- 1Ocean College, Yan Tai University, Yantai, China
- 2Jiaozhou Bay, National Marine Ecosystem Research Station, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 3Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 4College of Marine Science, University of Chinese Academy of Sciences, Beijing, China
In marine ecosystems, copepods (<2 mm) play an important role in the transfer of carbon and energy to higher tropic levels. Investigations in Jiaozhou Bay were carried out throughout 2017–2018, combined with laboratory experiments. The annual abundance, egg production, female prosome length, female carbon mass, clutch size, population composition, and development time of Oithona similis at different temperatures were analyzed to assess the life strategies and estimate generation cycles of O.similis in typical temperate regions at mid-latitude, represented by Jiaozhou Bay. The results include: 1) O.similis abundance was characterized by bimodal cycles, with the major peak in winter (1471 ± 206 ind/m³) and the minor peak in spring (740 ± 320 ind/m³). O. similis abundance was not subject to food limitations except in January. The lower abundance was mainly affected by temperature (>20°C) and low salinity in August. Predation from large size zooplankton was one of the factors in regulating O. similis abuncance in Jiaozhou Bay. 2) Over the year, the egg production rate, female prosome length, and female carbon mass of O.similis ranged from 0.03 ± 0.02 to 1.21 ± 0.25 eggs female-1d-1, 410 ± 6 to 472 ± 4 μm, and 0.35 ± 0.05 to 0.43 ± 0.03 μg c female-1 respectively. Female prosome length, and female carbon were both significantly negative correlated with temperature(P<0.01). The clutch size of O.similis varied between 4 and 21 and we did’t find it was related with other factors. 3) O. similis appeared at almost all stages, and reproduced continuously throughout the year. According to the sex ratio of O.similis and other factors, we inferred that O. similis produced four generations per year in Jiaozhou Bay.
Introduction
In marine pelagic ecosystems, zooplankton, especially copepods (<2 mm), which play an important role in carbon flow processes, can exploit microbial food webs efficiently (Nielsen and Sabatini, 1996; Turner, 2004). O.similis Claus 1866 (Cyclopoida), as a cosmopolitan copepod, is one of the most abundant species, with a distribution ranging from coastal areas to oceanic regions and from tropical areas to cold regions. It contributes to the natural diet of fish larvae and large zooplankton (Zamora-Terol et al., 2013). Different from other free-spawning copepods, O.similis prefers to feed on moving food such as ciliates, has lower fecundity, and has longer egg-hatching times (Kiørboe and Sabatini, 1994). O. similis can reproduce actively throughout the year without dispause, even in cold regions such as the arctic. It is more abundant than calanoid copepods, which need to diapause without reproduction in winter (Ashjian et al., 2003; Lischka and Hagen, 2005; Dvoretsky, 2007a; Dvoretsky and Dvoretsky, 2009a). In recent years, global ocean environments have undergone great changes, and although copepod populations have declined considerably across the North Atlantic, O. similis has always maintained stable populations (Cornwell et al., 2020).
Previous ecological studies about O. similis have mainly focused on seasonal distribution, abundance (Metz, 1995; Ashjian et al., 2003), reproductive characteristics such as egg production rate, feeding ability, community structure, long-term variations at a single site, and life cycle strategies (Metz, 1996; Cornwell et al., 2020). Previous investigations suggested that O.similis was highly abundant mainly related to its tolerance to relatively wide temperature ranges (Castellani et al., 2005a; Castellani et al., 2007), its low energy requirement to adapt to oligotrophic environments (Castellani et al., 2005b), and its low predation mortality compared with other copepod species (Logerwell and Ohman, 1999; Eiane and Ohman, 2004).
O.similis is more abundant in arctic areas and temperate latitudes than in tropical latitudes where high temperatures limit O. similis abundance (Castellani et al., 2007; Dvoretsky and Dvoretsky, 2009b; Weydmann et al., 2014). However, ecological research on O. similis seasonal distribution, abundance, and reproductive characteristics has mainly concentrated on high latitude areas such as polar and subpolar regions, and islands around western Europe. In temperate latitude region, the study of O. similis was relatively less. Arima’s investigaton was mainly focused on fatty acids in ocean areas around Japan (Arima et al., 2014). Also, there were some distribution and adaption studies on the congeneric species Oithona davisae in the black sea and aegean sea (Vetlichny et al., 2016; Besiktepe et al., 2022). Although the research of O. similis in temperate latitude region was relatively less, the abundance of O. similis was relatively high. In the Yellow sea of China, O. similis was one of the dominant species in Spring and Winter. The abundance of O. similis even reached 2496.5 ind/m³ in Spring in 2014, occupied 30.7% of the total zooplankton abundance(Wang et al., 2021). Our present study area located in Jiaozhou Bay, which is a semi-enclosed temperate bay located on the western side of the Yellow Sea at temperate latitude. The study is the first attempt to investigate annual variations of abundance, egg production rates, and the community structure of O.similis. The purpose of our study is to reveal the basic annual ecological index variations of O.similis, and to evaluate the environmental factors that may affect O. similis. The ultimate goal of this study is to speculate about the life strategy and probable generations of O. similis in typical temperate regions at mid-latitudes represented by Jiaozhou Bay.
Materials and methods
Sample collection
Jiaozhou Bay is a semi-enclosed temperate bay connected to the Yellow Sea through a narrow opening. The area of the bay is about 390 km2 with an average water depth of 7 m. It belongs to temperate monsoon climate, the annual average temperature is 12.2°C. More than 10 rivers flow into Jiaozhou Bay. Ruditapes philippinarum culture is very popular in the bay. With the intensive anthropogenic activities, such as aquaculture, wastewater discharge, Jiaozhou Bay is now generally described as a eutrophic ecosystem. Noctiluca scientillans bloom and Entermorpha outbreak appeared in March and August in recent years (Sun et al., 2012).
Annual samples were collected monthly by RV Chuang Xin at the same stations (Figure 1) in Jiaozhou Bay, from April 2017 to March 2018, using a conical plankton net (mouth diameter: 0.57m; mesh size: 63 μm) towed vertically from the bottom of the bay to surface. The sites were divided in three parts: inner zone (A3, C3, C4), estuarine zone (D1, D3, D5), and outer zone (D6, D7, D8).The environmental characteristics of each sampling site were shown in the table as a supplementary material. 500 mL filtered sea water was collected at each site in every month. All the samples were fixed with 5% formalin solution, and then counted under a dissecting microscope. Temperature and salinity were measured with an AAQ1183-1F CTD (Alec Electronics Co., Japan). To measure total Chl a concentrations, 500 mL of natural seawater was filtered through 0.45 μm of cellulose acetate (CA). The membranes were extracted with 90% acetone (v/v) in a refrigerator for 24 h (≤0°C), and then measured with a Turner Designs Model 7200 fluorometer.
All stages of O. similis, including naupliar (N1-N6), copepodite (C1-C5) and adults (female and male) were identified. 4 ml samples were taken to count at least 50 individuals of O. similis were found. When O. similis abundance was low, samples were divided into two parts, every individual of O. similis from one half was identified to an appropriate developmental stage.The morphology character of O. similis developental stages and the mesurements taken were both according to previous methods (Takahashi and Uchiyama, 2007; Jean-jose et al., 2016). The size and shape were chosen as characteristics to identify the different naupliar stages. In the copepodid stages, the identification characters such as: body length, development of pleopods, setal development in antennae, genital segment and appearance of cephalosome, metasome and urosome were used to record the stages. All the observations were carried out using a binocular dissection microscope (PhenixSMZ180) and optical microscope (Olympus BX53T-32P01).
The O. similis females with and without egg sacs were both quantified. The clutch size (the number of eggs per sac) was recorded including detached egg sac information. The egg sacs were transparent, not requiring dissection (Drif et al., 2010). Female prosome length (PL) measured from the margin of the prosome to the posterior of the 4th segment (Uye, 1982). We selected 10 females for measurement, then converted these measurements into female carbon mass (CF), using an equation developed by Uye (1982).
Laboratory incubation experiment
O. similis samples were collected from a shrimp culture pond in Longkou, Shan Dong province. After being brought back to the laboratory, the collected samples were transported to the plastic box in live condition by providing continuous supply of air using a portable aerator. About 300 numbers of egg-bearing O. similis were stocked in transparent pet bottle of 1L capacity. The pet bottle was arranged specially for their stocking for further experiments. The microalgae Isochrysis galbana was harvested from continuous cultures and provided as feed twice a day at a cell concentration of 105 cell ml-1. The microalgae cell concentration was determined daily using a optical microscope (Olympus BX53T-32P01). Under the premise that other environmental factors were consistent, six temperature gradients (0, 5, 10, 15, 20, and 25°C) were setted for incubation experiment. 10 female O. similis were seperated from the pet bottle as experimental samples in each group in a small beaker and three parallel groups were set up.Once the eggs sac break, we selected the O. similis to a new beaker and observed it everyday. At last, we recorded the whole time that the egg took to complete their development process at different temperatures.
