. Recently Olivares-Rubio and Espinosa-Aguirre (2021) reported on AChE inhibition in fish in response to crude oil and its derivatives; although currently no clear mechanism has been reported that allows understanding the variation in AChE activity when exposed to these contaminants. Future work could include further assessments in Cardigan Bay, for both biomarkers and chemical analysis in biota and sediment at the sampling station level, to help establish the spatial trend of this interaction.
In conclusion, the analysis of the CSEMP programme dataset, for 3 established biomarkers used as part of the UK biological effects of contaminants monitoring, allowed to better discern how factors, other than environmental contaminants, contribute to explain the variability in the response of these markers. As data on dab muscle AChE, becomes available through the CSEMP monitoring programme, status and trend assessment for the UK should be carried on for this marker to allow evaluating how the difference in average regional values are evolving. Furthermore, we suggest muscle AChE values should be analyzed and reported by sex, based on our results indicating that gender has some influence in dab muscle AChE response. Female dab had overall significant higher levels of hepatic EROD, however a strong and significant interaction effect between age and sex was uncovered in our long-term analysis, showing how EROD values might vary differently between male and female dab as age increases. The implication of these findings in terms of metabolizing and/or activation capability between gender for dab, and potential links with liver alterations and neoplasms prevalence should be further investigated. Age, sex, CF and GSI were not significant predictors of biliary 1 OH-pyrene levels variability in dab during the period covered by this study.
Correlations between the three biomarkers, at the regional level, might assist in discerning types of contamination prevailing over the studied period, however further correlation analysis, at the station level, namely coastal versus offshore stations, will help to evaluate further.
Publicly available datasets were analyzed in this study. This data can be found here: Marine Environment Monitoring and Assessment National database (MERMAN).
SD: database organization, statistical analysis, data interpretation, manuscript preparation and writing. AF: Design of the study, statistical analysis, data interpretation. MA: Conception and design of the study, data interpretation, manuscript writing and revision. All authors contributed to the article and approved the submitted version.
This study was carried out in part-fulfillment of the degree of Master of Science, School of Environmental Sciences (University of East Anglia) by Shannon Dalessandri, recipient of Across the Pond Scholarship for International Students and Simon Wharmby Postgraduate Scholarship in Environmental Sciences (2019). The CSEMP programme is funded by the Department for Environment, Food, and Rural Affairs (Defra), UK.
The authors would like to acknowledge and thank all those who have contributed to the CSEMP monitoring programme and the MERMAN database. A special acknowledgement goes to the staff members of the ECORA team (Cefas) for conducting the biomarkers analysis, and also to Paul Nelson (Cefas) for the assistance with the extracted dataset.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ariese F., Beyer J., Jonsson G., Visa C. P., Krahn M. M. (2005). Review of analytical methods for determining metabolites of polycyclic aromatic compunds (PACs) in fish bile. ICES Techniques Mar. Environ. Sci. 39, 49.
Askem C. E., Wright S. R., Vannoni M., Robinson C. D., White K., Lyons B. P., et al. (2018). Spatial and temporal analysis of biliary 1-hydroxypyrene, hepatic ethoxyresorufin-o-deethylase and muscle acetylcholinesterase activity in UK flatfish. Mar. pollut. Bull. 133, 872–880. doi: 10.1016/J.MARPOLBUL.2018.06.059
Bates D. M., Machler M., Bolker B. M., Walker S. C. (2014). Fitting linear mixed-effects models using lme4. J. Stat. Software 67, 1–48. doi: 10.48550/arXiv.1406.5823
BDOC (2016) MERMAN station dictionary. Available at: https://www.bodc.ac.uk/projects/data_management/uk/merman/project_specific/.
Bignell J. P., Etherton M., Nelson P., Stentiford G., Taylor N. G. H., Visconti V., Alves M. T. (In preparation). Significant decrease in fish hepatic neoplasms revealed by long-term environmental monitoring.
Bignell J. P., Robinson C., Taylor N. (2018) Trends and status of liver neoplasms in flatfish. UK marine online assessment tool. Available at: https://moat.cefas.co.uk/pressures-from-human-activities/contaminants/liver-neoplasms/.
Bocquene G., Galgani F. (1998). Biological effects of contaminants: Cholinesterase inhibition by organophosphate and carbamate compounds. ICES Techniques Mar. Environ. Sci. 22, 12.
Burgeot T., Bocquene G., Porte C., Dimeet J., RM S., LM G., et al. (1996). Bioindicators of pollutant exposure in the northwestern Mediterranean Sea. Mar. Ecol. Prog. Ser. 131, 125–141. doi: 10.3354/meps131125
Davies I. M., Vethaak A. D. (2012). “Integrated marine environmental monitoring of chemicals and their effects,” in ICES cooperative research report no. 315. Denmark: International Council for the Exploration of the Sea.