Data analysis
The O.similis community structure was made up of copepodites and adults. The percentage at each stage was calculated by the abundance of different stages. Egg production rate (EPR) of O. similis, based on the egg ratio method, was calculated from female and egg abundance. The egg abundance was calculated by divide the eggs number(the number of eggs per sac multiple by the female number) by the volume of the filtered sea water, The equation was calculated as follows:
EPR=E/F*HT HT=1504.5(T+7.6998)-2.05 (Checkley, 1980; Nielsen et al., 2002) E: egg abundance (individuals/m3), F: female abundance (individuals/m3) T: sea water surface temperature at sample site.
CF was calculated from the equation CF=10 ^ [1.45 × (Log PL) – 4.25] (Uye, 1982).
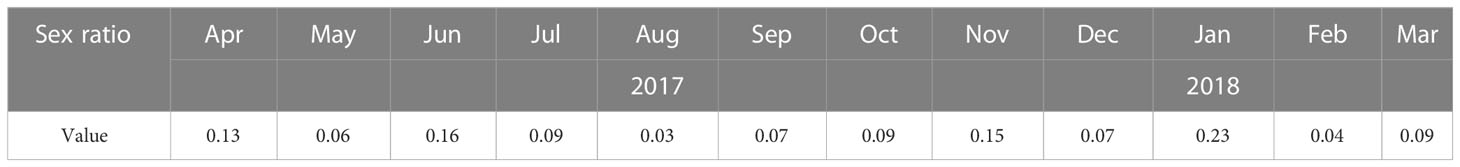
Sex ratio (males/females) was calculated in each month, the value of sex ratio ≥ 0.12 was used as a determinant of reproduction events as characteristic for the female-skewed Oithonidae family (Kiorboe, 2006).
Sample station map and contour maps were generated in Golden Software surfer 16.0. The O. similis abundance difference and environmental factors difference at different zones were evaluated statistically using Independant samples t -test with SPSS V19.0. Pearson correlation analysis was carried out between the ecological indicators of O. similis (abundance, EPR, Clutch size, PL) and environmental factors with SPSS V19.0. All data were presented as the means ± SD.
Results
Annual variations of environment factors in Jiaozhou Bay
The average Chl a concentration ranged between 0.43 ± 0.37 mg/m³and 1.82 ± 0.62 mg/m³ (Figure 2), with the exception of an average of 2.19 ± 0.26 mg/m³in February and an average of 1.93 ± 0.37 mg/m³in August. The Chl a concentration in outer zone was higer than in inner zone in August (P<0.05), the opposite trend occurred in December. Annual average surface sea temperature (SST) ranged from 3.14 ± 1.27°C to 27.33 ± 1.38°C (Figure 2), with the highest of 30.65°C observed in August and the lowest of 0.10°C observed in February.There is no significant difference in different zones. Annual average surface salinity ranged from 29.92 ± 1.14‰ to 31.80 ± 0.09‰ (Figure 2). Obvious dilution by fresh water was observed in August with the lowest salinity, of 29.40‰, at station A3, the difference was not significant in different zones.
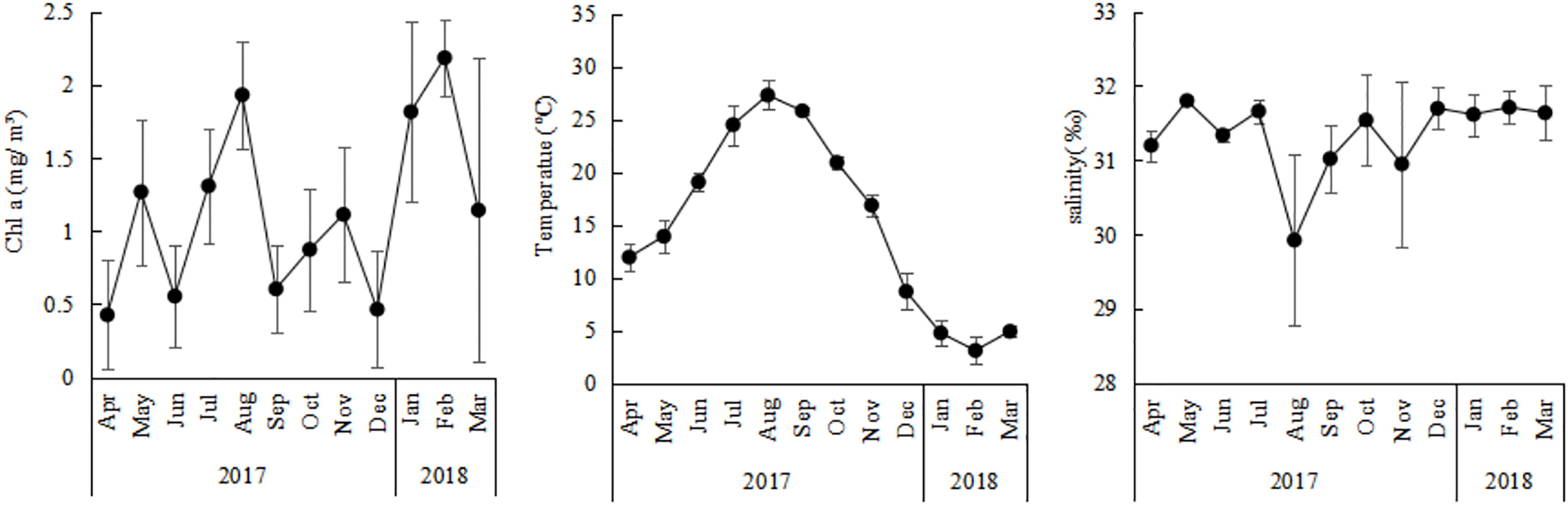
Figure 2 Annual variations of CHL a concerntration, temperature, and salinity in Jiaozhou Bay, vertical bars represent standard deviation (SD).
Annual variations of Oithona similis abundance in Jiaozhou Bay
O. similis abundance varied seasonally in Jiaozhou Bay (Figure 3). The average abundance was 500 ± 65 ind/m³ in spring (months 3–5), 198 ± 56 ind/m³ in summer (months 6–8), 116 ± 34 ind/m³ in autumn (months 9–11), and 1071 ± 232 ind/m³ in winter (months 12–2). Two peaks were observed, the major one was in January (1472 ± 206 ind/m³) and the minor one was in May (740 ± 320 ind/m³).The lowest abundance was observed in August (16 ± 15 ind/m³). We found O.similis abundance was negatively correlated (P<0.05) with zooplankton abundance which sampled using 500μm mesh during our investigation time in Jiaozhou Bay (Table 1).
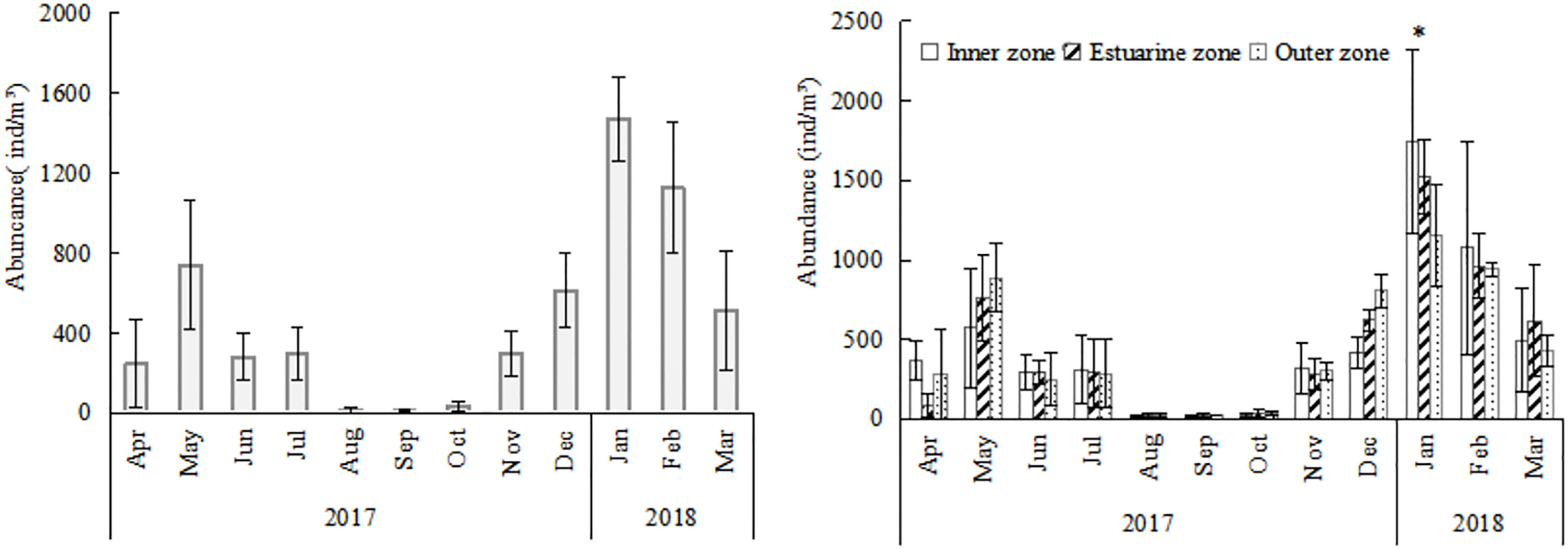
Figure 3 Annual variation and annual spatial variations of Oithona similis in Jiaozhou Bay, vertical bars represent deviation (SD). * means significantly difference (P<0.05).
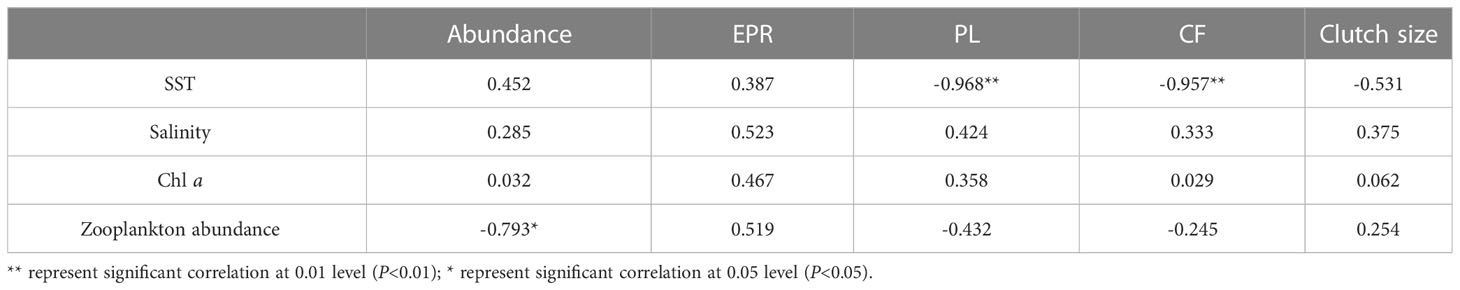
Table 1 Spearman correlation coefficients between ecological indicators of O. similis and environmental factors.
Annual spatial distribution of Oithona similis abundance
There were no regular differences in spatial distribution of O.similis abundance except in January (Figure 3). The O.similis abundance in the inner zone (1738 ± 577 ind/m³) was significantly higher than in the outer zone (1152± 322 ind/m³) in January (P<0.05).We did not find any relationship between O. similis abundance and Chl a concentration in January, but the abundance of O. similis was consistent with Chl a concentration in November, which also decreased from the inner zone sites to the outer zone sites (Figure 4).
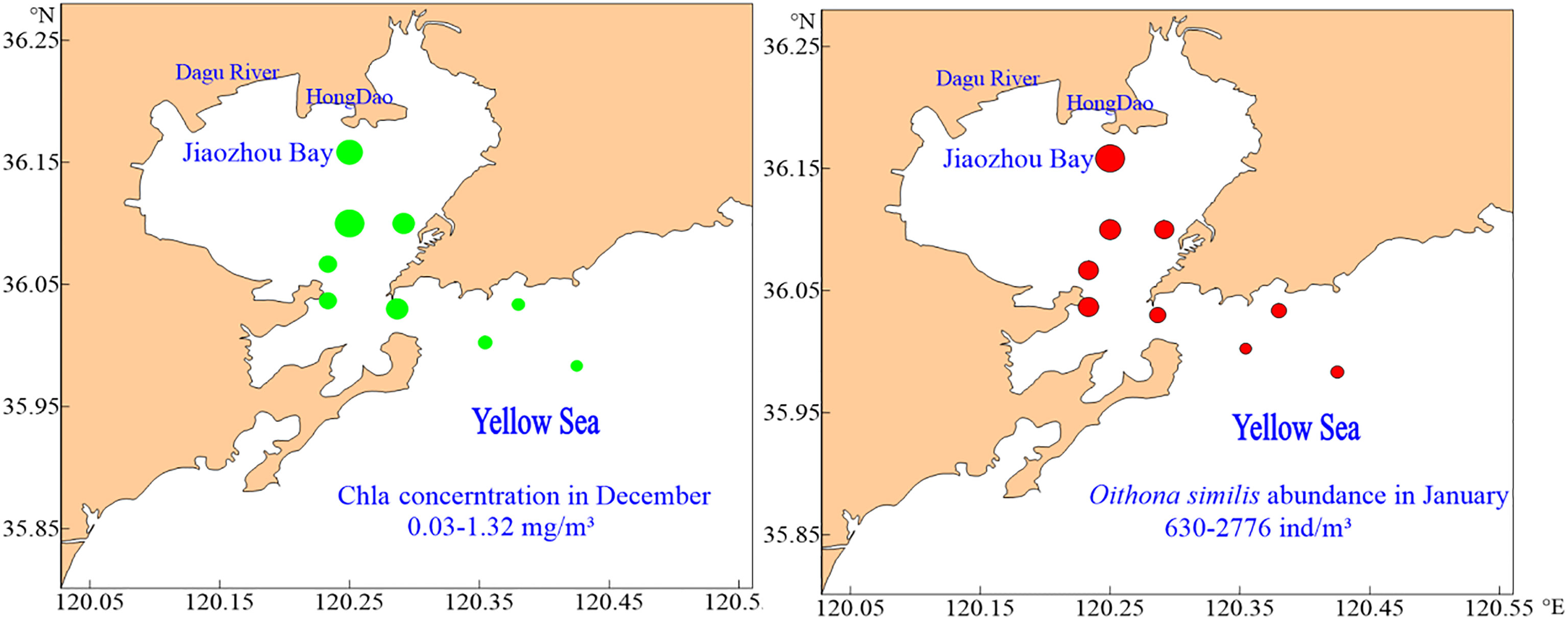
Figure 4 The spatial distribution of CHL a concentration and Oithona similis abundance in December and January in Jiaozhou Bay.
Annual variations of the O. similis EPR, female PL, CF, and clutch size in Jiaozhou Bay
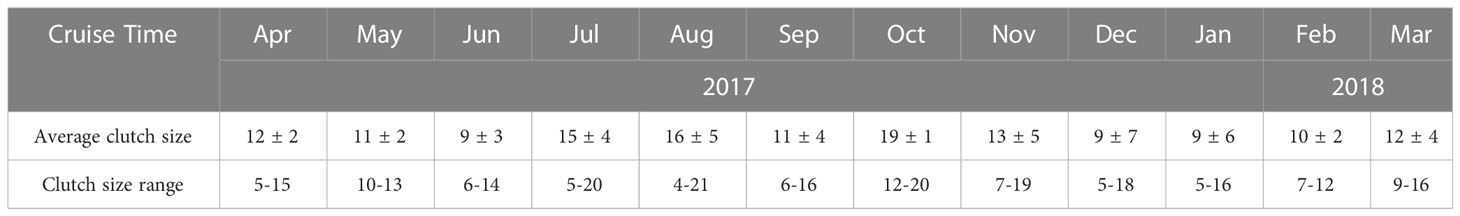
The EPR of O.similis varied monthly, with the highest EPR appearing in June (1.21 ± 0.25 eggs female-1 d-1), the lowest EPR in January (0.03 ± 0.02 eggs female-1 d-1), and the average EPR in other months varied between 0.11 ± 0.01 and 1.05 ± 0.12 eggs female-1d-1(Figure 5). We found that the average O. similis female PL ranged from 410 ± 6 μm to 472 ± 4 μm through the year (Figure 5), with the highest PL values in February 2018 and the lowest values in August 2017. The carbon mass value of females varied between 0.35 ± 0.05 and 0.43 ± 0.03 μg c female-1. The trend was similar to that of the female PL values. Both the O. similis female PL value and female carbon mass value were significantly negative correlated with temperature (P<0.01).The clutch size of O.similis fluctuated between 4–21 (Table 2). We did not find any relationship between O.similis clutch size and other environment factors such as temperature and Chl a concentration.