De Raedemaecker F., Brophy D., O’Connor I., O’Neill B. (2012). Dependence of RNA : DNA ratios and fulton’s K condition indices on environmental characteristics of plaice and dab nursery grounds. Estuarine Coast. Shelf Sci. 98, 60–70. doi: 10.1016/j.ecss.2011.11.033
Dévier M.-H., Le Dû-Lacoste M., Akcha F., Morin B., Peluhet L., Le Menach K., et al. (2013). Biliary PAH metabolites, EROD activity and DNA damage in dab (Limanda limanda) from seine estuary (France). Environ. Sci. pollut. Res. 20 (2), 708–722. doi: 10.1007/s11356-012-1345-7
European Comission (2020) The marine strategy framework directive. Available at: https://ec.europa.eu/environment/marine/eu-coast-and-marine-policy/marine-strategy-framework-directive/index_en.htm.
Farrington J. W., Takada H. (2014). Persistent organic pollutants (POPs), polycyclic aromatic hydrocarbons (PAHs), and plastics: Examples of the status, trend, and cycling of organic chemicals of environmental concern in the ocean. Oceanography 27 (1), 196–213. doi: 10.5670/oceanog.2014.23
Flammarion P., Garric J. (1999). A statistical approach for classifying the extent of EROD induction of fish sampled in clean and contaminated waters. Water Res. 33 (11), 2683–2689. doi: 10.1016/S0043-1354(98)00492-8
Fulton M. H., Key P. B. (2001). Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ. Toxicol. Chem. 20 (1), 37–45. doi: 10.1897/1551-5028(2001)020<0037:aiiefa>2.0.co;2
Galgani F., Bocquené G., Cadiou Y. (1992). Evidence of variation in cholinesterase activity in fish along a pollution gradient in the north Sea. Mar. Ecol. Prog. Ser. 91 (1/3), 77–82. doi: 10.3354/meps091077
Giltrap M., Ronan J., Bignell J. P., Lyons B. P., Collins E., Rochford H., et al. (2017). Integration of biological effects, fish histopathology and contaminant measurements for the assessment of fish health: A pilot application in Irish marine waters. Mar. Environ. Res. 129, 113–132. doi: 10.1016/J.MARENVRES.2017.04.004
Htun-Han M. (1978). The reproductive biology of the dab limanda limanda (L.) in the north Sea: Seasonal changes in the ovary. J. Fish Biol. 13 (3), 351–359. doi: 10.1111/j.1095-8649.1978.tb03443.x
Johnson L. L., Stehr C. M., Olson O. P., Myers M. S., Pierce S. M., Wigren C. A., et al. (1993). Chemical contaminants and hepatic lesions in winter flounder (Pleuronectes americanus) from the northeast coast of the united states. Environ. Sci. Technol. 27 (13), 2759–2771. doi: 10.1021/es00049a015
Kammann U. (2007). PAH metabolites in bile fluids of dab (Limanda limanda) and flounder (Platichthys flesus): Spatial distribution and seasonal changes. Environ. Sci. pollut. Res. - Int. 14 (2), 102–108. doi: 10.1065/espr2006.05.308
Kammann U., Lang T., Vobach M., Wosniok W. (2005). Ethoxyresorufin-o-deethylase (EROD) activity in dab (Limanda limanda) as biomarker for marine monitoring (6 pp). Environ. Sci. pollut. Res. 12 (3), 140–145. doi: 10.1065/espr2004.12.228
Kirby M. F., Morris S., Hurst M., Kirby S. J., Neall P., Tylor T., et al. (2000). The use of cholinesterase activity in flounder (Platichthys flesus) muscle tissue as a biomarker of neurotoxic contamination in {UK} estuaries. Mar. pollut. Bull. 40 (9), 780–791. doi: 10.1016/S0025-326X(00)00069-2
Lange U., Jedamski-Grymlas J., Siebers D., Karbe L. (1992). Ethoxyresorufin O-deethylase and cytochrome P450 in the liver of dab (Limanda limanda (L.)) from the central and southern north Sea. Mar. pollut. Bull. 24 (9), 446–451. doi: 10.1016/0025-326X(92)90344-6
Livingston D., Förlin L., George S. (1994) Molecular biomarkers and toxic consequences of impact by organic pollution in aquatic organisms. water quality and stress indicators in marine and freshwater systems: Linking levels of organisation. Available at: http://aquaticcommons.org/5314/.
MARG (2020). Green book - UK clean seas environmental monitoring programme-marine assessment and review group. United Kingdom: Marine Assessment and Review Group.
Myers M. S., Johnson L. L., Collier T. K. (2003). Establishing the causal relationship between polycyclic aromatic hydrocarbon (PAH) exposure and hepatic neoplasms and neoplasia-related liver lesions in English sole (Pleuronectes vetulus). Hum. Ecol. Risk Assessment: Int. J. 9 (1), 67–94. doi: 10.1080/713609853
Napierska D., Podolska M. (2003). Preliminary results of AChE and GST measurements in flounder platichthys flesus from the southern Baltic Sea. Bull. Sea Fisheries Ins. 2, 51–66.