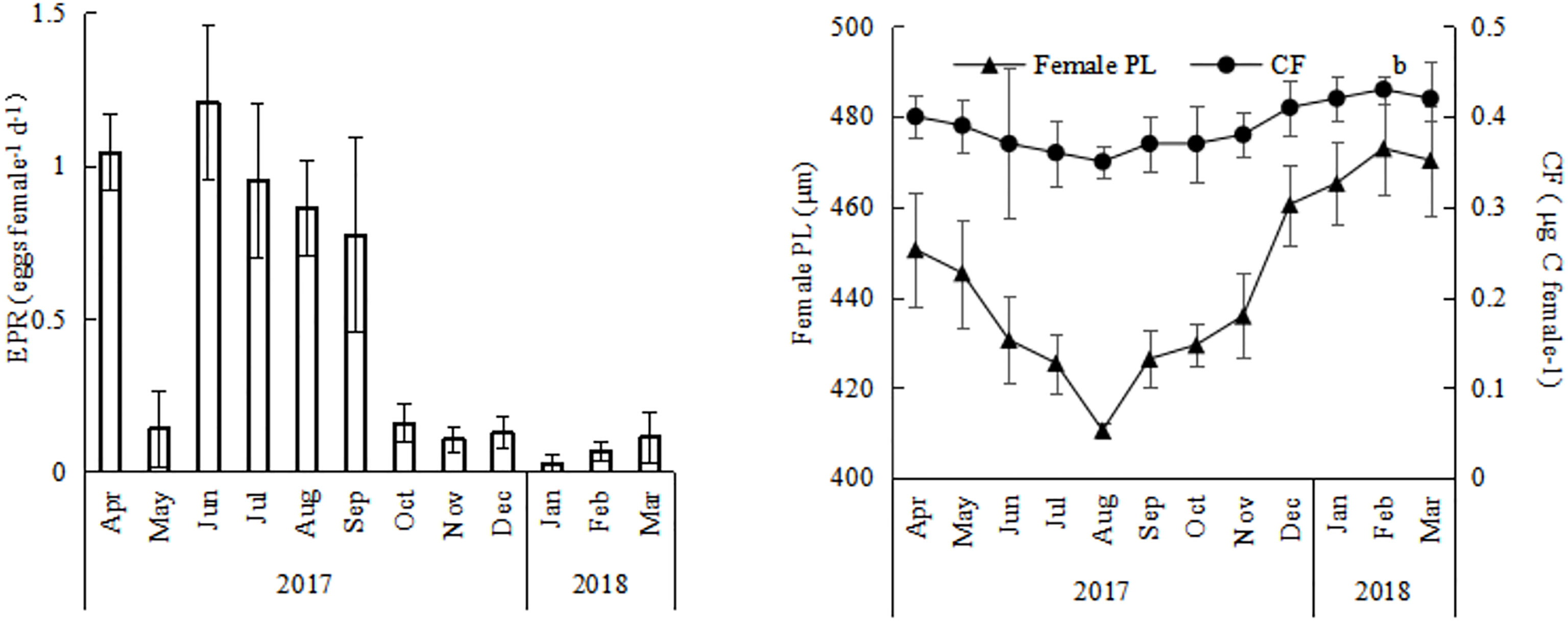
Figure 5 Annual variations of EPR, female PL, CF of Oithona similis in Jiaozhou Bay, vertical bars represent standard deviation (SD).
Annual compostion of O. similis community in Jiaozhou Bay
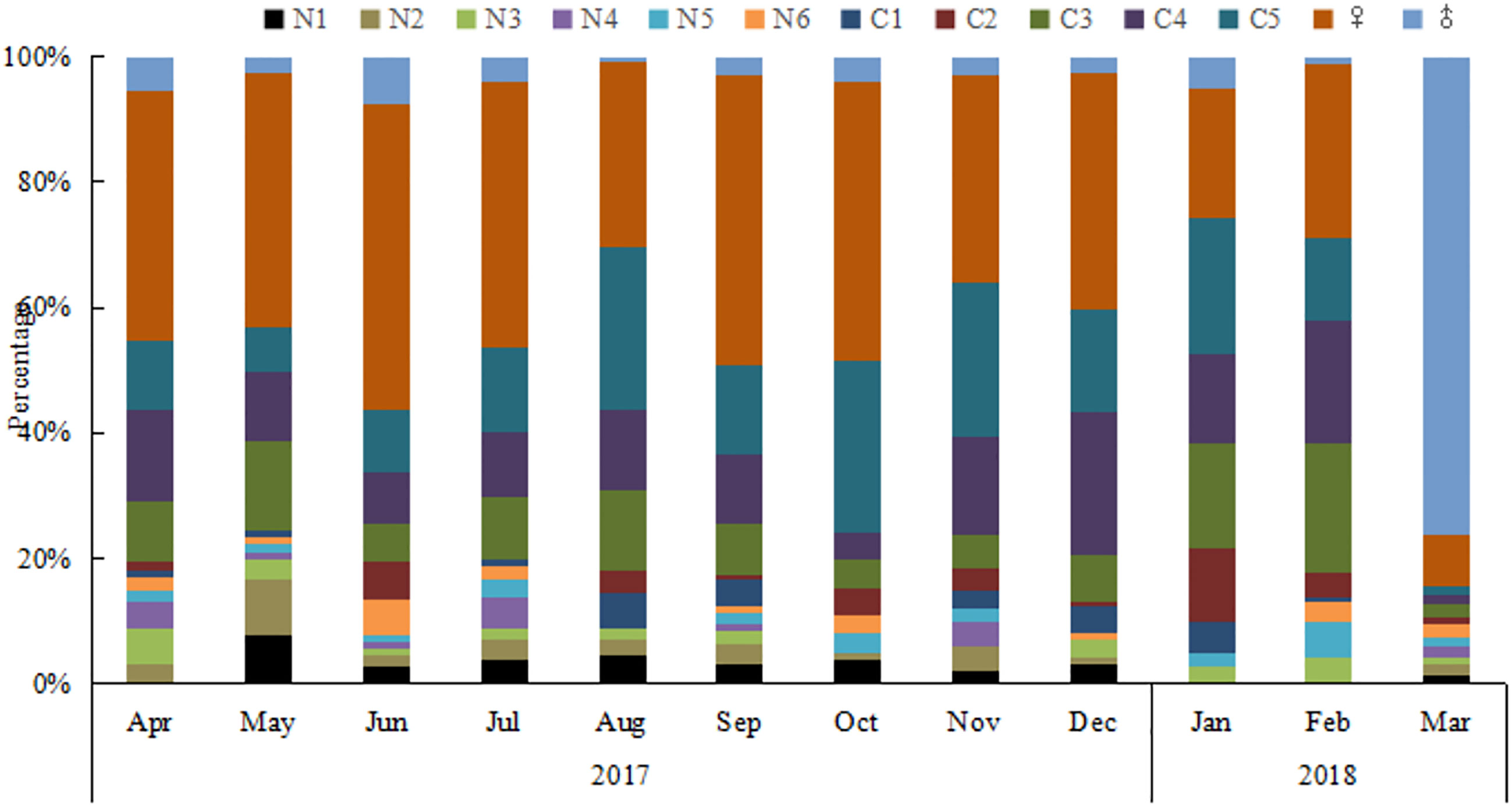
The number of O. similis from naupliar (N1-N6), copepodite (C1-C5), and adults (female and male) were counted separately. Individuals appeared at almost all stages throughout the year (Figure 6). The proportion of females was high throughout the year (21%–55%), with the highest value in June. The monthly sex ratio of O. similis was shown in the Table 3. The sex ratio of O. similis≥ 0.12 occurred in April, June, November and February of the next year. Nauplii proportion were relatively high in May(21%), July(19%) throughout the year.
Growth and development time of O. similis at different temperatures
In laboratory culture experiments, we setted up six temperature gradients (0, 5, 10, 15, 20, and 25°C). The results indicated that the span of the life cycle of O.similis after fertilization decreased with increasing temperature. The development time of O. similis lasted nearly 65 d at 0°C (Table 4). When the incubation temperature varied from 5 to 20°C, the development time lasted from 30 d to 50 d. With the incubation temperature increased to 25°C, the development period was reduced to 22 d.
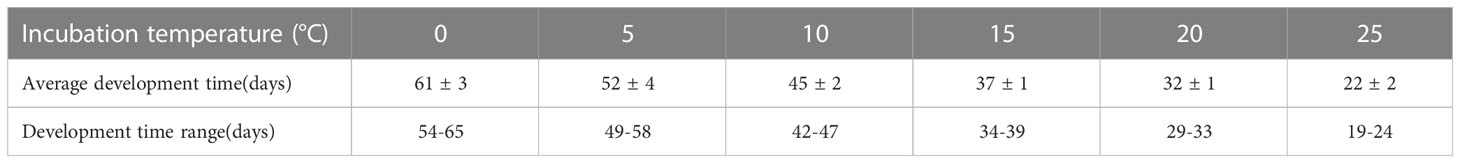
Table 4 The development time of O. similis at different temperature in laboratory culture experiment.
The life history of O. similis in Jiaozhou Bay
Based on the obtained results, the proportions of females was high and individuals appeared at almost all stages throughout the year. We believed that O. similis reproduced actively throughout the year without dispause in Jiaozhou Bay. In Spring, with the increasing sea temperature and Chl a concentration, O. similis EPR increased. The less incubation time make the number of O. similis increased over time. O. similis abundance minor peak appeared (740 ± 320 ind/m³) in May. When summer arrived, although there was no significant reduction of O.similis EPR, O. similis abundance reached the lowest value for the high temperature(>25°C) and low salinity (<30).When the autumn coming, the sea water temperature fell below 20°Cin November, O.similis abundance began to increase. In Winter, Chl a concentration first fell to 0.47 ± 0.40 mg/m³ in December, then arised to 1.83 ± 0.62 mg/m³ in January in next year, without food limitation, O.similis abundance reached the major peak in January (1472 ± 206 ind/m³), while the O. similis ERP fell to the lowest value for the cold weather. The value of sex ration≥ 0.12 was used as a determinant of reproduction events as characteristic for the female-skewed Oithonidae family. In Jiaozhou Bay, the sex ratio ≥ 0.12 occurred in April, June, November and February of the next year. We inferred that four generation formed in the whole year.
Discussion
Annual variations of O. similis abundance and environmental factors in Jiaozhou Bay
The abundance of O. similis in Jiaozhou Bay was characterized by bimodal cycles, with the major peak in January (1472 ± 206 ind/m³) and the minor one in May (740 ± 320 ind/m³). This bimodal cycle was in accordance with a previous study in the White and Barents sea (Dvoretsky, 2007b; Dvoretsky and Dvoretsky, 2009a) in the Southern Ocean (Fransz and Gonzalez, 1995). In our study, we found that the O. similis abundance was not correlated with SST, except when the temperature range was to the edge of its thermal range. In previous studies, temperature was an important driving dynamic for O. similis populations (Ward and Hirst, 2007; Dvoretsky and Dvoretsky, 2009b; Castellani et al., 2016), in terms of both abundance and biomass (Castellani et al., 2007). That study found that when the SST>19°C, O.similis abundance was limited (Castellani et al., 2005a). The basal metabolic rate of O. similis and other poikilotherms generally increases with temperature up to a maximum after which the organism dies. Increases in metabolic cost for basic organismal maintenance as a result of temperature increases means that less of the energy ingested or stored by the copepod can be allocated to reproduction and growth (Alcaraz et al., 2014). However, the importance of temperature in driving population abundance dynamics may depend on whether the temperature range was to the edge of the thermal range. Our study also confirmed this issue: from November to June in 2017 in Jiaozhou Bay, the SST was less than 20°C, gradually rising from July to October and being especially high in August where it exceeded 25°C (Figure 2). O.similis abundance decreased from the minor peak of 740 ± 320 ind/m³ ind/m³ to the lowest abundance of 16 ± 15 ind/m³in August. It’s worthing mentioned that, the depth of sea water in Jiaozhou Bay was about 10m-20m, so we used SST instead of mean values for the sampling layer. This might also influence our results.
Salinity was another factor that may affect the abundance of O. similis. All stages of O. similis were found to avoid low salinity in the Bornholm Basin in the Baltic Sea (Hansen et al., 2004), Aricic Kongsfjorden (Lischka and Hagen, 2005), and Hylsfjord in Norway (Nielsen and Andersen, 2002). In Jiaozhou Bay, the salinity level was around 29 ± 1‰ to 31 ± 0.4‰ throughout the year, except in August when rainfall was heavy and salinity decreased from 31 ± 0.2‰ in July to 29 ± 1.1‰ in August at some stations. Hence, the lowest abundance of O.similis in August was most likely a consequence of both high temperatures and low salinity.
In our study, O. similis abundance had no relationship with Chl a concentration. O. similis can exploit a wide range of prey sizes and prefers motile prey such as ciliates and dinoflagellates. Moreover, O. similis can survive with a relatively low metabolic rate, which allows it to prosper at food concentrations that would be limiting for other copepod species (Castellani et al., 2005b). However, in some regions, such as the North-East Atlantic and Mediterranean, Oithona similis abundance was positively related to Chl a concentration at relatively low Chl a concentrations (Castellani et al., 2016). In our study, the abundance of O. similis decreased from inner zone sites to outer zone sites in January, which was consistent with Chl a concentrations in December (Figure 4). It may be that food had a delayed effect on O. similis abundance. Meanwhile, the abundance of O. similis in Jiaozhou Bay was highest for the year in January, with food consumption became a limiting factor only at that time. With the Chl a concentration increased in January and February, food limitation disappeared.
We found O. similis abundance was negatively correlated with zooplankton abundance which sampled using 500μm mesh during our investigation time in Jiaozhou Bay (Table 1). This illustrated that predation was one of the factors in regulating O. similis abuncance in Jiaozhou Bay.
Annual variations of O. similis EPR and environmental factors in Jiaozhou Bay
The EPR of O. similis varied seasonally, with the highest EPR appearing in summer (June:1.21 ± 0.25 eggs female-1 d-1). EPR was mainly determined by temperature, female abundance, and egg abundance. In a previous study, scholars found that O. similis EPR was greatly determined by hatching time, which was at the same time dictated by temperature (Drif et al., 2010). Higher temperatures increase rates of embryonic development and consequently reduce hatching time. (Nielsen et al., 2002). In our study, the early EPR peak in April in 2017 possibly resulted from an accelerated development of the spring cohort due to the rapid rate of seasonal warming (Figure 5). In winter, when the temperature in the environment fell below 1°C, the EPR of O. similis dropped to 0.03 ± 0.02 eggs female-1d-1.
Food availability has been considered one of the most important factors in driving feeding and egg production rates in calanoid copepods (Kiørboe and Nielsen, 1994; Saiz and Calbet, 2011). At regions of high latitude, EPR was significantly and positively related to Chl a concentrations. Food limitation is the main reason why environmental variability can influence energy allocation in organisms, with the investment of energy under stressful conditions going towards survival, resulting in reduced fecundity (Kiørboe et al., 2015). In our study, we did not find a relationship between EPR and Chl a concentration, However, an increase in egg production was observed in June during the post-bloom phase (Figure 5), very likely due to a delay between coupling of food availability (which increased during the spring bloom) and EPR. These observations are in agreement with studies that covered a longer part of the reproductive season for O. similis and reported reproductive peaks in summer or early autumn (Lischka and Hagen, 2007; Madsen et al., 2008; Dvoretsky and Dvoretsky, 2009b).
In our study, the annual EPR and adult abundance maxima of O.similis were decoupled. Decoupled EPR and abundance seasonality has previously been reported for O.similis in the Arctic (Lischka and Hagen, 2005). EPR is a poor predictor of abundance in later developmental stages. A mismatch between seasonality in egg production and egg viability can lead to eggs being produced in sub-optimal conditions for peak egg fitness, with negative consequences on recruitment success (Varpe et al., 2007). Rate of maturation from egg to adult should determine the time period between maximum reproductive output and increased adult abundance, assuming high recruitment success of the population. The fact that adult female abundance did not increase until long after the period of maximum EPR, despite their relatively short development times, confirms that there are indeed other factors, such as mortality and advection (Irigoien and Harris, 2003; Hirst et al., 2007), influencing copepod abundance. Ohman and Hirche (2001) presented evidence for density-dependent mortality in an oceanic population of Calanus finmarchicus, whereby egg mortality rates were a function of adult female and copepodite abundance. Likewise, density dependence in egg mortality rates, with higher mortality observed at higher adult densities, has been reported for the Calanus helgolandicus population at station L4 (Hirst et al., 2007). Thus predation, by cannibalism or from other species, combined with egg hatching success (Maud et al., 2015), may also contribute to decoupled seasonality in egg production and copepod abundance. In Jiaozhou Bay, when the temperature was higher than 20°C, the conditions were good for egg hatching of O. similis, but adult survival was poor. Together with the predation and mortality factors, EPR and adult abundance maxima of O. similis were decoupled. This result is consistent with previous study.