Nicolaus E. E. M., Law R. J., Wright S. R., Lyons B. P. (2015). Spatial and temporal analysis of the risks posed by polycyclic aromatic hydrocarbon, polychlorinated biphenyl and metal contaminants in sediments in UK estuaries and coastal waters. Mar. Pollut. Bull. 95 (1), 469–479. doi: 10.1016/J.MARPOLBUL.2015.03.012
Nicolaus E. E. M., Robinson C. D., Fryer R. (2018a) Status assessment for biological effects (Bile meatbolite) in fish. UK marine online assessment tool. Available at: https://moat.cefas.co.uk/pressures-from-human-activities/contaminants/bile-metabolites/.
Nicolaus E. E. M., Robinson C. D., Fryer R. (2018b) Status assessment for biological effects (EROD enzyme activity) in fish. UK marine online assessment tool. Available at: https://moat.cefas.co.uk/pressures-from-human-activities/contaminants/erod/.
Nicolaus E. E. M., Wright S. R., Bolam T. P. C., Barber J. L., Bignell J. P., Lyons B. P. (2016). Spatial and temporal analysis of the risks posed by polychlorinated biphenyl and metal contaminants in dab (Limanda limanda) collected from waters around England and Wales. Mar. pollut. Bull. 112 (1–2), 399–405. doi: 10.1016/j.marpolbul.2016.07.048
Olivares-Rubio H. F., Espinosa-Aguirre J. J. (2021). Acetylcholinesterase activity in fish species exposed to crude oil hydrocarbons: A review and new perspectives. Chemosphere 264, 128401. doi: 10.1016/j.chemosphere.2020.128401
OSPAR Comission (2013) Background document and technical annexes for biological effects monitoring, update 2013. Monitoring and assessment series. Available at: https://www.ospar.org/documents?v=7316.
OSPAR Comission (2020) (CEMP). Available at: https://www.ospar.org/work-areas/cross-cutting-issues/cemp.
Peters L. D., Livingstone D. R. (1995). Studies on cytochrome P4501A in early and adult life stages of turbot (Scophthalmus maximus l.). Mar. Environ. Res. 39 (1), 5–9. doi: 10.1016/0141-1136(94)00064-V
R Core Team (2019). R: A languange and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.r-project.org/.
Richardson D. M., Davies I. M., Moffat C. F., Pollard P., Stagg R. M. (2001). Biliary PAH metabolites and EROD activity in flounder (Platichthys flesus) from a contaminated estuarine environment. J. Environ. Monit. 3 (6), 610–615. doi: 10.1039/B106353G
Schneider R., Schiedek D., Petersen G. I. (2000). Baltic Cod reproductive impairment: ovarian organo-chlorine levels, hepatic EROD activity, muscular AChE activity, developmental sucess of egg, larvae, challenge tests. ICES CM 2000/S:09. Denmark: International Council for the Exploration of the Sea.
Sleiderink H. M., Beyer J., Scholtens E., Goksøyr A., Nieuwenhuize J., Van Liere J. M., et al. (1995a). Influence of temperature and polyaromatic contaminants on CYP1A levels in north Sea dab (Limanda limanda). Aquat. Toxicol. 32 (2–3), 189–209. doi: 10.1016/0166-445X(94)00080-A
Sleiderink H. M., Oostingh I., Goksøyr A., Boon J. P. (1995b). Sensitivity of cytochrome P450 1A induction in dab (Limanda limanda) of different age and sex as a biomarker for environmental contaminants in the southern north Sea. Arch. Environ. Contamination Toxicol. 28 (4), 423–430. doi: 10.1007/BF00211623
Stagg R., McIntosh A., Gubbins M. J. (2016). Determination of CYP1A-dependent mono-oxygenase activity in dab by fluorimetric measurement of EROD activity in S9 or microsomal liver fractions. ICES Techniques Mar. Environ. Sci. 57, 21. doi: 10.17895/ices.pub.5085
Vethaak A. D., Baggelaar P. K., van Lieverloo J. H. M., Ariese F. (2016). Decadal trends in polycyclic aromatic hydrocarbon (PAH) contamination assessed by 1-hydroxypyrene in fish bile fluid in the Netherlands: Declining in marine waters but still a concern in estuaries. Front. Mar. Sci. 3. doi: 10.3389/fmars.2016.00215
Keywords: flatfish, monitoring programmes, biological effects, acetylcholinesterase AChE, 7-ethoxyresorufin-O-deethylase (EROD), biliary 1-hydroxypyrene (1-OH pyrene)
Citation: Dalessandri S, Franco AMA and Assunção MGL (2023) UK long-term monitoring dataset: assessing factors affecting biomarkers commonly used in environmental programmes. Front. Mar. Sci. 10:1124952. doi: 10.3389/fmars.2023.1124952
Received: 15 December 2022; Accepted: 01 March 2023;
Published: 03 May 2023.
Edited by:
Lucía Viñas Diéguez, Spanish Institute of Oceanography (IEO), SpainReviewed by:
Annalisa Zaccaroni, University of Bologna, ItalyCopyright © 2023 Dalessandri, Franco and Assunção. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta G. L. Assunção, bWFydGEuYXNzdW5jYW9AY2VmYXMuZ292LnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.