The relationship between environment factors and O. similis female PL, CF, clutch size
In the present study, O. similis female PL values and CF values were significantly negative correlated with temperature through the year(P<0.01) (Table1). In previous study, the authors also found that O.similis adult female body size conformed to the temperature size rule (Atkinson, 1994), decreasing from a maximum in spring following the low winter temperatures to a minimum in autumn after the annual temperature maximum (Cornwell et al., 2018). The basal metabolic rate of O. similis and other poikilotherms generally increases with temperature, so less energy is allocated to body length growth. So a decline in PL and CF value was found in July-September (Figure 5) in Jiaozhou Bay. O. similis clutch size did not show a systematic trend in Jiaozhou Bay. We also did not find a relationship between O.similis clutch size and female PL. For most copepods, especially broadcast spawners such as Acartia clausi, Temora longicornis, and Centropages hamatus, clutch size could be highly predictable from female size (Halsband and Hirche, 2001). However, for O. similis, the body size of females was not an important factor. The lifespan of O. similis females can be up to 50 d, so the female mean prosome length is the product of various overlapping generations and of adults of different ages (Nancy et al., 2009). The age of an adult has a profound impact on its clutch size (Kimoto et al., 1986). However, we did not find any relationship between clutch size and other environmental factors such as temperature and Chl a concentration. Temperature only influences O. similis hatching time (Drif et al., 2010). Microzooplankton can play a greater role in its diet (Castellani et al., 2008), which may explain the lack of a relationship between O. similis clutch size and Chl a concentration. This concurs with the findings of previous studies (Dvoretsky and Dvoretsky, 2009a; Temperoni et al., 2011).
The annual composition of O. similis community and the approximate generations in Jiaozhou Bay
Individuals of O. similis appeared at almost all stages throughout the year (Figure 6).The proportion of female adults was high throughout the year (21%–55%). Hence, our data on O. similis support the concept of continuous reproduction to maintain a stable population structure with a wide stage distribution, as suggested previously (Fransz, 1988). According to the results of our laboratory culture experiment, the life cycle of O. similis lasted from 22 ± 2 d to 61 ± 3 d at different temperatures (Table 4). The incubation time was shorten with the increasing temperature. According to the sex ratio of O. similis and other factors, we inferred that O.similis produced four generations in Jiaozhou Bay, they were in April, June, November and January of the next year. In spring,with the increasing temperature, EPR valaue and female propotion increased in April, the minor O. similis abundance peak in May resulted from a new generation. In June, EPR was the highest in the whole year, Nauplii occupied 19% of the whole O. similis abundance in July. A new generation formed. From August to October, SST was >20°C, O. similis abundance decreased with the high temperature. In November, with the declining temperature and relatively high Chl a concentration, O. similis which was influenced by high temperature from August to October formed a new generation. The developmental time of the Winter (November)generation was about 2 months with relatively lower temperature.The major peak of O. similis adult abundance occurred, a new generation formed in January. Nauplii proportion increased to 12% in February.
The comparisons of O. similis traits with other regions
O.similis is one of the most common, often dominating copepods in artic and subartic regions. (Dvoretsky and Dvoretsky, 2009b, 2011; Balazy et al., 2021).The annual cycle investigation of O. similis population dynamics were mainly conducted in north-western Svalbard waters (Lischka and Hagen, 2005), Isfjorden(Balazy et al., 2021), and Barents sea (Dvoretsky and Dvoretsky, 2009a). In north-western Svalbard, there were two peaks of O. similis abundance in the whole year,the maximum abundance was in November (704,633 ind/m-2), the minor one was in February(about 300000ind/m-2), the other researchers found that the two peaks of O. similis were in July and September in Barents Sea(560 ind/m³) and Arctic Isfjorden (about 4500 ind/m³). In arctic and subarctic regions, the temperature was around 0°C in most month, even lower than 0°C in Winter. The maximum abundance was mainly determined by temperature which influence the development rate of the Oithona spp. In north-western Svalbard waters, the temperature was above 8°C in November, the relatively high value in the whole year. In Barents Sea and Arctic Isfjorden, the temperaure was also relatively high in June and September (>5°C). High abundance of O. similis observed in September in Arctic Isfjorden may also be related to the presences of its preferred food (ciliates/heterotrophic protists) after periods of high primary production. Recent studies have indicated that during the periods of complete darkness in the arctic winter when primary production was close to zero. We concluded that food and temperature were the main factors which influenced O. similis abundance peak in Arctic and Subarctic areas. In Jiaozhou Bay, O. similis abundance was also characterized by bimodal cycles, one was in January(1472 ± 206 ind/m³), another was in May (740± 320 ind/m³). For the different mesh size and different calculation of O. similis abundance (including nauplii or not) in the investigations of different region, the peak value of O. similis abundance was not comparable. Different with the arctic and subarctic regions, in temperate region of Jiaozhou Bay, the lower abundance was mainly affected by high temperature (>20°C) and low salinity(29‰) in August. Predation from large size zooplankton was another factor in regulating O. similis abundance.
EPR range was around 0-4 eggs female-1 day-1 in most studies carried out in arctic waters and subarctic areas, except some report in warmer areas (5.2, 5.6, and 6 eggs female-1 day-1. (Uye and Sano, 1995; Nielsen and Sabatini, 1996; Temperoni et al., 2011). The highest value mostly occurred in Summer. In general, reproductive characteristics of Oithona spp.in high-latitude environments have reported temperature as a major factor limiting their fecundity (Metz, 1995; Ward and Hirst, 2007; Dvoretsky and Dvoretsky, 2009a; Dvoretsky and Dvoretsky, 2009b). In Jiaozhou Bay, EPR varied between 0.03 ± 0.02 and 1.21 ± 0.25 eggs female-1 d-1. To some extent, EPR value was increased with temperature except for high value (>20°C) in August to October. The EPR peak value(1.21 ± 0.25 eggs female-1 day-1) in June was lower than in high latitude region. During our investigation, Noctiluca scientillans bloom occurred in April, large number of O.similis eggs and nauplii might be eaten by Noctiluca scientillans, that might influence the peak value of EPR in June. The PL of O.similis in Jiaozhou Bay ranged from 410 ± 6 μm to 472 ± 4 μm, lower than in arctic and subarctic regions, such as in the Barent sea (mean value:482μm), in White Sea (460-470μm), in Okhotsk Sea(520–530 μm) for the reason of relatively high average temperature (Dvoretsky and Dvoretsky, 2009a, 2009b). Because of smaller body length, the clutch size (4-21) of O. similis in Jiaozhou Bay was also lower than O.similis in high-latitude regions, such as 18-29 in Barent sea (Dvoretsky and Dvoretsky, 2009a), 14-38 in Western Greenland (Zamora-Terol et al., 2013).
In high-latitude regions, two generations were found with two abundance peaks in Barent sea (July and September) (Dvoretsky and Dvoretsky, 2009a), In Kongsfjorden (May-June, August-September)(Lischka and Hagen, 2005) and in Canadian Arctic waters (Hopcroft et al., 2005). In seas with harsher conditions, such as the White and Laptev seas, there is only one new generation per year (Dvoretsky, 2007a, 2007b). In these areas, temperatures and salinity influenced population dynamics and generations times. Elevated temperatures can accelerate development of O. similis and increase the number of generations in a year. Ice cover was usually present during most of the year although intensive melting takes place in spring in high latitude regeion. The lower salinity result from melting ice prolonged the time of O. similis abundance peak. In the Japan Sea, O. similis populations produced at least four to five generations during the year (at temperature range from 6°Cto 22°C (Kasyan, 2001). In the warm Andaman Sea (Thailand waters), Oithona spp.produced five generations in all seasons (at 15°C). If water were warmer, then the number of generations occurring with in a year increases. In Jiaozhou Bay, O. similis produced four generations (at temperature range from 3°C to 27°C). It was mostly determined by temperature and predation, partly influenced by salinity and Noctiluca scientillans bloom in particular month.
Conclusion
In typical temperate regions at mid-latitude, as represented by Jiaozhou Bay, the abundance of O. similis is characterized by bimodal cycles, with the major peak (1472 ± 206 ind/m³) in winter and the minor peak (740 ± 320 ind/m³ ind/m³) in spring. O. similis inhabiting in Jiaozhou Bay is not subject to food limitations except in January when their abundance is particularly high. Their lower abundance was mainly affected by temperature (>20°C) and low salinity. Predation from large size zooplankton was another factor in regulating O. similis abuncance. The EPR of O. similis ranged from 0.03 ± 0.02 to 1.21 ± 0.25 eggs female-1d-1 over the year and reproduced actively throughout the year without dispause. O. similis female PL values (410 ± 6–472 ± 4 μm) and CF values (0.35 ± 0.05–0.43 ± 0.03 μg c female-1) were significantly negative correlated with temperature through the year (P<0.01). The clutch size of O. similis varied from 4 to 21, and it did not related to other environmental factors. According to the sex ratio of O. similis and other factors, we inferred that O. similis produced four generations of per year in Jiaozhou Bay.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JY: Conceptualization, Methodology, Formal analysis and Writing Original Draft preparation. CW and PS: Methodology, Sampling design and field performance, Validation and Visualization. AW and ZT: Data Curation, Investigation, Validation and Resources. YW: Conceptualization, Methodology, Writing - Reviewing and Editing. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (No. 42090044, 41506153, 41976114) and Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA23050301).
Acknowledgments
The environmental data were shared by Jiaozhou Bay Marine Ecosystem Research station. We thank the crew on the research vessel of RV Chuang Xin in Jiaozhou Bay for their support of the field sampling.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alcaraz M., Felipe J., Grote U., Arashkevich E., Nikishina A. (2014). Life in a warming ocean: Thermal thresholds and metabolic balance of Arctic zooplankton. J. Plankton Res. 36, 3–10. doi: 10.1093/plankt/fbt111
Arima D., Yamaguchi A., Abe Y., Matsuno K., Saito R., Asami H., et al. (2014). Seasonal changes in body size and oil sac volume ot the three planktonic copepods, Paracalanus parvus (Claus,1863), Pseudocalanus newmani (Frost,1989) and Oithona similis(Claus,1866), in a temperate embayment: What controls their seasonality? Crustaceana 87 (3), 364–375. doi: 10.1163/15685403-00003287
Ashjian C. J., Campbell R. G., Welch H. E., Butler M., Van Keuren D. (2003). Annual cycle in abundance, distribution, and size in relation to hydrography of important copepod species in the western Arctic ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 50, 1235–1261. doi: 10.1016/S0967-0637(03)00129-8
Atkinson D. (1994). Temperature and organism size: A biological law for ectotherms? Adv. Ecol. Res. 25, 1–58. doi: 10.1016/S0065-2504(08)60212-3
Balazy K., Boehnke. R., Trudnowska E., Søreide Janne E., BłachowiakSamołyk K. (2021). Phenology of oithona similis demonstrates that ecological flexibility may be a winning trait in the warming Arctic. Sci.Rep 11, 18599. doi: 10.1038/S41598-021-98068-8
Besiktepe S., Kurt T. T., Gubanova A. (2022). Mesozooplankton composition and distribution in İzmir bay, Aegean Sea: With special emphasis on copepods. Reg. Stud. Mar. Sci 55, 102567. doi: 10.1016/j.rsma.2022.102567
Castellani C., Irigoien X., Harris R. P. (2005b). Feeding and egg production of oithona similis in the north Atlantic. Mar. Ecol. Prog. Ser. 288, 173–182. doi: 10.3354/meps288173
Castellani C., Irigoien X., Harris R. P., Holliday N. P. (2007). Regional and temporal variation of Oithona spp. biomass, stage structure and productivity in the irminger Sea, north Atlantic. J. Plankton Res. 29, 1051–1070. doi: 10.1093/plankt/fbm079
Castellani C., Irigoien X., Mayor D. J., Harris R. P., Wilson D. (2008). Feeding of Calanus finmarchicus and Oithona similis on the microplankton assemblage in the irminger Sea, north Atlantic. J. Plankton Res. 30, 1095–1116. doi: 10.1093/plankt/fbn074
Castellani C., Licandro P., Fileman E., Di Capua I., Mazzocchi M. G. (2016). Oithona similis likes it cool: evidence from two long-term time series. J. Plankton Res. 38, 703–717. doi: 10.1093/plankt/fbv104
Castellani C., Robinson C., Smith T., Lampitt R. S. (2005a). Temperature affects respiration rate of. Oithona similis. Mar. Ecol. Prog. Ser. 285, 129–135. doi: 10.3354/meps285129
Checkley D. M. J. (1980). The egg production of a marine planktonic copepod in relation to its food supply: Laboratory studies. Limnol. Oceanogr. 25, 430–446. doi: 10.4319/lo.1980.25.3.0430
Cornwell L. E., Fileman E. S., Bruun J. T., Hirst A. G., Tarran G. H., Findly H. S, et al. (2020). Resilience of the copepod Oithona similis to climatic variability: Egg production, mortality, and vertical habitat partitioning. Front. Mar. Sci. 7 (29), 1–15. doi: 10.3389/FMARS.2020.00029
Cornwell L. E., Findlay H. S., Fileman E. S., Smyth T. J., Hirst A. G., Bruun J. T., et al. (2018). Seasonality of Oithona similis and Calanus helgolandicus reproduction and abundance: contrasting responses to environmental variation at a shelf site. J. Plankton Res. 40, 295–310. doi: 10.1093/plankt/fby007
Drif K., Hirst ,. A. G., Hay S. (2010). Seasonal abundance and egg production rates of Oithona similis and Pseudocalanus elongatus in the northern north Sea: a first comparison of egg-ratio and incubation methods. Mar. Ecol. Prog. Ser. 415, 159–175. doi: 10.3354/meps08748
Dvoretsky V. G. (2007a). Characteristics of the Oithona similis (Copepoda: Cyclopoida) in the white and barents seas. Dokl. Biol. Sci. 414, 223–225. doi: 10.1134/S0012496607030167
Dvoretsky V. G. (2007b). Peculiarities of population structure of Oithona similis (Copepoda: Cyclopoida) in the white and barents seas. Dokl. RAN 414 (4), 1–4. doi: 10.1134/S0012496607030167
Dvoretsky V. G., Dvoretsky A. G. (2009a). Life cycle of Oithona similis (Copepoda: Cyclopoida) in kola bay (Barents Sea). Mar. Biol. 156, 1433–1446. doi: 10.1007/s00227-009-1183-4
Dvoretsky V. G., Dvoretsky A. G. (2009b). Spatial variations in reproductive characteristics of the small copepod. Oithona similis Barents Sea.Mar. Ecol. Prog. Ser. 386, 133–146. doi: 10.3354/meps08085
Dvoretsky V. G., Dvoretsky A. G. (2011). The mortality of the planktonic copepod Oithona similis Claus, 1866 (Copepoda: Cyclopoida) in the Barents and White seas. Russ. J. Mar. Biol 37, 123–131. doi: 10.1134/s106307401102005
Eiane K., Ohman M. D. (2004). Stage-specific mortality of Calanusfinmarchicus, Pseudocalanus elongatus and Oithona similis on fladen ground, north Sea, during a spring bloom. Mar. Ecol. Prog. Ser. 268, 183–193. doi: 10.3354/meps268183
Fransz G. P. (1988). Vernal abundance, structure and development of epipelagic copepod populations of the eastern weddell Sea (Antarctica). Polar Biol. 9, 107–114. doi: 10.1007/BF00442037
Fransz H. G., Gonzalez S. R. (1995). The production of Oithona similis (Copepoda: Cyclopoida) in the southern ocean. ICES J. Mar. Sci. 52, 549–555. doi: 10.1016/1054-3139(95)80069-7
Halsband C., Hirche H. J. (2001). Reproductive cycles of dominant calanoid copepods in the north Sea. Mar. Ecol. Prog. Ser. 209, 219–229. doi: 10.3354/meps209219
Hansen F. C., Möllmann C., Schütz U., Hinrichsen H. H. (2004). Spatio temporal distribution of Oithona similis in the bornholm Basin(Central Baltic Sea). J. Plankton Res. 26 (6), 659–668. doi: 10.1093/plankt/fbh061
Hirst A. G., Bonnet D., Harris R. P. (2007). Seasonal dynamics and mortality rates of Calanus helgolandicus over two years at a station in the English channel. Mar. Ecol. Prog. Ser. 340, 189–205. doi: 10.3354/meps340189
Hopcroft R. R., Clarke C., Nelson R. J., Raskoff K. A. (2005). Zooplankton communities of the arctic’s Canada basin: The contribution by smaller taxa. Polar. Biol. 28, 198–206. doi: 10.1007/s00300-004-0680-7
Irigoien X., Harris R. P. (2003). Interannual variability of Calanus helgolandicus in the English channel. Fish. Oceanogr. 12, 317–326. doi: 10.1046/j.1365-2419.2003.00247
Jean-jose J., Lincy A., Lipton A. P., Chandran A. (2016). Developmental stages observed during experimental culture of the egg bearing cyclopoid copepod Oithona similis (Claus 1866). Indian.J. Geo-Mar. Sci. 45 (2), 333–337.
Kasyan V. V. (2001). Distribution and dynamics of Oithona similis Claus (Copepoda: Cyclopoida) in the amur bay of the Japan Sea. Izv.TINRO 139, 271–281.
Kiørboe T., Ceballos S., Thygesen U. H. (2015). Interrelations between senescence, life-history traits, and behavior in planktonic copepods. Ecology 96, 2225–2235. doi: 10.1890/14-2205.1
Kiørboe T., Nielsen T. G. (1994). Regulation of zooplankton biomass and production in a temperate, coastal ecosystem. I. Copepods. Limnol. Oceanogr. 39, 493–507. doi: 10.4319/lo.1994.39.3.0493
Kiørboe T., Sabatini M. (1994). Reproductive life cycle strategies in egg-carrying cyclopoid and free-spawning calanoid copepods. J. Plankton Res. 16, 1353–1364. doi: 10.1093/plankt/16.10.1353
Kimoto K., Uye S. I., Onbé T. (1986). Egg production of a brackish-water calanoid copepod Sinocalanus tennellus in relation to food abundance and temperature. Bull. Plankton Soc.Japan. 33, 133–145.
Kiorboe T. (2006). Sex, sex-ratios, and the dynamics of pelagic copepod populations. Oecol 148, 40–50. doi: 10.1007/s00442-005-0346-3
Lischka S., Hagen W. (2005). Life histories of the copepods Pseudocalanus minutus, Pseudocalanus acuspes (Calanoida) and Oithona similis (Cyclopoida) in the Arctic kongsfjorden (Svalbard). Polar Biol. 28, 910–921. doi: 10.1007/s00300-005-0017-1
Lischka S., Hagen W. (2007). Seasonal lipid dynamics of the copepods Pseudocalanus minutus (Calanoida) and Oithona similis (Cyclopoida) in the Arctic kongsfjorden (Svalbard). Mar. Biol. 150, 443–454. doi: 10.1007/s00227-006-0359-4
Logerwell E. A., Ohman M. D. (1999). Egg-brooding, body size and predation risk in planktonic marine copepods. Oecologia 121, 426–431. doi: 10.1007/s004250948
Madsen S., Nielsen T., Hansen B. (2008). Annual population development and production by small copepods in disko bay, western Greenland. Mar. Biol. 155, 63–77. doi: 10.1007/s00227-008-1007-y
Maud J. L., Atkinson A., Hirst A. G., Lindeque P. K., Widdicombe C. E., Harmer R. A., et al. (2015). How does Calanus helgolandicus maintain its population in a variable environment? analysis of a 25-year time series from the English channel. Prog. Oceanogr. 137, 513–523. doi: 10.1016/j.pocean.ocean.04.028
Metz C. (1995). Seasonal variation in the distribution and abundance of Oithona and Oncaea species (Copepoda, Crustacea) in the south eastern weddell Sea, Antarctica. Polar Biol. 15, 187–194. doi: 10.1007/BF00239058
Metz C. (1996). Life strategies of dominant Antarctic Oithonidae (Cyclopoida, copepoda) and Oncaedae (Poecilostomatoida, copepoda) in the bellingshausen Sea. Ber. Polarforsch. 207, 123.
Nancy F. S., Gasparini S., Petersen F., Mayzaud P. (2009). Seasonal and individual variability of lipid reserves in Oithona similis (Cyclopoida) in Arctic fjord. Polar Biol. 32 (2), 233–242. doi: 10.1016/j.polar.2012.09.001
Nielsen T. G., Andersen C. M. (2002). Plankton community structure and production along a freshwater-influenced Norwegian fjord system. Mar. Biol. 141 (4), 707–724. doi: 10.1007/s00227-002-0868-8
Nielsen T. G., Møller E. F., Satapoomin S., Ringuette M., Hopcroft R. R. (2002). Egg hatching rate of the cyclopoid copepod Oithona similis in Arctic and temperate waters. Mar. Ecol. Prog. Ser. 236, 301–306. doi: 10.3354/meps236301
Nielsen T. G., Sabatini M. (1996). Role of cyclopoid copepods oithona spp. in north Sea plankton communities. Mar. Ecol. Prog. Ser. 139, 79–93. doi: 10.1016/j.polar2012.09.001
Ohman M. D., Hirche H. J. (2001). Density-dependent mortality in an oceanic copepod population. Nature 412, 638–641. doi: 10.1038/35088068
Saiz E., Calbet A. (2011). Copepod feeding in the ocean: Scaling patterns, composition of their diet and the bias of estimates due to microzooplankton grazing during incubations. Hydrobiologia 666, 181–196. doi: 10.1007/s10750-010-0421-6
Sun S., Li Y. H., Sun X. X. (2012). Changes in the small-jellyfish community in recent decades in jiaozhou bay, China. Chin. J. Oceanol. Limnol. 30 (4), 507–518. doi: 10.1007/s00343-012-1179-7
Takahashi T., Uchiyama I. (2007). Morphology of the naupliar stages of some Oithona species (Copepoda:Cyclopoida) occurring in toyama bay, southern Japan Sea. Plank. Benth. Res. 2 (1), 12–27. doi: 10.3800/pbr.2.12
Temperoni B., Viñas M. D., Diovisalvi N., Negri R. (2011). Seasonal production of oithona nana giesbrecht 1893.(Copepoda: Cyclopoida) in temperate coastal waters off Argentina. J. Plankton Res. 33, 729–740. doi: 10.1093/plankt/fbq141
Turner J. T. (2004). The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool. stud. 43, 255-266. uye, S.I.(1982). length–weight relationships of important zooplankton from the inland Sea of Japan. J. Oceanogr. Soc Jpn. 38, 149–158. doi: 10.1007/BF02110286
Uye S. I. (1982). Length–weight relationships of important zooplankton from the Inland Sea of Japan. J. Oceanogr. Soc. Jpn. 38, 149–158. doi: 10.1007/BF02110286
Uye S. I., Sano K. (1995). Seasonal reproductive biology of the small cyclopoid copepod oithona davisae in a temperate eutrophicinlet. Mar. Ecol. Prog. Ser. 118, 121–128. doi: 10.3354/meps118121
Varpe Ø., Jørgensen C., Tarling G. A., Fiksen Ø. (2007). Early is better: Seasonal egg fitness and timing of reproduction in a zooplankton life-history model. Oikos 116, 1331–1342. doi: 10.1111/j.0030-1299.2007.15893.x
Vetlichny L., Hubareva E., Khanaychenko A., Gubanova A., Altukhov D., Besiktepe S. (2016). Adaptive strategy of thermophilic Oithona davisae in the cold black sea environment. Turk. J. Fish. Aquat. Sci. 16, 77–90. doi: 10.4194/1303-2712-v16_1_09
Wang J., Li H. R., Chen H. J., Liu G. X., Zhuang Y. Y. (2021). Community characteristics of zooplankton in the yellow Sea and bohai Sea in spring 2014. Mar.Sci 46 (5), 17–29.
Ward P., Hirst A. G. (2007). Oithona similis in a high latitude ecosystem: abundance, distribution and temperature limitation of fecundity rates in a sac spawning copepod. Mar. Biol. 151, 1099–1110. doi: 10.1007/s00227-006-0548-1
Weydmann A., Carstensen J., Goszczko I., Dmoch K., Olszewska A., Kwasniewski S. (2014). Shift towards the dominance of boreal species in the Arctic: Inter-annual and spatial zooplankton variability in the West spitsbergen current. Mar. Ecol. Prog. Ser. 501, 41–52. doi: 10.3354/meps10694
Keywords: Oithona similis, abundance, egg production, community structure, generations, development time
Citation: Yin J, Wu C, Shen P, Wan A, Tao Z and Wang Y (2023) Annual variations of abundance, egg production rate, and community structure of Oithona similis in Jiaozhou Bay, the Yellow Sea. Front. Mar. Sci. 10:1119883. doi: 10.3389/fmars.2023.1119883
Received: 09 December 2022; Accepted: 10 February 2023;
Published: 24 February 2023.
Edited by:
Yang Liu, Ocean University of China, ChinaReviewed by:
Vladimir G. Dvoretsky, Murmansk Marine Biological Institute, RussiaIole Di Capua, Anton Dohrn Zoological Station Naples, Italy
Copyright © 2023 Yin, Wu, Shen, Wan, Tao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yantao Wang, d2FuZ3l0QHFkaW8uYWMuY24=
 Jiehui Yin
Jiehui Yin Cunchao Wu
Cunchao Wu Pingping Shen
Pingping Shen Aiyong Wan2
Aiyong Wan2 Zhencheng Tao
Zhencheng Tao Yantao Wang
Yantao Wang